Summary
Background
Although HDL cholesterol concentrations are strongly and inversely associated with risk of coronary heart disease, interventions that raise HDL cholesterol do not reduce risk of coronary heart disease. HDL cholesterol efflux capacity—a prototypical measure of HDL function—has been associated with coronary heart disease after adjusting for HDL cholesterol, but its effect on incident coronary heart disease risk is uncertain.
Methods
We measured cholesterol efflux capacity and assessed its relation with vascular risk factors and incident coronary heart disease events in a nested case-control sample from the prospective EPIC-Norfolk study of 25 639 individuals aged 40–79 years, assessed in 1993–97 and followed up to 2009. We quantified cholesterol efflux capacity in 1745 patients with incident coronary heart disease and 1749 control participants free of any cardiovascular disorders by use of a validated ex-vivo radiotracer assay that involved incubation of cholesterol-labelled J774 macrophages with apoB-depleted serum from study participants.
Findings
Cholesterol efflux capacity was positively correlated with HDL cholesterol concentration (r=0·40; p<0·0001) and apoA-I concentration (r=0·22; p<0·0001). It was also inversely correlated with type 2 diabetes (r=–0·18; p<0·0001) and positively correlated with alcohol consumption (r=0·12; p<0·0001). In analyses comparing the top and bottom tertiles, cholesterol efflux capacity was significantly and inversely associated with incident coronary heart disease events, independent of age, sex, diabetes, hypertension, smoking and alcohol use, waist:hip ratio, BMI, LDL cholesterol concentration, log-triglycerides, and HDL cholesterol or apoA-I concentrations (odds ratio 0·64, 95% CI 0·51–0·80). After a similar multivariable adjustment the risk of incident coronary heart disease was 0·80 (95% CI 0·70–0·90) for a per-SD change in cholesterol efflux capacity.
Interpretation
HDL cholesterol efflux capacity might provide an alternative mechanism for therapeutic modulation of the HDL pathway beyond HDL cholesterol concentration to help reduce risk of coronary heart disease.
Funding
US National Institutes of Health, UK Medical Research Council, Cancer Research UK.
Introduction
HDL cholesterol is log-linearly and inversely associated with risk of coronary heart disease.1, 2 Direct antiatherogenic effects of HDL cholesterol or its major protein apoA-I have been shown in preclinical studies, including prevention or regression of lesions in animal models of atherosclerosis after administration of HDL cholesterol or apoA-I.3, 4 However, clinical trials and genetic studies have yielded conflicting evidence, suggesting that interventions or genetic variants that raise HDL cholesterol concentration do not necessarily decrease risk of coronary heart disease.5, 6, 7, 8, 9, 10 This discrepancy has encouraged further studies of the properties of HDL other than static mass-based measures to help understand the mechanisms that link HDL-related pathways with coronary heart disease.
Cholesterol efflux capacity is a measure of the ability of HDL to promote cholesterol removal from lipid-laden macrophages, the first step in the process of reverse cholesterol transport.11 HDL cholesterol efflux capacity was inversely associated with prevalent risk of coronary heart disease in a cross-sectional study.12 However, this design has inherent limitations because of the inability to rule out any changes in efflux capacity or other relevant traits (ie, HDL cholesterol concentration) that could occur because of ongoing atherosclerosis or an acute event in a population with prevalent disease.13 Indeed, although a subsequent report14 confirmed a significant inverse relationship between cholesterol efflux capacity and prevalent coronary heart disease, it suggested the potential for a positive association with incident cardiovascular events. In a population-based US cohort study of 132 participants with incident cardiovascular disease events (54 with coronary heart disease events), cholesterol efflux capacity (measured with a different assay) was inversely associated with incident cardiovascular disease events independent of HDL cholesterol concentration.15
Here, we report findings from a study of the association between HDL cholesterol efflux capacity and incident coronary heart disease in a larger cohort in the UK.
Research in context.
Evidence before this study
Evidence from observational, genetic, and interventional studies has been inconsistent about the atheroprotective effects of HDL cholesterol.
