Abstract
Background:
Although progress has been made in the detection and characterization of neural plasticity in depression, it has not been fully understood in individual synaptic changes in the neural circuits under chronic stress and antidepressant treatment.
Methods:
Using electron microscopy and Western-blot analyses, the present study quantitatively examined the changes in the Gray’s Type I synaptic ultrastructures and the expression of synapse-associated proteins in the key brain regions of rats’ depressive-related neural circuit after chronic unpredicted mild stress and/or escitalopram administration. Meanwhile, their depressive behaviors were also determined by several tests.
Results:
The Type I synapses underwent considerable remodeling after chronic unpredicted mild stress, which resulted in the changed width of the synaptic cleft, length of the active zone, postsynaptic density thickness, and/or synaptic curvature in the subregions of medial prefrontal cortex and hippocampus, as well as the basolateral amygdaloid nucleus of the amygdala, accompanied by changed expression of several synapse-associated proteins. Chronic escitalopram administration significantly changed the above alternations in the chronic unpredicted mild stress rats but had little effect on normal controls. Also, there was a positive correlation between the locomotor activity and the maximal synaptic postsynaptic density thickness in the stratum radiatum of the Cornu Ammonis 1 region and a negative correlation between the sucrose preference and the length of the active zone in the basolateral amygdaloid nucleus region in chronic unpredicted mild stress rats.
Conclusion:
These findings strongly indicate that chronic stress and escitalopram can alter synaptic plasticity in the neural circuits, and the remodeled synaptic ultrastructure was correlated with the rats’ depressive behaviors, suggesting a therapeutic target for further exploration.
Keywords: Synapse, plasticity, depression, rat
Introduction
There is growing evidence that major depressive disorder (MDD) is associated with impairment in neural circuits related to emotion and cognition (Drevets et al., 2008; Duman and Voleti, 2012; Price and Drevets, 2012). The medial prefrontal cortex (mPFC) network, composed of mPFC and its associated brain regions (eg, amygdala and hippocampus), is one of the most important circuits regulating the responses to stress in either animal models (Moustafa et al., 2013; Chang et al., 2014) or MDD patients (Lorenzetti et al., 2009; Price and Drevets, 2012).
Neuroplasticity, including synaptogenesis and its associated functional alternations, is a key feature of the adult brain, enabling it to make adaptive responses in the continuously changing environment (Gu et al., 2013). Dysfunction of the processes occurs in some brain regions in animal models. For example, repeated restraint or chronic unpredicted mild stress (CUMS) can induce structural remodeling of neurons (Alfarez et al., 2003; McLaughlin et al., 2007; Morales Rico et al., 2014), a deficit in long-term potentiation, and facilitation in long-term depression in the rat hippocampus (Pavlides et al., 2002; Artola et al., 2006; Qiao et al., 2014). Based on those findings, the “Synaptogenesis Hypothesis” was proposed, considering there was destabilization and loss of synaptic connections in mood and emotion circuits in depression (Duman and Voleti, 2012).
Although structural remodeling of neurons occurs after stress, there are inconsistent reports in different studies. For example, Morales Rico et al. (2014) showed that chronic restraint induced a decrease in dendritic spine density in the ventral CA1 neurons in female rats, but there were no changes in male rats, while others groups have observed that synaptic plasticity in the CA1 region was affected by stress in male rats and monkeys (Fuchs et al., 1995; Sousa et al., 2000; Alfarez et al., 2003). Thus, specific structural remodeling of neurons may depend not only on the animal species and sex, but also on the brain regions and stress types (Vyas et al., 2002; Alfarez et al., 2003; McLaughlin et al., 2007; Morales Rico et al., 2015). However, how would the ultrastructure of an individual synapse be affected by chronic stress or treatment? Are the effects similar among different regions of neural circuits? Is there any relationship between changed synaptic ultrastructure and depressive-like behaviors? Elucidating ultrastructural changes of individual synapses will help to reveal the responses and modification of a synapse and, at least in part, estimate the size and correlated activity of dendrites or spines during chronic stress and antidepressant treatment.
Based on Gray’s concept, the Type I synapses are the most common subtype of synapses and are located mainly on dendritic spines and shafts in the central nervous system of animals (Gray, 1959; Klemann and Roubos, 2011). Proteins in their well-developed postsynapstic density (PSD) mediate the communication of neurons, which is related to the function and plasticity of synapse (Boeckers, 2006; Klemann and Roubos, 2011). A lot of evidence has shown the Type I synapses are the site of glutamate transmission (Waselus and Van Bockstaele, 2007; Klemann and Roubos, 2011). The morphological plasticity of synapses is known to be accompanied by changed expression of some synapse-associated proteins, which have apparent correlation with the size of the PSD and the efficacy of signal transmission (Boeckers, 2006; Sheng and Kim, 2011). However, until now, the expression of these proteins in different brain regions during a chronic depressive-like procedure or after long-term antidepressant treatment is still largely unexplored.
CUMS is a highly valid animal model with similarity to the effects of environmental factors on depression in humans (Willner, 2005). Escitalopram is one of the first line of drugs used for the treatment of MDD, and it belongs to the family of selective serotonin reuptake inhibitors (Kirino, 2012). Chronic treatment with escitalopram can restore the hippocampal long-term potentiation in an animal model of depression (Bhagya et al., 2011). To understand the synaptic plasticity that may occur in the mPFC circuit, we investigated the effects of stress and escitalopram treatment on the morphology of Gray’s Type I synapses in several subfields of prefrontal cortex, hippocampus, and amygdala in CUMS rats. The synapse-associated proteins are involved in the regulation of neurotransmission and synaptic plasticity (Chi et al., 2001; Kwon and Chapman, 2011). Thus, the expression of several important synapse-associated proteins, including presynaptic proteins (synapsin I and synaptophysin) and postsynaptic proteins (PSD93, PSD95 and spinophilin), was also determined.
Materials and Methods
Animals
Adult male Sprague-Dawley rats (Experimental Animal Center, Shanghai, China) were maintained in a temperature- and humidity-controlled environment. The standard 12-h-light/-dark cycle (lights on at 7:00 am) was changed only during the course of stimulation. Food and water were provided ad libitum except when food or water deprivation was applied as a stressor. After a 1-week acclimation period, rats were divided into 2 groups (CUMS and normal control groups). Rats that received stress were housed individually (Jayatissa et al., 2006; Wang et al., 2009) except when grouping was applied as a stressor. Chronic stress lasted for 8 consecutive weeks. The normal controls were housed 4 to 5 animals per cage in a separate room under standard conditions.
After the 4-week experimental period, rats in the CUMS group were selected according to their performance in sucrose preference, forced swimming test (FST), and open-field test (OFT). Only the stressed rats that exhibited changed behaviors in at least 2 of the tests (decreased sucrose preference and changes in OFT or FST) were entered in the following experiments, while the remaining rats were excluded and sacrificed. The depressive-like rats were divided into the CUMS with saline treatment subgroup (CS; n=12), and the CUMS with escitalopram treatment subgroup (CE; n=12). Meanwhile, the controls were divided into 2 matched subgroups: normal rats with saline treatment (NS; n=12) and normal rats with escitalopram treatment (NE; n=12). One-half of the rats in each subgroup were used for electron microscope analysis and the other one-half for Western-blot analysis. The procedures used in the present study were in strict compliance with the NIH laboratory animal care guidelines.
CUMS
The CUMS protocol was adapted from previous studies with some modifications (Willner, 1997; Jayatissa et al., 2006). The CUMS procedure consisted of 8 different stressors applied randomly every week for 8 weeks. These stressors included food deprivation, water deprivation, grouped housing, cage tilt, soiled cage, tail clamping, continuous lighting, and cold swimming (Table 1).
Table 1.
Stressors Used for Inducing Chronic Unpredicted Mild Stress in Rats
| Day | Food deprivation | Water deprivation | Soiled cage | Continuous lighting | Tilt cage | Clamping tail | Grouped housing | Cold swimming |
|---|---|---|---|---|---|---|---|---|
| 1 | 5:00 pm | 5:00 pm | 3:00 pm | 9:00 am | 5:00 pm | 3:00 pm (1min) | 9:00 am | 3:00 pm (5min) |
| 2 | 10:00 am | 10:00 am | 9:00 am | 9:00 pm | 9:00 am | 9:00 am |
The stressors lasted for 8 weeks and each animal received one stressor every day. The stressors were randomly changed through the weeks.
