Abstract
In the title compound, C24H21NO3S, the dihedral angles between the indole ring system (r.m.s. deviation = 0.030 Å) and the sulfur and ethylene-bonded benzene rings are 80.2 (2) and 49.29 (15)°, respectively. The dihedral angle between the pendant benzene rings is 37.7 (2)°. In the crystal, molecules are linked by C—H⋯O hydrogen bonds and weak C—H⋯π and π–π [centroid-to-centroid distances = 3.549 (2) and 3.743 (3) Å] interactions, forming a three-dimensional network.
Keywords: crystal structure, phenylsulfonyl, 1H-indole, hydrogen bonding, C—H⋯π interactions, π–π interactions
Related literature
For the biological activity of indole derivatives, see: Andreani et al. (2001 ▸); Kolocouris et al. (1994 ▸). For the structures of related compounds, see: Chakkaravarthi et al. (2007 ▸, 2008 ▸).
Experimental
Crystal data
C24H21NO3S
M r = 403.48
Monoclinic,

a = 27.373 (4) Å
b = 12.7232 (16) Å
c = 12.0881 (13) Å
β = 102.827 (6)°
V = 4104.9 (9) Å3
Z = 8
Mo Kα radiation
μ = 0.18 mm−1
T = 295 K
0.28 × 0.24 × 0.20 mm
Data collection
Bruker Kappa APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▸) T min = 0.951, T max = 0.964
26597 measured reflections
4852 independent reflections
2432 reflections with I > 2σ(I)
R int = 0.120
Refinement
R[F 2 > 2σ(F 2)] = 0.064
wR(F 2) = 0.231
S = 1.00
4852 reflections
264 parameters
H-atom parameters constrained
Δρmax = 0.28 e Å−3
Δρmin = −0.37 e Å−3
Data collection: APEX2 (Bruker, 2004 ▸); cell refinement: SAINT (Bruker, 2004 ▸); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▸); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▸); molecular graphics: PLATON (Spek, 2009 ▸); software used to prepare material for publication: SHELXL97 and PLATON.
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S2056989015016631/hb7496sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989015016631/hb7496Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989015016631/hb7496Isup3.cml
. DOI: 10.1107/S2056989015016631/hb7496fig1.tif
The molecular structure of (I), with 30% probability displacement ellipsoids for non-H atoms.
b . DOI: 10.1107/S2056989015016631/hb7496fig2.tif
The crystal packing of the title compound viewed along the b axis. The hydrogen bonds are shown as dashed lines (see Table 1), and C-bound H atoms have been omitted for clarity.
CCDC reference: 1422542
Additional supporting information: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (, ).
Cg2, Cg3 and Cg4 are the centroids of the C1C6, C7C12 and C18C23 rings, respectively.
| DHA | DH | HA | D A | DHA |
|---|---|---|---|---|
| C6H6O2i | 0.93 | 2.46 | 3.249(5) | 143 |
| C15H15C Cg4ii | 0.96 | 2.82 | 3.759(4) | 167 |
| C24H24A Cg3iii | 0.96 | 2.84 | 3.634(6) | 140 |
| C24H24C Cg2iii | 0.96 | 2.88 | 3.520(5) | 125 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
Acknowledgments
The authors acknowledge the SAIF, IIT, Madras, for the data collection.
supplementary crystallographic information
S1. Comment
Indole derivatives exhibit antitumour (Andreani et al., 2001) and antiviral (Kolocouris et al., 1994) activities. The molecular structure of the title compound is illustrated in Fig. 1. The geometric parameters of the title molecule agree well with the reported similar structures (Chakkaravarthi et al. 2007, 2008). The torsion angles O1—S1—N1—C7 and O2—S1—N1—C14 [-48.0 (3)° and 38.3 (3)°, respectively] indicate the syn-conformation of the sulfonyl moiety.
