Abstract
Background
Oxidative stress, inducing cardiomyocyte apoptosis or myocardial ischemia, is the major denominator of many cardiac diseases. In this study, we intended to explore the regulatory function of microRNA-137 (miR-137) in oxidative stress-induced cardiomyocyte apoptosis.
Material/Methods
Cardiomyocytes were extracted from newborn C57BL/6 mice and cultured in vitro. Apoptosis was induced by H2O2, and evaluated by TUNEL assay. The effect of cardiomyocyte apoptosis on gene expression of miR-137 was evaluated by qRT-PCR. Lentivirus was used to stably down-regulate miR-137, and the subsequent effects of miR-137 down-regulation on cardiomyocyte apoptosis, its targeted gene CDC42, and caspase pathway were evaluated by TUNEL assay, dual-luciferase reporter assay, and Western blot assay, respectively. Finally, CDC42 was down-regulated by siRNA and its effect on miR-137-mediated cardiomyocyte apoptosis protection was examined.
Results
H2O2 induced significant apoptosis and up-regulated miR-137 in cardiomyocytes, whereas lentivirus-mediated miR-137 down-regulation protected against apoptosis. CDC42 was the direct target gene of miR-137 and proteins of CDC42, caspase-3, and caspase-9 were all regulated by miR-137 down-regulation in cardiomyocyte apoptosis. SiRNA-mediated CDC42 down-regulation reversed the protection of miR-137 down-regulation against cardiomyocyte apoptosis.
Conclusions
Our work demonstrated miR-137 and CDC42 are critical regulators in cardiomyocyte apoptosis. It may help to identify the molecular targets to prevent myocardial injury in human patients.
MeSH Keywords: Apoptosis; MicroRNAs; Myocytes, Cardiac
Background
Oxidative stress is one of the major molecular denominators in cardiovascular disease [1]. In acute myocardial infarction (AMI), commonly known as heart attack, oxidative stress induces the generation of large amounts of reactive oxygen species and increases blood vessel permeability, severely damaging vessel tissues and cardiomyocytes [1,2]. Although tremendous progress has been made in understanding the underlying mechanisms of AMI and developing efficient treatments, it still remains the major cause of cardiovascular disease and contributes significantly to cardiac-related mortality [3–6]. Thus, understanding the intricate underlying mechanisms and exploring biomarkers or molecular targets of oxidative stress-induced AMI is key to the battle against cardiovascular disease.
MicroRNAs (miRNAs) are families of small (~20 nucleotides long), non-coding RNAs that mediate posttranscriptional gene expression by binding the 3′-untranslated regions (UTRs) of target genes, thus inhibiting protein translation or inducing gene degradation [7]. Many of the miRNA families are important regulators in AMI, playing protective or negative roles in regulating myocardial injury [8]. For example, miR-24 was shown to protect against heart degradation and cardiomyocyte apoptosis by maintaining the L-type Ca2+ channels-ryanodine receptor tandem and suppressing apoptotic Bim signaling pathway [9,10]. Conversely, the miR-15 family was shown to promote cardiomyocyte apoptosis by down-regulating NAD-dependent protein deacetylase sirtuin-1 (SIRT1) [11–13]. Among the many cardiac miRNAs, miR-137 was identified to be differentially expressed in cardiomyocytes at different developmental stages [14]. However, little is known about whether miR-137 has regulatory mechanisms in myocardial injury.
Therefore, the aim of our study was to evaluate the molecular profile and functional mechanism of miR-137 in oxidative stress-induced myocardial injury. In addition, the possible molecular targets of miR-137 during the process of cardiomyocyte apoptosis regulation were explored. The results of our work could expand understanding of miRNA regulation in myocardial injury, and help identify biomarkers to predict or prevent heart attack.
Material and Methods
Ethic statement
All protocols and experimental procedures were approved by the Ethics Committee and Animal Research Study Board at the Second Hospital of Jilin University (Protocol # 14-04-562).
