Abstract
Background:
A recent Monographs Working Group of the International Agency for Research on Cancer concluded that there is limited evidence for a causal association between exposure to asbestos and stomach cancer.
Methods:
We performed a meta-analysis to quantitatively evaluate this association. Random effects models were used to summarise the relative risks across studies. Sources of heterogeneity were explored through subgroup analyses and meta-regression.
Results:
We identified 40 mortality cohort studies from 37 separate papers, and cancer incidence data were extracted for 15 separate cohorts from 14 papers. The overall meta-SMR for stomach cancer for total cohort was 1.15 (95% confidence interval 1.03–1.27), with heterogeneous results across studies. Statistically significant excesses were observed in North America and Australia but not in Europe, and for generic asbestos workers and insulators. Meta-SMRs were larger for cohorts reporting a SMR for lung cancer above 2 and cohort sizes below 1000.
Conclusions:
Our results support the conclusion by IARC that exposure to asbestos is associated with a moderate increased risk of stomach cancer.
Keywords: asbestos, meta-analysis, occupation, stomach cancer
The most recent IARC monograph on asbestos (Straif et al, 2009; IARC, 2011) concluded that all forms of asbestos (chrysotile, crocidolite, amosite, tremolite, actinolite and anthophyllite) are carcinogenic to humans (Group 1). They concluded that asbestos causes mesothelioma and cancer of the lung, larynx and ovary (Group1), and note that positive associations have been observed between asbestos and cancer of the pharynx, stomach and colorectum (group 2A). However, no quantitative estimates of these associations were carried out, except for ovarian cancer (Camargo et al, 2011).
We conducted a meta-analysis of the results on stomach cancer of cohort studies of workers exposed to asbestos, as part of our work estimating the burden of occupational cancer in the United Kingdom (Rushton et al, 2010). The present analysis was built on the US IOM report published in 2006 (IOM, 2006); we have updated their results and extended the analyses by gender and subcategory (geography, industry and type of asbestos).
Materials and methods
Literature search
A search of the literature was performed to find all published reports of asbestos-exposed cohorts according to the MOOSE guideline (Stroup et al, 2000). As stomach cancer was not generally the primary disease of concern in those studies, each paper was read and those reporting mortality from or incidence of cancer of the stomach were selected. Searches of Medline and Embase were conducted for papers published worldwide in English between 1964 and 2010. Only cohorts of workers with predominant exposure to asbestos were included. For example, although workers in the rubber industry are exposed to asbestos, the causal role of this specific carcinogen cannot be established (IARC, 1998). When several publications relating to the same cohort were available, we used the most recent report. References of identified papers were examined for additional relevant publications, and a check was made with previous reviews to ensure all cohorts were identified.
For each study, we extracted the following data (when the information was available): observed and expected numbers of cases due to stomach cancer and/or the SMR/SIR and its associated confidence interval (CI), the total number of cases, the lung cancer SMR/SIR, the dates when the study was carried out, inclusion and exclusion criteria, the comparison population, the percentage of men, the average duration of employment, the geographical area, the industry sector, the type of asbestos. For the studies that reported results based on latency period, latency periods were defined as the time since the first exposure or employment. We extracted both sets of results with and without latency.
Methods for quantitative syntheses
Overall pooled estimates of the SMR/SIR (meta-SMR/SIR) with associated 95% CI were obtained using random- and fixed-effects methods (Sutton et al, 2000). When not provided, 95% CI of SMR/SIR were obtained via Byar's approximation (Breslow and Day, 1987). For studies in which there were zero observed cases, 1 was added to both observed and expected cases. Sensitivity analyses to this approach were undertaken in which either studies with zero observed case were excluded from the analysis or the observed number of cases was set to equal to the expected number of cases (Alder et al, 2006).
A test for heterogeneity between study results was performed as a χ2-test with degrees of freedom equal to the number of studies minus one and associated P-value was reported. As this test is susceptible to the number of studies included in the meta-analysis, Higgins and Thompson (2002) developed an alternative approach that quantifies the effect of heterogeneity, providing a measure of the degree of inconsistency in the studies' results. This quantity I2 describes the proportion of total variation in study estimates that is due to heterogeneity. Negative values of I2 are put equal to zero so that I2 lies between 0 and 100%. A value of 0% indicates no observed heterogeneity and larger values show increasing heterogeneity. This quantity was also reported with its associated 95% CI; a value >50% was considered to indicate substantial heterogeneity (Higgins et al, 2003).
The influence of individual studies on the overall meta-SMR was assessed visually via radial plots, by re-estimating the overall effect omitting each study in turn. In addition, we used common influence diagnostics to highlight outlying influential studies (Viechtbauer and Cheung, 2010). Meta-regression techniques and stratified analyses were used to explore the influence of cohort and study characteristics. Publication bias was also assessed graphically with a funnel plot and by using Egger's test (Egger et al, 1997).
Analyses were performed separately for men and women, and for both genders combined. We also analysed the data according to the latency, that is, the time since the first exposure: studies were categorised as to whether they had carried out a lagged analysis or not, with the definition of a lagged category being an exposure lag of at least 10 years after the first exposure/employment. Separate subgroup analyses were carried out by geography (Europe, North America and Australia, China and Russia together) and by occupation/industry. The latter contained six categories as defined in the IOM reports (IOM, 2006): insulators, generic asbestos workers (where no occupation or industry was specified), textile asbestos workers, cement asbestos workers, miners and other occupations with substantial exposure to asbestos (such as shipyard workers). We also provided a pooled estimated by type of asbestos, sample size and publication year.
To analyse the dose–response effect of asbestos exposure, we used two different methods. The first one was based on the RR for the highest category of exposure, as the categories for the dose–response relationships were not comparable. In the second approach, studies were divided according to the magnitude of the lung cancer SMR (below or above 2), corresponding to low and high occupational exposure to asbestos. Lung cancer mortality/incidence was used as a substitute for the exposure measurements, because of the clear relationship between asbestos exposure and lung cancer (IARC, 2011).
All the analyses described above were carried out using the Metafor package (Viechtbauer, 2010) for R software.
Results
Characteristics of the studies
The literature search identified 70 references that contained potentially relevant information for the meta-analysis. Mortality was the outcome in most of the cohort studies reviewed. Data on mortality were extracted for 40 cohorts from 37 separate papers, and data on cancer incidence were extracted for 15 separate cohorts from 14 papers. Table 1 summarises the study characteristics. Unique cohorts are numbered 1–55.
Table 1. Study characteristics–mortality and incidence studies.
