Abstract
Aims
In atrial fibrillation (AF), abnormalities in Ca2+ release contribute to arrhythmia generation and contractile dysfunction. We explore whether ryanodine receptor (RyR) cluster ultrastructure is altered and is associated with functional abnormalities in AF.
Methods and results
Using high-resolution confocal microscopy (STED), we examined RyR cluster morphology in fixed atrial myocytes from sheep with persistent AF (N = 6) and control (Ctrl; N = 6) animals. RyR clusters on average contained 15 contiguous RyRs; this did not differ between AF and Ctrl. However, the distance between clusters was significantly reduced in AF (288 ± 12 vs. 376 ± 17 nm). When RyR clusters were grouped into Ca2+ release units (CRUs), i.e. clusters separated by <150 nm, CRUs in AF had more clusters (3.43 ± 0.10 vs. 2.95 ± 0.02 in Ctrl), which were more dispersed. Furthermore, in AF cells, more RyR clusters were found between Z lines. In parallel experiments, Ca2+ sparks were monitored in live permeabilized myocytes. In AF, myocytes had >50% higher spark frequency with increased spark time to peak (TTP) and duration, and a higher incidence of macrosparks. A computational model of the CRU was used to simulate the morphological alterations observed in AF cells. Increasing cluster fragmentation to the level observed in AF cells caused the observed changes, i.e. higher spark frequency, increased TTP and duration; RyR clusters dispersed between Z-lines increased the occurrence of macrosparks.
Conclusion
In persistent AF, ultrastructural reorganization of RyR clusters within CRUs is associated with overactive Ca2+ release, increasing the likelihood of propagating Ca2+ release.
Keywords: Atrial fibrillation, Atrial myocytes, Sarcoplasmic reticulum, Ryanodine receptor, Super-resolution microscopy
1. Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia.1 The resultant loss of atrial contraction leads to atrial thrombi; embolization and stroke are major causes of morbidity and mortality. In persistent AF, antiarrhythmic drug and catheter ablation therapies are less effective.2 Further understanding of the cellular processes involved in the generation and maintenance of AF may help to identify novel targets for antiarrhythmic treatment.3
Recently, evidence has emerged for a major role of dysregulation of ryanodine receptor (RyR) function and Ca2+ release from the sarcoplasmic reticulum (SR). An increase in RyR activity, attributed to an up-regulation of CaMKII, was reported in patients with persistent AF.4,5 This may lead to an increased propensity for spontaneous Ca2+ waves and subsequent activation of NCX. An up-regulation of NCX was also reported and this, coupled with the spontaneous release, led to membrane depolarization producing delayed afterdepolarizations.5 This agrees with the observation of an earlier study showing an increased frequency of spontaneous Ca2+ waves and sparks in patients with AF.6 While changes in RyR phosphorylation have been emphasized, reduced expression has also been reported.7 However, there is no data on a possible change in the organization of RyR. Data from animal models similarly highlight a role for altered RyR function in AF, though studies in permanent and persistent AF are limited. In the dog with heart failure and with AF, abnormal Ca2+ release has been reported as well as increased RyR phosphorylation.8 In sheep with persistent AF, T-tubule re-organization was evident, but we also found that RyR expression was reduced, although confocal immunofluorescence imaging revealed no change in RyR distribution and density.9 This hints at an underlying remodelling of RyR ultrastructural organization.
RyR clusters have previously been studied using electron microscopy of freeze fracture in thin slice preparations of skeletal and cardiac tissue. From these measurements, RyR clusters were shown as contiguous crystalline arrays of single RyR molecules. Recently developed methods allow the measurement of intracellular structures based on immunostaining in myocytes, below the traditional fluorescence resolution limits, the so-called super-resolution microscopy, including STED (STimulated Emission Depletion) and dSTORM (direct Stochastic Optical Reconstruction Microscopy).10–12 Study of RyR clusters in ventricular cardiac myocytes has shown that RyR clusters have a higher, though fragmented organization, with small and large clusters grouped together in formations termed ‘superclusters’.13,14 These may act together to release Ca2+, observed as a Ca2+ spark, and such grouping of RyRs is also referred to as a Ca2+ release unit (CRU).13,15,16 In the case of atrial cells as studied here, these are frequently not coupled with the sarcolemma.9,17 Modelling has revealed that RyR cluster behaviour may relate to their size; small clusters are proposed to have lower levels of allosteric regulation via coupled gating and so fire more readily, contributing more to diastolic release.16,18 Similarly, preferential inhibition of small RyR clusters has highlighted their involvement in the initiation of spontaneous Ca2+ waves.19 In this study, it was hypothesized that small clusters would act to relay Ca2+ release to neighbouring clusters and that this was particularly important in gaining critical mass for the initiation of a Ca2+ wave. It is conceivable that abnormal fragmentation of RyR clusters within CRUs may assist in the process of inter-cluster activation which initiates Ca2+ waves, but so far this has not been studied.
In the present study, we have investigated RyR cluster morphology in an established sheep model of persistent AF.9,20 To this end, we carried out super-resolution measurements of RyR, to explore cluster size, shape, and fragmentation using STED microscopy. We defined functional CRUs as a group of clusters within 150 nm distance of each other, allowing cluster–cluster interaction.13,15 In the same cells, we measured subcellular Ca2+ release to assess the functional changes that occur in AF. We use computational modelling to predict the impact of the altered organization of RyR clusters and CRUs on Ca2+ release, comparing predictions to observations of spark properties. We demonstrate that CRU morphology is a crucial determinant of the diastolic Ca2+ release process, which may further potentiate aberrant Ca2+ release in persistent AF.
2. Methods
A detailed description is available in the Supplementary material online.
The sheep model and atrial myocyte isolation were as described before.9
For STED microscopy, myocytes were fixed immediately after isolation in 2% paraformaldehyde.
Fixed myocytes were labelled with a primary RyR2 antibody and Alexa 647N secondary. Samples were imaged on a custom-built STED microscope21 (see Supplementary material online, Figure S1) and analysed using custom software written in Python.
