Abstract
Aim:
Lipolysis in fat tissue plays an important role in the development of metabolic disturbances, a characteristic feature of chronic kidney disease (CKD). In the present study, we tested the hypothesis that the inhibition of endoplasmic reticulum (ER) stress could alleviate lipolysis in white adipose tissue in a rat model of CKD.
Methods:
A rat model of CKD was established by a method of reduced renal mass (RRM). Lipolysis was measured as the release of glycerol in ex vivo fat pads and cultured primary adipocytes. The activity of lipases and markers of ER stress were measured by Western blotting and immunoprecipitation.
Results:
Our data showed that lipolysis in visceral white adipose tissue was increased in RRM rats compared with control rats. In addition, increased phosphorylation of hormone-sensitive lipase (HSL) and binding of adipose triglyceride lipase (ATGL) to comparative gene identification-58 (CGI-58) protein were observed in the RRM rats. The phosphorylation of ER stress markers, including IRE1α, PERK, and eukaryotic initiation factor (eIF) 2α, and the expression of ER stress marker 78 kDa glucose-regulated protein (GRP78) were significantly increased in RRM rats. Treatment with an inhibitor of ER stress partially but significantly alleviated lipolysis, and this alleviation was accompanied by reduced binding of ATGL to CGI-58.
Conclusion:
Our results showed that enhanced lipolysis and ER stress occurred in visceral white adipose tissue in a rat model of CKD. Moreover, inhibition of ER stress significantly alleviated lipolysis. These findings suggest that ER stress is a potential therapeutic target for the metabolic disturbances associated with CKD.
Keywords: lipolysis, white adipose tissue, ER stress, chronic kidney disease
Introduction
Metabolic disturbances, such as protein-energy wasting1, dyslipidemia2, and lipid redistribution3, have been frequently demonstrated in chronic kidney disease (CKD). However, the underlying mechanisms of these disturbances remain elusive. Several in vitro studies4,5,6 have shown that white adipocyte dysfunctions may occur in the micro-environment of uremia.
White adipocyte tissue plays an important role in the regulation of lipid metabolism, energy homeostasis and insulin sensitivity via lipolysis, lipogenesis and the secretion of several cytokines7. Circulating metabolic profiling has demonstrated increased lipolysis in patients with CKD8. We and others have also found that uremic toxins induce lipolysis in cultured adipocytes5,9. Indeed, a loss of body fat has been identified in patients with CKD (particularly advanced CKD) and has been linked with increased mortality10. However, the direct evaluation of lipolysis in white adipose tissue in the setting of CKD has not received much attention.
The role of endoplasm reticulum (ER) stress in the pathogenesis of metabolic disease has been increasingly recognized11,12. In obesity and diabetic mellitus, which are both associated with metabolic disturbances, ER stress has been repeatedly demonstrated in white adipose tissue13,14. Additionally, ER stress has been shown to induce an intracellular inflammatory cascade and insulin resistance in adipocytes15. Our work and other previous studies have shown that ER stress can trigger lipolysis in cultured adipocytes9,16. This information suggests that ER stress may be one key pathway leading to metabolic disease states. Although ER stress has been demonstrated in the kidney17 and aorta18 in the context of CKD, whether ER stress is activated in white adipose tissue in this setting remains unclear.
In the present study, we tested the hypothesis that the inhibition of ER stress could alleviate lipolysis in white adipose tissue under the conditions of CKD. Our data showed that ER stress was activated and lipolysis was enhanced in visceral white adipose tissue in rats with reduced renal mass (RRM), a model of CKD. Furthermore, the inhibition of ER stress significantly alleviated lipolysis. This information suggests that ER stress is a potential therapeutic target for the metabolic disturbances observed in CKD.
Materials and methods
Animal preparation
Male Sprague-Dawley rats initially weighing 180–200 g (Southern Medical University Animal Experiment Center, Guangzhou, China) were maintained under standardized conditions with a standard rodent diet. Rats were housed in temperature-controlled, light-cycled quarters with ad libitum access to food and water. The rats were subjected either to a five-sixths nephrectomy (a right nephrectomy with surgical resection of two-thirds of the left kidney) or a sham operation under anesthesia. Ten weeks after the surgery, plasma creatinine, blood urea nitrogen and renal pathology were evaluated to confirm the establishment of chronic renal failure. Eighteen weeks after the surgery, the rats were subjected to analysis of biochemical and physical parameters. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No 85–23, revised 1996). All animal procedures were approved by the Animal Experiment Committee of Southern Medical University, Guangzhou, China.
