Abstract
PURPOSE
We examined the effects of participating in a “train-the-trainer” program and being a peer supporter on metabolic and cognitive/psychological/behavioral parameters in Chinese patients with type 2 diabetes.
METHODS
In response to our invitation, 79 patients with fair glycemic control (HbA1c <8%) agreed to participate in a “train-the-trainer” program to become peer supporters. Of the 59 who completed the program successfully, 33 agreed to be peer supporters (“agreed trainees”) and were each assigned to support 10 patients for 1 year, with a voluntary extension period of 3 additional years, while 26 trainees declined to be supporters (“refused trainees”). A group of 60 patients with fair glycemic control who did not attend the training program and were under usual care were selected as a comparison group. The primary outcome was the change in average HbA1c levels for the 3 groups from baseline to 6 months.
RESULTS
At 6 months, HbA1c was unchanged in the trainees (at baseline, 7.1 ± 0.3%; at 6 months, 7.1 ± 1.1%) but increased in the comparison group (at baseline, 7.1 ± 0.5%; at 6 months, 7.3 ± 1.1%. P = .02 for between-group comparison). Self-reported self-care activities including diet adherence and foot care improved in the trainees but not the comparison group. After 4 years, HbA1c remained stable among the agreed trainees (at baseline, 7.0 ± 0.2%; at 4 years: 7.2 ± 0.6%), compared with increases in the refused trainees (at baseline, 7.1 ± 0.4%; at 4 years, 7.8 ± 0.8%) and comparison group (at baseline, 7.1 ± 0.5%; at 4 years, 8.1 ± 0.6%. P = .001 for between-group comparison).
CONCLUSIONS
Patients with diabetes who engaged in providing ongoing peer support to other patients with diabetes improved their self-care while maintaining glycemic control over 4 years.
Keywords: type 2 diabetes mellitus, peer support, diabetes self-care, social support
INTRODUCTION
Diabetes self-management is often emotionally and physically taxing, demanding lifelong commitment to medication adherence and lifestyle modification.1 Health care professionals such as diabetes nurses can effectively deliver diabetes self-management education (DSME), especially for the initial acquisition of knowledge and skills.2,3 In the United States, the Medicare system provides for 10 hours of initial diabetes education in the first year for patients who have diabetes, with 2 hours of follow-up education for each subsequent year.4 In a meta-analysis of 31 randomized controlled trials, DSME programs decreased HbA1c by 0.76% more than in the comparison groups at immediate follow-up, and by 0.26% at 1 to 3 months, with a 1% reduction in HbA1c associated with every additional 23.6 hours of contact.2 Given the importance of contact time to maintain learned behaviors, peer support has been recommended as a means to improve long-term self-management.5
Peer support refers to the transfer of experiential knowledge of a specific behavior or coping strategy for a stressor between people who share a particular characteristic.6,7 Thus, people with a common illness can share knowledge and experience in a less hierarchical and more reciprocal relationship than that between patients and health care professionals.8,9 Recent studies support the use of expert patients as peer supporters for patients with chronic diseases.7,10,11 To date, most studies have focused on the effects of peer support on the recipients,12–14 while the effects of being a peer supporter have been systematically examined in only a few studies. These studies, which have involved conditions other than diabetes, have reported improvements in health behaviors and self-efficacy,15,16 depression,16 and even mortality risk among peer supporters.17,18
We previously reported a randomized trial conducted to evaluate the effect of receiving peer support in patients with type 2 diabetes.19 In an integrated care setting that incorporated specialized diabetes clinics in Hong Kong, receiving peer support did not further improve cardiometabolic well-being within 1 year, but a subgroup of patients with negative emotions benefited from peer support to the extent of having improved psychological health and reduced hospitalization. In this part of the same study, we prospectively evaluated the effects of providing peer support on metabolic, cognitive, and psychological parameters in peer supporters themselves.
METHODS
Participant Recruitment and Selection
Between February 1 and May 31 of 2009, participants from 3 hospitals (Ruttonjee Hospital, Alice Ho Miu Ling Nethersole Hospital, and Prince of Wales Hospital, all in Hong Kong) were identified and recruited by their nurses during routine medical visits. Patients with type 2 diabetes aged 18 to 75 years with fair glycemic control (HbA1c <8%), good understanding of living with diabetes, clear communication skills, and a desire to serve were invited to attend a “train-the-trainer” program as potential peer supporters. Exclusion criteria included illiteracy, physical impairment, and mental illness impairing communication with others. Those who completed the training program and passed assessments were invited to be peer supporters. Those who agreed (“agreed trainees”) were compared with those who declined (“refused trainees”). A group of patients from the same sites under usual care who had similar glycemic control but did not attend the training program were selected as comparison group subjects. All patients gave written informed consent for research and publication purposes. The study was approved by the Chinese University of Hong Kong New Territories East Cluster Clinical Research Ethics Committee.
