Abstract
Aims
Inhibitory G (Gi) proteins have been proposed to be cardioprotective. We investigated effects of Gαi2 knockout on cardiac function and survival in a murine heart failure model of cardiac β1-adrenoceptor overexpression.
Methods and results
β1-transgenic mice lacking Gαi2 (β1-tg/Gαi2−/−) were compared with wild-type mice and littermates either overexpressing cardiac β1-adrenoceptors (β1-tg) or lacking Gαi2 (Gαi2−/−). At 300 days, mortality of mice only lacking Gαi2 was already higher compared with wild-type or β1-tg, but similar to β1-tg/Gαi2−/−, mice. Beyond 300 days, mortality of β1-tg/Gαi2−/− mice was enhanced compared with all other genotypes (mean survival time: 363 ± 21 days). At 300 days of age, echocardiography revealed similar cardiac function of wild-type, β1-tg, and Gαi2−/− mice, but significant impairment for β1-tg/Gαi2−/− mice (e.g. ejection fraction 14 ± 2 vs. 40 ± 4% in wild-type mice). Significantly increased ventricle-to-body weight ratio (0.71 ± 0.06 vs. 0.48 ± 0.02% in wild-type mice), left ventricular size (length 0.82 ± 0.04 vs. 0.66 ± 0.03 cm in wild types), and atrial natriuretic peptide and brain natriuretic peptide expression (mRNA: 2819 and 495% of wild-type mice, respectively) indicated hypertrophy. Gαi3 was significantly up-regulated in Gαi2 knockout mice (protein compared with wild type: 340 ± 90% in Gαi2−/− and 394 ± 80% in β1-tg/Gαi2−/−, respectively).
Conclusions
Gαi2 deficiency combined with cardiac β1-adrenoceptor overexpression strongly impaired survival and cardiac function. At 300 days of age, β1-adrenoceptor overexpression alone had not induced cardiac hypertrophy or dysfunction while there was overt cardiomyopathy in mice additionally lacking Gαi2. We propose an enhanced effect of increased β1-adrenergic drive by the lack of protection via Gαi2. Gαi3 up-regulation was not sufficient to compensate for Gαi2 deficiency, suggesting an isoform-specific or a concentration-dependent mechanism.
Keywords: Adrenergic receptor, Inhibitory G protein, Cardiomyopathy, Heart failure, Cardioprotection
1. Introduction
Norepinephrine concentrations and sympathetic drive are increased in human heart failure.1–3 Although this helps to maintain contractile force, blood pressure, and blood flow to vital organs, it becomes detrimental in the long run.4,5 β1- and β2-adrenoceptors are the most abundant cardiac adrenoceptors with the expression of β1-adrenoceptors exceeding that of β2-adrenoceptors by four-fold in the normal (human) heart.4 β1-adrenoceptors exclusively couple with stimulatory G proteins (Gs), whereas β2-adrenoceptors have been shown to directly interact with both Gs and inhibitory G (Gi) proteins.6,7 In human heart failure, expression of sarcolemmal β1-adrenoceptors and their coupling with Gs decreases.8 On the other hand, an increase of ‘promiscuous’ β2- relative to β1-adrenoceptors and an increased expression of Gi (particularly the isoform Gi2) are observed.8,9 This latter finding can be interpreted as an attempt to shield the heart against catecholamines since Rau et al.10 have shown that a Gi2 increase similar to that found in human heart failure is sufficient to significantly reduce adenylyl cyclase-mediated cAMP production. This and other findings support the hypothesis that signalling via Gi proteins is cardioprotective.11–14 Of interest, overexpression of both β1- and β2-adrenoceptors has been shown to induce cardiac hypertrophy and heart failure in mice, respectively.15,16 However, levels of overexpression necessary to cause heart failure seem to be higher for β2-adrenoceptors that in contrast to β1-adrenoceptors couple with both Gs and Gi.15,16 Nonetheless, cardioprotective effects mediated by Gi proteins are still a matter of debate, particularly regarding the cardiac condition, e.g. normal vs. pathological. To address this issue, we investigated whether lack of the catalytic Gi2 subunit (Gαi2) affects cardiac function and survival in the murine heart failure model of cardiac β1-adrenoceptor overexpression compared with wild-type mice and mice either lacking Gαi2 or overexpressing β1. Parts of this work have already been published as a conference abstract.17
2. Methods
2.1. Mouse models used
Animals were kept in individually ventilated cages with a 12/12 h dark/light cycle and food and water ad libitum. Mice ubiquitary lacking Gαi2 (Gαi2−/−) have originally been generated on a 129Sv background, but subsequently been backcrossed to C57BL/6J.13,18,19 Mice with a cardiac-specific overexpression of the human β1-adrenoceptor (β1-tg) have originally been generated on a FVB/N background.15 For the current study, we backcrossed this line on a C57BL/6J background (>10 generations) to allow for mating with Gαi2 knockout mice to generate β1-transgenic animals deficient of Gαi2−/− (β1-tg/Gαi2−/−). As control animals we used age-matched wild-type littermates. Animals of both sexes were used for our study. For genotyping, tail-clips from 3-week-old mice were used. Genomic DNA was prepared and genotyping PCR for Gαi2 and the β1-receptor was performed as described previously.13,20 Animals were killed by cervical dislocation. Animal breeding, maintenance, and experiments were approved by the responsible federal state authority (Landesamt fuer Natur-, Umwelt- und Verbraucherschutz Nordrhein-Westfalen; reference: 87-51.04.2010.A078). All animal experiments comply with the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes.
2.2. Survival analysis
All genotypes were monitored for a minimum period of 2 years. Kaplan–Meier survival curves were used to determine mean survival time of each mouse line. Spontaneous death was defined as the event of interest, while killing an animal was a censored event. Mice used for breeding were excluded from our analysis. Numbers of mice included in our survival analysis were 200, 34, 37, and 192 for C57BL/6 (wild type), Gαi2−/−, β1-tg/Gαi2−/−, and β1-tg mice, respectively. Numbers of spontaneous deaths that occurred during an observation period were 9 (C57BL/6), 10 (Gαi2−/−), 21 (β1-tg/Gαi2−/−), and 22 (β1-tg).
2.3. Echocardiography
At an age of 302 ± 19 days, mice (n = 5–7 of every genotype) were examined by echocardiography under light inhalation anaesthesia with oxygen and 1.5% isoflurane through a nose cap. Chests were epilated and the animals were placed on a heating table to prevent hypothermia and cardiodepressive effects. For the experiments, a commercial echocardiography system (Philips iE33 ultrasonic system, ‘Qlab Cardiac Analysis’ Software; Philips Healthcare, Hamburg, Germany) equipped with a 15 MHz linear array transducer (L15-io7) allowing frame rates of 270 Hz was used. The transducer was moved along the parasternal long and short axis of the left ventricle, and loops of 3 s duration were recorded in one-dimensional (M-mode) and two-dimensional planes. To monitor the heart rate of the animals and thus anaesthesia during measurements, an ECG was derived. For reconstructive three-dimensional echocardiography, multiple short-axis slices were recorded every 500 µm using a millimetre screw-tripod.21,22
2.4. Ventricle-to-body weight ratio
Before killing a mouse, its body weight was measured. For determining ventricular weight, hearts were excised immediately after killing by cervical dislocation, atria were cut, and intraventricular blood removed. We analysed 11, 8, 7, and 14 hearts of C57BL/6 (wild-type), Gαi2−/−, β1-tg/Gαi2−/−, and β1-tg mice, respectively, including those from mice examined by echocardiography.