Rats were starved overnight (12 h), and blood was drawn by transcutaneous cardiac puncture into a heparinized syringe after anesthesia. At the end of the experiment, blood was collected and centrifuged at 10 000×g to collect plasma.
Adipose tissue organ culture
We performed adipose tissue organ culture to evaluate ex vivo lipolysis as previously described19. Freshly isolated visceral white adipose tissue samples (epididymal, omental, and retroperitoneal adipose tissue) were washed in PBS and placed in 60-mm dishes at 100 mg total tissue (wet weight) per dish. The fresh tissue was minced into 1-mm pieces and incubated in Krebs-Ringer Bicarbonate (KRB) buffer containing 1% fatty acid–free BSA (Sigma-Aldrich, St Louis, MO, USA). The samples were incubated at 37 °C under 5% CO2 with mild shaking. After 120 min, glycerol release in the medium was measured using a glycerol determination kit. The fat pads were homogenized in PBS. Glycerol levels were then normalized to 100 grams of tissue protein for each sample.
Primary adipocyte isolation and culture
We isolated primary adipocytes to determine the in vitro lipolysis as previously reported20. Briefly, epididymal, omental, and retroperitoneal fat pads (approximately 2 g) from sham and chronic renal failure rats were extracted, weighed, and then finely minced using microscissors at room temperature. The minced tissue was transferred to plastic vials containing KRB buffer supplemented with 30 mmol/L HEPES (pH 7.4) and collagenase (1 g/mL). Tissue was incubated for 30 min at 37 °C with gentle agitation. Subsequently, digested tissue was filtered through a nylon mesh, and cells were collected into plastic 50-mL tubes and allowed to stand for 5 min. The infra-natant containing the collagenase solution was carefully removed using a long needle and syringe. The floating layer of adipocytes was washed three times with 10 mL of adipocyte incubation solution that contained KRB buffer supplemented with 4% (w/v) fatty acid-free bovine albumin fraction V. The adipocyte solution was centrifuged at 800 rounds per minute. Finally, 200 μL of packed cells was resuspended in 5 mL of adipocyte incubation solution for subsequent distribution into assay tubes. The purity of the isolation procedure was tested by investigating 200 cells from each rat under a light microscope. The number of isolated cells not resembling fat cells or cell material that was stuck to a fat cell was always below 10 per 200 counted cells. Lipolysis was measured by assaying glycerol release after cell incubation at 37 °C with 5% CO2 for 1 h. Cell protein content was measured, and all data were normalized per microgram cell protein.
Western blotting
For whole tissue samples, fat depots were extracted and immediately snap frozen in liquid nitrogen. Tissue samples (approximately 100 mg) were subsequently homogenized in a lysis buffer. For isolated adipocytes, cells were homogenized in a lysis buffer, and the lysates were centrifuged. Western blotting was conducted as described elsewhere9. Antibodies used included the following: anti-HSL, anti-phosphor-HSL serine 563, anti-phosphor-HSL serine 660, anti-ATGL, anti-CGI-58, anti-PERK, anti-phospho-PERK (Thr 981), anti-eIF2α, anti-phospho-eIF2α (Ser51), anti-JNK1/3, anti-phospho-JNK (Thr 183/Tyr 185), anti-IRE1α, and anti-phospho-IRE1α (Ser724) (Cell Signaling, Danvers, MA, USA). β-Actin was used as an internal control.
Statistical analysis
All experiments were performed in triplicate. Data are presented as the mean±standard deviation (SD) of three independent experiments as indicated. All data were analyzed with SPSS 11.0 for Windows. The difference in mean values between groups was tested using one-way ANOVA. To identify significant differences between two groups, comparisons were made using Student's t-test. P values less than 0.05 were considered significant.