In the crystal, the molecules are linked by C—H···O hydrogen bonds (Table 1 & Fig. 2) and the packing also features weak C—H···π (Table 1) and π–π [Cg1···Cg1i distance 3.549 (2) Å; Cg2···Cg2ii distance 3.743 (3) Å; (i) 1/2 - x,1/2 - y,1 - z; 1 - x,-y,1 - z; Cg1 and Cg2 are the centroids of the rings (N1/C7/C12/C13/C14) and (C1—C6), respectively] interactions in a three-dimensional network.
S2. Experimental
To a suspension of sodium hydride (0.22 g, 4.74 mmol) in dry THF (10 ml) at -10°C, the solution of diethyl (3-methyl-1-(phenylsulfonyl)-1H-indol-2-yl)methylphosphonate (1 g, 2.37 mmol) in dry THF (10 ml) was slowly added under nitrogen atmosphere and stirred for 1 h at -10°C. Then, the solution of p-anisaldehyde (0.31 ml, 2.61 mmol) in dry THF (5 ml) was added and the stirring was continued at -10 to 0°C for another 2 h. After completion of the reaction (monitored by TLC), the yellow solution was poured over crushed ice (80 g) containing Conc. HCl (5 ml). The solid obtained was filtered, dried and recrystallized from methanol solution to afford the title compound in the form of colourless blocks.
S3. Refinement
H atoms were positioned geometrically and refined using riding model, with C—H = 0.93 Å and Uiso(H) = 1.2Ueq(C) for C—H and C—H = 0.96 Å and Uiso(H) = 1.5Ueq(C) for methyl. The reflections (2 0 0) and (1 1 0) were omitted during refinement which were owing poor agreement.
Figures
Fig. 1.

The molecular structure of (I), with 30% probability displacement ellipsoids for non-H atoms.
Fig. 2.
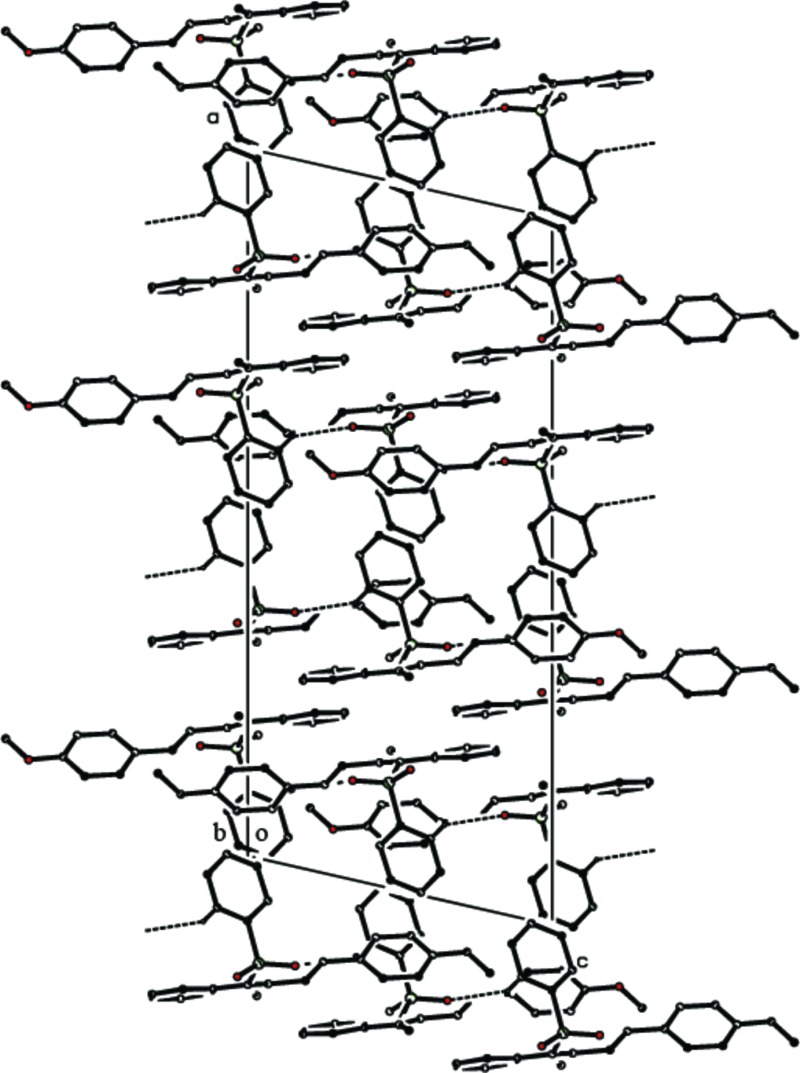
The crystal packing of the title compound viewed along the b axis. The hydrogen bonds are shown as dashed lines (see Table 1), and C-bound H atoms have been omitted for clarity.