Drug Treatment
Animals were administered saline or escitalopram oxalate (kindly provided by H. Lundbeck A/S. Copenhagen-Valby, Denmark) every day from 5 weeks until the end of the experiment (28 total days). The drug was freshly prepared in saline solution and was administered intraperitoneally at 1mL/kg body weight at a dose of 10mg/kg body weight.
Behavior Tests
Body Weight Measuring and Sucrose Preference Test
Body weights were measured every day at 9:00 am. A sucrose preference test was employed weekly to operationally define anhedonia (Katz, 1981; Overstreet, 2012). The test procedure lasted for 24 hours with bottles containing 1% (wt/vol) sucrose solution or water, and their positions were changed after 12 hours. Intake was measured by weighing the bottles at the beginning and end of each test. Sucrose preference was calculated according the following ratio: sucrose preference=sucrose intake/(sucrose intake+water intake) (Overstreet, 2012).
FST
Rats were individually placed into a cylinder (30×60cm; Shanghai Yishu Company, China) filled with water (25±2°C, 30cm deep), and their behaviors were recorded by a video camera placed in front of the cylinder. The test lasted for 6 minutes, and the immobility during only the last 5 minutes was analyzed. Each rat was judged to be immobile when no additional activity was observed other than that required to keep the rat’s head above water (Cryan et al., 2005).
OFT
Locomotor activity of the animals was analyzed using the OFT between 2:00 and 5:00 pm each time. The apparatus (Shanghai Yishu Company) consisted of a wooden box measuring 100×100×50cm. The total distance and rearing times of each rat were counted in a 5-minute session and recorded by a video camera. The apparatus was cleaned with detergent and dried after each rat was tested.
The FST and OFT were performed 3 times: at the beginning, middle, and end of the experimental period. All of the final behavioral tests were carried out 48 hours later from the last dose of escitalopram administration.
Tissue Preparation
All the rats were sacrificed 5 days after the last saline or escitalopram treatment.
Tissue Preparation for Transmission Electron Microscopic Analysis
Rats from the 4 groups (n=6/group) were anesthetized with an overdose of 7% chloral hydrate in saline and then perfused with 200mL saline through the ascending aorta followed by 5% glutaraldehyde dissolved in phosphate buffer (0.1M, pH 7.4). Two subareas of the mPFC, prelimbic cortex (PrL) and area 1 of the cingulate cortex (Cg1), were removed (Kodama et al., 2004; Mattinson et al., 2011) from the Bregma 3.70mm to Bregma 2.20mm. The area of the basolateral amygdaloid nucleus (BLA) was obtained from Bregma -2.30mm to Bregma -3.30mm. The structure from the Bregma -3.30mm to Bregma -4.30mm of the dorsal hippocampus was subsequently analyzed for structural changes using electron microscopy. The stratum radiatum of the Cornu Ammonis 1 (CA1sr), stratum radiatum/stratum lacunosum of Cornu Ammonis 3 (CA3sl/sr), and stratum moleculare of Dentate Gyrus (DGsm) were removed using brain matrices and a stereoscopic anatomic microscope.
The samples were trimmed to be about 0.5 mm×1 mm×1mm and postfixed overnight in the 3% glutaraldehyde dissolved in phosphate buffer (0.1M, pH 7.4). Samples were then washed, dehydrated with ascending concentrations of ethanol, incubated in 1% osmium tetroxide for 1 hour at room temperature, and embedded in Epon media. Ultra-thin sections (70nm) were subsequently collected on formvar-coated grids, contrasted with uranyl acetate and lead citrate. Six sections of each brain region were viewed using a transmission electron microscope (H-7650, Hitachi High-Technologies Corporation). Ten electron micrographs were taken randomly from each field-of-view in each section. At least 180 synapses were analyzed in each brain area of one rat.
Tissue Preparation for Western Blot
Six rats from each group were deeply anesthetized, perfused with 200mL saline perfusion through the ascending aorta, and decapitated. Their brains were rapidly removed and promptly stored at -80°C until further analysis.
Ultrastructure Analysis
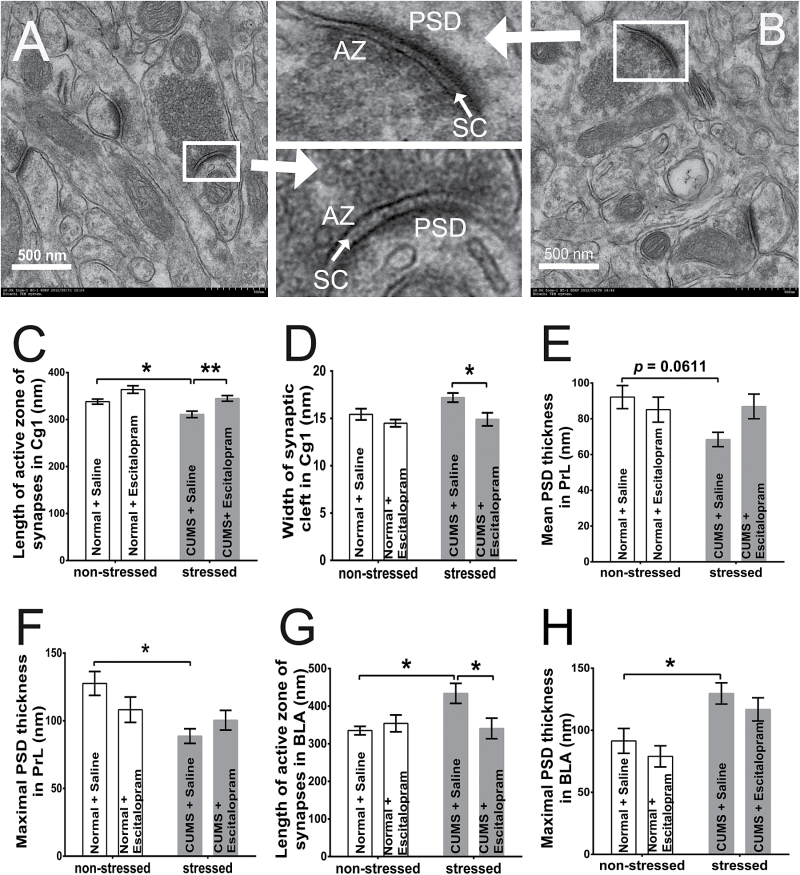
Criteria for ultrastructural analysis of Gray’s Type I included: (1) synapses must be asymmetric, transverse, and not tangentially cut, and (2) synapses must show a clear PSD and synaptic cleft (Christoffel et al., 2011) (Figure 3A-B). Image-Pro Plus 6.0 software was used to measure the mean width of the synaptic cleft, the mean and maximal PSD thickness, the length of the active zone, and the curvature of the synapses. The synaptic curvature was classified as follows: (1) concave, a protrusion of presynaptic terminal into the postsynaptic button; (2) convex, a protrusion of postsynaptic button into the presynaptic terminal; and (3) flat, no discernable curvature (Marrone and Petit, 2002; Connor et al., 2006).
Figure 3.
Changed excitatory synaptic structures in area 1 of the cingulate cortex (Cg1) (C-D), prelimbic cortex (PrL) (E-F), and basolateral amygdaloid nucleus (BLA) (G-H) regions in different groups (n=6/group). A and B were 2 sections’ electron microscopic images of Cg1 region in the normal rats with saline treatment (NS). The upper small image between A and B was a concave-formed synapse from B and the lower a convex-formed synapse from A. In the Cg1 region, chronic unpredicted mild stress (CUMS) reduced the length of the active zone (AZ) in CUMS with saline treatment subgroup (CS) rats (vs NS rats, P=.0427) (C) and escitalopram increased it (P=.0089) (C) and decreased the width of the synaptic cleft (SC) (P=.0363) (D) in CUMS with escitalopram treatment subgroup (CE) rats compared with CS rats, without any effect on those of NE rats (P=.9053 and .0579). In the PrL region, the maximal postsynapstic density (PSD) thickness in CS rats sharply decreased (P=.0113) (E), and the mean PSD thickness showed a similar trend (P=.0611) (F) compared with that of the NS group. In the BLA area, the length of the AZ (P=.029) (G) and the maximal PSD thickness (P=.036) (H) increased significantly in CS rats compared with the NS group, and escitalopram significantly reversed the change of AZ in the CE group (vs CS rats, P=.0419) but not in the NE group (vs NS rats, P=.934) (G). *P<.05, **P<.005. Error bars represent SEM.
