Abstract
Background:
Growing evidence indicates that inflammation has a crucial role in the development and progression of cancer. We developed a novel systemic inflammation score (SIS) based on preoperative serum albumin and lymphocyte-to-monocyte ratio (LMR), and examined its prognostic value for patients with clear-cell renal cell carcinoma (ccRCC) after surgery.
Methods:
The study comprised 441 ccRCC patients undergoing nephrectomy between 2008 and 2009 in a single centre. The SIS was developed and its associations with clinicopathological features and overall survival (OS) were evaluated.
Results:
The SIS consisted of serum albumin and LMR that were both retained as independent indicators adjusting for other haematological and laboratory markers of systemic inflammation responses and traditional clinicopathological features. A high SIS was significantly associated with aggressive tumour behaviours and served as an independent prognostic factor of reduced OS. Furthermore, the SIS could significantly stratify patient prognosis in different tumour stages and Mayo Clinic stage, size, grade and necrosis scores. Incorporation of the SIS into a prognostic model including TNM stage, Fuhrman grade and lymphovascular invasion generated a nomogram, which predicted accurately 3- and 5-year survival for ccRCC patients.
Conclusions:
The SIS as a potentially powerful prognostic biomarker might improve traditional clinicopathological analysis to refine clinical outcome prediction for ccRCC patients after surgery.
Keywords: renal cell carcinoma, inflammation, biomarker, prognosis, nomogram
Clear-cell renal cell carcinoma (ccRCC) is the most common type of kidney cancer and accounts for ∼60% to 70% of all renal tumours. Despite advances in diagnosis especially for widespread use of abdominal imaging, 20%–30% of all patients are diagnosed with metastatic disease and another 20% of patients undergoing curative nephrectomy will have a relapse and develop metastatic RCC with a poor prognosis during follow-up (Ljungberg et al, 2011). By far, several clinicopathological prognostic models have been commonly used to predict prognosis for postoperative RCC patients, such as the TNM staging system (Edge and Compton, 2010), the Fuhrman grading system (Fuhrman et al, 1982) and the Mayo Clinic stage, size, grade and necrosis (SSIGN) score (Frank et al, 2002); however, accurate prediction of individual tumour biology remains difficult. It is expected that a combination of specific RCC biomarkers into conventional clinicopathological characteristics will allow better prediction of prognosis (Shariat and Xylinas, 2012).
Cancer-related inflammation is currently recognised as the seventh hallmark of cancer, which often present with the features of an inflamed tissue, including inflammatory cell infiltration and an activated stroma (Mantovani, 2009). In addition to local inflammatory symptoms, cancer patients frequently present with systemic inflammation responses, including increased peripheral blood cell amounts and decreased haemoglobin and serum albumin levels (Chechlinska et al, 2010). Thus, several circulating blood cell-based prognostic biomarkers have been developed to predict patient outcome in various tumours, such as neutrophil-to-lymphocyte ratio (NLR) (Pichler et al, 2013; Hermanns et al, 2014), platelet-to-lymphocyte ratio (PLR) (Fox et al, 2013; Krenn-Pilko et al, 2014) and lymphocyte-to-monocyte ratio (LMR) (Hutterer et al, 2014; Szkandera et al, 2014). Preoperative haemoglobin and serum albumin levels are also reported as prognostic indicators for cancer clinical prognosis (Karakiewicz et al, 2007; McMillan, 2013). These markers are inexpensive to test and routinely performed in clinical setting, and hence potentially provide readily available and objective information to help clinicians to estimate patient outcome. However, there are few reports about the values of these markers, as independent indicators analysed all together and the extent how to integrate them to refine outcome prediction for ccRCC patients.
In this study, we developed a novel prognostic score named systemic inflammation score (SIS) based on preoperative serum albumin and LMR, which were both proven to be independently associated with ccRCC patients outcome adjusting for NLR, PLR, haemoglobin and traditional clinical and pathologic variables, and investigated the correlations of SIS with clinicopathologcal parameters and the prognostic value of SIS in ccRCC patients. Furthermore, a nomogram-combined SIS with TNM stage, Fuhrman grade and lymphovascular invasion was established to predict 3- and 5-year survival for ccRCC patients after surgery.
Patients and methods
Patients
This retrospective study included 441 consecutive patients with ccRCC, who underwent nephrectomy in our institute from 2008 to 2009. The exclusion criteria were as follows: non-ccRCC confirmed histopathologically, a history of previous anti-cancer therapies and other malignancies, bilateral renal cancer, perioperative mortality and preoperative routine laboratory tests unavailable. For each patient, the following clinical and pathologic information was gathered: age at surgery, gender, TNM stage (Edge and Compton, 2010), Fuhrman grade (Fuhrman et al, 1982), tumour size, tumour necrosis and lymphovascular invasion. Histopathologic review on each of the tumour specimens was performed by a single pathologist to confirm reported pathologic findings. Tumour size was recorded as the longest diameter described in pathologic reports. Tumour necrosis was defined as the presence of microscopic coagulative necrosis. Lymphovascular invasion was defined as the presence of tumour cells within an endothelium-lined space without underlying muscular walls. The presence of nodal metastases was defined by pathologic examinations and the presence of distant metastases was defined according to radiographic findings. The haematological and laboratory parameters were obtained within 1 week before surgery. The SSIGN score was applied to classify patients into three risk levels: 0–3, 4–7 and ⩾8 scores based on tumour stage, size, grade and necrosis (Frank et al, 2002).
