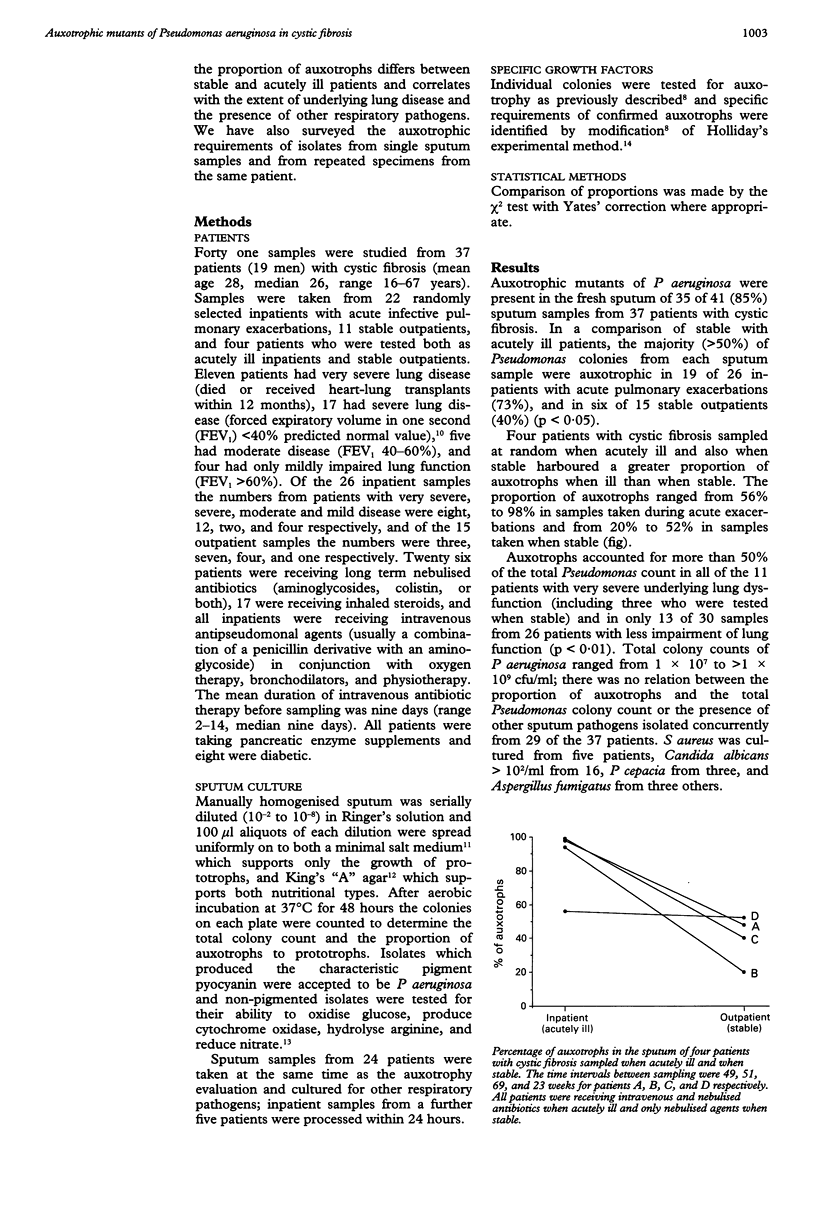
Abstract
BACKGROUND--Pseudomonas aeruginosa has been located in the endobronchiolar spaces of patients with cystic fibrosis where nutrients may be limited. In these sites it is thought that adaptation of the pathogen might occur and growth factors, present in relative excess, may thus promote survival of the organism. Auxotrophy of pulmonary isolates of P aeruginosa has previously been shown to be a feature of cystic fibrosis and chronic lung sepsis; auxotrophic isolates have additional nutritional requirements to the prototrophic "wild types" of the species. A study was therefore carried out to determine whether the proportion of auxotrophs differs between stable and acutely ill patients, or correlates with the extent of underlying disease. METHODS--Sputum samples were cultured for P aeruginosa and tested for auxotrophy by spreading serial dilutions of homogenised sputum on to a minimal medium which supports only prototrophs, and a complete medium which supports both nutritional types. The proportion of auxotrophs to prototrophs was determined and growth factors of confirmed auxotrophs were identified. RESULTS--Thirty two (86%) of 37 adults with cystic fibrosis infected with P aeruginosa harboured auxotrophs; methionine dependent mutants were isolated from seven of 16 patients tested (44%). More than 50% of the total number of colonies were auxotrophic in 19 of 26 samples (73%) from patients with acute exacerbations and in only six of 15 samples (40%) from clinically stable patients. In four patients from whom samples in both the acute and stable states were available, the proportion of auxotrophs fell in the sample taken when stable. Auxotrophs predominated in all samples from 11 of those patients with very severe underlying lung disease, in contrast to 13 of 30 samples from patients with less severe disease. There was no association between the percentage of auxotrophs and the presence of other respiratory pathogens. CONCLUSIONS--The majority of adults with cystic fibrosis infected with P aeruginosa harbour auxotrophs in the sputum. A significant proportion of acutely ill patients and those with severe underlying disease have a preponderance of auxotrophs in the sputum compared with stable patients and those with less severe disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore R. S., Christie C. D., Smith G. J. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis. Implications for the pathogenesis of progressive lung deterioration. Am Rev Respir Dis. 1989 Dec;140(6):1650–1661. doi: 10.1164/ajrccm/140.6.1650. [DOI] [PubMed] [Google Scholar]
