Abstract
Background:
Phyllodes tumors are rare fibroepithelial neoplasms of the breast, which carry the potential risk of local recurrence and metastasis. Phyllodes tumors share several histological features with fibroadenomas, and no widely accepted markers for distinguishing these lesions have been identified.
Methods:
We analyzed molecular abnormalities related to telomere elongation in tumors, including TERT promoter mutations, as well as loss of expression of ATRX and DAXX, in a total of 104 phyllodes tumors and fibroadenomas.
Results:
Sequencing analyses showed that TERT promoter mutations were frequent in phyllodes tumors (30/46, 65%), but rare in fibroadenomas (4/58, 7%). Among phyllodes tumors, the mutations were more frequent in borderline tumors (13/15, 87%), but were also common in benign (9/18, 50%) and malignant tumors (8/13, 62%). Remarkably, all but one TERT promoter-mutated tumor also contained MED12 mutations, indicating that these mutations are strongly associated (P=8.4 × 10−6). Expression of ATRX and DAXX, as evaluated by immunohistochemistry, was retained in all tumors.
Conclusions:
Our observations suggest a critical role of TERT promoter mutations, in cooperation with MED12 mutations, in the development of phyllodes tumors. Because TERT promoter mutations are rare among fibroadenomas, their detection may be of potential use in discriminating between phyllodes tumors and fibroadenomas.
Keywords: TERT, MED12, phyllodes tumor, fibroadenoma, breast
Fibroadenomas and phyllodes tumors constitute the major histological types of fibroepithelial tumors of the breast (Brogi, 2009; Tavassoli and Eusebi, 2009; Tan et al, 2012). Fibroadenomas are one of the most common benign breast neoplasms, mainly affecting young adults. Phyllodes tumors are rare, accounting for <1% of all breast tumors, and are associated with a risk of local recurrence and metastasis. Despite the distinct clinical behavior of these tumors, there is significant overlap in their morphology, particularly between benign phyllodes tumors and intracanalicular-type fibroadenomas, and biopsy diagnosis of these lesions is often challenging (Tsang et al, 2011; Yasir et al, 2014).
Recent studies have begun to address the genetic backgrounds of fibroepithelial tumors of the breast. We and other researchers previously reported that MED12 mutations are very common in both phyllodes tumors and fibroadenomas (Lim et al, 2014; Cani et al, 2015; Yoshida et al, 2015). The identification of a common genetic alteration in these tumors implies a histogenetic relationship. Malignant phyllodes tumors have also been reported to be associated with aberrations in several classic oncogenes and tumor-suppressor genes, including TP53, RB1, CDKN2A and EGFR (Kersting et al, 2006; Jones et al, 2008; Cani et al, 2015). However, despite their different biological behavior, no molecular abnormalities that distinguish benign and borderline phyllodes tumors from fibroadenomas have been reported.
Telomeres are repetitive nucleotide sequences at the end of chromosomes that are required for chromosomal integrity (Aubert and Lansdorp, 2008). In the absence of telomerase activity, telomeres shorten with each cell division, eventually leading to senescence or cell death. Therefore, tumor cells require a mechanism to maintain telomere length to acquire immortality. Most commonly, tumor cells exhibit the increased telomerase activity, which synthesizes telomeric DNA sequences (Kim et al, 1994; Shay and Bacchetti, 1997). TERT encodes the catalytic subunit of telomerase, which is a major determinant of telomerase activity (Aubert and Lansdorp, 2008). Its transcription is repressed in the most quiescent somatic cells but is activated in the majority of malignant tumors (Shay and Roninson, 2004). Recent studies have shown that mutations in the TERT promoter are responsible for overexpression of TERT in a range of tumors, including melanomas, hepatocellular carcinomas and urothelial carcinomas (Horn et al, 2013; Huang et al, 2013; Killela et al, 2013; Heidenreich et al, 2014). Another subset of tumors uses a different mechanism for telomere maintenance, which is known as the alternative lengthening of telomeres (ALT; Cesare and Reddel, 2010). Interestingly, loss of ATRX and DAXX, both of which are involved in chromatin remodeling, has been shown to be closely associated with the ALT phenotype in several types of tumors, including pancreatic neuroendocrine tumors, glioblastomas and uterine leiomyosarcomas (Cesare and Reddel, 2010; Liau et al, 2015).