Western Blot
The procedure for Western-blot analysis used in this study followed a previously reported protocol (Li et al., 2007). Briefly, frozen cerebrum tissues were collected, homogenized, and centrifuged at 12,000 g for 20 minutes at 4°C. The supernatant was phase collected. Proteins were separated by SDS-PAGE and transferred to a PVDF membrane (Millipore). Membranes were blocked with 5% skimmed milk and incubated with primary antibodies specific for PSD93 (1:2000, Abcam), PSD95 (1:2000, Abcam), spinophilin (1:1000, Abcam), synapsin I (1:2000, Abcam), or synaptophysin (1:1000, Millipore). After incubation with goat anti-rabbit or goat anti-mouse IgG, the membranes were developed using an electrochemiluminescence (ECL) chemiluminiscence kit (Millipore). The protein levels were quantified using Quantity One software (Bio-Rad) and normalized to GAPDH expression.
Statistical Analysis
Statistical analyses were carried out using GraphPad Prism 6.01 and IBM SPSS Statistics 20 (for analysis of covariance). All of the graphic representations were performed using GraphPad Prism 6.01. Abnormal behaviors were defined when the value was 2 SD lower than that of the controls. Two-way ANOVA was used for analyses of the data and was followed by Tukey posthoc tests. ANCOVA was used to determine the effects of body weight on OFT and FST. A Pearson correlation analysis was performed to estimate the relationship between synaptic morphological changes and behaviors. Data are reported as means ± SEM. P<.05 was considered significant.
Results
Effects of CUMS and Escitalopram Treatment on Rats’ Body Weight and Sucrose Preference
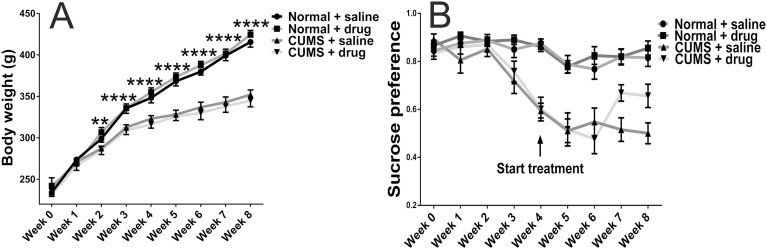
The body weight of stressed rats was significantly less than that of normal controls (F=17.01, P=.0002, n=24/group), and the difference was maintained to the last measurement (F=135.2, P<.0001, n=12/group). However, body weight remained unaffected by chronic escitalopram treatment throughout the experimental procedure in both the unstressed and stressed groups (F=0.004, P=.95, n=12/group) (Figure 1A).
Figure 1.
The body weight (A) and sucrose preference (B) of rats in the 4 groups during 8 weeks. Chronic unpredicted mild stress (CUMS) significantly reduced the increase of rats’ body weight after 2 weeks of stress (P=.0046, n=24/group), and the influence remained to the end of experiment (P<.0001, n=12/group). However, chronic escitalopram administration did not affect the body weight in both stressed and control animals (A). After 3 weeks of initial exposure to CUMS, sucrose preference was significantly decreased in stressed rats compared with controls (P=.0002, n=24/group), and the difference continued to the end of experiment at week 8 (the normal rats with saline treatment [NS] group vs the CUMS with saline treatment subgroup (CS) group, P<.0001, n=12/group). After 3 weeks of escitalopram treatment, the sucrose preference of rats in the CUMS with escitalopram treatment subgroup (CE) group was significantly increased compared with that of the CS group (P=.0473). The significance increased further at the end of experiment for the CE group compared with the CS group (P=.0157). **P<.05 and ****P<.0001, the differences in body weight between control and stressed rats. Error bars represent SEM.
Sucrose preference decreased significantly during 4 weeks of stress compared with the normal controls (F=39.94, P<.0001). After 3 weeks of treatment with escitalopram, the sucrose preference was significantly increased in CUMS rats (F=4.133, P 2-way ANOVA=.0481, P Tukey=.0473), and this increase was further significant at the end of the experimental period (F=6.044, P 2-way ANOVA=.018, P Tukey=.0157). Escitalopram did not affect sucrose preference in unstressed controls (NE group; Figure 1B).
Effects of CUMS and Escitalopram on FST of Rats in the 4 Groups
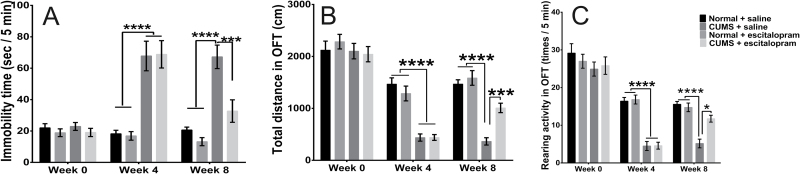
Body weight did not affect the immobility time of rats in FST either at week 4 (F=1.895, P=.175, n=24/group) or at the end point of the experiment (F=0.839, P=.365, n=12/group). CUMS significantly increased the immobility time of FST (F=57.97, P 2-way ANOVA < .0001, P Tukey < .0001, n=24/group), and the difference was maintained to the end point of the measurement (F=37.28, P 2-way ANOVA < .0001, P Tukey < .0001, n=12/group). A 4-week treatment substantially decreased the immobility time of CUMS rats (F=15.08, P 2-way ANOVA=.0003, P Tukey=.0003, n=12/group) (Figure 2A). No difference was seen between the NE and NS groups (P Tukey=.763, n=12/group).
Figure 2.
Note the results of the forced swimming test (FST) (A) and open-field test (OFT) (B-C) throughout the experimental period. (A) Chronic unpredicted mild stress (CUMS) significantly increased the immobility time in the FST. After escitalopram treatment, the immobility time of CUMS with saline treatment subgroup (CS) rats substantially decreased (n=12/group). (B) Distance travelled by CS rats was significantly reduced in the OFT. Chronic escitalopram treatment increased the distance travelled by CUMS with escitalopram treatment subgroup (CE) rats (n=12/group). (C) Meanwhile, the rearing times of CUMS rats decreased compared with that of the control rats. After 4 weeks of treatment, escitalopram significantly increased the rearing times of CUMS rats (n=12/group). *P<.05, ***P<.001, ****P<.0001. Error bars represent SEM.
Effects of CUMS and Escitalopram on OFT of Rats in the 4 Groups
ANCOVA analysis showed that body weight did not affect either the total distance or the rearing times of rats in the OFT. CUMS significantly reduced the total distance rats ran at week 4 (F=40.70, P<.0001, n=24/group) or at the end point of the measurement (F=71.38, P 2-way ANOVA < .0001, P Tukey < .0001, n=12/group), while escitalopram increased the total distance in the CE group (F=14.62, P 2-way ANOVA=.0004, P Tukey=.0002) but had no effect in the NE group (P Tukey=.8402, n=12/group) (Figure 2B).
The rearing times of stressed rats were sharply decreased at week 4 (F=47.99, P<.0001, n=24/group) and at the end point of the experiment (F=44.57, P 2-way ANOVA<.0001, P Tukey<.0001, n=12/group). Chronic escitalopram treatment significantly increased the rearing times of CUMS rats (F=8.187, P 2-way ANOVA=.0064, P Tukey=.0002) but had no effect on NE rats (P Tukey=.9357, n=12/group) (Figure 2C).
Comparison of Synaptic Morphology in Brain Regions of Rats in the 4 Groups
Comparison of Synaptic Morphology in the Rat’s Cg1 Region
In the Cg1 region, chronic stress decreased and escitalopram treatment increased the length of the synaptic active zone significantly (F=11.88, P=.0025 and F=20.02, P=.0002, respectively; n=6/group). There was a trend for an increase in the length of the active zone in the NE group (P=.0579). Meanwhile, escitalopram treatment remarkably decreased the synaptic cleft in stressed rats (F=8.611, P=.0082) (Figure 3C-D).
