Abstract
Background
The aim of the trial is to demonstrate that with the use of modern IMRT/IGRT and reduction of safety margins postoperative wound complications can be reduced.
Methods/ Design
The trial is designed as a prospective, monocentric clinical phase II trial. The treatment is performed with helical IMRT on the Tomotherapy HiArt System© or with RapidArc© IMRT as available. All treatments are performed with 6 MV photons and daily online CT-based IGRT.
A dose of 50 Gy in 2 Gy single fractions (5 fractions per week) is prescribed. Restaging including MRI of the primary tumor site as well as CT of the thorax/abdomen is planned 4 weeks after RT. PET-examinations or any other imaging can be performed as required clinically. In cases of R1 resection, brachytherapy is anticipated in the 2nd postoperative week. Brachytherapy catheters are implanted into the tumor bed depending on the size and location of the lesion. Surgery is planned 5–6 weeks after completion of neoadjuvant RT. All patients are seen for a first follow-up visit 2 weeks after wound healing is completed, thereafter every 3 months during the first 2 years. The endpoints of the study are evaluated in detail during the first (2 weeks) and second (3 months) follow-up. Functional outcome and QOL are documented prior to treatment and at year 1 and 2. Treatment response and efficacy will be scored according to the RECIST 1.1 criteria. A total patient number of 50 with an expected 20 % rate of wound complications were calculated for the study, which translates into a 95 % confidence interval of 10.0-33.7 % for wound complication rate in a binomial distribution.
Discussion
The present study protocol prospectively evaluates the use of IMRT/IGRT for neoadjuvant RT in patients with soft tissue sarcomas of the extremity with the primary endpoint wound complications, which is the major concern with this treatment sequence. Besides complications rates, local control rates and survival rates, as well as QOL, functional outcome and treatment response parameters (imaging and pathology) are part of the protocol. The data of the present PREMISS study will enhance the current literature and support the hypothesis that neoadjuvant RT with IMRT/IGRT offers an excellent risk-benefit ratio in this patient population.
Trial registration
Background
Treatment of soft tissue sarcomas of the extremities is a challenge for the interdisciplinary team. In general, radiation therapy (RT) is indicated in stage II and III (not T1a). There are two approaches for RT in this situation, either neoadjuvant or adjuvant. For preoperative RT usually lower doses are applied, and a dose of 50 Gy has been established; in the postoperative setting higher doses between 60-66Gy are required. Thus, preoperative RT seems to be more beneficial in terms of long-term RT-associated side effects such as edema, joint stiffness, nerve lesions or bone fractures. On the other hand, preoperative RT is associated with a higher rates of wound complications after surgery (35 % vs. 17 %). This is more or less independently of the location, i.e. shoulder, upper or lower extremity and of the histology, which can be very heterogeneous including liposarcoma, leiomyosarcoma, undifferentiated sarcoma or synovial sarcoma. Thus, due to the required expertise of all disciplines, patients with such tumors should be treated at a specialized sarcoma unit [1–6], since it has been shown that treatment at a high volume center is associated with a significantly increased survival and better functional outcome [7].
Surgery is the mainstay of treatment in sarcomas and should be evaluated in every case. If surgery with a complete removal of the tumor is not possible, RT is a curative alternative; local control rates range between 20–45 % [8]. Generally, function-preserving treatment is a main goal, whereas in the past radical excisions with compartment resections leading to a loss of function, or amputations, were performed regularly; today, function-preserving treatment is anticipated with complete removal where possible, and combination with RT when necessary.
Few randomized studies are available which is mainly due to the low incidence of soft tissue sarcomas: An older trial compared limb amputation with a combination of extremity-conserving resection plus postoperative RT; no difference in disease-free survival and overall survival was observed [9].
Two other trials assessed postoperative RT or interstitial brachytherapy, both studies showed a clear advantage for local control compared to surgery alone [10, 11]. A large number of retrospective analyses have confirmed the positive value of postoperative RT for extremity sarcomas [12–23].
Today, extremity-conserving surgical treatment is possible in 80–95 % of all patients [24–28]. This requires, however, a well-functioning interdisciplinary team consisting of orthopaedic surgeons, radiation oncologists, plastic surgeons, oncologists, pathologists and radiologists [29, 30].
In detail, two main concepts exist for the application of RT: preoperative vs. postoperative. As in other indications such as esophageal, pancreatic or rectal cancer, there are clear arguments in favor of preoperative RT: The treatment volume is generally much smaller, since postoperative changes as well as intraoperatively manipulated tissue including the surgical entry channel and scar do not need to be treated [31]. Compared to postoperative RT, only doses of around 50 Gy are required. The smaller treatment volume together with the lower RT dose result in lower rates of treatment-related side effects. Moreover, tumor as well as normal tissue oxygenation is not impaired due to postoperative scarring; this leads to a higher sensitivity to radiation due to the oxygen enhancement ratio (OER; [23]).
