Abstract
Interventions targeting physical activity may be valuable as an adjunct to alcohol treatment, but have been relative untested. In the current study, alcohol dependent, physically sedentary patients were randomized to: a 12-week moderate-intensity, group aerobic exercise intervention (AE; n = 25) or a brief advice to exercise intervention (BA-E; n=23). Results showed that individuals in AE reported significantly fewer drinking and heavy drinking days, relative to BA-E during treatment. Furthermore adherence to AE strengthened the beneficial effect of intervention on alcohol use outcomes. While high levels of moderate-intensity exercise appeared to facilitate alcohol recovery regardless of intervention arm, attending the group-based AE intervention seemed to further enhance the positive effects of exercise on alcohol use. Study findings indicate that a moderate intensity, group aerobic exercise intervention is an efficacious adjunct to alcohol treatment. Improving adherence to the intervention may enhance its beneficial effects on alcohol use.
Introduction
While treatments for alcohol dependence have demonstrated efficacy (W. R. Miller & Wilbourne, 2002; Read, Kahler, & Stevenson, 2001), relapse remains problematic with rates of relapse in the first year following treatment ranging from 60 – 90% (Brownell, Marlatt, Lichtenstein, & Wilson, 1986; Maisto, Connors, & Zywiak, 2000; W.R. Miller, Walters, & Bennett, 2001; Weisner, Matzger, & Kaskutas, 2003). Over the years, stagnant rates of relapse in alcohol use and other addictive disorders have garnered much needed attention (Marlatt & Donovan, 2005; Moos & Moos, 2006), which led to new approaches to relapse prevention. The work of Marlatt and colleagues’ (Marlatt & Donovan, 2005) is among the most prominent and relapse prevention strategies based on this model have shown promise in the treatment for alcohol use disorders (Carroll, 1996; Irvin, Bowers, Dunn, & Wang, 1999; Witkiewitz & Marlatt, 2004). However, unequal attention has been paid to each component of this model. In particular, the lifestyle modification component, one of the primary domains of Marlatt’s model, has received the least emphasis in relapse prevention programs for alcohol dependence (Marlatt & Witkiewitz, 2005; Witkiewitz & Marlatt, 2004), and as a result, rigorous empirical evaluation is lacking.
Among approaches to lifestyle modification, exercise holds particular promise for relapse prevention. Exercise has been described as “a highly recommended lifestyle change activity” for relapse prevention (Marlatt & Gordon, 1985, P 309), and the potential value of exercise and fitness in the prevention and treatment of addictive disorders has been widely noted (Agne & Paolucci, 1982; Taylor, Sallis, & Needle, 1985; Tkachuk & Martin, 1999). A growing body of empirical research has begun to explore potential treatment applications of exercise for a variety of clinical problems (USDHHS, 1996). Exercise also has the potential to be cost-effective, flexible and accessible; many forms of exercise may be conducted independently at little expense. Moreover, exercise has minimal side effects and far less risk of adverse events than the use of psychotropic medication (Broocks et al., 1998). In short, exercise appears to offer decided advantages as a treatment strategy for alcohol dependent individuals.
Despite its potential value, only two controlled studies have examined the effects of an exercise intervention in individuals with excessive alcohol use. A study involving inpatients in alcohol rehabilitation treatment (Sinyor, Brown, Rostant, & Seraganian, 1982) revealed that participants in the exercise group demonstrated better abstinence outcomes post-treatment than did non-exercising participants. A later study (Murphy, Pagano, & Marlatt, 1986) found that heavy drinking college students assigned to either running or yoga/meditation demonstrated significant decreases in quantity of alcohol consumption relative to no exercise control participants. Findings from both studies are consistent in supporting a positive relationship between exercise and drinking outcomes. However, both studies also suffer from methodological limitations, including non-random assignment to treatment (i.e., using different treatment site as a comparison group) in the former study (Sinyor, et al., 1982), and use of a non-clinical population (i.e., participants were undergraduates who qualified as heavy social drinkers) (Murphy, et al., 1986).
In addition, no studies to date have examined potential mediators of the relationship between exercise and alcohol use in a controlled exercise study. There are a number of proposed mechanisms by which exercise may facilitate recovery from alcohol problems; in particular, the positive impacts of exercise on symptoms of depression and anxiety have been well documented (Babyak et al., 2000; Carek, Laibstain, & Carek, 2011; Craft & Landers, 1998; Dinas, Koutedakis, & Flouris, 2011; Dunn, Trivedi, & O’Neal, 2001; Gill, Womack, & Safranek, 2010; Lawlor & Hopker, 2001; Mead et al., 2009; Perraton, Kumar, & Machotka, 2010). Such evidence suggests that exercise may reduce relapse risk by alleviating symptoms of depression and anxiety, especially given that depressive and anxiety symptoms are common among alcohol dependent patients and are often associated with relapse and poor treatment outcome (R. A. Brown et al., 1998; Gill, et al., 2010; Suter, Strik, & Moggi, 2011). Research has also supported the role of self-efficacy in alcohol recovery, with higher self-efficacy to avoid drinking consistently predicting better treatment outcomes (Greenfield et al., 2000; Ilgen, Tiet, Finney, & Moos, 2006; Rychtarik, Prue, Rapp, & King, 1992; Vielva & Iraurgi, 2001). Engaging in regular exercise may indirectly increase self-efficacy via its beneficial effects on positive and negative affect (Peluso & Guerra de Andrade, 2005; Penedo & Dahn, 2005; Reed & Ones, 2006) and craving (Ussher et al., 2004), and in turn, reduce alcohol intake or risk of relapse. In summary, there is theoretical and empirical support for the proposition that exercise interventions may lead to positive outcomes for alcohol dependent patients through several underlying mechanisms. However, to date standardized, structured, exercise-based interventions for alcohol use have not been evaluated in methodologically rigorous clinical trials.
