Summary
Background
To investigate potential cardiovascular and other effects of long-term pharmacological interleukin 1 (IL-1) inhibition, we studied genetic variants that produce inhibition of IL-1, a master regulator of inflammation.
Methods
We created a genetic score combining the effects of alleles of two common variants (rs6743376 and rs1542176) that are located upstream of IL1RN, the gene encoding the IL-1 receptor antagonist (IL-1Ra; an endogenous inhibitor of both IL-1α and IL-1β); both alleles increase soluble IL-1Ra protein concentration. We compared effects on inflammation biomarkers of this genetic score with those of anakinra, the recombinant form of IL-1Ra, which has previously been studied in randomised trials of rheumatoid arthritis and other inflammatory disorders. In primary analyses, we investigated the score in relation to rheumatoid arthritis and four cardiometabolic diseases (type 2 diabetes, coronary heart disease, ischaemic stroke, and abdominal aortic aneurysm; 453 411 total participants). In exploratory analyses, we studied the relation of the score to many disease traits and to 24 other disorders of proposed relevance to IL-1 signalling (746 171 total participants).
Findings
For each IL1RN minor allele inherited, serum concentrations of IL-1Ra increased by 0·22 SD (95% CI 0·18–0·25; 12·5%; p=9·3 × 10−33), concentrations of interleukin 6 decreased by 0·02 SD (−0·04 to −0·01; −1·7%; p=3·5 × 10−3), and concentrations of C-reactive protein decreased by 0·03 SD (−0·04 to −0·02; −3·4%; p=7·7 × 10−14). We noted the effects of the genetic score on these inflammation biomarkers to be directionally concordant with those of anakinra. The allele count of the genetic score had roughly log-linear, dose-dependent associations with both IL-1Ra concentration and risk of coronary heart disease. For people who carried four IL-1Ra-raising alleles, the odds ratio for coronary heart disease was 1·15 (1·08–1·22; p=1·8 × 10−6) compared with people who carried no IL-1Ra-raising alleles; the per-allele odds ratio for coronary heart disease was 1·03 (1·02–1·04; p=3·9 × 10−10). Per-allele odds ratios were 0·97 (0·95–0·99; p=9·9 × 10−4) for rheumatoid arthritis, 0·99 (0·97–1·01; p=0·47) for type 2 diabetes, 1·00 (0·98–1·02; p=0·92) for ischaemic stroke, and 1·08 (1·04–1·12; p=1·8 × 10−5) for abdominal aortic aneurysm. In exploratory analyses, we observed per-allele increases in concentrations of proatherogenic lipids, including LDL-cholesterol, but no clear evidence of association for blood pressure, glycaemic traits, or any of the 24 other disorders studied. Modelling suggested that the observed increase in LDL-cholesterol could account for about a third of the association observed between the genetic score and increased coronary risk.
Interpretation
Human genetic data suggest that long-term dual IL-1α/β inhibition could increase cardiovascular risk and, conversely, reduce the risk of development of rheumatoid arthritis. The cardiovascular risk might, in part, be mediated through an increase in proatherogenic lipid concentrations.
Funding
UK Medical Research Council, British Heart Foundation, UK National Institute for Health Research, National Institute for Health Research Cambridge Biomedical Research Centre, European Research Council, and European Commission Framework Programme 7.
Introduction
Interleukin 1 (IL-1), a cytokine that acts as a master regulator of inflammation, triggers a cascade of inflammatory mediators by activation of the IL-1 receptor.1 Drugs that inhibit IL-1 are licensed for treatment of inflammatory disorders, such as rheumatoid arthritis. Trials are in progress for a broad range of additional indications,1 such as cardiometabolic disorders,2, 3, 4 because some evidence suggests that persistent inflammation increases cardiovascular risk and that IL-1 signalling is increased in the pancreatic islet cells of patients with type 2 diabetes.5 However, potential concerns exist about the safety of long-term IL-1 inhibition. The IL-1 receptor is present on nearly all human cells, and IL-1 has key roles in host defence, wound healing, and many other processes.6 Potential cardiovascular and other effects of sustained inhibition therefore need to be understood.7
One important approach to gain such information is through randomised trials of IL-1-inhibiting drugs. However, although trials of IL-1 inhibitors have shown rapid reductions in disease severity and symptoms in inflammatory disorders,1, 8 they have been too brief or insufficiently powered to assess the effect on cardiovascular and other disease outcomes. A complementary approach is to study genetic variants known to result in IL-1 inhibition. Because genotypes are fixed at conception, human genetic studies could help to predict the effects of long-term IL-1 inhibition.9, 10
We aimed to create a genetic score that combines information on rs6743376 and rs1542176, two uncorrelated variants located upstream of ILRN, the gene encoding the IL-1 receptor antagonist (IL-1Ra). These variants are the strongest known genetic determinants of circulating IL-1Ra protein concentrations.11 IL-1Ra is an endogenous inhibitor of IL-1 that blocks activation of the IL-1 receptor by either IL-1α or IL-1β.1 This genetic score could thus mimic the effects of IL-1 inhibitors (eg, anakinra) that have the same mechanism of action as IL-1Ra (appendix p 41). By contrast, this genetic score would not necessarily be expected to mimic the effects of drugs that selectively inhibit either IL-1α (eg, MABp1) or IL-1β (eg, canakinumab). We investigated this genetic score in relation to rheumatoid arthritis and four cardiovascular disorders (type 2 diabetes, coronary heart disease, ischaemic stroke, and abdominal aortic aneurysm), and, in exploratory analyses, in relation to additional disorders and disease traits.
Methods
Study design and procedures
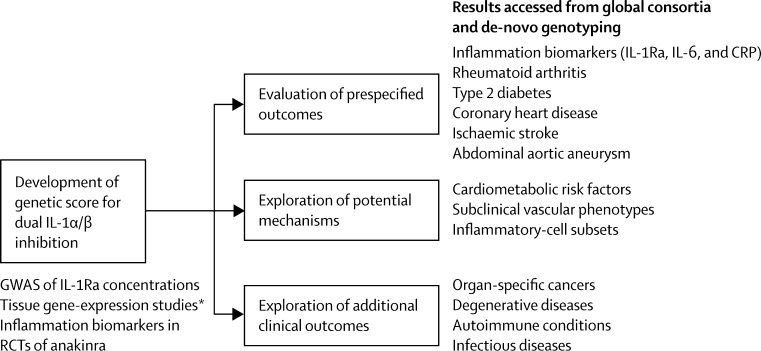
Figure 1 summarises the study approach, and the table provides definitions and sources of data used. First, we constructed a score containing two genetic variants (rs6743376 and rs1542176) previously robustly linked with IL-1Ra concentration,11 and then investigated the biological relevance of the score through analysis of gene expression data (figure 1, appendix pp 1–4). Second, we assessed the effects of our genetic score on circulating concentrations of IL-1Ra, interleukin 6 (IL-6), and C-reactive protein (CRP; table, appendix pp 5–10). We then compared the score's effects on these inflammation biomarkers with those of anakinra, the recombinant form of IL-1Ra, from existing randomised trial data (appendix p 10). Third, because anakinra is licensed for treatment of rheumatoid arthritis, we assessed the score in patients with rheumatoid arthritis and in healthy controls (table, appendix p 11). Fourth, we assessed the score in relation to type 2 diabetes, coronary heart disease, ischaemic stroke, and abdominal aortic aneurysm (table, appendix pp 11–12) because each of these disorders is either being investigated as an outcome in trials of IL-1 inhibitors,1 or has been previously robustly linked with a functional genetic variant for inflammation,31 or both. Fifth, to gain insight into mechanisms that might link IL-1α/β signalling with cardiovascular disorders, we did exploratory analyses of the score in relation to disease traits (appendix pp 13–15). Sixth, to help predict the broad effects of long-term dual IL-1α/β inhibition, we explored the score in relation to several additional disorders (appendix p 12).
Figure 1.
Study design
CRP=C-reactive protein. GWAS=genome-wide association study. IL-1Ra=interleukin 1 receptor antagonist. IL-6=interleukin 6; RCT=randomised controlled trial. *Refers to the Multiple Tissue Human Expression Resource and Genotype-Tissue Expression project.
