Abstract
Background:
We report final results with extended follow-up from a global, expanded-access trial that pre-regulatory approval provided sunitinib to metastatic renal cell carcinoma (mRCC) patients, ineligible for registration-directed trials.
Methods:
Patients ⩾18 years received oral sunitinib 50 mg per day on a 4-weeks-on–2-weeks-off schedule. Safety was assessed regularly. Tumour measurements were scheduled per local practice.
Results:
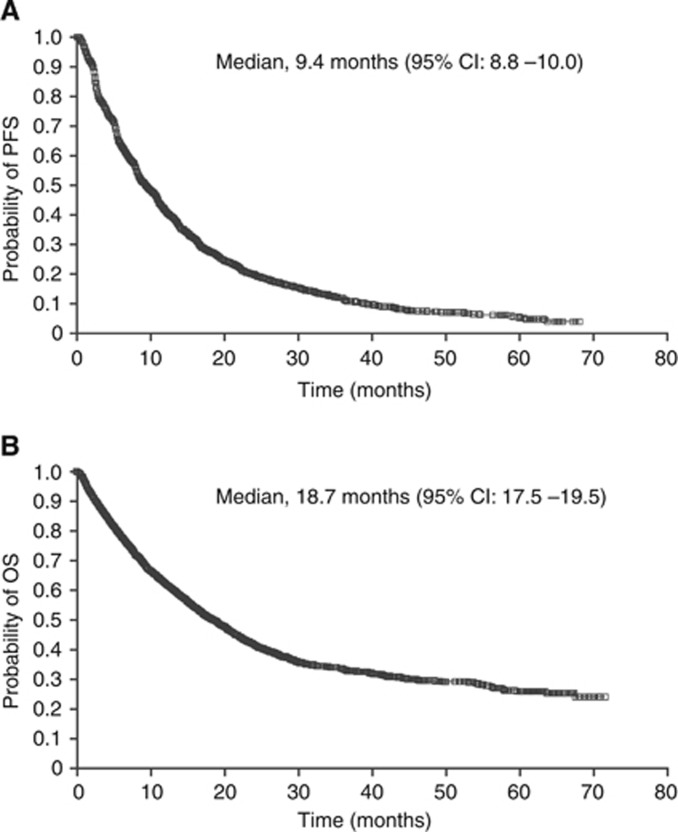
A total of 4543 patients received sunitinib. Median treatment duration and follow-up were 7.5 and 13.6 months. Objective response rate was 16% (95% confidence interval (CI): 15–17). Median progression-free survival (PFS) and overall survival (OS) were 9.4 months (95% CI: 8.8–10.0) and 18.7 months (95% CI: 17.5–19.5). Median PFS in subgroups of interest: aged ⩾65 years (33%), 10.1 months; Eastern Cooperative Oncology Group performance status ⩾2 (14%), 3.5 months; non-clear cell histology (12%), 6.0 months; and brain metastases (7%), 5.3 months. OS was strongly associated with the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model (n=4065). The most common grade 3/4 treatment-related adverse events were thrombocytopenia (10%), fatigue (9%), and asthenia, neutropenia, and hand–foot syndrome (each 7%).
Conclusion:
Final analysis of the sunitinib expanded-access trial provided a good opportunity to evaluate the long-term side effects of a tyrosine kinase inhibitor used worldwide in mRCC. Efficacy and safety findings were consistent with previous results.
Keywords: renal cell carcinoma, sunitinib, tyrosine kinase inhibitor, expanded-access trial, prognosis
Sunitinib is an orally administered multitargeted inhibitor of vascular endothelial growth factor receptors (VEGFRs), platelet-derived growth factor receptors, and other receptor tyrosine kinases (Abrams et al, 2003; Mendel et al, 2003; O'Farrell et al, 2003). The efficacy of sunitinib demonstrated in two phase II trials of patients with cytokine-refractory advanced renal cell carcinoma (RCC) led to its conditional approval (Motzer et al, 2006a, 2006b). In a subsequent pivotal phase III registration trial of treatment-naive patients with metastatic RCC (mRCC), sunitinib demonstrated a significant improvement in progression-free survival (PFS) compared with interferon-alfa (median PFS 11 vs 5 months; hazard ratio (HR): 0.42, P<0.001) and in objective response rate (ORR; 31% vs 6%, P<0.001; Motzer et al, 2007); in addition, overall survival (OS) was longer with sunitinib (median 26.4 vs 21.8 months; HR: 0.821, P=0.051; Motzer et al, 2009). Given the lack of active agents available in 2005 to treat advanced RCC, a global, expanded-access trial was implemented to provide sunitinib to patients in countries where its approval had not yet been granted and to those ineligible for registration-directed trials. Initial results from this expanded-access programme confirmed the activity of sunitinib in a broader ‘real-world' population (Gore et al, 2009).
Here we report final results with extended follow-up of the 4543 patients treated in the expanded-access trial. This extensive database was also used in an exploratory analysis to provide further external validation of the RCC prognostic model from the International Metastatic Renal-Cell Carcinoma Database Consortium (IMDC; Heng et al, 2009, 2013).
Patients and Methods
Patients
All patients were aged 18 years or older with histologically confirmed mRCC that was either treatment-naive or previously treated. Other requirements were as follows: resolution of prior treatment toxicities, adequate organ function, no major comorbidities, the potential to derive clinical benefit from sunitinib as judged by the treating physician, and ineligibility for other sunitinib studies. Any Eastern Cooperative Oncology Group (ECOG) performance status and asymptomatic brain metastases were permitted. Full details for eligibility criteria have been previously reported (Gore et al, 2009). All patients gave written, informed consent.
