Abstract
Background
High- and low-flux hemodialysis (HFHD and LFHD, respectively) are dialysis procedures designed to eliminate blood toxins that accumulate in end-stage renal disease. HFHD may reduce vascular calcification by removing serum fibroblast growth factor 23 (FGF-23). However, whether HFHD is better than LFHD is still under debate. We therefore compared the efficacy of HFHD and LFHD in controlling FGF-23 and vascular calcification.
Material/Methods
Fifty hemodialysis patients were recruited and randomly treated with either HFHD or LFHD. Fasting venous blood was collected at baseline, six months, and twelve months after the treatment. We then measured levels of FGF-23, calcium, phosphorus, parathyroid hormone, and alkaline phosphatase. Further, abdominal lateral radiographs were taken to calculate aorta abdominalis calcification scores (AACs).
Results
Compared to the LFHD group, FGF-23 and AACs in the HFHD group significantly decreased after 12 months treatment (p=0.049 and p=0.002, respectively). AACs were positively correlated with FGF-23 in all patients (p=0.004), the HFHD group alone (p=0.040), and the LFHD group alone (p=0.037). We also found that older patients, patients with higher blood phosphorus levels, and higher FGF-23 levels had an increased risk of aorta abdominalis calcification (p=0.048, p=0.003, p=0.001, respectively). HFHD was more able to reduce the risk of aorta abdominalis calcification than LFHD (p=0.003).
Conclusions
FGF-23 is an independent risk factor for the development of vascular calcification. HFHD may benefit hemodialysis patients by reducing serum FGF-23 levels and controlling vascular calcification.
MeSH Keywords: Fibroblast Growth Factors; Hemodialysis Solutions; Renal Insufficiency, Chronic; Vascular Calcification
Background
It is reported that nearly 40% of patients with ESRD (end stage renal disease) die of cardiovascular disease [1,2]. Cardiovascular disease is also a major challenge in renal transplantation [3]. A major factor leading to cardiovascular disease in hemodialysis patients is vascular calcification [4,5], which causes vascular stiffness, higher pulse wave velocities, and elevated pulse pressures [6]. It is thought that vascular calcification is caused by disrupted mineral metabolism and abnormal regulation of phosphorus and calcium [7,8].
Fibroblast growth factor 23 (FGF-23) is an osteocyte-derived hormone that participates in phosphate and vitamin D metabolism [9,10]. Elevated FGF-23 levels are observed in phase 2 of chronic kidney disease and are inversely correlated with glomerular filtration rate [11]. Patients with ESRD may reach FGF-23 blood levels around 10–1000 times higher than normal [12]. Even within the normal range, a strong association was observed between high serum FGF-23 levels and atherosclerotic burden, endothelial dysfunction, and arterial stiffness [13,14]. Elevated FGF-23 serum levels are particularly implicated in the calcification of peripheral vasculature and the coronary artery [7,15,16]. FGF-23 may therefore be an independent predictor of cardiovascular disease in dialysis patients [17,18].
High-flux hemodialysis (HFHD) is a dialysis procedure designed to eliminate blood toxins that accumulate in ESRD. Previous studies show that HFHD may reduce vascular stiffness [19] and remove FGF-23 [20]. Whether HFHD is better than low-flux hemodialysis (LFHD) is still under debate. In this study, we compared the efficacy of HFHD and LFHD in reducing vascular calcification and serum FGF-23 levels in patients receiving maintenance hemodialysis.
Material and Methods
The study was conducted according to the Declaration of Helsinki and was approved by the ethics committee of Xiangya Hospital, Zhongnan University. All participants signed informed consent forms. This study was registered as a clinical trial (Chinese Clinical Trial Registry, www.chictr.org.cn, ChiCTR-OPC-15006249).
From June 2013 to June 2014, fifty patients receiving maintenance hemodialysis were recruited to participate. Inclusion criteria was as follows: 18–80 years of age, previously treated with hemodialysis for at least 3 months (3× per week; 4 h per session), dry weight was relatively stable, arteriovenous fistula was created for hemodialysis, and blood flow rate was ≥200 ml/min. Exclusion criteria were as follows: patients with severe infections in the past three months, hepatic disease, or previous hemodiafiltration or peritoneal dialysis treatments.