The current study is the second in a series of studies intended to address this gap in the literature. In the first study (R. A. Brown et al., 2009), we described the development of a 12-week, supervised group aerobic exercise program as an adjunctive intervention for alcohol dependent patients in recovery. The exercise intervention also taught participants cognitive-behavioral strategies to help them incorporate exercise into their daily lives and offered financial incentives for program adherence. We also presented preliminary data from a small pilot study demonstrating the feasibility of the intervention and yielding significant reductions in alcohol consumption and improvements in cardiorespiratory fitness (R. A. Brown, et al., 2009). In the present study, we report the results of a preliminary, randomized controlled trial of this adjunctive exercise intervention for individuals in early recovery from alcohol dependence. In addition to providing a more rigorous test of this novel intervention for alcohol dependence, the present study addresses methodological limitations of previous studies. Specifically, participants were randomly assigned to treatment condition, and the exercise sessions were supervised, with duration and intensity carefully monitored and recorded to ensure patient participation and accurate delivery of the intervention.
First, we hypothesized that, as an adjunct to treatment among physically sedentary alcohol dependent patients in early recovery, a 12-week moderate-intensity group aerobic exercise intervention (AE) would be more effective than a brief advice to exercise comparison condition (BA-E) in reducing the quantity and frequency of alcohol use due to the exercise supervision, group support, cognitive-behavioral strategies and financial incentives offered by the AE intervention. Secondly, we predicted that regardless of intervention conditions, higher levels of exercise at follow-up assessments would be associated with lower levels of alcohol use. Thirdly, we hypothesized that AE would yield greater improvements in depressive and anxiety symptoms, and self-efficacy (potential mediators/secondary outcomes) relative to BA-E. Finally, we predicted that greater levels of exercise as well as cardiorespiratory fitness would be observed in AE compared to BA-E.
Method
Participants
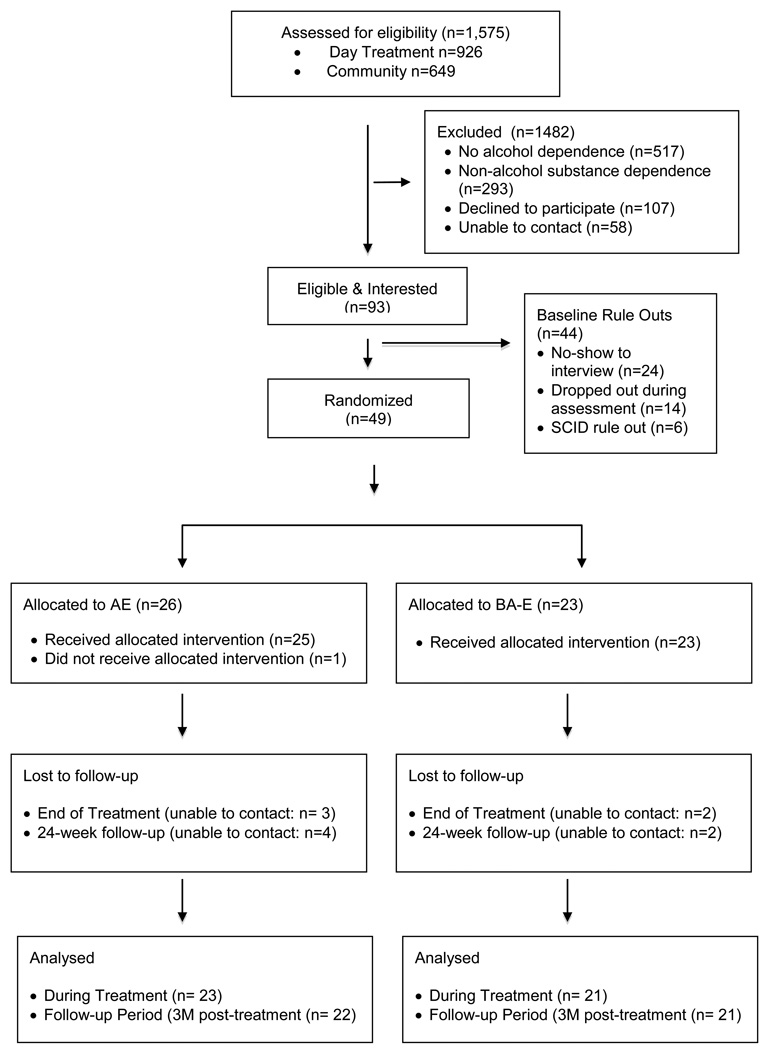
A total of 49 participants were recruited from alcohol and drug day treatment services program at Butler Hospital (n = 20) and from the community via media advertisements (n = 29). Patients at the Butler Hospital program had an attending psychiatrist, a counselor whom they met with daily to coordinate their treatment and they participated in cognitive-behavioral groups aimed at teaching sobriety and relapse prevention coping skills. Study participants met the following inclusion criteria; 1) being between 18 and 65 years of age, 2) meeting DSM-IV-TR criteria for alcohol dependence, 3) currently sedentary (i.e., exercising less than 60 minutes per week for the past 6 months), 4) being in early alcohol recovery (i.e., currently in alcohol treatment and abstinent from alcohol for less than 90 days), and 5) having been medically cleared to engage in moderate intensity exercise by the study physician. The exclusion criteria included: 1) non-alcohol, substance dependence (except nicotine dependence), 2) anorexia or bulimia nervosa, 3) bipolar disorder, 4) a history of psychotic disorder or current psychotic symptoms, 5) current suicidality, 6) marked organic impairment, 7) physical disabilities, medical problems, or use of medications that would interfere with participation in a program of moderate exercise, and 8) current pregnancy or intent to become pregnant during the next 12 weeks. The consort diagram is shown in Figure 1. Participants were recruited from an intensive alcohol treatment program and from the community, and provided informed consent. Demographic data and baseline drinking variables are presented in Table 1, and indicate that control and treatment groups were equivalent in age, gender, ethnicity and education, marital status, employment status and baseline drinking variables.