Table.
Definitions and sources of contributing data for main study outcomes
| Participants* | Assessment method or endpoint definition | |
|---|---|---|
| Interleukin 1 receptor antagonist | ||
| CHS11 | 3081 | Validated, commercially available ELISA-based system (Mesoscale, Maryland, USA) |
| Interleukin 6 | ||
| CHS11 | 2917 | Validated, commercially available ELISA-based systems (eg, R&D systems, Minnesota, USA, or Millipore, Missouri, USA) |
| SardiNIA12 | 5924 | |
| UK10K consortium | 7311 | |
| C-reactive protein | ||
| CHS11 | 3181 | Validated, commercially available ELISA-based systems (eg, Millipore), nephelometric systems (Dade Behring, Illinois, USA), or turbidimetric systems (eg, Dako, Glostrup, Denmark) |
| CCHS13 | 3806 | |
| CGPS13 | 2728 | |
| EPIC-CVD14 | 14 100 | |
| SardiNIA12 | 5716 | |
| UK10K consortium | 33 911 | |
| Rheumatoid arthritis | ||
| Okada et al15 | 14 361/43 923 | 1987 criteria of the ACR |
| Type 2 diabetes | ||
| DIAGRAM and EPIC-InterAct16, 17, 18 | 18 715/61 692 | ADA, WHO criteria, or similar |
| Coronary heart disease | ||
| C4D19 | 11 733/11 816 | MI and other major coronary events (about 90% of cases); angiographic stenosis only (about 10% of cases) |
| CARDIoGRAM20 | 22 149/52 247 | |
| Studies in CARDIoGRAM20 with de-novo genotyping (deCODE,21 GerMIFS I-V,22 and OHGS/CCGB)23 | 14 703/38 817 | |
| Studies with de-novo genotyping in a central laboratory (BRAVE,† CCHS,13 CIHDS/CGPS,13 EPIC-CVD,14 and PROMIS)24 | 21 947/23 494 | |
| Ischaemic stroke | ||
| METASTROKE25 | 12 389/62 004 | Clinical with radiological confirmation, subtyping done with TOAST classification system |
| Abdominal aortic aneurysm | ||
| AAA Genetics Consortium26, 27, 28, 29, 30 | 4682/38 739 | Infrarenal aortic diameter of less than 30 mm, ascertained by ultrasonography or cross-sectional imaging, and patients who presented with acute rupture |
CHS=Cardiovascular Health Study. CCHS=Copenhagen City Heart Study. CGPS=Copenhagen General Population Study. EPIC-CVD=European Prospective Investigation into Cancer and Nutrition-Cardiovascular Disease study. ACR=American College of Rheumatology. DIAGRAM=Diabetes Genetics Replication and Meta-analysis consortium. ADA=American Diabetes Association. C4D=Coronary Artery Disease Genetics consortium. MI=myocardial infarction. CARDIoGRAM=transatlantic Coronary Artery Disease Genome-wide Replication and Meta-analysis consortium. deCODE=deCODE genetics coronary heart disease study. GerMIFS I-V=German Myocardial Infarction Family studies. OHGS=Ottawa Heart Genomics Study. CCGB=Cleveland Clinic GeneBank. BRAVE=Bangladesh Risk of Acute Vascular Events study. CIHDS=Copenhagen Ischaemic Heart Disease Study. PROMIS=Pakistan Risk of Myocardial Infarction Study. TOAST=Trial of Org 10172 in Acute Stroke Treatment. AAA=abdominal aortic aneurysm.
Data are n or cases/controls.
Chowdhury R, et al, unpublished.
Samples and data collection
To investigate the biological relevance of the genetic score, we investigated associations with mRNA concentrations of genes in the vicinity of rs6743376 and rs1542176. We accessed information from the Multiple Tissue Human Expression Resource, which contains information on adipose tissue, skin, and lymphoblastoid cell lines from 850 people, and from the Genotype-Tissue Expression project, which includes information on 13 different tissue types derived from 60–170 people.
In up to 63 442 participants, we quantified the effects of the genetic score on concentrations of IL-1Ra, IL-6, and CRP using data from the Cardiovascular Health Study, Copenhagen City Heart Study, Copenhagen General Population Study, European Prospective Investigation into Cancer and Nutrition-Cardiovascular Disease Study, SardiNIA study, and UK10K consortium (table, appendix pp 5–10, 21–22, and 29).
To compare the effects on inflammation biomarkers of IL-1Ra-raising alleles with those of anakinra, we did a systematic review of published randomised trials, including data for 1125 patients in eight trials (appendix pp 10, 38, 44). We calculated standardised treatment effects for anakinra doses of 75 mg or 100 mg (the most widely used doses in treatment of rheumatoid arthritis) on concentrations of inflammation biomarkers, and then pooled the results by fixed-effect inverse-variance weighted meta-analysis.
In 453 411 total participants, we investigated the genetic score in relation to rheumatoid arthritis and four cardiovascular disorders (type 2 diabetes, coronary heart disease, ischaemic stroke, and abdominal aortic aneurysm). For each disorder, we sought results from the largest available consortium. For rheumatoid arthritis, we accessed results from Okada and colleagues;15, 32 for type 2 diabetes, from the Diabetes Genetics Replication and Meta-analysis consortium and European Prospective Investigation into Cancer and Nutrition-InterAct;16, 17, 18 for abdominal aortic aneurysm, from the Abdominal Aortic Aneurysm Genetics Consortium;26, 27, 28, 29, 30 and ischaemic stroke, from the METASTROKE consortium (appendix pp 26 and 29).25
For coronary heart disease, we had access to study-level data for 70 532 patients and 126 374 controls. For 98 961 of these participants (36 650 patients and 62 311 controls), we did de-novo genotyping of rs6743376 and rs1542176 (table and appendix pp 5–8). We did genotyping using customised arrays in a central laboratory by technicians masked to the phenotypic status of the participants' samples for the following five studies contributing participant-level data: the Bangladesh Risk of Acute Vascular Events Study, Copenhagen City Heart Study, Copenhagen Ischaemic Heart Disease/Copenhagen General Population Study, European Prospective Investigation into Cancer and Nutrition-Cardiovascular Disease Study, and Pakistan Risk of Myocardial Infarction Study (appendix pp 9 and 23–25). Similar methods were used in three studies that did de-novo genotyping in local laboratories (deCODE, the German Myocardial Infarction Family Study, and the Ottawa Heart Genomics Study/Cleveland Clinic GeneBank). We supplemented genotyping with existing tabular data from the transatlantic Coronary Artery Disease Genome-wide Replication and Meta-analysis and Coronary Artery Disease Genetics consortia,19, 20 which enabled us to ascertain cases and controls within each allele count category of the genetic score (appendix p 12). About 90% of patients had myocardial infarction or other major acute coronary events; the remainder had angiographic evidence alone (eg, >50% coronary stenosis; appendix pp 35–36).
In up to 116 937 participants, we did exploratory analyses of the genetic score in relation to different disease traits to gain insight into mechanisms that might link IL-1α/β signalling with cardiometabolic disorders. We first examined 18 conventional cardiometabolic risk factors (total cholesterol, HDL cholesterol, triglycerides, LDL cholesterol,33, 34 apolipoprotein A1 and B, lipoprotein[a], systolic and diastolic blood pressure, fasting glucose,35 HbA1c,36 fasting insulin,35 2 h glucose,37 fasting proinsulin,38 height, BMI, waist circumference, and waist to hip ratio).39, 40 For each trait, we sought results from the largest available consortium (appendix p 33), supplemented with data from de-novo genotyping from the five studies mentioned previously providing participant-level data (appendix pp 5–8 and 21–22). When we noted suggestive associations between the genetic score and specific proatherogenic lipid concentrations, we extended this exploration to further traits, including metabolic profiles (eg, nuclear magnetic resonance spectroscopy metabolomics), subclinical cardiovascular phenotypes (eg, carotid intima-media thickness), and inflammatory cell subsets (eg, regulatory CD4 T-cell count). Appendix pp 33–34 provide a full account of the biological traits that we explored.