Patients with localised RCC were treated with radical or partial nephrectomy. Patients with metastatic RCC were treated with cytoreductive nephrectomy followed by interferon-α-based immunotherapy or tyrosine kinase inhibitors therapy. Patients were followed up postoperatively with physical examination, laboratory studies, chest imaging and abdominal ultrasound or computed tomography every 6 months for the first 2 years and annually thereafter. Follow-up was terminated in January 2015. Overall survival (OS) was defined as the time from surgery to death from all causes or censored at the last follow-up date. The clinicopathological characteristics of patients are shown in Table 1. Overall survival rates were 91.2% and 85.5% at 3 and 5 years, respectively. After a median follow-up of 66 months (interquartile range, 63–69 months), 65 patients (14.7%) had died from all causes. This study was approved by the Zhongshan hospital's Ethics Committee and informed consent was obtained from each patient.
Table 1. Patient and tumour characteristics.
| Characteristics | No. | % |
|---|---|---|
|
Age, years | ||
| Median (IQR) | 56 (46–63) | |
|
Sex | ||
| Male | 318 | 72.1 |
| Female | 123 | 27.9 |
|
T stage | ||
| T1 | 301 | 68.3 |
| T2 | 32 | 7.3 |
| T3 | 107 | 24.3 |
| T4 | 1 | 0.2 |
|
N stage | ||
| N0 | 434 | 98.4 |
| N1 | 7 | 1.6 |
|
M stage | ||
| M0 | 435 | 98.6 |
| M1 | 6 | 1.4 |
|
TNM stage | ||
| I | 297 | 67.3 |
| II | 30 | 6.8 |
| III | 108 | 24.5 |
| IV | 6 | 1.4 |
|
Fuhrman grade | ||
| 1 | 72 | 16.3 |
| 2 | 202 | 45.8 |
| 3 | 106 | 24.0 |
| 4 | 61 | 13.8 |
|
Tumour size, cm | ||
| <5 | 299 | 67.8 |
| ⩾5 | 142 | 32.2 |
|
Tumour necrosis | ||
| Absent | 348 | 78.9 |
| Present | 93 | 21.1 |
|
Lymphovascular invasion | ||
| Absent | 323 | 73.2 |
| Present | 118 | 26.8 |
|
SSIGN score | ||
| 0–3 | 320 | 72.6 |
| 4–7 | 105 | 23.8 |
| ⩾8 | 16 | 3.6 |
|
Haemoglobin, g l−1 | ||
| Median (IQR) | 139 (126–148) | |
|
Albumin, g l−1 | ||
| Median (IQR) | 42 (39–44) | |
|
NLR | ||
| Median (IQR) | 2.0 (1.5–2.6) | |
|
PLR | ||
| Median (IQR) | 110 (84–142) | |
|
LMR | ||
| Median (IQR) | 4.4 (3.2–5.8) | |
Abbreviations: IQR=interquartile range; LMR=lymphocyte to monocyte ratio; NLR=neutrophil to lymphocyte ratio; No.=number of patients; PLR=platelet to lymphocyte ratio; SSIGN=the Mayo Clinic stage, size, grade, and necrosis.
Statistical analysis
Analysis was performed with SPSS 21.0 (IBM Corporation, Armonk, NY, USA) and R software version 3.0.2 and the ‘rms' package (R Foundation for Statistical Computing, Vienna, Austria). Pearson χ2-test or Fisher's exact test was used to compare categorical variables and continuous variables were analysed by Wilcoxon rank-sum test or Kruskal–Wallis test. The Kaplan–Meier method with log-rank test was used to compare survival curves. The Cox proportional hazards regression model was applied to perform univariate and multivariate analyses, and those variables that achieved statistical significance in the univariate analysis were entered into the multivariable analysis. We first evaluated these haematological and laboratory markers including NLR, PLR, LMR, haemoglobin and serum albumin as continuous variables, together with traditional clinicopathological variables in the univariate and multivariate analyses, and identified that LMR and serum albumin were independent prognostic factors of OS. Next, the two markers were analysed as categorical variables. Dichotomisation of serum albumin was based on the lower range of normal measurement at 40 g l−1 (normal range, 40–55 g l−1). Owing to no widely accepted cutpoint of LMR, we used the median value at 4.44 as the cutoff for dichotomisation. The SIS was established based on the combination of different serum albumin and LMR levels. The SIS as well as traditional clinicopathological variables was assessed in the multivariate analysis. A nomogram was created by R software using ‘rms' package. Calibration plots were generated to examine the performance characteristics of the predictive nomogram. The Harrell's Concordance index (C-index) was used to quantify the predictive accuracy (Harrell et al, 1996), which ranges from 0.5 (no predictive power) to 1 (perfect prediction). All statistical tests were two-sided and were performed at a significance level of 0.05.
