Abstract
Background:
Structural magnetic resonance imaging techniques are powerful tools for examining the effects of drug use on the brain. The nicotine and cannabis literature has demonstrated differences between nicotine cigarette smokers and cannabis users compared to controls in brain structure; however, less is known about the effects of co-occurring cannabis and tobacco use.
Methods:
We used voxel-based morphometry to examine gray matter volume differences between four groups: (1) cannabis-dependent individuals who do not smoke tobacco (Cs); (2) cannabis-dependent individuals who smoke tobacco (CTs); (3) cannabis-naïve, nicotine-dependent individuals who smoke tobacco (Ts); and (4) healthy controls (HCs). We also explored associations between gray matter volume and measures of cannabis and tobacco use.
Results:
A significant group effect was observed in the left putamen, thalamus, right precentral gyrus, and left cerebellum. Compared to HCs, the Cs, CTs, and Ts exhibited larger gray matter volumes in the left putamen. Cs also had larger gray matter volume than HCs in the right precentral gyrus. Cs and CTs exhibited smaller gray matter volume than HCs in the thalamus, and CTs and Ts had smaller left cerebellar gray matter volume than HCs.
Conclusions:
This study extends previous research that independently examined the effects of cannabis or tobacco use on brain structure by including an examination of co-occurring cannabis and tobacco use, and provides evidence that cannabis and tobacco exposure are associated with alterations in brain regions associated with addiction.
Keywords: Cannabis, dependence, gray matter volume, nicotine, voxel-based morphometry
Introduction
Cannabis dependence (now cannabis use disorder), like all drug addictions, is a chronic, relapsing disorder marked by compulsive drug-taking despite negative consequences. Cannabis use has been associated with a range of adverse effects, including negative health outcomes (Kalant, 2004), acute and chronic mental health problems (Moore et al., 2007), and psychosocial and cognitive impairments (Crean et al., 2011). According to a recent review (Batalla et al., 2013), adult chronic cannabis users exhibit altered brain activity compared to healthy controls during cognitive tasks that involve sustained attention (Chang et al., 2006; Abdullaev et al., 2010), working memory (Jager et al., 2006, 2007), inhibitory control (Hester et al., 2009), decision-making skills (Wesley et al., 2011), and psychomotor function (King et al., 2011). These alterations in neural function and associated behaviors may contribute to continued use and relapse. The compulsion to use cannabis is often driven by stress (Hyman and Sinha, 2009) and craving (Filbey and DeWitt, 2012), whereas cannabis use alleviates these negative feelings by reducing anxiety and craving (Fokos and Panagis, 2010). When cannabis is smoked, the main psychoactive component, Δ9-tetrahydrocannabinol (THC; Gaoni and Mechoulam, 1971), is absorbed through the lungs and rapidly enters the bloodstream and crosses the blood-brain barrier within minutes (Ashton, 2001). Once in the brain, THC acts as a partial agonist at the endogenous cannabinoid 1 (CB1) receptor and interferes with the endogenous cannabinoid system (Hoffman et al., 2007). CB1 receptors are located throughout the brain, but are mainly located in the hippocampus, amygdala, cerebellum, prefrontal cortex, and striatum (Lawston et al., 2000; Downer et al., 2001; Burns et al., 2007).
Preclinical studies have shown that THC exposure induces dose-dependent toxicity and structural changes in brain regions rich in CB1 receptors (Hoffman et al., 2007); however, it remains unclear whether these preclinical findings are transferable to humans. Human structural magnetic resonance imaging (sMRI) studies have explored the effects of cannabis use on brain morphology by comparing gray matter differences between cannabis users and non-using controls, and provide insight into the potential effects of THC exposure on the brain (for reviews see Lorenzetti et al., 2010, 2014; Batalla et al., 2013; Rocchetti et al., 2013). The most consistent findings have been smaller gray matter volume and lower gray matter density in the hippocampus/parahippocampus of cannabis users compared to non-using controls (Matochik et al., 2005; Yucel et al., 2008; Ashtari et al., 2011; Demirakca et al., 2011) with hippocampal gray matter volume in cannabis users inversely correlating with the total number of joints (Ashtari et al., 2011) and cones smoked (Yucel et al., 2008). Other gray matter volume findings include smaller amygdalar gray matter volume (Yucel et al., 2008) and larger anterior cerebellar gray matter volume (Cousijn et al., 2012) of cannabis users than controls; however, some studies report no structural alterations in cannabis users (Block et al., 2000; Tzilos et al., 2005; Yucel et al., 2008). Furthermore, Battistella et al. (2014) explored gray matter volume differences between occasional and regular cannabis users to better understand the long-term effects of cannabis use on brain structure and found that regular cannabis users exhibited smaller gray matter volume in the medial temporal cortex, temporal pole, parahippocampal gyrus, left insula, and the orbitofrontal cortex, and larger gray matter volume in areas of the cerebellum. The discrepancies across these studies have been attributed to several factors, such as heterogeneity in sample characteristics and methodological differences in data processing and analyses (Lorenzetti et al., 2010), but could also be related to co-occurring use of cannabis and tobacco in the study populations.
