Abstract
Background
Diabetic nephropathy (DN) is one of the most significant long-term complications of diabetes mellitus (DM), and it is a primary risk factor for end-stage renal disease. MicroRNAs (miRNAs) play important roles in regulating the expression of genes, including interleukin-6R (IL-6R), which has been reported to be involved in the development of DNDN. The aim of this study was to identify the dysregulation of miRNA and its target responsible for the development of DN in DM.
Material/Methods
We collected the kidney tissues from patients with DN (N=36) and control patients (N=28), and performed real-time PCR and Western blot analysis to determine the expression of IL-6R. Computational analysis and luciferase assay were used to identify the miRNA that regulates IL-6R. To explore the association between rs12976445 polymorphism and risk of DN, we enrolled 594 DM patients with (N=282) or without DN (N=312), and studied the association between a variant in miR-125a and risk of DN in DM.
Results
The expression of IL-6R was barely detected in the control groups, while in the DN group, the IL-6R was clearly detectable. Next, miR-125a was identified as a regulator of IL-6R by using informatics analysis and luciferase assay. A single-nucleotide polymorphism (rs12976445) in pri-miR-125a has been shown to compromise the mature processing of miR-125a, and we showed that the expression levels of miR-125a was comparable between individuals carrying TT and CT, and when combined into 1 group, the miR-125a expression was approximately 3 times lower than in the CC group. We found significant differences regarding rs12976445 genotype distribution between the DN and the control (OR=1.45, 95% C.I.=1.02–2.08, p<0.05) with the possible confounding factors adjusted for by using logistic regression analysis.
Conclusions
We identified miR-125a as a direct regulator of IL-6R, and the genotype of rs12976445 might be a novel predictor of the development of DN in DM.
MeSH Keywords: Cytokine Receptor gp130, Diabetic Nephropathies, MicroRNAs
Background
Diabetic nephropathy (DN) is one of the most important long-term complications of diabetes, and it is the primary cause of end-stage renal disease and deaths in diabetic patients [1]. The major clinical characteristics of DN includes progressively decreasing glomerular filtration rate (GFR) and persistent albuminuria, with macroalbuminuria (>300mg/day) indicating DN progression and microalbuminuria (30 to 300 mg of albumin in urine per day) representing early DN [2]. The main pathological characteristics of DN are expansion and hypertrophy in the tubular compartments and glomerular mesangium, as well as buildup of extracellular matrix (ECM) proteins and dysfunction of podocytes. There are several mechanisms, such as protein kinase C, advanced glycation end-products, hyperglycemia, oxidative stress, poly (ADP-ribose) polymerase activation, and inflammation, which are thought to account for the development and pathogenesis of DN [3]. Unfortunately, the hidden molecular pathogenesis is not completely identified.
Interleukin 6 (IL-6) is a multi-potent cytokine; its main function appears to induce proliferation and differentiation of B lymphocyte and acute inflammatory responses. IL-6 has been shown to enhance the differentiation and growth of a wide range of cells, including renal mesangial cells [4]. The aberrantly enhanced proliferation of renal mesangial cells may lead to basement membrane thickening and mesangial matrix expansion, which is a major histological characteristic of diabetic nephropathy [5–7]. Actually, thickening of basement membrane and mesangial proliferation can be observed in early-stage nephropathy [8]. IL-6 may be very important in mesangial proliferation in patients who have glomerular renal disease [4,9]. IL-6 acts on a receptor that consists of 2 subunits, gp130 and IL-6 receptor (IL-6R). First, IL-6 binds to the IL-6R, and this complex subsequently binds to the gp130 subunit [10,11]. Elevated concentrations of IL-6R have been observed in both DN [12] and type II diabetes mellitus (DM) [13].
MicroRNAs (miRNAs) consist of short non-coding RNAs that regulate physiological and pathological processes by suppressing expression of target gene via blocking protein translation or promoting degradation of mRNA. Recently, studies have discovered key properties for specific miRNAs in a battery of biological and cellular processes, such as proliferation, development, and differentiation [14]. Although the role of miRNAs has been identified in the pathogenesis of many human diseases, their function in diabetes and DN is less understood. Earlier reports have shown that single-nucleotide polymorphisms (SNPs) in miRNA genes may interfere with the interaction between miRNAs and mRNAs of target genes, or compromise the processing of mature miRNA [15–18], which may contribute to susceptibility to certain human diseases [19,20].
In this study, we confirmed the differential expression of IL-6R in DN and identified miR-125a as a regulator of IL-6R. It has been previously shown that a SNP (rs12976445) is present in the pre-miR-125a gene, and compromises the mature processing of the miRNA, which leads to an increased risk of autoimmune thyroid conditions [21] and repeated pregnancy loss [22]. Based on the evidence mentioned above, we studied the association between rs12976445 polymorphism and risk of DN in the patients with DM.
