Abstract
Aim:
Methyl eugenol is a major active component extracted from the Chinese herb Asari Radix et Rhizoma, which has been used to treat toothache and other pain. Previous in vivo studies have shown that methyl eugenol has anesthetic and antinociceptive effects. The aim of this study was to determine the possible mechanism underlying its effect on nervous system disorders.
Methods:
The direct interaction of methyl eugenol with Na+ channels was explored and characterized using electrophysiological recordings from Nav1.7-transfected CHO cells.
Results:
In whole-cell patch clamp mode, methyl eugenol tonically inhibited peripheral nerve Nav1.7 currents in a concentration- and voltage-dependent manner, with an IC50 of 295 μmol/L at a −100 mV holding potential. Functionally, methyl eugenol preferentially bound to Nav1.7 channels in the inactivated and/or open state, with weaker binding to channels in the resting state. Thus, in the presence of methyl eugenol, Nav1.7 channels exhibited reduced availability for activation in a steady-state inactivation protocol, strong use-dependent inhibition, enhanced binding kinetics, and slow recovery from inactivation compared to untreated channels. An estimation of the affinity of methyl eugenol for the resting and inactivated states of the channel also demonstrated that methyl eugenol preferentially binds to inactivated channels, with a 6.4 times greater affinity compared to channels in the resting state. The failure of inactivated channels to completely recover to control levels at higher concentrations of methyl eugenol implies that the drug may drive more drug-bound, fast-inactivated channels into drug-bound, slow-inactivated channels.
Conclusion:
Methyl eugenol is a potential candidate as an effective local anesthetic and analgesic. The antinociceptive and anesthetic effects of methyl eugenol result from the inhibitory action of methyl eugenol on peripheral Na+ channels.
Keywords: sodium channel blocker, methyl eugenol, peripheral Nav1.7 channels, local anesthetics, analgesic, asarum, Xixin
Introduction
Xixin (Asari Radix et Rhizoma) is a traditional herbal medicine that originates in the roots and rhizomes of Asarum heterotropoides Fr Schmidt var mandshuricum (Maxim) Kitag, A sieboldii Miq var seoulense Nakai and A sieboldii Miq1. It has been widely used as a local anesthetic and a remedy for toothache, headache, and inflammatory diseases in China, Japan, and Korea2. Xixin, applied by chewing its roots, is well known in China for its control of toothache.
Methyl eugenol (4-allyl-1,2-dimethoxybenzene) is a major active component isolated from Xixin and other plants3,4. Many biological actions of methyl eugenol have been reported. In addition to its actions as a fly attractant, it is also known for its anesthetic, antinociceptive, anti-epileptic properties, hypothermic and myorelaxant properties5,6,7,8,9. The anesthetic property of methyl eugenol has been demonstrated by a loss of the righting reflex and the decreased sensitivity to a tail pinch in rats and mice, and a loss of the corneal reflex in rabbits5,7,10. Methyl eugenol and methyl eugenol-enriched plant essential oil also display anti-epileptic action by inhibiting electroshock- or pentylenetetrazol-induced convulsions6,9,11. More recently, antinociceptive effects of methyl eugenol have been shown through its relief of the secondary phase of formalin-induced pain in mice12. Methyl eugenol displays antinociceptive and anesthetic effects similar to Xixin, implying that methyl eugenol is the main antinociceptive component of Xixin. Although the antinociceptive and anesthetic effects of methyl eugenol and Xixin have been reported, the pharmacological basis underlying these actions has not been elucidated.
Na+ channels are a common target for anesthetics and analgesics13. Local anesthetics and analgesics prevent the transmission of nerve impulses via their binding to Na+ channels14,15,16. Anticonvulsants, such as carbamazepine (Tegretol, CBZ), are also used to treat neuropathic pain due to their interaction with Na+ channels17. Two main types of Na+ currents, termed tetrodotoxin (TTX)-sensitive and TTX-resistant, have been identified in the dorsal root ganglion (DRG)18. The TTX-sensitive Na+ channel isoform Nav1.7 is highly expressed in the majority of small DRG neurons19. Studies have demonstrated a greater involvement of Nav1.7 in inflammatory pain20 and in mediating pain signaling21. TTX-resistant Na+ channels diminish following axotomy and are replaced by an abnormally high density of TTX-sensitive Na+ channels, illustrating that TTX-sensitive Na+ channels play a role in pathological pain development22. The involvement of Nav1.7 in propagating pain information has been strongly indicated by the fact that a Nav1.7 congenital channelopathy results in the inability to experience pain23,24,25. Recently, a study demonstrated an increase in Nav1.7 expression in painful human dental pulp26. Based on the antinociceptive and anesthetic actions of methyl eugenol in vivo and of Xixin in dental clinics, the peripheral TTX-sensitive channel isoform, Nav1.7, represents a good candidate for the channel involved in the effects of methyl eugenol.
Here, we characterize the inhibitory effect of methyl eugenol on Na+ channels using the whole-cell patch clamp technique in transfected cells that transiently express the human peripheral Nav1.7 channel isoform.
Materials and methods
Cell culture and transient transfections
The cDNA clone of pNaEx8 plasmid encoding the Nav1.7 α subunit27(gift of Dr Franz HOFMANN, Institut für Pharmakologie und Toxikologie der Technischen Universität München, Germany) was transiently expressed in Chinese hamster ovary (CHO) cells. The cells were maintained in Ham's F12 Media (Sigma, St Louis, MO, USA) fortified with 10% fetal bovine serum (Gibco, Grant Island, NY, USA), 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, Grant Island, NY, USA) and kept in a humidified incubator at 37 °C and 5% CO2.
Transient transfection was performed as follows: CHO cells were plated at 2.5×105 cells/35-mm dish for 24 h prior to the transfection. The cells were exposed to 1 mL Opti-MEM (Gibco, Grant Island, NY, USA) media containing 0.8 μg of the α subunit plasmid DNA, 0.2 μg of a GFP indicator plasmid, and 6 μL of Lipofectamine reagent (Gibco, Grant Island, NY, USA) that was pre-incubated for 30 min. After incubation for 4 h, the cells were washed 3 times with control media and grown for 24 h under standard culturing conditions. The transfected cells were then plated on glass coverslips for the following day's experiments. Only cells expressing GFP were selected for electrophysiological recordings.