Study design and treatment
This was an international, open-label, expanded-access trial of sunitinib (SUTENT; Pfizer Inc., New York, NY, USA) that treated patients from participating centres in 50 countries. The primary objective of the study was to provide sunitinib to patients with mRCC and no access to the drug who might benefit from treatment. Secondary objectives included assessment of efficacy and toxicity in the overall population, as well as in subgroups of interest.
The first patient was enrolled in June 2005. Accrual discontinued on a country-by-country basis according to treatment availability, with the last patient enrolled in December 2007. All patients received oral sunitinib at a starting dose of 50 mg per day for 4 weeks on treatment followed by 2 weeks off treatment in repeated 6-week cycles (schedule 4/2). Provision was made for dose reduction to 37.5 mg per day and if needed to 25 mg per day on the basis of individual tolerance. A protocol amendment in May 2006 provided investigators with the option of administering sunitinib on a continuous daily dosing schedule (usual starting dose 37.5 mg per day). Treatment continued until disease progression, unacceptable toxicity, or withdrawal of consent. The study was approved by the institutional review board or independent ethics committee at each participating centre, and was run in accordance with the International Conference on Harmonization Good Clinical Practice Guidelines.
Study assessments
Screening evaluations included disease assessment, physical examination, biochemistry and haematology tests, and a record of concomitant medications. Monitoring safety was mandatory at regular intervals (on days 1, 14, and 28 of cycle 1, and days 1 and 28 of subsequent cycles, until a protocol amendment (May 2006) removed the day 28 assessment in cycles ⩾3) by physical examination, ECOG performance status, haematology and biochemistry tests and cardiac function (12-lead electrocardiogram), and by recording and grading all adverse events (AEs) according to the National Cancer Institute Common Terminology Criteria for Adverse Events (Cancer Therapy Evaluation Program, 2006).
No specific schedule was dictated by the protocol; however, tumour measurement was assessed by Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0 (Therasse et al, 2000). Assessments were performed as per the local standard of care for mRCC, with data on tumour response, PFS, and OS collected when possible. Objective response rate was defined as the number of confirmed complete plus partial responses according to RECIST (Therasse et al, 2000). Progression-free survival was defined as the time from start of therapy to disease progression or death from any cause (whichever occurred first). Only deaths that occurred within 28 days of the last dose were counted as PFS events; however, disease progression was not restricted to the treatment period plus the 28-day follow-up period. OS was defined as the time from start of therapy to death from any cause. Patients who were not known to be dead at the time of analysis or on the data cutoff date (September 2008) were censored on the date that they were last known to be alive.
Statistical analysis
Because of the nature of this study, there was no pre-determined sample size nor were inferential analyses preplanned or any pre-specified hypotheses tested. All patients who received at least one dose of sunitinib were included in the current analyses (modified intention-to-treat population), except for patients with non-RECIST tumour measurements (n=324), who were excluded from efficacy analyses based on tumour response data. Objective response rate was calculated with corresponding exact 95% two-sided confidence interval (CI) using standard methods based on the binomial distribution. The Kaplan–Meier method was used to estimate PFS and OS, with 95% CI calculated for the median.
In an exploratory analysis, patients were grouped into favourable (0 adverse factors), intermediate (1–2 adverse factors), and poor (⩾3 adverse factors) risk categories according to the IMDC model (Heng et al, 2009, 2013), with median OS for each group estimated by the Kaplan–Meier method and compared by the log-rank test. Adverse factors were ECOG performance status >1, time from diagnosis to study treatment <1 year (calculated as the time from original diagnosis to the date of the first sunitinib dose in the current study), haemoglobin < lower limit of normal (LLN), calcium > upper limit of normal (ULN), neutrophil count >ULN, and platelet count >ULN. In addition, a Cox multivariate analysis was used to explore the association between OS and the IMDC prognostic factors. All P-values were considered exploratory.
Results
Patients
In total, 4577 patients were enrolled into the study, including 4543 patients who received at least one dose of sunitinib and comprised the population for analysis purposes. Baseline patient characteristics are described in Table 1. The broad trial population included patient subgroups of interest, including patients age ⩾65 years (33%) and those with ECOG performance status ⩾2 (14%), non-clear cell RCC (12%), and brain metastases (7%). Overall, 26% of patients were classified as having a poor prognosis (Table 1), according to modified risk groups based on published Memorial Sloan-Kettering Cancer Center (MSKCC) criteria (Motzer et al, 2002, 2004).
Table 1. Baseline patient characteristics.
| Characteristic | Sunitinib (N=4543) |
|---|---|
| Median age (range), years | 59.0 (19.0–89.0) |
| Age ⩾65 years, n (%) | 1485 (33) |
| Male/female, n (%) | 3364/1179 (74/26) |
|
ECOG performance status,
n
(%)a | |
| 0 | 1868 (41) |
| 1 | 1949 (43) |
| 2 | 547 (12) |
| 3 | 80 (2) |
| 4 | 7 (<1) |
|
Histology,
n
(%)a | |
| Clear cell | 4010 (88) |
| Non-clear cell | 532 (12) |
| Prior nephrectomy, n (%)a | 4044 (89) |
|
Disease sites,
n
(%) | |
| Lung | 3469 (76) |
| Lymph nodes | 2333 (51) |
| Bone | 1593 (35) |
| Liver | 1236 (27) |
| Brain | 338 (7) |
|
Prior systemic therapy,
n
(%) | |
| Antiangiogenicb | 440 (10) |
| Cytokine | 3096 (68) |
|
Modified risk groups based on published MSKCC data,
n
(%)a,c | |
| Favourable | 915 (20) |
| Intermediate | 1495 (33) |
| Poor | 1177 (26) |
|
Risk groups based on the IMDC prognostic model,
n
(%)a,d | |
| Favourable | 988 (22) |
| Intermediate | 2188 (48) |
| Poor | 889 (20) |
Abbreviations: ECOG=Eastern Cooperative Oncology Group; IMDC=International Metastatic Renal-Cell Carcinoma Database Consortium; LLN=lower limit of normal; MSKCC=Memorial Sloan-Kettering Cancer Center; ULN=upper limit of normal.