Material and Methods
Preparation of human kidney sample
Renal biopsies were performed to collect renal tissue samples from 36 DM patients who had DN, and 28 controls in our hospital. The controls included in this study were the patients confirmed with renal carcinoma who underwent nephrectomy and did not have diabetes, hypertension, and other co-existing conditions. After all participants signed informed consent, renal biopsies (DN patients) or nephrectomy surgery (controls, mainly kidney cortexes) were performed to collect kidney tissues for real-time polymerase chain reaction (PCR) and Western blot analysis. In addition, peripheral blood samples were collected from 594 DM patients with (N=282) or without (N=312) DN. The demographic and clinicopathological features are described in Table 1. Written consent was obtained from each participant, and the approval of this study was obtained from the Ethics Committee on Human Research of The People ‘s Hospital in Hanzhong (Hanzhong, China).
Table 1.
Demographic and clinicopathological features of the participants recruited in this study.
| DM with nephropathy | DM without nephropathy | |
|---|---|---|
| N | 282 | 312 |
| Sex (Male) | 227 | 249 |
| Age (years) | 52.63±8.6 | 51.37±9.3 |
| Smoking (%) | 34.12 | 32.39 |
| Duration of DM (years) | 7.78±7.89 | 8.89±8.14 |
| Duration of DMN (months) | 13.21±10.23 | 0 |
| eGFR (ml/min) | 59.35±23.64 | 104.35±25.78 |
| TG (mmol/L) | 2.15±1.54 | 1.78±1.48 |
| CHOL (mmol/L) | 5.8±1.9 | 5.3±2.1 |
| HDL (mmol/L) | 1.64±1.23 | 1.72±1.13 |
| LDL (mmol/L) | 3.34±1.34 | 3.56±1.43 |
| Urinary protein excretion (g/24 h) | 3.34±2.56 | 0.053±0.012 |
| HbA1C (%) | 8.32±2.34 | 8.12±2.56 |
| Serum albumin (g/L) | 29.34±12.26 | 48.49±13.98 |
| Serum creatinine (umol/L) | 148.65±78.28 | 68.12±29.32 |
DM – diabetes mellitus; DMN – diabetic nephropathy.
Real-time PCR analysis
Total RNA was isolated from renal tissue samples or the cultured cells by using TRIzol (Invitrogen, Carlsbad, CA). Complementary DNA (cDNA) was then synthesized by using SuperScript III (Invitrogen, Carlsbad, CA). The expression of miR-125a and IL-6R mRNA was measured in the human tissue samples and cultured cells. An ABI PRISM7500 system (Applied Biosystems, Foster City, CA) was used to perform a quantitative PCR. Each experiment was repeated 3 times.
Rs12976445 polymorphism genotyping
DNA extraction kit (Sangong, Shanghai, China) was used to extract genomic DNA from the peripheral blood samples. NanoDrop 1000 Spec-trophotometer (Thermo Fisher Scientific, DE) was used to determine the quantity and quality of the isolated genomic DNA. Next, the target chromosome segment was PCR-amplified using the primer set: 5′-CCA TCG TGT GGG TCT CAA G-3′ and 5′-TCT TTC ACA GTG GAT CCT CTG AC-3′. Subsequently, the genotype of rs12976445 polymorphism was determined by using direct Sanger sequencing.
Cell culture
Human glomerular mesangial IP15 cell line were purchased from Chinese Academy of Sciences Cell Bank (Shanghai, China). Cells were incubated in RPMI-1640 medium containing 10% fetal bovine serum (FBS) and were placed in a humidified incubator at 37°C with an atmosphere of 95% air and 5% CO2. All experiments used cells at passages from 3 and 8.
Transient transfection
Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) was used to perform the transient transfections as specified in the manufacturer’s protocol. miRNA oligonucleotide was transfected for function loss experiment as per a designated protocol. In brief, cells were plated in 6-well plates and grew to 80% confluency. Next, the culture media was supplemented with miR-125a mimics, IL-6R specific siRNA or a matched miRNA negative control (Genepharma, Shanghai, China) at a final concentration of 50 nM. At 6 h after transfection, the medium was replenished with RPMI-1640.
In vitro cell proliferation assay
The colorimetric procedure was performed to assess cell viability by using the Cell Counting Kit-8 (Dojindo, Shanghai, China), following the manufacturer’s instruction. The absorbance was measured at 450 nm using a microplate reader. Five duplicate wells were determined for all experiments for each group.