Whole-cell patch-clamp recordings
Na+ currents were recorded using the whole-cell patch clamp recording technique. Cells were cultured on coverslips and then transferred to a handmade recording chamber and continuously perfused at room temperature with extracellular solution containing (in mmol/L): 130 NaCl, 4 KCl, 1.5 CaCl2, 1.5 MgCl2, 5 glucose, 5 HEPES, 20 sucrose, pH 7.4 adjusted with NaOH. The recording chamber volume was approximately 0.4 mL, and the flow rate was 0.6 mL/min. An MP-285 micromanipulator (Sutter Instrument Co, Novato, CA, USA) was used to place the electrode onto the cell. Patch pipettes were pulled from borosilicate glass capillaries (Drummond Scientific Co, Broomall, PA, USA) on an electrode puller (Model P-97, Sutter Instrument Co) and were filled with a 0.2 μmol/L filtered internal solution containing (in mmol/L): 90 CsF, 60 CsCl, 10 NaCl, 5 HEPES, pH 7.4 adjusted with NaOH. The pipettes had an input resistance of 0.8–1.4 MΩ. Recordings were performed at room temperature (22 °C) using a patch clamp EPC 9 (HEKA Elektronik GmbH, Germany) and were filtered at 5 kHz. Leakage currents were subtracted using a P/4 or P/2 protocol. Pulse (HEKA elektronik GmbH, Germany) was used for the experimental control and basic data analysis. The methods of Na+ current recorded from Nav1.7 expressing cells are similar to our previously published methods28. Cells with a series resistance of less than 2.5 MΩ were used for the drug test experiments, and only cells with a whole-cell maximal Na+ current of at least 1 nA were used in the analysis. To test for tonic inhibition evoked by the drug, the Na+ currents were recorded at 8 min after switching the perfusion solution to a solution containing methyl eugenol.
Drug application
Methyl eugenol was supplied by the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Stock solutions of 500 mmol/L methyl eugenol were prepared in dimethyl sulfoxide (DMSO) and then diluted to the desired concentration for the experiments in the perfusion buffer. For all experiments, drugs were applied via perfusion. Control recordings showed that 0.2% DMSO had no detectable effect on the Na+ current in cells expressing Nav1.7. The concentration of methyl eugenol used for most of the experiments was less than 625 μmol/L, which resulted in the perfusion buffer containing 0.125% DMSO.
Statistical analysis
The data were analyzed using a combination of PulseFit (HEKA Elektronik GmbH, Germany) and SigmaPlot 9.0 (Jandel Scientific, Corte Madera, CA, USA) software. All results are presented as the mean±SEM.
Results
In most culture preparations, approximately 30% of the GFP expressing cells displayed a fast, transient inward current following depolarization. The maximal peak inward current of Nav1.7 expressing cells ranged from 0.8 to 7 nA; however, in a few cells, it was up to 12 nA. These currents were completely blocked by 0.5 μmol/L tetrodotoxin, confirming their identity as uncontaminated tetrodotoxin-sensitive Na+ currents under our recording conditions.
Methyl eugenol tonically inhibits Nav1.7 Na+ currents
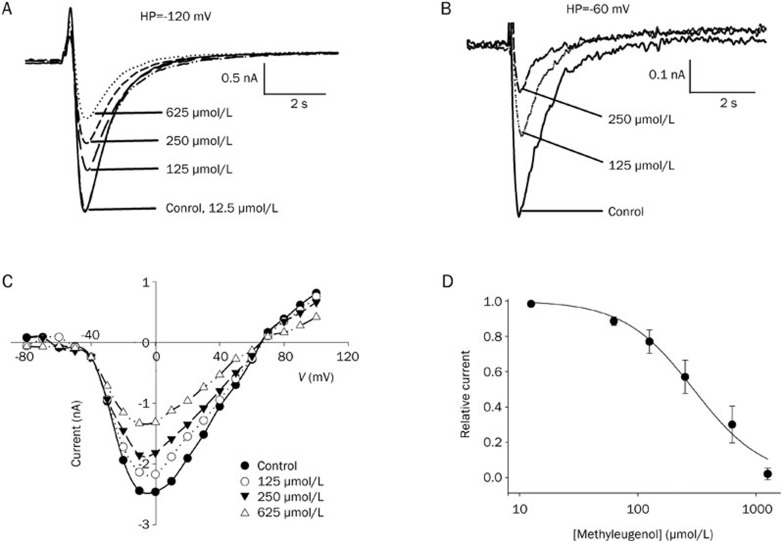
The application of methyl eugenol reversibly inhibited the peak amplitude of the Na+ current (Figure 1). The current traces of Nav1.7 channels recorded at a variety of methyl eugenol concentrations are superimposed at a holding potential of −120 mV and −60 mV, respectively; the results indicate a tonic inhibition of Nav1.7 channels by methyl eugenol in a concentration- and voltage-dependent manner (Figure 1A, 1B). Most of the channels are in the resting state at a holding potential of −120 mV and are inactivated at a holding potential of −60 mV. These characteristics are observed by measuring the inactivation curve of Nav1.7 (Figure 2A).
Figure 1.
Tonic inhibition by methyl eugenol of Nav1.7 channels. (A, B) Concentration-dependent tonic inhibition by methyl eugenol of Nav1.7 channels in the resting state (holding potential at −120 mV) and inactivated state (holding potential at −60 mV). The current traces of Nav1.7 channels recorded in control conditions and in the presence of varying concentrations of methyl eugenol are superimposed for the resting and inactivated states, respectively. The data are from the same representative cell. The currents were elicited using a 30-ms pulse to 0 mV from a holding potential of −120 mV or −60 mV. (C) Current-voltage relationships in the absence or presence of various concentrations of methyl eugenol were determined by stepping to various depolarized potentials (ranging from −80 to +100 mV in 10 mV increments) for 9 ms from a holding potential of −100 mV. (D) Concentration-response curve for the inhibition of Na+ current by methyl eugenol. The cells were held at −100 mV and stepped to 0 mV for 10 ms. The peak current in the presence of methyl eugenol were normalized to the control peak current, and then averaged. Each point was the mean±SEM of 4–8 cells. The lines represent the best fit for the data to the equation: y=1−xn/(Kdn+xn), where y is the fractional current, Kd is the apparent dissociation constant for methyl eugenol, and n is the Hill coefficient. Kd and n were estimated using a Marquadt nonlinear least-squares procedure.