The “Train-the-Trainer” Program
The train-the-trainer program was designed to empower trainees to provide basic knowledge and emotional support to their peers with type 2 diabetes. The program consisted of 4 monthly workshops, each lasting 8 hours, for a total of 32 hours. Health care experts led the workshops, which included both didactic components and interactive components such as role playing and group sharing. The main components of the syllabus were these:
Effective communication, focusing on positive thinking, empathetic listening, and appropriate questioning, taught by a neurolinguistic programming expert
Diabetes diet review, with cooking tips, education on common misconceptions of the diabetic diet, and suggestions for weight management, taught by an accredited dietician
Physical activity training, including precautions to take during exercise, stretching exercises, and sustaining motivation for daily physical activity, delivered by a nurse qualified in fitness training
Behavioral psychology, with emphasis on positive thinking, goal setting, decision making, and coping with negative emotions, delivered by a qualified psychologist At the end of the training program, all trainees underwent formal evaluation using case scenarios and questionnaires to assess their competency as potential peer supporters.
Peer Support Delivery
Each agreed trainee was assigned 10 patients of the same gender to support. Agreed trainees were introduced to their patient groups in several meetings where the rationale, purpose, and expectations for this study were explained. The meeting was hosted by 1 attending doctor, 1 nurse, and the project coordinator. The peer supporters were asked to provide structured peer support for at least 1 year, with provision for a voluntary extension of 3 more years.
We have described elsewhere how peer support was delivered during the 1-year structured program.19 Briefly, the peer supporters were asked to give each of their assigned patients a 15- to 20-minute telephone call biweekly for the first 3 months, monthly for the second 3 months, and every 2 months for the last 6 months. Peer supporters were given a checklist to use in reviewing specific self-management skills, including medication adherence, healthy diet, regular exercise, sick day management, foot care, and glucose monitoring. They were also encouraged to provide psychological support based on their own experiences. Peer supporters submitted their phone call checklists every 3 months for documentation of their discussion items, duration of each call, and relevant remarks. Additional electronic communication and group gatherings were left to the discretion of the participants. During the voluntary extension period, the peer supporters were asked to maintain contact with their assigned patients every 1 to 2 months for another 3 years. They were also required to document the calls and return the checklists to the project coordinator every year.
Providing Ongoing Support to Peer Supporters
In the 1-year structured program, peer supporters were reviewed by the doctor-nurse team and a project coordinator in 3 half-day debriefing meetings to share experiences, troubleshoot, and provide mutual support. Peer supporters were given opportunities to express their feelings and frustrations with their patient groups and to develop follow-up actions. Peer supporters anxious about their performance were reminded that, as nonprofessionals, they should not expect themselves to perform at the professional level, and they were reminded to encourage their patients to seek medical advice for uncertain issues. They were also asked to share sensitive information only with the medical team.
In the voluntary 3-year extension period, peer supporters met every 6 months with the project team, including at least 1 nurse, in a less formal group setting, such as hiking or having lunch together. They were encouraged to build a community among themselves and contact each other to share experiences and provide mutual support. They were also reminded to seek help from the project coordinator or the nurses if they encountered any problems.
Outcome Measurements
The primary outcome was change in HbA1c at 6 months. Secondary outcomes included changes in blood pressure, lipid profile, and cognitive/psychological/behavioral measures. Changes in the latter were assessed using validated instruments in Chinese, including the Depression Anxiety and Stress Scale (DASS) for emotional health,20 the EuroQol-5D (EQ-5D) for health-related quality of life,21 the Diabetes Empowerment Scale (DES) for self-efficacy,22 the Patient Health Questionnaire (PHQ) for depression,23 the General Health Questionnaire (GHQ) for psychological health in general population,24 and the Summary of Diabetes Self Care Activities (SDSCA) for self-care activities.25 At the end of the 3-year extension period, metabolic parameters were retrieved from the Hong Kong Hospital Authority Clinical Management System, which is shared by all public hospitals.
Clinical Care
All 3 groups were managed in the usual care setting of their hospital or community-based clinic. Hong Kong has a heavily subsidized health care system, and all patients have access to medications, investigations, and consultations for a nominal fee (US $10 per clinic visit, US $1.50 per drug for a 3–4–month supply). All individuals had access to professional diabetes education at their hospital and were usually followed up every 3–4 months at their clinics.
Sample Size Estimation and Statistical Analysis
As we said earlier, this training program was part of a trial to evaluate the effect of receiving peer support on glycemic control in patients with type 2 diabetes.19 Based on power calculations for the main study, we needed 30 peer supporters for a 1:10 ratio of peer supporters to peers. Expecting that one-half of the qualified trainees might agree to become peer supporters, we needed to train 60 subjects. Assuming a 25% attrition rate, we enrolled 79 patients in the training program.
All data were expressed as mean ± SD, median (interquartile range [IQR]), or percentage, as appropriate. Paired t-tests for continuous variables and McNemar tests for categorical variables were used for within-group comparisons. For between-group comparisons at baseline, independent t-tests and chi-square tests were used, while analysis of covariance was used for comparing the change from baseline to the 6th month between groups. We adjusted for gender when comparing the trainees and comparison group. For comparison of metabolic control after 4 years, we compared the entire group of agreed trainees, refused trainees and comparison group members using one-way ANOVA. All statistical analysis was performed using SPSS Statistics, version 20.0 (IBM).