Added value of the study
We estimated the effect of cholesterol efflux capacity, a parameter of HDL function, on incident coronary heart disease risk. We investigated more than 13-times more coronary heart disease cases than did previous studies; hence, estimating the association of cholesterol efflux capacity with disease risk with greater confidence. Our study also examines for the first time associations of efflux capacity with a range of vascular and non-vascular traits. We also assessed the associations of HDL cholesterol or apoA-I concentrations with incident coronary heart disease risk after adjusting for cholesterol efflux capacity.
Implications of all the available evidence
Cholesterol efflux capacity is significantly and inversely associated with incident coronary heart events independent of established cardiovascular risk factors and even after adjusting for HDL cholesterol or apoA-I concentrations. If cholesterol efflux capacity is shown to be causally associated with coronary heart disease risk through genetic or interventional studies, it will provide a new mechanism to alter the HDL pathway beyond static HDL concentration to reduce the risk of primary coronary events.
Methods
Study design and participants
We did this nested case-control study with patients from the EPIC-Norfolk study,16 a prospective population-based investigation of 25 693 men and women aged 40–79 years who live in Norfolk (UK). Participants were recruited from general practices. Anthropometric characteristics, blood pressure, and lipids were similar to those in a typical UK population.16 In a baseline survey done between 1993 and 1997, participants completed a detailed health and lifestyle questionnaire and visited a clinic. Non-fasting blood samples were obtained, spun, and separated for long-term storage in liquid nitrogen or –80°C freezers. Participants were identified as having coronary heart disease during follow-up if they were admitted to hospital or died with coronary heart disease as an underlying cause, or both.17 Coronary heart disease was defined by codes 410–414 from the International Classification of Diseases (ninth revision),18 and included participants with unstable angina, stable angina, and myocardial infarction (fatal or not). The Norwich District Health Authority ethics committee approved the study, and all participants gave written informed consent.
We identified 1745 initially healthy people who later developed fatal or non-fatal coronary heart disease up to March, 2009. Control participants were initially healthy and remained free of any cardiovascular disease during follow-up. One control participant per patient with coronary heart disease was randomly selected by computerised algorithm from the cohort to enable a control sample population which broadly matched the age–sex distribution of the case population. Controls had to have at least as much follow-up time as cases and had to be alive during follow-up. We selected 1749 controls for whom information was available for all cardiovascular risk factors and a serum sample was available for measurement of cholesterol efflux capacity. All participants were of European ancestry.
Procedures
For measurement of cholesterol efflux capacity, we used a 0·5 mL serum sample, which was stored at –80°C at baseline and thawed for re-aliquoting before being sent to the University of Pennsylvania on dry ice. All samples were shipped (and consequently assayed) in two batches with a similar number of cases and controls within each batch; all analyses were adjusted for batch number.
We quantified cholesterol efflux capacity with use of a previously published protocol.12, 19 J774 cells, derived from a mouse macrophage cell line, were plated and radiolabelled with 2 μCi of 3H-cholesterol (Perkin Elmer, Waltham, USA) per mL. ABCA1 was upregulated by 6 h incubation with 0·3 mmol/L 8-(4-chlorophenylthio)-cyclic AMP (Sigma-Aldrich, St Louis, USA), because ABCA1-mediated efflux is an important pathway of cholesterol efflux from macrophages and basal J774 cells have very low levels of ABCA1 activity. Subsequently, efflux medium containing 2·8% apoB-depleted serum was added and the efflux period was for 4 h. To prepare apoB-depleted serum, samples were thawed before apoB precipitation. Briefly, 40 parts polyethylene glycol solution (20% polyethylene glycol 8000 molecular weight in 200 mmol/L glycine buffer [Fisher Scientific, Pittsburg, USA], pH 7·4) was added to 100 parts serum and mixed by pipetting, then incubated at room temperature for 20 min before spinning in a microcentrifuge at 10 000 revolutions per min for 30 min at 4°C. ApoB-containing lipoproteins were pelleted by this procedure and the supernatant, which contained the HDL fraction, was recovered and diluted in 14 mmol/L MEM-HEPES (no bicarbonate; Fisher Scientific, Pittsburg, USA) and 0·15 mmol/L cAMP (Sigma-Aldrich, St Louis, USA) to 1·4% (equivalent to 1% serum).