Patients were randomly allocated into either the HFHD group or the LFHD group (25 per group). Patients received respective treatments for 12 months, which was performed 4 h per session three times per week. The hemodialysis machines utilized in this study were a Fresenius 4008S (Bad Homburg, Germany) and a Gambro AK96 (Lund, Sweden). The dialysate flow rate was set at 500 ml/min, blood flow was set at 250–300 ml/min, and the urea clearance index (Kt/V) was ≥1.2. A CA-HP170 dialyzer (Baxter, Deerfield, USA) was used for LFHD. The surface area of the tri-cellulose acetate (TCA) membrane was 1.7 m2 and the ultrafiltration coefficient was 10.0 ml/hr/mmHg. A CT-190G dialyzer (Baxter, Deerfield, USA) was utilized for HFHD. The surface area of the TCA membrane was 1.9 m2 and the ultrafiltration coefficient was 36.0 ml/hr/mmHg.
Fasting venous blood was collected at baseline, six months, and twelve months after treatment. Samples were centrifuged at 3000 rpm/min for 10 min. Serum was then collected and stored at −80°C. FGF-23 levels were measured by commercial ELISA kits (Millipore Inc, USA) according to the manufacturer’s instructions. Calcium, phosphorus, AKP, albumin, creatinine, and lipid levels were measured by standard methods. PTH was measured by an electrochemiluminescence immunoassay (Roche Diagnostics, Germany).
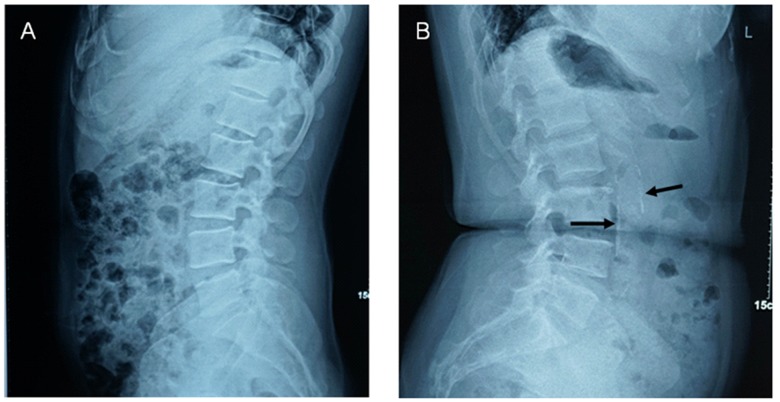
Aorta abdominalis calcification was evaluated by lateral radiography of the abdomen. Aorta abdominalis calcification scores (AACs, Figure 1) were calculated according to the length of calcified deposits on the anterior and posterior walls of the aorta abdominalis, which corresponds to lumbar vertebrae 1–4. Anterior and posterior calcification scores were recorded separately for each vertebra (i.e. eight separate scores were recorded). Scoring was performed blindly by two independent doctors as follows: 0 point, no calcification; 1 point, calcification of <1/3 of the vascular wall; 2 points, calcification of 1/3–2/3 of the vascular wall; 3 points, calcification of >2/3 of the vascular wall [21–23]. Final AACs were calculated by summing the eight separate scores, ranging from 0 to 24 points. The mean score between the two doctors was considered as the final score.
Figure 1.
Examples of lateral abdominal radiographs. A. 45-year-old man; AACs=0. B. 62-year-old woman; AACs=8. Arrows point to calcified plaque.
Statistical analyses were performed in SPSS 16.0. T-tests were performed for comparing between different groups of the patients’ characteristics data. Chi-squared tests were used for enumeration data. Repeated measures analysis of variance was used to examine changes in serum calcium, phosphorus, PTH, AKP, FGF-23, and AACs from baseline to 12 months after treatment. Pearson correlation analysis was applied to compute the correlation between FGF-23 with AACs. Multiple regression analyses were also carried out to screen potential factors influencing the development of vascular calcification. These factors included age, duration of hemodialysis, blood pressure, FGF-23 levels, therapy alternatives, blood calcium levels, and blood phosphorus levels. P < 0.05 was considered to be statistically significant.
