Abstract
Background:
From 2013, once-only flexible sigmoidoscopy (FS) at age 55 is being phased into the England National Health Service Bowel Cancer Screening Programme (NHSBCSP), augmenting biennial guaiac faecal occult blood testing (gFOBT) at ages 60–74. Here, we project the impact of this change on colorectal cancer (CRC) cases and deaths prevented in England by mid-2030.
Methods:
We simulated the life-course of English residents reaching age 55 from 2013 onwards. Model inputs included population numbers, invitation rates and CRC incidence and mortality rates. The impact of gFOBT and FS alone on CRC incidence and mortality were derived from published trials, assuming an uptake of 50% for FS and 57% for gFOBT. For FS plus gFOBT, we assumed the gFOBT effect to be 75% of the gFOBT alone impact.
Results:
By mid-2030, 8.5 million individuals will have been invited for once-only FS screening. Adding FS to gFOBT screening is estimated to prevent an extra 9627 (−10%) cases and 2207 (−12%) deaths by mid-2030. If FS uptake is 38% or 71%, respectively, an extra 7379 (−8%) or 13 689 (−15%) cases and 1691 (−9%) or 3154 (−17%) deaths will be prevented by mid-2030.
Conclusions:
Adding once-only FS at age 55 to the NHSBCSP will prevent ∼10 000 CRC cases and ∼2000 CRC deaths by mid-2030 if FS uptake is 50%. In 2030, one cancer was estimated to be prevented per 150 FS screening episodes, and one death prevented per 900 FS screening episodes. The actual reductions will depend on the FS invitation schedule and uptake rates.
Keywords: colorectal cancer, England, flexible sigmoidoscopy, guaiac faecal occult blood test, mass screening, simulation study
Individual bowel screening strategies, including stool-based tests and sigmoidoscopy have been shown to decrease mortality from colorectal cancer (CRC) (Holme et al, 2013). Furthermore, modelling studies demonstrated that various combined strategies can be cost-effective in reducing CRC mortality when compared with no screening (Jeong and Cairns, 2013). Because there is no single best bowel screening strategy, programmes differ between countries.
In 2006, the English National Health Service Bowel Cancer Screening Programme (NHSBCSP) rolled out biennial guaiac faecal occult blood testing (gFOBT) for individuals aged 60–74 (Morris et al, 2012). Once-only flexible sigmoidoscopy (FS) at age 55 is currently being phased into the NHSBCSP, in addition to the biennial gFOBT testing; in 2013, six screening centres had started and full invitation is expected in 2018 (NHSBCSP, personal communication).
The issue of the likely health impact of adding FS to the NHSBCSP is an important one. The individual impact of once-only FS and biennial gFOBT on incidence of and mortality from CRC has been studied in five and four trials, respectively, but no single trial has assessed the combined efficacy of these approaches (Holme et al, 2013). In this study we simulate the effect of adding once-only FS at age 55 to the NHSBCSP on the number of CRC cases and deaths in England by mid-2030 using published population and trial data.
Materials and Methods
Model scenarios
We estimated projected CRC cases and deaths in the absence of screening and for three screening scenarios: (1) biennial gFOBT at ages 60–74, (2) once-only FS at age 55, and (3) once-only FS at age 55 plus biennial gFOBT at ages 60–74. For the three screening scenarios, we also estimated deaths as a result of the screening intervention, that is, death from perforation of the large bowel during endoscopic examination.
Model parameters
The estimation requires a number of inputs, including, among others, population numbers in the relevant age group, screening invitation rates, incidence of and mortality from CRC, and effects of the screening modalities on these.
Population size. Expected English mid-year population sizes for persons aged 55 in 2013–2030 were derived from the projections made by the Office of National Statistics (Office of National Statistics, 2011, 2014a). The NHSBCSP invites all persons at relevant age groups if registered with a general practitioner in England (Morris et al, 2012). Persons identified with an increased risk of CRC are offered regular colonoscopy screening, and are unlikely to experience an additional benefit from the mass screening programme (Cancer Research UK, 2013b). Therefore, we considered 1% of the population ineligible, in line with estimations from Macafee et al (2008). Population numbers at ages 56+ were based on the projections at age 55 and the 2010–2012 England national life tables, that is, age-specific all-cause mortality probabilities (Office of National Statistics, 2014b). For simplicity, we assumed age-specific all-cause mortality to be constant over the projected study period, as the expected increase in life expectancy is only 0.2% per year (Office of National Statistics, 2013, 2014b).
Underlying CRC incidence and mortality rates. The CRC incidence and mortality rates in the absence of screening were derived from the England population. England CRC incidence and mortality rates by 5-year age group were provided by Cancer Research UK. In line with recent trends, we assumed a constant underlying CRC incidence rate for 2013–2030 and used the 2005 population estimate, that is, prior to the gFOBT screening roll-out (Morris et al, 2012). We reduced the incidence rates by 5% to exclude high-risk persons who account for ∼5% of CRC cases and follow a different surveillance programme (Cancer Research UK, 2014b).
In the absence of screening, the England CRC mortality rates for 2013–2030 were estimated by taking the 2005 rates (prior to the NHSBCSP roll-out) and assuming a reduction of 2% per calendar year, equal to the average annual decrease in mortality at ages 55–72 observed during 2000–2005. CRC mortality rates were adjusted for CRC deaths occurring from CRC cases detected before 2013 by taking the underlying CRC survival curve into account, assuming a 1-, 5-, and 10-year survival probability of 74, 54 and 50%, respectively (Cancer Research UK, 2012). The mortality rates were reduced by 2%, taking into account the effect of colonoscopy screening in high-risk groups (5% (incidence) × 0.35 (mortality reduction by colonoscopy screening) (Atkin et al, 2010; National Institute for Clinical Excellence (NICE), 2011)).
The age-specific incidence and mortality rates were interpolated linearly from the 5-year categories. The expected rates in the absence of screening are presented in Table 1.
