Abstract
Background:
A think tank sponsored by the Collegium Internationale Neuropsychopharmacologium (CINP) debated the status and prospects of biological markers for psychiatric disorders, focusing on schizophrenia and major depressive disorder.
Methods:
Discussions covered markers defining and predicting specific disorders or domains of dysfunction, as well as predicting and monitoring medication efficacy. Deliberations included clinically useful and viable biomarkers, why suitable markers are not available, and the need for tightly-controlled sample collection.
Results:
Different types of biomarkers, appropriate sensitivity, specificity, and broad-based exploitability were discussed. Whilst a number of candidates are in the discovery phases, all will require replication in larger, real-life cohorts. Clinical cost-effectiveness also needs to be established.
Conclusions:
Since a single measure is unlikely to suffice, multi-modal strategies look more promising, although they bring greater technical and implementation complexities. Identifying reproducible, robust biomarkers will probably require pre-competitive consortia to provide the resources needed to identify, validate, and develop the relevant clinical tests.
Keywords: Animal studies, biomarkers, clinical samples, imaging, psychiatric disorders
Introduction
In 2013, the CINP Think Tank considered the potential of “clinically useful biomarkers to define target populations,” with the concept that they would have the capacity to improve therapeutic outcomes or facilitate the development of novel, more focused treatments. To date, there is only one commercial biological test for clinical use in psychiatric disorders, MDDScore, which augments standardized diagnostic interviews in identifying people with major depressive disorder (Bilello et al., 2013). This was the basis on which the CINP Think Tank brought together interested parties from industry and academia to debate how best to strategize and make practical plans to search for biomarkers for psychiatric disorders. The debate was wide-ranging, moving from why there are so few clinical tests in psychiatry to the types of biomarkers that would be most useful clinically, either for patient stratification or monitoring treatment efficacy. Rather than an exhaustive review of the literature, this report summarizes the Think Tank discussion of opportunities to be seized and challenges to be overcome in the discovery and validation of biomarkers for psychiatric disorders and their treatment.
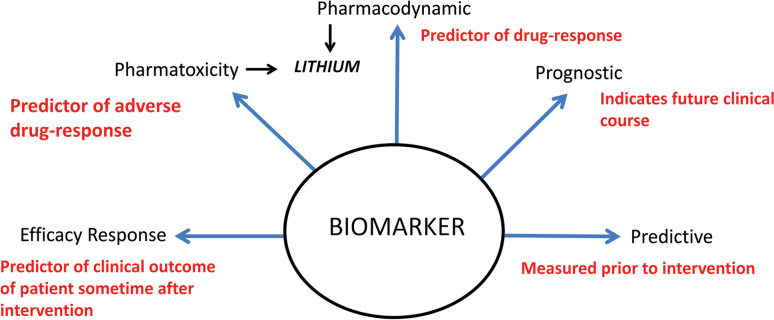
Whilst there has been particular focus on diagnostic biomarkers for psychiatric disorders, the reality is that this is only one example of a biomarker (see Figure 1). Other areas of medicine use markers in a number of ways. For example, human epidermal growth factor (Her2) and oestrogen and progesterone receptors are used as markers for disease stratification in breast cancer (Vaz-Luis et al., 2013). Although no biomarker for Alzheimer’s disease has been universally recognized, decreased extracellular levels of beta amyloid in the brain are considered an early indicator of the risk of developing this neurodegenerative disorder (Buchhave et al., 2012), whilst the extent of cardiac reperfusion following a cardiac arrest is used as a marker of prognosis (Neumar et al., 2008). Markers can also be used to guide therapeutic decisions; it is recommended that people of Asian descent are tested for the human leukocyte antigen B*1502 (HLAB*1502) allele prior to commencing treatment with a range of anticonvulsants to avoid the risk of toxic epidermal necrolysis (US Food and Drug Administration, 2013). In psychiatry, lithium dosage varies considerably because the goal is to attain a specific plasma level which is known to be associated with a clinical effect (see Malhi et al., 2011), making plasma lithium an effective theranostic. Given the range of potential uses, in order to identify suitable markers that will be clinically useful in psychiatry and determine how they will be used we must first decide what we want to achieve.
Figure 1.
Potential clinical uses for biomarkers in psychiatry.
The discovery and development of biomarkers has greatly impacted the management and treatment of a number of medical conditions, such as diabetes and cancer. Conversely, the discovery of a biomarker doesn’t invariably lead to improved clinical outcomes. Indeed, there are potential disadvantages and ethical issues associated with biomarkers that need to be considered as the field progresses. Unless there is a distinct benefit associated with using the biomarker, it will have little, if any, appeal to clinicians or patients. This is best illustrated by the fact that less than 20% of people with a family history of Huntington’s disease request testing to determine whether or not they will develop the disease (Meiser and Dunn, 2001). Since there is no therapeutic intervention available they perceive no benefit in knowing in advance that they will develop the disease. Indeed, a survey found that 15% of people at risk considered suicide to be an option should they develop symptoms (Mastromauro et al., 1987), providing a powerful reason not to test people at risk of the disease. Conversely, a test with a clinical consequence can also have drawbacks. This is demonstrated by the use of prostate-specific antigen (PSA) levels as a screen for prostate cancer; PSA can be elevated by benign events, thereby reducing the diagnostic specificity of the test. Approximately a quarter of the men with elevated PSA levels have prostate cancer and, of these, only a small number of cases would be fatal (Barry, 2001). Thus, the use of PSA levels alone would lead to the over-diagnosis of prostate cancer, an increase in health care costs, and unnecessary anxiety for patients. Equally importantly, it has been reported that depression and suicide increases in men who are known to have an elevated PSA rather than purely in men who have prostate cancer (Lehman, 2014). Thus, whilst the desired outcome in men with elevated PSA would be an evaluation for prostate cancer, the test itself is having detrimental outcomes in some individuals. This is an excellent demonstration that careful consideration needs to be given to the use of biomarkers for psychiatric disorders if and when they become available.