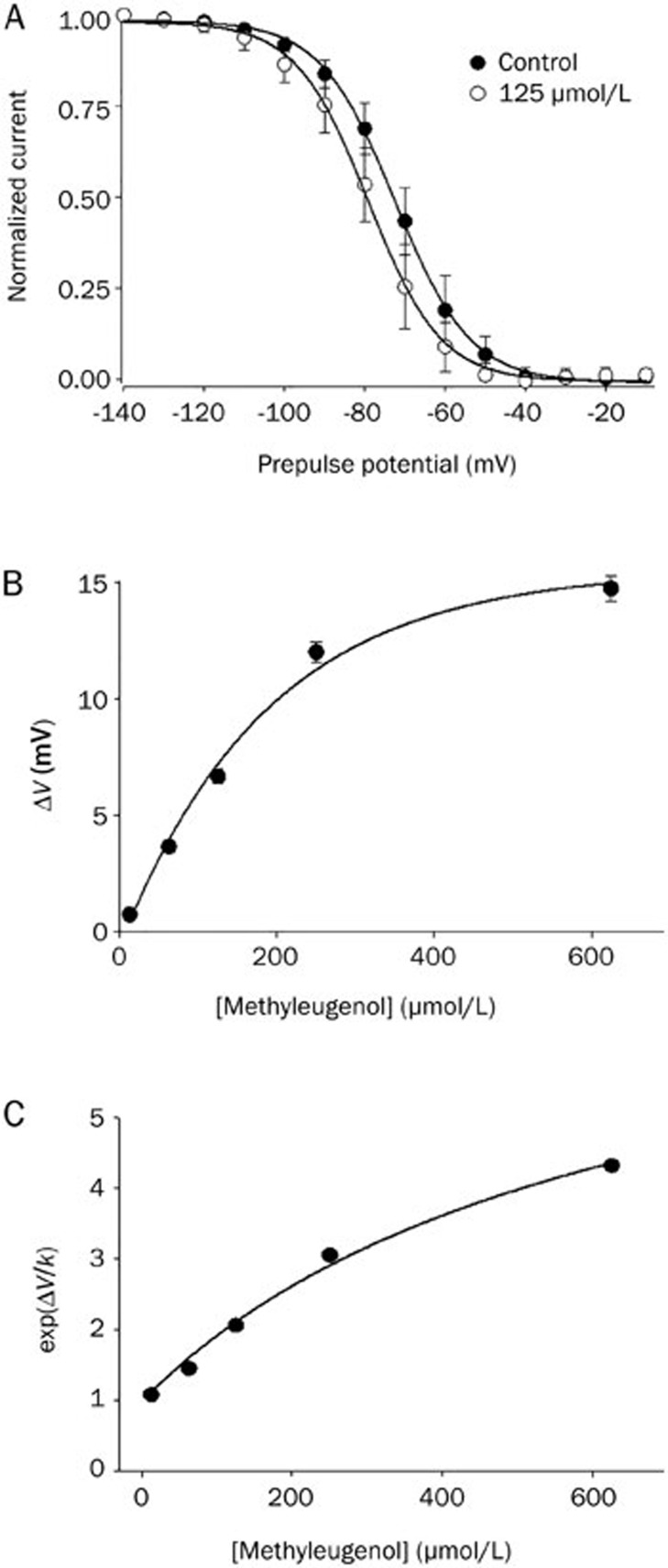
Figure 2.
The effects of methyl eugenol on the voltage-dependent inactivation of Nav1.7 channels and the estimation of methyl eugenol affinities. (A) The shift in the inactivation curve of Nav1.7 by 125 μmol/L methyl eugenol (n=5) is shown. The voltage dependence of steady-state inactivation (h∞) was examined by applying 500-ms prepulse potentials from −140 mV to −10 mV in 10-mV increments from a holding potential of −100 mV before stepping to the test potential (0 mV) for 35 ms. The peak current (I) for each cell was normalized with respect to the first value measured at the test potential (0 mV). The line through the data conforms to the equation: y=1−1/{1−exp[(V−Vh)/k]}, where V is the membrane potential, Vh is the prepulse potential where the current is half-maximal, and k is the slope factor. (B) Concentration-dependent shift of the midpoint of inactivation (ΔV) caused by 12.5, 62.5, 125, 250, and 625 μmol/L methyl eugenol for Nav1.7 (n=4–9). The line fits to a monoexponential equation. The mean Vh at different concentrations of methyl eugenol was obtained by fitting a Boltzmann relationship as described above. (C) The data were fitted by: exp(ΔV/k)=[1+(D/KI)]/[1+(D/KR)], where ΔV and k are the shift in the midpoint of the inactivation curve and the slope factor, D is the concentration of methyl eugenol, and KI and KR are the dissociation constants for the inactivated and resting states.
Figure 1C shows the current-voltage (I–V) relationship for Nav1.7 in control cells and in those exposed to various concentrations of methyl eugenol. The shape of the I–V curve was unaffected by concentrations of methyl eugenol up to 625 μmol/L. This result suggests that at the concentrations tested, methyl eugenol had no effect on the voltage-dependence of Na+ channel activation. To further examine the tonic inhibition by methyl eugenol, we determined the concentration–response relationship at a holding potential of −100 mV, where most channels are in a resting state under control conditions.
Clinically, the control of toothache using the herbal medicine Asarum Xixin is usually mediated through chewing the herb or via topical administration, which allows for the usage of a higher concentration of the drug (in its alcohol extracted form). To link the above tested methyl eugenol concentrations with those showing therapeutic effects in the clinic, methyl eugenol concentrations up to 1.2 mmol/L were used to determine the concentration-response relationship. The average tonic inhibition by methyl eugenol at varying concentrations fit well to the Hill equation (Figure 1D). The fitted Hill coefficient (n) value was calculated to be 1.27, and it appeared that the stoichiometry of the drug and receptor interaction was 1:1. The IC50 value was obtained by fitting the data to the Hill equation at −100 mV and estimated to be 295 μmol/L.
Methyl eugenol shifts the steady-state inactivation curve
In previous studies using Na+ channel blockers such as phenytoin and carbamazepine, these drugs affected the voltage-dependent availability of Na+ channels, as seen by a shift in the steady-state inactivation curve to more negative potentials29,30. The larger tonic inhibition observed at a holding potential of −60 mV compared to −120 mV (Figure 1A, 1B) suggests that methyl eugenol preferentially interacts with channels in the inactivated state. Methyl eugenol shifted the inactivation curve toward more negative potentials (Figure 2A). The shift evoked by methyl eugenol was concentration-dependent. The current for each cell was normalized and averaged, then fit to a single Boltzmann relationship from which the mean Vh and k values were calculated. The slope factor (k) of the curve was not affected at the concentrations of methyl eugenol that we tested (12.5–625 μmol/L). Figure 2B shows the overall shift of the midpoint of the inactivation curve for Nav1.7 induced by different concentrations of methyl eugenol.
Estimation of methyl eugenol affinity for the resting versus inactivated state of Na+ channels
The above results indicate that methyl eugenol preferentially binds to Nav1.7 channels when they are in an inactivated state (Figure 2). We also observed a concentration-dependent tonic inhibition of methyl eugenol at negative holding potentials (Figure 1A, 1B), suggesting that methyl eugenol also interacts with channels in their resting state. To further analyze the effects of methyl eugenol on Nav1.7, we estimated the affinity of methyl eugenol for the resting and inactivated states of the channel using curve shift analysis (ΔV) as described by Kuo and Bean31. The data in Figure 2B were transformed to Exp(ΔV/k), where k is the Boltzmann inactivation curve slope factor obtained from the experiments on the shift of the inactivation curves, and then plotted against the methyl eugenol concentration (Figure 2C). The slope factor, k, of the curves was not affected by methyl eugenol under our experimental conditions and concentrations tested (Figure 2A, 2B). By fitting the data, the values of KR and KI, where KR and KI are the equilibrium dissociation constants for the resting and inactivated states, respectively, were determined. The affinity of methyl eugenol for the resting and inactivated states is expressed as 1/KR and 1/KI. The calculated values of KR and KI were 525 μmol/L and 82.0 μmol/L for the resting and inactivated states of Nav1.7 (Figure 2C), respectively. These values support our hypothesis that methyl eugenol binds preferentially to channels in the inactivated state.