Results
Associations of LMR, serum albumin and SIS with OS
Results from the univariate analysis indicated that NLR, PLR, LMR, haemoglobin and serum albumin as continuous variables were prognostic factors of OS as well as TNM stage, Fuhrman grade, tumour size, tumour necrosis and lymphovascular invasion (Table 2), whereas age at surgery and gender had no prognostic significance for OS. Based on our multivariate analysis, the serum albumin and LMR were independent prognostic factors for OS (HR, 0.909; 95% CI, 0.840–0.985; P=0.019; HR, 0.839; 95% CI, 0.705–0.998; P=0.047, respectively), together with TNM stage, Fuhrman grade and lymphovascular invasion (Table 2).
Table 2. Univariate and multivariate Cox proportional hazards regression analysis for OS.
| Univariate |
Multivariatea |
Multivariateb |
|||||
|---|---|---|---|---|---|---|---|
| Variables | P-values | HR | 95% CI | P-values | HR | 95% CI | P-values |
| Age | 0.185 | ||||||
| Sex | 0.236 | ||||||
| TNM stage | <0.001 | <0.001 | <0.001 | ||||
| I | Reference | Reference | |||||
| II | 1.331 | 0.500–3.541 | 0.567 | 1.277 | 0.472–3.458 | 0.630 | |
| III | 2.222 | 1.232–4.008 | 0.008 | 2.348 | 1.302–4.232 | 0.005 | |
| IV | 24.483 | 5.197–115.332 | <0.001 | 14.644 | 4.651–46.106 | <0.001 | |
| Fuhrman grade | <0.001 | 0.017 | 0.023 | ||||
| 1 | Reference | Reference | |||||
| 2 | 1.215 | 0.401–3.686 | 0.731 | 1.374 | 0.455–4.150 | 0.573 | |
| 3 | 1.263 | 0.401–3.983 | 0.690 | 1.483 | 0.477–4.613 | 0.496 | |
| 4 | 3.267 | 1.041–10.253 | 0.042 | 3.519 | 1.133–10.931 | 0.030 | |
| Tumour size, cm | <0.001 | 0.428 | 0.540 | ||||
| <5 | Reference | Reference | |||||
| ⩾5 | 1.255 | 0.716–2.200 | 1.192 | 0.680–2.091 | |||
| Tumour necrosis | <0.001 | 0.096 | 0.199 | ||||
| Absent | Reference | Reference | |||||
| Present | 1.621 | 0.918–2.862 | 1.449 | 0.823–2.550 | |||
| LVI | <0.001 | <0.001 | <0.001 | ||||
| Absent | Reference | Reference | |||||
| Present | 2.959 | 1.657–5.286 | 3.033 | 1.699–5.414 | |||
| Albuminc | <0.001 | 0.909 | 0.840–0.985 | 0.019 | |||
| Haemoglobinc | <0.001 | 0.993 | 0.977–1.009 | 0.406 | |||
| LMRc | <0.001 | 0.839 | 0.705–0.998 | 0.047 | |||
| PLRc | <0.001 | 0.997 | 0.994–1.001 | 0.138 | |||
| NLRc | <0.001 | 0.971 | 0.838–1.124 | 0.691 | |||
| SIS | <0.001 | 0.001 | |||||
| 0 | Reference | ||||||
| 1 | 2.109 | 1.020–4.362 | 0.044 | ||||
| 2 | 4.149 | 1.980–8.695 | <0.001 | ||||
Abbreviations: CI=confidence interval; HR=hazard ratio; LMR=lymphocyte to monocyte ratio; LVI=lymphovascular invasion; NLR=neutrophil to lymphocyte ratio; OS=overall survival; PLR=platelet to lymphocyte ratio; SIS=systemic inflammation score.
Adjustment for all variables listed in the table, except for age, sex and SIS.
Adjustment for all variables listed in the table, except for age, sex, albumin, haemoglobin, LMR, PLR and NLR.
Analysed as a continuous variable.