Luciferase reporter gene assay
Human genomic DNA was used for amplification of full-length 3′-UTR of IL-6D by PCR and the clone into luciferase reporter vector (Promega, Madison, WI). The Quick Change XL site-directed mutagenesis kit (Stratagene, La Jolla, CA) was used to introduce the mutations into the putative binding sites at 91–99 bp and 621–627 bp of the 3′UTR of IL-6R. The reporter constructs and miR-negative control or miR-125a mimic were used for co-transfection of renal mesangial cells. The cells were incubated for 24 h, and then the activities of luciferase were determined in a luminometer using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) as per the manufacturer’s instructions.
Western blot
RIPA buffer was used to lyse the human tissues or cells, and total cellular protein was centrifuged and obtained. The concentration was determined by the BCA method. After separation by SDS-PAGE, proteins were transferred to PVDF membranes (0.45 mm, Millipore), and then wash with TBST, blockage with 5% skim milk powder dissolved in TBST for 1 h, the PVDF membranes were cultured with primary antibodies against IL-6R or β-actin (Santa Cruz, CA, USA) (Dilution 1:1500 for both primary antibodies) at 4°C overnight with dilutions as per the instruction of the manufacturer. The internal reference was β-actin. After washing with TBST, the PVDF membranes were cultured with secondary antibodies conjugated with horseradish peroxidase (HRP) for 1 h at 37°C. Chemiluminescence (ECL) reagent (Pierce, Thermo Scientific, Rockford, IL,) was used to detect the protein bands.
Statistical analysis
A goodness-of-fit χ2 test in controls was used to assess Hardy-Weinberg equilibrium (HWE) for the genotype. The differences in distributions of demographic characteristics between cases and controls were determined using Pearson’s Tχ2 test. The strength of correlation between the polymorphism and DN risk odds ratios (ORs) and 95% confidence intervals (95% CIs) were determined by univariate logistic regression model. After adjustment for age, sex, smoking status, duration of DM, or DN (as described in Table 1), a multivariate logistic regression model was used for the calculation of adjusted ORs and 95% CIs. Statistical significance was set at P<0.05 that was 2-sided, and SPSS 19.0 (SPSS, Chicago, IL) was used to perform all statistical analyses.
Results
In this study, we collected the kidney tissues from the patients with DN (N=36) and the control patients (N=28), and performed realtime PCR and Western blot analysis to determine the expression of IL-6R that had been previously reported to be functionally involved in the development of diabetic nephropathy [23]. As expected, the expression of IL-6R was barely detected in the controls groups, while in the DN groups, the IL-6R was clearly detectable (Figure 1A). Similarly, the mRNA expression level of IL-6R was substantially upregulated in the tissue samples from DN patients compared with the controls (Figure 1B).
Figure 1.
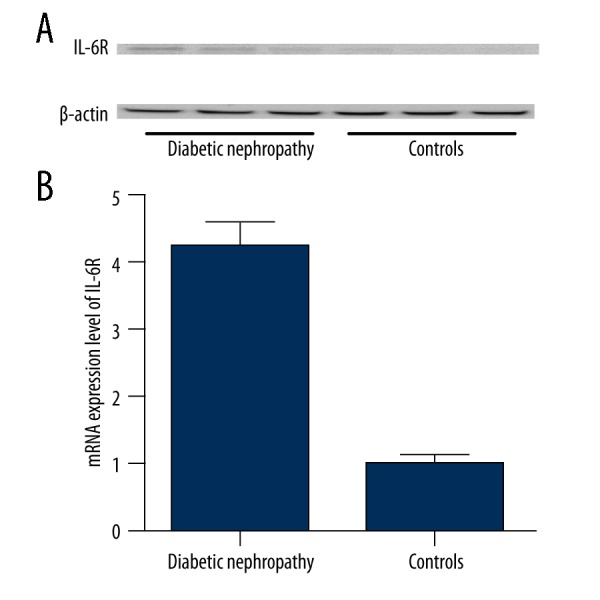
(A) The expression of IL-6R was barely detectable in the controls groups, but was clearly detectable in the DN groups; (B) The mRNA expression level of IL-6R was substantially upregulated in the tissue samples from DN patients compared with the controls.
To identify the potential regulator of IL-6R in the kidneys, we searched the miRNA database (www.mirdb.org) and identify miR-125a as a possible regulator of IL-6R with 2 putative binding sites (91–99 bp and 621–627 bp) within the 3′UTR of the gene. To test it, a luciferase assay reporter system was set up by amplifying and inserting the 3′UTR of IL-6R into pGL3 that contained downstream firefly luciferase. To identify the real binding site responsible for the interaction, the 2 putative binding sites were mutated individually (mutant-1 and mutant-2) and combinatively (mutant-3) as described in Figure 2. Next, the constructs carrying either wild-type or the mutated 3′UTR of IL-6R were transfected into the cultured mesangial cells that overexpressed miR-125a or the scramble control sequence. As presented in Figure 2, we found that the luciferase activity was significantly decreased in the cells transfected with miR-125a mimics and wild-type, mutant-1, and mutant-2 IL-6R 3′UTR, whereas the luciferase activity was similar between the cells transfected with mutant-3 IL-6R 3′UTR and the control (Figure 3), suggesting that either of the binding sites was sufficient for a full interaction between miR-125a and IL-6R.
