Abstract
A series of glycoconjugates with defined connectivity were synthesized to investigate the impact of coupling Salmonella typhimurium O-antigen to different amino acids of CRM197 protein carrier. In particular, two novel methods for site-selective glycan conjugation were developed to obtain conjugates with single attachment site on the protein, based on chemical modification of a disulfide bond and pH-controlled transglutaminase-catalyzed modification of lysine, respectively. Importantly, conjugation at the C186-201 bond resulted in significantly higher anti O-antigen bactericidal antibody titers than coupling to K37/39, and in comparable titers to conjugates bearing a larger number of saccharides. This study demonstrates that the conjugation site plays a role in determining the immunogenicity in mice and one single attachment point may be sufficient to induce high levels of bactericidal antibodies.
Keywords: carbohydrates, glycoproteins, protein modification, solmonella, vaccines
Glycoconjugate vaccines are composed of a sugar antigen covalently linked to a protein carrier, and can elicit specific long-lasting immunogenic response toward poorly immunogenic carbohydrates.[1] Currently, almost all conjugate vaccines are prepared by random conjugation at lysines or aspartates/glutamates.[2] Recent studies have highlighted a possible role for the saccharide–protein connectivity in determining immunogenicity.[3] Emerging site-selective conjugation methods can offer new tools to study this phenomenon.[4] The first attempts at linking saccharide antigens to define sites on the carrier protein have been performed using synthetic oligosaccharides.[5]
Our previous findings[5b–d encouraged us to further elucidate a comprehensive relationship between connectivity and immunogenicity, and to define the minimal glycan loading for a defined regioisomeric glycoconjugate.
Meanwhile, we are interested to develop a glycoconjugate vaccines against Salmonella enterica serovar typhimurium, which is one of the most common serovars responsible for invasive nontyphoidal Salmonella (iNTS) disease in Africa,[6] resulting in case fatality rates up to 25 %.[7] A vaccine against S. typhimurium is urgently needed, but not yet available.
The lipopolysaccharide O-antigen (OAg) of Salmonella (Figure 1) has been implicated as a target of protective immunity.[9] Here we present two novel methods for site-selective glycan conjugation providing conjugates with single attachment sites on the protein, based on disulfide bond chemical modification and pH-controlled transglutaminase-catalyzed reaction at lysine,[10] respectively. By these methods and others previously reported,[5b,c] a diverse set of site-selective derivatizations at disulfide, lysines, tyrosines, and glutamates/aspartates of CRM197 (Scheme 1) was prepared and a survey of immunogenicity of various regioisomeric OAg-based glycoconjugate vaccines against S. typhimurium was conducted.[9d, 11]
Figure 1.
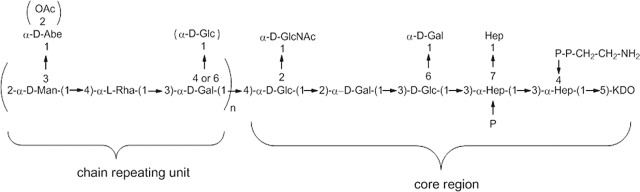
Structure of the O-antigen chain linked to the core region of 2192 S. typhimurium lipopolysaccharide.[8]
Scheme 1.
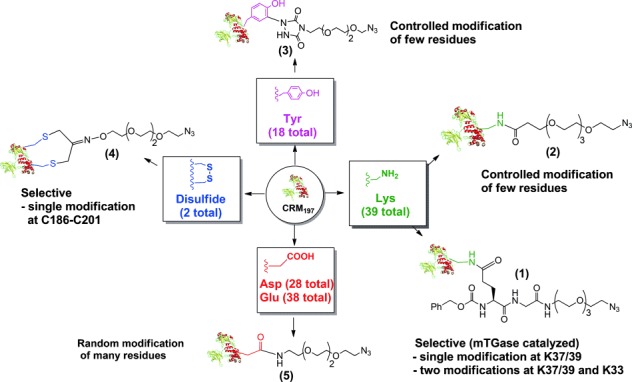
Amino acids of CRM197 were derivatized with a different average number of linkers per protein. Appropriate bifunctional azido linkers were used according to the amino acids targeted on the protein. See the Supporting Information for detailed reaction conditions.
CRM197 was selected as the carrier protein in the study, because of its defined structure and well-proven clinical validation.[12] The preparation of its mutants is challenging, we hence seek for diverse site-controlled conjugation of CRM197 to enable a comprehensive structure–activity relationship (SAR) evaluation.