Table 1. Model input: CRC incidence and mortality rates in the absence of screening and hazard rate ratios for the three screening scenarios when compared with no screening.
| Cycle |
CRC incidence |
CRC mortality |
||||||
|---|---|---|---|---|---|---|---|---|
| Year since age 55 | No screening (Hazard rate)a | gFOBT (HRR) | FSb (HRR) | FSb plus FOBT (HRR) | No sceening (Hazard rate, 2013)c | gFOBT (HRR) | FSb (HRR) | FSb plus FOBT (HRR) |
| 1 | 0.0006 | 1 | 2.05 | 2.05 | 0.0001 | 1 | 0.78 | 0.78 |
| 2 | 0.0007 | 1 | 0.77 | 0.77 | 0.0001 | 1 | 0.78 | 0.78 |
| 3 | 0.0007 | 1 | 0.77 | 0.77 | 0.0002 | 1 | 0.78 | 0.78 |
| 4 | 0.0008 | 1 | 0.47 | 0.47 | 0.0002 | 1 | 0.78 | 0.78 |
| 5 | 0.0009 | 1 | 0.82 | 0.82 | 0.0002 | 1 | 0.78 | 0.78 |
| 6 | 0.0010 | 1.64 | 0.65 | 0.96 | 0.0002 | 0.87 | 0.78 | 0.70 |
| 7 | 0.0010 | 0.77 | 0.77 | 0.63 | 0.0002 | 0.87 | 0.78 | 0.70 |
| 8 | 0.0010 | 1.13 | 0.65 | 0.72 | 0.0003 | 0.87 | 0.78 | 0.70 |
| 9 | 0.0012 | 0.86 | 0.77 | 0.68 | 0.0003 | 0.87 | 0.78 | 0.70 |
| 10 | 0.0013 | 1.08 | 1.00 | 1.06 | 0.0003 | 0.87 | 0.78 | 0.70 |
| 11 | 0.0014 | 0.69 | 0.53 | 0.41 | 0.0004 | 0.87 | 0.78 | 0.70 |
| 12 | 0.0015 | 1.11 | 0.65 | 0.70 | 0.0004 | 0.87 | 0.78 | 0.70 |
| 13 | 0.0017 | 0.71 | 0.75d | 0.58 | 0.0004 | 0.87 | 0.78 | 0.70 |
| 14 | 0.0018 | 0.94 | 0.75d | 0.71 | 0.0005 | 0.87 | 0.78 | 0.70 |
| 15 | 0.0020 | 1.00 | 0.75d | 0.75 | 0.0005 | 0.87 | 0.78 | 0.70 |
| 16 | 0.0021 | 1.06 | 0.75d | 0.78 | 0.0005 | 0.87 | 0.78 | 0.70 |
| 17 | 0.0023 | 1.06 | 0.75d | 0.78 | 0.0006 | 0.87 | 0.78 | 0.70 |
Abbreviations: CRC=colorectal cancer; FS=flexible sigmoidoscopy; gFOBT=guaiac faecal occult blood testing; HRR=hazard rate ratio.
CRC incidence is assumed to be constant in the years 2013–2030.
Presented for FS uptake 50%.
Adjusted for follow-up time, presented for cohort 2013. For cohort years 2014–2030, an annual 2% decrease in CRC mortality is assumed.
Extrapolations from follow-up years 8–12.
Invitation rates. Invitation to biennial gFOBT at ages 60–74 was assumed to be 100% in 2013–2030. According to the current Public Health England delivery plan, invitation for FS at age 55 was 3% in 2013, and will at best be 10% in 2014, 20% in 2015, 50% in 2016, 70% in 2017 and 100% in 2018–2030 (NHSBCSP, personal communication).
Impact of screening approaches on CRC incidence. The effect of biennial gFOBT on CRC incidence as of age 60 was estimated on the basis of the Nottingham gFOBT screening trial where uptake was 57% in those randomised (Scholefield et al, 2012). This trial randomised 152 850 participants aged 45–74 in 1981–1991 to receive either biennial gFOBT screening (intervention group) or no screening (control group). Median follow-up was 20 years. We calculated the year-specific hazard rate ratios (HRR) between the CRC incidence curves for the intervention and control group: the CRC incidence HRR was >1 for the screening years and <1 for the intermittent years (Table 1).
For once-only FS, the year-specific HRRs for CRC incidence were estimated on the basis of UK FS screening trial where uptake was 71% (Atkin et al, 2010). The FS trial randomised 170 432 men and women aged 55–64 in 1994–1999 who showed an interest in FS screening into receiving either once-only FS (intervention group) or no screening (control group) (Atkin et al, 2010). The participants were followed for up to 12 years. The HRR of CRC incidence rates were decreased by 30% to adjust for the lower FS uptake rate, that is, 50% instead of 71% (Table 1). For follow-up years 13+, we assumed a HRR of 0.75, which is equal to the average HRR in follow-up years 8–12. In the trials, the HRR's varied considerably between individual years, which is reflected in Table 1.
No trial data were available on the effect of once-only FS followed by biennial gFOBT. We assumed CRC incidence rates to be equal to the FS-alone scenario at ages 55–59. To estimate the effect of adding gFOBT at ages 60–74 to FS alone, we estimated the proportion of CRC cases not prevented by FS. FS examines the distal part of the bowel, that is, the rectum and the sigmoid colon. Atkin et al observed that in participants undergoing FS, the incidence rate of distal CRCs in the screened group 5 years after randomisation was ∼25% of the hazard of the control group, whereas incidence for proximal CRC did not change between the intervention and control group. We therefore assumed that adding gFOBT to FS screening detects 75% (34%(proximal)+50%(non-attendees) × 66%(distal)+50%(attendees) × 25%(HRR) × 66%(distal)) of the cases detected by gFOBT alone, in addition to the ones prevented by FS alone (Table 1). This follows from the fact that in the control arm of the UK FS trial, 34% of detected CRCs were proximal and 66% distal (including rectal), and FS uptake was 50% (Atkin et al, 2010; Robb et al, 2010).
Impact of screening approaches on CRC mortality. For CRC mortality, we assumed a constant HRR over time between the three screening scenarios and no screening. In the Nottingham gFOBT trial, a HRR of 0.87 was observed for participants aged 60+ (Scholefield et al, 2012). The UK FS trial (71% uptake) showed that once-only FS reduced CRC mortality by 31% (HRR=0.69) (Atkin et al, 2010). For an FS uptake of 50%, a HRR of 0.78 is assumed (1-(1-0.69) × (50%/71%)). In line with CRC incidence, we assumed that the CRC mortality HRR of gFOBT following once-only FS would be 75% (see above) of the effect of gFOBT alone, that is, 1-(1-0.87) × 0.75=0.90, therefore obtaining a HRR of 0.70 (i.e. 0.78 × 0.90) as a base-case scenario for the effect of once-only FS followed by gFOBT (Table 1).
When evaluating the effect of biennial gFOBT screening on CRC mortality, whether preceded by FS or not, we recalibrated the model at the start of gFOBT screening (t0=age 60) to avoid any reduction in CRC deaths between age 55 and 60 as a result of gFOBT screening.