Number (%) of patients with missing data: ECOG performance status=92 (2), histology=1 (<1), prior nephrectomy =199 (4), modified risk groups based on published MSKCC data (Motzer et al, 2002, 2004)=956 (21), risk groups based on the IMDC prognostic model (Heng et al, 2009, 2013)=478 (11).
Included sorafenib and bevacizumab.
Risk factors were ECOG performance status ⩾2, haemoglobin <LLN, and corrected serum calcium >10 mg dl−1; patients without prior cytokine treatment also had lactose dehydrogenase >1.5 × ULN and time to interferon-alfa use of <1 year as risk factors (Motzer et al, 2002, 2004). Patients with prior cytokine therapy were assigned to favourable, intermediate, or poor risk groups if 0, 1, or >1 risk factors were present, respectively. Patients without prior cytokine treatment were assigned to favourable, intermediate, or poor risk groups if 0, 1 or 2, or >2 risk factors were present, respectively.
Risk factors were ECOG performance status >1, time from diagnosis to study treatment <1 year, haemoglobin<LLN, calcium>ULN, neutrophil count >ULN, and platelet count > ULN (Heng et al, 2009, 2013). Patients with 0, 1 or 2, or >2 risk factors were assigned to the favourable, intermediate, or poor risk groups, respectively.
Treatment exposure
Patients received a median of six cycles (range: 1–57), with median treatment duration of 7.5 months (95% CI: 6.9–7.8). Median follow-up (the time from the start of therapy until the patient was censored for survival or died, whichever occurred first) was 13.6 months (range: <1–71.3 months), and was similar for those who previously had or had not received cytokine therapy. At the time of analysis, 4298 patients (95%) had discontinued treatment. Among these patients, the most common reasons for stopping therapy were lack of efficacy (39%), death (21%), AE (16%), consent withdrawn (9%), and lost to follow-up (3%). Overall, 49% of patients required a dose reduction of sunitinib. The dose was reduced to 37.5 mg per day in 34% of patients, to 25 mg per day in 15% of patients, and to 12.5 mg per day in <1% of patients. (Seventy patients (2%) were assigned to and received 37.5 mg per day on a continuous daily dosing schedule.) Dose reductions of sunitinib occurred at a higher frequency in patients who received prior cytokine treatment for advanced RCC, compared with those who had not received cytokines (51% vs 47%).
Safety
Approximately 95% of patients reported treatment-related AEs of any grade, the most frequent of which were diarrhoea (47%), fatigue (40%), nausea (36%), and decreased appetite (31% Table 2). All-grade hypothyroidism was reported as a treatment-related AE in 11% of patients, and proteinuria was reported in 1% of patients overall. (Routine testing of thyroid function was not required; however, urinalysis (dipstick protein urinalysis) to monitor proteinuria was done at screening, day 1 of cycle 2, as clinically indicated, and at the end of treatment.) The most commonly reported treatment-related grade 3/4 AEs included thrombocytopenia (10%), fatigue (9%), asthenia, hand–foot syndrome, and neutropenia (each 7%), hypertension (6%), and diarrhoea (5% Table 2). In this large population, the reported overall incidence of cardiac disorders considered treatment related was 6% (grade 3/4<2%). Rates of all-grade cardiac failure or congestive cardiac failure were <1%. Twelve patients (<1%) died as a result of a treatment-related cardiac event, including cardiac failure (n=4), myocardial infarction (n=5), and cardiac arrest, cardiopulmonary failure, and myocarditis (each n=1).
Table 2. Treatment-related adverse events of interest and those that occurred in ⩾10% of the modified intent-to-treat population (N=4543).
| Adverse event | Grade 1/2, n (%) | Grade 3/4, n (%) | Total, N (%)a |
|---|---|---|---|
|
Non-haematologic | |||
| Diarrhoea | 1885 (41) | 237 (5) | 2122 (47) |
| Fatigue | 1406 (31) | 403 (9) | 1809 (40) |
| Nausea | 1517 (33) | 111 (2) | 1629 (36)b |
| Decreased appetite | 1295 (29) | 102 (2) | 1398 (31)b |
| Mucosal inflammation | 1195 (26) | 137 (3) | 1332 (29) |
| Stomatitis | 1144 (25) | 133 (3) | 1277 (28) |
| Vomiting | 1107 (24) | 143 (3) | 1250 (28) |
| Hand–foot syndrome | 909 (20) | 311 (7) | 1221 (27)b |
| Dysgeusia | 1124 (25) | 28 (1) | 1152 (25) |
| Hypertension | 837 (18) | 267 (6) | 1104 (24) |
| Asthenia | 713 (16) | 306 (7) | 1021 (22)b,c |
| Dyspepsia | 828 (18) | 16 (<1) | 844 (19) |
| Rash | 734 (16) | 38 (1) | 772 (17) |
| Constipation | 628 (14) | 12 (<1) | 641 (14)b |
| Epistaxis | 585 (13) | 31 (1) | 616 (14) |
| Yellow skin | 588 (13) | 5 (<1) | 593 (13) |
| Headache | 495 (11) | 26 (1) | 521 (11) |
| Hypothyroidism | 489 (11) | 27 (1) | 516 (11) |
| Skin discolouration | 487 (11) | 4 (<1) | 491 (11) |
| Hair colour changes | 481 (11) | 9 (<1) | 490 (11) |
| Dry skin | 458 (10) | 3 (<1) | 461 (10) |
| Pain in extremity | 413 (9) | 42 (1) | 455 (10) |
| ALT increased | 94 (2) | 23 (1) | 117 (3) |
| Cardiac failure | 0 | 13 (<1) | 17 (<1) |
| Congestive cardiac failure | 1 (<1) | 13 (<1) | 14 (<1) |
|
Haematologicd | |||
| Thrombocytopenia | 741 (16) | 440 (10) | 1182 (26) |
| Neutropenia | 486 (11) | 315 (7) | 801 (18) |
| Anaemia | 594 (13) | 203 (4) | 798 (18)b |
| Leukopenia | 414 (9) | 97 (2) | 511 (11) |
Abbreviation: ALT=alanine aminotransferase.