| ID | Reference (related papers) | O | Year | Country | Industry | Asbestos type | Gender | Employment | End of follow-up | No of subjects |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Selikoff 1979 (Selikoff et al, 1979) (Selikoff et al, 1964; Selikoff and Seidman, 1991; Selikoff et al, 1980) | M | 1979 | USA | Insulation workers (union) | Ch, Am | Men | Union before 1943 | 1976 | 632 |
| 2 | Acheson 1982. I (Acheson et al, 1982) | M | 1982 | UK | Manufacture of gas masks | Ch | Women | From 1939 | 1980 | 570 |
| 3 | Acheson 1982.I (Acheson et al, 1982) | M | 1982 | UK | Manufacture of gas masks | Cr | Women | From 1939 | 1980 | 757 |
| 4 | Acheson 1984 (Acheson et al, 1984) | M | 1984 | UK | Manufacture of insulation board | Am | Men | 1947–1978 | 1980 | 5969 |
| 5 | Olshon 1984 (Ohlson et al, 1984) | M | 1984 | Sweden | Railroad shop | Mixed | Men | 1939–1980 | 1980 | 3442 |
| 6 | Peto 1985.I (Peto et al, 1985) | M | 1985 | UK | Asbestos textile workers | Ch, Cr | Men | 1916–1983 | 1983 | 145 |
| 7 | Peto 1985.II (Peto et al, 1985) | M | 1985 | UK | Asbestos textile workers | Ch, Cr | Women | 1916–1983 | 1983 | 283 |
| 8 | Peto 1985.III (Peto et al, 1985) | M | 1985 | UK | Asbestos textile workers | Ch, Cr | Men | 1916–1983 | 1983 | 3211 |
| 9 | Gardner 1986 (Gardner et al, 1986) | M | 1986 | UK | asbestos cement factory | Ch | Men and women | 1941–1983 | 1984 | 2167 |
| 10 | Seidman 1986 (Seidman et al, 1986) | M | 1986 | USA | Insulation workers | Am | Men | 1941–1945 | 1985 | 820 |
| 11 | Amandus 1987 (Amandus and Wheeler, 1987) | M | 1987 | USA | Vermiculite miners and millers | Tr-Ac | Men | Until 1970 | 1981 | 575 |
| 12 | Enterline 1987 (Enterline et al, 1987) | M | 1987 | USA | Asbestos products company | Ch, Cr, Am | Men | 1941–1967 | 1980 | 1074 |
| 13 | Hughes 1987 (Hughes et al, 1987) | M | 1987 | USA | Asbestos cement factory | Ch, Cr, Am | Men | Until 1970 | 1982 | 6931 |
| 14 | Sanden 1987 (Sandén and Järvholm, 1987) | I | 1987 | Sweden | Shipyard workers | Ch | Men | 1977–1979 | 1983 | 3787 |
| 15 | Tola 1988 (Tola et al, 1988) | I | 1988 | Finland | Shipyard workers | Mixed | Men | 1945–1960 | 1981 | 7775 |
| 16 | Melkild 1989 (Melkild et al, 1989) | I | 1989 | Norway | Shipyard workers | Ch | Men | 1946–1977 | 1986 | 4778 |
| 17 | Raffn 1989 (Raffn et al, 1989) | I | 1989 | Denmark | Asbestos cement factory | Mixed | Men | 1928–1984 | 1984 | 7996 |
| 18 | Neuberger 1990 (Neuberger and Kundi, 1990) | M | 1990 | Austria | Asbestos cement factory | Ch, Cr | Men and women | 1950–1981 | 1987 | 2816 |
| 19 | Botta 1991 (Botta et al, 1991) | M | 1991 | Italy | Asbestos cement factory | Ch, Cr | Men and women | 1950–1980 | 1986 | 3367 |
| 20 | Selikoff 1991 (Selikoff and Seidman, 1991) (Selikoff et al, 1964; Selikoff et al, 1979; Selikoff et al, 1980) | M | 1991 | USA/Canada | Insulation workers (union) | Ch, Am | Men | In union 1967 | 1987 | 17 800 |
| 21 | Cheng 1992 (Cheng and Kong, 1992) | M | 1992 | China | Chrysolite asbestos products workers | Ch | Men and women | Present in 1972 | 1987 | 1172 |
| 22 | Sanden 1992 (Sandén et al, 1992) | M | 1992 | Sweden | Shipyard workers | Ch | Men | 1977–1979 | 1987 | 3893 |
| 23 | Danielsen 1993 (Danielsen et al, 1993) | I | 1993 | Norway | Shipyard production workers | Ch | Men | 1940–1979 | – | 4571 |
| 24 | Kogan 1993 (Kogan et al, 1993) | M | 1993 | Russia | Friction products | Ch | Men and women | – | 1988 | 2834 |
| 25 | Zhu 1993 (Zhu and Wang, 1993) | M | 1993 | China | Asbestos factory | Ch | Men and women | – | 1986 | 5893 |
| 26 | Meurman 1994 (Meurman et al, 1994) | I | 1994 | Finland | Asbestos miners | An | Men and women | 1953–1967 | 1991 | 903 |
| 27 | Liddell 1997 (Liddell et al, 1997) (McDonald et al, 1993; McDonald et al, 1980) | M | 1997 | Canada | Miners and millers | Ch | Men | – | 1992 | 9780 |
| 28 | Pang 1997 (Pang et al, 1997) | M | 1997 | China | Asbestos factory | Ch | Men and women | Until 1972 | 1994 | 530 |
| 29 | Levin 1998 (Levin et al, 1998) | M | 1998 | USA | Manufacture of asbestos pipe insulation | Am | Men | Alive in 1964 | 1993 | 1121 |
| 30 | Battista 1999 (Battista et al, 1999) | M | 1999 | Italy | Rail carriage construction and repair | Ch, Cr | Men | 1945–1969 | 1997 | 734 |
| 31 | Germani 1999 (Germani et al, 1999) | M | 1999 | Italy | Women compensated for asbestosis | Ch, Cr | Women | Alive in 1979 | 1997 | 631 |
| 32 | Karjalainen 1999.I (Karjalainen et al, 1999) | I | 1999 | Finland | Patients with asbestos-related pulmonary | Mixed | Men and women | 1964–1995 | 1995 | 1376 |
| 33 | Karjalainen 1999.II (Karjalainen et al, 1999) | I | 1999 | Finland | Patients with pleural fibrosis | Mixed | Men and women | 1964–1995 | 1995 | 4887 |
| 34 | Berry 2000 (Newhouse et al, 1985; Berry et al, 2000) | M | 2000 | UK | Asbestos factory | Ch, Cr, Am | Men and women | 1933–1964 1936–1942 | 1980 | 5100 |
| 35 | Szeszenia 2000 (Szeszenia-Dabrowska et al, 2000) | M | 2000 | Poland | Asbestos cement factory | Ch, Cr | Men | Until 1980 | 1991 | 2525 |
| 36 | Puntoni 2001 (Puntoni et al, 2001) | M | 2001 | Italy | Shipyard workers | Mixed | Men | 1960–1981 | 1996 | 3984 |
| 37 | LaProvote 2002 (de La Provôté et al, 2002) | I | 2002 | France | Manufacture fireproof textiles and friction materials | Mixed | Men and women | Until 1978 | 1995 | 1820 |
| 38 | Ulvestad 2002 (Ulvestad et al, 2002) | I | 2002 | Norway | Asbestos cement factory | Mixed | Men | 1942–1976 | 1999 | 541 |
| 39 | Szeszenia 2002 (Szeszenia-Dabrowska et al, 2002) | M | 2002 | Poland | Workers compensated for asbestosis | Mixed | Men | 1970–1997 | 1999 | 907 |
| 40 | Koskinen 2003 (Koskinen et al, 2003) | I | 2003 | Finland | Asbestos workers (screening campaign) | Mixed | Men and women | – | 1992 | 24 215 |
| 41 | Finkelstein 2004 (Finkelstein and Verma, 2004) | M | 2004 | Canada | Pipe trades workers (union) | Mixed | Men | From 1949 | 1999 | 25 285 |
| 42 | Reid 2004 (Armstrong et al, 1988; Reid et al, 2004) | I | 2004 | Australia | Crocidolite miners and millers | Cr | Men | 1943–1966 | 1999 | 5685 |
| 43 | Smailyte 2004 (Smailyte et al, 2004) | I | 2004 | Lithuania | Asbestos cement factory | Ch | Men and women | Until 1978 | 2000 | 1887 |
| 44 | Ulvestad 2004 (Ulvestad et al, 2004) | I | 2004 | Norway | insulation workers (union) | Ch, Am | Men | 1930–1975 | 1999 | 1116 |
| 45 | Wilczynska 2005 (Wilczynska et al, 2005) (Szeszenia-Dabrowska et al, 1991; Szeszenia-Dabrowska et al, 1988a; Szeszenia-Dabrowska et al, 1988b) | M | 2005 | Poland | Asbestos processing plant | Mixed | Men and women | 1945–1980 | 1999 | 4187 |
| 46 | Hein 2007 (Hein et al, 2007) (Brown et al, 1994; Dement et al, 1994; Dement et al, 1983) | M | 2007 | USA | Asbestos textile workers | Ch | Men and women | 1940–1965 | 2001 | 3072 |
| 47 | Krstev 2007 (Krstev et al, 2007) | M | 2007 | USA | Shipyard production workers | Mixed | Men and women | 1950–1964 | 2001 | 4702 |
| 48 | Pira 2007 (Pira et al, 2005, 2007) | M | 2007 | Italy | Asbestos textile workers | Mixed | Men and women | 1946–1984 | 2004 | 1966 |
| 49 | Frost 2008 (Frost et al, 2008) | M | 2008 | GB | Asbestos removal workers (campaign) | Mixed | Men and women | From 1971 | 2005 | 52 387 |
| 50 | Musk2008 (Musk et al, 2008) (Armstrong et al, 1988; Reid et al, 2004) | M | 2008 | Australia | crocidolite miners and millers | Cr | Men | 1943–1966 | 2000 | 6943 |
| 51 | Clin 2009 (Clin et al, 2009) | I | 2009 | France | Asbestos reprocessing plant (textile, friction) | Mixed | Men and women | Before 1978 | 2004 | 2024 |
| 52 | Harding 2009 (Hutchings et al, 1995; Harding et al, 2009) | M | 2009 | UK | Asbestos survey | Mixed | Men and women | 2005 | 9811 | |
| 53 | Loomis 2009 (Loomis et al, 2009) | M | 2009 | USA | Asbestos textile workers | Ch | Men and women | 1950–1973 | 2003 | 5770 |
| 54 | Pira 2009 (Pira et al, 2009) (Piolatto et al, 1990; Rubino et al, 1979) | M | 2009 | Italy | Chrysotile asbestos miners | Ch | Men | 1930–1975 | 1946 | 2003 |
| 55 | Pesch 2010 (Pesch et al, 2010) | M | 2010 | Germany | Asbestos survey | Mixed | Men | 1993–1995 | 2007 | 576 |
Abbreviations: -=not applicable; Am=Amosite; An=Anthophyllite; Ch=Chrysotile; Cr=Crocidolite; I=Incidence; M=Mortality; O=Outcome.