Cardiomyocytes culture
Newborn cardiomyocytes were prepared from mice (C57BL/6, postnatal 1–3 days) based on the method described previously [15]. Briefly, mice were sacrificed and the hearts were quickly extracted into ice-cold phosphate-buffered saline (PBS, Invitrogen, USA). Right-side ventricles were cut and minced into small pieces. The cardiomyocytes were dissociated by trituration in 0.05% trypsin-EDTA (Invitrogen, USA) for 10 min. After centrifuging, supernatant was discard and the cardiomyocyte pellets were resuspended in DMEM/F12 (1:1) medium supplemented with 10% fetal calf serum (FBS, Invitrogen, USA), penicillin (100 U/ml), and streptomycin (100 mg/ml), 2 mM l-glutamine (Invitrogen, USA), 0.1 mM nonessential amino acids (Invitrogen, USA), and 3 mM sodium pyruvate (Invitrogen, USA). Cells were incubated in 95% O2/5% CO2 at 37°C overnight. The floating cardiomyocytes were removed and re-plated in 6-well plates at a density of 5×105 cells/well. The culture medium was replenished every 2 or 3 days. To induce apoptosis, culture cardiomyocytes were incubated with 100 μM hydrogen peroxide (H2O2) for 24 h, based on the methods described previously [16,17].
Apoptosis assay
A terminal deoxyribonucleotidyl transferase (TDT)-mediated dUTP-digoxigenin nick end labeling (TUNEL) assay (Roche Diagnostics, USA) was used to evaluate cardiomyocyte apoptosis according to manufacturer’s instruction. Briefly, after washing the cultured cardiomyocytes with ice-cold PBS, the cells were fixed with 4% PFA for 1 h at RT. Cardiomyocyte nuclei were then immune-stained with DAPI antibody (blue), and apoptotic cells stained with TUNEL (green). Images were then taken on a Zeiss LSM 710 confocal microscope with 100× objective (Zeiss, Germany). For quantification, the percentages of non-apoptotic cardiomyocyte were evaluated by counting DAIP-positive but TUNEL-negative cardiomyocytes against all DAIP-positive cardiomyocytes.
RNA isolation and quantitative real-time PCR (qRT-PCR)
The cultured cardiomyocytes were suspended and collected. Total RNA was extracted using a Trizol Kit (Invitrogen, USA) according to manufacturer’s instructions. Reverse transcription was then performed with a TaqMan Reverse Transcription Kit (Qiagen, USA). To detect gene expression, quantitative real-time PCR (qRT-PCR) was performed using an iCycler iQ System with the iQ SYBR Green Super Mix (BioRad, USA) according to manufacturer’s instructions. GAPDH and U6 snRNA were used as internal controls. Gene fold changes were calculated using (2−ΔΔCt) method [18].
Lentiviral transfection
The murine mature miR-137 inhibitor lentivirus (miR-137-In) and its negative control lentivirus (miR-C) were commercially obtained from SunBio Biotech (SunBio Medical Biotechnology, Shanghai, China). Lentiviral transfection was performed using Lipofectamine 2000 (Qiagen, USA) according to manufacturer’s instructions. Culture medium was replenished 24 h after transfection.
Luciferase reporter assay
The wild-type 3′-UTR of cell division control protein 42, CDC42 (CDC42-WT) was amplified from mouse cDNA library, and cloned into a pGL3-basic firefly luciferase reporter vector (Promega, USA) according to manufacturer’s instruction. The putative mmu-miR-137 binding site on CDC42 3′-UTR was mutated using a QuickchangeXL mutagenesis kit (Strategene, USA), then cloned into firefly luciferase reporter vector (CDC42-MU). In HEK293T culture, mouse miR-137 mimic lentivirus (miR-137, SunBio Biotech, China) and its negative control lentivirus (miR-NC) were co-transfected with either CDC42-WT or CDC42-MU luciferase reporter vector. Twenty-four hours after transfection, a Dual Luciferase Reporter Assay System (Promega, USA) was performed according to manufacturer’s instruction. The relative luciferase activities were normalized to the value with CDC42-MU and miR-NC co-transfection.
Western blot
Cardiomyocytes were lysed in 50mM Tris-HCl (Sigma-Aldrich, USA), and denatured to shear DNA (5 min, 100°C). The proteins were resolved in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE), and transferred to polyvinylidene difluoride (PVDF) membranes (Sigma-Aldrich, USA). After blocking treatment of 1% BSA for 1 h, the membrane was washed 3 times in PBS-0.05% Tween (Sigma-Aldrich, USA), and incubated with primary antibodies against CDC42, Caspase-3, and Caspase-9 (Santa Cruz Biotechnology, Santa Cruz, USA) at 4°C overnight. After another 3 washes, the blot membrane was incubated with LI-COR Odyssey secondary antibodies (1:10,000, Bio-Rad, USA) at room temperature for 1 h. The blotting was visualized using an Odyssey infrared imager (Bio-Rad, USA).
