Abstract
The mechanisms linking hepatitis B virus (HBV) and hepatitis C virus (HCV) infection to hepatocellular carcinoma (HCC) remain largely unknown. Natural killer (NK) cells account for 25%–50% of the total number of liver lymphocytes, suggesting that NK cells play an important role in liver immunity. The number of NK cells in the blood and tumor tissues of HCC patients is positively correlated with their survival and prognosis. Furthermore, a group of NK cell-associated genes in HCC tissues is positively associated with the prolonged survival. These facts suggest that NK cells and HCC progression are strongly associated. In this review, we describe the abnormal NK cells and their functional impairment in patients with chronic HBV and HCV infection, which contribute to the progression of HCC. Then, we summarize the association of NK cells with HCC based on the abnormalities in the numbers and phenotypes of blood and liver NK cells in HCC patients. In particular, the exhaustion of NK cells that represents lower cytotoxicity and impaired cytokine production may serve as a predictor for the occurrence of HCC. Finally, we present the current achievements in NK cell immunotherapy conducted in mouse models of liver cancer and in clinical trials, highlighting how chemoimmunotherapy, NK cell transfer, gene therapy, cytokine therapy and mAb therapy improve NK cell function in HCC treatment. It is conceivable that NK cell-based anti-HCC therapeutic strategies alone or in combination with other therapies will be great promise for HCC treatment.
Keywords: natural killer cell, hepatocellular carcinoma, HBV, HCV, immunotherapy, gene therapy, cytokine therapy, mAb therapy
Introduction
Hepatocellular carcinoma (HCC) is currently the fifth most common malignancy and the third leading cause of cancer mortality1. The highest HCC incidence rates are reported from developing countries, where hepatitis B virus (HBV) and hepatitis C virus (HCV) infection are endemic. The main risk factors for HCC include HBV/HCV infection, alcoholism, nonalcoholic steatohepatitis and diabetes2. Chronic HBV infection accounts for approximately 50% of HCC cases worldwide3,4. Despite the accumulated clinical and epidemiological evidence showing that the development of liver cancer is highly related to chronic HBV infection, the mechanisms linking HBV infection and HCC remain largely unresolved. Natural killer (NK) cells, which are innate lymphoid cells that have natural cytotoxicity and regulatory functions, form the first line of defense against viral infections and tumors. The proportion of innate immune lymphocytes [including NK, NK T-cells (NKT) and γδT cells] accounts for 50% of the total number of liver lymphocytes, of which NK cells account for a large part5. The percentage of NK cells among liver cells is at least five times as high as the percentages among spleen or peripheral blood, suggesting that NK cells may play an important role in the immune function of the liver. NK cells constitutively express a large number of immune recognition receptors (NKRs) that recognize ligands on hepatocytes, liver sinusoidal endothelial cells (LSEC), stellate cells and Kupffer cells to maintain the balance between the immune response and immune tolerance6,7. The number of NK cells in the peripheral blood of patients with HCC is significantly positively correlated with survival rates and the prognosis of liver cancer8,9. Despite these associations, the mechanisms linking NK cells and HCC remain unclear. Here, we describe the abnormal numbers, phenotypes and dysfunctions of NK cells in patients with chronic HBV or HCV infection and HCC. Then, we summarize the current achievements in HCC immunotherapy by improving NK cell functions.
Abnormal frequency and phenotype of NK cells in chronic hepatitis B (CHB) and chronic hepatitis C (CHC) patients
Chronic HBV and HCV infections are the leading causes of liver disease progression, resulting in hepatic cirrhosis and HCC. The incidence of HCC in the West and Japan is clearly rising due to chronic HCV infection. In developing countries, HBV infection comprises 70% of the risk factors for HCC4. Decreased NK cell activity in HCC patients might be related to chronic hepatitis B and C virus infection. The chronic inflammation induced by persistent HBV or HCV infection impaired NK cell function, thereby contributing to the progression of HCC.
NK cells are usually abundant and play an important role in early viral clearance in acute HBV- or HCV-infected patients; however, the numbers of NK cells are lower in both peripheral blood and the liver in chronically infected HBV and HCV patients compared with healthy individuals. Moreover, the frequency of NK cells appears to decrease concomitantly with disease progression10,11,12. The total proportion of NK cells in PBMCs from HCV patients was remarkably decreased compared with healthy controls (8.6% vs 13.3%, respectively). The frequency of CD56bright cells was increased (10.0% vs 6.0%, respectively), while the frequency of CD56dim cells was reduced (90.0% vs 94.0%, respectively)12. Another study found that the frequencies of circulating NK cells were reduced and the phenotypes were altered in 22 HBV+ and 35 HCV+ patients compared with healthy controls11. The percentage of peripheral blood NK cells was approximately 30% lower in the 28 HCV patients compared with the HCV-negative subjects. The reduction was mainly derived from the CD56dim NK cells10. In HCV patients, the proportion of intrahepatic CD56+ NK cells was dramatically lower compared with their proportion in the peripheral blood (5.1% vs 8.6%, respectively). Similar reduced ratios of NK subsets in the liver and blood demonstrated that the decreased proportion of peripheral NK cells in HCV patients was not caused by their accumulation in the liver13.