Harm by death due to perforation. To determine the expected number of endoscopy-related deaths in England, we multiplied the expected rate of deaths owing to perforation by the number of English residents invited for FS and gFOBT screening. Death owing to perforation rates were assumed to be 0.35 per 100 000 persons invited for FS screening if FS uptake is 50% and 0.08 per 100 000 persons invited for gFOBT screening if gFOBT uptake is 57% (Whyte et al, 2012). These estimates represent lifetime risk of death due to perforation following polypectomy performed during screening FS, and follow-up and surveillance colonoscopy (compliance assumed 79% and 82%, respectively).
Population uptake rates. The uptake of gFOBT screening in the English population was close to the Nottingham gFOBT trial finding, that is, 57% (Logan et al, 2012; Moss et al, 2012; Lo et al, 2014). For FS, however, the uptake was 71% in UK FS trial and is expected to be ∼50% in the English population (Robb et al, 2010). To reflect screening practice, we used an FS uptake of 50% as base-case scenario.
Analysis
The main outcomes were: the number of CRC cases and deaths by scenario and calendar year (2014–2030). We estimated these based on the age- and year-specific England population size, the CRC incidence and mortality rates, and effects of the screening modalities on these, taking the FS invitation rates and timing of gFOBT testing into account. We also estimated the number of deaths owing to perforation on the basis of the number of people invited for gFOBT tests and FS exams.
We start our simulation at age 55, when English residents would get invited to FS screening. Invitation for gFOBT starts at age 60. As a result, all individuals will experience an initial period of 5 years during which only FS can impact CRC mortality. During the subsequent years, both screening tests will impact CRC incidence and mortality rates. Over the 17-year simulation period, all individuals will have been invited for FS, but only 12 out of the 17 cohorts (71%) will have been invited for gFOBT screening by the end of the simulation period.
To assess the stability of the results, we determined the impact of alternative estimates of several uncertain parameters in one-way sensitivity analyses. An overview of the sensitivity analyses is presented in Table 2.
Table 2. Model parameters: base-case and sensitivity analyses.
| Model parameter | Base-case analysis | Sensitivity analysis | ||
|---|---|---|---|---|
| 1 | Trend in underlying CRC incidence rates | Constant | A | Annual decrease of 0.5% |
| B | Annual increase of 0.5% | |||
| Trend in underlying CRC mortality rates | Annual decrease of 2% | A | Annual decrease of 3% | |
| B | Constant | |||
| 2 | Invitation rates FS | 3% in 2013, 10% in 2014, 20% in 2015, 50% in 2016, 70% in 2017 and 100% in 2018–2030 | 3% in 2013, 6% in 2014, 12% in 2015, 20% in 2016, 40% in 2017, 60% in 2018, 75% in 2019 and 100% in 2020–2030 | |
| 3 | Uptake FS | 50% | A | 38% |
| B | 71% | |||
| 4 | HRR CRC incidence and mortality | Point estimate HRRs | A | HRR gFOBT -1 s.d. |
| B | HRR gFOBT +1 s.d. | |||
| C | HRR FS -1 s.d. | |||
| D | HRR FS +1 s.d. | |||
| 5 | Effect of gFOBT following FS | 75% of the effect of gFOBT alone | 70% of the effect of gFOBT alone |
Abbreviations: CRC=colorectal cancer; FS=flexible sigmoidoscopy; gFOBT=guaiac faecal occult blood testing; HRR=hazard rate ratio; s.d.=standard deviation.
Trend in underlying CRC incidence and mortality rates. We assumed the CRC incidence rate to decrease and increase by 0.5% per year as of 2005. CRC mortality rates were alternatively assumed to decrease by 3% per year and to be constant as of 2005.
Invitation rates FS. As an alternative invitation schedule for FS at age 55, we delayed full invitation by 2 years: 3% in 2013, 6% in 2014, 12% in 2015, 20% in 2016, 40% in 2017, 60% in 2018, 75% in 2019 and 100% for 2020 onwards, respectively.
Uptake FS. FS uptakes rates of 38% and 71% were used as lower and upper limit, respectively (Atkin et al, 2010; Holme et al, 2013).
Impact of screening approaches on CRC incidence and mortality. In the base-case scenario we included the point estimates of the HRRs. Alternatively, we used the point estimate ±1 s.d., which was 0.03 and 0.05 for gFOBT and 0.02 and 0.05 for FS, for CRC incidence and mortality, respectively (Atkin et al, 2010; Scholefield et al, 2012).
gFOBT at ages 60–74 following FS at age 55. In the base-case scenario, we followed the results from the UK FS trial and assumed an FS uptake of 50% instead of 71% (Atkin et al, 2010). As an alternative scenario we assumed that in participants who undergo FS, 90% of distal cancers and adenomas are detected by FS and no development of new distal adenomas occur. Indeed, miss rates for adenomas at colonoscopy were 28% for adenomas 1–5 mm, 13% for adenomas 5–10 mm and 2% for adenomas ⩾10 mm based on six studies where participants underwent two same-day colonoscopies with follow-up polypectomy (van Rijn et al, 2006). We kept the assumption that FS only detects distal cancers. Then the alternative estimate of the effect of gFOBT following FS in the sensitivity analysis was 70% (34%(proximal)+50%(non-attendees) × 66%(distal)+50%(attendees) × 10%(miss rate distal lesions) × 66%(distal)).
Results
It is expected that yearly ∼610 000–650 000 English residents will be invited for FS screening at age 55 (Table 3). By mid-2030, 8.5 million individuals will have been invited for once-only FS screening.