Eighty patients (2%) died from treatment-related adverse events (data not shown, except for cardiac failure (n=4) and asthenia and thrombocytopenia (both n=1)).
Grade missing for one patient.
Includes one patient with grade 5 asthenia, a 55-year-old female with a medical history of hypertension and Hodgkin's disease, who had baseline Eastern Cooperative Oncology Group performance status of 2, and massive liver metastases, and pulmonary and mediastinal metastases before the start of the study; in addition to asthenia, other treatment-related serious adverse events experienced by the patient included dyspnoea, thrombopenia, hypotension, and hypothermia.
Related haematological adverse events with different preferred terms were collected and pooled.
Sixty-eight patients (1%) died from other treatment-related (non-cardiac) AEs, the most frequent of which were cerebral haemorrhage (n=6), death (n=5), hepatic and renal failure (each n=4), and gastrointestinal haemorrhage, general physical health deterioration, pulmonary embolism, respiratory failure, and septic shock (each n=3). Among the more commonly reported AEs, two patients died as a result of thrombocytopenia and asthenia (each n=1), as reported by the investigators.
Serum chemistry laboratory tests showed a transient increase in median alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and lactate dehydrogenase levels during cycles 1–3 that stabilised at values near or below baseline in subsequent cycles (data not shown). Clinically significant elevations in ALT or AST were each reported as all-grade treatment-related AEs in 3% of patients, with similar incidences of both and ALP reported as all-grade AEs of any cause (Supplementary Table 1).
The incidences of non-haematological, sunitinib-related AEs of any grade in subgroups of interest were comparable to (for patients aged ⩾65 years; Supplementary Table 2; and those with RCC of non-clear histology (data not shown)) or apparently lower than (for those with brain metastases (data not shown), and ECOG performance status ⩾2; Supplementary Table 3) that in the overall population. The overall incidence of non-haematological grade 3/4 AEs was broadly similar across the subgroups and when compared with the overall population, with the exception of the elderly subgroup (⩾65 years), in which the total incidence appeared slightly higher (61%) than in patients <65 years of age (51%). Otherwise, the incidences of the most commonly reported non-haematological AEs were generally similar in both age groups (Supplementary Table 2).
Of interest, the overall incidence of grade 3/4 AEs appeared to be slightly lower in patients with ECOG performance status ⩾2 (46%) than in patients with ECOG performance status 0 or 1 (56%). Otherwise, the incidences of the most commonly reported grade 3/4 AEs were generally similar, with more apparent variation in the incidences of grade 1/2 AEs according to performance status (Supplementary Table 3).
The safety profile of sunitinib appeared generally unchanged with long-term follow-up. When the final data reported here were compared with interim results reported in 2009 (Gore et al, 2009), the frequency and grade of common AEs were similar, except for a numerical increase in all-grade hypothyroidism (11% vs 6%).
Efficacy
A total of 4219 mRCC patients were included in the analysis of tumour response, of whom 63 patients achieved a complete response (1%) and 597 patients a partial response (14%), yielding an ORR of 16% (95% CI: 15–17; Table 3). The ORR was similar in patients with and without previous cytokine treatment, and also similar in elderly patients (⩾65 years) compared with younger patients (<65 years; Table 3). Responses were reported among patients in all subpopulations of interest (Table 3). Patients at favourable risk based on the modified MSKCC prognostic criteria fared better than those at poor risk, with ORRs of 26%, 16%, and 9% in the favourable, intermediate, and poor risk groups, respectively (as originally classified before the exploratory analysis using the IMDC model). Approximately 45% of patients in the overall population had stable disease for at least 3 months, with similar rates regardless of prior cytokine treatment, among elderly patients, and in those with RCC of non-clear cell histology; patients with ECOG performance status ⩾2 or brain metastases were less likely to achieve durable stable disease (Table 3). Progressive disease or stable disease <3 months was found in 19% of patients.
Table 3. Tumour response according to RECIST (version 1.0) and clinical benefit.
|
Prior cytokine treatment |
Patient subgroups |
|||||||
|---|---|---|---|---|---|---|---|---|
| All patientsa (N=4219) | Yes (n=2907) | No (n=1312) | Age ⩾65 years (n=1386) | Age <65 years (n=2833) | ECOG PS ⩾2 (n=587) | Non-clear cell histology (n=505) | Brain metastases (n=324) | |
| Number of evaluable patients | 3353 | 2343 | 1010 | 1030 | 2323 | 300 | 379 | 215 |
| Objective response, n (%) | 660 (16) | 444 (15) | 216 (16) | 195 (14) | 465 (16) | 32 (5) | 42 (8) | 30 (9) |
| Complete response, n (%) | 63 (1) | 34 (1) | 29 (2) | 8 (1) | 55 (2) | 1 (<1) | 4 (1) | 3 (1) |
| Partial response, n (%) | 597 (14) | 410 (14) | 187 (14) | 187 (13) | 410 (14) | 31 (5) | 38 (8) | 27 (8) |
| Stable disease ⩾3 months, n (%) | 1893 (45) | 1347 (46) | 546 (42) | 596 (43) | 1297 (46) | 149 (25) | 217 (43) | 107 (33) |
| Progressive disease or stable disease <3 months, n (%) | 800 (19) | 552 (19) | 248 (19) | 239 (17) | 561 (20) | 119 (20) | 120 (24) | 78 (24) |
| Clinical benefit,b n (%) | 2553 (61) | 1791 (62) | 762 (58) | 791 (57) | 1762 (62) | 181 (31) | 259 (51) | 137 (42) |
Abbreviations: ECOG PS=Eastern Cooperative Oncology Group performance status; RECIST=Response Evaluation Criteria in Solid Tumors.