Although previous studies have reported telomerase activity in a small number of phyllodes tumors and fibroadenomas (Hiyama et al, 1996; Mokbel et al, 1999), the mechanisms underlying telomere maintenance in these tumors have not been previously examined. In the present study, we analyzed the molecular abnormalities involved in telomere elongation, including TERT promoter mutations, as well as loss of expression of ATRX and DAXX, in a series of phyllodes tumors and fibroadenomas.
Materials and methods
Tissue samples
This study was approved by the ethics committee of the National Cancer Center, Tokyo, Japan. All the tissue samples were obtained from the National Cancer Center Hospital, Tokyo, Japan. The resected specimens were routinely fixed in 10% formalin and embedded in paraffin. This study examined 46 phyllodes tumors and 58 fibroadenomas that had previously been analyzed to determine their MED12 mutation status (Yoshida et al, 2015). The phyllodes tumors were subclassified according to the WHO classification as benign, borderline or malignant lesions (Tan et al, 2012). Fibroadenomas were classified as intracanalicular-type, pericanalicular-type and complex-type (Tan et al, 2012).
Mutational analysis
Ten-micrometer-thick sections of paraffin-embedded specimens were deparaffinized, stained briefly with hematoxylin and subjected to DNA extraction. The lesions were microdissected using sterilized toothpicks under a microscope. The dissected samples were incubated in DNA extraction buffer at 50 °C overnight, heated at 100 °C for 10 min to inactivate proteinase K, and then directly subjected to PCR using a pair of primers encompassing the core promoter region of TERT (5′-CAGCGCTGCCTGAAACTC-3′ and 5′-GTCCTGCCCCTTCACCTT-3′) as described previously (Horn et al, 2013; Yoshida et al, 2015). The PCR products were purified and sequenced bidirectionally using an Applied Biosystems 3130 Genetic Analyzer (Applied Biosystems, Foster, CA, USA). All mutations were confirmed by re-analysis of the respective specimens, including DNA extraction.
A microdissection-based analysis was performed in selected cases with a TERT promoter mutation. The epithelial and stromal components were microdissected separately using a laser microdissection system (MMI CellCut system; Molecular Machines and Industries, Glattbrugg, Switzerland). The isolated samples were then examined for the presence of TERT promoter mutations, as described above.
Histology and immunohistochemistry
Histological analysis was performed in accordance with WHO classification (Tan et al, 2012). Two pathologists (MY and SS) independently scored each case for tumor border, stromal cellularity, stromal atypia, mitosis, stromal overgrowth and malignant heterologous component (Supplementary Table 1). Mitotic counts were quantified per 10 high-power fields with the field size adjusted to 0.196 mm2. Discrepant cases were reviewed together to achieve consensus.
Immunohistochemical analysis was performed on formalin-fixed paraffin-embedded specimens. Antigen retrieval was performed by autoclaving in a 10 mM citrate buffer (pH 6.0) for 10 min. Anti-ATRX (HPA001906; 1:200 dilution; Sigma-Aldrich, St. Louis, MO, USA) and anti-DAXX (HPA008736; 1:250 dilution; Sigma-Aldrich) antibodies were used as the primary antibodies (Heaphy et al, 2011). For staining, we used an automated stainer (Dako, Glostrup, Denmark), according to the manufacturer's protocol. ChemMate EnVision (Dako) methods were used for detection. Tumors showing uniformly negative staining were regarded as showing loss of expression, using lymphocytes and endothelial cells as internal positive controls.
Statistical analysis
Fisher's exact test or an extended Fisher's exact test was used to analyze categorical variables. A Mann-Whitney's U-test was used for analysis of continuous variables. P-values<0.05 were considered to indicate statistical significance.
Results
Sequencing analyses revealed TERT promoter mutations in 30 of 46 phyllodes tumors (65%) and in 4 of 58 fibroadenomas (7% Figure 1A, Table 1, Supplementary Table 1), indicating a substantial difference in these two tumor types (P=1.5 × 10−10). Remarkably, all but one of the TERT promoter-mutated tumors concurrently harbored MED12 mutations (P=8.4 × 10−6). Non-tumor tissue specimens were available for 17 of the phyllodes tumors and three of the fibroadenomas with TERT promoter mutations, and sequencing analyses of these samples did not identify any mutations, indicating the somatic nature of TERT promoter mutations.