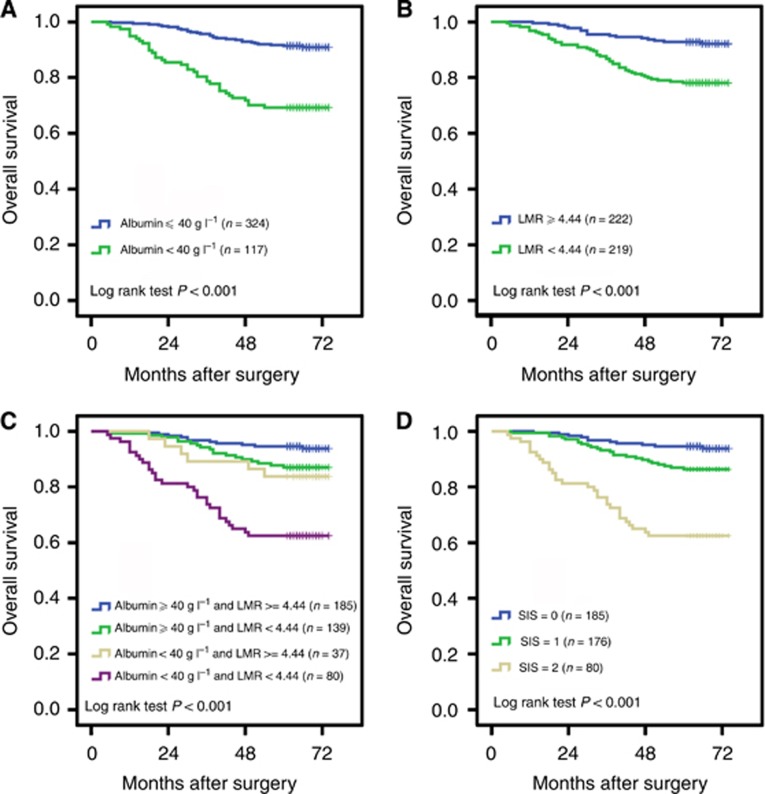
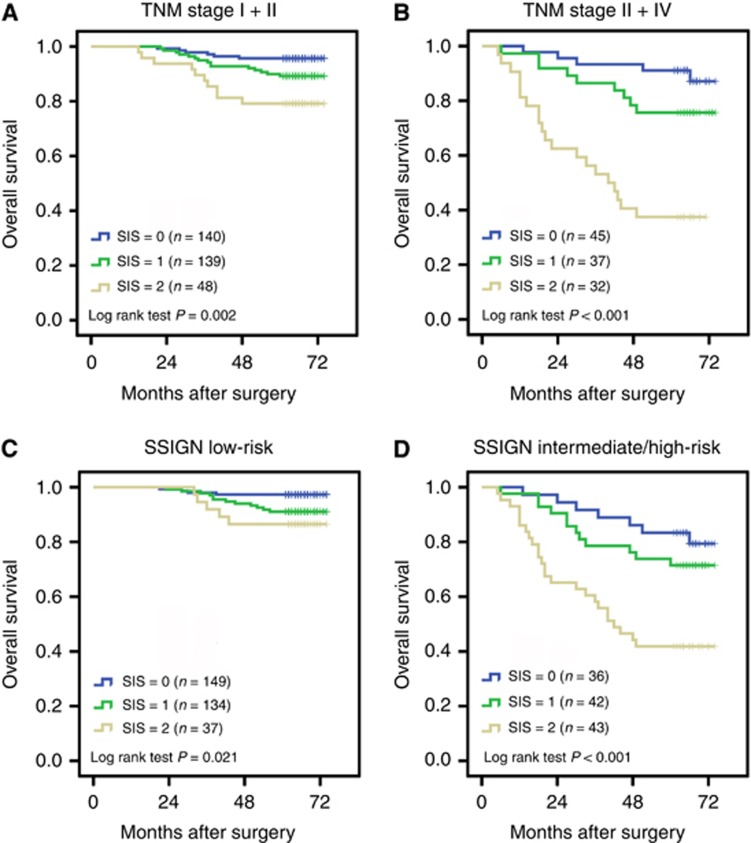
As mentioned above, the continuously coded serum albumin was stratified into <40 or ⩾40 g l−1 and the continuously coded LMR was stratified into <4.44 or ⩾4.44 for subsequent analyses. Kaplan–Meier analysis indicated that the decreased serum albumin and LMR were both associated with shorter OS (P<0.001 for both) (Figures 1A and B). To further discriminate patients with different outcome, we combined serum albumin and LMR levels to generate four subgroups. We found significant differences exist among the four subgroups (Figure 1C). In subgroups of either serum albumin ⩾40 g l−1 or LMR ⩾4.44, the OS was quite similar (HR, 1.282; 95% CI, 0.509–3.230; P=0.598). Thus, we combined the two subgroups to establish the SIS defined as follows: patients with both decreased serum albumin and decreased LMR (<40 g l−1 and <4.44, respectively) were assigned score 2; patients with either decreased serum albumin or decreased LMR were assigned score 1 and patients with both elevated serum albumin and elevated LMR (⩾40 g l−1 and ⩾4.44, respectively) were assigned score 0. Kaplan–Meier curves showed that high SIS was associated with shorter OS (P<0.001) (Figure 1D). To investigate further the effect of SIS in stratifying patients with different TNM stages and SSIGN scores, we considered TNM stages I and II as early-stage tumour and TNM stage III and IV as advanced-stage tumour. The SSIGN 0–3 scores, 4–7 scores and ⩾8 scores were grouped as low-risk, intermediate-risk and high-risk, respectively. By Kaplan–Meier analysis, we found that the three subgroups of SIS differed significantly in both early-stage and advanced-stage (P=0.002 and P<0.001, respectively) (Figures 2A and B), and in both SSIGN low-risk level and intermediate/high-risk level (P=0.021 and P<0.001, respectively) (Figures 2C and D).
