Nakamura-Ishizu et al. report that megakaryocytes function as a niche to maintain HSC quiescence through CLEC-2–mediated production of Thpo and other key regulators of HSC function. These findings could enable manipulation of HSCs for clinical application.
Abstract
Hematopoietic stem cells (HSCs) depend on the bone marrow (BM) niche for their maintenance, proliferation, and differentiation. The BM niche is composed of nonhematopoietic and mature hematopoietic cells, including megakaryocytes (Mks). Thrombopoietin (Thpo) is a crucial cytokine produced by BM niche cells. However, the cellular source of Thpo, upon which HSCs primarily depend, is unclear. Moreover, no specific molecular pathway for the regulation of Thpo production in the BM has been identified. Here, we demonstrate that the membrane protein C-type lectin-like receptor-2 (CLEC-2) mediates the production of Thpo and other factors in Mks. Mice conditionally deleted for CLEC-2 in Mks (Clec2MkΔ/Δ) produced lower levels of Thpo in Mks. CLEC-2–deficient Mks showed down-regulation of CLEC-2–related signaling molecules Syk, Lcp2, and Plcg2. Knockdown of these molecules in cultured Mks decreased expression of Thpo. Clec2MkΔ/Δ mice exhibited reduced BM HSC quiescence and repopulation potential, along with extramedullary hematopoiesis. The low level of Thpo production may account for the decline in HSC potential in Clec2MkΔ/Δ mice, as administration of recombinant Thpo to Clec2MkΔ/Δ mice restored stem cell potential. Our study identifies CLEC-2 signaling as a novel molecular mechanism mediating the production of Thpo and other factors for the maintenance of HSCs.
Maintenance of hematopoietic stem cells (HSCs) within the adult BM is crucial for the healthy production of hematopoietic cells (Orkin and Zon, 2008). HSCs reside in a specialized microenvironment in the BM called the niche (Schofield, 1978). Along with cell-intrinsic programs, the niche influences the cell fate of HSCs, which in turn govern the homeostasis of the hematopoietic system (Nakamura-Ishizu et al., 2014a). The HSC niche is chiefly composed of nonhematopoietic cells, including immature osteoblasts (OBLs; Arai and Suda, 2007), endothelial cells (ECs; Butler et al., 2010; Ding et al., 2012), perivascular cells (Sugiyama et al., 2006; Ding et al., 2012), mesenchymal stem cells (MSCs; Méndez-Ferrer et al., 2010), sympathetic nervous cells (Katayama et al., 2006), adipocytes (Naveiras et al., 2009), and nonmyelinating Schwann cells (Yamazaki et al., 2011). Nonetheless, mature hematopoietic cells such as macrophages/monocytes (Chow et al., 2011), osteoclasts (Kollet et al., 2006), and regulatory T cells (Fujisaki et al., 2011) also regulate HSCs, albeit mainly in an indirect manner, through the modulation of nonhematopoietic niche cells. Recently, mature megakaryocytes (Mks) were described as hematopoietic progeny that directly regulate HSC quiescence (Heazlewood et al., 2013; Bruns et al., 2014; Zhao et al., 2014; Nakamura-Ishizu et al., 2014b); one of the mechanisms underlying Mk niche function is the production of the cytokine thrombopoietin (Thpo) by Mks themselves (Nakamura-Ishizu et al., 2014b). However, among the Mk-related niche factors reported to date, no molecular mechanism that is specific to Mks has been identified.
Thpo is a crucial cytokine for both the maturation of Mks and the maintenance of quiescent HSCs (Zucker-Franklin and Kaushansky, 1996; Qian et al., 2007; Yoshihara et al., 2007). Thpo is produced in multiple organs, including the liver, kidney, spleen, and muscle (Nomura et al., 1997). Baseline production of serum Thpo is thought to be maintained by the liver and regulated in response to inflammatory stress or changes in glycosylation of aged platelets (Kaser et al., 2001; Stone et al., 2012; Grozovsky et al., 2015). Serum Thpo levels also fluctuate according to circulating platelet number: platelets sequester Thpo via the myeloproliferative leukemia virus oncogene (c-Mpl), the receptor for Thpo (Kuter and Rosenberg, 1995; de Graaf et al., 2010), thereby lowering Thpo levels. Thus, platelet number is not as tightly regulated by Thpo production as erythrocyte number is by erythropoietin production (Fandrey and Bunn, 1993). It is likely that BM HSCs depend on Thpo, which is produced in the BM by niche cells. Depletion of circulating platelets by neuraminidase does not affect HSCs (Bruns et al., 2014), indicating that serum Thpo up-regulation through thrombocytopenia does not affect HSC maintenance. Moreover, HSCs reside near bone-lining OBLs and mature Mks, which both support HSCs by producing Thpo (Yoshihara et al., 2007; Nakamura-Ishizu et al., 2014b). However, the main cellular source of Thpo, upon which BM HSCs depend, and the molecular signaling pathway that mediates BM Thpo production remain elusive.
Recent studies showed that signals mediated through C-type lectin-like domain-containing receptors (CLEC-4H1 and CLEC-4H2; also known as Ashwell–Morell receptor) stimulate Thpo production in hepatocytes through recognition of desialylated platelets (Grozovsky et al., 2015). Platelets and Mks express CLEC-2 (Suzuki-Inoue et al., 2006, 2007), which is among the top 25 genes specifically expressed on Mks (Senis et al., 2007). Activation of platelet CLEC-2 through binding to sialylated podoplanin is essential for the segregation of lymphatic and blood vessels during development (Bertozzi et al., 2010; Suzuki-Inoue et al., 2010). CLEC-2–podoplanin signaling also functions in maintenance of lymphocyte- and dendritic cell–related responses in the stroma of lymph nodes (Acton et al., 2012, 2014; Herzog et al., 2013).
The significance of CLEC-2 expression on Mks in BM hematopoiesis, and whether it is involved in Thpo production in Mks, has not been previously explored. Here, we demonstrate that Mk-specific deficiency of CLEC-2 disrupts HSC quiescence and alters HSC potential as a result of defective Mk niche function. Moreover, we demonstrate that CLEC-2 signaling is involved in various molecular pathways for production of niche factors, including Thpo in Mks. Through the identification of CLEC-2, a novel Mk-specific factor, our data elucidate the organ-dependent production and function of Thpo and reinforce the idea that Mks contribute to a niche that regulates HSC quiescence.
RESULTS
CLEC-2 is highly expressed on BM Mks
CLEC-2 expression was detected in platelets, dendritic cells, and liver sinusoidal endothelia (Suzuki-Inoue et al., 2011); however, the expression of CLEC-2 in BM has not been previously investigated. Using immunohistochemistry (IHC), we observed expression of CLEC-2 protein on the surface of Mks and ECs in the BM (Fig. 1 A). Hematopoietic stem and progenitor cell (HSPC) fractions also expressed Clec2 transcripts (Fig. 1 B). Flow cytometry analysis confirmed surface CLEC-2 expression on HSPCs (Fig. 1 C). CLEC-2 protein expression was also compared in various hematopoietic (Fig. 1 D) and niche cells (Fig. 1 E). Among the hematopoietic and niche cells, CLEC-2 expression on CD41+ Mks and ECs was significantly high.
Figure 1.
CLEC-2 is expressed in Mks and is specifically deleted in Mks of Clec2MkΔ/Δ mice. (A) IHC of BM of 10-wk-old wild-type mice. CLEC-2 stains for VE-cadherin–positive ECs (arrowheads) and VE-cadherin–negative Mks (arrows). Enlargement of the dotted box is shown in the top right panel. CLEC-2 stains for CD41-positive Mks (bottom right). (B) Relative expression of CLEC-2 (Clec1b) transcripts in various hematopoietic cells. (C) Calculation of mean fluorescence ratios of CLEC-2 in various HSPC populations as measured by flow cytometry. (D and E) Mean fluorescence intensity of various hematopoietic (D) and niche (E) cells. (B–E) Means ± SD. n = 4; two independent experiments. *, P < 0.05; and **, P < 0.01 by Tukey’s test. (F) Detection of Clec2 deletion by genomic PCR. An intact exon yields a 2,500-bp product, whereas removal of floxed exon 1 results in a 1,200-bp product. (G) Expression of CLEC-2 in platelets and LT-HSCs from Clec2MkΔ/Δ (Δ/Δ) and Clec2+/+ (+/+) mice. Red, CLEC-2 antibody-stained; blue, isotype control. Means ± SD. n = 5; two independent experiments. P = 0.0078 for platelets and P = 0.69 for LT-HSCs by Student’s t test. (H) IHC of BM staining CLEC-2 and VE-cadherin in Clec2MkΔ/Δ (Δ/Δ) mice. Bars: (A and H) 100 µm; (A, top right) 25 µm. (I) Measurement of PB parameters in Clec2MkΔ/Δ (Δ/Δ) and Clec2+/+ (+/+) mice. Means ± SD. n = 5; three independent experiments. P-values as indicated by Student’s t test.
We investigated mice deficient in CLEC-2 specifically in the Mk lineage (PF4-Cre:Clec2flox/flox mice [Clec2MkΔ/Δ]), which are born and survive through adulthood (Osada et al., 2012). PCR analysis confirmed that Clec2 deletion was specific to Mk lineages: deletion was detected in genomic DNA from CD41+ (glycoprotein IIb+) cells, but not LSK (Lineage negative, Sca-1+, c-Kit+) cells, in the BM of Clec2MkΔ/Δ mice (Fig. 1 F). CLEC-2 protein level was significantly reduced in platelets from Clec2MkΔ/Δ mice but retained in their long-term HSCs (LT-HSCs; Fig. 1 G). Deletion of CLEC-2 from Mks was also confirmed with IHC (Fig. 1 H). Clec2MkΔ/Δ mice exhibited mild thrombocytopenia along with anemia (Fig. 1 I).