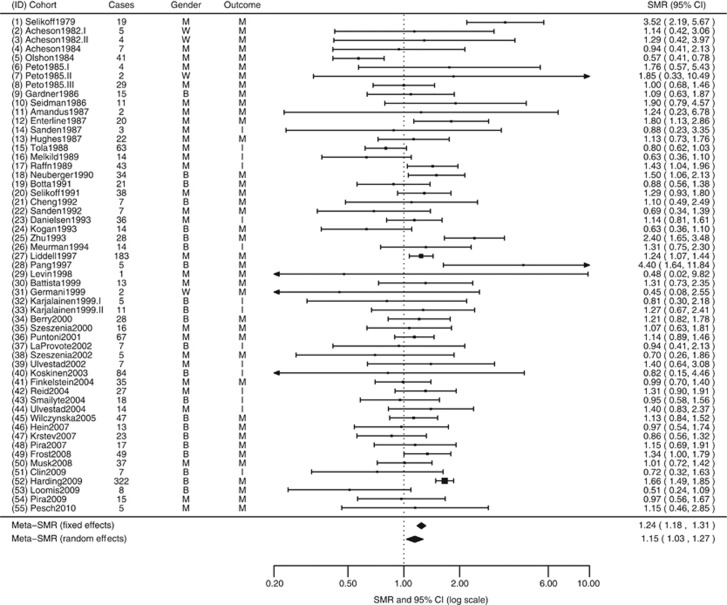
Mortality cohort studies have been carried out mainly in Europe (23 studies, 58%) and North America (12 studies, 30%). Three mortality cohorts were Chinese, one was Russian and one was Australian. Study mortality cohorts ranged in size between 145 and 52 387 workers. Thirteen (33%) of the mortality cohorts included women, although in most women were a small proportion of the total. Four studies involved only women (Acheson et al, 1982; Peto et al, 1985; Germani et al, 1999), and four reported results for the total cohort (Gardner et al, 1986; Zhu and Wang, 1993; Frost et al, 2008; Harding et al, 2009). The most common occupations were insulators (20%), generic asbestos workers (20%), textile asbestos workers (15%), cement asbestos workers (13%) and miners (10%). The latency (exposure lag) ranged between 10 and 20 years. The earliest follow-up period started in 1941 and the latest ended in 2007. The average length of follow-up was 29.9 years (range=9–49). The largest overall cohort RRs were among the earliest insulation workers (Selikoff et al, 1979) with a RR of 3.52 (Figure 1), and among two sets of workers in Chinese asbestos factories (Zhu and Wang, 1993; Pang et al, 1997): RRs were 4.4 and 2.2, respectively. Two studies carried out in Canada (Liddell et al, 1997) and the United Kingdom (Harding et al, 2009), involving 183 and 322 deaths from stomach cancer, showed consistent RR estimates with narrow 95% CI (1.24 and 1.66, respectively).
Figure 1.
Meta-analysis of stomach cancer mortality and incidence for total cohort, all exposure lags.
Incidence studies have been carried out in Northern Europe (11 studies, 73%), in France (2 studies), in Lithuania (1 study) and in Australia (1 study) and included fewer than 900 subjects to over 24 200. Half of the studies included women, in a small proportion of the total cohort. The largest overall cohort RR was among Danish asbestos cement workers (Raffn et al, 1989) with a RR of 1.43 (95% CI 1.03–1.93). All the other studies reported RRs close to one.
Quantitative synthesis
Table 2 summarises all the meta-SMRs and meta-SIRs obtained for men and women separately, and by consideration of an exposure lag or not. The meta-SIR for stomach cancer incidence was 1.09 (95% CI 0.94–1.26; 14 studies) and 1.10 (95% CI 0.52–2.33; 6 studies) for men and women, respectively, with homogenous results (P=0.16 and 0.99, respectively).
Table 2. Pooled analysis for stomach cancer mortality and incidence by exposure lag (latency) and type of outcome using random effects model.
| Outcome | na | SMR | 95% CI | τ2 b | PQc | I2 (%)d | |
|---|---|---|---|---|---|---|---|
| Men | |||||||
| All exposure lags | I | 14 | 1.09 | (0.94–1.26) | 0.019 | 0.16 | 28.0 |
| M | 30 | 1.16 | (1.00–1.34) | 0.085 | <0.001 | 63.5 | |
| M+I | 44 | 1.13 | (1.02–1.26) | 0.057 | <0.001 | 54.7 | |
| At least 10 yr exposure lag | I | 2 | – | – | – | – | – |
| M | 9 | 1.16 | (0.79–1.69) | 0.213 | <0.001 | 75.3 | |
| M+I | 11 | 1.09 | (0.77–1.53) | 0.197 | <0.001 | 71.8 | |
| No exposure lag | I | 14 | 1.09 | (0.94–1.26) | 0.019 | 0.16 | 28.0 |
| M | 26 | 1.18 | (0.99–1.40) | 0.111 | <0.001 | 69.6 | |
| M+I | 40 | 1.14 | (1.01–1.28) | 0.069 | <0.001 | 59.7 | |
| Women | |||||||
| All exposure lags | I | 6 | 1.1 | (0.52–2.33) | 0 | 0.99 | – |
| M | 13 | 0.93 | (0.67–1.30) | 0 | 0.90 | 0 | |
| M+I | 19 | 0.96 | (0.71–1.30) | 0 | 0.99 | – | |
| No exposure lag | I | 6 | 1.1 | (0.52–2.33) | 0 | 0.99 | – |
| M | 12 | 0.89 | (0.62–1.26) | 0 | 0.90 | 0 | |
| M+I | 18 | 0.92 | (0.67–1.27) | 0 | 0.99 | – | |
| Total cohort | |||||||
| All exposure lags | I | 15 | 1.07 | (0.91–1.25) | 0.022 | 0.26 | 25.5 |
| M | 40 | 1.17 | (1.03–1.33) | 0.087 | <0.001 | 69.1 | |
| M+I | 55 | 1.15 | (1.03–1.27) | 0.069 | <0.001 | 61.9 | |
| At least 10 yr exposure lag | I | 0 | – | – | – | – | – |
| M | 10 | 1.12 | (0.80–1.56) | 0.182 | <0.001 | 81.6 | |
| M+I | 12 | 1.07 | (0.79–1.44) | 0.169 | <0.001 | 78.5 | |
| No exposure lag | I | 15 | 1.07 | (0.91–1.25) | 0.022 | 0.26 | 25.5 |
| M | 36 | 1.18 | (1.03–1.36) | 0.102 | <0.001 | 72.7 | |
| M+I | 51 | 1.15 | (1.03–1.28) | 0.078 | <0.001 | 64.8 | |
Abbreviations: – = not applicable; CI=confidence interval; I=incidence, M=mortality.
