Abstract
Background:
Alcohol consumption has been suggested to increase risk of breast cancer through a mechanism that also increases mammographic density. Whether the association between alcohol consumption and mammographic density is modified by background breast cancer risk has, however, not been studied.
Methods:
We conducted a population-based cross-sectional study of 53 060 Swedish women aged 40–74 years. Alcohol consumption was assessed using a web-based self-administered questionnaire. Mammographic density was measured using the fully-automated volumetric Volpara method. The Tyrer–Cuzick prediction model was used to estimate risk of developing breast cancer in the next 10 years. Linear regression models were used to evaluate the association between alcohol consumption and volumetric mammographic density and the potential influence of Tyrer–Cuzick breast cancer risk.
Results:
Overall, increasing alcohol consumption was associated with higher absolute dense volume (cm3) and per cent dense volume (%). The association between alcohol consumption and absolute dense volume was most pronounced among women with the highest (⩾5%) Tyrer–Cuzick 10-year risk. Among high-risk women, women consuming 5.0–9.9, 10.0–19.9, 20.0–29.9, and 30.0–40.0 g of alcohol per day had 2.6 cm3 (95% confidence interval (CI), 0.2–4.9), 2.9 cm3 (95% CI, −0.6 to 6.3), 4.6 cm3 (95% CI, 1.5–7.7), and 10.8 cm3 (95% CI, 4.8–17.0) higher absolute dense volume, respectively, as compared with women abstaining from alcohol. A trend of increasing alcohol consumption and higher absolute dense volume was seen in women at low (⩽3%) risk, but not in women at moderate (3.0–4.9%) risk.
Conclusion:
Alcohol consumption may increase breast cancer risk through increasing mammographic density, particularly in women at high background risk of breast cancer.
Keywords: alcohol consumption, mammographic density, background breast cancer risk, Tyrer-Cuzick prediction model
Mammographic density refers to the amount of fibroglandular and fat tissue of the breast, and is one of the strongest risk factors of breast cancer. The risk of developing breast cancer is 4–6 times higher among women with high mammographic density compared with women with low density (Boyd et al, 1995; McCormack and dos Santos Silva, 2006). Alcohol consumption is also associated with an increased risk of breast cancer (Smith-Warner et al, 1998; Singletary and Gapstur, 2001; Chen et al, 2011), and its effect could be mediated through altered mammographic density. Women with high alcohol consumption have been shown to have higher mammographic density than abstainers (Vachon et al, 2000b; Flom et al, 2009; Cabanes et al, 2011; Voevodina et al, 2013). However, it is unclear if the association between alcohol consumption and mammographic density is modified by other factors. The Tyrer–Cuzick prediction model estimates risk of developing breast cancer within 10 years based on several established risk factors of breast cancer, and can be used to categorise women into low, moderate, and high risk of breast cancer (Tyrer et al, 2004).
To our knowledge, the influence of background breast cancer risk on the association between alcohol consumption and mammographic density has not been studied. We therefore investigated the association between alcohol consumption and volumetric mammographic density and the potential effect measure modification by the Tyrer–Cuzick breast cancer risk in 53 060 Swedish women.
Materials and methods
Study population
The KARolinska MAmmography Project for Risk reduction of Breast Cancer (KARMA) is a population-based prospective cohort study of women attending one of four Swedish mammography units in the national mammography screening program in Sweden. The participants responded to a web-based questionnaire covering information on breast cancer risk factors. Raw and processed full-field digital mammograms have been stored.
Women within the age range for mammography screening in Sweden, that is, 40–74 years, and had a baseline mammogram (n=67 388) were included. Women were excluded if they had missing information on body mass index (BMI; n=3238), previous cancers other than non-melanoma skin cancer (n=4486), breast surgery (n=3363), recent pregnancy (n=49), a mammogram from only one breast (n=1892), or incomplete alcohol consumption data (n=909). Women reporting more than 40 bottles of beer per week (n=4) or had an alcohol consumption of >40 g per day (n=387) were considered as ‘outliers' and excluded. The final analyses included 53 060 women.
The ethical review board at the Karolinska Institutet approved the study. Written informed consent was obtained from each participant.
Mammographic density measurement
Mammograms were obtained using full-field digital mammography systems. We used raw mammograms from the mediolateral oblique view, which is the routine projection during mammography screening in Sweden.
Volumetric mammographic density was measured using the fully-automated Volpara method. Technical details of the method has been described elsewhere (Highnam et al, 2010). Briefly, the algorithm computes the thickness of dense tissue at each pixel using the X-ray attenuation of an entirely fatty region as an internal reference. The absolute dense volume (cm3) is calculated by integrating the dense thickness at each pixel over the whole mammogram, and the total breast volume (cm3) is obtained by multiplying the breast area by breast thickness, with an appropriate correction at the breast edge. Per cent dense volume (%) is calculated as the ratio of these two measures. For analyses, we calculated the mean mammographic density of the left and right breasts. We have recently shown that Volpara density measures are highly correlated with those of Cumulus method (Brand et al, 2014) and predict risk of breast cancer (Brand et al, 2014).
Assessment of exposure
Alcohol consumption was assessed using a web-based self-administered questionnaire based on the validated food frequency questionnaire MiniMeal-Q (Christensen et al, 2013). The participants provided information on frequency and amount of beverages consumed at least once per month during the months before study entry.
