Abstract
Background:
Limited data describe patient-reported outcomes (PROs) of localised oesophageal cancer treated with definitive chemoradiotherapy(CRT). The phase 2/3 SCOPE-1 trial assessed the effectiveness of CRT±cetuximab. The trial for the first time provided an opportunity to describe PROs from a multi-centre group of patients treated with CRT that are presented here.
Methods:
Patients undergoing CRT±cetuximab within the SCOPE-1 trial (258 patients from 36 UK centres) completed generic-, disease- and treatment-specific health-related quality of life (HRQL) questionnaires (EORTC QLQ-C30, QLQ-OES18, Dermatology Life-Quality Index (DLQI)) at baseline and at 7, 13, 24, 52 and 104 weeks. Mean EORTC functional scale scores (>15 point change significant), DLQI scores (>4 point change significant) and proportions of patients (>15% significant) with ‘minimal' or ‘severe' symptoms are presented.
Results:
Questionnaire response rates were good. At baseline, EORTC functional scores were high (>75%) and few symptoms were reported except for severe problems with fatigue, insomnia and eating-related symptoms (e.g., appetite loss, dysphagia, dry mouth) in both groups(>15%). Functional aspects of health deteriorated and symptoms increased with treatment and by week 13 global quality of life, physical, role and social function significantly deteriorated and more problems with fatigue, dyspnoea, appetite loss and trouble with taste were reported. Recovery occurred by 6 months (except severe fatigue and insomnia in >15% of patients) and maintained at follow-up with no differences between groups.
Conclusions:
CRT for localised oesophageal cancer has a significant detrimental impact on many aspects of HRQL; however, recovery is achieved by 6 months and maintained with the exception of persisting problems with severe fatigue and insomnia. The data suggest that the HRQL recovery after definitive CRT is quicker, and there is little lasting deficit compared with treatment including surgery. These data need to be compared with HRQL data from studies evaluating treatments including surgery for oesophageal cancer.
Keywords: oesophgeal, cancer, chemoradiotherapy, patient reported outcomes, cetuximab
Oesophageal cancer remains difficult to cure with overall 5-year survival rates <10%. (Jemal et al, 2011) Radical treatment, often including surgery, is undertaken in 20–30% of patients, although <25% of patients survive 5 years (MRC Oesophageal Cancer Working Party, 2002; Ychou et al, 2011). Oesophagectomy is associated with significant in-hospital risk, with a mortality of 2–5%, major morbidity in 10–20% and a detrimental impact on health-related quality of life (HRQL) (Jacobs et al, 2013). The addition of neo-adjuvant chemotherapy or chemoradiotherapy (CRT) before surgery can improve long-term survival, (Ronellenfitsch et al, 2013a, 2013b), but it is associated with additional postoperative morbidity and deterioration of HRQL during treatment (Derogar et al, 2012; Jacobs et al, 2014).
Radical non-surgical treatment with definitive radiotherapy may also be used, but evidence shows that concurrent CRT is more effective than radiotherapy alone (Wong and Malthaner, 2006; Kranzfelder et al, 2011). Five-year survival rates after definitive CRT are similar to those achieved by surgery although sufficiently robust studies comparing these two approaches are lacking (Malthaner et al, 2006; Wong and Malthaner, 2006; Kranzfelder et al, 2011; Ronellenfitsch et al, 2013b). Evaluation of radical treatment for oesophageal cancer typically includes measures of long-term survival, treatment toxicity and surgical morbidity. In addition, there is an imperative to describe the patient perspective with measures of HRQL that assess functions and symptoms directly reported by those individuals receiving treatment (Food and Drug Administration, 2009). Studies using generic-, disease- and treatment-specific measures can provide a comprehensive assessment of the effects of therapy on patient experience, and these data contribute to clinical decision making and support informed consent.
Patient-reported outcome (PRO) data are particularly relevant during and after treatment for oesophageal malignancy because of the overall poor outcomes observed, even if patients receive radical interventions. A number of studies have described the effect of surgical treatments with or without neoadjuvant therapy on HRQL (Blazeby et al, 2005; Parameswaran et al, 2008; Jacobs et al, 2012). However, the impact of definitive CRT on HRQL is less well described, and the available data are mainly from small, single-centre studies (Avery et al, 2007; Bedenne et al, 2007; Yamashita et al, 2009a). A recent randomised trial of CRT with or without cetuximab (the SCOPE-1 trial, a Cancer Research UK funded trial co-ordinated by the Wales Cancer Trial Unit in Cardiff University) included a comprehensive assessment of HRQL and recruited 258 patients from 36 UK centres. No differences in the prespecified HRQL domains were observed between the treatment groups and the addition of cetuximab to CRT could not be recommended for localised oesophageal cancer as there were increased numbers of treatment failures in the CRT plus cetuximab group (Crosby et al, 2013). However, the detailed trajectory of generic-, disease- and treatment-specific PROs were not described, and this report aims to provide the largest comprehensive description of the impact of definitive CRT treatment for localised oesophageal cancer on PROs, including HRQL, functions and symptoms.
Materials and methods
Participants and methods
SCOPE-1 was a multi-centre, open-label, randomised, parallel, two-arm, phase 2/3 trial, which did not continue past the phase 2 component. Full details of inclusion and exclusion criteria, recruitment and randomisation procedures and therapy have been reported (Hurt et al, 2011; Crosby et al, 2013). Briefly, patients with non-metastatic, histologically confirmed carcinoma of the oesophagus (adenocarcinoma, squamous cell or undifferentiated carcinoma) or gastro-oesophageal junction were eligible. Patients had disease either unsuitable for surgical therapy or individual patients had elected not to undergo surgery. All had full discussion at a specialist upper gastro-intestinal cancer multi-disciplinary team meeting. Patients were randomised 1:1 to CRT with cetuximab or CRT alone by stratified minimisation with a random element (80:20). Stratification was by recruiting hospital, primary reason for not having surgery, tumour histology and stage. All participants signed an informed consent. Multi-centre ethical approval was obtained, and the protocol was approved by the UK Medicines and Healthcare products Regulatory Agency.
