Abstract
Background
The purpose of this study was to examine whether short-term ingestion of a powdered tart cherry supplement prior to and following intense resistance-exercise attenuates muscle soreness and recovery strength loss, while reducing markers of muscle damage, inflammation, and oxidative stress.
Methods
Twenty-three healthy, resistance-trained men (20.9 ± 2.6 yr, 14.2 ± 5.4 % body fat, 63.9 ± 8.6 kg FFM) were matched based on relative maximal back squat strength, age, body weight, and fat free mass. Subjects were randomly assigned to ingest, in a double blind manner, capsules containing a placebo (P, n = 12) or powdered tart cherries [CherryPURE®] (TC, n = 11). Participants supplemented one time daily (480 mg/d) for 10-d including day of exercise up to 48-h post-exercise. Subjects performed ten sets of ten repetitions at 70 % of a 1-RM back squat exercise. Fasting blood samples, isokinetic MVCs, and quadriceps muscle soreness ratings were taken pre-lift, 60-min, 24-h, and 48-h post-lift and analyzed by MANOVA with repeated measures.
Results
Muscle soreness perception in the vastus medialis (¼) (p = 0.10) and the vastus lateralis (¼) (p = 0.024) was lower in TC over time compared to P. Compared to pre-lift, TC vastus medialis (¼) soreness was significantly attenuated up to 48-h post-lift with vastus lateralis (¼) soreness significantly lower at 24-h post-lift compared to P. TC changes in serum creatinine (p = 0.03, delta p = 0.024) and total protein (p = 0.018, delta p = 0.006) were lower over time and smaller from pre-lift levels over time compared to P Significant TC group reductions from pre-lift levels were found for AST and creatinine 48-h post-lift, bilirubin and ALT 60-min and 48-h post-lift. No significant supplementation effects were observed for serum inflammatory or anti-inflammatory markers. None of the free radical production, lipid peroxidation, or antioxidant capacity markers (NT, TBARS, TAS, SOD) demonstrated significant changes with supplementation. Changes in TC whole blood lymphocyte counts (p = 0.013) from pre-lift were greater compared to P, but TC lymphocyte counts returned to pre-lift values quicker than P.
Conclusion
Short-term supplementation of Montmorency powdered tart cherries surrounding a single bout of resistance exercise, appears to be an effective dietary supplement to attenuate muscle soreness, strength decrement during recovery, and markers of muscle catabolism in resistance trained individuals.
Keywords: Tart cherry, Resistance exercise, Recovery, Antioxidants, Anti-inflammatory, Muscle damage
Background
A large volume, high intensity strength training workout activates a load-induced stress response characterized by structural muscle damage, oxidative stress, and inflammation that facilitates the release of intramuscular proteins into systemic circulation that are usually associated with cardiovascular dysfunction, invasive surgery, and disease [1–4]. The repetitive nature of muscle contractions during bouts of high intensity exercise will induce muscular injury as a result of ultrastructural disruptions [3, 5–7] that ultimately lead to a muscular repair sequence of events: degeneration, inflammation, regeneration, and fibrosis [3, 8]. Muscle soreness following exercise is not the direct result of inflammation, but rather a product of high nociceptor and mechanoreceptor sensitivity to the potent chemicals and by-products released during muscular degeneration [5, 9, 10]. Millions of people, including athletes, use non-steroidal anti-inflammatory drugs (NSAIDs) to help reduce pain and inflammation [3, 5]. NSAID use mitigates the inflammatory response via non-specific inhibition of the cyclooxygenase (COX-1 and COX-2) enzymes that regulate production of inflammatory-stimulating prostaglandins [3, 5, 11]. However, NSAIDs remain controversial as some research has demonstrated that muscle protein synthesis and the function of satellite cells in skeletal muscle hypertrophy are compromised when COX enzymes are inhibited [12–16], while other studies have found no NSAID effect on post-exercise anabolic processes within muscle [17–19].
Due to the debate surrounding the use of NSAIDs in sport and exercise applications, performance nutrition research has more recently shifted focus toward phytochemical-containing fruits and other functional foods that seem to provide a beneficial anti-inflammatory and antioxidant effect [20, 21]. A wide variety of antioxidant and polyphenol-containing functional foods such as purple sweet potatoes [22–24], beet root juice [25–27], cranberries [28, 29], and blueberries [30, 31] have verified health, performance-enhancing, and exercise recovery benefits. However, compared to other functional foods, the high anthocyanin content of both tart (e.g. Montmorency cherries) and sweet (e.g. Bing cherries) [21, 32–34] have proven beneficial in health [35–38], inflammatory-related disease states (e.g. cardiovascular disease, diabetes, osteoarthritis, gout) [35, 36, 38–40], and sleep quality [41, 42]. Clinical supplementation success with cherries, particularly tart cherry whole fruit, concentrates and cultivar juice blends, spurred an increase in exercise-based research to prove beneficial effects in mitigating muscle damage, oxidative stress, inflammation, and muscle pain with an impetus to increase performance [21, 43, 44].
We are aware of only two studies that have evaluated the effects of tart cherry supplementation on responses to resistance-based exercise. The first study examined 8-d of tart cherry cultivar-blended juice supplementation on exercise-induced muscle pain surrounding a bout of 40 maximal eccentric elbow flexion contractions (2 sets × 20 repetitions) [20]. As a result of tart cherry supplementation, strength losses and exercise-induced muscle pain were attenuated compared to a placebo over 4-d of maximal eccentric elbow flexion exercise [20]. No hematological analysis was performed to decipher potential physiological effects of supplementation. A second study examined the effects of 10-d tart cherry concentrate supplementation on physiological markers of muscle damage, oxidative stress, inflammation, muscle function, and muscle pain surrounding an intensive single-leg 100 repetition knee extensor training session (10 sets × 10 repetitions) [4]. Similar to the first study, functional recovery of isometric muscle strength was greater as a result of tart cherry supplementation compared to placebo [4]. Hematological analysis demonstrated reduced oxidative stress following the leg extension protocol that supported the beneficial functional recovery results in the tart cherry trial [4]. Interestingly, a recent endurance-based study with blended Montmorency tart cherry juice supplementation evaluating stress, inflammation, and upper respiratory symptoms following a marathon run demonstrated an attenuation of post-race incidence and severity of upper respiratory tract symptoms and inflammation (serum CRP) [45]. The primary aim of the current study was to determine if this powdered supplement derived from tart cherry skins would promote similar attenuation of muscle soreness, strength losses, and oxidative damage as the tart cherry cultivar-blended juice and juice concentrate supplemented in the two previous resistance exercise studies. The secondary objective of this study was to determine whether short-term (10-d) ingestion of the powdered tart cherry formulation prior to and following a lower body strength exercise protocol of high volume and intensity will effectively reduce markers of muscle damage, muscle soreness, inflammation, oxidative stress, and attenuate strength losses during subsequent exercise performance.
Methods
Subjects
Twenty-three healthy, resistance-trained males (20.9 ± 2.6 yr, 81.7 ± 10.3 kg 14.2 ± 5.4 % body fat, 63.9 ± 8.6 kg FFM) participated as subjects in this study. Subjects were recruited through paper and electronically distributed study advertisements at Texas A&M University. Entrance criteria required subjects to have been involved in a progressive resistance training program that included regular squat exercise for at least 6 months prior to study recruitment and be able to perform a standard barbell back squat in a Smith machine rack of at least 1.5 times their body weight. Proper performance of barbell back squat technique was determined by a National Strength and Conditioning Association (NSCA) Certified Strength and Conditioning Coach (CSCS) during baseline testing. Figure 1 demonstrates the breakdown of the subject population as it pertains to the study progression. Discontinuation of any subject participation was not related to any aspect of the supplementation or testing protocol.
Fig. 1.
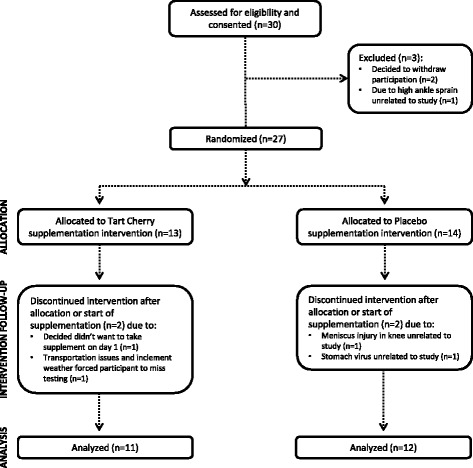
Consort diagram breakdown of the subject population as it pertains to the various stages of study progression
All subjects signed informed consent documents and the study was approved by the Texas A&M University Institutional Review Board prior to any data collection. Subjects were not allowed to participate in this study if they reported any of the following: 1) any metabolic disorders or taking any thyroid, hyperlipidemic, hypoglycemic, anti-hypertensive, anti-inflammatory (e.g. NSAIDs), and/or androgenic medications; 2) history of hypertension, hepatorenal, musculoskeletal, autoimmune, and/or neurological disease(s); 3) allergy to cherries or any cherry components (e.g. polyphenols, anthocyanins, anthocyanidins).
Experimental design
Figure 2 shows the experimental design used in this study. The study was conducted in a randomized, double-blind, and placebo-controlled manner. All subjects eligible to participate in the study completed a morning familiarization (FAM) session where they were provided detailed information regarding the study design, testing procedures, and supplementation protocols. Informed consent, medical history, and endurance training history questionnaires were also completed during the FAM session. A research nurse reviewed medical history documents and performed a physical exam on each subject to ensure safety and participation eligibility. A fasting blood sample was taken at the end of the FAM session if the subject met entrance criteria. Subjects were asked to not change their dietary habits in any way throughout the study (upon start of supplementation). This was monitored by subject documentation of dietary intake for 4-d (3 weekdays and 1 weekend day) of the first seven supplementation days prior to the resistance exercise challenge. Approximately 10-d prior to the resistance exercise intervention, eligible subjects returned to the lab for a morning baseline testing session to determine body weight, height, and body composition. The subjects then were familiarized with the maximal voluntary contraction (MVC) protocol using their dominant leg on an isokinetic knee extension dynamometer followed by a 5-min recovery before determination of their barbell back squat 1-repetition maximum (1-RM) in a Smith machine rack. Following baseline measurements, subjects were matched based on relative maximal back squat strength, fat free mass, body weight, and age accompanied by a random separation into two groups: 1) a placebo group or 2) a powdered tart cherry group.
Fig. 2.
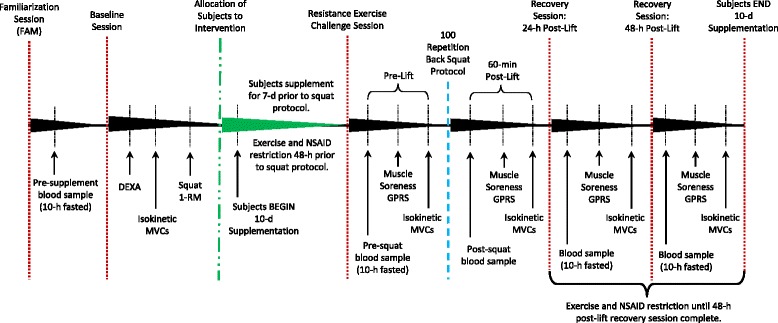
Experimental study design. DEXA = dual-energy X-Ray absorptiometer, MVC = maximal voluntary contraction, 1-RM = 1-repetition maximum, NSAID = non-steroidal anti-inflammatory drugs, GPRS = graphic pain rating scale, 7-d = 7-day, 48-h = 48-hour
Subjects were instructed to begin supplementation 7-d prior to the resistance exercise challenge (Day 0). Subjects were asked to fast overnight for 10-h to account for diurnal variation as well as refrain from exercise and consumption of NSAIDs for 48-h prior to all testing days. Due to the large variety of NSAIDs commonly available with half-life data ranging from approximately 1.6 to 30-h [46], the 48-h restriction was implemented to help reduce any potential concurrent effects. Exercise logs were not implemented to track subject activity patterns over the study protocol. On the day of the resistance exercise challenge, subjects reported to the lab where body weight, resting heart rate, and resting blood pressure were measured. Subjects then donated a fasting venous blood sample (approximately 20 ml) using standard clinical procedures and rated perceptions of muscle soreness to a standardized application of pressure on their dominant thigh at three designed locations using a graphic pain rating scale (GPRS). The subjects then completed the MVC protocol using their dominant leg on an isokinetic knee extension dynamometer followed by a 5-min recovery before the barbell back squat protocol utilizing a Smith machine rack was completed with a Tendo power and speed analyzer attached to the bar. Fasting (except 60-min post-lift) blood samples, GPRS ratings of quadriceps muscle soreness, and the isokinetic leg extension MVC protocol were completed at 60-min, 24-h and 48-h of post-lift recovery. The last day of supplementation correlated with 48-h post-lift recovery.
Exercise protocol
Isokinetic knee extension maximal voluntary contractions
At baseline, before the squat exercise, 60-min after the squat exercise, 24-h and 48-h post-lift exercise, subjects performed an isokinetic knee extension MVC test using their dominant leg on a Kin-Kom 125AP Isokinetic Dynamometer (Chattanooga-DJO Global Inc., Vista, CA, USA). During the baseline familiarization of the isokinetic leg extension MVC protocol, standard procedures were used to determine and record proper subject positioning on the Kin-Kom to ensure accurate repeatability of the testing protocol across all five sessions. The subject warmed-up on the isokinetic dynamometer by performing three-sets of five repetitions of dominant knee extension/flexion at 50 % of their MVC (determined during the FAM) with 1-min recovery between sets. After the 1-min recovery following the third warm-up set, the subject performed 3 MVCs in succession with no rest between contractions. Subjects were allotted a 5-min recovery between the isokinetic MVC and barbell back squat protocols. Test to test variability of performing this test yielded average CV values of ±8.47 % with a test retest correlation of r = 0.88.
Barbell back squat 1-repetition maximum determination
At baseline, following the 5-min recovery after the isokinetic MVCs, subjects performed three-sets of five repetitions of a barbell back squat on a Smith machine rack (Life Fitness, Schiller Park, IL, USA, model SSM) at 50 % of their anticipated 1-RM with 2-min recovery between sets. Following the warm-up protocol, the barbell back squat 1-RM was determined on the Smith machine rack using a standard 1-RM protocol defined by the NSCA with 2-min recovery periods between 1- repetition sets of increasing weight. All 1-RM determinations were made within 3–5 sets beyond the warm-up. In order to successfully complete each set, the subject was required to reach thigh parallel depth as determined by a NSCA Certified Strength and Conditioning Coach (CSCS) providing a verbal “up” cue during spotting.
