Background
Elevated cardiac troponin (cTn) in the absence of acute coronary syndromes (ACS) is associated with increased mortality in critically ill patients. There are no evidence-based interventions that reduce mortality in this group.
Objectives
We performed a retrospective investigation of the Veterans Administration Inpatient Evaluation Center database to determine whether drugs used in ACS (β-blockers, aspirin, and statins) are associated with reduced mortality in critically ill patients.
Methods
Thirty-day mortality was determined for non-ACS patients admitted to any Veterans Administration Intensive Care Unit between October 1, 2007, and September 30, 2008, adjusted for severity of illness. Troponin assay values were normalized across institutions.
Results
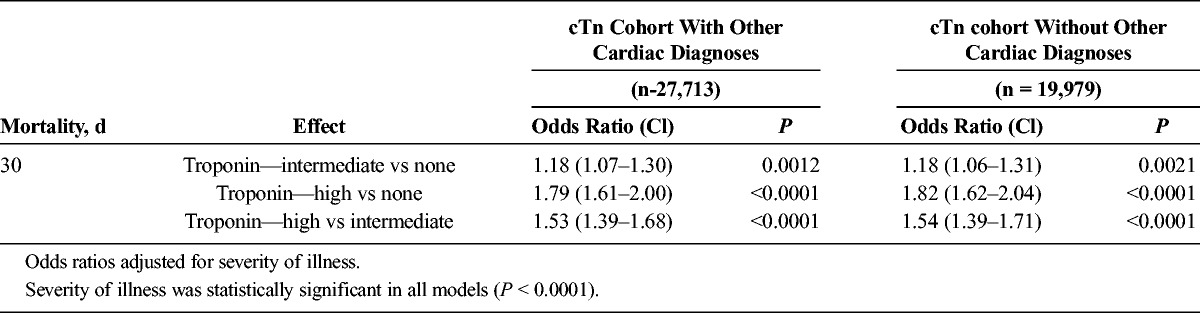
Multivariate analyses for 30-day mortality showed an odds ratio (OR) of 1.82 for patients with high cTn (P < 0.0001, cTn > 10% coefficient of variation) and 1.18 for intermediate cTn (P = 0.0021, cTn between lowest limit detectable and 10% coefficient of variation) compared with patients with no elevation, adjusting for severity of illness (n = 19,979). Logistic regression models showed that patients with no or intermediate elevations of cTn taking statins within 24 hours of cTn measurement had a lower mortality than patients not taking statins (OR, 0.66; 95% confidence interval [95% CI], 0.53–0.82; P = 0.0003), whereas patients with high cTn had a lower mortality if they were taking β-blockers or aspirin within 24 hours of cTn measurement compared to patients not taking β-blockers or aspirin (β-blockers: OR, 0.80; 95% CI, 0.68–0.94; P = 0.0077; aspirin: OR, 0.81;95% CI, 0.69–0.96; P = 0.0134).
Conclusions
This retrospective study confirms an association between elevated troponin and outcomes in critically ill patients without ACS and identifies statins, β-blockers, and aspirin as potential outcome modifiers in a cTn-dependent manner.
Key Words: cardiac troponin, critically ill, β-blocker, aspirin, statin
Critically ill patients who have elevated cardiac troponin (cTn) levels and no evidence of acute coronary syndrome (ACS) have a 2- to 4-fold increase in early mortality as compared with critically ill patients who do not have elevated cTn.1–5 Increased short-term mortality in patients with elevated cTn is observed in patients with sepsis,2,5,6 head trauma,7 and other critical injuries.7 This trend is also observed in critically ill children.8 The mechanism of cTn release in critically ill patients is poorly understood.9 The major concern when cTn is found to be elevated is whether rupture of a coronary arterial