In a total of 205 329 patients and 423 905 controls, we did exploratory analyses of the genetic score in relation to 24 additional disorders of proposed relevance to IL-1 signalling, including autoimmune, degenerative, neoplastic, and infectious diseases. Again, we sought results from the largest available disease-specific consortia. Appendix pp 13–15, 27–28, and 30–34 provide a full account of the disorders that we explored.
Statistical analysis
We constructed a genetic score for IL-1 inhibition by counting the number of IL-1Ra-increasing alleles—ie, the C-alleles at rs6743376 and rs1542176 (appendix p 2). Our objective was to investigate the relevance of a biologically meaningful genetic score to eight prespecified outcomes—ie, three soluble inflammation biomarkers, rheumatoid arthritis, and four cardiometabolic disorders. To reduce the possibility of artifactual results and enable analysis of additional data, we constructed an alternative score consisting of two other IL-1Ra-increasing alleles (rs6759676 and rs4251961, which are each correlated with one of the variants used in the main score) that were identified in a separate genome-wide association study of IL-1Ra concentration41 (appendix p 3). We made allowances for study of eight main outcomes by using a Bonferroni-corrected significance threshold guideline of p=0·006 (ie, 0·05/8). For exploratory analyses (figure 1), we used as guidelines to help interpretation: p=0·003 (0·05/18) for analyses of 18 conventional cardiovascular risk factors and p=0·002 (0·05/24) for analyses of the additional 24 disorders. We could not use data from gene arrays that did not have information on both rs6743376 and rs1542176, or suitable proxies (eg, CardioMetabochip, Immunochip, ITMAT-Broad-CARE, or Exome array [Illumina, California, USA]). We included information on participants of European or south Asian ancestry, but not on east Asians (rs6743376 and rs1542176 are correlated variants in east Asians, preventing creation of an appropriate genetic score; appendix pp 3 and 42).
To analyse summary-level data from consortia, we did a fixed-effect meta-analysis of the separate effects of rs6743376 and rs1542176 because these two variants are independent in European and south Asian ancestry populations (r2=0·00; D'=0·03 in 1000 Genomes).42 In an analysis of available individual participant data, we natural log transformed values of inflammation biomarkers and other continuous traits that had skewed distributions. We regressed standardised trait values on the genetic score, adjusting for age, sex, and ancestry-informative principal components. To help comparisons across markers, we primarily expressed associations as SD differences in concentrations and, secondarily, as percentage differences. For loge-transformed variables, we obtained percentage changes as the exponent of the pooled loge-transformed differences. For non-loge-transformed variables, we obtained percentage change with reference to the pooled mean of each variable across studies. For estimates derived from consortia, the mean value and SD corresponded to that of the largest study. For all analyses, we used complete participant analysis—ie, we excluded participants with missing data.
To assess associations of the genetic score with dichotomous disease outcomes, we used logistic regression models that adjusted for age, sex, and ancestry-informative principal components. In the analysis of tabular data, we used logistic regression models to estimate the per-allele odds ratio, or calculated odds ratios within each score category compared with the reference category. We assessed dose–response relations for the genetic score with IL-1Ra concentrations or coronary heart disease risk, irrespective of an arbitrarily chosen reference group by attributing a floating variance estimate to each category, including the reference group, based on Plummer's method.43 We did analyses separately by study, and pooled β coefficients across studies using fixed-effect inverse-variance-weighted meta-analysis. We assessed heterogeneity with the I2 statistic. To test for deviation from a linear dose–response association of the genetic score with coronary heart disease risk, we compared the fit of models that assumed a linear trend of the genetic score with those that made no assumption about the shape of the association, using a likelihood ratio test. We used Stata 13.1 for statistical analyses.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. DF and JD had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
The frequency of IL-1Ra-raising alleles was about 30% for rs6743376 and 50% for rs1542176. Analysis of the Encyclopedia of DNA Elements (ENCODE) suggested that the locus around rs6743376 and rs1542176 is a gene-regulatory region (appendix p 42). In the Multiple Tissue Human Expression Resource, we noted significant associations between the genetic score and IL1RN mRNA concentrations in subcutaneous adipose tissue (p=3·9 × 10−6) and lymphoblastoid cell lines (p=1·7 × 10−4; appendix pp 20 and 42–43). However, in these tissues (and in about ten further tissues we studied in the Genotype-Tissue Expression project; appendix p 4), we did not note significant associations between this score and mRNA concentrations of other genes in this region (data not shown).
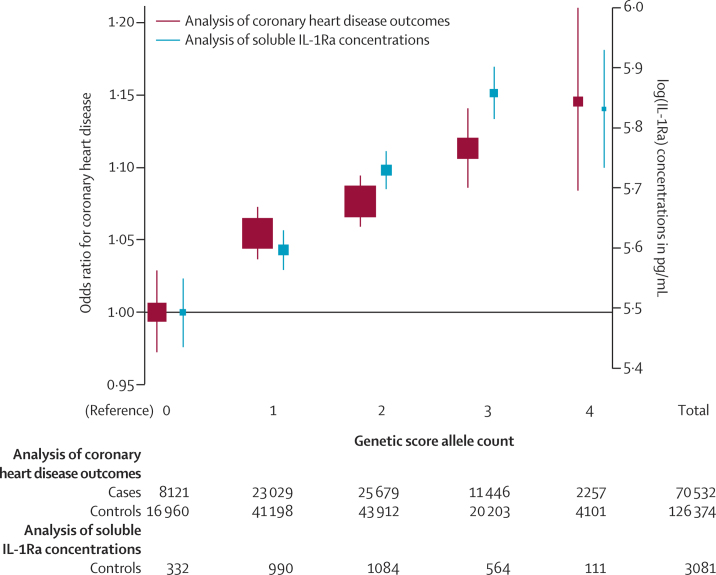
We noted a roughly log-linear, dose-dependent association of the genetic score with IL-1Ra concentration (figure 2; appendix p 43). For each IL1RN C-allele inherited, IL-1Ra concentrations increased by 0·22 SD (95% CI 0·18 to 0·25; 12·5%; p=9·3 × 10−33), IL-6 concentrations decreased by 0·02 SD (−0·04 to −0·01; −1·7%; p=3·5 × 10−3), and CRP concentrations decreased by 0·03 SD (−0·04 to −0·02; −3·4%; p=7·7 × 10−14; figure 3). The effects of the genetic score on these inflammation biomarkers were directionally concordant with those observed in anakinra trials. However, the absolute per-allele effects of the genetic score were much weaker than the effects of anakinra. Per-allele odds ratios with the score were 0·97 (0·95–0·99; p=9·9 × 10−4) for rheumatoid arthritis, 0·99 (0·97–1·01; p=0·47) for type 2 diabetes, 1·03 (1·02–1·04; p=3·9 × 10−10) for coronary heart disease, 1·00 (0·98–1·02; p=0·92) for ischaemic stroke, and 1·08 (1·04–1·12; p=1·8 × 10−5) for abdominal aortic aneurysm (figure 4).
Figure 2.
Relation of the genetic score allele count with soluble IL-1Ra concentrations and risk of coronary heart disease
Box sizes correspond to the number of coronary heart disease cases or participants with IL-1Ra concentration measurements contributing to analyses. Error bars represent 95% CIs, calculated with the method of Plummer.43 Estimates are derived from fixed-effect meta-analyses of study-specific estimates. Contributing studies are listed in the appendix pp 35–36. The underlying study-specific results used to construct this figure are provided in the appendix p 48, which includes study-specific tabulations of cases and controls within each score category, study-specific odds ratios, and the pooling of these results across contributing studies. IL-1Ra=interleukin 1 receptor antagonist.
Figure 3.
Effects on inflammation biomarkers of the genetic score compared with administration of 75 mg or 100 mg anakinra in eight randomised trials
Error bars show 95% CIs. To enable comparison of the magnitude of associations across several different markers, we did analyses with standardised units of measurement for each marker. Associations are presented as per 75 mg or 100 mg dose of anakinra compared with placebo, or per-allele change in the biomarker expressed as SDs. Study descriptions, individual study estimates, and meta-analysis results are provided for trial results in the appendix pp 16, 38, and 45. Genetic analyses are also provided in the appendix p 46. CRP=C-reactive protein. IL-1Ra=interleukin 1 receptor antagonist. IL-6=interleukin 6.