In the CS group, a significant decrease of convex-formed synapses (42.25%) was observed compared with those of the NS group (48.65%; P=.0444), while the flat ones increased to 48.8% compared with 34.47% in the NS group (P<.0001). Compared with the CS group, escitalopram increased the convex-formed synapses (53.02%, P=.01) and decreased flat-formed ones (34.73%, P<.0001) in the CE group (Table 2). However, escitalopram did not affect the proportions in the NE group.
Table 2.
Synaptic Curvatures in Several Brain Subregions among Different Groups
| Mean ± SEM | |||||
|---|---|---|---|---|---|
| Normal + saline | Normal + drug | CUMS + saline | CUMS + drug | ||
| CX (%) | 48.65±2.04 | 54.65±3.13 | 42.25±0.94* | 53.02±1.58# | |
| Cg1 | CC (%) | 16.88±3.08 | 11.04±3.39 | 8.95±1.62* | 12.25±2.19# |
| FL (%) | 34.47±2.70 | 34.31±1.04 | 48.8±1.16* | 34.73±1.70# | |
| CX (%) | 44.75±2.43 | 52.66±2.80* | 43.64±2.73 | 52.29±1.81# | |
| PrL | CC (%) | 14.92±1.98 | 8.73±2.22 | 10.81±1.90 | 10.13±1.68 |
| FL (%) | 40.33±2.37 | 38.62±1.82 | 45.56±2.57 | 37.58±2.37# | |
| CX (%) | 46.34±2.82 | 50.81±2.80 | 54.95±3.59* | 46.82±3.29# | |
| BLA | CC (%) | 15.44±3.58 | 9.61±1.95 | 31.21±1.85 | 15.21±2.83 |
| FL (%) | 41.88±2.61 | 38.74±3.06 | 31.83±1.98* | 44.64±2.74# | |
| CX (%) | 46.31±3.94 | 42.99±1.69 | 36.07±2.45* | 50.42±2.70# | |
| CA1 | CC (%) | 16.25±2.68 | 16.90±3.07 | 10.45±2.91 | 10.44±2.90 |
| FL (%) | 37.44±2.82 | 40.11±4.47 | 53.67±3.64* | 39.13±3.45# | |
| CX (%) | 48.36±0.97 | 48.76±1.74 | 39.73±1.44* | 55.31±4.86# | |
| CA3 | CC (%) | 17.47±3.11 | 13.57±3.36 | 17.03±2.21 | 12.73±2.98 |
| FL (%) | 34.17±2.89 | 37.67±3.22 | 43.24±2.40* | 31.95±3.30# | |
| CX (%) | 47.36±1.81 | 50.89±2.99 | 38.06±2.08* | 56.71±4.97*,# | |
| DG | CC (%) | 16.53±3.05 | 16.23±3.14 | 21.4±3.37 | 13.27±3.80 |
| FL (%) | 36.11±2.08 | 32.87±3.44 | 40.37±1.93 | 30.02±2.10# | |
Abbreviations: CC, concave-formed synapse; CX, convex-formed synapse; FL, flat-formed synapse; Cg1, area 1 of the cingulate cortex; PrL, prelimbic cortex; BLA, area of the basolateral amygdaloid nucleus; CA1, Cornu Ammonis 1; CA3, Cornu Ammonis 3; DG, Dentate Gyrus.
*P < .05 compared with the NS group. #P < .05 compared with the CS group.
Comparison of Synaptic Morphology in the Rats’ PrL Region
The main effect of stress on the synaptic morphology in the PrL was changes in the maximal PSD thickness (F=8.786, P=.0077, n=6/group). An analysis of PrL synaptic curvature showed no significant changes in CS rats compared with that of NS controls. However, chronic escitalopram treatment increased the proportion of convex-formed synapses in CE (43.64% to 52.29%, P=.0087) and NE (44.75% to 52.66%, P=.0159) rats and decreased the proportion of flat-formed synapses in CE rats (45.56% to 37.58%, P=.0151), respectively (Table 2).
Comparison of Synaptic Morphology in the Rats’ BLA Region
Stress significantly increased the maximal PSD thickness in rats (F=17.32, P=.0005, n=6/group). There was a trend for an increase in the length of the active zone in the BLA after stress (F=3.499, P=.0761). A significant interaction of stress and escitalopram was observed on the length of the active zone (F=6.037, P=.0233) in the BLA.
Interestingly, the convex-formed synapses in the BLA of CS rats increased to 54.95% compared with 46.34% in the NS rats (P=.0346), and decreased to 46.82% in the CE rats (P=.0454). There was a significant decrease of flat-formed synapses in the BLA of CS rats (31.83%) compared with those in the NS group (41.88%, P=.0143), while escitalopram increased the proportion significantly (44.64%, P=.0022) in the CE rats (Table 2). However, there was no significant effect of chronic escitalopram treatment on synaptic curvatures in the NE group.
Comparison of Synaptic Morphology in the 3 Subregions (CA1sr, CA3sl/sr, and DGsm) of Rats
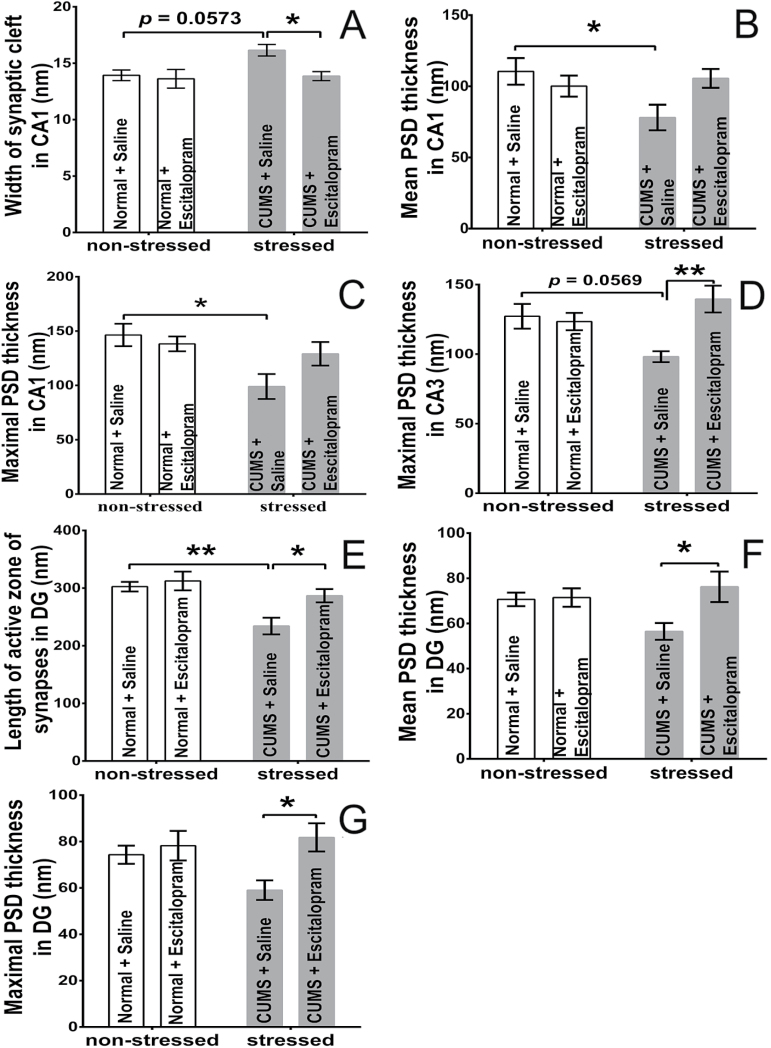
In the CA1sr region, the width of the synaptic cleft in the rats was increased by CUMS (F=4.581, P=.0448, n=6/group) or significantly decreased escitalopram treatment (F=5.102, P=.0352) (Figure 4A). CUMS significantly diminished the maximal PSD thickness in the stressed rats (F=7.963, P=.0105) (Figure 4B-C). In the CA3sl/sr, the maximal PSD thickness of the Type I synapse was increased by escitalopram (F=6.277, P=.021, n=6/group), and the interaction effect of stress and escitalopram was observed (F=9.059, P=.0069) (Figure 4D). In the DGsm region, CUMS diminished the length of the active zone (F=13.00, P=.0018, n=6/group) and escitalopram reversed it significantly (F=5.731, P=.0266). The PSD thickness in DGsm was affected by escitalopram in the CE group but not stress (F=4.972, P=.0374 for the mean; F=6.408, P=.0199 for the maximal) (Figure 4E-G).