2.5. Quantitative real-time PCR
For quantitative real-time PCR (qPCR), we used ventricles that were stored at −80°C immediately after excision. qPCR analysis was performed to determine relative ventricular mRNA expression levels of the cardiomyopathy markers atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), the Gi proteins Gαi2 and Gαi3, and the cardiac protein kinase A (PKA) targets ryanodine receptor 2 (RYR2), troponin I (TnI, TNNI3), and phospholamban (PLB). All steps of analysis were performed following the manufacturer's protocol by QIAGEN (Hilden, Germany). mRNA isolation was performed with the RNeasy® Fibrous Tissue Kit (QIAGEN). Quality and quantity of the purified mRNA were controlled using a NanoDrop 8000 Spectrophotometer (Thermo Scientific, Waltham, MA, USA). For reverse transcription, the QuantiTect® Reverse Transcription Kit was used (QIAGEN). qPCR was run in triple repeats with the QuantiTect SYBR® Green PCR Kit (QIAGEN). Specific primer pairs for Gαi2, BNP, RYR2, TNNI3, and PLB were designed using Roche Assay Design Center: Gαi2: 5′-AAG ACC TGT CCG GTG TCA T-3′ for sense and 5′-GGG ATG TAG TCA CTC TGT GC-3′ for antisense. BNP: 5′-GTC AGT CGT TTG GGC TGT AAC-3′ for sense and 5′-AGA CCC AGG CAG AGT CAG AA-3′ for antisense. RYR2: 5′-TTC ACA CCT GTT CCT GTG GA-3′ for sense and 5′-TTT CTC TTA TCC TTT CCA GGT GA-3′ for antisense. TNNI3: 5′-GAG CCA CAC GCC AAG AAA-3′ for sense and 5′-GCC CCT TCT CTC CAC GTC-3′ for antisense. PLB: 5′-CTG TGA CGA TCA CCG AAG C-3′ for sense and 5′-TGG TCA AGA GAA AGA TAA AAA GTT GA-3′ for antisense. Primer pairs for Gαi3 and ANP were reported previously.23–25 S29 served as a housekeeping gene. The qPCR was started with an initial step of incubation at 95°C for 15 min. Next, 45 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 25 s, and elongation at 72°C for 10 s were run with a transition rate of 20°C per second. Finally, a melting curve analysis was applied to check for product purity at 64°C for 1 min with a transition rate of 0.1°C per second.
2.6. Western blot analysis
Ventricles from three different animals per genotype were isolated, separately homogenized, and individually analysed. Tissue was homogenized in protein lysis buffer containing 21 mM Tris–HCl, pH 8.3, 0.67% SDS, 238 mM β-mercapoethanol, and 0.2 mM PMSF. Gα protein isoform separation was performed in gels containing 6 M urea. In total, 20 µg per lysate was loaded. To verify Gαi3 antibody specificity, ventricle lysates isolated from Gαi3-deficient mice were loaded.26 The proteins were visualized by immunodetection using the following primary antibodies described elsewhere:27–30 rabbit anti-Gαi2 (1 : 8000) and rabbit anti-Gαi3 (1 : 5000). Equal loading was verified with antibodies against rabbit anti-β-actin (1 : 10 000). The protein levels of Gαi2 and Gαi3 were quantified using the densitometric analysis software (Image Lab; Bio-Rad, Gräfelfing, Germany) and were normalized to the β-actin levels of the same samples. For each ventricle, immune blots were run in triplicates or quadruplicates. Homogenates of the four different genotypes were always loaded on the same gel and analysed in parallel.
2.7. Radioligand-binding experiments
Hearts from 4- to 6-month-old mice of both sexes were isolated in ice-cold phosphate buffer saline, frozen in liquid nitrogen, and stored at −80°C until membrane preparation. Ventricular tissue was homogenized in a 0.32 M sucrose solution and centrifuged for 11 min at 300 × g. Supernatants were centrifuged for 41 min at 80 000 × g and pellets resuspended in aqua destillata. All preparation steps were carried out at 4°C. Finally, aliquots of 0.5 mL were frozen in liquid nitrogen and stored at −80°C. Protein content was determined according to Lowry and ranged from 5.4 to 7.7 mg/mL. For saturation-binding experiments, membranes were incubated in Tris–HCl buffer (Tris 50 mM, pH 7.4; EDTA 5 mM) of a final volume of 1.5 mL at 23°C containing 50–300 μg protein. Eight concentrations (0.025–1.5 nM) of [3H]CGP 12177 (specific activity: 37.7 Ci/mmol; PerkinElmer, Rodgau, Germany) were used to quantify β-adrenoceptor expression. Non-specific binding was measured in the presence of 10 µM propranolol or atropine (Sigma-Aldrich, Steinheim, Germany). Incubation was stopped after 90 min by rapid filtration through polyethylenimine (0.1%)-pretreated glass microfiber sheets using a Brandel cell harvester. Filter-bound radioactivity was detected by liquid scintillation counting. Fraction of β2-adrenoceptor binding of [3H]CGP 12177 was estimated in the presence of 70 nM ICI 118,551 (Sigma-Aldrich). Quality of the used homogenates was similar, as confirmed by muscarinic receptor levels determined with six concentrations (0.5–1.5 nM) of [3H]N-methylscopolamine (specific activity: 84.1 Ci/mmol; PerkinElmer). Binding curves were analysed by non-linear curve fitting using the software GraphPad Prism®. Data points were fitted by a one-site-specific binding model.
2.8. Statistical analysis
Data are given as mean ± S.E.M. Survival times were calculated by Kaplan–Meier estimation. Differences were determined by the log-rank test. Cardiac functional parameters obtained from echocardiography and ventricle-to-body weight ratios were compared by ANOVA followed by post hoc tests (Bonferroni). mRNA expression ratios using Ct values obtained by qPCR were compared using the REST-2009® software.31 Distribution between sexes was compared by Fisher's exact test. P-values <0.05 were defined to indicate statistically significant differences.
3. Results
3.1. Gross phenotype
Genotypes were distributed as expectable according to the Mendelian law (not shown). Overall distribution between sexes was nearly equal (51% male and 49% female) and did not differ between genotypes (not shown). All mice behaved normally and no overt phenotype was observed. Deaths occurred suddenly and were only in single cases preceded by unspecific symptoms like reduced movement or seclusive behaviour. Gastrointestinal symptoms as described previously for mice lacking Gαi2 were not seen in our study, presumably due to keeping the mice in individually ventilated cages.32
3.2. Survival analysis
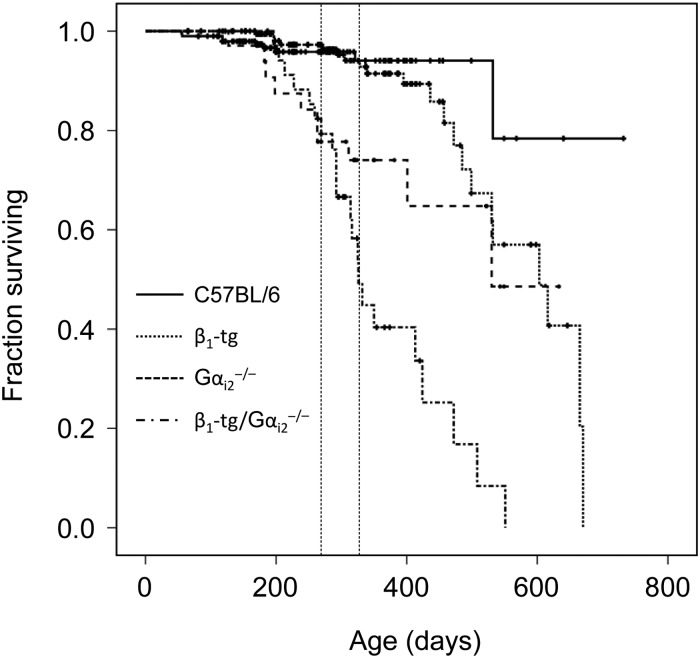
Kaplan–Meyer estimation revealed a significantly enhanced mortality of β1-transgenic mice deficient of Gαi2−/− (β1-tg/Gαi2−/−) compared with all other genotypes (Figure 1). Mean survival time was reduced to 363 ± 21 days (22 deaths out of 37 mice in total) compared with 669 ± 31 for wild-type mice (9/200), 489 ± 37 for Gαi2−/− (10/34), and 561 ± 20 for β1-tg mice (21/192). Though survival time of mice only lacking Gαi2 was significantly reduced, too, this effect was significantly more dramatic in β1-tg/Gαi2−/− mice (P = 0.02 in a log-rank test compared with Gαi2−/−). Of note, survival curves of β1-tg/Gαi2−/− and mice only lacking Gαi2 were indistinguishable up to an age of ∼300 days. Similar to recent findings by another group, survival curves of wild-type and β1-tg mice started to differ around an age of 13 months,33 though in our study the difference in mean survival times derived from Kaplan–Meier analysis did not reach statistical significance. Fifty per cent of deaths in the β1-tg/Gαi2−/− group already occurred up to an age of 300 days. Since survival curves thus indicated a rather early onset of detrimental effects, we defined 300 days as the target age for further experiments. Moreover, this allowed for a sufficient number of animals available for in vivo examination. Of note, the choice of an age of 300 days excluded a considerable number of β1-tg/Gαi2−/− (and to a lesser extent Gαi2−/−) animals from further analysis due to early death. This might have biased the obtained data, e.g. if animals were still living at Day 300 because they have been less affected than those that had already died.