Figure 4.
Associations of the genetic score with rheumatoid arthritis and four cardiometabolic disorders
Box sizes correspond to the number of cases contributing to analyses. Error bars show 95% CI. Summary statistics for individual single-nucleotide polymorphisms in the genetic score are provided in the appendix p 56. Coronary heart disease analyses are based on fixed-effects meta-analyses of study-level tabular data, assuming a per-allele model. Individual study estimates and meta-analysis results are provided in the appendix p 47. The I2 value for between-study heterogeneity for coronary heart disease analyses was 28·2% (p=0·113). For the other endpoints, however, a measure of between-study heterogeneity could not be calculated because each of the odds ratios derive from a single result provided by a consortium. Analyses of ischaemic stroke subtypes are provided in the appendix p 52. IL-1=interleukin 1. NA=not applicable. *Contributing data include, in part, rs6761276[T] as a proxy for rs6743376[C].
In subsidiary analyses, we noted that rs6743376 and rs1542176 each had similar-sized effects, both on IL-1Ra concentration and coronary heart disease risk (appendix pp 46–47). We used an alternative score described in the methods section consisting of two further SNPs (rs6759676 and rs4251961, which each are correlated with one of the SNPs used in our main score) (appendix pp 17 and 50–51). Per-allele odds ratios with the alternative score were similar to those in the primary score for rheumatoid arthritis (odds ratio 0·96 [0·94–0·98; p=4·7 × 10−4]), type 2 diabetes (1·00 [0·98–1·01; p=0·53]), coronary heart disease (1·03 [1·02–1·04; p=3·7 × 10−8]), and abdominal aortic aneurysm (1·04 [1·01–1·08; p=0·011]; appendix pp 50–51).
We noted a roughly log-linear and dose-dependent association between the score and coronary heart disease risk (figure 2), with no evidence to support the existence of a non-linear association (p=0·59). For the 3% of people who carried four IL-1Ra-raising alleles, the odds ratio for coronary heart disease was 1·15 (1·08–1·22; p=1·8 × 10−6). Results were similar in analyses restricted to studies of myocardial infarction (appendix p 49). We did not attempt dose–response analyses for other main outcomes studied because we did not have sufficient power.
We noted that, per allele, the genetic score was associated with a 0·016 SD (0·009–0·022) increase (0·3%; p=5·8 × 10−7) in total cholesterol concentration, a 0·014 SD (0·007–0·022) increase in LDL cholesterol concentration (0·3%; p=9·5 × 10−5), and a 0·009 SD (0·003–0·015) increase in triglyceride concentration (0·4%; p=2·6 × 10−3; figure 5; appendix p 18). To estimate how much of the association we observed between the genetic score and coronary heart disease could be accounted for by LDL cholesterol concentration, we estimated the causal effect of life-long change in LDL cholesterol concentration in two ways. First, our genetic estimation used published reports that identified variants that were robustly and exclusively associated with LDL cholesterol concentrations.44 Second, our phenotypic estimation used individual participant data for long-term average serum lipid concentrations from 302 430 participants in the Emerging Risk Factors Collaboration.45 These complementary approaches yielded broadly concordant findings, suggesting that LDL cholesterol concentration could account for 20–40% of the association we observed between the score and risk of coronary heart disease (appendix pp 18–19).
Figure 5.
Associations of the genetic score with cardiovascular and metabolic risk factors
Box sizes correspond to the number of participants contributing to analyses. Error bars represent 95% CIs. To enable comparison of the magnitude of associations across several different traits, we did analyses with standardised units of measurement for each trait. Associations are presented as per-allele changes in the traits expressed as SDs. Further information on contributing studies and a wider range of risk factors and biomarkers than shown in the figure is provided in the appendix pp 17–18, 21–22, 33–34, 37, and 53. The I2 value shows between-study heterogeneity. For LDL cholesterol, insulin, 2 h glucose, and proinsulin, estimates are derived from summary results from one single consortium for which heterogeneity statistics are not available. For most cohorts (about which relevant data were available), analyses of lipids and blood pressure excluded individuals taking lipid-lowering or blood pressure-lowering drugs. Analyses of glycaemic traits excluded individuals with known diabetes. NA=not applicable. *Contributing data include, in part, data on rs6761276[T] as a proxy for rs6743376[C]. †Not applicable because effect estimates derive from a single result.
We did not observe clear evidence of associations between the score and apolipoprotein B concentration, perhaps because available data on apolipoprotein B were only about a fifth as great as those for total cholesterol. Similarly, we did not observe clear evidence of associations between the score and several glycaemic traits. Perhaps because we generally had low power to study some traits, we did not note any clear associations of the genetic score with a range of proatherogenic lipid subclasses, metabolites, and other intermediate traits (eg, carotid intima-media thickness, presence of carotid plaque, or carotid-femoral pulse wave velocity; appendix p 37). For these traits, we typically analysed data for about a tenth of the number of participants we studied for total cholesterol.
We did not observe clear evidence of associations between the score and any of the 24 additional disorders that we studied, including a range of autoimmune, neoplastic, degenerative, and infectious disease outcomes. However, we noted substantial variation in the amount of available data (and, hence, power to detect associations) across the different disorders that we explored (appendix pp 54–56).
Discussion
Drugs such as anakinra that produce dual IL-1α/β inhibition are licensed for treatment of inflammatory disorders, but the long-term effects of such treatment on cardiovascular and other outcomes remain unknown (panel). Our powerful and multilayered analysis of human genetic data has suggested the surprising conclusion that sustained dual IL-1α/β inhibition could increase the risk of cardiovascular diseases, in part via increased proatherogenic lipids. These findings provide new insights into the clinical and biological effects of IL-1α/β signalling.
Panel. Research in context.
Systematic review
We searched Medline for randomised clinical trials published before Feb 18, 2014, without any language restrictions, by combining keywords related to IL-1α/β-blocking drugs and randomised clinical trials—eg, “anakinra”, “rilonacept”, “IL-1R1”, and “AMG108” (the full search strategy is provided in the appendix p 10). We identified 29 randomised, placebo-controlled trials of dual IL-1α/β inhibitors, such as anakinra, which have mainly been done in patients with rheumatoid arthritis and other inflammatory disorders. 22 of these trials reported on serious adverse events, but none specifically assessed cardiovascular safety. Investigators of only a few small trials46, 47, 48 have reported on glycosylated haemoglobin values and other metabolic measures, and they have reported conflicting results. Neither of two small trials of anakinra46, 47 have reported increases in proatherogenic lipid concentrations, but they did not report numerical results.
Interpretation
Our powerful and multilayered analysis of human genetic data has suggested the surprising conclusion that long-term IL-1α/b inhibition could increase the risk of cardiovascular diseases, in part, via increased proatherogenic lipids. These findings provide new insights into the clinical and biological effects of IL-1α/β signalling, and could have implications for patients taking anakinra and related drugs.
We anchored our investigation in a genetic score that was associated with upregulation of IL-1Ra and that produced effects on soluble biomarkers consistent with IL-1α/β inhibition. The score's biological relevance was suggested by its exclusive association with IL1RN mRNA concentrations in two tissues, by its roughly log-linear dose–response relation with soluble IL-1Ra concentration, and by its effects on soluble inflammation biomarkers that were directionally concordant with those of anakinra. We studied this genetic score in relation to rheumatoid arthritis, a disorder already treated with anakinra. We noted that our score was associated with a decreased risk of rheumatoid arthritis, a new finding that reinforces the relevance of our score to the known clinical effects of IL-1α/β inhibition. This finding raises the possibility that long-term IL-1α/β inhibition could prevent (or at least delay) development of rheumatoid arthritis. Moreover, this finding provides a further example of the overlap noted between targets related to genes implicated in rheumatoid arthritis and treatments already approved for it.15
Contrary to expectation, however, several of our findings suggest that long-term IL-1α/β inhibition could increase the risk of cardiovascular diseases. First, our genetic score was associated with an increased risk of coronary heart disease in a roughly log-linear and dose-dependent manner, analogous to the association we observed between the same score and IL-1Ra concentration. However, even taken together, investigators of trials of anakinra have recorded fewer than 40 coronary heart disease outcomes (appendix pp 16 and 39–40), and none have specifically addressed cardiovascular safety. Thus, we could not compare our results on cardiovascular risk with those from randomised trials.