Table 3. Annual number of persons modelled, total and aged 55, and numbers invited for gFOBT tests and FS exams.
|
Model cohort size |
gFOBT test, no. invited | FS exam, no. invited | ||
|---|---|---|---|---|
| Calendar year | Total | Age 55 | gFOBT/FS plus gFOBT | FS/FS plus gFOBT |
| 2013 | 623 519 | 623 519 | 0 | 18 706 |
| 2014 | 1 253 248 | 632 308 | 0 | 63 231 |
| 2015 | 1 888 841 | 641 096 | 0 | 128 219 |
| 2016 | 2 522 649 | 642 506 | 0 | 321 253 |
| 2017 | 3 154 379 | 643 915 | 0 | 450 741 |
| 2018 | 3 783 720 | 645 325 | 607 843 | 645 325 |
| 2019 | 4 410 325 | 646 734 | 616 411 | 646 734 |
| 2020 | 5 033 888 | 648 143 | 1 224 481 | 648 143 |
| 2021 | 5 646 237 | 641 776 | 1 234 305 | 641 776 |
| 2022 | 6 247 145 | 635 409 | 1 834 003 | 635 409 |
| 2023 | 6 836 169 | 629 042 | 1 845 047 | 629 042 |
| 2024 | 7 412 926 | 622 675 | 2 434 774 | 622 675 |
| 2025 | 7 976 761 | 616 307 | 2 446 993 | 616 307 |
| 2026 | 8 531 801 | 614 575 | 3 016 845 | 614 575 |
| 2027 | 9 077 660 | 612 844 | 3 022 601 | 612 844 |
| 2028 | 9 613 476 | 611 112 | 3 570 459 | 611 112 |
| 2029 | 10 138 410 | 609 381 | 3 569 795 | 609 381 |
| Total | 94 151 154 | 10 716 667 | 25 423 557 | 8 515 471 |
Abbreviations: FS=flexible sigmoidoscopy; gFOBT=guaiac faecal occult blood testing.
In the absence of screening, the predicted cumulative numbers of CRC cases are 40 726 by mid-2025 and 93 158 by mid-2030 (Table 4). When compared with no screening, gFOBT alone is expected to increase the number of CRC cases by 1498 (+4%) by mid-2025 and 522 (+1%) by mid-2030. FS alone and FS plus gFOBT are expected to decrease the number of CRC cases by 1615 (−4%) and 654 (−2%) by mid-2025 and by 9009 (−10%) and 9105 (−10%) by mid-2030, respectively, when compared with no screening.
Table 4. Annual and cumulative change in the number of CRC cases, and deaths by screening scenario and calendar year compared with no screening, with five-year milestones marked in bold.
|
No screening |
gFOBT |
FS |
FS plus gFOBT |
|||||
|---|---|---|---|---|---|---|---|---|
| Mid-year | Annual number | Cumulative number | Annual change | Cumulative change | Annual change | Cumulative change | Annual change | Cumulative change |
|
CRC cases | ||||||||
| 2014 | 354 | 354 | 0 | 0 | +11 | +11 | +11 | +11 |
| 2015 | 770 | 1124 | 0 | 0 | +35 | +46 | +35 | +46 |
| 2016 | 1189 | 2313 | 0 | 0 | +64 | +110 | +64 | +110 |
| 2017 | 1666 | 3979 | 0 | 0 | +155 | +265 | +155 | +265 |
| 2018 | 2200 | 6179 | 0 | 0 | +172 | +437 | +172 | +437 |
| 2019 | 2790 | 8969 | +365 | +365 | +201 | +638 | +560 | +997 |
| 2020 | 3435 | 12 404 | +226 | +591 | +48 | +686 | +257 | +1254 |
| 2021 | 4078 | 16 482 | +312 | +903 | −97 | +589 | +181 | +1435 |
| 2022 | 4825 | 21 307 | +209 | +1112 | −294 | +295 | −172 | +1263 |
| 2023 | 5619 | 26 926 | +269 | +1381 | −448 | −153 | −287 | +976 |
| 2024 | 6459 | 33 385 | +11 | +1392 | −641 | −794 | −788 | +188 |
| 2025 | 7341 | 40 726 | +106 | +1498 | −821 | −1615 | −842 | −654 |
| 2026 | 8316 | 49 042 | −176 | +1322 | −992 | −2607 | −1288 | −1942 |
| 2027 | 9330 | 58 372 | −243 | +1079 | −1215 | −3822 | −1513 | −3455 |
| 2028 | 10 434 | 68 806 | −251 | +828 | −1435 | −5257 | −1713 | −5168 |
| 2029 | 11 569 | 80 375 | −188 | +640 | −1739 | −6996 | −1875 | −7043 |
| 2030 | 12 783 | 93 158 | −118 | +522 | −2013 | −9009 | −2062 | −9105 |
|
CRC deaths | ||||||||
| 2014 | 52 | 52 | 0 | 0 | −1 | −1 | −1 | −1 |
| 2015 | 129 | 181 | 0 | 0 | −2 | −3 | −2 | −3 |
| 2016 | 223 | 404 | 0 | 0 | −4 | −7 | −4 | −7 |
| 2017 | 333 | 737 | 0 | 0 | −12 | −19 | −12 | −19 |
| 2018 | 454 | 1191 | 0 | 0 | −23 | −42 | −23 | −42 |
| 2019 | 584 | 1775 | −9 | −9 | −40 | −82 | −49 | −91 |
| 2020 | 723 | 2498 | −24 | −33 | −61 | −143 | −84 | −175 |
| 2021 | 870 | 3368 | −41 | −74 | −84 | −227 | −124 | −299 |
| 2022 | 1031 | 4399 | −61 | −135 | −110 | −337 | −168 | −467 |
| 2023 | 1209 | 5608 | −83 | −218 | −137 | −474 | −216 | −683 |
| 2024 | 1398 | 7006 | −108 | −326 | −167 | −641 | −266 | −949 |
| 2025 | 1596 | 8602 | −134 | −460 | −198 | −839 | −319 | −1268 |
| 2026 | 1802 | 10 404 | −162 | −622 | −232 | −1071 | −376 | −1644 |
| 2027 | 2022 | 12 426 | −192 | −814 | −268 | −1339 | −437 | −2081 |
| 2028 | 2254 | 14 680 | −224 | −1038 | −307 | −1646 | −502 | −2583 |
| 2029 | 2496 | 17 176 | −257 | −1295 | −348 | −1994 | −570 | −3153 |
| 2030 | 2745 | 19 921 | −291 | −1586 | −391 | −2385 | −640 | −3793 |
Abbreviations: CRC=colorectal cancer; FS=flexible sigmoidoscopy; gFOBT=guaiac faecal occult blood testing.
The predicted reductions in CRC deaths for gFOBT, FS and FS plus gFOBT compared with no screening were 460 (−5%), 839 (−10%) and 1268 (−15%) by mid-2025 and 1586 (−8%), 2385 (−12%) and 3793 (−19%) by mid-2030, respectively.