Globally, cannabis and tobacco are commonly mixed together and smoked concurrently in a practice called mulling (European Monitoring Centre for Drugs and Drug Addiction, 2011). A recent study in Australia reported that nearly two-thirds of cannabis users combine cannabis and tobacco for smoking (Banbury et al., 2013). In the United States, co-occurring use is also prevalent among individuals over the age of 18, with 36% of current cigarette smokers reporting cannabis use during the past 30 days and 64% of current cannabis users reporting cigarette use during the past 30 days (United States Department of Health and Human Services and Substance Abuse and Mental Health Services Administration, 2011). Although co-occurring use of cannabis and tobacco is prevalent, the extant literature examining the effects of cannabis and tobacco use on the brain focuses on their independent effects without sufficiently considering the effects of co-occurring use. Within the cigarette/tobacco smoking literature, sMRI studies indicate that smokers exhibit smaller gray matter volume and/or lower gray matter density than nonsmoking controls in the prefrontal, cingulate, insular, parietal, temporal, and occipital cortices, as well as in the thalamus and cerebellum (Brody et al., 2004; Gallinat et al., 2006; Almeida et al., 2008; Yu et al., 2011; Zhang et al., 2011a, 2011b; Liao et al., 2012; Morales et al., 2012; Franklin et al., 2014; Hanlon et al., 2014). Smokers have also been found to have larger gray matter volume/density than nonsmokers in several regions, including the insula (Zhang et al., 2011a), putamen, and parahippocampus (Franklin et al., 2014). Despite similarities in their route of administration, apparent effects on gray matter, and prevalence of co-occurring use, previous research has not fully assessed and/or controlled for cannabis use within the cigarette smoking studies. Similarly, only two cannabis-related studies statistically controlled for cigarette use (Jager et al., 2007; Gilman et al., 2014). Given the high rates of co-occurring cigarette and cannabis use and the potential effects of cannabis and cigarette smoking on brain structure, cigarette smoking and cannabis use should be considered in each of these disorders separately in order to ensure that gray matter differences are not incorrectly attributed to one drug or the other.
In the current study, we aimed to: (1) separate the effects of cigarette smoking from those of cannabis use and the effects of cannabis use from cigarette smoking on gray matter volume; (2) examine the effects of co-occurring cannabis and cigarette use on gray matter volume; (3) explore whether gray matter volume in cannabis users is associated with years of cannabis use; and (4) determine whether gray matter volume in cigarette smokers is associated with pack years, a measure of nicotine exposure. Unlike the majority of sMRI studies, which have primarily predefined regions of interest, we used a whole-brain voxel-wise approach and compared gray matter volumetric differences between cannabis-dependent individuals who have never smoked tobacco/cigarettes (Cs), cannabis-dependent individuals who smoke tobacco/cigarettes (CTs), nicotine-dependent, cannabis-naïve individuals (Ts), and healthy, non-using controls (HCs) using voxel-based morphometry (VBM). As mentioned above, the most consistent finding within the cannabis literature is smaller parahippocampal/hippocampal gray matter volume (Matochik et al., 2005; Yucel et al., 2008; Ashtari et al., 2011; Demirakca et al., 2011), so we hypothesized that Cs and CTs would exhibit lower gray matter volume in the parahippocampus/hippocampus than Ts and HCs. In a recent study, we found that nicotine-dependent cigarette smokers exhibited smaller gray matter volume in the thalamus and cerebellum, yet larger gray matter volume in the putamen and parahippocampus than controls (Franklin et al., 2014). We therefore hypothesized that Ts and CTs would show smaller gray matter volume in the thalamus and cerebellum and larger gray matter volume in the putamen compared to Cs and HCs.
Methods
Participants and Recruitment
All study procedures adhered to the Declaration of Helsinki and were approved by the University of Pennsylvania Institutional Review Board. Physically healthy individuals were recruited via media advertisements and referrals who are: (1) cannabis-dependent and do NOT smoke tobacco (C); (2) cannabis-dependent and smoke tobacco (CT); (3) cannabis-naïve, nicotine-dependent, and smoke tobacco (T); and (4) non-using healthy controls (HC). After completing an initial telephone screen, individuals received a description of their respective study, provided written informed consent, and completed a screening visit (i.e. physical examination and psychological assessment) to ensure that they fulfilled all study criteria. Exclusion criteria included current DSM-IV Axis I diagnoses (other than cannabis or nicotine dependence), lifetime history of head injury with loss of consciousness for more than 3min, contraindications for magnetic resonance imaging, current treatment for cannabis dependence, clinically significant medical conditions, lifetime history of illicit drug use other than cannabis, and use of medication interacting with the central nervous system. Further details regarding the inclusion procedure are described in previous studies (Franklin et al., 2014; Wetherill et al., 2014b). Approximately 45 minutes prior to scan acquisition, CTs and Ts were provided the opportunity to smoke a cigarette to ensure that they were not experiencing nicotine withdrawal symptoms during data acquisition. Self-report of last cannabis use in Cs and CTs was obtained (mean time since last use = 0.69 days, standard deviation [SD] = 0.51). The final population selected for this study consists of 19 Cs (mean age = 28.0 years; SD = 6.9), 21 CTs (mean age = 30.9 years; SD = 8.8), 21 Ts (mean age = 34.3 years; SD = 9.4), and 21 HCs (mean age = 30.5 years; SD = 8.8). Demographic characteristics are shown in Table 1.
Table 1.