No results for women for at least 10 year exposure lag as only one mortality study reported a SMR.
Number of cohorts included.
Variance (amount of heterogeneity).
P-value for the heterogeneity test.
Percentage of total variability due to heterogeneity.
The pooled analysis for stomach cancer mortality yielded a meta-SMR of 1.16 (95% CI 1.00–1.34; 30 studies) for men, with large heterogeneity of results (P<0.001, I2=63.5%); a meta-SMR of 0.93 (95% CI 0.67–1.30, 13 studies) was found for women, with homogeneous results across studies (P=0.90). For the total cohort, the meta-SMR was similar to that found for men only (meta-SMR=1.17, 95% CI 1.03–1.33, 40 studies).
Because mortality is a relatively accurate measure of disease incidence as stomach cancer has a low survival rate, and because of the very limited numbers of primary studies in which incidence data were reported, pooled analyses are also reported using mortality and incidence combined. In this situation, the meta-SMRs were similar to those found using only mortality data, with a slight reduction in heterogeneity (I2=54.7%). Figure 1 presents the individual study results and the overall meta-SMR for total cohort.
As the meta-SMRs from studies reporting results with exposure lag did not differ substantially from the overall results, the meta-SMRs below are reported for all exposure lag group and for mortality and incidence combined, unless specified otherwise.
Between study heterogeneity and influence of individual studies
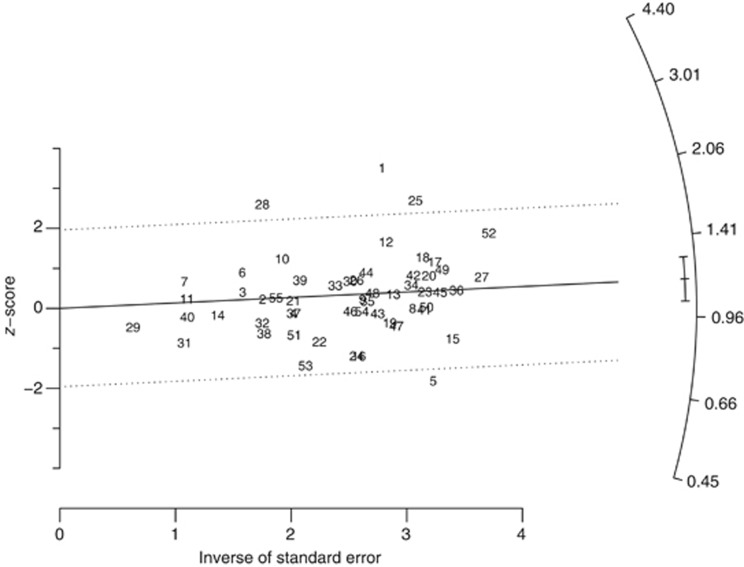
Table 2 also shows the heterogeneity (P-value) for each analysis. There was no evidence of heterogeneity in women but some in men. A few specific studies contributed to this heterogeneity, as illustrated by outlying points in the radial plot for stomach cancer for men (Figure 2): cohort 1 (Selikoff et al, 1979) was conducted in the earliest period, cohort 5 (Ohlson et al, 1984) was the only study to find a significant decrease in risk, cohort 28 (Pang et al, 1997) was carried out in China. For the total cohort, another cohort in China, cohort 25 (Zhu and Wang, 1993) also contributed to the heterogeneity.
Figure 2.
Radial plot for SMRs in a meta- analysis of stomach cancer mortality and incidence for total cohort, all exposure lags.
The covariates listed in the Methods section were explored as potential sources of heterogeneity using meta-regression methods. Table 3 gives the meta-SMR by subgroup for men and women. No significant predictor of the meta-SMR for women was found. Apart for type of asbestos and publication year, all the variables were a significant predicator for men, with some heterogeneity. The meta-SMRs for men showed elevated risks in the United States and Australia (meta-SMR=1.30, 95% CI 1.10–1.55), and China and Russia (meta-SMR=1.91, 95% CI 1.03–3.56). The pooled analysis within occupational strata demonstrated the highest meta-SMR for stomach cancer among generic asbestos workers (meta-SMR=1.41, 95% CI 1.10–1.82), followed by insulators (meta-SMR= 1.27, 95% CI 1.05–1.53). Meta-regression also showed positive associations for stomach cancer for the cohort sizes below 1000 compared with cohort size above 1000. Similar results were found for the total cohort (Supplementary Table 1).
Table 3. Stratification of cohort studies by subgroups, for men and women, mortality and incidence combined, all exposure lags (random effects model).
|
Men |
Women |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| na | SMR | 95% CI | τ2 b | PQEc | PQMd | na | SMR | 95% CI | τ2 b | PQEc | PQMd | |
| Geography | ||||||||||||
| Europe | 28 | 1.03 | (0.91–1.16) | 0.042 | <0.001 | <0.001 | 14 | 1.1 | (0.77–1.57) | 0 | 1 | 0.48 |
| North America + Australia | 13 | 1.3 | (1.10–1.55) | 2 | 0.37 | (0.04–3.13) | ||||||
| China+Russia | 3 | 1.91 | (1.03–3.56) | 3 | 0.69 | (0.38–1.28) | ||||||
| Occupation | ||||||||||||
| Cement asbestos workers | 6 | 1.12 | (0.88–1.42) | 0.028 | <0.001 | <0.001 | 2 | 1.27 | (0.59–2.72) | 0 | 0.95 | 0.97 |
| Generic asbestos workers | 7 | 1.41 | (1.10–1.82) | 5 | 0.87 | (0.44–1.73) | ||||||
| Insulators | 10 | 1.27 | (1.05–1.53) | 1 | 0.63 | (0.03–12.89) | ||||||
| Miners | 6 | 1.18 | (0.95–1.47) | 1 | 0.67 | (0.05–9.13) | ||||||
| Textile asbestos workers | 4 | 1.15 | (0.83–1.61) | 3 | 1.22 | (0.53–2.79) | ||||||
| Other occupation | 11 | 0.87 | (0.73–1.04) | 7 | 0.87 | (0.56–1.34) | ||||||
| SMR for lung cancer | ||||||||||||
| ⩽2 | 25 | 1.02 | (0.91–1.15) | 0.039 | <0.001 | <0.001 | 5 | 0.88 | (0.54–1.42) | 0 | 0.98 | 0.86 |
| >2 | 17 | 1.46 | (1.22–1.77) | 13 | 1.02 | (0.69–1.52) | ||||||
| Type of asbestos | ||||||||||||
| Amosite | 3 | 1.25 | (0.64–2.44) | 0.058 | <0.001 | 0.32 | – | – | – | 0 | 0.97 | 0.95 |
| Chrysotile | 11 | 1.09 | (0.87–1.37) | 6 | 0.84 | (0.52–1.35) | ||||||
| Crocidolite | 2 | 1.14 | (0.75–1.74) | 1 | 1.14 | (0.42–3.06) | ||||||
| Mixed | 27 | 1.13 | (0.99–1.29) | 11 | 1.05 | (0.68–1.64) | ||||||
| Sample size | ||||||||||||
| <1000 | 12 | 1.68 | (1.32–2.15) | 0.034 | 0.001 | <0.001 | 15 | 1.15 | (0.77–1.71) | 0 | 1 | 0.56 |
| 1000–1500 | 6 | 1.19 | (0.88–1.61) | 3 | 0.79 | (0.38–1.62) | ||||||
| >1500 | 26 | 1.04 | (0.93–1.16) | 1 | 0.7 | (0.37–1.33) | ||||||
| Publication year | ||||||||||||
| Before 1999 | 26 | 1.16 | (1.00–1.34) | 0.057 | <0.001 | 0.07 | 11 | 0.94 | (0.63–1.40) | 0 | 0.98 | 0.95 |
| After 1999 | 18 | 1.1 | (0.94–1.29) | 8 | 0.99 | (0.62–1.59) | ||||||
Abbreviations: – = not applicable; CI=confidence interval.