Another factor is the possibility of sterilization around the tumor using preoperative RT, leading to an improved resectability and higher rates of R0-resections [19]. This might be explained by a thickening of the tumor capsule which has been shown in experimental settings. On the other hand, in spite of the clear advantages of preoperative RT, higher rates of surgery-related wound complications have been shown by several groups [21, 32, 33].
A number of comparative analyses between pre- and postoperative RT are currently available in patients with extremity soft tissue sarcomas. A randomized prospective trial by O’Sullivan and colleagues randomized 50 Gy preoperative RT to 66 Gy postoperative RT. In the preoperative group, which consisted of 94 patients, 10 patients received an additional boost up to 16–20 Gy in cases of R1 resections. Initial data showed a slightly improved local control and survival in the preoperative group, however wound complications were 35 % compared to 17 % in the postoperative RT group; function of the extremity was comparable in both groups [13, 18]. Long term data support the beneficial risk-benefit ratio of preoperative RT [14]. In agreement with several retrospective reports lower rates of long-term side effects such as edema, fibrosis, fracture, joint stiffness or nerve toxicity as well as better functional outcome are observed [14, 15, 17, 22, 23, 32]. A meta-analysis including 1098 patients from 5 studies confirmed a higher local control and a higher overall survival of 76 % vs. 67 % for pre-operative RT [34]. A multi-institutional matched-pairs analysis including 821 patients also reported an improved overall survival after preoperative RT [20].
However, it has to be kept in mind that wound complication rates might be higher after preoperative RT with median complication rates of 16–35 %, depending on the series [12, 17–19, 21, 32, 35]. The rate of wound complications is dependent on RT dose, patient age, comorbidities, tumor and resection volume as well as tumor location [18, 21, 32, 36]: For example, patients with wound complications generally have a much larger resection volume than those without complications (919 cm3 vs. 456 cm3) [33].
Regarding the RT technique, 3D-conformal RT was standard over many years. Modern techniques such as intensity modulated radiotherapy (IMRT) offer improved dose conformality even for long and complex shaped volumes. Treatment planning comparisons analyzing 3D vs. IMRT could show that dose coverage as well as reduction of dose to normal tissue (bone, soft tissue) are better with IMRT [37–39]. Thus, since the implementation of IMRT for the treatment of soft tissue sarcomas, positive results were reported [40]. With helical IMRT as Tomotherapy© dose distributions often are even more conformal, longer volumes can be treated, and the treatment machine offers online MV-CT imaging for position verification. Early reports on Tomotherapy© for sarcomas reported excellent results as well as improved sparing of normal tissue [41–44]. For daily repositioning image-guidance as well as positioning devices are necessary. For extremity tumors, positioning inaccuracies of 1 cm or more have been observed [45]. This can be compensated by adequate treatment volumes as well as IGRT approaches. The improvements of RT techniques enable the radiation oncologist to reduce and adapt treatment volumes. For soft tissue sarcomas, in the past, uncertainties in positioning as well as in target volume definition depending e.g. on insufficient imaging have led to very large safety margins of ≥ 5 cm proximal/distal and 2 cm circumferentially around the visible tumor volume [31, 38, 45, 46]. Since optimized imaging including magnetic resonance imaging (MRI) as well as CT or PET-diagnostics are available, these safety margins can be reduced and IGRT approaches assure high precision of treatment delivery. Results from brachytherapy series have shown that local dose application to the tumor with small margins of 1–2 cm are excellent with local control rates of 79–87 (−100) % [10, 40, 47–50]. The main factor, however, is exact definition of the target volume and precise dose delivery. Since complication rates are dependent on the irradiated volume, the rationale for smaller safety margins is an optimization of the risk-benefit ratio [33, 51]; initial clinical data on IMRT for soft tissue sarcomas of the extremity have shown local control of 96 % at 3 years with small margins of 2 cm [52]. Intraoperative Radiotherapy (IORT) or brachytherapy can offer an enhanced therapeutic ratio: With both techniques local dose escalations directly to the target tissue are possible, without irradiation of large areas of normal tissue.
For neoadjuvant RT, doses of 50 Gy have been established, however, in some cases incomplete tumor resection with R1 margins requires individualized approaches. It has been shown that local dose escalation as a boost treatment up to 16–20 Gy with conventional fractionation can be performed, however, series from the literature show controversial results [18, 53].
Combination of percutaneous RT (40–50 Gy) and a brachytherapy boost (15–32 Gy) has been reported to be superior to percutaneous RT or brachytherapy alone [25, 49, 54–58]. Thus, combination treatments are considered as optimal for soft tissue sarcomas with positive resection margins [29, 59].