Figure 4.
Morphological changes of synaptic configuration in the stratum radiatum of the Cornu Ammonis 1 (CA1sr) (A-C), stratum radiatum/stratum lacunosum of Cornu Ammonis 3 (CA3sl/sr) (D), and stratum moleculare of Dentate Gyrus (DGsm) (E-G) areas of hippocampus in different groups (n=6/group). In the chronic unpredicted mild stress (CUMS) with saline treatment subgroup (CS) rats, CUMS increased the width of the synaptic cleft (P=.0573) (A), decreased the mean (P=.049) (B) and maximal (P=.0157) (C) postsynapstic density (PSD) thickness in the CA1sr, and decreased the length of the active zone (P=.007) (E) in the DGsm compared with normal rats with saline treatment (NS) rats. In the CA3sl/sr region, there was a downward trend of maximal PSD thickness in CS rats (vs NS rats, P=.0569) (D). Chronic escitalopram administration decreased the width of the synaptic cleft in the CA1sr (P=.0483) and increased the maximal PSD thickness in the CA3sl/sr (P=.0045) (D) and the length of the active zone (P=.0448) (E) as well as the mean (P=.0389) (F) and maximal (P=.0293) (G) PSD thickness in the DGsm of the CUMS with escitalopram treatment subgroup (CE) compared with that of the CS group. However, no changes were observed in the normal rats with escitalopram treatment (NE) group among all 3 subareas of hippocampus. *P<.05, **P<.005. Error bars represent SEM.
In all 3 subregions of the hippocampus, the proportion of convex-formed synapses in the CS rats was significantly decreased (36.07% in CA1sr, 39.73% in CA3sl/sr, 38.06% in DGsm, respectively) compared with that of the NS rats (46.31% in CA1sr, P=.0245, 48.36% in CA3sl/sr, P=.0385, and 47.36% in DGsm, P=.0343), whereas chronic stress increased the proportion of flat-formed synapses in CA1sr (53.67%) and CA3sl/sr (43.24%) compared with that of NS rats (37.44% in CA1sr, P=.0005; 34.17% in CA3sl/sr, P=.0299). Escitalopram increased the proportion of convex-formed synapses (50.42% in CA1sr, P=.002, 55.31% in CA3sl/sr, P=.0003, and 56.71% in DGsm, P<.0001) and decreased the proportion of flat-formed synapses (39.13% in CA1sr, P=.018, 31.95% in CA3sl/sr, P=.0075, and 30.02% in DGsm, P=.019) in the CE rats but did not affect the proportions in the NE group (Table 2).
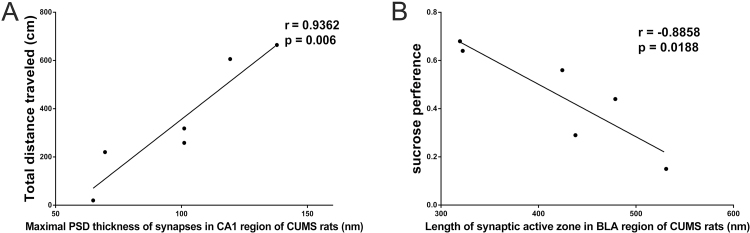
Correlation between the Behaviors and Synaptic Ultrastructure in CUMS Rats
Pearson correlation analysis revealed that there was a positive correlation between the total distance in the OFT and the maximal PSD thickness in the CA1sr region (r=0.9362, P=.006, n=6; Figure 5A) and a negative correlation between the sucrose preference and the length of the active zone of synapses in BLA region in the CS group (r=-0.8858, P=.0188, n=6; Figure 5B).
Figure 5.
Pearson correlation analysis of the synaptic ultrastructure and behaviors in chronic unpredicted mild stress (CUMS) rats. (A) High correlation between total distances CUMS rats run in the open-field test (OFT) and the synaptic maximal postsynapstic density (PSD) thickness in the stratum radiatum of the Cornu Ammonis 1 (CA1sr) region (n=6). (B) Correlation between the sucrose preference and the length of active zone of Gray’s Type I synapses in basolateral amygdaloid nucleus (BLA) region of CUMS rats (n=6).
Altered Expression of Synapse-Associated Proteins in Different Brain Regions of Rats in the 4 Groups
Altered Expression of Synapse-Associated Proteins in the Rats’ Cg1 Region in the 4 Groups
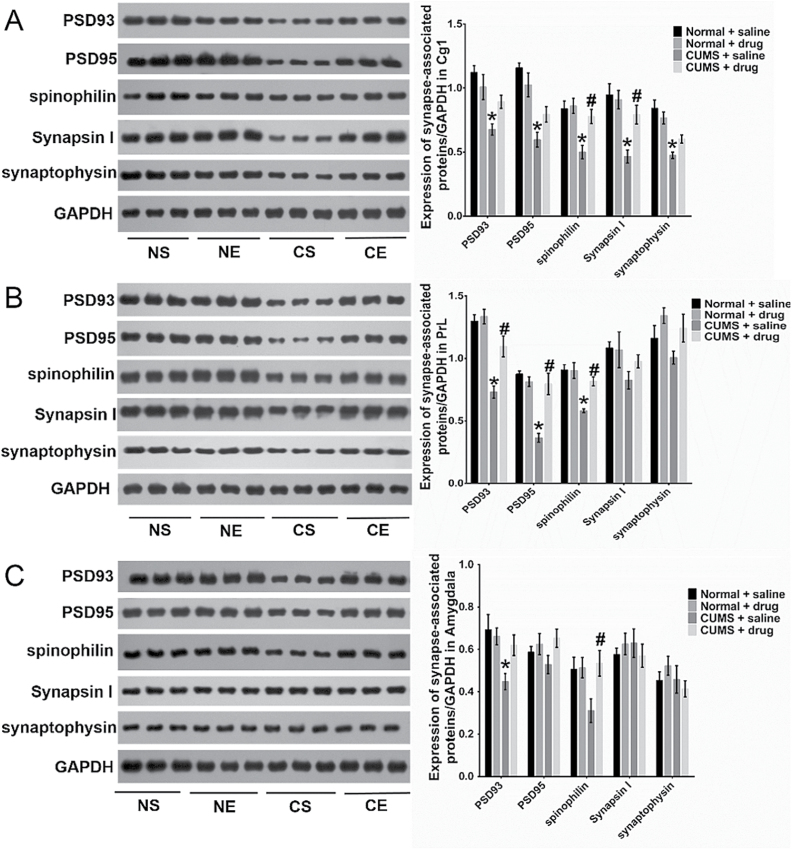
Two-way ANOVA analysis revealed that stress significantly decreased the expression of the tested proteins in the rat Cg1 region (F=18.5, P=.0003 for PSD93; F=36.6, P<.0001 for PSD95; F=13.69, P=.0014 for spinophilin; F=17.39, P=.0005 for synapsin I; F=35.24; P<.0001 for synaptophysin, respectively; n=6/group). Meanwhile, chronic escitalopram administration increased the expression of spinophilin in the stressed rats (F=6.917, P=.016) but not in the control animals. The interaction between stress and escitalopram was observed in the expression of all of the tested proteins in the rats (F=6.471, P=.0193 for PSD93; F=6.378, P=.0201 for PSD95; F=5.006, P=.0368 for spinophilin; F=6.466, P=.0194 for synapsin I; F=5.07; P=.0357 for synaptophysin, respectively) (Figure 6A).
Figure 6.