RESULTS
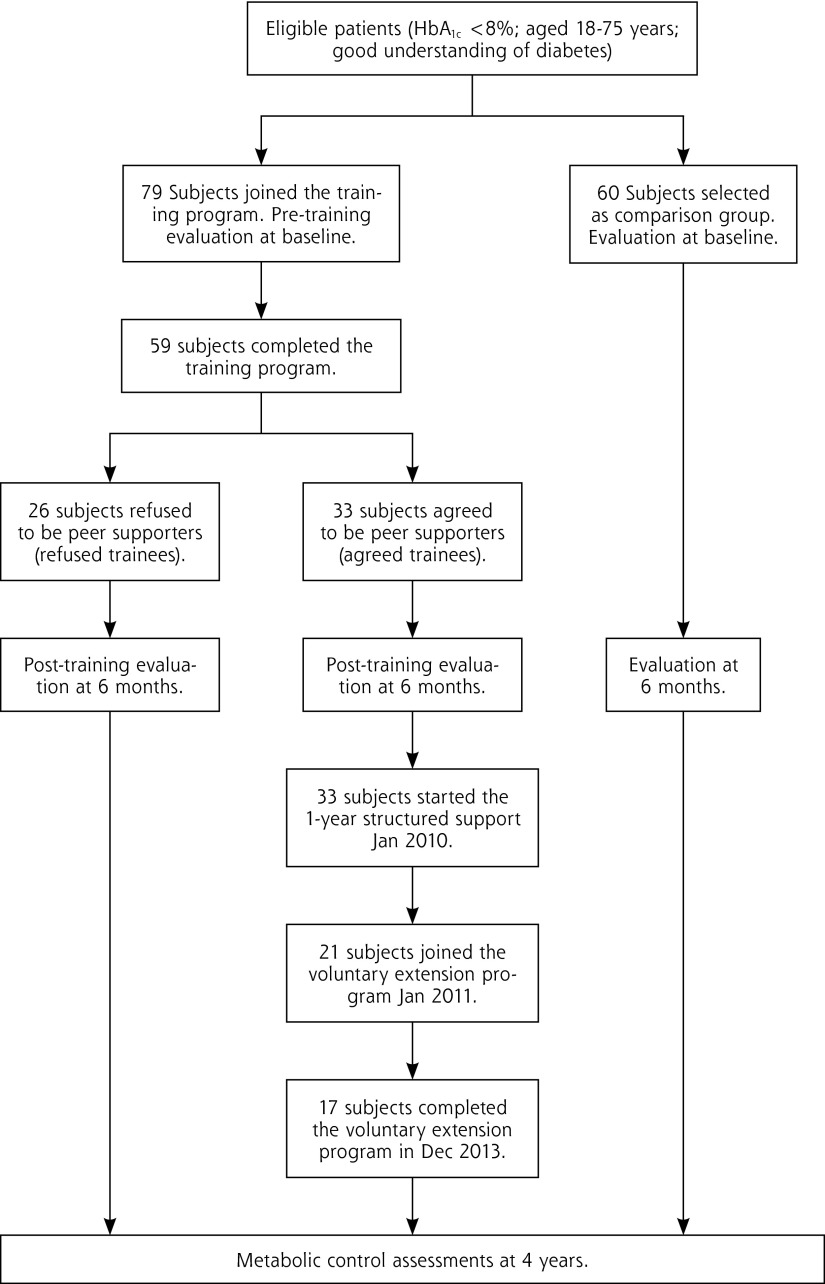
From February through May 2009, 79 eligible patients with fair glycemic control, age 55.6 ± 11.5 years, with disease duration of 11.0 ± 6.7 years, 35% male, consented to attend the train-the-trainer program, and another group of 60 patients with similar HbA1c levels who did not obtain the training were selected as the comparison group. Of the 59 trainees who completed the training program and passed their assessments, 33 agreed to be peer supporters and 26 refused. Two-thirds (21/33) of the peer supporters continued the 3-year voluntary extension and 17 completed the entire extension period (Figure 1).
Figure 1.
Recruitment and assessments of participants.
Comparison Between the Trainees and Comparison Group
At baseline, while the comparison group was more heavily male than the trainee group (65% vs 35%), the 2 groups did not differ in age, disease duration, education, or risk factor control. The trainees had higher SDSCA scores in the domains of glucose monitoring and medication adherence than the comparison group (Table 1).
Table 1.