Treatment protocol
CRT consisted of cisplatin 60 mg m−2 (day 1) and capecitabine 625 mg m−2 twice daily (days 1–21) for four cycles; cycles three and four were given concurrently with radiotherapy. The total dose of radiotherapy was 50 Gy in 25 fractions over 5 weeks (Monday–Friday) using 3-D conformal planning. The dose of Cetuximab was 400 mg m−2 on day 1 followed by 250 mg m−2 weekly given during induction chemotherapy as well as in the concurrent CRT phase. No adjuvant treatment was planned following CRT.
Study outcomes
The primary end point of the phase 2 component of the study was proportion of patients who were treatment failure-free at week 24; for the phase 3 component, overall survival was the primary end point. Secondary end points included progression-free survival, PROs (see below), health economics, toxicity (as per CTCAE version 3.0), treatment compliance and feasibility of recruitment.
Patient-reported outcomes
PROs were secondary end points in this trial and were assessed using the EORTC core questionnaire, the EORTC QLQ-C30 (version 3.0) (Aaronson et al, 1993), the disease specific module for oesophageal cancer, the QLQ-OES18 (Blazeby et al, 2003) and the treatment-specific validated Dermatology Life-Quality Index (DLQI) (Finlay and Khan, 1994) in paper format. The QLQ-C30 is a widely used and validated generic cancer questionnaire that assesses global quality of life, functional domains (physical, emotional, social, role and cognitive) and symptoms (fatigue, nausea and vomiting, pain, dyspnoea, insomnia, appetite loss, constipation, diarrhoea, financial difficulty) that commonly occur in patients with cancer. The QLQ-OES18 has four symptom scales assessing dysphagia, eating restrictions, reflux and pain and six single symptom items (trouble with saliva, choking, dry mouth, taste, cough and speech). All functional QLQ-C30 and QLQ-OES18 responses were linearly transformed to scores from 0 to 100 (Fayers et al, 2002). In the functional scales, high scores represent better quality of life (better function). Responses to the symptom items and scales in the EORTC questionnaires were shown to be skewed, and to appropriately summarise these data, the responses were categorised as either ‘minimal' or ‘severe'. Responses of ‘not at all' and ‘a little' being categorised as ‘minimal' and responses ‘quite a bit' or ‘very much' categorised as ‘severe' (Parameswaran et al, 2010; Rees et al, 2012).
The DLQI is a 10-item instrument that produces scores from 0 to 30 where the higher the score, the greater the impairment. Data from the DQLI were analysed according to the developer's instructions (Finlay and Khan, 1994). Nine of the 10 questions are scored on a four-point scale from zero to three and question number 7 has a dichotomous response scored zero or three. If a single question was not completed then this question was scored zero; if more than one question was incomplete the questionnaire was considered void. If more than one response was marked, then the highest score was used in the summation. If a single marked response covered two options, the lower score was used for the overall summation.
Assessment points were chosen to describe PRO changes across time from before randomisation (baseline–2 weeks before treatment start), at anticipated low points during therapy (7 weeks and 13 weeks) and at intervals that captured PROs during recovery (26 weeks) and in follow-up (52 weeks and 106 weeks). Time windows of ±3 weeks were applied for each assessment, which coincided with attendance for clinical follow-up visits. Questionnaires were checked at clinic visits to minimise missing items and assessments.
Data analyses
The primary trial was designed as a phase 2/3 with the independent data monitoring committee reviewing phase 2 data after 180 patients had a primary outcome (Crosby et al, 2013). The primary outcome in phase 2 was the proportion of patients who were treatment failure-free at 24 weeks. This was defined using the number who died or progressed before 24 weeks with those with a valid 24-week assessment as the denominator. All randomly assigned patients who met the eligibility criteria in the analysis of their allocated group were included. In the primary paper, the prespecified PROs were compared by treatment group. In this study, the full PRO data are described.
For EORTC functional scales, the mean scores and 95% confidence intervals (CIs) at each time point are presented and a change of ⩾15 points was considered clinically significant (Osoba et al, 1998). For EORTC symptoms scales, proportions of patients with severe/minimal symptoms were calculated with 95% Wilson CIs for each item at each time point owing to the skewed nature of the raw data, if proportions changed by ⩾15% this was considered clinically significant (Parameswaran et al, 2010; Rees et al, 2012).
To maximise the available data, all fully completed questionnaires were included as were questionnaires if half or more of the items within a scale were completed (Fayers et al, 2002). Missing data were imputed according to EORTC guidance where it is assumed that the missing items have values equal to the average of those items which were present for that respondent. Where data were missing from more than half the items within any scale, these scales were excluded from the analyses (Fayers et al, 2002). When a complete questionnaire was missing, the reason for the missing questionnaire was ascertained and categorised as (i) administrative failure, (ii) refusal, (iii) ill health, or (iv) due to an unknown or other reason.
For the DLQI, each score was summed to a final total out of 30 and was interpreted using the developer's validated recommendations, where a higher score reflects a greater impairment to quality of life, 0–1=no effect, 2–5=small effect, 6–10=moderate effect, 11–20=large effect, and 21–30=extremely large effect. A change in DQLI score of ⩾4 points is considered clinically significant (Basra et al, 2008). In addition, an exploratory analysis of the scales that constitute the overall DLQI measure under the headings symptoms and feelings, daily activities, leisure, work and school, personal relationships and treatment was undertaken with each heading reflecting a single item or two items forming a scale of the DLQI. Mean scores and 95% CIs were calculated and reported for the overall DLQI result and the scales. All analyses were undertaken and graphs produced using the STATA statistical software version 13.0 (Stata, College Station, TX, USA).