Results
Enhanced lipolysis in white adipose tissue in RRM rats
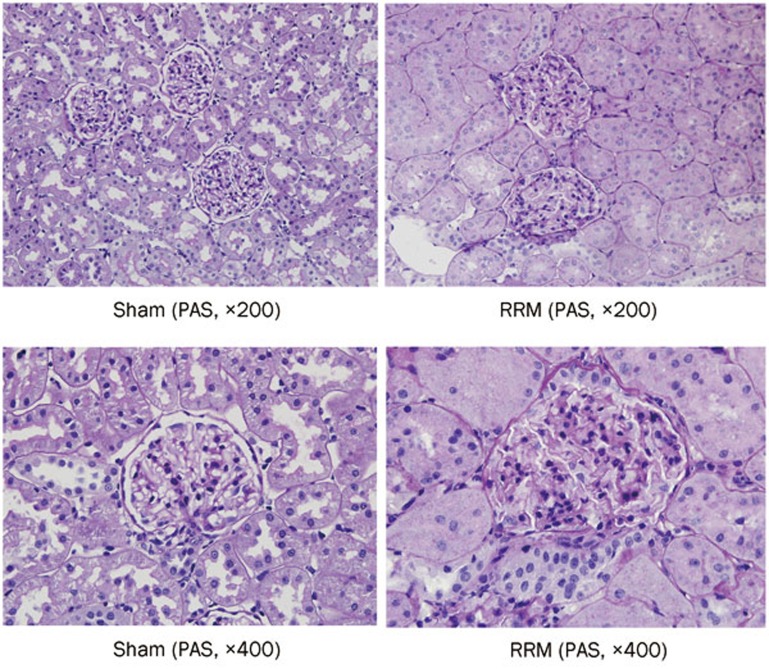
To confirm the establishment of chronic renal failure, we evaluated renal pathology. As presented in Figure 1, glomerulosclerosis developed in RRM rats 10 weeks after surgery. The characteristic features of the animals at the end of the study are shown in Table 1.
Figure 1.
Glomerulosclerosis in RRM rats. Images of PAS staining of renal pathology in sham and RRM rats 10 weeks after surgery are shown.
Table 1. Biochemical and physical parameters in the experimental rats.
| Sham | RRM | RRM+vehicle | RRM+PBA | |
|---|---|---|---|---|
| Body weight (g) | 513±11 | 435±26c | 433±23 | 475±28e |
| Renal function | ||||
| Plasma creatinine (μmol/L) | 60±3.8 | 133±10.2c | 132±10.3 | 125±10.7 |
| BUN (mmol/L) | 6.7±1.0 | 14.2±2.4c | 14.1±2.4 | 12.6±1.1 |
| Plasma TG (mmol/L) | 1.05±0.52 | 2.45±0.51c | 2.37±0.51 | 1.54±0.33e |
| Plasma glycerol (μmol/L) | 1.95±0.32 | 3.49±0.54c | 3.61±0.54 | 2.62±0.37e |
| Plasma FFAs (mmol/L) | 0.52±0.06 | 1.33±0.17c | 1.36±0.19 | 0.95±0.14e |
Data are expressed as mean±SD. n=6.
cP<0.01 vs sham.
eP<0.05 vs RRM+vehicle.
RRM, reduced renal mass; PBA, 4-phenyl butyric acid; BUN, blood urea nitrogen; TG, triglyceride; FFAs, free fatty acids.
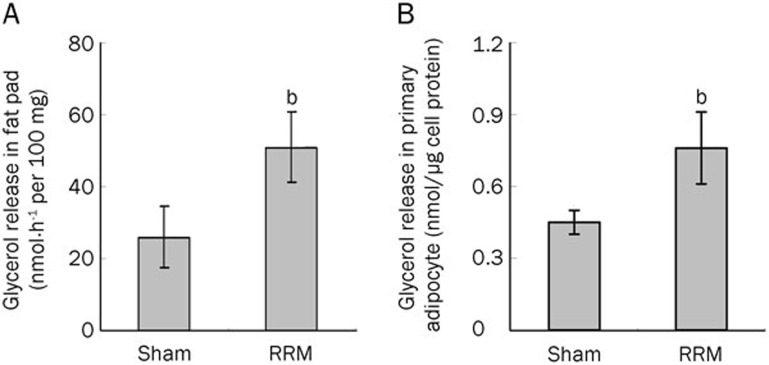
To evaluate lipolysis in visceral white adipose tissue, we measured glycerol release in the tissue pad and in primary white adipocytes. As shown in Figure 2A, glycerol release was significantly increased in the visceral fat pads of RRM rats. Similarly, glycerol release was also increased in the visceral primary adipocytes of RRM rats (Figure 2B).
Figure 2.