SiRNA transfection
Gene down-regulating siRNA against mouse CDC-42 (CDC42-siRNA) and its negative control siRNA (C-siRNA) were commercially obtained from RiboBio (RiboBio, China). The transfection of siRNA into cardiomyocytes was performed using Lipofectamine 2000 according to the manufacturer’s instructions. Twenty-four hours after transfection, culture medium was replenished.
Statistical analysis
All experiments were performed in triplicate, and results are shown as mean ±S.E.M. Statistical analysis was performed using a 2-tailed, unpaired Student’s t-test in EXCEL (Microsoft, USA). Significant difference was marked at P<0.05.
Results
H2O2 induced cardiomyocyte apoptosis and miR-137 upregulation
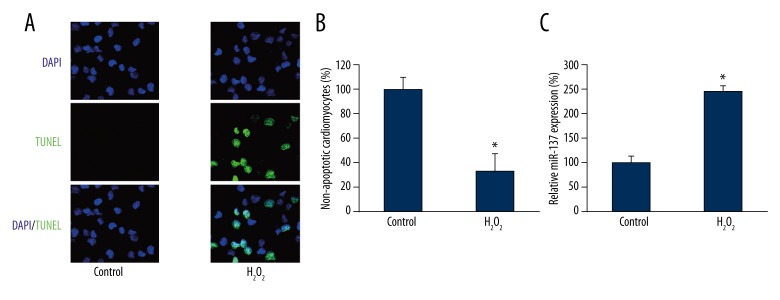
We first examined the effect of H2O2 on cardiomyocyte apoptosis and miRNA regulation. Newborn C57BL/6 mice were sacrificed and cardiomyocytes were cultured in vitro. To induce apoptosis, we took advantage of an established in vitro model to culture cardiomyocytes with 100 μM H2O2 for 1 h [16,17]. After culture medium was replenished, cardiomyocytes were cultured for another 24 h, followed by a TUNEL assay to examine apoptosis. The results showed that 100 μM H2O2 induced significant apoptosis, whereas no TUNEL positive cells were seen under control condition (without H2O2 treatment) (Figure 1A). Quantitative evaluation showed that the averaged percentage of healthy or non-apoptotic cardiomyocytes was reduced to ~35% by H2O2 treatment (Figure 1B, * P<0.05). We then evaluated the gene expression levels of miR-137 upon H2O2-induced apoptosis in cardiomyocytes. The results showed that miR-137 was significantly upregulated by 100 μM H2O2 (Figure 1C, * P<0.05).
Figure 1.
The effect of H2O2 on cardiomyocyte apoptosis and gene expression of miR-137. Cardiomyocytes were extracted from P1–P3 mice and incubated with 100 μM H2O2 for 24 h. (A) Cardiomyocyte apoptosis was evaluated by a TUNEL assay (green). The nuclei were stained with DAPI (blue). (B) The percentages of non-apoptotic cardiomyocytes (DAPI-positive/TUNEL-negative) were compared between Control and H2O2 treatment (* P<0.05). (C) The gene expression levels of miR-137 were also compared between Control and H2O2 treatment (* P<0.05).
MiR-137 down-regulation inhibited H2O2-induced cardiomyocyte apoptosis
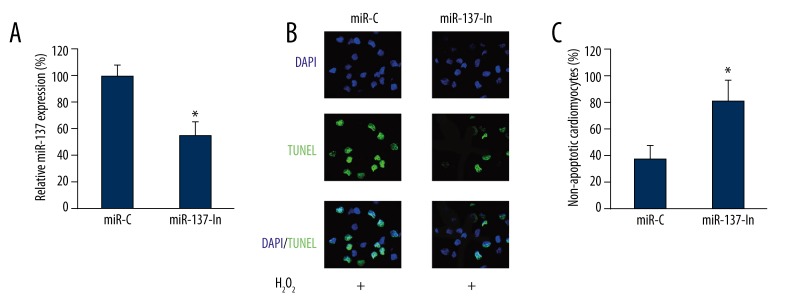
As we discovered that miR-137 was upregulated by H2O2-induced cardiomyocyte apoptosis, we wondered whether miR-137 had a functional role in it. We infected cardiomyocytes with miR-137 inhibitor lentivirus (miR-137-In). In control cardiomyocytes, we infected them with a negative control miRNA lentivirus (miR-C). We found that 24 h after lentiviral infection, the endogenous gene expression level of miR-137 was significantly down-regulated by miR-137-In as compared to miR-C (Figure 2A, * P<0.05). Then, the effect of miR-137 down-regulation on H2O2-induced cardiomyocyte apoptosis was examined by treating the cardiomyocytes with 100 μM H2O2 (24 h) following lentiviral infection. The results of TUNEL staining showed that significantly fewer cardiomyocytes had apoptosis, while miR-137 was down-regulated (Figure 2B). Quantitative evaluation further confirmed that miR-137 down-regulation inhibited H2O2-induced cardiomyocyte apoptosis (Figure 2C, * P<0.05).