Results
Seventy-three patients were initially screened in the study. However, 7 patients declined to participate and 16 patients were excluded (2 cases for severe infection within 3 months, 11 cases for hepatic disease, and 3 cases for previous hemodiafiltration or peritoneal dialysis treatments). The remaining 50 patients were randomly allocated into either the HFHD or LFHD group (25 in each).
The HFHD group included 23 chronic glomerulonephritis patients, one diabetic nephropathy patient, and one obstructive nephropathy patient. The LFHD group was comprised of 19 chronic glomerulonephritis patients, four diabetic nephropathy patients, and two hypertensive nephropathy patients. Over the one-year follow-up period, one HFHD participant was excluded for receiving a kidney transplant. In the LFHD group, one patient was excluded for kidney transplants, one patient died, and three others dropped out of the study.
No significant differences in basic patient characteristics (e.g. age, sex, co-morbidity diseases, blood pressure, lipid levels, and Kt/V) were detected between the HFHD and LFHD groups (p>0.05; Table 1).
Table 1.
Patients’ characteristics.
| HFHD (n=24) | LFHD (n=20) | p | |
|---|---|---|---|
| Age (years) | 48.17±13.48 | 47.55±14.72 | 0.885 |
| Male patients | 14 (58.3%) | 10 (50.0%) | 0.580 |
| Duration of hemodialysis (years) | 2.74±2.08 | 2.56±1.99 | 0.773 |
| Dry weight (kg) | 59.15±13.05 | 57.85±13.73 | 0.750 |
| Co-morbidity | |||
| Coronary artery disease | 10 (41.7%) | 7 (35.0%) | 0.651 |
| Stroke | 1 (4.2%) | 2 (10.0%) | 0.870 |
| Hypertension | 18 (75.0%) | 16 (80.0%) | 0.974 |
| Diabetes | 5 (20.8%) | 3 (15.0%) | 0.915 |
| Systolic pressure (mmHg) | 139.79±14.33 | 147.00±12.29 | 0.084 |
| Diastolic pressure (mmHg) | 82.29±9.55 | 87.00±8.18 | 0.090 |
| Total cholesterol (mmol/l) | 4.51±1.18 | 4.55±1.52 | 0.922 |
| LDL cholesterol (mmol/l) | 2.44±0.96 | 2.80±1.11 | 0.252 |
| HDL cholesterol (mmol/l) | 1.33±0.42 | 1.36±0.44 | 0.795 |
| Triglycerides (mmol/l) | 1.95±1.45 | 1.65±0.70 | 0.402 |
| Hemoglobin (g/l) | 98.63±9.80 | 93.15±12.21 | 0.106 |
| Albumin (g/l) | 11.03±3.67 | 12.57±2.37 | 0.115 |
| Creatinine (mg/dl) | 41.54±3.84 | 39.76±2.31 | 0.077 |
| Kt/V | 1.34±0.14 | 1.33±0.12 | 0.688 |
HFHD – high-flux hemodialysis; LFHD – low-flux hemodialysis; LDL – low-density lipoprotein; HDL – high-density lipoprotein; Kt/V – urea clearance index.