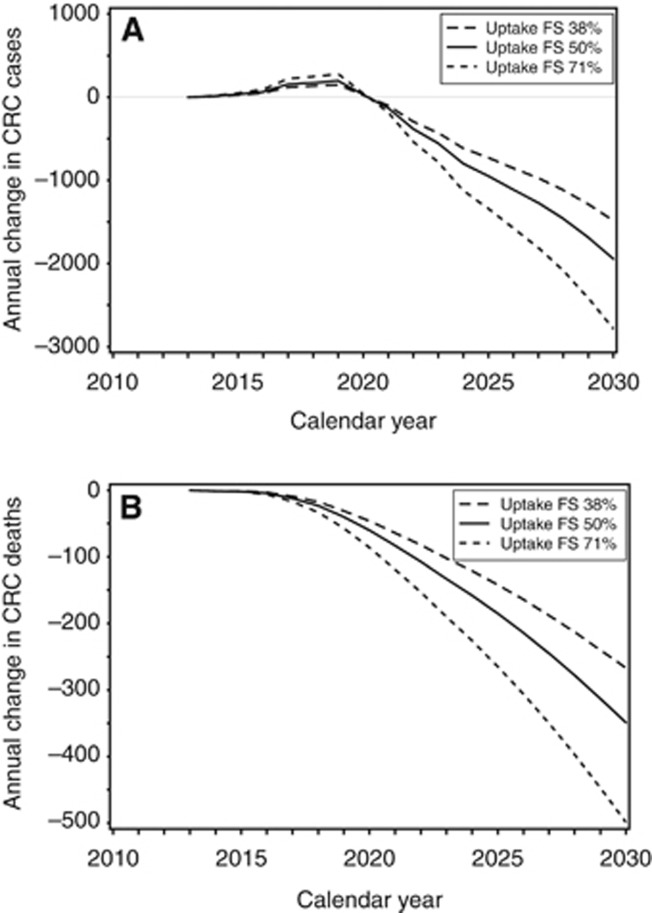
When compared with gFOBT alone, FS plus gFOBT was estimated to prevent 1944 (−15%) cases and 349 (−14%) deaths annually in 2030 (Figure 1). Adding FS to the English NHSBCSP would reduce the cumulative number of CRC cases and deaths by mid-2030, respectively, by 9627 (−10%) and 2207 (−12%) (Table 5).
Figure 1.
Annual change in number of colorectal cancer cases and deaths by adding once-only flexible sigmoidoscopy to the England NHS Bowel Cancer Screening Programme, presented for FS uptake of 38% (– –), 50% (–) and 71% (- - -).
Table 5. Sensitivity analyses: cumulative number of CRC cases and deaths prevented by adding FS to the England NHS Bowel Cancer Screening Programme.
|
CRC cases |
CRC deaths |
|||||||
|---|---|---|---|---|---|---|---|---|
| 2025 | 2030 | 2025 | 2030 | |||||
| N | % | N | % | N | % | N | % | |
|
Base-case: | ||||||||
| −2152 | −9627 | −808 | −2207 | |||||
|
Alternatives: | ||||||||
| 1. Trend in underlying CRC incidence and mortality rates | ||||||||
| A. Incidence −0.5% py, mortality −3% py | −2044 | −5% | −9002 | −6% | −759 | −6% | −2137 | −3% |
| B. Incidence +1.0% py, mortality contant | −2262 | +5% | −10 294 | +7% | −1133 | +40% | −3328 | +51% |
| 2. Alternative invitation rates FS | −1029 | −52% | −7204 | −25% | −572 | −29% | −1725 | −22% |
| 3. Alternative uptake rates FS | ||||||||
| A. FS uptake 38% | −1662 | −23% | −7379 | −23% | −619 | −23% | −1691 | −23% |
| B. FS uptake 71% | −3016 | +40% | −13 689 | +42% | −1155 | +43% | −3154 | +43% |
| 4. HRR mortality | ||||||||
| A. Lower HRR gFOBT | −2070 | −4% | −9217 | −4% | −796 | −1% | −2139 | −3% |
| B. Upper HRR gFOBT | −2231 | +4% | −10 037 | +4% | −821 | +2% | −2276 | +3% |
| C. Lower HRR FS | −2562 | +19% | −10 742 | +12% | −945 | +17% | −2594 | +18% |
| D. Upper HRR FS | −1740 | −19% | −8512 | −12% | −673 | −17% | −1824 | −17% |
| 5. Alternative impact of gFOBT following FS | −2180 | +1% | −9660 | +0% | −802 | −1% | −2172 | −2% |
Abbreviations: CRC=colorectal cancer; FS=flexible sigmoidoscopy; gFOBT=guaiac faecal occult blood testing; HRR=hazard rate ratio; py=per year.
If CRC incidence changes with ±0.5% per year, reductions change by 5% in 2025 and 6–7% in 2030 (Table 5). If CRC mortality decreases by 3% per year, then the projected reductions in CRC deaths are overestimated by 6% by mid-2025 and 3% by mid-2030. If CRC mortality stays constant, then higher reductions in CRC deaths are expected, that is, 40% by mid-2025 and 51% by mid-2030.
A 2-year delay in full FS invitation reduced the expected reductions in CRC cases by 52% by mid-2025 and by 25% by mid-2030. The impact on CRC deaths was smaller, that is, −29% by mid-2025 and −22% by mid-2030.
If FS uptake is 38%, then the projected reduction in CRC cases and deaths decreases by 23% when compared with an FS uptake of 50%. If FS uptake is 71%, then the projected reductions in CRC cases and deaths increases with 40–43%.
Varying the estimates of the HRR of the screening tests on CRC incidence by one s.d. resulted in reductions by mid-2030 ranging between 8512 (−12%) and 10 742 (+12%) CRC cases and 1824 (−17%) and 2594 (+18%) CRC deaths.
Uncertainty about the added effect of gFOBT following FS changed the predicted reduction by 0–2%.
The impact of the alternative assumptions on the annual reductions in CRC cases and deaths are presented in the Supplementary Appendix.
Death owing to perforation was estimated to be 0 for no screening, 4 for gFOBT alone, 21 for FS alone and 25 for FS plus gFOBT by mid-2025 and 0 for no screening, 11 for gFOBT alone, 30 for FS alone and 41 for FS plus gFOBT by mid-2030, respectively.
Discussion
Our model predicted that adding once-only FS at age 55 to the NHSBCSP, that is, biennial gFOBT testing at ages 60–74, will prevent an additional 2000 CRC deaths in England by mid-2030 if gFOBT uptake is 57% and FS uptake is 50%. This reduction is preceded by 8.5 million FS and 25.4 million gFOBT invitations and accompanied by a decrease of 10 000 CRC cases. In the year 2030, it is estimated that 300 000 FS episodes will take place, and that 2000 additional cases and 350 additional deaths will be prevented. These numbers translate to 150 FS episodes per CRC case prevented and 900 per CRC death prevented. The estimates confirm that a crucial benefit of FS screening is the prevention of CRC incidence, which has major implications for quality of life. Sensitivity analyses revealed that uncertainty about the FS invitation and uptake rates have a substantial impact on the predicted number of CRC cases and deaths prevented.