Overall, 324 patients were excluded from the modified intent-to-treat population for objective response due to non-RECIST tumour assessments. A further 866 patients were included in the analysis but were not assessed (n=250), not evaluable (n=19), or had missing data (n=597).
Clinical benefit=objective response+stable disease for ⩾3 months.
Within the limits of the lack of strictly standardised criteria for the timing and methodology of assessment of disease status, in the evaluable population, median PFS was 9.4 months (95% CI: 8.8–10.0) and median OS was 18.7 months (95% CI: 17.5–19.5; Figure 1 and Table 4). More than 20% of patients were still alive at 5 years. Median survival times were unaffected by prior cytokine status and were also similar in elderly patients compared with younger patients; however, it may have been affected by inclusion of patients with brain metastases or non-clear cell RCC (Table 4). In patients with either RCC of non-clear cell histology, brain metastases, or ECOG performance status ⩾2, both PFS and OS were substantially shorter than in the overall population (Table 4). PFS and OS also varied in patients classified according to the modified MSKCC prognostic criteria. In the favourable, intermediate, and poor risk groups, respectively, median PFS was 15.0 months (95% CI: 13.8–16.3), 10.6 months (95% CI: 9.4–11.1), and 5.4 months (95% CI: 5.1–5.7), while median OS was 56.5 months (95% CI: 41.6 to not reached), 20.0 months (95% CI: 18.4–21.3), and 9.1 months (95% CI: 8.4–9.7). The efficacy of sunitinib was maintained with long-term follow-up and was not markedly different, when final data were compared with interim results reported in 2009 (Gore et al, 2009; data not shown).
Figure 1.
Kaplan–Meier estimates of (A) PFSa and (B) OS for the overall population. aIn the PFS plot, 324 modified intent-to-treat patients were excluded from the evaluable population due to non-RECIST tumour assessment.
Table 4. Summary of median progression-free survival and overall survival.
|
Prior cytokine treatment |
Patient subgroups |
|||||||
|---|---|---|---|---|---|---|---|---|
| All patients (N=4543) | Yes (n=3096) | No (n=1447) | Age ⩾65 years (n=1485) | Age <65 years (n=3058) | ECOG PS ⩾2 (n=634) | Non-clear cell histology (n=532) | Brain metastases (n=338) | |
| Included in PFS analysis,a n | 4219 | 2907 | 1312 | 1386 | 2833 | 587 | 505 | 324 |
| Median PFS (95% CI), months | 9.4 (8.8–10.0) | 9.3 (8.6–10.1) | 9.7 (8.4–10.8) | 10.1 (8.8–10.9) | 9.2 (8.5–9.8) | 3.5 (2.8–4.2) | 6.0 (5.4–7.0) | 5.3 (4.4–5.6) |
| Median OS (95% CI), months | 18.7 (17.5–19.5) | 18.4 (17.1–19.5) | 19.0 (17.2–21.0) | 18.1 (16.5–20.3) | 18.8 (17.4–19.8) | 5.7 (4.9–6.4) | 12.2 (10.2–14.2) | 8.2 (7.4–9.6) |
Abbreviations: CI=confidence interval; ECOG PS=Eastern Cooperative Oncology Group performance status; OS=overall survival; PFS=progression-free survival; RECIST=Response Evaluation Criteria in Solid Tumors.
Overall, 324 patients were excluded from the modified intent-to-treat population for PFS analysis because of non-RECIST tumour assessment. Survival events including all deaths were collected up to September 2008.
IMDC prognostic model
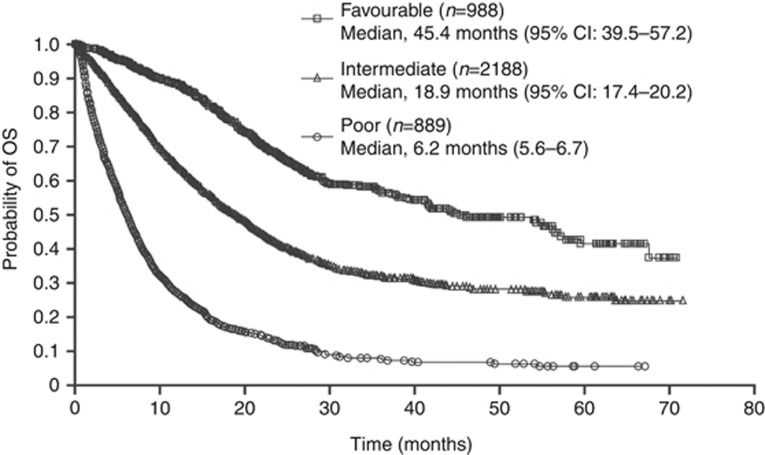
Data on the IMDC prognostic risk factors were available for a total of 4065 patients. In a multivariate Cox analysis of OS, all six factors (ECOG performance status >1, time from diagnosis to treatment <1 year, haemoglobin < LLN, calcium > ULN, neutrophil count > ULN, and platelet count > ULN) were significantly associated with reduced OS (each P<0.001; Table 5). OS was significantly different between patient subpopulations classified as having poor (n=889; median 6.2 months), intermediate (n=2188; median 18.9 months), and favourable risk (n=988; median 45.4 months), according to the IMDC model (Figure 2; for favourable vs intermediate or poor subgroups: HR: 0.3593, P<0.001; for poor vs favourable or intermediate subgroups: HR: 3.5153, P<0.001). Therefore, despite the inclusion of patients with brain metastases and non-clear cell RCC, this large sunitinib database validated external data for prognostic criteria according to the IMDC factors.