Figure 2.
The effect of miR-137 down-regulation on H2O2 -induced apoptosis in cardiomyocytes. Cardiomyocytes were transfected with lentiviruses of mmu-miR-137 inhibitor (miR-137-In) or its negative control miRNA (miR-C). (A) Twenty-four hours after lentiviral transfection, qRT-PCR was performed to measure the efficacy of lentiviral infection (* P<0.05). (B) Cardiomyocytes were then treated with 100 μM H2O2 for another 24 h, followed by a TUNEL assay to evaluate the effect of miR-137 down-regulation on cardiomyocyte apoptosis. (C) The percentages of non-apoptotic cardiomyocytes (DAPI-positive/TUNEL-negative) were compared between the cardiomyocytes transfected with miR-C and the ones transfected with miR-137-In (* P<0.05).
CDC42 and apoptosis pathway were regulated by miR-137 during H2O2-induced cardiomyocyte apoptosis
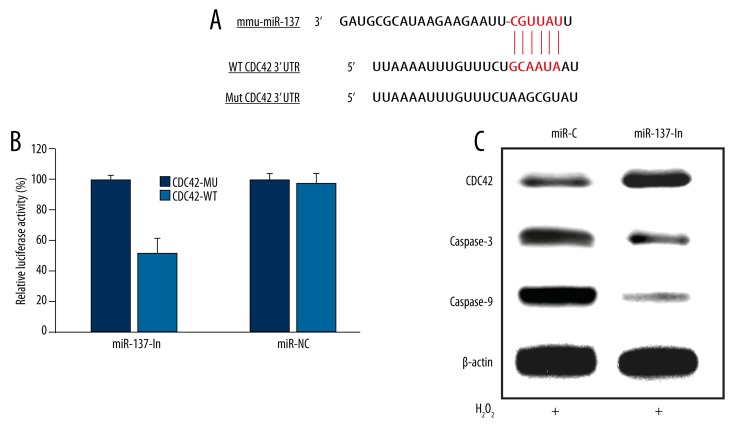
We then investigated the underlying molecular pathways associated with miR-137 in regulating H2O2 -induced cardiomyocyte apoptosis. First, we searched online miRNA target databases such as TargetScan, miRBase and miRanda, and found that CDC42 was a candidate target gene of miR-137 (Figure 3A). Second, we constructed firefly Luciferase reporter vectors containing either wild-type CDC42 3′UTR (CDC42-WT), or mutated CDC42 3′UTR with modified miR-137 binding site (CDC42-MU). We transfected HEK293T cells with either CDC42-WT or CDC42-MU, along with lentivirus containing miR-137 mimics (miR-137) or its negative control lentivirus (miR-NC). Twenty-four hours after luciferase transfection, we used a dual-luciferase reporter assay to examine the relative luciferase activities. The results showed that miR-137 significantly reduced the luciferase activity in HEK293T cells while CDC42-WT was co-transfected, as compared to the cells co-transfected with CDC42-MU (Figure 3B, * P<0.05), confirming that CDC42 was a direct target of miR-137. We also examined the CDC42 protein level in cardiomyocytes during the process of miR-137 down-regulation on H2O2-induced apoptosis. The Western blotting result demonstrated that down-regulating miR-137 induced the upregulation of CDC42 (Figure 3C). It also showed that caspase-3 and caspase-9, which are key caspase pathway proteins closely associated with CDC42 regulation, were subsequently down-regulated (Figure 3C).
Figure 3.