Rationale for the PREMISS study
Since the techniques of RT have been improved over the last decades, novel concepts for the treatment of soft tissue sarcomas are possible. This includes IGRT approaches with reduced safety margins to improve the therapeutic window in terms of reduction of long-term side effects. Since theoretical advantages of IMRT/IGRT in this patient population have been shown, and initial clinical data confirm this hypothesis, a prospective evaluation of preoperative IMRT/IGRT is necessary. Thus, reduction of safety margins around the visible tumor on MRI of 3 cm longitudinally and 1.5 cm circumferentially is possible based on previously published data [16]. For optimal surgical treatment, three treatment paths are defined for optimal surgical results.
Since in < 30 % of all patients treated with function and extremity preserving RT a R1 resection is present, local dose escalation in analogy to the randomized trial by O’Sullivan is part of this PREMISS trial.
The aim of the trial is to demonstrate that using modern IMRT/IGT and reduction of safety margins, postoperative wound complications can be reduced.
Endpoints of the study
The primary endpoint is the hypothesis that with preoperative IMRT/IRGT using small safety margins in combination with local dose escalation with brachytherapy in the R1 situation a wound complication rate of 20 % can be achieved.
Thus, the rate of wound complications up to 90 days after surgery is scored. Wound complications are defined as
any surgery for wound treatment requiring local or general anesthesia including debridement, operative drainage, secondary or repeated wound closure including rotational plastic, any free tissue transfer or skin transplantations exceeding the procedures included into the protocol
invasive procedures without anesthesia, e.g. 3 x aspiration of seroma
in-patient wound treatment e.g. intravenous antibiotics
<90 days treatments with wound dressing materials
Secondary endpoints of the study are determination of R0-resections, local control, metastases-free survival, overall survival, as well as acute and late toxicities of RT. This includes rates of extremity preservation, function of the extremity as well as quality of life (QOL).
Study design
The trial is designed as a prospective, monocentric clinical phase II trial. The study design is depicted in Fig. 1.
Fig. 1.
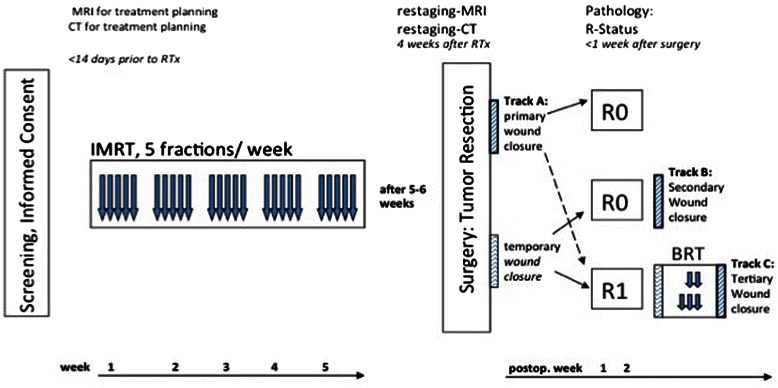
shows the study diagram of the PREMISS Study
Treatment planning for preoperative RT
The extremity will be positioned in a stable and reproducible position using vacuum mats or mask material as necessary. For the planning CT all scars are to be marked with wire. If necessary, bolus material is added and fixed in a reproducible manner.
Treatment planning is based on a CT with 3 mm slice thickness, including the visible tumor and the adjacent joint regions, at least 20 cm beyond the visible tumor. Fusion with MRI is performed within the treatment planning system. MR imaging should include coronal T2 stir, axial T2 with and without contrast, T1 stir with contrast enhancement.
Target Volume definition
The treatment volumes are defined on the planning CT including the following volumes:
primary tumor (PT): macroscopic tumor on contrast-enhanced MRI
gross tumor volume (GTV): PT plus surrounding pseudo capsule, i.e. edema and edematous changes tissue including tumor cell contamination
clinical target volume (CTV): GTV plus safety margins – 1 cm in lateral and ventro-dorsal direction, as well as 2.5 cm in proximal-distal direction. Natural borders are respected, i.e. skin or non-infiltrated bony structures as well as uninvolved compartments.
planning target volume (PTV): CTV plus a circumferential safety margin of 0.5 cm.
Additionally, all relevant organs at risk (OAR) and normal tissue structures are contoured.
Treatment technique and dose prescription
The treatment is performed with helical IMRT on the Tomotherapy HiArt System© or with RapidArc© IMRT as available. All treatments are performed with 6MV-photons and daily online CT-based IGRT.
A dose of 50 Gy in 2 Gy single fractions (5 fractions per week) is prescribed to the median in accordance with ICRU 83, with D50% = 50.0 Gy. At least 95 % of the PTV must receive 95 % of the prescribed dose, i.e. D95% > 47.5 Gy.
Surgical treatment
Surgery is planned 5–6 weeks after completion of neoadjuvant RT. Re-staging including MRI as well as CT of the thorax is planned 4 weeks after RT. PET-examinations or any other imaging can be performed as required clinically.