A strain-promoted azide–alkyne click reaction (SPAAC) was planned for the introduction of large glycan in the final step. Therefore, azido linkers were introduced at diverse sites on CRM197 (Scheme 1).
With regard to lysine selective modification, it was found that a Cbz-Gln-Gly (ZQG) linker bearing azide is compatible to microbial transglutaminase (mTGase) catalyzed labeling.[10] The Gln was selectively activated by mTGase and enabled the selective acylation of only one lysine at pH 8 after 18 h. The peptide mapping analysis following trypsin digestion indicated only one of K37/39 was labeled. K37/39 is indistinguishable in the limitation of the analytic method. Lowering the reaction pH to 6 and increasing the reaction time from 18 to 72 h led to an increased labeling level, and K33 was found as the additional labeling site besides K37/39 out of a total of 39 lysines (Scheme 2; Figures S1 and S2).
Scheme 2.
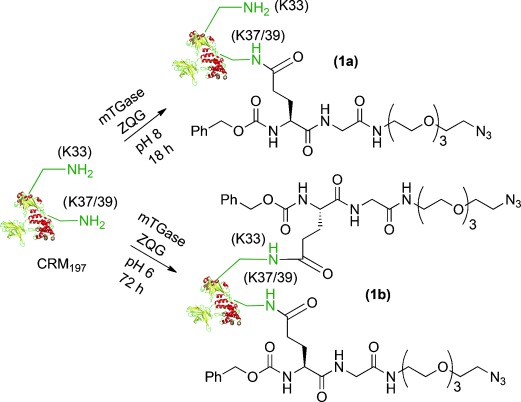
Selective introduction of azide linkers on K37/39 or K37/39 and K33 of CRM197.
We next explored single modifications at cysteines, which are paired in two disulfides in CRM197. The C461-C471 bond appeared to be buried inside the protein, and the C186-C201 was surface exposed. CRM197 was selectively partial reduced to release C186 and C201 in the presence of tris(2-carboxyethyl)phosphine (TCEP, 6 equiv) in sodium phosphate buffer (pH 7.4) at room temperature.
The subsequent incubation with 1,3-dichloroacetone (10 equiv) yielded the modified CRM197. The peptide mapping analysis following trypsin digestion gave 80 % sequence coverage of CRM197 and confirmed that a bridge-bearing ketone functionality was exclusively formed between C186 and C201, without any detectible C461-C471 crosslinking (see Figure S1 in the Supporting Information).[13] The azide was introduced to the modified CRM197 by oxime formation reaction with an aminooxy-azide bifunctional reagent (Scheme 3; Figure S3).
Scheme 3.
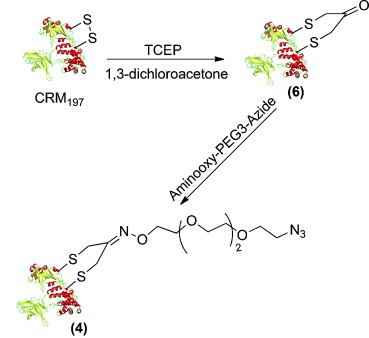
Selective introduction of the azide linker on C186-C201 of CRM197.
Then we envisaged the modification of few residues. Controlled conjugation to the more surface-exposed lysines, by activation of the amine groups with NHS-PEG4-N3 leads to the introduction of an average number of 7.1 and 3.8 linkers, respectively, onto CRM197 (NHS-PEG4-N3=azide- and N-hydroxysuccinimide-functionalized poly(ethylene glycol); see the Supporting Information).[5b] In agreement with a previous report,[5b] LC-ESI MS/MS analysis of the digested peptides showed that surface-exposed lysine residues were modified more readily than non-surface-exposed ones, with preferential modification of a subset of CRM197 residues (namely K103, K221, K242, with the further modification of K236, K498 and K526, for the product with 3.8 and 7.1 linkers respectively; LC=liquid chromatography, ESI=electrospray ionization, MS/MS=tandem mass spectrometry). Investigating further the reasons of this selectivity we also found some exceptions. For example K244 is also surface exposed and minimal modification was observed in contrast to the nearby K242. We noticed that K244 forms a salt bridge with adjacent aspartic acid and has limited flexibility and low nucleophilicity because of the protonated status. Hence, the availability of the lysine residues appears influenced by the amino acidic contour in addition to the surface exposition.