Crystal data
| C24H21NO3S | F(000) = 1696 |
| Mr = 403.48 | Dx = 1.306 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -C 2yc | Cell parameters from 5639 reflections |
| a = 27.373 (4) Å | θ = 2.6–24.6° |
| b = 12.7232 (16) Å | µ = 0.18 mm−1 |
| c = 12.0881 (13) Å | T = 295 K |
| β = 102.827 (6)° | Block, colourless |
| V = 4104.9 (9) Å3 | 0.28 × 0.24 × 0.20 mm |
| Z = 8 |
Data collection
| Bruker Kappa APEXII CCD diffractometer | 4852 independent reflections |
| Radiation source: fine-focus sealed tube | 2432 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.120 |
| ω and φ scan | θmax = 27.9°, θmin = 2.4° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −36→35 |
| Tmin = 0.951, Tmax = 0.964 | k = −16→16 |
| 26597 measured reflections | l = −15→15 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.064 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.231 | H-atom parameters constrained |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.109P)2 + 3.4059P] where P = (Fo2 + 2Fc2)/3 |
| 4852 reflections | (Δ/σ)max < 0.001 |
| 264 parameters | Δρmax = 0.28 e Å−3 |
| 0 restraints | Δρmin = −0.37 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.40832 (12) | 0.0851 (3) | 0.4987 (3) | 0.0558 (9) | |
| C2 | 0.44297 (16) | 0.1492 (3) | 0.5659 (4) | 0.0830 (12) | |
| H2 | 0.4371 | 0.1778 | 0.6326 | 0.100* | |
| C3 | 0.48806 (19) | 0.1704 (4) | 0.5304 (7) | 0.117 (2) | |
| H3 | 0.5124 | 0.2133 | 0.5741 | 0.140* | |
| C4 | 0.4957 (2) | 0.1274 (5) | 0.4313 (7) | 0.121 (2) | |
| H4 | 0.5254 | 0.1412 | 0.4083 | 0.145* | |
| C5 | 0.4609 (2) | 0.0657 (5) | 0.3678 (5) | 0.1072 (18) | |
| H5 | 0.4667 | 0.0369 | 0.3011 | 0.129* | |
| C6 | 0.41692 (15) | 0.0444 (3) | 0.3994 (3) | 0.0697 (10) | |
| H6 | 0.3928 | 0.0024 | 0.3536 | 0.084* | |
| C7 | 0.30101 (11) | 0.1982 (2) | 0.3861 (3) | 0.0467 (7) | |
| C8 | 0.29070 (13) | 0.1401 (3) | 0.2867 (3) | 0.0583 (9) | |
| H8 | 0.2952 | 0.0676 | 0.2882 | 0.070* | |
| C9 | 0.27364 (14) | 0.1922 (4) | 0.1857 (3) | 0.0712 (11) | |
| H9 | 0.2671 | 0.1542 | 0.1183 | 0.085* | |
| C10 | 0.26607 (15) | 0.2987 (4) | 0.1820 (3) | 0.0748 (11) | |
| H10 | 0.2537 | 0.3314 | 0.1126 | 0.090* | |
| C11 | 0.27647 (13) | 0.3574 (3) | 0.2794 (3) | 0.0642 (10) | |
| H11 | 0.2713 | 0.4297 | 0.2761 | 0.077* | |