CLEC-2–deficient mice have impaired Mk cell population
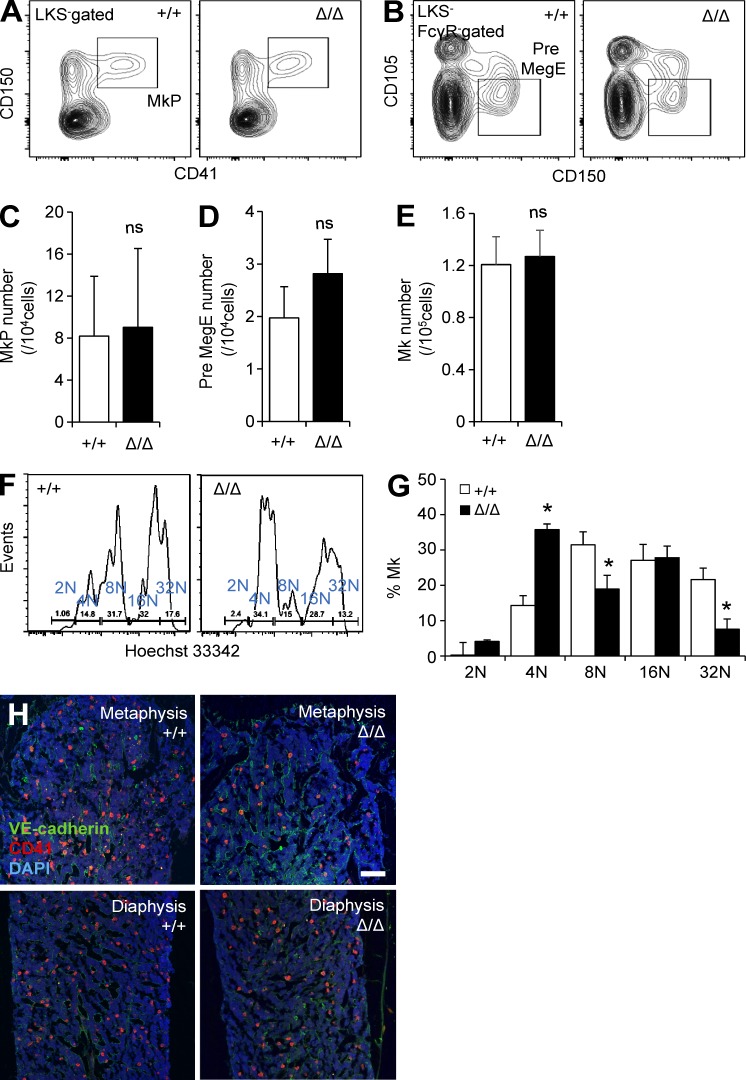
Because CLEC-2 is highly expressed in Mk lineages, we asked whether CLEC-2 deficiency altered the frequency and BM functions of Mk lineage cells. The frequencies and numbers of mature Mks and Mk progenitors (MkP and Pre MegE cells) were unaltered in Clec2MkΔ/Δ mice (Fig. 2, A–D), and total CD41+ Mk cell number was unaltered in Clec2MkΔ/Δ mice (Fig. 2 E). However, ploidy analysis revealed a reduced frequency of mature polyploid Mks with CLEC-2 deficiency (Fig. 2, F and G). IHC revealed a significant reduction in the number of Mks in the metaphyseal region of the BM in Clec2MkΔ/Δ mice (Fig. 2 H). These observations indicate that CLEC-2 deficiency modestly affects the maturation of Mks but drastically alters the distribution of Mks within the BM.
Figure 2.
Altered distribution of Mks in BM of Clec2MkΔ/Δ mice. (A and B) Representative flow cytometric plots showing Mk progenitor cell population (MkP: Lin−cKit+Sca-1−[LKS−] CD150+CD41+; Pre-MegE: LKS−FcγR−CD105+CD150+) in Clec2MkΔ/Δ (Δ/Δ) and Clec2+/+ (+/+) mice. (C–E) Number of MkP (C), Pre-MegE (D), and CD41+ Mks (E) in Δ/Δ and +/+ mice. Means ± SD. n = 5; two independent experiments. ns, P = 0.46 for C, P = 0.24 for D, and P = 0.24 for E by Student’s t test. (F and G) Representative flow cytometric plot showing Mks (F) and Mk frequency within the total Mk population (G), based on ploidy analysis of +/+ and Δ/Δ mice. Means ± SD. n = 4; two independent experiments. *, P < 0.05 by Tukey’s test. (H) IHC of BM, staining for ECs (VE-cadherin), Mks (CD41), and DAPI in the metaphysis and diaphysis of +/+ and Δ/Δ mice. Bar, 100 µm.
CLEC-2–deficient Mks exhibit impaired Thpo production and niche function
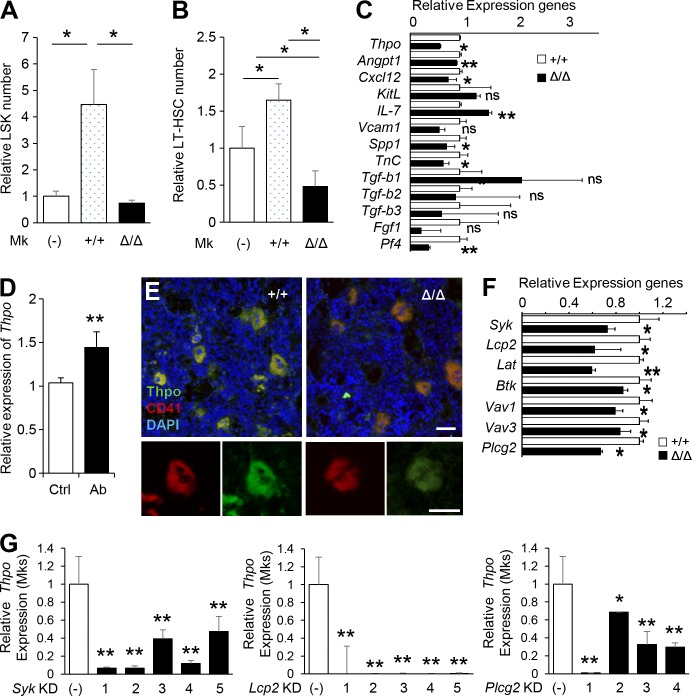
Mks can support HSC expansion in vitro, chiefly through the production of Thpo (Nakamura-Ishizu et al., 2014b). Hence, we asked whether CLEC-2 deficiency would alter the capacity of Mks to support HSCs in vitro. Addition of Mks to HSC cultures resulted in a Thpo-dependent increase in HSC number (Nakamura-Ishizu et al., 2014b). Accordingly, addition of Clec2+/+ Mks to cultured HSCs promoted proliferation, as indicated by expansion of LSK cells and LT-HSCs (Fig. 3, A and B). Clec2-deficient Mks exhibited a reduced capacity to stimulate HSPC proliferation and could not maintain LT-HSC populations (Fig. 3 B).
Figure 3.
CLEC-2–positive Mks produce Thpo. (A and B) Effect of 3 d of co-culture with Mks from Δ/Δ or +/+ mice on LSK cells (A) and LT-HSCs (B). Means ± SD. n = 4; two independent experiments. *, P < 0.05 by Tukey’s test. (C) Relative levels of niche factor (Thpo, Angpt1, Cxcl12, KitL, IL-7, Vcam1, Spp1, TnC, Tgf-b1, Tgf-b2, Tgf-b3, Fgf1, and Pf4) transcripts in Mks from Δ/Δ mice compared with +/+ mice. (D) Relative levels of Thpo transcripts were significantly up-regulated in cultured Mks treated with a CLEC-2 stimulatory antibody. (E) IHC of BM from +/+ and Δ/Δ mice. Mks, which are CD41, exhibit lower Thpo levels in CLEC-2–deficient Mks. Magnification of a representative Mk is shown in the bottom panels. Bars: (top) 50 µm; (bottom) 100 µm. (F) Gene expression of CLEC-2 downstream pathway molecules in Mks from +/+ and Δ/Δ mice. (G) Knockdown of Syk, Lcp2, and Plcg2 results in reduced expression of Thpo transcript in Mks. (C, D, F, and G) Means ± SD. n = 4; two independent experiments. ns, P > 0.05; *, P < 0.05; and **, P < 0.01 by Student’s t test.
Next, we assessed whether Clec2-deficient Mks exhibited changes in the production of various niche factors. Clec2-deficient Mks exhibited a broad range of decrease in the gene expression of various niche factors, including Angpt1, Cxcl12, IL-7, Spp1, TnC, and Pf4 (Fig. 3 C). Characteristically, Clec2-deficient Mks secreted less Thpo and altered Mk niche function. Clec2-deficient Mks exhibited significantly lower Thpo expression than Mks from Clec2+/+ mice (Fig. 3 C). Furthermore, wild-type Mks treated with CLEC-2–stimulating antibodies expressed significantly higher levels of Thpo transcripts (Fig. 3 D). The decrease of Thpo protein expression in CLEC-2–deficient Mks was confirmed by IHC (Fig. 3 E).
Transcript expression of CLEC-2 signaling pathway genes were decreased in CLEC-2–deficient Mks (Fig. 3 F). To confirm that CLEC-2 signaling affected Thpo production in Mks, we used inducible shRNAs to knock down three downstream molecules involved in CLEC-2 signaling: Syk, Lcp2, and Plcg2 (Suzuki-Inoue et al., 2011). Cultured LT-HSCs were transduced with lentiviral clones that inducibly express each shRNA and then cultured to obtain CD41+Ter119− Mks. Knockdown of Syk, Lcp2, and Plcg2 was highly efficient: transcripts of the targeted genes were nearly absent in sorted Mks (not depicted). Knockdown of Syk, Lcp2, or Plcg2 significantly decreased the expression of Thpo in the sorted Mks (Fig. 3 G). These data confirmed that CLEC-2 signaling is indeed critical for Thpo production in Mks.