Lipolysis in fat pads and primary adipocytes from RRM rats. Visceral white adipose tissue samples (epididymal, omental, and retroperitoneal adipose tissue) and primary adipocytes were cultured as described in the Materials and methods section. Glycerol release in the medium was measured. The glycerol release in the fat pads (A) and primary adipocytes (B) was significantly increased in the RRM rats. Data are expressed as the mean±SD of three independent experiments. bP<0.05 vs sham (n=6).
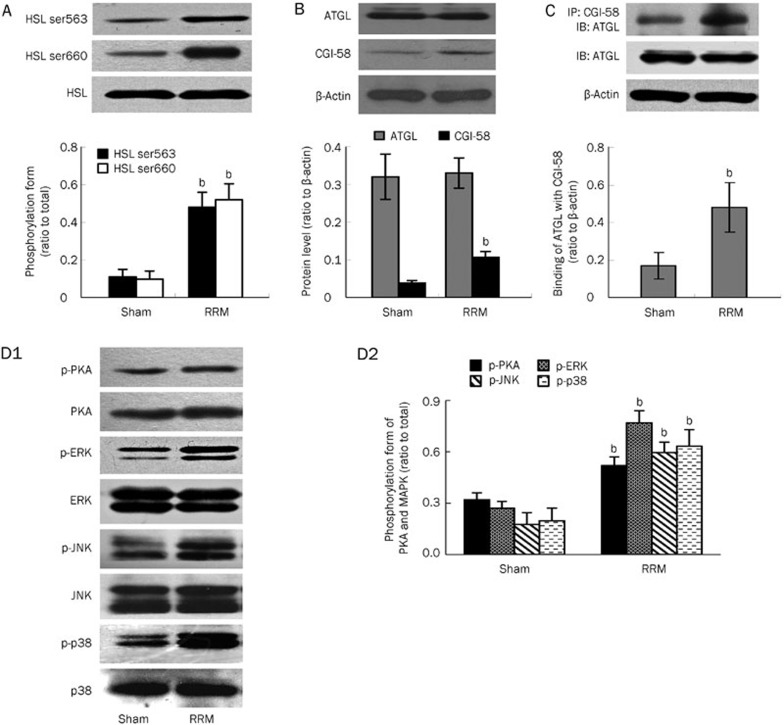
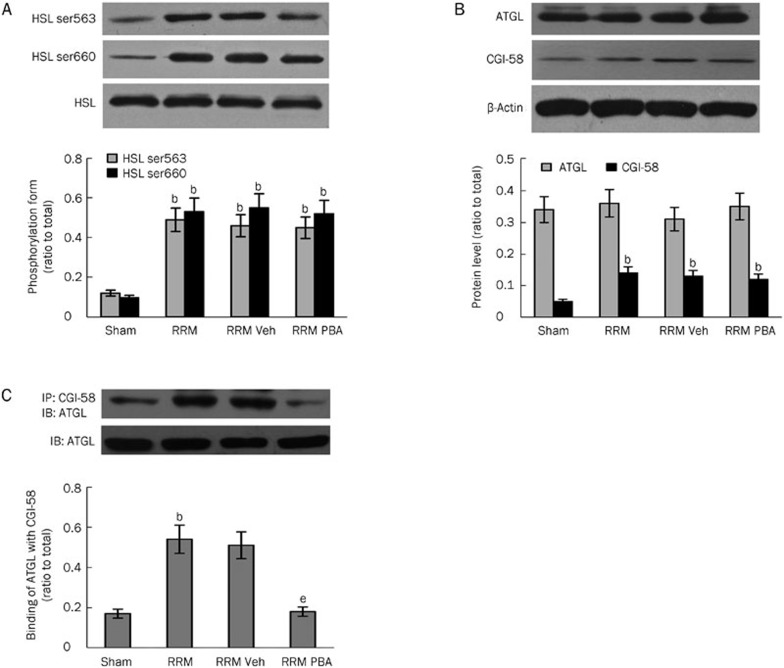
Hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) are the two key enzymes involved in the regulation of lipolysis21,22,23. We measured their activities and content in visceral white adipose tissue to confirm that excessive lipolysis was occurring. Phosphorylation of serine residues 563 and 660 is necessary for the activation of HSL. As shown in Figure 3A, phosphorylation of HSL was significantly enhanced in visceral white adipose tissue of RRM rats. Meanwhile, its abundance remained comparable between control and RRM rats.
Figure 3.