Figure 1.
Kaplan–Meier analysis for OS of ccRCC patients according to preoperative serum albumin and LMR. Kaplan–Meier analysis for OS according to (A) preoperative serum albumin, (B) preoperative LMR, (C) combination of preoperative serum albumin and LMR, and (D) SIS.
Figure 2.
Kaplan–Meier analysis for OS of ccRCC patients according to SIS in different tumour stages and SSIGN scores. Kaplan–Meier analysis for OS of ccRCC patients according to SIS in (A) early-stage (TNM stage I+II), (B) advanced-stage (TNM III+IV), (C) SSIGN low-risk (score 0–3) and (D) SSIGN intermediate/high-risk (score 4–7/score ≥8).
In the univariate analysis, the SIS had prognostic significance for OS (P<0.001). The multivariate analysis demonstrated the SIS (P=0.001), TNM stage (P<0.001), Fuhrman grade (P=0.023) and lymphovascular invasion (P<0.001) were independent prognostic factors of OS in ccRCC (Table 2).
Associations of LMR, serum albumin and SIS with clinicopathological characteristics
The associations of LMR and serum albumin with clinicopathological characteristics are shown in Table 3. Decreased serum albumin and LMR were both associated with older age at surgery (P<0.001 for both), high Fuhrman grade (P=0.049 and P=0.009, respectively), tumour size ⩾5cm (P=0.017 and P=0.002, respectively), the presence of tumour necrosis (P<0.001 and P=0.001, respectively), the presence of lymphovascular invasion (P=0.034 and P=0.025, respectively) and high SSIGN score (P<0.001 for both). In addition, decrease serum albumin was associated with high TNM stage (P<0.001).
Table 3. Associations of albumin, LMR and SIS with clinicopathological characteristics.
|
Albumin (g/l) |
LMR |
SIS |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <40 | ≥40 | P-values | <4.44 | ≥4.44 | P-values | 0 | 1 | 2 | P-values | |
| Characteristics | n=117 | n=324 | n=219 | n=222 | n=185 | n=176 | n=80 | |||
| Age, years | <0.001 | <0.001 | <0.001 | |||||||
| Median | 58 | 55 | 58 | 53 | 52 | 58 | 59 | |||
| IQR | 51–70 | 45–62 | 50–67 | 45–60 | 44–59 | 50–66 | 50–71 | |||
| Sex | 0.742 | 0.032 | 0.071 | |||||||
| Male | 83 | 235 | 168 | 150 | 124 | 137 | 57 | |||
| Female | 34 | 89 | 51 | 72 | 61 | 39 | 23 | |||
| TNM stage | <0.001 | 0.143 | <0.001 | |||||||
| I | 63 | 234 | 141 | 156 | 130 | 130 | 37 | |||
| II | 12 | 18 | 19 | 11 | 10 | 9 | 11 | |||
| III | 37 | 71 | 54 | 54 | 44 | 37 | 27 | |||
| IV | 5 | 1 | 5 | 1 | 1 | 0 | 5 | |||
| Fuhrman grade | 0.049 | 0.009 | 0.001 | |||||||
| 1 | 15 | 57 | 28 | 44 | 36 | 29 | 7 | |||
| 2 | 47 | 155 | 93 | 109 | 94 | 76 | 32 | |||
| 3 | 31 | 75 | 58 | 48 | 36 | 51 | 19 | |||
| 4 | 24 | 37 | 40 | 21 | 19 | 20 | 22 | |||
| Tumour size, cm | 0.017 | 0.002 | 0.001 | |||||||
| <5 | 69 | 230 | 133 | 166 | 139 | 118 | 42 | |||
| ⩾5 | 48 | 94 | 86 | 56 | 46 | 58 | 38 | |||
| Tumour necrosis | <0.001 | 0.001 | <0.001 | |||||||
| Absent | 79 | 269 | 158 | 190 | 160 | 139 | 49 | |||
| Present | 38 | 55 | 61 | 32 | 25 | 37 | 31 | |||
| LVI | 0.034 | 0.025 | 0.005 | |||||||
| Absent | 77 | 246 | 150 | 173 | 143 | 133 | 47 | |||
| Present | 40 | 78 | 69 | 49 | 42 | 43 | 33 | |||
| SSIGN score | <0.001 | <0.001 | <0.001 | |||||||
| 0–3 | 65 | 255 | 143 | 177 | 149 | 134 | 37 | |||
| 4–7 | 40 | 65 | 61 | 44 | 35 | 39 | 31 | |||
| ⩾8 | 12 | 4 | 15 | 1 | 1 | 3 | 12 | |||
Abbreviations: IQR=interquartile range; LMR=lymphocyte to monocyte ratio; LVI=lymphovascular invasion; SIS=systemic inflammation score; SSIGN=the Mayo Clinic stage, size, grade, and necrosis.