Clinical, Psychological, and Behavioral Characteristics at Baseline and After 6 Months for Patients With Type 2 Diabetes Who Joined the Training Program (Trainee Group) and Those Under Usual Care (Comparison Group)
| Trainee Group n = 79 | Comparison Group n = 60 | Between Groups P Valuea | |||
|---|---|---|---|---|---|
|
| |||||
| Baseline | Month 6 | Baseline | Month 6 | ||
| Demographics and lifestyle | |||||
| Age, y, mean ± SD | 55.6 ± 11.5 | 56.5 ± 10.9 | |||
| Male (%) | 35b | 65 | |||
| High school or above (%) | 12.5 | 20.6 | |||
| Disease duration, y, mean ± SD | 11.0 ± 6.7 | 8.3 ± 6.6 | |||
| Non-smoker (%) | 93.6 | 87.9 | |||
| Clinical characteristics | |||||
| HbA1c, mean ± SD | 7.1 ± 0.3 | 7.1 ± 1.1 | 7.1 ± 0.5 | 7.3 ± 1.1 | .02 |
| Fasting plasma glucose, mg/dL, mean ± SD | 137 ± 43 | 137 ± 20 | 142 ± 42 | 144 ± 43 | .56 |
| Systolic blood pressure, mmHg, mean ± SD | 127 ± 13.6 | 123 ± 14.8 | 129 ± 29.5 | 122 ± 54.0 | .28 |
| Diastolic blood pressure, mmHg, mean ± SD | 71 ± 7.0 | 72 ± 12.5 | 77 ± 17.0 | 79 ± 11.2 | .15 |
| Total cholesterol, mg/dL, mean ± SD | 180 ± 30 | 170 ± 39c | 170 ± 25 | 170 ± 20 | .04 |
| Triglyceride, mg/dL, mean (IQR) | 124 (70–168) | 124 (53–160) | 124 (62–186) | 132 (88–204) | .39 |
| HDL-cholesterol, mg/dL, mean (IQR) | 58 (31–77) | 58 (35–81) | 46 (31–77 | 46 (31–77) | .20 |
| LDL-cholesterol, mg/dL, mean ± SD | 100 ± 27 | 93 ± 31c | 97 ± 23 | 93 ± 23 | .15 |
| Questionnaires | |||||
| DES total score, mean ± SD | 37.9 ± 6.71 | 37.0 ± 7.38 | 41.0 ± 8.02 | 40.4 ± 9.77 | .42 |
| GHQ total score, mean ± SD | 44.4 ± 3.93 | 43.3 ± 3.67 | 45.5 ± 4.63 | 45.1 ± 4.06 | .09 |
| DASS total score, mean (IQR) | 4 (2–9) | 3 (0–7) | 6 (2–9) | 5 (1–8) | .68 |
| PHQ total score, mean (IQR) | 2 (1–3) | 1 (0–2)c | 2 (0–3) | 1 (0–3) | .21 |
| EQ-5D index, mean (IQR) | 1 (0.80–1) | 1 (0.82–1) | 1 (0.80–1) | 1 (0.81–1) | .34 |
| EQ-5D VAS, mean ± SD | 81.6 ± 11.5 | 81.8 ± 10.7 | 81.6 ± 12.4 | 79.0 ± 11.5 | .25 |
| SDSCA | |||||
| General diet, mean ± SD | 4.73 ± 1.63 | 5.57 ± 1.32c | 4.6 ± 1.8 | 4.62 ± 1.88 | .01 |
| Special diet,d mean ± SD | 4.55 ± 1.27 | 5.32 ± 1.3c | 4.42 ± 1.7 | 4.46 ± 1.5 | .008 |
| Exercise, mean ± SD | 4.55 ± 2.15 | 4.63 ± 2.07 | 3.83 ± 2.32 | 3.65 ± 2.07 | .07 |
| Glucose monitoring, mean ± SD | 2.58 ± 1.81b | 2.89 ± 1.77 | 2.33 ± 2.08 | 2.52 ± 2.21 | .47 |
| Foot care, mean ± SD | 4.97 ± 1.78 | 5.66 ± 1.44c | 4.31 ± 2.18 | 4.27 ± 1.95 | .001 |
| Medication adherence, mean ± SD | 6.81 ± 1.06b | 6.59 ± 1.41 | 5.95 ± 1.84 | 6.3 ± 1.6 | .75 |
Between-group comparison of change from baseline to month 6 adjusted for gender.
P <.05 between-group comparison at baseline.
P <.05 within-group comparison.
Special diet: fruit/vegetable and high-fat food consumption.
Note: These are the score ranges for assessment tools:
DES: 20-item Diabetes Empowerment Scale, range 20–100; higher score means better self-efficacy.
GHQ: 12-item General Health Questionnaire, range 0–36; higher score means poorer psychological health.
DASS: 21-item Depression Anxiety Stress Scale, range 0–63; higher score means more depression, anxiety and stress.
PHQ: 9-item Patient Health Questionnaire, range 0–27; higher score means more depression.
EQ-5D index score: 5-item Euroqol; UK traffic was used; range -0.594 to 1; higher score means better health-related quality of life.
EQ-5D VAS: Visual Analogue Scale of EQ-5D, range 0–100; higher score means better self-rated health status.
SDSCA: (14-item Summary of Diabetes Self Care Assessment, range: 0–98; higher score means better self-care.
After 6 months, HbA1c had increased in the comparison group from 7.1 ± 0.3% to 7.3 ± 1.1% (P = .19) while remaining unchanged in the trainee group (7.1 ± 0.3% to 7.1 ± 1.1%, P = .81; between-group P = .02). The trainee group also had reductions in total cholesterol (180 ± 30 mg/dL to 170 ± 39 mg/Dl [4.7 ± 0.9 mmol/L to 4.3 ± 1.0 mmol/L], P = .01), low density-lipoprotein cholesterol (LDL-C) (100 ± 27 mg/dL to 93 ± 31 mg/dL, P = .03), and improvements in self-reported self-care activities, which were not seen in the comparison group.