Activation of HSL, ATGL, PKA and MAPK in visceral white adipose tissue in RRM rats. The activation and abundance of HSL, ATGL, PKA, and MAPK were determined by Western blotting and immunoprecipitation. The activity of HSL (as detected by phosphorylation form) was significantly increased (A) in RRM rats. The abundance of ATGL (B) was comparable between control and RRM rats. CGI-58 (B) and the binding of ATGL to CGI-58 (C) were significantly increased in RRM rats. PKA and MAPK were over-activated in RRM rats (D1 and D2). Data are expressed as the mean±SD of three independent experiments. bP<0.05 vs sham (n=6).
The activity of ATGL is not only regulated by its content but is also greatly affected by comparative gene identification 58 (CGI-58)24, a lipid droplet-associated protein that promotes the hydrolysis of triglycerides by activating ATGL. Therefore, we measured the abundance of ATGL and CGI-58. As shown in Figure 3B, the abundance of ATGL remained unchanged in the visceral white adipose tissue of the RRM rats. Meanwhile, the abundance of CGI-58 was significantly increased in the RRM rats compared with the control rats. We then measured the binding of ATGL to CGI-58, an essential step to enforce its function. As shown in Figure 3C, the binding of ATGL to CGI-58 was significantly increased in RRM rats. This finding suggests that the activation of ATGL under conditions of CKD was mainly regulated by association with its co-activator.
The activation of protein kinase A (PKA) and mitogen-activated protein kinase (MAPK)16 has been shown to be involved in the activation of lipolysis signaling, and we therefore evaluated the activity of these kinases in the tissue samples. As shown in Figure 3D1 and 3D2, the phosphorylation of PKA and MAPK was significantly increased, indicating that these kinases were activated in CKD.
In summary, lipolysis was increased in visceral white adipose tissue under the conditions of CKD. Moreover, this increase was accompanied by increased phosphorylation of HSL, binding of ATGL to CGL-58, and expression of CGI-58.
The effect of inhibition of ER stress on lipolysis in white adipose tissue in RRM rats
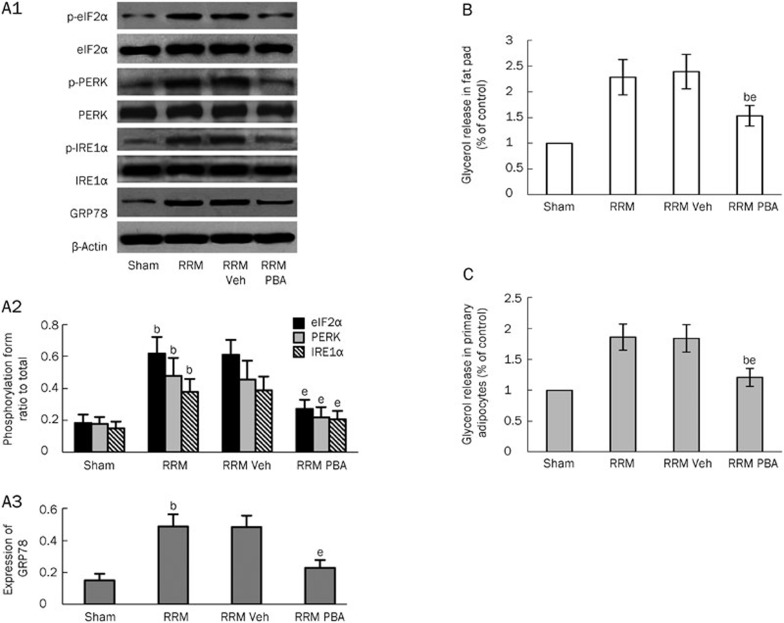
To investigate whether inhibition of ER stress alleviated lipolysis in white adipose tissue in CKD rats, we first evaluated whether ER stress was activated in the tissue. As shown in Figure 4A1–4A3, several markers of ER stress — phosphorylation of IRE1α, PERK, and eukaryotic initiation factor (eIF) 2α and expression of 78 kDa glucose-regulated protein (GRP78) — were significantly increased in RRM rats compared with sham rats, indicating that ER stress occurred in visceral white adipose tissue in the setting of CKD. To investigate whether inhibition of ER stress affected the enhanced lipolysis, we treated RRM rats with 4-phenyl butyric acid (PBA), a chemical chaperone that is known to reduce ER stress in vitro and in vivo25. Fourteen weeks after surgery, the RRM rats were divided into the following groups: RRM, RRM+vehicle (phosphate buffered saline, PBS), and RRM+PBA (400 μg/kg daily intragastric administration) for 4 additional weeks. As shown in Figure 4A1–4A3, the expression of ER stress markers in the visceral white adipose tissue was completely inhibited by treatment with PBA. Moreover, the inhibition of ER stress was associated with a significant reduction in lipolysis (Figure 4B and 4C). To further confirm the inhibitory effect of PBA treatment on lipolysis, we evaluated the activation of HSL and ATGL in RRM rats with or without PBA treatment. As shown in Figure 5A and 5B, treatment with PBA had no effect on the phosphorylation of HSL or the expression of CGI-58 or ATGL. However, inhibition of ER stress with PBA completely blocked the binding of ATGL to CGI-58 (Figure 5C).