Number of cohorts included.
residual variance (residual amount of heterogeneity).
P-value for the residual heterogeneity test.
P-value for the test of moderators (if the SMRs are different or not within the subgroup).
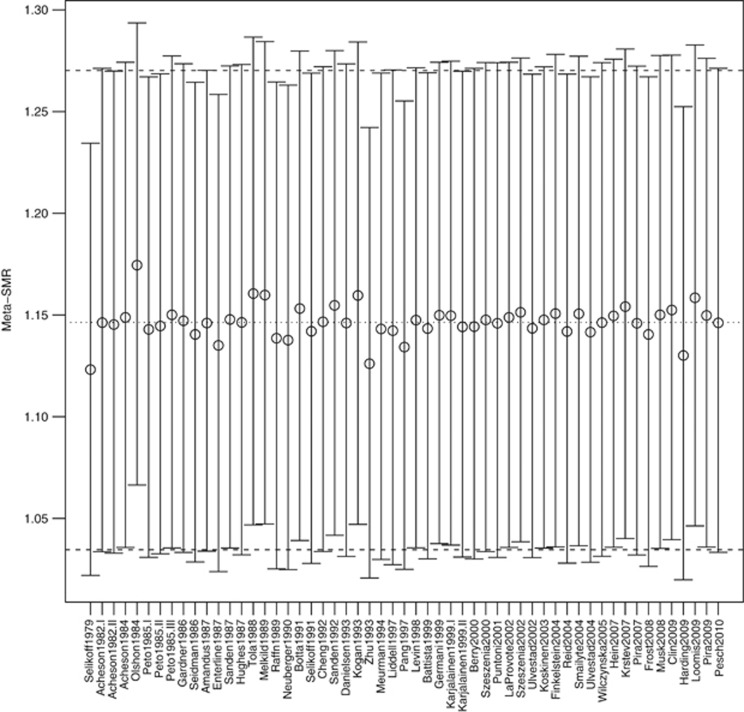
Figure 3 shows, for men, the investigation of the influence of individual studies via systematic ‘leave one out' exclusion. The studies appearing to contribute to heterogeneity also influence the meta-SMR. Using the other diagnostics, only Selikoff et al (1979) and Ohlson et al (1984) were influential (Supplementary Figure 1). The meta-SMR for stomach cancer excluding these 2 studies were 1.13 (95% CI 1.05–1.22), relatively similar to the one found with all the studies for men. The exclusion of the 3 influential studies (Selikoff et al, 1979; Ohlson et al, 1984; Pang et al, 1997) led to a meta-SMR of 1.12 (95% CI 1.04–1.20) and eliminated completely the heterogeneity (P=0.59, I2=7.3%) as well as the residual heterogeneity in the meta-regressions (P>0.44). The associations were generally attenuated (Supplementary Table 2), except for the miners (meta-SMR=1.21, 95% CI 1.07–1.36) compared with the other occupations.
Figure 3.
Influence of excluding each individual cohort for men, mortality and incidence combined, all exposure lags. Meta-SMRs and associated 95% CI (random-effects model). Dotted and dash lines represent the overall meta-SMR and its 95% CI.
Dose–response associations
Estimates of cumulative or duration of exposure among asbestos-exposed workers were reported for only 11 studies (Supplementary Table 3). The pooled SMR estimate of stomach cancer for men was 1.40 (95% CI 0.81–2.40), with a large degree of heterogeneity (I2= 67.7%).
Using a low/high exposure categorisation based on the lung cancer SMR, cohorts that reported a lung cancer SMR above 2.0 had higher meta-SMRs (SMR=1.46; 95% CI 1.22–1.77) compared with other cohorts (SMR=1.02; 95% CI 0.91–1.15).
Assessment of publication bias
For men and women, there was no evidence of publication bias from plots and statistical tests. However, for the total cohort, there is an evidence of publication bias (funnel plot in Supplementary Figure 2), with a suggestive lack of studies in the top right-hand corner of the plot, that is, large cohorts with large associations.
Zero cases
Four studies reported no deaths from stomach cancer for women; (Cheng and Kong, 1992; Pang et al, 1997; Hein et al, 2007; Krstev et al, 2007); only one study with men was concerned with this issue (Levin et al, 1998) Therefore, the investigation of the influence of approaches to handling zero cases was carried out only for women. Both excluding studies for which observed cases are zero and setting observed equal to expected values resulted in an increase in meta-SMRs and a slight widening of the confidence intervals compared with the default method of adding 1 to both observed and expected values. Whatever the latency, the meta-SMRs were 1.00 (95% CI 0.73–1.36) and 1.03 (95% CI 0.77–1.39) with the exclusion approach and imputation approach, respectively, compared with a meta-SMR of 0.96 (95% CI 0.71–1.30) with the default method.
Discussion
The association between asbestos and stomach cancer has been estimated in a meta-analysis of studies of workers in which a major portion of the cohort is presumed to have been exposed to asbestos. Our results demonstrated an increase in the pooled estimate in men (meta-SMR=1.13, 95% CI 1.02–1.26) for stomach cancer in relation to exposure to asbestos. Our meta-analysis provided an update of studies, compared with previous reviews and quantitative estimates and also thoroughly explored heterogeneity and publication bias.
The magnitude of the association in our meta-analysis was similar to that reported in the IOM report that included 42 cohorts (meta-SMR=1.17, 95% CI 1.07–1.28). More recently, Gamble (2008) reported that point estimates for cancer of the stomach mortality tended towards 1, with an overall RR estimate of 1.01 (95% CI 0.94–1.08), results more similar to those obtained by Goodman et al (1999).
Our analysis addressed heterogeneity and was based on studies included in the published meta-analyses and more recent publications. The observed overall heterogeneity among studies seemed to be explained by four cohorts. The cohort by Selikoff et al (1979) considered an early exposure period (up to 1962). Ohlson et al (1984) were the only ones to find a significant decrease in risk (SMR=0.57, 95% CI 0.42–0.79). Two studies (Zhu and Wang, 1993; Pang et al, 1997) were conducted in China, where asbestos production and exposure can be very high (LaDou, 2004).
We carried out meta-regression to investigate the influence of several variables. Positive and statistically significant associations were observed for non-European cohorts, generic asbestos workers, cohorts reporting a SMR for lung cancer above 2, and cohort size below 1000.