plaque has occurred and if the patient requires urgent coronary angiography and revascularization. Despite the prevalence of elevated cTn in this setting, obstructive coronary disease is infrequently found in the adult1 and is not thought to be a likely occurrence in pediatric patients. Furthermore, comorbidities found in these cohorts (hypotension, renal failure, sepsis) often preclude invasive testing. In fact, there are no evidence-based treatments known to reduce mortality in this high risk population.10 Current practice is to medically manage patients with elevated cTn using medications known to reduce mortality in ischemic myocardial injury—β-blockers, statins, aspirin—however, there is little evidence to support this practice.10 The aim of this investigation was to determine 30-day mortality in critically ill patients with elevated cTn. We hypothesized that treatment with β-blockers, aspirin, or statins would be associated with reduced 30-day mortality.
METHODS
Patients and Study Design
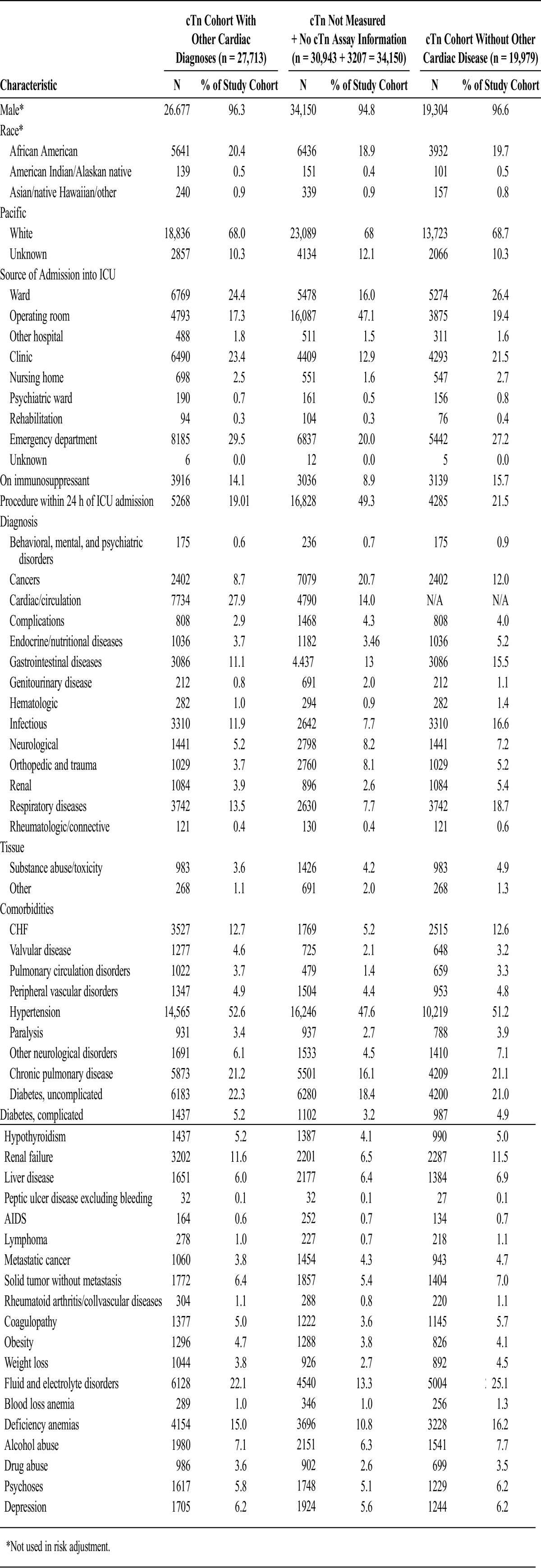
The protocol for this investigation was approved by the University of Cincinnati Investigational Review Board. This retrospective cohort study utilized the Veterans Affairs Inpatient Evaluation Center (IPEC) centralized database. The IPEC is a national quality management program developed to measure risk adjusted mortality and evidence-based processes in inpatient care. The cohort consisted of patients admitted to any VA medical or surgical intensive care unit (ICU) between October 1, 2007, and September 30, 2008. Patients were excluded if they were admitted for an ACS, or if they had any prior ICU admission for an ACS within 1 year of ICU admission (Fig. 1). The ACS patients were excluded because mortality is known to be improved by the use of β-blockers, aspirin, or statins in this group. When patients had multiple ICU stays within the year, only the first ICU stay was analyzed. The overall severity of illness was determined for this cohort as described below (n = 61,863). Patients who had other known cardiac disease were excluded from the mortality analysis as treatment often involves β-blockers, aspirin, or statins (n = 7734). Lastly, we focused on patients who had cTn measured and for whom cTn ranges could be determined (n = 19,979) for the mortality analysis. Surgical procedures were assessed as part of the severity of illness. Barcode Medication Administration files were used to create indicators to determine if a patient was given β-blockers, aspirin, and/or statins within 24 hours of troponin elevation. Appendix 1 provides a list of the medications identified by this method. The backward elimination logistic regression model was used to statistically adjust for drug interactions among these 3 classes of medication.