Persistent HBV or HCV infection often leads to changes in the phenotype of NK cells. In HCV patients, the frequencies of the HLA class I-specific receptors CD158a, h+ and CD158b, j+ on NK cells in liver infiltrating lymphocytes were significantly reduced, whereas intrahepatic NKG2A+ NK cells were more obviously decreased in HBV patients12. The phenotypic changes observed in chronic HCV patients are controversial. Earlier reports analyzed NK cell phenotypes from peripheral blood. In contrast, most later reports analyzed intrahepatic NK cells or compared intrahepatic NK cells with blood NK cells, thereby showing different phenotypic characteristics between intrahepatic and blood NK cells. Most data showed that the expression of activating receptors (eg, NKp30, NKp46, NKG2D, and NKG2C) was increased, while the expression of inhibitory receptors (such as NKG2A) was decreased on intrahepatic NK cells from persistently infected HCV patients11,12,13,14. However, one report showed a significant reduction in the proportion of activating NK cell receptors (eg, NKp46 and NKp30)-expressing NK cells accompanied by an increased proportion of NKG2A-expressing NK cells in chronic HCV patients compared with healthy and HBV-infected subjects15. The controversy concerning phenotypic features might derive from patients with different stages of disease (acute or chronic infection), viral loads, HCV genotypes, sampling sites (derived from blood or liver tissue), or populations. Some reports analyzed smaller numbers of subjects, and some reports failed to include appropriate control groups. Indeed, the evaluation of intrahepatic NK cells in healthy donors is limited by obvious ethical reasons. For HBV persistence, most reports showed reduced expression of activating receptors and increased expression of inhibitory receptors on hepatic or peripheral NK cells. For example, NKG2D/DAP10 and 2B4/SAP expression on NK cells was found to be decreased, while NKG2A expression was significantly increased in patients chronically infected with HBV16,17. The expression of the co-inhibitory receptor Tim-3 was reported to be significantly increased on circulating NK cells and liver-infiltrating lymphocytes from 40 CHB patients compared with 18 healthy controls and nine patients with fatty liver disease18. Another co-inhibitory receptor (PD-1) was also found to be up-regulated on intrahepatic NK cells and other immune cells from patients chronically infected with HBV19.
Functional impairment of NK cells in CHB and CHC patients
The phenotypic changes in NK cells induced by chronic HBV or HCV infection are usually accompanied by, or lead to, NK cell dysfunction16,20. Most observations demonstrated that the cytotoxicity and production of IFN-γ and TNF-α by NK cells were reduced during chronic HCV infection. However, some results showed that phenotypic changes did not necessarily reflect altered functions. The functional dichotomy of NK cells has also been reported in chronic HBV and HCV infections. For example, the expression of activating receptors (particularly NKG2D) was increased and the expression of inhibitory receptors was decreased in HCV patients, while the expression of activating receptors (particularly NKG2C) was increased and normal expression of inhibitory receptors was observed in chronic HBV patients; nevertheless, peripheral NK cells displayed enhanced cytotoxicity, whereas the production of IFN-γ and TNF-α was reduced in both chronic HBV and HCV patients11. CD107a degranulation and TNF-related apoptosis inducing ligand (TRAIL) expression were increased during chronic HBV and HCV infection, along with suppressed production of IFN-γ and TNF-α11,14,21. These functional features of NK cells in combination with conserved or enhanced cytolytic activity and dysfunctional cytokine production might contribute to chronic liver immunopathology and virus persistence. Anti-viral therapy or blockade of immunosuppressive cytokines (IL-10+/− TGF-β) restored NK cell activation and IFN-γ production21,22. However, another report showed significantly dysfunctional intrahepatic NK cell cytotoxicity associated with reduced expression of TRAIL and CD107a in chronic HCV patients compared with healthy controls23. Significant production of IL-10 and normal concentrations of IFN-γ by freshly separated NK cells from HCV patients were also reported24. Based on comparisons of the characteristics of the patients studied, the discrepancies concerning NK cell functions in chronic HCV patients might be associated with differences in viral loads, HCV genotypes, control groups, or detection methods, similar to the controversial reports concerning NK cell phenotypes. Regardless, most reports demonstrated impaired function, and even functional exhaustion, of NK cells during persistent chronic HBV or HCV infection that contributed to the progression of HCC.
Abnormal proportion or frequency of NK cells in HCC
Abnormal NK cytolytic functions have been described in various mouse models of cancer and in human patients25,26,27,28. NK cell exhaustion is highly correlated with the progression and metastasis of a variety of tumors29,30. Earlier observations about the role of NK cells in HCC progression were derived by detecting the proportion and absolute number of NK cells in peripheral blood. A reduction in the proportion of peripheral blood NK cells has been found in HCC patients at various stages compared with healthy controls. Specifically, peripheral CD56dimCD16pos NK subsets displayed a dramatic reduction, which resulted in a dramatically increased ratio of CD56bright and CD56dim NK cells in HCC patients31. Reduced absolute counts of CD56bright and CD56dim NK cells were also observed in the peripheral blood from 20 Egyptian patients with hepatitis C-related HCC compared with 152 healthy control subjects32. Later investigations focused on liver-resident NK cells in HCC patients to reflect the physiological nature of NK cells in the tumor environment. The lack of healthy livers meant that most of these studies compared tumor infiltrating lymphocytes (TILs) with non-tumor infiltrating lymphocytes (NILs). Cai et al analyzed 110 HCC patients at various stages (I/II/III) and found that NK cells largely accumulated in the liver tissues of HCC patients whose total peripheral NK cell frequencies were significantly decreased, whereas more NK cells were detected in NILs (approximately 19.7%) than in TILs (approximately 5.34%). There was no difference in the proportion of CD56brightCD16neg NK cells between NILs and TILs in these HCC patients. The dramatic reduction in the frequency of tumor-infiltrating NK cells mainly reflected the reduction in CD56dimCD16pos NK cells31. Another study reported a similar result: a decreased intrahepatic NK cell frequency in TILs (20.6%±10.4%) compared to NILs (27.9%±13.5%) in 50 HCC patients in various stages of disease (I/II/III/IV: 15/23/8/4)33. The NK proportion in TILs tends to decrease in advanced stage HCC patients. Another interesting study investigated the infiltration of NK cells in normal human livers, chronic hepatitis livers, intratumoral livers and their paired nontumoral livers using immunohistochemical staining. The results showed that NK cells were predominant in the normal liver, chronic hepatitis liver and nontumoral liver compared with the intratumoral tissue. The reduction in NK cells was particularly noticeable in patients with advanced stage HCC34. Furthermore, the density of the infiltrating CD56+ NK cells was correlated with HCC patient survival9. These data demonstrated a reduction in the frequency of circulating and intrahepatic NK cells in HCC patients that might contribute to the tumor's escape from immune surveillance.