Daily alcohol consumption (g per day) was calculated for each beverage by multiplying the frequency with the amount and the ethanol concentration of the beverage. The beverage-specific ethanol concentration was based on a report from the Swedish National Food Agency (2014). For beer with different alcohol contents, the ethanol concentrations ranged from 2.8 to 6.4 g per 100 ml. For different types of wine, the ethanol concentrations ranged from 9.5 to 9.9 g per 100 ml. The ethanol concentration of spirits was calculated as 28.1 g per 100 ml, which was the averaged ethanol concentration of the 10 hard liquors available in the nutrient database. Alcohol consumptions from all beverages were summed into total alcohol consumption. Non-drinkers were defined as women who reported no drinking or drinking less than once per month.
Covariates
The self-reported questionnaire includes extensive information on factors suggested to be related to mammographic density and alcohol consumption. Factors used for adjustment are described in ‘Statistical analyses' section. Women reporting menstruations in the 12 months before study entry were considered premenopausal. Those who had no menstruations over the past year or reported oophorectomy were considered postmenopausal. Women with missing menstruation status or having no menstruations because of gynaecological surgery other than oophorectomy were considered premenopausal if they were ⩽55 years or postmenopausal if >55 years. The individual risk of developing breast cancer in the next 10 year was calculated using the Tyrer–Cuzick prediction model (Tyrer et al, 2004). The model incorporates information on family history of breast cancer and personal characteristics including age at menarche, parity and age at first birth (if parous), age at menopause (if postmenopausal), proliferative benign breast disease, atypical hyperplasia, lobular carcinoma in situ, height, and BMI.
Statistical analyses
We used linear regression to study the association between alcohol consumption (exposure) and absolute and per cent dense volumes (outcomes). We considered two exposure definitions: continuous and categorised alcohol consumption. For the latter, alcohol consumption was categorised in accordance with a previous large cohort study (Chen et al, 2011), as 0 (non-drinkers), 0.1–4.9, 5.0–9.9, 10.0–19.9, 20–29.9, and 30.0–40.0 g per day. For both exposure definitions, we fitted two models: one unadjusted and one adjusted for covariates. The following covariates were included in the adjusted models as categorical variables: age at mammography screening, BMI, family history of breast cancer in mother or sisters, age at menarche, parity and age at first birth, oral contraceptive use, menopausal status, hormone replacement therapy (HRT) use, education level, smoking status, physical activity, and ethnicity (see the footnote in Table 2). As a sensitivity analysis, we also fitted the models using age, BMI, age at menarche, and physical activity as continuous variables. These analyses produced similar results (data not shown).
We calculated P-values for testing the null hypothesis of no exposure-outcome association, referred to as ‘Ptrend' (1 d.f.) and ‘Pglobal' (5 d.f.) for the continuous and categorised exposure models, respectively.
To study the dose–response pattern in closer detail, we refitted the adjusted model for absolute dense volume using natural cubic splines (De Boor, 2001). These splines model the influence of alcohol consumption as a sequence of third-degree polynomials, which are forced together at prespecified ‘knots'. We used two knots: one at 10.0 g per day and one at 20.0 g per day. To obtain an adjusted exposure–response curve, we used the fitted spline model to calculate standardised means (Rothman et al, 2008). This method averages the predicted (from the model) absolute dense volumes for all individuals, replacing the observed alcohol consumption for each individual with a fixed level, taken to be 5.0 g per day. If there are no unmeasured confounders, then the obtained standardised mean can be interpreted as the mean absolute dense volume in a population where everybody has an alcohol consumption of 5.0 g per day. The procedure is then repeated for different levels of alcohol consumption to produce an adjusted exposure–response curve.
To investigate the potential effect measure modification by the Tyrer–Cuzick 10-year breast cancer risk, we refitted the regression models stratified by breast cancer risk. We categorised Tyrer–Cuzick 10-year risk based on established cut-points (Evans et al, 2013). The cut-point for the highest risk group was, however, set as ⩾5.0% to have sufficient numbers for stratified analyses. Tyrer–Cuzick risk was thus categorised as <3.0, 3.0–4.9, and ⩾5.0%. A formal test for interaction was performed by adding a product term between alcohol consumption and breast cancer risk into the non-stratified models.
To study the dose–response pattern within levels of breast cancer risk, we refitted the spline models including a product term between alcohol consumption and risk.
We used two-sided tests with 5% significance level. To avoid assuming normally distributed error terms and homoscedastic variance of the outcomes, we used robust ‘sandwich' standard errors in confidence intervals (CIs) and P-values (Newey and McFadden, 1994). The statistical software R version 3.1.0 was used for analyses.
Results
The mean age at mammography screening was 54.8 (s.d. 9.7) years and the mean BMI was 25.2 (s.d. 4.2) kg m−2 (Table 1). Approximately 54% of the participants were postmenopausal and 5.4% of them reported currently using HRT. A majority (84.7%) of the participants had used oral contraceptives. The mean absolute dense volume was 62.8 cm3 (95% CI, 62.6–63.1) and the mean per cent dense volume was 9.1% (95% CI, 9.0–9.1). Compared with non-drinkers, alcohol consumers were older, of lower BMI, higher education levels, more likely to have used oral contraceptives, to be current HRT users, smokers, and less physically active.
