Abstract
Stereospecific nucleophilic substitution at the C-4α position of 2α-chloro-2′(2′,6′)-(di)halogenopicropodophyllotoxin derivatives with carboxylic acids mediated by BF3·Et2O was described. Interestingly, this stereoselective products were completely controlled by the reaction time. That is, if the reaction time was prolonged to 24.5–31 h, the resulting compounds were all transformed into the unusual C-ring aromatization products. Additionally, it demonstrated that BF3·Et2O and reaction temperature were the important factors for C-ring aromatization, and AlCl3 could be substituted for BF3·Et2O as a lewis acid for C-ring aromatization. Halogenation of E-ring of 2β-chloropodophyllotoxins with NCS or NBS also led to the same C-ring aromatization compounds. Especially compounds 5c, 6g and 7b exhibited insecticidal activity equal to that of toosendanin.
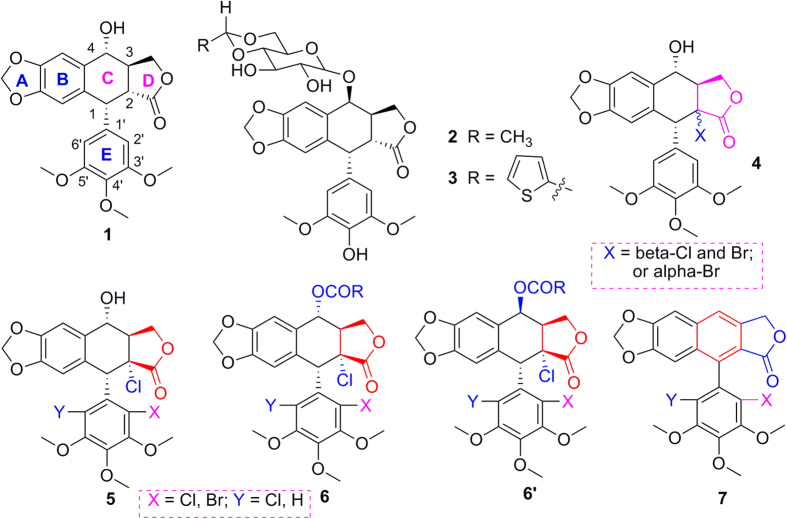
Podophyllotoxin (1, Fig. 1), an important naturally occurring cyclolignan isolated from the roots and rhizomes of Podophyllum species, has been widely used as a lead compound for preparation of potential anticancer agents such as etoposide (VP-16, 2, Fig. 1), teniposide (VM-26, 3, Fig. 1), etoposide phosphate, GL331 and TOP531,2. On the other hand, compound 1 also exhibited its interesting insecticidal and antifungal activities3,4,5. To find more potent podophyllotoxin derivatives, besides total synthesis of podophyllotoxin and its derivatives6,7,8,9,10,11, structural modifications of them have also attracted much attention in recent years12. To solve the instable trans-fused lactone of podophyllotoxin when exposed to mild base, a chlorine/bromine atom was introduced at its C-2α/β position to give 2α/β-halogenopodophyllotoxins (4, Fig. 1)13,14,15,16. More recently, we found that once a chlorine or bromine atom was firstly introduced at the C-2′ position on the E-ring of podophyllotoxin, 2α-chloro-2′(2′,6′)-(di)halogenopicropodophyllotoxins (5, Fig. 1) were then stereoselectively produced, and especially some 4α-acyloxy-2α-chloro-2′(2′,6′)-(di)halogenopicropodophyllotoxins (6, Fig. 1) displayed more potent insecticidal activity than toosendanin, a commercial insecticide derived from Melia azedarach17. Additionally, during the long period of plants evolution, plant secondary metabolites are produced due to the interaction between plants and environment (life and non-life). It is considered that pesticides originating from plant secondary metabolites may result in less or slower resistance development and lower pollution18,19. Recently, discovery of new insecticidal components directly from plant secondary metabolites, or by using them as the lead-compounds for structural modifications has been one of the important procedures for research and development of new pesticides20,21,22,23.
Figure 1. Chemical structures of podophyllotoxin (1) and its derivatives (2–7).
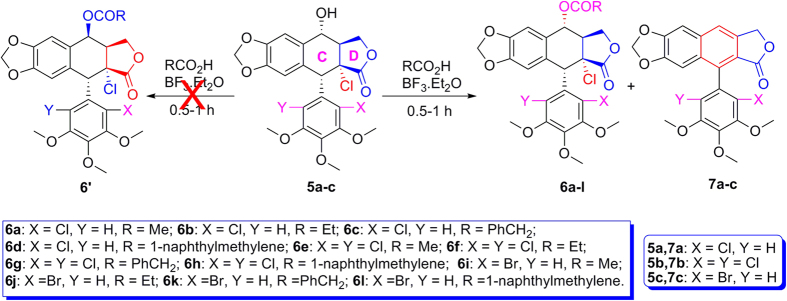
In continuation of our program aimed at the discovery and development of natural-product-based insecticidal agents, we envisaged to prepare a series of 4β-acyloxy-2α-chloro-2′(2′,6′)-(di)halogenopicropodophyllotoxins (6′) by SN1 reaction of 5 with carboxylic acids in the presence of BF3·Et2O. To our surprise, only compounds 6 were obtained, whereas compounds 6′ were not produced at all. Moreover, the above BF3·Et2O-mediated reaction was completely controlled by the reaction time. If the reaction time was prolonged, compounds 6 were all transformed into the unusual C-ring aromatization compounds 7.
Methods
Materials and Instruments
All chemical reagents and solvents were of reagent grade or purified according to standard methods before use. Analytical thin-layer chromatography (TLC) and preparative thin-layer chromatography (PTLC) were performed with silica gel plates using silica gel 60 GF254 (Qingdao Haiyang Chemical Co., Ltd., Qingdao, China). Silica gel column chromatography was performed with silica gel 200–300 mesh (Qingdao Haiyang Chemical Co., Ltd., Qingdao, China). Melting points were determined on a XT-4 digital melting-point apparatus (Beijing Tech Instrument Co., Ltd., Beijing, China) and were uncorrected. Infrared spectra (IR) were recorded on a Bruker TENSOR 27 spectrometer. Proton nuclear magnetic resonance spectra (1H NMR) and carbon nuclear magnetic resonance spectra (13C NMR) were recorded in CDCl3 or DMSO-d6 on a Bruker Avance DMX 400 or 500 MHz instrument using tetramethylsilane (TMS) as the internal standard. High-resolution mass spectra (HR-MS) were carried out with IonSpec 4.7 Tesla FTMS instrument. Compounds 5a–c were prepared in the same way as in our previous report14.