CLEC-2 deficiency in Mks directly affects HSC cycle quiescence
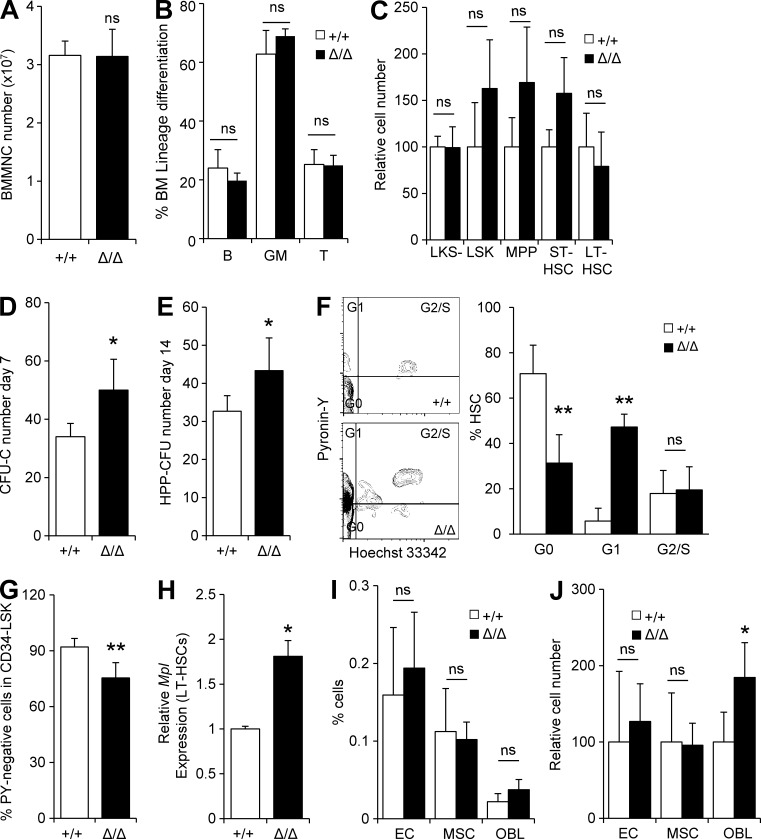
Because CLEC-2–deficient Mks exhibited diminished Thpo production and could not support cultured HSCs, we next assessed whether HSCs in Clec2MkΔ/Δ mice were affected in vivo. Lineage and HSPC differentiation were unchanged in the BM of Clec2MkΔ/Δ mice (Fig. 4, A–C). Functionally, HSPCs from Clec2MkΔ/Δ mice displayed a significant elevation in colony-forming capacity, indicating an increase in the proliferation capacity of HSPCs (Fig. 4, D and E). Cell cycle analysis of HSCs, as assessed by Pyronin Y staining, indicated loss of HSC quiescence (Pyronin Y–negative CD34− LSK cells) in Clec2MkΔ/Δ mice (Fig. 4, F and G). c-Mpl expression was significantly up-regulated in LT-HSCs from Clec2MkΔ/Δ mice, suggestive of deficient Thpo signaling (Fig. 4 H). Loss of nonhematopoietic niche cells (ECs, MSCs, and OBLs) was not observed in BM of Clec2MkΔ/Δ mice (Fig. 4, I and J).
Figure 4.
HSCs from Clec2MkΔ/Δ mice exhibit reduced stem cell quiescence. (A–C) Number of BM MNCs (A), lineage composition in the BM (B), and relative number of BM HSPCs (C) in +/+ and Δ/Δ mice. (D and E) CFU-C number (D) and HPP-CFC number (E) of 500 HSCs from Δ/Δ and +/+ mice. (A–E) Means ± SD. n = 6; two independent experiments. ns, P > 0.05; and *, P < 0.05 by Student’s t test. (F) Cell cycle analysis using Pyronin Y and Hoechst 33342 staining of CD34− LSK cells in +/+ and Δ/Δ mice. (G) Cell cycle state of CD34− LSK cells with Pyronin Y staining in +/+ and Δ/Δ mice. (H) Relative levels of Mpl transcripts in LT-HSCs from +/+ and Δ/Δ mice. (I and J) Number (I) and frequency (J) of ECs (CD45−Ter119−CD31+), MSCs (CD45−Ter119−Sca-1+ALCAM−), and OBLs (CD45−Ter119−CD31−Sca-1−ALCAM+) in Δ/Δ and +/+ mice. (F–J) Means ± SD. n = 4; two independent experiments. ns, P > 0.05; *, P < 0.05; and **, P < 0.01 by Student’s t test.
Although CLEC-2 is also expressed on ECs, EC-specific CLEC-2 deletion did not alter hematopoiesis: VE-cadherin-Cre:Clec2floxed/floxed mice exhibited no change in peripheral blood (PB) parameters (not depicted), HSPC numbers, HSC quiescence, or repopulation potential (not depicted). These observations indicate that Mk-specific Clec2 deficiency stimulates HSCs to exit the G0 phase of the cell cycle.
HSCs from Clec2MkΔ/Δ mice exhibit reduced stem cell potential
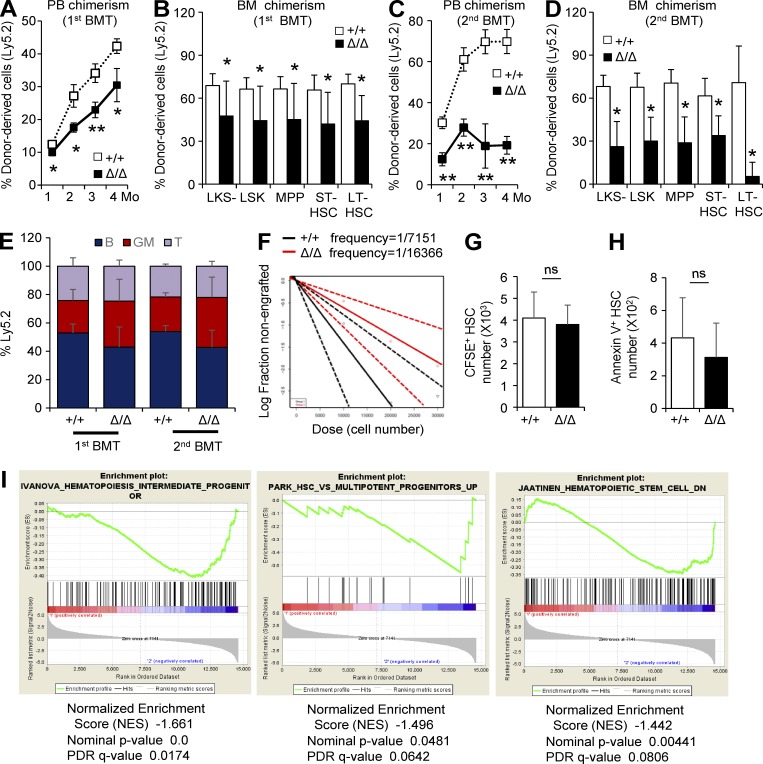
To further investigate the stem cell potential of HSCs from Clec2MkΔ/Δ mice, we performed a competitive repopulation assay, using BM transplantation (BMT) of LT-HSCs from Clec2+/+ or Clec2MkΔ/Δ mice (Ly5.2) into C57BL/6-Ly5.1 recipients. HSCs from Clec2MkΔ/Δ mice had lower engraftment rates, as assessed by chimerism in PB and BM of various HSPC fractions 4 mo after primary and secondary BMT (Fig. 5, A–D). No significant difference was noted in the lineage differentiation of engrafted HSCs from Clec2+/+ or Clec2MkΔ/Δ mice (Fig. 5 E). Limiting dilution analysis revealed a significantly lower frequency of repopulating HSCs in the BM of Clec2MkΔ/Δ mice (Fig. 5 F). HSCs from Clec2MkΔ/Δ mice did not exhibit defective homing to the BM (Fig. 5 G). In addition, HSCs from Clec2MkΔ/Δ mice did not exhibit higher rates of apoptosis, as assessed by Annexin V staining (Fig. 5 H). Gene set enrichment analysis of LT-HSCs from Clec2MkΔ/Δ mice revealed altered HSC-related gene expression profiles, notably an enrichment in progenitor cell–related genes, relative to LT-HSCs from Clec2+/+ mice, suggesting enhanced differentiation and a loss of stem cell character of HSCs from Clec2MkΔ/Δ mice (Fig. 5 I). These results indicate a loss of stem cell potential in Clec2MkΔ/Δ mice.
Figure 5.
HSCs from Clec2MkΔ/Δ mice exhibit reduced repopulation capacity. (A and C) Percentage of donor-derived cells (Ly5.2) in PB after the first and second BMT at the indicated time intervals (in months). (B and D) Percentage of donor-derived cells (Ly5.2) in BM HSPCs after the first and second BMT at the indicated time intervals (in months). (A–D) Means ± SEM. n = 6; two independent experiments. *, P < 0.05; and **, P < 0.01 by Student’s t test. (E) Lineage composition of Ly5.2 MNCs in recipient mice. Means ± SEM. n = 6; two independent experiments. P > 0.05 in all groups by Student’s t test. (F) Extreme limiting dilution assay of BM MNCs from Δ/Δ and +/+ mice. n = 7; two independent experiments. P = 0.0432 by Pearson’s χ2 test. (G) Number of CFSE staining LT-HSCs from Δ/Δ and +/+ mice homing to the BM 24 h after BMT. Note that no significant difference was present in homing capacity of HSCs from Δ/Δ and +/+ mice. (H) Number of Annexin V+ HSCs in Δ/Δ and +/+ mice. (G and H) Means ± SD. n = 4; two independent experiments. ns, P = 0.677 (G) or P = 0.48 (H) by Student’s t test. (I) Gene set enrichment analysis of LT-HSCs from Δ/Δ and +/+ mice (n = 10). Genes expressed by intermediate and multipotent progenitors were significantly up-regulated in LT-HSCs from Δ/Δ mice (plots are second and third from left). Statistical analysis is as shown in figure.
