Mutations in the catalytic subunit of the γ-secretase complex, Presenilin, cause familial Alzheimer’s disease. Analysis of patients’ brains shows that these mutations do not result in loss of enzymatic function but in qualitative changes in Aβ product profiles.
Abstract
Presenilin (PSEN) pathogenic mutations cause familial Alzheimer’s disease (AD [FAD]) in an autosomal-dominant manner. The extent to which the healthy and diseased alleles influence each other to cause neurodegeneration remains unclear. In this study, we assessed γ-secretase activity in brain samples from 15 nondemented subjects, 22 FAD patients harboring nine different mutations in PSEN1, and 11 sporadic AD (SAD) patients. FAD and control brain samples had similar overall γ-secretase activity levels, and therefore, loss of overall (endopeptidase) γ-secretase function cannot be an essential part of the pathogenic mechanism. In contrast, impaired carboxypeptidase-like activity (γ-secretase dysfunction) is a constant feature in all FAD brains. Significantly, we demonstrate that pharmacological activation of the carboxypeptidase-like γ-secretase activity with γ-secretase modulators alleviates the mutant PSEN pathogenic effects. Most SAD cases display normal endo- and carboxypeptidase-like γ-secretase activities. However and interestingly, a few SAD patient samples display γ-secretase dysfunction, suggesting that γ-secretase may play a role in some SAD cases. In conclusion, our study highlights qualitative shifts in amyloid-β (Aβ) profiles as the common denominator in FAD and supports a model in which the healthy allele contributes with normal Aβ products and the diseased allele generates longer aggregation-prone peptides that act as seeds inducing toxic amyloid conformations.
Early-onset familial Alzheimer’s disease (AD [FAD]), starting before age 65, is mainly caused by mutations in the Presenilin 1/2 (PSEN1/2) or the amyloid precursor protein (APP) genes and represents less than 0.1% of the total AD cases (Campion et al., 1999). Although rare, FAD offers a unique model to gain insights into the molecular mechanisms and etiology of sporadic AD (SAD).
PSEN is the catalytic subunit of the γ-secretase complex (De Strooper et al., 1998; Wolfe et al., 1999), an intramembrane multimeric protease involved in the processing of many type 1 transmembrane proteins; among them, the Notch receptors and APP have received much attention because of their association with crucial cell signaling events or with AD pathogenesis, respectively (for a review see Jurisch-Yaksi et al. [2013]). Nicastrin (Nct), PSEN enhancer 2 (Pen2), and anterior pharynx defective 1 (APH1) are, together with PSEN, essential components of the protease complex (De Strooper, 2003).
More than 150 pathogenic mutations in PSEN1 have been reported so far (http://www.molgen.ua.ac.be/ADMutations); and notably, the vast majority are missense substitutions distributed throughout the primary structure of PSEN1. PSEN/γ-secretase hydrolyzes peptide bonds in a process called regulated intramembrane proteolysis, which allows translation of extracellular signals into the cell. Compelling evidence indicates that γ-secretase cuts membrane proteins sequentially: the first endopeptidase cleavage (ε) releases a soluble intracellular domain (ICD), which may translocate to the nucleus to regulate gene expression while the remaining N-terminal transmembrane domain (TMD) fragment is successively cut by the carboxypeptidase-like activity of γ-secretase (γ-cleavages). Endopeptidase products, either amyloid-β49 (Aβ49) or Aβ48, are then processed along two major product lines: Aβ49 → Aβ46 → Aβ43 → Aβ40 or Aβ48 → Aβ45 → Aβ42 → Aβ38 (Takami et al., 2009). Every γ-cleavage removes a short C-terminal peptide from the TMD, reducing its hydrophobicity and increasing the probability of release. Secretion of an N-terminal fragment into the extracellular/luminal space terminates this sequence (Qi-Takahara et al., 2005; Yagishita et al., 2008; Takami et al., 2009). Importantly, the efficiency of the endopeptidase cleavage determines ICD product levels, which acquires high physiological relevance in the case of the Notch substrate. The carboxypeptidase-like efficiency, the number of cuts per substrate, determines the length of the N-terminal products; the level of efficiency is pathologically very relevant in the case of the APP substrate, as lower efficiency results in the production of longer and more aggregation-prone Aβ peptides (Chávez-Gutiérrez et al., 2012).
How mutations in the PSENs cause FAD remains a hotly debated topic in the field. Because FAD is an autosomal-dominant disorder (patients carry both healthy and mutant alleles), a major unknown in the discussion remains the role of the healthy allele and, to a lesser extent, the role of the brain environment on the total (normal + mutant proteases) γ-secretase activity. To what degree do normal and mutant complexes contribute to total γ-secretase activity in the patient brain? Does the healthy allele compensate for the disease allele effects? Despite their relevance, those questions have not been addressed.
Only one group, i.e., Potter et al. (2013), have estimated the Aβ production kinetics in the FAD brain by measuring isotope-labeled Aβ peptides in the cerebrospinal fluid of patients (stable isotope-labeled kinetics [SILK]). Feeding the in vivo metabolic labeling patient data into a mathematical model, specifically generated for their approach, they described higher Aβ42 production rates in the central nervous system of PSEN mutation carriers (Potter et al., 2013). Accordingly, Potter et al. (2013) suggest that increments in Aβ42 play a decisive pathogenic role in AD.
In contrast, a “revised” loss-of-function (LOF) hypothesis has recently been proposed by Xia et al. (2015). In this view, loss of PSEN/γ-secretase physiological cell signaling function causes neurodegeneration, whereas changes in Aβ peptides are only secondary byproducts that arise from but do not trigger the disease (Xia et al., 2015). The idea is only tenable if FAD-linked PSEN mutations exert a LOF effect on PSEN/γ-secretase and, in addition, a dominant-negative effect on the healthy PSEN allele (normal γ-secretase) in patients, a key part of this hypothesis (Heilig et al., 2013; Xia et al., 2015). However, it should be stressed that γ-secretase haploinsufficiency caused by nonsense, frameshift, and splice site mutations in genes coding for essential subunits of γ-secretase (Nct, Pen2, and PSEN) is pathogenic in nature; such haploinsufficiency causes a chronic inflammatory disease of hair follicles known as familial acne inversa. Most importantly, no clinical association between this disorder and AD has been reported (for a review see Pink et al. [2013]). Furthermore, if FAD-linked PSEN mutations were truly LOF mutations, resulting in “inactive” γ-secretase complexes, homozygous individuals for the disease allele would not be viable because of disturbances in Notch signaling during embryonic development. However, six individuals with homozygous PSEN1 E280A gene mutation have been identified (Kosik et al., 2015).
An alternative view to both hypotheses is that pathogenic mutations in PSEN cause disease by qualitative shifts in Aβ profile production (γ-secretase dysfunction; Chávez-Gutiérrez et al., 2012). We have demonstrated that loss of endopeptidase activity is not necessarily observed in γ-secretase complexes containing PSEN1/2 FAD-linked mutations, but reduced carboxypeptidase-like efficiency (γ-secretase dysfunction) is the constant denominator. Furthermore, FAD PSEN mutations may affect the carboxypeptidase-like γ-secretase activity at multiple turnovers, resulting in increased Aβ43 and Aβ42 levels as well as in other longer Aβ peptides, such as Aβ45 and Aβ46 (Quintero-Monzon et al., 2011; Chávez-Gutiérrez et al., 2012; Fernandez et al., 2014). These data support a model in which relative, rather than absolute, changes in Aβ product profiles are at the basis of PSEN/γ-secretase–mediated pathogenicity. However, these findings were based on studies conducted in PSEN1/2-deficient MEFs, which does not fully recapitulate the in vivo heterozygous situation in the FAD patient’s brain.