Barbell back squat exercise protocol
On the day of the resistance exercise challenge, following the 5-min recovery after the isokinetic MVCs, subjects performed one warm-up set of ten repetitions of the barbell back squat at 50 % 1-RM (determined during baseline). After 3-min recovery, the subjects performed ten sets of ten repetitions of a barbell back squat at 70 % 1-RM with 3-min recovery between sets. If necessary, the barbell back squat resistance was adjusted such that each subject completed the 100 repetition (10 sets × 10 repetitions) volume to thigh parallel depth, keeping the training volume consistent across the study population. This protocol is closely replicated after the repeated leg extension exercise implemented previously by Bowtell et al. [4]. A Tendo Power and Speed Analyzer (TENDO Sports Machines, Trencin, Slovak Republic, model PSA310) was attached to the bar during all 100 repetitions completed during the squat protocol to determine peak power, average force, and total work for each repetition. Following the squat protocol, subjects were only permitted to stretch with limited ambulation until the 60-min post-lift testing protocol was completed.
Supplementation protocol
Subjects were assigned in a double-blinded and randomized manner to ingest either a rice flour placebo (P, n = 12) or powdered tart cherry (TC, n = 11). Subjects were matched into one of the two groups according to relative maximal back squat strength, fat free mass, body weight, and age. Subjects were instructed to ingest 480 mg of the supplements with breakfast at 0800 7-d prior to, the day of, and 2-d following the resistance exercise challenge for a total supplementation timeline of 10-d. The tart cherry supplements contained 480 mg of freeze dried Montmorency tart cherry skin powder derived from tart cherry skins obtained after juicing (CherryPURE™ Freeze Dried Tart Cherry Powder, Shoreline Fruit, LLC, Transverse City, MI, USA). Prior analytical testing conducted in 2012 by Atlas Bioscience (Tuscon, AZ, USA) demonstrated that 290 mg of CherryPURE™ provides approximately 600 mg of phenolic compounds and 40 mg of anthocyanins, which is equivalent to consuming 10.5 fluid ounces of tart cherry juice. The supplements were prepared for distribution by Shoreline Fruit, LLC and sent to Advanced Laboratories (Salt Lake City, UT, USA) to quantify total phenolic compound and anthocyanin content of the powdered tart cherry supplements. Supplements were prepared in standardized color capsules and packaged in generic bottles by Shoreline Fruit, LLC for double blind administration.
Procedures
Dietary inventories
Within the first 7-d of supplementation, subjects were instructed to record all food and fluid intake over a 4-d period (3 weekdays, 1 weekend day), which was reflective of their normal dietary intake. Dietary inventories were then reviewed by a registered dietician and analyzed for average energy, macronutrient, and dietary antioxidant intake using ESHA Food Processor (Version 8.6) Nutritional Analysis software (ESHA Research Inc., Salem, OR, USA).
Anthropometrics and body composition
At the beginning of every testing session, subjects had their height and body mass measured according to standard procedures using a Healthometer Professional 500KL (Pelstar LLC, Alsip, IL, USA) self-calibrating digital scale with an accuracy of ± 0.02 kg. Whole body bone density and body composition measures (excluding cranium) were determined with a Hologic Discovery W Dual-Energy X-ray Absorptiometer (DEXA; Hologic Inc., Waltham, MA, USA) equipped with APEX Software (APEX Corporation Software, Pittsburg, PA, USA) by using procedures previously described [47, 48]. Mean test-retest reliability studies performed on male athletes in our lab with this DEXA machine have revealed mean coefficients of variation for total bone mineral content and total fat free/soft tissue mass of 0.31–0.45 % with a mean intraclass correlation of 0.985 [49]. On the day of each test, the equipment was calibrated following the manufacturer’s guidelines.
Muscle soreness perception measurement
Pressure application to the three specified areas of the quadriceps muscle group on the subject’s dominant leg was standardized to 50 N of pressure using a handheld Commander Algometer (JTECH Medical, Salt Lake City, UT, USA). Force was applied to each site of muscle contact with a cylindrical metal probe through a 1 cm diameter head. The standard amount of pressure was applied to the vastus lateralis (VL) at both 25 and 50 % of the distance between the superior border of the patella and the greater trochanter of the femur at the hip and to the vastus medalis (VM) at 25 % of the distance between the aforementioned landmarks. These three specific locations were measured and marked with a permanent marker on each subject during the initial muscle soreness perception measurement before the resistance exercise challenge. The participants were asked to maintain these three marked locations between testing sessions to avoid error with secondary measurement. The subject was seated in a reclined supine position and given the GPRS sheet to evaluate the perception of muscle soreness at each of the three quadriceps locations. The order of pressure application was standardized across all sessions and participants: 25 % VM, 25 % VL, and 50 % VL. The 50 N of pressure was applied to a relaxed quadriceps at each of the three locations using the algometer for a period of 3-sec to give the subject enough time to record their soreness evaluation on the GPRS. Perceptions of muscle soreness were recorded by measuring the distance (centimeters) of the participant mark on the GPRS from 0 cm (no pain). Test-to-test reliability for this protocol revealed a mean intraclass correlation of 0.90.
Blood collection
Subjects were required to fast (except 60-min post-lift) for 10-h prior to donating approximately four teaspoons (20 mL) of venous blood from an antecubital vein using standard phlebotomy procedures at each of the testing sessions. Blood analyzed for markers of muscle damage, oxidative stress, inflammation, and clinical chemistry panels were collected in two 7.5 mL BD Vacutainer® serum separation tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), left at room temperature for 15-min, and then centrifuged at 3500 rpm for 10-min using a standard, refrigerated (4 °C) bench top Thermo Scientific Heraeus MegaFuge 40R Centrifuge (Thermo Electron North America LLC, West Palm Beach, FL, USA). Serum supernatant was removed and stored at −80 °C in polypropylene microcentrifuge tubes for later analysis. Blood was also collected in a single 3.5 mL BD Vacutainer® lavender top tube containing K2 EDTA (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), left at room temperature for 15-min, and refrigerated for approximately 3–4 h before subsequent complete blood count analysis.
Clinical chemistry analysis
Whole blood samples were analyzed for complete blood count with platelet differentials (hemoglobin, hematocrit, red blood cell counts (RBC), white blood cell counts (WBC), lymphocytes, granulocytes (GRAN), and mid-range absolute count (MID)) using an Abbott Cell Dyn 1800 (Abbott Laboratories, Abbott Park, IL, USA) automated hematology analyzer. Internal quality control for the Abbott Cell Dyn 1800 was performed using three levels of control fluids purchased from the manufacturer to calibrate acceptable standard deviation (SD) and coefficients of variation (CV) values for all aforementioned whole blood cell counts. Serum samples were analyzed using a Cobas c111 (Roche Diagnostics GmbH, Indianapolis, IN, USA) automated clinical chemistry analyzer that was calibrated and optimized according to manufacturer guidelines. This analyzer has been known to be highly valid and reliable in previously published reports [50]. Each serum sample was assayed for a standard partial metabolic panel [(aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin)] and clinical markers of protein and fatty acid metabolism [(uric acid, creatinine, blood urea nitrogen (BUN), total protein, and creatine kinase (CK)]. The internal quality control for the Cobas c111 was performed using two levels of control fluids purchased from the manufacturer to calibrate acceptable SD and CV values for all aforementioned assays. Samples were re-run if the observed values were outside control values and/or clinical norms according to standard procedures.
Markers of anabolic/catabolic hormone status
Serum samples were assayed using standard commercially available enzyme-linked immunosorbent assay kits (ELISAs) for cortisol and testosterone (ALPCO Diagnostics, Salem, NH, USA) hormone analysis. Serum concentrations were determined calorimetrically using a BioTek ELX-808 Ultramicroplate reader (BioTek Instruments Inc., Winooski, VT, USA) at an optical density of 450 nm against a known standard curve using manufacturer recommended procedures. Samples were run in duplicate according to standard procedures to ensure validity of measurement. Test to test variability of performing these assays yielded average CV values for the aforementioned markers of: CORT (±6.85 %), and TEST (±4.47 %) with a test retest correlation for the same markers of: CORT (r = 0.92), TEST (r = 0.98).
Markers of oxidative stress
Serum samples were assayed using standard commercially available ELISA kits for Superoxide Dismutase (SOD Activity Assay kit), Total Antioxidant Status (TAS, Antioxidant Assay kit), Thiobarbituric Acid Reactive Substance (TBARS, Malondialdehyde-MDA, TCA method kit) (Cayman Chemical Company, Ann Arbor, MI, USA), and Nitrotyrosine (ALPCO Diagnostics, Salem, NH, USA). Serum concentrations for SOD and Nitrotyrosine were determined calorimetrically using the previously stated instrumentation at the same optical density (450 nm) against a known standard curve using standard procedures. Serum concentrations for TAS were also determined calorimetrically using the aforementioned instrumentation at an optical density of 405 nm against a known standard curve using standard procedures. Lastly, serum concentrations for TBARS were also determined fluorometrically using a SpectraMax Gemini multimode plate reader (Molecular Devices LLC, Sunnyvale, CA, USA) at an excitation wavelength of 530 nm and an emission wavelength of 550 nm against a known standard curve using standard procedures. Samples were run in duplicate according to standard procedures to ensure validity of measurement. Test to test variability of performing these assays yielded average CV values for the aforementioned markers of: SOD (±8.35 %), TAS (±14.24 %), TBARS (±8.30 %), and NT (±10.03 %) with a test retest correlation for the same markers of: SOD (r = 0.83), TAS (r = 0.85), TBARS (r = 0.94), and NT (r = 0.99).
Cytokine/Chemokine markers of inflammation
Serum markers of inflammation [(interleukin-1β (IL-1β), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and granulocyte-macrophage colony-stimulating factor (GM-CSF)] were measured by using a commercially available Milliplex MAP 13-Plex Human High Sensitivity T-Cell Magnetic Bead Panel kit (EMD Millipore Corporation, St. Charles, MO, USA) that was optimized for human serum samples. A minimum of 100 positive beads for each cytokine/chemokine was acquired with a Luminex MagPix instrument (Luminex Corporation, Austin, TX, USA). This instrument has been proven to be highly valid and reliable in previously published reports across many disciplines [51–54]. Manufacturer supplied controls were used to monitor the coefficients of variation. Samples were run in duplicate according to standard procedures to ensure validity of measurement. Test to test variability of performing these assays yielded average CV values for the aforementioned markers of: IL-1β (±4.37 %), IL-2 (±5.52 %), IL-4 (±4.59 %), IL-5 (±4.30 %), IL-6 (±4.26 %), IL-7 (±5.05 %), IL-8 (±4.55 %), IL-10 (±5.05 %), IL-12p70 (±4.92 %), IL-13 (±4.99 %),TNF-α (±6.05 %), IFN-γ (±4.42 %), and GM-CSF (±5.22 %) with a test retest correlation for the same markers of: IL-1β (r = 0.98), IL-2 (r = 0.99), IL-4 (r = 0.99), IL-5 (r = 0.99), IL-6 (r = 0.99), IL-7 (r = 0.99), IL-8 (r = 0.99), IL-10 (r = 1.00), IL-12p70 (r = 1.00), IL-13 (r = 0.99), TNF-α (r = 0.95), IFN-γ (r = 1.00), and GM-CSF (r = 1.00).
Statistical analysis
Individual group and time data are presented throughout the text and in all tables as means (± SD), while group effects are presented as means (± SEM). All related variables were grouped and analyzed using repeated measures MANOVA in IBM SPSS Statistics Software version 22.0 for Windows (IBM Corporation, Armonk, NY, USA) to assess values observed and changes from pre-lift levels in response to the supplement administered. Post-hoc LSD pairwise comparisons were used to analyze any significance among groups where needed with Cohen’s d calculations employed to determine effect magnitude. Data was considered statistically significant when the probability of error was less than 0.05 and considered to be trending when the probability of error was less than 0.10. Statistical trends (p < 0.05 to p < 0.10) were noted as is common practice in studies with relatively small sample size [55].
Results
Subject baseline characteristics
A total of 23 healthy, resistance-trained men completed the protocol for this study. Participants were 20.9 ± 2.6 years, 81.7 ± 10.3 kg, 14.2 ± 5.4 % body fat, and 63.9 ± 8.6 kg fat free mass. Participant demographic data are presented in Table 1. One-way ANOVA revealed no significant differences (p > 0.05) in baseline demographic markers. No significant differences in back squat 1-RM (p = 0.70) and relative squat strength ratio (p = 0.85) were reported across groups, hence the groups were generally well matched. One-way ANOVA analysis also demonstrated no differences in total work performed (p = 0.79) across groups, indicating that similar work was performed during the exercise intervention and differences can likely be attributed to the nutritional intervention.
Table 1.
Demographics by Study Group
| Variable | Group | Mean | Group (SEM) | p-value |
|---|---|---|---|---|
| N | P | 12 | n/a | n/a |
| TC | 11 | n/a | ||
| Total | 23 | n/a | ||
| Age | P | 20.58±1.78 | 0.51 | 0.593 |
| TC | 21.18±3.34 | 1.01 | ||
| Total | 20.87±2.60 | 0.54 | ||
| Height (cm) | P | 177±5.28 | 1.52 | 0.651 |
| TC | 178±10.71 | 3.23 | ||
| Total | 177±8.17 | 1.70 | ||
| Weight (kg) | P | 82.62±7.39 | 2.13 | 0.659 |
| TC | 80.64±13.16 | 3.97 | ||
| Total | 81.67±10.35 | 2.16 | ||
| Baseline HR (bpm) | P | 61.17±10.40 | 3.00 | 0.195 |
| TC | 66.09±6.67 | 2.01 | ||
| Total | 63.52±8.98 | 1.87 | ||
| BMD (g/cm2) | P | 1.16±0.10 | 0.03 | 0.389 |
| TC | 1.12±0.14 | 0.04 | ||
| Total | 1.14±0.12 | 0.02 | ||
| LM (kg) | P | 61.41±4.87 | 1.41 | 0.970 |
| TC | 61.55±11.16 | 3.36 | ||
| Total | 61.48±8.27 | 1.73 | ||
| FFM (kg) | P | 63.83±5.06 | 1.46 | 0.981 |
| TC | 63.92±11.64 | 3.51 | ||
| Total | 63.87±8.62 | 1.80 | ||
| FM (kg) | P | 11.81±5.44 | 1.57 | 0.595 |
| TC | 14.10±13.58 | 4.09 | ||
| Total | 12.90±10.00 | 2.08 | ||
| Body Fat (%) | P | 15.31±6.27 | 1.81 | 0.338 |
| TC | 13.09±4.31 | 1.30 | ||
| Total | 14.25±5.42 | 1.13 | ||
| Back Squat 1-RM (lbs) | P | 318±62 | 18.03 | 0.696 |
| TC | 306±82 | 24.72 | ||
| Total | 313±71 | 14.81 | ||
| Relative Squat Strength Ratio | P | 1.75±0.27 | 0.08 | 0.855 |
| TC | 1.73±0.34 | 0.10 | ||
| Total | 1.74±0.30 | 0.06 | ||
| Workout Total Work (kJ) | P | 0.68±0.14 | 0.04 | 0.798 |
| TC | 0.70±0.23 | 0.07 | ||
| Total | 0.69±0.18 | 0.04 |
Mean data expressed as means ± SD
HR heart rate, BMD bone mineral density, LM lean mass, FFM free-fat mass, FM fat mass, 1-RM 1-Repetition maximum
Nutritional intake and compliance
Nutritional intake was monitored over a 4-d period within the first 7-d of supplementation. Relevant nutritional components analyzed are listed in Table 2. No statistically significant interactions were observed across groups with respect to dietary intake.