Clec2MkΔ/Δ mice have major developmental vascular defects, chiefly in the lymphatic system (Suzuki-Inoue et al., 2010; Finney et al., 2012). To verify that the loss of stem cell potential resulted specifically from Clec2 deletion in BM Mk lineages, we transplanted BM mononuclear cells (MNCs) from Clec2+/+ or Clec2MkΔ/Δ mice (Ly5.2) along with BM MNCs from Ly5.1 mice at a 4:1 ratio into lethally irradiated Ly5.1 wild-type mice to test the effect of Clec2-deleted Mks on wild-type HSCs (Fig. 6 A). We observed a high frequency of Ly5.2+ Mks in recipient BM (Fig. 6 B). The number of engrafted Ly5.1 LT-HSCs (CD34−Flt-3− HSCs and CD150+CD48−CD41− [SLAM] HSCs) was significantly lower in transplants from Clec2MkΔ/Δ mouse donors (Fig. 6 C). Furthermore, the percentage of Ly5.1+ Pyronin Y–negative HSCs was significantly lower when Clec2MkΔ/Δ mice served as donors (Fig. 6, D and E). Also, both Ly5.1+ and Ly5.2+ HSCs in the recipient of Clec2MkΔ/Δ mouse donors stained higher levels of Ki67 (Fig. 6 F), indicating loss of cell cycle quiescence. These data suggest that the HSC defects exhibited by Clec2MkΔ/Δ mice are a direct consequence of Clec2 depletion in Mks.
Figure 6.
Loss of HSC quiescence in HSCs in Clec2MkΔ/Δ mice is Mk specific. (A) Scheme of the experimental model for BMT. 8 × 106 BM MNCs from Δ/Δ and +/+ mice were transplanted into lethally irradiated (Ly5.1) mice along with Ly5.1-positive 2 × 106 BM MNCs. (B) Percentage of Ly5.2-positive Mks in BMs of recipient mice. (C) Relative number of Ly5.1-positive HSPCs in recipient mice. (B and C) Means ± SD. n = 10; two independent experiments. ns, P = 0.167; *, P < 0.05; and **, P < 0.01 by Student’s t test. (D) Ly5.1+ Pyronin Y–negative cells in CD34− LSK cells in the BMs of recipient mice. (E) Ly5.1+ Pyronin Y–negative cells in CD34+ LSK cells in the BMs of recipient mice. (D and E) Means ± SD. n = 5; two independent experiments. **, P < 0.01 by Student’s t test. (F) Ratio of fluorescence intensity of Ki67 and TOTO-3 on single sorted cells in Ly5.1+ or Ly5.2+ HSCs in chimeric mice. Means ± SEM. n = 50; two independent experiments. **, P < 0.01 by Student’s t test.
Mk-specific CLEC-2 depletion results in extramedullary hematopoiesis
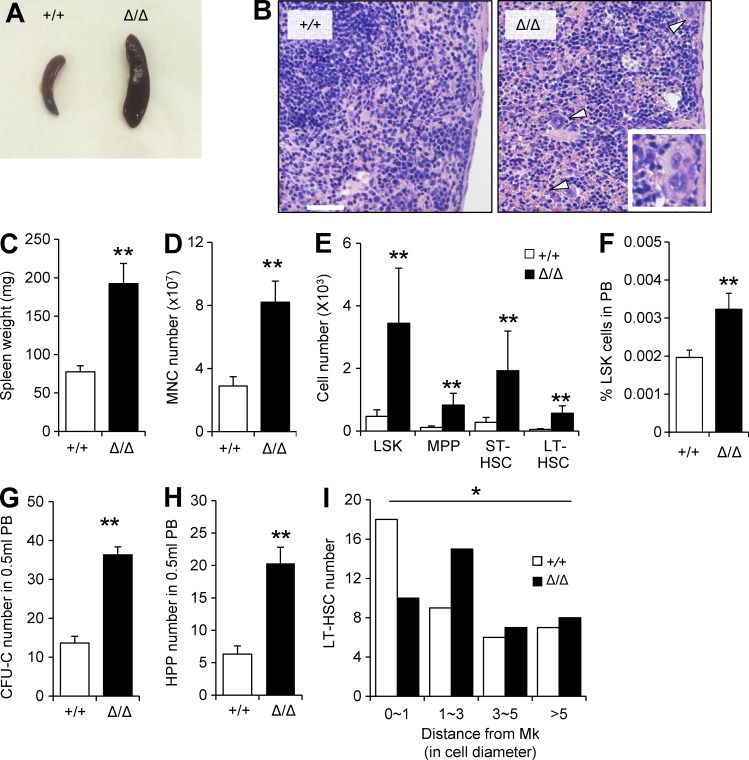
We next asked whether CLEC-2 deficiency affected HSC retention in the BM. Clec2MkΔ/Δ mice exhibited massive splenomegaly (Fig. 7, A and B). Both HSC and HSPC number and frequency were elevated in Clec2MkΔ/Δ mice (Fig. 7, C–E), indicating that the splenomegaly was caused by extramedullary hematopoiesis. PB of HSC Clec2MkΔ/Δ mice exhibited increased numbers of LSKs and LT-HSCs (Fig. 7 F). Furthermore, a significant increase in CFU-C and HPC-CFC was observed in the PB of Clec2MkΔ/Δ mice (Fig. 7, G and H), and the distance between LT-HSCs and Mks was significantly higher in Clec2MkΔ/Δ mice than in Clec2+/+ mice (Fig. 7 I). These data show that chronic loss of CLEC-2–expressing Mks decreased HSC retention in BM and induced extramedullary hematopoiesis in the spleen.
Figure 7.
Clec2MkΔ/Δ mice exhibit extramedullary hematopoiesis and elevated mobilization of HSCs into PB. (A and B) Gross appearance (A) and hematoxylin and eosin staining (B) of spleens from +/+ and Δ/Δ mice. Arrowheads indicate the presence of Mks in Clec2Mk+/+ spleens. Magnification of an Mk is shown in the inset. Bar, 200 µm. (C–E) Analysis of spleen of Δ/Δ and +/+ mice. Spleen weight (C), total MNC number (D), and absolute populations of various HSPC fractions (E) are significantly higher in spleens of Clec2MkΔ/Δ compared with Clec2Mk+/+ mice. (F–H) Percentage of LSK cells (F), CFU-C number (G), and HPP-CFC number (H) in PB of +/+ and Δ/Δ mice. (C–H) Means ± SD. n = 6; two independent experiments. **, P < 0.01 by Student’s t test. (I) Quantitation of distance of LT-HSCs from Mks in +/+ and Δ/Δ mice. Means ± SD. n = 6; two independent experiments. *, P = 0.038 by Tukey’s test.
Administration of Thpo rescues HSC phenotypes in Clec2MkΔ/Δ mice
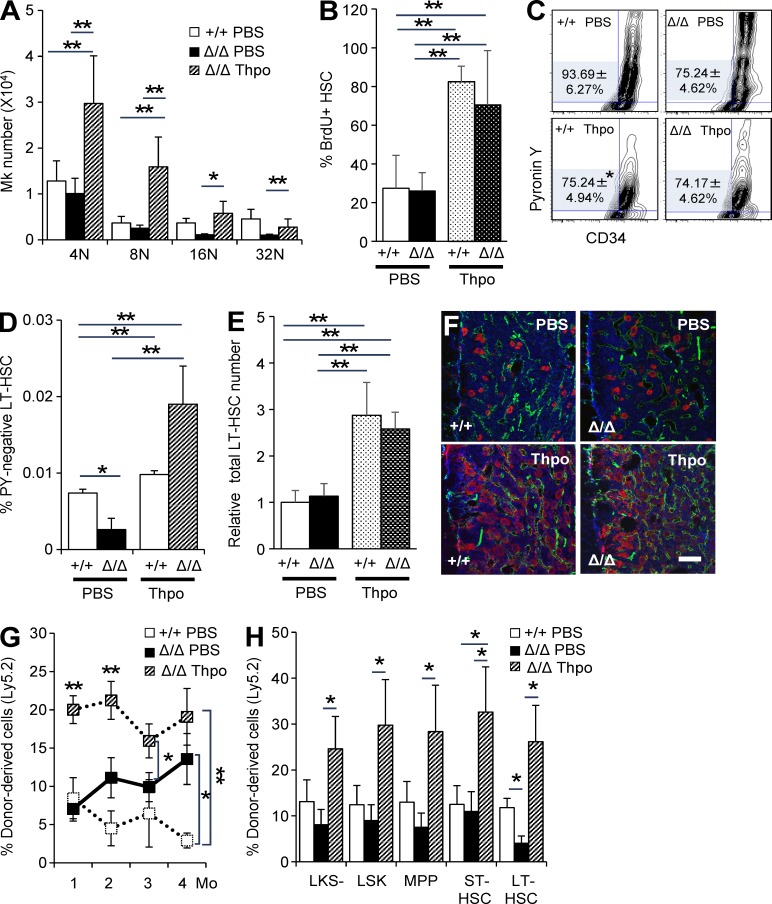
To determine whether the functional defects in HSCs from Clec2MkΔ/Δ mice could be attributed to reduced Thpo levels, we administered recombinant Thpo (PEG-rHuMGDF) to both Clec2+/+ and Clec2MkΔ/Δ mice. Intravenous Thpo injection for four consecutive days restored the number and frequency of polyploid Mks in Clec2MkΔ/Δ mice (Fig. 8 A). In agreement with a previous study (Walter et al., 2015), administration of Thpo stimulated proliferation of HSCs, as shown by the increased the percentage of BrdU-positive HSCs and the percentage of Pyronin Y–negative cells in CD34-LSK cells in both Clec2+/+ and Clec2MkΔ/Δ mice (Fig. 8, B and C). However, as Thpo administration instigates self-renewal of HSCs, the absolute number and frequency of CD34− Pyronin Y–negative HSCs within the BM were restored (Fig. 8, D and E). Thpo administration also significantly increased the number of Mks within the BM in both Clec2+/+ and Clec2MkΔ/Δ mice (Fig. 8 F). The repopulation potential of HSCs from Clec2MkΔ/Δ mice significantly increased with Thpo injection, as confirmed by competitive BMT (Fig. 8, G and H). Thus, the loss of HSC potential in Clec2MkΔ/Δ mice depended on the defective Thpo production of Mks.