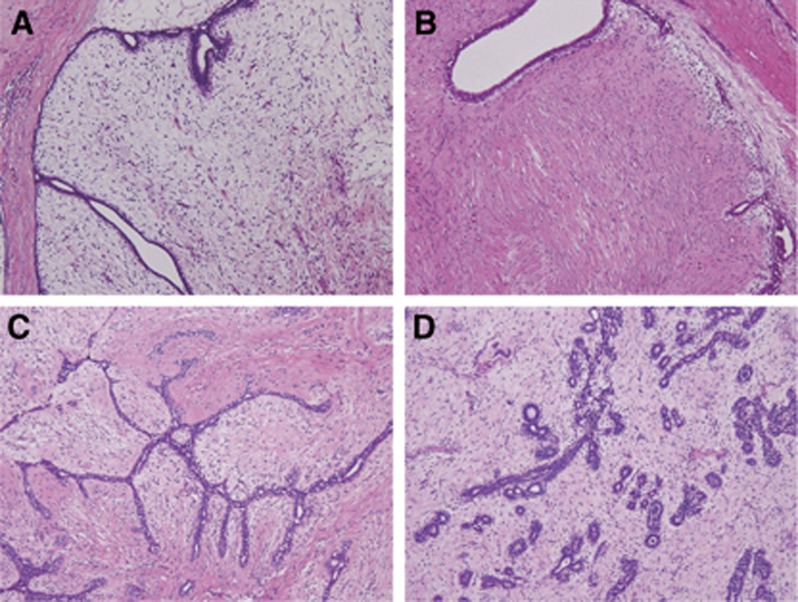
Figure 1.
Histology of phyllodes tumors and fibroadenomas. (A) Benign phyllodes tumor with a TERT promoter mutation. A distended and compressed duct lined by epithelium and expansion of moderately cellular and edematous stroma. (B) Benign phyllodes tumor without TERT promoter mutation. Compressed and partly dilated ducts and relatively fibrous stroma. (C) Intracanalicular-type fibroadenoma with a TERT mutation. Slit-like ducts compressed by proliferating stroma. (D) Pericanalicular-type fibroadenoma. Tubular ducts surrounded by edematous stroma.
Table 1. TERT promoter mutations in phyllodes tumors and fibroadenomas of the breast.
| Histology | Number of cases | Total mutated | Nucleotide change | Cases mutated |
|---|---|---|---|---|
| Phyllodes tumor | 46 | 30 (65%) | ||
| Benign | 18 | 9 (50%) | c.-146C>T | 3 |
| c.-124C>T | 5 | |||
| c.-124C>A | 1 | |||
| Borderline | 15 | 13 (87%) | c.-124C>T | 13 |
| Malignant | 13 | 8 (62%) | c.-146C>T | 1 |
| c.-124C>T | 7 | |||
| Fibroadenoma | 58 | 4 (7%) | ||
| Intracanalicular | 32 | 2 (6%) | c.-124C>T | 2 |
| Pericanalicular | 20 | 2 (10%) | c.-124C>T | 2 |
| Complex | 6 | 0 |
Among the phyllodes tumors, the presence of TERT promoter mutations was significantly associated with older age, but not with tumor size (Table 2). Analysis of tumor grade showed that borderline tumors more frequently possessed mutations, but the difference was not significant when the three different tumor grades were compared. The association with MED12 mutations was significant among phyllodes tumors. Histological evaluation showed that TERT promoter mutational status was significantly correlated with stromal cellularity, but not with the other morphological features (Table 2, Supplementary Table 1). In addition, when a comparison was made among benign phyllodes tumors, lesions with a TERT promoter mutation tended to show an edematous stroma, compared with TERT mutation-negative tumors, which showed a more fibrous stroma (Figure 1A and B).