If possible, the tumor will be resected surrounded by a layer of healthy tissue „en bloc“ in terms of an oncological radical resection ("wide/radical resection"). The resection entry channel from the diagnostic biopsy has to be included completely into the resection including the skin. An incomplete or reductive surgery is to be avoided. Reconstructive surgery for function preservation is anticipated. Curative approaches are the primary goal in situations when function and extremity preservation is not feasible.
The resection specimen must be clipped and marked so that correct anatomical reconstruction and correlation with imaging is possible. The surgeon will clip areas of potential incomplete resection on the resected tissue as well as in the tumor bed.
In cases of lymph node involvement on re-staging examinations in the area of the lymphatic spread of the tumor lymphadenectomy is performed. In cases of lung metastases after neoadjuvant RT or at the time of re-staging local control is still a priority, thus tumor resection is performed. Thereafter, any other measures necessary are taken, such as resection of lung lesions, chemotherapy, RT or other.
In cases of initial complete resection of the tumor direct closure of the wound is performed (Track A). If intraoperative rupture of the tumor occurs or if indication for hypobaric treatment or plastic surgery is present, vacuseal will be brought into the resection cavity and the wound is closed secondarily (Track B and C).
Within 5 days after tumor resection results of the pathological evaluation are available.
If the tumor is resected completely (R0), vacuseal is removed and the wound is closed (track B), if necessary with plastic surgery. If pathology reveals R1 status, secondary resection should be evaluated. If this is not possible with a function-preserving approach, local brachytherapy treatment in the resection cavity is performed. Thereafter, the wound is closed.
Pathology assessment
For precise pathological evaluation precise orientation of the resected specimen is necessary, thus, it is recommended that a pathologist is present at the time of tumor resection. Classification of tumor resection margins is of high importance since the indication for local boost dose escalation is dependent on this result. Boost treatment should be performed on day 6–8 after resection. Pathological classification should therefore be performed within 5 days after surgery and resection margins (R0, R1, Rx) have to be communicated to the orthopaedic surgeon and the radiation oncologist.
Besides resection margins, tumor grading as well as further immunohistochemical stainings for exact pathological diagnosis will be performed. The tumor will be measured in all dimensions (in cm). Response to RT according to the established pathological protocol for osteosarcomas according to Salzer-Kuntschik will be evaluated [60]. Vital tumor cells will be evaluated as established also for osteosarcomas [61].
Local dose escalation
In cases of R1 resection brachytherapy is anticipated in the 2. postoperative week. Brachytherapy catheters are implanted into the tumor bed depending on the size and location of the lesion.
Treatment planning is based on 3D-CT imaging with 3 mm slice thickness as well as the most recent MRI available.
The CTVBRT for the brachytherapy application includes the R1-area plus a 5 mm safety margin, or a boost the complete resection cavity plus 5 mm safety margin. No additional PTVBRT is added since the catheters are implanted directly into the target area.
Brachytherapy is performed using Iridium-192 High-Dose Rate (HDR)-afterloading. A dose of 12–15 Gy with 3 Gy single doses and 2 fractions per day (≥6 h between fractions) with D90% for the CTV/PTVBRT is applied.
Further evaluations
To characterize the effectivity of neoadjuvant IMRT/IGRT for extremity sarcomas, the following evaluations will be performed:
comparison of “conventional safety margins” and reduced safety margins within the protocols on treatment planning comparisons and calculation of dose reduction to normal tissue
evaluation of tumor response on MRT as well as statement on resectability of the operating orthopaedic surgeon prior to resection based on imaging only
histopathological characterization of the tumor and tumor response to treatment
correlation of tumor response with outcome and prognosis.
Inclusion criteria:
histologically confirmed and imaging defined soft tissue sarcoma of the extremities
AJCC-Stage II or III (without T1a-tumos, no N1)
primary or recurrent tumor
after biopsy or previous R2 resection
based on imaging, „primary resectability“ or potential resectability after neoadjuvant RT must be present
age ≥ 18 years
ECOG Performance Status 0–2
informed consent
Main exclusion criteria
extraskeletal tumors of the Ewing-/PNET-group
extraskeletal osteo- or chondrosarcoma
aggressive fibromatosis (desmoid tumors)
dermatofibrosarcoma protuberans
presence of lymph node metastases (N1) or distant metastases (M1)
expected survival < 1 year
pregnancy, adequate contraception until 3 months after RT
severe comorbidities impairing study treatment
severe wound infections or recurrent skin infections
known positive HIV-Status
surgery of the primary tumor or chemotherapy within the last two weeks prior to study treatment
persistent toxicity of other tumor treatments in the treatment region
simultaneous chemotherapy, targeted therapy or experimental tumor therapy
previous RT in the treatment region
medication with steroids or immuno-suppressants
Follow-up
All patients are seen for a first follow-up visit 2 weeks after wound healing is completed, thereafter every 3 months during the first 2 years. The endpoints of the study are evaluated in detail during the first (2 weeks) and second (3 months) follow-up.