| C12 | 0.29493 (11) | 0.3081 (3) | 0.3841 (3) | 0.0496 (8) | |
| C13 | 0.31014 (12) | 0.3465 (2) | 0.4972 (3) | 0.0516 (8) | |
| C14 | 0.32558 (11) | 0.2641 (2) | 0.5673 (3) | 0.0480 (8) | |
| C15 | 0.30517 (15) | 0.4586 (3) | 0.5297 (4) | 0.0736 (11) | |
| H15A | 0.3076 | 0.4632 | 0.6100 | 0.110* | |
| H15B | 0.2732 | 0.4853 | 0.4902 | 0.110* | |
| H15C | 0.3314 | 0.4994 | 0.5097 | 0.110* | |
| C16 | 0.34235 (13) | 0.2629 (3) | 0.6895 (3) | 0.0537 (8) | |
| H16 | 0.3298 | 0.2110 | 0.7298 | 0.064* | |
| C17 | 0.37458 (13) | 0.3316 (3) | 0.7474 (3) | 0.0556 (8) | |
| H17 | 0.3887 | 0.3794 | 0.7054 | 0.067* | |
| C18 | 0.38995 (12) | 0.3393 (3) | 0.8702 (3) | 0.0534 (8) | |
| C19 | 0.42576 (14) | 0.4133 (3) | 0.9200 (3) | 0.0675 (10) | |
| H19 | 0.4405 | 0.4553 | 0.8736 | 0.081* | |
| C20 | 0.43979 (15) | 0.4256 (3) | 1.0354 (3) | 0.0734 (11) | |
| H20 | 0.4635 | 0.4762 | 1.0657 | 0.088* | |
| C21 | 0.41924 (13) | 0.3642 (3) | 1.1070 (3) | 0.0619 (9) | |
| C22 | 0.38457 (12) | 0.2888 (3) | 1.0604 (3) | 0.0589 (9) | |
| H22 | 0.3707 | 0.2457 | 1.1075 | 0.071* | |
| C23 | 0.37042 (13) | 0.2769 (3) | 0.9445 (3) | 0.0602 (9) | |
| H23 | 0.3470 | 0.2255 | 0.9148 | 0.072* | |
| C24 | 0.4123 (2) | 0.3287 (4) | 1.2952 (4) | 0.1073 (17) | |
| H24A | 0.3772 | 0.3454 | 1.2805 | 0.161* | |
| H24B | 0.4278 | 0.3481 | 1.3717 | 0.161* | |
| H24C | 0.4164 | 0.2547 | 1.2852 | 0.161* | |
| N1 | 0.31802 (9) | 0.16882 (19) | 0.5006 (2) | 0.0477 (7) | |
| O1 | 0.32587 (9) | −0.02048 (17) | 0.4661 (2) | 0.0633 (7) | |
| O2 | 0.35975 (11) | 0.0507 (2) | 0.65544 (19) | 0.0733 (8) | |
| O3 | 0.43465 (10) | 0.3842 (3) | 1.2199 (2) | 0.0862 (9) | |
| S1 | 0.35144 (3) | 0.06038 (6) | 0.53598 (7) | 0.0512 (3) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0529 (19) | 0.0443 (19) | 0.068 (2) | 0.0044 (15) | 0.0089 (17) | 0.0051 (16) |
| C2 | 0.071 (3) | 0.058 (3) | 0.112 (3) | 0.002 (2) | 0.005 (2) | −0.013 (2) |
| C3 | 0.064 (3) | 0.071 (3) | 0.204 (7) | −0.011 (2) | 0.008 (4) | 0.002 (4) |
| C4 | 0.082 (4) | 0.097 (4) | 0.199 (7) | 0.008 (3) | 0.065 (4) | 0.028 (4) |
| C5 | 0.088 (3) | 0.114 (4) | 0.138 (5) | 0.007 (3) | 0.064 (3) | 0.021 (4) |
| C6 | 0.066 (2) | 0.076 (3) | 0.071 (3) | 0.0089 (19) | 0.0230 (19) | 0.004 (2) |