Demographic Characteristics
| C (n = 19) | CT (n = 21) | T (n = 21) | HC (n = 21) | p-values | |
|---|---|---|---|---|---|
| Sex, n(%), male | 10 (53) | 16 (76) | 12 (57) | 14 (67) | 0.41 |
| Age | 28 (7) | 31 (9) | 34 (9) | 31 (9) | 0.15a |
| Years of education | 13 (2) | 13 (1) | 14 (1) | 13 (1) | 0.17 |
| Nicotine dependence (FTND) | - | 4.6 (1.3) | 4.2 (1.6) | - | 0.51b |
| Cigarettes per day | - | 7.6 (6.7) | 14.1 (4.4) | - | 0.001b |
| Pack years | - | 4.5 (4.9) | 10.2 (8.2) | - | 0.009b |
| Age of onset of weekly cannabis use | 20 (6) | 17 (12) | - | - | 0.36c |
| Cannabis use, years | 8 (6) | 14 (9) | - | - | 0.02c |
| Cannabis use, past 30 days | 27 (4) | 25 (6) | - | - | 0.11c |
| Cannabis use, grams/week | 14 (11) | 20 (11) | - | - | 0.07c |
a p-value for Kruskal-Wallis nonparametric test of difference between groups
b p-values for comparison between CT and T groups
c p-values for comparison between C and CT groups
Values are shown as means (standard deviation). C, cannabis-dependent individual who does not smoke tobacco; CT, cannabis-dependent individual who smokes tobacco; FTND, Fagerstrom Test of Nicotine Dependence; HC, healthy control; T, cannabis-naïve, nicotine-dependent individual who smokes tobacco.
For all participants, urine drug screens verified the absence of illicit drugs (e.g. cocaine, opiates, amphetamines) and the absence of nicotine and its major metabolite, cotinine, in C and HC groups. The cannabis groups completed urine and saliva tests during the screening process to confirm the regular use of cannabis consumption. The Timeline Follow-Back (Sobell and Sobell, 1992) quantified cannabis, nicotine, alcohol, and opiate use during the past 30 days, and the Addiction Severity Index (McLellan et al., 1992) assessed lifetime cannabis, cocaine, amphetamine, heroin, opiate, barbiturate, hallucinogen, sedative, alcohol, and inhalant use. The Fagerstrom Test for Nicotine Dependence (FTND; Fagerstrom and Schneider, 1989) assessed severity of nicotine dependence among CTs and Ts.
Magnetic Resonance Acquisition
Imaging data were acquired on a Siemens 3 Tesla Trio whole-body scanner (Erlangen) at the Hospital of the University of Pennsylvania using a product 8-channel head coil. High-resolution T1-weighted anatomical images were obtained using a 3D-magnetization prepared rapid acquisition gradient echo sequence (repetition time = 1620ms, echo time = 3ms, flip angle = 15°, 160 contiguous slices of 1.0mm).
Data Processing
Data were preprocessed and analyzed using the Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology) VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm) implemented in MatlabR2013 (MathWorksInc.). Each subject’s T1-weighted images were checked for structural abnormalities and reoriented to the anterior-posterior commissure line. Reoriented images were segmented into gray matter, white matter, and cerebral spinal fluid in native space using the New Segmentation algorithm. The resulting rigidly aligned and resliced gray matter tissue maps were imported into DARTEL (Diffeomorphic Anatomical Registration Using Exponentiated Lie Algebra), an advanced high-dimensional diffeomorphic registration algorithm shown to improve spatial precision (Ashburner, 2007). Subsequently, a study-specific gray matter template was created from the imported maps of all participants. The final average gray matter template was generated after six iterations by averaging all of the aligned images. During successive iterations, flow fields for each participant’s images were yielded to parameterized deformations by warping to the template. The warped gray matter maps were transformed into Montreal Neurological Institute space and were scaled by the Jacobian determinants of the deformations. Finally, the normalized modulated maps were smoothed with a Gaussian kernel of 8mm full width half maximum.
Data Analysis
Demographics and questionnaire scores were examined for normality, and non-normal data (i.e. pack years, days of cannabis use) were log transformed. A Kruskal-Wallis nonparametric test examined group differences in age, and all other group differences were assessed using an analysis of variance. Voxel-based inferential statistics were performed on the smoothed modulated gray matter images using an analysis of covariance (ANCOVA) for investigating the main effect of group (F-test). Age and sex were included as covariates of no interest. Given that gray matter differences between adults with cannabis and tobacco co-use, adults who only use cannabis, and adults who are cannabis-naïve but smoke tobacco have never been reported, the data were analyzed using the voxel-wise threshold of p < 0.001 with a family-wise error correction for multiple comparisons (p < 0.05). To conduct post hoc pairwise comparisons and to explore associations between gray matter volume and measures of cannabis (e.g. years of cannabis use, age of cannabis use onset, grams of use per week, days of cannabis use in the past month) and tobacco use (e.g. years of cigarette use, age of cigarette use onset, cigarettes per day, pack years, and nicotine dependence), gray matter volume within each significant cluster was extracted for each participant using the MarsBaR tool in SPM (http://marsbar.sourceforge.net/) and exported to IBM SPSS Statistics 19.0. In SPSS, a series of ANCOVAs tested for main effect of group and confirmed SPM findings. Subsequent post hoc pairwise comparisons were conducted (e.g. Cs vs. CTs; Cs vs. Ts; Cs vs. HCs, etc.) to determine whether group differences were significant.