In the current study, we investigated processing of APP by the γ-secretase complex in postmortem human brain samples from FAD and SAD patients and healthy control subjects. Our investigation is the first to directly assess γ-secretase activity in brain material from FAD mutant carriers and to address how the FAD-linked mutant heterozygous situation in patients affects γ-secretase function in brain.
RESULTS AND DISCUSSION
Aβ production rates in FAD and SAD brains
We aimed to evaluate the effects of pathogenic PSEN1 mutants on total γ-secretase activity (healthy and disease PSEN1 alleles) in human brain samples from FAD patients. As a first step, we sought to determine and contrast the production rates of Aβ peptides in (a) human control brains, i.e., containing two healthy PSEN1 alleles; (b) FAD brains carrying pathogenic mutations in PSEN1, heterozygous for the PSEN1 alleles; and (c) SAD brains, with two healthy PSEN1 alleles. Aβ peptides are generated from APP-C99 membrane peptide by consecutive γ-secretase proteolytic cleavages. The first endopeptidase cut (ε) releases a soluble ICD (AICD) and generates a long membrane-associated Aβ peptide, either Aβ49 or Aβ48, which are then processed along two major product lines (Takami et al., 2009).
Active γ-secretase is associated with detergent-resistant membranes (DRMs; Wahrle et al., 2002) and DRMs prepared from brain or cells are a bona-fide source of γ-secretase activity (Matsumura et al., 2014). Thus, we prepared DRMs from the prefrontal cortices of 15 control brain samples, 22 FAD brain samples carrying nine different pathological PSEN1 mutations, V89L (1 case), intron 4 (2 cases), E120G (1 case), M139T (3 cases), I202F (1 case), P264L (2 cases), R278I (1 case), E280A (10 cases), and L286P (1 case; Table 1); and 11 SAD brain samples. DRMs were used as a source of the enzyme in in vitro activity assays. Importantly, DRMs contain γ-secretase in its native environment (the membrane) while maintaining the lipid composition of their origin (cells or brain).
Table 1. Clinical data of FAD patients whose brains were analyzed in this study.
| Mutation | Sex | Diagnosis | Age of onset | Age at death | APOE |
|---|---|---|---|---|---|
| yr | yr | ||||
| V89L | M | FAD | 48 | 57 | 23 |
| Intron 4 | F | FAD | 35 | 51.9 | 44 |
| Intron 4 | F | FAD | 36 | 41.6 | 33 |
| E120G | M | FAD | 34 | 44 | 33 |
| M139T | M | FAD | 47 | 64 | 33 |
| M139T | M | FAD | 48 | 57 | 33 |
| M139T | M | FAD | 45 | 53 | 33 |
| I202F | F | FAD | 48 | 59.3 | 44 |
| P264L | F | FAD | 45 | 56 | 44 |
| P264L | M | FAD | 53 | 60 | 34 |
| R278I | F | FAD | 46 | 65.6 | 34 |
| L286P | F | FAD | 35 | 56 | 33 |
| E280A | F | FAD | 47 | 54 | 33 |
| E280A | M | FAD | 44 | 52 | 33 |
| E280A | M | FAD | 54 | 63 | 34 |
| E280A | M | FAD | 47 | 56 | 33 |
| E280A | F | FAD | 46 | 67 | 34 |
| E280A | F | FAD | 48 | 64 | 33 |
| E280A | F | FAD | 43 | 48 | 33 |
| E280A | F | FAD | 50 | 60 | 33 |
| E280A | F | FAD | 52 | 68 | 33 |
| E280A | M | FAD | 47 | 58 | 33 |
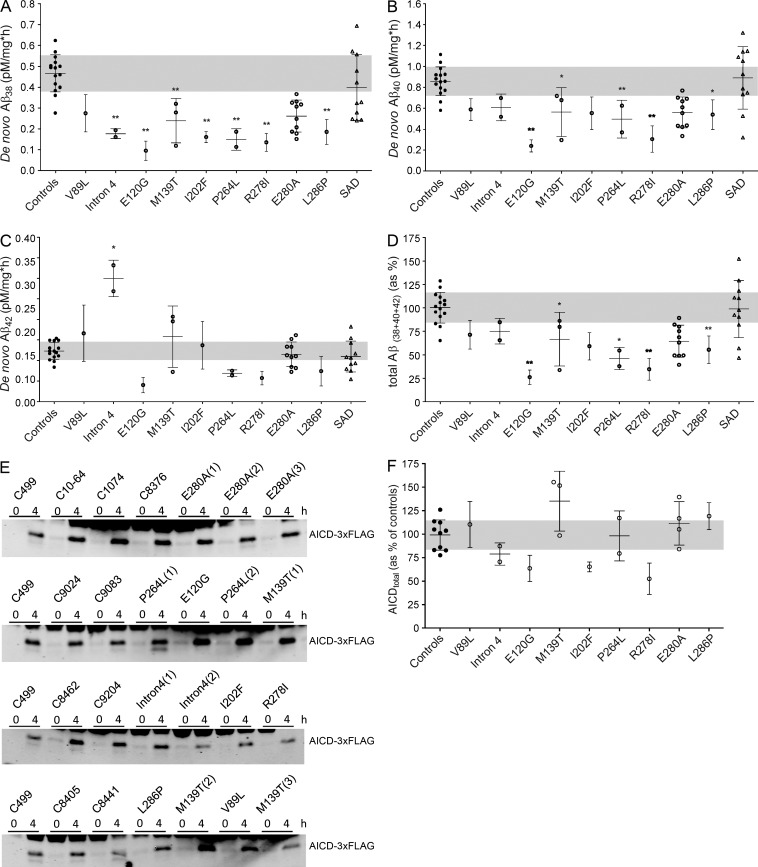
The production rates of the Aβ38, Aβ40, and Aβ42 peptides (de novo) were determined by incubation of equivalent amounts of brain DRMs with the purified APPC99-3×FLAG γ-secretase substrate, under saturating conditions for 0 and 4 h. Our results show a reduction in the Aβ38 production rate in the majority of FAD cases and a reduction in Aβ40 generation in five of nine FAD cases (Fig. 1, A and B). In contrast, Aβ production in SAD samples did not show significant alterations (Fig. 1, A–C). However, the dispersion observed in the SAD group may be an indication of changes in γ-secretase activity in a fraction of late-onset AD cases (see next section for further discussion). Most interestingly, there were no differences in Aβ42 production rates in most of the FAD brain samples, except for the intron 4 mutation cases, which display, on average, a 2.5-fold increment over controls (Fig. 1 C). A mild reduction in Aβ42 production rate was observed in the E120G-, P264L-, and R278I-PSEN1 brain samples, but the differences did not reach statistical significance. The γ-secretase inhibitor X (a transition state analogue) abolished the production of Aβ peptides (not depicted), demonstrating the specificity of the reaction. Unfortunately, the concentration of “de novo” generated Aβ37 and Aβ43 peptides in control and most of the mutant samples were below the detection limits.