Biomarkers in Psychiatry
Although clinical tests are well-established in other areas of medicine, their development in the field of psychiatry has been slow. There are a number of reasons associated with this, which have previously been dealt with in detail (Kapur et al., 2012). Essentially, although the Diagnostic and Statistical Manual of Mental Disorders and the International Classification of Diseases provide common languages for clinical evaluations, neither provide information about the detailed (or individual) phenomenological presentation or their underlying causes. Furthermore, their diagnostic frameworks define syndromes (Tamminga, 2008), making it unlikely that any biomarker will align with these descriptors; rather, it is more likely that markers will be predictive of subsets of individuals that may display specific symptoms or symptom clusters. This in itself is not an undesirable outcome; most people visit a clinician to have symptoms treated rather than to obtain a diagnosis, but it requires a shift in our conceptualization of the role of biomarkers in the “diagnosis” of psychiatric disorders. Rather than pursuing markers for diagnoses, it may be preferable to develop markers to stratify groups within the syndrome, which in turn may lead to more focused treatment options, possibly traversing nosological boundaries. This would be particularly important if we develop the capacity to identify people who will not respond well to standard first-line treatment options prior to treatment commencement. This approach is exemplified by the Research Domain Criteria (RDoC) established by the USA National Institute of Mental Health, which seeks to resolve the issues associated with mental illness independently of the current classification system used for diagnoses. This concept has the potential to provide ideal opportunities to develop biomarkers for specific behaviors, although to date none have been published.
Compounding the problem of trying to identify biomarkers for syndromes are issues with current study designs. Firstly, many studies assess a marker in a cohort with a uniform diagnosis and compare it to a control group (normally with no psychiatric or neurological history). This is an example of extreme comparisons; whilst this approach is useful in a discovery cohort at the start of a program to identify potential markers, many do not take the next step and assess the marker in a more clinically-relevant cohort presenting with a variety of diagnoses. In addition, even these early phase studies do not control for issues around blood collection, such as fasting status, time of day (diurnal rhythms), and phase of oestrous cycle, which can be critical when measuring highly responsive markers such as cytokines (Dean, 2011b). These are some reasons why markers that appear to have great promise often fail to perform adequately in clinical testing, where they lack the refinement necessary to distinguish between psychiatric disorders. Furthermore, many studies have relatively small cohorts; whilst this is understandable given the issues associated with recruiting people with psychiatric conditions, it means that most, if not all, are underpowered in statistical terms unless the marker has a very high signal-to-noise ratio. The lack of statistical power is especially problematic when coupled with small effect sizes (Cumming, 2008), resulting in a field littered with discordant reports for the majority of markers. When these issues are combined with the problem of dealing with the heterogeneous population described by a syndrome, it is unsurprising that many follow-up studies fail to confirm the original finding. Indeed, often the original finding is not replicated but a similar finding is made. In psychology this is referred to as an approximate replication; whilst such studies do not reject the original finding, they do not support it either. The problem is that neither measure is going to be a useful biomarker. The question then is how to identify robust and reliable markers with a strong enough discriminative power to be clinically relevant? The consensus of the Think Tank was that not only is it necessary to identify measures that can be replicated with a high degree of precision, but mechanisms to rapidly replicate such findings need to be established.
Although they are not factors given much consideration when designing studies to test specific hypotheses, the reality is that clinical tests, irrespective of the underlying science, have to be both easy to implement and inexpensive. This latter point is illustrated by VeriPsych, a blood-based diagnostic aid to confirm the diagnosis of recent-onset schizophrenia and marketed by Rules Based Medicine (Schwarz et al., 2010). The blood-based assay determined levels of 51 analytes and distinguished people diagnosed with schizophrenia from people with no history of psychiatric disorders with a sensitivity of 85% and a specificity of 84%. VeriPsych was marketed in 2010 to aid with the initial diagnosis, where over 30% of people with bipolar disorder are misdiagnosed as having schizophrenia or a psychotic disorder (Gonzalez-Pinto et al., 1998). In 2011, Rules Based Medicine became Myriad RBM and in 2013 VeriPsych was withdrawn, primarily due to cost, but also because of a desire for greater sensitivity and specificity and a market drive for a test that would aid in making differential diagnoses. Thus, in addition to the enormous task and technological challenges of identifying biomarkers, a number of other factors have to be taken into consideration in the development and marketing of such tools for clinical deployment.
Biomarkers at the Discovery Phase
Despite the problems associated with the identification, development, and marketing of biomarkers for psychiatric disorders, research is very active at the discovery level, using a range of modalities. One approach is the use of neuroimaging techniques to aid in diagnosis, drug response, and potentially drug development in psychiatric disorders. Using machine-based learning in a small cohort, the pattern of gray matter volume changes from magnetic resonance images (MRIs) retrospectively predicted the transition of at-risk mental state participants with attenuated psychotic symptoms to psychosis with a sensitivity of 0.83 and a specificity of 0.80 (Koutsouleris et al., 2009). This was replicated in a second, small but independent clinical sample, with a sensitivity of 0.81 and specificity of 0.87 (Koutsouleris et al., 2012a). Whilst the replication makes this approach promising, the authors only replicated the prediction accuracy within the same setting, leaving the complex question of how it is standardized across centers and imaging platforms. In addition, the time and costs associated with MRIs make it a difficult technique to market for routine tests, as required for biomarkers. Therefore, while MRIs are used clinically to identify contributing factors—for example, silent clinical infarcts in late-onset depression (Wu et al., 2014)—researchers are engaged in generating robust, clinically-replicated evidence for its utility as a biomarker.
Alternative complementary approaches include magnetic resonance spectroscopy to measure the response of combined levels of glutamate and glutamine (Glx) to ketamine infusion in healthy volunteers, as well as assessing differences between people with schizophrenia and control participants (see Poels et al., 2014 for a review). Using the Glx response to ketamine as a basis for developing drugs for schizophrenia has so far been unsuccessful; although the metabotropic glutamate 2/3 agonist Pomaglumetad methionil reversed the ketamine-evoked glutamate release and showed positive phase II results, it failed to show a significant effect as an adjunct in phase III trials (Stauffer et al., 2013). By contrast, high doses of D-cycloserine, which was introduced as a drug for tuberculosis, have been shown to be effective in some people with treatment-resistant depression when used as an adjunct to the current antidepressant (Heresco-Levy et al., 2013). People who had plasma glycine levels greater than 300 uM showed a better response to the adjunctive therapy. Combined with the report that poor responses to selective serotonin reuptake inhibitors are associated with high plasma glycine levels (Ji et al., 2011), this raises the possibility that plasma glycine might be a marker for the stratification of patients with depression. However, further work is required to determine if this measure clearly delineates between responders and non-responders.