Table 2.
Dietary Analysis by Study Group
| Variable | Group | Mean | Group (SEM) | p-value |
|---|---|---|---|---|
| Average Daily Caloric Consumption (kcal) | P | 2617±721 | 208 | 0.605 |
| TC | 2455±753 | 227 | ||
| Total | 2540±724 | 151 | ||
| Dietary Protein (g) | P | 147.86±43.13 | 12.45 | 0.871 |
| TC | 153.06±100.39 | 30.27 | ||
| Total | 150.35±74.28 | 15.49 | ||
| Dietary Carbohydrates (g) | P | 244.47±62.58 | 18.07 | 0.460 |
| TC | 223.52±70.90 | 21.38 | ||
| Total | 234.45±66.01 | 13.76 | ||
| Dietary Fat (g) | P | 84.54±23.55 | 6.80 | 0.427 |
| TC | 95.23±38.55 | 11.62 | ||
| Total | 89.65±31.35 | 6.54 | ||
| Dietary Beta-Carotene (mcg) | P | 2954±3436 | 992 | 0.651 |
| TC | 2111±5260 | 1586 | ||
| Total | 2551±4320 | 901 | ||
| Dietary Vitamin C [Ascorbic Acid] (mg) | P | 80.79±65.99 | 19.05 | 0.199 |
| TC | 50.56±38.41 | 11.58 | ||
| Total | 66.33±55.55 | 11.58 | ||
| Dietary Vitamin E [Alpha-Tocopherol] (mg) | P | 5.91±6.04 | 1.74 | 0.910 |
| TC | 6.24±7.44 | 2.24 | ||
| Total | 6.07±6.59 | 1.37 |
Mean data expressed as means ± SD
Muscle soreness perception assessment
Table 3 presents perceptions of muscle soreness across the resistance exercise protocol. The overall MANOVA analysis revealed a significant overall Wilks’ Lambda time (p < 0.001) interaction and a non-significant overall group x time effect (p = 0.20). Perception of muscle soreness in all three muscle testing locations irrespective of group significantly increased over time, peaking 48-h post-lift, indicating the onset of muscle soreness as a result of the lifting protocol. A significant difference between groups over time was found in vastus lateralis (¼) soreness perception (p = 0.024) where ratings increased by 55–170 % from pre-lift values in P, but only 35–104 % in TC over the recovery. Post-hoc analysis indicated a significantly attenuated increase in muscle soreness perception 24-h post-lift for TC supplementing subjects compared to P (see Fig. 3). Effects of supplementation tended to be different in the perception of vastus medialis (¼) soreness (p = 0.10) with significantly lower muscle soreness in TC versus P up to 48-h post-lift (see Fig. 3). Soreness perception ratings in the vastus medialis (¼) increased by 28–83 % from pre-lift values in P, but ranged from a 6 % decrease to only a 58 % increase in TC over the recovery.
Table 3.
Quadriceps Muscle Soreness Perception
| Variable | Group | Pre-Lift | 60-min Post | 24-hr Post | 48-hr Post | Group Mean | p-value (GG) | p-value (WSC) |
|---|---|---|---|---|---|---|---|---|
| Algo I (cm) | P | 4.34±2.52 | 5.53±3.15 | 7.78±2.93 | 7.94±2.92 | 6.40±0.76 | G=0.139 | |
| TC | 3.80±2.86 | 3.59±2.76 | 5.50±2.68 | 5.99±3.59 | 4.72±0.79 | T<0.001* | T L <0.001* | |
| Time Mean | 4.07±0.56 | 4.56±0.62 | 6.64±0.59Ψ◊ | 6.96±0.68Ψ◊ | G X T=0.236 | G X T q =0.172 | ||
| Algo II (cm) | P | 2.44±1.60 | 3.76±2.24Ψ | 6.60±2.99^Ψ◊ | 6.20±3.64Ψ◊ | 4.75±0.69 | G=0.773 | |
| TC | 2.98±2.70 | 4.03±2.93Ψ | 4.76±3.03Ψ | 6.07±3.67Ψ◊# | 4.46±0.72 | T<0.001* | T L <0.001* | |
| Time Mean | 2.71±0.46 | 3.90±0.54Ψ | 5.68±0.63Ψ◊ | 6.14±0.76Ψ◊ | G X T=0.187 | G X T q =0.024* | ||
| Algo III (cm) | P | 3.25±2.65 | 3.42±3.24 | 6.55±3.80 | 6.18±3.36 | 4.85±0.81 | G=0.609 | |
| TC | 2.68±2.67 | 3.76±3.18 | 4.89±3.87 | 5.64±3.74 | 4.24±0.85 | T<0.001* | T L <0.001* | |
| Time Mean | 2.96±0.55 | 3.59±0.67 | 5.72±0.80Ψ◊ | 5.91±0.74Ψ◊ | G X T=0.427 | G X T L =0.690 |
Individual group and time data expressed as means ± SD, while group effects are presented as means ± SEM. Data represents the participant soreness perception in the quadriceps muscle group at each test session during the 10 day intervention. MANOVA analysis revealed overall Wilks' Lambda time (p<0.001) and group x time (p=0.199). Univariate ANOVA p-levels from MANOVA analysis are presented for each variable. Univariate ANOVA p-levels are listed first by the Greenhouse-Geisser (GG) analysis and then by the within-subjects contrasts (WSC) to demonstrate the potential shape of the time or group x time interaction with significance indicated by the following super/subscripts: * indicates p<0.05 p-level significance, § indicates p<0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p<0.05 difference between groups, Ψ represents p<0.05 difference from pre-lift, ◊ represents p<0.05 difference from 60-min post, # represents p<0.05 difference from 24-hr post. Algo I = Algometer location #1: Vastus Medalis 1/4; Algo II = Algometer location #2: Vastus Lateralis 1/4; Algo III = Algometer location #3: Vastus Lateralis 1/2; G = group p-level; T = time p-level; G x T = interaction; q = quadratic p-level; L = linear p-level
Fig. 3.
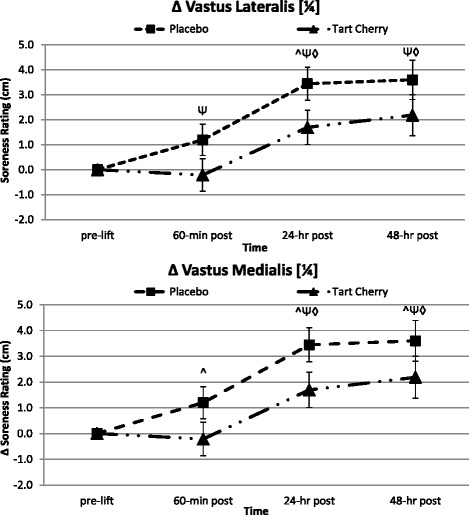
Perceptions of muscle soreness. Data expressed as means ± SE and significance indicated by the following super/subscripts: * indicates p < 0.05 p-level significance, § indicates p < 0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p < 0.05 difference between groups, Ψ represents p < 0.05 difference from pre-lift, ◊ represents p < 0.05 difference from 60-min post, # represents p < 0.05 difference from 24-h post
Isokinetic maximal voluntary contraction performance assessment
Table 4 reports the total work performed during the 3-repetition isokinetic flexion/extension MVC test. The overall MANOVA analysis revealed both a non-significant Wilks’ Lambda time interaction (p = 0.96) and a non-significant group x time effect (p = 0.75). The 3-repetition work summation univariate measures for extension (p < 0.001), flexion (p = 0.022), and total (p < 0.001) demonstrated significant changes over time, with the lowest work attained 60-min post-lift in all three measures. No aspect of the isokinetic strength recovery work performed demonstrated a significant group effect over time. While changes from pre-lift were not statistically significant between groups across time, the drop in 3-repetition summation of flexion (p = 0.21; d = 0.45), extension (p = 0.23; d = 0.45), and total work (p = 0.15; d = 0.55) performed 60-min post-lift may have been attenuated in TC compared to P based upon the moderate to large Cohen’s d effect size calculations. Average recovery decreases in flexion, extension, and total work performance from pre-lift values were 5, 19, and 14 % respectively in P, but only 2, 16, and 10 % in TC.
Table 4.
Isokinetic Maximal Voluntary Contraction Knee Extension/Flexion Total Work Performance
| Variable | Group | Baseline | Pre-Lift | 60-min Post | 24-hr Post | 48-hr Post | Group Mean | p-value (GG) | p-value (WSC) |
|---|---|---|---|---|---|---|---|---|---|
| IK Total Ext Work (N) | P | 1791±207 | 1682±235 | 1278±275 | 1408±296 | 1387±291 | 1509±90 | G=0.870 | |
| TC | 1757±378 | 1612±437 | 1298±422 | 1418±463 | 1352±383 | 1487±94 | T<0.001* | T L <0.001* | |
| Time Mean | 1774±295 | 1648±340† | 1288±345†Ψ | 1413±376†Ψ◊ | 1370±331†Ψ | G X T=0.792 | G X T q =0.662 | ||
| IK Total Flex Work (N) | P | 971±126^ | 962±141^ | 913±174^ | 925±153^ | 910±113^† | 936±58 | G=0.181 | |
| TC | 1073±252 | 1060±277 | 1064±225 | 1058±305 | 1010±289† | 1053±61 | T=0.087§ | T L =0.022* | |
| Time Mean | 1020±199 | 1009±218 | 986±210 | 989±242 | 958±217†Ψ | G X T=0.663 | G X T q =0.206 | ||
| IK Total Work (N) | P | 2762±293 | 2644±354 | 2191±388 | 2333±407 | 2296±373 | 2445±141 | G=0.646 | |
| TC | 2829±611 | 2673±687 | 2363±603 | 2476±733 | 2362±644 | 2540±148 | T<0.001* | T L <0.001* | |
| Time Mean | 2794±462 | 2658±527† | 2273±498†Ψ | 2401±577†Ψ◊ | 2328±509†Ψ | G X T=0.692 | G X T q =0.470 |
Individual group and time data expressed as means ± SD, while group effects are presented as means ± SEM. Data represents the isokinetic MVC knee extension and flexion performance each test session during the 10 day intervention. MANOVA analysis revealed overall Wilks' Lambda time (p<0.960) and group x time (p=0.748). Univariate ANOVA p-levels from MANOVA analysis are presented for each variable. Univariate ANOVA p-levels are listed first by the Greenhouse-Geisser (GG) analysis and then by the within-subjects contrasts (WSC) to demonstrate the potential shape of the time or group x time interaction with significance indicated by the following super/subscripts: * indicates p<0.05 p-level significance, § indicates p<0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p<0.05 difference between groups, † represents p<0.05 difference from baseline value, Ψ represents p<0.05 difference from pre-lift, ◊ represents p<0.05 difference from 60-min post, # represents p<0.05 difference from 24-hr post. Ext = extension; Flex = flexion; IK = isokinetic; MVC = maximal voluntary contraction; G = group p-level; T = time p-level; G x T = interaction; q = quadratic p-level; L = linear p-level
Markers of mechanical damage and physiological stress
Table 5 presents the serum mechanical damage and physiological stress markers tested in the standard clinical safety panel. The overall MANOVA analysis revealed a significant overall Wilks’ Lambda time (p < 0.001) effect, but no difference between groups over time (p = 0.50). Univariate measures for uric acid (p < 0.001), total bilirubin (p = 0.026), creatinine (p = 0.003), and total protein (p < 0.001) demonstrated significant changes over time, peaking 60-min post-lift. CK (p = 0.025) and AST (p = 0.003) also significantly changed over time, peaking 24-h post-lift. Significant group differences over time and group by time changes from pre-lift were reported for creatinine (p = 0.030, delta p = 0.024) and total protein (p = 0.018, delta p = 0.006). Analyzing the change from pre-lift levels, significant differences across groups for creatinine (p = 0.007) and total protein (p = 0.004) were also evident. Serum creatinine increased on average 2 % over pre-lift values during the recovery in P, but actually decreased 7 % below pre-lift in TC. Serum total protein increased 2–9 % over pre-lift values during the recovery in P, but decreased 0–4 % below pre-lift in TC. Subsequent post-hoc analysis indicated a significantly attenuated TC creatinine response 48-h post-lift and a TC total protein level that never differed from pre-lift measures 60-min and 48-h post-lift compared to deviations in P (see Fig. 4). Increases in serum CK levels tended to be lower in TC over time compared to P (p = 0.10). Serum CK levels increased on average 135 % over pre-lift values during the recovery in P, but increased only 98 % in TC. No other significant differences between groups over time or deviations from normal human clinical ranges were observed for kidney/liver enzymes [e.g. AST (p = 0.16), ALT (p = 0.27)] or markers of protein catabolism [bilirubin (p = 0.12), BUN:creatinine ratio (p = 0.39), uric acid (p = 0.15)]. However, further post-hoc analyses revealed TC facilitated post-lift attenuations in bilirubin, AST, and ALT 48-h post-lift compared to P (see Figs. 4 and 5).
Table 5.