Figure 8.
Maintenance of HSC quiescence requires Thpo produced by CLEC-2–positive Mks. (A) Changes in Mk number based on ploidy analysis after intravenous Thpo injection in Clec2MkΔ/Δ (Δ/Δ) and Clec2Mk+/+ (+/+) mice. Controls were injected with PBS. (B) Change in percentage of BrdU-positive HSCs after intravenous Thpo injection in Δ/Δ and +/+ mice. (A and B) Means ± SD. n = 4 (B) or 5 (A); two independent experiments. **, P < 0.01 by Tukey’s test. (C) Representative flow cytometry plot of Pyronin Y and Hoechst 33342 for CD34-LSK cells from Δ/Δ and +/+ mice after administration of PBS or Thpo. Numbers indicate percentage of Pyronin Y–negative cells in CD34-LSK cells. Means ± SD. n = 4; two independent experiments. *, P < 0.05 by Student’s t test. (D and E) Total number (D) and frequency (E) of Pyronin Y–negative CD34-LSK cells. Means ± SD. n = 4; two independent experiments. **, P < 0.01 by Tukey’s test. Note that Pyronin Y–negative CD34-LSK cell number is restored in Clec2MkΔ/Δ (Δ/Δ) BM after Thpo injection. (F) IHC of BMs from Δ/Δ and +/+ mice after administration of PBS or Thpo. Bar, 100 µm. (G and H) Percentage of donor-derived cells in PB (G) and BM HSPCs (H) at the indicated intervals after BMT. Means ± SEM. n = 6; two independent experiments. *, P < 0.05; and **, P < 0.01 by Tukey’s test.
DISCUSSION
In this study, we characterized the niche function of a C-type lectin-like family member, CLEC-2, in Mks. Mks from Clec2MkΔ/Δ mice exhibited reduced Thpo expression at both the gene and protein level. Knockdown of the CLEC-2 downstream pathway affected the expression of a broad range of niche factors in Mks, characteristically one being Thpo. Mk-specific deletion of CLEC-2 caused a subtle reduction in Mk number but significantly affected HSC quiescence and repopulation potentials. Administration of recombinant Thpo rescued HSC defects in Clec2MkΔ/Δ mice, indicating that defective Thpo production impaired HSC function in Clec2MkΔ/Δ mice. Thus, we demonstrated that CLEC-2 is an upstream factor essential for Mk-mediated maintenance of HSCs.
Our results identify Mk CLEC-2 signaling as a novel signaling pathway involved in the production of Thpo in the BM. Systemic Thpo levels depend on sequestration of Thpo by myeloproliferative leukemia protein (c-Mpl), a Thpo receptor, expressed on platelets (Kuter and Rosenberg, 1995). However, Mks in Clec2MkΔ/Δ mice produced lower levels of Thpo, despite the presence of thrombocytopenia, indicating that systemic Thpo levels determined by the availability of c-Mpl did not affect Mk-produced Thpo for the maintenance of HSCs. It remains controversial whether liver production of Thpo can compensate for reduced systemic levels of this cytokine (McCarty et al., 1995; Qian et al., 1998). Thpo transcript levels in the liver increase with inflammatory stress and accumulation of desialylated platelets (Wolber et al., 2001; Grozovsky et al., 2015) but does not change in thrombocytopenic mouse models (Cohen-Solal et al., 1996). Clec2MkΔ/Δ mice exhibited no change in liver Thpo transcript levels (unpublished data), indicating that CLEC-2 deficiency did not stimulate liver Thpo production. Therefore, our data indicate that the BM Thpo level is loosely associated with serum and liver Thpo levels and that Mks supply a critical amount of Thpo for maintenance of HSCs in the BM. However, our data do not eliminate the possibility that Thpo production by the BM stroma is necessary to maintain HSCs in the BM. In fact, OBLs produce Thpo (Yoshihara et al., 2007), and the platelet α-granule proteins PDGF and FGF-2 can stimulate expression of Thpo in BM stromal cells in vitro (Sungaran et al., 2000). Further studies are necessary to clarify the degree to which HSCs rely on locally or systemically produced Thpo.
Although HSCs from Clec2MkΔ/Δ mice were responsive to exogenous Thpo, indicating that the loss of stem cell potential of HSCs in Clec2MkΔ/Δ mice was caused by deficiency of Thpo, our data cannot differentiate whether Thpo directly or indirectly affected HSCs through the modulation of Mk number and function. Indeed, exogenous Thpo administration robustly enhances Mk proliferation and Mks can produce various factors other than Thpo that can affect HSC stem cell potential (Bruns et al., 2014; Zhao et al., 2014). Supporting this, although our analysis revealed Thpo as a prominent niche factor affected by CLEC-2 depletion, CLEC-2 deficiency also affected other niche-related gene expressions (such as Cxcl12, Angpt1, Vcam1, and Pf4) in Mks. In addition, CLEC-2–deficient Mks exhibited decrease in various CLEC-2 downstream pathways. These data may indicate that CLEC-2 signaling may be involved in the modulation of a broad range of Mk functions. Our data further position Mks as a potent niche cell but emphasize the need to elucidate the complex niche regulation on HSCs exerted by Mks.
Recent studies showed that specific depletion of Mks disrupts HSC quiescence through different mechanisms. Induced depletion of Mks for 7 d in PF4-Cre:iDTR mice chronically activates HSCs to proliferate and self-renew, through either platelet factor 4 (PF4; Bruns et al., 2014) or transforming growth factor-β1 (TGF-β1; Zhao et al., 2014). In contrast, we showed that acute depletion of Mks in PF4-Cre:iMos-Csp mice resulted in disruption of HSC quiescence and repopulation potentials without HSC self-renewal (Nakamura-Ishizu et al., 2014b). These three studies all reported direct Mk regulation of HSCs, yet portrayed Mks as acting in different modes, according to the time point after induction of Mk deletion. Confusing matters further, Mk depletion affected different subsets of HSCs in conflicting ways: CD34−Flt3−LSK cells exhibit modest or no increase in number, whereas CD150+CD105+LSK cells expanded up to 15-fold (Bruns et al., 2014; Zhao et al., 2014). The differences in the HSC niche functions of Mks may be attributed to the fact that depletion of Mks and platelets dramatically changes the levels of multiple factors. CLEC-2 depletion in Mks did not instigate self-renewal of HSCs or significant changes in platelet lineage-biased CD150+CD105+LSK cells (Pronk et al., 2007; unpublished data), but it did reduce the stem cell potential of LT-HSCs (CD34−Flt3−LSK; Christensen and Weissman, 2001). Our study indicates that CLEC-2–mediated Mk regulation of HSCs is not committed to the self-renewal of Mk lineage-biased HSCs, but instead specifically influences the quiescence and stem cell potential of a broader spectrum of HSCs. CLEC-2 may be an Mk-specific niche regulatory factor that shows the long-term effect of Mk regulation on HSC maintenance.
Constitutive deletion of CLEC-2 and conditional deletion of CLEC-2 from Mk/platelet lineages reportedly results in blood and lymphatic vessel dis-separation phenotype (Bertozzi et al., 2010; Suzuki-Inoue et al., 2010). Recently, the role of CLEC-2 in lymph node development and adult lymph node maintenance was reported (Herzog et al., 2013; Bénézech et al., 2014). Chimeric mice in which BMs of WT mice were reconstituted with BMs from Clec2MkΔ/Δ mice have been reported to present hemorrhage in the lymph nodes, indicating that platelet CLEC-2 maintains the integrity of vessels in the adult lymph node. No apparent hemorrhages in the lymph nodes were observed in the chimeric mice in which BMs of Clec2MkΔ/Δ mice and Clec2+/+ mice was transplanted in a 4:1 ratio, presumably because of the mixture of Clec2+/+ mouse–derived BM (unpublished data). The gross morphology of vasculature in the BM was unaffected in Clec2MkΔ/Δ mice, and no hemorrhage was observed in the BMs (unpublished data). Moreover, EC-specific deletion of CLEC-2 did not affect hematopoiesis. However, whether deficiency of CLEC-2 in platelets affected the function of BM ECs, especially in association to immune response, should be investigated in the future.
A characteristic finding in the Clec2MkΔ/Δ mice was the presence of extramedullary hematopoiesis. Thpo is known as a cytokine to induce HSC mobilization (Murray et al., 1998). Accordingly, administration of exogenous Thpo to Clec2MkΔ/Δ mice increased mobilization of HSCs to PB and did not rescue the extramedullary hematopoiesis phenotype. Therefore, decreased levels of Thpo in Clec2MkΔ/Δ mice are not causative of the extramedullary hematopoiesis phenotype. The mechanism of how Mks retain HSCs within the BM and how CLEC-2 signaling associates with HSC retention is yet to be investigated.