| C7 | 0.0452 (17) | 0.0462 (19) | 0.0502 (19) | 0.0007 (14) | 0.0141 (14) | 0.0035 (15) |
| C8 | 0.065 (2) | 0.048 (2) | 0.061 (2) | −0.0047 (16) | 0.0105 (17) | −0.0074 (17) |
| C9 | 0.078 (3) | 0.081 (3) | 0.051 (2) | −0.007 (2) | 0.0066 (19) | 0.001 (2) |
| C10 | 0.079 (3) | 0.074 (3) | 0.068 (3) | −0.003 (2) | 0.009 (2) | 0.017 (2) |
| C11 | 0.063 (2) | 0.053 (2) | 0.076 (3) | 0.0011 (17) | 0.0119 (19) | 0.016 (2) |
| C12 | 0.0437 (17) | 0.0437 (19) | 0.063 (2) | 0.0011 (14) | 0.0156 (15) | 0.0046 (16) |
| C13 | 0.0476 (18) | 0.0409 (19) | 0.069 (2) | 0.0003 (14) | 0.0194 (16) | −0.0030 (17) |
| C14 | 0.0498 (18) | 0.0418 (18) | 0.057 (2) | −0.0044 (14) | 0.0227 (15) | −0.0097 (15) |
| C15 | 0.075 (3) | 0.044 (2) | 0.102 (3) | 0.0026 (18) | 0.021 (2) | −0.008 (2) |
| C16 | 0.063 (2) | 0.047 (2) | 0.057 (2) | −0.0032 (16) | 0.0262 (17) | −0.0063 (15) |
| C17 | 0.059 (2) | 0.053 (2) | 0.056 (2) | −0.0041 (16) | 0.0173 (16) | −0.0039 (16) |
| C18 | 0.0542 (19) | 0.046 (2) | 0.062 (2) | −0.0012 (15) | 0.0176 (16) | −0.0080 (16) |
| C19 | 0.074 (2) | 0.067 (2) | 0.066 (2) | −0.018 (2) | 0.026 (2) | −0.0078 (19) |
| C20 | 0.068 (2) | 0.079 (3) | 0.074 (3) | −0.025 (2) | 0.018 (2) | −0.019 (2) |
| C21 | 0.057 (2) | 0.066 (2) | 0.062 (2) | 0.0018 (18) | 0.0109 (18) | −0.0069 (19) |
| C22 | 0.057 (2) | 0.057 (2) | 0.063 (2) | −0.0004 (17) | 0.0147 (17) | 0.0087 (18) |
| C23 | 0.058 (2) | 0.050 (2) | 0.071 (2) | −0.0051 (16) | 0.0119 (18) | −0.0014 (18) |
| C24 | 0.148 (5) | 0.098 (4) | 0.067 (3) | −0.013 (3) | 0.005 (3) | 0.018 (3) |
| N1 | 0.0532 (15) | 0.0406 (15) | 0.0509 (16) | −0.0033 (12) | 0.0149 (12) | −0.0032 (12) |
| O1 | 0.0731 (16) | 0.0360 (13) | 0.0815 (17) | −0.0048 (11) | 0.0189 (13) | −0.0054 (11) |
| O2 | 0.113 (2) | 0.0603 (16) | 0.0497 (15) | 0.0094 (14) | 0.0244 (14) | 0.0132 (12) |
| O3 | 0.0832 (19) | 0.109 (2) | 0.0646 (18) | −0.0207 (17) | 0.0131 (15) | −0.0117 (16) |
| S1 | 0.0654 (6) | 0.0367 (5) | 0.0536 (5) | 0.0005 (4) | 0.0176 (4) | 0.0033 (4) |
Geometric parameters (Å, º)
| C1—C2 | 1.372 (5) | C14—C16 | 1.447 (4) |
| C1—C6 | 1.375 (5) | C15—H15A | 0.9600 |
| C1—S1 | 1.742 (4) | C15—H15B | 0.9600 |
| C2—C3 | 1.420 (7) | C15—H15C | 0.9600 |
| C2—H2 | 0.9300 | C16—C17 | 1.326 (4) |
| C3—C4 | 1.375 (8) | C16—H16 | 0.9300 |