FIGURE 1.
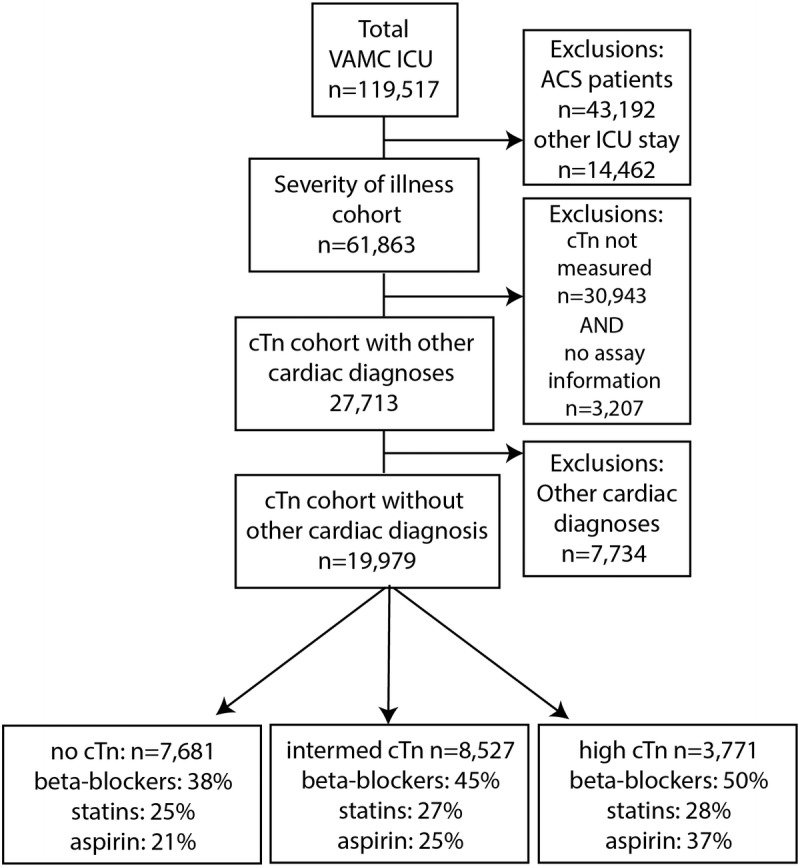
Branching algorithm describing VA-IPEC ICU patient population October 2007 to September 2008.
Troponin Measurements
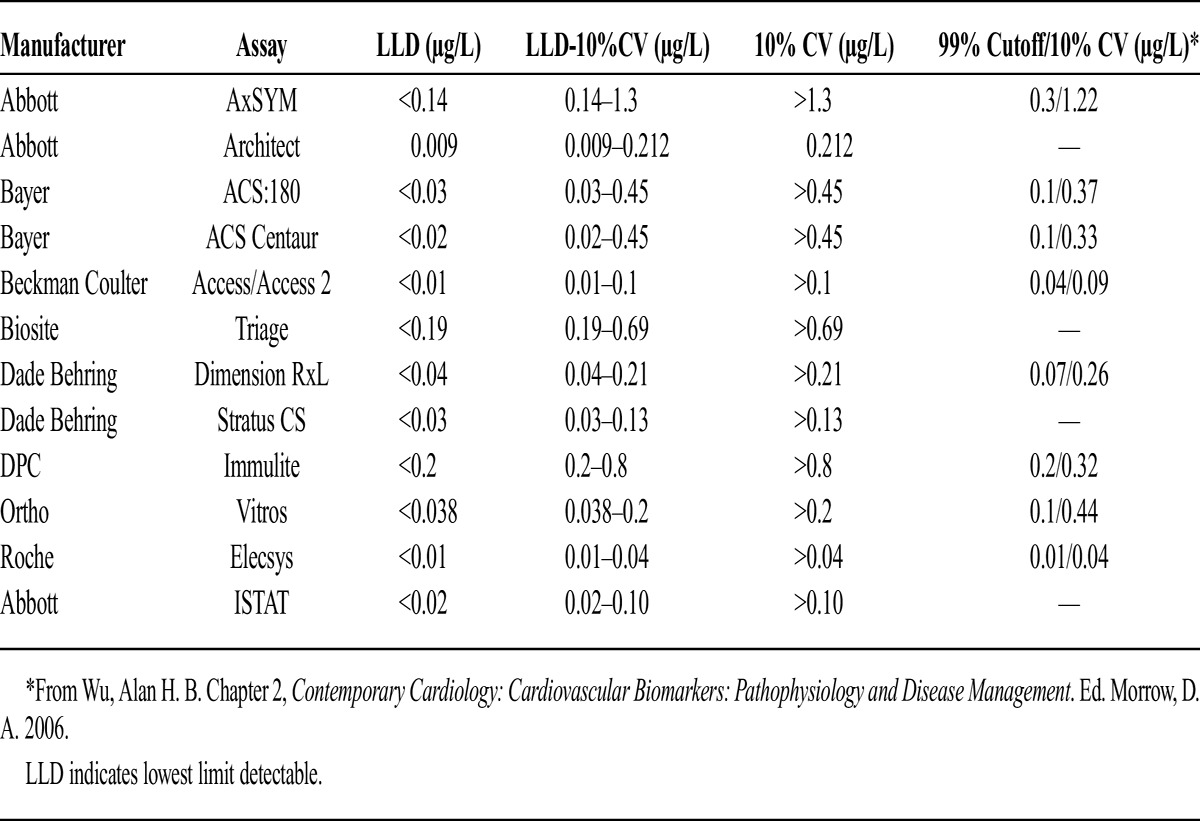
Both cTn T (cTnT) and cTn I (cTnI), components of a protein complex required for myocardial contraction, are well documented indicators of myocardial damage.11 The cTnT assays are standardized across institutions, however, cTnI assays are not. We therefore normalized values for cTnI using the appropriate manufacturer assay information. Appendix 2 provides a detailed list of the assays used and their reference ranges (μg/L). The peak cTnI or cTnT measured within 24 hours from admission to discharge from the ICU was extracted. Facilities were sent a survey asking them to identify which troponin assay they were using. Standard ranges for each assay were used across all facilities. If this information was not available, these patients were excluded from the mortality analysis (n = 3207). The cTnI below the lowest limit detectable was classified as no elevation, levels between the lowest limit detectable and the 10% coefficient of variation (CV) were classified as intermediate elevation, and levels greater than the 10% CV were classified as high elevation. This classification was used to normalize cTn values across a range of values familiar to practitioners, not to classify patients according to the presence or absence of myocardial infarction. The 10% CV for cTnI is the lowest concentration of cTnI in which the assay has a CV of 10% or less. The CV is a measure of variability that adjusts for the magnitude of the mean between assays, as compared to the standard deviation. The 10% CV is determined by assay manufacturers using samples of cTnI of known concentrations and is a measure of precision of the assay. For assays contemporary with this investigation, cTnI concentrations at the 99th percentile (accepted definition of myocardial infarction)11 were within the “intermediate range” (see Appendix 2), and the cTnI concentration for the 10% CV was greater than the 99th percentile cTnI concentration.
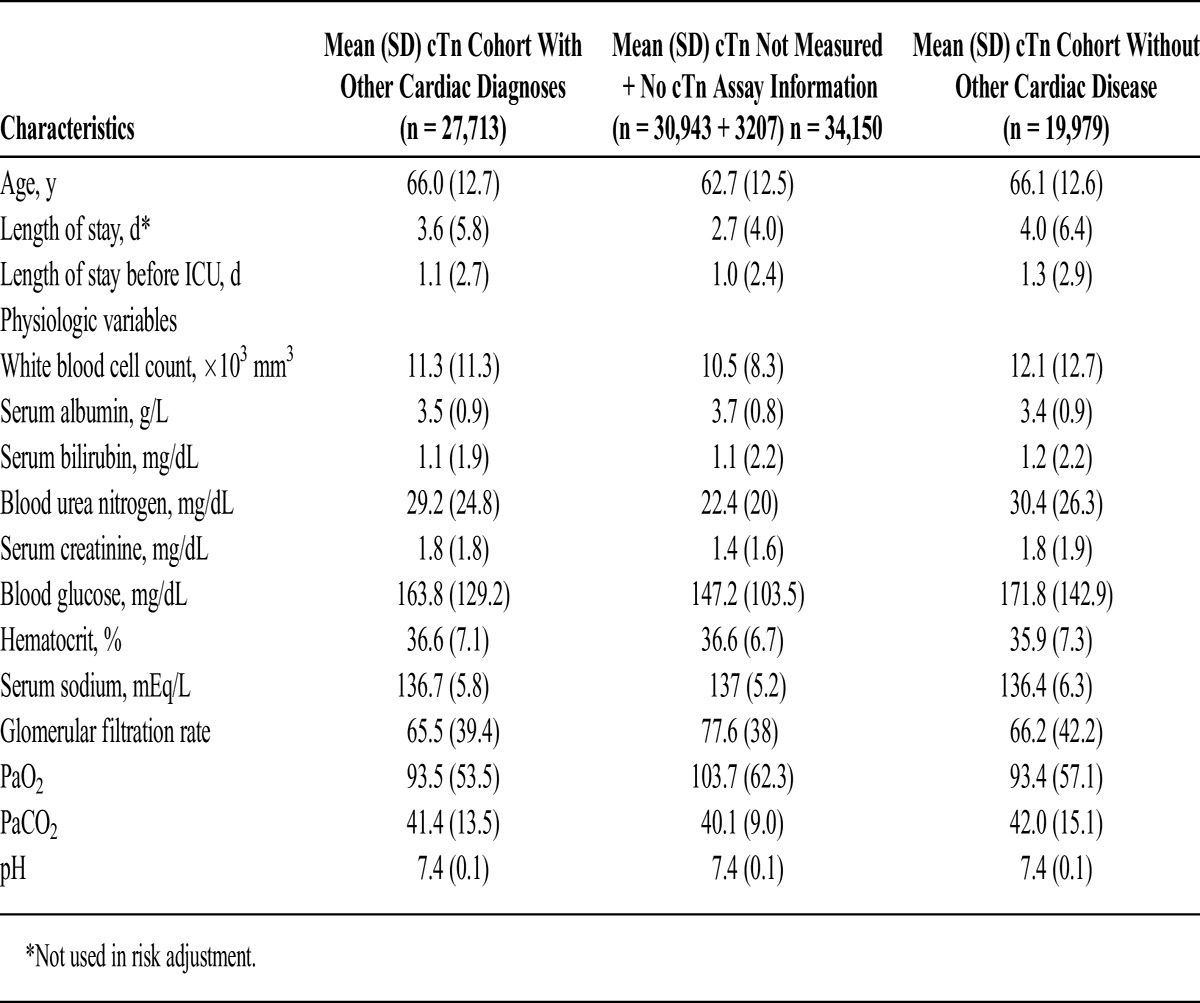
A large group of patients (30,943) did not have cTn measured and were not included in the mortality analysis; ramifications of this are discussed in the Limitations section. Appendices 3 and 4 present baseline characteristics between those with nondefinable troponin (n = 34,150) and those with definable troponin (n = 27,713).