Functional impairment of NK cells in HCC patients
Although there was a striking positive correlation between NK cell density in the intratumoral region and survival, NK cells often exhibited reduced infiltration and impaired functional activities (ie, lower production of TNF-α and IFN-γ) in advanced-stage HCC patients31. Increasing evidence strongly suggested that the dysfunction or exhaustion of NK cells in advanced tumor sites in HCC patients contributed to the pathogenesis of liver cancer. Several studies have shown that NK cells from PBMCs and TILs in HCC patients were defective in cytotoxicity and cytokine secretion compared with NK cells from healthy donors, and decreased NK cell activity might be associated with the development and invasion of HCC8,31,35,36. The defect in NK cell activity was inversely correlated with the patient's age, and HCC patients with lower NK cell activity usually presented venous invasion or involvement of both lobes36. The cytotoxicity of NK cells from both the peripheral blood and tumor sites of HCC patients was notably reduced compared with healthy controls8. The production of cytoplasmic granules (ie, granzyme A, granzyme B and perforin) and the secretion of IFN-γ by NK cells from PBMCs were also substantially decreased in advanced HCC patients (stage II and III) compared with healthy donors. Similar decreased activity was also found in NK cells from TILs compared with NILs. Significant reductions in granzyme B and perforin were also noted when stage II patients were compared with stage I patients. The reduced NK cell activity was associated with increased CD4+CD25+ T regulatory cells in the tumor environment of HCC patients31. Myeloid-derived suppressor cells (MDSCs), monocytes/macrophages and fibroblasts derived from HCC also trigger NK cell dysfunction via the NKp30 receptor, CD48/2B4 or PGE2 and IDO separately8,34,37.
NK cell-based immunotherapy for HCC
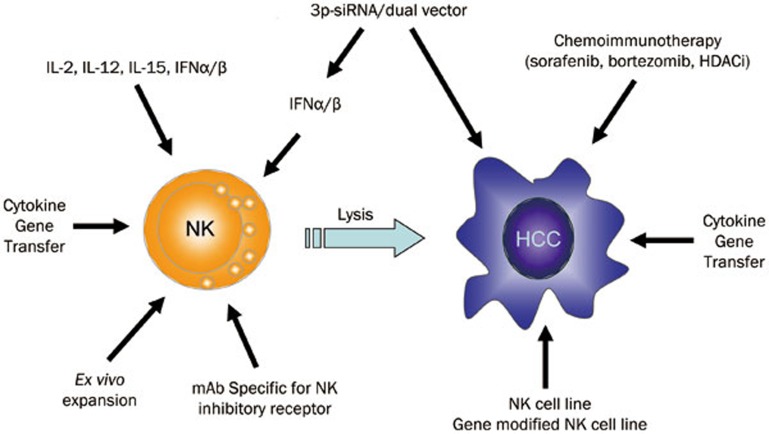
The development and progression of tumors is dependent on not only the intrinsic genomic instability of the tumor but also is the result of co-evolution of the immune system and tumor cells. The critical role of NK cells in the defense against viral infections and tumors has been confirmed. Massive tumor growth has been observed in NKG2D-deficient mice, providing critical evidence for the surveillance of tumors by NK cells and their receptors26. The therapeutic strategy of targeting NK cells in human cancer immunotherapy has displayed an efficient effect, particularly in hematological malignancies. This approach also shows promise in solid cancers, such as metastatic melanoma, kidney cancer and lung cancer in addition to HCC. Due to the rich frequency and unique features of NK cells in the liver, they exert important effects on the development and progression of HCC, in particular by restricting HBV or HCV infection and preventing disease progression from chronic hepatitis to HCC38,39. NK cells accumulate within the liver and can directly (or indirectly through the production of cytokines) kill infected cells, stressed hepatocytes and tumor cells. NK cells can also kill activated hepatic stellate cells (HSCs), thereby alleviating liver fibrogenesis. Accordingly, NK cells play crucial roles in not only eradicating HBV or HCV from the infected hepatocytes but also suppressing the development of fibrosis, cirrhosis and HCC; however, their functions (ie, cellular cytotoxicity and cytokine production) are disrupted in patients with prolonged HBV/HCV infection and during tumor transformation. Therefore, NK cell-based immunotherapy to overcome the mechanism of NK cell dysfunction and to induce efficient NK cell immune responses has been exploited as a promising therapeutic strategy. The therapeutic strategies mainly focus on inducing NK cell activation and reversing the dysfunction of NK cells, and have shown exciting potential for HCC immunotherapy (Figure 1).
Figure 1.
Natural killer (NK) cell-based immunotherapy for hepatocellular carcinoma (HCC). NK cell-based immunotherapy to overcome the mechanism of NK cell dysfunction and to induce efficient NK cell immune responses is a promising therapeutic strategy. The strategy involves chemoimmunotherapy, adoptive transfer of NK cells or gene-modified NK cells, gene therapy and cytokine therapy, and therapy with a mAb specific for NK inhibitory receptors. Combined therapy that boosts NK cell activation and blocks inhibitory signaling pathways (eg, PD-1, CTLA-4, and KIR) has been shown to be more effective at reversing tumor-induced immune tolerance. A recent dual functional therapy strategy comprising both the stimulation of NK cell activation by inducing type I IFN production and the targeted inhibition of tumor growth-related genes has shown great promise for HCC therapy.