Figure 1.
APP processing in human brain samples from FAD, SAD, and control patients. (A–D) Aβ production rate in brains of FAD or SAD patients compared with nondemented subjects. To determine de novo production of Aβ peptides, CHAPSO-resistant membranes prepared from brain tissue of patients were incubated with 1.5 µM C99-3×FLAG substrate and quantified using MSD ELISA technology. Graphs show mean ± SD for groups with one case or mean of means ± SD for groups with number of cases greater than one. (E) SDS-PAGE/Western blot showing AICD product levels in reactions with human control and FAD brains. The molecular mass of AICD-3×FLAG is ∼10 kD. (F) De novo AICD product levels (endopeptidase activity levels) in human control and FAD brain samples. Graph shows mean ± SE for groups with one case or mean of means ± SE for groups with number of cases greater than one. All experiments were repeated at least three times, and statistical significance was tested with one-way ANOVA and Dunnett's post test, taking the corresponding WT set as the control group (**, P < 0.01; *, P < 0.05).
To correct for any potential differences in protein concentrations in our assays, we also normalized Aβ production to flotilin-1 levels, determined by immunoblot, which did not change our observations (not depicted). Collectively, our data support a model in which relative changes in Aβ production in FAD are more important for disease than absolute increments in Aβ42 levels (Tanzi and Bertram, 2005; De Strooper, 2007; Kuperstein et al., 2010; Chávez-Gutiérrez et al., 2012). Thus, our findings contrast with those of Potter et al. (2013). The differences may arise from the fact that the SILK method measures Aβ released in the interstitial fluid, which provides an indirect and perhaps not so accurate assessment of γ-secretase activity in human brain. Although their mathematical model should in principle correct for these potential issues, several not yet experimentally verified assumptions were made with regard to production, secretion, and clearance mechanisms of Aβ peptides (for further discussion of the model see Edland and Galasko [2011]). For instance, it is assumed that different Aβs are generated independently from each other (Potter et al., 2013), which is not in line with the current knowledge showing that consecutive γ-secretase cleavages generate Aβ peptides (Takami et al., 2009). Furthermore, we would like to draw attention to the heterogeneous behavior of the mutation carrier cohort, reported as a proof of concept in Potter et al. (2013): the Aβ42 production rates were actually only elevated in three out of seven FAD-linked PSEN mutation carriers.
De novo production of AICD in FAD-PSEN brain samples
Our results indicate that Aβ38, Aβ40, and Aβ42 are the main secreted products, and the sum of “de novo” Aβ38, Aβ40, and Aβ42 products reveals lower Aβ production in brain samples carrying pathogenic mutations in PSEN1 in five out of nine cases, relative to control and SAD cases (Fig. 1 D). The observed effect on Aβ production could be caused by a reduction in the endopeptidase activity or an impaired carboxypeptidase-like efficiency in FAD brain samples. Therefore, we analyzed the production of AICD in our samples, which provides a relative indication for the efficiency of the γ-secretase endopeptidase activity in the tested human brain samples. Decreased γ-secretase endopeptidase efficiency may result in alterations in cell signaling events involved in cellular communication (LOF). We found no significant differences in the production rates of AICD (de novo AICD) in controls and FAD brain samples, although a slight reduction in AICD production rate was observed in the R278I brain sample (Fig. 1, E and F). These data clearly indicate that the overall γ-secretase endoprotease activity is unaffected in most of the FAD brain samples, and therefore, this effect cannot be an essential part of the pathogenic mechanism.
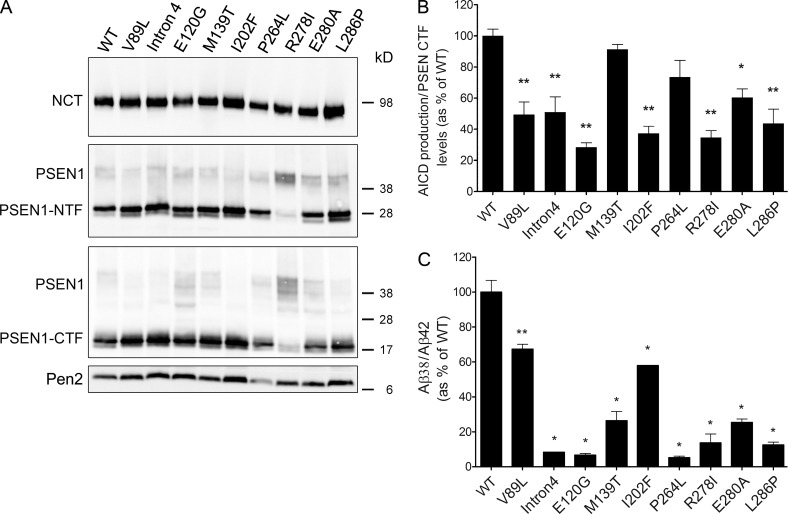
We have previously shown that pathogenic PSEN1 mutants display variable effects (including no effect) on the endopeptidase efficiency of mutant γ-secretase complexes but consistently reduce the γ-secretase carboxypeptidase-like efficiency (γ-secretase dysfunction), all relative to the normal enzyme (Chávez-Gutiérrez et al., 2012). To evaluate the effects of the pathogenic mutations analyzed here on γ-secretase activity, we stably expressed the different clinical mutant PSENs in a Psen1/2 knockout background (Fig. 3). With the exception of the R278I PSEN1 mutant, which severely impairs activation of the γ-secretase complex, Psen1/2 knockout MEFs transduced with normal or mutant PSEN1s express comparable levels of the mature γ-secretase complex (Fig. 3 A). Specific endopeptidase activities, defined as “de novo AICD” normalized against PSEN1-CTF subunit levels in the in vitro reactions, revealed variable effects (including no effect) of the PSEN1 mutations on the endopeptidase function of γ-secretase (Fig. 3 B). Interestingly, comparison of the endopeptidase activities of samples containing either normal or mutant complexes (Fig. 3 B) with FAD brain samples (normal + mutant enzymes; Fig. 1 F) demonstrates that the healthy allele (normal enzyme) compensates for (if any) decrements in the endopeptidase cleavage rates caused by the disease allele (mutant γ-secretase complex). The above implies that normal and mutant proteases contribute to γ-secretase endopeptidase activity. Undoubtedly, our data do not support a mutant-mediated “dominant-negative effect” on the healthy allele and, therefore, contrast with the hypothesis proposed recently by Heilig et al. (2013) and Xia et al. (2015).
Figure 3.
FAD-PSEN1 mutations show variable effects on the endopeptidase cleavage, but all impair the fourth enzymatic turnover of γ-secretase. (A) Nct, PSEN1-NTF, PSEN1-CTF, and Pen2 protein levels in Psen1/2−/− MEFs transduced to express human WT or FAD-PSEN1. (B) Measurement of AICD production for WT and mutant PSEN1 γ-secretase complexes. To determine specific activities for WT and FAD complexes, AICD products were normalized to PSEN1-CTF fragment levels quantified by Western blot. (C) FAD-PSEN1 mutations consistently impair the fourth catalytic cleavage seen as Aβ38/Aβ42 (product/substrate) ratio, relative to WT activity. All experiments were repeated three to five times. Graphs show mean ± SE, and statistical significance was tested with one-way ANOVA and Dunnett's post test, taking the corresponding WT set as the control group (**, P < 0.01; *, P < 0.05).