The triazolidinone-ene reaction in tris(hydroxymethyl)aminomethane (Tris) buffer was employed to selectively target tyrosine residues, resulting in the insertion of an azide moiety at defined sites (see the Supporting Information).[5d] By changing the ratio of linker to CRM197, an average of 1.5, 2.6, or 3.8 alkyne functional groups were introduced, respectively. LC-ESI MS/MS analysis of proteolytic digests indicated that the surface-exposed Y27, Y46, Y358, and Y380 were the major residues modified out of a total 18 tyrosines.[5c]
For the preparation of a random conjugate at E/D (CRM197 provides 38 glutamates and 28 aspartates), protein carboxyl groups were derivatized with an amine-PEG-azide linker after random activation with 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM). Even if the reaction was performed with an excess of DMT-MM and a linker to carboxyl groups ratio of 1 (see the Supporting Information), only an average of 4.8 amino acids were modified per CRM197.
For all samples, the recovery of purified protein was consistently larger than 80 %, and analysis by HPLC-SEC showed the presence of only one peak with a similar retention time to underivatized CRM197.
For coupling with the protein, OAg was modified at the terminal KDO sugar. After introduction of an adipic acid dihydrazide (ADH) molecule by reductive amination,[11, 14] a second linker was introduced with a terminal alkyne group (Scheme 4; see the Supporting Information).
Scheme 4.
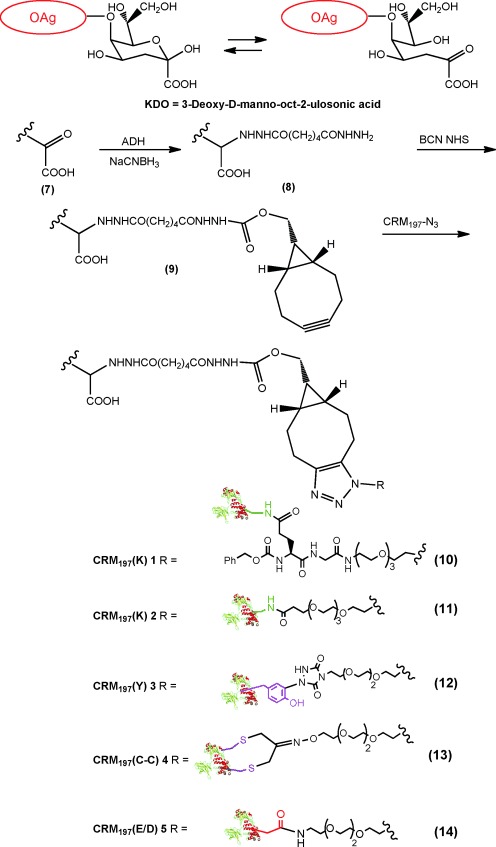
Conjugation strategy used for the synthesis of glyconjugates. The terminal KDO unit of the OAg chain (7) was activated with ADH (8) and then linked to BCN NHS I (9) to introduce the alkyne functionality. The activated OAg was finally conjugated with the azido-derivatized CRM197 by copper-free click chemistry (10–14).
Based on the recently published observation that large polysaccharides cannot be efficiently condensed by copper-mediated click chemistry,[15] the strain-promoted azide–alkyne cycloaddition variant of this reaction was used for the incorporation of saccharide.[15–16] Both these steps were characterized by sugar recoveries larger than 80 % and activation degrees larger than 90 %.
Copper-free click chemistry allowed linkage of the terminal sugar of the OAg chain to CRM197 even when few or only one linker were present on the protein (Table S1). Even when the number of linkers was increased to an average of seven lysine residues on CRM197, no more than an average of two OAg chains per protein was incorporated, as indicated by the molar OAg to CRM197 ratio of the obtained conjugate (11 b, Table 1). OAg-CRM197 10 with one or two glycan moieties were characterized by a similar sugar-to-protein ratio, close to 0.7 (Table 1), indicating that even when two linkers were introduced on the protein, only an average of one saccharide chain per CRM197 was linked.
Table 1.