Chemoimmunotherapy
Currently, a number of useful therapies are available to patients diagnosed with liver cancer. The therapeutic options for advanced HCC include surgical resection, liver transplantation and percutaneous ablation. Sorafenib, a multikinase inhibitor, is the first and only molecularly targeted drug shown to have a survival benefit in patients and has become a standard treatment for advanced HCC40. In addition to its direct suppressive effect on the growth of hepatoma cells, sorafenib has recently been shown to trigger hepatic NK cell activation and induce NK cell antitumor responses by triggering the proinflammatory activity of tumor-associated macrophages (TAM)41. Sorafenib also enhanced NK cell-mediated cytotoxic activity against HCC by increasing the expression of membrane-bound MICA and decreasing levels of soluble MICA from HCC cells42. These studies suggested an important effect of NK cell activation on the therapeutic efficacy of sorafenib in HCC patients. Similarly, the proteasome inhibitor Bortezomib not only mediates an antitumor effect in HCC by inhibiting tumor cell proliferation but also stimulates the cytotoxicity and IFN-γ production of NK cells by augmenting the cell surface expression of MICA/B on hepatoma cells43. Histone deacetylase inhibitors (HDACi) are a new class of anticancer agents that have shown promise in clinical trials for the treatment of human malignancies, including HCC. In addition to their direct proliferation inhibition and apoptosis induction of tumor cells, increasing evidence has shown that HDACis promote the recognition of tumor cells by immune cells. We and others have reported that HDACis promote MICA or MICB expression on hepatoma cells and increase the susceptibility of hepatoma cells to NK cell-mediated lysis44,45,46. The HDACi suberoylanilide hydroxamic acid (SAHA) not only directly augmented MICA/B transcription via promotion of MICA/B-associated histone acetylation but also epigenetically repressed the transcription of MICA/B-targeting miRNAs (miR-20a, miR-93, and miR-106b) by downregulating the host miRNA genes (miR-17-92 cluster and MCM7)46. This novel mechanism of action further supports the promise for HDACis in the therapy of HCC by promoting the efficacy of NK cell-mediated immunotherapy. Taken together, the synergistic chemoimmunotherapeutic strategy that relies on priming NK cell-mediated antitumor immune responses has shown great promise for HCC treatment.
NK cell transfer
Currently, using autologously or allogeneicly ex vivo expanded NK cells for tumor therapy is becoming more attractive to researchers47,48. NK cells from peripheral blood can be expanded by an average of 1600-fold using good manufacturing practices with compliant components49,50,51,52,53. Moreover, their cytotoxicity against primary autologous tumor targets is significantly increased with no significant cytotoxicity against normal cells. Cytokines such as IL-2, IL-12, IL-15, IL-21, and type I IFN further enhance the activation and cytolytic potency of NK cells during expansion48. The main limitation in using cytokines to stimulate endogenous NK cell activation or proliferation for cancer treatment is the toxicity of systemic cytokine administration. For example, adoptive transfer of autologous NK cells in combination with IL-2 often results in poor clinical outcomes due to the high toxicity of IL-2. Moreover, high IL-2 doses promote the expansion of regulatory T cells (Treg), thereby suppressing NK-cell functions, and inducing activation-induced cell death (AICD) of NK cells. IL-15 acts as an essential cytokine for NK cell development and survival that manifests antiapoptotic actions and inhibits IL-2-mediated AICD. Importantly, IL-15 shows potential for supporting large-scale expansion of clinical-grade NK cells with enhanced longer-term potency and preservation of activation receptors in therapy for malignancies without inducing the expansion of Tregs49. Another factor that impairs the efficacy of autologous NK cell transfer in cancer patients may be the MHC class I expression on tumor cells that suppresses the activation of autologous NK cells in vivo. Infusion of donor–recipient inhibitory KIR-HLA-mismatched allogeneic NK cells or blocking inhibitory receptors specific for MHC-I using anti-KIR Abs has shown strong clinical benefits with minimal toxicity in therapy for leukemia and solid cancers. A Phase I clinical trial on the safety and effectiveness of autologous NK and NKT cells to treat cancers such as HCC (registered at www.clinicaltrial.gov as trial NCT00909558) was sponsored in 2009, but was later suspended. A Phase II clinical trial (NCT02008929) to evaluate the safety and efficacy of MG4101 (ex vivo expanded allogeneic NK cells) as a secondary treatment after curative liver resection on patients with advanced HCC and a high risk of recurrence is about to begin. A safety study of liver NK cell therapy for hepatoma liver transplantation (NCT01147380) is also ongoing. Transfer of the NK92 cell line (the only NK cell line that has been approved by the FDA and has entered clinical trials) has shown efficient antitumor effects in patients with advanced malignant melanoma and renal cell carcinoma. NKG, a new NK cell line established in China, also exhibited strong cytotoxicity against human tumors in vitro and in a mouse xenograft model, showing promising adoptive immunotherapy for human cancer54.
Cytokine gene modification of NK cells could promote NK cell proliferation, increase NK cell survival and enhance the anti-tumor activity of NK cells55,56. Adoptive transfer of highly cytolytic NK cell lines and gene-modified NK cell lines has shown great promise in HCC therapy57,58 and the prevention of HCC recurrence59. In particular, a human IFN-α gene-modified NKL cell line (NKL-IFNα) showed strengthened cytolytic activity against human HCC cell lines and primary human hepatoma cancer cells compared with parental NKL cells. Importantly, adoptive transfer of NKL-IFNα cells significantly suppressed the tumor growth of HCC in a xenograft model and prolonged the survival of tumor-bearing nude mice57. Similarly, a human IL-15 gene-modified NKL cell line (NKL-IL-15) displayed a promising anti-HCC therapeutic effect in a xenograft model58. Adoptive transfer of TRAIL-expressing NK cells also improved the depressed immune status and prevented the recurrence of hepatocellular carcinoma after partial hepatectomy in a mouse model59. Similarly, adoptive transfer of granzyme H-overexpressing NK cells resulted in HBV eradication in HBV-infected mice60. Human allogeneic suicide gene-modified killer cells (aSGMKCs) were recently reported to show a high, rapid, interleukin-2-dependent, mainly NK cell-mediated cytotoxicity against human hepatoma cells, resulting in a marked, rapid and sustained regression of HCC in vivo after adoptive transfer61.