Figure 2.
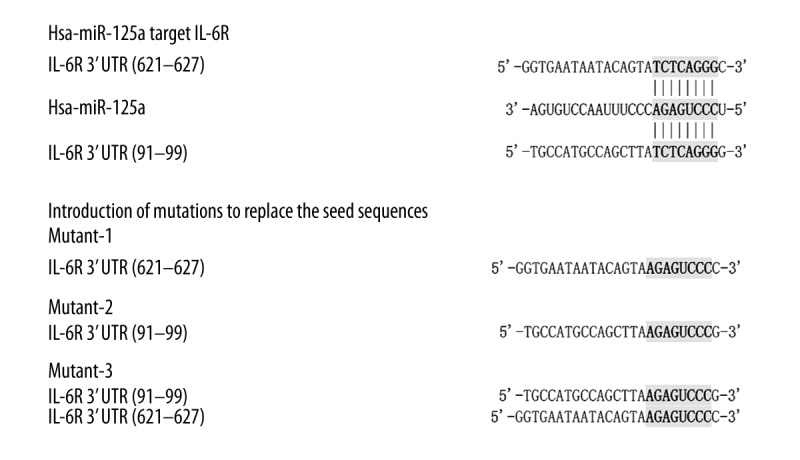
MiR-125a was identified as a possible regulator of IL-6R via 2 putative binding sites (91–99 bp and 621–627 bp) within the 3′UTR of the gene.
Figure 3.
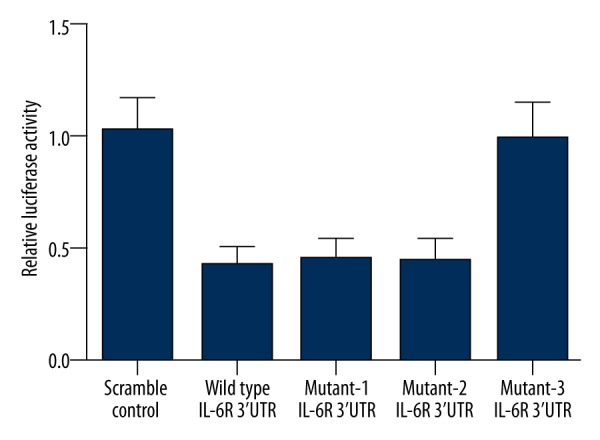
The luciferase activity was significantly decreased in the cells transfected with miR-125a mimics and wild-type, mutant-1, and mutant-2 IL-6R 3′UTR, whereas the luciferase activity was similar between the cells transfected with mutant-3 IL-6R 3′UTR and the controls.
To further verify the regulatory association between miR-125a and IL-6R, we examined the expression of miR-125a in the above-mentioned DN patients and controls, and we showed that the expression of miR-125a was significantly downregulated in DN patients compared with the controls (Figure 4A). We then regrouped the samples based the rs12976445 polymorphism genotypes, and we found that 35, 24, and 5 participants were genotyped as CC, CT, and TT, respectively. As shown in Figure 4B, the expression levels of miR-125a was comparable between TT and CT, so we merged those 2 groups into 1, which was significantly lower than the CC group.
Figure 4.
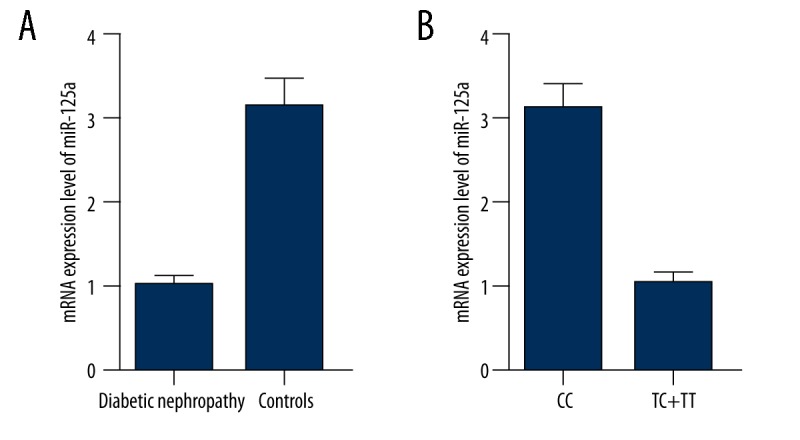
(A) The expression of miR-125a was significantly downregulated in DN patients compared with the controls; (B) The expression levels of miR-125a was comparable between TT and CT.