Characterization of OAg-CRM197 conjugates generated by copper-free click chemistry targeting different amino acids on CRM197
| Conjugate | Average CRM197 labeling | Modified sites on CRM197 | OAg chains per CRM197 |
|---|---|---|---|
| OAg-CRM197(K+1) 10 a | +1.0 | K37/K39 | 0.7 |
| OAg-CRM197(K+2) 10 b | +2.0 | K37/K39, K33 | 0.6 |
| OAg-CRM197(K+3.8) 11 a | +3.8 | K103, K221, K242 | 1.5 |
| OAg-CRM197(K+7.1) 11 b | +7.1 | K103, K221, K236, K242, K498, K526 | 2.0 |
| OAg-CRM197(Y+1.5) 12 a | +1.5 | Y27, Y46, Y358, Y380 | 2.0 |
| OAg-CRM197(Y+2.6) 12 b | +2.6 | Y27, Y46, Y358, Y380 | 2.4 |
| OAg-CRM197(Y+4.3) 12 c | +4.3 | Y27, Y46, Y358, Y380 | 3.7 |
| OAg-CRM197(C-C+1) 13 | +1.0 | C186-C201 | 1.2 |
| OAg-CRM197(E/D+4.8) 14 | +4.8 | random E/D | 1.4 |
Targeting of tyrosine residues for conjugation allowed better control of the OAg/CRM197 ratio in the corresponding glycoconjugates than targeting lysine residues, with the number of OAg chains introduced per CRM197 similar to the number of linkers introduced (OAg-CRM197 12, Table 1). In fact, although the linker loading on tyrosine residues was increased by just a few units, from 1.5 to 2.6 and 4.3, the corresponding carbohydrate loading enhanced from 2.0 to 2.4 to 3.8 respectively, in agreement with what was previously reported.[5c,d] This outcome could be related to the different surface distribution of involved lysine and tyrosine residues on CRM197 (Figure S1).[5b–d]
Particularly when the lysines were targeted, the incorporation of one OAg chain could challenge the insertion of a second large glycan moiety by steric hindrance (Figure S1). The carboxylic glutamate/aspartate chemistry was characterized by a low conjugation efficiency, even if an average number of 4.8 azido groups were introduced on the protein, and resulted in a conjugate with a low OAg-to-protein ratio (14, Table 1).
All the synthesized conjugates were tested in mice to investigate the impact of OAg–protein specific amino acid connectivity and saccharide-to-protein ratio on the immune response.
Groups of eight mice were immunized at days 0, 28, and 42 with 1 μg OAg-based dose of conjugates formulated with alhydrogel as adjuvant (Figure 2 and the Supporting Information). Notably, at day 56 10 b induced higher anti-OAg IgG responses than 10 a (p=0.0379), and at day 42 both 10 b and 13 gave significantly higher anti-OAg IgG responses than 10 a (p=0.0135 and p=0.0065 respectively). As these constructs possess the same OAg-to-protein ratio, this result strongly suggests that the position of glycosylation on the protein influences the immunogenicity of glycoconjugates. The anti-OAg IgG response obtained with 11 b was higher than that for 10 a both at day 42 and 56 (p=0.0153 and p=0.0353, respectively), but was not significantly different from 10 b and 11 a. Conjugates with higher OAg to protein ratio gave comparable immune responses, regardless of the conjugation site.
Figure 2.
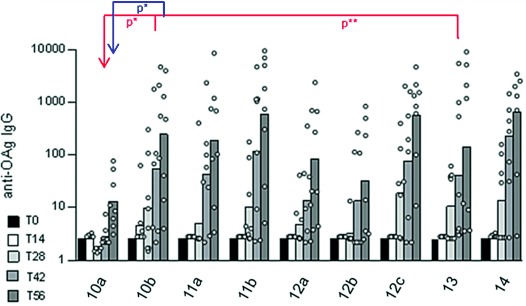
Anti-OAg IgG response induced in five-week old female C57BL/6 mice by OAg-CRM197 conjugate vaccines at 1 μg OAg dose. Individual animals are represented by the dots. Horizontal bars represent geometric means.
To test the functional activity of the elicited antibodies, individual mouse sera at day 56 were tested for serum bactericidal activity (SBA) against S. typhimurium D23580, the index invasive Malawian clinical isolate of the ST313 pathovar.[17] All conjugates induced bactericidal antibodies (Table 2 and the Supporting Information). The bactericidal activity of sera from mice immunized with conjugates with highest carbohydrate-to-protein ratios (11 b, 12 c, and 14) was similar, regardless of the amino acids involved and the OAg-to-CRM197 ratio. Importantly, OAg-CRM197 (13) induced sera with 10-fold greater bactericidal activity than 10 a, and comparable to the conjugates at higher OAg loading. The finding suggested the importance of the conjugation site on immunogenicity. In addition, the putative presented glycopeptide from 13 might bear two T-epitope peptides as the glycan was linked to two cysteines. This unique structural feature might contribute to the enhanced immunogenicity of the attached glycan.
Table 2.