Table 1. Characteristics of the study population by level of alcohol consumption.
|
Alcohol consumption (g per day) |
||||||
|---|---|---|---|---|---|---|
| Characteristics | All women | 0 | 0.1–9.9 | 10.0–19.9 | 20.0–29.9 | 30.0–40.0 |
| Number of participants, N (%) | 53 060 (100) | 9728 (18.3) | 33 096 (62.4) | 3538 (6.7) | 5635 (10.6) | 1063 (2.0) |
| Age at mammography, mean (s.d.), years | 54.8 (9.7) | 54.6 (10.0) | 54.4 (9.97) | 55.0 (9.3) | 56.6 (9.1) | 57.8 (9.0) |
| Body mass index, mean (s.d.), kg m−2 | 25.2 (4.2) | 26.4 (5.1) | 24.9 (4.0) | 25.0 (3.8) | 24.9 (3.7) | 25.3 (3.9) |
| Age at menarche, mean (s.d.), yearsa | 13.1 (1.5) | 13.0 (1.5) | 13.1 (1.5) | 13.1 (1.4) | 13.1 (1.4) | 13.2 (1.4) |
| Absolute dense volume, mean (95% CI), cm3 | 62.8 (62.6–63.1) | 63.8 (63.1–64.5) | 62.6 (62.2–63.0) | 63.0 (61.9–64.1) | 62.1 (61.3–63.0) | 65.4 (63.3–67.4) |
| Non-dense volume, mean (95% CI), cm3 | 781.3 (777.4–785.3) | 888.8 (878.6–899.1) | 750.8 (745.9–755.7) | 759.3 (74.8–773.7) | 779.0 (767.9–790.2) | 833.8 (806.8–860.8) |
| Total breast volume, mean (95% CI), cm3 | 844.2 (840.1–848.2) | 952.6 (942.1–963.1) | 813.4 (808.4–818.4) | 822.3 (807.4–837.1) | 841.1 (829.7–852.6) | 899.2 (871.6–926.9) |
| Per cent dense volume, mean (95% CI), % | 9.1 (9.0–9.1) | 8.3 (8.2–8.4) | 9.4 (9.3–9.4) | 9.3 (9.1–9.4) | 8.7 (8.6–8.9) | 8.6 (8.3–8.9) |
| Nulliparous, %a | 12.6 | 14.6 | 11.5 | 14.8 | 13.1 | 16.8 |
| Parous women only, N | 46 305 | 8280 | 29 239 | 3014 | 4890 | 882 |
| Age at first birth, mean (s.d.), yearsa | 27.2 (5.3) | 26.3 (5.5) | 27.5 (5.2) | 27.3 (5.2) | 27.2 (4.9) | 27.0 (5.0) |
| Number of live birth, mean (s.d.) | 2.2 (0.8) | 2.2 (0.9) | 2.2 (0.8) | 2.2 (0.8) | 2.1 (0.8) | 2.1 (0.8) |
| Family history of breast cancer, %a | 12.7 | 12.8 | 12.6 | 11.5 | 13.4 | 15.1 |
|
Education level, %a | ||||||
| Secondary school | 12.5 | 18.0 | 11.4 | 12.8 | 10.0 | 8.6 |
| High school | 30.6 | 36.2 | 29.7 | 29.9 | 27.5 | 27.7 |
| University or higher | 53.0 | 40.9 | 55.2 | 53.8 | 59.0 | 59.6 |
| Other | 3.6 | 4.3 | 3.4 | 3.4 | 3.4 | 3.9 |
| OC use (ever), %a | 84.7 | 77.9 | 85.7 | 87.7 | 87.8 | 87.6 |
| Postmenopausal women, N (%) | 28 579 (53.9) | 5201 (53.5) | 17 230 (52.1) | 1946 (55.0) | 3495 (62.0) | 707 (66.5) |
| Age at menopause, mean (s.d.), years | 49.9 (5.2) | 49.5 (5.7) | 50.0 (5.1) | 50.0 (5.1) | 50.2 (4.9) | 50.1 (4.8) |
| HRT use, %a | ||||||
| Never | 61.2 | 66.3 | 61.3 | 59.3 | 55.4 | 55.3 |
| Past | 23.8 | 20.4 | 23.7 | 24.2 | 27.8 | 29.1 |
| Current | 5.4 | 4.2 | 5.3 | 5.9 | 6.8 | 6.6 |
|
Smoking status, %a | ||||||
| Never | 47.7 | 53.1 | 50.9 | 38.6 | 30.4 | 22.4 |
| Past | 40.2 | 31.8 | 39.0 | 46.4 | 54.4 | 58.6 |
| Current | 11.8 | 14.8 | 9.8 | 14.8 | 15.0 | 19.0 |
|
Physical activity, %, (MET-h per day)a | ||||||
| <40.0 | 30.1 | 29.6 | 28.9 | 33.5 | 33.9 | 41.9 |
| 40.0–44.9 | 37.5 | 34.0 | 38.3 | 38.6 | 38.5 | 34.0 |
| 45.0–49.9 | 18.9 | 18.3 | 19.6 | 16.9 | 17.4 | 16.1 |
| ⩾50.0 | 9.9 | 12.6 | 9.8 | 8.0 | 7.4 | 5.4 |
|
Ethnicity (European ancestry), %a | ||||||
| No | 2.5 | 4.9 | 2.0 | 1.9 | 1.2 | 1.8 |
| Yes | 97.1 | 94.6 | 97.6 | 97.5 | 98.3 | 97.6 |
Abbreviations: CI=confidence interval; HRT=hormone replacement therapy; MET-h per day=metabolic equivalent hours per day; OC=oral contraceptives; s.d.=standard deviation.
Percentage of women with missing data on age at menarche (2.1%), parity status (0.2%), age at first birth (0.2%), family history of breast cancer (3.5%), education level (0.3%), OC use (1.2%), HRT use (6.8%), smoking status (0.3%), physical activity (3.7%), and ethnicity (0.4%).
Women with the highest (⩾5.0%) Tyrer–Cuzick 10-year breast cancer risk were older, had a higher BMI, were older at first birth, more likely to be nulliparous, to have a family history of breast cancer, to be postmenopausal, and to be current HRT users (Supplementary Table S1) as compared with women with low (<3.0%) Tyrer–Cuzick risk.