Trial management
The independent data monitoring committee met and reviewed the data five times at regular intervals during recruitment and, when reviewing the phase 2 results, made the decision to not recommend continuation of recruitment into phase 3 on 22 February 2012. All recruited patients were followed up to 24 weeks and PRO data were collected up to 104 weeks. This study is registered at ISRCTN, number 47718479. The protocol is published (Hurt et al, 2011) and a copy of full protocol can be accessed at: http://clinicaltrials.gov/ct2/show/NCT00509561?term=SCOPE1&rank=2.
Role of funding source
The trial was developed on behalf of the NCRI Upper GI Clinical Studies Group and Cancer Research UK's Clinical Trials Awards and Advisory Committee (CTAAC) approved the trial design. Merck provided the cetuximab and had no role in study design, data collection, analysis or interpretation or writing of this report. JR, JB and CH had full access to all the data and, along with the chief investigator (TC), had the final responsibility to submit for publication.
Results
Between February 2008 and 2012, 258 patients (129 assigned to each treatment group) from 36 UK centres were recruited, and as described above, the trial was stopped at the end of the phase 2 based on futility. Full details of the clinical outcomes are presented elsewhere (Crosby et al, 2013).
Questionnaire compliance and missing data
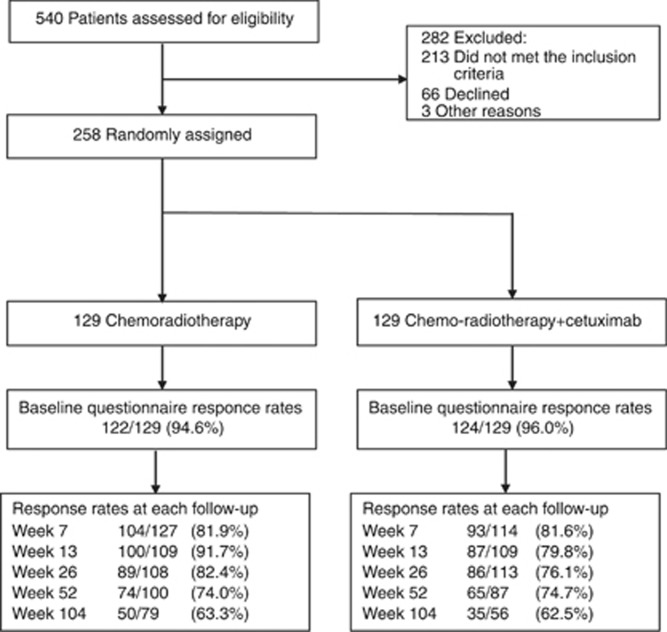
Questionnaire compliance was good throughout the study, initially being 122 out of 129 (94.6%) of those receiving CRT and 124 out of 129 (96.0%) of those receiving CRT plus cetuximab at baseline. Rates remained high and only reduced to 62.9% by the 24-month assessment in both groups (Figure 1). Details and reasons for missing questionnaires are in Table 1.
Figure 1.
CONSORT PRO flow diagram.
Table 1. Questionnaire compliance and reasons for missing data by treatment group.
|
Baseline |
7 weeks |
13 weeks |
26 weeks |
52 weeks |
104 weeks |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRT | CRT+C | CRT | CRT+C | CRT | CRT+C | CRT | CRT+C | CRT | CRT+C | CRT | CRT+C | |
| Eligible | 129 | 129 | 127 | 114 | 109 | 125 | 108 | 113 | 100 | 87 | 79 | 56 |
| Died | 0 | 0 | 1 | 0 | 6 | 2 | 15 | 9 | 20 | 33 | 36 | 61 |
| Withdrew | 0 | 0 | 1 | 15 | 14 | 2 | 6 | 7 | 9 | 9 | 10 | 7 |
| Questionnaire not due | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 5 |
| Returned (%) | 122 (94.6) | 124 (96.0) | 104 (81.9) | 93 (81.6) | 100 (91.7) | 87 (79.8) | 89 (82.4) | 86 (76.1) | 74 (74.0) | 65 74.7) | 50 (63.3) | 35 (62.5) |
| Administration | 0 | 0 | 8 | 7 | 2 | 8 | 12 | 10 | 10 | 10 | 10 | 8 |
| Patient declined | 0 | 0 | 3 | 0 | 1 | 4 | 3 | 1 | 2 | 1 | 2 | 2 |
| Patient too unwell | 0 | 0 | 0 | 2 | 4 | 4 | 2 | 2 | 3 | 4 | 1 | 2 |
| Other | 0 | 0 | 3 | 5 | 2 | 8 | 2 | 6 | 11 | 2 | 2 | 4 |
| No reason known | 7 | 5 | 9 | 7 | 0 | 14 | 0 | 8 | 0 | 5 | 14 | 5 |
Abbreviations: CRT=chemoradiotherapy; CRT+C=chemoradiotherapy plus cetuximab.