MiR-137 regulated CDC42 and caspase proteins in H2O2 -induced cardiomyocyte apoptosis. (A) The putative binding sites of murine miR-137 on wild-type (WT) CDC42 3′-UTR was highlighted. A mutated (Mut) CDC42 3′-UTR sequence was generated for the application of luciferase assay. (B) In a dual-luciferase reporter assay, HEK293T cells were transfected with firefly luciferase reporter inserted with WT CDC42 3′-UTR (CDC42-WT), or the reporter inserted with Mut CDC42 3′-UTR (CDC42-WT). Also, cells were co-transfected with either miR-137 mimics or its negative control miRNA (miR-NC). Twenty-four hours after transfection, relative luciferase activities were evaluated and normalized to the values in cells transfected with CDC42-MU (* P<0.05). (C) Cardiomyocytes were transfected with either miR-C or miR-137-In lentiviruses for 24 h, followed by another 24 h treatment of 100 μM H2O2. Western blotting analysis was used to compare the protein expression levels of CDC42, caspase-3 and caspase-9.
Inhibiting CDC42 reversed the protection of miR-137 down-regulation on H2O2-induced cardiomyocyte apoptosis
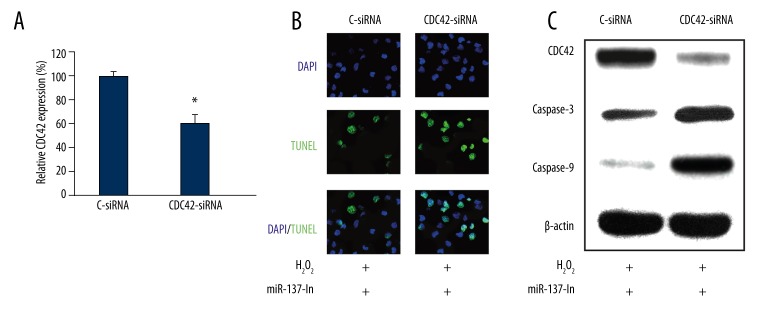
Finally, we investigated whether CDC42 were directly involved in the regulation of miR-137 on H2O2-induced cardiomyocyte apoptosis. We used siRNA technology to directly silence CDC42 gene in cardiomyocytes. The qRT-PCR result showed that endogenous CDC42 mRNA was significantly reduced in cardiomyocytes transfected with CDC42-specific siRNA (CDC42-siRNA), as compared to the cardiomyocytes transfected with a negative control siRNA (C-siRNA) (Figure 4A, * P<0.05). Then, we transfected the cultured cardiomyocytes with miR-137-In lentivirus for 24 h, followed by another transfection of either C-siRNA or CDC42-siRNA for 24 h. After replenishing the culture medium, cardiomyocytes were treated with 100 μM H2O2 for a 3rd period of 24 h, followed by TUNEL staining. The results of fluorescent imaging demonstrated that knocking down CDC42 increased the apoptosis in cardiomyocytes; even miR-137 was down-regulated (Figure 4B), suggesting that CDC42 was directly involved in the regulation of miR-137 on H2O2-induced apoptosis by negatively reversing the protective effect of miR-137 down-regulation on cardiomyocyte apoptosis. This was further confirmed by Western blot analysis, which showed that CDC42-siRNA not only down-regulated CDC42 protein production, but also up-regulated caspase-3 and caspase-9 to induce apoptosis in cardiomyocytes (Figure 4C).
Figure 4.
CDC42 down-regulation reversed the effect of miR-137 on H2O2-induced cardiomyocyte apoptosis. (A) Cardiomyocytes were transfected with CDC42 specific siRNA (CDC42-siRNA) or its negative control siRNA (C-siRNA) for 24 h. QRT-PCR was used to evaluate the transfection efficiency (* P<0.05). (B) Cardiomyocytes were transfected with miR-137-In for 24 h, followed by another 24 h transfection of either CDC42-siRNA or C-siRNA. Then, cardiomyocytes were treated with 100 μM H2O2 for 24 h, followed by a TUNEL assay to evaluate the effect of CDC42 down-regulation on cardiomyocyte apoptosis. (C) Western blotting analysis was ALSO used to compare the protein expression levels of CDC42, caspase-3 and caspase-9.
Discussion
In our work, we took advantage of an in vitro cardiomyocyte model to study the molecular mechanisms of miR-137 in regulating H2O2-induced cardiomyocyte apoptosis. We demonstrated that incubating neonatal cardiomyocytes with 100 μM H2O2 for 24 h induced significant apoptosis. This is in line with a previous report showing that 24-h treatment of moderate concentration H2O2 (100 μM) promoted apoptosis but not necrosis in adult cardiomyocytes [17].