Table 2. Correlation between TERT promoter mutation status and clinicopathological variables in phyllodes tumors of the breast.
|
TERT
promoter mutation |
|||
|---|---|---|---|
| Present | Absent | P-value | |
| Age, year-old | 54.5 (34–67) | 42.5 (26–58) | 0.0051 |
| Tumor size (mm) | 50 (10–130) | 42 (12–160) | 0.39 |
|
Grade | |||
| Benign | 9 | 9 | 0.086 |
| Borderline | 13 | 2 | |
| Malignant | 8 | 5 | |
|
Tumor border | |||
| Well-defined | 16 | 9 | 1.0 |
| Permeativea | 14 | 7 | |
|
Stromal cellularity | |||
| Low | 7 | 9 | 0.049 |
| Moderate-to-high | 23 | 7 | |
|
Stromal atypia | |||
| None or mild | 12 | 10 | 0.22 |
| Moderate-to-marked | 18 | 6 | |
|
Mitosis/10 HPFs | |||
| 0–4 | 18 | 10 | 1.0 |
| ⩽5 | 12 | 6 | |
|
Stromal overgrowth | |||
| Absent | 18 | 9 | 1.0 |
| Presentb | 12 | 7 | |
|
Malignant heterologous component | |||
| Absent | 29 | 14 | 0.27 |
| Present | 1 | 2 | |
|
MED12
mutation | |||
| Absent | 1 | 8 | 3.6 × 10−4 |
| Present | 29 | 8 | |
Abbreviation: HPF, high-power field.
Age and tumor size are indicated as the median (range).
Includes lesions with focally permeative borders.
Includes lesions with focal stromal overgrowth.
All four fibroadenomas with TERT promoter mutations, including two intracanalicular-type and two pericanalicular-type lesions, also possessed MED12 mutations (Supplementary Table 1). Histological re-examination confirmed the original diagnosis of the respective tumors, but we were unable to identify any distinct morphological features that distinguished them from TERT promoter mutation-negative tumors (Figure 1C and D).
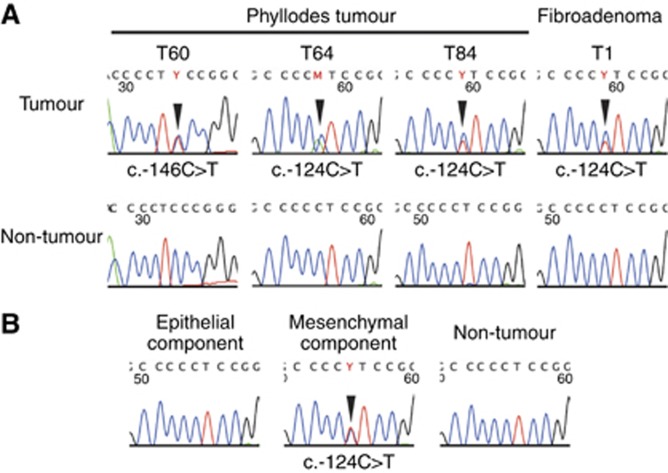
We performed a microdissection-based analysis of five phyllodes tumors and two fibroadenomas with TERT promoter mutations, to examine whether the epithelial component also harbored a TERT promoter mutation. The epithelial and stromal components were separately microdissected and subjected to sequencing analyses. The results showed that the TERT promoter mutations were only present in the stromal components, in all cases (Figure 2B).
Figure 2.
TERT promoter mutations in phyllodes tumors and a fibroadenoma. (A) TERT promoter mutations in phyllodes tumors and a fibroadenoma. (B) Microdissection-based analysis of TERT promoter mutations. Epithelial and stromal components of phyllodes tumors or fibroadenomas were separately microdissected and analyzed for the presence of TERT promoter mutations. The arrowheads indicate nucleotide substitutions.