Results
Demographic Characteristics
As shown in Table 1, groups did not differ in age, sex, or years of education. Comparisons between CT and T groups revealed that T individuals smoked more cigarettes per day and had greater pack years than CT individuals, but did not differ in nicotine dependence (FTND). Cannabis-dependent individuals (C vs CT groups) did not differ in age of cannabis use onset, cannabis use days (past 30 days), or amount of cannabis use (grams/week), but did differ in years of cannabis use.
VBM Analysis
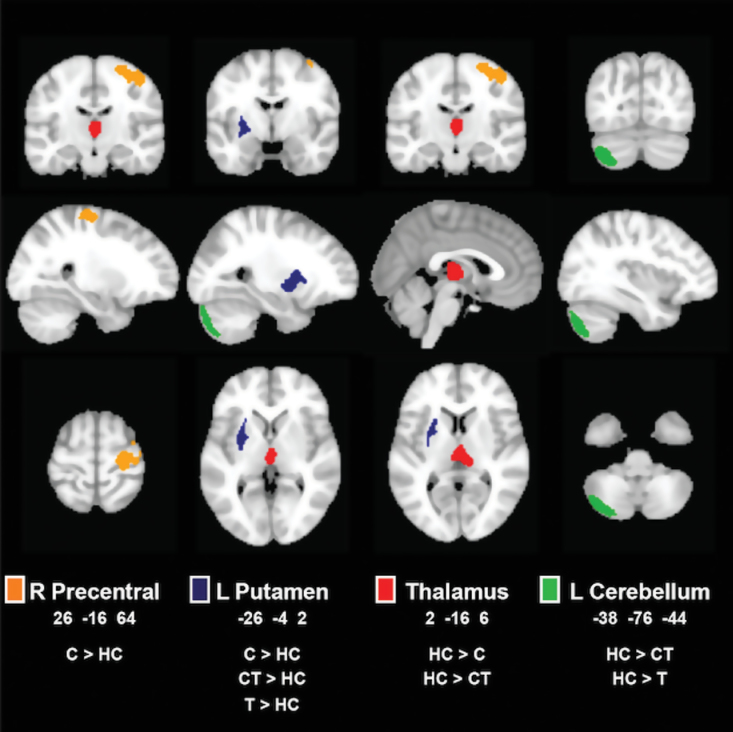
Whole-brain analyses revealed significant differences in gray matter volume between groups in the left putamen, right precentral gyrus, left cerebellum, and thalamus (Figure 1, Table 2). Pairwise comparisons within these clusters showed that the C group, CT group, and T group had greater gray matter volume in the left putamen compared to HCs, and Cs had greater gray matter volume in the right precentral gyrus compared to HCs. HCs showed greater gray matter volume in the thalamus compared to Cs and CTs. HCs also had greater gray matter volume in the left cerebellum than the CT and T groups. Finally, among those who use cannabis (i.e. Cs and CTs), partial correlations examined potential associations between gray matter volume and measures of cannabis use with age, sex, and pack years as covariates. No significant correlations were observed.
Figure 1.
Group differences in gray matter volume. Clusters of significant volume differences (p < 0.001, famiy-wise error (FWE) cluster-corrected at p < 0.05) are displayed on representative sagittal, coronal, and axial slices overlain on the standard Montreal Neurological Institute brain. Right side of the brain is depicted on the right side. C, cannabis-dependent individual who does not smoke tobacco; CT, cannabis-dependent individual who smokes tobacco; HC, healthy control; L, left; R, right; T, cannabis-naïve, nicotine-dependent individual who smokes tobacco.
Table 2.
Regional Gray Matter Volume Differences
| Brain region | MNI coordinates x y z | F-values | Cluster size (voxels) | Pairwise comparisons a | ||
|---|---|---|---|---|---|---|
| L Putamen | -26 | -4 | 2 | 17.84 | 387 | C > HC CT > HC T > HC |
| R Precentral | 26 | -16 | 64 | 11.97 | 667 | C > HC |
| L Cerebellum | -38 | -76 | -44 | 9.82 | 750 | HC > CT HC > T |
| Thalamus | 2 | -16 | 6 | 9.73 | 392 | HC > C HC > CT |
Voxel-wise height threshold of p < 0.001, p < 0.05 famiy-wise error cluster-corrected. C, cannabis-dependent individual who does not smoke tobacco; CT, cannabis-dependent individual who smokes tobacco; HC, healthy control; L, left; MNI, Montreal Neurological Institute brain; R, right; T, cannabis-naïve, nicotine-dependent individual who smokes tobacco.
Similar partial correlation analyses were conducted for CTs and Ts by exploring associations between gray matter volume and measures of tobacco cigarette use with age, sex, and years of cannabis use as covariates. No significant correlations were found between gray matter volume and measures of tobacco use.
Discussion
The current study provides evidence that cannabis and tobacco have differential effects on gray matter volume; however, co-occurring cannabis and tobacco use does not appear to have a distinct effect compared to only smoking cannabis or cigarettes. In general, our findings are largely consistent with research suggesting that long-term cannabis and tobacco smoking alters the brain structure in regions rich in cannabinoid 1 (CB1) receptors (Svizenska et al., 2008) and nicotinic acetylcholine receptors (Picard et al., 2013). Specifically, groups differed in gray matter volume in the left putamen, right precentral gyrus, thalamus, and left cerebellum. Compared to controls, Cs, CTs, and Ts exhibited larger gray matter volume in the left putamen, yet only Cs showed larger gray matter volume in the right precentral gyrus. Cannabis appeared to have a unique effect in the thalamus, with Cs and CTs having smaller gray matter volume than HCs, whereas nicotine appeared to have a unique effect within the left cerebellum, with CTs and Ts having smaller gray matter volume than HCs. Cannabis use and tobacco use measures did not correlate with gray matter volume in any of the above clusters.