The relationships between the SIS and clinicopathological features are also summarised in Table 3. High SIS was more likely to have older age at surgery (P<0.001), high TNM stage (P<0.001), high Fuhrman grade (P=0.001), large tumour size (P=0.001), the presence of tumour necrosis and lymphovascular invasion (P<0.001 and P=0.005, respectively), and high SSIGN score (P<0.001) (Table 3).
Predictive nomogram for OS
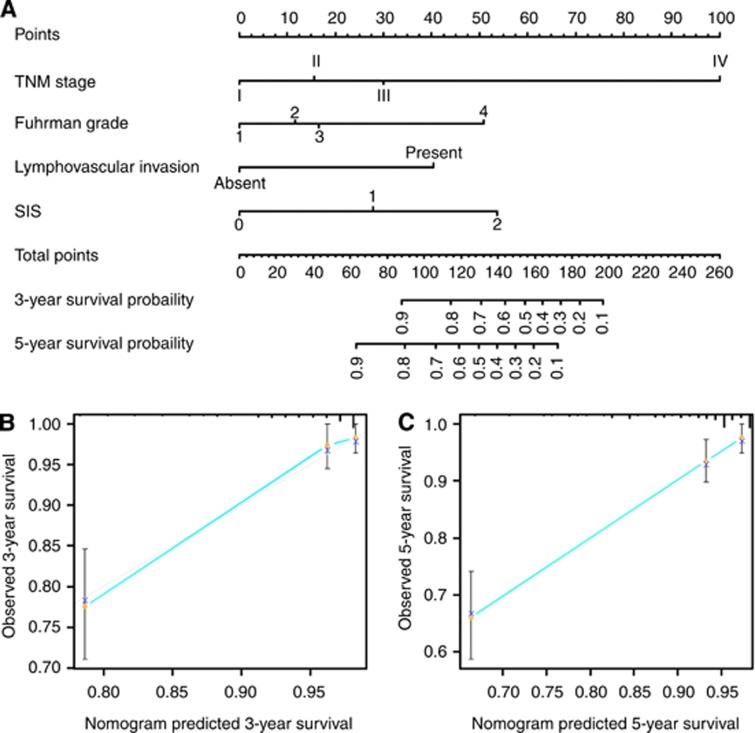
To provide a quantitative method for better outcome prediction, we constructed a nomogram that integrated the proven independent prognostic factors consisting of TNM stage, Fuhrman stage, lymphovascular invasion and SIS (Figure 3A). In this nomogram, a higher total point indicates a worse prognosis. For internal validation, calibration plots of the nomogram predicting 3- and 5-year survival performed well with the ideal model (Figures 3B and C). The C-index of the multivariate prognostic model based on TNM stage, Fuhrman stage and lymphovascular invasion was 0.80 and improved to 0.82 when the SIS (C-index 0.71) was incorporated, which showed a better predictive ability of OS than TNM stage (C-index 0.68) or SSIGN score (C-index 0.78).
Figure 3.
Nomogram for predicting 3- and 5-year OS of ccRCC patients after surgery. (A) Nomogram for predicting 3- and 5-year OS of ccRCC patients after surgery. Calibration plot of the nomogram for (B) 3-year and (C) 5-year survival. The dashed line represents the performance of an ideal nomogram. The blue line indicates the performance of the proposed nomogram. Orange circles are sub-cohorts of the data set; X is the bootstrapped corrected estimate of nomogram with 200 resamples. Vertical bars represent 95% CI. It seems that the nomogram predicts accurately 3- and 5-year OS.
Discussion
We investigated clinicopathological characteristics and prognosis of 441 ccRCC patients with haematological and laboratory markers of systemic inflammation responses. We demonstrated that decreased serum albumin and LMR levels before surgery were independent and adverse predictors of OS in multivariate analysis. Furthermore, we created a novel prognostic score named the SIS based on the combination of serum albumin and LMR after dichotomisation, and found that high SIS was associated with high TNM stage, high SSIGN risk level and poor outcome. Together with TNM stage, Fuhrman grade and lymphovascular invasion, the SIS was integrated in a prognostic nomogram that predicted OS with an accuracy of 0.82. The magnitude of the SIS was on the same order as that of Fuhrman grade system. Thus, our study serves as a proof of principal that systemic inflammatory information can provide important prognostic information that augments traditional clinicopathological analysis. It is hoped that additional, prospective and clinical validation studies will be conducted to confirm our findings and further basic research studies will be performed to identify the detailed mechanism that how inflammatory cells and mediators are involved in the pathogenesis and progression of renal cell cancer.
As Virchow originally made links between cancer and inflammation in the nineteenth century, contemporary studies have led to a general acceptance that inflammation has an important role in carcinogenesis (Balkwill and Mantovani, 2001). The hallmarks of cancer-related inflammation consist of the infiltration of inflammatory cells and the production of inflammatory mediators in tumour tissues, tissue remodelling, tissue repair and angiogenesis (Mantovani et al, 2008). Moreover, inflammation generates not only pro-tumoral microenvironment changes but also systemic changes that can facilitate tumour progression (Chechlinska et al, 2010). Therefore, the complex array of inflammatory cells and inflammatory mediators in tumour microenvironment may be reflected in the peripheral circulation.