| C3—H3 | 0.9300 | C17—C18 | 1.454 (5) |
| C4—C5 | 1.337 (8) | C17—H17 | 0.9300 |
| C4—H4 | 0.9300 | C18—C23 | 1.391 (5) |
| C5—C6 | 1.369 (6) | C18—C19 | 1.395 (5) |
| C5—H5 | 0.9300 | C19—C20 | 1.372 (5) |
| C6—H6 | 0.9300 | C19—H19 | 0.9300 |
| C7—C8 | 1.386 (4) | C20—C21 | 1.376 (5) |
| C7—C12 | 1.407 (5) | C20—H20 | 0.9300 |
| C7—N1 | 1.409 (4) | C21—O3 | 1.359 (4) |
| C8—C9 | 1.377 (5) | C21—C22 | 1.379 (5) |
| C8—H8 | 0.9300 | C22—C23 | 1.377 (5) |
| C9—C10 | 1.370 (6) | C22—H22 | 0.9300 |
| C9—H9 | 0.9300 | C23—H23 | 0.9300 |
| C10—C11 | 1.370 (5) | C24—O3 | 1.396 (5) |
| C10—H10 | 0.9300 | C24—H24A | 0.9600 |
| C11—C12 | 1.403 (5) | C24—H24B | 0.9600 |
| C11—H11 | 0.9300 | C24—H24C | 0.9600 |
| C12—C13 | 1.424 (5) | N1—S1 | 1.658 (3) |
| C13—C14 | 1.355 (4) | O1—S1 | 1.414 (2) |
| C13—C15 | 1.494 (5) | O2—S1 | 1.416 (2) |
| C14—N1 | 1.446 (4) | ||
| C2—C1—C6 | 120.7 (4) | H15A—C15—H15B | 109.5 |
| C2—C1—S1 | 119.6 (3) | C13—C15—H15C | 109.5 |
| C6—C1—S1 | 119.6 (3) | H15A—C15—H15C | 109.5 |
| C1—C2—C3 | 117.8 (5) | H15B—C15—H15C | 109.5 |
| C1—C2—H2 | 121.1 | C17—C16—C14 | 123.7 (3) |
| C3—C2—H2 | 121.1 | C17—C16—H16 | 118.2 |
| C4—C3—C2 | 120.0 (5) | C14—C16—H16 | 118.2 |
| C4—C3—H3 | 120.0 | C16—C17—C18 | 126.3 (3) |
| C2—C3—H3 | 120.0 | C16—C17—H17 | 116.9 |
| C5—C4—C3 | 120.4 (5) | C18—C17—H17 | 116.9 |
| C5—C4—H4 | 119.8 | C23—C18—C19 | 116.1 (3) |
| C3—C4—H4 | 119.8 | C23—C18—C17 | 123.7 (3) |
| C4—C5—C6 | 121.0 (5) | C19—C18—C17 | 120.2 (3) |
| C4—C5—H5 | 119.5 | C20—C19—C18 | 121.8 (3) |
| C6—C5—H5 | 119.5 | C20—C19—H19 | 119.1 |
| C5—C6—C1 | 120.1 (5) | C18—C19—H19 | 119.1 |
| C5—C6—H6 | 120.0 | C19—C20—C21 | 120.9 (4) |
| C1—C6—H6 | 120.0 | C19—C20—H20 | 119.5 |
| C8—C7—C12 | 121.0 (3) | C21—C20—H20 | 119.5 |
| C8—C7—N1 | 132.0 (3) | O3—C21—C20 | 116.5 (3) |
| C12—C7—N1 | 107.0 (3) | O3—C21—C22 | 124.9 (3) |
| C9—C8—C7 | 118.4 (3) | C20—C21—C22 | 118.6 (3) |
| C9—C8—H8 | 120.8 | C23—C22—C21 | 120.3 (3) |
| C7—C8—H8 | 120.8 | C23—C22—H22 | 119.9 |
| C10—C9—C8 | 121.6 (4) | C21—C22—H22 | 119.9 |
| C10—C9—H9 | 119.2 | C22—C23—C18 | 122.2 (3) |
| C8—C9—H9 | 119.2 | C22—C23—H23 | 118.9 |
| C9—C10—C11 | 120.7 (4) | C18—C23—H23 | 118.9 |
| C9—C10—H10 | 119.7 | O3—C24—H24A | 109.5 |
| C11—C10—H10 | 119.7 | O3—C24—H24B | 109.5 |