Figure 1.
Kaplan–Meier survival curves of wild-type, knockout, and transgenic mice. Vertical bars indicate a censoring event (usually representing killing of an animal for experimental purposes). Mean survival times were significantly decreased for Gαi2−/− and β1-tg/Gαi2−/− mice, each compared with all other genotypes. However, compared with Gαi2−/− mice, survival time was significantly lower in β1-tg/Gαi2−/− mice (log-rank test). The number of animals underlying survival analysis was 200, 34, 37, and 192 for wild-type (C57BL/6), Gαi2−/−, β1-tg/Gαi2−/−, and β1-tg mice, respectively. Vertical dashed lines label age range of mice used for further investigations (Figures 2, 3, 5–7).
3.3. Echocardiographic analysis of cardiac morphology and function
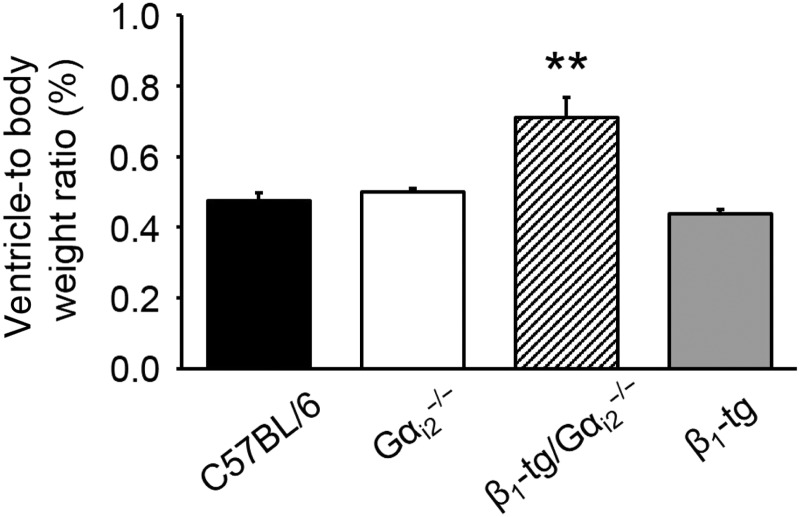
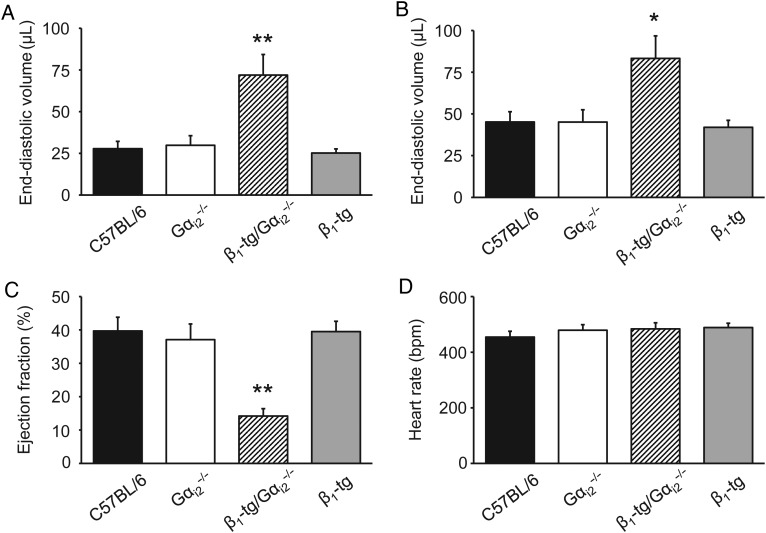
At a mean age of 300 days (range: 273–326), the ventricle-to-body weight ratio of β1-tg/Gαi2−/− mice was significantly increased (0.71 ± 0.06%, n = 5) compared with wild-type mice (0.48 ± 0.02%, n = 11), Gαi2−/− (0.50 ± 0.01%, n = 8), and β1-tg mice (0.44 ± 0.01%, n = 14), thus indicating cardiac hypertrophy in β1-transgenic mice lacking Gαi2 (Figure 2). This was confirmed in echocardiographic examinations of anaesthetized mice of the same age. Ventricles of β1-tg/Gαi2−/− mice appeared to be clearly enlarged (see Supplementary material online, Figure S1). End-systolic and end-diastolic ventricular volumes were similar when comparing wild-type, Gαi2−/−, and β1-tg mice, but significantly enhanced in β1-tg/Gαi2−/− mice compared with all other genotypes (Figure 3A and B). Furthermore, ejection fraction was significantly reduced in β1-tg/Gαi2−/− mice (Figure 3C). Significantly increased ventricular length and reduced myocardial thickness in β1-tg/Gαi2−/− mice indicated a dilative cardiomyopathy (data not shown; compare Supplementary material online, Figure S1).
Figure 2.
Ventricle-to-body weight ratios of knockout and transgenic mice indicate a significant cardiac hypertrophy of β1-tg/Gαi2−/− mice. The number of animals used for analysis was 11, 8, 5, and 14 for wild-type (C57BL/6), Gαi2−/−, β1-tg/Gαi2−/−, and β1-tg mice, respectively. Age range of examined mice was 301 ± 3 days. Asterisks indicate a significant difference compared with all other genotypes in Bonferroni post-tests following ANOVA (P < 0.01).
Figure 3.
Morphological and functional parameters obtained by echocardiography. End-systolic (A) and -diastolic (B) left ventricular volumes and the ejection fraction (C) were significantly impaired in β1-tg/Gαi2−/− mice compared with all other genotypes indicating overt heart failure. Heart rate (D) was similar for all investigated genotypes. The number of animals used for analysis was 5, 6, 6, and 7 for wild-type (C57BL/6), Gαi2−/−, β1-tg/Gαi2−/−, and β1-tg mice, respectively. Age range of examined mice was 307 ± 3 days. Asterisks indicate significant differences compared with all other genotypes in Bonferroni post-tests following ANOVA (*P < 0.05, **P < 0.01).