Study Outcomes
The primary outcome was 30-day mortality with respect to aspirin, β-blocker, or statin use in each group: patients with negative cTn, intermediate cTn, or high cTn.
Severity of Illness Model
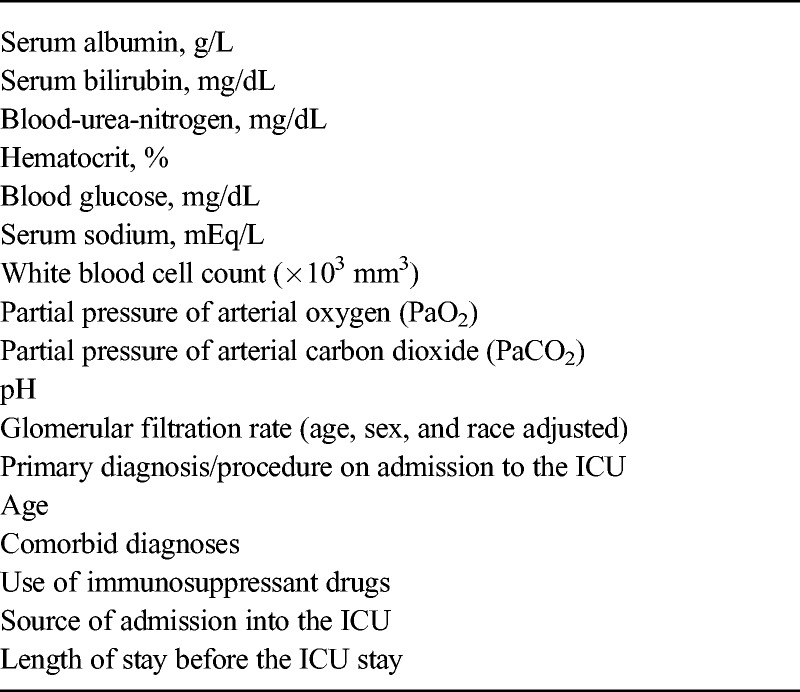
The IPEC uses a statistical model to predict the probability of mortality. This ICU risk-adjusted model was modeled after APACHE III and has been validated against and used with VA data since 1995.12–15 This model includes laboratory values primarily, but also the primary diagnosis/procedure on admission to the ICU, comorbid diagnoses, use of immunosuppressant drugs, source of admission into the ICU, and length of stay before the ICU stay (Table 1). For each laboratory value, the most abnormal value within 48 hours of admission (24 hours before or after) was used. If there was no laboratory value within this time frame, the mean of the normal range for that value was substituted.13
TABLE 1.
Variables Used to Determine the Severity of Illness in the IPEC Model
Statistical Methods
Severity of Illness
A logistic regression model, similar to the IPEC model described above, was run on the cohort to obtain the predicted mortality for each patient at 30 days. This model was run because it was important to get an estimate of the severity of illness in a population without ACS. The logistic regression model assigns the probability of mortality to each patient (between 0 and 1). The linear predictor from this 30-day mortality was used as a covariate in subsequent troponin analyses to adjust for severity of illness. Laboratory values were modeled using restricted cubic splines, with the exception of PaCO2 and pH which were combined and included as a categorical variable. Age was calculated at the time of hospital admission and modeled using a restricted cubic spline. Admission source was modeled as a categorical variable and included the following sources: outpatient clinic, emergency department, nursing home, operating room, other hospital ward, acute care ward, and other. Immunosuppressant drug use was modeled as a dichotomous variable (1, on immunosuppressants; 0, not on immunosuppressants) using a list of immunosuppressant drugs in the pharmacy package. The length of stay before ICU was calculated as the amount of time between hospital admission and ICU admission and was modeled using restricted cubic splines. A dichotomous variable was created to indicate whether or not the patient had an International Classification of Diseases-9 procedure code within the 24 hours surrounding ICU admission.
Descriptive statistics (frequencies and means) were generated for each variable included in the risk adjustment model. Appendices 3 and 4 contain the categorical and continuous variables used to perform the risk adjustments, respectively. The average probability of mortality, referred to as severity of illness in these analyses, was calculated among troponin elevation categories and by medication use.
Troponin Levels and Mortality
Univariate analyses examining the relationship between 30-day mortality and troponin elevation were conducted using χ2 tests. To determine if troponin is associated with mortality at 30 days after adjusting for severity of illness, a logistic regression model was run. This model included the linear predictor for each patient from the initial severity of illness calculation and troponin. Odds ratios (OR) and 95% confidence interval (95% CI) were calculated to compare mortality in patients with no troponin elevation to patients with high troponin or intermediate troponin elevation.
Medication Use and Outcome
Univariate analyses examining the relationship between mortality and medication use among each troponin elevation category were conducted using χ2 tests. Initial multivariate analyses suggested a significant interaction between cTn level and medication use (likelihood ratio test based on χ2 statistic of 9.837 with 3 degrees of freedom; P = 0.0200). Therefore, to determine if the use of β-blockers and/or statins and/or aspirin is associated with mortality reduction after adjusting for severity of illness, stratified logistic regression models were run. These models included the linear predictor for each patient from the initial severity of illness calculation, indicators for β-blockers, statins, and aspirin, and the interaction between β-blockers, statins, and/or aspirin. Stratified analyses were conducted among (1) those with no troponin elevation, (2) those with intermediate troponin elevation, and (3) those with high troponin elevation. All 3 logistic regression models were run for 30-day mortality. Both main effects and backward elimination models were conducted. The backward elimination model differs from the main effects model in that it includes not only the main effects of β-blockers, statins, and aspirin but also the interactions among these medications. In addition, terms are forced out one by one because they no longer achieve statistical significance at the 0.05 level. The inclusion significance level for the final backward elimination models was 0.05. Odds ratios and 95% CI were calculated for all covariates remaining in the backward elimination model. The final significance level used for all inferences was adjusted to account for the multiple tests as a result of stratification. Analyses were adjusted for severity of illness. SAS Version 9.3 was used for all analyses.
RESULTS
Characteristics of Patients
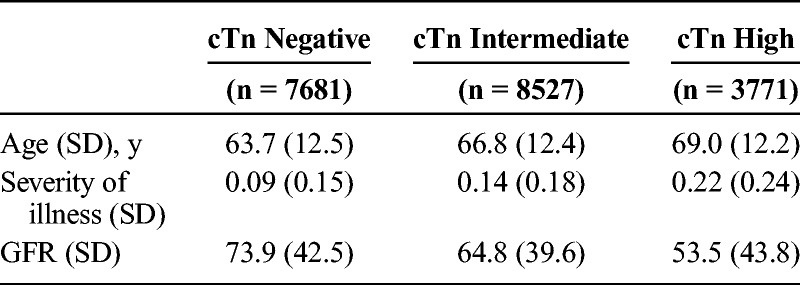
The cohort was predominantly men (96.6%) and white (68.7%). The majority of the patients were admitted for a respiratory diagnosis (18.7%), followed by infection (16.6%), gastrointestinal (15.5%), and cancer (12%) (Fig. 2). Patients who were cTn-negative were younger and had better renal function (Table 2), factors that were adjusted for in the mortality analysis.