General procedure for synthesis of 6a–l
To a mixture of 5a–c (0.15 mmol) and carboxylic acids (0.18 mmol) in dry CH2Cl2 (5 mL) at −15 °C, a solution of BF3.Et2O (0.18 mmol) in dry CH2Cl2 (5 mL) was added dropwise to keep the temperature below −15 °C. After adding, the reaction temperature was raised from −15 °C to −5 or 0 °C, and the reaction process was checked by TLC analysis. When the reaction was complete, the mixture was diluted by CH2Cl2 (30 mL), washed by water (20 mL), HCl (0.1 mol/L, 20 mL), 5% aq. NaHCO3 (20 mL) and brine (20 mL), dried over anhydrous Na2SO4, concentrated in vacuo, and purified by PTLC to give 6a–l (40–93% yields) and 7a–c (6–38% yields) as the white solids. The example data of 6a–c and 7a–c are shown as follows, whereas data of 6d–l can be found in the Supporting Information.
Data for 6a: White solid; m.p. 150–151 °C; [α]20D = −78 (c 3.5 mg/mL, CHCl3); IR cm−1 (KBr): 3050, 2936, 1786, 1735, 1487, 1399, 1109, 1016, 867; 1H NMR (500 MHz, CDCl3) δ: 6.68 (s, 1H, H-5), 6.64 (s, 1H, H-8), 6.58 (s, 1H, H-6′), 5.93–5.95 (m, 3H, H-4, OCH2O), 5.52 (s, 1H, H-1), 4.80–4.81 (m, 2H, H-11), 3.94 (s, 3H, 3′-OCH3), 3.88 (s, 3H, 5′-OCH3), 3.76 (s, 3H, 4′-OCH3), 2.97–2.98 (m, 1H, H-3), 2.16 (s, 3H, COCH3); 13C NMR (125 MHz, CDCl3) δ: 171.9, 170.6, 152.0, 149.9, 149.2, 148.2, 142.6, 132.1, 130.8, 123.9, 122.0, 108.8, 108.6, 108.3, 101.7, 75.1, 73.2, 66.8, 61.1, 61.1, 56.1, 49.3, 44.7, 21.1; HRMS (ESI): Calcd for C24H22O9Cl2Na ([M + Na]+), 547.0533; found, 547.0529.
Data for 6b: White solid; m.p. 179–180 °C; [α]20D = −80 (c 2.9 mg/mL, CHCl3); IR cm−1 (KBr): 3057, 2935, 1788, 1724, 1486, 1400, 1108, 1033, 868; 1H NMR (400 MHz, CDCl3) δ: 6.67 (s, 1H, H-5), 6.62 (s, 1H, H-8), 6.57 (s, 1H, H-6′), 5.93–5.94 (m, 3H, OCH2O, H-4), 5.51 (s, 1H, H-1), 4.80–4.81 (m, 2H, H-11), 3.93 (s, 3H, 3′-OCH3), 3.88 (s, 3H, 5′-OCH3), 3.75 (s, 3H, 4′-OCH3), 2.96–2.97 (m, 1H, H-3), 2.35–2.42 (m, 2H, CH3CH2), 1.20 (t, J = 7.6 Hz, 3H, CH3CH2); 13C NMR (100 MHz, CDCl3) δ: 174.2, 171.9, 152.1, 150.0, 149.2, 148.2, 142.8, 132.2, 130.7, 124.2, 122.1, 108.8, 108.6, 108.5, 101.7, 74.9, 73.1, 66.8, 61.1, 61.1, 56.2, 49.4, 44.7, 27.5, 9.0; HRMS (ESI): Calcd for C25H24O9Cl2Na ([M + Na]+), 561.0689; found, 561.0691.
Data for 6c: White solid; m.p. 79–80 °C; [α]19D = −66 (c 3.6 mg/mL, CHCl3); IR cm−1 (KBr): 3062, 2936, 1789, 1737, 1486, 1399, 1110, 1034, 864, 697; 1H NMR (400 MHz, CDCl3) δ: 7.27–7.39 (m, 5H), 6.67 (s, 1H, H-5), 6.57 (s, 1H, H-8), 6.51 (s, 1H, H-6′), 5.93 (d, J = 1.2 Hz, 2H, OCH2O), 5.89 (d, J = 3.2 Hz, 1H, H-4), 5.50 (s, 1H, H-1), 4.77–4.78 (m, 2H, H-11), 3.93 (s, 3H, 3′-OCH3), 3.89 (s, 3H, 5′-OCH3), 3.75 (s, 3H, 4′-OCH3), 3.67 (d, J = 6.8 Hz, 2H, PhCH2), 2.99–3.00 (m, 1H, H-3); 13C NMR (100 MHz, CDCl3) δ: 171.8, 171.6, 152.0, 150.0, 149.2, 148.2, 142.8, 132.6, 132.1, 130.6, 129.0, 128.9, 127.7, 123.9, 122.1, 108.7, 108.6, 108.4, 101.7, 75.5, 72.9, 66.7, 61.1, 61.1, 56.3, 49.2, 44.6, 41.1; HRMS (ESI): Calcd for C30H26O9Cl2Na ([M + Na]+), 623.0846; found, 623.0849.
Data for 7a: White solid; m.p. 168–169 °C; IR cm−1 (KBr): 3064, 2934, 2845, 1762, 1466, 1109, 1036, 1012, 883; 1H NMR (500 MHz, DMSO-d6) δ: 8.01 (s, 1H), 7.56 (s, 1H), 6.77 (s, 1H), 6.71 (s, 1H), 6.20 (s, 2H, OCH2O), 5.44–5.53 (m, 2H, H-11), 3.90 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 3.74 (s, 3H, OCH3); 13C NMR (125 MHz, DMSO-d6) δ: 168.8, 151.8, 149.7, 149.1, 148.6, 142.3, 140.0, 135.2, 134.2, 129.4, 128.7, 120.1, 118.8, 118.3, 109.9, 130.7, 102.1, 101.5, 68.2, 60.9, 60.6, 56.0; HRMS (ESI): Calcd for C22H17O7ClNa ([M + Na]+), 451.0555; found, 451.0570.