Our meta-analysis mainly represented studies conducted in developed geographical areas, particularly among European populations. It is possible that studies conducted in other geographic regions (e.g., developing countries) may be available through other biomedical literature databases. The meta-analysis (da Sun et al, 2008) published in Chinese with an abstract in English, which searched Chinese literature as well, found a meta-SMR of 1.20 (P<0.01) among workers exposed to chrysotile alone or mixed asbestos. The stomach cancer SMR was significantly increased in the asbestos cement workers, the screening mine workers and the insulators, (1.27, 1.21 and 2.13, respectively, P<0.05). These results seem consistent with the ones we observed. Another source of publication bias can arise from the lack of publications in parts of Asia, South America and the former Soviet Union where asbestos use is increasing (LaDou, 2004).
Some studies may have failed to take account of co-exposures that have been to be associated with excess risk of stomach cancer. The reported SMRs were not adjusted for known risk factors such as chronic infection with Helicobacter pylori, smoking and diet habits. Liddell et al (1997), for example, report that their finding of no trend of lung cancer with exposure up to 300 mpcf.y suggests that the 21% excess was due to some other factor, probably smoking, and that the effect of smoking on stomach cancer was twice as high as the effect of >300 mpcf.y. A recent study found statistically significant increased hazard ratios for gastric cancer and several asbestos exposure variables, adjusted for age and family history of gastric cancer, although, with the exception of long duration at high exposure, these associations tended to disappear after adjusting for smoking (Offermans et al, 2014).
Increases in stomach cancer have also been associated with work in a variety of dusty industries and from exposure to fumes and metal particles, for example, in foundry, steel and mining work (Cocco et al, 1996; Ji and Hemminki, 2006). A study in Swedish construction workers found exposure to silica exposure, but not asbestos, was significantly related to stomach cancer (Sjodhal et al, 2007). However, in our meta-analysis we restricted our studies to only those where the dominant exposure was asbestos.
We found a suggestive but nonsignificant association between asbestos type and the stomach cancer meta-SMR. Cohorts exposed to mixed asbestos showed larger SMRs than those exposed only to chrysotile asbestos. A meta-analysis by Li et al (2004) of 15 studies published before 2003 of workers exposed only to chrysotile found also a nonsignificant association (meta-SMR=1.24; 95% CI 0.95–1.62). Our risk estimate was slightly smaller as we did not include four cohorts, as they were published in Chinese. There has been a considerable controversy over the potency of asbestos fibre types with the risks of lung cancer and mesothelioma. As discussed in the review by Hodgson and Darnton (2000) some studies showed no difference in risk between these cancers and asbestos fibre types, while others have claimed a reduced potency for chrysotile, leading to a substantial heterogeneity in the findings. Our results tend to support a reduced risk for chrysotile and stomach cancer compared with the risk associated with other asbestos types.
In summary our results support the conclusion by IARC that exposure to asbestos is associated with a moderate increased risk of stomach cancer. Given the large number of workers exposed to asbestos worldwide, this may contribute to a substantial burden of mortality and morbidity.
Acknowledgments
This work was supported by the UK Health and Safety Executive (Grant JN3117). We thank Sally Hutchings for her valuable comments.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- Acheson ED, Gardner MJ, Pippard EC, Grime LP (1982) Mortality of two groups of women who manufactured gas masks from chrysotile and crocidolite asbestos: a 40-year follow-up. Br J Ind Med 39(4): 344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson ED, Gardner MJ, Winter PD, Bennett C (1984) Cancer in a factory using amosite asbestos. Int J Epidemiol 13(1): 3–10. [DOI] [PubMed] [Google Scholar]
- Alder N, Fenty J, Warren F, Sutton AJ, Rushton L, Jones DR, Abrams KR (2006) Meta-analysis of mortality and cancer incidence among workers in the synthetic rubber-producing industry. Am J Epidemiol 164(5): 405–420. [DOI] [PubMed] [Google Scholar]
- Amandus HE, Wheeler R (1987) The morbidity and mortality of vermiculite miners and millers exposed to tremolite-actinolite: Part II. Mortality. Am J Ind Med 11(1): 15–26. [DOI] [PubMed] [Google Scholar]
- Armstrong BK, de Klerk NH, Musk AW, Hobbs MS (1988) Mortality in miners and millers of crocidolite in Western Australia. Br J Ind Med 45(1): 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista G, Belli S, Comba P, Fiumalbi C, Grignoli M, Loi F, Orsi D, Paredes I (1999) Mortality due to asbestos-related causes among railway carriage construction and repair workers. Occup Med 49(8): 536–539. [DOI] [PubMed] [Google Scholar]
- Berry G, Newhouse ML, Wagner JC (2000) Mortality from all cancers of asbestos factory workers in east London 1933-80. Occup Environ Med 57(11): 782–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta M, Magnani C, Terracini B, Bertolone GP, Castagneto B, Cocito V, DeGiovanni D, Paglieri P (1991) Mortality from respiratory and digestive cancers among asbestos cement workers in Italy. Cancer Detect Prev 15(6): 445–447. [PubMed] [Google Scholar]
- Breslow NE, Day NE (1987) Statistical methods in cancer research. Volume II -The design and analysis of cohort studies. Vol. 82, IARC Scientific Publication. [PubMed] [Google Scholar]
- Brown DP, Dement JM, Okun A (1994) Mortality patterns among female and male chrysotile asbestos textile workers. J Occup Med 36(8): 882–888. [PubMed] [Google Scholar]
- Camargo MC, Stayner LT, Straif K, Reina M, Al-Alem U, Demers PA, Landrigan PJ (2011) Occupational exposure to asbestos and ovarian cancer: a meta-analysis. Environ Health Perspect 119(9): 1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WN, Kong J (1992) A retrospective mortality cohort study of chrysotile asbestos products workers in Tianjin 1972-1987. Environ Res 59(1): 271–278. [DOI] [PubMed] [Google Scholar]
- Clin B, Morlais F, Dubois B, Guizard AV, Desoubeaux N, Marquignon MF, Raffaelli C, Paris C, Galateau-Salle F, Launoy G, Letourneux M (2009) Occupational asbestos exposure and digestive cancers - a cohort study. Aliment Pharmacol Ther 30(4): 364–374. [DOI] [PubMed] [Google Scholar]
- Cocco P, Ward MH, Buiatti E (1996) Occupational risk factors of gastric cancer: an overview. Epidemiol Rev 18: 218–234. [DOI] [PubMed] [Google Scholar]
- da Sun T, Chen J-E, Zhang X-J, Li X-Y (2008) [Cohort studies on cancer mortality of digestive system among workers exposed to asbestos: a meta-analysis]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 26(10): 605–608. [PubMed] [Google Scholar]
- Danielsen TE, Langård S, Andersen A, Knudsen O (1993) Incidence of cancer among welders of mild steel and other shipyard workers. Br J Ind Med 50(12): 1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de La Provôté S, Desoubeaux N, Paris C, Letourneux M, Raffaelli C, Galateau-Salle F, Gignoux M, Launoy G (2002) Incidence of digestive cancers and occupational exposure to asbestos. Eur J Cancer Prev 11(6): 523–528. [DOI] [PubMed] [Google Scholar]