Larger Gray Matter Volume in Cannabis- and Nicotine-Dependent Individuals
Cs, CTs, and Ts had larger gray matter volume in the left putamen (dorsal striatum) than HCs. Striatal abnormalities are thought to underlie habitual, compulsive drug seeking and use despite negative consequences (Everitt et al., 2008; Koob and Volkow, 2010). Further, the striatum is an important part of the mesolimbic dopamine pathway, and as such, our findings suggest that the dopamine system may be a common mechanism for cannabis- and tobacco-related structural alterations. Similarly, previous studies have shown that methamphetamine-dependent individuals have greater gray matter volume within the putamen compared to controls (Chang et al., 2005; Jernigan et al., 2005; Churchwell et al., 2012). Enlarged striatal volume has also been reported in other psychiatric disorders characterized by repetitive, compulsive behaviors, such as autism (Hollander et al., 2005) and obsessive-compulsive disorder (Scarone et al., 1992). Further, severity of compulsivity has been associated with increased striatal gray matter volume (Radua and Mataix-Cols, 2009). As such, the increased striatal gray matter volume observed in our cannabis- and nicotine-dependent groups may be associated with the long-term, compulsive use of cannabis and tobacco. It is important to note, however, that recent research suggests that greater putamen gray matter volume may be present before the onset of substance use and could influence the development of substance use disorders (Ersche et al., 2012). Thus, gray matter volume differences in the putamen could contribute to and/or result from substance use disorders.
We also found that Cs differed from HCs in right precentral gray matter volume, with Cs exhibiting larger gray matter volume than HCs. The precentral gyrus, also known as the primary motor cortex, has been principally identified with motor function (Picard and Strick, 1996; Fink et al., 1997) and appears to play a role in cannabis dependence. Specifically, our finding is consistent with a previous study indicating that compared to men who do not smoke cannabis, men who smoke cannabis have greater gray matter density within the precentral gyus (Matochik et al., 2005). THC has been found to impair psychomotor function (King et al., 2011) and increase activation within brain areas involved in motor control and coordination, including the precentral gyrus (Weinstein et al., 2008a). This increase in brain metabolism during slower, inaccurate motor performance has been attributed to cannabis users having to “work harder” to complete a task (Kanayama et al., 2004); however, other work suggests that cannabis users recruit motor and attention areas more extensively (Weinstein et al., 2008b). Accordingly, one possible explanation for the larger gray matter volume within the precentral gyrus could be due to neuroadaptations associated with the repeated increase of blood flow and brain metabolism within the region due to chronic THC exposure (Hoffman et al., 2003; Sim-Selley, 2003), as recent research suggests that brain blood flow and gray matter volume are systematically linked (Varkuti et al., 2011). Future research is warranted given that only Cs, not CTs, exhibited this difference; however, at an uncorrected p < 0.001 threshold, CTs also exhibited greater gray matter volume in the right precentral gyrus.
Smaller Gray Matter Volume in Cannabis- and Nicotine-Dependent Individuals
We also found that Cs and CTs had smaller gray matter volume in the thalamus compared to HCs. Similar reductions in thalamic gray matter volume have been observed in individuals at high familial risk of psychosis who smoke cannabis (Welch et al., 2011); however, reductions in thalamic gray matter volume were not reported in other studies among adults who smoke cannabis, which is surprising given that the thalamus has been identified as a structure involved in mediating responses to cue-driven behaviors, especially in the context of addiction (Haight and Flagel, 2014). At the famiy-wise error-corrected threshold, Ts did not exhibit smaller gray matter volume in the thalamus compared to HCs, which contradicts some studies (Gallinat et al., 2006; Liao et al., 2012) but not others (Brody et al., 2004). Given that cigarette smoking behavior is complex and that various aspects of the behavior are modulated by both known (Bergen et al., 2009; Franklin et al., 2011; Wetherill et al., 2014a) and unknown factors, we speculate that differences in other characteristics (e.g. sex, genetics) are contributing to discrepancies across studies.
Unique Differences Between Cannabis- and Nicotine-Dependent Groups
The left cerebellum was identified as a brain region wherein tobacco smokers (CTs and Ts) showed smaller gray matter volume than HCs. This finding is consistent with previous studies demonstrating cerebellar gray matter differences between cigarette smokers and healthy controls (Yu et al., 2011; Kuhn et al., 2012; Franklin et al., 2014). Preclinical research suggests that long-term nicotine exposure results in a significant loss of cerebellar Purkinje cells (Chen et al., 2003), and as such, similar reductions could occur among human cigarette smokers and result in smaller cerebellar volume compared to HCs. It is important to note, however, that this interpretation is speculative and additional research on the effects of nicotine on the cerebellum is warranted.