We used liquid scintillation counting to quantify the efflux of radioactive cholesterol from J774 cells. We calculated the quantity of radioactive cholesterol incorporated into cellular lipids by means of isopropanol (Sigma-Aldrich, St Louis, USA) extraction of control wells not exposed to patient serum. We calculated percentage efflux by the formula: (μCi of 3H-cholesterol in mediums containing 2·8% apoB-depleted serum – μCi of 3H-cholesterol in serum-free mediums) / (μCi of 3H-cholesterol in cells) × 100. We did all assays in duplicate. To correct for interassay variation across plates, we included a pooled serum control from five healthy volunteers on each plate, and we normalised values for serum samples from patients to this pooled value in subsequent analyses. The interassay coefficient of variation was 4·5% and the intra-assay coefficient of variation was 7%. We measured serum concentrations of lipids, apoA-I, and apoB as previously described.17
Statistical analysis
We compared baseline characteristics between incident coronary heart disease cases and controls. We log-transformed triglyceride concentrations. We calculated partial correlation coefficients adjusted for age and sex to assess correlations between cholesterol efflux capacity and other traits. We further characterised associations between cholesterol efflux capacity and other characteristics by linear regression analyses adjusted for age, sex, and batch number. From each fitted regression model, we obtained overall adjusted mean values and 95% CIs for cholesterol efflux capacity within quintiles of continuous variables, or within each category for categorical variables. We calculated odds ratios (ORs) for risk of coronary heart disease by unconditional logistic regression, progressively adjusted for age, sex, history of diabetes, history of hypertension, cigarette use, alcohol intake, BMI, waist:hip ratio, LDL cholesterol concentration, log-triglyceride concentration, and HDL cholesterol or apoA-I concentration. We characterised the shapes of the associations by calculating ORs using tertiles of cholesterol efflux capacity; we estimated the corresponding 95% CIs from floating absolute variances that represent the amount of information underlying each group (including the reference group)20 and implemented fractional polynomial analyses (single level). We did statistical analyses with Stata (version 11). All p values were two-tailed, with a p value of less than 0·01 indicating statistical significance.
Role of the funding source
The funder had no role in study design, data collection, analysis, or interpretation, or the writing of the report. RS, SJ, and RL had access to all the data. DS, RS, NW, and DJR were responsible for the decision to submit for publication.
Results
Participants who developed incident coronary heart disease were slightly older and more overweight, were more likely to have a history of diabetes, hypertension, and smoking; had higher triglyceride, LDL cholesterol, and apoB concentrations; and had lower HDL cholesterol and apoA-I concentrations than did controls (table 1). Participants who developed coronary heart disease had significantly lower mean cholesterol efflux capacity (adjusted for age and sex) than did controls (1·17 vs 1·14; p=0·005).
Table 1.