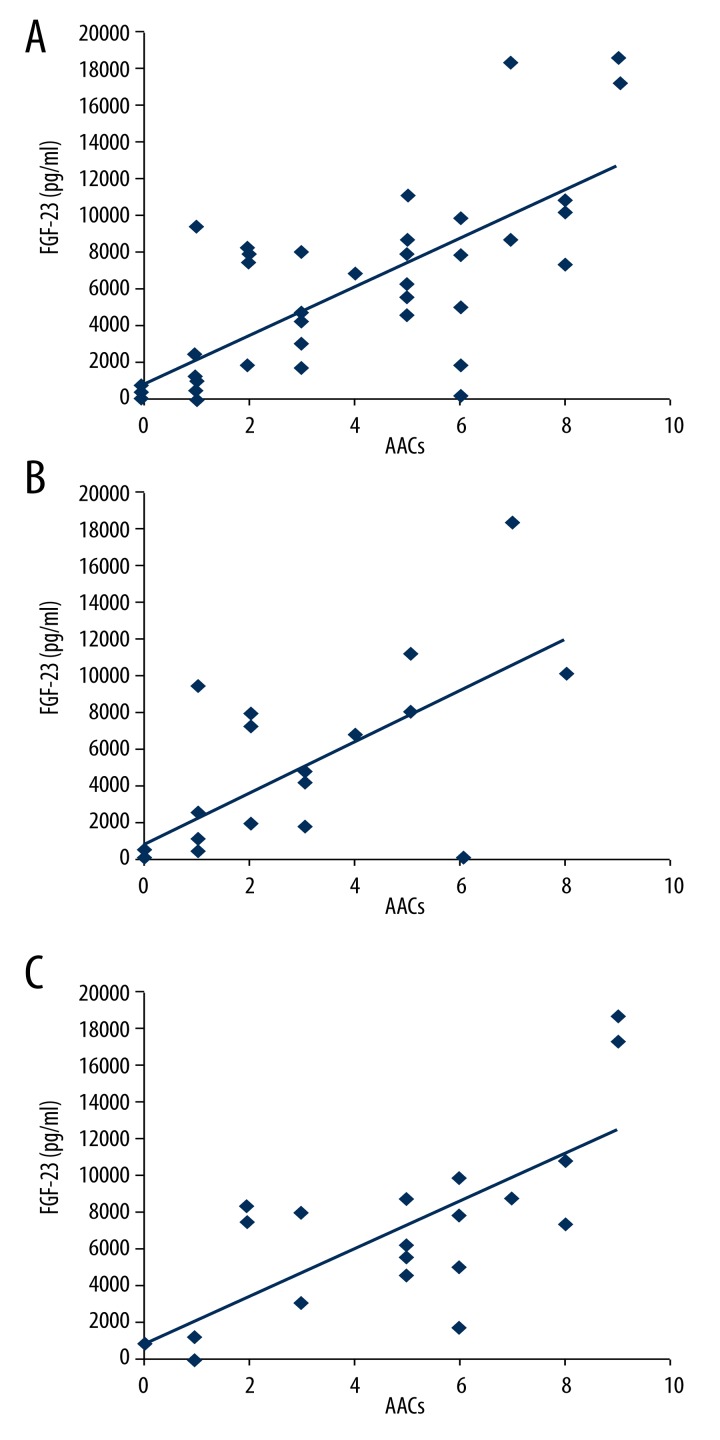
Six months after treatment, we found no significant difference in calcium, phosphorus, parathyroid hormone (PTH), alkaline phosphatase (AKP), FGF-23, or AACs between the two groups (p>0.05). After 12 months of treatment, FGF-23 levels and AACs significantly decreased in the HFHD group, but not the LFHD group (p=0.049 and p=0.002, respectively; Table 2). FGF-23 levels and AACs in the HFHD group decreased by 41.4±23.4% and 30.0±40.3% respectively, while they increased by 0.3±26.4% and 76.4±5.6% in the LFHD group, respectively. Pearson correlation analysis revealed that AACs were positively correlated with FGF-23 levels in all patients (p=0.004; Figure 2A), the HFHD group alone (p=0.040; Figure 2B), and the LFHD group alone (p=0.037, Figure 2C). Multiple regression analysis indicated that older patients (p=0.048), those with higher blood phosphorus levels (p=0.003), and higher FGF-23 levels (p=0.001) had significantly increased risks of aorta abdominalis calcification. It also showed that HFHD reduced the risk of aorta abdominalis calcification, compared to LFHD (p=0.003; Table 3).
Table 2.
Changes of clinical parameters during the treatment.