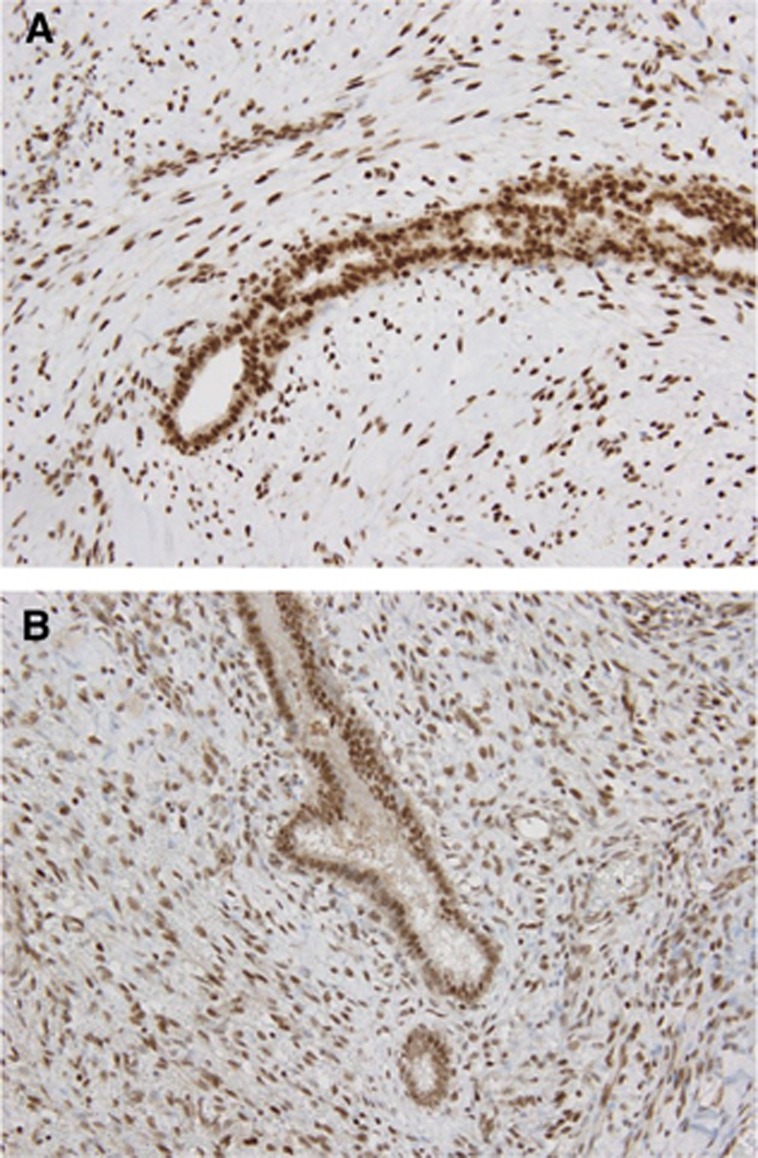
Immunohistochemical analysis showed diffuse nuclear expression of ATRX and DAXX in all phyllodes tumors and fibroadenomas (Figure 3A and B), suggesting that inactivation of these factors, which is related to the ALT phenotype, does not play a major role in the tumorigenesis of fibroepithelial tumors of the breast.
Figure 3.
ATRX and DAXX expression in phyllodes tumors. (A) ATRX expression in a borderline phyllodes tumor. Diffuse nuclear expression of ATRX in both epithelial and stromal cells. (B) DAXX expression in a borderline phyllodes tumor. Nuclear DAXX expression is retained in both epithelial and stromal components.
Discussion
The present study showed that TERT promoter mutations are frequent in phyllodes tumors, regardless of tumor grade. TERT promoter mutations are the second most frequent genetic aberration reported to date in phyllodes tumors, following MED12 mutations, which were identified in 80% of cases in the present cohort (Yoshida et al, 2015). These mutations have previously been reported in various tumors and have been shown to enhance promoter activity (Horn et al, 2013; Huang et al, 2013; Killela et al, 2013). Interestingly, TERT promoter mutations were identified almost exclusively in tumors with a MED12 mutation, indicating the synergistic roles of these two genetic abnormalities. TERT promoter mutation was also closely associated with older age. A similar association has been reported in thyroid carcinomas (Liu et al, 2014). As the telomere shortens with age, TERT promoter mutations have been suggested to have more major roles in preventing crisis caused by telomere shortening when oncogenic events take place in aged cells.
In contrast, none of the tumors showed loss of ATRX or DAXX, which are involved in ALT (Heaphy et al, 2011; Lovejoy et al, 2012). These observations suggest that the activation of telomerase by TERT promoter mutations plays a major role in telomere maintenance in phyllodes tumors.
The prevalence of TERT promoter mutations in malignant phyllodes tumors was lower than that in borderline tumors. This may simply be a bias due to the limited number of cases; alternatively, this observation might imply that a subset of malignant phyllodes tumors is directly derived from TERT mutation-negative precursors, rather than via stepwise progression from borderline tumors. Previous studies have shown that abnormal expression of some tumor-suppressor gene products, including p16, p53 and Rb, is almost exclusively observed in malignant phyllodes tumors (Tse et al, 2002; Tan et al, 2005; Cimino-Mathews et al, 2013). It seems possible that the acquisition of these molecular abnormalities is sufficient to gain malignant phenotypes, even in the absence of TERT promoter mutations.