To further validate IL-6R as a target of miR-125a, we examined the endogenous expression in mesangial cells transfected with miR-125a and IL-6R specific siRNA by using Western blot and real-time PCR analysis. We found that the protein expression level of IL-6R was significantly downregulated by introduction of miR-125a mimics and anti-IL-6R siRNA (Figure 5A); and, consistent with this, the mRNA of IL-6R was also substantially lower in the cells transfected with miR-125a mimics and anti-IL-6R siRNA compared with the control (Figure 5B). To test the effect of downregulation of IL-6R on the proliferation of mesangial cells, we evaluated the viability of the cells transfected with miR-125a and IL-6R specific siRNA. As shown in Figure 6, the viability of the cells transfected with miR-125a and IL-6R specific siRNA were significantly lower than that of the cells transfected with scramble control.
Figure 5.
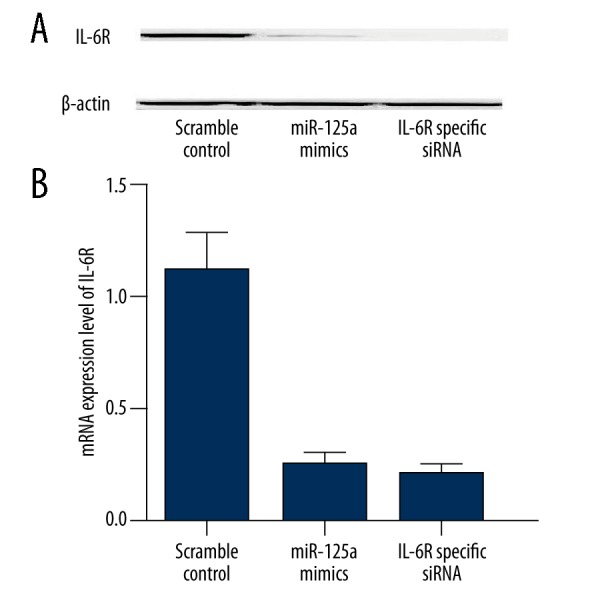
(A) Protein expression level of IL-6R was significantly downregulated by introduction of miR-125a mimics and anti-IL-6R siRNA; (B) The mRNA of IL-6R was also substantially lower in the cells transfected with miR-125a mimics and anti-IL-6R siRNA compared with the control.
Figure 6.
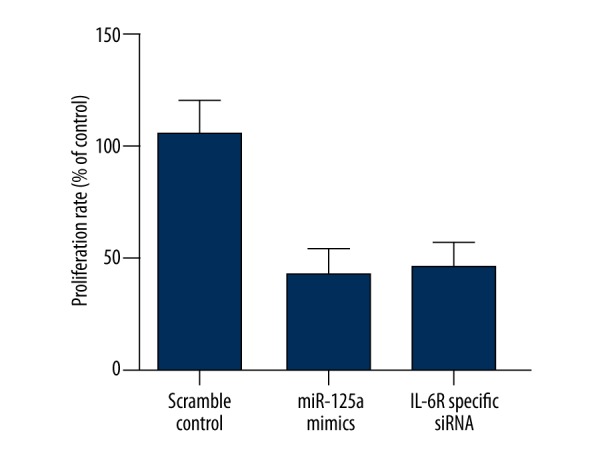
The viability of the cells transfected with miR-125a and IL-6R specific siRNA were significantly lower than that of the cells transfected with scramble control.
To explore the association between rs12976445 polymorphism and risk of DN, we enrolled 594 DM patients with (N=282) or without DN (N=312). The distributions of rs12976445 polymorphism were in Hardy-Weinberg equilibrium among the case group and the controls. As the expression levels of miR-125a was similar between TT and CT, and genotype frequency is low, we combined the 2 genotype groups together in comparison with the wild-type group (CC). The demographic and clinicopathological parameters in the 2 groups are presented in Table 1. As shown in Table 2, significant differences were noted regarding genotype distribution of rs12976445 between the DN and the controls (OR=1.45, 95% C.I.=1.02–2.08, p<0.05) with the possible confounding factors adjusted for by using logistic regression analysis.
Table 2.