PROs prior to treatment
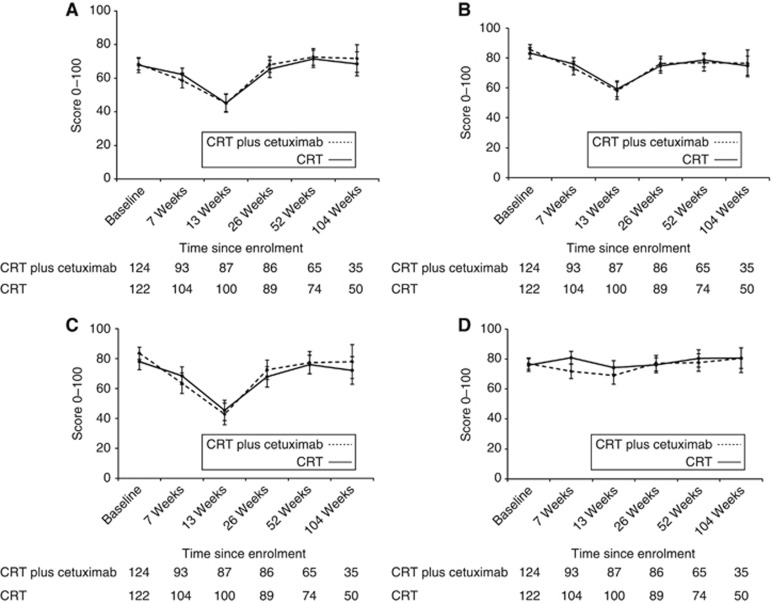
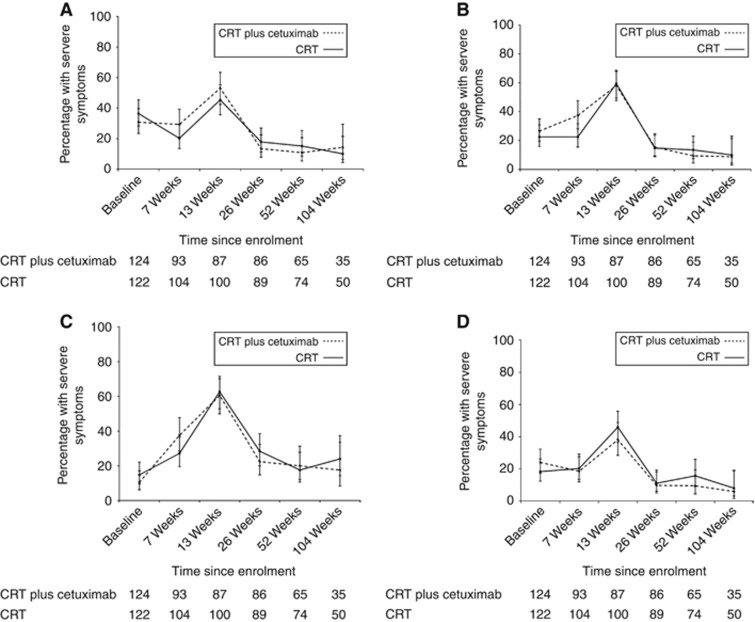
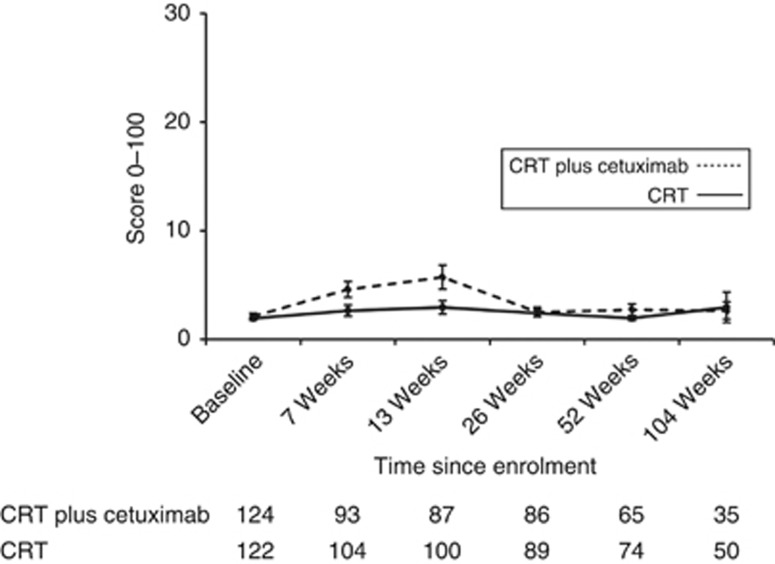
Mean baseline EORTC functional and global quality of life scores prior to treatment were >65 in both groups (Supplementary Table S1, Figures 2A–D). Figures for global quality of life, physical, role and emotional function are shown, and figures for social and cognitive function are shown in Supplementary Figures S1a and b, and similar patterns were observed. Baseline symptoms were minimal in most patients except that >15% of patients in both groups reported severe problems with fatigue, appetite loss, eating restrictions, dry mouth, trouble with saliva and dysphagia (Supplementary Table S1, Figures 3A–D). Figures for other EORTC symptom scores are not shown, but similar patterns were observed (Supplementary Table S2). Baseline DLQI scores were very low (few symptoms) with worst problems noted for ability to work (Figure 4; Supplementary Table S3).
Figure 2.
Line graphs showing the (A) Mean global quality of life scores and 95% CIs during treatment. (B) Mean physical function scores and 95% CIs during treatment. (C) Mean role function scores and 95% CIs during treatment. (D) Mean emotional function scores and 95% CIs during treatment.
Figure 3.
Line graphs showing the (A) Proportions of patients with severe eating restrictions and 95% CIs during treatment. (B) Proportions of patients with severe appetite loss and 95% CIs during treatment. (C) Proportions of patients with severe fatigue and 95% CIs during treatment. (D) Proportions of patients with severe dysphagia and 95% CIs during treatment.
Figure 4.
Line graph showing Dermatology Life Quality Index mean scores and 95% CIs during treatment.