Figure 1.
CONSORT diagram for enrollment and allocation of participants for AE and BA-E conditions.
Table 1.
Demographic characteristics for intent-to-treat and excluded sample.
| Intent to Treat | Excluded | ||||
|---|---|---|---|---|---|
| BA-E N=23 |
AE N=26 |
Treatment Period N=5 |
Follow-up Period N=6 |
||
|
M (SD) n (%) |
M (SD) n (%) |
M (SD) n (%) |
M (SD) n (%) |
||
| Age | 45.39 (9.99) | 43.46 (11.50) | 40.20 (8.58) | 37.00 (10.97) | |
| Female | 11 (42%) | 11 (48%) | 1 (20%) | 2 (33.3%) | |
| Race/Ethnicity | |||||
| Hispanic | 0 (0.0 %) | 1 (3.8%) | 0 (0.0 %) | 0 (0%) | |
| White | 21 (91.3%) | 24 (92.3%) | 4 (80%) | 5 (83.3%) | |
| African-American | 2 (8.7%) | 1 (3.8%) | 1 (20%) | 1 (16.7%) | |
| Education | |||||
| < High school graduate | 1 (4.3%) | 1 (3.8%) | 2 (40%) | 2 (33.3%) | |
| High school graduate | 4 (17.4%) | 8 (30.8%) | 1 (10%) | 1 (16.7%) | |
| Some College | 11 (47.8%) | 7 (26.9%) | 2 (40%) | 3 (50%) | |
| College degree | 7 (30.4%) | 10 (28.5%) | 0 (0%) | 0 (0%) | |
| Marital Status | |||||
| Married | 11 (47.8%) | 12 (46.2%) | 2 (40%) | 2 (33.3%) | |
| Divorced | 3 (13.0%) | 6 (23.1%) | 0 (0%) | 0 (0%) | |
| Never married | 8 (34.8%) | 8 (30.8%) | 3 (60%) | 4 (66.7%) | |
| Widowed | 1 (4.3%) | 0 (0.0%) | 0 (0%) | 0 (0.0%) | |
| Employment | |||||
| Full time | 15 (65.2%) | 16 (61.5%) | 2 (40%) | 2 (33.3%) | |
| Part time | 4 (17.4%) | 4 (15.4%) | 2 (40%) | 3 (50%) | |
| Retired/Disable | 1 (4.3%) | 5 (19.2%) | 0 (0%) | 0 (0%) | |
| Unemployed | 3 (13.0%) | 1 (3.8%) | 1 (20%) | 1 (16.7%) | |
| Percent Drinking Days | 51.6 (25.9) | 38.2 (27.6) | 32.7 (24.0) | 44.6 (25.9) | |
| Percent Heavy Drinking Days | 40.8 (29.7) | 29.0 (27.7) | 47.0 (29.2) | 33.0 (29.5) | |
| Number of Drinks per Day | 5.41 (4.12) | 4.51 (4.73) | 5.18 (4.90) | 4.75 (4.35) | |
Note: There was no difference in demographic characteristic or baseline alcohol variables between conditions (ps >.05).
Measures
Health Questionnaire & Physical Activity Screen
The health questionnaire assessed health history and status, including medical conditions that might complicate participation in a moderate intensity exercise program. The interview was used as a screen to determine sedentary status, and queried regular engagement in moderate intensity activity and assessed frequency and length of time of participation in exercise of this type.
Structured Clinical Interview for DSM-IV (SCID-P)
Relevant sections of the SCID-P (First, Spitzer, Gibbon & William, 1995) were administered to determine diagnostic criteria for inclusion/exclusion criteria.
Time-Line-Follow-Back (TLFB)
The TLFB interview was used to assess daily alcohol use at baseline and during follow-up. The TLFB has excellent reliability (Sobell, Maisto, Sobell, & Cooper, 1979) and validity (Sobell & Sobell, 1980). Data from the TLFB were summarized over 90 days prior to allocation, 12-week treatment, and 12-week post-treatment follow-up periods to yield the baseline alcohol variable (i.e., the average number of drinks per day) and primary alcohol outcome variables: number of drinking days (i.e., number of days any drinking was reported) and number of heavy drinking days (i.e., number of days heavy drinking was reported). A heavy drinking day was defined as 5 drinks per day for men and 4 drinks per day for women.
Breathanalysis
At baseline, each exercise session, and each follow-up assessment, breath was analyzed for alcohol to confirm abstinence prior to exercise participation and interview completion and to further corroborate self-report data.