Data for 7b: White solid; m.p. 218–219 °C; IR cm−1 (KBr): 3052, 2942, 2911, 1745, 1467, 1207, 1010, 895; 1H NMR (500 MHz, DMSO-d6) δ: 8.05 (s, 1H), 7.58 (s, 1H), 6.75 (s, 1H), 6.22 (s, 2H, OCH2O), 5.53 (s, 2H, H-11), 4.02 (s, 3H, 4′-OCH3), 3.89 (s, 6H, 3′, 5′-OCH3); 13C NMR (125 MHz, DMSO-d6) δ: 168.8, 149.9, 149.1, 148.8, 147.5, 140.1, 134.5, 132.3, 128.5, 128.2, 122.7, 120.8, 118.8, 103.9, 102.3, 100.7, 68.5, 61.2, 61.0; HRMS (ESI): Calcd for C22H16O7Cl2Na ([M + Na]+), 485.0165; found, 485.0170.
Data for 7c: White solid; m.p. 237–238 °C; IR cm−1 (KBr): 3062, 2920, 1758, 1619, 1460, 1249, 1101, 895. 1H NMR (400 MHz, CDCl3) δ: 7.74 (s, 1H), 7.22 (s, 1H), 6.84 (s, 1H), 6.59 (s, 1H), 6.09 (s, 2H, OCH2O), 5.35–5.45 (m, 2H, H-11), 4.01 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 3.79 (s, 3H, OCH3); 13C NMR (100 MHz, CDCl3) δ: 169.2, 152.7, 151.1, 150.1, 148.9, 143.0, 139.7, 138.4, 134.6, 131.5, 129.7, 119.6, 119.3, 110.3, 109.7, 103.8, 103.0, 101.9, 68.3, 61.3, 61.2, 56.1; HRMS (ESI): Calcd for C22H18O7Br ([M + H]+), 473.0230; found, 473.0235.
Biological assay17
The insecticidal activity of 5a–c, 6a–l and 7a–c against the pre-third-instar larvae of Mythimna separata Walker was assessed by leaf-dipping method. For each compound, 30 larvae (10 larvae per group) were used. Acetone solutions of compounds 5a–c, 6a–l, 7a–c, and toosendanin (used as a positive control) were prepared at the concentration of 1 mg/mL. Fresh wheat leaves were dipped into the corresponding solution for 3 s, then taken out, and dried in a room. Leaves treated with acetone alone were used as a blank control group. Several treated leaves were kept in each dish, where every 10 larvae were raised. If the treated leaves were consumed, additional treated leaves were added to the dish. After 48 h, untreated fresh leaves were added to all dishes until adult emergence. The experiment was carried out at 25 ± 2 °C and relative humidity (RH) 65–80%, and on 12 h/12 h (light/dark) photoperiod. The insecticidal activity of the tested compounds against the pre-third-instar larvae of M. separata was calculated by the following formula:
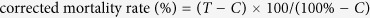 |
where T is the mortality rate in the treated group expressed as a percentage and C is the mortality rate in the untreated group expressed as a percentage.
Results and Discussion
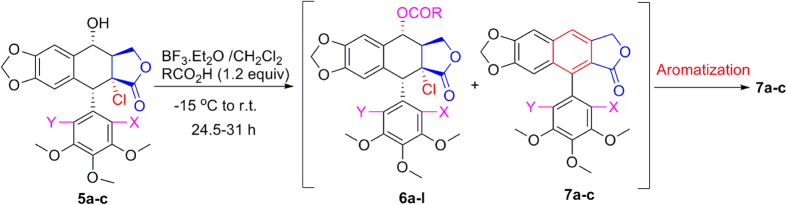
As shown in Fig. 2 and Table 1, when compounds 5a–c were allowed to react with carboxylic acids in the presence of BF3·Et2O at −15 °C to −5 °C for 0.5 h (or at −15 °C to 0 °C for 1 h), the expected compounds 6′ were not obtained, whereas compounds 6a–l were stereoselectively afforded in 40–93% yields. Meanwhile, the corresponding by-products 7a–c, containing the aromatized C-ring, were also afforded. Interestingly, the amount of 7a–c would be increased with the advance of time; on the contrary, the amount of 6a–l would be decreased accordingly. Subsequently, compounds 5a–c reacting with carboxylic acids in the presence of BF3·Et2O for a prolonged time was investigated as described in Fig. 3 and Table 2. It generally took 24.5–31 h for 6a–l all transformed into the corresponding compounds 7a–c in 40–99% yields. The assignment of configuration of acyloxy at the C-4 position of 6c, 6d, 6k and 6l (containing the cis-lactone, and an halogen atom on the E-ring) was according to our previous research results: if J3.4 ≈ 2.0 Hz, it indicates that H-3 and H-4 is trans relationship, that is, the substituent at the C-4 position of picropodophyllotoxin is α configuration15. The J3.4 values of H-4 of 6c, 6d, 6k and 6l were 3.2 Hz, therefore, the substituents at the C-4 position of 6c, 6d, 6k and 6l were α configuration. Because the NMR spectra of 6e–h (bearing the cis-lactone, and two chlorine atoms on the E-ring) were the same as those of 6e′-h′ (compounds 6e′–h′ were prepared from 5b in the presence of DCC and DMAP with acetic acid, propionic acid, phenylacetic acid, and 1-naphthylacetic acid, respectively. See Supporting Information), so the configuration of acyloxy at the C-4 position of 6e–h was α. The configuration of 6b, 6i, 6j, and 7a–c was confirmed by the X-ray crystallography (Figs 4, 5, 6, 7, 8, 9). Six crystallographic data (excluding structure factors) for the structures of 6b, 6i, 6j, and 7a–c have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 918891, 922185, 922186, 918697, 918890, and 922184, respectively. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB21EZ, UK [fax: + 44 (0)1223 336033 or e-mail: deposit@ccdc.cam.ac.uk]. It clearly demonstrated that the propionyloxy of 6b, acetyloxy of 6i, and propionyloxy of 6j at the C-4 position all were present in α configuration. The C-ring of 7a–c was all aromatized. Meanwhile, based on the X-ray crystallography (Figs 4, 5, 6), if the acyloxy group at the C-4 position adopted β configuration, big steric effects would be observed between the lactone and the acyloxy group. Hence the acyloxy group at the C-4 position of 6a–l were all present in α configuration.
Figure 2. Compounds 5a–c reacting with carboxylic acids in the presence of BF3·Et2O.