Figure 4.
The effect of the inhibition of ER stress on lipolysis in visceral white adipose tissue in RRM rats. ER stress markers and lipolysis were measured in visceral white adipose tissue in sham and RRM rats treated with or without an inhibitor of ER stress. The expression of ER stress markers was significantly increased in RRM rats (A1–A3). Treatment with PBA, an inhibitor of ER stress, inhibited the expression of these markers (A1–A3). The inhibition of ER stress led to a significant reduction in glycerol release from the fat pads (B) and primary adipocytes (C). Data are expressed as the mean±SD of three independent experiments. bP<0.05 vs sham. eP<0.05 vs vehicle-treated group (n=6).
Figure 5.
The effect of the inhibition of ER stress on the activation of HSL and ATGL. The activation and abundance of HSL and ATGL were measured in visceral white adipose tissue from sham and RRM rats treated with or without PBA. Treatment with PBA had no effect on the activation of HSL (A) or the abundance of AGTL or CGI-58 (B). However, PBA treatment blocked the binding of ATGL to CGI-58 (C). Data are expressed as the mean±SD of three independent experiments. bP<0.05 vs sham. eP<0.05 vs vehicle-treated group (n=6).
In summary, the inhibition of ER stress was shown to alleviate lipolysis, mainly by blocking the activation of ATGL.
Discussion
Lipolysis in white adipose tissue, the cleavage of triglycerides and the release of fatty acids and glycerol play essential roles not only in lipid and energy homeostasis26 but also in the development of lipotoxicity and fat redistribution27,28. Here, we have shown that lipolysis is enhanced in the visceral white adipose tissue in a rat model of CKD. Furthermore, our data demonstrated that ER stress occurred in the visceral white adipose tissue and that the inhibition of ER stress alleviated the enhanced lipolysis in this tissue. Thus, ER stress might be a potential therapeutic target for metabolic disturbances in patients with CKD.
White adipocyte dysfunction has been demonstrated under the conditions of CKD29,30, and previous in vitro studies showed that lipolysis was triggered in the micro-environment of uremia4,5,6. Here, we provided further evidence that lipolysis is increased in visceral white adipose tissue in CKD. The release of glycerol, a marker of lipolysis, was significantly increased in cultured fat pads and primary white adipocytes. In addition, the phosphorylation of HSL and binding of ATGL to CGI-58 were significantly increased in the tissue samples, suggesting that the two master enzymes required for lipolysis were over-activated in this rat model of CKD. In agreement with our results, fat loss is common in patients with CKD10.
The underlying mechanisms of enhanced lipolysis in visceral white adipose tissue remain unclear. A large number of hormonal signaling pathways and lipid droplet-associated protein factors have been demonstrated to regulate substrate access and the activity of lipase, and thereby to govern the lipolysis of white adipose tissue31,32. Our data showed that the phosphorylation of HSL and expression of CGI-58 were increased in the tissue of the RRM rats. Phosphorylation of HSL is required for the activity of the enzyme and is mainly regulated by protein kinase A. CGI-58 is localized to lipid droplets and potently activates ATGL by approximately 20-fold after interacting with ATGL24. This information suggests that multiple pathways might be involved in the activation of HSL and ATGL under conditions of CKD. However, the detailed mechanism needs further investigation.