Mks indirectly regulate HSCs by stimulating OBLs after transplantation (Olson et al., 2013). Mk numbers in Clec2MkΔ/Δ mice were reduced preferentially in the metaphyseal region of the BM. Podoplanin, an activating ligand of CLEC-2 (Bertozzi et al., 2010), was highly expressed in osteo-lineage cells (Schacht et al., 2005). Displacement of Mks in Clec2MkΔ/Δ mice from the bone-rich metaphysis suggests that CLEC-2 deficiency in Mks may impair Mk and OBL interactions. Furthermore, it indicates that OBLs may indirectly regulate Thpo production in Mks via their expression of podoplanin. Moreover, our findings suggest that CLEC-2–podoplanin signaling may be a novel molecular pathway for niche cell function. Indeed, CLEC-2–deficient Mks exhibited decreased gene expression of CLEC-2 downstream molecules, and knockdown of signals downstream of the CLEC-2–podoplanin interaction (Syk, Lcp2, and Plcg2) confirmed that CLEC-2 signaling is crucial for Mk Thpo production. Loss of sialic acid expression on platelets contributes to Thpo production in the liver (Grozovsky et al., 2015). Because the lectin-like properties of CLEC-2 allow it to interact with sialic acid residues on podoplanin (Pan et al., 2014), our findings provide insight into the involvement of glycosylation in Thpo production.
In summary, our study strongly indicates that Mks function as a niche to maintain HSC quiescence through CLEC-2. These findings could enable manipulation of HSCs and Mks for clinical applications, as well as therapies against diseases related to defects in HSCs and Mks.
MATERIALS AND METHODS
Mice
All mice were in the C57BL/6 background. Clec2 flox/flox mice were described previously (Osada et al., 2012). PF4-Cre transgenic mice were provided by R.C. Skoda (University Hospital, Basel, Switzerland; Tiedt et al., 2007). Clec2flox/flox mice were crossed with either PF4-Cre or VE-cadherin-Cre (VEC-Cre) transgenic mice (stock 006137 purchased from The Jackson Laboratory) to obtain PF4-Cre:Clec2flox/flox mice (Clec2MkΔ/Δ) or VEC-Cre:Clec2flox/flox mice (Clec2ECΔ/Δ), respectively. C57BL/6-Ly5.1 or C57BL/6-Ly5.2 mice were used for competitive repopulation assays. Unless specified, 10–12-wk-old mice were used in all experiments. All animal experiments were approved by Keio University and performed in accordance with the Guidelines of Keio University for Animal and Recombinant DNA experiments.
PB analysis and colony assays
PB was collected from the tail vein in a heparinized microtube (Drummond Scientific) and analyzed using CellTac (NIHON KOHDEN). For colony assays and assessment of PB mobilization of HSCs, PB was collected from the inferior vena cava of anesthetized mice using a 27G needle. MNCs from 0.5 ml PB were obtained by centrifugation using Lymphoprep (Axis-Shield) and then used for colony assays. Colony counts for CFU-C and HPP-CFC were assessed on days 7 and 14, respectively.
Antibodies
Primary antibodies used for IHC and flow cytometry were as follows: c-Kit (2B8; eBioscience), CD16/32 (93; eBioscience), VE-cadherin (eBioscience), c-Kit (R&D Systems), Sca-1 (E13-161.7; BioLegend), CD48 (HM48-1; BioLegend), CD150 (TC15-12F12.2; BioLegend), IL-7Rα (SB/199; BioLegend), endoglin (MJ7/18; BioLegend), CD4 (L3T4; BD), CD8 (53-6.72; BD), B220 (RA3-6B2; BD), TER-119 (BD), Gr-1 (RB6-8C5; BD), CD34 (RAM34; BD), Mac-1 (M/70; BD), Flt-3 (A2F10.1; BD), CD41 (MWReg30; BD), CD45.2 (104; BD), CD45.1 (A20; BD), GPIbα (Xia.G5; Emfret), Clec2 (AbD Serotec), and Thpo (Bioss). Secondary antibodies for IHC were Alexa Fluor 488–conjugated IgGs (Molecular Probes) or Cy3/Cy5/DyLight549/DyLight649-conjugated IgGs (Jackson ImmunoResearch Laboratories, Inc.). IHC specimens were treated with DAPI (Molecular Probes) for nuclear staining. Stimulatory rabbit anti–mouse CLEC-2 antibody was a gift of K. Suzuki-Inoue.
Immunostaining of BM
Decalcified BM sections were prepared and stained as described previously (Nakamura-Ishizu et al., 2012). Frozen sections prepared according to the Kawamoto method (Kawamoto, 2003) were used to stain Lin−CD41−CD48−CD150+ cells in the BM.
Confocal microscopy and quantification of fluorescent images
Fluorescence images were obtained using a confocal laser-scanning microscope (FV1000; Olympus). Scanning was performed in sequential laser emission mode to avoid scanning at other wavelengths. Images obtained from BM sections were analyzed using the TissueQuest image analysis software (TissueGnostics).
Flow cytometric analysis, cell cycle analysis, and competitive repopulation assays
Flow cytometric analysis and competitive repopulation assays were performed as described previously (Arai et al., 2004). Cell cycle analysis of hematopoietic cells was performed using Pyronin Y staining and short-term BrdU incorporation assays (Takubo et al., 2010). For Ki-67 staining, HSCs were sorted and attached to glass slides with sedimentation and subsequently stained for Ki-67 and TOTO-3. Cells were observed under a confocal laser-scanning microscope (FV1000) for measurement of single cell fluorescence intensity and calculated for their relative Ki-67 fluorescence against nuclear stain (TOTO-3).
BMT
BM MNCs (4 × 105 cells) from C57BL/6-Ly5.1 mice, together with LT-HSCs (5 × 102 cells) from the indicated mice (Ly5.2), were transplanted into lethally irradiated C57BL/6-Ly5.1 congenic mice. Secondary transplantations into lethally irradiated C57BL/6-Ly5.1 congenic mice were performed using 2 × 106 BM MNCs from primary recipients. Recipient mice were sacrificed for analysis 4 mo after BMT. For extreme limiting dilution assays (Hu and Smyth, 2009), lethally irradiated Ly5.1 mice were transplanted with 104, 2 × 104, or 3 × 104 BM MNCs from either Clec2+/+ or Clec2MkΔ/Δ mice along with 2 × 105 Ly5.1 competitor cells. PB chimerism for the limiting dilution assay was assessed 12 wk after transplantation.
In vitro HSC and Mk co-cultures
Mature Mks (B220−Mac-1−Gr-1−CD41+) were obtained from mouse BM as described previously (Heazlewood et al., 2013). LT-HSCs (Lin−c-Kit+Sca-1+Flt-3−CD34−) sorted from Ly5.1 mice were co-cultured for 3 d with Mks in SF-O3 medium (Sankyo Junyaku) supplemented with murine recombinant SCF (100 ng/ml) with or without human recombinant Thpo (100 ng/ml) and then analyzed (Kabaya et al., 1996). LT-HSCs and Mks were cultured at a 1:1 ratio. Mks were cultured for 2 d to obtain conditioned medium for the indicated experiments. To inhibit Thpo activity, a recombinant mouse ThpoR (Mpl) Fc chimera (0.4 µg/ml; R&D Systems) was added to the culture. An IgG Fc fragment (0.4 µg/ml; Jackson ImmunoResearch Laboratories, Inc.) served as a control. For knockdowns in Mks, MISSION custom vectors (Sigma-Aldrich) were used.
In vivo Thpo assays
For in vivo stimulation of Thpo signaling, recombinant human Thpo (PEG-rHuMGDF; Kabaya et al., 1996; donated by Kyowa Hakko Kirin Co., Ltd.) was administered. Mice were treated with either 100 µg/kg (i.v.) of PEG-rHuMGDF or human IgG Fc fragment (Jackson ImmunoResearch Laboratories, Inc.). For rescue experiments, Clec2MkΔ/Δ mice were treated for four consecutive days.
Quantitative PCR assay
Isolated RNA was reverse transcribed with Superscript VILO (Invitrogen). Quantitative PCR assays were performed using an ABI 7500 Fast Real-Time PCR System (Applied Biosystems), SYBR Premix Ex Taq (Takara Bio Inc.), and primer sets for each gene (Takara Bio Inc.). Values obtained were normalized to β-actin expression and expressed as fold induction relative to control samples.
Genomic PCR assay
Genomic DNA was isolated from sorted cells using NucleoSpin (Takara Bio Inc.), and PCR was conducted using 5′-ACGTATCTCTGAACATCCAAGAAAG-3′ and 5′-CTGATCTTACCTGCATTCCATTAGT-3′ as primers.