SBA titers (GMT) of sera at day 56 against S. typhimurium D23580 strain. SBA titer is defined as the reciprocal serum dilution needed to have 50 % of killing of the bacteria, compared to the time 0
| Conjugate | 10a | 10b | 11a | 11b | 12a | 12b | 12c | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|
| SBA GMT (×1000) | 0.73 | 3.26 | 3.96 | 10.6 | 10.87 | 3.60 | 11.42 | 7.76 | 5.64 |
Immunogenicity results obtained are consistent with the model recently proposed for glycoconjugate vaccines where glycopeptides have an important role in the B-T cell cooperation.[3] Alternatively, it is plausible to think that different attachment sites could result in 1) diverse exposure/presentation of the glycoconjugate to antigen presenting cells, 2) different processing of the vaccine inside B-cells, or 3) formation of different peptides after processing of the conjugates inside B-cells to be presented to T-cells. The importance of the amino acids targeted in the conjugation reaction is not apparent when random chemical methods are used. Anti-OAg IgG levels among some conjugates characterized by a similar OAg-to-protein ratio, but targeting different amino acids (lysines vs. glutamates/aspartates and lysines vs. tyrosines) were comparable (Figure 2). These results could be explained by an average response which masks the contribution of individual “optimal” positions.
In conclusion, we have developed a diverse set of site-selective conjugation methods suitable for the synthesis of glyco-CRM197, conjugates with various carbohydrate loading and attachment points. We found that site-selective single or double attachment of glycan antigens is sufficient to induce high levels of anti-OAg IgG antibodies with serum bactericidal activity. The coupling site of the saccharide to one defined point on the carrier protein affected the elicited immune response. The use of such highly selective chemical methods has advantages in terms of consistency of production and characterization, and allows a better investigation of the immunological mechanism of glycoconjugate vaccines.
Acknowledgments
We thank M. Carducci, G. Di Salvo, and F. Necchi (SBVGH) for ELISA and help with SBA. We also thank L. Salvini (Toscana Life Sciences Foundation Siena) for help with MALDI-TOF analysis.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
miscellaneous_information
References
- 1.Costantino P, Rappuoli R, Berti F. Expert Opin. Drug Discovery. 2011;6:1045–1066. doi: 10.1517/17460441.2011.609554. [DOI] [PubMed] [Google Scholar]
- 2.Adamo R, Nilo A, Castagner B, Boutureira O, Berti F, Bernardes GJL. Chem. Sci. 2013;4:2995–3008. doi: 10.1039/c3sc50862e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avci FY, Li X, Tsuji M, Kasper DL. Nat. Med. 2011;17:1602–1609. doi: 10.1038/nm.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Boutureira O, Bernardes GJ. Chem. Rev. 2015;115:2174–2195. doi: 10.1021/cr500399p. [DOI] [PubMed] [Google Scholar]
- 4b.Spicer CD, Davis BG. Nat. Commun. 2014;5:4740. doi: 10.1038/ncomms5740. [DOI] [PubMed] [Google Scholar]
- 5a.Grayson EJ, Bernardes GJ, Chalker JM, Boutureira O, Koeppe JR, Davis BG. Angew. Chem. Int. Ed. 2011;50:4127–4132. doi: 10.1002/anie.201006327. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2011;123 [Google Scholar]
- 5b.Crotti S, Zhai H, Zhou J, Allan M, Proietti D, Pansegrau W, Hu QY, Berti F, Adamo R. ChemBiochem. 2014;15:836–843. doi: 10.1002/cbic.201300785. [DOI] [PubMed] [Google Scholar]
- 5c.Hu Q-Y, Allan M, Adamo R, Quinn D, Zhai H, Wu G, Clark K, Zhou J, Ortiz S, Wang B, Danieli E, Crotti S, Tontini M, Brogioni G, Berti F. Chem. Sci. 2013;4:3827–3832. [Google Scholar]
- 5d.Adamo R, Hu QY, Torosantucci A, Crotti S, Brogioni G, Allan M, Chiani P, Bromuro C, Quinn D, Tontini M, Berti F. Chem. Sci. 2014;5:4302–4311. [Google Scholar]