In 2010, ∼33 000 CRC cases and 13 000 CRC deaths were observed in England (Cancer Research UK, 2013a, 2014a). In this study, we modelled the course of men and women turning 55 years between 2013 and 2030 in the absence of screening and simulated three screening scenarios: gFOBT, FS and FS plus gFOBT. This enabled us to estimate the impact of adding FS at age 55 to gFOBT at age 60 to 74 on screening results at population level. Men and women aged 56–74 in 2013 who are offered biennial gFOBT screening at ages 60–74 during the projected period were not simulated. Parkin et al (2008) estimated that the first NHSBCSP, comprising biennial gFOBT at ages 60–69 in 2007–2009 and at ages 60–74 as of 2010, would prevent 1800 CRC cases and 1800 CRC deaths annually by 2025. Our model showed that adding FS would prevent an extra 900 CRC cases and 200 CRC deaths if FS uptake is 50% (Figure 1). This suggests that the NHSBCSP will prevent ∼2700 CRC cases and 2000 CRC deaths. Both the reduction by FS and by gFOBT will not have reached their maximum in 2025. Assuming that the effect of the NHSBCSP will persist until age 90 and full coverage of FS is reached in 2018, then the maximum annual reduction in CRC cases and deaths is expected beyond 2050.
Our model estimates that 350 CRC deaths would be prevented per year in 2030 if FS uptake is 50%, in addition to the gFOBT only screening programme. This annual number is still increasing beyond 2030. In 1993, it was estimated that a once-only FS programme could prevent up to 3500 CRC deaths per year (Atkin et al, 1993). These two estimates are consistent if one considers the differences in the scenarios postulated. For example, the mortality rates used were those for the UK in 1987. Further, Atkin et al estimated the effect over 22 years for FS vs no screening, whereas we modelled 17 years and determined the impact of FS in addition to biennial gFOBT screening. Table 6 shows the effect of the major differences in the scenarios and how equalisation of these convert the Atkin et al figure to 700 deaths prevented per year, much closer to the 350 estimated in the current model. For the same reasons, we expect that the predicted reduction of 5500 CRC cases in steady state will, after adjustments, be of similar size.
Table 6. Application of parameters in current model to figures of Atkin et al, 1993.
| Parameter | Atkin et al | Geurts et al | Correction factor (ratio) | Application of Geurts et al's assumptions to the Atkin et al's estimates |
|---|---|---|---|---|
| Annual reduction in CRC deaths of FS vs no screening (based on data from 1987) | 3499 | |||
| Eligible population size | 602400 persons aged 58 in the UK in 1987 | On average 501 000 persons aged 55 in England in 2013–2030 (Table 3) | 0.83 | 2910 |
| Age at FS | 58 years | 55 years (20-year mortality rate 20% lower, CRUK) | 0.80 | 2328 |
| Case fatality rate | 60% | 38% | 0.63 | 1474 |
| Effect size | HRR CRC mortality FS vs no screening=0.67 | HRR CRC mortality FS vs no screening=0.69 (UK FS trial, Table 1) | 0.94 | 1385 |
| Uptake FS | 70% | 50% | 0.71 | 989 |
| Number of age cohorts | 22 (age 57–79) | 17 (age 55–72) | 0.77 | 764 |
| Net benefit after partial coverage by gFOBT | Not applicable | HRR FS vs no screening=0.78; HRR FS plus gFOBT vs gFOBT=0.70/0.87=0.80 (for gFOBT screening years) | 0.91 | 695 |
| Annual reduction in CRC deaths of FS plus gFOBT vs gFOBT in 2030 | 695 | 349 |
Abbreviations: CRUK=cancer research UK; FS=flexible sigmoidoscopy; gFOBT=guaiac faecal occult blood test; HRR=hazard rate ratio.
Colonoscopy surveillance after removal of initial lesions is part of the screening programme, and as such, part of the projected reductions (National Institute for Clinical Excellence (NICE), 2011). In the UK FS trial, for example, 5% of those screened with FS underwent subsequent colonoscopic surveillance (Atkin et al, 2010).
This paper addresses the effects of adding FS to the current UK programme on CRC incidence and mortality. Clearly, the combination of faecal immunochemical testing (FIT) with FS is also of interest: FIT is likely to be more sensitive than gFOBT (Brenner and Tao, 2013), and may also result in higher participation rates (Hol et al, 2010). These characteristics are likely to result in additional lives being saved, but there are currently no randomised trial estimates of the effect of FIT on CRC incidence and mortality.
The Nottingham gFOBT trial showed that gFOBT reduces CRC mortality, but has a small and non-significant impact on CRC incidence (Scholefield et al, 2012). FS decreases both CRC incidence and mortality (Atkin et al, 2010). Owing to the prevention of CRC cases, adding FS to the NHSBCSP will not only prevent CRC death but also prevent potential mutilating treatments and thereby increases participants quality of life, although this comes at a low but significant risk of deaths owing to perforation.
By definition, predictions are prone to uncertainty, especially for long-term projections. The trend in underlying CRC incidence and mortality rate is an important input parameter in this study. Annual CRC incidence rates have been increasing over the last decades (Cancer Research UK, 2013a). However, since the 2000s, a slight decrease in CRC incidence rates is observed. For CRC mortality, a clear and constant decrease is observed (Cancer Research UK, 2014a). Parkin et al (2008) predicted both underlying CRC incidence and mortality to decrease in 2013–2025. Other modelling studies on early detection of CRC assumed a constant CRC incidence and mortality rate, as the natural history models lack a time-dependent component (Macafee et al, 2008; Whyte et al, 2012; Jeong and Cairns, 2013). Uncertainty about the future trends can have a substantial impact on the predicted reductions. Our sensitivity analysis showed, for example, that if CRC mortality rates stay constant between 2005 and 2030, then the modelled reductions of adding FS to the NHSBCSP would increase by 1100 CRC deaths (51% of total reduction) by mid-2030.
The FS invitation scheme is another important factor in estimating the health effect of the new NHSBCSP, because a 2-year delay in full invitation would reduce the CRC cases prevented by adding FS to the NHSBCSP by mid-2025 with 52% and CRC deaths with 29%. The exact timing of full invitation becomes less important as follow-up time increases.