Another imaging modality is blood flow; a MRI study using gadolinium for contrast reported that high levels of basal cerebral blood volume in the hippocampal CA1 subfield could be used to predict the transition from ultra-high risk to psychosis (Schobel et al., 2009). However, there was no clear separation between groups at the individual level, making this difficult to implement clinically. An alternative approach is the use of infrared scans to monitor blood flow in the forehead during a verbal fluency task. The rate of change in blood flow patterns was different between patients with schizophrenia compared to patients with major depressive disorder (Kinou et al., 2013). Following validation, this imaging approach may enable the differential diagnosis between the two disorders, thereby rapidly initiating appropriate treatment for people with either disorder.
In addition to the neuroimaging strategies, a number of other clinical markers are being assessed for their diagnostic utility. With the current focus on the contribution of cognitive deficits to psychiatric disorders (Millan et al., 2012; Andreou and Bozikas, 2013; Trivedi and Greer, 2014), it is not surprising that neurocognitive data has been used to investigate whether specific deficits correlate with defined illness stages (Pukrop et al., 2006). To date, no individual test has achieved the level of sensitivity and specificity required for a diagnostic marker. However, using patterns of neurocognitive performance across premorbid verbal IQ, processing speed, working memory, verbal and visual memory, and executive function has been reported to distinguish healthy controls from people with an at-risk mental state- with a sensitivity of 0.96 and a specificity of 0.80 (Koutsouleris et al., 2012b). The same neurocognitive performance data also retrospectively identified people with at-risk mental states who transitioned to psychosis from those who did not with a sensitivity of 0.80 and a specificity of 0.75 (Koutsouleris et al., 2012b). As discussed for the gray matter volume analysis, these outcomes are not likely to be accurate enough to be used as diagnostic markers in their own right, but their predictive value might be enhanced by combining them with other markers, in line with recent exploration of multimodal markers and the risk of transition (Fusar-Poli et al., 2013).
Within psychiatric disorders there is a relatively high degree of heritability: 0.81 for schizophrenia, 0.75 for bipolar disorder, and 0.37 for major depressive disorder (Sullivan et al., 2012). Thus, one approach to biomarker discovery is to harness the heritability of these disorders and look for genetic markers. Following reports of a partial overlap in the genetic vulnerability between psychiatric disorders (The International Schizophrenia Consortium, 2009; Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013a), there has been a drive to identify genetic markers for phenotypes rather than continuing with the historic diagnostic labels. Using this approach, a role for calcium channel signaling has been identified in autism spectrum disorder, attention deficit-hyperactivity disorder, bipolar disorder, major depressive disorder, and schizophrenia (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013b). It remains to be determined whether these findings translate into either a better understanding of the causes of these disorders or novel drug targets, which might be of particular use during the pre-clinical phase of adult-onset disorders, allowing a non-specific intervention that trans-nosologically impedes conversion. The logical progression of these findings has resulted in studies to determine whether particular clinical features are associated with genetic markers. One of the first studies to assess this reported a significant impact of the catechol-O-methyl transferase (COMT) functional polymorphism [rs4680; Val(108/158)Met] with working memory as assessed by the N-back task (Bertolino et al., 2004). The methionine substitution, which confers lower activity of this enzyme responsible for the catabolism of dopamine, was associated with a better performance on the task. Furthermore, people with this allele also showed greater improvements in working memory and negative symptoms following treatment with olanzapine, raising the possibility it might serve as an indicator for treatment responsivity. Recent findings also link a functional polymorphism (rs6314) in the serotonin (5-hydroxytryptamine) 2A receptor to performance on cognitive tasks and response to olanzapine (Blasi et al., 2013). In this instance, the T allele was associated with lower levels of receptor expression, poorer performance on the trail making test, the prescription of higher doses, and an attenuated response to olanzapine. Together, the associations of these polymorphisms suggest that batteries of polymorphisms, rather than individual ones, might be required to understand the nuances of interactions between behavior, cortical circuitry, and, ultimately, treatment response across psychiatric disorders. This posit is in accordance with current notions of epistasis and the need for pathway analyses when examining patterns of genetic risk (Sullivan et al., 2012). The complexity of the picture is underlined by the fact that the COMT Met allele was not associated with performance on a working memory task in people with major depressive disorder (Opmeer et al., 2013); however, it should be noted that the task used was the Tower of London, not the N-back task. Given the levels of heritability for the psychiatric disorders, it is evident that genes are not the sole contributors to susceptibility for the disorders. With our increasing understanding of the role played by epigenetics in modifying gene expression as a response to environmental events, the impact of these dynamic mechanisms on the expression levels of genes has increasingly been examined (Millan, 2013). Investigations into the epigenetics of COMT have revealed that methylation of the valine at the rs4680 site in response to stress is associated with reduced enzyme expression and has a moderate, positive association with performance on the N-back task (Ursini et al., 2011). Thus, both genetic and epigenetic markers across heritable phenotypes are an active area of research. This is attested to by recent publications regarding polygenic risk scores in psychiatry and frontal brain activation (Whalley et al., 2015), schizophrenia and working memory (Kauppi et al., 2014), and stressful life events and depressive symptoms (Musliner et al., 2014). However, like other markers discussed in this report, they need to be properly validated.