Changed expression of synapse-associated proteins in area 1 of the cingulate cortex (Cg1) (A), prelimbic cortex (PrL) (B), and amygdala (C) of rats in the 4 groups (n=6/group). Tukey’s posthoc test showed the expression of the tested proteins was significantly decreased in the Cg1 of chronic unpredicted mild stress (CUMS) with saline treatment subgroup (CS) rats (vs normal rats with saline treatment (NS) rats (P=.0005 for PSD93, P<.0001 for PSD95, P=.0023 for spinophilin, P=.0007 for synapsin I, and P<.0001 for synaptophysin, respectively). After escitalopram treatment, the expression of spinophilin and synapsin I increased in CUMS with escitalopram treatment subgroup (CE) rats (vs CS rats, P=.0127 and .0206). In the PrL region (B), CUMS decreased the expressions of PSD93, PSD95, and spinophilin in the CS rats (vs NS rats, P<.0001, < .0001, and=.0002, respectively), and escitalopram increased their expression in the CE group (vs CS group, P=.0024, < .0001, and=.0047, respectively). In the amygdala, the expression of PSD93 was decreased in CS rats (vs NS rats, P=.0142). After chronic treatment, the expression of spinophilin was increased in the CE group (vs CS group, P=.0454). However, there were no significant changes in the expression of other tested proteins in the amygdala in any of the 4 groups (C). *P<.05 compared with the protein expression in NS groups. #P<.05 compared with the expression of protein in CS rats. Error bars represent SEM.
Altered Expression of Synapse-Associated Proteins in the Rats’ PrL Region in the 4 Groups
The stress significantly decreased the expression of postsynaptic proteins in the PrL regions (F=42.63, P<.0001 for PSD93; F=25.67, P<.0001 for PSD95; and F=23.11, P=.0001 for spinophilin, respectively; n=6/group). Escitalopram increased the expression of postsynaptic proteins in the stressed rats (F=7.07, P=.0004 for PSD93; F=12.72, P=.0019 for PSD95; and F=7.267, P=.0139 for spinophilin, respectively) without any effect in the control rats. Also, the combined effect of stress and escitalopram changed protein expression in the rat PrL region (F=10.56, P=.0151 for PSD93; F=22.33, P=.0001 for PSD95; and F=7.8, P=.0112 for spinophilin, respectively) (Figure 6B).
Altered Expression of Synapse-Associated Proteins in the Rats’ Amygdala in the 4 Groups
In the amygdala, stress significantly decreased the expression of PSD93 (F=7.867, P=.0109, n=6/group). Neither the treatment nor the interaction of stress and escitalopram influenced the expression of the proteins in the amygdala in the 4 groups (Figure 6C).
Altered Expression of Synapse-Associated Proteins in the Rats’ 3 Subfields of the Hippocampus in the 4 Groups
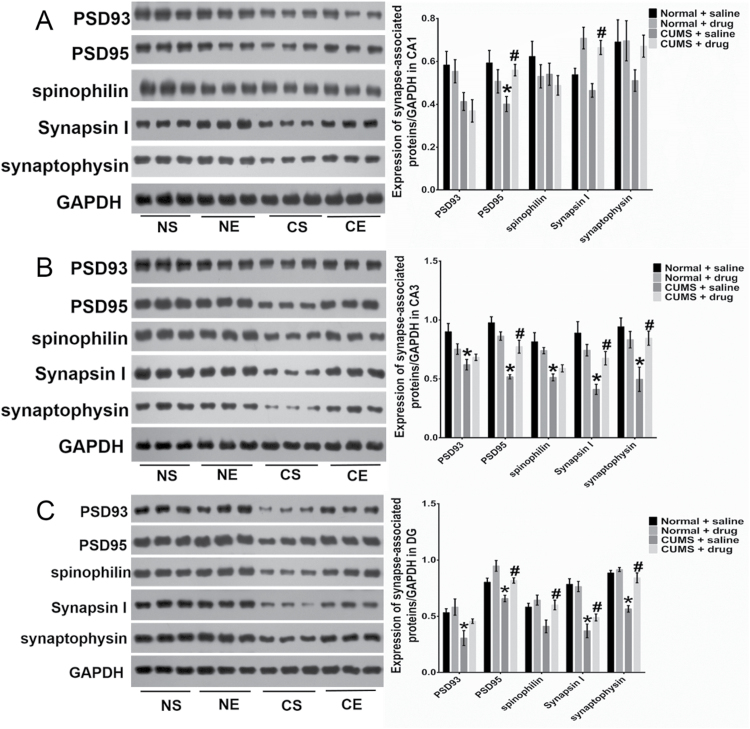
In the CA1 region, the stress decreased the expression of PSD93 (F=11.04, P=.0034, n=6/group), while escitalopram significantly increased the expression of synapsin I (F=2.48, P=.13) (Figure 7A). There was a significant interaction between stress and escitalopram on the expression of PSD95 (F=7.219, P=.0142). In the CA3 region (Figure 7B), stress significantly decreased the expression of the 5 proteins (F=12.77, P=.0019 for PSD93; F=42.21, P<.0001 for PSD95; F=24.07, P<.0001 for spinophilin; F=17.48, P=.0005 for synapsin I; and F=7.624, P=.012 for synaptophysin, respectively; n=6/group). There was a remarkable interaction between stress and escitalopram on the expression of 4 proteins in the rats’ CA3 region (F=4.585, P=.0448 for PSD93; F=19.01, P=.0003 for PSD95; F=9.881, P=.0051 for synapsin I; and F=8.545, P=.0084 for synaptophysin, respectively). In the DG area, stress diminished the expression of the tested proteins (F=11.25, P=.0032 for PSD93; F=14.19, P=.001 for PSD95; F=5.641, P=.0277 for spinophilin; F=50.85, P<.0001 for synapsin I; and F=41.57, P<.0001 for synaptophysin, respectively; n=6/group) (Figure 7C). Escitalopram increased the expression of PSD95 (F=14.9, P=.0004), spinophilin (F=5.641, P=.0277), and synaptophysin (F=25.27, P<.0001) in stressed rats. However, escitalopram treatment did not affect the protein expression in all 3 subfields of the hippocampus in the NE rats.
Figure 7.
Changed expression of synapse-associated proteins in the CA1 (A), CA3 (B), and DG (C) areas of rats in the 4 groups (n=6/group). In the CA1 region (A), the expression of PSD95 decreased significantly in the CS rats compared with that of the normal rats with saline treatment (NS) rats (P=.0339). Escitalopram treatment increased the expression of synapsin I in both normal rats with escitalopram treatment (NE) (P=.0199) and CUMS with escitalopram treatment subgroup (CE) rats (P=.0057). In the CA3 region (B), the expression of all proteins decreased significantly in the CS rats compared with that of the NS rats (P=.0033 for PSD93, P<.0001 for PSD95, P=.0009 for spinophilin, P=.0002 for synapsin I, and P=.0035 for synaptophysin, respectively). Chronic treatment improved the expression of PSD95, synapsin I, and synaptophysin (P=.0019, .0434, and .0243, respectively) of CE rats compared with the CS group. In the DG region (C), the expression of PSD93, PSD95, synapsin I, and synaptophysin was decreased in CS rats (vs NS rats, P=.0291,=.0417, < .0001, < .0001, respectively). The expression of PSD95, spinophilin, and synaptophysin was significantly increased in the CE group (vs the CS group: P=.0218,=.0352, and < .0001, respectively). Chronic escitalopram treatment did not affect the protein expression among the 3 hippocampal subfields. *P<.05 compared with the protein expression in NS groups. #P<.05 compared with the expression of proteins in CS rats. Error bars represent SEM.
Discussion
The main finding from the present study was that the Gray’s Type I synapses underwent considerable remodeling in depression-related circuits of rats after CUMS and escitalopram treatment. The results demonstrated that the specific and regional plasticity of individual synapses might play important roles in the depressive-like behaviors of stressed rats.
The subjects used in the CS and CE groups were carefully selected. Only the rats meeting the specific criteria outlined in the Methods section were used for the subsequent experiment, which provided high levels of homogeneity among rats. Escitalopram was given at a high dose of 10mg/kg, which was effective in producing significant antidepressant-like effects (Zomkowski et al., 2010). Final behavioral assessment were carried out 48 hours after the last injection to exclude the acute effect of escitalopram (O’Brien et al., 2013).