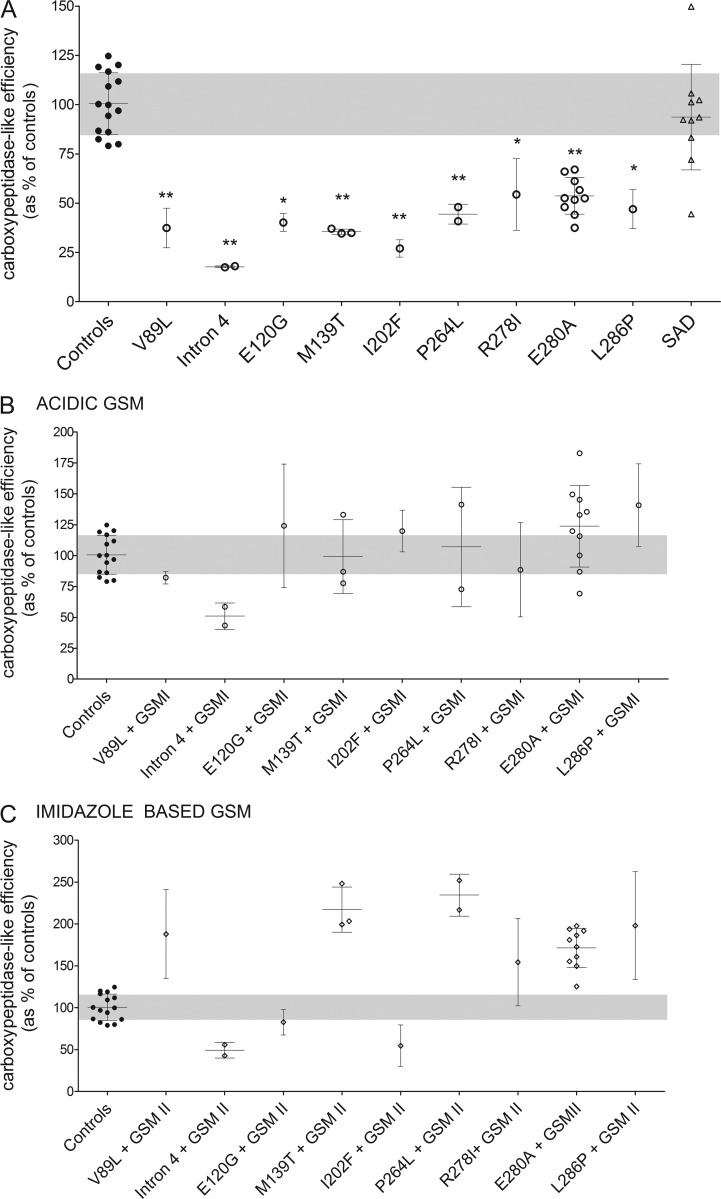
Carboxypeptidase-like efficiency in FAD and SAD brain samples
Interestingly, brain samples carrying pathogenic PSEN1 mutations consistently display lower “Aβ38 + Aβ40 + Aβ42” production rates (Fig. 1 D) than for AICD (Fig. 1 F), suggesting a higher production of longer Aβ peptides (>Aβ42) in FAD versus control brain samples, which may be indicative of impaired carboxypeptidase-like efficiency (γ-secretase dysfunction). γ-Cleavage efficiency can be assessed by determining the Aβ38/Aβ42 ratio, which represents the product/substrate ratio for the fourth catalytic turnover of the γ-secretase. Significantly, changes in this ratio correlate directly with this particular cleavage efficiency. Thus, we calculated the Aβ38/Aβ42 ratios of total γ-secretase in FAD, SAD, and control brain samples. In the FAD brain samples, the observed decrement in the short Aβ38 and Aβ40 peptides translated into a significant reduction in the Aβ38/Aβ42 ratio (Fig. 2 A). Remarkably, regardless of the nature and position of the mutation in PSEN1, the Aβ38/Aβ42 ratios revealed a consistent reduction in total γ-secretase carboxypeptidase-like efficiencies in FAD brain samples, relative to controls. Our data thus show no alterations in the total (normal + mutant complexes) γ-secretase endopeptidase activity and lower de novo production of Aβ38 and Aβ40 peptides. This is not accompanied by increased Aβ42 production levels in most of the FAD patient brain samples. These results strongly suggest that longer Aβ peptides (such as Aβ43 and Aβ45) are produced in patient brain samples. Unfortunately, technical limitations do not allow us currently to measure the production of these longer peptides.
Figure 2.
AD-causing PSEN1 mutants impair γ-cleavage efficiency in FAD human brain samples, and GSMs correct for the pathogenic effect. (A) Carboxypeptidase-like efficiency seen as Aβ38/Aβ42 (product/substrate) ratio. (B and C) The responses to GSMs observed in mutation carrier brain samples is contrasted with the carboxypeptidase-like efficiencies measured in brain samples from nondemented subjects (gray area, shown in panel A). To determine the response to GSMs, CHAPSO-resistant membranes prepared from brain tissue of patients were incubated with 1.5 µM C99-3×FLAG substrate in the presence of 1 µM GSM. Graphs show mean ± SD for groups with one case or mean of means ± SD for groups with number of cases greater than one. All experiments were repeated three to five times, and statistical significance was tested with one-way ANOVA and Dunnett's post test, taking the corresponding WT set as the control group (**, P < 0.01; *, P < 0.05).
The amplitude of the effects on the Aβ38/Aβ42 ratios did not correlate with the age at onset (Table 1), suggesting that additional genetic and/or environmental factors may play a role in the onset of FAD, and we speculate that altered processing of other substrates could contribute to this. We also looked at the Aβ42/Aβ40 ratio because increments in this (somewhat deliberately chosen) ratio have been used as hallmark of FAD mutations for decades. Our data show increased Aβ42/Aβ40 ratios in six (V89L, intron 4, M139T, I202F, R278I, and E280A) of the nine PSEN1 mutant cohorts analyzed.
With regard to SAD, our data reveal similar γ-secretase carboxypeptidase-like efficiencies relative to control brain samples. However, in accordance with the Aβ production velocities (Fig. 1, A–D), we observed a marked Aβ38/Aβ42 ratio dispersion among the SAD brain samples (Fig. 2 A). This may indicate alterations in γ-secretase carboxypeptidase-like efficiency in a subset of late-onset SAD cases but exclude the hypothesis that changes in γ-secretase efficiency play a major role in the majority of SAD patients. Application of in vivo metabolic labeling in SAD patients found no changes in Aβ production in late AD (Mawuenyega et al., 2010). However, analysis of other γ-secretase products in cerebrospinal fluid of late-onset patients revealed the apparent existence of subpopulations of SAD patients showing differential alterations in γ-secretase activity, including enzyme dysfunction (Hata et al., 2011, 2012). In addition, increments in the carboxypeptidase-like efficiency of γ-secretase in SAD brain samples have also been reported (Kakuda et al., 2012). Thus, our data support the idea that the causes of late-onset AD are heterogeneous and raise the possibility that SAD patients showing γ-secretase dysfunction could benefit from γ-secretase activation (see next section). However, the analysis of a larger cohort is needed to accurately evaluate the relevance of γ-secretase dysfunction in the sporadic form of AD.