Gene therapy and cytokine therapy
Some effective genetic manipulation approaches have been demonstrated to enhance the interaction of NK and tumor cells by expressing transgenic cytokines and overexpressing activating receptors. Intratumoral gene transfer of cytokine genes such as IL-2, IL-12, and IL-15 could induce NK cell proliferation, enhance their activation and further restore their cytotoxic capacity62. Gene therapy with an adenovirus carrying the IL-12 gene (AdCMVIL-12) for orthotopic HCC in rats demonstrated a significant inhibition of tumor growth. The key antitumor mechanism was the activation of NK cells by AdCMVIL-1263. Intratumoral gene transfer of IL-12 in a primary murine HCC model also significantly inhibited tumor growth, neovascularization and spontaneous lung metastasis. Similarly, the inhibition of tumor growth was almost entirely dependent on NK cells; this finding was confirmed by NK cell depletion64. The anti-tumor effect of adenovirus-mediated CD40L (AdCMVmCD40L) transfer in a rat HCC model was also associated with increased IL-12 serum levels and enhanced NK activity65. Adoptive transfer of IFN- or IL-15 gene-modified NK cells augmented anti-HCC effects in a xenograft model57,58. An experimental therapy for HCC comprising type I and type III IFNs in a BNL hepatoma model demonstrated the critical role of NK cells in the anti-tumor activity of IFNs66. A recombinant vesicular stomatitis virus expressing IFN-β, which may exert an anti-tumor effect by activating NK cells, has been proposed; the Phase I clinical trial (NCT01628640) is now in progress to treat patients with adult primary HCC and sorafenib refractory/intolerant HCC. Another novel therapeutic strategy for HCC using a dual-function vector with both immunostimulatory and pim-3-silencing effects has shown great promise with in vivo anti-tumor effects by arousing NK cell activation via ssRNA-primed immunostimulation and type I IFN production67. This dual functional therapy strategy involving both stimulating immune responses (particularly NK cells) by inducing type I IFN production and targeting inhibition of tumor growth-related genes has shown great promise for treating both chronic HBV infection and HCC68,69,70,71,72.
mAb therapy
Therapeutic approaches targeting co-inhibitory molecules using specific monoclonal antibodies have opened up an exciting new era of immunotherapy73,74,75. The effectiveness of cancer immunotherapy targeting the co-inhibitory molecules CTLA-4 and PD-1 has been fully validated in advanced melanoma and other solid tumors, which demonstrated that the reversal of tumor immune tolerance is an important new pathway for the treatment of cancer. Human anti-CTLA-4 monoclonal antibodies were approved by the FDA in 201176. The results from clinical trials of the use of anti-PD-L1 monoclonal antibodies to block the PD-1/PD-L1-mediated signaling pathway demonstrated that targeting co-inhibitory molecules represented an attractive immunotherapeutic approach77,78. Furthermore, a combination of anti-CTLA-4 and anti-PD-L1 antibodies was more effective at reversing tumor immune tolerance in mice79,80. Dual anti–LAG-3/anti–PD-1 antibody treatment resulted in decreased tumor growth and increased survival81. This therapeutic strategy has also begun clinical testing in advanced HCC patients and has shown promising effects. An anti-CTLA-4 human mAb (tremelimumab) has been used for advanced HCC and has completed its Phase II clinical trial (NCT01008358); this antibody displayed an acceptable safety profile as well as antitumor and antiviral activity82. A phase I trial of an anti-PD-1 mAb (nivolumab) is currently ongoing for patients with advanced HCC (NCT01658878)83.
This immune checkpoint blockade usually targets T cells and aims to rescue T-cell exhaustion and amplify T cell responses. NK cells also express a variety of co-inhibitory receptors that deliver inhibitory signals. NK cell activation is regulated by the balance between inhibitory and activating receptors, with inhibitory signals typically predominating over activation signals by blocking activating signaling. Increased levels of inhibitory receptors and decreased expression of activating receptors on NK cells limit NK cell-mediated tumor immunosurveillance in HCC patients. The expression of the co-inhibitory receptors PD-1 and Tim-3 on NK cells in patients with HBV infection were significantly increased and were associated with the exhaustion of immune cells84,85. Hence, these inhibitory receptors may also act as immune checkpoints in the regulation of NK cell activation and responses. Targeting co-inhibitory molecules on NK cells with specific monoclonal antibodies might represent a promising strategy for tumor therapy, in particular HCC. Therapeutic strategies that block inhibitory receptors (such as the killer immunoglobulin-like receptor, KIR) to enhance NK cell activity have been exploited in hematological diseases, such as leukemia, lymphoma and multiple myeloma (MM), and have shown efficacy and safety86,87. This therapy, alone or in combination with anti-PD-1 antibodies, is also being tested in patients with advanced solid tumors (NCT01714739). Our recent report showed that blocking the inhibitory receptor NKG2A displayed efficacy for the promotion of NK cell activity and clearance of HBV in HBV-persistent mice17. The T-cell immunoglobulin and ITIM domain (TIGIT) receptor was recently defined as an inhibitory receptor expressed on NK cells88. We recently demonstrated the negative regulatory role of TIGIT on NK cell activation in a murine acute viral hepatitis and liver regeneration model89,90. Blocking TIGIT enhanced NK cell activation and interferon-gamma (IFN-γ) production89. These results showed that TIGIT might be a candidate immune-checkpoint protein, and blockading TIGIT to reverse dysfunctional NK cells might be useful for HCC therapy. Collectively, an inhibitory blockade for NK cells might become a promising therapeutic strategy for HCC and chronic HBV or HCV persistent patients, although further study is required to confirm its potential value.