FIGURE 2.
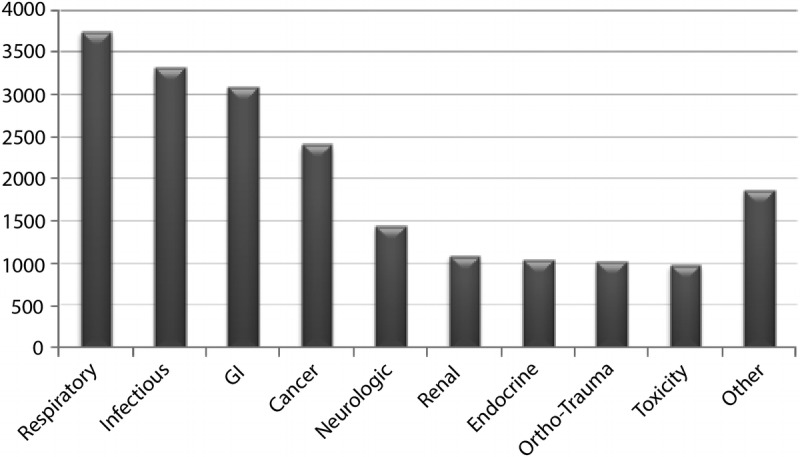
Distribution of primary diagnoses admitted to VA ICUs October 2007 to September 2008 (n = 19,979).
TABLE 2.
Descriptive Statistics Age and Severity of Illness Increase With cTn, Whereas GFR Decreases With cTn
Troponin and Mortality
Figure 3 shows the univariate analysis illustrating troponin level and 30-day mortality, Table 3 shows the tabulated results from each group. About 10.7% of the patients died at 30 days if they had no cTn elevation, whereas 16.6% of patients died if they had intermediate cTn, and 31.7% died at 30 days if they had high cTn (P < 0.0001). After adjusting for severity of illness, mortality among critically ill patients was proportional to cTn levels and statistically significant. Multivariate analysis showed that the odds of death were 1.82 times greater for patients with high cTn compared with no cTn elevation, and 1.18 times greater for patients with intermediate cTn compared with no cTn elevation (P < 0.0001), after adjusting for severity of illness. The differences between the intermediate and high cTn groups were also statistically significant. Patients who did not have a cTn level measured had a lower severity of illness than those patients who had the blood test: cTn not measured, mean severity of illness, 0.07 (SD 0.14, n = 34,150), cTn measured, mean severity of illness 0.13 (SD 0.19, n = 27,713).
FIGURE 3.
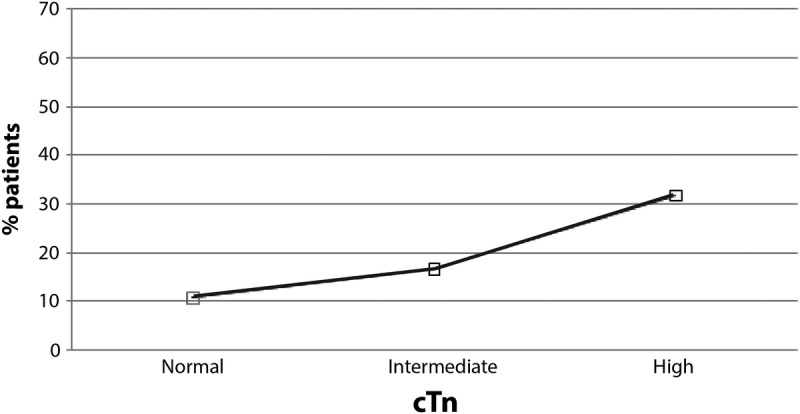
Relationship between 30-day mortality and troponin level: no cTn elevation, 10.7% 30-day mortality; intermediate cTn, 16.6% 30-day mortality; high cTn, 31.7% 30-day mortality.
TABLE 3.
Effect of Troponin Level on Mortality: cTn Cohorts With Other Cardiac Diagnoses (27,713) and Without Other Cardiac Diagnoses (19,979)
Medication Use and Outcome
Univariate analyses showed an increase in mortality proportional to cTn level within each medication class. Thirty-day mortality in those with no cTn elevation, intermediate cTn, and high cTN was 31.3%, 38.5%, and 40.6%, respectively, for patients on β-blockers (P < 0.0001); 14.9%, 19.0%, and 22.7%, respectively, for patients on statins (P < 0.0001); and 15.9%, 21.4%, and 31.2%, respectively, for patients on aspirin (P < 0.0009).
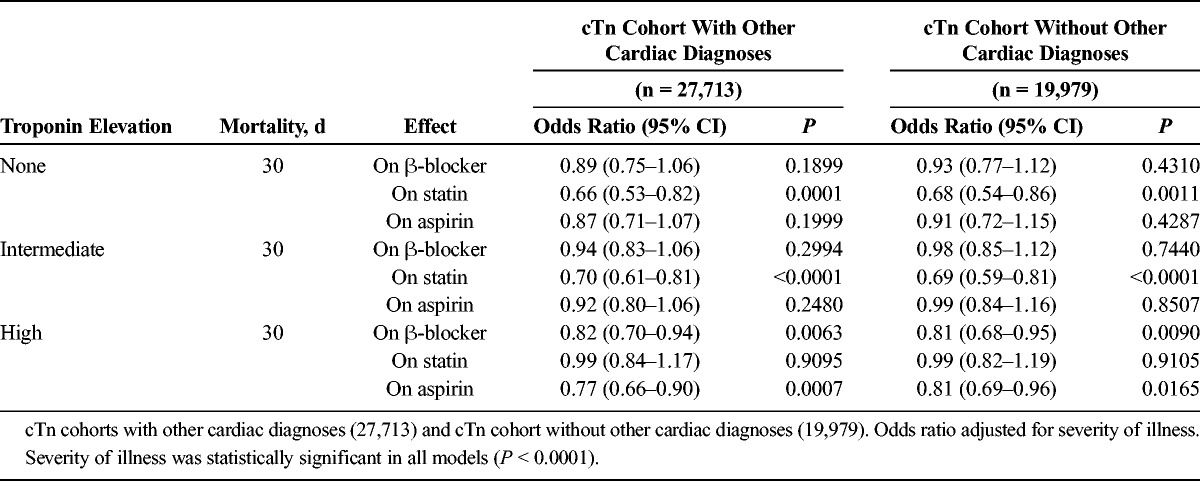
The results from the main effects model indicate that among those with no cTn elevation, statins were associated with lower mortality at 30 days (Table 4; OR, 0.68; 95% CI, 0.54–0.86). In those with an intermediate cTn elevation, statins were again associated with lower mortality at 30 days (OR, 0.69; 95% CI, 0.59–0.81). No relationships between mortality and β-blockers or aspirin were statistically significant for patients with negative or intermediate cTn. In contrast, among those with a high cTn elevation, statins were no longer statistically significant at 30 days, however, β-blockers (OR, 0.81;95% CI, 0.68–0.95) and aspirin (OR, 0.81; 95% CI, 0.69–0.96) were associated with lower mortality. All analyses were adjusted for severity of illness. Inferences from the stratified models held up to multiple testing criteria.