γ-Secretase modulators (GSMs) alleviate the FAD-associated effects of the majority of PSEN1 mutations
GSMs act as activators of the carboxypeptidase-like activity of the γ-secretase complex (Chávez-Gutiérrez et al., 2012; Takeo et al., 2014). Given the observed reduction in the γ-secretase carboxypeptidase-like efficiency in FAD patient brain samples, we hypothesized that incubation with GSMs would enhance the carboxypeptidase-like activity and thereby correct for the pathogenic Aβ profiles associated with FAD. Thus, we decided to investigate the effects of two different GSM families on the efficiency of the γ-secretase carboxypeptidase-like activity in FAD brain samples. Specifically, we tested an acid- and an imidazole-based modulator at 1 µM in our in vitro reactions (Fig. 2, B and C, respectively). Fig. 2 (B and C) shows that both GSMs restore the efficiency of the carboxypeptidase-like activity to control levels in eight out of nine FAD PSEN1 and seven out of nine FAD PSEN1 brain samples, respectively. Patient brain samples carrying the intron 4 mutation displayed limited responses to both GSMs. Similarly, the imidazole-based GSM did not restore the efficiency of the carboxypeptidase-like activity in the I202F case. The first extracellular loop of PSEN is part of an allosteric ligand-binding site within the N-terminal fragment of PSEN (Takeo et al., 2014); most likely, the intron 4 and I202F mutations disrupt the binding of GSMs to PSEN/γ-secretase. These data indicate that GSM treatment may be particularly useful in FAD, although the magnitude of the modulatory response may depend on the nature of the mutation and the GSM chemistry. A very recent study reached similar conclusions on the effects of GSMs on FAD, using as a model neuronal cultures derived from FAD patient induced pluripotent stem cells (Moore et al., 2015). We would like to point out that full documentation of GSM-mediated effects on Aβ profiles should be performed before any clinical applications. In particular, potential modulatory effects on the generation of long Aβ peptides (>42 amino acids) should be considered to prevent nondesired alterations at that level.
Collectively, these studies demonstrate no significant differences in the endopeptidase activity levels between FAD and control brain samples. The lack of effect on AICD production ascertains that the effects on the Aβ38/Aβ42 ratio are not simply caused by the extensive damage in the late-stage AD brains. Interestingly, when expressed in homozygous Psen1/2-deficient MEFs, some of the clinical mutants cause by themselves decrements in the overall γ-secretase endopeptidase activity (seven mutations cause a reduction ranging from 70% to ∼30% of the normal γ-secretase activity, and two mutations did not affect activity; Fig. 3 B). The presence of the normal PSEN1 allele in heterozygous FAD patients probably compensates for the decrease in γ-secretase endopeptidase activity observed for some of the pathogenic PSEN1 mutants in homozygous Psen1/2-deficient MEFs (Fig. 3 B). Thus, our investigation does not support a dominant-negative effect of the FAD allele over the healthy allele (normal PSEN1/γ-secretase), as proposed by others (Xia et al., 2015). However, we cannot discard the proposition that misprocessing of other γ-secretase substrates may contribute to disease symptoms (discussed in Chávez-Gutiérrez et al. [2012]) and help to explain the wide clinical spectrum observed in FAD patients (reviewed in Bergmans and De Strooper [2010]).
Significantly, this study is important in its demonstration that γ-secretase dysfunction is the common denominator in FAD patient brain samples (Fig. 2 A). These findings are entirely consistent with the effect of FAD-linked PSEN mutations on γ-secretase function shown in Fig. 3 C and in our previous work (Chávez-Gutiérrez et al., 2012). Our data highlight that qualitative shifts in Aβ product profiles, toward longer Aβ peptides, are the central feature in FAD pathogenesis, although elevated Aβ42 peptide could contribute to pathogenesis in some FAD cases.
The qualitative shifts in the Aβ profiles observed with the clinical mutations suggest that longer Aβ peptides (≥42) may promote neurotoxicity by providing the seeds for “toxic oligomers,” even at low concentrations. In this regard, a recent publication has put emphasis on the high amyloidogenicity and pathogenicity of Aβ43 (Saito et al., 2011). Although we could not quantify this peptide in our tests on brain samples, because the amounts generated did not reach the threshold of detection, our previous work with membranes from Psen1/2-deficient fibroblasts expressing WT or mutant PSENs clearly indicates that FAD-linked mutants elevate the relative production of Aβ43 (Chávez-Gutiérrez et al., 2012). Furthermore, we show that the Aβ40 production rate is consistently low in FAD patient brain samples. Depletion of this particular peptide may also contribute to pathogenesis (Wang et al., 2006; Kim et al., 2007). Notably, minor changes in Aβ profiles have been reported to have drastic effects on neurotoxicity (Kuperstein et al., 2010). A phase II clinical trial using a humanized antibody against different Aβ42 assemblies (crenezumab; Adolfsson et al., 2012) is currently being conducted on PSEN1-E280A patients in Colombia (http://www.clinicaltrials.gov/ct2/show/NCT01998841). According to the observed γ-secretase dysfunction in FAD, it would be of relevance to test the affinity of crenezumab for other long, aggregation-prone Aβ peptides.
With regard to SAD, our data reveal a heterogeneous group in terms of γ-secretase activity, suggesting that subpopulations of late-onset patients may present alterations in Aβ production, which could be of relevance for future “personalized” treatment strategies. However, the fact that most patients do not show altered γ-secretase activity supports the view that accumulation of Aβ peptides in the central nervous system of SAD patients is more frequently caused by impaired Aβ peptide clearance (Mawuenyega et al., 2010). The potential causes of alterations in γ-secretase function in these few late-onset cases are intriguing, and the implications of such changes on disease onset, progression/duration, and therapy are currently unknown.
In conclusion, our investigation is the first to assess how the heterozygous situation in patients actually affects γ-secretase function in human brain. We find no evidence for a loss of overall γ-secretase endopeptidase function. Alternatively, we propose that qualitative changes in Aβ product profiles are the basis of PSEN/γ-secretase–mediated pathogenicity. These findings imply that long Aβ peptides are potently pathogenic, and we speculate that a small alteration in the clearance of these long amyloidogenic peptides may contribute to late-onset AD. Finally, our findings may have direct implications in therapy, as they indicate that activation of the carboxypeptidase-like activity (while respecting the endopeptidase function) could be a promising therapeutic concept in FAD.