Depressive Symptoms
The Center for Epidemiological Studies-Depression scale (CES-D), which has demonstrated good reliability and validity (Radloff, 1977), was administered to assess level of depressive symptoms.
Anxiety Symptoms
The Spielberger State-Trait Anxiety Inventory (STAI; (Spielberger et al., 1988), which has strong psychometric properties (Spielberger, et al., 1988), was used to evaluate anxiety symptoms.
Self-Efficacy for Alcohol Abstinence
The Situational Confidence Questionnaire (SCQ;(Annis & Graham, 1988) was used to assess Bandura’s concept of self-efficacy for alcohol-related situations. The SCQ has been found to be a good predictor of treatment outcome (Burling, Reilly, Moltzen, & Ziff, 1989).
Level of Exercise
The TLFB, which has demonstrated reliability and validity for assessing levels of daily exercise over extended periods of time (Panza, Weinstock, Ash, & Pescatello, 2012), was employed to assess type, intensity level, and duration of daily exercise at baseline and during follow-up. TLFB for exercise was used to calculate the average number of minutes of exercise each week during the 12-weeks of active treatment and the 12-week post-treatment follow-up that was rated to be at least of moderate intensity (Rating of Perceived Exertion [RPE] ≥ 11).
Physical Fitness
The estimated maximal oxygen consumption (VO2 max) was obtained during the cardiorespiratory fitness test, conducted by the study physician or a masters-level exercise physiologist, using a submaximal graded exercise protocol on a motorized treadmill, at baseline and 12-week assessments. Increases in estimated VO2 max at follow-up assessment indicated improved cardiorespiratory fitness.
Procedure
The intervention is described in detail elsewhere (Brown, et al., 2009). Briefly, participants were randomized to either moderate intensity, group aerobic exercise (AE) or a brief advice to exercise (BA-E) comparison condition using urn randomization (Wei, 1978) to assure a balanced distribution according to gender and age. All participants completed baseline assessments and follow-up interviews at the end of treatment (12 weeks) and at 6-months after baseline assessment (6-month follow-up). Group Aerobic.
Exercise (AE)
The AE was comprised of three primary components: 1) moderate-intensity aerobic exercise, 2) group behavioral treatment and 3) incentive system. The exercise component involved 12 weekly aerobic exercise group sessions at the study fitness facility located on the grounds of Butler Hospital. Although occurring infrequently, participants with transportation difficulties were provided with taxicab vouchers to attend exercise sessions. Participants joined the group on a rolling admissions basis, and group size ranged from 2 – 5, with a modal group size of 3 participants. Under the supervision of a masters level exercise physiologist, participants at each group session exercised on a treadmill, elliptical machine or recumbent bicycle. At the beginning of each participant’s initial weekly exercise group, the exercise physiologist provided information regarding the psychological and physical benefits of moderate intensity exercise, and oriented participants on all aspects of the exercise intervention. In subsequent sessions, the exercise physiologist guided participants on the intensity and duration of the exercise to be performed. Exercise sessions began at 20 minutes per session and gradually progressed to 40 minutes per session by week 12. Participants exercised at a rate that achieved 55 – 69% (moderate-intensity) of age-predicted maximal heart rate. This exercise regimen is consistent with the guidelines offered by the American College of Sports Medicine (ACSM; (American College of Sports Medicine, 2000). Participants in the study were also given “prescriptions” from the exercise physiologist (tailored to their level of fitness) to engage in moderate-intensity aerobic exercise on a minimum of two to three other occasions during the week for the duration.
Group behavioral treatment involved weekly, brief (15–20 minutes) group interventions based on cognitive and behavioral techniques. Through these weekly groups, participants were guided as to how to increase overall fitness through behavioral changes in their daily lives. Each brief group session was focused on a certain topic designed to increase overall motivation resulting in improved exercise adherence and maintenance. Modules included: 1) Benefits of Exercise, 2) Benefits of Exercise for Alcohol Recovery, 3) Goal Setting, 4) Getting and Staying Motivated, 5) Getting Back on Track, 6) Exercise and Coping with Negative Moods, 7) Identifying and Overcoming Barriers, 8) Time Management, 9) Basic Information for Exercising Wisely, 10) Maintenance of Exercise, 11) Making Plans for Action, 12) Social Support. In order to maximize adherence to the exercise program and self-monitoring of daily exercise activities, participants earned incentives for various levels of adherence. The incentive consisted of providing participants with $5 for attending each weekly combined group/exercise session and an additional $5 for returning their completed exercise self-monitoring form (from the prior week) at that session. In addition, participants earned the opportunity to draw for a voucher (in amount ranging from $10 – $50) at each weekly session, if they had consecutive attendance (i.e., had attended the prior week’s group + exercise session).
Brief advice to exercise (BA-E)
In BA-E, a doctoral level clinical psychologist or an exercise physiologist informed participants that they had been medically cleared to begin a moderate exercise regimen, and engaged in a 15–20 minute discussion of the psychological and physical benefits of exercise. The intervention also included public health recommendations for exercise frequency (ACSM, 2000), duration, and intensity, and a written handout to summarize the information provided during this session. BA-E participants did not receive payments contingent upon attendance and self-monitoring and did not draw from a fish bowl, but rather were all paid an amount that was expected to be equivalent to the average amount of the contingent incentives earned in the AE condition. As participants were recruited on a rolling admissions basis and we could not know early in the study the average amount that AE participants would earn in this study, all BA-E participants were paid $194 (in gift cards) at their 12 week, end of treatment time point: the average amount paid to the AE group participants (with the same adherence contingencies) in a prior pilot study (R. A. Brown, et al., 2009).