Significant differences were noted regarding genotype distribution of rs12976445 between the subjects with or without DMN with the possible confounding factors adjusted by using logistic regression analysis.
| DM with nephropathy (N=282) | DM without nephropathy (N=312) | P value | Adjusted OR (95%CI) | |
|---|---|---|---|---|
| CC | 185 (65.62%) | 230 (73.71%) | ||
| CT | 91 (32.26%) | 78 (25.00%) | ||
| TT | 6 (2.12%) | 4 (1.29%) | ||
| Combined | ||||
| CC | 185 (65.62%) | 230 (73.71%) | 0.0384 | 1.00 |
| CT/TT | 97 (34.38%) | 82 (26.29%) | 1.45 (1.02–2.08) | |
Discussion
A major complication of DM is DN. A study reported that either environmental or genetic factors may cause various pathophysiological cascades correlated with development of DN [24]. In the past decade, studies have suggested that inflammation plays an important role in the occurrence and progression of such kidney injury [24,25]. Researchers first found inflammatory cytokines was associated with the pathogenesis of DN in 1991 [26]. Later, studies have shown that intrinsic renal cells (glomerular, mesangial, tubular epithelial, and endothelial cells) have the ability to synthesize proinflammatory cytokines [27]. As a major modulator of inflammation, Interleukin-6 (IL-6) is elevated in DM patients with DN, and is also presented at increased concentrations in patients with pronounced proteinuria when compared to patients with microalbuminuria [28]. The experimental animal models of DN showed over-activation of IL-6 signaling pathway in the kidney of the patients with DN [29]. In this study, we performed real-time PCR and Western blot analysis to determine the expression of IL-6R in the kidney tissues from the patients with DN and the control patients, and we found that the expression of IL-6R was barely detected in the controls groups, while in the DN groups, the IL-6R was clearly detectable. Similarly, the mRNA expression level of IL-6R was substantially upregulated in the tissue samples from DN patients compared with the controls. To identify the potential regulator of IL-6R in the kidneys, we searched the miRNA database online and identified miR-125a as a possible regulator of IL-6R, with 2 putative binding sites (91–99 bp and 621–627 bp) within the 3′UTR of the gene. To identify the real binding site responsible for the interaction, the 2 putative binding sites were mutated individually (mutant-1 and mutant-2) and combinatively (mutant-3), and we found that the luciferase activity was significantly decreased in the cells transfected with miR-125a mimics and wild-type, mutant-1, and mutant-2 IL-6R 3′UTR, whereas the luciferase activity was similar between the cells transfected with mutant-3 IL-6R 3′UTR and the controls (Figure 3), indicating that either of the binding sites was sufficient for a full interaction between miR-125a and IL-6R. To further validate IL-6R as a target of miR-125a, we examined the endogenous expression in mesangial cells transfected with miR-125a and IL-6R specific siRNA by using Western blot and real-time PCR analysis, and we found that protein and mRNA expression level of IL-6R was significantly downregulated by introduction of miR-125a mimics and anti-IL-6R siRNA.
Suzuki et al. [30] investigated renal samples from patients with DN and observed over-activation of the IL-6/IL-6R signaling pathway in interstitium, mesangium, tubules, and infiltrating cells, which might be responsible for the abnormally enhanced proliferation of mesangial cells [31]. In line with this, it has also been shown that stimulation of the IL-6/IL-6R signaling pathway promoted proliferation of mesangial cells and fibronectin, which subsequently increased the permeability of glomerular endothelial cell [24,25]. To test the effect of downregulation of IL-6R on the proliferation of mesangial cells, we evaluated the viability of the cells transfected with miR-125a and IL-6R specific siRNA, and we found that the viability of the cells transfected with miR-125a and IL-6R specific siRNA were significantly lower than that of the cells transfected with scramble control.
MiR-125a, located at chromosome 19q13.41, has been reported to be involved in pathogenesis of many human diseases as well as development of organs [32,33]. Accumulating studies have suggested that miR-125a is associated with the pathogenesis of human cancers, such as lung, gastric, and breast cancers [32,34–36]. miR-125a may act as either an oncogene or a tumor suppressor, depending on the cellular context. For instance, the downregulation of miR-125a was observed in some cancers, such as leukemia [32] and lung cancer [35,36], where the increased expression suppressed the proliferation of cancer cells and promoted apoptosis. Recent evidence showed that mature miRNA processing and mature miR-125a expression may be disrupted by the T allele of rs12976445, resulting in augmented production of target genes, including LIFR and ERBB2 [22, 38]. It has been previously shown that a SNP (rs12976445) is present in the pre-miR-125a gene, and it compromises the mature processing of the miRNA, which leads to an increased risk of autoimmune thyroid conditions [21] and repeated pregnancy loss [22]. In this study, we studied the association between rs12976445 polymorphism and risk of DN in a total of 594 DM patients with (N=282) or without DN (N=312), and we found that rs12976445 was significantly associated with DN (OR=1.45, 95% C.I.=1.02–2.08, p<0.05) after the possible confounding factors were adjusted for by using logistic regression analysis.