Methyl eugenol evokes large use-dependent inhibition
Methyl eugenol has been shown to display anesthetic, anti-epileptic and antinociceptive effects in vivo5,7,10,12. It is well known that use-dependent inhibition plays an important role in anti-neuropathic pain and anti-convulsion29.
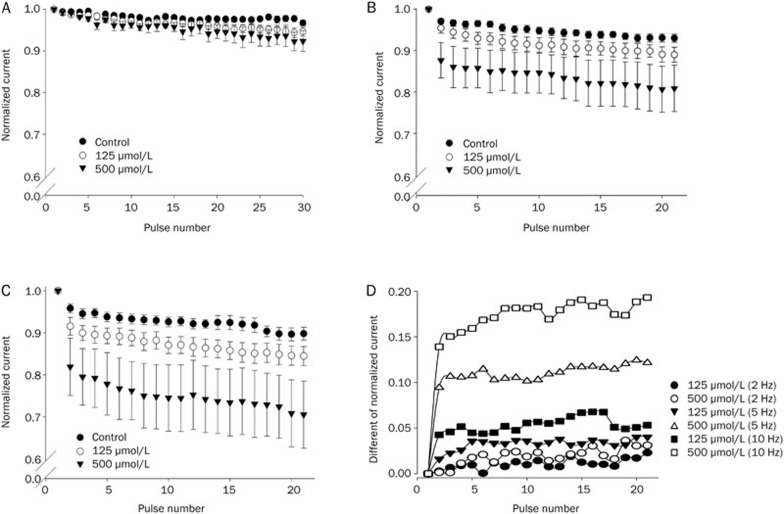
Different stimulus frequencies (2, 5, and 10 Hz with a 5-ms test duration) were used to test the use-dependence of methyl eugenol (Figure 3). At a lower pulse frequency (2 Hz), trains of stimulus pulses to 0 mV induced a modest use-dependent block in the presence of methyl eugenol that showed a gradual increase in the magnitude of the block (Figure 3A). At either 5 Hz or 10 Hz, methyl eugenol evoked a larger use-dependent inhibition with fast kinetics of the inhibition of Na+ currents (Figure 3B, 3C). The steady-state level of the use-dependent inhibition was reached after only a few test pulses (Figure 3B, 3C), suggesting that methyl eugenol may preferentially bind to channels in the open and/or inactivated states.
Figure 3.
Use-dependent inhibition of Nav1.7 by methyl eugenol. Cells were held at −100 mV and stimulated with a train of 5-ms pulses to 0 mV at a frequency of 2 Hz (A), 5 Hz (B), and 10 Hz (C) in the absence or presence of methyl eugenol. For each experiment, the current amplitudes were normalized with respect to the current evoked by the first pulse in the train [I(pulse n)/I(pulse 1)]. For all 3 stimulus frequencies (2, 5, and 10 Hz), n=8 (control), 8 (125 μmol/L) and 4 (500 μmol/L). (D) The differences between the normalized currents in methyl eugenol and control at 2, 5, and 10 Hz were calculated. The data are from (A, B, and C).
To more directly compare the potency of the methyl eugenol-mediated use-dependent inhibition at different stimulus frequencies, the difference between the normalized current in the absence and presence of methyl eugenol was calculated for each stimulus condition. Figure 3D shows the different potencies of the use-dependent inhibition and the kinetics of the block evoked by methyl eugenol (125 μmol/L and 500 μmol/L) on Nav1.7 channels. Methyl eugenol displayed greater frequency-dependent inhibition at a higher stimulus frequency. At the 15th pulse at 10 Hz, 125 μmol/L methyl eugenol displayed two times more inhibition than at 5 Hz, and 5.4 times more than at 2 Hz. Similarly, 500 μmol/L methyl eugenol evoked 8.5 times greater inhibition at the 15th pulse at 10 Hz compared to 2 Hz. Methyl eugenol also changed the kinetic behavior of the use-dependent inhibition, as observed in Figure 3D. Methyl eugenol induced faster kinetics of inhibition at higher stimulus frequencies and with higher concentrations of the drug.
Binding kinetics of drug-channel interactions: methyl eugenol appears to bind preferentially to the fast-inactivated state of Nav1.7
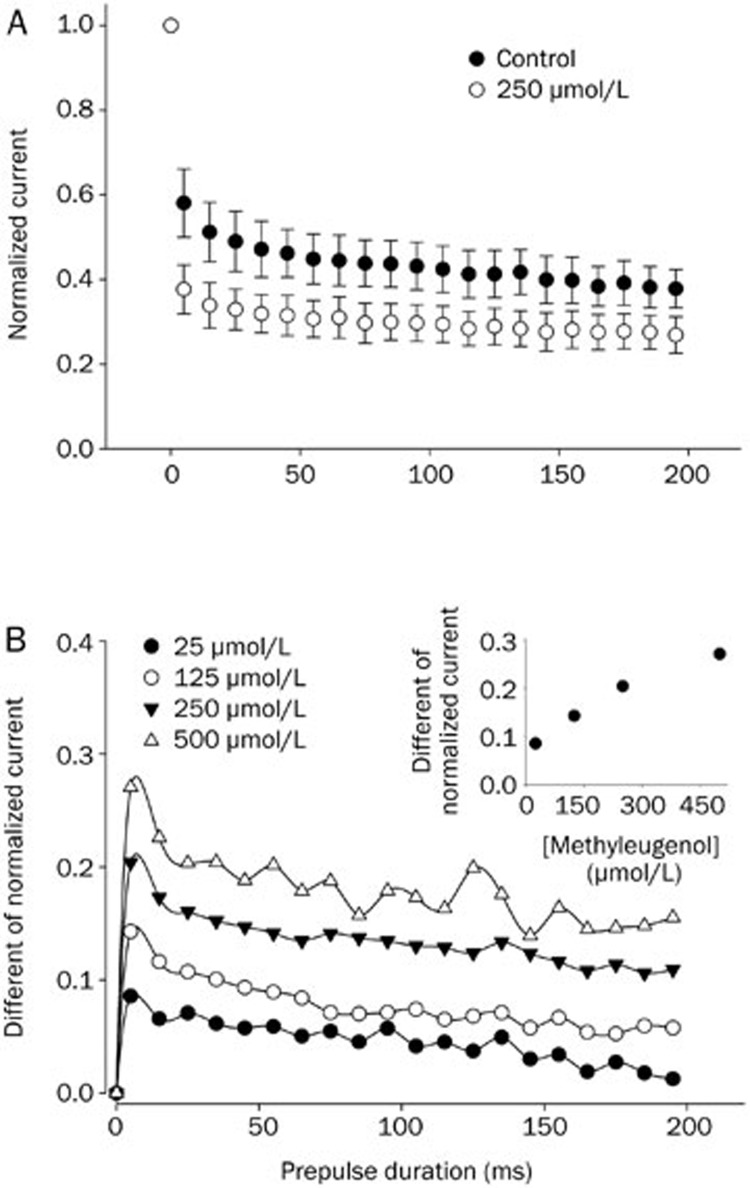
Figure 4 shows the effect of methyl eugenol binding on the development of the inactivation of Nav1.7 channels. The holding potential was −100 mV. In the control condition, the development of channel inactivation was seen by a rapid decay of the peak current that was evoked by increasing the prepulse (0 mV) duration (Figure 4A). With 250 μmol/L methyl eugenol, Nav1.7 currents decayed much faster and more prominently than in control conditions, presumably due to the binding of the drug to the inactivated channels (Figure 4A). The fraction of inactivated current, which developed as the duration of the prepulse increased, was fitted to a double exponential function. The Na+ channel inactivation evoked by a depolarization of short duration (ie, millisecond) is thought to represent classic “fast” inactivation32. Therefore, under the present experimental conditions, the enhanced decay of the Na+ current by methyl eugenol suggests that methyl eugenol may preferentially bind to a fast-inactivated state of the Nav1.7 channel.