PROs during treatment
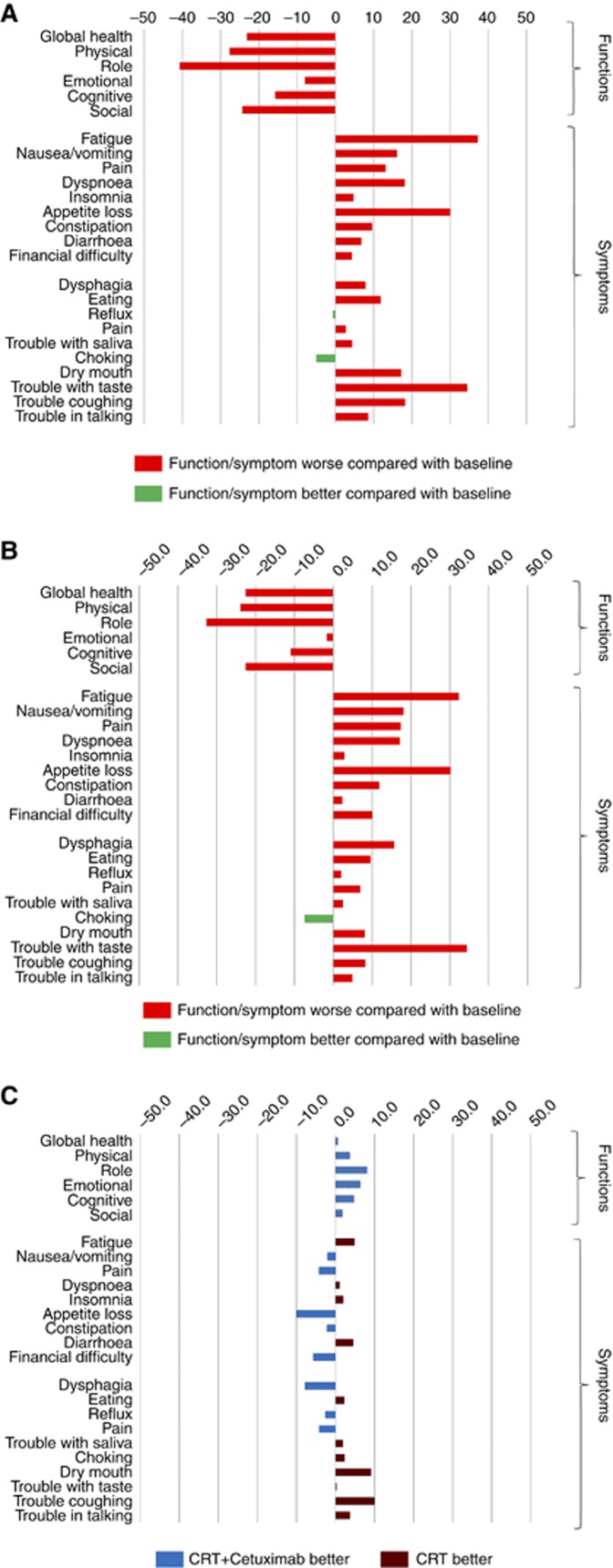
Most functional PROs deteriorated in both groups during treatment, and by 13 weeks reductions in mean scores of >15 points were observed in both groups for global quality of life and physical, social and role function (Figures 2A–C). Similarly, patients reported more symptoms (Figures 2A–C). Proportions of patients with severe symptoms were increased in both groups by >15% for fatigue, dyspnoea, appetite loss and trouble with taste at 13 weeks; increases were similar in both groups. Patients receiving cetuximab reported worse overall DLQI scores than those receiving CRT alone, although these did not reach clinical significance (mean score changes <4 points) (Figure 4; Supplementary Table S3).
PROs during follow-up
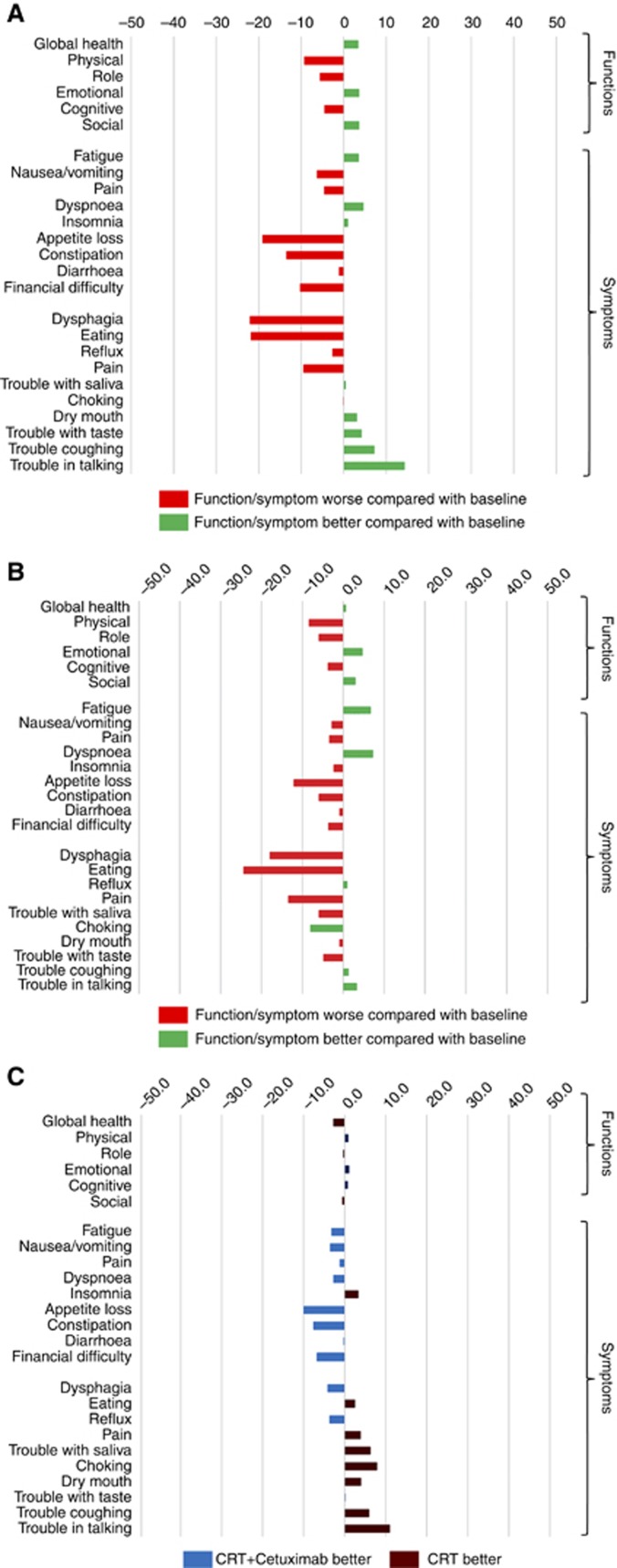
Most PROs recovered to baseline levels by 26 weeks (Supplementary Tables S1 and S2, Figures 2A–D and Figures 3A–D). Severe symptoms experienced during treatment all resolved and were maintained in follow-up except for on-going problems with fatigue and insomnia (reported by >15% of patients during follow-up). There were no clinically significant changes in mean function scores between baseline and the 104-week assessment (functional scores were similar to the start of treatment) and symptoms showed a similar pattern, although clinically significant improvements (>15% reduction) in appetite loss and eating restrictions were reported at the final assessment time. Scores did not differ between treatment groups (Supplementary Tables S1 and S2, Figures 5A–C and Figures 6A–C). Throughout follow-up, DLQI scores were low and stable (i.e., few problems) (Figure 4; Supplementary Table S3).