Data Analysis Plan
Primary aims of this preliminary randomized trial were evaluated using linear models appropriate for drinking and exercise outcomes. Primary drinking outcomes based upon TLFB were number of drinking days and heavy drinking days assessed during the 12-week treatment period and the 12-week follow-up. Poisson, negative binomial and zero-inflated regression models for count outcomes were fit separately for drinking outcomes during treatment and during follow-up. We proceeded with zero-inflated Poisson (ZIP) models, which allow for non-normally distributed counts of drinking events and frequent reports of no drinking (e.g., zero-inflation), given that the Vuong Non-Nested Hypothesis Test-Statistic (Long, 1997) of −3.53 (p<0.01) and −4.64 (p<0.01) consistently supported the improved fit of the zero-inflated over standard Poisson models for drinking during treatment and follow-up periods, respectively. Model testing did not support the use of less restricted, negative binomial models (Osgood, 2000; Paternoster & Brame, 1997) suggesting that count outcomes were not overdispersed and the log (Theta) parameter was not significantly different than zero in any model (ps >0.10) (Long, 1997). For the exercise outcomes, we used robust regression to reduce the influence of outliers (Rousseeuw & Yohai, 1984). Participants who provided at least 2/3 of self-report assessments (56 days) during the 12-week treatment and 12-week follow-up periods were included in the primary outcome analyses (Group, 1997). For each period, the count of drinking days and heavy drinking days was created by multiplying the valid percentage of reported drinking days and heavy drinking days (i.e., dividing the number of drinking days and heavy drinking days by the number of reported days) by 84 days (12 weeks).
Results
Treatment assignment and adherence
Recruitment, enrollment and treatment allocation information and accompanying sociodemographic characteristics of AE and BA-E participants are listed in the consort diagram (see Figure 1). A total of 1575 individuals screened for eligibility for the study were recruited from alcohol treatment program (n=926) and the community (n=649). Of those screened, 305 (32.9%) and 212 (32.7%) did not meet criteria for alcohol dependence and 162 (17.5%) and 131 (20.2%) were excluded due to reporting non-alcohol substance dependence from the alcohol and drug day treatment program and the community, respectively. There was no difference in the proportion of participants who were ruled out due to a lack of alcohol dependence or meeting criteria for non-alcohol substance dependence between the two recruitment venues (ps > .05).
During the recruitment period, 93 participants were scheduled for baseline assessments. Of these, 6 (6%) were excluded because of a SCID-diagnosed psychiatric rule out, 24 (26%) did not show up for their baseline appointment, and 14 (15%) did not complete the baseline assessment because they failed to show up for the submaximal graded exercise test. A total of 49 participants were eligible to participate in the 12-week aerobic exercise intervention. There were no serious adverse events during the study. Self-report assessments were available for 90% (n=44) and 88 % (n=43) of participants during treatment and at the final 24-week (6 months) assessment, respectively. Follow-up rates for cardiorespiratory fitness test was 61% at 6-months. No significant differences in adherence rates between conditions were observed (ps > 0.57).
Final sample
The sample of 49 participants included 22 (45%) females and 27 (55%) males. The mean age of participants was 44.37 (SD = 10.75) years. The sample was primarily Caucasian (75.51%) with an average of 14.6 (SD = 2.4) years of education. Current alcohol treatments in the 3-months prior to enrollment included inpatient or day hospital care (69%), AA/NA attendance (67%), pharmacotherapy for alcohol use (53%), and contacts with individual providers (73%). Treatment conditions did not differ significantly on demographics (age, gender, education level, marital status, and employment) and baseline drinking variables (i.e., the number of drinks per day, percent drinking days, percent heavy drinking days, ps > .094). In addition, continuous abstinent days prior to study enrollment (the first day of intervention) ranged from 1 to 55 days (mean = 19.12, SD = 12.79). As in other baseline alcohol variables, no difference in the number of continuous abstinent days between AE (mean = 18.48) and BA-E (mean = 19.83) was found (p = 0.72) while a shorter continuous abstinent period prior to the intervention predicted greater number of days drinking or heavy drinking during the treatment (ps ≤ .001), as expected.
Analyses during the treatment and follow-up periods are based upon the 44 and 43 participants, respectively, who provided sufficient data (2/3 of self-report assessments) for analyses. There were no significant differences in baseline characteristics between included and excluded participants, except for years of education. Included participants had greater years of education compared to those of the excluded participants (Table 1).
Of the final sample, 20 participants (AE = 11, BA-E = 9) were recruited from alcohol and drug day treatment services program and 29 participants (AE = 15, BA-E = 14) were from the community. The proportion of participants recruited from the two recruitment sites did not differ between treatment conditions. No differences in the demographics listed above were observed across recruitment sites. However, participants who were recruited from the day treatment reported significantly greater percentage of baseline drinking days and heavy drinking days (M = 58.6%, 45.8%, SD = 29.9, 22.0) than participants recruited from the community (M = 35.7%, 27.5%, SD = 35.8, 21.6); (t(45) = 3.02, 2.19 p = 0.004, 0.03, respectively). While those in the day treatment also reported higher average number of drinks per day (M = 6.3, SD = 5.8) than those from the community (M = 4.1, SD = 3.7), the difference did not reach statistical significance (t(45) = 1.71, p = 0.094).