Overall, increasing alcohol consumption was associated with higher absolute and per cent dense volumes after adjustment for potential confounders (Table 2). Women drinking 30.0–40.0 g per day of alcohol had an estimated 4.5 cm3 (95% CI, 2.2–6.8) higher absolute dense volume compared with non-drinkers. When alcohol consumption was used as a continuous exposure, each additional 10.0 g per day of alcohol was associated with 0.9 cm3 (95% CI, 0.5–1.3) increase in absolute dense volume. Alcohol consumption was also associated with higher per cent dense volume. Women drinking 30.0–40.0 g per day of alcohol had 0.5% (95% CI, 0.2–0.8) higher per cent dense volume compared with non-drinkers. Moreover, each additional 10.0 g per day of alcohol was associated with an increase of 0.1% (95% CI, 0.02–0.1) in per cent dense volume.
Table 2. Associations between alcohol consumption and volumetric mammographic density among all women.
|
β
(95% Confidence interval) |
||||||
|---|---|---|---|---|---|---|
| |
Absolute dense volume (cm3) |
Per cent dense volume (%) |
||||
| Alcohol consumption (g per day) | N | % | Unadjusted | Multivariable adjusteda | Unadjusted | Multivariable adjusteda |
| 0 | 9728 | 18.3 | Ref. | Ref. | Ref. | Ref. |
| 0.1–4.9 | 13 437 | 25.3 | −0.6 (−1.5, 0.3) | −0.2 (−0.8, 1.1) | 1.0 (0.9, 1.2) | 0.3 (0.2, 0.4) |
| 5.0–9.9 | 19 659 | 37.1 | −1.6 (−2.4, −0.8) | 0.5 (−0.4, 1.4) | 1.2 (1.0, 1.3) | 0.3 (0.2, 0.4) |
| 10.0–19.9 | 3538 | 6.7 | −0.8 (−2.1, 0.5) | 0.9 (−0.5, 2.2) | 1.0 (0.8, 1.2) | 0.4 (0.3, 0.6) |
| 20.0–29.9 | 5635 | 10.6 | −1.7 (−2.8, −0.6) | 1.3 (0.2, 2.5) | 0.5 (0.3, 0.6) | 0.2 (0, 0.3) |
| 30.0–40.0 | 1063 | 2.0 | 1.6 (−0.5, 3.7) | 4.5 (2.2, 6.8) | 0.3 (0, 0.6) | 0.5 (0.2, 0.8) |
| Pglobalb | <0.001 | <0.001 | <0.001 | <0.001 | ||
| For every 10 g per day increasec | −0.2 (−0.5, 0.2) | 0.9 (0.5, 1.3) | 0 (0, 0.1) | 0.1 (0.02, 0.1) | ||
| Ptrendd | 0.34 | <0.001 | 0.86 | 0.01 | ||
Abbreviations: β=regression coefficient; CI=confidence interval.
Regression coefficients were adjusted for age at mammography (5-year categories), body mass index (<25.0, 25.0–29.9, and ⩾30.0 kg m−2), family history of breast cancer in mother or sisters (yes, no), age at menarche (<13, 13, 14, and ⩾15 years), parity and age at first birth (nulliparous; 1–2 births, age at first birth<26 years; 1–2 births, age at first birth ⩾26 years, ⩾3 birth; age at first birth <26 years; ⩾3 births, age at first birth ⩾26 years), oral contraceptives use (never, ever), menopausal status (pre/postmenopausal), hormone replacement therapy use (never, past, current), education level (secondary school, high school, university or higher, other), smoking status (never, past, current), physical activity (<40.0, 40.0–44.9, 45.0–49.9, and ⩾50.0 metabolic equivalent hours per day), and ethnicity (having a European ancestry; yes or no).
Pglobal values were obtained from regression models using alcohol consumption as a categorical exposure.
Change in absolute dense volume for every 10 g per day increase in alcohol consumption, from regression models with alcohol consumption as a continuous exposure.
Ptrend values were obtained from regression models using alcohol consumption as a continuous exposure.
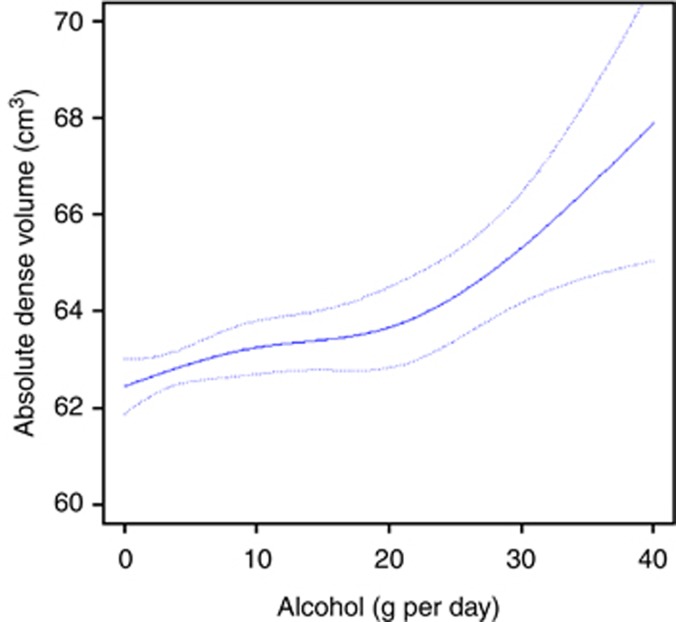
Figure 1 shows the dose–response relationship between alcohol consumption and absolute dense volume, together with 95% pointwise CIs (dashed line), among all women. The mean absolute dense volume would have been 62.4 cm3 (95% CI, 61.9–63.0) had all women been non-drinkers, and 67.9 cm3 (95% CI, 65.0–70.8) had all women had an alcohol consumption of 40 g per day, assuming no unmeasured confounders.