Figure 4.
The binging of methyl eugenol on the development of inactivation in Nav1.7 channels. (A) A conditioning prepulse was applied to 0 mV from −100 mV for varying durations (5–195 ms) followed by a 10-ms gap to −100 mV, then followed by the application of a test pulse to 0 mV (5 ms). The current elicited by the test pulses was normalized with respect to the first test current elicited by a 5-ms prepulse. The normalized currents were averaged and plotted against the prepulse duration. The fraction of current decay was fitted to a double exponential function (control: n=5; in methyl eugenol: n=5). (B) The difference between the averaged normalized current for Nav1.7 channels in the absence and presence of methyl eugenol is shown. The insert illustrates the relationship between the various concentrations of methyl eugenol and the corresponding maximal response with a 5-ms prepulse.
The contamination resulting from the normally inactivated channels under the experimental conditions was corrected by calculating the difference between the normalized current in the presence of methyl eugenol and the normalized current in the control conditions. Methyl eugenol evoked the largest current decay within a 10 ms prepulse duration, after which the decay tended to be relatively stable (Figure 4B). The decay of current due to methyl eugenol was concentration-dependent (see the insert in Figure 4B). The binding of methyl eugenol to Nav1.7 within the first 10 ms of the prepulse is consistent with the hypothesis that the binding of methyl eugenol exhibits rapid kinetics to induce use-dependent inhibition (Figure 3B, 3C).
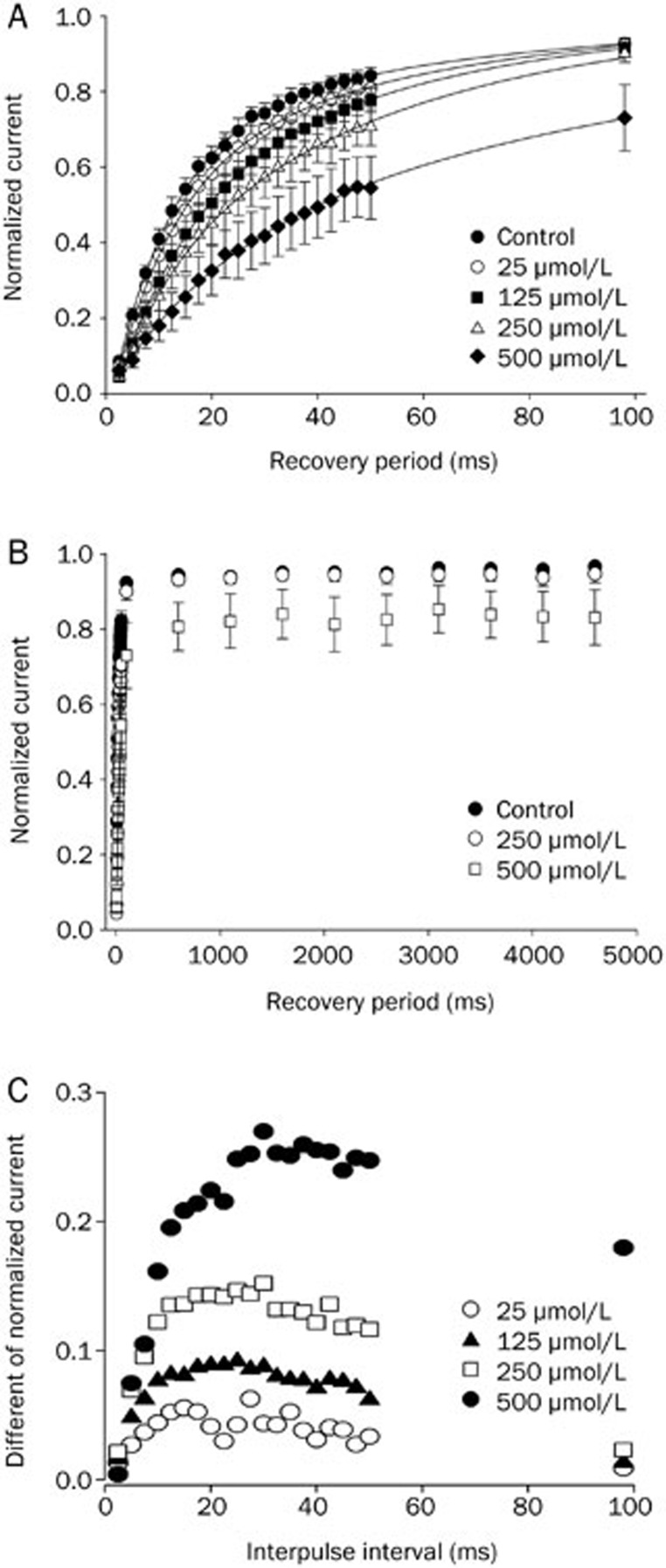
Effects of methyl eugenol on the recovery from inactivation
The recovery of inactivated Nav1.7 channels in the presence of methyl eugenol was tested using a two-pulse protocol from a −100 mV holding potential. An inactivating, conditioning prepulse duration of 100-ms was utilized to allow for a complete fast inactivation without inducing slow inactivation and to allow for the complete binding of the drug to the inactivated channels. Figure 5A demonstrates that methyl eugenol slowed the rate of recovery from inactivation for Nav1.7 at increasing concentrations. The higher the concentration of methyl eugenol, the longer the recovery period to control levels. Within a 100-ms recovery period, the methyl eugenol effect was completely reversed at 25–125 μmol/L and was approximately 97.5% reversed at 250 μmol/L. However, at 500 μmol/L methyl eugenol, the effect was only reversed to 80.3%. Recovery curves were further tested using longer recovery periods up to 5 (Figure 5B). Interestingly, cells perfused with 500 μmol/L methyl eugenol failed to completely recover to control levels even after a 5 s recovery period (∼86.7% recovery). To more directly compare the recovery rate of Nav1.7, the difference between the normalized currents in the presence and absence of methyl eugenol was calculated (Table 1). The maximal value of the methyl eugenol-evoked slowing of recovery involving fast-inactivated channels occurred when the interpulse interval was 15- to 30-ms (Figure 5C). The remainder of the methyl eugenol-bound inactivated channels did recover to control levels, albeit at a slower rate. The higher the concentration of methyl eugenol, the slower the second phase of the recovery period (Table 1).