Many of the miRNA families have been shown to play critical roles in myocardial ischemia [17]. A previous report showed that miR-137 was down-regulated as the cardiomyocyte differentiates and proliferates [14], suggesting that miR-137 may play a developmental role in cardiomyocyte. However, there has been no report on whether miR-137 is differentially expressed in pathological cardiomyocytes, or if there are any functional roles of miR-137 in regulating myocardial injury. In this work, we demonstrated that miR-137 was markedly upregulated in cardiomyocytes during the process of H2O2-induced apoptosis. Most importantly, we showed lentivirus-mediated miR-137 down-regulation reduced apoptosis in cardiomyocytes. Thus, this is the first report to show differential expression of miR-137 and the functional role of miR-137 in myocardial injury.
Many of the molecular pathways contribute to apoptosis protection in postmitotic cells, such as sympathetic neurons, cardiomyocytes, neurotrophin, and cell cycle genes [19–23]. CDC42, a key cell cycle regulator, is known to play an important role in regulating heart development [24–26], as well as in conditional knockout of CDC42 in heart-induced cardiac hypertrophy and cardiomyocyte apoptosis [27]. In this work, we first demonstrated, by dual-luciferase reporter assay, that CDC42 was the direct target of miR-137. Then, we showed that CDC42 was upregulated while miR-137 was down-regulated to reduce H2O2-induced cardiomyocyte apoptosis. Also, we demonstrated that key apoptosis pathway proteins, caspase-3 and caspase-9, were down-regulated during the process of miR-137 down-regulation-mediated protection against cardiomyocyte apoptosis. Most importantly, we showed that siRNA-mediated CDC42 down-regulation reversed the protection of miR-137 down-regulation on H2O2-induced cardiomyocyte apoptosis, suggesting that the underlying molecular pathway of miR-137 to regulate apoptosis is through the targeting of CDC42. It is interesting that while CDC42 was down-regulated, caspase pathway protein caspase-3 and caspase-9 were both up-regulated. It is known that CDC42 cleavage is the down-stream product of caspase in Fas-mediated apoptosis [28]. The result of our work showing up-stream regulation of CDC42 on caspase-3 and caspase-9 suggests that a very complex apoptotic signaling pathway may be involved in myocardial injury.
Conclusions
Our work demonstrated, for the first time, a functional role of miR-137/CDC42 signaling pathway in regulating oxidative stress-induced cardiomyocyte apoptosis. Although the current study was limited to an animal model, our results may help identify novel biomarkers and molecular targets to treat human heart attack patients in the future.
Footnotes
Conflict of Interest
None.
Source of support: Departmental sources
References
- 1.Misra MK, Sarwat M, Bhakuni P, et al. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15(10):RA209–19. [PubMed] [Google Scholar]
- 2.White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372(9638):570–84. doi: 10.1016/S0140-6736(08)61237-4. [DOI] [PubMed] [Google Scholar]
- 3.Reddy K, Khaliq A, Henning RJ. Recent advances in the diagnosis and treatment of acute myocardial infarction. World J Cardiol. 2015;7(5):243–76. doi: 10.4330/wjc.v7.i5.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibanez B, Heusch G, Ovize M, Van de Werf F. Evolving Therapies for Myocardial Ischemia/Reperfusion Injury. J Am Coll Cardiol. 2015;65(14):1454–71. doi: 10.1016/j.jacc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 5.Gu J, Hu W, Liu X. Pioglitazone improves potassium channel remodeling induced by angiotensin II in atrial myocytes. Med Sci Monit Basic Res. 2014;20:153–60. doi: 10.12659/MSMBR.892450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan A, Li G, Zhang X, Zhu B, Linghu H. Pro-survival effect of Dock180 overexpression on rat-derived H9C2 cardiomyocytes. Med Sci Monit Basic Res. 2013;19:12–19. doi: 10.12659/MSMBR.883738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–55. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 8.Boon RA, Dimmeler S. MicroRNAs in myocardial infarction. Nat Rev Cardiol. 2015;12(3):135–42. doi: 10.1038/nrcardio.2014.207. [DOI] [PubMed] [Google Scholar]