MATERIALS AND METHODS
Antibodies and reagents
Antibodies were purchased as follows: MAB5232 against human PSEN1-CTF from EMD Millipore, 18189 rabbit polyclonal against human Pen2 from Abcam, 612290 and 610820 mouse monoclonal anti–human NCT and anti–human flotillin-1 from BD. ELISA antibodies and GSMs were obtained through collaboration with Janssen Pharmaceutica NV, Beerse, Belgium: JRF AB038 for Aβ1-38, JRF/cAb40/28 for Aβ1-40, JRF/cAb42/26 for Aβ1-42, and detection antibody JRF/AbN/25 against the N terminus of Aβ. Acid-based ((2-[(1R,2S)-1-[4-methyl-1-[4-(trifluoromethyl)phenyl]pentyl]-2-[4-(trifluoromethyl)phenyl]-4-piperidyl] acetic acid) and imidazole-based (N-[2-fluoro-5-(trifluoromethyl)phenyl]-5-[3-methoxy-4-(4-methylimidazol-1-yl)phenyl]-2-methyl-1,2,4-triazol-3-amine) GSMs were synthesized according to described procedures (Crump et al., 2011; Velter et al., 2014). γ-Secretase inhibitor refers to inhibitor X purchased from EMD Millipore.
Expression and purification of C99-3×FLAG substrate
Substrate expression and purification was performed as previously described (Chávez-Gutiérrez et al., 2008). Purity was assessed by SDS-PAGE and Coomassie staining (gelcode reagent; Thermo Fisher Scientific).
Subjects
Human cortical specimens for quantification of γ-secretase activity were obtained from Brain Bank at Tokyo Metropolitan Institute of Gerontology, Queen Square Brain Bank for Neurological Disorders at University College London, throughout collaboration with the Neuroscience Group of Antioquia Brain Bank at University of Antioquia, Medellín, Colombia, and the Neurological Tissue Bank of the Biobanc-Hospital Clinic-IDIBAPS. All of the samples came from brains that were removed and placed in −80°C within 65 h postmortem (patients were moved to a cold room within 2 h after death). Samples were collected according to protocols approved by respective ethical boards, and written legal consents for the use of organs for medical research are available for each patient. A total of 48 brain samples were used for the reported project: 6 SAD cases and 10 controls from the Brain Bank at Tokyo (Brodmann areas 9–11); 4 different PSEN1 FAD mutations from the Queen Square Brain Bank at University College London (1 patient per mutation, Brodmann areas 9–11); 10 E280A FAD cases, 5 SAD cases, and 4 controls from D. Sepulveda-Falla (Brodmann area 11); 5 different PSEN1 FAD mutations (8 patients, Brodmann areas 9–11; Pera et al., 2013); and 1 control from the Neurological Tissue Bank of the Biobanc-Hospital Clinic-IDIBAPS. All human protocols were approved by Medical Ethics Committee UZ KU Leuven, Belgium.
Generation of MEFs
Psen1/Psen2−/− MEFs (Herreman et al., 2000) were cultured in Dulbecco’s modified Eagle’s medium/F-12 (Life Technologies) containing 10% fetal bovine serum. MEFs were transduced using pMSCV-puro, a replication-defective recombinant retroviral expression system (Takara Bio Inc.) harboring cDNA inserts coding for WT human PSEN1 or variants: V89L, intron 4 (p.L113_I114insT), E120G, M139T, I202F, I213T, P264L, R278I, and L286P. Stable cell lines were selected using 5 µg/ml puromycin (Sigma-Aldrich).
DRM preparation from human brains or MEFs
CHAPSO DRMs were prepared for human brain frontal cortices as previously described (Kakuda et al., 2012) with minor modifications or from MEFs expressing WT γ-secretase or mutant complexes containing PSEN1 mutations. In the first case, after careful removal of leptomeninges and blood vessels, <250 mg blocks of tissue were homogenized in ∼10 vol of 10% sucrose in MBS buffer (25 mM MES, pH 6.5, 150 mM NaCl) containing 1% CHAPSO (Sigma-Aldrich) and protease inhibitors (Complete; Roche). In the case of MEFs, total membranes were prepared from 12 big culture dishes (245 × 245 × 25), and membrane pellets were homogenized in ∼2.5 ml of 10% sucrose in MBS buffer (25 mM MES, pH 6.5, 150 mM NaCl) containing 1% CHAPSO and protease inhibitors. Each homogenate was mixed with equal volume of 70% sucrose in MBS buffer, and 4 ml was placed at the bottom of an ultracentrifuge tube (344059; Beckman Coulter) and successively overlaid with 4 ml of 35% sucrose and 4 ml of 5% sucrose, both in MBS buffer. Samples were centrifuged at 39,000 rpm for 20 h at 4°C on an SW 41 Ti rotor (Beckman Coulter). After centrifugation, the DRM fraction (interface of 5%/35% sucrose) was carefully collected, rinsed in 20 mM PIPES, pH 7.0, 250 mM sucrose, and 1 M EGTA, and recentrifuged twice (100,000 g, 60 min, 4°C). The resultant pellet was resuspended with the aforementioned buffer using a 26G syringe and stored at −80°C until use. All DRM fractions used in this study were set to 1 µg/µl with 20 mM PIPES, pH 7.0, 250 mM sucrose, and 1 mM EGTA. Protein levels were tested by immunoblot using anti–flotillin-1 antibody.
Quantification of Aβ production rates by MSD ELISA
To determine de novo production of Aβ peptides, 6 µg CHAPSO-resistant membranes were incubated for 0 or 4 h at 37°C with 1.5 µM C99-3×FLAG substrate. The activity assays were performed in the presence of 2.5% DMSO (or 1 µM GSM in DMSO), 1 mM EGTA, 0.3% CHAPSO, and protease inhibitors (Complete; Roche). Aβ38, Aβ40, and Aβ42 levels in reactions were quantified on Multi-Spot 96-well plates precoated with anti-Aβ38, -Aβ40, and -Aβ42 antibodies using multiplex MSD technology. MSD plates were blocked with 150 µl/well 0.1% casein buffer for 1.5 h at room temperature (600 rpm) and rinsed 5× with 200 µl/well washing buffer (PBS + 0.05% Tween-20). 25 µl SULFO-TAG JRF/AbN/25 detection antibody diluted in blocking buffer was mixed with 25 µl of standards (synthetic human Aβ1-38, Aβ1-40, and Aβ1-42 peptides) or reaction samples diluted in blocking buffer and loaded 50 µl per well.
After overnight incubation at 4°C, plates were rinsed with washing buffer and 150 µl/well of the 2× MSD Read Buffer T (Tris-based buffer containing tripropylamine, purchased from Meso Scale Discovery) was added. Plates were immediately read on a Sector Imager 6000 (Meso Scale Discovery). We determined the rates at which Aβ38, Aβ40, and Aβ42 are produced in each sample by subtracting the 0-h value from the 4-h value obtained by ELISA (Meso Scale Discovery) and normalizing Aβ amounts against time to express rates in pM/h. To address the effects of modulators, we performed reactions in the presence of 1 µM GSMs. The ratio Aβ38/42 was taken as an estimation of the efficiency of the fourth turnover of the γ-secretase complex.
Statistical analysis
All statistical analysis was performed using Prism 6 software (GraphPad Software). An ANOVA test was used to test the significance of the changes between groups.
Acknowledgments
We thank Dr. Amantha Thathiah for critical reading of the manuscript. We thank Dr. Francisco Lopera (University of Antioquia, Medellín, Colombia), Dr. Isidro Ferrer (Institut Neuropatologia, Hospital Universitari Bellvitge, Barcelona, Spain), Dr. Ellen Gelpi (Neurological Tissue Bank of the Biobanc-Hospital Clinic-IDIBAPS, Barcelona, Spain), Dr. Hiroyuki Hatsuta (Tokyo Metropolitan Institute of Gerontology, Tokyo, Japan), and Dr. Yasuo Ihara and Dr. Nobuto Kakuda (Doshisha University, Kyoto, Japan) for their invaluable assistance in the collection of postmortem human brain samples. We are grateful to all donors and their relatives for consent to autopsy and use of their tissues to advance scientific research. We thank Mark Mercken from Janssen Pharmaceutica for anti-Aβ monoclonal antibodies and Michel Vande Kerckhove for helpful discussions.