Ca2+ sparks were measured in permeabilized myocytes perfused with a mock intracellular solution containing Fluo-3 (20 µmol/L) with a free Ca2+ of 150 nmol/L. Ca2+ sparks were analysed using custom software based on the Cheng algorithm.22
Computer modelling of Ca2+ release was based on a modified version of a previous model23 using an Ultrafast Monte Carlo method, with the inclusion of localized RyR Ca2+-sensing domain.
Data are presented as mean ± SEM for RyR clusters and otherwise as scatter plots for individual cells. To include both N animals and n cells in the analysis, a hierarchical method was used.
3. Results
3.1. Near single RyR resolution by STED microscopy
Images of sub-resolution (20 nm) crimson beads were collected. The average of three aligned beads was calculated, and the profile was fitted with a Gaussian function to assess lateral resolution (Figure 1A). Given RyR size (30 nm), this translates to a potential resolution of 1–2 individual RyR tetramers. Figure 1B shows a typical myocyte imaged using confocal microscopy. Figure 1C–F shows the image of a 3-sarcomere-wide region, with RyRs visible along the Z-lines. Improvements in resolution are evident from conventional confocal (C) to the STED (D) and then deconvolved STED images (E). After deconvolution, automated thresholding was possible (F). Figure 1G shows a typical image of 10 clusters of contiguous RyRs resolved by this method, illustrating the highly variable size and shape. Cluster images were used as a mask, and the approximate RyR number that could fit into each cluster was calculated using a grid of RyR sized units (30 × 30 nm) (Figure 1H). Given the limits of resolution, this method had limited utility if clusters had less than two RyRs; therefore, the number of RyRs per cluster will be stated as approximate.
Figure 1.
Deconvolved STED microscopy resolves RyR sub-cluster formations in atrial myocytes. (A) Average of the same three fluorescent beads aligned on their peaks from confocal (i) and STED (ii) recordings, allowing a ∼4–6× improvement in resolution. (B) RyR antibody labelling in an atrial myocyte visualized using confocal microscopy. (C–F) Optical and software-based methods used to allow RyR cluster resolution in a region of an atrial myocyte (i), with further zoom in of the region outlined in red (ii). (C) Conventional confocal image; (D) the raw STED image; (E) after deconvolution noise is reduced with more defined edges of each sub-cluster; (F) RyR clusters are thresholded to allow morphology quantification. (G) Individual colours delineate 10 clusters taken from F (ii). (H) Method for RyR cluster size quantification: a grid of single RyRs (blue squares) are superimposed on the thresholded image. Scale bars: (B) 5 µm; (Ci–Fi) 250 nm; (Cii–Fii)100 nm.
3.2. Ryr cluster size is unaltered in Ctrl and AF
There were no immediately apparent differences in RyR clusters of Ctrl and AF myocytes (Figure 2A and B). To ascertain whether RyR clusters were different in AF, pertinent metrics of morphology were developed and examined in 14 myocytes from 4 animals in each group. Mean cluster size was similar, 15.4 ± 0.4 RyRs per cluster vs. 15.3 ± 0.6 (nclusters = 2581 and 1261 in Ctrl and AF, respectively, Figure 2C). The distribution of RyRs as a function of cluster size is shown in Figure 2D. The relationship is well fit with the sum of an exponential for small clusters and a Gaussian function for larger clusters (R2 = 0.60 in Ctrl and 0.59 in AF). This indicates that small and large RyR clusters belong to two sub-populations. The Gaussian peak at ∼50 RyR indicates that of the larger population, this is the most common cluster size in both AF and Ctrl. The cumulative histogram of RyR cluster size (Figure 2E) shows that most RyRs reside in small clusters, with over half of all RyR residing in clusters containing six RyRs or less. Assessment of the proximity between individual RyR clusters (Figure 2F) shows that clusters are closer together in AF. Mean centroid distance was reduced by 23% in AF.
Figure 2.
Quantification of RyR cluster size. (A and B) Typical deconvolved STED (i) and thresholded STED (ii) from Ctrl and AF cells; scale bars: upper panel 500 nm, lower 200 nm. (C) Mean RyR cluster size; nclusters = 2581 and 1261, ncells = 14 and 14 in 4 Ctrl and 4 AF animals, respectively. (D) Distribution histogram of RyRs (fraction of the total number of RyRs) as a function of RyR cluster size in Ctrl (black) and AF (red). The two distributions have been fit with the sum of an exponential and Gaussian distribution; right panel with bin size 10. (E) Cumulative histogram of the distribution of RyRs according to cluster size (same data as in D). The majority of RyRs reside in clusters containing <6 RyR. (F) Mean nearest neighbour distance between individual RyR clusters in Ctrl and AF, quantified by distances between the centre of each cluster (***P = 0.006). Data are mean ± SEM.