| C10—C11—C12 | 119.7 (4) | H24A—C24—H24B | 109.5 |
| C10—C11—H11 | 120.2 | O3—C24—H24C | 109.5 |
| C12—C11—H11 | 120.2 | H24A—C24—H24C | 109.5 |
| C11—C12—C7 | 118.6 (3) | H24B—C24—H24C | 109.5 |
| C11—C12—C13 | 133.0 (3) | C7—N1—C14 | 107.5 (2) |
| C7—C12—C13 | 108.4 (3) | C7—N1—S1 | 121.2 (2) |
| C14—C13—C12 | 108.6 (3) | C14—N1—S1 | 123.5 (2) |
| C14—C13—C15 | 127.4 (3) | C21—O3—C24 | 118.4 (3) |
| C12—C13—C15 | 123.8 (3) | O1—S1—O2 | 119.45 (15) |
| C13—C14—N1 | 108.3 (3) | O1—S1—N1 | 106.24 (14) |
| C13—C14—C16 | 129.2 (3) | O2—S1—N1 | 106.86 (14) |
| N1—C14—C16 | 122.3 (3) | O1—S1—C1 | 109.21 (16) |
| C13—C15—H15A | 109.5 | O2—S1—C1 | 109.19 (17) |
| C13—C15—H15B | 109.5 | N1—S1—C1 | 104.87 (14) |
| C6—C1—C2—C3 | −1.1 (6) | C23—C18—C19—C20 | −1.8 (5) |
| S1—C1—C2—C3 | −177.7 (3) | C17—C18—C19—C20 | 177.5 (3) |
| C1—C2—C3—C4 | 0.2 (7) | C18—C19—C20—C21 | 0.7 (6) |
| C2—C3—C4—C5 | 0.2 (9) | C19—C20—C21—O3 | −178.4 (4) |
| C3—C4—C5—C6 | 0.3 (9) | C19—C20—C21—C22 | 0.8 (6) |
| C4—C5—C6—C1 | −1.1 (7) | O3—C21—C22—C23 | 178.1 (3) |
| C2—C1—C6—C5 | 1.5 (6) | C20—C21—C22—C23 | −1.1 (5) |
| S1—C1—C6—C5 | 178.2 (3) | C21—C22—C23—C18 | −0.1 (5) |
| C12—C7—C8—C9 | −0.9 (5) | C19—C18—C23—C22 | 1.5 (5) |
| N1—C7—C8—C9 | 179.2 (3) | C17—C18—C23—C22 | −177.7 (3) |
| C7—C8—C9—C10 | −1.0 (6) | C8—C7—N1—C14 | 175.9 (3) |
| C8—C9—C10—C11 | 1.6 (6) | C12—C7—N1—C14 | −4.0 (3) |
| C9—C10—C11—C12 | −0.2 (6) | C8—C7—N1—S1 | 25.9 (4) |
| C10—C11—C12—C7 | −1.7 (5) | C12—C7—N1—S1 | −154.0 (2) |
| C10—C11—C12—C13 | 178.2 (3) | C13—C14—N1—C7 | 4.5 (3) |
| C8—C7—C12—C11 | 2.2 (5) | C16—C14—N1—C7 | 179.0 (3) |
| N1—C7—C12—C11 | −177.8 (3) | C13—C14—N1—S1 | 153.6 (2) |
| C8—C7—C12—C13 | −177.7 (3) | C16—C14—N1—S1 | −31.8 (4) |
| N1—C7—C12—C13 | 2.2 (3) | C20—C21—O3—C24 | 175.7 (4) |
| C11—C12—C13—C14 | −179.4 (3) | C22—C21—O3—C24 | −3.5 (6) |
| C7—C12—C13—C14 | 0.5 (4) | C7—N1—S1—O1 | −48.0 (3) |
| C11—C12—C13—C15 | 5.1 (6) | C14—N1—S1—O1 | 166.9 (2) |
| C7—C12—C13—C15 | −175.0 (3) | C7—N1—S1—O2 | −176.6 (2) |
| C12—C13—C14—N1 | −3.0 (3) | C14—N1—S1—O2 | 38.3 (3) |
| C15—C13—C14—N1 | 172.2 (3) | C7—N1—S1—C1 | 67.6 (3) |
| C12—C13—C14—C16 | −177.1 (3) | C14—N1—S1—C1 | −77.5 (3) |
| C15—C13—C14—C16 | −1.8 (5) | C2—C1—S1—O1 | −170.2 (3) |