Table 1. Investigation of 5a-c Reacting with Carboxylic Acids Mediated by BF3·Et2O.
| Entry | Compound | Acid | T[°C] | t[h] | Isolated yield [%] |
|
|---|---|---|---|---|---|---|
| 6 | 7 | |||||
| 1 | 5a | acetic acid | −15 ~ −5 | 0.5 | 6a (75) | 7a (27) |
| 2 | 5a | propionic acid | −15 ~ −5 | 0.5 | 6b (47) | 7a (27) |
| 3 | 5a | phenylacetic acid | −15 ~ −5 | 0.5 | 6c (66) | 7a (21) |
| 4 | 5a | 1-naphthylacetic acid | −15 ~ −5 | 0.5 | 6d (53) | 7a (22) |
| 5 | 5b | acetic acid | −15 ~ −5 | 0.5 | 6e (64) | 7b (14) |
| 6 | 5b | propionic acid | −15 ~ −5 | 0.5 | 6f (40) | 7b (23) |
| 7 | 5b | phenylacetic acid | −15 ~ −5 | 0.5 | 6g (78) | 7b (11) |
| 8 | 5b | 1-naphthylacetic acid | −15 ~ 0 | 1 | 6h (50) | 7b (38) |
| 9 | 5c | acetic acid | −15 ~ −5 | 0.5 | 6i (93) | 7c (trace) |
| 10 | 5c | propionic acid | −15 ~ −5 | 0.5 | 6j (66) | 7c (6) |
| 11 | 5c | phenylacetic acid | −15 ~ 0 | 1 | 6k (72) | 7c (27) |
| 12 | 5c | 1-naphthylacetic acid | −15 ~ 0 | 1 | 6l (50) | 7c (29) |
Figure 3. Compounds 5a-c reacting with carboxylic acids in the presence of BF3·Et2O for a prolonged time.
Table 2. Compounds 5a–c Reacting with Carboxylic Acids Mediated by BF3·Et2O for a Prolonged Time.
| Entry | Compound | Acid | t[h] | Isolated yield [%] |
|---|---|---|---|---|
| 1 | 5a | acetic acid | 25.5 | 7a (75) |
| 2 | 5a | propionic acid | 25.5 | 7a (80) |
| 3 | 5a | phenylacetic acid | 30.5 | 7a (47) |
| 4 | 5a | 1-naphthylacetic acid | 24.5 | 7a (93) |
| 5 | 5b | acetic acid | 30.5 | 7b (90) |
| 6 | 5b | propionic acid | 28.5 | 7b (40) |
| 7 | 5b | phenylacetic acid | 24.5 | 7b (41) |
| 8 | 5b | 1-naphthylacetic acid | 26 | 7b (91) |
| 9 | 5c | acetic acid | 25.5 | 7c (99) |
| 10 | 5c | propionic acid | 30.5 | 7c (50) |
| 11 | 5c | phenylacetic acid | 25 | 7c (78) |
| 12 | 5c | 1-naphthylacetic acid | 31 | 7c (64) |
Figure 4. X-ray crystal structure of compound 6b.
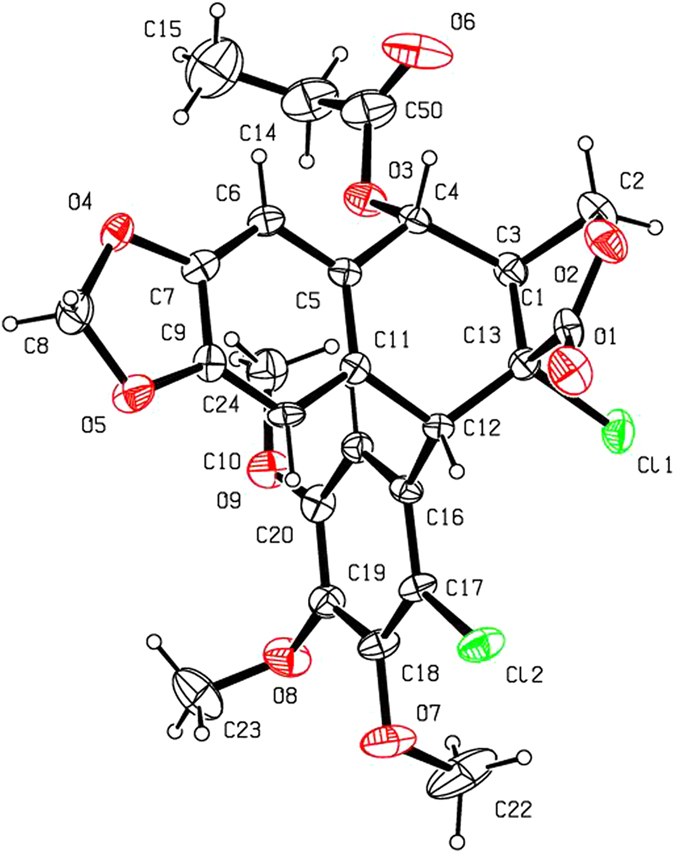
Drawing by Hui Xu.
Figure 5. X-ray crystal structure of compound 6i.
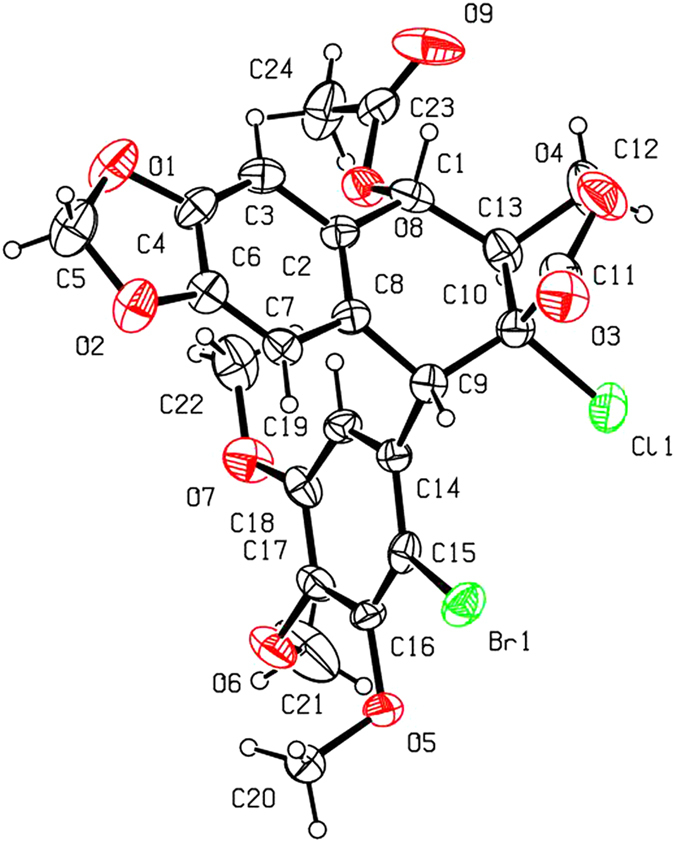
Drawing by Hui Xu.