- 6.Reddy EA, Shaw AV, Crump JA. Lancet Infect. Dis. 2010;10:417–432. doi: 10.1016/S1473-3099(10)70072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Lancet. 2012;379:2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Micoli F, Ravenscroft N, Cescutti P, Stefanetti G, Londero S, Rondini S, MacLennan CA. Carbohydr. Res. 2014;385:1–8. doi: 10.1016/j.carres.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 9a.Nagy G, Pal T. Biol. Chem. 2008;389:513–520. doi: 10.1515/bc.2008.056. [DOI] [PubMed] [Google Scholar]
- 9b.Trebicka E, Jacob S, Pirzai W, Hurley BP, Cherayil BJ. Clin. Vaccine Immunol. 2013;20:1491–1498. doi: 10.1128/CVI.00289-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9c.MacLennan CA. Semin. Immunol. 2013;25:114–123. doi: 10.1016/j.smim.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 9d.Rondini S, Micoli F, Lanzilao L, Gavini M, Alfini R, Brandt C, Clare S, Mastroeni P, Saul A, MacLennan CA. Infect. Immun. 2015;83:996–1007. doi: 10.1128/IAI.03079-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Rabuka D. Curr. Opin. Chem. Biol. 2010;14:790–796. doi: 10.1016/j.cbpa.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10b.Dennler P, Chiotellis A, Fischer E, Bregeon D, Belmant C, Gauthier L, Lhospice F, Romagne F, Schibli R. Bioconjugate Chem. 2014;25:569–578. doi: 10.1021/bc400574z. [DOI] [PubMed] [Google Scholar]
- 10c.Jeger S, Zimmermann K, Blanc A, Grunberg J, Honer M, Hunziker P, Struthers H, Schibli R. Angew. Chem. Int. Ed. 2010;49:9995–9997. doi: 10.1002/anie.201004243. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2010;122 [Google Scholar]
- 11.Stefanetti G, Rondini S, Lanzilao L, Saul A, MacLennan CA, Micoli F. Vaccine. 2014;32:6122–6129. doi: 10.1016/j.vaccine.2014.08.056. [DOI] [PubMed] [Google Scholar]
- 12a.Rennels MB, Edwards KM, Keyserling HL, Reisinger KS, Hogerman DA, Madore DV, Chang I, Paradiso PR, Malinoski FJ, Kimura A. Pediatrics. 1998;101:604–611. doi: 10.1542/peds.101.4.604. [DOI] [PubMed] [Google Scholar]
- 12b.Shinefield HR, Black S, Ray P, Chang I, Lewis N, Fireman B, Hackell J, Paradiso PR, Siber G, Kohberger R, Madore DV, Malinowski FJ, Kimura A, Le C, Landaw I, Aguilar J, Hansen J. Pediatr. Infect. Dis. J. 1999;18:757–763. doi: 10.1097/00006454-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 12c.Snape MD, Perrett KP, Ford KJ, John TM, Pace D, Yu LM, Langley JM, McNeil S, Dull PM, Ceddia F, Anemona A, Halperin SA, Dobson S, Pollard AJ. JAMA J. Am. Med. Assoc. 2008;299:173–184. doi: 10.1001/jama.2007.29-c. [DOI] [PubMed] [Google Scholar]
- 12d.Kamboj KK, King CL, Greenspan NS, Kirchner HL, Schreiber JR. J. Infect. Dis. 2001;184:931–935. doi: 10.1086/323342. [DOI] [PubMed] [Google Scholar]
- 13.Hu Q-Y, Imase H. 2014. PCT Int. Appl WO 2014083505 A1, 20140605.
- 14.Micoli F, Rondini S, Gavini M, Lanzilao L, Medaglini D, Saul A, Martin LB. PLoS One. 2012;7:e47039. doi: 10.1371/journal.pone.0047039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilo A, Allan M, Brogioni B, Proietti D, Cattaneo V, Crotti S, Sokup S, Zhai H, Margarit I, Berti F, Hu QY, Adamo R. Bioconjugate Chem. 2014;25:2105–2111. doi: 10.1021/bc500438h. [DOI] [PubMed] [Google Scholar]
- 16a.Jewett JC, Bertozzi CR. Chem. Soc. Rev. 2010;39:1272–1279. doi: 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16b.Machida T, Lang K, Xue L, Chin JW, Winssinger N. Bioconjugate Chem. 2015;26:802–806. doi: 10.1021/acs.bioconjchem.5b00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kingsley RA, Msefula CL, Thomson NR, Kariuki S, Holt KE, Gordon MA, Harris D, Clarke L, Whitehead S, Sangal V, Marsh K, Achtman M, Molyneux ME, Cormican M, Parkhill J, MacLennan CA, Heyderman RS, Dougan G. Genome Res. 2009;19:2279–2287. doi: 10.1101/gr.091017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
miscellaneous_information