Characteristics of participants with or without an incident coronary heart disease event
| Controls (n=1749) | Cases (n=1745) | p value | |
|---|---|---|---|
| Age (years)* | 65·0 (7·81) | 66·1 (7·48) | <0·0001 |
| Males | 1067 (61·0%) | 1187 (68·0%) | <0·0001 |
| Waist:hip ratio | 0·88 (0·09) | 0·91 (0·08) | <0·0001 |
| BMI | 26·08 (3·36) | 27·21 (3·89) | <0·0001 |
| History of diabetes | 88 (5·0%) | 269 (15·4%) | <0·0001 |
| History of hypertension | 259 (14·8%) | 642 (36·8%) | <0·0001 |
| Systolic blood pressure (mm Hg) | 138·06 (17·64) | 143·31 (18·76) | <0·0001 |
| Diastolic blood pressure (mm Hg) | 83·05 (10·72) | 85·90 (12·33) | <0·0001 |
| Current tobacco user | 149 (8·5%) | 204 (11·7%) | <0·0001 |
| Alcohol (units per week) | 7·56 (9·29) | 7·11 (10·20) | 0·17 |
| Statin use | 4 (0·2%) | 38 (2·2%) | <0·0001 |
| Triglyceride concentration (mmol/L) | 1·22 (0·19)† | 1·30 (0·19)† | <0·0001 |
| Total cholesterol concentration (mmol/L) | 6·23 (1·14) | 6·38 (1·13) | <0·0001 |
| LDL cholesterol concentration (mmol/L) | 4·02 (1·01) | 4·19 (1·00) | <0·0001 |
| ApoB concentration (mmol/L) | 0·99 (0·26) | 1·05 (0·26) | <0·0001 |
| HDL cholesterol concentration (mmol/L) | 1·41 (0·41) | 1·28 (0·36) | <0·0001 |
| ApoA-I concentration (mmol/L) | 1·58 (0·32) | 1·53 (0·36) | <0·0001 |
Data are mean (SD) or n (%).
Mean exact age.
Geometric mean (SD of log-triglycerides).
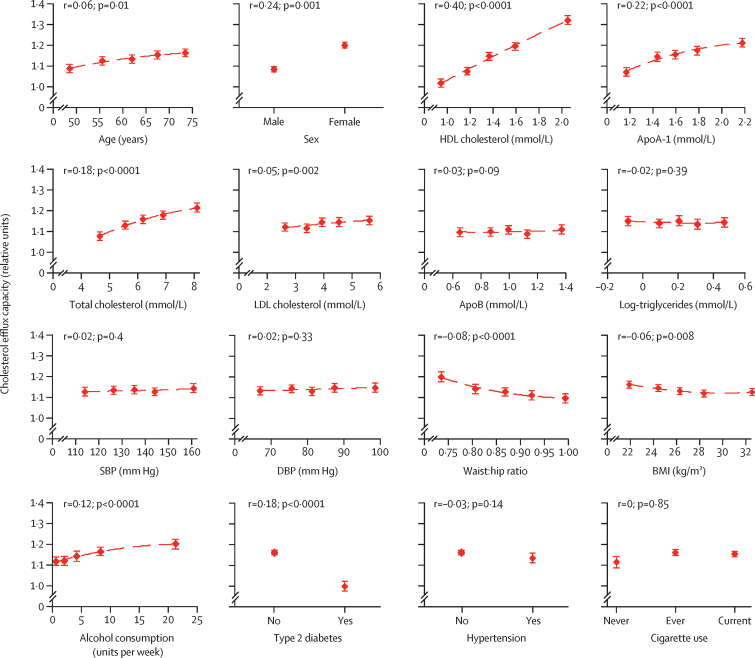
In control participants, we recorded a significant positive correlation between cholesterol efflux capacity and HDL cholesterol (r=0·40, 95% CI 0·36–0·42) and between cholesterol efflux capacity and apoA-I (r=0·22, 0·18–0·26; figure 1, appendix p 1). We detected a weaker positive correlation with total cholesterol (r=0·18, 0·15–0·22) and LDL cholesterol (r=0·05, 0·02–0·08). We also recorded significant positive correlations with female sex (r=0·24, 0·20–0·27) and alcohol intake (r=0·12, 0·08–0·05), whereas we detected significant inverse relationships for waist:hip ratio, BMI, and history of diabetes.
Figure 1.
Cross-sectional correlates of cholesterol efflux capacity in control participants (n=1749)
Assessed by linear regression analyses adjusted for age, sex, and batch number. From each fitted regression model, we obtained overall adjusted mean values and 95% CIs for cholesterol efflux capacity within quintiles of continuous markers, or within each category for categorical variables. SBP=systolic blood pressure. DBP=diastolic blood pressure.