Figure 5.
Waterfall plot showing (A) Mean change in PRO scores from baseline to 13 weeks in patients receiving chemo-radiotherapy and cetuximab. (B) Mean change in PRO scores from baseline to 13 weeks in patients receiving CRT alone. (C) Mean change in HRQL outcomes from baseline to 13 weeks. Positive values in the functional scales and negative values for the symptom scale denote overall benefit from CRT with cetuximab (CRT+Cetuximab) compared with CRT alone.
Figure 6.
Waterfall plot showing (A) Mean change in PRO scores from baseline to 104 weeks in patients receiving chemo-radiotherapy and cetuximab. (B) Mean change in PRO scores from baseline to 104 weeks in patients receiving CRT alone. (C) Mean change in HRQL outcomes from baseline to 104 weeks. Positive values in the functional scales and negative values for the symptom scale denote overall benefit from CRT with cetuximab (CRT+Cetuximab) compared with CRT alone.
Discussion
Although the SCOPE-1 trial was closed by the independent data monitoring committee because of futility, it showed that CRT with or without cetuximab is associated with 2-year survival similar to that reported by surgical randomised trials and large series (Macdonald et al, 2001; Cunningham et al, 2006; Gebski et al, 2007; Tepper et al, 2008). An important aspect of the SCOPE-1 trial was its detailed assessment of PRO in a multi-centre study setting in patients with localised oesophageal cancer undergoing definitive CRT. Generic-, disease- and treatment-specific PROs were completed, and good questionnaire response rates were obtained in both groups at baseline and follow-up to 24 months. Results show a significant deterioration in functional scores and increased symptoms during treatment particularly at 13 weeks, which should direct clinicians to particularly focus supportive care for patients at this time. Recovery of most PROs was achieved by 6 months, and by 104 weeks scores were similar or better than before treatment. These data, therefore, can be used in clinical decisionmaking to inform patients of the PRO during and following definitive CRT, and they suggest that recovery of PROs after CRT is better that that reported after treatments including surgery (Reynolds et al, 2006; Avery et al, 2007). This is an important and novel observation that requires further evaluation.
Previous research examining PROs in non-surgical radical treatment of oesophageal cancer has been limited to small, single-centre studies (Avery et al, 2007; Ariga et al, 2009; Jacobs et al, 2012). Although validated EORTC generic- and disease-specific measures were often used, many did not report baseline data or reasons for missing assessments. Recently, the clinical results from multi-centre RCT examining two CRT regimens has been reported. The study did assess PROs with EORTC generic- and disease-specific measures, and PRO data will be reported separately (Conroy et al, 2014). The separate reporting of clinical trial data and PROs may reduce the use of the PRO data in decisionmaking, because clinicians may read the initial report in a main stream journal but not be aware of the secondary publication of the detailed PRO information. This issue has been highlighted in the recent PRO CONSORT extension that recommends that trial papers report both sets of data to enable the use of the information in practice (Calvert et al, 2013).
Unlike definitive CRT, there has been extensive and in-depth study of PRO after surgical treatment for localised oesophageal cancer (Blazeby et al, 2000; Gillham et al, 2008; Yamashita et al, 2009b; Derogar et al, 2012; Jacobs et al, 2014). Generally studies show a marked deterioration in PRO following surgery that takes 6–9 months to recover, and patients who do not survive >2 years do not recover their quality of life. Studies of long-term survivors of surgery using EORTC measures show that persistent problems with dyspnoea, reflux and physical function occur at 104 weeks and beyond, but no deficits with eating restrictions are reported. Therefore, this current study that examines in detail PRO after definitive CRT treatment provides new information about PRO in long-term survivors of CRT that may be used in clinical decisionmaking for patients who may be suitable for a surgical or non-surgical radical approach for oesophageal cancer. Randomised comparative analyses of PRO between CRT and surgical treatments for oesophageal cancer are still needed, although this type of study will be difficult to conduct (Blazeby et al, 2014). This study has several limitations. Despite efforts, questionnaire return rates in the later stages of the study reduced to 62% in both groups. It is possible that non-responders had different PRO profiles to responders, and there is bias in the results. It also has limited long-term follow-up (to 104 weeks only), and 5-year survival and HRQL data following definitive CRT are still needed. It is also possible that the DLQI tool for assessing skin symptoms, which showed minimal change in this study, may lack the sensitivity necessary to detect skin problems related to the use of cetuximab. Finally, it is possible that patients with squamous cell cancer may have different responses to CRT compared with adenocarcinoma and this may be apparent in the PRO scores. These analyses have not been undertaken and could only be considered exploratory from this data set. We also acknowledge that the inclusion criteria allowed a heterogeneous population – patients with advanced inoperable disease, those who were medically unfit for surgery as well as patient who elected to have non-surgical treatment. These groups may have had different HRQL profiles and these differences have not been captured in this study. It should also be recognised that clinical trial outcomes do not always reflect that achievable in the community, for example, patients with severe dysphagia may have undergone primary stenting and could have been excluded from the trial. A final weakness is that the rates of interventions for dysphagia during follow-up (the need for dilatation) were not recorded. It is possible that the surviving patients (who reported few problems with dysphagia and eating restrictions) require several dilatations to maintain this – although this is speculation alone.