Comparison Between the Agreed Group and Refused Group
Of the 59 qualified trainees, 26 refused to be peer supporters due to lack of time (50%), lack of interest in the program (19%), feeling unprepared (15%), and other reasons (16%). Among the 33 agreed trainees, 9 (28%) were housewives, 15 (47%) were retirees, and 8 (25%) were non-manual workers. The two groups had similar clinical profiles at baseline except that the agreed trainees had a better self-rated health status based on GHQ and EQ-5D VAS (Table 2).
Table 2.
Clinical, Psychological, and Behavioral Characteristics at Baseline and 6 Months of Patients With Type 2 Diabetes Who Agreed to Become Peer Supporters (Agreed Trainees) And Patients Who Refused (Refused Trainees) After Attending The Training Program
| Agreed Trainees (n = 33) | Refused Trainees (n = 26) | Between Groups P Valuea | |||
|---|---|---|---|---|---|
|
| |||||
| Baseline | Month 6 | Baseline | Month 6 | ||
| Demographics and lifestyle | |||||
| Age, y, mean ± SD | 55.6 ± 11.5 | 53.8 ± 14.8 | |||
| Male (%) | 35 | 20 | |||
| High school or above (%) | 20.6 | 12.5 | |||
| Disease duration, y, mean ± SD | 11.3 ± 6.7 | 12.6 ± 6.4 | |||
| Non-smoker (%) | 93.8 | 92.9 | |||
| Clinical characteristics | 125.1 | ||||
| HbA1c, mean ± SD | 7.0 ±2 | 7.0 ± 0.6 | 7.1 ± 0.4 | 7.1 ± 0.5 | .38 |
| Fasting plasma glucose, mg/dL | 135 ± 41 | 117 ± 31b | 140 ± 61 | 137 ± 43 | .04 |
| Systolic blood pressure, mmHg, mean ± SD | 125 ± 12.3 | 123 ± 10. 7 | 127 ± 14.4 | 125 ± 19.9 | .17 |
| Diastolic blood pressure, mmHg, mean ± SD | 72 ± 9.5 | 73 ± 9.9 | 69 ± 6.2 | 72 ± 12.4 | .27 |
| Total cholesterol, mg/dL, mean ± SD | 174 ± 35 | 155 ± 50b | 182 ± 35 | 170 ± 39 | .81 |
| Triglyceride, mg/dL, mean (IQR) | 124 (97–160) | 142 (97–186) | 124 (53–160) | 124 (53–142) | .16 |
| HDL-cholesterol, mg/dL, mean (IQR) | 50 (42–58) | 46 (38–54) | 58 (46–66) | 58 (6–77) | .41 |
| LDL-cholesterol, mg/dL, mean ± SD | 97 ± 31 | 89 ± 35b | 104 ± 22 | 100 ± 23 | .69 |
| Questionnaires | |||||
| DES mean score, mean ± SD | 4.18 ± 0.35 | 4.13 ± 0.27 | 4.02 ± 0.35 | 3.97 ± 0.15 | .20 |
| GHQ total score, mean ± SD | 43.5 ± 3.8c | 43 ± 3.82 | 46.2 ± 4.2 | 44.3 ± 2.76 | .45 |
| DASS total score, mean (IQR) | 4 (2–9) | 3 (0–8) | 6 (3–14) | 3.5 (1.8–11.8) | .67 |
| PHQ total score, mean (IQR) | 2 (1–3) | 0 (0–2)b | 2 (0–4) | 2 (1–3) | .33 |
| EQ-5D index, mean (IQR) | 1 (0.80–1) | 1 (0.82–1) | 1 (0.80–1) | 0.80 (0.15–1) | .009 |
| EQ-5D VAS, mean ± SD | 84.6 ± 7.7c | 88.1 ± 8.9 | 74.5 ± 14.2 | 76.1 ± 15.2 | .95 |
| SDSCA | |||||
| General diet, mean ± SD | 4.59 ± 1.62 | 5.76 ± 0.95b | 4.11 ± 2.01 | 5.3 ± 2.11 | .52 |
| Special diet,d mean ± SD | 4.62 ± 1.28 | 5.78 ± 1.30b | 4.39 ± 1.39 | 5.17 ± 1.28 | .39 |
| Exercise, mean ± SD | 4.48 ± 1.95 | 4.76 ± 2.06 | 3.68 ± 2.28 | 4.4 ± 2.12 | .56 |
| Glucose monitoring, mean ± SD | 2.82 ± 1.94 | 3.17 ± 1.89 | 3.23 ± 2.47 | 2.5 ± 1.78 | .63 |
| Foot care, mean ± SD | 4.85 ± 1.82 | 5.79 ± 1.45b | 4.33 ± 1.87 | 5.4 ± 1.43 | .09 |
| Medication adherence, mean ± SD | 6.79 ± 1.11 | 6.54 ± 1.5 | 6.45 ± 1.48 | 7 ± 0.85 | .31 |
Between-group comparison of change from baseline to month 6.
P <.05 within-group comparison.
P <.05 between-group comparison at baseline.
Special diet: fruit/vegetable and high-fat food consumption.