In analyses adjusted for age, sex, and batch number, the OR for coronary heart disease was 0·56 (95% CI 0·46–0·62) in individuals with cholesterol efflux capacity in the top tertile compared with those in the bottom tertile (table 2). After progressive adjustment for various cardiovascular risk factors, cholesterol efflux capacity remained significantly associated with incident coronary heart disease events, including HDL cholesterol concentration (table 2, appendix p 2). For a per-SD increase in cholesterol efflux capacity, the OR for coronary heart disease was 0·80 (0·70–0·90) in the final multivariable model adjusted for HDL cholesterol concentration. The ORs for coronary heart disease progressively decreased across tertiles of cholesterol efflux capacity (figure 2). When we used apoA-I concentrations instead of HDL cholesterol concentrations in the final multivariable analyses, the OR for coronary heart disease risk did not change materially from the baseline model and cholesterol efflux capacity remained independently associated with incident coronary heart disease events independent of apoA-I concentration (appendix p 3–4; the models shown in appendix p 3–4 were restricted to participants who had information for apoA-1 concentration: 1428 cases and 1749 controls). Analyses using fractional polynomial curves suggested a dose-response association (although not a perfectly linear association) between cholesterol efflux capacity and coronary heart disease risk independent of HDL cholesterol or apoA-1 concentrations (appendix p 5).
Table 2.
Association of cholesterol efflux capacity with incident coronary heart disease events
| Bottom tertile | Middle tertile | Top tertile* | Per-SD increase | |
|---|---|---|---|---|
| Not adjusted | 1·00 (0·86–1·16) | 0·78 (0·67–0·9) | 0·58 (0·48–0·71) | 0·70 (0·64–0·78) |
| Adjusted for age, sex, batch number | 1·00 (0·86–1·17) | 0·79 (0·68–0·92) | 0·56 (0·46–0·68) | 0·70 (0·62–0·78) |
| Plus history of diabetes | 1·00 (0·85–1·17) | 0·83 (0·71–0·96) | 0·60 (0·49–0·73) | 0·75 (0·67–0·83) |
| Plus history of hypertension | 1·00 (0·85–1·18) | 0·82 (0·70–0·96) | 0·59 (0·48–0·72) | 0·75 (0·67–0·83) |
| Plus cigarette use | 1·00 (0·85–1·18) | 0·82 (0·71–0·96) | 0·59 (0·48–0·73) | 0·76 (0·68–0·84) |
| Plus alcohol use | 1·00 (0·85–1·18) | 0·84 (0·72–0·98) | 0·61 (0·50–0·75) | 0·76 (0·68–0·84) |
| Plus waist:hip ratio and BMI | 1·00 (0·85–1·18) | 0·84 (0·72–0·98) | 0·62 (0·51–0·77) | 0·80 (0·71–0·90) |
| Plus LDL cholesterol | 1·00 (0·85–1·18) | 0·83 (0·71–0·96) | 0·59 (0·48–0·73) | 0·78 (0·70–0·87) |
| Plus log-triglycerides | 1·00 (0·85–1·18) | 0·82 (0·70–0·96) | 0·58 (0·47–0·71) | 0·78 (0·70–0·87) |
| Plus HDL cholesterol | 1·00 (0·83–1·20) | 0·84 (0·73–0·97) | 0·64 (0·51–0·80) | 0·80 (0·70–0·90) |
Data are odds ratio (95% CI). Mean cholesterol efflux capacity was 0·83 (SD 0·09) in the bottom tertile, 1·13 (SD 0·09) in the middle tertile, and 1·40 (SD 0·09) in the top tertile. We calculated odds ratios for coronary heart disease by unconditional logistic regression analyses. The total number of participants was the same for all the models. We estimated 95% CIs from floating absolute variances that represent the amount of information underlying each group (including the reference group).20
For trend, p<0·0001.
Figure 2.