- Elborn J. S., Shale D. J. Cystic fibrosis. 2. Lung injury in cystic fibrosis. Thorax. 1990 Dec;45(12):970–973. doi: 10.1136/thx.45.12.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan P. H., Gage P. A., Welch D. F., Muszynski M. J., Wait K. R. Prevalence of thymidine-dependent Staphylococcus aureus in patients with cystic fibrosis. J Clin Microbiol. 1987 Jul;25(7):1258–1261. doi: 10.1128/jcm.25.7.1258-1261.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- King A., Phillips I. The identification of pseudomonads and related bacteria in a clinical laboratory. J Med Microbiol. 1978 May;11(2):165–176. doi: 10.1099/00222615-11-2-165. [DOI] [PubMed] [Google Scholar]
- MATTHEWS L. W., SPECTOR S., LEMM J., POTTER J. L. STUDIES ON PULMONARY SECRETIONS. I. THE OVER-ALL CHEMICAL COMPOSITION OF PULMONARY SECRETIONS FROM PATIENTS WITH CYSTIC FIBROSIS, BRONCHIECTASIS, AND LARYNGECTOMY. Am Rev Respir Dis. 1963 Aug;88:199–204. doi: 10.1164/arrd.1963.88.2.199. [DOI] [PubMed] [Google Scholar]
- Neijens H. J. Strategies and perspectives in treatment of respiratory infections. Acta Paediatr Scand Suppl. 1989;363:66–73. doi: 10.1111/apa.1989.78.s363.66. [DOI] [PubMed] [Google Scholar]
- Pitt T. L. Biology of Pseudomonas aeruginosa in relation to pulmonary infection in cystic fibrosis. J R Soc Med. 1986;79 (Suppl 12):13–18. [PMC free article] [PubMed] [Google Scholar]
- Reid L. M., Bhaskar K. R. Macromolecular and lipid constituents of bronchial epithelial mucus. Symp Soc Exp Biol. 1989;43:201–219. [PubMed] [Google Scholar]
- Speert D. P., Farmer S. W., Campbell M. E., Musser J. M., Selander R. K., Kuo S. Conversion of Pseudomonas aeruginosa to the phenotype characteristic of strains from patients with cystic fibrosis. J Clin Microbiol. 1990 Feb;28(2):188–194. doi: 10.1128/jcm.28.2.188-194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. F., Hodson M. E., Pitt T. L. Auxotrophy of Pseudomonas aeruginosa in cystic fibrosis. FEMS Microbiol Lett. 1992 May 1;71(3):243–246. doi: 10.1016/0378-1097(92)90716-2. [DOI] [PubMed] [Google Scholar]
- Tower P. A., Johnson L. L., Ferro A. J., Fitchen J. H., Riscoe M. K. Synergistic activity of 5-trifluoromethylthioribose and inhibitors of methionine synthesis against Klebsiella pneumoniae. Antimicrob Agents Chemother. 1991 Aug;35(8):1557–1561. doi: 10.1128/aac.35.8.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]