3.3. Organization of CRUs is different in AF
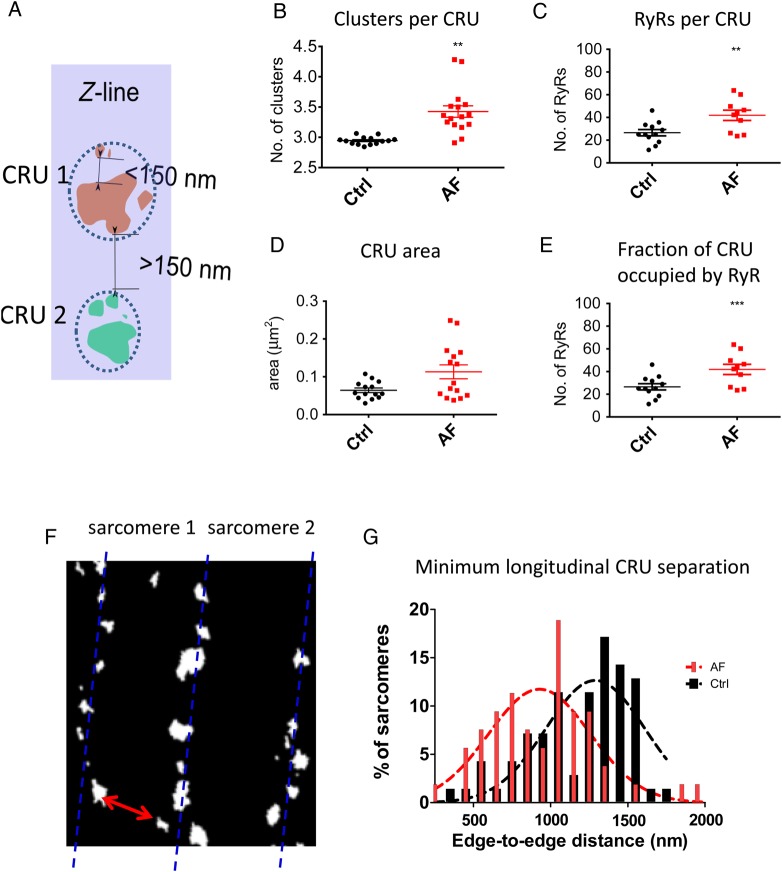
Experimental work has shown RyRs within clusters interact via coupled gating.24 Theoretical work has shown this would mean that small clusters behave differently18 and may have a role in the generation of Ca2+ waves.19,25 It has been speculated that clusters that are close together will also interact to trigger release if they are within 100 nm of each other, forming functional release units.13 Our modelling (below) indicated that a distance of ≤150 nm yields a high probability of cluster–cluster interaction; therefore, RyR clusters were grouped when edge-to-edge distances were ≤150 nm (Figure 3A). This enables additional quantification of CRU organization, relevant to their function. Using these grouping criteria, Figure 3B–D shows that CRUs are larger in AF with more clusters in a CRU and a higher total number of RyRs.
Figure 3.
Quantification of alterations of CRU morphology and separation in AF. (A) Criteria for cluster grouping within CRUs defined as functionally grouped clusters if within the 150 nm edge to edge of each other (shown all of similar colour). (B) Mean number of clusters per CRU (n cells and sheep as in Figure 2, **P = 0.015). (C) Number of individual RyRs per CRU (**P = 0.0015). (D) The area occupied by a CRU within minimal bounding polygon (see Supplementary material online, P = 0.14). (E) The fraction of CRU occupied by RyR was quantified as a ratio of RyR : total area per CRU, which is inversely related to the degree of CRU fragmentation (***P = 0.0001). (F) Quantification of the minimum separation of RyRs in the longitudinal direction (red arrow as an example of measured distances). (G) Histogram of the minimum separation in the longitudinal direction showing that in AF more sarcomeres have CRUs that are closer together in the longitudinal direction.
To assess the internal organization within a CRU, the ratio of cluster filled with RyR to the total CRU area was calculated (Figure 3E). CRU's in AF were less filled with RyR indicating a higher degree of fragmentation in AF.
3.4. A high proportion of RyR clusters are not along the Z-line in AF
The initiation of Ca2+ waves is highly sensitive to longitudinal sarcomeric CRU separation which may be reduced in hypertrophy.26 No such alteration in sarcomere length was observed here, but the presence of RyR clusters between Z-lines could facilitate propagation. To investigate this, the minimum separation of large (>10 RyR) clusters in the longitudinal axis was quantified (Figure 3F). Small clusters (<6 RyR) were excluded on the basis that their Ca2+ release would only allow propagation over a short distance (100–150 nm). A histogram of minimum RyR cluster separation in the longitudinal axis (Figure 3G) shows that many more clusters are <1 µm apart in AF; the median of the histogram is 920 nm in AF vs. 1300 nm in Ctrl. Similarly, in AF a larger proportion (11.0 ± 0.1 vs. 5.7 ± 0.9% in Ctrl) of RyR clusters were found between Z-lines (see Supplementary material online, Figure S2). This shows that dispersion of RyR clusters away from the Z-line is more pronounced in AF, narrowing the distance Ca2+ needs to diffuse across the sarcomere to activate neighbouring CRUs.
3.5. Functional alterations in spark frequency and kinetics in AF
To assess RyR cluster function, freshly isolated cells were permeabilized and perfused with a mock intracellular solution to enable the observation of spontaneous sparks (Figure 4A).
Figure 4.
More frequent Ca2+ sparks, with slowed kinetics in permeabilized AF myocytes. (A) Examples of line scan images of spark recording in Ctrl (i) and AF (ii); rectangles highlight macrosparks. (B) Examples of two types of macrosparks in AF: multisite (i, from left box in Aii) and single site (ii, from the right box in Aii); macrospark incidence was increased in AF (iii). (C) Mean spark parameters show increase in spark frequency (i, ****P = 0.045), a reduced width (ii, ***P = 0.001), a longer spark duration (iii, ***P = 0.0001), and time to peak (TTP, iv, ***P = 0.004) in AF. Ncells = 26 and 31 in 5 Ctrl and 6 AF animals, respectively. (Di) Average linescans of all sparks in a typical Ctrl and AF cell (N = 179 and 258) with their temporal and spatial profiles from the central three pixels (lines on left indicate regions taken). Scale bar = 10 µm and 100 ms in (A); 5 µm and 50 ms in (B); 20 ms and 2 µm in (Di), 0.2 ΔF/F0 and 20 ms in (Dii); 0.2 ΔF/F0 and 2 µm in (Diii).
Most notably, macrosparks and double release sparks were observed in AF, but not in Ctrl (Figure 4B). Single Ca2+ spark properties were also different (Figure 4C). Frequency was markedly higher in AF (Ci) and width was lower (Cii), implying a smaller Ca2+ flux per release event on average. Differences in spark kinetics were also evident; duration (Ciii) and time to peak (Civ) were prolonged in AF, by 15 and 18%, respectively, and illustrated by example (Figure 4D). However, SR content in permeabilized myocytes was not different (see Supplementary material online, Figure S3).