Conclusions
Our results showed that miR-125a is a key player in the development of DN via targeting IL-6R, and underexpression of miR-125a promoted proliferation of mesangial cells by releasing the inhibition on IL-6R. Downregulation of miR-125a could be, at least partially, attributed to the presence of rs12976445 polymorphism, which compromises the mature processing of the miRNA, demonstrating that miR-125a is a promising novel diagnostic and therapeutic target for DN.
Footnotes
Conflict of interest
No conflict of interest is claimed by the authors.
Source of support: Departmental sources
References
- 1.Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4:444–52. doi: 10.1038/ncpendmet0894. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez ML, Distefano JK. The role of non-coding RNAs in diabetic nephropathy: potential applications as biomarkers for disease development and progression. Diabetes Res Clin Pract. 2013;99:1–11. doi: 10.1016/j.diabres.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Sun YM, Su Y, Li J, Wang LF. Recent advances in understanding the biochemical and molecular mechanism of diabetic nephropathy. Biochem Biophys Res Commun. 2013;433:359–61. doi: 10.1016/j.bbrc.2013.02.120. [DOI] [PubMed] [Google Scholar]
- 4.Horii Y, Muraguchi A, Iwano M, et al. Involvement of IL-6 in mesangial proliferative glomerulonephritis. J Immunol. 1989;143:3949–55. [PubMed] [Google Scholar]
- 5.Heptinstall RH. Diabetes mellitus: Pathology of the Kidney. 3rd ed. Boston: Little, Brown; 1983. pp. 1397–453. [Google Scholar]
- 6.Hayashi H, Karasawa R, Inn H, et al. An electron microscopic study of glomeruli in Japanese patients with non-insulin dependent diabetes mellitus. Kidney Int. 1992;41:749–57. doi: 10.1038/ki.1992.117. [DOI] [PubMed] [Google Scholar]
- 7.Kida H, Yoshimura M, Ikeda K, et al. Pathogenesis of diabetic nephropathy in non-insulin-dependent diabetes mellitus. J Diabet Complications. 1991;5:82–83. doi: 10.1016/0891-6632(91)90025-k. [DOI] [PubMed] [Google Scholar]
- 8.Bangstad HJ, Osterby R, Dahl-Jorgensen K, et al. Improvement of blood glucose control in IDDM patients retards the progression of morphological changes in early diabetic nephropathy. Diabetologia. 1994;37:483–90. doi: 10.1007/s001250050136. [DOI] [PubMed] [Google Scholar]
- 9.van den Dobbelsteen ME, van der Woude FJ, Schroeijers WE, et al. Binding of dimeric and polymeric IgA to rat renal mesangial cells enhances the release of interleukin 6. Kidney Int. 1994;46:512–19. doi: 10.1038/ki.1994.302. [DOI] [PubMed] [Google Scholar]
- 10.Kishimoto T, Tanaka T, Yoshida K, et al. Cytokine signal transduction through a homo- or heterodimer of gp130. Ann NY Acad Sci. 1995;766:224–34. doi: 10.1111/j.1749-6632.1995.tb26670.x. [DOI] [PubMed] [Google Scholar]
- 11.Jones SA, Horiuchi S, Topley N, et al. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001;15:43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- 12.Pecoits-Filho R, Barany P, Lindholm B, et al. Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant. 2002;17:1684–88. doi: 10.1093/ndt/17.9.1684. [DOI] [PubMed] [Google Scholar]
- 13.Kado S, Nagase T, Nagata N. Circulating levels of interleukin-6, its soluble receptor and interleukin-6/interleukin-6 receptor complexes in patients with type 2 diabetes mellitus. Acta Diabetol. 1999;36:67–72. doi: 10.1007/s005920050147. [DOI] [PubMed] [Google Scholar]
- 14.Chang RC, Ying W, Bazer FW, Zhou B. MicroRNAs control macrophage formation and activation: the inflammatory link between obesity and cardiovascular diseases. Cells. 2014;3:702–12. doi: 10.3390/cells3030702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper JD, Smyth DJ, Bailey R, et al. The candidate genes TAF5L, TCF7, PDCD1, IL6 and ICAM1 cannot be excluded from having effects in type 1 diabetes. BMC Med Genet. 2007;8:71. doi: 10.1186/1471-2350-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristiansen OP, Nolsoe RL, Larsen L, et al. Association of a functional 17beta-estradiol sensitive IL6-174G/C promoter polymorphism with early-onset type 1 diabetes in females. Hum Mol Genet. 2003;12:1101–10. doi: 10.1093/hmg/ddg132. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes A. Standards of medical care in diabetes – 2013. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez JR, Armengol L, Sole X, et al. SNPassoc: an R package to perform whole genome association studies. Bioinformatics. 2007;23:644–45. doi: 10.1093/bioinformatics/btm025. [DOI] [PubMed] [Google Scholar]