The FS uptake rate is probably the most uncertain factor for the predicted number of CRC cases and deaths. Our sensitivity analysis showed that if the uptake is 38% or 71% instead of 50%, then our predictions are over- and underestimated by >20% and >40%, respectively.
In this modelling study, we used RCT results to estimate the impact of population screening. The use of trial data has the advantage of providing unbiased estimates for both the intervention group (once-only FS or biennial gFOBT screening) and the control group (no screening). Trial results are however generally more favourable than population results, because of participant selection and guidance, that is, the Hawthorne effect (McCambridge et al, 2014).
In contrast to, for example, breast cancer screening, selection bias does not seem to be an issue in bowel screening. In the UK FS trial, those who did not attend FS screening had exactly the same CRC incidence and mortality as the uninvited controls (Atkin et al, 2010). Also, CRC incidence in the control group was almost exactly the same as that of the general population, suggesting that the two-stage invitation procedure was related only to participation rate and not to underlying risk or to the effect of the intervention. Long-term follow-up of the pilot population will reveal whether the 50% compliant population are similarly representative in terms of underlying incidence and mortality.
The value of using trial estimates to predict the impact of screening on CRC incidence and mortality in the population depends on the similarity between the trial setup and execution and how it is done in screening practice. Age of entry for example differed between the trials (45–74 for biennial gFOBT and 55–64 for once-only FS) and the screening programme (60–74 for biennial gFOBT and 55 for once-only FS). Age-specific HRRs were only available for CRC mortality in the Nottingham gFOBT trial (Scholefield et al, 2012), and we assumed the HRRs to be independent of age in the present study.
Furthermore, the screening uptake rates may differ between trials and practice. For gFOBT, trial and population uptake rates were observed to be similar (Logan et al, 2012; Moss et al, 2012; Scholefield et al, 2012; Holme et al, 2013; Lo et al, 2014). FS uptake rates observed in the trials (58–84%) (Holme et al, 2013) were however much higher than expected (50%) (Robb et al, 2010).
No trial data were available for the uptake and effect of gFOBT screening following FS examination. In the Netherlands, FS non-attendees had a lower FIT uptake (25%) than primary FIT screening (62%) (Hol et al, 2012). It is unknown if this also applies to gFOBT and to the English residents, and what the uptake of FIT is in FS attendees. For now, we assumed that the uptake of gFOBT following FS is independent of the uptake of FS, and the same as for the gFOBT alone scenario. The effect of gFOBT following FS was estimated based on published literature. Our sensitivity analysis showed that, for example, assuming no development of new adenomas did not change the modelled results, which may be explained by the relatively short follow-up period modelled in this study, where the impact of gFOBT is small in comparison with FS.
The base-case and alternative scenarios for FS plus gFOBT were based on the observation by Atkin et al (2010) that 66% of the CRC cases are distal and that the incidence of proximal colon cancer was similar for the intervention and control group. Previous studies observed slightly higher FS sensitivity rates (72–86%) for all-site advanced neoplasia (Whitlock et al, 2008). The actual proportion of distal and proximal CRC detected by FS in the NHSBCSP will depend on the colonoscopy follow-up strategy after finding an adenoma during FS (Castells et al, 2013).
Projected reductions in CRC cases and deaths are expected to differ between men and women. Several input variables, like CRC incidence and mortality and screening uptake, are known to be sex- and age-specific (Cancer Research UK, 2012, 2013a; Massat et al, 2013; Lo et al, 2014). Further, age-and sex-specific estimates of CRC transition probabilities and test sensitivities are becoming available (Sung et al, 2003; Brenner et al, 2007). Full stochastic modelling, based on age-and sex-specific parameter estimates, may yield more precise and sex-specific predictions of the impact of adding FS to the NHSBCSP and should be the focus of future study. In addition, the impact of FIT alone and in combination with FS can be assessed in a Markov model simulating the adenoma-carcinoma sequence of CRC, and using the sensitivity and specificity values for FIT. Full stochastic modelling also enables long-term projections and determines the maximal annual reduction in CRC cases and deaths achieved by the English NHSBCSP.
In conclusion, adding once-only FS at age 55 to the NHSBCSP has the potential to prevent an additional 10 000 CRC cases and 2000 CRC deaths by mid-2030. This reduction can only be achieved if FS invitation is complete in 2018 and if the uptake of FS is 50%. A major benefit of FS is the prevention of the cancer occurring in the first place.
Acknowledgments
The Policy Research Unit in Cancer Awareness, Screening and Early Diagnosis receives funding for a research programme from the Department of Health Policy Research Programme. It is a collaboration between researchers from seven institutions (Queen Mary University of London, UCL, King's College London, London School of Hygiene and Tropical Medicine, Hull York Medical School, Durham University and Peninsula Medical School). We thank Sue Moss, Sophie Whyte and several employees from the English NHS Bowel Cancer Screening Programme for their helpful information and discussion.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License
Supplementary Material
References
- Atkin WS, Cuzick J, Northover JM, Whynes DK (1993) Prevention of colorectal cancer by once-only sigmoidoscopy. Lancet 341: 736–740. [DOI] [PubMed] [Google Scholar]
- Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JMA, Parkin DM, Wardle J, Duffy SW, Cuzick J UK Flexible Sigmoidoscopy Trial Investigators (2010) Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 375: 1624–1633. [DOI] [PubMed] [Google Scholar]
- Brenner H, Hoffmeister M, Stegmaier C, Brenner G, Altenhofen L, Haug U (2007) Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut 56: 1585–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H, Tao S (2013) Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer 49: 3049–3054. [DOI] [PubMed] [Google Scholar]
- Cancer Research UK (2012) Bowel cancer survival statistics http://www.cancerresearchuk.org/cancer-info/cancerstats/types/bowel/survival/bowel-cancer-survival-statistics Cancer Research UK (accessed 7 May 2014).
- Cancer Research UK (2013. a) Bowel cancer incidence statistics http://www.cancerresearchuk.org/cancer-info/cancerstats/types/bowel/incidence/uk-bowel-cancer-incidence-statistics Cancer Research UK (accessed 08 May 2014).
- Cancer Research UK (2013. b) Who is screened for bowel cancer http://www.cancerresearchuk.org/cancer-help/type/bowel-cancer/about/screening/who-is-screened-for-bowel-cancer#bc (accessed 23 May 2014).
- Cancer Research UK (2014. a) Bowel cancer mortality statistics http://www.cancerresearchuk.org/cancer-info/cancerstats/types/bowel/mortality/ Cancer Research UK (accessed 8 May 2014).