| 0 Month | 6 Month | 12 Month | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HFHD | LFHD | P | HFHD | LFHD | P | HFHD | LFHD | P | |
| Calcium (mg/dl) | 9.02 ±0.90 | 8.62 ±0.85 | 0.139 | 9.07 ±0.82 | 8.66 ±0.94 | 0.127 | 9.12 ±0.99 | 8.74 ±1.05 | 0.217 |
| Phosphorus (mg/dl) | 6.36 ±1.22 | 6.02 ±1.52 | 0.409 | 6.33 ±1.88 | 6.10 ±1.44 | 0.662 | 5.88 ±1.45 | 6.11 ±1.31 | 0.592 |
| PTH (pg/ml) | 468.87 ±282.78 | 434.41 ±244.13 | 0.671 | 524.49 ±331.25 | 454.11 ±381.26 | 0.516 | 487.10 ±394.94 | 552.02 ±442.42 | 0.610 |
| AKP (U/L) | 144.86 ±64.61 | 141.54 ±61.11 | 0.863 | 128.41 ±90.13 | 132.12 ±65.27 | 0.879 | 120.78 ±85.74 | 120.85 ±51.27 | 0.997 |
| FGF-23 (pg/ml) | 7042.93 ±6246.53 | 7072.11 ±6615.59 | 0.988 | 5701.58 ±5338.70 | 6569.73 ±5803.68 | 0.608 | 4125.07 ±4785.17 | 7090.53 ±4864.97 | 0.049 |
| AACs | 3.33 ±4.01 | 2.75 ±2.88 | 0.589 | 3.25 ±3.91 | 3.50 ±2.72 | 0.811 | 2.33 ±2.39 | 4.85 ±2.72 | 0.002 |
HFHD – high-flux hemodialysis; LFHD – low-flux hemodialysis; PTH – parathyroid Hormone; AKP – alkline phosphatase; FGF-23 – fibroblast growth factor 23; AACs – aorta abdominalis calcification scores.
Figure 2.
Correlations between AACs and FGF-23. (A) Correlation of all patients. (B) Correlation in the HFHD group. (C) Correlation in the LFHD group.
Table 3.
Multiple-regression analysis to screen potential factors influencing the development of vascular calcification.
| β | 95% CI | p | |
|---|---|---|---|
| Age | 0.044 | 0.000~0.088 | 0.048 |
| FGF-23 | <0.001 | 0.000~0.002 | 0.001 |
| Therapy alternatives | −1.818 | −2.976~−0.660 | 0.003 |
| Phosphorus | 0.637 | 0.232~1.042 | 0.003 |
| Systolic pressure | 0.001 | −0.039~0.041 | 0.951 |
| Dialysis duration | −0.147 | −0.414~0.120 | 0.272 |
| Calcium | 0.471 | −0.034~0.976 | 0.067 |
Discussion
Our study supports findings that serum FGF-23 levels are positively correlated with aorta abdominalis calcification. Further, it shows that HFHD is more effective than LFHD in clearing serum FGF-23 and reducing aorta abdominalis calcification. Moreover, multiple-regression analysis shows that advanced age, serum FGF-23, and blood phosphorus are risk factors for the development of vascular calcification. Further, we show that HFHD is more effective than LFHD.
FGF-23 is a mineral regulatory factor that regulates phosphate homeostasis in chronic kidney disease patients. FGF-23 decreases renal phosphate reabsorption and increases urinary phosphate waste by decreasing the expression of sodium-phosphate transporter in proximal tubular epithelial cells [20]. Compromised renal function may result in the accumulation of phosphorus and decreased clearance of blood FGF-23 levels [24,25]. Previous studies show that increased FGF-23 levels are correlated with increased mortality and vascular calcification in hemodialysis patient [15,18], which is supported by the results presented here.
It is reasonable to consider that increasing FGF-23 clearance benefits hemodialysis patients. Molecular weight cut-offs of traditional hemodialysis membranes range from 20 to 30 kDa. Thus, FGF-23 is not removed since it has a molecular weight of 32 kDa. Though we detected a small fluctuation of FGF-23 levels in the LFHD group, significant increases were not observed in our study. Torres et al. (2008) found that LFHD treatment did not decrease serum FGF-23 concentration [26]. In contrast, it has been shown that alpha1-microglobulin, which has a molecular weight of 33 kDa, is reduced by 21% using a high flux polyether-sulfone membrane [27]. Another study found that the FGF-23 clearance rate by HFHD reaches to 36.2±28.6%, which is substantially lower than on-line hemodiafiltration, which has a FGF-23 clearance rate of 55.7±25.2%) [20]. In the present study, the clearance rate of FGF-23 in the HFHD group was 41.4±23.4% after 12 months of treatment, while FGF-23 increased 0.3±26.4% in the LFHD group.