Unlike two previous studies among cannabis-using adult (Cousijn et al., 2012) and adolescent populations (Medina et al., 2010), we did not find cerebellar volume differences between cannabis-dependent adults who do not smoke tobacco and healthy controls. Discrepancies may be attributable to differences in methodology and cannabis use histories. Specifically, the previous studies used a priori regions of interest approaches and examined populations who were not cannabis dependent and who reported less cannabis use. Further, the earlier studies did not control for cigarette smoking, and as such, the cerebellar findings could actually be due to nicotine exposure rather than cannabis use. As such, additional studies are needed to assess the effects of cannabis use (without concurrent tobacco smoking) on cerebellar brain structure.
Contrary to our hypotheses, we did not find differences in hippocampal gray matter volume among Cs and CTs. It is important to note that at an uncorrected p < 0.001 threshold, Cs and CTs exhibited smaller gray matter volume, and as such, this null finding could be due to our conservative statistical approach, as CTs and Cs showed smaller hippocampal gray matter volume compared to HCs and Ts at a lower, more liberal statistical threshold.
Correlations Between Gray Matter Volume and Measures of Cannabis and Tobacco Use
We did not find significant correlations between cannabis and tobacco use measures and gray matter volume. Null findings may be related to our conservative statistical approach and focus on clusters showing significant differences. Thus, additional research is necessary.
Limitations
This study has several important strengths and limitations. It is the first study to explore the differential effects of cannabis, tobacco, and concurrent cannabis and tobacco use among adults. The groups were well-matched on demographic characteristics, and by including Cs, CTs, Ts, and HCs, we examined the unique effects of each substance and their co-occurring use on adult gray matter. This cross-sectional study design prohibits our ability to dissociate causal effects of cannabis and tobacco smoking from predisposing biological factors. Our sample size also precludes us from examining how other factors, such as sex and genetic vulnerabilities, may influence these findings.
Conclusion
This VBM, sMRI study provides new information on the effects of cannabis, cigarettes, and co-occurring cannabis and cigarette smoking on brain structure. Cannabis, tobacco, and their co-occurring use had similar effects within several brain regions associated with motivation and reward, yet differed in unique ways in the cerebellum and thalamus. Although longitudinal studies are needed, this study extends previous studies that independently examine the effects of cannabis or tobacco use on brain structure by including an examination of co-occurring cannabis and tobacco use, exclusive use of one or the other.
Statement of Interest
None.
Acknowledgments
This study was supported by grants received from the Pennsylvania Department of Health Commonwealth Universal Research Enhancement program awarded to Drs Childress and Franklin; the National Institutes of Health (R21 DA032022 and R01 HL102119 to Dr Rao; R01 DA029845 and R01 DA030394 to Dr Frankllin); Pfizer Pharmaceuticals (Dr Franklin); and a pilot grant from the Institute for Translational Medicine and Therapeutics of the University of Pennsylvania awarded to Dr Rao. The authors would like to thank the clinical and support staff at the Center for the Studies of Addiction, Perelman School of Medicine at the University of Pennsylvania, the MRI technical staff at the Hospital of the University of Pennsylvania, and all individuals who participated in this research.
References
- Abdullaev Y, Posner MI, Nunnally R, Dishion TJ. (2010) Functional MRI evidence for inefficient attentional control in adolescent chronic cannabis abuse. Behav Brain Res 215:45–57. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Garrido GJ, Lautenschlager NT, Hulse GK, Jamrozik K, Flicker L. (2008) Smoking is associated with reduced cortical regional gray matter density in brain regions associated with incipient Alzheimer disease. Am J Geriat Psychiatry 16:92–98. [DOI] [PubMed] [Google Scholar]
- Ashburner J. (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Avants B, Cyckowski L, Cervellione KL, Roofeh D, Cook P, Gee J, Sevy S, Kumra S. (2011) Medial temporal structures and memory functions in adolescents with heavy cannabis use. J Psychiatr Res 45:1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton CH. (2001) Pharmacology and effects of cannabis: a brief review. Br J Psychiatry 178:101–106. [DOI] [PubMed] [Google Scholar]
- Banbury A, Zask A, Carter SM, van Beurden E, Tokley R, Passey M, Copeland J. (2013) Smoking mull: a grounded theory model on the dynamics of combined tobacco and cannabis use among adult men. Health Promot J Austr 24:143–150. [DOI] [PubMed] [Google Scholar]
- Batalla A, Bhattacharyya S, Yucel M, Fusar-Poli P, Crippa JA, Nogue S, Torrens M, Pujol J, Farre M, Martin-Santos R. (2013) Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLOS One 8:e55821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella G, Fornari E, Annoni JM, Chtioui H, Dao K, Fabritius M, Favrat B, Mall JF, Maeder P, Giroud C. (2014) Long-term effects of cannabis on brain structure. Neuropsychopharmacology 39:2041–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AW, et al. (2009) Dopamine genes and nicotine dependence in treatment-seeking and community smokers. Neuropsychopharmacology 34:2252–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Ehrhardt JC, Augustinack JC, Ghoneim MM, Arndt S, Hall JA. (2000) Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport 11:491–496. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED. (2004) Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry 55:77–84. [DOI] [PubMed] [Google Scholar]