Figure 1.
Standardised mean absolute dense volume obtained from linear regression spline (solid line), as a function of alcohol consumption, together with pointwise 95% confidence interval (dashed lines) among all women.
After taking the Tyrer–Cuzick 10-year breast cancer risk into account, the association between alcohol consumption and absolute dense volume became stronger in women with the highest (⩾5.0%) Tyrer–Cuzick risk (Pinteraction=0.003; Table 3). Within high-risk women, as compared with non-drinkers, the estimated increase in absolute dense volume in women consuming 5.0–9.9, 10.0–19.9, 20.0–29.9, and 30.0–40.0 g per day of alcohol was 2.6 cm3 (95% CI, 0.2–4.9), 2.9 cm3 (95% CI, −0.6 to 6.3), 4.6 cm3 (95% CI, 1.5–7.7), and 10.8 cm3 (95% CI, 4.8–17.0), respectively. This corresponded to an increase of 2.4 cm3 (95% CI, 1.4–3.5) in absolute dense volume for each additional 10.0 g of alcohol consumed per day. There was a borderline statistically significant association between alcohol consumption and absolute dense volume among women at low (⩽3.0%) Tyrer–Cuzick risk, as evaluated by the trend test (Ptrend=0.05). No association was found between alcohol consumption and absolute dense volume among women with moderate (3.0–4.9%) Tyrer–Cuzick risk. We found no indication of interaction between alcohol consumption and breast cancer risk regarding per cent dense volume (Pinteraction=0.52; data not shown).
Table 3. Associations between alcohol consumption and absolute dense volume (cm3) stratified by 10-year breast cancer risk predicted by the Tyrer–Cuzick model.
|
Breast cancer risk <3.0% |
Breast cancer risk 3.0–4.9% |
Breast cancer risk ⩾5.0% |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Alcohol consumption (g per day) | N | % | β (95% CI)a | N | % | β (95% CI)a | N | % | β (95% CI)a |
| 0 | 5360 | 19.9 | Ref. | 3120 | 16.8 | Ref. | 1248 | 16.8 | Ref. |
| 0.1–4.9 | 7199 | 26.7 | 0 (−1.3, 1.3) | 4482 | 24.1 | 0.3 (−1.4, 1.9) | 1756 | 23.6 | 0.8 (−1.8, 3.4) |
| 5.0–9.9 | 9782 | 36.2 | 0 (−1.2, 1.3) | 7085 | 38.0 | 0.4 (−1.1, 1.9) | 2792 | 37.5 | 2.6 (0.2, 4.9) |
| 10.0–19.9 | 1721 | 6.4 | 0.7 (−1.3, 2.7) | 1307 | 7.0 | 0.3 (−1.9, 2.5) | 510 | 6.9 | 2.9 (−0.6, 6.3) |
| 20.0–29.9 | 2511 | 9.3 | 1.0 (−0.6, 2.7) | 2185 | 11.7 | 0.5 (−1.5, 2.4) | 939 | 12.6 | 4.6 (1.5, 7.7) |
| 30.0–40.0 | 424 | 1.6 | 3.0 (−0.3, 6.4) | 442 | 2.4 | 3.4 (−0.1, 6.9) | 197 | 2.6 | 10.8 (4.8, 17.0) |
| Pglobalb | 0.37 | 0.57 | <0.001 | ||||||
| For every 10 g per day increasec | 0.6 (0.1, 1.2) | 0.5 (−0.2, 1.1) | 2.4 (1.4, 3.5) | ||||||
| Ptrendd | 0.05 | 0.15 | <0.001 | ||||||
| Pinteractione | 0.003 | ||||||||
Abbreviations: β=regression coefficient; CI=confidence interval.
Regression coefficients were adjusted for covariates as listed in the footnote of Table 2.
Pglobal values were obtained from regression models using alcohol consumption as a categorical exposure.
Change in absolute dense volume for every 10 g per day increase in alcohol consumption, from regression models with alcohol consumption as a continuous exposure.
Ptrend values were obtained from regression models using alcohol consumption as a continuous exposure.
Pinteraction was obtained from the non-stratified regression model by adding a product term between alcohol consumption and the 10-year breast cancer risk, as predicted with the use of the Tyrer–Cuzick prediction model.
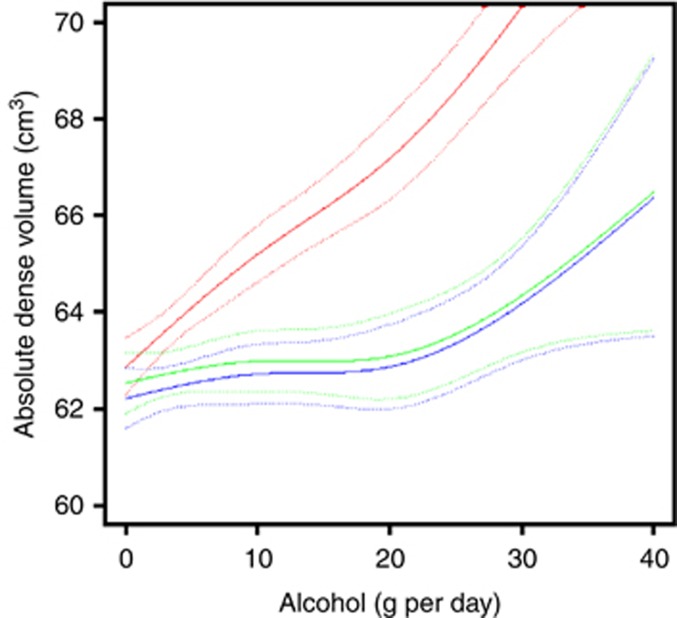
Figure 2 shows the dose–response relationship between alcohol consumption and absolute dense volume for those with <3.0% (green solid line), 3.0–4.9% (blue solid line), and ⩾5.0% (red solid line) breast cancer risk. There is clear modifying effect by background breast cancer risk, with considerably steeper dose–response curves for women with high risk compared with women with low and moderate risk.