Association of cholesterol efflux capacity tertiles with incident coronary heart disease risk
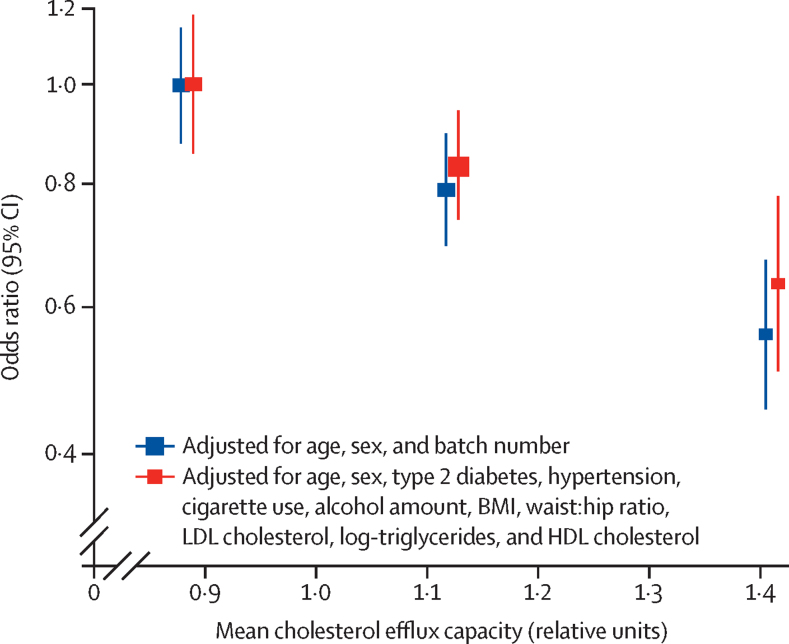
We investigated the association of HDL cholesterol and apoA-I concentrations with incident coronary heart disease events in the same participants in whom we measured cholesterol efflux capacity. As expected, comparing top versus bottom tertiles, both HDL cholesterol and apoA-I concentrations were significantly and inversely associated with coronary heart disease events independent of cardiovascular risk factors (figure 3). However, further adjustment for cholesterol efflux capacity attenuated the magnitude of these associations, making them non-significant (figure 3). By contrast, neither adjustment for HDL cholesterol nor apoA-I concentrations attenuated the association between cholesterol efflux capacity and coronary heart disease (figure 3).
Figure 3.
Association with coronary heart disease
*Age, sex, history of diabetes, history of hypertension, cigarette use, alcohol intake, BMI, waist:hip ratio, LDL cholesterol concentration, log-triglyceride concentration. CEC=cholesterol efflux capacity. CHD=coronary heart disease. CVD=cardiovascular disease.
When we analysed cholesterol efflux capacity in association with incident coronary heart disease events across subgroups of cardiovascular risk factors (at a significance threshold of p<0·01), we detected no significant subgroup interactions. We noted trends for interaction (p<0·05) for a stronger relationship of cholesterol efflux capacity with coronary heart disease events in men (compared with women) and in never smokers (compared with ever smokers; appendix p 6).
Discussion
We report here the first large-scale prospective population-based study that has investigated the association of HDL cholesterol efflux capacity with cardiovascular traits and incident coronary heart disease events. We found that cholesterol efflux capacity is significantly and inversely associated with incident coronary heart disease events independent of several established cardiovascular risk factors and even after adjusting for HDL cholesterol or apoA-I concentrations. Conversely, the significant inverse association of HDL cholesterol or apoA-I concentrations with incident coronary heart disease events became non-significant when analyses were adjusted for cholesterol efflux capacity. Additionally, we report that cholesterol efflux capacity was positively correlated with alcohol intake and inversely correlated with type 2 diabetes and measures of obesity.
These results help to clarify the relation between cholesterol efflux capacity and incident coronary events. Our results contrast with a previous report14 based on a study population ascertained at the time of coronary angiography, which concluded that cholesterol efflux capacity was positively associated with incident coronary heart disease events. However, our data are generally consistent with a report15 from the Dallas Heart Study that showed an inverse association of cholesterol efflux capacity with risk of incident coronary heart disease. Our study extends this previous report in several ways. First, our study is much larger than the Dallas study, involving 13 times the number of incident cardiovascular cases and 32 times the number of incident coronary heart disease cases, which enabled us to quantify the effect of cholesterol efflux capacity on incident chronic heart disease risk and to do robust subgroup analyses. Second, whereas the cohort in the Dallas Heart Study had a mixed ethnic origin profile and considerably younger participants, we included only participants of European descent who were older at baseline. Third, samples in the Dallas Heart Study were collected while fasting whereas we measured cholesterol efflux capacity in randomly collected samples.