3.6. Co-activation of RyR clusters within a functional CRU
A stochastic model of RyR was used to assess how the organization of RyRs affects the function of individual clusters and their interaction within functional CRUs. Here a temporal and spatial model of Ca2+ sparks with single channel kinetics was used, based on a previous model,27 adapted to the permeabilized atrial myocyte (see Supplementary material online). In a first simulation, the probability of one small cluster (5 RyRs) being triggered by the opening of a larger neighbouring cluster (49 RyRs) was assessed (Figure 5A). A pseudo-linescan image of the total Ca2+ (Figure 5Bi) highlights a mild asymmetry on the lower side of the image due to the activation of the small cluster. This is indiscernible on the resulting F/Fo image (Figure 5Bii), but is visible in spatial profiles of total Ca2+ (Figure 5Ci) and fluorescence (Figure 5Cii) at the time points indicated. Given this occurs after the peak of the main release, it would be undetectable by traditional spark analysis. The key finding from this simulation was the relationship describing the probability of small clusters being triggered (Ptrigger) as a function of their distance from the main cluster (Figure 5Di). The relatively steep drop at ∼150 nm from 0.937 to 0.315 indicates that small clusters ≥150 nm away are unlikely to be influenced by release from the main cluster. This demarcates an effective 150 nm sphere of influence of this mode of activation. The delay in activation of the small clusters (Figure 5Dii) was negligible (<2 ms) for distances ≤150 nm.
Figure 5.
Computational modelling of intra-CRU RyR interaction. (A) Schematic of model for simulation: release from one large RyR cluster within the CRU can activate the smaller RyR cluster by the diffusion of released Ca2+ from the larger cluster (depicted by the red arrows); the 49 RyR large cluster is 200 nm edge to edge away from the 5 RyR cluster. (B) Simulated linescan image of total Ca2+ (i): A smaller, more prolonged release from the small cluster is visible, after activation by Ca2+ released from the main cluster; however, this is not visible on the simulated linescan F/F0 image (ii). (C) Spatial profiles of total Ca2+ (i) and F/F0 (ii) at different time points after peak release (inset). Cluster interaction is only evident as asymmetry in the total Ca2+ plot at −0.1 and −0.2 distances. (D) Probability of release from a small cluster being triggered (Ptrigger) as function of the distance from the larger site (i) and the corresponding delay of activation (ii). There is a high likelihood of activation with <2 ms delay if small clusters are ≤150 nm away from a larger one. (E) Schematic of model for simulation: synchronous release from small (5 RyR) clusters can activate a larger (25 RyR) cluster 100 nm away by the diffusion of released Ca2+ (red arrows). (F) Simulated linescan images of total Ca2+ (i) and resultant F/F0 (ii). (G) Spatial profiles of total Ca2+ (i) and F/F0 (ii) at times indicated after the peak of release. (H) Simulated probability of triggering a central cluster with increasing numbers of satellite RyR clusters. Dotted lines indicate experimentally observed numbers of satellites in Ctrl (black) and AF (red). Scale bars: (Bi and Fi): 200 nm, 5 ms; (Bii and Fii): 500 nm, 20 ms.
3.7. Spark probability is increased in more fragmented CRUs
The probability of small clusters within a CRU to trigger larger neighbouring clusters (Ptrigger) was further investigated. A varying number of small (5 RyR) clusters were set to open simultaneously, the Ca2+ released then diffused to the larger cluster (25 RyR), triggering activation (Figure 5E). Simulated pseudo-linescans of total Ca2+ and ΔF/F0 from a two satellite-triggered release show a higher amplitude, longer duration release (Figure 5F) even more visible on the temporal profiles (Figure 5G), with F/Fo values remaining high 5 ms after the peak release, in contrast to the more rapid fall shown in Cii (compare traces at 10 ms).
In AF, cells showed increased fragmentation of CRUs. To explore the functional implications of this, the number of satellite clusters around a large cluster was increased. The probability of triggering release from the larger cluster by these satellite clusters had a very steep dependence on the number of satellite clusters (Figure 5H). In AF, the number of satellites increased from 2 to 2.5 (3 to 3.5 clusters per CRU); combined with the reduced buffering found in AF (see Supplementary material online, Figure S3), this increased the likelihood of release triggered in this way by ∼75% (dotted lines in Figure 5H), approximating the increase in spark frequency observed experimentally.
Modelling shows the spark frequency of small clusters to be comparatively higher (see Supplementary material online, Figure S5), thereby increasing the likelihood of all satellites synchronizing, activating release. Nevertheless, the 75% increase may represent more of an upper limit as satellites may be >100 nm away. Even given these caveats, higher CRU fragmentation and reduced buffering would both facilitate intra-cluster interaction, leading to an increased spontaneous spark rate.
3.8. Modelling sarcomeric Ca2+propagation
One notable difference in AF was the appearance of release events propagating across sarcomeres, macrosparks. Shorter sarcomere lengths can greatly increase the probability of this type of propagating release,11 but this was not observed here. However, higher incidence of clusters between Z-lines (see Supplementary material online, Figure S4) and shorter distance between clusters in the longitudinal axis (Figure 3F) may have similar effects.
To investigate this, we simulated four clusters with varying separation (Figure 6A, 400–700 nm). These clusters with 25 RyRs each had three small (5 RyR) clusters 100 nm apart, simulating CRU geometry in AF. With this configuration, the probability of propagation between the clusters was 100% when 400 nm apart, (Figure 6D). This fell rapidly as clusters were moved further than 500 nm apart but allowed for formation of macrosparks, as simulated in Figure 6B and C where four clusters were placed 600 nm apart. Triggering the two central clusters allowed propagation (with a ∼6% chance). The F/F0 image of this event had a FWHM of ∼5 µm, similar to that observed experimentally. This simulation highlights how RyR cluster dispersion could lead to the genesis of macrosparks by providing a relay system to allow propagation across sarcomeres.
Figure 6.
Simulation of neighbouring cluster activation during a macrospark. (A) Schematic of the model: four clusters, each with one central 25 RyR cluster and three clusters with 5 RyR, placed at variable edge-to-edge distances, from 400 to 700 nm. (B) Simulated linescan image of a macrospark event. Four clusters were placed 600 nm apart; the central release site from one was activated, releasing Ca2+ that diffused to raise Ca2+ local to the neighbouring site, triggering its release. Resultant total Ca2+(i) and F/F0 linescan images (ii) from this simulation are shown. (C) Spatial profiles of the ΔF/F0 and total Ca2+ are depicted at the timescales indicated. (D) Probability of propagation between adjacent clusters, analogous to the probability of macrospark formation, as a function of the longitudinal separation between them. Scale bars in B: 2 µm vertical (i and ii), 5 ms (i) and 20 ms (ii) horizontal.