Markers of Muscle Catabolism, Secondary Muscle Damage, and Physiological Stress
| Variable | Group | Baseline | Pre-Lift | 60-min Post | 24-hr Post | 48-hr Post | Group Mean | p-value (GG) | p-value (WSC) |
|---|---|---|---|---|---|---|---|---|---|
| AST (U/L) | P | 31.85±9.65 | 27.47±6.40 | 30.79±7.88 | 41.39±13.31 | 37.67±10.33 | 33.83±2.19 | G=0.919 | |
| TC | 37.70±17.65 | 28.39±7.62 | 29.53±6.46 | 40.59±17.59 | 31.32±11.57 | 33.51±2.29 | T=0.003* | T q =0.759 | |
| Time Mean | 34.78±2.93 | 27.93±1.46† | 30.16±1.51Ψ | 40.99±3.23Ψ◊ | 34.50±2.28Ψ# | G X T=0.316 | G X T L =0.162 | ||
| ALT (U/L) | P | 33.81±18.74 | 32.21±12.26 | 34.19±13.60 | 36.78±14.97 | 35.80±13.84 | 34.56±4.19 | G=0.455 | |
| TC | 31.78±13.22 | 30.01±19.33 | 28.32±15.83 | 31.08±19.58 | 28.54±17.41 | 29.94±4.38 | T=0.529 | T L =0.180 | |
| Time Mean | 32.79±3.41 | 31.11±3.34 | 31.26±3.07 | 33.93±3.61Ψ◊ | 32.17±3.27 | G X T=0.521 | G X T L =0.266 | ||
| Total Billirubin (umol/L) | P | 8.00±3.67 | 7.25±3.47 | 9.08±5.31 | 8.14±3.82 | 7.35±3.21 | 7.96±1.01 | G=0.395 | |
| TC | 9.65±3.85 | 9.98±4.95 | 9.58±4.01 | 9.79±5.20 | 7.19±3.21 | 9.24±1.06 | T=0.064§ | T q =0.026* | |
| Time Mean | 8.83±0.78 | 8.61±0.89 | 9.33±0.99 | 8.97±0.95 | 7.27±0.67†Ψ◊# | G X T=0.306 | G X T L =0.125 | ||
| Urea/BUN (mmol/L) | P | 6.59±1.54 | 6.16±1.74 | 6.07±1.59 | 6.48±1.96 | 5.92±2.33 | 6.24±0.44 | G=0.794 | |
| TC | 6.49±1.39 | 6.75±1.30 | 6.40±1.33 | 6.59±1.83 | 5.83±1.66 | 6.41±0.46 | T=0.061§ | T L =0.055§ | |
| Time Mean | 6.54±0.31 | 6.46±0.32 | 6.24±0.31 | 6.53±0.40 | 5.88±0.43†Ψ# | G X T=0.594 | G X T q =0.238 | ||
| Creatinine (umol/L) | P | 96.50±13.23 | 97.19±19.28 | 102.76±17.25^ | 98.91±21.16^ | 95.20±17.73^◊ | 98.11±4.05 | G=0.273 | |
| TC | 91.82±11.99 | 96.84±15.27 | 95.42±14.37 | 91.52±11.27 | 81.99±14.87†Ψ◊# | 91.52±4.23 | T=0.004* | T q =0.003* | |
| Time Mean | 94.16±2.64 | 97.02±3.65 | 99.09±3.33 | 95.21±3.58 | 88.60±3.43†Ψ◊# | G X T=0.178 | G X T L =0.030* | ||
| BUN/Creatinine Ratio | P | 17.11±4.38 | 15.97±3.99 | 14.82±3.66 | 16.66±5.67 | 15.50±5.26 | 16.01±1.08 | G=0.353 | |
| TC | 17.65±3.76 | 17.51±3.83 | 16.82±3.74 | 17.85±4.35 | 17.64±3.80 | 17.50±1.13 | T=0.214 | T q =0.225 | |
| Time Mean | 17.37±4.01 | 16.71±3.90 | 15.78±3.75†Ψ | 17.23±5.00 | 16.53±4.64 | G X T=0.721 | G X T L =0.388 | ||
| Uric Acid (umol/L) | P | 328±66 | 317±66 | 444±136 | 367±80 | 349±88 | 361.12±20.37 | G=0.647 | |
| TC | 306±52 | 310±54 | 470±117 | 350±93 | 302±79 | 347.44±21.28 | T<0.001* | T q <0.001* | |
| Time Mean | 317±12 | 314±13 | 457±27†Ψ | 358±18†Ψ◊ | 325±17◊# | G X T=0.298 | G X T q =0.146 | ||
| CK (U/L) | P | 468±288 | 322±243 | 451±267 | 967±700 | 855±981 | 613±82 | G=0.487 | |
| TC | 843±1156 | 259±146 | 355±198 | 770±470 | 415±213 | 529±86 | T=0.025* | T q =0.222 | |
| Time Mean | 656±172 | 291±42† | 403±49Ψ | 869±126Ψ◊ | 635±151Ψ | G X T=0.209 | G X T L =0.100§ | ||
| Total Protein (mmol/L) | P | 78.67±6.11 | 67.55±7.96^† | 72.23±6.47†Ψ | 68.67±9.45† | 73.67±11.11†Ψ# | 72.16±1.52 | G=0.305 | |
| TC | 80.53±4.60 | 74.13±2.62† | 74.59±4.18† | 72.43±6.72† | 70.66±7.92†Ψ◊ | 74.47±1.59 | T<0.001* | T q <0.001* | |
| Time Mean | 79.60±1.14 | 70.84±1.26† | 73.41±1.15†Ψ | 70.55±1.72† | 72.17±2.03† | G XT=0.091§ | G X T q =0.018* |
Individual group and time data expressed as means ± SD, while group effects are presented as means ± SEM. Data represents the response to muscle catabolism, mechanical damage, and physiological stress at each test session during the 10 day intervention. MANOVA analysis revealed overall Wilks' Lambda time (p<0.001) and group x time (p=0.504). Univariate ANOVA p-levels from MANOVA analysis are presented for each variable. Univariate ANOVA p-levels are listed first by the Greenhouse-Geisser (GG) analysis and then by the within-subjects contrasts (WSC) to demonstrate the potential shape of the time or group x time interaction with significance indicated by the following super/subscripts: * indicates p<0.05 p-level significance, § indicates p<0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p<0.05 difference between groups, † represents p<0.05 difference from baseline value, Ψ represents p<0.05 difference from pre-lift, ◊ represents p<0.05 difference from 60-min post, # represents p<0.05 difference from 24-hr post. AST Aspartate aminotransferase, ALT Alanine aminotransferase, BUN blood urea nitrogen, CK Creatine kinase, G group p-level, T time p-level, G x T interaction, q quadratic p-level, L linear p-level
Fig. 4.
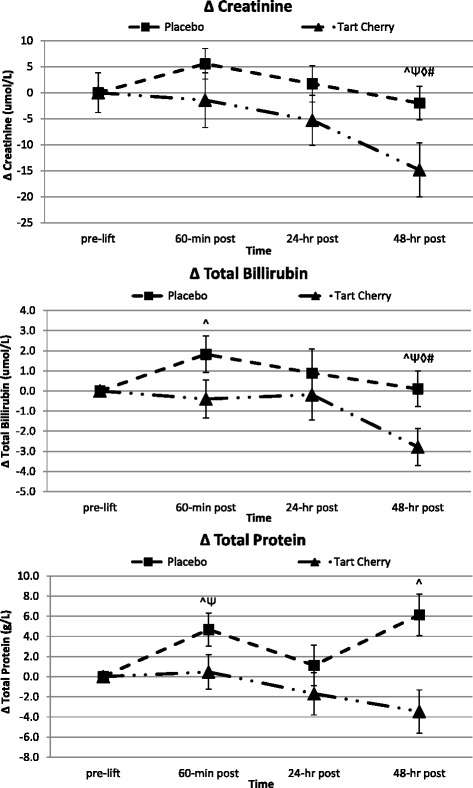
Markers of protein catabolism. Data expressed as means ± SE and significance indicated by the following super/subscripts: * indicates p < 0.05 p-level significance, § indicates p < 0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p < 0.05 difference between groups, Ψ represents p < 0.05 difference from pre-lift, ◊ represents p < 0.05 difference from 60-min post, # represents p < 0.05 difference from 24-h post
Fig. 5.
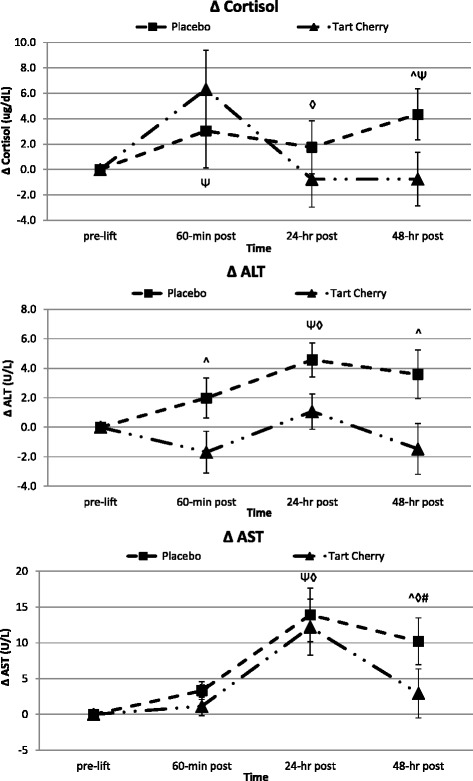
Markers of physiological stress and secondary indices of muscle damage. Data expressed as means ± SE and significance indicated by the following super/subscripts: * indicates p < 0.05 p-level significance, § indicates p < 0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p < 0.05 difference between groups, Ψ represents p < 0.05 difference from pre-lift, ◊ represents p < 0.05 difference from 60-min post, # represents p < 0.05 difference from 24-h post
Anabolic/catabolic hormone response markers
Table 6 shows the serum anabolic and catabolic hormone markers analyzed in response to exercise. The overall MANOVA analyses revealed both non-significant overall Wilks’ Lambda time (p = 0.29) and overall group changes across time (p = 0.46). Cortisol levels tended to change over time (p = 0.093). Serum cortisol levels were significantly different between groups over time (p = 0.029) and revealed how supplementation caused significant variability (p = 0.032) in cortisol changes from pre-lift levels across the recovery period. Serum cortisol levels increased on average 13 % over pre-lift values during the recovery in P, but only 8 % in TC. The delta post-hoc analysis revealed that TC cortisol levels were not different from pre-lift levels and were significantly lower at 48-h post-lift compared to rising cortisol levels in P (see Fig. 5).
Table 6.
Anabolic/Catabolic Hormone Response
| Variable | Group | Baseline | Pre-Lift | 60-min Post | 24-hr Post | 48-hr Post | Group Mean | p-value (GG) | p-value (WSC) |
|---|---|---|---|---|---|---|---|---|---|
| Cortisol (ug/dL) | P | 28.34±13.07^ | 23.33±8.80 | 26.37±7.33 | 25.08±10.21^ | 27.68±11.20^ | 26.16±2.18 | G=0.167 | |
| TC | 21.48±7.02 | 20.49±7.92 | 26.81±12.08† | 19.72±5.56◊ | 19.74±6.82◊ | 21.65±2.28 | T=0.093§ | T L =0.611 | |
| Time Mean | 24.91±2.22 | 21.91±1.75 | 26.59±2.06Ψ | 22.40±1.74◊ | 23.71±1.96 | G X T=0.170 | G X T q =0.029* | ||
| Testosterone (ng/mL) | P | 9.86±3.46 | 9.37±3.45 | 9.25±2.92 | 10.17±3.55 | 9.94±3.42 | 9.72±0.75 | G=0.025* | |
| TC | 7.24±2.22 | 7.17±2.00 | 6.97±1.95 | 7.20±2.00 | 6.91±1.70 | 7.10±0.79 | T=0.428 | T q =0.427 | |
| Time Mean | 8.55±0.61 | 8.27±0.60 | 8.11±0.52 | 8.68±0.61 | 8.42±0.57 | G X T=0.556 | G X T L =0.174 | ||
| Test/Cort Ratio | P | 0.037±0.009 | 0.043±0.014 | 0.037±0.013 | 0.045±0.019 | 0.040±0.018 | 0.040±0.003 | G=0.538 | |
| TC | 0.037±0.015 | 0.040±0.020 | 0.031±0.015 | 0.039±0.013 | 0.040±0.019 | 0.037±0.004 | T=0.152 | T L =0.516 | |
| Time Mean | 0.037±0.012 | 0.042±0.017 | 0.034±0.014 | 0.042±0.016 | 0.040±0.018 | G X T=0.815 | G X T q =0.196 |
Individual group and time data expressed as means ± SD, while group effects are presented as means ± SEM. Data represents the stress and sex hormone response at each test session during the 10 day intervention. MANOVA analysis revealed overall Wilks' Lambda time (p=0.293) and group x time (p=0.458). Univariate ANOVA p-levels from MANOVA analysis are presented for each variable. Univariate ANOVA p-levels are listed first by the Greenhouse-Geisser (GG) analysis and then by the within-subjects contrasts (WSC) to demonstrate the potential shape of the time or group x time interaction with significance indicated by the following super/subscripts: * indicates p<0.05 p-level significance, § indicates p<0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p<0.05 difference between groups, † represents p<0.05 difference from baseline value, Ψ represents p<0.05 difference from pre-lift, ◊ represents p<0.05 difference from 60-min post, # represents p<0.05 difference from 24-hr post. Test/Cort = Testosterone/Cortisol ratio G group p-level, T time p-level, G x T interaction, q quadratic p-level; L = linear p-level
Markers of free radical production and oxidative stress
Table 7 reports the serum markers of free radical production [reactive oxygen species (ROS) and reactive nitrogen species (RNS)] analyzed in response to exercise. The overall MANOVA analysis revealed no significant overall Wilks’ Lambda time (p = 0.32) interaction and no overall group differences over time (p = 0.76). None of the univariate measures for markers of free radical production, lipid peroxidation, and antioxidant capacity/activity (NT, TBARS, TAS, and SOD) reported main time effects or differences between groups over time.
Table 7.