Adherence to AE intervention
Out of 12 sessions, participants attended an average of 8.44 (SD = 4.12) in the AE condition and nine of the twenty-six participants (35%) in the AE condition completed all 12 sessions. A total of 16 participants (62%) met our criteria for adherence and attended 8 or more of the exercise sessions at the fitness facility. The average attendance and self-monitoring amount earned by AE participants was $60.77 (SD = 38.88) and the average fishbowl amount paid to AE participants was $103.46 (SD = 73.81). All payments were in gift cards to a local supermarket or department store. The total average amount paid to AE participants was $164.23 (SD = 111.11).
Primary Drinking Outcomes by Treatment
Primary drinking outcome models included age, gender, the average number of drinks per day during the 90-days prior to randomization, and average minutes of exercise each week, and evaluated whether AE compared to BA-E had fewer drinking/heavy drinking days (main effect of treatment). Table 2 and 3 list results for components of the models evaluating frequency of drinking (i.e., number of any and heavy drinking days) during treatment and follow-up periods, respectively. During the treatment period, zero-inflated Poisson models for drinking days (X2 (2) = 170.28, p < .01) and heavy drinking days (X2(2) = 116.98, p < .01) were each statistically significant. Results support a significant decrease in drinking days and heavy drinking days for participants in the AE relative to those in BA-E during treatment, an effect that was not maintained during the 12-week follow-up (ps > 0.40). Participants in AE reported −0.27 and −0.54 fewer log count of drinking days and heavy drinking days respectively during treatment while no significant difference in the number of no drinking days was found across treatments (ps > .70)
Table 2.
Results from zero-inflated count regression models for primary drinking outcomes during the 12-week treatment period. Models evaluate number of drinking days for AE relative to BA-E and in relation to weekly average minutes of exercise during the 12-week active treatment period.
| Any Drinking Days |
Heavy Drinking Days |
|||||
|---|---|---|---|---|---|---|
| b | SE | p | b | SE | p | |
| (Intercept) | 0.42 | 0.49 | 0.39 | 2.44 | 0.54 | 0.00 |
| Age | 0.04 | 0.01 | 0.00 | 0.01 | 0.01 | 0.36 |
| Female | −0.06 | 0.16 | 0.73 | 0.24 | 0.22 | 0.29 |
| Baseline Drinks per Day | 0.17 | 0.02 | 0.00 | 0.12 | 0.03 | 0.00 |
| Condition (AE) | −0.27 | 0.11 | 0.02 | −0.54 | 0.14 | 0.00 |
| Minutes of Exercise (sqrt) | 0.02 | 0.01 | 0.12 | −0.06 | 0.02 | 0.00 |
| Condition(AE) X Minutes of Exercise (sqrt)a | 0.23 | 0.03 | 0.00 | 0.16 | 0.04 | 0.00 |
Note: a = Interaction term evaluated in subsequent model. All other terms are derived from main effects models.
Table 3.
Results from zero-inflated count regression models for primary drinking outcomes during the 12-week follow-up period. Models evaluate number of drinking days for GE relative to SIIE and in relation to average minutes of exercise each week during the 12-week post-treatment period.
| Any Drinking Days |
Heavy Drinking Days |
|||||
|---|---|---|---|---|---|---|
| B | SE | p | b | SE | p | |
| (Intercept) | 1.27 | 0.32 | 0.00 | 0.52 | 0.41 | 0.20 |
| Age | 0.05 | 0.01 | 0.00 | 0.06 | 0.01 | 0.00 |
| Female | 0.68 | 0.10 | 0.00 | 1.32 | 0.12 | 0.00 |
| Baseline Drinks per Day | 0.03 | 0.01 | 0.01 | 0.06 | 0.01 | 0.00 |
| Condition (AE) | −0.11 | 0.08 | 0.14 | 0.04 | 0.10 | 0.70 |
| Minutes of Exercise (sqrt) | −0.04 | 0.01 | 0.00 | −0.10 | 0.01 | 0.00 |
| Condition(AE) X Minutes of Exercise (sqrt)a | −0.02 | 0.01 | 0.12 | 0.10 | 0.02 | 0.00 |
Note: a = Interaction term evaluated in subsequent model. All other terms are derived from main effects models.
Drinking outcomes by adherence to AE intervention
Exploratory regression analyses examined whether adherence to AE strengthened observed effects using dummy-coded indicators to compare among AE participants who were/were not adherent (e.g. attended 2/3 of sessions) to BA-E participants. Participants who were adherent to AE had significantly fewer drinking (b = −1.13, 95%CI =−1.53 – −0.73, p = 0.000) and heavy drinking days (b = −0.43, 95%CI =−0.83 – −0.04, p =0.03) than those in BA-E during treatment. Non-adherent participants did not differ from those in BA-E on drinking days during treatment but did have fewer heavy drinking days (b = −0.64, 95%CI = −1.03 – −0.25, p = 0.001). During the follow-up period, adherent participants continued to evidence fewer drinking days (b = −0.71, 95%CI =−0.92 – −0.50, p = 0.000), but had significantly greater heavy drinking days (b = 0.42, 95%CI = 0.09 −0.75 p =0.01) than BA-E participants.