Recent studies have shown the prognostic significance of NLR and LMR in postoperative patients with nonmetastatic RCC (Pichler et al, 2013; Hutterer et al, 2014) and that of PLR in patients with advanced RCC (Fox et al, 2013). Moreover, Morgan et al (2011) found that preoperative low serum albumin was significantly associated with reduced survival for 369 locoregional RCC patients. Another study for 1828 all-stages RCC patients found that preoperative haemoglobin was a significant predictor of RCC-specific mortality (Karakiewicz et al, 2007). However, the prognostic value of integrating these frequently requested haematological and laboratory markers into the traditional clinicopathological features remains obscure in RCC. In our study, we demonstrated that serum albumin and LMR were independent prognostic factors of OS in ccRCC patients. Although NLR, PLR and haemoglobin were significantly associated with survival in univariate analysis, they were not retained as independent indicators in the multivariate model. Furthermore, we created the SIS based on the combination of serum albumin and LMR. We found that both decreased serum albumin and decreased LMR levels (SIS score 2) were associated with advanced tumour stage and poor outcome, and elevated levels of both (SIS score 0) were associated with early tumour stage and favourable outcome, indicating that the SIS could be a more objective marker that reflects the balance between host inflammation and immune response status than indexes such as NLR, PLR and haemoglobin.
As an integrated indicator based on serum albumin and LMR, the biological reason behind the prognostic value of SIS might be elucidated by the function of the albumin, lymphocytes and monocytes. Recent evidence indicates that monocytes can be recruited in tumour tissues and differentiate into tumour-associated macrophages (TAMs) exerting pro-tumoral action (Qian and Pollard, 2010). Lymphocytes can enhance cancer immune-surveillance to inhibit tumour cell proliferation, invasion and metastasis (Dunn et al, 2004). Thus, a low circulating lymphocyte amount might be responsible for a weak and insufficient immune response to tumours and an elevated circulating monocyte level may reflect an increased production of TAMs as a marker of high tumour burden. Accordingly, a decrease LMR conveying poor prognosis was observed in a variety of malignancies including RCC (Hutterer et al, 2014). Serum albumin is known as a negative acute phase protein and is synthesised specially in the liver (Esper and Harb, 2005). Decreased serum albumin represents not only a malnutrition status but also a sustained systemic inflammation response (McMillan, 2009), therefore providing significant prognostic information for many types of cancer with or without integration into prognostic systems (McMillan, 2008; Gupta and Lis, 2010). In agreement with previous findings, we demonstrated that high SIS was an independent predictor of diminished survival for ccRCC patients. Moreover, high SIS was significantly correlated with aggressive tumour biological phenotypes such as advanced tumour stage, high Fuhrman grade, large tumour size and the presence of necrosis and lymphovascular invasion. These results partially reveal complex interactions of elevated systemic inflammation responses and tumour progression. In addition, we found that the SIS could further stratify patients into three risk subgroups in different tumour stages and SSIGN risk levels, suggesting that the SIS might provide additional prognostic information as a complement to the well-established clinicopathological prognostic models.
In tradition, the prediction of prognosis in RCC patients is based on clinical and pathological factors such as TNM stage and SSIGN score. By incorporating the SIS into TNM stages, Fuhrman grade and lymphovascular invasion, a nomogram was constructed and performed well in internal validation. When assessing OS, a higher predictive accuracy of the nomogram can be observed compared with that of TNM stage or SSIGN score. Nonetheless, it needs to be further investigated whether the predictive value of the nomogram is superior to that of SSIGN score for cancer-specific survival (CSS), because the SSIGN score is developed originally with the endpoint of CSS. Meanwhile, the magnitude of the SIS was on the same order as Fuhrman grade. Thus, our study indicates that the SIS can provide additional prognostic information to traditional clinicopathological features. These results may facilitate clinicians to better stratify patients who need adjuvant therapy, intensive postoperative follow-up or participating in clinical trials.
The strength of our study is that the measurement of SIS is based on standard laboratory tests of serum albumin, lymphocyte and monocyte counts, which are routinely applied in clinical practice. However, there are a few limitations in this study. First, owing to the retrospective nature and no external validation, the prognostic significance of the SIS in ccRCC patients remains to be investigated prospectively in other populations and larger cohorts in the future. Second, because C-reactive protein (CRP) is not routinely measured in our clinical practice, we did not add CRP in our analyses. Further studies should estimate the prognostic value of CRP in combination with SIS as well as clinicopathological parameters. Third, because of limited patients with metastasis (n=12), we did not specially assess the relationship between the SIS and metastatic disease.
In conclusion, we developed a novel and easily obtained prognostic score named SIS, which was based on preoperative serum albumin and LMR. The SIS served as an independent prognostic factor and should be incorporated into traditional clinical and pathologic variables to complement outcome prediction of ccRCC patients after surgery.