Gene set enrichment analysis
Total RNA was extracted from LT-HSCs (Lin−cKit+Sca-1+CD34−Flt-3−) from Clec2Mk+/+ and Clec2MkΔ/Δ mice. Total RNA was purified using an RNeasy Mini kit (QIAGEN). Microarray processing was performed by DNA Chip Research Inc. Normalized expression data were assessed using GSEA v2.0.13 software (Broad Institute). Gene sets used were PARK_HSC_VS_MULTIPOTENT_PROGENITORS_DN, PARK_HSC_VS_MULTIPOTENT_PROGENITORS_UP, PARK_HSC_MARKERS, PARK_HSC_AND_MULTIPOTENT_PROGENITORS, IVANOVA_HEMATOPOIESIS_STEM_CELL, GRAHAM_CML_QUIESCENT_VS_NORMAL_QUIESCENT_UP, GRAHAM_NORMAL_QUIESCENT_VS_NORMAL_DIVIDING_UP, BYSTRYKH_HEMATOPOIESIS_STEM_CELL_AND_BRAIN_QTL_TRANS, BAKKER_FOXO3_TARGETS_UP, IVANOVA_HEMATOPOIESIS_EARLY_PROGENITOR, IVANOVA_HEMATOPOIESIS_INTERMEDIATE_PROGENITOR, IVANOVA_HEMATOPOIESIS_LATE_PROGENITOR, IVANOVA_HEMATOPOIESIS_STEM_CELL_LONG_TERM, IVANOVA_HEMATOPOIESIS_STEM_CELL_SHORT_TERM, JAATINEN_HEMATOPOIETIC_STEM_CELL_UP, and JAATINEN_HEMATOPOIETIC_STEM_CELL_DN, obtained from the Molecular Signatures Database v4.0 available at the GSEA web site. The number of permutations was set at 1,000. Gene sets with nominal p-value <0.05 and a false discovery rate q-value (FDR-q) <0.25 were considered statistically significant.
Statistical analysis
All results are expressed as means ± SD unless otherwise specified. Statistical significance was determined by Tukey’s multiple comparison test. The two-tailed Student’s t test was used for two-group comparisons. All experiments were conducted and confirmed in at least two replicates.
Acknowledgments
The authors thank T. Muraki and T. Hirose for technical assistance.
T. Suda is supported by the National Medical Research Council (NMRC) grant of Singapore Translational Research Investigator (STaR) Award Investigator. A. Nakamura-Ishizu was supported in part by a fellowship from the Japan Society for the Promotion of Science, a Grants-in-Aid for Scientific Research (25-40030 and 26713035), a research grant from the SENSHIN medical research foundation, a research grant from the Takeda Foundation, and a grant from the Naito Foundation. K. Takubo was supported in part by a Ministry of Education, Culture, Sports, Science and Technology (MEXT) Grant-in-Aid for Young Scientists (A). T. Suda and K. Takubo were supported in part by a MEXT Grant-in-Aid for Scientific Research (A) and a MEXT Grant-in-Aid for Scientific Research on Innovative Areas. K. Suzuki-Inoue was supported in part by the NEXT program (LS052). K. Takubo was supported in part by a MEXT Grant-in-Aid for Scientific Research (15H04861,26115005) and a Grant of the National Center for Global Health and Medicine (H26-001).
The authors declare no competing financial interests.
Author contributions: T. Suda, K. Takubo, and A. Nakamura-Ishizu designed the project, analyzed the data, and wrote the manuscript. A. Nakamura-Ishizu and K. Takubo organized, performed, and analyzed all experiments. H. Kobayashi performed and analyzed the microarray gene expression experiment. K. Suzuki-Inoue provided mice and discussed and analyzed experiments. All authors read and approved the final manuscript.
Footnotes
Abbreviations used:
- BMT
- BM transplantation
- EC
- endothelial cell
- HSC
- hematopoietic stem cell
- HSPC
- hematopoietic stem and progenitor cell
- IHC
- immunohistochemistry
- LT-HSC
- long-term HSC
- Mk
- megakaryocyte
- MNC
- mononuclear cell
- MSC
- mesenchymal stem cell
- OBL
- osteoblast
- PB
- peripheral blood
- Thpo
- thrombopoietin
References
- Acton S.E., Astarita J.L., Malhotra D., Lukacs-Kornek V., Franz B., Hess P.R., Jakus Z., Kuligowski M., Fletcher A.L., Elpek K.G., et al. 2012. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity. 37:276–289. 10.1016/j.immuni.2012.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acton S.E., Farrugia A.J., Astarita J.L., Mourão-Sá D., Jenkins R.P., Nye E., Hooper S., van Blijswijk J., Rogers N.C., Snelgrove K.J., et al. 2014. Dendritic cells control fibroblastic reticular network tension and lymph node expansion. Nature. 514:498–502. 10.1038/nature13814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai F., and Suda T.. 2007. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann. N. Y. Acad. Sci. 1106:41–53. 10.1196/annals.1392.005 [DOI] [PubMed] [Google Scholar]
- Arai F., Hirao A., Ohmura M., Sato H., Matsuoka S., Takubo K., Ito K., Koh G.Y., and Suda T.. 2004. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 118:149–161. 10.1016/j.cell.2004.07.004 [DOI] [PubMed] [Google Scholar]
- Bénézech C., Nayar S., Finney B.A., Withers D.R., Lowe K., Desanti G.E., Marriott C.L., Watson S.P., Caamaño J.H., Buckley C.D., and Barone F.. 2014. CLEC-2 is required for development and maintenance of lymph nodes. Blood. 123:3200–3207. 10.1182/blood-2013-03-489286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertozzi C.C., Schmaier A.A., Mericko P., Hess P.R., Zou Z., Chen M., Chen C.-Y., Xu B., Lu M.-M., Zhou D., et al. 2010. Platelets regulate lymphatic vascular development through CLEC-2–SLP-76 signaling. Blood. 116:661–670. 10.1182/blood-2010-02-270876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns I., Lucas D., Pinho S., Ahmed J., Lambert M.P., Kunisaki Y., Scheiermann C., Schiff L., Poncz M., Bergman A., and Frenette P.S.. 2014. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat. Med. 20:1315–1320. 10.1038/nm.3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J.M., Nolan D.J., Vertes E.L., Varnum-Finney B., Kobayashi H., Hooper A.T., Seandel M., Shido K., White I.A., Kobayashi M., et al. 2010. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 6:251–264. 10.1016/j.stem.2010.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A., Lucas D., Hidalgo A., Méndez-Ferrer S., Hashimoto D., Scheiermann C., Battista M., Leboeuf M., Prophete C., van Rooijen N., et al. 2011. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J. Exp. Med. 208:261–271. 10.1084/jem.20101688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J.L., and Weissman I.L.. 2001. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc. Natl. Acad. Sci. USA. 98:14541–14546. 10.1073/pnas.261562798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Solal K., Villeval J.L., Titeux M., Lok S., Vainchenker W., and Wendling F.. 1996. Constitutive expression of Mpl ligand transcripts during thrombocytopenia or thrombocytosis. Blood. 88:2578–2584. [PubMed] [Google Scholar]
- de Graaf C.A., Kauppi M., Baldwin T., Hyland C.D., Metcalf D., Willson T.A., Carpinelli M.R., Smyth G.K., Alexander W.S., and Hilton D.J.. 2010. Regulation of hematopoietic stem cells by their mature progeny. Proc. Natl. Acad. Sci. USA. 107:21689–21694. 10.1073/pnas.1016166108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Saunders T.L., Enikolopov G., and Morrison S.J.. 2012. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 481:457–462. 10.1038/nature10783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandrey J., and Bunn H.F.. 1993. In vivo and in vitro regulation of erythropoietin mRNA: measurement by competitive polymerase chain reaction. Blood. 81:617–623. [PubMed] [Google Scholar]
- Finney B.A., Schweighoffer E., Navarro-Núñez L., Bénézech C., Barone F., Hughes C.E., Langan S.A., Lowe K.L., Pollitt A.Y., Mourao-Sa D., et al. 2012. CLEC-2 and Syk in the megakaryocytic/platelet lineage are essential for development. Blood. 119:1747–1756. 10.1182/blood-2011-09-380709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki J., Wu J., Carlson A.L., Silberstein L., Putheti P., Larocca R., Gao W., Saito T.I., Lo Celso C., Tsuyuzaki H., et al. 2011. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 474:216–219. 10.1038/nature10160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozovsky R., Begonja A.J., Liu K., Visner G., Hartwig J.H., Falet H., and Hoffmeister K.M.. 2015. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat. Med. 21:47–54. 10.1038/nm.3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heazlewood S.Y., Neaves R.J., Williams B., Haylock D.N., Adams T.E., and Nilsson S.K.. 2013. Megakaryocytes co-localise with hemopoietic stem cells and release cytokines that up-regulate stem cell proliferation. Stem Cell Res. (Amst.). 11:782–792. 10.1016/j.scr.2013.05.007 [DOI] [PubMed] [Google Scholar]
- Herzog B.H., Fu J., Wilson S.J., Hess P.R., Sen A., McDaniel J.M., Pan Y., Sheng M., Yago T., Silasi-Mansat R., et al. 2013. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature. 502:105–109. 10.1038/nature12501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., and Smyth G.K.. 2009. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods. 347:70–78. 10.1016/j.jim.2009.06.008 [DOI] [PubMed] [Google Scholar]
- Kabaya K., Akahori H., Shibuya K., Nitta Y., Ida M., Kusaka M., Kato T., and Miyazaki H.. 1996. In vivo effects of pegylated recombinant human megakaryocyte growth and development factor on hematopoiesis in normal mice. Stem Cells. 14:651–660. 10.1002/stem.140651 [DOI] [PubMed] [Google Scholar]
- Kaser A., Brandacher G., Steurer W., Kaser S., Offner F.A., Zoller H., Theurl I., Widder W., Molnar C., Ludwiczek O., et al. 2001. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 98:2720–2725. 10.1182/blood.V98.9.2720 [DOI] [PubMed] [Google Scholar]