Note: These are the score ranges for assessment tools:
DES: 20-item Diabetes Empowerment Scale, range 20–100; higher score means better self-efficacy.
GHQ: 12-item General Health Questionnaire, range 0–36; higher score means poorer psychological health.
DASS: 21-item Depression Anxiety Stress Scale, range 0–63; higher score means more depression, anxiety and stress.
PHQ: 9-item Patient Health Questionnaire, range 0–27; higher score means more depression.
EQ-5D index score: 5-item Euroqol; UK traffic was used; range -0.594 to 1; higher score means better health-related quality of life.
EQ-5D VAS: Visual Analogue Scale of EQ-5D, range 0–100; higher score means better self-rated health status.
SDSCA: (14-item Summary of Diabetes Self Care Assessment, range: 0–98; higher score means better self-care.
After 6 months, fasting plasma glucose had decreased from 135 ± 41 mg/dL to 117 ± 31 mg/dL, (P = .033) in the agreed trainees while not changing in the refused trainees (140 ± 61 mg/dL to 137 ± 43 mg/dL, P = .61; between-group P = .04). Health-related quality of life decreased in the refused trainees (1[0.8–1.0] vs 0.8[0.5–1.0], P = .35) but remained stable in the agreed trainees (1[0.8–1.0] vs 1[0.8–1.0], P = .57) (between-group P = .009) who also had improvements in total cholesterol, LDL-C, depressive symptoms, and self-care compared with baseline (Table 2).
Metabolic Control After 4 years
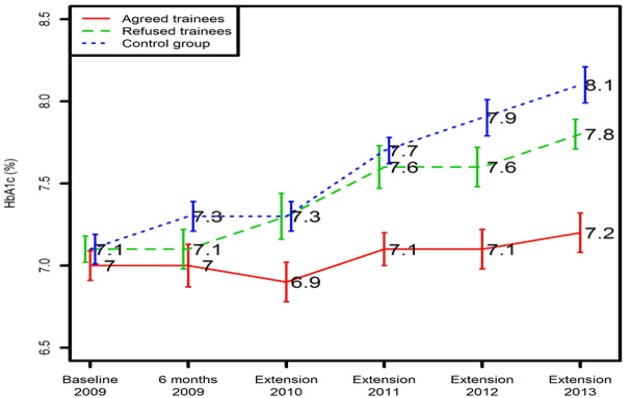
HbA1c remained unchanged in the agreed trainees after they had provided structured peer support for 4 years (baseline, 7.0 ± 0.2%; at 4 years, 7.2 ± 0.6%, P = .07) but increased in both the refused trainees (7.1 ± 0.4% to 7.8 ± 0.8%, P = .02) and the comparison group (7.1 ± 0.5% to 8.1 ± 0.6%, P = .01; among groups, P <.001) (Figure 2). No significant changes in blood pressure or LDL-C were observed in any group during the 4 years (data not shown).
Figure 2.
Comparison of glycemic control in agreed trainees, refused trainees, and the comparison group during a 4-year observational period.
Data were presented as mean ± SE.
P = .001: Comparison of HbA1c at extension year 2013 among the accepted trainees, refused trainees, and the comparison group.
DISCUSSION
Patients with type 2 diabetes and fair glycemic control who attended a peer support train-the-trainer program focusing on diet, exercise, psychology, and communication improved their own self-care behaviors and metabolic control. Those who agreed to become peer supporters had higher self-rated health status at baseline with further improvements in glycemic and lipid control as well as self-care behaviors at 6 months. After 4 years, the agreed peer supporters maintained their glycemic control while control deteriorated in the refused and comparison groups.
In a recent meta-analysis of quality improvement strategies within diabetes care, promotion of self-management had the largest positive effect on metabolic control.26 In our study, people who joined the train-the-trainer program emphasizing DSME improved their self-care behaviors and glycemic control compared with the comparison group at 6 months, lending further support to the effectiveness of self-management training in type 2 diabetes.3 Moreover, by attending the train-the-trainer program, these patients were empowered with coping skills to address the chronicity of their condition with a focus on positive thinking, goal setting, and stress management, which were associated with reduced depressive symptoms. These findings echo the importance of incorporating coping strategies for dealing with negative emotions in addition to providing medical knowledge and technical skills in a well-designed DSME program.27,28
Compared with patients who attended the train-the-trainer program but refused to be peer supporters, those who agreed to be peer supporters had very similar characteristics at baseline except higher self-rated health status, perhaps reflecting a happier and more optimistic group of individuals.29 Interestingly, the improvement in self-care behaviors and metabolic control after 6 months appeared to be limited to those who became peer supporters despite the fact thatthe refused group had completed the same training program. Thus, beyond the benefits of the education included in the training, subsequent differences between the agreed and refused trainees suggest benefits of actually providing peer support, not just being trained to do so. Moreover, the volunteer effect and willingness to help among peer supporters might have self-perpetuating and positive benefits.30 The intention to support others might have engaged the peer supporters to improve their own self-care behaviors and maintain good glycemic control over the long-term.31,32 Additionally, voluntary work has been associated with higher self-esteem,33 lower psychological distress, better quality of life,34 and reduced mortality,18 all findings consistent with the positive changes on emotions and self-management seen in our peer supporters.