Figure 2.
Standardised mean absolute dense volume obtained from linear regression spline (solid line), as a function of alcohol consumption, together with pointwise 95% CI (dashed lines), stratified by Tyrer–Cuzick 10-year breast cancer risk. Green line: women with <3.0% breast cancer risk; blue line: women with 3.0–4.9% breast cancer risk; red line: women with ⩾5.0% breast cancer risk. A full color version of this figure is available at British Journal of Cancer journal online.
Discussion
In the present study, we found increasing alcohol consumption to be associated with higher absolute and per cent volumetric mammographic densities. Intriguingly, we observed a clear interaction between alcohol consumption and the background risk of breast cancer according to the Tyrer–Cuzick prediction model, with a stronger association between alcohol consumption and absolute dense volume among women with the highest (⩾5.0%) Tyrer–Cuzick 10-year breast cancer risk. A borderline statistically significant association was seen among women at low (⩽3.0%) Tyrer–Cuzick risk, but no association was found among women at moderate (3.0–4.9%) Tyrer–Cuzick risk.
Breast cancer is the most common form of cancer occurring in women worldwide, with nearly 1.7 million new diagnoses in 2012 (Ferlay et al, 2014). Most of the established risk factors of breast cancer, such as having a family history of breast or ovarian cancer, early age at menarche, and number of births, are difficult to influence, thus making primary prevention of breast cancer a major challenge. Among the few modifiable factors associated with breast cancer, alcohol consumption has long been shown to increase the risk (Smith-Warner et al, 1998; Singletary and Gapstur, 2001; Chen et al, 2011). Every 10 g of alcohol consumed per day has been associated with 12% increase in breast cancer risk (Allen et al, 2009).
It is important to note that in all prior studies mammographic density was assessed using area-based methods, whereas we used volumetric measures of mammographic density. Volumetric measures take into account breast thickness, and hence are expected to better reflect the actual amount of fibroglandular tissue in the breast. Therefore, it is particularly challenging to make direct comparisons between our findings and others. However, our result of an association between alcohol consumption and per cent mammographic density is consistent with most previous studies (Vachon et al, 2000a; Flom et al, 2009; Cabanes et al, 2011; Voevodina et al, 2013), showing that women with high alcohol intake have higher per cent dense area compared with non-drinkers. A few studies did not observe this finding, perhaps because of limited sample size (Maskarinec et al, 2006) or low alcohol intake (Maskarinec et al, 2006; Qureshi et al, 2012). Two studies on alcohol consumption and absolute density have been conducted before ours. One of them supported our findings (Brand et al, 2013), but not the other (Qureshi et al, 2012), possibly because few women consumed higher amounts of alcohol (defined as ⩾10 g per day). Per cent mammographic density has been established as one of the strongest risk factors of breast cancer. In addition, a strong association between absolute mammographic density and breast cancer risk has also been shown (Vachon et al, 2007; Pettersson et al, 2014). Absolute mammographic density reflects the amount of fibroglandular and connective tissue in the breast, and therefore the number of breast cells susceptible to malignant transformation.
To our knowledge, this is the largest study that has examined the association between alcohol consumption and mammographic density. This study is based on a unique and rich data set with information on several breast cancer risk factors, permitting the calculation of breast cancer risk using the Tyrer–Cuzick prediction model. Additional strengths are the population-based setting and the fully automated volumetric Volpara method, thus reducing possible misclassification of mammographic density (Vachon et al, 2000b; Maskarinec et al, 2006; Flom et al, 2009; Cabanes et al, 2011; Qureshi et al, 2012; Voevodina et al, 2013). Moreover, we used a comprehensive and validated questionnaire for assessing alcohol consumption.
A limitation of our study is its cross-sectional design, which does not allow us to rule out reverse causation between alcohol consumption and mammographic density. However, it is unlikely that high mammographic density would induce higher alcohol consumption. Although self-reported alcohol consumption is prone to misclassification, particularly under-reporting, such bias would most likely be non-differential as women are typically not aware of their mammographic density.
Alcohol has been shown to influence circulating sex hormones in both premenopausal and postmenopausal women (Reichman et al, 1993; Dorgan et al, 2001), possibly through increased aromatase activity (Purohit, 2000) and expression (Monteiro et al, 2009), prolonged hepatic clearance of oestrogens (Ginsburg et al, 1995), and/or increased oestrogen receptor expression and signalling (Fan et al, 2000). Sex hormone levels have been shown to affect mammographic density (Boyd et al, 2011; Cuzick et al, 2011) and a dose–response relationship between alcohol consumption and breast cancer risk has repeatedly been described (Smith-Warner et al, 1998; Singletary and Gapstur, 2001; Chen et al, 2011).