| C13—C14—C16—C17 | −45.6 (5) | C6—C1—S1—O1 | 13.1 (3) |
| N1—C14—C16—C17 | 141.1 (3) | C2—C1—S1—O2 | −37.9 (3) |
| C14—C16—C17—C18 | 175.1 (3) | C6—C1—S1—O2 | 145.3 (3) |
| C16—C17—C18—C23 | −3.2 (5) | C2—C1—S1—N1 | 76.3 (3) |
| C16—C17—C18—C19 | 177.6 (3) | C6—C1—S1—N1 | −100.4 (3) |
Hydrogen-bond geometry (Å, º)
Cg2, Cg3 and Cg4 are the centroids of the C1–C6, C7–C12 and C18–C23 rings, respectively.
| D—H···A | D—H | H···A | D···A | D—H···A |
| C6—H6···O2i | 0.93 | 2.46 | 3.249 (5) | 143 |
| C15—H15C···Cg4ii | 0.96 | 2.82 | 3.759 (4) | 167 |
| C24—H24A···Cg3iii | 0.96 | 2.84 | 3.634 (6) | 140 |
| C24—H24C···Cg2iii | 0.96 | 2.88 | 3.520 (5) | 125 |
Symmetry codes: (i) x, −y, z−1/2; (ii) −x+1/2, y+3/2, −z−1/2; (iii) x, y, z+1.
Footnotes
Supporting information for this paper is available from the IUCr electronic archives (Reference: HB7496).
References
- Andreani, A., Granaiola, M., Leoni, A., Locatelli, A., Morigi, R., Rambaldi, M., Giorgi, G., Salvini, L. & Garaliene, V. (2001). Anticancer Drug. Des. 16, 167–174. [PubMed]
- Bruker (2004). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Chakkaravarthi, G., Dhayalan, V., Mohanakrishnan, A. K. & Manivannan, V. (2008). Acta Cryst. E64, o542. [DOI] [PMC free article] [PubMed]
- Chakkaravarthi, G., Ramesh, N., Mohanakrishnan, A. K. & Manivannan, V. (2007). Acta Cryst. E63, o3564.
- Kolocouris, N., Foscolos, G. B., Kolocouris, A., Marakos, P., Pouli, N., Fytas, G., Ikeda, S. & De Clercq, E. (1994). J. Med. Chem. 37, 2896–2902. [DOI] [PubMed]
- Sheldrick, G. M. (1996). SADABS. University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S2056989015016631/hb7496sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989015016631/hb7496Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989015016631/hb7496Isup3.cml
. DOI: 10.1107/S2056989015016631/hb7496fig1.tif
The molecular structure of (I), with 30% probability displacement ellipsoids for non-H atoms.
b . DOI: 10.1107/S2056989015016631/hb7496fig2.tif
The crystal packing of the title compound viewed along the b axis. The hydrogen bonds are shown as dashed lines (see Table 1), and C-bound H atoms have been omitted for clarity.
CCDC reference: 1422542
Additional supporting information: crystallographic information; 3D view; checkCIF report