Interestingly, we found that aorta abdominalis calcification is better controlled by HFHD than LFHD. Further, we find that advanced age, blood phosphorus levels, and serum FGF-23 levels are all independent risk factors for the development of vascular calcification, which agrees with a previous study [7]. Another study, including 1130 healthy males, also suggests that circulating FGF-23 is associated with mineral metabolism, including bone metabolism-regulating cytokines, and with severe AACs independent of traditional risk factors [28]. Mirza et al. (2009) report that higher serum FGF-23 levels, even within normal range, are independently correlated with increased arterial stiffness and impaired vasoreactivity [14]. These elevated FGF-23 concentrations may then stimulate vascular calcification by acting directly on the vascular wall to induce a local reduction of the enzyme Klotho [29]. Interestingly, a recent study indicates that hemodiafiltration appears to offer no extra benefit over HFHD in terms of controlling vascular stiffness [19]. This may due to the limited follow-up period or that the efficacies of hemodiafiltration and HFHD are similar in controlling vascular calcification. Here, we show that the improvement of AACs in HFHD compared to LFHD is not significant at 6 months, but becomes significant at 12 months (P=0.002).
Although the results presented here are encouraging, a few limitations should be considered. First, the reliability of the study may be impaired by the small cohort size and short follow-up time. Larger studies are needed to verify the present findings. Second, in 2009, KDIGO (Kidney Disease: Improving Global Outcomes) guidelines suggested that an echocardiogram should also be used to detect the valvular calcification in ESRD patients. This was not performed in the present study. Third, all patients were from a single center. Whether the cohort represents the whole population remains unknown.
Conclusions
FGF-23 is an independent risk factor for the development of vascular calcification. HFHD is superior to LFHD and benefits hemodialysis patients by reducing serum FGF-23 levels and reducing vascular calcification.
Acknowledgements
We also would like to thank all participants enrolled in this study.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare regarding the contents of this study.
Source of support: The study was supported by a Renal Research Grant from Baxter China (No. CHN-RENAL-IIS-2012-014)
References
- 1.Nakai S, Iseki K, Itami N, et al. Overview of regular dialysis treatment in Japan (as of 31 December 2009) Ther Apher Dial. 2012;16:11–53. doi: 10.1111/j.1744-9987.2011.01050.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang S, Qin L, Wu T, et al. Elevated cardiac markers in chronic kidney disease as a consequence of hyperphosphatemia-induced cardiacmyocyte injury. Med Sci Monit. 2014;20:2043–53. doi: 10.12659/MSM.890909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sagban TA, Baur B, Schelzig H, et al. Vascular challenges in renal transplantation. Ann Transplant. 2014;19:464–71. doi: 10.12659/AOT.890893. [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482–94. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 5.Kraus MA, Kalra PA, Hunter J, et al. The prevalence of vascular calcification in patients with end-stage renal disease on hemodialysis: a cross-sectional observational study. Ther Adv Chronic Dis. 2015;6:84–96. doi: 10.1177/2040622315578654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsao CW, Pencina KM, Massaro JM, et al. Cross-sectional relations of arterial stiffness, pressure pulsatility, wave reflection, and arterial calcification. Arterioscler Thromb Vasc Biol. 2014;34:2495–500. doi: 10.1161/ATVBAHA.114.303916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasrallah MM, Elshehaby AR, Salem MM, et al. Fibroblast growth factor-23 (FGF-23) is independently correlated to aortic calcification in haemodialysis patients. Nephrol Dial Transplant. 2010;25:2679–85. doi: 10.1093/ndt/gfq089. [DOI] [PubMed] [Google Scholar]
- 8.Karwowski W, Naumnik B, Szczepański M, Myśliwiec M. The mechanismof vascular calcification – a systematic review. Med Sci Monit. 2012;18(1):RA1–11. doi: 10.12659/MSM.882181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heine GH, Seiler S, Fliser D. FGF-23: the rise of a novel cardiovascularrisk marker in CKD. Nephrol Dial Transplant. 2012;27:3072–81. doi: 10.1093/ndt/gfs259. [DOI] [PubMed] [Google Scholar]