3.4. Expression of β-adrenoceptors and comparison to β-transgenic mice on an FVB/N background
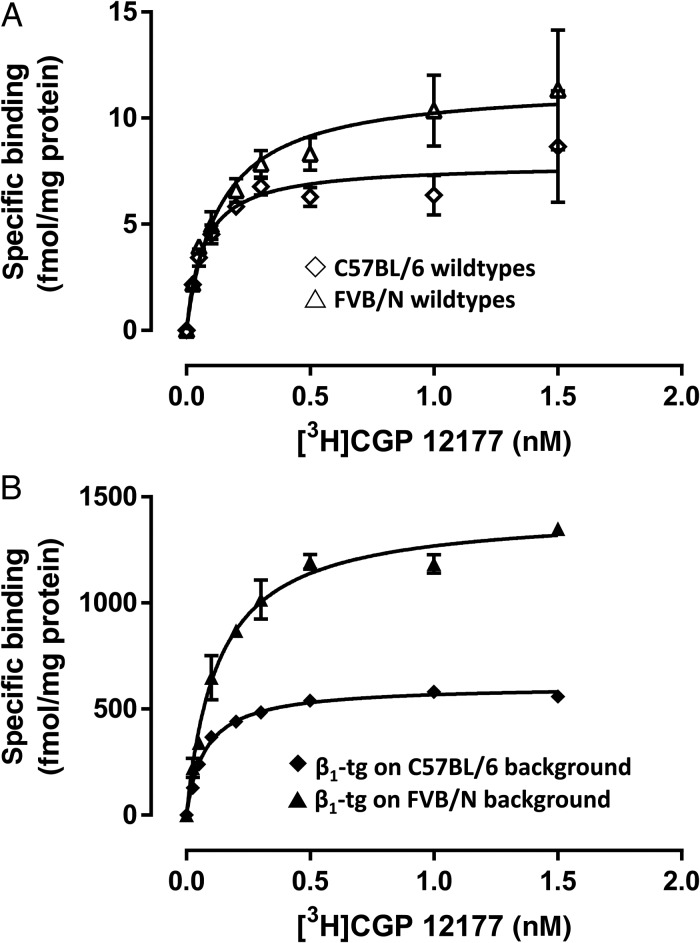
Using saturation radioligand-binding experiments (Figure 4), we confirmed significant overexpression of β-adrenoceptors in 4- to 6-month-old β1-tg mice (Bmax 609 ± 18 vs. 8.4 ± 1.6 fmol/mg in C57BL/6 wild-type mice). In wild-type ventricles, ∼80% of radioligand binding was due to β1-adrenoceptors, while in transgenic mice virtually all detected receptors were β1-adrenoceptors (data not shown). Of note, on the original FVB/N background, we found the overexpression level to be more than two-fold higher (Bmax: 1425 ± 68 fmol/mg) than on a C57BL/6 background. Furthermore, in FVB/N-based β1-transgenic mice, mean survival time was significantly reduced (402 ± 15 days). Similar to (C57BL/6-based) β1-tg/Gαi2−/− mice, ventricle-to-body weight ratio was increased (0.62 ± 0.03 vs. 0.52 ± 0.02% in FVB/N wild-type mice; P < 0.05; n = 10 and 7, respectively) and cardiac function impaired (e.g. ejection fraction: 21 ± 1 vs. 41 ± 2% in FVB/N wild-type mice; P < 0.01; n = 5 and 6, respectively) at a mean age of 307 ± 6 days (range 276–330).
Figure 4.
Ventricular β-adrenoceptor expression obtained by radioligand binding in (A) wild-type and (B) β1 overexpressing mice (β1-tg) at an age of 4–6 months. In both C57BL/6- and FVB/N-based β1-tg mice, β-adrenoceptor expression was strikingly increased compared with the corresponding wild-type. Of note, the (over-) expression level was higher in tissue from transgenic mice with an FVB/N background. Each homogenate (10–12 ventricles per genotype) was used in three independent experiments in duplicates.
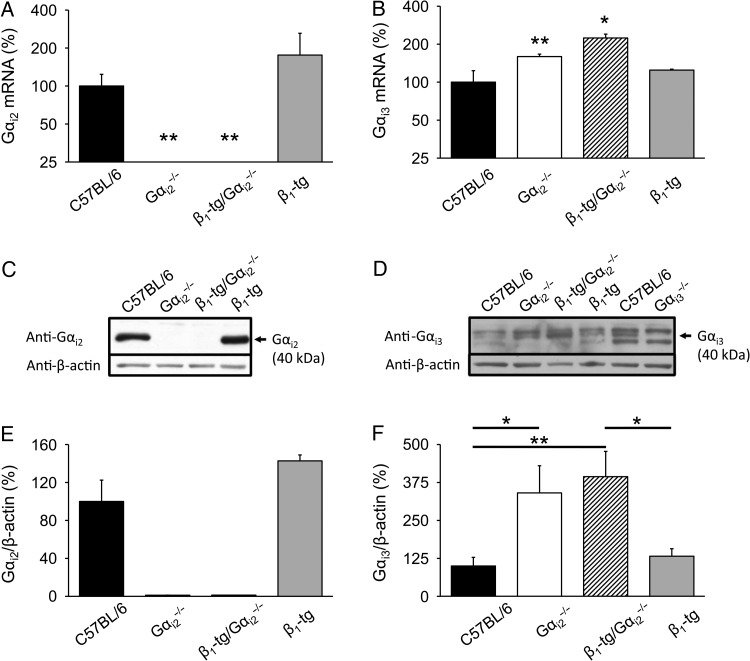
3.5. Expression of Gi proteins
Expression of Gαi2 mRNA was not detectable in Gαi2 knockout mice (Figure 5A). We found that the lack of Gαi2 was accompanied by an increased expression of Gαi3 mRNA, both on a wild-type and on a β1-transgenic background (Figure 5B). Expression of neither Gαi2 nor Gαi3 mRNA was significantly altered in hearts of mice being only transgenic for the β1-adrenoceptor. Immunoblot analysis of ventricle homogenates revealed Gαi2 protein expression in ventricles of C57BL/6 and β1-tg mice, whereas the Gαi2 protein was absent in Gαi2−/− and β1-tg/Gαi2−/− ventricles (Figure 5C and E). Gαi3 protein levels were significantly up-regulated in Gαi2−/− (340 ± 90%) and β1-tg/Gαi2−/− (394 ± 80%) ventricles compared with C57BL/6 and β1-tg animals and absent in Gαi3-deficient ventricles (Figure 5D and F).
Figure 5.
Ventricular expression of cardiac Gi. (A) Gαi2 mRNA was not detectable in knockout mice on either wild-type or β1-transgenic background and expressed at wild-type level in mice only transgenic for the β1-adrenoceptor. (B) Gαi3 mRNA expression was significantly increased compared with wild-type mice in animals lacking Gαi2 on both a wild-type and β1-transgenic background, respectively. The number of animals used for qPCR analysis was 3–4 for each genotype. Age range of examined mice was 302 ± 4 days. Asterisks indicate significant differences compared with mRNA expression levels of age-matched wild-type mice in an REST® analysis (*P < 0.05, **P < 0.01). (C) Representative immunoblots for the Gαi2 protein in ventricle homogenates. The Gαi2 protein was absent in Gαi2−/− and β1-tg/Gαi2−/− mice. (E) Statistical analysis of Gαi2 expression levels. (D) Representative immunoblot for the Gαi3 protein in ventricle homogenates. To verify Gαi3 antibody specificity, Gαi3-deficient ventricle homogenates were loaded in addition. (F) Statistical analysis of Gαi3 expression levels. A significant up-regulation of Gαi3 protein levels was detectable in Gαi2−/− and β1-tg/Gαi2−/− mice. For western blots, individual homogenates from three animals per genotype were analysed in 3–4 independent experiments. β-Actin was used to demonstrate equal loading of the gels. Asterisks indicate significant differences in Bonferroni post-tests following ANOVA (*P < 0.05; **P < 0.01).