Figure 5.
The effects of various concentrations of methyl eugenol on the recovery from inactivation of Na+ channels. The recovery from inactivation was measured with a two-pulse protocol that consisted of a 100-ms conditioning pulse to 0 mV from a −100 mV holding potential, followed by an interpulse interval of varying duration at a −100 mV holding potential, then a test pulse to 0 mV for 10 ms. The amplitude of the current elicited by the test pulses was normalized with respect to the current elicited by the conditioning pulses in each series and were plotted as a function of the recovery interval. (A) Methyl eugenol slowed the recovery rate from inactivation. An interpulse interval of 2.5–100 ms was used to show more clearly the initial slowing effect. The data fit to a double exponential function according to the equation y=1−A exp(t/τ1)−B exp(t/τ2), where y is the normalized current, A, B are the amplitudes of the corresponding components, t is the interpulse interval, and τ1 and τ2 are time constants for recovery. (B) Methyl eugenol slowed the rate of recovery from inactivation with an interpulse interval of varying duration (2.5–5000 ms), while a higher concentration of methyl eugenol (500 μmol/L) produced an incomplete recovery of inactivated Nav1.7 channels. (C) The differences between the normalized currents in the presence of various concentrations of methyl eugenol and the corresponding normalized control currents are shown. Data are from (A).
Table 1. Summary of methyl eugenol's effects on the recovery from inactivation of the Nav1.7 channel.
| Drug (μmol/L) | Methyl eugenol |
Control |
||
|---|---|---|---|---|
| τ1 (ms) | τ2 (s) | τ1 (ms) | τ2 (s) | |
| 25 | 20.1 | 3.8 | 16.5 | 3.8 |
| (n=4) | (n=4) | |||
| 125 | 27.4 | 7.1 | 19.8 | 3.3 |
| (n=8) | (n=8) | |||
| 250 | 31.3 | 10.2 | 18.2 | 3.2 |
| (n=5) | (n=5) | |||
| 500 | 40.4 | 23.5 | 20.2 | 4.1 |
| (n=5) | (n=5) | |||
For each concentration of methyl eugenol, the control was perfused with an equivalent concentration of DMSO.
Discussion
To clarify the mechanisms underlying the in vivo anesthetic and anti-nociceptive effects of methyl eugenol and the herbal medicine Xixin (Asari Radix et Rhizoma), we determined the effects of methyl eugenol on the Na+ channel isoform, Nav1.7, using the technique of whole-cell patch clamp recording. Methyl eugenol reversibly inhibited Nav1.7 current in a concentration- and voltage-dependent manner. Methyl eugenol shifted the steady state inactivation curve of Nav1.7 to more negative potentials, and in the presence of methyl eugenol, a train of stimuli induced a greater use-dependent current reduction. The binding kinetics of methyl eugenol indicated that methyl eugenol preferentially binds to Nav1.7 channels in the fast-inactivated state. Methyl eugenol also slowed the rate of recovery from inactivation of Nav1.7 channels. An estimation of the affinity of methyl eugenol for the resting and inactivated states of Nav1.7 also confirmed that methyl eugenol preferentially binds to channels in the inactivated state. These characteristics suggest that methyl eugenol may effectively limit the sustained firing or transient high-frequency bursts of firing that occur in peripheral nerves under conditions of neuropathic pain.
Methyl eugenol is a chemical derivative of eugenol. Cloves are used in Chinese medicine, and clove oil has been used for centuries in dentistry as an effective analgesic for dental emergencies33. Eugenol and clove oil also have anesthetic and analgesic effects in rodents10,34. Recently, the cellular mechanisms underlying eugenol's analgesic action have been proposed, including the inhibition of Ca2+, Na+, and K+ currents and the potentiation of GABAA receptors35,36,37,38. Although methyl eugenol and eugenol are chemically similar compounds, they can exhibit different pharmacological effects, eg, methyl eugenol has been shown to have hypothermic and muscle relaxant actions, while eugenol has not6. However, both compounds have analgesic effects in vivo5. By characterizing methyl eugenol's action on the Nav1.7 channel, we found that methyl eugenol displayed distinctly different characteristics compared to prior results obtained with eugenol. Cho et al37 reported that eugenol reduced the maximal Na+ current with no frequency or use-dependent inhibition in rat dorsal root ganglia neurons. In contrast, methyl eugenol displayed greater use-dependent inhibition (Figure 3) as well as a slower rate of recovery for the inactivated channels (Figure 5). By comparing their effects on Na+ channels, we propose that methyl eugenol would be a good candidate to replace eugenol as an analgesic in dentistry.
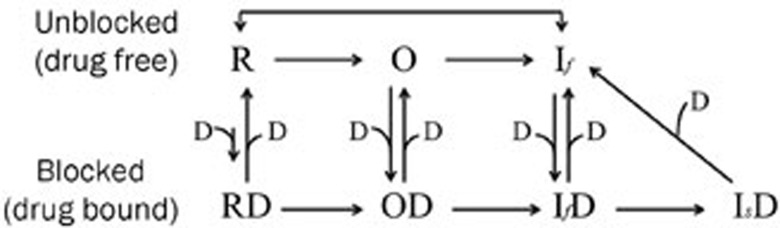
Numerous studies have established that various drugs inhibit Na+ channels in different ways through the selective interaction with distinct channel structures or functional states29,39. Our results indicate that methyl eugenol has a higher affinity for inactivated and/or open channels, with weaker binding to channels in the resting state. Through its rapid binding to open channels and stabilization of a nonconducting state, methyl eugenol can produce greater and faster use-dependent inhibition of Na+ current and slow the kinetics of recovery of channels in the inactivated state40. Methyl eugenol rapidly reaches a steady-state level in a use-dependent manner (Figure 3B, 3C), similar to the use-dependency evoked by lidocaine on Nav1.441,42, Nav1.743,44, Nav1.545, and Nav1.843. This similarity in use-dependency between methyl eugenol and lidocaine suggests that methyl eugenol also binds to open and/or inactivated states of the channel to produce a greater use-dependence40. Qualitatively, methyl eugenol appears to exhibit the same functional characteristics as lidocaine29,40. However, high concentrations of methyl eugenol strongly inhibited the rate of recovery of drug-bound inactivated channels (Figure 5). This suggests that high concentrations of methyl eugenol may drive fast-inactivated Nav1.7 channels into drug-bound, slow-inactivated channels31. Based on our results and pharmacological analyses, we provide the following model for the effect of methyl eugenol (Figure 6). This model is a modified version of the “modulated receptor hypothesis”46. In addition to fast inactivation (If), which takes place within milliseconds, voltage-gated Na+ channels may also undergo slow inactivation (Is), a mechanism that occurs over a period of seconds to minutes. In our experimental conditions (Figure 5), a 100-ms conditioning pulse duration was used that allowed for complete fast inactivation. This condition is not able to induce slow inactivation28. However, our results showed that higher concentrations of methyl eugenol resulted in a failure to completely recover to control function even after a 5 s recovery period, indicating that methyl eugenol can drive IfD directly into IsD. It appears that Is does not have to participate in this process. In this model, the interaction between methyl eugenol and Na+ channels is not a first-order binding reaction that can explain why recovery from the blocked state depends on the concentration of methyl eugenol (Figure 5).