Advances in high-throughput techniques are being harnessed to identify changes in the “omics” associated with psychiatric disorders. These approaches have two goals: firstly to understand more about the pathogenesis of the disorder and secondarily to detect markers that will help in the identification and treatment strategies for people with the disorder. It is now established that the cytokine system is disrupted in psychiatric disorders (Dean, 2011b), including major depressive disorder (Stelzhammer et al., 2014)—particularly the pro-inflammatory members of the family (Xu et al., 2012)—schizophrenia (de Witte et al., 2014; Fillman et al., 2013), and bipolar disorder (Brambilla et al., 2014; Bauer et al., 2014). Likewise, oxidative stress markers have been reported in major depressive disorder (Stelzhammer et al., 2014), schizophrenia (Liu et al., 2014), and bipolar disorder (Brown et al., 2014). Given the widespread changes reported in these factors, the potential use of specific members of these families as biomarkers remains to be determined, based on their specificity for the event in question. An additional advantage of these large-scale studies is that reports of markers that identify subgroups within the diagnostic category are gradually emerging. The most progress in this aspect has been made in schizophrenia, where subgroups have been identified on the basis of binding to the muscarinic M1 receptor (Scarr et al., 2009), levels of cytokines (Fillman et al., 2013; Schwarz et al., 2014), growth factors and hormonal pathways (Schwarz et al., 2014), and patterns of polymorphisms (Arnedo et al., 2015). Until the division of schizophrenia into biological subgroups has a clinical significance or outcome, the identification of these and similar markers will be of academic interest only. However, if therapies are developed that are more suitable for a subgroup or a specific subgroup is associated with a particular prognosis, then the ability to identify that group will be of great clinical interest.
Predicting and Tracking Medication Efficacy
Finally, the quest for biomarkers that will predict treatment response is attracting attention from clinicians, regulators, and pharmaceutical companies. However, research is still at an early phase so there are relatively few studies available. In major depressive disorder, high levels of histone deacetylase 5 prior to treatment initiation appear to be a robust marker for treatment response (Iga et al., 2007; Hobara et al., 2010; Belzeaux et al., 2010). Levels of cyclic-adenosine monophosphate (cAMP) response element binding protein 1 (Iga et al., 2007), histone deacetylase 2 (Hobara et al., 2010), serotonergic markers (Belzeaux et al., 2010), a panel of four gene expression profiles (Belzeaux et al., 2012), and interferon regulatory factor 7 (Mamdani et al., 2011) have variously been reported to change following treatment with antidepressants. Within schizophrenia, a small study reported that clinical improvement following treatment with unspecified second-generation antipsychotics was associated with a return to control levels of peripheral Dishevelled-Associated Activator Of Morphogenesis (Kuzman et al., 2009). Another study reported that mRNA levels of neuropilin and tolloid-like (NETO) and proto-oncogene AF4/Fragile X E mental retardation syndrome (AF4/FMR2) Family Member 3 (AFF3) were significantly altered after 3 weeks of treatment in responders to olanzapine or risperidone compared to non-responders (Mamdani et al., 2013). For bipolar disorder, there are no reports of clear indicators of treatment response to antipsychotic medications. However, vascular endothelial growth factor A was reported to be decreased following treatment with lithium in small groups of healthy individuals and patients with bipolar disorder (Kikuchi et al., 2011). Whilst this has potential for use as a marker of medication compliance, it provides no clue as to treatment response. By contrast, an investigation of gene expression profiles between depressed people with bipolar disorder who did (n = 10; 50+% reduction in initial score on the Hamilton depression rating scale) and did not (n = 10) respond to lithium reported that people who responded had an increased ratio of anti-apoptotic to pro-apoptotic B-cell lymphoma 2 (Bcl2) family members, whilst the converse was true for those who did not respond (Lowthert et al., 2012). Whether the same expression profiles will predict response of the manic phase to treatment remains to be determined. Whilst all these findings are of potential interest, for such markers to be truly useful they need to be prospectively, as well as retrospectively, accurate. In addition, many of these studies have been conducted in small cohorts. As previously discussed, under-powered sample sizes are a major contributor to the non-replication and pseudo-replication that dog this area of research. Thus follow-up studies in larger, independent cohorts are required to advance these findings beyond the discovery phase.
One tactic to identify markers for treatment stratification is to employ a “bottom up” scheme, identifying potential markers in animal models with relevant phenotypes and then assessing their validity in patient samples. One example of this approach is a mouse which overexpresses the SH3 and Multiple Ankyrin Repeat Domains 3 (SHANK3) gene (Han et al., 2013). This mouse was developed because of the association of loss and/or over-expression of this gene with several neuropsychiatric disorders. Duplications spanning SHANK3 result in its overexpression; transgenic animals exhibit hyperactivity, reduced pre-pulse inhibition, hyperphagia, and abnormal circadian rhythms, providing face validity for bipolar disorder. The hyperactivity and abnormal pre-pulse inhibition were reversed by valproate but not lithium. Thus, SHANK3 genotyping might be useful in identifying patients with bipolar disorder who will not respond well to lithium prior to treatment initiation. Examining the proteomes of mice that exhibited high and low scores on a test associated with anxiety led to the identification of single nucleotide polymorphisms in enolase phosphatase, resulting in a lower activity of the enzyme which forms part of the methionine salvage pathway (Ditzen et al., 2010). A product of this pathway, S-adenosyl-L-methionine, was tested as an antidepressant with varying outcomes; a meta-analysis determined that it has an efficacy similar to that of tricyclic antidepressants (Bressa, 1994). The preclinical studies suggest that genotyping patients prior to treatment might improve the clinical utility of this compound. The ability to clinically identify subgroups within syndromes that will respond preferentially to different types of treatment modalities and reliably predict drug effectiveness in individual patients has the potential to dramatically change treatment and drug development for psychiatric disorders from the current “one size fits all” approach to a much more personalized treatment plan.
How do We Move the Concept of Biomarkers Forward?
As can be seen from the preceding efforts to identify various biomarkers, the discovery phase of biomarker development is very active. There are two fundamental answers to the question of how we can expedite the development of biomarkers from discovery through validation and marketing to the clinic. Firstly, it is critical that the measure is rigorous, precise, and reproducible. For example; working memory is often used as a task for functional neuroimaging, but there are different forms of working memory: spatial, visual, and verbal modalities are all widely used. However, do they measure the same thing? How do scores on the N-back test relate to scores on the trail making test? Thus, to avoid generating approximate replications, if a particular neuropsychological tool has potential as a biomarker, the validation and eventual clinical use of it must employ the original protocol to retain the unique specificity and sensitivity of the test. The same principles also apply to biochemical markers, as peripheral levels of many gene products and metabolites will vary throughout the day and, furthermore, some may be influenced by external factors. For any biochemical entity to be assessed, it is vital that the physiological constraints in the general population are known so that collecting and processing samples can be optimized. It is then vital that a standardized protocol encompassing sample collection, storage, processing, and marker measurement is developed and strictly adhered to in order to retain the integrity of the test. Ideally, any workflow, from measuring the test to producing an answer, will have as few nodes as possible to keep variation to a minimum. In addition, as previously suggested (Dean, 2011a), it is critical for researchers in the field to develop networks to expedite the validation of potential biomarkers so that validated biomarkers can rapidly be moved into the clinic.