Figure 6. X-ray crystal structure of compound 6j.
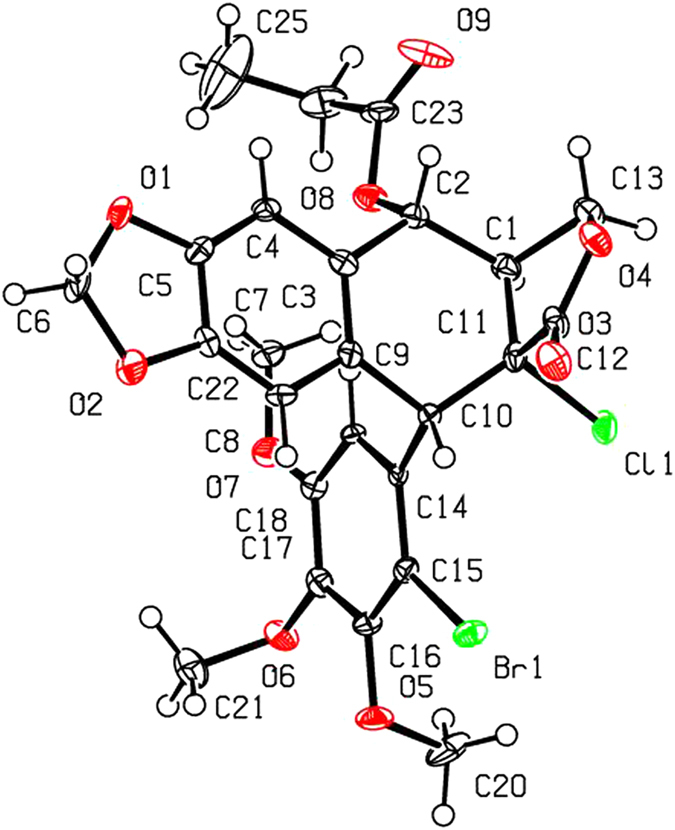
Drawing by Hui Xu.
Figure 7. X-ray crystal structure of compound 7a.
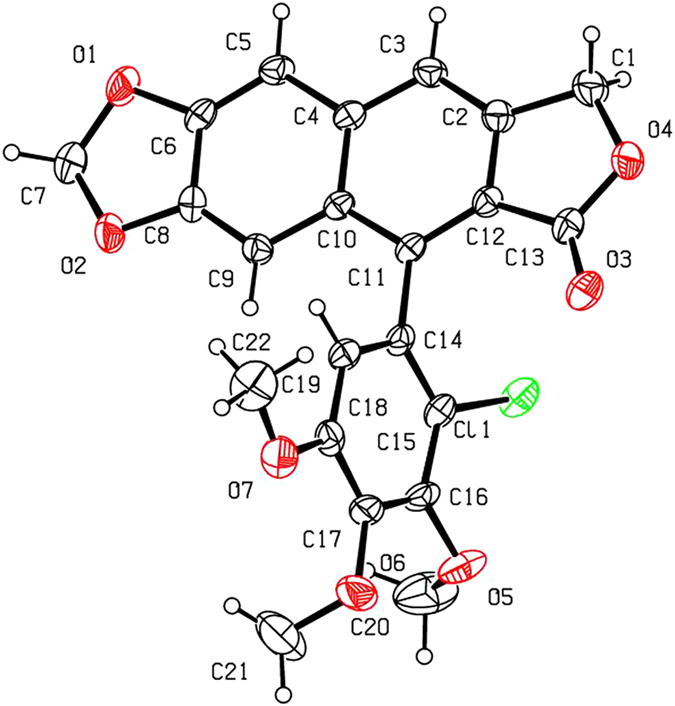
Drawing by Hui Xu.
Figure 8. X-ray crystal structure of compound 7b.
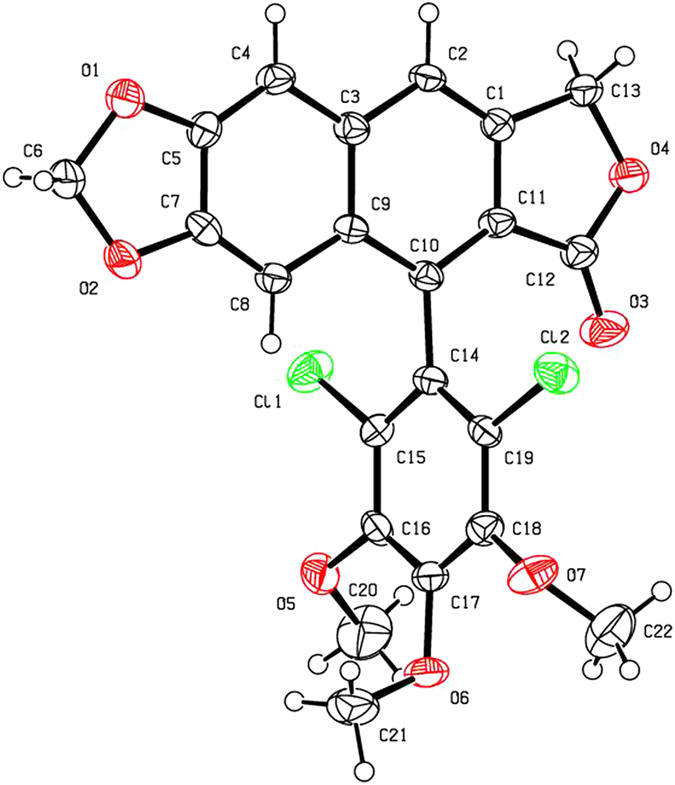
Drawing by Hui Xu.
Figure 9. X-ray crystal structure of compound 7c.