The Tyrer–Cuzick prediction model includes several hormonal factors that are associated with a woman's cumulative sex hormones exposure, such as parity, age at menarche, age at first childbirth, and age at menopause (Tyrer et al, 2004). We found women at higher Tyrer–Cuzick risk of breast cancer to have a stronger association between alcohol consumption and mammographic density than women at low or moderate risk. Ingestion of 0.7 g alcohol per kg body weight has been reported to be associated with a three-fold increase in oestradiol levels in women on HRT, but not in non-HRT users (Ginsburg et al, 1996). Furthermore, alcohol consumers who also use HRT have been found to have a more pronounced increase in breast cancer risk compared with consumers not using HRT (Chen et al, 2002; Nielsen and Gronbaek, 2008), suggesting that the effects of alcohol on breast carcinogenesis could be stronger in the presence of sex steroid hormones.
High volumetric mammographic density, measured using the Volpara method, has been associated with an increased risk of breast cancer among KARMA participants (Brand et al, 2014). Based on these results, women in the present study who consumed 30.0–40.0 g per day (3.0–4.0 drinks per day) had an estimated increase of ∼8.7% in relative breast cancer incidence rate as compared with non-drinkers.
In this study, women at high Tyrer–Cuzick breast cancer risk who consumed larger amounts of alcohol had higher absolute mammographic density, and thereby likely have a higher risk of breast cancer than non-drinking women at lower Tyrer–Cuzick risk. Higher density also increases the risk of ‘masking bias' leading to a false-negative result at mammography screening. Our results highlight the notion that women at high background risk of breast cancer should consider lowering their alcohol consumption to reduce mammographic density, and thereby breast cancer risk and the negative impact on mammographic sensitivity.
In conclusion, this is the first study evaluating the influence of background breast cancer risk when determining the effect of an established risk factor, alcohol consumption, on mammographic density. If confirmed, our findings have the potential to influence future risk counselling.
Acknowledgments
We thank all the participants in the KARMA study and the study personnel for their devoted work during data collection. We also would like to acknowledge Ralph Highnam and co-workers for technical support on the Volpara software for volumetric mammographic density measurement. This work was supported by the Märit and Hans Rausing's Initiative Against Breast Cancer. The funding resources had no role in the study design, data collection, analyses, data interpretation, writing the manuscript, or in the decision to submit the manuscript for publication.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, Green J, Million Women Study Collaborators (2009) Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst 101: 296–305. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, Lockwood GA, Tritchler DL, Yaffe MJ (1995) Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst 87: 670–675. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Melnichouk O, Martin LJ, Hislop G, Chiarelli AM, Yaffe MJ, Minkin S (2011) Mammographic density, response to hormones, and breast cancer risk. J Clin Oncol 29: 2985–2992. [DOI] [PubMed] [Google Scholar]
- Brand JS, Czene K, Eriksson L, Trinh T, Bhoo-Pathy N, Hall P, Celebioglu F (2013) Influence of lifestyle factors on mammographic density in postmenopausal women. PLoS One 8: e81876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand JS, Czene K, Shepherd JA, Leifland K, Heddson B, Sundbom A, Eriksson M, Li J, Humphreys K, Hall P (2014) Automated measurement of volumetric mammographic density: a tool for widespread breast cancer risk assessment. Cancer Epidemiol Biomarkers Prev 23: 1764–1772. [DOI] [PubMed] [Google Scholar]
- Cabanes A, Pastor-Barriuso R, Garcia-Lopez M, Pedraz-Pingarron C, Sanchez-Contador C, Vazquez Carrete JA, Moreno MP, Vidal C, Salas D, Miranda-Garcia J, Peris M, Moreo P, Santamarina MC, Collado-Garcia F, Gonzalez-Roman I, Ascunce N, Pollan M, Spain DDM (2011) Alcohol, tobacco, and mammographic density: a population-based study. Breast Cancer Res Treat 129: 135–147. [DOI] [PubMed] [Google Scholar]
- Chen WY, Colditz GA, Rosner B, Hankinson SE, Hunter DJ, Manson JE, Stampfer MJ, Willett WC, Speizer FE (2002) Use of postmenopausal hormones, alcohol, and risk for invasive breast cancer. Ann Intern Med 137: 798–804. [DOI] [PubMed] [Google Scholar]
- Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC (2011) Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA 306: 1884–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SE, Moller E, Bonn SE, Ploner A, Wright A, Sjolander A, Balter O, Lissner L, Balter K (2013) Two new meal- and web-based interactive food frequency questionnaires: validation of energy and macronutrient intake. J Med Internet Res 15: e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, Forbes JF, Warren RM (2011) Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case–control study. J Natl Cancer Inst 103: 744–752. [DOI] [PubMed] [Google Scholar]
- De Boor C (2001) A practical guide to splines, revised edition. Appl Math Sci 27. [Google Scholar]
- Dorgan JF, Baer DJ, Albert PS, Judd JT, Brown ED, Corle DK, Campbell WS, Hartman TJ, Tejpar AA, Clevidence BA, Giffen CA, Chandler DW, Stanczyk FZ, Taylor PR (2001) Serum hormones and the alcohol-breast cancer association in postmenopausal women. J Natl Cancer Inst 93: 710–715. [DOI] [PubMed] [Google Scholar]
- Evans DG, Graham J, O'Connell S, Arnold S, Fitzsimmons D (2013) Familial breast cancer: summary of updated NICE guidance. BMJ 346: f3829. [DOI] [PubMed] [Google Scholar]
- Fan S, Meng Q, Gao B, Grossman J, Yadegari M, Goldberg ID, Rosen EM (2000) Alcohol stimulates estrogen receptor signaling in human breast cancer cell lines. Cancer Res 60: 5635–5639. [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram II, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman DD, Bray F (2014) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- Flom JD, Ferris JS, Tehranifar P, Terry MB (2009) Alcohol intake over the life course and mammographic density. Breast Cancer Res Treat 117: 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg ES, Mello NK, Mendelson JH, Barbieri RL, Teoh SK, Rothman M, Gao X, Sholar JW (1996) Effects of alcohol ingestion on estrogens in postmenopausal women. JAMA 276: 1747–1751. [DOI] [PubMed] [Google Scholar]
- Ginsburg ES, Walsh BW, Shea BF, Gao X, Gleason RE, Barbieri RL (1995) The effects of ethanol on the clearance of estradiol in postmenopausal women. Fertil Steril 63: 1227–1230. [PubMed] [Google Scholar]
- Highnam R, Brady M, Yaffe MJ, Karssemeijer N, Harvey J (2010) Robust breast composition measurement – Volpara (TM). Dig Mammogr 6136: 342–349. [Google Scholar]
- KARMA KARMA – KARolinska MAmmography Project for Risk Prediction of Breast Cancer [Online]. Available at http://karmastudy.org/sources/ last accessed on 30 November 2014.