Table 5. Multivariate Cox analysis of overall survival using the IMDC prognostic factors.
| Parameter | Parameter estimate±s.e. | Hazard ratio | 95% CI | P-value |
|---|---|---|---|---|
| ECOG PS >1 | 0.79±0.05 | 2.20 | 1.98–2.44 | <0.0001 |
| Time from diagnosis to treatment <1 year | 0.28±0.04 | 1.32 | 1.21–1.44 | <0.0001 |
| Haemoglobin < LLN | 0.61±0.05 | 1.84 | 1.68–2.01 | <0.0001 |
| Calcium>ULN | 0.34±0.06 | 1.41 | 1.25–1.59 | <0.0001 |
| Neutrophil count > ULN | 0.71±0.05 | 2.03 | 1.83–2.25 | <0.0001 |
| Platelet count > ULN | 0.31±0.05 | 1.36 | 1.23–1.50 | <0.0001 |
Abbreviations: CI=confidence interval; ECOG PS=Eastern Cooperative Oncology Group performance status; IMDC=International Metastatic Renal-Cell Carcinoma Database Consortium; LLN=lower limit of normal; s.e.=standard error; ULN=upper limit of normal.
Figure 2.
Kaplan–Meier estimates of OS for the overall population, according to prognostic risk group based on the IMDC model (Heng et al, 2009, 2013). N=4065. a aIn total, 478 patients were excluded due to missing data for one or more risk factors.
Discussion
The final analysis of this global, expanded-access trial of sunitinib in advanced mRCC confirms the efficacy and safety of this agent in >4500 patients in a real-world setting. Although the results presented here are limited by the nature of the expanded-access study design, these findings are particularly valuable due to the large patient population. Combined with the extended duration of patient follow-up, this provides a good opportunity to evaluate sunitinib toxicity and long-term side effects. The sunitinib safety profile described here was consistent with the approved labelling for sunitinib (SUTENT (sunitinib malate) prescribing information) and provides an update to the preliminary findings from the interim report of data from this trial (Gore et al, 2009), with the exception of an apparent increase in the frequency of hypothyroidism (11% vs 6%) reported as an AE, potentially due to an increased awareness of this AE over time. The incidence of many commonly reported AEs also increased slightly, which is not unexpected given the increased treatment exposure (6 vs 5 cycles, respectively) and longer median follow-up (13.6 vs 11.6 months, respectively) in this final analysis. Low rates of all-grade, treatment-related cardiac events (6%) were observed. Efficacy outcomes were consistent with previously published findings from registration-directed phase II and III clinical trials (Motzer et al, 2006a, b, 2007, 2009). In those studies, the ORR was higher at 30–40%, compared with 16% here, in which tumour assessments were done as per the local standard of care for RCC. However, median PFS was 11 months in the first-line setting and 8.3 and 8.7 months in the cytokine-refractory setting, compared with 9.4 months here. OS was 26.4 months and 16.4 months in the first-line and cytokine-refractory settings, respectively, compared with our finding of 18.7 months. Notably, >20% of patients in the current study survived for 5 years or longer. In addition to subgroups of interest included in the expanded-access trial (notably patients with ECOG performance status ⩾2 or brain metastases), 68% of patients had received prior cytokine therapy and 10% had been previously treated with antiangiogenic agents. All of these factors might be expected to negatively impact on PFS and OS in the overall study population. In this context, however, it is worth emphasising that efficacy was in fact comparable in patients regardless of prior cytokine treatment.
The efficacy of sunitinib in patients with brain metastases, poor performance status, and non-clear cell RCC compares favourably with historical data for these subgroups. The safety profile of sunitinib in these three subgroups was similar to that overall, although the incidence of treatment-related AEs was consistently lower in the poor performance status and brain metastases subgroups. This may be due to shorter drug exposure; for example, patients with brain metastases received a median three cycles (range: 1–50), compared with six cycles (range: 1–57) in the overall population. Safety and efficacy in the fourth subgroup analysed, comprising elderly patients aged ⩾65 years, were broadly similar to that observed in younger patients (<65 years). This is an important finding, as this age group encompasses 64% of newly diagnosed RCC cases and accounts for >22% of deaths from this disease (Howlader et al, 2012). The observed activity of sunitinib in the elderly has been confirmed in an extensive retrospective analysis of data pooled from 1059 patients in six clinical trials of sunitinib as first-line or cytokine-refractory treatment for advanced RCC, which found no difference in efficacy between patients aged ⩾70 years and <70 years (Hutson et al, 2014). In the same analysis, although most treatment-emergent AEs occurred at similar rates in each age group, some were significantly more common in older vs younger patients, including fatigue, cough, peripheral oedema, anaemia, decreased appetite, and thrombocytopenia (all P<0.05). Despite these observations, advanced age alone is not a reason to withhold sunitinib treatment for advanced RCC, as older patients derive a similar efficacy benefit as younger ones.
Antitumour activity has also been observed in patients who received sunitinib with a primary tumour in place. In an interim analysis of data from this study, the safety profile of sunitinib was similar in patients with a prior nephrectomy and those without a prior nephrectomy (Szczylik et al, 2008). However, efficacy outcomes were more favourable in patients with a prior nephrectomy than in those without (e.g. median PFS, 12.0 vs 6.5 months, respectively; P=0.0021), thereby indirectly supporting the value of cytoreductive nephrectomy in the era of targeted agents, as also recently reported elsewhere (Aizer et al, 2014; Heng et al, 2014).