Functional outcome and QOL are documented prior to treatment and at year 1 and 2.
Treatment response and efficacy will be scored according to the RECIST 1.1 criteria.
Sample size calculation
A total patient number of 50 with an expected 20 % rate of wound complications was calculated for the study; the intent to treat (ITT) collective includes all patients included into the trial which signed informed consent and were allotted a patient study number. The per protocol collective (PP) includes only those patients, whose study treatment was applied completely without any severe protocol deviations.
Analysis for the primary and secondary endpoints are performed on the ITT collective, and re-evaluated in the PP group. The primary endpoint is the rate of wound complications 3 months after wound closure, including the 95 % confidence interval. The secondary endpoints are analyzed with an explorative approach. The rate of wound complications per treatment track is evaluated as means including the 95 % confidence interval. Survival rates are determined using the Kaplan-Meier Method.
Discussion
Neoadjuvant RT is an established treatment approach for extremity sarcomas, showing beneficial results compared to postoperative treatment. A major downside are increased rates of wound complications compared to postoperative RT. However, with modern RT approaches such as IMRT and IGRT, treatment precision is optimized with daily image guidance.
In the past, large safety margins were necessary to provide optimal oncological treatment, however, these safety margins most probably also contributed to the high rates of side effects since large amounts of normal tissue were exposed to RT.
The use of modern techniques enables the radiation oncologist to deliver precise RT doses, therefore margins around the tumor can be reduced which leads to sparing of normal tissue.
The present study protocol prospectively evaluates the use of IMRT/IGRT as neoadjuvant RT in patients with soft tissue sarcomas of the extremity with the primary endpoint wound complications, which is the major concern with this treatment sequence. Besides complications rates, local control rates and survival rates, as well as QOL and functional outcome as well as treatment response parameters (imaging and pathology) are part of the protocol. The data of the present PREMISS study will enhance the current literature and support the hypothesis that neoadjuvant RT with IMRT/IGRT offer an excellent risk-benefit ratio in this patient population.
Acknowledgement
This work is funded by the Wilhelm Sander Foundation, Grant application 2009.906.1. We thank our team of technicians for excellent patient care.
Abbreviations
- BRT
Brachytherapy
- CT
Computer Tomography
- CTV
Clinical Target Volume
- GTV
Gross Tumor Volume
- IGRT
Image Guided Radiotherapy
- IMRT
Intensity Modulated Radiotherapy
- IORT
Intraoperative Radiotherapy
- MRI
Magnetic Resonance Imaging
- PTV
Planning Target Volume
Footnotes
Competing interests
The authors declare they have no competing interests.
Authors’ contributions
BR–generation of the study protocol, patient treatment, study coordination and documentation. SEC–manuscript writing and finalization, patient treatment and supervision, study coordination. CH–patient treatment, study documentation, data analysis. VK–study protocol generation, sample size calculation, biometrics. HR - generation of the study protocol, patient treatment orthopaedic surgery. RvER - generation of the study protocol, patient treatment orthopaedic surgery. KS- generation of the study protocol, pathology evaluations. KW–generation of the study protocol, patient treatment, radiology evaluation
Contributor Information
Barbara Röper, Email: b.roeper@t-online.de.
Christine Heinrich, Email: christine.heinrich@lrz.tum.de.
Victoria Kehl, Email: victoria.kehl@tum.de.
Hans Rechl, Email: rechl@tum.de.
Katja Specht, Email: katja.specht@tum.de.
Klaus Wörtler, Email: klaus.woertler@tum.de.
Andreas Töpfer, Email: toepfer@tum.de.
Rüdiger von Eisenharth-Rothe, Email: r.eisenhart-rothe@ortho.med.tu-muenchen.