- Katayama Y., Battista M., Kao W.-M., Hidalgo A., Peired A.J., Thomas S.A., and Frenette P.S.. 2006. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 124:407–421. 10.1016/j.cell.2005.10.041 [DOI] [PubMed] [Google Scholar]
- Kawamoto T. 2003. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch. Histol. Cytol. 66:123–143. 10.1679/aohc.66.123 [DOI] [PubMed] [Google Scholar]
- Kollet O., Dar A., Shivtiel S., Kalinkovich A., Lapid K., Sztainberg Y., Tesio M., Samstein R.M., Goichberg P., Spiegel A., et al. 2006. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat. Med. 12:657–664. 10.1038/nm1417 [DOI] [PubMed] [Google Scholar]
- Kuter D.J., and Rosenberg R.D.. 1995. The reciprocal relationship of thrombopoietin (c-Mpl ligand) to changes in the platelet mass during busulfan-induced thrombocytopenia in the rabbit. Blood. 85:2720–2730. [PubMed] [Google Scholar]
- McCarty J.M., Sprugel K.H., Fox N.E., Sabath D.E., and Kaushansky K.. 1995. Murine thrombopoietin mRNA levels are modulated by platelet count. Blood. 86:3668–3675. [PubMed] [Google Scholar]
- Méndez-Ferrer S., Michurina T.V., Ferraro F., Mazloom A.R., Macarthur B.D., Lira S.A., Scadden D.T., Ma’ayan A., Enikolopov G.N., and Frenette P.S.. 2010. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 466:829–834. 10.1038/nature09262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L.J., Luens K.M., Estrada M.F., Bruno E., Hoffman R., Cohen R.L., Ashby M.A., and Vadhan-Raj S.. 1998. Thrombopoietin mobilizes CD34+ cell subsets into peripheral blood and expands multilineage progenitors in bone marrow of cancer patients with normal hematopoiesis. Exp. Hematol. 26:207–216. [PubMed] [Google Scholar]
- Nakamura-Ishizu A., Okuno Y., Omatsu Y., Okabe K., Morimoto J., Uede T., Nagasawa T., Suda T., and Kubota Y.. 2012. Extracellular matrix protein tenascin-C is required in the bone marrow microenvironment primed for hematopoietic regeneration. Blood. 119:5429–5437. 10.1182/blood-2011-11-393645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Ishizu A., Takizawa H., and Suda T.. 2014a The analysis, roles and regulation of quiescence in hematopoietic stem cells. Development. 141:4656–4666. 10.1242/dev.106575 [DOI] [PubMed] [Google Scholar]
- Nakamura-Ishizu A., Takubo K., Fujioka M., and Suda T.. 2014b Megakaryocytes are essential for HSC quiescence through the production of thrombopoietin. Biochem. Biophys. Res. Commun. 454:353–357. 10.1016/j.bbrc.2014.10.095 [DOI] [PubMed] [Google Scholar]
- Naveiras O., Nardi V., Wenzel P.L., Hauschka P.V., Fahey F., and Daley G.Q.. 2009. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 460:259–263. 10.1038/nature08099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S., Ogami K., Kawamura K., Tsukamoto I., Kudo Y., Kanakura Y., Kitamura Y., Miyazaki H., and Kato T.. 1997. Cellular localization of thrombopoietin mRNA in the liver by in situ hybridization. Exp. Hematol. 25:565–572. [PubMed] [Google Scholar]
- Olson T.S., Caselli A., Otsuru S., Hofmann T.J., Williams R., Paolucci P., Dominici M., and Horwitz E.M.. 2013. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood. 121:5238–5249. 10.1182/blood-2012-10-463414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S.H., and Zon L.I.. 2008. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 132:631–644. 10.1016/j.cell.2008.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada M., Inoue O., Ding G., Shirai T., Ichise H., Hirayama K., Takano K., Yatomi Y., Hirashima M., Fujii H., et al. 2012. Platelet activation receptor CLEC-2 regulates blood/lymphatic vessel separation by inhibiting proliferation, migration, and tube formation of lymphatic endothelial cells. J. Biol. Chem. 287:22241–22252. 10.1074/jbc.M111.329987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Yago T., Fu J., Herzog B., McDaniel J.M., Mehta-D’Souza P., Cai X., Ruan C., McEver R.P., West C., et al. 2014. Podoplanin requires sialylated O-glycans for stable expression on lymphatic endothelial cells and for interaction with platelets. Blood. 124:3656–3665. 10.1182/blood-2014-04-572107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk C.J.H., Rossi D.J., Månsson R., Attema J.L., Norddahl G.L., Chan C.K.F., Sigvardsson M., Weissman I.L., and Bryder D.. 2007. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 1:428–442. 10.1016/j.stem.2007.07.005 [DOI] [PubMed] [Google Scholar]
- Qian H., Buza-Vidas N., Hyland C.D., Jensen C.T., Antonchuk J., Månsson R., Thoren L.A., Ekblom M., Alexander W.S., and Jacobsen S.E.. 2007. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 1:671–684. 10.1016/j.stem.2007.10.008 [DOI] [PubMed] [Google Scholar]
- Qian S., Fu F., Li W., Chen Q., and de Sauvage F.J.. 1998. Primary role of the liver in thrombopoietin production shown by tissue-specific knockout. Blood. 92:2189–2191. [PubMed] [Google Scholar]
- Schacht V., Dadras S.S., Johnson L.A., Jackson D.G., Hong Y.-K., and Detmar M.. 2005. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am. J. Pathol. 166:913–921. 10.1016/S0002-9440(10)62311-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield R. 1978. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 4:7–25. [PubMed] [Google Scholar]
- Senis Y.A., Tomlinson M.G., García A., Dumon S., Heath V.L., Herbert J., Cobbold S.P., Spalton J.C., Ayman S., Antrobus R., et al. 2007. A comprehensive proteomics and genomics analysis reveals novel transmembrane proteins in human platelets and mouse megakaryocytes including G6b-B, a novel immunoreceptor tyrosine-based inhibitory motif protein. Mol. Cell. Proteomics. 6:548–564. 10.1074/mcp.D600007-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone R.L., Nick A.M., McNeish I.A., Balkwill F., Han H.D., Bottsford-Miller J., Rupairmoole R., Armaiz-Pena G.N., Pecot C.V., Coward J., et al. 2012. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med. 366:610–618. (published erratum appears in N. Engl. J. Med. 2012. 367:1768) 10.1056/NEJMoa1110352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Kohara H., Noda M., and Nagasawa T.. 2006. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 25:977–988. 10.1016/j.immuni.2006.10.016 [DOI] [PubMed] [Google Scholar]
- Sungaran R., Chisholm O.T., Markovic B., Khachigian L.M., Tanaka Y., and Chong B.H.. 2000. The role of platelet alpha-granular proteins in the regulation of thrombopoietin messenger RNA expression in human bone marrow stromal cells. Blood. 95:3094–3101. [PubMed] [Google Scholar]
- Suzuki-Inoue K., Fuller G.L.J., García A., Eble J.A., Pöhlmann S., Inoue O., Gartner T.K., Hughan S.C., Pearce A.C., Laing G.D., et al. 2006. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 107:542–549. 10.1182/blood-2005-05-1994 [DOI] [PubMed] [Google Scholar]
- Suzuki-Inoue K., Kato Y., Inoue O., Kaneko M.K., Mishima K., Yatomi Y., Yamazaki Y., Narimatsu H., and Ozaki Y.. 2007. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J. Biol. Chem. 282:25993–26001. 10.1074/jbc.M702327200 [DOI] [PubMed] [Google Scholar]
- Suzuki-Inoue K., Inoue O., Ding G., Nishimura S., Hokamura K., Eto K., Kashiwagi H., Tomiyama Y., Yatomi Y., Umemura K., et al. 2010. Essential in vivo roles of the C-type lectin receptor CLEC-2: embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J. Biol. Chem. 285:24494–24507. 10.1074/jbc.M110.130575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki-Inoue K., Inoue O., and Ozaki Y.. 2011. The novel platelet activation receptor CLEC-2. Platelets. 22:380–384. 10.3109/09537104.2011.556274 [DOI] [PubMed] [Google Scholar]
- Takubo K., Goda N., Yamada W., Iriuchishima H., Ikeda E., Kubota Y., Shima H., Johnson R.S., Hirao A., Suematsu M., and Suda T.. 2010. Regulation of the HIF-1α level is essential for hematopoietic stem cells. Cell Stem Cell. 7:391–402. 10.1016/j.stem.2010.06.020 [DOI] [PubMed] [Google Scholar]
- Tiedt R., Schomber T., Hao-Shen H., and Skoda R.C.. 2007. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 109:1503–1506. 10.1182/blood-2006-04-020362 [DOI] [PubMed] [Google Scholar]
- Walter D., Lier A., Geiselhart A., Thalheimer F.B., Huntscha S., Sobotta M.C., Moehrle B., Brocks D., Bayindir I., Kaschutnig P., et al. 2015. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature. 520:549–552. 10.1038/nature14131 [DOI] [PubMed] [Google Scholar]
- Wolber E.M., Fandrey J., Frackowski U., and Jelkmann W.. 2001. Hepatic thrombopoietin mRNA is increased in acute inflammation. Thromb. Haemost. 86:1421–1424. [PubMed] [Google Scholar]
- Yamazaki S., Ema H., Karlsson G., Yamaguchi T., Miyoshi H., Shioda S., Taketo M.M., Karlsson S., Iwama A., and Nakauchi H.. 2011. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 147:1146–1158. 10.1016/j.cell.2011.09.053 [DOI] [PubMed] [Google Scholar]
- Yoshihara H., Arai F., Hosokawa K., Hagiwara T., Takubo K., Nakamura Y., Gomei Y., Iwasaki H., Matsuoka S., Miyamoto K., et al. 2007. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 1:685–697. 10.1016/j.stem.2007.10.020 [DOI] [PubMed] [Google Scholar]
- Zhao M., Perry J.M., Marshall H., Venkatraman A., Qian P., He X.C., Ahamed J., and Li L.. 2014. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat. Med. 20:1321–1326. 10.1038/nm.3706 [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D., and Kaushansky K.. 1996. Effect of thrombopoietin on the development of megakaryocytes and platelets: an ultrastructural analysis. Blood. 88:1632–1638. [PubMed] [Google Scholar]