ER stress has been demonstrated to trigger lipolysis in cultured adipocytes. We provided several lines of evidence that ER stress may be an important player in the excessive lipolysis that occurs in CKD. The ER stress markers measured in this study were significantly increased, suggesting that ER stress occurred. Moreover, this finding is in accordance with our previous studies showing that uremic toxins induce ER stress in cultured adipocytes33. Because there are several cell types in white adipose tissue, ER stress may also occur in another cell types in the tissue. Additionally, the inhibition of ER stress led to a significant reduction in lipolysis. The inhibition of ER stress did not affect the phosphorylation of HSL or the content of HSL, ATGL, or CGI-58. However, treatment with an ER stress inhibitor completely blocked the binding of ATGL to CGI-58. This information suggests that ER stress regulates lipolysis mainly by promoting the interaction of ATGL with CGI-58 under conditions of CKD. However, the mechanism of this effect warrants further study. Although lipolysis in visceral white adipose tissue was significantly reduced by the inhibition of ER stress, the level was higher than that under normal conditions. This finding suggests there are other pathways involved in the enhanced lipolysis that occurs under conditions of CKD.
An increased level of blood triglycerides in CKD has been well documented. However, potential alterations in the uptake of free fatty acids and the synthesis of triglycerides in the liver and muscle under the conditions of CKD and the effect of lipolysis in fat cells on the circulating level of triglycerides require further study. Moreover, the detailed mechanisms that link lipolysis in white adipose tissue with metabolic disturbances remain poorly understood. Excess lipolysis in white adipose tissue can cause lipotoxicity and fat redistribution and lead to metabolic disturbances; therefore, it is essential to further examine the detailed mechanisms of enhanced lipolysis in CKD.
In conclusion, enhanced lipolysis and ER stress occurred in the visceral white adipose tissue in a rat model of CKD. Inhibition of ER stress alleriated lipolysis, suggesting that ER stress is a rational therapeutic target for the metabolic disturbances observed in CKD.
Author contribution
Qiu-gen ZHOU conceived and designed the experiments; Yan ZHU, Yu-ling CHEN, Xiao-yan DING, Cong LI, Guo-yu XU, and Li-li HU performed the experiments; Yan ZHU and Yu-ling CHEN analyzed the data; Qiu-gen ZHOU and Fan-fan HOU wrote the manuscript.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81070613 and 81270827) and the Research Fund for the Doctoral Program (No 20104433120004).
References
- 1Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 2008; 73: 391–8. [DOI] [PubMed] [Google Scholar]
- 2Keane WF, Tomassini JE, Neff DR. Lipid abnormalities in patients with chronic kidney disease. Contrib Nephrol 2011; 171: 135–42. [DOI] [PubMed] [Google Scholar]
- 3Ruan XZ, Moorhead JF, Varghese Z. Lipid redistribution in renal dysfunction. Kidney Int 2008; 74: 407–9. [DOI] [PubMed] [Google Scholar]
- 4Aminzadeh MA, Pahl MV, Barton CH, Doctor NS, Vaziri ND. Human uraemic plasma stimulates release of leptin and uptake of tumour necrosis factor-alpha in visceral adipocytes. Nephrol Dial Transplant 2009; 24: 3626–31. [DOI] [PubMed] [Google Scholar]
- 5Axelsson J, Astrom G, Sjolin E, Qureshi AR, Lorente-Cebrian S, Stenvinkel P, et al. Uraemic sera stimulate lipolysis in human adipocytes: role of perilipin. Nephrol Dial Transplant 2011; 26: 2485–91. [DOI] [PubMed] [Google Scholar]
- 6Kalbacher E, Koppe L, Zarrouki B, Pillon NJ, Fouque D, Soulage CO. Human uremic plasma and not urea induces exuberant secretion of leptin in 3T3-L1 adipocytes. J Ren Nutr 2011; 21: 72–5. [DOI] [PubMed] [Google Scholar]
- 7Wang S, Soni KG, Semache M, Casavant S, Fortier M, Pan L, et al. Lipolysis and the integrated physiology of lipid energy metabolism. Mol Genet Metab 2008; 95: 117–26. [DOI] [PubMed] [Google Scholar]
- 8Rhee EP, Souza A, Farrell L, Pollak MR, Lewis GD, Steele DJ, et al. Metabolite profiling identifies markers of uremia. J Am Soc Nephrol 2010; 21: 1041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Zhou QG, Zhou M, Hou FF, Peng X. Asymmetrical dimethylarginine triggers lipolysis and inflammatory response via induction of endoplasmic reticulum stress in cultured adipocytes. Am J Physiol Endocrinol Metab 2009; 296: E869–78. [DOI] [PubMed] [Google Scholar]