Figure 6.
The model of methyl eugenol action on peripheral Na+ channels. Drugs (D) can bind to Na+ channels in the resting (R), open (O) and fast-inactivated (If) states. The drug-bound fast-inactivated state can switch into a drug-bound slow-inactivated state (Is) under certain conditions.
Methyl eugenol induced a much slower recovery from channel inactivation, which implies that high concentrations of methyl eugenol may effectively limit or paralyze neuronal activity to exert a local anesthetic effect. In the dental clinic, the topical application or subcutaneous administration of methyl eugenol provides a means to produce a high concentration of the drug at a localized site for a certain period. Furthermore, a recent study has demonstrated an increased Nav1.7 expression in painful human dental pulp26. Therefore, given the inhibition of recovery by methyl eugenol, we propose that methyl eugenol could be a more effective analgesic for the treatment of toothache than clove oil. Additionally, understanding the differences in the patterns of Na+ channel inhibition by the chemical modification of eugenol to methyl eugenol should provide insights into the development of more potent compounds for clinical use.
In conclusion, the present results suggest that methyl eugenol inhibits peripheral nerve Nav1.7 channels with a higher affinity for inactivated and/or open channels and a weaker affinity to those in the resting state. Higher concentrations of the drug could drive more drug-bound, fast-inactivated channels into drug-bound, slow-inactivated channels that greatly delay the recovery to control levels of channel function. Our results provide a possible mechanism of the antinociceptive and anesthetic actions of methyl eugenol and the herbal medicine Xixin through the inhibition of peripheral Na+ channels.
Author contribution
Ze-Jun WANG proposed the study concepts, designed and performed the experiments and data analysis; Ze-Jun WANG, Boris TABAKOFF and Thomas HEINBOCKEL prepared the manuscript; Simon R LEVINSON designed the experiments and contributed chemical reagents and recording equipment.
Abbreviations
Methyl eugenol, 4-allyl-1,2-dimethoxybenzene; DMSO, dimethylsulfoxide; CHO, Chinese hamster ovary; DRG, dorsal root ganglia; CNS, central nervous system; TTX, tetrodotoxin.
Acknowledgments
We are grateful to Dr Franz HOFMANN, Institut für Pharmakologie und Toxikologie der Technischen Universität München, Germany, for his kindly gift of the pNaEx8 plasmid, and Dr Sergey P PRONK, Department of Pharmacology, University of Colorado Denver School of Medicine, Aurora, CO, USA for the preparation of the pNaEx8 plasmid. We thank the Foundation of Selected Item Support for Researcher Back from Overseas (sponsored by Ministry of Personnel, China) (Ze-Jun WANG), Banbury Fund (Boris TABAKOFF) and US PHS grants (GM08016, MD07597, Thomas HEINBOCKEL) for financial support.
References
- Chinese Pharmacopoeia Committee. Pharmacopoeia of the People's Republic of China. Vol I. Beijing: Chemical Industry Press; 2005. p 159.
- Li YL, Tian M, Yu J, Shang MY, Cai SQ. Studies on morphology and aristolochic acid analogue constituents of Asarum campaniflorum and a comparison with two official species of Asari Radix et Rhizoma. J Nat Med 2010; 64: 442–51. [DOI] [PubMed] [Google Scholar]
- Wang D, Wang X, Xia X. Analysis of season variation of methyleugenol and safrole in Asarum heterotropoides by gas chromatography. Se Pu 1997; 15: 85–6. [PubMed] [Google Scholar]
- De Vincenzi M, Silano M, Stacchini P, Scazzocchio B. Constituents of aromatic plants: I. Methyleugenol. Fitoterapia 2000; 71: 216–21. [DOI] [PubMed] [Google Scholar]
- Carlini EA, Dallmeier K, Zelger JL. Methyleugenol as a surgical anesthetic in rodents. Experientia 1981; 37: 588–9. [DOI] [PubMed] [Google Scholar]
- Dallmeier K, Carlini EA. Anesthetic, hypothermic, myorelaxant and anticonvulsant effects of synthetic eugenol derivatives and natural analogues. Pharmacology 1981; 22: 113–27. [DOI] [PubMed] [Google Scholar]
- Sousa MB, Ximenes MF, Mota MT, Moreira LF, Menezes AA. Circadian variation of methyleugenol anesthesia in albino rats. Braz J Med Biol Res 1990; 23: 423–5. [PubMed] [Google Scholar]
- Lima CC, Criddle DN, Coelho-de-Souza AN, Monte FJ, Jaffar M, Leal-Cardoso JH. Relaxant and antispasmodic actions of methyleugenol on guinea-pig isolated ileum. Planta Med 2000; 66: 408–11. [DOI] [PubMed] [Google Scholar]
- Sayyah M, Valizadeh J, Kamalinejad M. Anticonvulsant activity of the leaf essential oil of Laurus nobilis against pentylenetetrazole- and maximal electroshock-induced seizures. Phytomedicine 2002; 9: 212–6. [DOI] [PubMed] [Google Scholar]
- Sell AB, Carlini EA. Anesthetic action of methyleugenol and other eugenol derivatives. Pharmacology 1976; 14: 367–77. [DOI] [PubMed] [Google Scholar]
- Dallmeier Zelger KR, Zelger JL, Carlini EA. New anticonvulsants derived from 4-allyl-2-methoxyphenol (Eugenol): comparison with common antiepileptics in mice. Pharmacology 1983; 27: 40–9. [DOI] [PubMed] [Google Scholar]
- Yano S, Suzuki Y, Yuzurihara M, Kase Y, Takeda S, Watanabe S, et al. Antinociceptive effect of methyleugenol on formalin-induced hyperalgesia in mice. Eur J Pharmacol 2006; 553: 99–103. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Dib-Hajj S, Cummins TR, Black JA. Sodium channels and pain. Proc Natl Acad Sci U S A 1999; 96: 7635–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss HB, Kambouris NG, Marbán E, Tomaselli GF, Balser JR. Isoform-specific lidocaine block of sodium channels explained by differences in gating. Biophys J 2000; 78: 200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MD. Selective block of late Na+ current by local anaesthetics in rat large sensory neurones. Br J Pharmacol 2000; 129: 1617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier P, Vijayaragavan K, Chahine M. Differential modulation of Nav1.7 and Nav1.8 peripheral nerve sodium channels by the local anesthetic lidocaine. Br J Pharmacol 2004; 142: 576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewska JM, Patsalos PN. Drugs used in the management of trigeminal neuralgia. Oral Surg Oral Med Oral Pathol 1992; 74: 439–50. [DOI] [PubMed] [Google Scholar]