Plasticity of Gray’s Type I Synapses among Brain Regions in Depression Circuits after Stress and Escitalopram Treatment
The present study found that the ultrastructural remodeling of individual Gray’s Type I synapses had been affected by CUMS and escitalopram administration, suggesting that the dimensions of the synaptic ultrastructures determined the efficiency of neurotransmission and correlated with synapse strength (Arellano et al., 2007; Yong et al., 2014). Therefore, CUMS may reduce the Type I synaptic strength and neurotransmission efficiency in the neural circuit via decreasing the size of the PSD complex, active zone, or synaptic curvature and increasing the width of synaptic cleft in relevant brain regions in rats. Due to the site of glutamate neurotransmission in Type I synapses (Waselus and Van Bockstaele, 2007; Klemann and Roubos, 2011), the essential structural plasticity of synapses that occurred in stressed rats might result in the reduced synaptic strength and neurotransmission efficiency of glutamate in the mPFC network (Stewart et al., 2005; Donohue et al., 2006; Radley et al., 2008; Kim et al., 2014). Furthermore, chronic escitalopram administration carried out its antidepressant effect, at least partly, through improving the changes of excitatory synaptic morphology.
The present study found that CUMS rats possessed synaptic remodeling accompanied by a changed expression of synapse-associated proteins. From the perspective of structure the synapse-associated proteins, including pre- and postsynaptic proteins, played crucial roles in the development, maintenance, and plasticity of spines and synapse (Kwon and Chapman, 2011; Bambico and Belzung, 2013; Meyer et al., 2014). The fine-tuned expression of the proteins herein may therefore be essential for the regulated synaptic morphology after either stress or escitalopram treatment (Kwon and Chapman, 2011; Iasevoli et al., 2013).
The mPFC network, especially the hippocampus, mPFC, and amygdala, are closely interconnected and influence each other through direct and indirect neural activity (Leuner and Shors, 2013; Moustafa et al., 2013). The hippocampus-to-PFC connection is a glutamatergic monosynaptic pathway (Cerqueira et al., 2008) and would be imbalanced in CUMS rats, as suggested by our study. However, the synaptic remodeling was regionally specific, with significantly more changes in the rat CA1 compared with the CA3 after 56 consecutive days of stress. Although both the CA1 and CA3 were important hippocampal subfields, their response to stress may be different (Conrad et al., 1999; Christian et al., 2011). It was demonstrated that the CA3 dendrite atrophy was not permanent but reversible and cell-type specific 10 days after cessation of restraint stress (Conrad et al., 1999; Christian et al., 2011). Thus, CUMS caused the imbalanced connection within the rat hippocampus, and the changed synaptic morphology in the CA3 region may reverse somewhat 5 days after cessation of CUMS.
Interestingly, CUMS increased the length of the active zone and PSD thickness in the BLA region, suggesting increased size or complexity of Type I synapses in BLA as previous findings reported (Roozendaal et al., 2009). In the neural circuit, mPFC could suppress amygdala activity, and the mPFC-BLA pathway regulated emotional behavior and inhibited inappropriate responses from the effects of stress under normal circumstances (Sotres-Bayon et al., 2004). Meanwhile, the interactions between the BLA and hippocampus regulated emotional arousal effects on memory consolidation (Roozendaal et al., 2009). Thus, the synaptic changes in BLA might be the result of direct actions of chronic stress or from the reduced inhibition by synapse-remodeled mPFC (Sotres-Bayon et al., 2004; Sotres-Bayon and Quirk, 2010; Duman and Voleti, 2012), and these may also modulate the synaptic plasticity in other brain regions (Roozendaal et al., 2009).
Based on the interconnection of brain regions, it is likely that the structural remodeling in one core region may influence the functions of others (Roozendaal et al., 2009). Therefore, the changed synaptic plasticity among different regions might be in a mutual cause-effect relation and may result in an imbalance of the mPFC network, which contributes to the development of disrupted behaviors in CUMS rats. Escitalopram had its antidepressant effects on the CUMS rats via partly improving or reversing these disturbances (Bambico and Belzung, 2013).
The Relationship of the Changed Synaptic Plasticity with Depressive-Like Behaviors in CUMS Rats
Excitatory synapses play a crucial role in synaptic transmission, synaptic plasticity, and behavioral adaptation (Timmermans et al., 2013). The present study has revealed the relationship of the reduced synaptic PSD thickness in CA1 and CUMS rats’ locomotor activity, and the length of the synaptic active zone in BLA with the anhedonia of CUMS rat. Usually, the hippocampus is important for affective behaviors, such as hopelessness (Nestler et al., 2002). Airan et al. (2007) found that increased error signals from CA1 could be associated with the depressive-like behavior in the FST, resulting in the failure to adapt to environmental changes. The amygdala played important roles in the reward circuit in both patients and animals (Russo and Nestler, 2013; Stuhrmann et al., 2013). Glutamatergic activity in the BLA is necessary for motivated behavior and partly mediates the incentive properties of rewards (Der-Avakian and Markou, 2012). Chronic stress could increase reward thresholds reflecting a reward deficit (ie, anhedonia), whereas antidepressant treatments had the opposite effect (Chartoff et al., 2012; Der-Avakian and Markou, 2012). Therefore, there might be a close connection between the changed plasticity of Type I synapses in the core brain regions of the neural circuit and depressive-like behaviors when undergoing chronic stress.
In conclusion, chronic depressive-like rats suffered from changed synaptic plasticity in the neural circuit of emotion. However, chronic treatment with escitalopram partly normalized the synaptic remodeling. Of course, there are several limitations in the present study. For example, quantitative determination was not performed for the volume of subfields and the synaptic density. In addition, our study did not examine time-depended changes in synaptic plasticity in the neural circuits during exposure to chronic stress. Therefore, further comprehensive studies of the mechanisms of synaptic plasticity are required to develop treatment for depressive disorders.
Statement of Interest
None
Acknowledgements
We are grateful to H. Lundbeck A/S (Copenhagen-Valby, Denmark) for kindly providing escitalopram oxalate. This work was supported by the National Natural Science Foundation of China (grant numbers 81071101, 31371074, and 81402910), the Fundamental Research Funds for the Central Universities and the Research Innovation Program for College and University Graduates of Jiangsu Province (CXLX12_0125), and grants from National Basic Research Program of China (2013CB835103) and Strategic Priority Research Program of Chinese Academy of Science (XDB02020002).