In summary, this study has investigated PRO in localised oesophageal cancer; this detailed analysis highlights significant new findings not described in the initial clinical paper (Crosby et al, 2013) and demonstrates that there is a severe detrimental impact on function and symptoms during treatment. These problems resolve by 6 months and in survivors few symptoms are reported 2 years after treatment. The data suggest that the HRQL recovery after definitive CRT is quicker and there is little lasting deficit compared with treatment including surgery. This underlines the need for a well-designed and conducted RCT comparing definitive CRT with treatment including surgery for localised oesophageal cancer (Blazeby et al, 2014).
Acknowledgments
The SCOPE1 trial was sponsored by Velindre NHS Trust, funded by Cancer Research UK Grant number C20177/A7256, and Cancer Research UK core funding at the Wales Cancer Trials Unit (WCTU). Merck provided free cetuximab, labelling and distribution for the study. The radiotherapy trials quality assurance (RTTQA) was funded by Cancer Research UK and the Cardiff National Cancer Research Institute RTTQA Centre at Velindre NHS Trust, which are funded by the National Institute of Social Care and Health Research (NISCHR; Wales) and the Department of Health (England). JM Blazeby is supported by the MRC ConDuCT-II Hub (Collaboration and innovation for Difficult and Complex randomised controlled Trials In Invasive procedures – MR/K025643/1. David Cunningham is funded by the NIHR Biomedical Research Centre at the Royal Marsden and Institute of Cancer Research. S Mukherjee is partly funded by the NIHR Biomedical Research Unit, Oxford. Dr Tom Crosby is part funded by the NISCHR Academic Health Sciences Collaboration research fellowship scheme.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- Aaronson N, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez N, Filiberti A, Flechtner H, Fleishman S, de Haes J, Kaasa S, Klee M, Rofe P, Schraub S, Sneeuw K, Sullivan M, Takeda F (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85: 365–376. [DOI] [PubMed] [Google Scholar]
- Ariga H, Nemoto K, Miyazaki S, Yoshioka T, Ogawa Y, Sakayauchi T, Jingu K, Miyata G, Onodera K, Ichikawa H, Kamei T, Kato S, Ishioka C, Satomi S, Yamada S (2009) Prospective comparison of surgery alone and chemoradiotherapy with selective surgery in resectable squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys 75(2): 348–356. [DOI] [PubMed] [Google Scholar]
- Avery KN, Metcalfe C, Barham CP, Alderson D, Falk SJ, Blazeby JM (2007) Quality of life during potentially curative treatment for locally advanced oesophageal cancer. Br J Surg 94(11): 1369–1376. [DOI] [PubMed] [Google Scholar]
- Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY (2008) The Dermatology Life Quality Index 1994-2007: a comprehensive review of validation data and clinical results. Br J Dermatol 159(5): 997–1035. [DOI] [PubMed] [Google Scholar]
- Bedenne L, Michel P, Bouche O, Milan C, Mariette C, Conroy T, Pezet D, Roullet B, Seitz JF, Herr JP, Paillot B, Arveux P, Bonnetain F, Binquet C (2007) Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 25(10): 1160–1168. [DOI] [PubMed] [Google Scholar]
- Blazeby JM, Alderson D, Farndon JR (2000) Quality of life in patients with oesophageal cancer. Recent Results Cancer Res 155: 193–204. [DOI] [PubMed] [Google Scholar]
- Blazeby JM, Conroy T, Hammerlid E, Fayers P, Sezer O, Koller M, Arraras J, Bottomley A, Vickery CW, Etienne PL, Alderson D European Organisation for R Treatement of Cancer G Quality of Life G (2003) Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer 39(10): 1384–1394. [DOI] [PubMed] [Google Scholar]
- Blazeby JM, Sanford E, Falk SJ, Alderson D, Donovan JL (2005) Health-related quality of life during neoadjuvant treatment and surgery for localized esophageal carcinoma. Cancer 103(9): 1791–1799. [DOI] [PubMed] [Google Scholar]
- Blazeby JM, Strong S, Donovan JL, Wilson C, Hollingworth W, Crosby T, Nicklin J, Falk SJ, Barham CP, Hollowood AD, Streets CG, Titcomb D, Krysztopik R, Griffin SM, Brookes ST (2014) Feasibility RCT of definitive chemoradiotherapy or chemotherapy and surgery for oesophageal squamous cell cancer. Br J Cancer 111(2): 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD CONSORT PRO Group (2013) Reporting of patient-reported outcomes in randomized trials: The consort pro extension. JAMA 309(8): 814–822. [DOI] [PubMed] [Google Scholar]
- Conroy T, Galais M-P, Raoul J-L, Bouché O, Gourgou-Bourgade S, Douillard J-Y, Etienne P-L, Boige V, Martel-Lafay I, Michel P, Llacer-Moscardo C, François E, Créhange G, Abdelghani MB, Juzyna B, Bedenne L, Adenis A (2014) Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol 15(3): 305–314. [DOI] [PubMed] [Google Scholar]
- Crosby T, Hurt CN, Falk S, Gollins S, Mukherjee S, Staffurth J, Ray R, Bashir N, Bridgewater JA, Geh JI, Cunningham D, Blazeby J, Roy R, Maughan T, Griffiths G (2013) Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol 14(7): 627–637. [DOI] [PubMed] [Google Scholar]
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJH, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ the MAGIC Trial Participants (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355(1): 11–20. [DOI] [PubMed] [Google Scholar]
- Derogar M, Orsini N, Sadr-Azodi O, Lagergren P (2012) Influence of major postoperative complications on health-related quality of life among long-term survivors of esophageal cancer surgery. J Clin Oncol 30(14): 1615–1619. [DOI] [PubMed] [Google Scholar]
- Fayers P, Aaronson N, Bjordal K, Groenvold M, Curran D, Bottomley A (2002) EORTC QLQ-C30 Scoring Manual 2nd edn. EORTC Publications: Brussels, Belgium. [Google Scholar]