Markers of Free Radical Production and Oxidative Stress
| Variable | Group | Baseline | Pre-Lift | 60-min Post | 24-hr Post | 48-hr Post | Group Mean | p-value (GG) | p-value (WSC) |
|---|---|---|---|---|---|---|---|---|---|
| Nitrotyrosine (nM) | P | 305±267 | 306±311 | 297±289 | 297±330 | 294±305 | 300±74 | G=0.940 | |
| TC | 303±193 | 319±254 | 277±190 | 275±202 | 283±203 | 292±78 | T=0.280 | T L =0.146 | |
| Time Mean | 304±230 | 312±279 | 288±241 | 287±270 | 289±256Ψ | G X T=0.613 | G X T L =0.483 | ||
| TBARS (uM) | P | 7.84±2.69 | 7.27±2.91 | 8.60±2.67 | 8.03±3.34 | 8.43±3.74 | 8.03±0.81 | G=0.913 | |
| TC | 7.74±4.24 | 8.24±4.03 | 7.44±3.84 | 8.19±3.46 | 7.91±3.35 | 7.90±0.84 | T=0.937 | T L =0.539 | |
| Time Mean | 7.79±3.44 | 7.73±3.44 | 8.05±3.26 | 8.10±3.32 | 8.18±3.49 | G X T=0.569 | G X T L =0.649 | ||
| TAS (mM) | P | 2.90±1.34 | 2.83±1.77 | 3.02±1.85 | 2.95±1.47 | 2.80±1.81 | 2.90±0.39 | G=0.752 | |
| TC | 2.69±1.17 | 2.39±1.14 | 2.99±1.07 | 2.73±1.36 | 2.81±1.34 | 2.72±0.41 | T=0.323 | T q =0.419 | |
| Time Mean | 2.80±1.23 | 2.62±1.48 | 3.01±1.49 | 2.84±1.39 | 2.81±1.57 | G X T=0.706 | G X T L =0.442 | ||
| SOD (U/mL) | P | 0.58±0.07 | 0.59±0.05 | 0.59±0.09 | 0.56±0.08 | 0.58±0.06 | 0.58±0.02 | G=0.668 | |
| TC | 0.55±0.12 | 0.60±0.09 | 0.54±0.09 | 0.57±0.10 | 0.57±0.11 | 0.57±0.02 | T=0.264 | T q =0.827 | |
| Time Mean | 0.56±0.10 | 0.60±0.07† | 0.57±0.09 | 0.57±0.08 | 0.58±0.09 | G X T=0.335 | G X T L =0.784 |
Individual group and time data expressed as means ± SD, while group effects are presented as means ± SEM. Data represents the response to reactive oxygen and nitrogen species production in addition to antioxidant activity at each test session during the 10 day intervention. MANOVA analysis revealed overall Wilks' Lambda time (p=0.321) and group x time (p=0.756). Univariate ANOVA p-levels from MANOVA analysis are presented for each variable. Univariate ANOVA p-levels are listed first by the Greenhouse-Geisser (GG) analysis and then by the within-subjects contrasts (WSC) to demonstrate the potential shape of the time or group x time interaction with significance indicated by the following super/subscripts: * indicates p<0.05 p-level significance, § indicates p<0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p<0.05 difference between groups, † represents p<0.05 difference from baseline value, Ψ represents p<0.05 difference from pre-lift, ◊ represents p<0.05 difference from 60-min post, # represents p<0.05 difference from 24-hr post
TBARS Thiobarbituric acid reactive substances, TAS Total antioxidant status, SOD superoxide dismutase, G group p-level, T time p-level, G x T interaction, q quadratic p-level, L linear p-level
Inflammatory response markers
Table 8 shows the serum inflammatory cytokine and chemokine markers analyzed in response to exercise. The overall MANOVA analysis revealed a significant overall Wilks’ Lambda time (p < 0.001) interaction, but a non-significant overall group x time effect (p = 0.30). Univariate measures for TNF-α (p = 0.001), IL-1β (p = 0.030), IL-6 (p = 0.023), and IL-8 (p = 0.018) demonstrated significant changes over time and from baseline measures, peaking 24-h post-lift. No significant differences between groups were observed over time for any of the inflammatory cytokines or chemokines.
Table 8.
Pro-inflammatory Cytokines and Chemokines
| Variable | Group | Baseline | Pre-Lift | 60-min Post | 24-hr Post | 48-hr Post | Group Mean | p-value (GG) | p-value (WSC) |
|---|---|---|---|---|---|---|---|---|---|
| TNF-α (pg/mL) | P | 2.82±1.33 | 3.30±1.59 | 3.33±1.88 | 2.98±1.45 | 2.85±1.41 | 3.05±0.36 | G=0.212 | |
| TC | 2.12±0.92 | 2.55±1.15 | 2.61±1.00 | 2.44±0.82 | 2.23±0.94 | 2.39±0.37 | T=0.003* | T q =0.001* | |
| Time Mean | 2.47±0.24 | 2.92±0.29† | 2.97±0.32† | 2.71±0.25◊ | 2.54±0.25Ψ◊ | G X T=0.873 | G X T L =0.649 | ||
| IFN-γ (pg/mL) | P | 14.92±31.51 | 18.91±44.08 | 18.99±44.51 | 21.33±51.78 | 21.56±51.82 | 19.14±9.41 | G=0.365 | |
| TC | 6.19±5.69 | 6.46±5.01 | 7.12±5.19 | 7.11±5.98 | 5.78±6.02 | 6.53±9.83 | T=0.287 | T q =0.136 | |
| Time Mean | 10.56±4.83 | 12.68±6.70 | 13.06±6.77 | 14.22±7.87 | 13.67±7.88# | G X T=0.314 | G X T L =0.295 | ||
| IL-1β (pg/mL) | P | 0.77±0.32 | 0.84±0.35 | 0.87±0.39 | 0.83±0.38 | 0.83±0.35 | 0.83±0.10 | G=0.048* | |
| TC | 0.75±0.35 | 0.75±0.34 | 0.82±0.26 | 0.85±0.30 | 0.82±0.34 | 0.80±0.10 | T=0.047* | T q =0.030* | |
| Time Mean | 0.76±0.07 | 0.80±0.07† | 0.85±0.07† | 0.84±0.07 | 0.82±0.07 | G X T=0.411 | G X T q =0.488 | ||
| IL-2 (pg/mL) | P | 1.86±1.86 | 2.00±2.18 | 1.97±2.16 | 1.91±2.15 | 1.87±2.09 | 1.92±0.71 | G=0.956 | |
| TC | 1.70±2.96 | 1.73±2.73 | 2.06±2.65 | 2.26±3.13 | 2.15±3.03 | 1.98±0.74 | T=0.292 | T L =0.214 | |
| Time Mean | 1.78±0.51 | 1.87±0.51 | 2.02±0.50 | 2.08±0.56 | 2.01±0.54 | G X T=0.230 | G X T L =0.163 | ||
| IL-6 (pg/mL) | P | 1.19±0.95 | 1.34±1.36 | 1.61±1.60 | 1.33±1.38 | 1.26±1.21 | 1.35±0.37 | G=0.724 | |
| TC | 0.87±1.34 | 1.09±1.31 | 1.54±1.38 | 1.20±1.49 | 1.07±1.43 | 1.16±0.39 | T=0.020* | T q =0.023* | |
| Time Mean | 1.03±0.24 | 1.22±0.28 | 1.57±0.31†Ψ | 1.27±0.30† | 1.17±0.28◊# | G X T=0.761 | G X T L =0.512 | ||
| IL-8 (pg/mL) | P | 4.25±3.99 | 5.07±5.74 | 5.04±6.16 | 5.16±6.41 | 4.94±6.32 | 4.89±1.23 | G=0.496 | |
| TC | 2.95±1.56 | 3.45±1.75 | 4.14±1.29 | 4.02±1.78 | 3.74±1.91 | 3.66±1.28 | T=0.042* | T q =0.018* | |
| Time Mean | 3.60±0.64 | 4.26±0.90† | 4.59±0.95† | 4.59±1.00 | 4.34±0.99 | G X T=0.697 | G X T L =0.736 | ||
| IL-12p70 (pg/mL) | P | 2.27±4.13 | 3.03±6.51 | 3.05±6.63 | 3.01±6.42 | 2.78±5.68 | 2.83±1.33 | G=0.577 | |
| TC | 1.70±2.67 | 1.76±2.59 | 1.68±2.15 | 1.91±2.76 | 1.66±2.61 | 1.74±1.39 | T=0.279 | T q =0.264 | |
| Time Mean | 1.99±0.73 | 2.39±1.05 | 2.37±1.05 | 2.46±1.05 | 2.22±0.94 | G X T=0.377 | G X T L =0.351 |
Individual group and time data expressed as means ± SD, while group effects are presented as means ± SEM. Data represents the pro-inflammatory response at each test session during the 10 day intervention. MANOVA analysis revealed overall Wilks' Lambda time (p<0.001) and group x time (p=0.302). Univariate ANOVA p-levels from MANOVA analysis are presented for each variable. Univariate ANOVA p-levels are listed first by the Greenhouse-Geisser (GG) analysis and then by the within-subjects contrasts (WSC) to demonstrate the potential shape of the time or group x time interaction with significance indicated by the following super/subscripts: * indicates p<0.05 p-level significance, § indicates p<0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p<0.05 difference between groups, † represents p<0.05 difference from baseline value, Ψ represents p<0.05 difference from pre-lift, ◊ represents p<0.05 difference from 60-min post, # represents p<0.05 difference from 24-hr post
TNF-α tumor necrosis factor alpha, IFN-γ interferon gamma, IL interleukin, G group p-level, T time p-level, G x T interaction, q quadratic p-level, L linear p-level
Anti-inflammatory response markers
Table 9 presents the serum anti-inflammatory cytokine markers analyzed in response to exercise. The overall MANOVA analysis revealed a significant overall Wilks’ Lambda time (p < 0.001) interaction, but a non-significant overall effect of treatment over time (p = 0.45). Univariate measures for IL-4 (p = 0.001) and IL-7 (p = 0.033) reported significant main time effects with IL-13 levels approaching significance across time (p = 0.055), peaking 60-min post-lift. No significant group effects over time were reported for any of the anti-inflammatory cytokine markers.
Table 9.
Anti-inflammatory Cytokines
| Variable | Group | Baseline | Pre-Lift | 60-min Post | 24-hr Post | 48-hr Post | Group Mean | p-value (GG) | p-value (WSC) |
|---|---|---|---|---|---|---|---|---|---|
| IL-4 (pg/mL) | P | 6.03±3.18 | 7.86±5.06 | 8.26±5.22 | 7.44±4.95 | 7.83±5.10 | 7.48±1.05 | G=0.273 | |
| TC | 4.16±1.91 | 5.45±2.04 | 6.38±2.49 | 6.36±2.45 | 6.54±3.01 | 5.78±1.09 | T=0.001* | T q =0.001* | |
| Time Mean | 5.10±0.55 | 6.65±0.82† | 7.32±0.87† | 6.90±0.83†◊ | 7.19±0.88† | G X T=0.421 | G X T L =0.405 | ||
| IL-5 (pg/mL) | P | 0.69±0.37 | 0.70±0.41 | 0.70±0.46 | 0.64±0.37 | 0.65±0.40 | 0.68±0.11 | G=0.749 | |
| TC | 0.75±0.43 | 0.72±0.40 | 0.71±0.37 | 0.77±0.43 | 0.70±0.42 | 0.73±0.12 | T=0.716 | T L =0.325 | |
| Time Mean | 0.72±0.08 | 0.71±0.09 | 0.70±0.09 | 0.71±0.08 | 0.68±0.09 | G X T=0.438 | G X T L =0.582 | ||
| IL-7 (pg/mL) | P | 4.73±2.19 | 5.41±2.49 | 5.94±3.71 | 5.52±2.75 | 5.46±2.57 | 5.41±0.63 | G=0.414 | |
| TC | 4.15±1.86 | 4.45±2.00 | 5.31±2.18 | 4.91±1.61 | 4.47±1.54 | 4.66±0.65 | T=0.041* | T q =0.033* | |
| Time Mean | 4.44±0.43 | 4.93±0.48† | 5.62±0.64† | 5.21±0.48† | 4.97±0.45† | G X T=0.843 | G X T L =0.757 | ||
| IL-10 (pg/mL) | P | 4.52±3.85 | 3.69±1.82 | 4.21±1.92 | 3.48±1.70 | 3.45±1.74 | 3.87±0.56 | G=0.869 | |
| TC | 2.77±1.74 | 3.49±2.03 | 6.02±5.70 | 3.33±2.54 | 3.07±2.54 | 3.74±0.59 | T=0.103§ | T q =0.157 | |
| Time Mean | 3.65±0.63 | 3.59±0.40 | 5.12±0.87Ψ | 3.41±0.45 | 3.26±0.45 | G X T=0.182 | G X T q =0.120 | ||
| IL-13 (pg/mL) | P | 3.30±2.89 | 3.33±3.30 | 3.40±3.48 | 3.24±3.64 | 3.27±3.56 | 3.31±0.78 | G=0.449 | |
| TC | 1.96±1.74 | 2.26±1.66 | 2.75±2.09 | 2.78±2.18 | 2.45±2.34 | 2.44±0.81 | T=0.393 | T q =0.055§ | |
| Time Mean | 2.63±0.50 | 2.80±0.55 | 3.08±0.61 | 3.01±0.63 | 2.86±0.64 | G X T=0.402 | G X T q =0.133 |
Individual group and time data expressed as means ± SD, while group effects are presented as means ± SEM. Data represents the anti-inflammatory response at each test session during the 10 day intervention. MANOVA analysis revealed overall Wilks' Lambda time (p<0.001) and group x time (p=0.447). Univariate ANOVA p-levels from MANOVA analysis are presented for each variable. Univariate ANOVA p-levels are listed first by the Greenhouse-Geisser (GG) analysis and then by the within-subjects contrasts (WSC) to demonstrate the potential shape of the time or group x time interaction with significance indicated by the following super/subscripts: * indicates p<0.05 p-level significance, § indicates p<0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p<0.05 difference between groups, † represents p<0.05 difference from baseline value, Ψ represents p<0.05 difference from pre-lift, ◊ represents p<0.05 difference from 60-min post, # represents p<0.05 difference from 24-hr post
IL interleukin, G group p-level, T time p-level, G x T interaction, q quadratic p-level, L linear p-level
Clinical markers of immune-related complete blood counts
Table 10 displays immune response-related complete blood count markers analyzed in response to exercise. The overall MANOVA analysis demonstrated significant overall Wilks’ Lambda time interaction (p < 0.001), but a non-significant overall difference between groups over time (p = 0.68). Univariate measures for lymphocytes (p < 0.001) and MID (p < 0.001) demonstrated significant changes over time and from baseline measures, most depressed 60-min post-lift. WBC (p = 0.004) and GRAN (p = 0.003) also significantly changed over time, with lowest values 48-h post-lift. MID levels tended to be different between groups over time (p = 0.062). Significant group changes over time (p = 0.015) and differences in group effects from pre-lift levels over the recovery period (p = 0.013) were reported for lymphocytes. Lymphocyte levels decreased on average 23 % below pre-lift values during the recovery in P, but only 11 % in TC. Subsequent post-hoc analysis showed a significantly greater lymphocyte counts 24-h and 48-h post-lift in TC compared to P, but unlike those supplementing with P, TC lymphocyte counts returned to pre-lift values by the end of the recovery (48-h post-lift). A significant group effect over time was also reported for WBC (p = 0.020). WBC decreased on average 8 % below pre-lift values during the recovery in P, but only 3 % in TC. Post-hoc analysis demonstrated significant WBC differences pre-lift, 24-h, and 48-h post-lift between TC and P that became insignificant once changes were calculated from pre-lift levels.
Table 10.