References
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. (2007) High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science 317:819–823. [DOI] [PubMed] [Google Scholar]
- Alfarez DN, Joëls M, Krugers HJ. (2003) Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci 17:1928–1934. [DOI] [PubMed] [Google Scholar]
- Arellano JI, Benavides-Piccione R, Defelipe J, Yuste R. (2007) Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front Neurosci 1:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artola A, Von Frijtag JC, Fermont PCJ, Gispen WH, Schrama LH, Kamal A, Spruijt BM. (2006) Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. Eur J Neurosci 23:261–272. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Belzung C. (2013) Novel insights into depression and antidepressants: a synergy between synaptogenesis and neurogenesis? Curr Top Behav Neurosci 15:243–291. [DOI] [PubMed] [Google Scholar]
- Bhagya V, Srikumar BN, Raju TR, Rao BSS. (2011) Chronic escitalopram treatment restores spatial learning, monoamine levels, and hippocampal long-term potentiation in an animal model of depression. Psychopharmacology 214:477–494. [DOI] [PubMed] [Google Scholar]
- Boeckers TM. (2006) The postsynaptic density. Cell Tissue Res 326:409–422. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Almeida OFX, Sousa N. (2008) The stressed prefrontal cortex. Left? Right! Brain Behav Immun 22:630–638. [DOI] [PubMed] [Google Scholar]
- Chang CH, Chen MC, Qiu MH, Lu J. (2014) Ventromedial prefrontal cortex regulates depressive behavior and rapid eye movement sleep in the rat. Neuropharmacology 86:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. (2012) Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology 62:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi P, Greengard P, Ryan TA. (2001) Synapsin dispersion and reclustering during synaptic activity. Nat Neurosci 4:1187–1193. [DOI] [PubMed] [Google Scholar]
- Christian KM, Miracle AD, Wellman CL, Nakazawa K. (2011) Chronic stress-induced hippocampal dendritic retraction requires CA3 NMDA receptors. Neuroscience 174:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, Eisch AJ, Dietz DM, Ferguson D, Neve RL, Greengard P, Kim Y, Morrison JH, Russo SJ. (2011) IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci 31:314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor S, Williams PT, Armstrong B, Petit TL, Ivanco TL, Weeks AC. (2006) Long-term potentiation is associated with changes in synaptic ultrastructure in the rat neocortex. Synapse 59:378–382. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magariños AM, McEwen BS. (1999) Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci 113:902–913. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. (2005) Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev 29:547–569. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. (2012) The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci 35:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue HS, Gabbott PL, Davies H, Rodríguez JJ, Cordero MI, Sandi C, Medvedev NI, Popov VI, Colyer FM, Peddie CJ, Stewart MG. (2006) Chronic restraint stress induces changes in synapse morphology in stratum lacunosum-moleculare CA1 rat hippocampus: a stereological and three-dimensional ultrastructural study. Neuroscience 140:597–606. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. (2008) Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 213:93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Voleti B. (2012) Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci 35:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Uno H, Flügge G. (1995) Chronic psychosocial stress induces morphological alterations in hippocampal pyramidal neurons of the tree shrew. Brain Res 673:275–282. [DOI] [PubMed] [Google Scholar]
- Gray EG. (1959) Axo-somatic and axo-dendritic synapses of the cerebral cortex. J Anat 93:420–433. [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Janoschka S, Ge S. (2013) Neurogenesis and hippocampal plasticity in adult brain. Curr Top Behav Neurosci. 15:31–48 [DOI] [PubMed] [Google Scholar]
- Iasevoli F, Tomasetti C, de Bartolomeis A. (2013) Scaffolding proteins of the post-synaptic density contribute to synaptic plasticity by regulating receptor localization and distribution: relevance for neuropsychiatric diseases. Neurochem Res 38:1–22. [DOI] [PubMed] [Google Scholar]
- Jayatissa MN, Bisgaard C, Tingström A, Papp M, Wiborg O. (2006) Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology 31:2395–2404. [DOI] [PubMed] [Google Scholar]
- Katz RJ. (1981) Animal models and human depressive disorders. Neurosci Biobehav Rev 5:231–246. [DOI] [PubMed] [Google Scholar]
- Kim H, Yi JH, Choi K, Hong S, Shin KS, Kang SJ. (2014) Regional differences in acute corticosterone-induced dendritic remodeling in the rat brain and their behavioral consequences. BMC Neurosci 15:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino E. (2012) Escitalopram for the management of major depressive disorder: a review of its efficacy, safety, and patient acceptability. Patient Prefer Adherence 6:853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemann CJHM, Roubos EW. (2011) The gray area between synapse structure and function-Gray’s synapse types I and II revisited. Synapse 65:1222–1230. [DOI] [PubMed] [Google Scholar]
- Kodama M, Fujioka T, Duman RS. (2004) Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol Psychiatry 56:570–580. [DOI] [PubMed] [Google Scholar]
- Kwon SE, Chapman ER. (2011) Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron 70:847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. (2013) Stress, anxiety, and dendritic spines: what are the connections? Neuroscience 251:108–119. [DOI] [PubMed] [Google Scholar]
- Li X-L, Zhang W, Zhou X, Wang X-Y, Zhang H-T, Qin D-X, Zhang H, Li Q, Li M, Wang T-H. (2007) Temporal changes in the expression of some neurotrophins in spinal cord transected adult rats. Neuropeptides 41:135–143. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V, Allen NB, Fornito A, Yücel M. (2009) Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord 117:1–17. [DOI] [PubMed] [Google Scholar]
- Marrone DF, Petit TL. (2002) The role of synaptic morphology in neural plasticity: structural interactions underlying synaptic power. Brain Res Brain Res Rev 38:291–308. [DOI] [PubMed] [Google Scholar]
- Mattinson CE, Burmeister JJ, Quintero JE, Pomerleau F, Huettl P, Gerhardt GA. (2011) Tonic and phasic release of glutamate and acetylcholine neurotransmission in sub-regions of the rat prefrontal cortex using enzyme-based microelectrode arrays. J Neurosci Methods 202:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. (2007) The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res 1161:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Bonhoeffer T, Scheuss V. (2014) Balance and stability of synaptic structures during synaptic plasticity. Neuron 82:430–443. [DOI] [PubMed] [Google Scholar]
- Rico AM, Mendoza AL, Durán DA, Torres Hde L, Mendoza GA, Gómez AB (2015) The effects of chronic restraint on the morphology of ventral CA1 neurons in female Long Evans rats. Stress 18(1): 67–75. [DOI] [PubMed] [Google Scholar]
- Moustafa AA, Gilbertson MW, Orr SP, Herzallah MM, Servatius RJ, Myers CE. (2013) A model of amygdala-hippocampal-prefrontal interaction in fear conditioning and extinction in animals. Brain Cogn 81:29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. (2002) Neurobiology of depression. Neuron 34:13–25. [DOI] [PubMed] [Google Scholar]
- O’Brien FE, O’Connor RM, Clarke G, Dinan TG, Griffin BT, Cryan JF. (2013) P-glycoprotein inhibition increases the brain distribution and antidepressant-like activity of escitalopram in rodents. Neuropsychopharmacology 38:2209–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH. (2012) Modeling depression in animal models. Methods Mol Biol 829:125–144. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Nivón LG, McEwen BS. (2002) Effects of chronic stress on hippocampal long-term potentiation. Hippocampus 12:245–257. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. (2012) Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci 16:61–71. [DOI] [PubMed] [Google Scholar]
- Qiao H, An S-C, Ren W, Ma X-M. (2014) Progressive alterations of hippocampal CA3-CA1 synapses in an animal model of depression. Behav Brain Res 275C:191–200. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. (2008) Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol 507:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. (2009) Stress, memory and the amygdala. Nat Rev Neurosci 10:423–433. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. (2013) The brain reward circuitry in mood disorders. Nat Rev Neurosci 14:609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Kim E. (2011) The postsynaptic organization of synapses. Cold Spring Harb Perspect Biol 3(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE a, LeDoux JE. (2004) Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem 11:525–535. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. (2010) Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol 20:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov N V, Madeira MD, Almeida OF, Paula-Barbosa MM. (2000) Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience 97:253–266. [DOI] [PubMed] [Google Scholar]
- Stewart MG, Davies HA, Sandi C, Kraev I V, Rogachevsky V V, Peddie CJ, Rodriguez JJ, Cordero MI, Donohue HS, Gabbott PL a, Popov VI. (2005) Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: a three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience 131:43–54. [DOI] [PubMed] [Google Scholar]
- Stuhrmann A, Dohm K, Kugel H, Zwanzger P, Redlich R, Grotegerd D, Rauch AV, Arolt V, Heindel W, Suslow T, Zwitserlood P, Dannlowski U. (2013) Mood-congruent amygdala responses to subliminally presented facial expressions in major depression: associations with anhedonia. J Psychiatry Neurosci 38:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans W, Xiong H, Hoogenraad CC, Krugers HJ. (2013) Stress and excitatory synapses: from health to disease. Neuroscience 248:626–636. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. (2002) Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22:6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Zhang Z, Guo YJ, Zhou H, Teng GJ, Chen BA. (2009) Anhedonia and activity deficits in rats: impact of post-stroke depression. J Psychopharmacol 23:295–304. [DOI] [PubMed] [Google Scholar]
- Waselus M, Van Bockstaele EJ. (2007) Co-localization of corticotropin-releasing factor and vesicular glutamate transporters within axon terminals of the rat dorsal raphe nucleus. Brain Res 1174:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. (1997) Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology 134:319–329. [DOI] [PubMed] [Google Scholar]
- Willner P. (2005) Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52:90–110. [DOI] [PubMed] [Google Scholar]
- Yong Z, Yan L, Gao X, Gong Z, Su R. (2014) Effects of thienorphine on synaptic structure and synaptophysin expression in the rat nucleus accumbens. Neuroscience 274C:53–58. [DOI] [PubMed] [Google Scholar]
- Zomkowski ADE, Engel D, Gabilan NH, Rodrigues ALS. (2010) Involvement of NMDA receptors and L-arginine-nitric oxide-cyclic guanosine monophosphate pathway in the antidepressant-like effects of escitalopram in the forced swimming test. Eur Neuropsychopharmacol 20:793–801. [DOI] [PubMed] [Google Scholar]