- Burns HD, et al. (2007) [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc Natl Acad Sci USA 104:9800–9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. (2005) Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry 57:967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T. (2006) Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain 129:1096–1112. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Edwards RB, Romero RD, Parnell SE, Monk RJ. (2003) Long-term nicotine exposure reduces Purkinje cell number in the adult rat cerebellar vermis. Neurotoxicol Teratol 25:329–334. [DOI] [PubMed] [Google Scholar]
- Churchwell JC, Carey PD, Ferrett HL, Stein DJ, Yurgelun-Todd DA. (2012) Abnormal striatal circuitry and intensified novelty seeking among adolescents who abuse methamphetamine and cannabis. Dev Neurosci 34:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. (2012) Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage 59:3845–3851. [DOI] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ. (2011) An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med 5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirakca T, Sartorius A, Ende G, Meyer N, Welzel H, Skopp G, Mann K, Hermann D. (2011) Diminished gray matter in the hippocampus of cannabis users: possible protective effects of cannabidiol. Drug Alcohol Depend 114:242–245. [DOI] [PubMed] [Google Scholar]
- Downer E, Boland B, Fogarty M, Campbell V. (2001) Delta 9-tetrahydrocannabinol induces the apoptotic pathway in cultured cortical neurones via activation of the CB1 receptor. Neuroreport 12:3973–3978. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. (2012) Abnormal brain structure implicated in stimulant drug addiction. Science 335:601–604. [DOI] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (2011) 2011 annual report on the state of the drugs problem in Europe. Lisbon, Portugal: EMCDDA. [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. (2008) Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Phil Trans R Soc B 363:3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom KO, Schneider NG. (1989) Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med 12:159–182. [DOI] [PubMed] [Google Scholar]
- Filbey FM, DeWitt SJ. (2012) Cannabis cue-elicited craving and the reward neurocircuitry. Prog Neuropsychopharmacol Biol Psychiatry 38:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE. (1997) Multiple nonprimary motor areas in the human cortex. J Neurophysiol 77:2164–2174. [DOI] [PubMed] [Google Scholar]
- Fokos S, Panagis G. (2010) Effects of delta9-tetrahydrocannabinol on reward and anxiety in rats exposed to chronic unpredictable stress. J Psychopharmacol 24:767–777. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Li Y, Suh JJ, Goldman M, Lohoff FW, Cruz J, Hazan R, Jens W, Detre JA, Berrettini W, O’Brien CP, Childress AR. (2011) Dopamine transporter genotype modulation of neural responses to smoking cues: confirmation in a new cohort. Addict Biol 16:308–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wetherill RR, Jagannathan K, Johnson B, Mumma J, Hager N, Rao H, Childress AR. (2014) The effects of chronic cigarette smoking on gray matter volume: influence of sex. PLOS One 9:e104102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedtgen M. (2006) Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci 24:1744–1750. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. (1971) The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J Am Chem Soc 93:217–224. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Kuster JK, Lee S, Lee MJ, Kim BW, Makris N, van der Kouwe A, Blood AJ, Breiter HC. (2014) Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci 34:5529–5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight JL, Flagel SB. (2014) A potential role for the paraventricular nucleus of the thalamus in mediating individual variation in Pavlovian conditioned responses. Front Behav Neurosci 8:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Owens MM, Joseph JE, Zhu X, George MS, Brady KT, Hartwell KJ. (2014) Lower subcortical gray matter volume in both younger smokers and established smokers relative to non-smokers. Addict Biol. Retrieved 20 Sep 2014. http://dx.doi.org/10.1111/adb.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. (2009) Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology 34:2450–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Caulder T, Lupica CR. (2003) Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J Neurosci 23:4815–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Yang R, Lichtman AH, Lupica CR. (2007) Opposing actions of chronic Delta9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation. Learn Mem 14:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, Licalzi E, Wasserman S, Soorya L, Buchsbaum M. (2005) Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biol Psychiatry 58:226–232. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Sinha R. (2009) Stress-related factors in cannabis use and misuse: implications for prevention and treatment. J Subst Abuse Treat 36:400–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G, Kahn RS, Van Den Brink W, Van Ree JM, Ramsey NF. (2006) Long-term effects of frequent cannabis use on working memory and attention: an fMRI study. Psychopharmacology (Berl) 185:358–368. [DOI] [PubMed] [Google Scholar]
- Jager G, Van Hell HH, De Win MM, Kahn RS, Van Den Brink W, Van Ree JM, Ramsey NF. (2007) Effects of frequent cannabis use on hippocampal activity during an associative memory task. Eur Neuropsychopharmacol 17:289–297. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. (2005) Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psych 162:1461–1472. [DOI] [PubMed] [Google Scholar]
- Kalant H. (2004) Adverse effects of cannabis on health: an update of the literature since 1996. Prog Neuropsychopharmacol Biol Psychiatry 28:849–863. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. (2004) Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 176:239–247. [DOI] [PubMed] [Google Scholar]
- King GR, Ernst T, Deng W, Stenger A, Gonzales RM, Nakama H, Chang L. (2011) Altered brain activation during visuomotor integration in chronic active cannabis users: relationship to cortisol levels. J Neurosci 31:17923–17931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Romanowski A, Schilling C, Mobascher A, Warbrick T, Winterer G, Gallinat J. (2012) Brain grey matter deficits in smokers: focus on the cerebellum. Brain Struct Funct 217:517–522. [DOI] [PubMed] [Google Scholar]