TERT promoter mutations were rare among fibroadenomas. Considering that MED12 mutations were commonly detected in both fibroadenomas and phyllodes tumors, the difference in the prevalence of TERT promoter mutations may potentially explain the differences in the biological behavior of these tumors. The growth of fibroadenomas is usually self-limited, and they often undergo involution (Tavassoli and Eusebi, 2009; Tan et al, 2012). Conceivably, the lack of telomerase activity in fibroadenomas may eventually lead to senescence and/or cell death of tumor cells and prevent continuous growth. On the other hand, continuous growth and local recurrence appear to reflect the immortality of tumor cells, which require a mechanism for telomere elongation, in phyllodes tumors.
The presence of TERT promoter mutations in a small subset of fibroadenomas is of potential interest. Although these included two pericanalicular-type fibroadenomas, which are morphologically dissimilar to phyllodes tumors, some of these lesions may represent early-stage phyllodes tumors. Our review failed to identify morphological features specific to TERT promoter-mutated fibroadenomas. In addition, TERT promoter mutations do not appear to be associated with older age or larger tumor size in fibroadenomas. However, this could be owing to the limited number of cases analyzed, and future studies may determine the clinicopathological significance of TERT promoter mutations in fibroadenomas.
Microdissection-based analysis showed that TERT promoter mutations were only present in the stromal component, and not in the epithelial component, in both phyllodes tumors and fibroadenomas. This observation is consistent with observations of MED12 mutations in previous studies (Lim et al, 2014; Yoshida et al, 2015), and supports the idea that stromal cells are the principal neoplastic component of these fibroepithelial tumors. However, the consistent presence of epithelial components in these tumors implies the requirement for epithelium-derived factors in the proliferation of tumoral stromal cells, as previously suggested (Sawhney et al, 1992; Sawyer et al, 2002). Phyllodes tumors and fibroadenomas may be developed through interactions between neoplastic stroma and reactive epithelium.
The clinical management of phyllodes tumors and fibroadenomas differs significantly. Because of their self-limited nature, many fibroadenomas are treated by simple excision; follow-up alone, without surgical intervention, is sometimes a valid option (Cant et al, 1995; Brogi, 2009). However, phyllodes tumors need to be removed with sufficient margins, to reduce the risk of recurrence (Chen et al, 2005; Brogi, 2009). Therefore, preoperative diagnosis has a significant impact on the determination of appropriate clinical management. However, distinction of phyllodes tumors from fibroadenomas is often challenging (Tsang et al, 2011; Yasir et al, 2014), and there are no reliable molecular markers are currently available to distinguish between these tumors. On the basis of the difference in prevalence observed between these two types of tumors, TERT promoter mutations might be of potential use as an ancillary diagnostic tool.
Acknowledgments
We are grateful to Ms. Sachiko Miura, Ms. Toshiko Sakaguchi and Ms. Chizu Kina for technical assistance and to Taichi Shimazu for helpful comments on the statistical analysis.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- Aubert G, Lansdorp PM (2008) Telomeres and aging. Physiol Rev 88(2): 557–579. [DOI] [PubMed] [Google Scholar]
- Brogi E (2009) Fibroepithelial Neoplasms. In: Hoda SA, Brogi E, Koerner FC, Rosen PR (eds) 4th edn. Rosen's breast pathology pp 213–270. Lippincot Williams and Willkins: Philadelphia, PA, USA. [Google Scholar]