Secondly, the search, validation, and subsequent marketing of biomarkers will require funding in excess of that generally available from the current funding sources. The process will also need to bring people with very divergent expertise together in a collaborative atmosphere. Ideally there would be clinical and basic researchers, ready access to well-curated samples, coordination of the research effort, knowledge of the requirements of diagnostic kits, and eventually expertise in marketing the product. The best model currently available for this type of venture is a pre-competitive consortium, of which Novel Methods leading to New Medications in Depression and Schizophrenia (NEWMEDS) (Tansey et al., 2012) is an example. NEWMEDS was established by the Innovative Medicines Initiative, which is a public/private undertaking between the European Union and the European Federation of Pharmaceutical Industries and Associations to find new methods of developing drugs for schizophrenia and depression (http://www.newmeds-europe.com/index.php). The consortium has 19 partners from 12 countries, including 10 pharmaceutical companies, seven academic institutions, and two small- and medium-sized enterprises, with management being handled by a third such enterprise. As part of their research agenda, the consortium has determined that genotyping cannot be used to predict responses to antidepressant drugs (Tansey et al., 2012) and assessed factors that predict antipsychotic drug response (Rabinowitz et al., 2014). These and other outcomes demonstrate that it is possible for a large disparate collection of interests to work collaboratively to achieve a common goal. A pre-competitive initiative, the P1vital CNS Experimental Medicine Consortium, has recently been formed with the express intention of “validating the next generation of biomarkers in schizophrenia.” This consortium has five pharmaceutical companies and collaborates with five academic institutions. To date, they have assessed the effects of atypical antipsychotic drugs and nicotine on four biomarker tasks in people who were high schizotypes, as well as performance and brain activation of high schizotypes on virtual mazes (see Dourish and Dawson, 2014). It remains to be determined how well these markers perform in real-life clinical populations. Since this initiative closely matches one of the suggestions arising from the 2013 Think Tank, it will be interesting to see how it performs.
Summary
During the two days of intense discussion that constituted the 2013 Think Tank, a number of significant points were made and endorsed by the attendees. The following are key messages resulting from this meeting:
There is great potential for biomarkers within psychiatry. Potential uses include diagnosis, prognosis (risk), prediction and evaluation of responses to treatment, avoidance of adverse events, and identification of specific subgroups within diagnostic syndromes.
In order to identify useful biomarkers, it is necessary to recognize that genes and their products do not code for symptoms or disorders, therefore we should not expect them to be specific to diagnoses. Furthermore, epigenetic changes may overwrite the genetic blueprint.
Predictors need to have accuracy at the individual level in real life, not just statistically significant differences between group means in experimental studies.
It is vital to standardize measurements and tests across groups or centers around the world to ensure the reproducibility, reliability, and robustness of tests.
Since the “all or none” effect seen in specific types of cancer is unlikely to occur in psychiatric disorders, it will be necessary to develop normative data for the markers.
We need simple, cost-effective readouts if biomarkers are to be used on a broad-based scale for risk estimation and predicting responses to treatment by specialists or in general practice.
Single biomarkers may not be enough; for example, in predicting the risk of psychosis or depression, multi-modal approaches may be more reliable. There is a diversity of potential readouts that can be combined, including biochemical, cognitive, electrophysiological, genetic, and neuroimaging tests.
Longitudinal studies, assessing the expression of a battery of markers over a significant period of time, might prove to be more discriminating than cross-sectional studies.
A large number of potential markers are under investigation at the discovery stage. In order to move these forward, replication studies in much larger, independent cohorts are essential.
There is a need for pre-competitive partnerships, possibly academic, industry, or government, to successfully identify and validate markers so that reliable clinical tests can be developed. Including patients and regulators in discussions would assist in setting targets since market forces and consumer needs do not necessarily align with clinical or research questions.
Despite formidable challenges, much progress has been made and will continue to be made towards the clinically-important goal of developing valid, reliable, and broadly-usable biomarkers for psychiatric disorders and their treatment. However, we have to be realistic about the time it takes to identify, validate, and market biological tests. It might be that a test may not be perfect initially, with its true value and application only emerging once it is in wider use in the clinic. The question to be answered is how good a test has to be to get the chance to make it into the clinic. In essence, addressing that question will require the identification of universally accepted parameters that biomarkers would be useful for. Once these have been established, all aspects of sample collection, processing, and storage, whether they are biological material, or physiological readouts, need to be standardized. Potential markers will require rigorous evaluation of their sensitivity, specificity, predictive value, and likelihood ratio before being tested in a larger validation cohort that reflects a normal clinical population. Normative data will also need to be generated for these markers. If successful, the significant final steps will be to develop a cost-effective, easily implementable diagnostic tool that does not require access to highly specialized equipment that would restrict its use. CINP has already supported this process by sponsoring a forum, in the form of the Think Tanks, for the unencumbered exchange of ideas necessary for such an ambitious program to be implemented. Programs that promote cross-discipline collaborations, such as RDoC, and the specialized research consortia that already exist can also make significant contributions to the development and implementation of such a scheme.
Statement of Interest
Dr Scarr has received honorarium from Astra-Zeneca and travel support from GSK. Dr Millan is a full-time employee of Institut de Recherche Servier and has no other interests to declare. Dr Bahn is a consultant for Myriad Genetics and a director of Psynova Neurotech. Dr Bertolino is an employee of Hoffman - La Roche with no other interests to declare. Dr Turck reports no financial support or conflicts of interest. Dr Kapur has received grant support from GSK, GW, and Roche, has served as a one-off consultant and/or speaker for AstraZeneca, Bristol Meyers Squibb, Eli Lilly, Envivo, Janssen - Johnson and Johnson, Otsuka, Pfizer, and Takeda, and serves on the Scientific Advisory Boards for Lundbeck and Roche. Dr Möller has received honoraria for lectures or advisory activities or received grants from Astra-Zeneca, Lilly, Lundbeck, Pfizer, Schwabe, and Servier. Dr Dean has received travel support from GSK and honoraria from Pfizer, Eli Lilly, and MSD.