3.6. Expression of hypertrophy markers and PKA targets
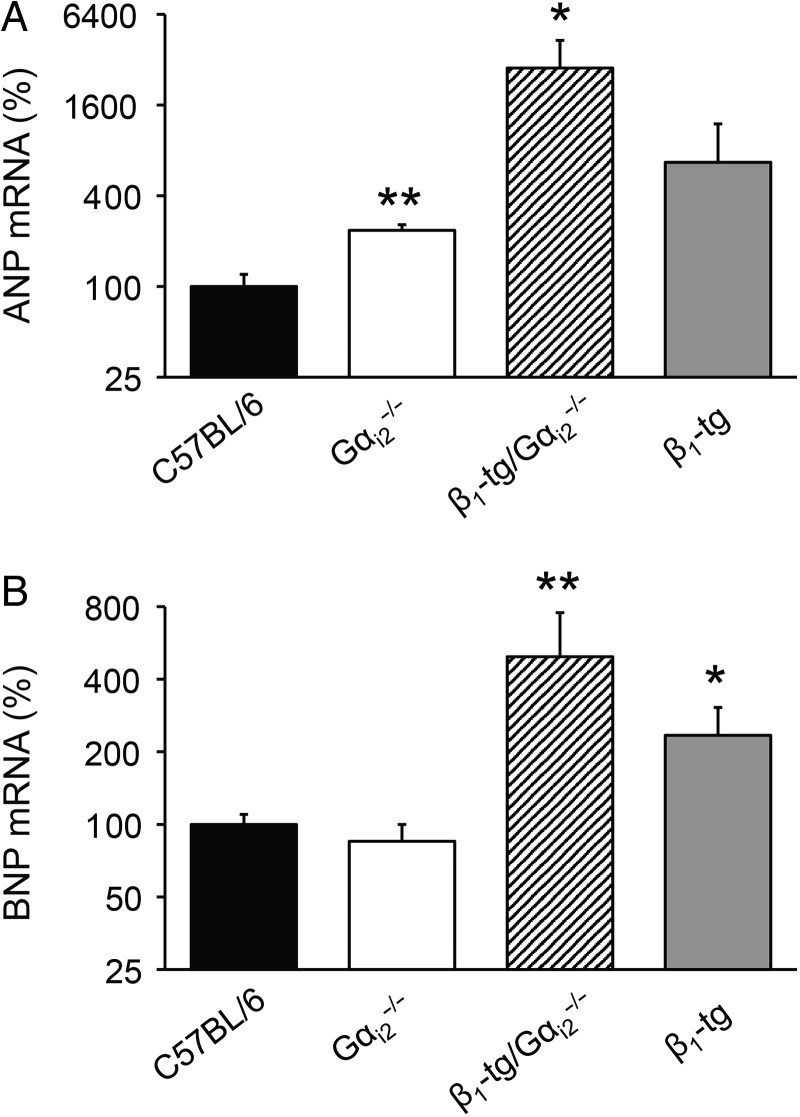
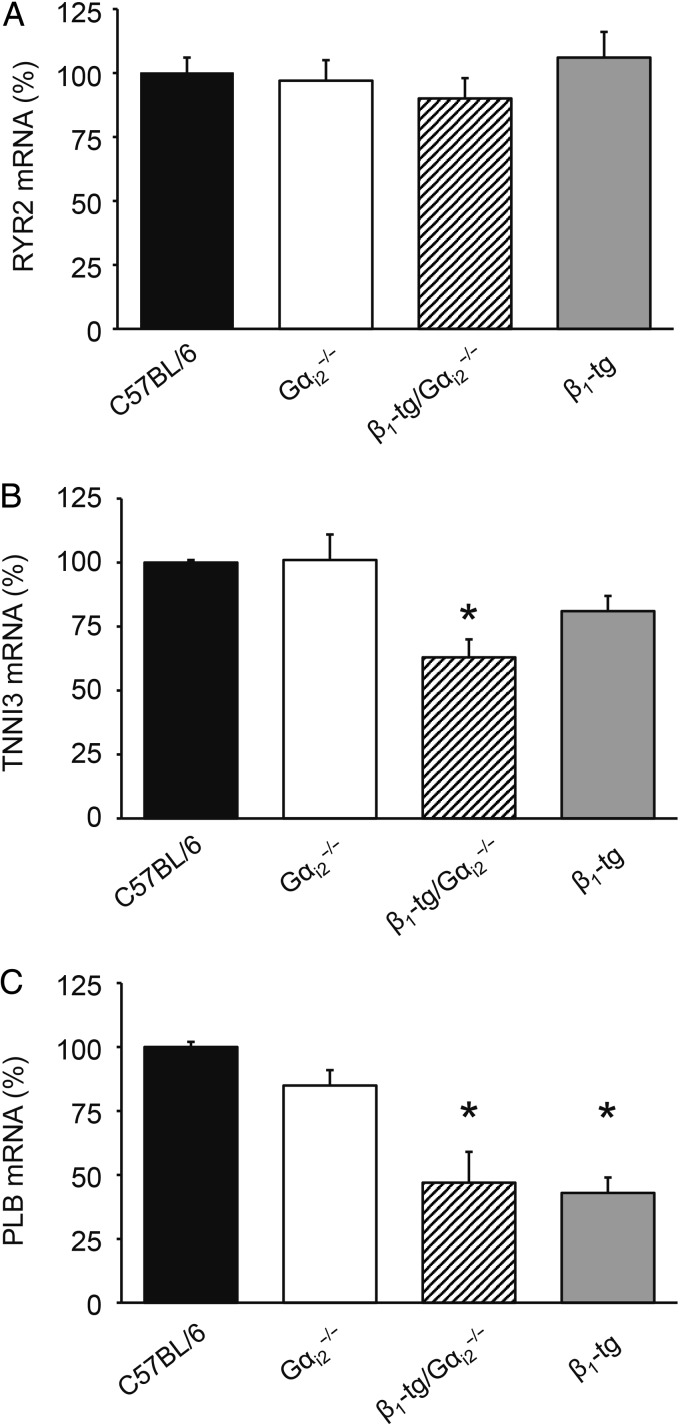
According to our morphological and functional findings, qPCR analysis revealed a significant up-regulation of the cardiomyopathy markers ANP (Figure 6A) and BNP (Figure 6B) in ventricular tissue of β1-tg/Gαi2−/− compared with wild-type mice. However, mRNA expression levels of ANP were also significantly increased in Gαi2−/− mice and showed a tendency to be higher in β1-tg animals (Figure 6A). BNP levels were unchanged in Gαi2−/−, but significantly increased in β1-tg mice (Figure 6B). We tested for mRNA expression of the calcium release channel (ryanodine receptor) RYR2, the cardiac TnI TNNI3, and PLB as known targets of PKA-mediated phosphorylation (Figure 7). Compared with C57BL/6 wild-type mice, no differences were seen for RYR2 mRNA, but there was a significantly reduced expression of TnI in ventricles of β1-tg/Gαi2−/− (63 ± 7% of wild type), and of PLB in ventricles of both β1-tg and β1-tg/Gαi2−/− (43 ± 6 and 47 ± 12% of wild type, respectively).
Figure 6.
Expression of cardiac hypertrophy markers in murine ventricles. Increased mRNA expression of ANP (A) and BNP (B) compared with wild-type levels confirmed morphological parameters indicating cardiac hypertrophy in β1-tg/Gαi2−/− mice. The number of animals used for analysis was 3–4 for each genotype. Age range of examined mice was 302 ± 4 days. Asterisks indicate significant differences compared with mRNA expression levels of age-matched wild-type mice in an REST® analysis (*P < 0.05, **P < 0.01).
Figure 7.
Ventricular mRNA expression of the PKA targets RYR2, TnI, and PLB. (A) RYR2 expression was similar in all genotypes. (B) TnI expression was significantly reduced in β1-tg/Gαi2−/− ventricles compared with wild-type (C57BL/6) tissue. (C) In both β1-adrenoceptor overexpressing mouse lines (β1-tg and β1-tg/Gαi2−/−), ventricular PLB expression was significantly reduced compared with wild-type (C57BL/6) tissue. The number of animals used for analysis was 3–4 for each genotype. Age range of examined mice was 288 ± 9 days. Asterisks indicate significant differences compared with mRNA expression levels of age-matched wild-type mice in an REST® analysis (*P < 0.05).