- Elliott AA, Elliott JR. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. J Physiol 1993; 463: 39–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Aral JJ, Moss BL, He ZJ, Koszowski AG, Whisenand T, Levinson SR, et al. Identification of PN1, a predominant voltage-dependent sodium channel expressed principally in peripheral neurons. Proc Nat Acad Sci U S A 1997; 94: 1527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JA, Liu S, Tanak M, Cumminsc TR, Waxman SG. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain 2004; 108: 237–47. [DOI] [PubMed] [Google Scholar]
- Nassar MA, Stirling LC, Forlani G, Baker MD, Matthews EA, Dickenson AH, et al. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci U S A 2004; 101: 12706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo MA, Kocsis JD, Waxman SG. Selective loss of slow and enhancement of fast Na+ currents in cutaneous afferent dorsal root ganglion neurons following axotomy. Neurobiol Dis 1995; 2: 87–96. [DOI] [PubMed] [Google Scholar]
- Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature 2006; 444: 894–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S, Dahllund L, Eriksson AB, Hellgren D, Karlsson U, Lund PE, et al. A stop codon mutation in SCN9A causes lack of pain sensation. Hum Mol Genet 2007; 16: 2114–21. [DOI] [PubMed] [Google Scholar]
- Goldberg YP, MacFarlane J, MacDonald ML, Thompson J, Dube MP, Mattice M, et al. Loss-of-function mutations in the Nav1.7 gene underlie congenital indifference to pain in multiple human populations. Clin Genet 2007; 71: 311–9. [DOI] [PubMed] [Google Scholar]
- Luo S, Perry GM, Levinson SR, Henry MA. Nav1.7 expression is increased in painful human dental pulp. Mol Pain 2008; 4: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugbauer N, Lacinova L, Flockerzi V, Hofmann F. Structure and functional expression of a new member of the tetrodotoxin-sensitive voltage-activated sodium channel family from human neuroendocrine cells. EMBO J 1995; 14: 1084–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZJ, Snell LD, Tabakoff B, Levinson SR. Inhibition of neuronal Na+ channels by the novel antiepileptic compound DCUKA: identification of the diphenylureido moiety as an inactivation modifier. Exp Neurol 2002; 178: 129–38. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, Scheuer T, Catterall WA. Frequency and voltage-dependent inhibition of type IIA Na+ channels, expressed in a mammalian cell line, by local anesthetic, antiarrhythmic, and anticonvulsant drugs. Mol Pharmacol 1991; 40: 756–65. [PubMed] [Google Scholar]
- Schwartz JR, Grigat G. Phenytoin and carbamazepine: potential- and frequency-dependent block of Na currents in mammalian myelinated nerve fibers. Epilepsia 1989; 30: 286–94. [DOI] [PubMed] [Google Scholar]
- Kuo CC, Bean BP. Slow binding of phenytoin to inactivated Na+ channels in rat hippocampal neurons. Mol Pharmacol 1994; 46: 716–25. [PubMed] [Google Scholar]
- Vedantham V, Cannon SC. Slow inactivation does not affect movement of the fast inactivation gate in voltagegated Na+ channels. J Gen Physiol 1998; 111: 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo T, Shibata M. The selective capsaicin antagonist capsazepine abolishes the antinociceptive action of eugenol and guaiacol. J Dent Res 1997; 76: 848–51. [DOI] [PubMed] [Google Scholar]
- Park CK, Kim K, Jung SJ, Kim MJ, Ahn DK, Hong SD, et al. Molecular mechanism for local anesthetic action of eugenol in the rat trigeminal system. Pain 2009; 144: 84–94. [DOI] [PubMed] [Google Scholar]
- Park CK, Li HY, Yeon KY, Jung SJ, Choi SY, Lee SJ, et al. Eugenol inhibits sodium currents in dental afferent neurons. J Dent Res 2006; 85: 900–4. [DOI] [PubMed] [Google Scholar]
- Lee MH, Yeon KY, Park CK, Li HY, Fang Z, Kim MS, et al. Eugenol inhibits calcium currents in dental afferent neurons. J Dent Res 2005; 84: 848–51. [DOI] [PubMed] [Google Scholar]
- Cho JS, Kim TH, Lim JM, Song JH. Effects of eugenol on Na+ currents in rat dorsal root ganglion neurons. Brain Res 2008; 1234: 53–62. [DOI] [PubMed] [Google Scholar]
- Sensch O, Vierling W, Brandt W, Reiter M. Effects of inhibition of calcium and potassium currents in guinea-pig cardiac contraction: comparison of beta-caryophyllene oxide, eugenol, and nifedipine. Br J Pharmacol 2000; 131: 1089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci U S A 1996; 93: 9270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GK, Strichartz GR. State-dependent inhibition of sodium channels by local anesthetics: a 40-year evolution. Biochem (Mosc) Suppl Ser A Membr Cell Biol 2012; 6: 120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stein RT, Soderlund DM. Role of the local anesthetic receptor in the state-dependent inhibition of voltage-gated sodium channels by the insecticide metaflumizone. Mol Pharmacol 2012; 81: 366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcisio-Miranda M, Muroi Y, Chowdhury S, Chanda B. Molecular mechanism of allosteric modification of voltage-dependent sodium channels by local anesthetics. J Gen Physiol 2010; 136: 541–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler A, Reiprich A, Mohapatra DP, Nau C. Use-dependent block by lidocaine but not amitriptyline is more pronounced in tetrodotoxin (TTX)-resistant Nav1.8 than in TTX-sensitive Na+ channels. J Pharmacol Exp Ther 2007; 320: 354–64. [DOI] [PubMed] [Google Scholar]
- Leffler A, Reckzeh J, Nau C. Block of sensory neuronal Na+ channels by the secreolytic ambroxol is associated with an interaction with local anesthetic binding sites. Eur J Pharmacol 2010; 630: 19–28. [DOI] [PubMed] [Google Scholar]
- Wang DW, Mistry AM, Kahlig KM, Kearney JA, Xiang J, George AL Jr. Propranolol blocks cardiac and neuronal voltage-gated sodium channels. Front Pharmacol 2010; 1: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondeghem LM, Katzung BG. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta 1977; 472: 373–98. [DOI] [PubMed] [Google Scholar]