4. Discussion
Using STED microscopy, RyR cluster morphology in atrial myocytes could be analysed in detail, revealing fragmentation of CRUs in persistent AF. This nanoscale remodelling impacts on unitary Ca2+ signalling, as shown experimentally and through simulation.
4.1. Ryr cluster organization in atrial cells and remodelling with AF
STED microscopy allowed us to visualize immunostained RyR clusters at a resolution of around 60 nm; deconvolution allowed further improvements in image resolution and quality. Similar methods have recently been used to resolve T-tubule structures in living ventricular myocytes.28 This compares somewhat less favourably to the resolution of 20–30 nm obtained using STORM.12 STED offers the advantage of allowing access to the centre of the cell, with reduced acquisition time, and is less prone to artefacts of densely stained samples; however, the experimental setup is more complex.
In their pioneering work using STORM imaging, Baddeley et al.13 reported on RyR organization in peripheral clusters close to the membrane surface. The assumption was that sampling of peripheral clusters implied a more flat geometry, therefore facilitating measurement of complete clusters. As we are sampling more at the centre, this assumption cannot be made. Given the axial resolution of STED, we can assume we are actually sampling from a layer of ∼0.5 µm. Despite these differences in technique, in depth within the cell, in species and cardiac region, there is a remarkable similarity in the ultrastructural properties of cluster size and organization between the two studies. Likewise we find that on average there are ∼15 RyRs per cluster, but with a broad distribution of cluster sizes. This similarity between studies is surprising, but may highlight a common mechanism governing the process of cluster formation. Further analysis in the current study suggests two sub-populations of RyR clusters. The most numerous consists of small clusters containing six or less RyRs; the second has a broader distribution with predominance at ∼50 RyR clusters. This value is slightly higher than a recent 3-D analysis of Ca2+ sparks in cat atrial myocytes, which calculated the flux produced from typical sparks originated from 20–30 RyRs,29 but lower than the ∼80 RyRs estimated from deconvolved confocal images30 or ∼63 RyRs using dSTORM14 in rat ventricular myocytes. This disparity between the studies may be due to incomplete activation of all RyR clusters per CRU during each spark event. However, species differences in CRU morphology cannot be discounted.
Remarkably, the primary assembly of RyR clusters appears to be unaltered in AF, as cluster size was unchanged. However, the organization of the individual clusters into functional CRUs is different. In AF, there are more RyRs per CRU, yet within CRUs these clusters are less densely packed, resulting in a larger CRU area with a more fragmented CRU morphology. Of note, total RyR protein expression levels are slightly lower9 (see Supplementary material online, Figure S6), which could contribute to more dispersed clusters. It is tempting to speculate that changes in CRU morphology may relate to the fragmentation of the T- and axial tubular (TAT) structure in AF9 or atria from heart failure.17 Proteins associated with RyR such as junctophilin-2 (JPH2) and more recently BIN-131 have been implicated in the TAT organization,32,33 and it is conceivable that these play a role in the secondary RyR organization. Low levels of JPH2 have been implicated in AF.34 Alternatively, alterations in SR geometry may occur in AF and affect RyR cluster formation. SR geometry changes have been reported in ventricular myocytes from mice with genetically altered SR proteins.35,36 This could also have implications for sparks properties by altering SR Ca2+ depletion rates.37 Further study is required to assess these processes.
4.2. Cluster re-organization leads to increased RyR release activity
In the current study, functional properties of RyR were measured in permeabilized cells at 150 nmol/L Ca2+. The permeabilized cell preparation allows a high degree of control over the intracellular environment without confounding factors of surface membrane transport, sub-membrane Ca2+ microdomains, or CaMKII activation.38 It is particularly suited to study the properties of RyR clusters during direct activation by the prevailing Ca2+ while preserving cluster structure and interaction, as opposed to bilayer experiments. Our findings in AF myocytes of an elevated spark frequency and high occurrence of locally propagating Ca2+ release events (macrosparks) therefore can be most likely attributed to the changes in RyR cluster organization. Computational modelling supports this hypothesis.
A major element appears to be the close proximity of multiple small clusters within a CRU which could drastically increase the probability of a CRU to fire. Indeed, our modelling suggests that over half of all sparks may be due to triggering by smaller clusters. RyR cluster size has been predicted to affect release; coupled gating and allosteric interaction via FKBP12.6 may regulate the probability of RyR cluster opening.24 A configuration with more small clusters per CRU may underlie repetitive or hyperactive spark sites.39–41 However, recent evidence has emphasized that local Ca2+ release causing CICR from neighbours within each cluster is the prime determinant of RyR behaviour, influencing Ca2+ spark frequency and release duration.42 Thus, although the mode of interaction is presently under debate, the consensus is that RyRs in close proximity interact and influence CRU behaviour.
Previous modelling and experimental data have suggested that small RyR clusters could underlie non-spark-mediated loss of Ca2+ in the regulation of SR content18,43 and participate in the initiation of spontaneous Ca2+ waves.19 The current modelling and experimental data indicate that the presence of more small RyR clusters within CRUs also contributes to Ca2+ sparks, leading to a higher Ca2+ spark rate in AF compared with Ctrl. Primarily this is due to smaller clusters within the functional group opening (>20×) more often than larger clusters. When these cluster openings synchronize, they can trigger release from clusters close by. In AF, this fragmented CRU geometry is more prevalent, given that the mean number of clusters per CRU increases from 3 to ∼3.5.
Sarcomeres with clusters bridging the sarcomeric separation and in close proximity of each other were more common in AF. Using a simplified model for cross-sarcomere propagation, this predicted a high probability of propagation. Bridging of the sarcomere through clusters away from the Z-line could thus explain the higher macrospark frequency observed. For a macrospark, recruitment of >2 clusters <700 nm apart would be required. Since minimum edge-to-edge sarcomeric distances were around this order in AF, this form of secondary recruitment would have a high likelihood. Prolonged or slow sparks have been described recently in a murine model of heart failure and were hypothesized to occur due to more subdivision in each CRU.44 The current study finds more striking structural and functional measurements highlighting the relevance of CRU remodelling to cardiac disease.