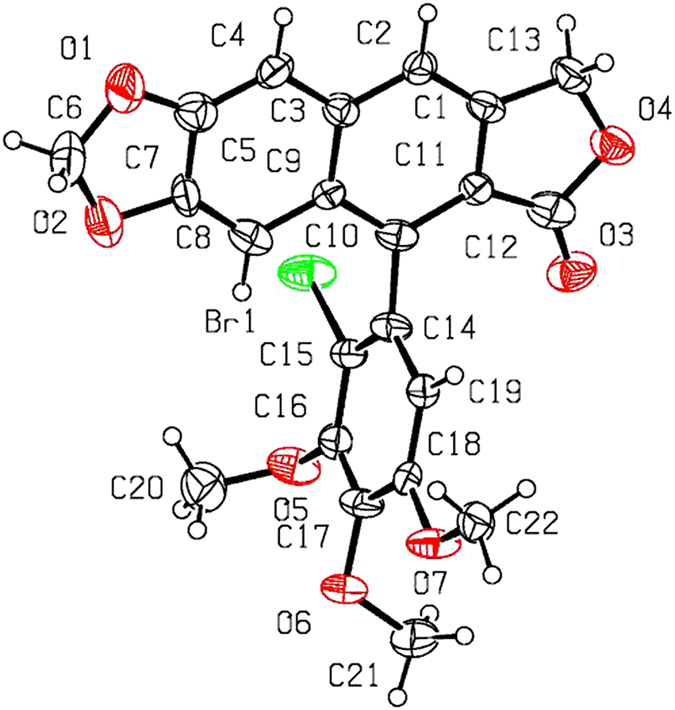
Drawing by Hui Xu.
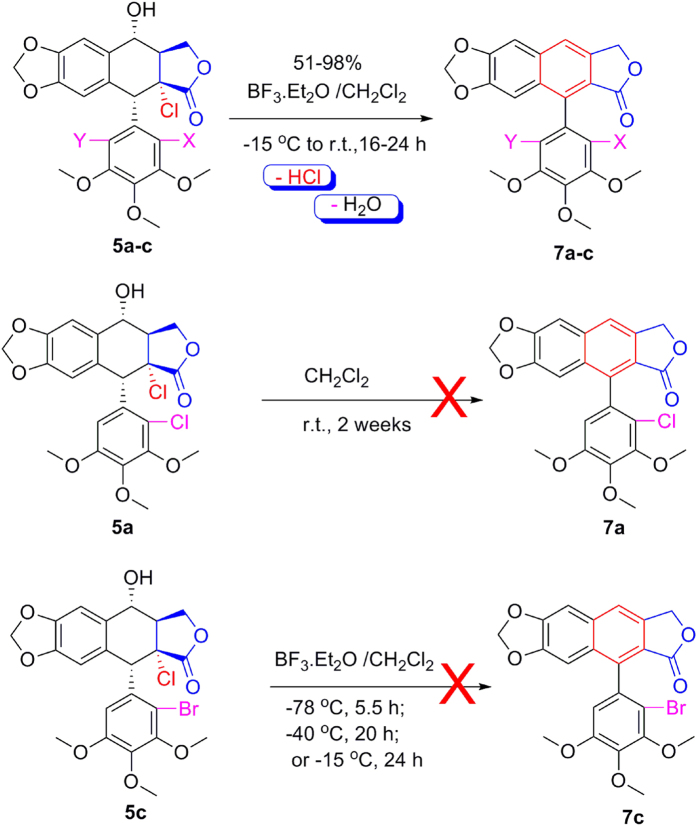
Furthermore, compounds 5a, 5b or 5c with BF3·Et2O in CH2Cl2 was also examined. As described in Fig. 10, when the reaction time was prolonged to 16–24 h, compounds 5a–c were smoothly transformed into the corresponding compounds 7a-c in 51–98% yields by C-ring aromatization. However, when only compound 5a in CH2Cl2 was stirred at room temperature for two weeks, except 5a, no product was produced. Additionally, when compound 5c with BF3·Et2O in CH2Cl2 was stirred at −78 °C for 5.5 h, −40 °C for 20 h, or −15 °C for 24 h, except 5c, the target compound 7c was not obtained. It demonstrated that BF3·Et2O and reaction temperature were the important factors for C-ring aromatization of 5a–c.
Figure 10. Investigation of 5a–c mediated by BF3·Et2O or not.
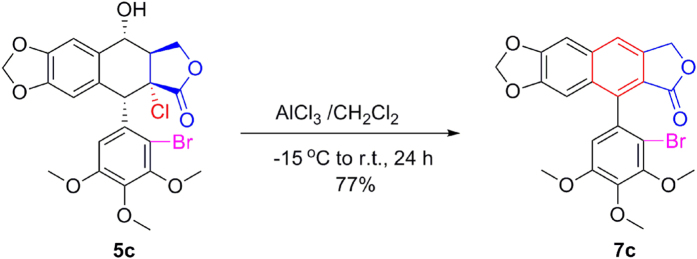
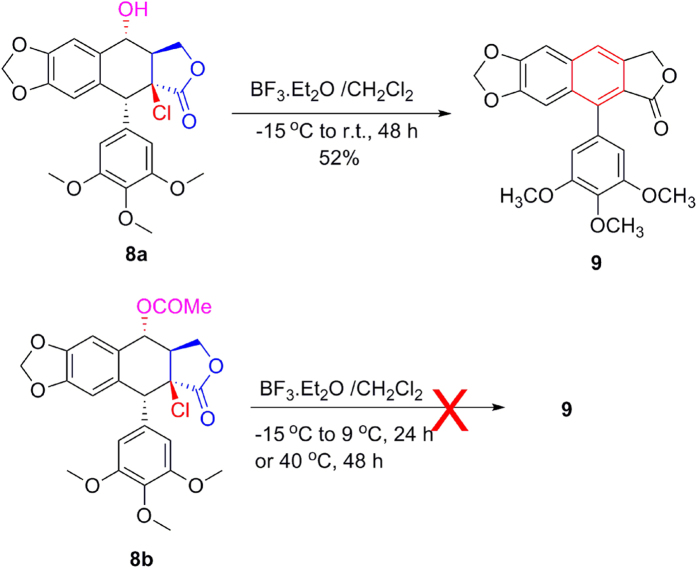
Subsequently, as shown in Fig. 11, when compound 5c with AlCl3 in CH2Cl2 was stirred at −15 °C to r.t. for 24 h, compound 7c was obtained in 77% yield. It suggested that AlCl3 could be substituted for BF3·Et2O as a lewis acid for C-ring aromatization. It might involve a BF3·Et2O-promoted dehydrochlorination, followed by a BF3·Et2O-promoted dehydration or dehydroacylation. Meanwhile, as shown in Fig. 12, when 2β-chloropodophyllotoxin (8a) in the presence of BF3·Et2O was stirred at −15 °C to r.t. for 48 h, C-ring aromatization product 9 was obtained in 52% yield (see Supporting Information). However, 4α-acetyloxy-2β-chloropodophyllotoxin (8b) in the presence of BF3·Et2O was stirred at −15 °C to 9 °C for 24 h, or at 40 °C for 48 h, the proposed product 9 was not detected.