- 9.Li RC, Tao J, Guo YB, et al. In vivo suppression of microRNA-24 prevents the transition toward decompensated hypertrophy in aortic-constricted mice. Circ Res. 2013;112(4):601–5. doi: 10.1161/CIRCRESAHA.112.300806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian L, Van Laake LW, Huang Y, et al. miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J Exp Med. 2011;208(3):549–60. doi: 10.1084/jem.20101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hullinger TG, Montgomery RL, Seto AG, et al. Inhibition of miR-15 protects against cardiac ischemic injury. Circ Res. 2012;110(1):71–81. doi: 10.1161/CIRCRESAHA.111.244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishi H, Ono K, Iwanaga Y, et al. MicroRNA-15b modulates cellular ATP levels and degenerates mitochondria via Arl2 in neonatal rat cardiac myocytes. J Biol Chem. 2010;285(7):4920–30. doi: 10.1074/jbc.M109.082610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu H, Yang Y, Wang Y, et al. MicroRNA-195 promotes palmitate-induced apoptosis in cardiomyocytes by down-regulating Sirt1. Cardiovasc Res. 2011;92(1):75–84. doi: 10.1093/cvr/cvr145. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Li TS, Lee ST, et al. Dedifferentiation and proliferation of mammalian cardiomyocytes. PloS One. 2010;5(9):e12559. doi: 10.1371/journal.pone.0012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sreejit P, Kumar S, Verma RS. An improved protocol for primary culture of cardiomyocyte from neonatal mice. In Vitro Cell Dev Biol Anim. 2008;44(3–4):45–50. doi: 10.1007/s11626-007-9079-4. [DOI] [PubMed] [Google Scholar]
- 16.Aikawa R, Nitta-Komatsubara Y, Kudoh S, et al. Reactive oxygen species induce cardiomyocyte apoptosis partly through TNF-alpha. Cytokine. 2002;18(4):179–83. doi: 10.1006/cyto.2001.1007. [DOI] [PubMed] [Google Scholar]
- 17.Kwon SH, Pimentel DR, Remondino A, et al. H(2)O(2) regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. J Mol Cell Cardiol. 2003;35(6):615–21. doi: 10.1016/s0022-2828(03)00084-1. [DOI] [PubMed] [Google Scholar]
- 18.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caporali A, Sala-Newby GB, Meloni M, et al. Identification of the prosurvival activity of nerve growth factor on cardiac myocytes. Cell Death Differ. 2008;15(2):299–311. doi: 10.1038/sj.cdd.4402263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong M, Brugeaud A, Edge AS. Regenerated synapses between postnatal hair cells and auditory neurons. J Assoc Res Otolaryngol. 2013;14(3):321–29. doi: 10.1007/s10162-013-0374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caporali A, Emanueli C. Cardiovascular actions of neurotrophins. Physiol Rev. 2009;89(1):279–308. doi: 10.1152/physrev.00007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikenishi A, Okayama H, Iwamoto N, et al. Cell cycle regulation in mouse heart during embryonic and postnatal stages. Dev Growth Differ. 2012;54(8):731–38. doi: 10.1111/j.1440-169X.2012.01373.x. [DOI] [PubMed] [Google Scholar]
- 23.Adachi S, Ito H, Tamamori-Adachi M, et al. Cyclin A/cdk2 activation is involved in hypoxia-induced apoptosis in cardiomyocytes. Circ Res. 2001;88(4):408–14. doi: 10.1161/01.res.88.4.408. [DOI] [PubMed] [Google Scholar]
- 24.Vogler G, Liu J, Iafe TW, et al. Cdc42 and formin activity control non-muscle myosin dynamics during Drosophila heart morphogenesis. J Cell Biol. 2014;206(7):909–22. doi: 10.1083/jcb.201405075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swope D, Kramer J, King TR, et al. Cdc42 is required in a genetically distinct subset of cardiac cells during Drosophila dorsal vessel closure. Dev Biol. 2014;392(2):221–32. doi: 10.1016/j.ydbio.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian L, Wythe JD, Liu J, et al. Tinman/Nkx2-5 acts via miR-1 and upstream of Cdc42 to regulate heart function across species. J Cell Biol. 2011;193(7):1181–96. doi: 10.1083/jcb.201006114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maillet M, Lynch JM, Sanna B, et al. Cdc42 is an antihypertrophic molecular switch in the mouse heart. J Clin Invest. 2009;119(10):3079–88. doi: 10.1172/JCI37694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tu S, Cerione RA. Cdc42 is a substrate for caspases and influences Fas-induced apoptosis. J Biol Chem. 2001;276(22):19656–63. doi: 10.1074/jbc.M009838200. [DOI] [PubMed] [Google Scholar]