4.3. Relevance of changes in RyR cluster structure and function to the pathology of AF
Dysregulation of Ca2+ release and RyR, observed as increased spark activity and Ca2+ waves in patients with AF,4–6 are considered contributing factors in AF. Altered calcium release has been attributed to secondary modification of RyR function through CaMKII-dependent phosphorylation;45 oxidation or nitrosylation, described in heart failure,46,47 may also be present. The current study demonstrates that remodelling at the nanoscale level may be an additional factor. The relative importance for RyR activity and AF in vivo is at present difficult to gauge as there are no tools that allow a direct intervention changing cluster organization/size and evaluating the impact on AF in vivo, as opposed to the pharmacological tools, or genetic tools in mice, that can be applied to alter phosphorylation.
An increased spark probability due to ultrastructural changes may induce a positive feedback loop of CaMKII activation in the vicinity of increased release activity. Additional phosphorylation or oxidation processes would sensitize the fragmented clusters to prevailing Ca2+. Since smaller RyR clusters are proposed to be more sensitive to phosphorylation processes,18 it is likely that indeed organization at the nanoscale level and functional modifications will interact in facilitating abnormal Ca2+ release in AF.
Reduced cytosolic buffering in AF would facilitate free diffusion of released Ca2+ allowing activation of adjacent CRUs. This form of inter-CRU recruitment may play an important role in the recruitment of adjacent sites in physiological Ca2+ waves, which occur as part of the E–C coupling process in the atria. In persistent AF, this would infer an increase in the effective gain of the Ca2+ release system. This effective increase in gain and reduced cellular buffering may partially compensate for the reduced L-type current and reduced T-tubule density observed, but may have detrimental effects by inducing spontaneous Ca2+ waves which may generate DADs, especially when NCX activity is increased as is the case in AF.5,9 Whether spontaneous depolarization plays a role in the substrate of persistent AF is a matter of debate. A study on maintenance of stretch-related AF suggested interplay between rotors and focal discharges.48 Multiple small release events that are not triggering an AP may contribute to membrane instability by a small increase in net inward NCX current.
Regarding the relative role of RyR function within the overall changes in Ca2+ handling in AF, further computer modelling and experimental study is needed to uncover the involvement in the arrhythmic burden and contractile dysfunction. Of note, however, a number of reports highlight the occurrence of AF in patients with primary genetic RyR dysfunction.49
4.4. Limitations
While the resolution of the measurements here is sufficient to study RyR CRU morphology, single RyR measurements are not possible. The CRU's measured here are from >2 µm deep within the cell and therefore are not flat. Peripheral clusters are proposed to be more flat;12 however, myocyte morphology will never allow perfect alignment with the coverslip. Similarly, the axial resolution of STED microscopy, while better than highly inclined TIRF mode, is still ∼0.5 µm. These confounding factors may limit more accurate RyR cluster quantification. Alternative approaches to gain information on cluster size which include out of focus RyR, using a method previously used for confocal measurements,30 are presented and discussed in the Supplementary material online, Figure S6.
To study Ca2+ sparks in the absence of Ca2+ waves, a moderate level (350 µM) of EGTA was used. The addition of cytosolic buffers is known to affect Ca2+ release, and recent studies43,50 suggest that this may happen with ≥360 µM EGTA, similar to that used here. If experiments had been carried out at lower buffering levels, the observed differences in spark frequency and TTP may have been more pronounced.
Differences in RyR phosphorylation, but not in FKBP12.6 or PP1 protein expression, can be detected in tissue homogenates (see Supplementary material online, Figure S7). However, in permeabilized cells, modification of RyR function by CaMKII (or NOS) is assumed to be absent as the intracellular milieu is clamped at [Ca] below levels necessary for activation (see Supplementary material online) and comparable for CTRL and AF. Likewise redox conditions should be similar for both groups. However, we cannot exclude microdomains maintaining differences in redox near RyR as redox potential was not clamped as in Shirokova et al.51
Coupled gating was used in the simulations here. No clear consensus on the importance of coupled gating on RyR function has yet been reached. However, it is clear that RyR opening increases the likelihood of triggering neighbours by Ca2+-induced Ca2+ release and that this nanoscale influence has a similar effect.52 This is discussed further in the Supplementary material online.
4.5. Conclusions
RyR clusters are highly variable in size and can be further described in a second-order organization into functional CRUs. The current data underscore the role of RyR cluster morphology and buffering in the generation of spontaneous Ca2+ release.
In persistent AF, more fragmented CRUs, with more clusters that are in close proximity to each other, lead to more inter-cluster interaction, potentiated by reduced Ca2+ buffering. Together, this leads to an overactive Ca2+ release with increased chance of propagating release events.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the Belgian Science Policy Program P6/31 (K.R.S) and P6/27 (J.H.); European Union grant Health-F2-2009-241526 EUTrigTreat (K.R.S.); the FP7 Marie Fellowship PIEF-GA-2009-255264 (N.M.); Flemish research council (FWO) grant G060212N (K.R.S. and N.M.), G.0366.06 (J.H.), clinical researcher fellowship (R.W.), postdoctoral research fellowship (I.L.); Medtronic Belgium (R.W.); JST, PRESTO grant (J.H.); IWT scholarship (W.S.); Flemish Government longterm structural funding-Methusalem (J.H.); National Institutes of Health grant R01HL105239 and R01HL105239 (M.S.J.). Funding to pay the Open Access publication charges for this article was provided by European Union grant Health-F2-2009-241526 EUTrigTreat (K.R.S.).
Acknowledgements
The authors thank Godfrey Smith for careful reading of the manuscript. NVIDIA Corporation is gratefully acknowledged for providing GPUs for computation to M.S.J.
Conflict of interest: none declared.