In an exploratory analysis, we found a strong association between OS and the IMDC prognostic factors (Heng et al, 2009, 2013), validating this model in >4000 patients receiving treatment in the tyrosine kinase inhibitor era. (In the original Heng et al. (2009), median OS was not reached in the favourable risk group; however, the 2-year OS rate was 75%, which is similar to the results in this study.) Numerous prognostic models for RCC have been developed over the past decades, in parallel with new treatment options for this disease. To our knowledge, the data reported here represent the largest contemporary patient population evaluated to date using an RCC prognostic model. That said, given the nature of our study, the characteristics of these patients, especially those in the intermediate and poor risk groups, may not be entirely equivalent to those of patients in the original Heng et al. (or MSKCC) model (Motzer et al, 2002; Heng et al, 2009).
The landscape for managing advanced RCC is rapidly changing, with a range of targeted agents now approved for the treatment of this disease. Despite limitations such as lack of a comparator and lack of independent review of tumour response, expanded-access trials are a practical way of offering a particular treatment to registration-directed, trial-ineligible patients and of gathering data about its activity in a real-world setting of very diverse patients. To date, the sunitinib expanded-access trial is the largest reported of its kind globally in the mRCC population. Two expanded-access trials have also been conducted with sorafenib in patients with advanced RCC, one based in the United States (n=2504) and one in Europe (n=1150; Stadler et al, 2010; Beck et al, 2011). The European trial limited patients to those in whom at least one previous line of systemic therapy had failed, or who were unable to tolerate cytokine therapy, whereas the US trial included treatment-naive patients. In each case, the safety and efficacy of sorafenib was consistent with that reported for the pivotal phase III trial (Escudier et al, 2007), and activity was seen in subgroups of interest similar to those included in the sunitinib expanded-access trial. Likewise, no new safety issues were identified in an international expanded-access programme of everolimus in a restricted population of patients with mRCC after failure of initial VEGFR-directed therapy (n=1367) (Grünwald et al, 2012). In this trial, median treatment duration was only 14 weeks, which may reflect the treatment setting, but which also means that safety and efficacy data gathered were limited in comparison with the sunitinib trial.
This expanded-access trial in mRCC has considerably extended our knowledge of the efficacy and safety of sunitinib in a real-world setting, and enabled over 4500 patients with wide-ranging disease states to receive sunitinib treatment. The sunitinib safety profile was consistent with prior reports, no unexpected long-term AEs were reported, and clinical benefit was seen in both treatment-naive and previously treated patients, in older as well as younger patients, and in those with traditionally poor prognosis.
Acknowledgments
We thank all of the participating patients and their families, as well as the global network of investigators, research nurses, study coordinators, and operations staff. MEG is funded by the NHIR Biomedical Research Centre at the Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, London, UK. This trial was sponsored by Pfizer Inc. Medical writing support was provided by Andy Gannon and Jean Scott at ACUMED part of the KnowledgePoint360 Group, an Ashfield company (New York, NY, USA), with funding from Pfizer Inc.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
Prior presentations: Gore ME, Bukowski R, Porta C et al. Sunitinib global expanded-access trial in metastatic renal cell carcinoma (mRCC): final results. Presented at the European Society for Medical Oncology Congress, Vienna, Austria, 28 September 2012 to 2 October 2012. Gore ME, Porta C, Bracarda S et al. Updated safety and efficacy results for sunitinib from a global, expanded-access trial in metastatic renal cell carcinoma. Presented at the 2011 European Multidisciplinary Cancer Congress, Stockholm, Sweden, 23–27 September 2011.
MEG reported receiving consultancy fees from Pfizer and Astellas, and honoraria from Roche, Pfizer, Novartis, and Bristol-Myers Squibb. CS reported receiving consultancy fees from Pfizer, Bayer, GlaxoSmithKline, and Wyeth Pharmaceuticals. CP reported receiving consultancy fees from Pfizer, Bayer Schering Pharma, GlaxoSmithKline, Novartis, Boehringer-Ingelheim, and AVEO/Astellas, honoraria from Pfizer, Bayer Schering Pharma, GlaxoSmithKline, Novartis, and Astellas, and research funding from Pfizer, Bayer Schering Pharma, and Novartis. SB reported receiving consultancy fees from Pfizer, Bayer Schering Pharma, GlaxoSmithKline, Novartis, Boehringer-Ingelheim, AVEO/Astellas, and Genentech, and honoraria from Novartis, Pfizer, Bayer Schering Pharma, and GlaxoSmithKline. GAB reported receiving consultancy fees and honoraria from Pfizer, Novartis, and GlaxoSmithKline, and research funding from Pfizer. SO reported receiving consultancy fees and honoraria from Pfizer, Novartis, GlaxoSmithKline, Bayer Schering Pharma, and Boehringer-Ingelheim. S-HL reported receiving consultancy fees from Pfizer, Novartis, Bayer, and Roche, honoraria from Pfizer, Novartis, GlaxoSmithKline, and Bayer, and research funding from Pfizer, AstraZeneca, and Novartis. JH reported receiving consultancy fees from Pfizer. EV reported receiving consultancy fees and honoraria from Pfizer, Novartis, GlaxoSmithKline, and Roche, and research funding from Pfizer. PS reported receiving consultancy fees and honoraria from Pfizer. PM reported receiving consultancy fees from Janssen. REH reported receiving honoraria from Pfizer, Novartis, GlaxoSmithKline, and Bristol-Myers Squibb, and research funding from Pfizer, Novartis, and GlaxoSmithKline. GC reported receiving honoraria and research funding from Pfizer, Novartis, and GlaxoSmithKline. WEEE reported receiving consultancy fees and honoraria from Pfizer, Novartis, GlaxoSmithKline, Roche, Bayer, Boehringer Ingelheim, Astellas, and Bristol-Myers Squibb. KZ, KF, EM, MJL, and SH are full-time employees of Pfizer and hold Pfizer stock. RB reported receiving consultancy fees from Pfizer, Novartis, GlaxoSmithKline, Genentech, and Exelixis, honoraria from Pfizer, Novartis, GlaxoSmithKline, Bayer, and Genentech, and expert testimony fees from Pfizer and Novartis. DC, TMK, and LC declared no conflict of interest.