4. Discussion
In line with the idea of cardioprotective signalling mediated by Gi proteins, we show here that the lack of Gαi2 in mice overexpressing cardiac β1-adrenoceptors was detrimental: cardiac contractility was significantly depressed and survival time dramatically reduced. Mice deficient for either Gαi2 or Gαi3 have been reported to have no overt cardiac phenotype in vivo (echocardiography) and ex vivo (isolated whole hearts and myocytes).34 This is in perfect agreement with our current data obtained from Gαi2-deficient mice in the same range of age. Survival time of Gαi2-deficient mice was reduced, but reduction was significantly more pronounced in β1-tg/Gαi2−/− mice. Of interest, survival curves of β1-tg/Gαi2−/− and mice only lacking Gαi2 were indistinguishable up to an age of ∼300 days. Since survival curves of β1-tg mice start to drop rather late (this study and Lee et al.33), it is tempting to speculate that the early effect on mortality (up to Day 300) is due to Gαi2 deficiency while the detrimental effect observed beyond 300 days of age is related to the cardiac overexpression of β1-adrenoceptors. Given that cardiac function of mice only lacking Gαi2 was unaffected at an age of 300 days, a cause of death other than reduced pump function has to be supposed. In a previous study, mice with both a Gαi2 knockout and a cardiac-specific overexpression of β2-adrenoceptors have been shown to be not viable.13 This fatal outcome might be explained by direct coupling of β2-adrenoceptors to Gi proteins. The drastic effect of Gαi2 deficiency on top of cardiac β1-adrenoceptor overexpression seen in our current study is rather surprising given that β1-adrenoceptors are thought to exclusively couple with Gs proteins. However, recent work suggests a role for Gi in modulation of β1-mediated effects. Melsom et al.35 showed that inhibition of Gi proteins by pertussis toxin (PTX) not only enhanced cAMP accumulation following selective stimulation of either β1- or β2-adrenoceptors, but also increased inotropic potency of β1- and β2-adrenergic agonists, respectively. Another work by the same group indicates that Gi exerts intrinsic receptor-independent inhibitory activity on adenylyl cyclase.36
4.1. β1-Adrenoceptor overexpression
Like in mice overexpressing the cardiac β2-adrenoceptor, the phenotype of β1-transgenic mice seems to depend on the extent of overexpression. A higher expression level of β1-adrenoceptors led to an earlier and more pronounced hypertrophy of cardiomyocytes and a higher sensitivity of contractile response to dobutamine.15 We found that after back-crossing on a C57BL/6 background, cardiac β-adrenoceptor overexpression was lower compared with FVB/N-based β1-transgenic mice. This might explain why survival curves of β1-transgenic mice on a C57BL/6 background start to drop later (in accordance to findings of another group that independently backcrossed the β1-transgeni mice mouse of Engelhardt et al. on a C57BL/6 background).15,33 In good agreement, cardiac function was already impaired at an age of 300 days in FVB/N-, but not in C57BL/6-based β1-transgenic mice.
4.2. cAMP-dependent adrenergic signalling
That there was overt cardiomyopathy in (C57BL/6-based) β1-transgenic mice additionally lacking Gαi2 thus might be explained by a dual mechanism: increased β1-adrenergic drive and lack of protection by Gαi2. Engelhardt et al.37 found an increased expression of the cardiac Na+-/H+-exchanger NHE1 in β1-adrenoceptor transgenic mice and showed that NHE1 inhibition prevented heart failure in this mouse model. Since NHE1 activity is modulated by cAMP, one might speculate that, in the β1-overexpression model, lack of Gαi2 further aggravates NHE1-dependent effects leading to accelerated development of cardiac dysfunction and eventually mortality in our study.38 Though only an indirect hint, we looked at the expression of other potential phosphorylation targets at the mRNA level. Altered phosphorylation of the calcium release channel RYR2 has been attributed to heart failure and cardiac arrhythmia, though there is an ongoing controversial discussion.39 In our mouse models, RYR2 mRNA expression was unchanged, but we cannot exclude that enhanced phosphorylation due to a maintained increase of β-adrenergic tone might be involved in the early mortality we observed in β1-transgenic mice lacking Gαi2. TnI seems to mediate both lusitropic and inotropic responses following β-adrenergic stimulation in murine hearts,40 though the role of TnI and its phosphorylation in healthy and failing hearts is matter of debate.41 We found a significant reduction of TnI expression at the mRNA level in β1-transgenic mice lacking Gαi2. This might be related to their detrimental outcome since it has been shown that TnI knockout leads to heart failure in adult mice.42 Both β1-transgenic mouse lines investigated in our study (β1-tg and β1-tg/Gαi2−/−) showed a significant reduction of PLB mRNA expression. Engelhardt et al.43 found no alteration of PLB expression in β1-transgenic mice at an age of 2 months, but an increased PLB phosphorylation indicating enhanced β-adrenergic signalling. The decrease of PLB expression observed at an age of ∼9–10 months in our study might be compensatory by restoring the function of the SR calcium pump SERCA2A.44 Indeed, knockout of PLB in β-transgenic mouse hearts has been shown to rescue its heart failure phenotype.45 However, there seems to be a critical difference between mice and men because human PLB null has been shown to result in lethal cardiomyopathy.46 Of note, PKA-dependent phosphorylation of β2-adrenoceptors has been shown to drive a switch from Gs to Gi signalling,47 though this mechanism is matter of debate. β1-Adrenoceptor overexpression thus might enhance β2-adrenoceptor-mediated Gi signalling. This would explain the pronounced effect of Gαi2 deficiency in β1-transgenic mice compared with mice with an otherwise wild-type background seen in our study.
4.3. Gi-mediated adrenergic signalling
Irrespective of the (disputed) role of β2-adrenoceptor regulation by β1-adrenoceptors, the accepted role of Gi proteins for β2-adrenoceptor signalling should be considered. Communal et al.48 have described opposing β-adrenoceptor-mediated effects on apoptosis in rat cardiomyocytes in terms of enhancement via β1- but attenuation via β2-adrenoceptors. Indeed, the combination of β1-adrenoceptor blockers and β2-adrenoceptor agonists has been shown to be superior to only β1-adrenoceptor antagonists in treating cardiomyopathy in rodents.49,50 Though the above-mentioned anti-apoptotic effect by β2-adrenergic signalling was attributed to Gi-dependent activation of p38, the finding that overexpression of a dominant negative p38 isoform rescued cardiomyopathy of β2- but not β1-adrenoceptor transgenic mice argues against a role of this mechanism in our study.51,52 Kohler et al.14 have recently shown that following an ischaemia/reperfusion (I/R) protocol, cardiac infarct size was significantly increased in mice deficient for Gαi2. Also in a murine I/R model, similar findings have been obtained with an induced cardiac expression of a Gi inhibitor peptide.11 Taken together with our current study on β1-adrenoceptor overexpressing mice, these data suggest that the cardioprotective role of Gi proteins might become evident under certain circumstances only, i.e. cardiac stress and/or disease. Not all studies support the idea of Gi proteins being protective in cardiomyopathy and heart failure. The beneficial effect of combined treatment with a β1-adrenoceptor blocker and a β2-adrenoceptor agonist following myocardial infarction in rats was also seen when using fenoterol that has been shown to mediate β2-adrenergic effects independent of Gi proteins.50,53 Of note, the reported G protein selectivity of stereoisomers of fenoterol has recently been challenged.54 In a mouse model of dilative cardiomyopathy due to cre-recombinase overexpression, an increase of Gi signalling by knock-in of a mutated Gαi2 did not improve but even worsen ventricular hypertrophy and mortality.55 In a pathophysiologically more relevant model, Hussain et al.56 found no change in Gi activity in rat heart failure following myocardial infarction: despite a significant increase of Gαi2 protein by 50% Gi inhibition by PTX treatment, here did neither change baseline contractility nor inotropic response following β-adrenergic stimulation in ventricular strips from failing hearts.
4.4. Role of Gi2 vs. Gi3
The putative differential roles of the most abundant cardiac Gi isoforms Gαi2 and Gαi3 are still a matter of debate. As mentioned above, mice with cardiac-specific overexpression of β2-adrenoceptors were not viable, if they in addition were deficient of Gαi2.13 In the same study, heterozygous knockout of Gαi2 in β2-transgenic mice caused a reduced activity of ventricular L-type Ca2+ channels that was attributed to an increased expression of Gαi3. These data led us to the hypothesis that Gαi2 is cardioprotective, whereas Gαi3 is rather of regulatory relevance. In a subsequent study, this hypothesis was supported by our finding that, in Gαi2 knockout mice, cardiac Gαi3 expression was up-regulated and accordingly ventricular Ca2+-current density was decreased while, in mice lacking Gαi3, Ca2+-current density was enhanced despite an increased Gαi2 expression.24 Kohler et al.14 confirmed an increased cardiac Gαi2 expression in mice lacking Gαi3 and found ventricular infarct size following ischaemia to be reduced here compared with wild-type mice or mice lacking Gαi2.