- Cani AK, Hovelson DH, McDaniel AS, Sadis S, Haller MJ, Yadati V, Amin AM, Bratley J, Bandla S, Williams PD, Rhodes K, Liu CJ, Quist MJ, Rhodes DR, Grasso CS, Kleer CG, Tomlins SA (2015) Next-gen sequencing exposes frequent MED12 mutations and actionable therapeutic targets in phyllodes tumors. Mol Cancer Res 13(4): 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant PJ, Madden MV, Coleman MG, Dent DM (1995) Non-operative management of breast masses diagnosed as fibroadenoma. Br J Surg 82(6): 792–794. [DOI] [PubMed] [Google Scholar]
- Cesare AJ, Reddel RR (2010) Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet 11(5): 319–330. [DOI] [PubMed] [Google Scholar]
- Chen WH, Cheng SP, Tzen CY, Yang TL, Jeng KS, Liu CL, Liu TP (2005) Surgical treatment of phyllodes tumors of the breast: retrospective review of 172 cases. J Surg Oncol 91(3): 185–194. [DOI] [PubMed] [Google Scholar]
- Cimino-Mathews A, Hicks JL, Sharma R, Vang R, Illei PB, De Marzo A, Emens LA, Argani P (2013) A subset of malignant phyllodes tumors harbors alterations in the Rb/p16 pathway. Hum Pathol 44(11): 2494–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaphy CM, de Wilde RF, Jiao Y, Klein AP, Edil BH, Shi C, Bettegowda C, Rodriguez FJ, Eberhart CG, Hebbar S, Offerhaus GJ, McLendon R, Rasheed BA, He Y, Yan H, Bigner DD, Oba-Shinjo SM, Marie SK, Riggins GJ, Kinzler KW, Vogelstein B, Hruban RH, Maitra A, Papadopoulos N, Meeker AK (2011) Altered telomeres in tumors with ATRX and DAXX mutations. Science 333(6041): 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich B, Rachakonda PS, Hemminki K, Kumar R (2014) TERT promoter mutations in cancer development. Curr Opin Genet Dev 24: 30–37. [DOI] [PubMed] [Google Scholar]
- Hiyama E, Gollahon L, Kataoka T, Kuroi K, Yokoyama T, Gazdar AF, Hiyama K, Piatyszek MA, Shay JW (1996) Telomerase activity in human breast tumors. J Natl Cancer Inst 88(2): 116–122. [DOI] [PubMed] [Google Scholar]
- Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, Schadendorf D, Kumar R (2013) TERT promoter mutations in familial and sporadic melanoma. Science 339(6122): 959–961. [DOI] [PubMed] [Google Scholar]
- Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA (2013) Highly recurrent TERT promoter mutations in human melanoma. Science 339(6122): 957–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Mitter R, Springall R, Graham T, Winter E, Gillett C, Hanby AM, Tomlinson IP, Sawyer EJ Phyllodes Tumour C (2008) A comprehensive genetic profile of phyllodes tumours of the breast detects important mutations, intra-tumoral genetic heterogeneity and new genetic changes on recurrence. J Pathol 214(5): 533–544. [DOI] [PubMed] [Google Scholar]
- Kersting C, Kuijper A, Schmidt H, Packeisen J, Liedtke C, Tidow N, Gustmann C, Hinrichs B, Wulfing P, Tio J, Boecker W, van Diest P, Brandt B, Buerger H (2006) Amplifications of the epidermal growth factor receptor gene (egfr) are common in phyllodes tumors of the breast and are associated with tumor progression. Lab Invest 86(1): 54–61. [DOI] [PubMed] [Google Scholar]
- Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA Jr, Friedman AH, Friedman H, Gallia GL, Giovanella BC, Grollman AP, He TC, He Y, Hruban RH, Jallo GI, Mandahl N, Meeker AK, Mertens F, Netto GJ, Rasheed BA, Riggins GJ, Rosenquist TA, Schiffman M, Shih IeM, Theodorescu D, Torbenson MS, Velculescu VE, Wang TL, Wentzensen N, Wood LD, Zhang M, McLendon RE, Bigner DD, Kinzler KW, Vogelstein B, Papadopoulos N, Yan H (2013) TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA 110(15): 6021–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW (1994) Specific association of human telomerase activity with immortal cells and cancer. Science 266(5193): 2011–2015. [DOI] [PubMed] [Google Scholar]