Second, we noted potential associations between the genetic score and increased concentrations of LDL cholesterol and triglycerides. Our modelling analysis suggested that such associations could explain about a third of the observed association between the score and increased coronary heart disease risk.45, 49 However, we were again unable to compare our findings meaningfully with results from relevant randomised trials. Findings from two small anakinra trials (together consisting of fewer than 100 participants)46, 47 showed no significant increases in proatherogenic lipid concentrations, but the magnitude of lipid increases detectable in these trials is unknown because the studies did not report numerical results. By contrast, investigators of a trial of canakinumab (a selective IL-1β inhibitor) in about 500 participants50 reported significant elevations in triglyceride (but not LDL cholesterol) concentrations. However, the relevance of our genetic score to canakinumab is uncertain because although our score should mimic the effects of dual IL-1α/β inhibition, it does not necessarily mirror selective IL-1β inhibition. Indeed, we note that suitable genetic scores do not exist that distinguish the effects of long-term inhibition of IL-1α from those of long-term inhibition of IL-1β, and the two cytokines seem distinct and non-redundant.6, 51 Hence, our results do not necessarily have implications for trials such as the Canakinumab Anti-inflammatory Thrombosis Outcome Study, which is designed to test selective IL-1β inhibition in secondary prevention of cardiovascular disease.4, 52
Third, our genetic score was associated with increased risk of abdominal aortic aneurysm. Whereas our findings show that inhibition of IL-1α/β signalling could increase the risk of coronary heart disease and abdominal aortic aneurysm, findings from previous human genetic studies (including our own) have shown that inhibition of IL-6 signalling could actually reduce the risk of these disorders.53, 54, 55 Together, these findings highlight the complexity of inflammatory pathways underlying cardiovascular diseases. Studies are needed to help to understand mechanisms that account for the divergent effects of these two interrelated inflammation pathways.
Our findings also challenge studies in animals that have reported reductions in atherosclerosis, or slowing of aneurysm growth, after pharmacological dual IL-1α/β inhibition or genetic deletion of components of the IL-1α/β system.56, 57 For example, findings from some studies of mice without the IL-1 receptor have suggested a protective role of IL-1α/β signalling in atherosclerosis.58 The contrast between most previous animal studies and the results from our study of human genetic data might be due, at least in part, to the limited ability of model organisms to fully represent the human immune system and human cardiovascular diseases.59
Fourth, we noted no evidence of associations between our genetic score and the risk of type 2 diabetes, insulin sensitivity, other glycaemic traits, blood pressure, or adiposity. By comparison, investigators of three small randomised trials of anakinra (collectively comprising about 150 participants)46, 47, 48 reported conflicting results in relation to effects on HbA1c values. Furthermore, findings from a trial of canakinumab in about 500 participants did not show significant reductions in HbA1c values.50 Nevertheless, our genetic study had substantially less power to assess type 2 diabetes and measures of glycaemia than it did to assess coronary heart disease and proatherogenic lipids. Hence, although our results suggest that long-term IL-1α/β inhibition is unlikely to prevent type 2 diabetes or improve metabolic features associated with the disease,46, 47 further studies might be needed to assess any moderate effects. Similar considerations apply to the null association between our genetic score and the risk of ischaemic stroke.
Our study had major strengths and potential limitations. One strength was that we used a prespecified analysis plan. We also replicated our main findings using an alternative genetic score that contained different variants from those used in our main score. We accessed results from about 1 million people in worldwide consortia of relevant diseases and traits, which we supplemented with data from de-novo genotyping in nearly a further 100 000 participants. Because we showed that our genetic score was exclusively associated with IL1RN mRNA concentrations in adipose tissue and lymphoblastoid cell lines, the associations that we observed of the genetic score with cardiovascular diseases and traits were unlikely to be driven by neighbouring genes or variants. However, we did not have access to data for other potentially relevant tissues (eg, primary leucocytes or hepatocytes). Because our genetic score should provide information about the effects of lifelong IL-1α/β inhibition, reduced IL-1α/β signalling from early life could lead to compensatory changes that affect cardiovascular risk. However, our genetic score was associated with reduced concentrations of both IL-6 and CRP in adults, consistent with the expected downstream effects of uncompensated IL-1 inhibition.
One limitation of our study is that its findings can suggest only qualitative concordance of the effects on inflammation biomarkers of our genetic score and anakinra. Genetic and pharmacological IL-1 inhibition differ with respect to the magnitude and duration of inhibition, shown by the 5–10-times weaker effects that we observed of our genetic score on inflammation biomarkers compared with those of anakinra. Few people in randomised trials of anakinra have had IL-1Ra concentrations measured. In addition, whereas anakinra has mainly been studied in trials of people with pre-existing inflammatory disorders, we related our genetic score to inflammation biomarkers mainly in healthy people.
For the aforementioned reasons, the data in this report are difficult to use to estimate the magnitude of potential cardiovascular hazard associated with dual IL-1α/β inhibition. Nevertheless, the robust but moderate associations that we identified in this study between genetic IL-1α/β inhibition and cardiovascular risk do not preclude a substantial clinical effect because the size of an odds ratio conferred by natural variation in a particular gene bears no necessary relation to the size of hazard or benefit that might accrue from intervention directed at the pathway that the gene identifies.10 For example, statins confer substantial reductions in cardiovascular risk, despite slight associations between common variants in genes that are the target of statins (LDLR and HMGCR) and coronary heart disease.9 In summary, our study—which has introduced the concept of use of a wide-angle genetic approach to predict the broad phenotypic effects of perturbation of a biological pathway—has provided new insights into the clinical and biological effects of dual IL-1α/β signalling in relation to several cardiometabolic disorders and disease traits.
Correspondence to: Dr Daniel Freitag or Prof John Danesh, The Interleukin 1 Genetics Consortium Coordinating Centre, Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge CB1 8RN, UK IL1GC@medschl.cam.ac.uk
For the Diabetes Genetics Replication and Meta-analysis consortium see http://diagram-consortium.org
This online publication has been corrected. The corrected version first appeared at thelancet.com/diabetes-endocrinology on May 21, 2014
Acknowledgments
Acknowledgments
This work was funded by the UK Medical Research Council (G0800270), British Heart Foundation (SP/09/002), UK National Institute for Health Research Cambridge Biomedical Research Centre, European Research Council (268834), and European Commission Framework Programme 7 (HEALTH-F2-2012-279233). Funding for the component studies in this analysis is provided in the appendix. Murray Clarke, Philip De Jager, Peter Libby, Ziad Mallat, Nadeem Sarwar, and John Todd provided helpful comments on an earlier version of the manuscript. Collaborators are listed in the appendix pp 65–86. A full set of acknowledgments is provided in the appendix pp 87–96.