- 19.Melendez-Ramirez LY, Richards RJ, Cefalu WT. Complications of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:625–40. doi: 10.1016/j.ecl.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 20.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 21.Inoue Y, Watanabe M, Inoue N, et al. Associations of single nucleotide polymorphisms in precursor-microRNA (miR)-125a and the expression of mature miR-125a with the development and prognosis of autoimmune thyroid diseases. Clin Exp Immunol. 2014;178:229–35. doi: 10.1111/cei.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Y, Liu CM, Qi L, et al. Two common SNPs in pri-miR-125a alter the mature miRNA expression and associate with recurrent pregnancy loss in a Han-Chinese population. RNA Biol. 2011;8:861–72. doi: 10.4161/rna.8.5.16034. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Zhang Z, Chu W, et al. Molecular screening and association analyses of the interleukin 6 receptor gene variants with type 2 diabetes, diabetic nephropathy, and insulin sensitivity. J Clin Endocrinol Metab. 2005;90:1123–29. doi: 10.1210/jc.2004-1606. [DOI] [PubMed] [Google Scholar]
- 24.Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, Garcia-Perez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327–40. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 25.Lim AKH, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm. 2012;2012:1–12. doi: 10.1155/2012/146154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasegawa G, Nakano K, Sawada M, et al. Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int. 1991;40:1007–12. doi: 10.1038/ki.1991.308. [DOI] [PubMed] [Google Scholar]
- 27.Sugimoto H, Shikata K, Wada J, et al. Advanced glycation end products-cytokine-nitric oxide sequence pathway in the development of diabetic nephropathy: aminoguanidine ameliorates the overexpression of tumour necrosis factor-alpha and inducible nitric oxide synthase in diabetic rat glomeruli. Diabetologia. 1999;42:878–86. doi: 10.1007/s001250051241. [DOI] [PubMed] [Google Scholar]
- 28.Saraheimo M, Teppo AM, Forsblom C, et al. Diabetic nephropathy is associated with low-grade inflammation in Type 1 diabetic patients. Diabetologia. 2003;46:1402–7. doi: 10.1007/s00125-003-1194-5. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Nino MD, Bozic M, Cordoba-Lanus E, et al. Beyond proteinuria: VDR activation reduces renal inflammation in experimental diabetic nephropathy. Am J Physiol Renal Physiol. 2012;302:F647–57. doi: 10.1152/ajprenal.00090.2011. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki D, Miyazaki M, Naka R, et al. In situ hybridization of interleukin 6 in diabetic nephropathy. Diabetes. 1995;44:1233–38. doi: 10.2337/diab.44.10.1233. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura A, Suzuki T, Kohsaka T. Renal tubular function modulates urinary levels of interleukin-6. Nephron. 1995;70:416–20. doi: 10.1159/000188638. [DOI] [PubMed] [Google Scholar]
- 32.Shaham L, Binder V, Gefen N, et al. MiR-125 in normal and malignant hematopoiesis. Leukemia. 2012;26:2011–18. doi: 10.1038/leu.2012.90. [DOI] [PubMed] [Google Scholar]
- 33.Leotta M, Biamonte L, Raimondi L, et al. A p53-dependent tumor suppressor network is induced by selective miR-125a-5p inhibition in multiple myeloma cells. J Cell Physiol. 2014;229:2106–16. doi: 10.1002/jcp.24669. [DOI] [PubMed] [Google Scholar]
- 34.Nishida N, Mimori K, Fabbri M, et al. MicroRNA-125a-5p is an independent prognostic factor in gastric cancer and inhibits the proliferation of human gastric cancer cells in combination with trastuzumab. Clin Cancer Res. 2011;17:2725–33. doi: 10.1158/1078-0432.CCR-10-2132. [DOI] [PubMed] [Google Scholar]
- 35.Zhu WY, Luo B, An JY, et al. Differential expression of miR-125a-5p and let-7e predicts the progression and prognosis of non-small cell lung cancer. Cancer Invest. 2014l;32:394–401. doi: 10.3109/07357907.2014.922569. [DOI] [PubMed] [Google Scholar]
- 36.Jiang L, Chang J, Zhang Q, et al. MicroRNA hsa-miR-125a-3p activates p53 and induces apoptosis in lung cancer cells. Cancer Invest. 2013;31:538–44. doi: 10.3109/07357907.2013.820314. [DOI] [PubMed] [Google Scholar]
- 37.Lehmann TP, Korski K, Ibbs M, et al. rs12976445 variant in the pri-miR-125a correlates with a lower level of hsa-miR-125a and ERBB2 overexpression in breast cancer patients. Oncol Lett. 2013;5:569–73. doi: 10.3892/ol.2012.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]