Stephanie E. Combs, Email: Stephanie.combs@tum.de
References
- 1.DeLaney TF. Optimizing radiation therapy and post-treatment function in the management of extremity soft tissue sarcoma. Curr Treat Options Oncol. 2004;5(6):463–476. doi: 10.1007/s11864-004-0035-1. [DOI] [PubMed] [Google Scholar]
- 2.Engstrom K, et al. Liposarcoma: outcome based on the Scandinavian Sarcoma Group register. Cancer. 2008;113(7):1649–1656. doi: 10.1002/cncr.23784. [DOI] [PubMed] [Google Scholar]
- 3.Hueman MT, et al. Management of extremity soft tissue sarcomas. Surg Clin North Am. 2008;88(3):539–557. doi: 10.1016/j.suc.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Palesty JA, Kraybill WG. Developments in the management of extremity soft tissue sarcomas. Cancer Invest. 2005;23(8):692–699. doi: 10.1080/07357900500359984. [DOI] [PubMed] [Google Scholar]
- 5.Patel SR, Zagars GK, Pisters PW. The follow-up of adult soft-tissue sarcomas. Semin Oncol. 2003;30(3):413–416. doi: 10.1016/S0093-7754(03)00101-5. [DOI] [PubMed] [Google Scholar]
- 6.Swallow CJ, Catton CN. Local management of adult soft tissue sarcomas. Semin Oncol. 2007;34(3):256–269. doi: 10.1053/j.seminoncol.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez JC, et al. Should soft tissue sarcomas be treated at high-volume centers? An analysis of 4205 patients. Ann Surg. 2007;245(6):952–958. doi: 10.1097/01.sla.0000250438.04393.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kepka L, et al. Results of radiation therapy for unresected soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2005;63(3):852–859. doi: 10.1016/j.ijrobp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196(3):305–315. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison LB, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in the management of completely resected soft tissue sarcomas of the extremity and superficial trunk. Int J Radiat Oncol Biol Phys. 1993;27(2):259–265. doi: 10.1016/0360-3016(93)90236-O. [DOI] [PubMed] [Google Scholar]
- 11.Yang JC, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16(1):197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 12.Brant TA, et al. Preoperative irradiation for soft tissue sarcomas of the trunk and extremities in adults. Int J Radiat Oncol Biol Phys. 1990;19(4):899–906. doi: 10.1016/0360-3016(90)90010-H. [DOI] [PubMed] [Google Scholar]
- 13.Davis AM, et al. Function and health status outcomes in a randomized trial comparing preoperative and postoperative radiotherapy in extremity soft tissue sarcoma. J Clin Oncol. 2002;20(22):4472–4477. doi: 10.1200/JCO.2002.03.084. [DOI] [PubMed] [Google Scholar]
- 14.Davis AM, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75(1):48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Hui AC, et al. Preoperative radiotherapy for soft tissue sarcoma: the Peter MacCallum Cancer Centre experience. Eur J Surg Oncol. 2006;32(10):1159–1164. doi: 10.1016/j.ejso.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Kim B, et al. An effective preoperative three-dimensional radiotherapy target volume for extremity soft tissue sarcoma and the effect of margin width on local control. Int J Radiat Oncol Biol Phys. 2010;77(3):843–850. doi: 10.1016/j.ijrobp.2009.06.086. [DOI] [PubMed] [Google Scholar]
- 17.Kuklo TR, et al. Preoperative versus postoperative radiation therapy for soft-tissue sarcomas. Am J Orthop (Belle Mead NJ) 2005;34(2):75–80. [PubMed] [Google Scholar]
- 18.O'Sullivan B, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 19.Robinson MH, et al. Preoperative radiotherapy for initially inoperable extremity soft tissue sarcomas. Clin Oncol (R Coll Radiol) 1992;4(1):36–43. doi: 10.1016/S0936-6555(05)80772-1. [DOI] [PubMed] [Google Scholar]
- 20.Sampath S, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma: multi-institutional analysis of 821 patients. Int J Radiat Oncol Biol Phys. 2011;81(2):498–505. doi: 10.1016/j.ijrobp.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 21.Tseng JF, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13(9):1209–1215. doi: 10.1245/s10434-006-9028-6. [DOI] [PubMed] [Google Scholar]
- 22.Wolfson AH. Preoperative vs postoperative radiation therapy for extremity soft tissue sarcoma: controversy and present management. Curr Opin Oncol. 2005;17(4):357–360. doi: 10.1097/01.cco.0000161745.24887.82. [DOI] [PubMed] [Google Scholar]
- 23.Zagars GK, et al. Preoperative vs. postoperative radiation therapy for soft tissue sarcoma: a retrospective comparative evaluation of disease outcome. Int J Radiat Oncol Biol Phys. 2003;56(2):482–488. doi: 10.1016/S0360-3016(02)04510-8. [DOI] [PubMed] [Google Scholar]