5. Conclusion
In conclusion, our data support the idea of the Gi protein Gαi2 to be cardioprotective. Of interest, this was observed in β1-adrenoceptor overexpressing mice, i.e. a mouse model of cardiac stress not directly involving Gi signalling. The lack of Gαi2 seemed to aggravate rather than cause cardiac dysfunction. The observed up-regulation of Gαi3 was not sufficient to compensate for Gαi2 deficiency, suggesting an isoform-specific or a concentration-dependent mechanism to be elucidated in further studies.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This study was supported in part by the Intramural Research Program of the NIH (Project Z01-ES-101643) to L.B. and by Deutsche Forschungsgemeinschaft (DFG; He 1578/13-1 and 2) to S.H.
Acknowledgements
We highly appreciate the technical assistance of Sigrid Kirchmann-Hecht, Anna Wellermann, and Jens Reifenrath.
Conflict of interest: none declared.
References
- 1.Cohn JN. The sympathetic nervous system in heart failure. J Cardiovasc Pharmacol 1989;14(Suppl 5):S57–S61. [PubMed] [Google Scholar]
- 2.Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, Kubo SH, Rudin-Toretsky E, Yusuf S. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD). Circulation 1990;82:1724–1729. [DOI] [PubMed] [Google Scholar]
- 3.Francis GS, Goldsmith SR, Ziesche SM, Cohn JN. Response of plasma norepinephrine and epinephrine to dynamic exercise in patients with congestive heart failure. Am J Cardiol 1982;49:1152–1156. [DOI] [PubMed] [Google Scholar]
- 4.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res 2014;114:1815–1826. [DOI] [PubMed] [Google Scholar]
- 5.El-Armouche A, Eschenhagen T. Beta-adrenergic stimulation and myocardial function in the failing heart. Heart Fail Rev 2009;14:225–241. [DOI] [PubMed] [Google Scholar]
- 6.Ahles A, Engelhardt S. Polymorphic variants of adrenoceptors: pharmacology, physiology, and role in disease. Pharmacol Rev 2014;66:598–637. [DOI] [PubMed] [Google Scholar]
- 7.Xiao RP, Zhu W, Zheng M, Cao C, Zhang Y, Lakatta EG, Han Q. Subtype-specific alpha1- and beta-adrenoceptor signaling in the heart. Trends Pharmacol Sci 2006;27:330–337. [DOI] [PubMed] [Google Scholar]
- 8.Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S, Stinson EB. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res 1986;59:297–309. [DOI] [PubMed] [Google Scholar]
- 9.Eschenhagen T. G proteins and the heart. Cell Biol Int 1993;17:723–749. [DOI] [PubMed] [Google Scholar]
- 10.Rau T, Nose M, Remmers U, Weil J, Weissmuller A, Davia K, Harding S, Peppel K, Koch WJ, Eschenhagen T. Overexpression of wild-type Galpha(i)-2 suppresses beta-adrenergic signaling in cardiac myocytes. FASEB J 2003;17:523–525. [DOI] [PubMed] [Google Scholar]
- 11.DeGeorge BR Jr, Gao E, Boucher M, Vinge LE, Martini JS, Raake PW, Chuprun JK, Harris DM, Kim GW, Soltys S, Eckhart AD, Koch WJ. Targeted inhibition of cardiomyocyte Gi signaling enhances susceptibility to apoptotic cell death in response to ischemic stress. Circulation 2008;117:1378–1387. [DOI] [PubMed] [Google Scholar]
- 12.El-Armouche A, Zolk O, Rau T, Eschenhagen T. Inhibitory G-proteins and their role in desensitization of the adenylyl cyclase pathway in heart failure. Cardiovasc Res 2003;60:478–487. [DOI] [PubMed] [Google Scholar]
- 13.Foerster K, Groner F, Matthes J, Koch WJ, Birnbaumer L, Herzig S. Cardioprotection specific for the G protein Gi2 in chronic adrenergic signaling through beta 2-adrenoceptors. Proc Natl Acad Sci USA 2003;100:14475–14480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohler D, Devanathan V, Bernardo de Oliveira Franz C, Eldh T, Novakovic A, Roth JM, Granja T, Birnbaumer L, Rosenberger P, Beer-Hammer S, Nurnberg B. Galphai2- and Galphai3-deficient mice display opposite severity of myocardial ischemia reperfusion injury. PLoS ONE 2014;9:e98325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci USA 1999;96:7059–7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liggett SB, Tepe NM, Lorenz JN, Canning AM, Jantz TD, Mitarai S, Yatani A, Dorn GW II. Early and delayed consequences of beta(2)-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation 2000;101:1707–1714. [DOI] [PubMed] [Google Scholar]
- 17.Keller K, Maaß M, Dizayee S, Müller-Ehmsen J, Birnbaumer L, Engelhardt S, Herzig S, Matthes J. Reduction of survival and development of cardiac hypertrophy by Gi2 deficiency in beta1-adrenoceptor overexpressing mice. Naunyn Schmiedebergs Arch Pharmacol 2014;387:S56. [Google Scholar]
- 18.Rudolph U, Brabet P, Hasty P, Bradley A, Birnbaumer L. Disruption of the G(i2) alpha locus in embryonic stem cells and mice: a modified hit and run strategy with detection by a PCR dependent on gap repair. Transgenic Res 1993;2:345–355. [DOI] [PubMed] [Google Scholar]
- 19.Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, Boulay G, Bradley A, Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet 1995;10:143–150. [DOI] [PubMed] [Google Scholar]
- 20.Foerster K, Kaeferstein T, Groner F, Engelhardt S, Matthes J, Koch WJ, Lohse MJ, Herzig S. Calcium channel function and regulation in beta 1- and beta 2-adrenoceptor transgenic mice. Naunyn Schmiedebergs Arch Pharmacol 2004;369:490–495. [DOI] [PubMed] [Google Scholar]
- 21.Ghanem A, Troatz C, Elhafi N, Dewald O, Heeschen C, Nickenig G, Stypmann J, Tiemann K. Quantitation of myocardial borderzone using reconstructive 3-D echocardiography after chronic infarction in rats: incremental value of low-dose dobutamine. Ultrasound Med Biol 2008;34:559–566. [DOI] [PubMed] [Google Scholar]
- 22.Kanno S, Lerner DL, Schuessler RB, Betsuyaku T, Yamada KA, Saffitz JE, Kovacs A. Echocardiographic evaluation of ventricular remodeling in a mouse model of myocardial infarction. J Am Soc Echocardiogr 2002;15:601–609. [DOI] [PubMed] [Google Scholar]
- 23.Bai Y, Morgan EE, Giovannucci DR, Pierre SV, Philipson KD, Askari A, Liu L. Different roles of the cardiac Na+/Ca2+-exchanger in ouabain-induced inotropy, cell signaling, and hypertrophy. Am J Physiol Heart Circ Physiol 2013;304:H427–H435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dizayee S, Kaestner S, Kuck F, Hein P, Klein C, Piekorz RP, Meszaros J, Matthes J, Bjrnbaumer L, Nurnberg B, Herzig S. Galphai2- and Galphai3-specific regulation of voltage-dependent L-type calcium channels in cardiomyocytes. PLoS ONE 2011;6:e24979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiege K, Le DD, Syed SN, Ali SR, Novakovic A, Beer-Hammer S, Piekorz RP, Schmidt RE, Nurnberg B, Gessner JE. Defective macrophage migration in Galphai2- but not Galphai3-deficient mice. J Immunol 2012;189:980–987. [DOI] [PubMed] [Google Scholar]
- 26.Jiang M, Spicher K, Boulay G, Martin-Requero A, Dye CA, Rudolph U, Birnbaumer L. Mouse gene knockout and knockin strategies in application to alpha subunits of Gi/Go family of G proteins. Methods Enzymol 2002;344:277–298. [DOI] [PubMed] [Google Scholar]