- 10Kalantar-Zadeh K, Kuwae N, Wu DY, Shantouf RS, Fouque D, Anker SD, et al. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr 2006; 83: 202–10. [DOI] [PubMed] [Google Scholar]
- 11Basseri S, Austin RC. Endoplasmic reticulum stress and lipid metabolism: mechanisms and therapeutic potential. Biochem Res Int 2012; 2012: 841362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Gregor MF, Hotamisligil GS. Thematic review series: adipocyte biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res 2007; 48: 1905–14. [DOI] [PubMed] [Google Scholar]
- 13Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, et al. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 2008; 57: 2438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004; 306: 457–61. [DOI] [PubMed] [Google Scholar]
- 15Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature 2002; 420: 333–6. [DOI] [PubMed] [Google Scholar]
- 16Deng J, Liu S, Zou L, Xu C, Geng B, Xu G. Lipolysis response to endoplasmic reticulum stress in adipose cells. J Biol Chem 2012; 287: 6240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Cybulsky AV. Endoplasmic reticulum stress in proteinuric kidney disease. Kidney Int 2010; 77: 187–93. [DOI] [PubMed] [Google Scholar]
- 18Zhou QG, Fu XJ, Xu GY, Cao W, Liu HF, Nie J, et al. Vascular insulin resistance related to endoplasmic reticulum stress in aortas from a rat model of chronic kidney disease. Am J Physiol Heart Circ Physiol 2012; 303: H1154–65. [DOI] [PubMed] [Google Scholar]
- 19Huang ZH, Reardon CA, Mazzone T. Endogenous ApoE expression modulates adipocyte triglyceride content and turnover. Diabetes 2006; 55: 3394–402. [DOI] [PubMed] [Google Scholar]
- 20Gaidhu MP, Anthony NM, Patel P, Hawke TJ, Ceddia RB. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am J Physiol Cell Physiol 2010; 298: C961–71. [DOI] [PubMed] [Google Scholar]
- 21Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis — a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res 2011; 50: 14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res 2009; 50: 3–21. [DOI] [PubMed] [Google Scholar]
- 23Ryden M, Jocken J, van Harmelen V, Dicker A, Hoffstedt J, Wiren M, et al. Comparative studies of the role of hormone-sensitive lipase and adipose triglyceride lipase in human fat cell lipolysis. Am J Physiol Endocrinol Metab 2007; 292: E1847–55. [DOI] [PubMed] [Google Scholar]
- 24Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab 2006; 3: 309–19. [DOI] [PubMed] [Google Scholar]
- 25Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006; 313: 1137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, et al. FAT SIGNALS — lipases and lipolysis in lipid metabolism and signaling. Cell Metab 2012; 15: 279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Arner P, Bernard S, Salehpour M, Possnert G, Liebl J, Steier P, et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature 2011; 478: 110–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Frayn K, Bernard S, Spalding K, Arner P. Adipocyte triglyceride turnover is independently associated with atherogenic dyslipidemia. J Am Heart Assoc 2012; 1: e 003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Roubicek T, Bartlova M, Krajickova J, Haluzikova D, Mraz M, Lacinova Z, et al. Increased production of proinflammatory cytokines in adipose tissue of patients with end-stage renal disease. Nutrition 2009; 25: 762–8. [DOI] [PubMed] [Google Scholar]
- 30Pelletier CC, Koppe L, Croze ML, Kalbacher E, Vella RE, Guebre-Egziabher F, et al. White adipose tissue overproduces the lipid-mobilizing factor zinc alpha2-glycoprotein in chronic kidney disease. Kidney Int 2013; 83: 878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res 2009; 48: 275–97. [DOI] [PubMed] [Google Scholar]
- 32Granneman JG, Moore HP, Granneman RL, Greenberg AS, Obin MS, Zhu Z. Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem 2007; 282: 5726–35. [DOI] [PubMed] [Google Scholar]
- 33Zhou QG, Zhou M, Lou AJ, Xie D, Hou FF. Advanced oxidation protein products induce inflammatory response and insulin resistance in cultured adipocytes via induction of endoplasmic reticulum stress. Cell Physiol Biochem 2010; 26: 775–86. [DOI] [PubMed] [Google Scholar]