- Maskarinec G, Takata Y, Pagano I, Lurie G, Wilkens LR, Kolonel LN (2006) Alcohol consumption and mammographic density in a multiethnic population. Int J Cancer 118: 2579–2583. [DOI] [PubMed] [Google Scholar]
- McCormack VA, dos Santos Silva I (2006) Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 15: 1159–1169. [DOI] [PubMed] [Google Scholar]
- Monteiro R, Soares R, Guerreiro S, Pestana D, Calhau C, Azevedo I (2009) Red wine increases adipose tissue aromatase expression and regulates body weight and adipocyte size. Nutrition 25: 699–705. [DOI] [PubMed] [Google Scholar]
- Newey WK, McFadden D (1994) Large sample estimation and hypothesis testing. In Handbook of Econometrics, Robert FE, Daniel LM (eds) Chapter 36, Vol. 4, pp 2111–2145 Elsevier: Amsterdam. [Google Scholar]
- Nielsen NR, Gronbaek M (2008) Interactions between intakes of alcohol and postmenopausal hormones on risk of breast cancer. Int J Cancer 122: 1109–1113. [DOI] [PubMed] [Google Scholar]
- Pettersson A, Graff RE, Ursin G, Santos Silva ID, McCormack V, Baglietto L, Vachon C, Bakker MF, Giles GG, Chia KS, Czene K, Eriksson L, Hall P, Hartman M, Warren RM, Hislop G, Chiarelli AM, Hopper JL, Krishnan K, Li J, Li Q, Pagano I, Rosner BA, Wong CS, Scott C, Stone J, Maskarinec G, Boyd NF, Van Gils CH, Tamimi RM (2014) Mammographic density phenotypes and risk of breast cancer: a meta-analysis. J Natl Cancer Inst 106: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit V (2000) Can alcohol promote aromatization of androgens to estrogens? A review. Alcohol 22: 123–127. [DOI] [PubMed] [Google Scholar]
- Qureshi SA, Couto E, Hofvind S, Wu AH, Ursin G (2012) Alcohol intake and mammographic density in postmenopausal Norwegian women. Breast Cancer Res Treat 131: 993–1002. [DOI] [PubMed] [Google Scholar]
- Reichman ME, Judd JT, Longcope C, Schatzkin A, Clevidence BA, Nair PP, Campbell WS, Taylor PR (1993) Effects of alcohol consumption on plasma and urinary hormone concentrations in premenopausal women. J Natl Cancer Inst 85: 722–727. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL (eds) (2008) Modern Epidemiology, p 386. Lippincott Williams & Wilkins: USA.
- Singletary KW, Gapstur SM (2001) Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA 286: 2143–2151. [DOI] [PubMed] [Google Scholar]
- Smith-Warner SA, Spiegelman D, Yaun SS, van den Brandt PA, Folsom AR, Goldbohm RA, Graham S, Holmberg L, Howe GR, Marshall JR, Miller AB, Potter JD, Speizer FE, Willett WC, Wolk A, Hunter DJ (1998) Alcohol and breast cancer in women: a pooled analysis of cohort studies. JAMA 279: 535–540. [DOI] [PubMed] [Google Scholar]
- Swedish National Food Agency (2014) Swedish Food Composition Database [Online]. Swedish National Food Agency. Available at http://www.livsmedelsverket.se/livsmedelsdatabasen (last accessed 30 November 2014).
- Tyrer J, Duffy SW, Cuzick J (2004) A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 23: 1111–1130. [DOI] [PubMed] [Google Scholar]
- Vachon CM, Brandt KR, Ghosh K, Scott CG, Maloney SD, Carston MJ, Pankratz VS, Sellers TA (2007) Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiol Biomarkers Prev 16: 43–49. [DOI] [PubMed] [Google Scholar]
- Vachon CM, Kuni CC, Anderson K, Anderson VE, Sellers TA (2000. a) Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States). Cancer Causes Control 11: 653–662. [DOI] [PubMed] [Google Scholar]
- Vachon CM, Kushi LH, Cerhan JR, Kuni CC, Sellers TA (2000. b) Association of diet and mammographic breast density in the Minnesota breast cancer family cohort. Cancer Epidemiol Biomarkers Prev 9: 151–160. [PubMed] [Google Scholar]
- Voevodina O, Billich C, arand B, NageL G (2013) Association of Mediterranean diet, dietary supplements and alcohol consumption with breast density among women in South Germany: a cross-sectional study. BMC Public Health 13: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.