- 24.Bauer HC, et al. Monitoring referral and treatment in soft tissue sarcoma: study based on 1,851 patients from the Scandinavian Sarcoma Group Register. Acta Orthop Scand. 2001;72(2):150–159. doi: 10.1080/000164701317323408. [DOI] [PubMed] [Google Scholar]
- 25.Beltrami G, et al. Limb salvage surgery in combination with brachytherapy and external beam radiation for high-grade soft tissue sarcomas. Eur J Surg Oncol. 2008;34(7):811–816. doi: 10.1016/j.ejso.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Bray PW, et al. Limb salvage surgery and adjuvant radiotherapy for soft tissue sarcomas of the forearm and hand. J Hand Surg Am. 1997;22(3):495–503. doi: 10.1016/S0363-5023(97)80019-6. [DOI] [PubMed] [Google Scholar]
- 27.Eilber FC, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237(2):218–226. doi: 10.1097/01.SLA.0000048448.56448.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papagelopoulos PJ, et al. Current concepts for management of soft tissue sarcomas of the extremities. J Surg Orthop Adv. 2008;17(3):204–215. [PubMed] [Google Scholar]
- 29.Delannes M, Thomas L. Brachytherapy for soft tissue sarcomas. Technique and therapeutic indications. Cancer Radiother. 2006;10(1–2):63–67. doi: 10.1016/j.canrad.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Wunder JS, et al. Opportunities for improving the therapeutic ratio for patients with sarcoma. Lancet Oncol. 2007;8(6):513–524. doi: 10.1016/S1470-2045(07)70169-9. [DOI] [PubMed] [Google Scholar]
- 31.Lartigau E, et al. Definitions of target volumes in soft tissue sarcomas of the extremities. Cancer Radiother. 2001;5(5):695–703. doi: 10.1016/S1278-3218(01)00120-2. [DOI] [PubMed] [Google Scholar]
- 32.Cannon CP, et al. Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer. 2006;107(10):2455–2461. doi: 10.1002/cncr.22298. [DOI] [PubMed] [Google Scholar]
- 33.Geller DS, et al. Soft tissue sarcoma resection volume associated with wound-healing complications. Clin Orthop Relat Res. 2007;459:182–185. doi: 10.1097/BLO.0b013e3180514c50. [DOI] [PubMed] [Google Scholar]
- 34.Al-Absi E, et al. A systematic review and meta-analysis of oncologic outcomes of pre- versus postoperative radiation in localized resectable soft-tissue sarcoma. Ann Surg Oncol. 2010;17(5):1367–1374. doi: 10.1245/s10434-009-0885-7. [DOI] [PubMed] [Google Scholar]
- 35.Akudugu JM, et al. Wound healing morbidity in STS patients treated with preoperative radiotherapy in relation to in vitro skin fibroblast radiosensitivity, proliferative capacity and TGF-beta activity. Radiother Oncol. 2006;78(1):17–26. doi: 10.1016/j.radonc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Spierer MM, et al. Tolerance of tissue transfers to adjuvant radiation therapy in primary soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys. 2003;56(4):1112–1116. doi: 10.1016/S0360-3016(03)00200-1. [DOI] [PubMed] [Google Scholar]
- 37.Griffin AM, et al. Radiation planning comparison for superficial tissue avoidance in radiotherapy for soft tissue sarcoma of the lower extremity. Int J Radiat Oncol Biol Phys. 2007;67(3):847–856. doi: 10.1016/j.ijrobp.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 38.Hong L, et al. Intensity-modulated radiotherapy for soft tissue sarcoma of the thigh. Int J Radiat Oncol Biol Phys. 2004;59(3):752–759. doi: 10.1016/j.ijrobp.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 39.Stewart AJ, Lee YK, Saran FH. Comparison of conventional radiotherapy and intensity-modulated radiotherapy for post-operative radiotherapy for primary extremity soft tissue sarcoma. Radiother Oncol. 2009;93(1):125–130. doi: 10.1016/j.radonc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Alektiar KM, et al. Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol. 2008;26(20):3440–3444. doi: 10.1200/JCO.2008.16.6249. [DOI] [PubMed] [Google Scholar]
- 41.Donnay L, et al. Radiotherapy for soft tissue sarcomas of extremities. Preliminary comparative dosimetric study of 3D conformal radiotherapy versus helical tomotherapy. Cancer Radiother. 2008;12(8):809–816. doi: 10.1016/j.canrad.2008.08.275. [DOI] [PubMed] [Google Scholar]
- 42.Pezner RD, et al. Dosimetric comparison of helical tomotherapy treatment and step-and-shoot intensity-modulated radiotherapy of retroperitoneal sarcoma. Radiother Oncol. 2006;81(1):81–87. doi: 10.1016/j.radonc.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 43.Plowman PN, Cooke K, Walsh N. Indications for tomotherapy/intensity-modulated radiation therapy in paediatric radiotherapy: extracranial disease. Br J Radiol. 2008;81(971):872–880. doi: 10.1259/bjr/14878999. [DOI] [PubMed] [Google Scholar]