Figure 11. Investigation of 5c mediated by AlCl3.
Figure 12. Investigation of 8a and 8b mediated by BF3·Et2O.
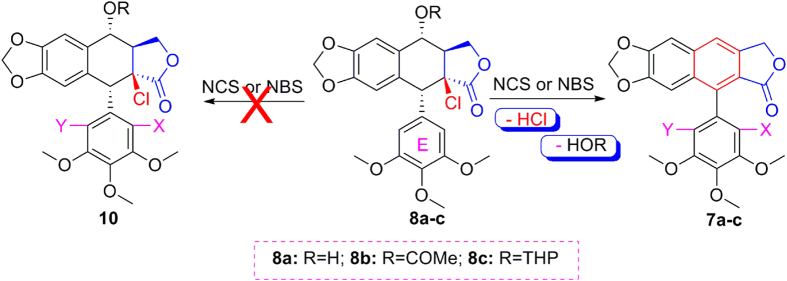
On the other hand, as shown in Fig. 13, we envisaged whether 2β-chloro-2′(2′,6′)-(di)halogenopodophyllotoxin derivatives (10, the diastereoisomers of 6), could be prepared by direct halogenation of E-ring of 2β-chloropodophyllotoxins. As described in Table 3, the reaction of 2β-chloropodophyllotoxins (8a–c) with NCS or NBS was further examined. When compound 8a reacted with 1.1 equiv of NCS at 25 °C for 24 h, no product was obtained (entry 1). When the reaction temperature was raised to 30 °C, the target E-ring halogenation product was not produced; interestingly, whereas the C-ring aromatization compounds such as 7a and 7b were obtained in 35% and 15% yields, respectively (entry 2). And when at 40 °C for 4 h, compounds 7a and 7b were obtained in 54% and 12% yields, respectively (entry 3). However, when the reaction temperature was raised to 50 °C, the yields of 7a and 7b were not increased (entry 4). When compound 8a reacted with 2.2 equiv of NCS at 40 °C for 11 h, only 7b was obtained in 52% yield (entry 5). Similarly, when compound 8a reacted with 1.1 equiv of NBS at 40 °C for 5 h, compound 7c was obtained in 50% yield (entry 7); when compound 8c reacted with 1.1 equiv of NCS at 40 °C for 19 h, compounds 7a and 7b were obtained in 34% and 25% yields, respectively (entry 12). But when compound 8b reacted with NCS or NBS, the reaction temperature should be raised. For example, when compound 8b reacted with 1.1 equiv of NCS at 40 °C for 48 h, compound 7a was obtained only in 12% yield (entry 8); whereas the reaction temperature was raised to 60 °C for 24 h, compound 7a was obtained in 94% (entry 9). When compound 8b reacted with 2.2 equiv of NCS for 17 h or 1.1 equiv of NBS for 24 h at 60 °C, compounds 7b and 7c were obtained in 87% and 91% yields, respectively (entries 10 and 11). All in all, the reaction temperature was very important for 2β-chloropodophyllotoxins reacting with NCS or NBS to give 7a–c. According to our previous results14, reaction of 8a–c with NCS or NBS might involve E-ring halogenation of 2β-chloropodophyllotoxins, followed by the dehydrochlorination and dehydration or dehydroacylation.
Figure 13. 2β-Chloropodophyllotoxins (8a–c) reacting with NCS or NBS.
Table 3. Investigation of 2β-Chloropodophyllotoxins (8a–c) Reacting with NCS or NBS.
| Entry | Compound | Amount of NCS orNBS | T[°C] | t[h] | Isolated yield [%] |
|---|---|---|---|---|---|
| 1 | 8a | NCS (1.1 equiv) | 25 | 24 | 7a (0) |
| 2 | 8a | NCS (1.1 equiv) | 30 | 7 | 7a (35) + 7b (15) |
| 3 | 8a | NCS (1.1 equiv) | 40 | 4 | 7a (54) + 7b (12) |
| 4 | 8a | NCS (1.1 equiv) | 50 | 4 | 7a (40) + 7b (7) |
| 5 | 8a | NCS (2.2 equiv) | 40 | 11 | 7b (52) |
| 6 | 8a | NBS (1.1 equiv) | 25 | 24 | 7c (0) |
| 7 | 8a | NBS (1.1 equiv) | 40 | 5 | 7c (50) |
| 8 | 8b | NCS (1.1 equiv) | 40 | 48 | 7a (12) |
| 9 | 8b | NCS (1.1 equiv) | 60 | 24 | 7a (94) + 7b (trace) |
| 10 | 8b | NCS (2.2 equiv) | 60 | 17 | 7b (87) |
| 11 | 8b | NBS (1.1 equiv) | 60 | 24 | 7c (91) |
| 12 | 8c | NCS (1.1 equiv) | 40 | 19 | 7a (34) + 7b (25) |
Finally, compounds 5a–c, 6a–l, and 7a–c were evaluated as insecticidal agents against the pre-third-instar larvae of oriental armyworm, Mythimna separata (Walker) at the concentration of 1 mg/mL. Among 5a–c and 6a–l, as described in our previous paper17, only compounds 5c and 6g exhibited insecticidal activity equal to that of toosendanin, and the final corrected mortality rates of 5c, 6g and toosendanin were 51.9%, 55.6% and 51.9%, respectively. The final corrected mortality rates of 1 and 7a–c were 37%, 40.7%, 51.9%, and 37%, respectively.
Conclusion
In summary, we have developed a BF3·Et2O-mediated stereoselective synthesis 4α-acyloxy-2α-chloro-2′(2′,6′)-(di)halogenopicropodophyllotoxin derivatives, which was completely controlled by the reaction time. If the reaction time was prolonged to 24.5–31 h, the target compounds were all transformed into the C-ring aromatization compounds. Additionally, it demonstrated that BF3·Et2O and reaction temperature were the important factors for C-ring aromatization, and AlCl3 could be substituted for BF3·Et2O as a lewis acid for C-ring aromatization. However, in the presence of NCS or NBS, 2β-chloropodophyllotoxins could also be transformed into the same C-ring aromatization compounds. Notably, compounds 5c, 6g and 7b exhibited insecticidal activity equal to that of toosendanin.
Additional Information
How to cite this article: Fan, L. et al. Insight into 2α-Chloro-2′(2′,6′)-(Di)Halogenopicropodophyllotoxins Reacting with Carboxylic Acids Mediated by BF3·Et2O. Sci. Rep. 5, 16285; doi: 10.1038/srep16285 (2015).