Markers of Immune-Related Complete Blood Counts
| Variable | Group | Baseline | Pre-Lift | 60-min Post | 24-hr Post | 48-hr Post | Group Mean | p-value (GG) | p-value (WSC) | |
|---|---|---|---|---|---|---|---|---|---|---|
| LYMPH (K/uL) | P | 1.93±0.54 | 2.21±0.69 | 1.45±0.43†Ψ | 1.86±0.71^Ψ◊ | 1.78±0.48^Ψ◊ | 1.85±0.20 | G=0.311 | ||
| TC | 2.09±0.86 | 2.36±1.05† | 1.49±0.58†Ψ | 2.62±1.51†Ψ◊ | 2.15±0.78◊# | 2.14±0.21 | T<0.001* | T q =0.136 | ||
| Time Mean | 2.01±0.15 | 2.28±0.18† | 1.47±0.11†Ψ | 2.24±0.24◊ | 1.97±0.13Ψ◊ | G X T=0.143 | G X T L =0.015* | |||
| WBC (K/uL) | P | 5.88±1.23 | 6.61±2.21^ | 6.38±1.45 | 6.16±1.62^ | 5.75±1.61^ | 6.16±0.40 | G=0.337 | ||
| TC | 5.54±1.25 | 7.18±2.03† | 6.98±2.04† | 7.40±2.17† | 6.55±1.23†# | 6.73±0.42 | T=0.004* | T q =0.004* | ||
| Time Mean | 5.71±0.26 | 6.90±0.44† | 6.68±0.37† | 6.78±0.40† | 6.15±0.30Ψ | G X T=0.207 | G X T L =0.020* | |||
| MID (K/uL) | P | 0.43±0.12 | 0.64±0.19 | 0.43±0.14 | 0.53±0.13 | 0.48±0.13 | 0.50±0.03 | G=0.866 | ||
| TC | 0.42±0.10 | 0.61±0.24 | 0.38±0.10 | 0.60±0.19 | 0.55±0.16 | 0.51±0.03 | T<0.001* | T L =0.100§ | ||
| Time Mean | 0.43±0.02 | 0.63±0.05† | 0.41±0.03Ψ | 0.56±0.03†◊ | 0.51±0.03†Ψ◊ | G X T=0.324 | G X T L =0.062§ | |||
| GRAN (K/uL) | P | 3.50±0.97 | 3.76±1.47 | 4.51±1.20 | 3.78±1.33 | 3.48±1.32 | 3.80±0.33 | G=0.577 | ||
| TC | 3.04±0.48 | 4.22±1.77 | 5.11±2.35 | 4.17±1.61 | 3.84±0.98 | 4.08±0.35 | T<0.001* | T q =0.002* | ||
| Time Mean | 3.27±0.16 | 3.99±0.34† | 4.81±0.38†Ψ | 3.97±0.31†◊ | 3.66±0.03◊ | G X T=0.332 | G X T L =0.119 | |||
| GM-CSF (pg/mL) | P | 8.12±8.23 | 8.18±8.33 | 7.87±7.38 | 7.54±7.67 | 7.39±7.49 | 7.82±2.71 | G=0.512 | ||
| TC | 10.37±11.40 | 10.51±10.83 | 10.52±10.48 | 10.67±11.19 | 10.09±10.89 | 10.43±2.83 | T=0.446 | T L =0.277 | ||
| Time Mean | 9.24±2.06 | 9.34±2.00 | 9.20±1.88 | 9.10±1.99 | 8.74±1.93 | G X T=0.624 | G X T L =0.450 | |||
Individual group and time data expressed as means ± SD, while group effects are presented as means ± SEM. Data represents immune cellular response markers at each test session during the 10 day intervention. MANOVA analysis revealed overall Wilks' Lambda time (p<0.001) and group x time (p=0.684). Univariate ANOVA p-levels from MANOVA analysis are presented for each variable. Univariate ANOVA p-levels are listed first by the Greenhouse-Geisser (GG) analysis and then by the within-subjects contrasts (WSC) to demonstrate the potential shape of the time or group x time interaction with significance indicated by the following super/subscripts: * indicates p<0.05 p-level significance, § indicates p<0.10 p-level significance. LSD post hoc analysis is indicated by the following superscripts: ^ represents p<0.05 difference between groups, † represents p<0.05 difference from baseline value, Ψ represents p<0.05 difference from pre-lift, ◊ represents p<0.05 difference from 60-min post, # represents p<0.05 difference from 24-hr post
LYMPH lymphocytes, WBC white blood cell, MID mid-range absolute count, GRAN granulocyte absolute count, GM-CSF granulocyte-macrophage colony-stimulating factor, G group p-level, T time p-level, G x T interaction, q quadratic p-level, L linear p-level
Discussion
This is the first study to investigate the effect of freeze dried Montmorency tart cherry skin powder on acute resistance exercise performance and recovery. It was hypothesized that supplementation with this novel powdered tart cherry skin supplement surrounding an acute bout of intense resistance exercise would reduce perceptions of muscle soreness, markers of muscle damage, oxidative stress, and inflammation, thus better maintaining subsequent performance within the first 48-h of recovery. The results of the present study demonstrate that this powered tart cherry formulation is effective in promoting decreased perceptions of muscle soreness following intense resistance exercise. Tart cherry powder reduced perceptions of muscle soreness in the distal vastus medalis and lateralis. In accordance with decreased perceptions of muscle soreness, tart cherry powder supplementation reduced serum markers of muscle catabolism and physiological stress over the 48-h post-lift period compared to placebo. As a result of the resistance exercise, markers of oxidative stress, lipid peroxidation, and antioxidant activity did not significantly change over time as was not affected by differences in supplementation. The inflammatory response deviated from pre-lift values during the recovery period, but the change was not different between supplement groups.
The attenuation of quadriceps muscle soreness in conjunction with reduced hemodynamic markers of muscle catabolism following the resistance training bout indicates that the consumption of tart cherry powder may have dampened the effects of the secondary muscle damage response. The initial injury to the muscle microstructure is defined by a mechanical disruption of the myofibrils, particularly in response to high volume and intensity eccentric exercise [9, 44, 56]. The damage to the muscle microstructure triggers a local inflammatory response characterized by infiltration of fluid, plasma proteins, and free radicals that all exacerbate the initial mechanical muscle damage, creating a secondary injury incidence [9, 44, 57–60]. Decreased perceptions of quadriceps muscle soreness and muscle catabolism indices reveal that supplementation with powdered tart cherry may aid in blunting the secondary muscle damage effects compared to placebo.
The apparent beneficial effect of tart cherry powder supplementation on the perception of muscle soreness after a resistance exercise bout is consistent with some of the previously published findings. Connolly et al. [20] reported that consumption of a Montmorency tart cherry juice in healthy college-aged males significantly reduced pain in the elbow flexors after a bout of eccentric exercise using a visual analog scale (VAS). Peak muscle pain was achieved 24-h post-exercise in the tart cherry group compared to a continued increase in pain 48-h post-exercise in the placebo group. In contrast, Connolly et al. [20] reported no difference in pressure pain threshold (PPT) scores between the two experimental groups. Using a strenuous single leg knee extension protocol in a cohort of well-trained male athletes, Bowtell et al. [4] demonstrated a trend in post-exercise muscle pain reduction via PPT with Montmorency tart cherry juice supplementation up to 48-h post-exercise compared to placebo. The positive results in the current study are similar to previous tart cherry supplementation research findings, showing that powdered tart cherry supplementation significantly attenuates muscle soreness perceptions throughout the 48-h post-lift recovery compared to placebo. Slight differences in study outcomes may lie in the disparity of muscle pain or soreness measures, tart cherry supplements, quantity of musculature involvement, and/or exercise modality. Measurement of muscle soreness perception in the present study utilizing both an algometer and a GRPS was implemented to help ameliorate the purely subjective nature of a VAS as the only measure of pain or soreness.
The attenuation in post-lift muscle soreness with powdered tart cherry supplementation may be described by underlying catabolic mechanisms. Similar to the results of the current study, Bowtell et al. [4], following exhaustive leg extension exercise, demonstrated a faster post-exercise recovery of knee extensor force coupled with a trend for a smaller CK percent change from pre-exercise levels up to 48-h post-exercise with tart cherry supplementation compared to placebo [43]. Following a treadmill incremental exercise protocol with thoroughbred horses, Ducharme et al. [61] reported an attenuation trending toward significance in CK levels during exercise recovery when supplementing with a tart cherry juice blend compared to placebo. Similar to the post-lift attenuations of ALT (60-min and 48-h post) and AST (48-h post) as secondary markers of muscle damage in the current study, Ducharme et al. [61] reported that AST was significantly mitigated during and following an incremental exercise protocol in the tart cherry-treated horses compared to placebo [43]. In an endurance-based crossover study examining the effects of acute ibuprofen (8400 mg) ingestion surrounding a 45-min downhill treadmill run, Donnelly et al. [62] reported an increase in serum CK, AST, and lactate dehydrogenase post-run with maximal activity of CK and AST occurring at 24-h post-run, while lactate dehydrogenase, creatinine, and urea peaked 6-h post-run. Donnelly et al. [62] revealed that serum CK and urea levels were significantly higher after ibuprofen supplementation compared to placebo throughout the post-run recovery. Analyzing hemodynamic clinical chemistry makers before, 4-h, and 24-h post-Boston marathon, Kratz et al. [63] reported significant increases in total CK, AST, ALT, total protein, uric acid, and creatinine 4-h post-race and confirmed that CK, creatinine, uric acid, ALT, and AST remained significantly elevated over pre-race values 24-h post-race. The attenuation of these markers in the current study, demonstrates beneficial powdered tart cherry supplementation effects on the post-resistance exercise catabolic, muscle damage, and stress response.
Acute, intensive resistance exercise (>60 % 1-RM) has been reported to significantly increase muscular mechanical trauma as the initial phase of injury [4, 64] coupled with a secondary inflammatory injury phase that occurs as a result of plasma protein and inflammatory cell infiltration of the damaged tissue [4, 9, 57]. Howatson et al. [44] reported significantly lower IL-6 concentrations after running a marathon that coincided with quicker recovery of knee extensor maximal strength following the marathon in Montmorency tart cherry juice supplemented subjects compared to placebo [44]. As an inflammatory marker, IL-6 was significantly attenuated immediately post-race with tart cherry consumption compared to placebo [43, 44]. In a strength-based supplement study with resistance trained subjects, Bowtell et al. [4] was also not able to detect any significant effects on IL-6 as a marker of inflammation. Further, Trombold et al. [65] reported similar non-significant inflammatory (IL-6) findings in a study involving non-resistance trained subjects performing repeated bouts of eccentric elbow flexor muscle contractions with consumption of pomegranate-derived ellagitannin [4]. Supplementation in the current study did not affect the inflammatory response to muscular trauma as markers peaked 60-min post-lift with no response differences between supplementation groups. Attenuation of muscle soreness perceptions and markers of muscle damage are not likely attributed to changes in inflammation following a single resistance exercise bout. Differences in results between studies are likely attributed to variation in intensity, duration, and modality of the aforementioned exercise protocols.
The increase in inflammatory-related cytokines and chemokines such as TNF-α, IL-1β, IL-6, and IL-8 during and immediately following exercise has been shown in previous research [66–68] and closely resembles the inflammatory response in the current study. As described in previous literature, the increase in serum IL-6 within both groups 60-min post-lift in the current study can likely be attributed to the large release of muscle-derived IL-6 as a result of the contracting muscle fibers [66, 69]. The influx of muscle-derived IL-6 within the systemic circulation may be one of the triggers for subsequent cortisol release in response to exercise-induced stress [66, 70]. Glucocorticoids, specifically cortisol, and anti-inflammatory cytokines such as IL-4, IL-10, and IL-13 are released during strenuous exercise and typically demonstrate an immunosuppressive influence to help modulate the immune response balance [71]. However, extended immunodepression due to elevated plasma cortisol during the post-exercise period, particularly following long bouts of intense training in the fasted state [66], may hinder recovery and subsequent performance. Previous research conducted by McAnulty et al. [72] in trained endurance athletes reported a significant cortisol increase over time, but no significant differences in immediate and 1.5-h post-race cortisol levels with 2-month vitamin E supplementation compared to placebo. Comparing 14-d supplementation of N-acetyl-cysteine (NAC), epigallocatechin gallate (EGCG), or a placebo in active males surrounding a single bout of eccentric knee-extensor exercise, Kerksick et al. [73] found a significant decrease in serum cortisol levels 6-h post-exercise, but no differences between groups up to 72-h post-exercise despite a significant blunting of muscle soreness perception 24-h post exercise in the two supplement groups compared to placebo. The current study demonstrated an increase in cortisol levels 60-min post-lift compared to pre-lift in both groups. However, unlike the cortisol and muscle soreness perception results reported by Kerksick et al. [73], the current study demonstrated significantly elevated cortisol levels and muscle soreness in the placebo group compared to the powdered tart cherry group 24-h and 48-h post-lift.
The placebo group elevations in serum cortisol levels in the latter recovery period may be linked to differences in the immune response and the degree of secondary muscle injury. Previous research by McCarthy et al. [74] and Robson et al. [75] demonstrate that in response to repetitive muscular contraction during and immediately after acute, intense exercise, there is a large increase in circulating neutrophils (GRAN) and lymphocytes. According to data collected by Robson et al. [75], plasma neutrophil levels tend to peak 2–3 hours post-exercise, but remain elevated above pre-exercise levels for up to 10-h post-exercise due to elevations in plasma cortisol (e.g. perception of stress) that facilitate the release of neutrophils from the bone marrow. The current study demonstrated a plasma neutrophil (GRAN) peak 60-min post-lift, but effects of supplementation could not be determined as levels returned to pre-lift concentrations by 24-h. Unlike the neutrophil response to acute exercise, lymphocytes respond in a biphasic pattern, where plasma levels are significantly elevated during and immediately post-exercise followed by a drop below pre-exercise levels during the initial recovery period due to a efflux from the blood circulation, and a slow return to baseline levels as equilibrium is reached again [71, 75]. Robson et al. [75] demonstrated that serum cortisol levels influence both the influx of neutrophils and efflux of lymphocytes from systemic circulation. The results of the current study demonstrated that lymphocyte levels in the placebo group never returned to pre-lift levels during the 48-h recovery. This may be linked to the elevated plasma cortisol levels in the placebo group representing an increased physiological perception of exercise stress 48-h post-lift compared to pre-lift and powdered tart cherry group levels. As previously mentioned, the secondary injury phase occurs as a result of significant plasma protein and inflammatory cell influx within the damaged tissue [4, 9, 57]. Powdered tart cherry supplementation may have aided in reducing secondary muscular injury through an initial dampening of the immune response paired with the attenuated cortisol response later in exercise recovery. However, immune cell count changes in response to supplementation and the resistance exercise stimulus are speculative due to the lack of cellular count data corresponding to typical patterns of immune cell responses during and immediately following the barbell back squat challenge.