Exercise and Physical Fitness Outcomes by Treatment
Levels of exercise and physical fitness by treatment
Participants in AE and BA-E reported a median of 120 minutes (Interquartile range [IQR] = 60 – 247) and 93.75 minutes (IQR= 0 – 158) respectively during the 12-week active treatment. During the post-treatment follow-up, participants in AE and BA-E reported a median of 154.11 minutes (IQR = 43.75–295.89) and 129.64 minutes (IQR= 88.57– 281.79), respectively. Robust linear regression models for non-normal data that controlled for baseline levels of exercise (during the past 90 days) suggested that AE and BA-E did not differ significantly in levels of moderate exercise during the 12-week active treatment period (b = 62.57, 95%CI =−.82 – 128.96, p = 0.07), or during the 12-week follow-up (p = 0.98).
The estimated VO2 max of ten participants in each condition were not assessed at 12-weeks and baseline values were carried forward. Changes in VO2 max were evaluated in regression models with controls for planned covariates of age, gender, and baseline VO2 max. Participants in AE relative to BA-E had gains in VO2 max after 12-weeks that were not statistically significant (p = 0.53). Average VO2 at baseline of 28.6 (SD=8.3) and 23.1 (SD=4.7) increased to 32.7 (SD=10.6) and 25.9 (SD=7.3) for participants in AE and BA-E, respectively.
Levels of exercise and physical fitness by adherence to AE intervention
Follow-up analyses suggested that adherent participants in AE (b = 110.80, 95%CI =32.01 – 189.59, p = 0.008), but not non-adherent AE participants (p = 0.93), reported greater minutes of exercise than BA-E during treatment. No such difference was observed during the 12-week follow-up (ps >0.10). VO2 max increased significantly more among those adherent to AE compared to those in BA-E (b=4.97, 95%CI = 0.78 – 9.17, p = 0.025).
Primary Drinking Outcomes by Exercise Level
We also evaluated whether level of moderate-intensity exercise was related to both drinking days and heavy drinking days regardless of treatment condition, and whether effects of level of exercise on drinking days and heavy drinking days varied across treatment (treatment by exercise interaction). These models, which included age, gender, intervention condition and baseline drinking days, indicated that while level of exercise (i.e., average minutes of weekly exercise) was related to decreases in drinking days only during the 12-week follow-up period, level of exercise was associated with decreases in heavy drinking days both during treatment and during the 12-week follow-up (ps < 0.01; see Tables 2 & 3). There were also significant interactions suggesting that the effects of exercise on the count of drinking days and heavy drinking days were stronger for AE than BA-E (ps < 0.01) during the treatment and follow-up periods, respectively (Table 2 & Table 3). Participants in AE who exercised more frequently had significantly fewer drinking days during treatment and fewer heavy drinking days during treatment and follow-up periods, compared to BA-E participants exercising at the equivalent level.
Secondary Outcomes: Depression, Anxiety, and Self-efficacy
Levels of depressive and anxiety symptoms, and abstinence self-efficacy were assessed at baseline, end of the 12-week treatment, and at the 12-week follow-up. Regression models with planned covariates and control for corresponding scores at baseline suggested that at the end of the treatment, there were no significant treatment differences (AE vs. BA-E) in depressive (p = 0.29), anxiety symptoms (p = 0.11) or self-efficacy for abstinence (p = 0.76). Moreover, no significant differences in depression, anxiety, or self-efficacy between AE and BA-E were observed at the 12-week follow-up (ps >.50). Changes in symptoms of depression, anxiety, and self-efficacy were similar for those who were adherent or non-adherent to AE (ps >0.10) during treatment and the follow-up period.
Discussion
In this study, we aimed to test the efficacy of an aerobic exercise intervention as an adjunct treatment in reducing alcohol use among physically sedentary alcohol dependent patients in early recovery. We further examined the effects of the exercise intervention on symptoms of depression, anxiety, self-efficacy, as well as levels of exercise and cardiorespiratory fitness. The results supported our primary hypothesis; the group aerobic exercise intervention (AE) demonstrated efficacy in reducing alcohol use, compared to the brief advice to exercise intervention (BA-E) although the beneficial effects depended on timeframe and treatment adherence. While levels of exercise did not significantly differ across conditions, the AE may enhance the positive impact of exercise on alcohol use. Findings on the secondary outcomes were mixed, however.
As predicted, frequency of alcohol use was reduced in the AE condition, independent of levels of exercise, suggesting that the amount of moderate-intensity exercise did not fully account for observed decreases in alcohol consumption in AE. Active ingredients of the group exercise intervention may not only be exercise-related components, but may also include nonspecific factors (e.g., receiving supervision and support, accountability to others, frequent contact with study staff and other patients or receipt of contingent incentives). This effect was, however, observed only during the intervention, indicating that the group exercise may be helpful for the duration of the program, but may not have lasting effects on alcohol use.
Follow-up analyses also revealed that the benefit of AE on the frequency of drinking was evident only for those who attended sufficient aerobic exercise sessions (i.e., 8 or more out of 12 sessions). In other words, the treatment effects on abstinent days were mainly driven by adherent AE participants. However, it is important to note that the AE and BA-E interventions were not equated for therapist interaction. Those adherent to the AE intervention were exposed to much more social interaction from both the treatment staff as well as other research participants. Given the discrepancy in contact time, treatment effects of the adherent AE group cannot be attributed solely to engaging in actual physical exercise but may have also been due to the additional interaction. In order to isolate the effect of exercise, future studies should be designed such that contact time between conditions is controlled.