TABLE 4.
Effect of Medication Use on Mortality According to cTn Level—Main Effects Model
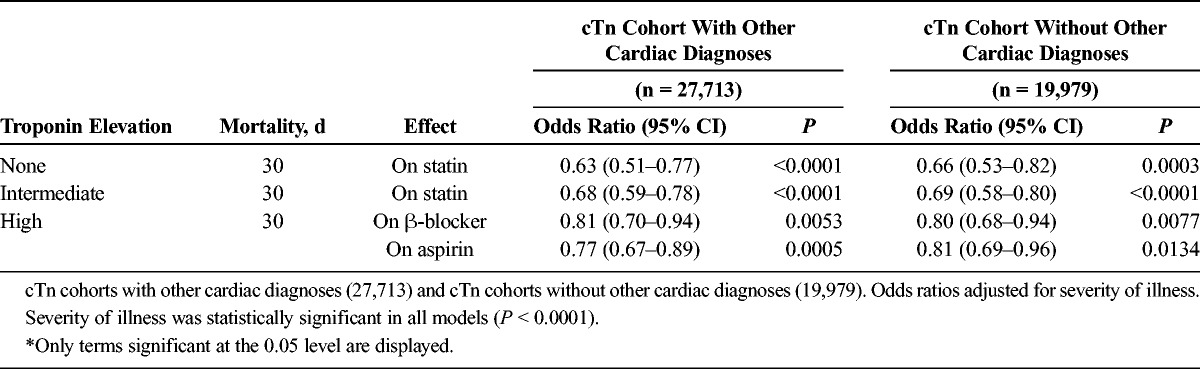
The results from the backward elimination model were similar to the results from the main effects model (Table 5). In patients with no cTn or intermediate cTn elevation, statins alone were associated with lower mortality at 30 days (negative cTn: OR, 0.66; 95% CI, 0.53–0.82; intermediate cTn: OR, 0.69; 95% CI, 0.58–0.80). No relationships between mortality and β-blockers or aspirin were statistically significant for these groups. Patients with high cTn elevation, however, demonstrated an associated reduction in mortality with β-blockers (OR, 0.80; 95% CI, 0.68–0.94) and aspirin (OR, 0.81; 95% CI, 0.69–0.96) at 30 days, with no relationship between mortality and statin use.
TABLE 5.
Effect of Medication Use on Mortality According to cTn Level—Backward Elimination Model
DISCUSSION
The purpose of this investigation was to determine whether treatments administered to patients with cTn elevation in the setting of critical illness are associated with improved short-term mortality. This large retrospective study demonstrated 2 major findings: first, that cTn is associated with 30-day mortality in critically ill patients in the absence of ACS, and second, that statins, aspirin, and β-blockers were associated with decreased mortality in a cTn-dependent manner.
Troponin as an Independent Marker of Mortality
The relationship between cTn and mortality in critically ill patients is not new,1,3,4,8,16–19 but not all published work is in agreement.20 A limitation of studies using cTnI as a marker is that there are many cTnI assay manufacturers. The decision to use the 99th percentile of cTnI or T to consider a sample suggestive of myocardial infarction has been set by consensus,11 but the actual value of cTnI can vary based on the assay used. Cardiac troponin T values, however, are uniform because the assay is produced by a single manufacturer. One strength of this analysis is that cTnI values were normalized based on established cutoffs determined by the assay used, and this provided a large sample of patients within each cTnI range. This work and the majority of published studies support the finding that cTn elevation in critically ill patients is associated with increased mortality. Increasing mortality risk from no cTn elevation to intermediate to high cTn elevation also suggests that cTn may be a continuous marker of mortality risk, as it is in patients with an ACS.21
The timing of cTn assessment may impact the observed result. This analysis used the highest cTn level that occurred on admission to the ICU. In a recent prospective observational study of septic patients, cTnI was highest on the first day of admission and decreased thereafter.22 It is likely that our analysis included the earliest, therefore highest, cTn measurement.
The role of high sensitivity cTnT testing (hs-cTnT) in identifying ICU patients at increased mortality risk is not clear. The high sensitivity assays can detect as little as 10 ng/L of troponin in the serum as opposed to a lower limit of detection of 32 ng/L in contemporary cTnI testing.23 It is expected that high sensitivity testing would identify a larger group of at-risk patients who may benefit from the addition of aspirin, β-blocker, and statin. In a recent prospective observational study, hs-cTnT was measured in 144 consecutive ICU patients.24 Sixty-two patients (43%) had evidence of hs-cTnT elevation without findings of an ACS, a larger group than was found in a previous study using the contemporary assays (19%).16 The non-ACS patients identified with hs-cTnT testing had a 23% risk of dying in the ICU as compared to patients with no hs-cTnT elevation (23 patients, 4.4% risk of ICU mortality). The hs-cTnT investigation, however, did not adjust for severity of illness (cTn levels correlate with severity of illness as found in this investigation) nor exclude for prior cardiac disease (16% of patients with hs-cTnT elevation had known ischemic heart disease). Patients with known ischemic heart disease should already have aspirin, β-blockers, and statin prescribed, as tolerated. It is not clear, therefore, that high sensitivity troponin levels would increase the number of at risk patients identified, or add further refinement to this question. Prospective analyses that exclude for prior ischemic heart disease and adjust for severity of illness will be needed to address whether hs-cTnT adds additional information to identify at-risk patients in the ICU.