Acknowledgments
We thank Ms Lingli Chen (Department of Pathology, Zhongshan Hospital of Fudan University) for technical assistance. This study was funded by grants from the National Natural Science Foundation of China (31100629, 31270863, 81372755, 81471621, 81472227, 81402082 and 81402085), Program for New Century Excellent Talents in University (NCET-13-0146) and the Shanghai Rising-Star Program (13QA1400300). All these study sponsors have no roles in the study design, in the collection, analysis and interpretation of data.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License
References
- Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357(9255): 539–545. [DOI] [PubMed] [Google Scholar]
- Chechlinska M, Kowalewska M, Nowak R (2010) Systemic inflammation as a confounding factor in cancer biomarker discovery and validation. Nat Rev Cancer 10(1): 2–3. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD (2004) The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21(2): 137–148. [DOI] [PubMed] [Google Scholar]
- Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17(6): 1471–1474. [DOI] [PubMed] [Google Scholar]
- Esper DH, Harb WA (2005) The cancer cachexia syndrome: a review of metabolic and clinical manifestations. Nutr Clin Pract 20(4): 369–376. [DOI] [PubMed] [Google Scholar]
- Fox P, Hudson M, Brown C, Lord S, Gebski V, De Souza P, Lee CK (2013) Markers of systemic inflammation predict survival in patients with advanced renal cell cancer. Br J Cancer 109(1): 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H (2002) An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol 168(6): 2395–2400. [DOI] [PubMed] [Google Scholar]
- Fuhrman SA, Lasky LC, Limas C (1982) Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 6(7): 655–663. [DOI] [PubMed] [Google Scholar]
- Gupta D, Lis CG (2010) Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 9: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell FE Jr., Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15(4): 361–387. [DOI] [PubMed] [Google Scholar]
- Hermanns T, Bhindi B, Wei Y, Yu J, Noon AP, Richard PO, Bhatt JR, Almatar A, Jewett MA, Fleshner NE, Zlotta AR, Templeton AJ, Kulkarni GS (2014) Pre-treatment neutrophil-to-lymphocyte ratio as predictor of adverse outcomes in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Br J Cancer 111(3): 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutterer GC, Stoeckigt C, Stojakovic T, Jesche J, Eberhard K, Pummer K, Zigeuner R, Pichler M (2014) Low preoperative lymphocyte-monocyte ratio (LMR) represents a potentially poor prognostic factor in nonmetastatic clear cell renal cell carcinoma. Urol Oncol 32(7): 1041–1048. [DOI] [PubMed] [Google Scholar]
- Karakiewicz PI, Trinh QD, Lam JS, Tostain J, Pantuck AJ, Belldegrun AS, Patard JJ (2007) Platelet count and preoperative haemoglobin do not significantly increase the performance of established predictors of renal cell carcinoma-specific mortality. Eur Urol 52(5): 1428–1436. [DOI] [PubMed] [Google Scholar]
- Krenn-Pilko S, Langsenlehner U, Thurner EM, Stojakovic T, Pichler M, Gerger A, Kapp KS, Langsenlehner T (2014) The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer 110(10): 2524–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, Kiemeney LA (2011) The epidemiology of renal cell carcinoma. Eur Urol 60(4): 615–621. [DOI] [PubMed] [Google Scholar]
- Mantovani A (2009) Cancer: inflaming metastasis. Nature 457(7225): 36–37. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454(7203): 436–444. [DOI] [PubMed] [Google Scholar]
- McMillan DC (2008) An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc 67(3): 257–262. [DOI] [PubMed] [Google Scholar]
- McMillan DC (2009) Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 12(3): 223–226. [DOI] [PubMed] [Google Scholar]
- McMillan DC (2013) The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 39(5): 534–540. [DOI] [PubMed] [Google Scholar]
- Morgan TM, Tang D, Stratton KL, Barocas DA, Anderson CB, Gregg JR, Chang SS, Cookson MS, Herrell SD, Smith JA Jr., Clark PE (2011) Preoperative nutritional status is an important predictor of survival in patients undergoing surgery for renal cell carcinoma. Eur Urol 59(6): 923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler M, Hutterer GC, Stoeckigt C, Chromecki TF, Stojakovic T, Golbeck S, Eberhard K, Gerger A, Mannweiler S, Pummer K, Zigeuner R (2013) Validation of the pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in a large European cohort of renal cell carcinoma patients. Br J Cancer 108(4): 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141(1): 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariat SF, Xylinas E (2012) Biomarkers in personalised treatment of renal-cell carcinoma. Lancet Oncol 13(8): 751–752. [DOI] [PubMed] [Google Scholar]
- Szkandera J, Gerger A, Liegl-Atzwanger B, Absenger G, Stotz M, Friesenbichler J, Trajanoski S, Stojakovic T, Eberhard K, Leithner A, Pichler M (2014) The lymphocyte/monocyte ratio predicts poor clinical outcome and improves the predictive accuracy in patients with soft tissue sarcomas. Int J Cancer 135(2): 362–370. [DOI] [PubMed] [Google Scholar]