This work was funded by the Fund for Scientific Research, Flanders, the KU Leuven, a Methusalem grant from the KU Leuven and the Flemisch Government, IWT Agency for Innovation by Science and Technology, Janssen Pharmaceutica, Interuniversity Attraction Poles Program of the Belgian Federal Science Policy Office, Instituto de Salud Carlos III (PI10/00018 to A. Lleo), and CIBERNED. The London Neurodegenerative Diseases Brain Bank received funding from the Medical Research Council and from the Brains for Dementia Research project (funded by the Alzheimer’s Society and Alzheimer’s Research UK). We acknowledge support from the National Institute for Health Research (NIHR) Queen Square Biomedical Unit in Dementia and the Leonard Wolfson Experimental Neurology Centre. N.C. Fox is an NIHR Senior Investigator. D. Sepulveda-Falla is supported by the Hamburg State Ministry of Science and Research, Landesforschungsförderung “Molekulare mechanismen der netzwerkmodifizierung.” N.S. Ryan is supported by a Brain Exit Fellowship. T. Lashley is supported by an Alzheimer’s Research UK fellowship. B. De Strooper is supported by the Arthur Bax and Anna Vanluffelen chair for Alzheimer’s disease.
H. Gijsen is an employee of Janssen Pharmaceutica NV and holds stock in Johnson & Johnson. B. De Strooper is a consultant for Janssen Pharmaceutica, Envivo Pharmaceuticals, and Remynd NV. The authors declare no further competing financial interests.
Footnotes
Abbreviations used:
- Aβ
- amyloid-β
- AD
- Alzheimer’s disease
- APP
- amyloid precursor protein
- DRM
- detergent-resistant membrane
- FAD
- familial AD
- GSM
- γ-secretase modulator
- ICD
- intracellular domain
- LOF
- loss-of-function
- Nct
- Nicastrin
- PSEN
- Presenilin
- SAD
- sporadic AD
References
- Adolfsson O., Pihlgren M., Toni N., Varisco Y., Buccarello A.L., Antoniello K., Lohmann S., Piorkowska K., Gafner V., Atwal J.K., et al. . 2012. An effector-reduced anti-β-amyloid (Aβ) antibody with unique aβ binding properties promotes neuroprotection and glial engulfment of Aβ. J. Neurosci. 32:9677–9689. 10.1523/JNEUROSCI.4742-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmans B.A., and De Strooper B.. 2010. γ-secretases: from cell biology to therapeutic strategies. Lancet Neurol. 9:215–226. 10.1016/S1474-4422(09)70332-1 [DOI] [PubMed] [Google Scholar]
- Campion D., Dumanchin C., Hannequin D., Dubois B., Belliard S., Puel M., Thomas-Anterion C., Michon A., Martin C., Charbonnier F., et al. . 1999. Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am. J. Hum. Genet. 65:664–670. 10.1086/302553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez-Gutiérrez L., Tolia A., Maes E., Li T., Wong P.C., and de Strooper B.. 2008. Glu332 in the Nicastrin ectodomain is essential for γ-secretase complex maturation but not for its activity. J. Biol. Chem. 283:20096–20105. 10.1074/jbc.M803040200 [DOI] [PubMed] [Google Scholar]
- Chávez-Gutiérrez L., Bammens L., Benilova I., Vandersteen A., Benurwar M., Borgers M., Lismont S., Zhou L., Van Cleynenbreugel S., Esselmann H., et al. . 2012. The mechanism of γ-secretase dysfunction in familial Alzheimer disease. EMBO J. 31:2261–2274. 10.1038/emboj.2012.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump C.J., Fish B.A., Castro S.V., Chau D.M., Gertsik N., Ahn K., Stiff C., Pozdnyakov N., Bales K.R., Johnson D.S., and Li Y.M.. 2011. Piperidine acetic acid based γ-secretase modulators directly bind to Presenilin-1. ACS Chem. Neurosci. 2:705–710. 10.1021/cn200098p [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B. 2003. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active γ-secretase complex. Neuron. 38:9–12. 10.1016/S0896-6273(03)00205-8 [DOI] [PubMed] [Google Scholar]
- De Strooper B. 2007. Loss-of-function presenilin mutations in Alzheimer disease. Talking Point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 8:141–146. 10.1038/sj.embor.7400897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B., Saftig P., Craessaerts K., Vanderstichele H., Guhde G., Annaert W., Von Figura K., and Van Leuven F.. 1998. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 391:387–390. 10.1038/34910 [DOI] [PubMed] [Google Scholar]
- Edland S.D., and Galasko D.R.. 2011. Fractional synthesis and clearance rates for amyloid β. Nat. Med. 17:1178–1179. 10.1038/nm.2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez M.A., Klutkowski J.A., Freret T., and Wolfe M.S.. 2014. Alzheimer presenilin-1 mutations dramatically reduce trimming of long amyloid β-peptides (Aβ) by γ-secretase to increase 42-to-40-residue Aβ. J. Biol. Chem. 289:31043–31052. 10.1074/jbc.M114.581165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S., Fujishige S., Araki Y., Taniguchi M., Urakami K., Peskind E., Akatsu H., Araseki M., Yamamoto K., Martins R.N., et al. . 2011. Alternative processing of γ-secretase substrates in common forms of mild cognitive impairment and Alzheimer’s disease: evidence for γ-secretase dysfunction. Ann. Neurol. 69:1026–1031. 10.1002/ana.22343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S., Taniguchi M., Piao Y., Ikeuchi T., Fagan A.M., Holtzman D.M., Bateman R., Sohrabi H.R., Martins R.N., Gandy S., et al. ; Japanese Alzheimer’s Disease Neuroimaging Initiative . 2012. Multiple γ-secretase product peptides are coordinately increased in concentration in the cerebrospinal fluid of a subpopulation of sporadic Alzheimer’s disease subjects. Mol. Neurodegener. 7:16 10.1186/1750-1326-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig E.A., Gutti U., Tai T., Shen J., and Kelleher R.J. III. 2013. Trans-dominant negative effects of pathogenic PSEN1 mutations on γ-secretase activity and Aβ production. J. Neurosci. 33:11606–11617. 10.1523/JNEUROSCI.0954-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreman A., Serneels L., Annaert W., Collen D., Schoonjans L., and De Strooper B.. 2000. Total inactivation of γ-secretase activity in presenilin-deficient embryonic stem cells. Nat. Cell Biol. 2:461–462. 10.1038/35017105 [DOI] [PubMed] [Google Scholar]
- Jurisch-Yaksi N., Sannerud R., and Annaert W.. 2013. A fast growing spectrum of biological functions of γ-secretase in development and disease. Biochim. Biophys. Acta. 1828:2815–2827. 10.1016/j.bbamem.2013.04.016 [DOI] [PubMed] [Google Scholar]