Outcome Modification Associated With Medical Therapy
This analysis suggests that measurement of cTn in critically ill patients identifies a treatment pathway associated with reduced 30-day mortality (Fig. 4), although a prospective analysis including all at-risk patients and excluding all potentially confounding cardiac diseases is necessary to confirm this result. Critically ill patients with either no elevation of cTn or those with an intermediate value who are treated with statins had a significantly reduced mortality risk than those patients not treated with statins. In addition, patients with high cTn had lower associated 30-day mortality if they were treated with aspirin, β-blockers, or both, than cTn high patients who were not treated with aspirin or β-blockers. This suggests that cTn measurement not only identifies a mortality risk, but also indicates a potential treatment plan that is associated with reduced 30-day mortality. To our knowledge, this has not been previously reported.
FIGURE 4.
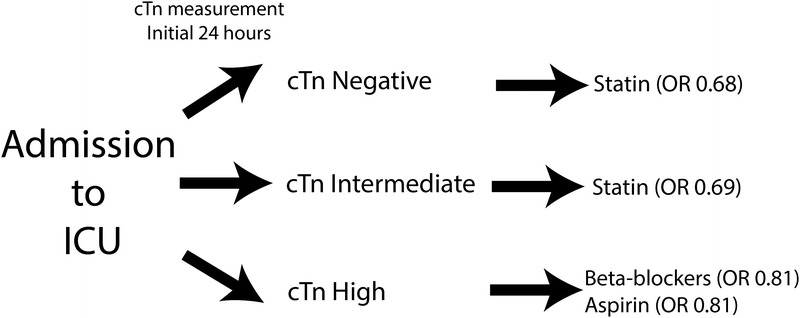
Measurement of cTn in critically ill patients may help guide potentially life-saving therapy.
Our findings are supported indirectly in the literature. A prospective, randomized, open-label study of 154 patients in septic shock showed that the β-blocker esmolol, titrated to keep heart rate between 80 and 94 beats per minute, resulted in an overall survival benefit (HR, 0.392; 95% CI, 0.261–0.590; P < 0.001), after adjustment for severity of illness.25 Although cTn levels were not measured in this study, the Simplified Acute Physiology Score II was 52 in the treatment group, a value that predicts 50% mortality.26 In our investigation, the degree of troponin elevation correlated with severity of illness. The high cTn cohort had a mortality of 31.7%, a severity of illness that was similar, if not more favorable, than the septic shock group of the Morelli et al investigation. Aspirin is also associated with reduced mortality in 2 large retrospective investigations of patients presenting with severe sepsis.27,28 Had cTn levels been measured in these subjects, it is likely that the majority would have fallen into the high cTn group.
A meta-analysis of randomized controlled clinical trials of statins in septic patients,29 and a systematic review that included observational studies,30 provide conflicting results. Observational studies suggest a benefit from statin use, whereas randomized controlled clinical trials show no benefit in severe sepsis. It is possible that patients should first be assessed for cTn, as our data suggests. In a randomized, double blind, placebo controlled study of statin use in septic patients on the ward,31 40 mg of atorvastatin prevented conversion of sepsis to severe sepsis (4% vs 24%, P = 0.007). The mean APACHE II score for this group correlated to an anticipated mortality rate of 15%. This was similar to the cTn none and cTn intermediate groups in our investigation (mortality rates 10.7% and 16.6%, respectively). Statins may therefore benefit a lower-risk group.
That statins may benefit patients who are less severely ill, whereas β-blockers and aspirin may benefit patients who are more severely ill, suggesting different mechanisms of disease process intervention in each group. Unpublished studies from this group suggest microvascular obstruction as a cause for end-organ damage in extremely ill patients. Thus, benefit from aspirin would make sense in that the antiplatelet effect may mitigate microvascular obstruction. The benefits from β-blockers may be largely cardioprotective by reducing cardiac work and myocardial oxygen demand, as is observed in patients presenting with a myocardial infarction.32,33 Significant cTn release indicates myocardial demand in excess of oxygen supply. β-blockers probably assist in reducing myocardial oxygen demand, thus may be cardioprotective. Benefit from statins in less severely ill patients points to intervention in an inflammatory process, before significant end organ damage has occurred.
Lastly, our investigation indicates that medications should be considered in all studies of mortality in critically ill patients, as our data clearly show that medications are associated with outcome modification.
CONCLUSIONS
This large retrospective observational cohort study suggests that elevated cTn in critically ill patients without an ACS is an independent risk factor of 30-day mortality. Using 2 statistical models, our data further show lower 30-day mortality in both cTn-positive or -negative groups, depending on pharmacologic treatment. Patients with no or intermediate cTn elevation had a significantly lower associated risk of death if treated with statins, whereas cTn positive patients had a significantly lower associated risk of death if treated with aspirin and/or β-blockers. The presence of cTn in the blood of critically ill patients may identify populations of patients who benefit from different treatments, and in addition suggests a different pathophysiologic mechanism of increased mortality in each group. This work supports the need for prospective translational studies that investigate the mechanisms of improved mortality in each group.
LIMITATIONS
There are several important limitations to this investigation. This is a retrospective investigation of all patients who happened to get a cTn level drawn during admission. It is possible that some of the patients who had the test drawn may have had some cardiovascular complaint or sign reflecting a higher degree of illness. This is supported by our data. Patients who did not have cTnI measured were less severely ill than patients who had cTnI measured. Addition of patients with a lower severity of illness might impact our finding that statins improved the outcome in patients with no cTn; however, this remains to be shown in a prospective clinical trial. We were only able to assay medication use during the hospital stay. We do not know whether the medications were being taken before admission, or for how long the medications were administered after the cTn assay. We cannot conclude from our work that cTn is a continuous variable. A large prospective clinical trial could clarify this and may also provide cutoff values. Lastly, high sensitivity assays were not available for wide use during the period of data collection. As discussed, hs-cTnT assays may improve the resolution at the lower cTn levels, but this remains to be investigated.
ACKNOWLEDGMENT
The author thanks Dr. Paul Succop for his contributions.
Appendix 1.
Medications

Appendix 2.
Troponin Assays
Appendix 3.
Categorical Variables Used in Risk Adjustment
Appendix 4.
Continuous Variables Used in Risk Adjustment
Footnotes
The contents of this manuscript do not represent the view of the Department of Veterans Affairs or the U.S. Government. This material is the result of work supported by use of facilities at the VA Inpatient Evaluation Center, and fulfilled partial requirements for a PhD (Dr. Poe) in the Department of Environmental Health at the University of Cincinnati.