The increase in antioxidant bioavailability from tart cherries containing high levels of flavonoids and anthocyanins [76, 77] has been hypothesized to be one of the main benefits of tart cherry supplementation. Antioxidant bioavailability is important in maintaining adequate redox balance to support the endogenous antioxidant systems following excessive ROS-producing strenuous exercise. Ducharme et al. [61] investigated the effects of Montmorency tart cherry juice supplementation on oxidative stress in thoroughbred horses following a stepwise incremental treadmill running protocol [43]. Overall indications of oxidative stress as a result of exercise were reported through significant elevations in lipid hydroperoxidation (TBARS) [61], but similar to the current study, no differences between groups were shown. In an endurance study examining the effects of tart cherry juice supplementation on oxidative stress following a marathon run, Howatson et al. [44] demonstrated significantly lower TBARS levels in the tart cherry supplemented group versus placebo 48-h post-marathon. As a highly reactive oxide metabolite of nitric oxide, peroxynitrate-bound tyrosine residues forming nitrotyrosine (NT) [78] were measured by Sureda et al. [79] following supplementation of vitamin C + E surrounding a half-marathon. Suerda et al. [79] reported a significant increase in both NT immediately post-race and 3-h post-race in the placebo group compared to the vitamin C + E supplemented group, indicating that antioxidant supplementation may have an attenuating effect on the oxidation of nitrogen-containing compounds surrounding endurance exercise. Contrarily, both Bowtell et al. [4] and the current study found no difference between supplementation groups with regard to oxidation of nitric oxide (NT) in the post-strength exercise period. A difference in outcome may be attributable to the difference in exercise modality and thus the minimal reliance on aerobic metabolic demands during resistance exercise. Further, evidence in the literature utilizing lipid peroxidation (TBARS) analyses have presented a potential lack of oxidative damage detection specificity in human studies that may also explain the variability in results between the current and previous studies [11, 43, 80, 81].
In a study analyzing the recovery from a marathon run, Howatson et al. [44] analyzed plasma TAS, as a measure of antioxidant or antiradical activity [82], and found that TAS fell below baseline 48-h post-marathon in the placebo group [43]. Unlike the tart cherry group, the placebo group failed to maintain TAS or redox balance following the endurance exercise, demonstrating possible antioxidant effectiveness on excessive ROS production with bouts of endurance exercise [43]. Childs et al. [83] analyzing TAS following arm eccentric exercise-induced injury demonstrated significantly greater TAS levels with vitamin C + NAC supplementation compared to placebo 48-h through 7-d post-exercise in healthy, untrained males. A resistance training-related study conducted by Lafay et al. [84] investigating the effects of a polyphenol-rich grape extract on repeated jumping force capacity, found a significant jumping force capacity improvement in the grape extract group over placebo. However, similar to the current study, Lafay et al. [84] found no significant difference in SOD activity between the groups. The current resistance training study also did not demonstrate any significant change in TAS activity across time or between groups possibly attributed to differences in study population and the low metabolic cost associated with resistance/eccentric exercise versus endurance exercise.
The strengths of this particular study revolve around the analysis of a large cohort of muscle soreness, performance, muscle damage, inflammatory, and oxidative damage measures to contribute a comprehensive analysis to the existing body of published literature. This type of comprehensive hemodynamic analysis has not been conducted in a tart cherry supplement study paired with resistance exercise. Further, the current study employed one of the largest subject cohorts among recent studies examining polyphenolic, vitamin, or NSAID supplementation effects on performance and related hemodynamic markers. The utilization of this supplement within the free-living resistance trained population demonstrated its effectiveness under normative training, diet, and performance conditions. The ease associated with consuming an encapsulated powdered supplement versus a potentially inconvenient or unpalatable juice most likely enhanced subject compliance with supplementation. Potential limitations and weakness of the current study should also be considered. The placebo-control matched design of this study was effective in equalizing study subject exposure to the resistance protocol irrespective of supplement group, however compared to a cross-over design, there might have been some variability associated with subject pairing. Differences in resistance training experience and overall state of training beyond the study inclusion/exclusion criteria may also have been a source of variability in study cohort recruitment. Due to the large number of hemodynamic markers measured in this study, the five selected time points of blood draws over the course of the experimental period may have not captured the entire pharmacokinetic profile for each marker. Despite controlling 48-h training activity and NSAID intake with a 10-h fast before each session, nutritional intake and hydration status of each subject was not controlled nor tested. Differences in nutrition and hydration among study subjects could have been a potential source of hemodynamic marker or performance measure variability in a cohort of this size. Further, the 48-hour NSAID ingestion limitation may not have been a sufficient washout time for all NSAID-type medications considering the large range in reported half-life of common NSAIDs (1.6 to 30-h). The major overriding strengths of this study are that this is the first practical study to be conducted surrounding an acute bout of strength exercise incorporating significant muscle mass recruitment and it is the first study to be conducted utilizing a powdered form of cherries rather than a juice or concentrate.
Conclusions
The current study demonstrated that consumption of a Montmorency powdered tart cherry supplement 7-d before, the day of, and 2-d after completing a single bout of high volume, high-intensity resistance exercise, appears to be an effective dietary supplement in reducing muscle soreness across the most biomechanically loaded region of the quadriceps near the distal patellar attachment and markers of muscle catabolism in resistance trained individuals. Short-term supplementation with powdered tart cherry surrounding a single bout of resistance training did not demonstrate any definitive effect on markers of oxidative damage or inflammation. Due to the inconclusive oxidative damage and inflammatory evidence, mechanisms of short-term powdered tart cherry and other related phytochemical-containing nutritional supplements surrounding bouts of high intensity, anaerobic and resistance exercise need to be further investigated. Additional examination of powdered tart cherry supplementation with other forms of exercise that are known to promote a more pronounced effect on inflammation and oxidative stress (e.g. endurance exercise) is also needed. However, the initial effectiveness in reducing perceptions of muscle soreness and markers of muscle catabolism in resistance-trained men demonstrates that powdered tart cherry supplementation provides similar benefits as previously studied tart cherry juices or concentrates following acute bouts of lower body strength-based exercise.
Acknowledgements
We would like to thank the subjects that participated in this study as well as all of the students and laboratory assistants in the Exercise & Sport Nutrition Laboratory at Texas A&M University who assisted with data collection. We would also like to thank Mike Vaughn and Katherine Kelly for their valuable assistance in sample analysis.
Footnotes
Competing interests
This study was funded by Anderson Global Group, LLC (Irvine, CA, USA) and Shoreline Fruit, LLC (Traverse City, MI, USA) through an unrestricted research grant to Texas A&M University. All researchers involved independently collected, analyzed, and interpreted the results from this study and have no financial interests concerning the outcome of this investigation. RBK serves as a university approved scientific consultant for Anderson Global Group, LLC and has served as a scientific and legal consultant related to exercise and nutrition. The results from this study do not constitute endorsement by the authors and/or the institution concerning the nutrients investigated.
Authors’ contributions
KL assisted in designing the study, writing the grant, study coordination, data collection, data analysis, and manuscript preparation. RD, CG, AO, SS, and NB assisted in data collection and sample analysis. EG assisted with data collection, sample analysis, and reviewed dietary inventories as the Registered Dietician on staff. CR is the director of the Exercise & Sport Nutrition Lab. MG assisted in research design and consultation. SMT, SR, and SC assisted in study consultation. RBK (corresponding author) assisted in the design of the study, data analysis, manuscript preparation, obtained the grant, and served as the study PI. All authors read and approved the final manuscript.
Contributor Information
Kyle Levers, Email: klevers@hlkn.tamu.edu.
Ryan Dalton, Email: ryanldalton@hlkn.tamu.edu.
Elfego Galvan, Email: egalvan@hlkn.tamu.edu.
Chelsea Goodenough, Email: chelsea.goodenough@hlkn.tamu.edu.
Abigail O’Connor, Email: oconnora7@hlkn.tamu.edu.
Sunday Simbo, Email: sysimbo@hlkn.tamu.edu.
Nicholas Barringer, Email: nicholas.d.barringer.mil@mail.mil.
Susanne U. Mertens-Talcott, Email: smtalcott@tamu.edu
Christopher Rasmussen, Email: crasmussen@hlkn.tamu.edu.
Mike Greenwood, Email: mgreenwood@hlkn.tamu.edu.
Steven Riechman, Email: sriechman@hlkn.tamu.edu.
Stephen Crouse, Email: crouse@hlkn.tamu.edu.
Richard B. Kreider, Email: rkreider@hlkn.tamu.edu
References
- 1.Fallon KE. The acute phase response and exercise: the ultramarathon as prototype exercise. Clin J Sport Med. 2001;11(1):38–43. doi: 10.1097/00042752-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Mastaloudis A, Morrow JD, Hopkins DW, Devaraj S, Traber MG. Antioxidant supplementation prevents exercise-induced lipid peroxidation, but not inflammation, in ultramarathon runners. Free Radic Biol Med. 2004;36(10):1329–41. doi: 10.1016/j.freeradbiomed.2004.02.069. [DOI] [PubMed] [Google Scholar]
- 3.Urso ML. Anti-inflammatory interventions and skeletal muscle injury: benefit or detriment? J Appl Physiol. 2013;115(6):920–8. doi: 10.1152/japplphysiol.00036.2013. [DOI] [PubMed] [Google Scholar]
- 4.Bowtell JL, Sumners DP, Dyer A, Fox P, Mileva KN. Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Med Sci Sports Exerc. 2011;43(8):1544–51. doi: 10.1249/MSS.0b013e31820e5adc. [DOI] [PubMed] [Google Scholar]
- 5.Schoenfeld BJ. The Use of Nonsteroidal anti-inflammatory drugs for exercise-induced muscle damage. Sports Med. 2012;42(12):1017–28. doi: 10.1007/BF03262309. [DOI] [PubMed] [Google Scholar]
- 6.Friden J, Sjöström M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med. 1983;4(3):170–6. doi: 10.1055/s-2008-1026030. [DOI] [PubMed] [Google Scholar]
- 7.Lieber RL, Fridén J. Mechanisms of muscle injury after eccentric contraction. Aust. J. Sci. Med. Sport. 1999;2(3):253–65. doi: 10.1016/S1440-2440(99)80177-7. [DOI] [PubMed] [Google Scholar]
- 8.Tidball JG. Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc. 1995;27(7):1022–32. doi: 10.1249/00005768-199507000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002;81(11):S52–S69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- 10.Proske U, Morgan D. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001;537(2):333–45. doi: 10.1111/j.1469-7793.2001.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell PG, Walshe IH, Davison GW, Stevenson E, Howatson G. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients. 2014;6(2):829–43. doi: 10.3390/nu6020829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trappe TA, White F, Lambert CP, Cesar D, Hellerstein M, Evans WJ. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am. J. Physiol. Endocrinol. Metab. 2002;282(3):E551–E6. doi: 10.1152/ajpendo.00352.2001. [DOI] [PubMed] [Google Scholar]
- 13.Bondesen BA, Mills ST, Kegley KM, Pavlath GK. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am. J. Physiol. Cell Physiol. 2004;287(2):C475–C83. doi: 10.1152/ajpcell.00088.2004. [DOI] [PubMed] [Google Scholar]
- 14.Mikkelsen U, Langberg H, Helmark I, Skovgaard D, Andersen L, Kjaer M, et al. Local NSAID infusion inhibits satellite cell proliferation in human skeletal muscle after eccentric exercise. J Appl Physiol. 2009;107(5):1600–11. doi: 10.1152/japplphysiol.00707.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodemann H, Goldberg A. Arachidonic acid, prostaglandin E and F2a influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem. 1982;257:1632–8. [PubMed] [Google Scholar]
- 16.Palmer R. Prostaglandins and the control of muscle protein synthesis and degradation. Prostaglandins Leukot Essent Fat Acids. 1990;39(2):95–104. doi: 10.1016/0952-3278(90)90017-F. [DOI] [PubMed] [Google Scholar]
- 17.Mikkelsen U, Schjerling P, Helmark IC, Reitelseder S, Holm L, Skovgaard D, et al. Local NSAID infusion does not affect protein synthesis and gene expression in human muscle after eccentric exercise. Scand J Med Sci Sports. 2011;21(5):630–44. doi: 10.1111/j.1600-0838.2010.01170.x. [DOI] [PubMed] [Google Scholar]
- 18.Burd NA, Dickinson JM, LeMoine JK, Carroll CC, Sullivan BE, Haus JM, et al. Effect of a cyclooxygenase-2 inhibitor on postexercise muscle protein synthesis in humans. Am. J. Physiol. Endocrinol. Metab. 2010;298(2):E354–E61. doi: 10.1152/ajpendo.00423.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen SG, Miller BF, Hansen M, Kjaer M, Holm L. Exercise and NSAIDs: effect on muscle protein synthesis in patients with knee osteoarthritis. Med Sci Sports Exerc. 2011;43(3):425–31. doi: 10.1249/MSS.0b013e3181f27375. [DOI] [PubMed] [Google Scholar]
- 20.Connolly DA, McHugh MP, Padilla-Zakour OI, Carlson L, Sayers SP. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br J Sports Med. 2006;40(8):679–83. doi: 10.1136/bjsm.2005.025429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bell PG, Gaze DC, Davison GW, George TW, Scotter MJ, Howatson G. Montmorency tart cherry (Prunus cerasus L.) concentrate lowers uric acid, independent of plasma cyanidin-3-O-glucosiderutinoside. J Funct Foods. 2014;11:82–90. doi: 10.1016/j.jff.2014.09.004. [DOI] [Google Scholar]