- 27.Exner T, Jensen ON, Mann M, Kleuss C, Nurnberg B. Posttranslational modification of Galphao1 generates Galphao3, an abundant G protein in brain. Proc Natl Acad Sci USA 1999;96:1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gohla A, Klement K, Piekorz RP, Pexa K, vom Dahl S, Spicher K, Dreval V, Haussinger D, Birnbaumer L, Nurnberg B. An obligatory requirement for the heterotrimeric G protein Gi3 in the antiautophagic action of insulin in the liver. Proc Natl Acad Sci USA 2007;104:3003–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leiss V, Flockerzie K, Novakovic A, Rath M, Schonsiegel A, Birnbaumer L, Schurmann A, Harteneck C, Nurnberg B. Insulin secretion stimulated by l-arginine and its metabolite l-ornithine depends on Galphai2. Am J Physiol Endocrinol Metab 2014;307:E800–E812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leopoldt D, Harteneck C, Nurnberg B. G proteins endogenously expressed in Sf 9 cells: interactions with mammalian histamine receptors. Naunyn Schmiedebergs Arch Pharmacol 1997;356:216–224. [DOI] [PubMed] [Google Scholar]
- 31.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 2002;30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiege K, Ali SR, Gewecke B, Novakovic A, Konrad FM, Pexa K, Beer-Hammer S, Reutershan J, Piekorz RP, Schmidt RE, Nurnberg B, Gessner JE. Galphai2 is the essential Galphai protein in immune complex-induced lung disease. J Immunol 2013;190:324–333. [DOI] [PubMed] [Google Scholar]
- 33.Lee GJ, Yan L, Vatner DE, Vatner SF. Mst1 inhibition rescues beta1-adrenergic cardiomyopathy by reducing myocyte necrosis and non-myocyte apoptosis rather than myocyte apoptosis. Basic Res Cardiol 2015;110:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain M, Lim CC, Nagata K, Davis VM, Milstone DS, Liao R, Mortensen RM. Targeted inactivation of Galpha(i) does not alter cardiac function or beta-adrenergic sensitivity. Am J Physiol Heart Circ Physiol 2001;280:H569–H575. [DOI] [PubMed] [Google Scholar]
- 35.Melsom CB, Hussain RI, Orstavik O, Aronsen JM, Sjaastad I, Skomedal T, Osnes JB, Levy FO, Krobert KA. Non-classical regulation of beta1- and beta 2-adrenoceptor-mediated inotropic responses in rat heart ventricle by the G protein Gi. Naunyn Schmiedebergs Arch Pharmacol 2014;387:1177–1186. [DOI] [PubMed] [Google Scholar]
- 36.Melsom CB, Orstavik O, Osnes JB, Skomedal T, Levy FO, Krobert KA. Gi proteins regulate adenylyl cyclase activity independent of receptor activation. PLoS ONE 2014;9:e106608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engelhardt S, Hein L, Keller U, Klambt K, Lohse MJ. Inhibition of Na(+)-H(+) exchange prevents hypertrophy, fibrosis, and heart failure in beta(1)-adrenergic receptor transgenic mice. Circ Res 2002;90:814–819. [DOI] [PubMed] [Google Scholar]
- 38.Leineweber K, Heusch G, Schulz R. Regulation and role of the presynaptic and myocardial Na+/H+ exchanger NHE1: effects on the sympathetic nervous system in heart failure. Cardiovasc Drug Rev 2007;25:123–131. [DOI] [PubMed] [Google Scholar]
- 39.Dobrev D, Wehrens XH. Role of RyR2 phosphorylation in heart failure and arrhythmias: controversies around ryanodine receptor phosphorylation in cardiac disease. Circ Res 2014;114:1311–1319; discussion 1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Layland J, Grieve DJ, Cave AC, Sparks E, Solaro RJ, Shah AM. Essential role of troponin I in the positive inotropic response to isoprenaline in mouse hearts contracting auxotonically. J Physiol 2004;556:835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marston SB, de Tombe PP. Troponin phosphorylation and myofilament Ca2+-sensitivity in heart failure: increased or decreased? J Mol Cell Cardiol 2008;45:603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang X, Pi Y, Lee KJ, Henkel AS, Gregg RG, Powers PA, Walker JW. Cardiac troponin I gene knockout: a mouse model of myocardial troponin I deficiency. Circ Res 1999;84:1–8. [DOI] [PubMed] [Google Scholar]
- 43.Engelhardt S, Boknik P, Keller U, Neumann J, Lohse MJ, Hein L. Early impairment of calcium handling and altered expression of junctin in hearts of mice overexpressing the beta1-adrenergic receptor. FASEB J 2001;15:2718–2720. [DOI] [PubMed] [Google Scholar]
- 44.Marks AR. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J Clin Invest 2013;123:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engelhardt S, Hein L, Dyachenkow V, Kranias EG, Isenberg G, Lohse MJ. Altered calcium handling is critically involved in the cardiotoxic effects of chronic beta-adrenergic stimulation. Circulation 2004;109:1154–1160. [DOI] [PubMed] [Google Scholar]
- 46.Haghighi K, Kolokathis F, Pater L, Lynch RA, Asahi M, Gramolini AO, Fan GC, Tsiapras D, Hahn HS, Adamopoulos S, Liggett SB, Dorn GW II, MacLennan DH, Kremastinos DT, Kranias EG. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest 2003;111:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature 1997;390:88–91. [DOI] [PubMed] [Google Scholar]
- 48.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis : role of a pertussis toxin-sensitive G protein. Circulation 1999;100:2210–2212. [DOI] [PubMed] [Google Scholar]
- 49.Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, Talan MI. Beneficial effects of chronic pharmacological manipulation of beta-adrenoreceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation 2004;110:1083–1090. [DOI] [PubMed] [Google Scholar]
- 50.Ahmet I, Lakatta EG, Talan MI. Pharmacological stimulation of beta2-adrenergic receptors (beta2AR) enhances therapeutic effectiveness of beta1AR blockade in rodent dilated ischemic cardiomyopathy. Heart Fail Rev 2005;10:289–296. [DOI] [PubMed] [Google Scholar]
- 51.Peter PS, Brady JE, Yan L, Chen W, Engelhardt S, Wang Y, Sadoshima J, Vatner SF, Vatner DE. Inhibition of p38 alpha MAPK rescues cardiomyopathy induced by overexpressed beta 2-adrenergic receptor, but not beta 1-adrenergic receptor. J Clin Invest 2007;117:1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Communal C, Colucci WS, Singh K. p38 mitogen-activated protein kinase pathway protects adult rat ventricular myocytes against beta-adrenergic receptor-stimulated apoptosis. Evidence for Gi-dependent activation. J Biol Chem 2000;275:19395–19400. [DOI] [PubMed] [Google Scholar]
- 53.Xiao RP, Zhang SJ, Chakir K, Avdonin P, Zhu W, Bond RA, Balke CW, Lakatta EG, Cheng H. Enhanced G(i) signaling selectively negates beta2-adrenergic receptor (AR)—but not beta1-AR-mediated positive inotropic effect in myocytes from failing rat hearts. Circulation 2003;108:1633–1639. [DOI] [PubMed] [Google Scholar]
- 54.Reinartz MT, Kalble S, Littmann T, Ozawa T, Dove S, Kaever V, Wainer IW, Seifert R. Structure-bias relationships for fenoterol stereoisomers in six molecular and cellular assays at the beta2-adrenoceptor. Naunyn Schmiedebergs Arch Pharmacol 2015;388:51–65. [DOI] [PubMed] [Google Scholar]
- 55.Kaur K, Parra S, Chen R, Charbeneau RA, Wade SM, Jay PY, Neubig RR. Galphai2 signaling: friend or foe in cardiac injury and heart failure? Naunyn Schmiedebergs Arch Pharmacol 2012;385:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hussain RI, Aronsen JM, Afzal F, Sjaastad I, Osnes JB, Skomedal T, Levy FO, Krobert KA. The functional activity of inhibitory G protein (G(i)) is not increased in failing heart ventricle. J Mol Cell Cardiol 2013;56:129–138. [DOI] [PubMed] [Google Scholar]