Supplementary Material
Acknowledgments
The present research was supported by National Natural Science Foundation of China (No. 31071737), and the Special Funds of Central Colleges Basic Scientific Research Operating Expenses, Northwest A&F University (No. YQ2013008, 2452015096).
Footnotes
Author Contributions L.F. and X.Z. performed experiments, and analysed data; C.Z. analysed data; H.X. designed experiments, analysed data and wrote the manuscript.
References
- You Y. J. Podophyllotoxin derivatives: Current synthetic approaches for new anticancer agents. Curr. Pharm. Des. 11, 1695–1717 (2005). [DOI] [PubMed] [Google Scholar]
- Gordaliza M., Garcia P. A., Miguel Del Corral J. M., Castro M. A. & Gomez-Zurita M. A. Podophyllotoxin: distribution, sources, applications and new cytotoxic derivatives. Toxicon 44, 441–459 (2004). [DOI] [PubMed] [Google Scholar]
- Kumar K. A., Singh S. K., Kumar B. S. & Doble M. Synthesis, anti-fungal activity evaluation and QSAR studies on podophyllotoxin derivatives. Cent. Eur. J. Chem. 5, 880–897 (2007). [Google Scholar]
- Miyazawa M., Fukuyama M., Yoshio K., Kato T. & Ishikawa Y. Biologically active components against Drosophila melanogaster from Podophyllum hexandrum. J. Agric. Food Chem. 47, 5108−5110 (1999). [DOI] [PubMed] [Google Scholar]
- Xu H., Lv M. & Tian X. A review on hemisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins: 2003-2007. Curr. Med. Chem. 16, 327–349 (2009). [DOI] [PubMed] [Google Scholar]
- Stadler D. & Bach T. Concise stereoselective synthesis of (−)-podophyllotoxin by an intermolecular iron (III)-catalyzed Friedel-Crafts alkylation. Angew. Chem. Int. Ed. 47, 7557−7559 (2008). [DOI] [PubMed] [Google Scholar]
- Engelhardt U., Sarkar A. & Linker T. Efficient enantioselective total synthesis of (−) epipodophyllotoxin. Angew. Chem. Int. Ed. 42, 2487−2489 (2003). [DOI] [PubMed] [Google Scholar]
- Reynolds A. J., Scott A. J., Turner C. I. & Sherburn M. S. The intramolecular carboxyarylation approach to podophyllotoxin. J. Am. Chem. Soc. 125, 12108−12109 (2003). [DOI] [PubMed] [Google Scholar]
- Jeedimalla N., Johns J. & Roche S. P. Mechanistic investigation and implications of a sacrificial aniline for the tandem cascade synthesis of 4-aza-podophyllotoxin analogues. Tetrahedron Lett. 54, 5845−5848 (2013). [Google Scholar]
- Merchan Arenas D. R., Rojas Ruíz F. A. & Kouznetsov V. V. Highly diastereoselective synthesis of new heterolignan-like 6,7-methylendioxy-tetrahydroquinolines using the clove bud essential oil as raw material. Tetrahedron Lett. 52, 1388−1391 (2011). [Google Scholar]
- Ting C. P. & Maimone T. J. C-H bond arylation in the synthesis of aryltetralin lignans: A short total synthesis of podophyllotoxin. Angew. Chem. Int. Ed. 53, 3115−3119 (2014). [DOI] [PubMed] [Google Scholar]
- Lv M. & Xu H. Recent advances in semisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins: an update (2008–2010). Mini-Rev. Med. Chem. 11, 901−909 (2011). [DOI] [PubMed] [Google Scholar]
- Glinski M. B., Freed J. C. & Durst T. Preparation of 2-substituted podophyllotoxin derivatives. J. Org. Chem. 52, 2749–2753 (1987). [Google Scholar]
- Che Z. P., Yu X., Zhi X. Y., Fan L. L. & Xu H. Synthesis of novel 4α-(acyloxy)-2′(2′,6′)-(di)halogenopodophyllotoxin derivatives as insecticidal agents. J. Agric. Food Chem. 61, 8148−8155 (2013). [DOI] [PubMed] [Google Scholar]
- Xu H., Wang Q. T. & Guo Y. Stereoselective synthesis of 4α-alkyloxy-2-α/β-bromopodophyllotoxin derivatives as insecticidal agents. Chem. Eur. J. 17, 8299–8303 (2011). [DOI] [PubMed] [Google Scholar]
- Xu H., Xiao X. & Wang Q. T. Natural products-based insecticidal agents 7. Semisynthesis and insecticidal activity of novel 4α-alkyloxy-2-chloropodophyllotoxin derivatives against Mythimna separata Walker in vivo. Bioorg. Med. Chem. Lett. 20, 5009−5012 (2010). [DOI] [PubMed] [Google Scholar]
- Fan, L. L., Guo Y., Zhi X. Y., Y X. & Xu H. Stereoselective synthesis of 2α-chloropicropodophyllotoxins and insecticidal activity of their esters against oriental armyworm, Mythimna separata Walker. J. Agric. Food Chem. 62, 3726–3733 (2014). [DOI] [PubMed] [Google Scholar]
- Isman M. B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 51, 45–66 (2006). [DOI] [PubMed] [Google Scholar]
- Seiber J. N., Coats J., Duke S. O. & Gross A. D. Biopesticides: state of the art and future opportunities. J. Agric. Food Chem. 62, 11613–11619 (2014). [DOI] [PubMed] [Google Scholar]
- Caboni P. et al. Nematicidal activity of mint aqueous extracts against the root-knot nematode meloidogyne incognita. J. Agric. Food Chem. 61, 9784–9788 (2013). [DOI] [PubMed] [Google Scholar]
- Meepagala K. M., Bernier U. R., Burandt C. & Duke S. O. Mosquito repellents based on a natural chromene analogue with longer duration of action than N,N-diethyl-meta-toluamide (DEET). J. Agric. Food Chem. 61, 9293–9297 (2013). [DOI] [PubMed] [Google Scholar]
- Oliver M. P., Crouse G. D., Demeter D. A. & Sparks T. C. Synthesis and insecticidal activity of spinosyns with C9-O-benzyl bioisosteres in place of the 2′,3′,4′-tri-O-methyl rhamnose. J. Agric. Food Chem. 63, 5571–5577 (2015). [DOI] [PubMed] [Google Scholar]
- Gerwick B. C. & Sparks T. C. Natural products for pest control: an analysis of their role, value and future. Pest Manag. Sci. 70, 1169–1185 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.