Contributors
The writing committee was composed of DFF, ASB, PW, JMMH, SBu, SKap, AMW, MSw, JRS, AR, DCr, BGN, DS, SGT, and JD. DFF, ASB, PW, SBu, SKap, SGT, and JD contributed to the study concept and design. PG, MJB, GTJ, AvR, DRC, MA-K, GFM, IBW, CDL, LB, SW, AF, SAL, MM-N, SDT, EZ, AS, HH, DFE, TE, HG, RSH, SN, SSa, NS, HSM, NJW, DJR, MR, TA, TBH, VG, AH, OHF, RT, BMP, MF, HW, ASH, NJS, WM, RCl, RCo, JSK, JCC, SKat, RM, JE, AK, HS, KS, UT, JDW, AT-H, DSA, AM, EDA, RCh, BGN, DS, and JD recruited and characterised participants. ASB, JMMH, RY, WHK, SSp, SFN, LAL, MJB, GTJ, RAS, SBe, PIWdB, and JE worked in the laboratory and managed data. DFF, PW, JMMH, SKap, RY, WHK, EH, SFN, LAL, MJB, GTJ, RAS, SBe, EP, GTh, TK, LZ, MN, RD, WZ, JCH, MK, GED, CPN, AG, JCB, AD, SL, AVS, LQ, AFB, FNGvH, GTr, HK, MDR, SSV, DCC, JM, MdA, IJK, PLP, CAM, EPB, OG, DRC, DSC, LJR, JAP, JH, NJT, MA-K, JK, GFM, AP, MG, YLi, NF, MFK, SKG, CDL, LB, MAB, DME, SBa, MAF, HB, SAL, MM-N, JFF, NLS, MSu, EZ, KP, MAN, AS, CP, JPB, HH, DFE, DT, IPT, MD, KH, GM, TE, HG, JMA, MH, NW, YW, DCh, RSH, MMI, DTB, MHL, NKH, YLu, SN, and MB contributed results. DFF, ASB, JMMH, SBu, SKap, and SGT were responsible for data and statistical analyses. DFF, ASB, PW, JMMH, SBu, SKap, AMW, MSw, JRS, AR, DCr, BGN, DS, SGT, and JD drafted the manuscript. All authors contributed to, read, and approved the final version of the report.
The Interleukin 1 Genetics Consortium
Daniel F Freitag PhD, Adam S Butterworth PhD, Peter Willeit MD, Joanna M M Howson PhD, Stephen Burgess PhD, Stephen Kaptoge PhD, Robin Young PhD, Weang Kee Ho PhD, Angela M Wood PhD, Michael Sweeting PhD, Sarah Spackman MMath, James R Staley MSc, Anna Ramond DPharm, Eric Harshfield MPH (University of Cambridge, Cambridge, UK); Sune F Nielsen PhD, Peer Grande MD (Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark); Leslie A Lange PhD (University of North Carolina, Chapel Hill, NC, USA); Matthew J Bown FRCS (University of Leicester and National Institute for Health Research Leicester Cardiovascular Biomedical Research Unit, Leicester, UK); Gregory T Jones PhD (University of Otago, Dunedin, New Zealand); Robert A Scott PhD (Medical Research Council Epidemiology Unit, Cambridge, UK); Steve Bevan PhD (University of Cambridge, Cambridge, UK); Eleonora Porcu PhD (Istituto di Ricerca Genetica e Biomedica, Monserrato, Italy); Gudmar Thorleifsson PhD (deCODE Genetics/Amgen, Reykjavik, Iceland); Lingyao Zeng MSc, Thorsten Kessler MD (Deutsches Herzzentrum München, Technische Universität München, and German Centre for Cardiovascular Research, partner site Munich Heart Alliance, Munich, Germany); Majid Nikpay PhD (Ruddy Canadian Cardiovascular Genetics Centre, University of Ottawa Heart Institute, Ottawa, Canada); Ron Do PhD (Broad Institute, Cambridge and Massachusetts General Hospital, Boston, MA, USA); Weihua Zhang PhD (Imperial College, London, UK); Jemma C Hopewell PhD (University of Oxford, Oxford, UK); Marcus Kleber PhD, Graciela E Delgado MSc (University of Heidelberg, Heidelberg, Germany); Christopher P Nelson PhD (University of Leicester and National Institute for Health Research Leicester Cardiovascular Biomedical Research Unit, Leicester, UK); Anuj Goel MSc (University of Oxford, Oxford, UK); Joshua C Bis PhD (University of Washington, Seattle, WA, USA); Abbas Dehghan PhD, Symen Ligthart MD (Erasmus Medical Center, University Medical Center Rotterdam, Rotterdam, Netherlands); Albert V Smith PhD (Icelandic Heart Association, Kópavogur, Iceland, and University of Iceland, Reykjavík, Iceland); Liming Qu MS (University of Pennsylvania, Philadelphia, PA, USA); Femke NG van 't Hof MD, Paul I W de Bakker PhD, Annette F Baas PhD (University Medical Center Utrecht, Utrecht, Netherlands); Andre van Rij FRACS (University of Otago, Dunedin, New Zealand); Gerard Tromp PhD, Helena Kuivaniemi PhD (Sigfried and Janet Weis Center for Research, Geisinger Health System, Danville, PA, USA); Marylyn D Ritchie PhD, Shefali S Verma MS (Pennsylvania State University, University Park, PA, USA); Dana C Crawford PhD (Case Western Reserve University, Cleveland, OH, USA); Jennifer Malinowski PhD (Yale University, New Haven, CT, USA); Mariza de Andrade PhD, Iftikhar J Kullo MD (Mayo Clinic, Rochester, MN, USA); Peggy L Peissig PhD (Marshfield Clinic Research Foundation, Marshfield, WI, USA); Catherine A McCarty PhD (Research Division Essentia Institute of Rural Health, Duluth, MN, USA); Erwin P Böttinger MD, Omri Gottesman MD (Icahn School of Medicine Mount Sinai, New York, NY, USA); David R Crosslin PhD (University of Washington, Seattle, WA, USA); David S Carrell PhD (Group Health Research Institute, Seattle, WA, USA); Laura J Rasmussen-Torvik PhD, Jennifer A Pacheco BA (Northwestern University Feinberg School of Medicine, Chicago, IL, USA); European Prospective Investigation into Cancer and Nutrition Cardiovascular Disease study consortium; Aneurysm Consortium; Electronic Medical Records and Genomics Network; Jie Huang MD (Wellcome Trust Sanger Institute, Hinxton, Cambridge, UK); Nicholas J Timpson PhD (Medical Research Council Integrative Epidemiology Unit, University of Bristol, Bristol, UK); UK 10K consortium; European Prospective Investigation into Cancer and nutrition-InterAct consortium; METASTROKE consortium; Johannes Kettunen PhD (University of Oulu, Oulu, Finland, and National Institute for Health and Welfare, Helsinki, Finland); Mika Ala-Korpela PhD (University of Oulu, Oulu, Finland, and University of Bristol, Bristol, UK); Gary F Mitchell MD (Cardiovascular Engineering, Norwood, MA, USA); Afshin Parsa MD (University of Maryland School of Medicine, Baltimore, MD, USA); Ian B Wilkinson FRCP (University of Cambridge, Cambridge, UK); Mathias Gorski Dipl.-Inf. (University of Regensburg, Regensburg, Germany); Yong Li MD (University Hospital Freiburg, Freiburg, Germany); Chronic Kidney Disease Genetics Consortium; Nora Franceschini MD (University of North Carolina, Chapel Hill, NC, USA); Margaux F Keller PhD (National Institute on Aging, National Institutes of Health, Bethesda, MD, USA); Santhi K Ganesh MD (University of Michigan, Ann Arbor, MI, USA); Cohorts for Heart and Aging Research in Genomic Epidemiology Haematology Working Group; Carl D Langefeld PhD (Wake Forest School of Medicine, Winston-Salem, NC, USA); Lucie Bruijn PhD (Amyotrophic Lateral Sclerosis Association, Washington, DC, USA); Matthew A Brown MD, David M Evans PhD (University of Queensland Diamantina Institute, Translational Research Institute, Princess Alexandra Hospital, Brisbane, Australia); Australo-Anglo-American Spondyloarthritis Consortium; Svetlana Baltic PhD (University of Western Australia, Perth, WA, Australia); Manuel A Ferreira PhD (Queensland Institute of Medical Research-Berghofer Medical Research Institute, Brisbane, QLD, Australia); Australian Asthma Genetics Consortium; Hansjörg Baurecht MSc, Stephan Weidinger MD (University Hospital Schleswig-Holstein, Kiel, Germany); Andre Franke PhD (Christian-Albrechts-University of Kiel, Kiel, Germany); Steven A Lubitz MD (Massachusetts General Hospital, Boston, MA, USA); Martina Müller-Nurasyid PhD (Institute