- Kakuda N., Shoji M., Arai H., Furukawa K., Ikeuchi T., Akazawa K., Takami M., Hatsuta H., Murayama S., Hashimoto Y., et al. ; Japanese Alzheimer’s Disease Neuroimaging Initiative . 2012. Altered γ-secretase activity in mild cognitive impairment and Alzheimer’s disease. EMBO Mol. Med. 4:344–352. 10.1002/emmm.201200214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Onstead L., Randle S., Price R., Smithson L., Zwizinski C., Dickson D.W., Golde T., and McGowan E.. 2007. Aβ40 inhibits amyloid deposition in vivo. J. Neurosci. 27:627–633. 10.1523/JNEUROSCI.4849-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K.S., Muñoz C., Lopez L., Arcila M.L., García G., Madrigal L., Moreno S., Ríos Romenets S., Lopez H., Gutierrez M., et al. . 2015. Homozygosity of the autosomal dominant Alzheimer disease presenilin 1 E280A mutation. Neurology. 84:206–208. 10.1212/WNL.0000000000001130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperstein I., Broersen K., Benilova I., Rozenski J., Jonckheere W., Debulpaep M., Vandersteen A., Segers-Nolten I., Van Der Werf K., Subramaniam V., et al. . 2010. Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 29:3408–3420. 10.1038/emboj.2010.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura N., Takami M., Okochi M., Wada-Kakuda S., Fujiwara H., Tagami S., Funamoto S., Ihara Y., and Morishima-Kawashima M.. 2014. γ-Secretase associated with lipid rafts: multiple interactive pathways in the stepwise processing of β-carboxyl-terminal fragment. J. Biol. Chem. 289:5109–5121. 10.1074/jbc.M113.510131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawuenyega K.G., Sigurdson W., Ovod V., Munsell L., Kasten T., Morris J.C., Yarasheski K.E., and Bateman R.J.. 2010. Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science. 330:1774 10.1126/science.1197623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S., Evans L.D., Andersson T., Portelius E., Smith J., Dias T.B., Saurat N., McGlade A., Kirwan P., Blennow K., et al. . 2015. APP metabolism regulates tau proteostasis in human cerebral cortex neurons. Cell Reports. 11:689–696. 10.1016/j.celrep.2015.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera M., Alcolea D., Sánchez-Valle R., Guardia-Laguarta C., Colom-Cadena M., Badiola N., Suárez-Calvet M., Lladó A., Barrera-Ocampo A.A., Sepulveda-Falla D., et al. . 2013. Distinct patterns of APP processing in the CNS in autosomal-dominant and sporadic Alzheimer disease. Acta Neuropathol. 125:201–213. 10.1007/s00401-012-1062-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pink A.E., Simpson M.A., Desai N., Trembath R.C., and Barker J.N.. 2013. γ-Secretase mutations in hidradenitis suppurativa: new insights into disease pathogenesis. J. Invest. Dermatol. 133:601–607. 10.1038/jid.2012.372 [DOI] [PubMed] [Google Scholar]
- Potter R., Patterson B.W., Elbert D.L., Ovod V., Kasten T., Sigurdson W., Mawuenyega K., Blazey T., Goate A., Chott R., et al. . 2013. Increased in vivo amyloid-β42 production, exchange, and loss in presenilin mutation carriers. Sci. Transl. Med. 5:189ra77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi-Takahara Y., Morishima-Kawashima M., Tanimura Y., Dolios G., Hirotani N., Horikoshi Y., Kametani F., Maeda M., Saido T.C., Wang R., and Ihara Y.. 2005. Longer forms of amyloid β protein: implications for the mechanism of intramembrane cleavage by γ-secretase. J. Neurosci. 25:436–445. 10.1523/JNEUROSCI.1575-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero-Monzon O., Martin M.M., Fernandez M.A., Cappello C.A., Krzysiak A.J., Osenkowski P., and Wolfe M.S.. 2011. Dissociation between the processivity and total activity of γ-secretase: implications for the mechanism of Alzheimer’s disease-causing presenilin mutations. Biochemistry. 50:9023–9035. 10.1021/bi2007146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Suemoto T., Brouwers N., Sleegers K., Funamoto S., Mihira N., Matsuba Y., Yamada K., Nilsson P., Takano J., et al. . 2011. Potent amyloidogenicity and pathogenicity of Aβ43. Nat. Neurosci. 14:1023–1032. 10.1038/nn.2858 [DOI] [PubMed] [Google Scholar]
- Takami M., Nagashima Y., Sano Y., Ishihara S., Morishima-Kawashima M., Funamoto S., and Ihara Y.. 2009. γ-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of β-carboxyl terminal fragment. J. Neurosci. 29:13042–13052. 10.1523/JNEUROSCI.2362-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo K., Tanimura S., Shinoda T., Osawa S., Zahariev I.K., Takegami N., Ishizuka-Katsura Y., Shinya N., Takagi-Niidome S., Tominaga A., et al. . 2014. Allosteric regulation of γ-secretase activity by a phenylimidazole-type γ-secretase modulator. Proc. Natl. Acad. Sci. USA. 111:10544–10549. 10.1073/pnas.1402171111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi R.E., and Bertram L.. 2005. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 120:545–555. 10.1016/j.cell.2005.02.008 [DOI] [PubMed] [Google Scholar]
- Velter A.I., Bischoff F.P., Berthelot D., De Cleyn M., Oehlrich D., Jaroskova L., Macdonald G., Minne G., Pieters S., Rombouts F., et al. . 2014. Anilinotriazoles as potent gamma secretase modulators. Bioorg. Med. Chem. Lett. 24:5805–5813. 10.1016/j.bmcl.2014.10.024 [DOI] [PubMed] [Google Scholar]
- Wahrle S., Das P., Nyborg A.C., McLendon C., Shoji M., Kawarabayashi T., Younkin L.H., Younkin S.G., and Golde T.E.. 2002. Cholesterol-dependent γ-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol. Dis. 9:11–23. 10.1006/nbdi.2001.0470 [DOI] [PubMed] [Google Scholar]
- Wang R., Wang B., He W., and Zheng H.. 2006. Wild-type presenilin 1 protects against Alzheimer disease mutation-induced amyloid pathology. J. Biol. Chem. 281:15330–15336. 10.1074/jbc.M512574200 [DOI] [PubMed] [Google Scholar]
- Wolfe M.S., Xia W., Ostaszewski B.L., Diehl T.S., Kimberly W.T., and Selkoe D.J.. 1999. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature. 398:513–517. 10.1038/19077 [DOI] [PubMed] [Google Scholar]
- Xia D., Watanabe H., Wu B., Lee S.H., Li Y., Tsvetkov E., Bolshakov V.Y., Shen J., and Kelleher R.J. III. 2015. Presenilin-1 knockin mice reveal loss-of-function mechanism for familial Alzheimer’s disease. Neuron. 85:967–981. 10.1016/j.neuron.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagishita S., Morishima-Kawashima M., Ishiura S., and Ihara Y.. 2008. Aβ46 is processed to Aβ40 and Aβ43, but not to Aβ42, in the low density membrane domains. J. Biol. Chem. 283:733–738. 10.1074/jbc.M707103200 [DOI] [PubMed] [Google Scholar]