Of note, although the refused trainees had a 0.2% lower HbA1c at 6 months after the training program than the comparison group, HbA1c had increased by 1% in both these groups after 4 years, in contrast to the observed maintenance of glycemic control of the agreed trainees. These findings underscore the importance of ongoing reinforcement to maintain the short-term improvement after a typical DSME program.2,35,36 The mutual learning and ongoing support among peers, peer supporters, and healthcare professionals might have further improved the ability of peer supporters to control their diabetes, solve problems, and cope with negative emotions associated with living with diabetes.37,38
This study had several limitations. Participants in the study group and comparison group were not randomized, but selected based on their having similar glycemic control. The selection process resulted in a mismatch in gender distribution between the groups, for which we adjusted in our statistical comparisons. A self-selection bias in the makeup of the agreed and refused trainee groups is likely. Although we collected reasons for refusal, we did not capture factors like employment status that may have affected patients’ decisions.
Both groups had similar clinical profiles, however, and both received similar clinical care in the same settings. The substantial difference in glycemic control after 4 years supports the hypothesis that engagement as peer supporters, not merely self-selection bias, contributed to the improvements seen in the agreed trainees.
The small sample size in each group precluded more refined analyses such as repeated-measures analysis. We also acknowledge that error may have resulted from multiple comparisons. We did not measure psychosocial status at the end of the 4-year period and were not able to evaluate the longitudinal impact of providing peer support on emotional status. Further, we did not capture detailed information about treatments, such as antidiabetic drug dosage changes, the addition of insulin, etc. Lastly, this study was conducted in hospital-based diabetes clinics and enlisted a multidisciplinary team to train the peer supporters, which might not be generalizable to primary care settings.
Conclusion
This study prospectively reported the long-term effects of providing ongoing peer support to others on patients with type 2 diabetes. It captured multidimensional outcomes and provides longitudinal evidence that by providing ongoing help to others, patients with diabetes benefited in regards to self-care, psychological health, and glycemic control over 4 years. Engaging patients to become peer supporters may be a useful strategy for long-term diabetes management.
Acknowledgments
Special thanks to all staff at the Asia Diabetes Foundation for their support.
Footnotes
Conflicts of interest: authors report none.
Funding support: Funding for this research was provided by the American Academy of Family Physicians Foundation through the Peers for Progress program with support from the Eli Lilly and Company Foundation and by the Asia Diabetes Foundation. The funders had no role in the study design, data collection, data analysis, or preparation of the manuscript.
Previous presentation: The preliminary results of this study were accepted as a poster presentation at the World Diabetes Congress 2011; December 8, 2011; Dubai, United Arab Emirates.
REFERENCES
- 1.Ahola AJ, Groop PH. Barriers to self-management of diabetes. Diabet Med. 2013;30(4):413–420. [DOI] [PubMed] [Google Scholar]
- 2.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care. 2002; 25(7):1159–1171. [DOI] [PubMed] [Google Scholar]
- 3.Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24(3):561–587. [DOI] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services. Centers for Medicare & Medicaid Services. Medicare’s Coverage of Diabetes Supplies & Services. http://www.medicare.gov/Pubs/pdf/11022.pdf. CMS Product No. 11022. Revised September 2013.
- 5.Fisher EB, Earp JA, Maman S, Zolotor A. Cross-cultural and international adaptation of peer support for diabetes management. Fam Pract. 2010;27(Suppl 1):i6–i16. [DOI] [PubMed] [Google Scholar]
- 6.Dennis CL. Peer support within a health care context: a concept analysis. Int J Nurs Stud. 2003;40(3):321–332. [DOI] [PubMed] [Google Scholar]
- 7.Boothroyd RI, Fisher EB. Peers for progress: promoting peer support for health around the world. Fam Pract. 2010;27(Suppl 1):i62–i68. [DOI] [PubMed] [Google Scholar]
- 8.Brownson CA, Heisler M. The role of peer support in diabetes care and self-management. Patient. 2009;2(1):5–17. [DOI] [PubMed] [Google Scholar]
- 9.Solomon P. Peer support/peer provided services underlying processes, benefits, and critical ingredients. Psychiatr Rehabil J. 2004; 27(4):392–401. [DOI] [PubMed] [Google Scholar]
- 10.Tattersall RL. The expert patient: a new approach to chronic disease management for the twenty-first century. Clin Med. 2002;2(3): 227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher EB, Boothroyd RI, Coufal MM, et al. Peer support for self-management of diabetes improved outcomes in international settings. Health Aff (Millwood). 2012;31(1):130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorig K, Ritter PL, Villa FJ, Armas J. Community-based peer-led diabetes self-management: a randomized trial. Diabetes Educ. 2009; 35(4):641–651. [DOI] [PubMed] [Google Scholar]