Conclusion and perspectives
NK cells play crucial roles in the clearance of HBV or HCV infection and suppression of the development of liver fibrosis, cirrhosis and hepatocarcinogenesis. NK-cell-based anti-HCC therapeutic strategies are becoming increasingly attractive. However, most attempts are being explored in animal models or in ongoing human clinical trials. Assessment of the long-term anti-tumor effects and safety require further observation. An enhanced understanding of tumor recognition by NK cells and the mechanisms used by HCC to evade NK cells within the liver-specific microenvironment will yield valuable insights for the treatment of HCC. The selection of NK cells with a predominant activating profile (eg, the selection of KIR-mismatched NK donors or the blockade of inhibitory checkpoint molecules on NK cells) is critical for delivering successful anti-HCC activity. Immunotherapy based on specific NK subsets (particularly liver-specific NK cells) may represent an attractive approach for HCC patients. Importantly, strategies to combine NK cell-based immunotherapy with conventional chemotherapy or other multiple therapies will hold greater promise for HCC and HBV/HCV persistent patients.
Acknowledgments
This work was supported by grants from the Chinese Government Department of Science & Technology of China (2012ZX10002006, 2012ZX10002014, 2013ZX10002002, 2012AA020901, 2010CB911901, and 2013CB944901) and the Natural Science Foundation of China (81273220, 81472646, and 91442114).
References
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011; 365: 1118–27. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132: 2557–76. [DOI] [PubMed] [Google Scholar]
- Ferenci P, Fried M, Labrecque D, Bruix J, Sherman M, Omata M, et al. Hepatocellular carcinoma (HCC): a global perspective. J Clin Gastroenterol 2010; 44: 239–45. [DOI] [PubMed] [Google Scholar]
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142: 1264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Jeong WI, Tian Z. Liver: an organ with predominant innate immunity. Hepatology 2008; 47: 729–36. [DOI] [PubMed] [Google Scholar]
- Li F, Tian Z. The liver works as a school to educate regulatory immune cells. Cell Mol Immunol 2013; 10: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Sun C, Tian Z, Xiao W. NK cells in immunotolerant organs. Cell Mol Immunol 2013; 10: 202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009; 50: 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew V, Chen J, Lee D, Loh E, Lee J, Lim KH, et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut 2012; 61: 427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima C, Paschal DM, Wang CC, Yoshihara CS, Wood BL, Yeo AE, et al. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology 2006; 43: 573–80. [DOI] [PubMed] [Google Scholar]
- Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology 2009; 137: 1151–60. [DOI] [PubMed] [Google Scholar]
- Bonorino P, Ramzan M, Camous X, Dufeu-Duchesne T, Thelu MA, Sturm N, et al. Fine characterization of intrahepatic NK cells expressing natural killer receptors in chronic hepatitis B and C. J Hepatol 2009; 51: 458–67. [DOI] [PubMed] [Google Scholar]
- Meier UC, Owen RE, Taylor E, Worth A, Naoumov N, Willberg C, et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol 2005; 79: 12365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, et al. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology 2010; 138: 325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut 2006; 55: 869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Fu B, Gao Y, Liao X, Sun R, Tian Z, et al. TGF-beta1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog 2012; 8: e 1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Wei H, Wei H, Gao Y, Xu L, Yin W, et al. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology 2013; 144: 392–401. [DOI] [PubMed] [Google Scholar]
- Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao D, et al. T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B. J Hepatol 2010; 52: 322–9. [DOI] [PubMed] [Google Scholar]
- Cao D, Xu H, Guo G, Ruan Z, Fei L, Xie Z, et al. Intrahepatic expression of programmed death-1 and its ligands in patients with HBV-related acute-on-chronic liver failure. Inflammation 2013; 36: 110–20. [DOI] [PubMed] [Google Scholar]
- Shabani Z, Bagheri M, Zare-Bidaki M, Hassanshahi G, Arababadi MK, Mohammadi Nejad M, et al. NK cells in hepatitis B virus infection: a potent target for immunotherapy. Arch Virol 2014; 159: 1555–65. [DOI] [PubMed] [Google Scholar]
- Peppa D, Micco L, Javaid A, Kennedy PT, Schurich A, Dunn C, et al. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathog 2010; 6: e 1001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjwa ET, van Oord GW, Hegmans JP, Janssen HL, Woltman AM. Viral load reduction improves activation and function of natural killer cells in patients with chronic hepatitis B. J Hepatol 2011; 54: 209–18. [DOI] [PubMed] [Google Scholar]
- Varchetta S, Mele D, Mantovani S, Oliviero B, Cremonesi E, Ludovisi S, et al. Impaired intrahepatic natural killer cell cytotoxic function in chronic hepatitis C virus infection. Hepatology 2012; 56: 841–9. [DOI] [PubMed] [Google Scholar]
- De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P, et al. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol 2007; 37: 445–55. [DOI] [PubMed] [Google Scholar]
- Farnault L, Sanchez C, Baier C, Le Treut T, Costello RT. Hematological malignancies escape from NK cell innate immune surveillance: mechanisms and therapeutic implications. Clin Dev Immunol 2012; 2012: 421702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 2008; 28: 571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, et al. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood 2002; 99: 3661–7. [DOI] [PubMed] [Google Scholar]
- Sanchez CJ, Le Treut T, Boehrer A, Knoblauch B, Imbert J, Olive D, et al. Natural killer cells and malignant haemopathies: a model for the interaction of cancer with innate immunity. Cancer Immunol Immunother 2011; 60: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol 2001; 1: 41–9. [DOI] [PubMed] [Google Scholar]
- Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med 2001; 7: 94–100. [DOI] [PubMed] [Google Scholar]
- Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol 2008; 129: 428–37. [DOI] [PubMed] [Google Scholar]
- Fathy A, Eldin MM, Metwally L, Eida M, Abdel-Rehim M. Diminished absolute counts of CD56dim and CD56bright natural killer cells in peripheral blood from Egyptian patients with hepatocellular carcinoma. Egypt J Immunol 2009; 16: 17–25. [PubMed] [Google Scholar]
- Guo CL, Yang HC, Yang XH, Cheng W, Dong TX, Zhu WJ, et al. Associations between infiltrating lymphocyte subsets and hepatocellular carcinoma. Asian Pac J Cancer Prev 2012; 13: 5909–13. [DOI] [PubMed] [Google Scholar]
- Wu Y, Kuang DM, Pan WD, Wan YL, Lao XM, Wang D, et al. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology 2013; 57: 1107–16. [DOI] [PubMed] [Google Scholar]
- Jinushi M, Takehara T, Tatsumi T, Hiramatsu N, Sakamori R, Yamaguchi S, et al. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. J Hepatol 2005; 43: 1013–20. [DOI] [PubMed] [Google Scholar]
- Chuang WL, Liu HW, Chang WY. Natural killer cell activity in patients with hepatocellular carcinoma relative to early development and tumor invasion. Cancer 1990; 65: 926–30. [DOI] [PubMed] [Google Scholar]
- Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, et al. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett 2012; 318: 154–61. [DOI] [PubMed] [Google Scholar]
- Tian Z, Chen Y, Gao B. Natural killer cells in liver disease. Hepatology 2013; 57: 1654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Q, Zhang J, Sun X, Zhang C, Han Q, Tian Z. NK cells are the crucial antitumor mediators when STAT3-mediated immunosuppression is blocked in hepatocellular carcinoma. J Immunol 2014; 193: 2016–23. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–90. [DOI] [PubMed] [Google Scholar]
- Sprinzl MF, Reisinger F, Puschnik A, Ringelhan M, Ackermann K, Hartmann D, et al. Sorafenib perpetuates cellular anticancer effector functions by modulating the crosstalk between macrophages and natural killer cells. Hepatology 2013; 57: 2358–68. [DOI] [PubMed] [Google Scholar]
- Kohga K, Takehara T, Tatsumi T, Ishida H, Miyagi T, Hosui A, et al. Sorafenib inhibits the shedding of major histocompatibility complex class I-related chain A on hepatocellular carcinoma cells by down-regulating a disintegrin and metalloproteinase 9. Hepatology 2010; 51: 1264–73. [DOI] [PubMed] [Google Scholar]
- Armeanu S, Krusch M, Baltz KM, Weiss TS, Smirnow I, Steinle A, et al. Direct and natural killer cell-mediated antitumor effects of low-dose bortezomib in hepatocellular carcinoma. Clin Cancer Res 2008; 14: 3520–8. [DOI] [PubMed] [Google Scholar]
- Zhang C, Wang Y, Zhou Z, Zhang J, Tian Z. Sodium butyrate upregulates expression of NKG2D ligand MICA/B in HeLa and HepG2 cell lines and increases their susceptibility to NK lysis. Cancer Immunol Immunother 2009; 58: 1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armeanu S, Bitzer M, Lauer UM, Venturelli S, Pathil A, Krusch M, et al. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res 2005; 65: 6321–9. [DOI] [PubMed] [Google Scholar]
- Yang H, Lan P, Hou Z, Guan Y, Zhang J, Xu W, et al. Histone deacetylase inhibitor SAHA epigenetically regulates miR-17-92 cluster and MCM7 to upregulate MICA expression in hepatoma. Br J Cancer 2015; 112: 112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M, Zhang J, Jiang W, Chen Y, Tian Z. Natural killer cell lines in tumor immunotherapy. Front Med 2012; 6: 56–66. [DOI] [PubMed] [Google Scholar]
- Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol 2013; 10: 230–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suck G, Oei VY, Linn YC, Ho SH, Chu S, Choong A, et al. Interleukin-15 supports generation of highly potent clinical-grade natural killer cells in long-term cultures for targeting hematological malignancies. Exp Hematol 2011; 39: 904–14. [DOI] [PubMed] [Google Scholar]
- Garg TK, Szmania SM, Khan JA, Hoering A, Malbrough PA, Moreno-Bost A, et al. Highly activated and expanded natural killer cells for multiple myeloma immunotherapy. Haematologica 2012; 97: 1348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EK, Ahn YO, Kim S, Kim TM, Keam B, Heo DS. Ex vivo activation and expansion of natural killer cells from patients with advanced cancer with feeder cells from healthy volunteers. Cytotherapy 2013; 15: 231–41. [DOI] [PubMed] [Google Scholar]
- Lim O, Lee Y, Chung H, Her JH, Kang SM, Jung MY, et al. GMP-compliant, large-scale expanded allogeneic natural killer cells have potent cytolytic activity against cancer cells in vitro and in vivo. PLoS One 2013; 8: e 53611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luevano M, Madrigal A, Saudemont A. Generation of natural killer cells from hematopoietic stem cells in vitro for immunotherapy. Cell Mol Immunol 2012; 9: 310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M, Ma J, Chen Y, Zhang J, Zhao W, Zhang J, et al. Establishment, characterization, and successful adaptive therapy against human tumors of NKG cell, a new human NK cell line. Cell Transplant 2011; 20: 1731–46. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang J, Tian Z. Functional characterization of interleukin-15 gene transduction into the human natural killer cell line NKL. Cytotherapy 2008; 10: 265–74. [DOI] [PubMed] [Google Scholar]
- Zhang J, Sun R, Wei H, Tian Z. Characterization of interleukin-15 gene-modified human natural killer cells: implications for adoptive cellular immunotherapy. Haematologica 2004; 89: 338–47. [PubMed] [Google Scholar]
- Jiang W, Zhang C, Tian Z, Zhang J. hIFN-alpha gene modification augments human natural killer cell line anti-human hepatocellular carcinoma function. Gene Ther 2013; 20: 1062–9. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang C, Tian Z, Zhang J. hIL-15 gene-modified human natural killer cells (NKL-IL15) augments the anti-human hepatocellular carcinoma effect in vivo. Immunobiology 2014; 219: 547–53. [DOI] [PubMed] [Google Scholar]