of Genetic Epidemiology, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany, and Ludwig-Maximilians-University Munich, and German Centre for Cardiovascular Research, partner site Munich Heart Alliance, Munich, Germany); Atrial Fribrillation Genetics Consortium; Janine F Felix PhD (Erasmus Medical Center, University Medical Center Rotterdam, Rotterdam, Netherlands); Nicholas L Smith PhD (University of Washington, Seattle, WA, USA); Cohorts for Heart and Aging Research in Genomic Epidemiology Heart Failure Working Group; Marc Sudman BA, Susan D Thompson PhD (Cincinnati Children's Hospital Medical Center, Cincinnati, OH, USA); Consortium for Juvenile Arthritis Genetics; Eleftheria Zeggini PhD, Kalliope Panoutsopoulou PhD (Wellcome Trust Sanger Institute, Hinxton, Cambridge, UK); Arthritis Research UK Osteoarthritis Genetics consortium; Mike A Nalls PhD, Andrew Singleton PhD (National Institute on Aging, National Institutes of Health, Bethesda, MD, USA); International Parkinson's Disease Consortium; Constantin Polychronakos MD (McGill University Health Centre, Montreal, QC, Canada); Jonathan P Bradfield PhD, Hakon Hakonarson MD (Children's Hospital Philadelphia, Philadelphia, PA, USA); Douglas F Easton PhD, Deborah Thompson PhD (University of Cambridge, Cambridge, UK); Breast Cancer Association Consortium; Ian P Tomlinson MD (Wellcome Trust Centre for Human Genetics, Oxford, UK); Malcolm Dunlop FMedSci (University of Edinburgh, and Medical Research Council Human Genetics Unit, Edinburgh, UK); Kari Hemminki MD (German Cancer Research Centre, Heidelberg, Germany, and Lund University, Malmö, Sweden); Gareth Morgan FRCP (University of Arkansas for Medical Sciences, Little Rock, AR, USA); Timothy Eisen FRCP (Cambridge University Health Partners, Cambridge, UK); Hartmut Goldschmidt MD (Heidelberg University, and National Center for Tumor Diseases, Heidelberg, Germany); James M Allan PhD (Northern Institute for Cancer Research, Newcastle University, Newcastle upon Tyne, UK); Marc Henrion PhD, Nicola Whiffin MA, Yufei Wang PhD, Daniel Chubb PhD (Institute of Cancer Research, Sutton, UK), Richard S Houlston FMedSci (Institute of Cancer Research, London, UK); Mark M Iles PhD, D Timothy Bishop PhD (Leeds Cancer Research UK Centre, University of Leeds, Leeds, UK); Matthew H Law PhD, Nicholas K Hayward PhD (Queensland Institute of Medical Research-Berghofer Medical Research Institute, Brisbane, QLD, Australia); Melanoma Genetics consortium; Yang Luo PhD (Wellcome Trust Sanger Institute, Hinxton, Cambridge, UK); Sergey Nejentsev MD (University of Cambridge, Cambridge, UK); Maja Barbalic PhD (Human Genetics Center, University of Texas Health Science Center, Houston, TX, USA); Cohorts for Heart and Aging Research in Genomic Epidemiology inflammation working group; David Crossman FRCP (University of St Andrews, St Andrews, UK); Serena Sanna PhD (Istituto di Ricerca Genetica e Biomedica, Monserrato, Italy); Nicole Soranzo PhD (Wellcome Trust Sanger Institute, Hinxton, Cambridge, UK); Hugh S Markus FRCP (University of Cambridge, Cambridge, UK); Nicholas J Wareham FRCP (Medical Research Council Epidemiology Unit, Cambridge, UK); Daniel J Rader MD, Muredach Reilly MD (Institute for Translational Medicine and Therapeutics, and Cardiovascular Institute, Perleman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA); Themistocles Assimes MD (Stanford University, Stanford, CA, USA); Tamara B Harris MD (Laboratory of Epidemiology and Population Sciences, National Institute on Aging, Bethesda, MD, USA); Albert Hofman MD, Oscar H Franco MD (Erasmus Medical Center, University Medical Center Rotterdam, Rotterdam, Netherlands); Vilmundur Gudnason MD (Icelandic Heart Association, Kópavogur, Iceland, and University of Iceland, Reykjavík, Iceland); Russell Tracy PhD (University of Vermont College of Medicine, Burlington, VT, USA); Bruce M Psaty MD (University of Washington, Seattle, WA, USA); Martin Farrall PhD, Hugh Watkins FMedSci (University of Oxford, Oxford, UK); Alistair S Hall FRCP (Leeds Institute of Genetics, Health and Therapeutics, University of Leeds, Leeds, UK); Nilesh J Samani FRCP (University of Leicester and National Institute for Health Research Leicester Cardiovascular Biomedical Research Unit, Leicester, UK); Winfried März MD (Synlab Academy, Synlab Services GmbH, Mannheim, Germany); Robert Clarke FRCP, Rory Collins FMedSci (University of Oxford, Oxford, UK); Jaspal S Kooner FRCP, John C Chambers PhD (Imperial College, London, UK); Myocardial Infarction Genetics Consortium; Sekar Kathiresan MD (Broad Institute, Cambridge and Massachusetts General Hospital, Boston, MA, USA); Ruth McPherson FRCP(C) (Ruddy Canadian Cardiovascular Genetics Centre, University of Ottawa Heart Institute, Ottawa, Canada); Jeanette Erdmann PhD (Institute for Integrative and Experimental Genomics, University of Lübeck, and German Research Centre for Cardiovascular Research, Lübeck, Germany); Adnan Kastrati MD, Heribert Schunkert MD (Deutsches Herzzentrum München, Technische Universität München, and German Centre for Cardiovascular Research, partner site Munich Heart Alliance, Munich, Germany); Kári Stefánsson MD, Unnur Thorsteinsdottir PhD (deCODE Genetics/Amgen, and University of Iceland, Reykjavik, Iceland); Jeremy D Walston MD (Johns Hopkins University School of Medicine, Baltimore, MD, USA); Anne Tybjærg-Hansen MD (Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark); Dewan S Alam PhD (Centre for Control of Chronic Diseases, International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, Bangladesh); Abdullah Al Shafi Majumder FRCP (National Institute of Cardiovascular Diseases, Dhaka, Bangladesh); Emanuele Di Angelantonio MD, Rajiv Chowdhury MD (University of Cambridge, Cambridge, UK); Børge G Nordestgaard MD (Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark); Danish Saleheen MD (University of Pennsylvania, Philadelphia, USA, and University of Cambridge, Cambridge, UK); Simon G Thompson FMedSci, and John Danesh FRCP (University of Cambridge, Cambridge, UK)
Declaration of interests
The work of DFF, ASB, SGT, JRS, AR, JMMH, SKap, AMW, SB, and JD has been supported by grants awarded to JD from the UK Medical Research Council, British Heart Foundation, UK National Institute for Health Research, National Institute for Health Research Cambridge Biomedical Research Centre, European Research Council, European Commission Framework Programme 7, US National Institutes of Health, Merck, Novartis, GlaxoSmithKline, and Pfizer. JD has received consulting fees from Merck, Novartis, GlaxoSmithKline, and Pfizer, and grants from Denka, diaDexus, Fogarty International Centre, Roche, UK Biobank, Merck, Pfizer, GlaxoSmithKline, and Novartis outside the submitted work. ASB reports grants from Pfizer, Merck, and Novartis, all outside of the submitted work. DCr has IL genetics patents related to detection and treatment of early onset of ageing-related disorders, and diagnostics for cardiovascular disorders. DCr has also received grants from Novartis for research outside of the submitted work. All other members of the writing committee declare no competing interests.