- Finlay AY, Khan GK (1994) Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol 19(3): 210–216. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (2009) Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to support Labeling Claims. Food and Drug Administration, US Department of Health and Human Services: Rockville, MD, USA. [Google Scholar]
- Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J (2007) Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 8(3): 226–234. [DOI] [PubMed] [Google Scholar]
- Gillham CM, Aherne N, Rowley S, Moore J, Hollywood D, O'Byrne K, Reynolds JV (2008) Quality of life and survival in patients treated with radical chemoradiation alone for oesophageal cancer. Clin Oncol (R Coll Radiol) 20(3): 227–233. [DOI] [PubMed] [Google Scholar]
- Hurt CN, Nixon LS, Griffiths GO, Al-Mokhtar R, Gollins S, Staffurth JN, Phillips CJ, Blazeby JM, Crosby TD (2011) SCOPE1: a randomised phase II/III multicentre clinical trial of definitive chemoradiation, with or without cetuximab, in carcinoma of the oesophagus. BMC Cancer 11: 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M, Macefield RC, Blazeby JM, Korfage IJ, van Berge Henegouwen MI, de Haes HC, Smets EM, Sprangers MA (2012) Systematic review reveals limitations of studies evaluating health-related quality of life after potentially curative treatment for esophageal cancer. Qual Life Res 22(7): 1787–1803. [DOI] [PubMed] [Google Scholar]
- Jacobs M, Macefield RC, Blazeby JM, Korfage IJ, van Berge Henegouwen MI, de Haes HC, Smets EM, Sprangers MA (2013) Systematic review reveals limitations of studies evaluating health-related quality of life after potentially curative treatment for esophageal cancer. Qual Life Res 22(7): 1787–1803. [DOI] [PubMed] [Google Scholar]
- Jacobs M, Macefield RC, Elbers RG, Sitnikova K, Korfage IJ, Smets EMA, Henselmans I, van Berge Henegouwen MI, de Haes JCJM, Blazeby JM, Sprangers MAG (2014) Meta-analysis shows clinically relevant and long-lasting deterioration in health-related quality of life after esophageal cancer surgery. Qual Life Res 23(4): 1155–1176. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2): 69–90. [DOI] [PubMed] [Google Scholar]
- Kranzfelder M, Schuster T, Geinitz H, Friess H, Buchler P (2011) Meta-analysis of neoadjuvant treatment modalities and definitive non-surgical therapy for oesophageal squamous cell cancer. Br J Surg 98(6): 768–783. [DOI] [PubMed] [Google Scholar]
- Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345(10): 725–730. [DOI] [PubMed] [Google Scholar]
- Malthaner RA, Collin S, Fenlon D (2006) Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database Syst Rev 3: CD001556. [DOI] [PubMed] [Google Scholar]
- MRC Oesophageal Cancer Working Party (2002) Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 359(9319): 1727–1733. [DOI] [PubMed] [Google Scholar]
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J (1998) Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16(1): 139–144. [DOI] [PubMed] [Google Scholar]
- Parameswaran R, Blazeby JM, Hughes R, Mitchell K, Berrisford RG, Wajed SA (2010) Health-related quality of life after minimally invasive oesophagectomy. Br J Surg 97(4): 525–531. [DOI] [PubMed] [Google Scholar]
- Parameswaran R, McNair A, Avery KN, Berrisford RG, Wajed SA, Sprangers MA, Blazeby JM (2008) The role of health-related quality of life outcomes in clinical decision making in surgery for esophageal cancer: a systematic review. Ann Surg Oncol 15(9): 2372–2379. [DOI] [PubMed] [Google Scholar]
- Rees JR, Blazeby JM, Fayers P, Friend EA, Welsh FK, John TG, Rees M (2012) Patient-reported outcomes after hepatic resection of colorectal cancer metastases. J Clin Oncol 30(12): 1364–1370. [DOI] [PubMed] [Google Scholar]
- Reynolds JV, McLaughlin R, Moore J, Rowley S, Ravi N, Byrne PJ (2006) Prospective evaluation of quality of life in patients with localized oesophageal cancer treated by multimodality therapy or surgery alone. Br J Surg 93(9): 1084–1090. [DOI] [PubMed] [Google Scholar]
- Ronellenfitsch U, Schwarzbach M, Hofheinz R, Kienle P, Kieser M, Slanger TE, Burmeister B, Kelsen D, Niedzwiecki D, Schuhmacher C, Urba S, van de Velde C, Walsh TN, Ychou M, Jensen K (2013. a) Preoperative chemo(radio)therapy versus primary surgery for gastroesophageal adenocarcinoma: systematic review with meta-analysis combining individual patient and aggregate data. Eur J Cancer 49(15): 3149–3158. [DOI] [PubMed] [Google Scholar]
- Ronellenfitsch U, Schwarzbach M, Hofheinz R, Kienle P, Kieser M, Slanger TE, Jensen K Group GEAM-a (2013. b) Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst Rev 5: CD008107. [DOI] [PubMed] [Google Scholar]
- Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, Kiel K, Willett C, Sugarbaker D, Mayer R (2008) Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 26(7): 1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R, Malthaner R (2006) Combined chemotherapy and radiotherapy (without surgery) compared with radiotherapy alone in localized carcinoma of the esophagus. Cochrane Database Syst Rev 1: CD002092. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Okuma K, Seto Y, Mori K, Kobayashi S, Wakui R, Ohtomo K, Nakagawa K (2009. a) A retrospective comparison of clinical outcomes and quality of life measures between definitive chemoradiation alone and radical surgery for clinical stage II-III esophageal carcinoma. J Surg Oncol 100(6): 435–441. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Okuma K, Seto Y, Mori K, Kobayashi S, Wakui R, Ohtomo K, Nakagawa K (2009. b) A retrospective comparison of clinical outcomes and quality of life measures between definitive chemoradiation alone and radical surgery for clinical stage II–III esophageal carcinoma. J Surg Oncol 100(6): 435–441. [DOI] [PubMed] [Google Scholar]
- Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Geneve J, Lasser P, Rougier P (2011) Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 29(13): 1715–1721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.