- Dement JM, Brown DP, Okun A (1994) Follow-up study of chrysotile asbestos textile workers: cohort mortality and case-control analyses. Am J Ind Med 26(4): 431–447. [DOI] [PubMed] [Google Scholar]
- Dement JM, Harris RL, Symons MJ, Shy CM (1983) Exposures and mortality among chrysotile asbestos workers. Part II: mortality. Am J Ind Med 4(3): 421–433. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109): 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enterline PE, Hartley J, Henderson V (1987) Asbestos and cancer: a cohort followed up to death. Br J Ind Med 44(6): 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein MM, Verma DK (2004) A cohort study of mortality among Ontario pipe trades workers. Occup Environ Med 61(9): 736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost G, Harding AH, Darnton A, McElvenny D, Morgan D (2008) Occupational exposure to asbestos and mortality among asbestos removal workers: a Poisson regression analysis. Br J Cancer 99(5): 822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble J (2008) Risk of gastrointestinal cancers from inhalation and ingestion of asbestos. Regul Toxicol Pharmacol 52(1 Suppl): S124–S153. [DOI] [PubMed] [Google Scholar]
- Gardner MJ, Winter PD, Pannett B, Powell CA (1986) Follow up study of workers manufacturing chrysotile asbestos cement products. Br J Ind Med 43(11): 726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germani D, Belli S, Bruno C, Grignoli M, Nesti M, Pirastu R, Comba P (1999) Cohort mortality study of women compensated for asbestosis in Italy. Am J Ind Med 36(1): 129–134. [DOI] [PubMed] [Google Scholar]
- Goodman M, Morgan RW, Ray R, Malloy CD, Zhao K (1999) Cancer in asbestos-exposed occupational cohorts: a meta-analysis. Cancer Causes Control 10(5): 453–465. [DOI] [PubMed] [Google Scholar]
- Harding AH, Darnton A, Wegerdt J, McElvenny D (2009) Mortality among British asbestos workers undergoing regular medical examinations (1971-2005). Occup Environ Med 66(7): 487–495. [DOI] [PubMed] [Google Scholar]
- Hein MJ, Stayner LT, Lehman E, Dement JM (2007) Follow-up study of chrysotile textile workers: cohort mortality and exposure-response. Occup Environ Med 64(9): 616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11): 1539–1558. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ (Clin Res Ed) 327(7414): 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson JT, Darnton A (2000) The quantitative risks of mesothelioma and lung cancer in relation to asbestos exposure. Ann Occup Hyge 44(8): 565–601. [PubMed] [Google Scholar]
- Hughes JM, Weill H, Hammad YY (1987) Mortality of workers employed in two asbestos cement manufacturing plants. Br J Ind Med 44(3): 161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings S, Jones J, Hodgson J (1995) Asbestos related diseases. In Occupational health decennial supplement, The Registrar General's decennial supplement for England and Wales Drever F (ed) pp 127–152. HMSO: London. [Google Scholar]
- IARC (1998) Monographs on the Evaluation of Carcinogenic Risks to Humans Vol. 28, IARC: Lyon, France. [Google Scholar]
- IARC (2011) Monographs on the Evaluation of Carcinogenic Risks to Humans Vol. 100C, IARC: Lyon, France. [Google Scholar]
- IOM (2006) Asbestos: Selected Cancers. The National Academies Press. [PubMed] [Google Scholar]
- Ji J, Hemminki K (2006) Socio-economic and occupational risk factors for gastric cancers: a cohort study in Sweden. Eur J Cancer Prev 15: 391–397. [DOI] [PubMed] [Google Scholar]
- Karjalainen A, Pukkala E, Kauppinen T, Partanen T (1999) Incidence of cancer among Finnish patients with asbestos-related pulmonary or pleural fibrosis. Cancer Causes Control 10(1): 51–57. [DOI] [PubMed] [Google Scholar]
- Kogan PM, Yatsenko AS, Tregubov ES, Gurvich EB, Kuzina LE (1993) Evaluation of carcinogenic risk in friction product workers. La Medicina del lavoro 84(4): 290–296. [PubMed] [Google Scholar]
- Koskinen K, Pukkala E, Reijula K, Karjalainen A (2003) Incidence of cancer among the participants of the Finnish Asbestos Screening Campaign. Scand J Work Environ Health 29(1): 64–70. [DOI] [PubMed] [Google Scholar]
- Krstev S, Stewart P, Rusiecki J, Blair A (2007) Mortality among shipyard Coast Guard workers: a retrospective cohort study. Occup Environ Med 64(10): 651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDou J (2004) The asbestos cancer epidemic. Environ Health Perspect 112(3): 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin JL, McLarty JW, Hurst GA, Smith AN, Frank AL (1998) Tyler asbestos workers: mortality experience in a cohort exposed to amosite. Occup Environ Med 55(3): 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Sun T-D, Zhang X, Lai R-N, Li X-Y, Fan X-J, Morinaga K (2004) Cohort studies on cancer mortality among workers exposed only to chrysotile asbestos: a meta-analysis. Biomed Environ Sci 17(4): 459–468. [PubMed] [Google Scholar]
- Liddell FD, McDonald AD, McDonald JC (1997) The 1891-1920 birth cohort of Quebec chrysotile miners and millers: development from 1904 and mortality to 1992. Ann Occup Hyg 41(1): 13–36. [DOI] [PubMed] [Google Scholar]
- Loomis D, Dement JM, Wolf SH, Richardson DB (2009) Lung cancer mortality and fibre exposures among North Carolina asbestos textile workers. Occup Environ Med 66(8): 535–542. [DOI] [PubMed] [Google Scholar]
- McDonald JC, Liddell FD, Dufresne A, McDonald AD (1993) The 1891-1920 birth cohort of Quebec chrysotile miners and millers: mortality 1976-88. Br J Ind Med 50(12): 1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JC, Liddell FD, Gibbs GW, Eyssen GE, McDonald AD (1980) Dust exposure and mortality in chrysotile mining, 1910-75. Br J Ind Med 37(1): 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkild A, Langård S, Andersen A, Tønnessen JN (1989) Incidence of cancer among welders and other workers in a Norwegian shipyard. Scand J Work Environ Health 15(6): 387–394. [DOI] [PubMed] [Google Scholar]
- Meurman LO, Pukkala E, Hakama M (1994) Incidence of cancer among anthophyllite asbestos miners in Finland. Occup Environ Med 51(6): 421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musk AW, de Klerk NH, Reid A, Ambrosini GL, Fritschi L, Olsen NJ, Merler E, Hobbs MST, Berry G (2008) Mortality of former crocidolite (blue asbestos) miners and millers at Wittenoom. Occup Environ Med 65(8): 541–543. [DOI] [PubMed] [Google Scholar]
- Neuberger M, Kundi M (1990) Individual asbestos exposure: smoking and mortality—a cohort study in the asbestos cement industry. Br J Ind Med 47(9): 615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse ML, Berry G, Wagner JC (1985) Mortality of factory workers in east London 1933-80. Br J Ind Med 42(1): 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermans NSM, Vermeulen R, Burdoff A, Goldbohm RA, Kerzei AP, Peters S, Kauppinen T, Kromhout H, van den Brandt PA (2014) Occupational asbestos exposure and the risk of oesophageal gastric and colorectal cancer in the prospective Netherlands Cohort Study. Int J Cancer 40: 420. [DOI] [PubMed] [Google Scholar]
- Ohlson CG, Klaesson B, Hogstedt C (1984) Mortality among asbestos-exposed workers in a railroad workshop. Scand J Work Environ Health 10(5): 283–291. [DOI] [PubMed] [Google Scholar]
- Pang ZC, Zhang Z, Wang Y, Zhang H (1997) Mortality from a Chinese asbestos plant: overall cancer mortality. Am J Ind Med 32(5): 442–444. [DOI] [PubMed] [Google Scholar]