- Lawston J, Borella A, Robinson JK, Whitaker-Azmitia PM. (2000) Changes in hippocampal morphology following chronic treatment with the synthetic cannabinoid WIN 55,212-2. Brain Res 877:407–410. [DOI] [PubMed] [Google Scholar]
- Liao Y, Tang J, Liu T, Chen X, Hao W. (2012) Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study. Addict Biol 17:977–980. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V, Lubman DI, Whittle S, Solowij N, Yucel M. (2010) Structural MRI findings in long-term cannabis users: what do we know? Subst Use Misuse 45:1787–1808. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V, Solowij N, Fornito A, Lubman DI, Yucel M. (2014) The association between regular cannabis exposure and alterations of human brain morphology: an updated review of the literature. Curr Pharm Des 20:2138–2167. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI. (2005) Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend 77:23–30. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. (1992) The fifth edition of the addiction severity index. J Subst Abuse Treat 9:199–213. [DOI] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Tapert SF. (2010) Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Res 182:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. (2007) Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 370:319–328. [DOI] [PubMed] [Google Scholar]
- Morales AM, Lee B, Hellemann G, O’Neill J, London ED. (2012) Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend 125:230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Sadaghiani S, Leroy C, Courvoisier DS, Maroy R, Bottlaender M. (2013) High density of nicotinic receptors in the cingulo-insular network. Neuroimage 79:42–51. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. (1996) Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 6:342–353. [DOI] [PubMed] [Google Scholar]
- Radua J, Mataix-Cols D. (2009) Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry 195:393–402. [DOI] [PubMed] [Google Scholar]
- Rocchetti M, Crescini A, Borgwardt S, Caverzasi E, Politi P, Atakan Z, Fusar-Poli P. (2013) Is cannabis neurotoxic for the healthy brain? A meta-analytical review of structural brain alterations in non-psychotic users. Psychiatry Clin Neurosci 67:483–492. [DOI] [PubMed] [Google Scholar]
- Scarone S, Colombo C, Livian S, Abbruzzese M, Ronchi P, Locatelli M, Scotti G, Smeraldi E. (1992) Increased right caudate nucleus size in obsessive-compulsive disorder: detection with magnetic resonance imaging. Psychiatry Res 45:115–121. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ. (2003) Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol 15:91–119. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. (1992) Timeline follow-back: a technique for assessing self-reported ethanol consumption. In: Measuring alcohol consumption: psychosocial and biological methods (Allen J, Litten RZ, eds), pp 41–72. Totowa, NJ: Humana Press. [Google Scholar]
- Svizenska I, Dubovy P, Sulcova A. (2008) Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures--a short review. Pharmacol Biochem Behav 90:501–511. [DOI] [PubMed] [Google Scholar]
- Tzilos GK, Cintron CB, Wood JB, Simpson NS, Young AD, Pope HG, Jr., Yurgelun-Todd DA. (2005) Lack of hippocampal volume change in long-term heavy cannabis users. Am J Addict 14:64–72. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration (2012) National survey on drug use and health, 2012. ICPSR34933-v2. Ann Arbor, MI: Inter-university Consortium for Political and Social Research. [Google Scholar]
- Varkuti B, Cavusoglu M, Kullik A, Schiffler B, Veit R, Yilmaz O, Rosenstiel W, Braun C, Uludag K, Birbaumer N, Sitaram R. (2011) Quantifying the link between anatomical connectivity, gray matter volume and regional cerebral blood flow: an integrative MRI study. PLOS One 6:e14801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein A, Brickner O, Lerman H, Greemland M, Bloch M, Lester H, Chisin R, Mechoulam R, Bar-Hamburger R, Freedman N, Even-Sapir E. (2008a) Brain imaging study of the acute effects of Delta9-tetrahydrocannabinol (THC) on attention and motor coordination in regular users of marijuana. Psychopharmacology (Berl) 196:119–131. [DOI] [PubMed] [Google Scholar]
- Weinstein A, Brickner O, Lerman H, Greemland M, Bloch M, Lester H, Chisin R, Sarne Y, Mechoulam R, Bar-Hamburger R, Freedman N, Even-Sapir E. (2008b) A study investigating the acute dose-response effects of 13mg and 17mg Delta 9- tetrahydrocannabinol on cognitive-motor skills, subjective and autonomic measures in regular users of marijuana. J Psychopharmacol 22:441–451. [DOI] [PubMed] [Google Scholar]
- Welch KA, Stanfield AC, McIntosh AM, Whalley HC, Job DE, Moorhead TW, Owens DG, Lawrie SM, Johnstone EC. (2011) Impact of cannabis use on thalamic volume in people at familial high risk of schizophrenia. Br J Psychiatry 199:386–390. [DOI] [PubMed] [Google Scholar]
- Wesley MJ, Hanlon CA, Porrino LJ. (2011) Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res 191:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Jagannathan K, Lohoff FW, Ehrman R, O’Brien CP, Childress AR, Franklin TR. (2014a) Neural correlates of attentional bias for smoking cues: modulation by variance in the dopamine transporter gene. Addict Biol 19:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Childress AR, Jagannathan K, Bender J, Young KA, Suh JJ, O’Brien CP, Franklin TR. (2014b) Neural responses to subliminally presented cannabis and other emotionally evocative cues in cannabis-dependent individuals. Psychopharmacology (Berl) 231:1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Zhao L, Lu L. (2011) Regional grey and white matter changes in heavy male smokers. PLOS One 6:e27440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI. (2008) Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry 65:694–701. [DOI] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. (2011a) Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage 54:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Gu H, Geng X, Yang Y, Stein EA. (2011b) Anatomical differences and network characteristics underlying smoking cue reactivity. Neuroimage 54:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]