Acknowledgements
This work was supported by the Australian Research Council (FT100100689 to Dr Scarr), BMBF QuantPro grant and the Max Planck Society (Dr Turck), and the National Health and Medical Research Council (#APP1002240 to Dr Dean). The authors would like to thank all participants of the Think Tank 2013 for their stimulating discussions and insightful questions, particularly Professor Angelos Halaris for his cogent summation. The 2013 Think Tank was sponsored by the International College of Neuropsychopharmacology (CINP).
References
- Andreou C, Bozikas VP. (2013) The predictive significance of neurocognitive factors for functional outcome in bipolar disorder. Curr Opin Psychiatry 26:54–59. [DOI] [PubMed] [Google Scholar]
- Arnedo J, Svrakic DM, Del VC, Romero-Zaliz R, Hernandez-Cuervo H, Fanous AH, Pato MT, Pato CN, de Erausquin GA, Cloninger CR, Zwir I. (2015) Uncovering the hidden risk architecture of the schizophrenias: confirmation in three independent genome-wide association studies. Am J Psych 172:139–153. [DOI] [PubMed] [Google Scholar]
- Barry MJ. (2001) Prostate-specific antigen testing for early diagnosis of prostate cancer. N Engl J Med 344:1373–1377. [DOI] [PubMed] [Google Scholar]
- Bauer IE, Pascoe MC, Wollenhaupt-Aguiar B, Kapczinski F, Soares JC. (2014) Inflammatory mediators of cognitive impairment in bipolar disorder. J Psychiatr Res 56:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzeaux R, Formisano-Tréziny C, Loundou A, Boyer L, Gabert J, Samuelian JC, Féron F, Naudin J, Ibrahim EC. (2010) Clinical variations modulate patterns of gene expression and define blood biomarkers in major depression. J Psychiatr Res 44:1205–1213. [DOI] [PubMed] [Google Scholar]
- Belzeaux R, Bergon A, Jeanjean V, Loriod B, Formisano-Treziny C, Verrier L, Loundou A, Baumstarck-Barrau K, Boyer L, Gall V, Gabert J, Nguyen C, Azorin JM, Naudin J, Ibrahim EC. (2012) Responder and nonresponder patients exhibit different peripheral transcriptional signatures during major depressive episode. Transl Psychiatry 2:e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Caforio G, Blasi G, De CM, Latorre V, Petruzzella V, Altamura M, Nappi G, Papa S, Callicott JH, Mattay VS, Bellomo A, Scarabino T, Weinberger DR, Nardini M. (2004) Interaction of COMT (Val(108/158)Met) genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psych 161:1798–1805. [DOI] [PubMed] [Google Scholar]
- Bilello JA, Thurmond LM, Smith KM. (2013) Use of the MDDScore biomarker panel for the detection of major depressive order in centralized intractable pain. Biol Psychiatry 75:261S. [Google Scholar]
- Blasi G, De VC, Papazacharias A. (2013) Converging evidence for the association of functional genetic variation in the serotonin receptor 2a gene with prefrontal function and olanzapine treatment. JAMA Psychiatry 70:921–930. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Bellani M, Isola M, Bergami A, Marinelli V, Dusi N, Rambaldelli G, Tansella M, Maria Finardi A, Martino G, Perlini C, Furlan R. (2014) Increased M1/decreased M2 signature and signs of Th1/Th2 shift in chronic patients with bipolar disorder, but not in those with schizophrenia. Transl Psychiatry 4:e406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressa GM. (1994) S-adenosyl-l-methionine (SAMe) as antidepressant: meta-analysis of clinical studies. Acta Neurol Scand 89:7–14. [DOI] [PubMed] [Google Scholar]
- Brown NC, Andreazza AC, Young LT. (2014) An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res 218:61–68. [DOI] [PubMed] [Google Scholar]
- Buchhave P, Minthon L, Zetterberg H, Wallin Å, Blennow K, Hansson O. (2012) Cerebrospinal fluid levels of ß-amyloid 1–42, but not of tau, are fully changed already 5 to 10 years before the onset of alzheimer dementia. Arch Gen Psychiatry 69:98–106. [DOI] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium (2013a) Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet 45:984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium (2013b) Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381:1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming G. (2008) Replication and p intervals: p values predict the future only vaguely, but confidence intervals do much better. Perspect Psychol Sci 3:286–300. [DOI] [PubMed] [Google Scholar]
- de Witte L, Tomasik J, Schwarz E, Guest PC, Rahmoune H, Kahn RS, Bahn S. (2014) Cytokine alterations in first-episode schizophrenia patients before and after antipsychotic treatment. Schizophr Res 154:23–29. [DOI] [PubMed] [Google Scholar]
- Dean B. (2011a) Dissecting the syndrome of schizophrenia: progress toward clinically useful biomarkers. Schizophr Res Treatment Retrieved 29 Aug 2011. doi:10.1155/2011/614730:Article ID 614730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B. (2011b) Understanding the role of inflammatory-related pathways in the pathophysiology and treatment of psychiatric disorders: evidence from human peripheral studies and CNS studies. Int J Neuropsychop 14:997–1012. [DOI] [PubMed] [Google Scholar]
- Ditzen C, Varadarajulu J, Czibere L, Gonik M, Targosz BS, Hambsch B, Bettecken T, Kesler MS, Frank E, Bunck M, Teplytska L, Erhardt A, Holsboer F, Muller-Myhsok B, Landgraf R, Turck CW. (2010) Proteomic-based genotyping in a mouse model of trait anxiety exposes disease-relevant pathways. Mol Psychiatry 15:702–711. [DOI] [PubMed] [Google Scholar]
- Dourish CT, Dawson GR. (2014) Precompetitive consortium approach to validation of the next generation of biomarkers in schizophrenia (editorial). Biomark Med 8:5–8. [DOI] [PubMed] [Google Scholar]
- Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, Cairns M, Weickert CS. (2013) Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry 18:206–214. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Bechdolf A. (2013) The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry 70:107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Pinto A, Gutierrez M, Mosquera F, Ballesteros J, Lopez P, Ezcurra J, Figuerido JL, de LJ. (1998) First episode in bipolar disorder: misdiagnosis and psychotic symptoms. J Affect Disord 50:41–44. [DOI] [PubMed] [Google Scholar]