The Dallas Heart Study used a newer fluorescently labelled (BODIPY) cholesterol method to assay cholesterol efflux capacity, in contrast to the more established radiolabelled cholesterol assay that we used in the present study. A comprehensive analysis12 of the radiolabelled cholesterol assay we used showed that roughly a third of cholesterol efflux is mediated by ABCA1. By contrast, analysis of the fluorescently labelled cholesterol assay suggests that a much greater proportion of efflux is mediated by ABCA1.21 Differences in the assay methods probably account for the differences in the correlation of cholesterol efflux capacity with HDL cholesterol, which in the report of the Dallas Heart Study was only 0·07 (consistent with a previous study21), whereas in our study it was 0·40 (consistent with other studies using a similar assay12, 19). Data for apoA-I concentrations were not reported by the Dallas Heart Study; in our study, the correlation of cholesterol efflux capacity with apoA-I concentration was lower than correlation with HDL cholesterol, and cholesterol efflux capacity remained strongly inversely associated with incident coronary heart disease events after adjusting for apoA-I concentrations. Overall, despite the differences in the study populations and the cholesterol efflux capacity assay methods, the consistency of the major findings of the two studies strengthens the conclusion that cholesterol efflux capacity measured in initially healthy individuals is inversely associated with incident coronary heart disease events independent of HDL cholesterol concentration. The relation between cholesterol efflux capacity and incident events in patients with pre-existing coronary heart disease might be different, and further studies are needed to address this issue.
Our study has some limitations. It was not adequately powered to assess the shape of the relation between cholesterol efflux capacity across smaller subdivisions such as quintiles in association with coronary heart disease risk. Our subgroup analyses provide evidence suggestive of effect modification by HDL cholesterol (p<0·05 but >0·01); the association of cholesterol efflux capacity with coronary heart disease events seemed to be non-linear across tertiles of HDL cholesterol concentration. We also recorded suggestive interactions for apoA-I, sex, and smoking status (all p<0·05 but >0·01); these subgroup analyses show the need for even larger studies to characterise in detail the association of efflux capacity with coronary heart disease events across various subgroups with greater confidence. In the EPIC-Norfolk cohort, lipid concentrations and cholesterol efflux capacity were measured in random (generally non-fasting) samples. However, analyses in which we stratified for time since last meal showed no differences in cholesterol efflux capacity across quartiles of fasting time (appendix p 6), suggesting that cholesterol efflux capacity was not affected by postprandial state. We did not measure cholesterol efflux capacity in repeat samples in the same participants at different times and thus we could not correct for regression dilution, which could have led to underestimation of the association between cholesterol efflux capacity and incident coronary heart disease risk.
Although we have shown an inverse relationship between cholesterol efflux capacity and risk of incident coronary heart disease, the causal nature of this relationship is uncertain. Further studies with information about genotypes and other traits are needed to exclude residual confounding and assess the cause of this association through a mendelian randomisation study. One such study7 has suggested that HDL cholesterol concentrations are not causally relevant in coronary heart disease; it is plausible that other functional properties of HDL (such as cholesterol efflux capacity) might have causal relevance in coronary heart disease. If cholesterol efflux capacity is causally related to coronary heart disease, then interventions to increase cholesterol efflux capacity could be a new treatment approach to reduce the risk of coronary heart disease.
Acknowledgments
Acknowledgments
We thank the participants of the EPIC-Norfolk cohort. The EPIC Norfolk cohort is supported by programme grants from the UK Medical Research Council and Cancer Research UK. Cholesterol efflux measurements in the EPIC-Norfolk study and the analyses were funded by a US National Institutes of Health grant (R01-HL111398) awarded to DJR and DS.