- Pesch B, Taeger D, Johnen G, Gross IM, Weber DG, Gube M, Müller-Lux A, Heinze E, Wiethege T, Neumann V, Tannapfel A, Raithel H-J, Brüning T, Kraus T (2010) Cancer mortality in a surveillance cohort of German males formerly exposed to asbestos. Int J Hyg Environ Health 213(1): 44–51. [DOI] [PubMed] [Google Scholar]
- Peto J, Doll R, Hermon C, Binns W, Clayton R, Goffe T (1985) Relationship of mortality to measures of environmental asbestos pollution in an asbestos textile factory. Ann Occup Hyg 29(3): 305–355. [DOI] [PubMed] [Google Scholar]
- Piolatto G, Negri E, Vecchia CL, Pira E, Decarli A, Peto J (1990) An update of cancer mortality among chrysotile asbestos miners in Balangero, northern Italy. Br J Ind Med 47(12): 810–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pira E, Pelucchi C, Buffoni L, Palmas A, Turbiglio M, Negri E, Piolatto PG, Vecchia CL (2005) Cancer mortality in a cohort of asbestos textile workers. Br J Cancer 92(3): 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pira E, Pelucchi C, Piolatto PG, Negri E, Bilei T, La Vecchia C (2009) Mortality from cancer and other causes in the Balangero cohort of chrysotile asbestos miners. Occup Environ Med 66(12): 805–809. [DOI] [PubMed] [Google Scholar]
- Pira E, Pelucchi C, Piolatto PG, Negri E, Discalzi G, Vecchia CL (2007) First and subsequent asbestos exposures in relation to mesothelioma and lung cancer mortality. Br J Cancer 97(9): 1300–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntoni R, Merlo F, Borsa L, Reggiardo G, Garrone E, Ceppi M (2001) A historical cohort mortality study among shipyard workers in Genoa, Italy. Am J Ind Med 40(4): 363–370. [DOI] [PubMed] [Google Scholar]
- Raffn E, Lynge E, Juel K, Korsgaard B (1989) Incidence of cancer and mortality among employees in the asbestos cement industry in Denmark. Br J Ind Med 46(2): 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid A, Ambrosini G, de Klerk N, Fritschi L, Musk B (2004) Aerodigestive and gastrointestinal tract cancers and exposure to crocidolite (blue asbestos): incidence and mortality among former crocidolite workers. Int J Cancer 111(5): 757–761. [DOI] [PubMed] [Google Scholar]
- Rubino GF, Piolatto G, Newhouse ML, Scansetti G, Aresini GA, Murray R (1979) Mortality of chrysotile asbestos workers at the Balangero Mine, Northern Italy. Br J Ind Med 36(3): 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton L, Bagga S, Bevan R, Brown TP, Cherrie JW, Holmes P, Fortunato L, Slack R, Van Tongeren M, Young C, Hutchings SJ (2010) Occupation and cancer in Britain. Br J Cancer 102(9): 1428–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandén A, Järvholm B (1987) Cancer morbidity in Swedish shipyard workers 1978-1983. Int Arch Occup Environ Health 59(5): 455–462. [DOI] [PubMed] [Google Scholar]
- Sandén A, Järvholm B, Larsson S, Thiringer G (1992) The risk of lung cancer and mesothelioma after cessation of asbestos exposure: a prospective cohort study of shipyard workers. Eur Respir J 5(3): 281–285. [PubMed] [Google Scholar]
- Seidman H, Selikoff IJ, Gelb SK (1986) Mortality experience of amosite asbestos factory workers: dose-response relationships 5 to 40 years after onset of short-term work exposure. Am J Ind Med 10(5-6): 479–514. [DOI] [PubMed] [Google Scholar]
- Selikoff IJ, Churg J, Hammond EC (1964) Asbestos exposure and neoplasia. JAMA 188: 22–26. [DOI] [PubMed] [Google Scholar]
- Selikoff IJ, Hammond EC, Seidman H (1979) Mortality experience of insulation workers in the United States and Canada, 1943—1976. Ann N Y Acad Sci 330: 91–116. [DOI] [PubMed] [Google Scholar]
- Selikoff IJ, Seidman H (1991) Asbestos-associated deaths among insulation workers in the United States and Canada, 1967-1987. Ann N Y Acad Sci 643: 1–14. [DOI] [PubMed] [Google Scholar]
- Selikoff IJ, Seidman H, Hammond EC (1980) Mortality effects of cigarette smoking among amosite asbestos factory workers. J Natl Cancer Inst 65(3): 507–513. [PubMed] [Google Scholar]
- Sjodhal K, Jansson C, Bergdahl IA, Adam J, Bofetta P, Lagergren J (2007) Airborne exposures and risk of gastric cancer: a prospective cohort study. Int J Cancer 120: 2013–2018. [DOI] [PubMed] [Google Scholar]
- Smailyte G, Kurtinaitis J, Andersen A (2004) Cancer mortality and morbidity among Lithuanian asbestos-cement producing workers. Scand J Work Environ Health 30(1): 64–70. [DOI] [PubMed] [Google Scholar]
- Straif K, Benbrahim-Tallaa L, Baan R, Grosse Y, Secretan B, Ghissassi FE, Bouvard V, Guha N, Freeman C, Galichet L, Cogliano V W HO International Agency for Research on Cancer Monograph Working Group (2009) A review of human carcinogens—part C: metals, arsenic, dusts, and fibres. Lancet Oncol 10(5): 453–454. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15): 2008–2012. [DOI] [PubMed] [Google Scholar]
- Sutton AJ, Abams KR, Jones DR, Sheldon TA, Song F (2000) Methods for Meta-analysis in Medical Research. John Wiley and Sons: Chichester. [Google Scholar]
- Szeszenia-Dabrowska N, Urszula W, Szymczak W, Strzelecka A (2002) Mortality study of workers compensated for asbestosis in Poland, 1970-1997. Int Arch Occup Environ Health 15(3): 267–278. [PubMed] [Google Scholar]
- Szeszenia-Dabrowska N, Wilczynska U, Kaczmarek T, Szymczak W (1991) Cancer mortality among male workers in the Polish rubber industry. Pol J Occup Med Environ Health 4(2): 149–157. [PubMed] [Google Scholar]
- Szeszenia-Dabrowska N, Wilczynska U, Szymczak W (1988. a) Mortality among female workers in an asbestos factory in Poland. Pol J Occup Med 1(3): 203–212. [PubMed] [Google Scholar]
- Szeszenia-Dabrowska N, Wilczynska U, Szymczak W (1988. b) A mortality study among male workers occupationally exposed to asbestos dust in Poland. Pol J Occup Med 1(1): 77–87. [PubMed] [Google Scholar]
- Szeszenia-Dabrowska N, Wilczynska U, Szymczak W (2000) Mortality of workers at two asbestos-cement plants in Poland. Int Arch Occup Environ Health 13(2): 121–130. [PubMed] [Google Scholar]
- Tola S, Kalliomäki PL, Pukkala E, Asp S, Korkala ML (1988) Incidence of cancer among welders, platers, machinists, and pipe fitters in shipyards and machine shops. Br J Ind Med 45(4): 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulvestad B, Kjaerheim K, Martinsen JI, Damberg G, Wannag A, Mowe G, Andersen A (2002) Cancer incidence among workers in the asbestos-cement producing industry in Norway. Scand J Work Environ Health 28(6): 411–417. [DOI] [PubMed] [Google Scholar]
- Ulvestad B, Kjaerheim K, Martinsen JI, Mowe G, Andersen A (2004) Cancer incidence among members of the Norwegian trade union of insulation workers. J Occup Environ Med 46(1): 84–89. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W (2010) Conducting Meta-Analyses in R with the metafor Package. J Stat Softw 36(3): 1–48. [Google Scholar]
- Viechtbauer W, Cheung MWL (2010) Outlier and influence diagnostics for meta-analysis. Res Syn Methods 1(2): 112–125. [DOI] [PubMed] [Google Scholar]
- Wilczynska U, Szymczak W, Szeszenia-Dabrowska N (2005) Mortality from malignant neoplasms among workers of an asbestos processing plant in Poland: results of prolonged observation. Int Arch Occup Environ Health 18(4): 313–326. [PubMed] [Google Scholar]
- Zhu H, Wang Z (1993) Study of occupational lung cancer in asbestos factories in China. Br J Ind Med 50(11): 1039–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.