- Cancer Research UK (2014. b) High risk groups for bowel cancer http://www.cancerresearchuk.org/cancer-help/type/bowel-cancer/about/risks/high-risk-groups-for-bowel-cancer (accessed 23 May 2014).
- Castells A, Bessa X, Quintero E, Bujanda L, Cubiella J, Salas D, Lanas A, Carballo F, Morillas JD, Hernandez C, Jover R, Montalvo I, Arenas J, Cosme A, Hernandez V, Iglesias B, Castro I, Cid L, Sala T, Ponce M, Andres M, Teruel G, Peris A, Roncales MP, Gonzalez-Rubio F, Seoane-Urgorri A, Grau J, Serradesanferm A, Pellise M, Ono A, Cruzado J, Perez-Riquelme F, Alonso-Abreu I, Carrillo-Palau M, De La Vega-Prieto M, Iglesias R, Amador J, Blanco JM, Sastre R, Ferrandiz J, Gonzalez-Hernandez MJ, Andreu M COLONPREV study investigators (2013) Risk of advanced proximal neoplasms according to distal colorectal findings: comparison of sigmoidoscopy-based strategies. J Natl Cancer Inst 105: 878–886. [DOI] [PubMed] [Google Scholar]
- Hol L, de Jonge V, van Leerdam ME, van Ballegooijen M, Looman CW, van Vuuren AJ, Reijerink JC, Habbema JD, Essink-Bot ML, Kuipers EJ (2010) Screening for colorectal cancer: comparison of perceived test burden of guaiac-based faecal occult blood test, faecal immunochemical test and flexible sigmoidoscopy. Eur J Cancer 46:: 2059–2066. [DOI] [PubMed] [Google Scholar]
- Hol L, Kuipers EJ, van Ballegooijen M, van Vuuren AJ, Reijerink JC, Habbema DJ, van Leerdam ME (2012) Uptake of faecal immunochemical test screening among nonparticipants in a flexible sigmoidoscopy screening programme. Int J Cancer 130: 2096–2102. [DOI] [PubMed] [Google Scholar]
- Holme O, Bretthauer M, Fretheim A, Odgaard-Jensen J, Hoff G (2013) Flexible sigmoidoscopy versus faecal occult blood testing for colorectal cancer screening in asymptomatic individuals. Cochrane Database Syst Rev 9: CD009259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong KE, Cairns JA (2013) Review of economic evidence in the prevention and early detection of colorectal cancer. Health Econ Rev 3: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SH, Halloran S, Snowball J, Seaman H, Wardle J, von Wagner C (2014) Colorectal cancer screening uptake over three biennial invitation rounds in the English bowel cancer screening programme. Gut 64: 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RFA, Patnick J, Nickerson C, Coleman L, Rutter MD, von Wagner C English Bowel Cancer Screening Evaluation Committee (2012) Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 61: 1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macafee DAL, Waller M, Whynes DK, Moss S, Scholefield JH (2008) Population screening for colorectal cancer: the implications of an ageing population. Br J Cancer 99: 1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massat NJ, Moss SM, Halloran SP, Duffy SW (2013) Screening and primary prevention of colorectal cancer: a review of sex-specific and site-specific differences. J Med Screen 20: 125–148. [DOI] [PubMed] [Google Scholar]
- McCambridge J, Witton J, Elbourne DR (2014) Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol 67: 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EJA, Whitehouse LE, Farrell T, Nickerson C, Thomas JD, Quirke P, Rutter MD, Rees C, Finan PJ, Wilkinson JR, Patnick J (2012) A retrospective observational study examining the characteristics and outcomes of tumours diagnosed within and without of the English NHS Bowel Cancer Screening Programme. Br J Cancer 107: 757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SM, Campbell C, Melia J, Coleman D, Smith S, Parker R, Ramsell P, Patnick J, Weller DP (2012) Performance measures in three rounds of the English bowel cancer screening pilot. Gut 61: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Clinical Excellence (NICE) (2011) Colonoscopic surveillance for prevention of colorectal cancer in people with ulcerative colitis, Crohn's disease or adenomas. http://www.guidance.nice.org.uk/cg118. [PubMed]
- Office of National Statistics (2011) National Population Projections, 2010-Based Statistical Bulletin http://www.ons.gov.uk/ons/dcp171778_235886.pdf (accessed 9 April 2014).
- Office of National Statistics (2013) 2012-based Expectation of Life, 1981-2062, Principal Projection, England www.ons.gov.uk (accessed 25 April 2014).
- Office of National Statistics (2014. a) Annual mid-year population estimates for England http://www.ons.gov.uk/ (accessed 25 March 2014).
- Office of National Statistics (2014. b) England, National Life Tables, 1980-82 to 2010-12 http://www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-352834 (accessed 9 April 2014).
- Parkin DM, Tappenden P, Olsen AH, Patnick J, Sasieni P (2008) Predicting the impact of the screening programme for colorectal cancer in the UK. J Med Screen 15: 163–174. [DOI] [PubMed] [Google Scholar]
- Robb K, Power E, Kralj-Hans I, Edwards R, Vance M, Atkin W, Wardle J (2010) Flexible sigmoidoscopy screening for colorectal cancer: uptake in a population-based pilot programme. J Med Screen 17: 75–78. [DOI] [PubMed] [Google Scholar]
- Scholefield JH, Moss SM, Mangham CM, Whynes DK, Hardcastle JD (2012) Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut 61: 1036–1040. [DOI] [PubMed] [Google Scholar]
- Sung JJY, Chan FKL, Leung WK, Wu JCY, Lau JYW, Ching J, To KF, Lee YT, Luk YW, Kung NNS, Kwok SPY, Li MKW, Chung SCS (2003) Screening for colorectal cancer in Chinese: comparison of fecal occult blood test, flexible sigmoidoscopy, and colonoscopy. Gastroenterology 124: 608–614. [DOI] [PubMed] [Google Scholar]
- van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E (2006) Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol 101: 343–350. [DOI] [PubMed] [Google Scholar]
- Whitlock EP, Lin JS, Liles E, Beil TL, Fu R (2008) Screening for colorectal cancer: a targeted, updated systematic review for the US Preventive Services Task Force. Ann Intern Med 149: 638–658. [DOI] [PubMed] [Google Scholar]
- Whyte S, Chilcott J, Halloran S (2012) Reappraisal of the options for colorectal cancer screening in England. Colorectal Dis 14: e547–e561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.