Supplementary Material
References
- 1.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- 3.Morton AC, Rothman AM, Greenwood JP. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur Heart J. 2014 doi: 10.1093/eurheartj/ehu272. published online July 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ridker PM, Luscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. 2014;35:1782–1791. doi: 10.1093/eurheartj/ehu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maedler K, Sergeev P, Ris F. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rader DJ. IL-1 and atherosclerosis: a murine twist to an evolving human story. J Clin Invest. 2012;122:27–30. doi: 10.1172/JCI61163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen SB, Moreland LW, Cush JJ. A multicentre, double blind, randomised, placebo controlled trial of anakinra (Kineret), a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Ann Rheum Dis. 2004;63:1062–1068. doi: 10.1136/ard.2003.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plenge RM, Scolnick EM, Altshuler D. Validating therapeutic targets through human genetics. Nat Rev Drug Discov. 2013;12:581–594. doi: 10.1038/nrd4051. [DOI] [PubMed] [Google Scholar]
- 10.Gregory AP, Dendrou CA, Attfield KE. TNF receptor 1 genetic risk mirrors outcome of anti-TNF therapy in multiple sclerosis. Nature. 2012;488:508–511. doi: 10.1038/nature11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matteini AM, Li J, Lange EM. Novel gene variants predict serum levels of the cytokines IL-18 and IL-1ra in older adults. Cytokine. 2014;65:10–16. doi: 10.1016/j.cyto.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naitza S, Porcu E, Steri M. A genome-wide association scan on the levels of markers of inflammation in Sardinians reveals associations that underpin its complex regulation. PLoS Genet. 2012;8:e1002480. doi: 10.1371/journal.pgen.1002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 14.Danesh J, Saracci R, Berglund G. EPIC-Heart: the cardiovascular component of a prospective study of nutritional, lifestyle and biological factors in 520,000 middle-aged participants from 10 European countries. Eur J Epidemiol. 2007;22:129–141. doi: 10.1007/s10654-006-9096-8. [DOI] [PubMed] [Google Scholar]
- 15.Okada Y, Wu D, Trynka G. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506:376–381. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris AP, Voight BF, Teslovich TM. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44:981–990. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voight BF, Scott LJ, Steinthorsdottir V. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010;42:579–589. doi: 10.1038/ng.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langenberg C, Sharp S, Forouhi NG. Design and cohort description of the InterAct Project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC Study. Diabetologia. 2011;54:2272–2282. doi: 10.1007/s00125-011-2182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peden JF, Hopewell JC, Saleheen D. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat Genet. 2011;43:339–344. doi: 10.1038/ng.782. [DOI] [PubMed] [Google Scholar]
- 20.Schunkert H, Konig IR, Kathiresan S. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helgadottir A, Thorleifsson G, Manolescu A. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 22.Erdmann J, Grosshennig A, Braund PS. New susceptibility locus for coronary artery disease on chromosome 3q22.3. Nat Genet. 2009;41:280–282. doi: 10.1038/ng.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies RW, Wells GA, Stewart AF. A genome-wide association study for coronary artery disease identifies a novel susceptibility locus in the major histocompatibility complex. Circ Cardiovasc Genet. 2012;5:217–225. doi: 10.1161/CIRCGENETICS.111.961243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saleheen D, Zaidi M, Rasheed A. The Pakistan Risk of Myocardial Infarction Study: a resource for the study of genetic, lifestyle and other determinants of myocardial infarction in south Asia. Eur J Epidemiol. 2009;24:329–338. doi: 10.1007/s10654-009-9334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Traylor M, Farrall M, Holliday EG. Genetic risk factors for ischaemic stroke and its subtypes (the METASTROKE collaboration): a meta-analysis of genome-wide association studies. Lancet Neurol. 2012;11:951–962. doi: 10.1016/S1474-4422(12)70234-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bown MJ, Jones GT, Harrison SC. Abdominal aortic aneurysm is associated with a variant in low-density lipoprotein receptor-related protein 1. Am J Hum Genet. 2011;89:619–627. doi: 10.1016/j.ajhg.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gretarsdottir S, Baas AF, Thorleifsson G. Genome-wide association study identifies a sequence variant within the DAB2IP gene conferring susceptibility to abdominal aortic aneurysm. Nat Genet. 2010;42:692–697. doi: 10.1038/ng.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones GT, Bown MJ, Gretarsdottir S. A sequence variant associated with sortilin-1 (SORT1) on 1p13·3 is independently associated with abdominal aortic aneurysm. Hum Mol Genet. 2013;22:2941–2947. doi: 10.1093/hmg/ddt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibamura H, Olson JM, van Vlijmen-Van KC. Genome scan for familial abdominal aortic aneurysm using sex and family history as covariates suggests genetic heterogeneity and identifies linkage to chromosome 19q13. Circulation. 2004;109:2103–2108. doi: 10.1161/01.CIR.0000127857.77161.A1. [DOI] [PubMed] [Google Scholar]
- 30.Gottesman O, Kuivaniemi H, Tromp G. The Electronic Medical Records and Genomics (eMERGE) Network: past, present, and future. Genet Med. 2013;15:761–771. doi: 10.1038/gim.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira RC, Freitag DF, Cutler AJ. Functional IL6R 358Ala allele impairs classical IL-6 receptor signaling and influences risk of diverse inflammatory diseases. PLoS Genet. 2013;9:e1003444. doi: 10.1371/journal.pgen.1003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Software and data sources used in Okada and colleagues http://plaza.umin.ac.jp/∼yokada/datasource/software.htm (accessed Feb 2, 2014).
- 33.Willer CJ, Schmidt EM, Sengupta S. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Global Lipids Genetics Consortium Results. http://www.sph.umich.edu/csg/abecasis/public/lipids2013 (accessed July 23, 2014). [DOI] [PMC free article] [PubMed]
- 35.Dupuis J, Langenberg C, Prokopenko I. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soranzo N, Sanna S, Wheeler E. Common variants at 10 genomic loci influence hemoglobin A(1)(C) levels via glycemic and nonglycemic pathways. Diabetes. 2010;59:3229–3239. doi: 10.2337/db10-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saxena R, Hivert MF, Langenberg C. Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet. 2010;42:142–148. doi: 10.1038/ng.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strawbridge RJ, Dupuis J, Prokopenko I. Genome-wide association identifies nine common variants associated with fasting proinsulin levels and provides new insights into the pathophysiology of type 2 diabetes. Diabetes. 2011;60:2624–2634. doi: 10.2337/db11-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Randall JC, Winkler TW, Kutalik Z. Sex-stratified genome-wide association studies including 270,000 individuals show sexual dimorphism in genetic loci for anthropometric traits. PLoS Genet. 2013;9:e1003500. doi: 10.1371/journal.pgen.1003500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Genetic Investigation of ANthropometric Traits (GIANT) consortium http://www.broadinstitute.org/collaboration/giant (accessed July 25, 2014).
- 41.Herder C, Nuotio ML, Shah S. Genetic determinants of circulating interleukin-1 receptor antagonist levels and their association with glycemic traits. Diabetes. 2014;63:4343–4359. doi: 10.2337/db14-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abecasis GR, Auton A, Brooks LD. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plummer M. Improved estimates of floating absolute risk. Stat Med. 2004;23:93–104. doi: 10.1002/sim.1485. [DOI] [PubMed] [Google Scholar]
- 44.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Angelantonio E, Sarwar N, Perry P. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsen CM, Faulenbach M, Vaag A. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 47.van Asseldonk EJ, Stienstra R, Koenen TB, Joosten LA, Netea MG, Tack CJ. Treatment with anakinra improves disposition index but not insulin sensitivity in nondiabetic subjects with the metabolic syndrome: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2011;96:2119–2126. doi: 10.1210/jc.2010-2992. [DOI] [PubMed] [Google Scholar]
- 48.Moran A, Bundy B, Becker DJ. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;38:1905–1915. doi: 10.1016/S0140-6736(13)60023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 50.Ridker PM, Howard CP, Walter V. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–2748. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 51.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 53.Sarwar N, Butterworth AS, Freitag DF. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–1213. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. Hingorani AD, Casas JP. Interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379:1214–1224. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harrison SC, Smith AJ, Jones GT. Interleukin-6 receptor pathways in abdominal aortic aneurysm. Eur Heart J. 2013;34:3707–3716. doi: 10.1093/eurheartj/ehs354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheedy FJ, Moore KJ. IL-1 signaling in atherosclerosis: sibling rivalry. Nat Immunol. 2013;14:1030–1032. doi: 10.1038/ni.2711. [DOI] [PubMed] [Google Scholar]
- 58.Alexander MR, Moehle CW, Johnson JL. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest. 2012;122:70–79. doi: 10.1172/JCI43713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seok J, Warren HS, Cuenca AG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.