- 13.Dale J, Caramlau I, Sturt J, Friede T, Walker R. Telephone peer-delivered intervention for diabetes motivation and support: the telecare exploratory RCT. Patient Educ Couns. 2009;75(1):91–98. [DOI] [PubMed] [Google Scholar]
- 14.Lorig K, Ritter PL, Laurent DD, et al. Online diabetes self-management program: a randomized study. Diabetes Care. 2010;33(6):1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz CE, Sendor M. Helping others helps oneself: response shift effects in peer support. Soc Sci Med. 1999;48(11):1563–1575. [DOI] [PubMed] [Google Scholar]
- 16.Arnstein P, Vidal M, Wells-Federman C, Morgan B, Caudill M. From chronic pain patient to peer: benefits and risks of volunteering. Pain Manag Nurs. 2002;3(3):94–103. [DOI] [PubMed] [Google Scholar]
- 17.Musick MA, Herzog AR, House JS. Volunteering and mortality among older adults: findings from a national sample. J Gerontol B Psychol Sci Soc Sci. 1999;54(3):S173–S180. [DOI] [PubMed] [Google Scholar]
- 18.Brown SL, Nesse RM, Vinokur AD, Smith DM. Providing social support may be more beneficial than receiving it: results from a prospective study of mortality. Psychol Sci. 2003;14(4):320–327. [DOI] [PubMed] [Google Scholar]
- 19.Chan JC, Sui Y, Oldenburg B, et al. ; JADE and PEARL Project Team. Effects of telephone-based peer support in patients with type 2 diabetes mellitus receiving integrated care: a randomized clinical trial. JAMA Intern Med. 2014;174(6):972–981. [DOI] [PubMed] [Google Scholar]
- 20.Taouk MLP, Laube R. Psychometic properties of a Chinese version of the short Depression Anxiety Stress Scale (DASS21). Report for New South Wales Transcultural Mental Health Centre, Cumberland Hospital, Sydney. 2001. [Google Scholar]
- 21.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–1108. [DOI] [PubMed] [Google Scholar]
- 22.Shiu AT, Martin CR, Thompson DR, Wong RY. Psychometric properties of the Chinese version of the diabetes empowerment scale. Psychol Health Med. 2006;11(2):198–208. [DOI] [PubMed] [Google Scholar]
- 23.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg DP, Blackwell B. Psychiatric illness in general practice. A detailed study using a new method of case identification. Br Med J. 1970;1(5707):439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–950. [DOI] [PubMed] [Google Scholar]
- 26.Tricco AC, Ivers NM, Grimshaw JM, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet. 2012;379(9833):2252–2261. [DOI] [PubMed] [Google Scholar]
- 27.Brown SA. Interventions to promote diabetes self-management: state of the science. Diabetes Educ. 1999;25(6)(Suppl):52–61. [DOI] [PubMed] [Google Scholar]
- 28.Mensing C, Boucher J, Cypress M, et al. ; Task Force to Review and Revise the National Standards for Diabetes Self-Management Education Programs. National standards for diabetes self-management education. Diabetes Care. 2000;23(5):682–689. [DOI] [PubMed] [Google Scholar]
- 29.Miilunpalo S, Vuori I, Oja P, Pasanen M, Urponen H. Self-rated health status as a health measure: the predictive value of self-reported health status on the use of physician services and on mortality in the working-age population. J Clin Epidemiol. 1997;50(5):517–528. [DOI] [PubMed] [Google Scholar]
- 30.Barlow JH, Bancroft GV, Turner AP. Volunteer, lay tutors’ experiences of the Chronic Disease Self-Management Course: being valued and adding value. Health Educ Res. 2005;20(2):128–136. [DOI] [PubMed] [Google Scholar]
- 31.Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Proc. 1991;50(2):179–211. [Google Scholar]
- 32.Ajzen I, Driver BL. Prediction of leisure participation from behavioral, normative, and control beliefs - an application of the theory of planned behavior. Leis Sci. 1991;13(3):185–204. [Google Scholar]
- 33.Omoto AM, Snyder M, Martino SC. Volunteerism and the life course: Investigating age-related agendas for action. Basic Appl Soc Psych. 2000;22(3):181–197. [Google Scholar]
- 34.Wheeler JA, Gorey KM, Greenblatt B. The beneficial effects of volunteering for older volunteers and the people they serve: a meta-analysis. Int J Aging Hum Dev. 1998;47(1):69–79. [DOI] [PubMed] [Google Scholar]
- 35.Brown SA, Blozis SA, Kouzekanani K, Garcia AA, Winchell M, Hanis CL. Dosage effects of diabetes self-management education for Mexican Americans: the Starr County Border Health Initiative. Diabetes Care. 2005;28(3):527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wing RR, Goldstein MG, Acton KJ, et al. Behavioral science research in diabetes: lifestyle changes related to obesity, eating behavior, and physical activity. Diabetes Care. 2001;24(1):117–123. [DOI] [PubMed] [Google Scholar]
- 37.Tang TS, Gillard ML, Funnell MM, et al. Developing a new generation of ongoing: Diabetes self-management support interventions: a preliminary report. Diabetes Educ. 2005;31(1):91–97. [DOI] [PubMed] [Google Scholar]
- 38.Funnell MM, Nwankwo R, Gillard ML, Anderson RM, Tang TS. Implementing an empowerment-based diabetes self-management education program. Diabetes Educ. 2005;31(1):53, 55,–56, 61. [DOI] [PubMed] [Google Scholar]