REFERENCES
- 1. Ammann P, Maggiorini M, Bertel O, et al. Troponin as a risk factor for mortality in critically ill patients without acute coronary syndromes. J Am Coll Cardiol. 2003; 41: 2004– 2009. [DOI] [PubMed] [Google Scholar]
- 2. John J, Woodward DB, Wang Y, et al. Troponin-I as a prognosticator of mortality in severe sepsis patients. J Crit Care. 2010; 25: 270– 275. [DOI] [PubMed] [Google Scholar]
- 3. Reynolds T, Cecconi M, Collinson P, et al. Raised serum cardiac troponin I concentrations predict hospital mortality in intensive care unit patients. Br J Anaesth. 2012; 109: 219– 224. [DOI] [PubMed] [Google Scholar]
- 4. Stein R, Gupta B, Agarwal S, et al. Prognostic implications of normal (<0.10 ng/ml) and borderline (0.10 to 1.49 ng/ml) troponin elevation levels in critically ill patients without acute coronary syndrome. Am J Cardiol. 2008; 102: 509– 512. [DOI] [PubMed] [Google Scholar]
- 5. Vasile VC, Chai HS, Abdeldayem D, et al. Elevated cardiac troponin T levels in critically ill patients with sepsis. Am J Med. 2013; 126: 1114– 1121. [DOI] [PubMed] [Google Scholar]
- 6. Ammann P, Fehr T, Minder EI, et al. Elevation of troponin I in sepsis and septic shock. Intensive Care Med. 2001; 27: 965– 969. [DOI] [PubMed] [Google Scholar]
- 7. Martin M, Mullenix P, Rhee P, et al. Troponin increases in the critically injured patient: mechanical trauma or physiologic stress? J Trauma. 2005; 59: 1086– 1091. [DOI] [PubMed] [Google Scholar]
- 8. Hassan B, Morsy S, Siam A, et al. Myocardial injury in critically ill children: a case control study. ISRN Cardiol. 2014; 2014: 919150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamilton MA, Toner A, Cecconi M. Troponin in critically ill patients. Minerva Anestesiol. 2012; 78: 1039– 1045. [PubMed] [Google Scholar]
- 10. Alpert JS, Thygesen KA, White HD, et al. Diagnostic and therapeutic implications of type 2 myocardial infarction: review and commentary. Am J Med. 2014; 127: 105– 108. [DOI] [PubMed] [Google Scholar]
- 11. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012; 60: 1581– 1598. [DOI] [PubMed] [Google Scholar]
- 12. Render ML, Welsh DE, Kollef M, et al. Automated computerized intensive care unit severity of illness measure in the Department of Veterans Affairs: preliminary results. SISVistA Investigators. Scrutiny of ICU Severity Veterans Health Systems Technology Architecture. Crit Care Med. 2000; 28: 3540– 3546. [DOI] [PubMed] [Google Scholar]
- 13. Render ML, Kim HM, Welsh DE, et al. ; VA ICU Project (VIP) Investigators. Automated intensive care unit risk adjustment: results from a National Veterans Affairs study. Crit Care Med. 2003; 31: 1638– 1646. [DOI] [PubMed] [Google Scholar]
- 14. Render ML, Kim HM, Deddens J, et al. Variation in outcomes in Veterans Affairs intensive care units with a computerized severity measure. Crit Care Med. 2005; 33: 930– 939. [DOI] [PubMed] [Google Scholar]
- 15. Render ML, Deddens J, Freyberg R, et al. Veterans Affairs intensive care unit risk adjustment model: validation, updating, recalibration. Crit Care Med. 2008; 36: 1031– 1042. [DOI] [PubMed] [Google Scholar]
- 16. Lim W, Cook DJ, Griffith LE, et al. Elevated cardiac troponin levels in critically ill patients: prevalence, incidence, and outcomes. Am J Crit Care. 2006; 15: 280– 288. [PubMed] [Google Scholar]
- 17. Babuin L, Vasile VC, Rio Perez JA, et al. Elevated cardiac troponin is an independent risk factor for short- and long-term mortality in medical intensive care unit patients. Crit Care Med. 2008; 36: 759– 765. [DOI] [PubMed] [Google Scholar]
- 18. Salim A, Hadjizacharia P, Brown C, et al. Significance of troponin elevation after severe traumatic brain injury. J Trauma. 2008; 64: 46– 52. [DOI] [PubMed] [Google Scholar]
- 19. Vasile VC, Chai HS, Khambatta S, et al. Significance of elevated cardiac troponin T levels in critically ill patients with acute respiratory disease. Am J Med. 2010; 123: 1049– 1058. [DOI] [PubMed] [Google Scholar]
- 20. Tiruvoipati R, Sultana N, Lewis D. Cardiac troponin I does not independently predict mortality in critically ill patients with severe sepsis. Emerg Med Australas. 2012; 24: 151– 158. [DOI] [PubMed] [Google Scholar]
- 21. Antman EM, Tanasijevic MJ, Thompson B, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996; 335: 1342– 1349. [DOI] [PubMed] [Google Scholar]
- 22. Klouche K, Jonquet O, Cristol JP. The diagnostic challenge of myocardial infarction in critically ill patients: do high-sensitivity troponin measurements add more clarity or more confusion? Crit Care. 2014; 18: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Lemos JA. Increasingly sensitive assays for cardiac troponins: a review. JAMA. 2013; 309: 2262– 2269. [DOI] [PubMed] [Google Scholar]
- 24. Ostermann M, Forni LG. Measuring biomarkers of acute kidney injury during renal replacement therapy: wisdom or folly? Crit Care. 2014; 18: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morelli A, Ertmer C, Westphal M, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013; 310: 1683– 1691. [DOI] [PubMed] [Google Scholar]
- 26. Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993; 270: 2957– 2963. [DOI] [PubMed] [Google Scholar]
- 27. Otto GP, Sossdorf M, Boettel J, et al. Effects of low-dose acetylsalicylic acid and atherosclerotic vascular diseases on the outcome in patients with severe sepsis or septic shock. Platelets. 2013; 24: 480– 485. [DOI] [PubMed] [Google Scholar]
- 28. Sossdorf M, Otto GP, Boettel J, et al. Benefit of low-dose aspirin and non-steroidal anti-inflammatory drugs in septic patients. Crit Care. 2013; 17: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomas G, Hraiech S, Loundou A, et al. Statin therapy in critically-ill patients with severe sepsis: a review and meta-analysis of randomized clinical trials. Minerva Anestesiol. 2015; 81: 921– 930. [PubMed] [Google Scholar]
- 30. Tralhão AF, Cés de Souza-Dantas V, Salluh JI, Póvoa PM. Impact of statins in outcomes of septic patients: a systematic review. Postgrad Med. 2014; 126: 45– 58. [DOI] [PubMed] [Google Scholar]
- 31. Patel JM, Snaith C, Thickett DR, et al. Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (ASEPSIS Trial). Crit Care. 2012; 16: R231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions, O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013; 61: e78– e140. [DOI] [PubMed] [Google Scholar]
- 33.2012 Writing Committee Members, Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/Non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2012; 126: 875– 910. [DOI] [PubMed] [Google Scholar]