References
- 1.Wakili R, Voigt N, Kaab S, Dobrev D, Nattel S. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest 2011;121:2955–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camm AJ, Lip GY, De CR, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation. Eur Heart J 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 3.Dobrev D, Carlsson L, Nattel S. Novel molecular targets for atrial fibrillation therapy. Nat Rev Drug Discov 2012;11:275–291. [DOI] [PubMed] [Google Scholar]
- 4.Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schondube FA, Hasenfuss G, Maier LS. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res 2010;106:1134–1144. [DOI] [PubMed] [Google Scholar]
- 5.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012;125:2059–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hove-Madsen L, Llach A, Bayes-Genis A, Roura S, Rodriguez FE, Aris A, Cinca J. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation 2004;110:1358–1363. [DOI] [PubMed] [Google Scholar]
- 7.Ohkusa T, Ueyama T, Yamada J, Yano M, Fujumura Y, Esato K, Matsuzaki M. Alterations in cardiac sarcoplasmic reticulum Ca2+ regulatory proteins in the atrial tissue of patients with chronic atrial fibrillation. J Am Coll Cardiol 1999;34:255–263. [DOI] [PubMed] [Google Scholar]
- 8.Zhao ZH, Zhang HC, Xu Y, Zhang P, Li XB, Liu YS, Guo JH. Inositol-1,4,5-trisphosphate and ryanodine-dependent Ca2+ signaling in a chronic dog model of atrial fibrillation. Cardiology 2007;107:269–276. [DOI] [PubMed] [Google Scholar]
- 9.Lenaerts I, Bito V, Heinzel FR, Driesen RB, Holemans P, D'hooge J, Heidbuchel H, Sipido KR, Willems R. Ultrastructural and functional remodeling of the coupling between Ca2+ influx and sarcoplasmic reticulum Ca2+ release in right atrial myocytes from experimental persistent atrial fibrillation. Circ Res 2009;105:876–885. [DOI] [PubMed] [Google Scholar]
- 10.Hell SW, Wichmann J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt Lett 1994;19:780–782. [DOI] [PubMed] [Google Scholar]
- 11.Izu LT, Means SA, Shadid JN, Chen-Izu Y, Balke CW. Interplay of ryanodine receptor distribution and calcium dynamics. Biophys J 2006;91:95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soeller C, Baddeley D. Super-resolution imaging of EC coupling protein distribution in the heart. J Mol Cell Cardiol 2013;58:32–40. [DOI] [PubMed] [Google Scholar]
- 13.Baddeley D, Jayasinghe ID, Lam L, Rossberger S, Cannell MB, Soeller C. Optical single-channel resolution imaging of the ryanodine receptor distribution in rat cardiac myocytes. Proc Natl Acad Sci USA 2009;106:22275–22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou Y, Jayasinghe I, Crossman DJ, Baddeley D, Soeller C. Nanoscale analysis of ryanodine receptor clusters in dyadic couplings of rat cardiac myocytes. J Mol Cell Cardiol 2015;80:45–55. [DOI] [PubMed] [Google Scholar]
- 15.Franzini-Armstrong C, Protasi F, Ramesh V. Shape, size, and distribution of Ca(2+) release units and couplons in skeletal and cardiac muscles. Biophys J 1999;77:1528–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng H, Lederer WJ. Calcium sparks. Physiol Rev 2008;88:1491–1545. [DOI] [PubMed] [Google Scholar]
- 17.Dibb KM, Clarke JD, Horn MA, Richards MA, Graham HK, Eisner DA, Trafford AW. Characterization of an extensive transverse tubular network in sheep atrial myocytes and its depletion in heart failure. Circ Heart Fail 2009;2:482–489. [DOI] [PubMed] [Google Scholar]
- 18.Sobie EA, Guatimosim S, Gomez-Viquez L, Song LS, Hartmann H, Saleet JM, Lederer WJ. The Ca2+ leak paradox and rogue ryanodine receptors: SR Ca2+ efflux theory and practice. Prog Biophys Mol Biol 2006;90:172–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacQuaide N, Ramay HR, Sobie EA, Smith GL. Differential sensitivity of Ca(2)+ wave and Ca(2)+ spark events to ruthenium red in isolated permeabilised rabbit cardiomyocytes. J Physiol 2010;588:4731–4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anne W, Willems R, Holemans P, Beckers F, Roskams T, Lenaerts I, Ector H, Heidbuchel H. Self-terminating AF depends on electrical remodeling while persistent AF depends on additional structural changes in a rapid atrially paced sheep model. J Mol Cell Cardiol 2007;43:148–158. [DOI] [PubMed] [Google Scholar]
- 21.Dedecker P, Muls B, Hofkens J, Enderlein J, Hotta J. Orientational effects in the excitation and de-excitation of single molecules interacting with donut-mode laser beams. Opt Express 2007;15:3372–3383. [DOI] [PubMed] [Google Scholar]
- 22.Cheng H, Song LS, Shirokova N, Gonzalez A, Lakatta EG, Rios E, Stern MD. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys J 1999;76:606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams GS, Chikando AC, Tuan HT, Sobie EA, Lederer WJ, Jafri MS. Dynamics of calcium sparks and calcium leak in the heart. Biophys J 2011;101:1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marx SO, Gaburjakova J, Gaburjakova M, Henrikson C, Ondrias K, Marks AR. Coupled gating between cardiac calcium release channels (ryanodine receptors). Circ Res 2001;88:1151–1158. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Wasserstrom JA, Shiferaw Y. Role of coupled gating between cardiac ryanodine receptors in the genesis of triggered arrhythmias. Am J Physiol Heart Circ Physiol 2009;297:H171–H180. [DOI] [PubMed] [Google Scholar]
- 26.Chen-Izu Y, Ward CW, Stark W Jr, Banyasz T, Sumandea MP, Balke CW, Izu LT, Wehrens XH. Phosphorylation of RyR2 and shortening of RyR2 cluster spacing in spontaneously hypertensive rat with heart failure. Am J Physiol Heart Circ Physiol 2007;293:H2409–H2417. [DOI] [PubMed] [Google Scholar]