- Liau JY, Tsai JH, Jeng YM, Lee JC, Hsu HH, Yang CY (2015) Leiomyosarcoma with alternative lengthening of telomeres is associated with aggressive histologic features, loss of ATRX expression, and poor clinical outcome. Am J Surg Pathol 39(2): 236–244. [DOI] [PubMed] [Google Scholar]
- Lim WK, Ong CK, Tan J, Thike AA, Ng CC, Rajasegaran V, Myint SS, Nagarajan S, Nasir ND, McPherson JR, Cutcutache I, Poore G, Tay ST, Ooi WS, Tan VK, Hartman M, Ong KW, Tan BK, Rozen SG, Tan PH, Tan P, Teh BT (2014) Exome sequencing identifies highly recurrent MED12 somatic mutations in breast fibroadenoma. Nat Genet 46(8): 877–880. [DOI] [PubMed] [Google Scholar]
- Liu T, Wang N, Cao J, Sofiadis A, Dinets A, Zedenius J, Larsson C, Xu D (2014) The age- and shorter telomere-dependent TERT promoter mutation in follicular thyroid cell-derived carcinomas. Oncogene 33(42): 4978–4984. [DOI] [PubMed] [Google Scholar]
- Lovejoy CA, Li W, Reisenweber S, Thongthip S, Bruno J, de Lange T, De S, Petrini JH, Sung PA, Jasin M, Rosenbluh J, Zwang Y, Weir BA, Hatton C, Ivanova E, Macconaill L, Hanna M, Hahn WC, Lue NF, Reddel RR, Jiao Y, Kinzler K, Vogelstein B, Papadopoulos N, Meeker AK Consortium ALTSC (2012) Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS Genet 8(7): e1002772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokbel K, Ghilchik M, Parris CN, Newbold RF (1999) Telomerase activity in phyllodes tumours. Eur J Surg Oncol 25(4): 352–355. [DOI] [PubMed] [Google Scholar]
- Sawhney N, Garrahan N, Douglas-Jones AG, Williams ED (1992) Epithelial—stromal interactions in tumors. A morphologic study of fibroepithelial tumors of the breast. Cancer 70(8): 2115–2120. [DOI] [PubMed] [Google Scholar]
- Sawyer EJ, Hanby AM, Rowan AJ, Gillett CE, Thomas RE, Poulsom R, Lakhani SR, Ellis IO, Ellis P, Tomlinson IP (2002) The Wnt pathway, epithelial-stromal interactions, and malignant progression in phyllodes tumours. J Pathol 196(4): 437–444. [DOI] [PubMed] [Google Scholar]
- Shay JW, Bacchetti S (1997) A survey of telomerase activity in human cancer. Eur J Cancer 33(5): 787–791. [DOI] [PubMed] [Google Scholar]
- Shay JW, Roninson IB (2004) Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene 23(16): 2919–2933. [DOI] [PubMed] [Google Scholar]
- Tan PH, Jayabaskar T, Yip G, Tan Y, Hilmy M, Selvarajan S, Bay BH (2005) p53 and c-kit (CD117) protein expression as prognostic indicators in breast phyllodes tumors: a tissue microarray study. Mod Pathol 18(12): 1527–1534. [DOI] [PubMed] [Google Scholar]
- Tan PH, Tse G, Lee A, Simpson JF, Hanby AM (2012) Fibroepithelial tumours. In: Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ (eds) WHO Classification of Tumours of the Breast pp 142–147. IARC Press: Lyon, France. [Google Scholar]
- Tavassoli FA, Eusebi V (2009) Biphasic tumors. In: Tavassoli FA, Eusebi V (eds) vol 10, Afip Atlas Of Tumor Pathology. American Registry of Pathology: Washington, DC, USA, pp 315–340. [Google Scholar]
- Tsang AK, Chan SK, Lam CC, Lui PC, Chau HH, Tan PH, Tse GM (2011) Phyllodes tumours of the breast—differentiating features in core needle biopsy. Histopathology 59(4): 600–608. [DOI] [PubMed] [Google Scholar]
- Tse GM, Putti TC, Kung FY, Scolyer RA, Law BK, Lau TS, Lee CS (2002) Increased p53 protein expression in malignant mammary phyllodes tumors. Mod Pathol 15(7): 734–740. [DOI] [PubMed] [Google Scholar]
- Yasir S, Gamez R, Jenkins S, Visscher DW, Nassar A (2014) Significant histologic features differentiating cellular fibroadenoma from phyllodes tumor on core needle biopsy specimens. Am J Clin Pathol 142(3): 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Sekine S, Ogawa R, Yoshida H, Maeshima A, Kanai Y, Kinoshita T, Ochiai A (2015) Frequent MED12 mutations in phyllodes tumors of the breast. Br J Cancer 112(10): 1703–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.