- 10.Zeng Y, Feng S, Han OY, et al. Role of fibroblast growth factor-23 in the pathogenesis of atherosclerosis in peritoneal dialysis patients. Genet Mol Res. 2015;14:719–29. doi: 10.4238/2015.January.30.15. [DOI] [PubMed] [Google Scholar]
- 11.Ix JH, Shlipak MG, Wassel CL, Whooley MA. Fibroblast growth factor-23 and early decrements in kidney function: the Heart and Soul Study. Nephrol Dial Transplant. 2010;25:993–97. doi: 10.1093/ndt/gfp699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakanishi S, Kazama JJ, Nii KT, et al. Serum fibroblast growth factor-23 levels predict the future refractory hyperparathyroidism in dialysis patients. Kidney Int. 2005;67:1171–78. doi: 10.1111/j.1523-1755.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- 13.Mirza MA, Hansen T, Johansson L, et al. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant. 2009;24:3125–31. doi: 10.1093/ndt/gfp205. [DOI] [PubMed] [Google Scholar]
- 14.Mirza MA, Larsson A, Lind L, Larsson TE. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205:385–90. doi: 10.1016/j.atherosclerosis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Jean G, Bresson E, Terrat JC, et al. Peripheral vascular calcification in long-haemodialysis patients: associated factors and survival consequences. Nephrol Dial Transplant. 2009;24:948–55. doi: 10.1093/ndt/gfn571. [DOI] [PubMed] [Google Scholar]
- 16.Gutiérrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–52. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jean G, Terrat JC, Vanel T, et al. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant. 2009;24:2792–96. doi: 10.1093/ndt/gfp191. [DOI] [PubMed] [Google Scholar]
- 18.Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–92. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charitaki E, Davenport A. Does hemodiafiltration reduce vascular stiffness measured by aortic pulse wave velocity compared with high-flux hemodialysis? Hemodial Int. 2014;18:391–95. doi: 10.1111/hdi.12119. [DOI] [PubMed] [Google Scholar]
- 20.Patrier L, Dupuy AM, Granger VA, et al. FGF-23 removal is improved by on-line high-efficiency hemodiafiltration compared to conventional highflux hemodialysis. J Nephrol. 2013;26:342–49. doi: 10.5301/jn.5000150. [DOI] [PubMed] [Google Scholar]
- 21.Kauppila LI, Polak JF, Cupples LA, et al. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132:245–50. doi: 10.1016/s0021-9150(97)00106-8. [DOI] [PubMed] [Google Scholar]
- 22.Honkanen E, Kauppila L, Wikström B, et al. Abdominal aortic calcification in dialysis patients: results of the CORD study. Nephrol Dial Transplant. 2008;23:4009–15. doi: 10.1093/ndt/gfn403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogawa FN, Yamaguchi T, Yamamoto M, et al. Serum osteocalcin levels are inversely associated with abdominal aortic calcification in men with type 2 diabetes mellitus. Osteoporos Int. 2013;24:2223–30. doi: 10.1007/s00198-013-2289-6. [DOI] [PubMed] [Google Scholar]
- 24.Yamada S, Tsuruya K, Tokumoto M, et al. Fibroblast growth factor 23, but not parathyroid hormone, is associated with urinary phosphate regulation in patients on peritoneal dialysis. Ther Apher Dial. 2015;19:73–80. doi: 10.1111/1744-9987.12221. [DOI] [PubMed] [Google Scholar]
- 25.Gutierrez OM, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–15. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 26.Urena TP, Friedlander G, Devernejoul MC, Silve C, Prie D. Bone mass does not correlate with the serum fibroblast growth factor 23 in hemodialysis patients. Kidney Int. 2008;73:102–7. doi: 10.1038/sj.ki.5002622. [DOI] [PubMed] [Google Scholar]
- 27.Maduell F, Sanchez CJ, Blasco JA, et al. Middle molecules removal bey ond beta2-microglobulin. Nefrologia. 2006;26:469–75. [PubMed] [Google Scholar]
- 28.Schoppet M, Hofbauer LC, Brinskelle SN, et al. Serum level of the phosphaturic factor FGF23 is associated with abdominal aortic calcification in men: the STRAMBO study. J Clin Endocrinol Metab. 2012;97:575–83. doi: 10.1210/jc.2011-2836. [DOI] [PubMed] [Google Scholar]
- 29.Donate CJ, Mora FC, Martínez SR, et al. Expression of FGF23/KLOTHO system in human vascular tissue. Int J Cardiol. 2013;165:179–83. doi: 10.1016/j.ijcard.2011.08.850. [DOI] [PubMed] [Google Scholar]