Supplementary Material
References
- Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM (2003) SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther 2: 471–478. [PubMed] [Google Scholar]
- Aizer AA, Urun Y, McKay RR, Kibel AS, Nguyen PL, Choueiri TK (2014) Cytoreductive nephrectomy in patients with metastatic non-clear-cell renal cell carcinoma (RCC). BJU Int 113: E67–E74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J, Procopio G, Bajetta E, Keilholz U, Negrier S, Szczylik C, Bokemeyer C, Bracarda S, Richel DJ, Staehler M, Strauss UP, Mersmann S, Burock K, Escudier B (2011) Final results of the European Advanced Renal Cell Carcinoma Sorafenib (EU-ARCCS) expanded-access study: a large open-label study in diverse community settings. Ann Oncol 22: 1812–1823. [DOI] [PubMed] [Google Scholar]
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM on behalf of the TARGET Study Group (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356: 125–134. [DOI] [PubMed] [Google Scholar]
- Cancer Therapy Evaluation Program (2006) Common Terminology Criteria for Adverse Events (CTCAE). Version 3.0, DCTD, NCI, NIH, DHHS. 9 August 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf (accessed 28 May 2015).
- Gore ME, Szcylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S, Hariharan S, Lee SH, Haanen J, Castellano D, Vrdoljak E, Schöffski P, Mainwaring P, Nieto A, Yuan J, Bukowski R (2009) Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol 10: 757–763. [DOI] [PubMed] [Google Scholar]
- Grünwald V, Karakiewicz PI, Bavbek SE, Miller K, Machiels JP, Lee SH, Larkin J, Bono P, Rha SY, Castellano D, Blank CU, Knox JJ, Hawkins R, Anak O, Rosamilia M, Booth J, Pirotta N, Bodrogi I on behalf of the REACT Study Group (2012) An international expanded-access programme of everolimus: addressing safety and efficacy in patients with metastatic renal cell carcinoma who progress after initial vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapy. Eur J Cancer 48: 324–332. [DOI] [PubMed] [Google Scholar]
- Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, Mackenzie M, Wood L, Donskov F, Tan MH, Rha SY, Agarwal N, Kollmannsberger C, Rini BI, Choueiri TK (2013) External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol 14: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, Venner P, Knox JJ, Chi KN, Kollmannsberger C, McDermott DF, Oh WK, Atkins MB, Bukowski RM, Rini BI, Choueiri TK (2009) Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 27: 5794–5799. [DOI] [PubMed] [Google Scholar]
- Heng DY, Wells JC, Rini BI, Beuselinck B, Lee JL, Knox JJ, Bjarnason GA, Pal SK, Kollmannsberger CK, Yuasa T, Srinivas S, Donskov F, Bamias A, Wood LA, Ernst DS, Agarwal N, Vaishampayan UN, Rha SY, Kim JJ, Choueiri TK (2014) Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol 66(4): 704–710. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) (2012) SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations). National Cancer Institute: Bethesda, MD, USA. http://seer.cancer.gov/csr/1975_2009_pops09/. [Google Scholar]
- Hutson TE, Bukowski RM, Rini BI, Gore ME, Larkin JMG, Figlin RA, Barrios CH, Escudier B, Lin X, Fly K, Martell B, Matczak E, Motzer RJ (2014) Efficacy and safety of sunitinib in elderly patients with metastatic renal cell carcinoma. Br J Cancer 110: 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, Murray LJ, Carver J, Chan E, Moss KG, Haznedar JO, Sukbuntherng J, Blake RA, Sun L, Tang C, Miller T, Shirazian S, McMahon G, Cherrington JM (2003) In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 9: 327–337. [PubMed] [Google Scholar]
- Motzer RJ, Bacik J, Mazumdar M (2004) Prognostic factors for survival of patients with stage IV renal cell carcinoma: Memorial Sloan-Kettering Cancer Center experience. Clin Cancer Res 10: 6302S–6303S. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M (2002) Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 20: 289–296. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, Garcia-del-Muro X, Sosman JA, Solska E, Wilding G, Thompson JA, Kim ST, Chen I, Huang X, Figlin RA (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27: 3584–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356: 115–124. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE, Li JZ, Bello CL, Theuer CP, George DJ, Rini BI (2006. a) Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 24: 16–24. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Merchan JR, Wilding G, Ginsberg MS, Bacik J, Kim ST, Baum CM, Michaelson MD (2006. b) Sunitinib in patients with metastatic renal cell carcinoma. JAMA 295: 2516–2524. [DOI] [PubMed] [Google Scholar]
- O'Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, Wong LM, Hong W, Lee LB, Town A, Smolich BD, Manning WC, Murray LJ, Heinrich MC, Cherrington JM (2003) SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood 101: 3597–3605. [DOI] [PubMed] [Google Scholar]
- Stadler WM, Figlin RA, McDermott DF, Dutcher JP, Knox JJ, Miller WH Jr, Hainsworth JD, Henderson CA, George JR, Hajdenberg J, Kindwall-Keller TL, Ernstoff MS, Drabkin HA, Curti BD, Chu L, Ryan CW, Hotte SJ, Xia C, Cupit L, Bukowski RM on behalf of the ARCCS Study Investigators (2010) Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer 116: 1272–1280. [DOI] [PubMed] [Google Scholar]
- Szczylik C, Porta C, Bracarda S, Hawkins R, Bjarnason GA, Oudard S, Lee S, Carteni G, Hariharan S, Gore ME (2008) Sunitinib in patients with or without prior nephrectomy in an expanded access trial of metastatic renal cell carcinoma (mRCC). J Clin Oncol 26(Suppl 15S): Abstract 5124. [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.