- Ohira M, Ohdan H, Mitsuta H, Ishiyama K, Tanaka Y, Igarashi Y, et al. Adoptive transfer of TRAIL-expressing natural killer cells prevents recurrence of hepatocellular carcinoma after partial hepatectomy. Transplantation 2006; 82: 1712–9. [DOI] [PubMed] [Google Scholar]
- Tang H, Li C, Wang L, Zhang H, Fan Z. Granzyme H of cytotoxic lymphocytes is required for clearance of the hepatitis B virus through cleavage of the hepatitis B virus X protein. J Immunol 2012; 188: 824–31. [DOI] [PubMed] [Google Scholar]
- Leboeuf C, Mailly L, Wu T, Bour G, Durand S, Brignon N, et al. In vivo proof of concept of adoptive immunotherapy for hepatocellular carcinoma using allogeneic suicide gene-modified killer cells. Mol Ther 2014; 22: 634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CH, Chang CM, Tang SW, Pan WY, Fang CC, Chen Y, et al. Differential antitumor effect of interleukin-12 family cytokines on orthotopic hepatocellular carcinoma. J Gene Med 2010; 12: 423–34. [DOI] [PubMed] [Google Scholar]
- Barajas M, Mazzolini G, Genove G, Bilbao R, Narvaiza I, Schmitz V, et al. Gene therapy of orthotopic hepatocellular carcinoma in rats using adenovirus coding for interleukin 12. Hepatology 2001; 33: 52–61. [DOI] [PubMed] [Google Scholar]
- Harada N, Shimada M, Okano S, Suehiro T, Soejima Y, Tomita Y, et al. IL-12 gene therapy is an effective therapeutic strategy for hepatocellular carcinoma in immunosuppressed mice. J Immunol 2004; 173: 6635–44. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Carmona MA, Lukacs-Kornek V, Timmerman A, Shabani S, Kornek M, Vogt A, et al. CD40 ligand-expressing dendritic cells induce regression of hepatocellular carcinoma by activating innate and acquired immunity in vivo. Hepatology 2008; 48: 157–68. [DOI] [PubMed] [Google Scholar]
- Abushahba W, Balan M, Castaneda I, Yuan Y, Reuhl K, Raveche E, et al. Antitumor activity of type I and type III interferons in BNL hepatoma model. Cancer Immunol Immunother 2010; 59: 1059–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Lan P, Yu X, Han Q, Zhang J, Tian Z, et al. Immunotherapy for hepatoma using a dual-function vector with both immunostimulatory and pim-3-silencing effects. Mol Cancer Ther 2014; 13: 1503–13. [DOI] [PubMed] [Google Scholar]
- Lan P, Zhang C, Han Q, Zhang J, Tian Z. Therapeutic recovery of hepatitis B virus (HBV)-induced hepatocyte-intrinsic immune defect reverses systemic adaptive immune tolerance. Hepatology 2013; 58: 73–85. [DOI] [PubMed] [Google Scholar]
- Han Q, Zhang C, Zhang J, Tian Z. Reversal of hepatitis B virus-induced immune tolerance by an immunostimulatory 3p-HBx-siRNAs in a retinoic acid inducible gene I-dependent manner. Hepatology 2011; 54: 1179–89. [DOI] [PubMed] [Google Scholar]
- Ebert G, Poeck H, Lucifora J, Baschuk N, Esser K, Esposito I, et al. 5' Triphosphorylated small interfering RNAs control replication of hepatitis B virus and induce an interferon response in human liver cells and mice. Gastroenterology 2011; 141: 696–706, e1–3. [DOI] [PubMed] [Google Scholar]
- Han Q, Zhang C, Zhang J, Tian Z. The role of innate immunity in HBV infection. Semin Immunopathol 2013; 35: 23–38. [DOI] [PubMed] [Google Scholar]
- Han Q, Lan P, Zhang J, Zhang C, Tian Z. Reversal of hepatitis B virus-induced systemic immune tolerance by intrinsic innate immune stimulation. J Gastroenterol Hepatol 2013; 28 Suppl 1: 132–7. [DOI] [PubMed] [Google Scholar]
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480: 480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364: 2517–26. [DOI] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer 2011; 11: 805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366: 2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med 2012; 366: 2517–9. [DOI] [PubMed] [Google Scholar]
- Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, et al. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res 2012; 72: 887–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010; 107: 4275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 2012; 72: 917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangro B, Gomez-Martin C, de la Mata M, Inarrairaegui M, Garralda E, Barrera P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013; 59: 81–8. [DOI] [PubMed] [Google Scholar]
- Hato T, Goyal L, Greten TF, Duda DG, Zhu AX. Immune checkpoint blockade in hepatocellular carcinoma: current progress and future directions. Hepatology 2014; 60: 1776–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology 2012; 56: 1342–51. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Shi F, Zhou L, Zhang MN, Chen Y, Chang XJ, et al. Upregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma. PLoS One 2011; 6: e 23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DM Jr, Hofmeister CC, Padmanabhan S, Suvannasankha A, Jagannath S, Abonour R, et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood 2012; 120: 4324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vey N, Bourhis JH, Boissel N, Bordessoule D, Prebet T, Charbonnier A, et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood 2012; 120: 4317–23. [DOI] [PubMed] [Google Scholar]
- Li M, Xia P, Du Y, Liu S, Huang G, Chen J, et al. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-gamma production of natural killer cells via beta-arrestin 2-mediated negative signaling. J Biol Chem 2014; 289: 17647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi J, Zhang Q, Liang D, Xiong L, Wei H, Sun R, et al. T-cell Ig and ITIM domain regulates natural killer cell activation in murine acute viral hepatitis. Hepatology 2014; 59: 1715–25. [DOI] [PubMed] [Google Scholar]
- Bi J, Zheng X, Chen Y, Wei H, Sun R, Tian Z. TIGIT safeguards liver regeneration through regulating natural killer cell-hepatocyte crosstalk. Hepatology 2014; 60: 1389–98. [DOI] [PubMed] [Google Scholar]