- Han K, Holder JL, Jr, Schaaf CP, Lu H, Chen H, Kang H, Tang J, Wu Z, Hao S, Cheung SW, Yu P, Sun H, Breman AM, Patel A, Lu HC, Zoghbi HY. (2013) SHANK3 overexpression causes manic-like behaviour with unique pharmacogenetic properties. Nature 503:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heresco-Levy U, Gelfin G, Bloch B, Levin R, Edelman S, Javitt DC, Kremer I. (2013) A randomized add-on trial of high-dose D-cycloserine for treatment-resistant depression. Int J Neuropsychop 16:501–506. [DOI] [PubMed] [Google Scholar]
- Hobara T, Uchida S, Otsuki K, Matsubara T, Funato H, Matsuo K, Suetsugi M, Watanabe Y. (2010) Altered gene expression of histone deacetylases in mood disorder patients. J Psychiatr Res 44:263–270. [DOI] [PubMed] [Google Scholar]
- Iga Ji, Ueno Si, Yamauchi K, Numata S, Kinouchi S, Tayoshi-Shibuya S, Song H, Ohmori T. (2007) Altered HDAC5 and CREB mRNA expressions in the peripheral leukocytes of major depression. Prog Neuropsychopharmacol Biol Psychiatry 31:628–632. [DOI] [PubMed] [Google Scholar]
- The International Schizophrenia Consortium (2009) Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Hebbring S, Zhu H, Jenkins GD, Biernacka J, Snyder K, Drews M, Fiehn O, Zeng Z, Schaid D, Mrazek DA, Kaddurah-Daouk R, Weinshilboum RM. (2011) Glycine and a glycine dehydrogenase (GLDC) SNP as citalopram/escitalopram response biomarkers in depression: pharmacometabolomics-informed pharmacogenomics. Clin Pharmacol Ther 89:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Phillips AG, Insel TR. (2012) Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it[quest]. Mol Psychiatry 17:1174–1179. [DOI] [PubMed] [Google Scholar]
- Kauppi K, Westlye LT, Tesli M, Bettella F, Brandt CL, Mattingsdal M, Ueland T, Espeseth T, Agartz I, Melle I, Djurovic S, Andreassen OA. (2014) Polygenic risk for schizophrenia associated with working memory-related prefrontal brain activation in patients with schizophrenia and healthy controls. Schizophr Bull Retrieved 16 Dec 2014. doi:10.1093/schbul/sbu152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Iga Ji, Tayoshi S, Nakataki M, Watanabe S, Numata S, Ohmori T. (2011) Lithium decreases VEGF mRNA expression in leukocytes of healthy subjects and patients with bipolar disorder. Hum Psychopharmacol Clin Exp 26:358–363. [DOI] [PubMed] [Google Scholar]
- Kinou M, Takizawa R, Marumo K, Kawasaki S, Kawakubo Y, Fukuda M, Kasai K. (2013) Differential spatiotemporal characteristics of the prefrontal hemodynamic response and their association with functional impairment in schizophrenia and major depression. Schizophr Res 150:459–467. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N, Meisenzahl EM, Davatzikos C, Bottlender R, Frodl T, Scheuerecker J, Schmitt G, Zetzsche T, Decker P, Reiser M, Möller HJ, Gaser C. (2009) Use of neuroanatomical pattern classification to identify subjects in at-risk mental states of psychosis and predict disease transition. Arch Gen Psychiatry 66:700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris N, Borgwardt S, Meisenzahl EM, Bottlender R, Möller HJ, Riecher-Rössler A. (2012a) Disease prediction in the at-risk mental state for psychosis using neuroanatomical biomarkers: results from the FePsy study. Schizophr Bull 38:1234–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris N, Davatzikos C, Bottlender R, Patschurek-Kliche K, Scheuerecker J, Decker P, Gaser C, Möller HJ, Meisenzahl EM. (2012b) Early recognition and disease prediction in the at-risk mental states for psychosis using neurocognitive pattern classification. Schizophr Bull 38:1200–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzman MR, Medved V, Terzic J, Krainc D. (2009) Genome-wide expression analysis of peripheral blood identifies candidate biomarkers for schizophrenia. J Psychiatr Res 43:1073–1077. [DOI] [PubMed] [Google Scholar]
- Lehman S. (2014) Sometimes, ignorance really is bliss. In: The Globe and Mail, June 19th 2014 (Hayward K, ed.) pp L5 Toronto, Ontario: Phillip Crawley. [Google Scholar]
- Liu ML, Zheng P, Liu Z, Xu Y, Mu J, Guo J, Huang T, Meng HQ, Xie P. (2014) GC-MS based metabolomics identification of possible novel biomarkers for schizophrenia in peripheral blood mononuclear cells. Mol Biosyst. Retrieved 30 Jul 2014. doi: 10.1039/c4mb00157e [DOI] [PubMed] [Google Scholar]
- Lowthert L, Leffert J, Lin A, Umlauf S, Maloney K, Muralidharan A, Lorberg B, Mane S, Zhao H, Sinha R, Bhagwagar Z, Beech R. (2012) Increased ratio of anti-apoptotic to pro-apoptotic Bcl2 gene-family members in lithium-responders one month after treatment initiation. Biol Mood Anxiety Disord 2:15. Retrieved 31 Jul 2014. doi:10.1186/2045-5380-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi GS, Tanious M, Gershon S. (2011) The lithiumeter: a measured approach. Bipolar Disord 13:219–226. [DOI] [PubMed] [Google Scholar]
- Mamdani F, Berlim MT, Beaulieu MM, Labbe A, Merette C, Turecki G. (2011) Gene expression biomarkers of response to citalopram treatment in major depressive disorder. Transl Psychiatry 1:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamdani F, Martin MV, Lencz T, Rollins B, Robinson DG, Moon EA, Malhotra AK, Vawter MP. (2013) Coding and noncoding gene expression biomarkers in mood disorders and schizophrenia. Dis Markers 35:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastromauro C, Myers RH, Berkman B, Opitz JM, Reynolds JF. (1987) Attitudes toward presymptomatic testing in Huntington disease. Am J Med Genet 26:271–282. [DOI] [PubMed] [Google Scholar]