- 44.Whitelaw GL, et al. A dosimetric comparison between two intensity-modulated radiotherapy techniques: tomotherapy vs dynamic linear accelerator. Br J Radiol. 2008;81(964):333–340. doi: 10.1259/bjr/67084583. [DOI] [PubMed] [Google Scholar]
- 45.Ray M, McGinn C. Chapter 81: Soft Tissue Sarcomas. In: Halperin EC, Perez CA, Brady LW, editors. Perez and Brady's principles and practice of radiation oncology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. pp. 1808–1821. [Google Scholar]
- 46.Mundt AJ, et al. Conservative surgery and adjuvant radiation therapy in the management of adult soft tissue sarcoma of the extremities: clinical and radiobiological results. Int J Radiat Oncol Biol Phys. 1995;32(4):977–985. doi: 10.1016/0360-3016(95)00111-B. [DOI] [PubMed] [Google Scholar]
- 47.Laskar S, et al. Interstitial brachytherapy for childhood soft tissue sarcoma. Pediatr Blood Cancer. 2007;49(5):649–655. doi: 10.1002/pbc.21118. [DOI] [PubMed] [Google Scholar]
- 48.Rosenblatt E, et al. Interstitial brachytherapy in soft tissue sarcomas: the Rambam experience. Isr Med Assoc J. 2003;5(8):547–551. [PubMed] [Google Scholar]
- 49.Viani GA, et al. High-dose-rate brachytherapy for soft tissue sarcoma in children: a single institution experience. Radiat Oncol. 2008;3:9. doi: 10.1186/1748-717X-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zelefsky MJ, et al. Limb Salvage in Soft-Tissue Sarcomas Involving Neurovascular Structures Using Combined Surgical Resection and Brachytherapy. Int J Radiat Oncol Biol Phys. 1990;19(4):913–918. doi: 10.1016/0360-3016(90)90012-9. [DOI] [PubMed] [Google Scholar]
- 51.van Kampen M, et al. Correlation of intraoperatively irradiated volume and fibrosis in patients with soft-tissue sarcoma of the extremities. Int J Radiat Oncol Biol Phys. 2001;51(1):94–99. doi: 10.1016/S0360-3016(01)01620-0. [DOI] [PubMed] [Google Scholar]
- 52.Krasin MJ, Davidoff AM, Xiong X, Wu S, Hua CH, Navid F, Rodriguez-Galindo C, Rao BN, Hoth KA, Neel MD, Merchant TE, Kun LE, Spunt SL. Preliminary results from a prospective study using limited margin radiotherapy in pediatric and young adult patients with high-grade nonrhabdomyosarcoma soft-tissue sarcoma. Int J Radiat Oncol Biol Phys. 2010; 76(3):874-8 [DOI] [PMC free article] [PubMed]
- 53.Al Yami A, et al. Positive surgical margins in soft tissue sarcoma treated with preoperative radiation: is a postoperative boost necessary? Int J Radiat Oncol Biol Phys. 2010;77(4):1191–1197. doi: 10.1016/j.ijrobp.2009.06.074. [DOI] [PubMed] [Google Scholar]
- 54.Lazzaro G, et al. Pulsed dose-rate perioperative interstitial brachytherapy for soft tissue sarcomas of the extremities and skeletal muscles of the trunk. Ann Surg Oncol. 2005;12(11):935–942. doi: 10.1245/ASO.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Livi L, et al. Late treatment-related complications in 214 patients with extremity soft-tissue sarcoma treated by surgery and postoperative radiation therapy. Am J Surg. 2006;191(2):230–234. doi: 10.1016/j.amjsurg.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 56.Martinez-Monge R, et al. Perioperative high-dose-rate brachytherapy in soft tissue sarcomas of the extremity and superficial trunk in adults: initial results of a pilot study. Brachytherapy. 2005;4(4):264–270. doi: 10.1016/j.brachy.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 57.Petera J, et al. Perioperative hyperfractionated high-dose rate brachytherapy for the treatment of soft tissue sarcomas: multicentric experience. Ann Surg Oncol. 2010;17(1):206–210. doi: 10.1245/s10434-009-0684-1. [DOI] [PubMed] [Google Scholar]
- 58.Rudert M, et al. A new modification of combining vacuum therapy and brachytherapy in large subfascial soft -tissue sarcomas of the extremities. Strahlenther Onkol. 2010;186(4):224–228. doi: 10.1007/s00066-010-2046-0. [DOI] [PubMed] [Google Scholar]
- 59.Alekhteyar KM, et al. The effect of combined external beam radiotherapy and brachytherapy on local control and wound complications in patients with high-grade soft tissue sarcomas of the extremity with positive microscopic margin. Int J Radiat Oncol Biol Phys. 1996;36(2):321–324. doi: 10.1016/S0360-3016(96)00331-8. [DOI] [PubMed] [Google Scholar]
- 60.Salzer-Kuntschik M, et al. Morphological grades of regression in osteosarcoma after polychemotherapy - study COSS 80. J Cancer Res Clin Oncol. 1983;106(Suppl):21–24. doi: 10.1007/BF00625047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kotz R, et al. Advances in bone tumour treatment in 30 years with respect to survival and limb salvage. A single institution experience. Int Orthop. 2002;26(4):197–202. doi: 10.1007/s00264-002-0365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