- 27.Sobie EA, Dilly KW, dos Santos CJ, Lederer WJ, Jafri MS. Termination of cardiac Ca(2+) sparks: an investigative mathematical model of calcium-induced calcium release. Biophys J 2002;83:59–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner E, Lauterbach MA, Kohl T, Westphal V, Williams GS, Steinbrecher JH, Streich JH, Korff B, Tuan HT, Hagen B, Luther S, Hasenfuss G, Parlitz U, Jafri MS, Hell SW, Lederer WJ, Lehnart SE. Stimulated emission depletion live-cell super-resolution imaging shows proliferative remodeling of T-tubule membrane structures after myocardial infarction. Circ Res 2012;111:402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shkryl VM, Blatter LA, Rios E. Properties of Ca2+ sparks revealed by four-dimensional confocal imaging of cardiac muscle. J Gen Physiol 2012;139:189–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soeller C, Crossman D, Gilbert R, Cannell MB. Analysis of ryanodine receptor clusters in rat and human cardiac myocytes. Proc Natl Acad Sci USA 2007;104:14958–14963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caldwell JL, Smith CE, Taylor RF, Kitmitto A, Eisner DA, Dibb KM, Trafford AW. Dependence of cardiac transverse tubules on the BAR domain protein Amphiphysin II (BIN-1). Circ Res 2014;115:986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landstrom AP, Kellen CA, Dixit SS, van Oort RJ, Garbino A, Weisleder N, Ma J, Wehrens XH, Ackerman MJ. Junctophilin-2 expression silencing causes cardiocyte hypertrophy and abnormal intracellular calcium-handling. Circ Heart Fail 2011;4:214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell 2000;6:11–22. [DOI] [PubMed] [Google Scholar]
- 34.Beavers DL, Wang W, Ather S, Voigt N, Garbino A, Dixit SS, Landstrom AP, Li N, Wang Q, Olivotto I, Dobrev D, Ackerman MJ, Wehrens XH. Mutation E169K in junctophilin-2 causes atrial fibrillation due to impaired RyR2 stabilization. J Am Coll Cardiol 2013;62:2010–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knollmann B, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BEC, Horton KD, Weissman NJ, Holinstat I, Zhang W, Roden DM, Jones LR, Franzini-Armstrong C, Pfeifer K. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest 2006;116:2510–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swift F, Franzini-Armstrong C, Oyehaug L, Enger UH, Andersson KB, Christensen G, Sejersted OM, Louch WE. Extreme sarcoplasmic reticulum volume loss and compensatory T-tubule remodeling after Serca2 knockout. Proc Natl Acad Sci USA 2012;109:3997–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Picht E, Zima AV, Shannon TR, Duncan AM, Blatter LA, Bers DM. Dynamic calcium movement inside cardiac sarcoplasmic reticulum during release. Circ Res 2011;108:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacQuaide N, Dempster J, Smith GL. Measurement and modeling of Ca2+ waves in isolated rabbit ventricular cardiomyocytes. Biophys J 2007;93:2581–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science 1993;262:740–744. [DOI] [PubMed] [Google Scholar]
- 40.Parker I, Wier WG. Variability in frequency and characteristics of Ca2+ sparks at different release sites in rat ventricular myocytes. J Physiol 1997;505(Pt 2):337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang SQ, Song LS, Xu L, Meissner G, Lakatta EG, Rios E, Stern MD, Cheng H. Thermodynamically irreversible gating of ryanodine receptors in situ revealed by stereotyped duration of release in Ca(2+) sparks. Biophys J 2002;83:242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillespie D, Fill M. Pernicious attrition and inter-RyR2 CICR current control in cardiac muscle. J Mol Cell Cardiol 2013;58:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zima AV, Bovo E, Bers DM, Blatter LA. Ca(2)+ spark-dependent and -independent sarcoplasmic reticulum Ca(2)+ leak in normal and failing rabbit ventricular myocytes. J Physiol 2010;588:4743–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Louch WE, Hake J, Mork HK, Hougen K, Skrbic B, Ursu D, Tonnessen T, Sjaastad I, Sejersted OM. Slow Ca(2)(+) sparks de-synchronize Ca(2)(+) release in failing cardiomyocytes: evidence for altered configuration of Ca(2)(+) release units? J Mol Cell Cardiol 2013;58:41–52. [DOI] [PubMed] [Google Scholar]
- 45.Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, Danilo P, Ravens U, Rosen MR, Marks AR. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation 2005;111:2025–2032. [DOI] [PubMed] [Google Scholar]
- 46.Terentyev D, Gyorke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Gyorke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res 2008;103:1466–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalez DR, Treuer AV, Castellanos J, Dulce RA, Hare JM. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem 2010;285:28938–28945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamazaki M, Vaquero LM, Hou L, Campbell K, Zlochiver S, Klos M, Mironov S, Berenfeld O, Honjo H, Kodama I, Jalife J, Kalifa J. Mechanisms of stretch-induced atrial fibrillation in the presence and the absence of adrenocholinergic stimulation: interplay between rotors and focal discharges. Heart Rhythm 2009;6:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhuiyan ZA, van den Berg MP, van Tintelen JP, Bink-Boelkens MT, Wiesfeld AC, Alders M, Postma AV, van Langen I, Mannens MM, Wilde AA. Expanding spectrum of human RYR2-related disease: new electrocardiographic, structural, and genetic features. Circulation 2007;116:1569–1576. [DOI] [PubMed] [Google Scholar]
- 50.Brochet DX, Xie W, Yang D, Cheng H, Lederer WJ. Quarky calcium release in the heart. Circ Res 2011;108:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shirokova N, Kang C, Fernandez-Tenorio M, Wang W, Wang Q, Wehrens XH, Niggli E. Oxidative stress and Ca(2+) release events in mouse cardiomyocytes. Biophys J 2014;107:2815–2827. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Cannell MB, Kong CH, Imtiaz MS, Laver DR. Control of sarcoplasmic reticulum Ca2+ release by stochastic RyR gating within a 3D model of the cardiac dyad and importance of induction decay for CICR termination. Biophys J 2013;104:2149–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]