Contributors
DS and DJR designed the study with RS, RL, SMB, K-TK, and NW. DS, RS, SJ, WZ, and RL did the statistical analyses. DS, RS, AR, AP, DL, MLM, JB, and DJR were responsible for study conduct and coordination of cholesterol efflux measurements. DS, DJR, RS, JB, JJPK, SMB, K-TK, and NW were involved in data review and interpretation, report preparation, and subsequent revisions.
Declaration of interests
DJR is a founder of VascularStrategies, which does assays of HDL function (including cholesterol efflux capacity) as a service. JJPK has received personal fees from Cerenis, The Medicines Company, CSL Behring, Amgen, Regeneron, Eli Lilly, Genzyme, Aegerion, Esperion, AstraZeneca, Omthera, Pronova, Vascular Biogenics, Boehringer Ingelheim, Catabasis, AtheroNova, UniQure, Novartis, Merck Pharmaceuticals, Kowa, Dezima Pharmaceuticals, and Sanofi, outside the submitted work. The other authors declare no competing interests.
Contributor Information
Danish Saleheen, Email: saleheen@upenn.edu.
Daniel J Rader, Email: rader@mail.med.upenn.edu.
Supplementary Material
References
- 1.Lewington S, Whitlock G, Clarke R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55 000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 2.Di Angelantonio E, Sarwar N, Perry P. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest. 1990;85:1234–1241. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tangirala RK, Tsukamoto K, Chun SH, Usher D, Pure E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- 5.Barter PJ, Caulfield M, Eriksson M. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz GG, Olsson AG, Abt M. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 7.Boden WE, Probstfield JL, Anderson T. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 8.HPS2-THRIVE Collaborative Group. Landray MJ, Haynes R. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 9.Frikke-Schmidt R, Nordestgaard BG, Stene MC. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 2008;299:2524–2532. doi: 10.1001/jama.299.21.2524. [DOI] [PubMed] [Google Scholar]
- 10.Voight BF, Peloso GM, Orho-Melander M. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothblat GH, de la Llera-Moya M, Atger V, Kellner-Weibel G, Williams DL, Phillips MC. Cell cholesterol efflux: integration of old and new observations provides new insights. J Lipid Res. 1999;40:781–796. [PubMed] [Google Scholar]
- 12.Khera AV, Cuchel M, de la Llera-Moya M. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parish S, Peto R, Palmer A. The joint effects of apolipoprotein B, apolipoprotein A1, LDL cholesterol, and HDL cholesterol on risk: 3510 cases of acute myocardial infarction and 9805 controls. Eur Heart J. 2009;30:2137–2146. doi: 10.1093/eurheartj/ehp221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li XM, Tang WH, Mosior MK. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol. 2013;33:1696–1705. doi: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohatgi A, Khera A, Berry JD. HDL Cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day N, Oakes S, Luben R. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer. 1999;80(suppl 1):95–103. [PubMed] [Google Scholar]
- 17.Boekholdt SM, Kuivenhoven JA, Wareham NJ. Plasma levels of cholesteryl ester transfer protein and the risk of future coronary artery disease in apparently healthy men and women: the prospective EPIC (European Prospective Investigation into Cancer and nutrition)-Norfolk population study. Circulation. 2004;110:1418–1423. doi: 10.1161/01.CIR.0000141730.65972.95. [DOI] [PubMed] [Google Scholar]
- 18.ICD-9-CM Official Guidelines for Coding and Reporting http://www.cdc.gov/nchs/data/icd/icd9cm_guidelines_2011.pdf (accessed May 15, 2015).
- 19.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30:796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med. 1991;10:1025–1035. doi: 10.1002/sim.4780100703. [DOI] [PubMed] [Google Scholar]
- 21.Sankaranarayanan S, Kellner-Weibel G, de la Llera-Moya M. A sensitive assay for ABCA1-mediated cholesterol efflux using BODIPY-cholesterol. J Lipid Res. 2011;52:2332–2340. doi: 10.1194/jlr.D018051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.