- Meiser B, Dunn S. (2001) Psychological effect of genetic testing for Huntington’s disease: an update of the literature. West J Med 174:336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. (2013) An epigenetic framework for neurodevelopmental disorders: from pathogenesis to potential therapy. Neuropharmacology 68:2–82. [DOI] [PubMed] [Google Scholar]
- Millan MJ, et al. (2012) Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov 11:141–168. [DOI] [PubMed] [Google Scholar]
- Musliner KL, Seifuddin F, Judy JA, Pirooznia M, Goes FS, Zandi PP. (2015) Polygenic risk, stressful life events and depressive symptoms in older adults: a polygenic score analysis. Psychol Med. 45; 1709–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumar RW, et al. (2008) Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication a consensus statement from the international liaison committee on resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 118:2452–2483. [DOI] [PubMed] [Google Scholar]
- Opmeer EM, Kortekaas R, van Tol MJ, van der Wee NJ, Woudstra S, van Buchem MA, Penninx BW, Veltman DJ, Aleman A. (2013) Influence of COMT val158met genotype on the depressed brain during emotional processing and working memory. PLOS ONE 8:e73290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels EMP, Kegeles LS, Kantrowitz JT, Slifstein M, Javitt DC, Lieberman JA, Abi-Dargham A, Girgis RR. (2014) Imaging glutamate in schizophrenia: review of findings and implications for drug discovery. Mol Psychiatry 19:20–29. [DOI] [PubMed] [Google Scholar]
- Pukrop R, Schultze-Lutter F, Ruhrmann S, Brockhaus-Dumke A, Tendolkar I, Bechdolf A, Matuschek E, Klosterkotter J. (2006) Neurocognitive functioning in subjects at risk for a first episode of psychosis compared with first- and multiple-episode schizophrenia. J Clin Exp Neuropsychol 28:1388–1407. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J, Werbeloff N, Caers I, Mandel FS, Stauffer V, Ménard F, Kinon BJ, Kapur S. (2014) Determinants of antipsychotic response in schizophrenia: implications for practice and future clinical trials. J Clin Psychiatry 75:e308–e316. [DOI] [PubMed] [Google Scholar]
- Scarr E, Cowie TF, Kanellakis S, Sundram S, Pantelis C, Dean B. (2009) Decreased cortical muscarinic receptors define a subgroup of subjects with schizophrenia. Mol Psychiatry 14:1017–1023. [DOI] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM. (2009) DIfferential targeting of the ca1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry 66:938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E, et al. (2010) Validation of a blood-based laboratory test to aid in the confirmation of a diagnosis of schizophrenia. Biomark Insights 5:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E, van Beveren NJM, Ramsey J, Leweke FM, Rothermundt M, Bogerts B, Steiner J, Guest PC, Bahn S. (2014) Identification of subgroups of schizophrenia patients with changes in either immune or growth factor and hormonal pathways. Schizophr Bull 40:787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer VL, Millen BA, Andersen S, Kinon BJ, LaGrandeur L, Lindenmayer JP, Gomez JC. (2013) Pomaglumetad methionil: no significant difference as an adjunctive treatment for patients with prominent negative symptoms of schizophrenia compared to placebo. Schizophr Res 150:434–441. [DOI] [PubMed] [Google Scholar]
- Stelzhammer V, Haenisch F, Chan MK, Cooper JD, Steiner J, Steeb H, Martins-de-Souza D, Rahmoune H, Guest PC, Bahn S. (2014) Proteomic changes in serum of first onset, antidepressant drug-naïve major depression patients. Int J Neuropsychop 17:1599–1608. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Daly MJ, O’Donovan M. (2012) Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet 13:537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA. (2008) Accelerating New Knowledge in Schizophrenia. Am J Psych 165:949–951. [DOI] [PubMed] [Google Scholar]
- Tansey KE, et al. (2012) Genetic predictors of response to serotonergic and noradrenergic antidepressants in major depressive disorder: a genome-wide analysis of individual-level data and a meta-analysis. PLOS Med 9:e1001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL. (2014) Cognitive dysfunction in unipolar depression: implications for treatment. J Affect Disord 152–154:19–27. [DOI] [PubMed] [Google Scholar]
- Ursini G, Bollati V, Fazio L, Porcelli A, Iacovelli L, Catalani A, Sinibaldi L, Gelao B, Romano R, Rampino A, Taurisano P, Mancini M, Di Giorgio A, Popolizio T, Baccarelli A, De Blasi A, Blasi G, Bertolino A. (2011) Stress-related methylation of the catechol-o-methyltransferase Val158 allele predicts human prefrontal cognition and activity. J Neurosci 31:6692–6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food and Drug Administration (2013) Information for healthcare professionals: dangerous or even fatal skin reactions - carbamazepine (marketed as Carbatrol, Equetro, Tegretol, and generics). Silver Spring, MD: US Food and Drug Administration. [Google Scholar]
- Vaz-Luis I, Winer EP, Lin NU. (2013) Human epidermal growth factor receptor-2-positive breast cancer: does estrogen receptor status define two distinct subtypes? Ann Oncol 24:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley HC, Hall L, Romaniuk L, Macdonald A, Lawrie SM, Sussmann JE, McIntosh AM. (2014) Impact of cross-disorder polygenic risk on frontal brain activation with specific effect of schizophrenia risk. Schizophr Res 161: 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RH, Feng C, Xu Y, Hua T, Liu XY, Fang M. (2014) Late-onset depression in the absence of stroke: associated with silent brain infarctions, microbleeds and lesion locations. Int J Med Sci 11:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HB, Zhang RF, Luo D, Zhou Y, Wang Y, Fang L, Li WJ, Mu J, Zhang L, Zhang Y, Xie P. (2012) Comparative proteomic analysis of plasma from major depressive patients: identification of proteins associated with lipid metabolism and immunoregulation. Int J Neuropsychop 15:1413–1425. [DOI] [PubMed] [Google Scholar]