- 22.Cho J, Kang JS, Long PH, Jing J, Back Y, Chung K-S. Antioxidant and memory enhancing effects of purple sweet potato anthocyanin and cordyceps mushroom extract. Arch Pharm Res. 2003;26(10):821–5. doi: 10.1007/BF02980027. [DOI] [PubMed] [Google Scholar]
- 23.Teow CC, Truong V-D, McFeeters RF, Thompson RL, Pecota KV, Yencho GC. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007;103(3):829–38. doi: 10.1016/j.foodchem.2006.09.033. [DOI] [Google Scholar]
- 24.Suda I, Oki T, Masuda M, Kobayashi M, Nishiba Y, Furuta S. Physiological functionality of purple-fleshed sweet potatoes containing anthocyanins and their utilization in foods. JARQ. 2003;37(3):167–73. doi: 10.6090/jarq.37.167. [DOI] [Google Scholar]
- 25.Wootton-Beard PC, Moran A, Ryan L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin–Ciocalteu methods. Food Res Int. 2011;44(1):217–24. doi: 10.1016/j.foodres.2010.10.033. [DOI] [Google Scholar]
- 26.Wootton-Beard PC, Ryan L. A beetroot juice shot is a significant and convenient source of bioaccessible antioxidants. J Funct Foods. 2011;3(4):329–34. doi: 10.1016/j.jff.2011.05.007. [DOI] [Google Scholar]
- 27.Zielińska‐Przyjemska M, Olejnik A, Dobrowolska‐Zachwieja A, Grajek W. In vitro effects of beetroot juice and chips on oxidative metabolism and apoptosis in neutrophils from obese individuals. Phytother Res. 2009;23(1):49–55. doi: 10.1002/ptr.2535. [DOI] [PubMed] [Google Scholar]
- 28.Ruel G, Pomerleau S, Couture P, Lamarche B, Couillard C. Changes in plasma antioxidant capacity and oxidized low-density lipoprotein levels in men after short-term cranberry juice consumption. Metabolism. 2005;54(7):856–61. doi: 10.1016/j.metabol.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 29.Liburt N, McKeever K, Streltsova J, Franke W, Gordon ME, Manso Filho H, et al. Effects of ginger and cranberry extracts on the physiological response to exercise and markers of inflammation in horses. Comp. Exercise Physiol. 2009;6(04):157–69. doi: 10.1017/S175525401000005X. [DOI] [Google Scholar]
- 30.McAnulty LS, Nieman DC, Dumke CL, Shooter LA, Henson DA, Utter AC, et al. Effect of blueberry ingestion on natural killer cell counts, oxidative stress, and inflammation prior to and after 2.5 h of running. Appl Physiol Nutr Metab. 2011;36(6):976–84. doi: 10.1139/h11-120. [DOI] [PubMed] [Google Scholar]
- 31.Sánchez-Moreno C, Kimler VA, Cordts FL, Cady JA, Weller MA, Dumper JW, et al. Effect of a blueberry nutritional supplement on macronutrients, food group intake, and plasma vitamin E and vitamin C in US athletes. Int J Food Sci Nutr. 2008;59(4):327–38. doi: 10.1080/09637480701550176. [DOI] [PubMed] [Google Scholar]
- 32.Chandra A, Nair MG, Iezzoni A. Evaluation and characterization of the anthocyanin pigments in tart cherries (Prunus cerasus L.) J Agric Food Chem. 1992;40(6):967–9. doi: 10.1021/jf00018a010. [DOI] [Google Scholar]
- 33.Wang H, Nair MG, Iezzoni AF, Strasburg GM, Booren AM, Gray JI. Quantification and characterization of anthocyanins in Balaton tart cherries. J Agric Food Chem. 1997;45(7):2556–60. doi: 10.1021/jf960896k. [DOI] [Google Scholar]
- 34.Chandra A, Nair MG, Iezzoni AF. Isolation and stabilization of anthocyanins from tart cherries (Prunus cerasus L.) J Agric Food Chem. 1993;41(7):1062–5. doi: 10.1021/jf00031a009. [DOI] [Google Scholar]
- 35.McCune LM, Kubota C, Stendell-Hollis NR, Thomson CA. Cherries and health: a review. Crit Rev Food Sci Nutr. 2010;51(1):1–12. doi: 10.1080/10408390903001719. [DOI] [PubMed] [Google Scholar]
- 36.Kelley DS, Rasooly R, Jacob RA, Kader AA, Mackey BE. Consumption of Bing sweet cherries lowers circulating concentrations of inflammation markers in healthy men and women. J Nutr. 2006;136(4):981–6. doi: 10.1093/jn/136.4.981. [DOI] [PubMed] [Google Scholar]
- 37.Traustadottir T, Davies SS, Stock AA, Su Y, Heward CB, Roberts LJ, 2nd, et al. Tart cherry juice decreases oxidative stress in healthy older men and women. J Nutr. 2009;139(10):1896–900. doi: 10.3945/jn.109.111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacob RA, Spinozzi GM, Simon VA, Kelley DS, Prior RL, Hess-Pierce B, et al. Consumption of cherries lowers plasma urate in healthy women. J Nutr. 2003;133(6):1826–9. doi: 10.1093/jn/133.6.1826. [DOI] [PubMed] [Google Scholar]
- 39.Schumacher HR, Pullman-Mooar S, Gupta SR, Dinnella JE, Kim R, McHugh MP. Randomized double-blind crossover study of the efficacy of a tart cherry juice blend in treatment of osteoarthritis (OA) of the knee. Osteoarthr. Cart. 2013;21(8):1035–41. doi: 10.1016/j.joca.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Blau L. Cherry diet control for gout and arthritis. Tex. Rep. Biol. Med. 1950;8:309–11. [PubMed] [Google Scholar]
- 41.Howatson G, Bell PG, Tallent J, Middleton B, McHugh MP, Ellis J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur J Nutr. 2012;51(8):909–16. doi: 10.1007/s00394-011-0263-7. [DOI] [PubMed] [Google Scholar]
- 42.Pigeon WR, Carr M, Gorman C, Perlis ML. Effects of a tart cherry juice beverage on the sleep of older adults with insomnia: a pilot study. J Med Food. 2010;13(3):579–83. doi: 10.1089/jmf.2009.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bell P, McHugh M, Stevenson E, Howatson G. The role of cherries in exercise and health. Scand J Med Sci Sports. 2013;24(3):477–90. doi: 10.1111/sms.12085. [DOI] [PubMed] [Google Scholar]
- 44.Howatson G, McHugh MP, Hill JA, Brouner J, Jewell AP, van Someren KA, et al. Influence of tart cherry juice on indices of recovery following marathon running. Scand J Med Sci Sports. 2010;20(6):843–52. doi: 10.1111/j.1600-0838.2009.01005.x. [DOI] [PubMed] [Google Scholar]
- 45.Dimitriou L, Hill JA, Jehnali A, Dunbar J, Brouner J, McHugh MP, et al. Influence of a montmorency cherry juice blend on indices of exercise-induced stress and upper respiratory tract symptoms following marathon running—a pilot investigation. J Int Soc Sports Nutrition. 2015;12(1):1–7. doi: 10.1186/s12970-015-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee Y-A, Hong S-J, Lee S-H, Yang H-I. Perioperative medication management in arthritic patients. J Korean Rheumatism Association. 2008;15(2):101–9. doi: 10.4078/jkra.2008.15.2.101. [DOI] [Google Scholar]
- 47.DIagnostic and therapeutic technology assessment (datta). JAMA. 1992;267(2):286–94. doi:10.1001/jama.1992.03480020096040 [PubMed]
- 48.Klesges RC, Ward KD, Shelton ML, Applegate WB, Cantler ED, Palmieri GM, et al. Changes in bone mineral content in male athletes: mechanisms of action and intervention effects. Jama. 1996;276(3):226–30. doi: 10.1001/jama.1996.03540030060033. [DOI] [PubMed] [Google Scholar]
- 49.Cureton KJ, Tomporowski PD, Singhal A, Pasley JD, Bigelman KA, Lambourne K, et al. Dietary quercetin supplementation is not ergogenic in untrained men. J Appl Physiol. 2009;107(4):1095–104. doi: 10.1152/japplphysiol.00234.2009. [DOI] [PubMed] [Google Scholar]
- 50.Bowling JL, Katayev A. An evaluation of the Roche Cobas c 111. Lab Medicine. 2010;41(7):398–402. doi: 10.1309/LM6T8D1LKQXVNCAC. [DOI] [Google Scholar]
- 51.Dunbar SA, Hoffmeyer MR. Microsphere-Based Multiplex 2.9 Immunoassays: Development and Applications Using Luminex® xMAP® Technology. The Immunoassay Handbook: Theory and applications of ligand binding, ELISA and related techniques. 2013. p. 157. [Google Scholar]
- 52.Litteljohn D, Hayley S. Cytokines as potential biomarkers for Parkinson’s disease: a multiplex approach. Psychoneuroimmunology. Springer; 2012. p. 121–44. [DOI] [PubMed]
- 53.Wang K, Wadhwa PD, Culhane JF, Nelson EL. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: determinations of a panel of nine cytokines in clinical sample culture supernatants. J Reprod Immunol. 2005;66(2):175–91. doi: 10.1016/j.jri.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacobson JW, Oliver KG, Weiss C, Kettman J. Analysis of individual data from bead-based assays (“bead arrays”) Cytometry Part A. 2006;69(5):384–90. doi: 10.1002/cyto.a.20293. [DOI] [PubMed] [Google Scholar]
- 55.Schumm WR, Pratt KK, Hartenstein JL, Jenkins BA, Johnson GA. Determining statistical signifi cance (alpha) and reporting statistical trends: controversies, issues, and facts 1. Compr. Psychol. 2013;2(1):10. doi: 10.2466/03.CP.2.10. [DOI] [Google Scholar]
- 56.Enoka RM, Fuglevand AJ. Motor unit physiology: some unresolved issues. Muscle Nerve. 2001;24(1):4–17. doi: 10.1002/1097-4598(200101)24:1<4::AID-MUS13>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 57.MacIntyre DL, Reid WD, McKenzie DC. Delayed muscle soreness. Sports Med. 1995;20(1):24–40. doi: 10.2165/00007256-199520010-00003. [DOI] [PubMed] [Google Scholar]
- 58.Cheung K, Hume PA, Maxwell L. Delayed onset muscle soreness. Sports Med. 2003;33(2):145–64. doi: 10.2165/00007256-200333020-00005. [DOI] [PubMed] [Google Scholar]
- 59.Armstrong R. Initial events in exercise-induced muscular injury. Med Sci Sports Exerc. 1990;22(4):429–35. [PubMed] [Google Scholar]
- 60.Smith LL. Acute inflammation: the underlying mechanism in delayed onset muscle soreness? Med Sci Sports Exerc. 1991;23(5):542–51. doi: 10.1249/00005768-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Ducharme NG, Fortier LA, Kraus MS, Hobo S, Mohammed HO, McHugh MP, et al. Effect of a tart cherry juice blend on exercise-induced muscle damage in horses. Am J Vet Res. 2009;70(6):758–63. doi: 10.2460/ajvr.70.6.758. [DOI] [PubMed] [Google Scholar]
- 62.Donnelly A, Maughan R, Whiting P. Effects of ibuprofen on exercise-induced muscle soreness and indices of muscle damage. Br J Sports Med. 1990;24(3):191–5. doi: 10.1136/bjsm.24.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kratz A, Lewandrowski KB, Siegel AJ, Chun KY, Flood JG, Van Cott EM, et al. Effect of marathon running on hematologic and biochemical laboratory parameters, including cardiac markers. Am J Clin Pathol. 2002;118(6):856–63. doi: 10.1309/14TY-2TDJ-1X0Y-1V6V. [DOI] [PubMed] [Google Scholar]
- 64.Hudson MB, Hosick PA, McCaulley GO, Schrieber L, Wrieden J, Mcanulty SR, et al. The effect of resistance exercise on humoral markers of oxidative stress. Med Sci Sports Exerc. 2008;40(3):542. doi: 10.1249/MSS.0b013e31815daf89. [DOI] [PubMed] [Google Scholar]
- 65.Trombold JR, Barnes JN, Critchley L, Coyle EF. Ellagitannin consumption improves strength recovery 2–3 d after eccentric exercise. Med Sci Sports Exerc. 2010;42(3):493–8. doi: 10.1249/MSS.0b013e3181b64edd. [DOI] [PubMed] [Google Scholar]
- 66.Gleeson M. Immune function in sport and exercise. J Appl Physiol. 2007;103(2):693–9. doi: 10.1152/japplphysiol.00008.2007. [DOI] [PubMed] [Google Scholar]
- 67.Pedersen BK, Toft AD. Effects of exercise on lymphocytes and cytokines. Br J Sports Med. 2000;34(4):246–51. doi: 10.1136/bjsm.34.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK. Pro‐and anti‐inflammatory cytokine balance in strenuous exercise in humans. J Physiol. 1999;515(1):287–91. doi: 10.1111/j.1469-7793.1999.287ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steensberg A, Hall G, Osada T, Sacchetti M, Saltin B, Pedersen BK. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise‐induced increase in plasma interleukin-6. J Physiol. 2000;529(1):237–42. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol-Endocr Metabol. 2003;285(2):E433–E7. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- 71.Gleeson M, Bishop NC. The T cell and NK cell immune response to exercise. Ann. Transplant. 2005;10(4):44. [PubMed] [Google Scholar]
- 72.McAnulty SR, McAnulty LS, Nieman DC, Morrow JD, Shooter LA, Holmes S, et al. Effect of alpha-tocopherol supplementation on plasma homocysteine and oxidative stress in highly trained athletes before and after exhaustive exercise. J Nutr Biochem. 2005;16(9):530–7. doi: 10.1016/j.jnutbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 73.Kerksick CM, Kreider RB, Willoughby DS. Intramuscular adaptations to eccentric exercise and antioxidant supplementation. Amino Acids. 2010;39(1):219–32. doi: 10.1007/s00726-009-0432-7. [DOI] [PubMed] [Google Scholar]
- 74.McCarthy D, Dale M. The leucocytosis of exercise. Sports Med. 1988;6(6):333–63. doi: 10.2165/00007256-198806060-00002. [DOI] [PubMed] [Google Scholar]
- 75.Robson P, Blannin A, Walsh N, Castell LM, Gleeson M. Effects of exercise intensity, duration and recovery on in vitro neutrophil function in male athletes. Int J Sports Med. 1999;20(2):128–30. doi: 10.1055/s-2007-971106. [DOI] [PubMed] [Google Scholar]
- 76.Wang H, Nair MG, Strasburg GM, Chang Y-C, Booren AM, Gray JI, et al. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J Nat Prod. 1999;62(2):294–6. doi: 10.1021/np980501m. [DOI] [PubMed] [Google Scholar]
- 77.Seeram NP, Bourquin LD, Nair MG. Degradation products of cyanidin glycosides from tart cherries and their bioactivities. J Agric Food Chem. 2001;49(10):4924–9. doi: 10.1021/jf0107508. [DOI] [PubMed] [Google Scholar]
- 78.Vinten-Johansen J. Physiological effects of peroxynitrite potential products of the environment. Circ Res. 2000;87(3):170–2. doi: 10.1161/01.RES.87.3.170. [DOI] [PubMed] [Google Scholar]
- 79.Sureda A, Ferrer MD, Mestre A, Tur JA, Pons A. Prevention of neutrophil protein oxidation with vitamins C and e diet supplementation without affecting the adaptive response to exercise. Int J Sport Nutr Exerc Metab. 2013;23(1):31–9. doi: 10.1123/ijsnem.23.1.31. [DOI] [PubMed] [Google Scholar]
- 80.Bell PG, Walshe IH, Davison GW, Stevenson EJ, Howatson G. Recovery facilitation with Montmorency cherries following high-intensity, metabolically challenging exercise. Appl Physiol Nutr Metab. 2015;40(4):414–23. doi: 10.1139/apnm-2014-0244. [DOI] [PubMed] [Google Scholar]
- 81.Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189(1):41–54. doi: 10.1016/S0300-483X(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 82.Janaszewska A, Bartosz G. Assay of total antioxidant capacity: comparison of four methods as applied to human blood plasma. Scand J Clin Lab Investig. 2002;62(3):231–6. doi: 10.1080/003655102317475498. [DOI] [PubMed] [Google Scholar]
- 83.Childs A, Jacobs C, Kaminski T, Halliwell B, Leeuwenburgh C. Supplementation with vitamin C and N-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Radic Biol Med. 2001;31(6):745–53. doi: 10.1016/S0891-5849(01)00640-2. [DOI] [PubMed] [Google Scholar]
- 84.Lafay S, Jan C, Nardon K, Lemaire B, Ibarra A, Roller M, et al. Grape extract improves antioxidant status and physical performance in elite male athletes. J sports sci med. 2009;8(3):468. [PMC free article] [PubMed] [Google Scholar]