In addition, although the main effects of group exercise were limited to the treatment period, this was not true for adherent individuals in AE. Adherent AE participants reported significantly fewer drinking days, relative to those in BA-E, during the follow-up period. This finding deserves attention, especially since there was no difference in levels of exercise between adherent AE participants and those in BA-E during the follow-up period. This suggests that regular participation in a group intervention may have a lasting impact on alcohol abstinence, and highlights the importance of treatment adherence. Identifying factors that facilitate or hinder adherence to an exercise program may be crucial for developing and delivering effective interventions. However, the finding that adherent AE participants had significantly greater heavy drinking days than BA-E participants during the follow-up was unexpected. This finding may be related to reported findings of a positive, dose-response relationship between alcohol consumption and physical activity (Piazza-Gardner & Barry, 2012) or it may be an anomaly due to the small number of adherent participants in the comparison. More research on the effects of aerobic exercise intervention on subsequent alcohol use in larger samples of alcohol dependent individuals should help to clarify this issue.
While there is much room for improvement, it is worth noting that adherence to AE intervention in the current study (over 70%) was comparable to, if not greater than, what was expected from the extant exercise intervention literature in both general and clinical populations (e.g., (Cannistra, Balady, O’Malley, Weiner, & Ryan, 1992; Moore, Ruland, Pashkow, & Blackburn, 1998; Trivedi et al., 2011). For instance, even among those who completed the exercise programs, the overall mean adherence rates reported in exercise intervention studies is around 65% (Dishman, 1988). The rate of adherence to exercise intervention ranging from 50% to 65% were reported in community-based exercise programs (Lee et al., 1996), including those who are part of a prescribed cardiac rehabilitation (Cannistra, et al., 1992; Moore, et al., 1998). In addition, dropout rates of 18–50% have been reported in various exercise interventions (Chinn, White, Harland, Drinkwater, & Raybould, 1999; Linke, Gallo, & Norman, 2011), suggesting that engaging physically sedentary individuals in regular exercise continues to be challenging.
One of the components that may have helped enhance adherence rate in this study may be the cognitive behavioral intervention provided in AE weekly sessions. Past exercise intervention studies that involved behavioral interventions (e.g., goal setting and self-management techniques, problem solving and behavioral contracting) (Coleman et al., 1999; Jakicic, Wing, Butler, & Robertson, 1995) and other components (i.e., self-monitoring, phone calls to check progress and to answer their questions) (DeBusk, Stenestrand, Sheehan, & Haskell, 1990; Macfarlane, Taylor, & Cuddihy, 2006) showed considerably low attrition rates (7–12%). Moreover, our incentive system may have also successfully enhanced motivation to attend exercise sessions, as contingent incentives have been shown to increase exercise participation (Irons, Pope, Pierce, Van Patten, & Jarvis, 2013). External rewards may be useful, especially if short-term adherence to prescribed exercise/intervention has lasting benefits on alcohol use as seen in this study. However, a more generalizable strategy to enhance adherence is needed.
Finally, interventions did not significantly predict changes in potential mediators (i.e., depressive and anxiety symptoms and self-efficacy), as well as level of exercise and physical fitness, contrary to our hypotheses. Improved physical fitness level was found in adherent AE participants who engaged in a greater amount of moderate-intensity exercise, compared to the BA-E group or non-adherent AE participants. However, to our surprise, there were no differences in depressive and anxiety symptoms or self-efficacy between adherent AE participants and BA-E participants. There are several possible explanations for the present findings. One may be that a period of alcohol abstinence (prior to entry in this study) had already alleviated depressive and anxiety symptoms (S. A. Brown & Schuckit, 1988; Heinz et al., 1996; Lejoyeux, 1996; West & Gocka, 1986) and enhanced abstinence self-efficacy, above and beyond the effects of the intervention. Another possibility is that the intervention and exercise influenced drinking behaviors through alternative unexamined mechanisms such as decreases in the urge to drink (Ussher, Sampuran, Doshi, West, & Drummond, 2004), increases in positive affect (Reed & Ones, 2006), enhanced cognitive functioning (Colcombe & Kramer, 2003; Williams & Lord, 1997) or improved sleep (Driver & Taylor, 2000; King et al., 2008).
In addition, it is important to note that although no significant difference in exercise was found between conditions, both conditions showed an increase in levels of exercise from their baseline levels. In fact, we were surprised to find how much those in the minimal contact control group initiated and continued to exercise throughout the study given that they only received psychoeducation and brief advice to exercise. This may partially be a result of selection bias. That is, those who enrolled in the study may have already been interested in increasing levels of exercise.
Lack of adherence to treatment, including prescribed exercise, has been one of the obstacles in delivering effective treatment to various populations, including those suffering from diabetes, cardiovascular and pulmonary disease (Dishman, 1988; Kelly, 1995; Oldridge, 1984; Schneider, Khachadurian, Amorosa, Clemow, & Ruderman, 1992; Woodard & Berry, 2001). Future studies should focus not only on examining the effects of interventions, but also on identifying ways to enhance effectiveness of existing interventions by increasing treatment adherence. Innovative strategies using emerging technologies (e.g., computer-based interventions, interactive voice response, and smart phones) to improve treatment adherence have been explored and findings have been promising (Annesi, 1998; Pop-Eleches et al., 2011; Rodrigues et al., 2012). Integrating cost-effective strategies to enhance attendance/adherence rates may be a crucial step to increase the effectiveness of alcohol treatment.
Several limitations in this study warrant discussion. First, the small sample size demands caution when interpreting the results and “small” effects may not be detected due to limited power. Second, the lack of objective measure of exercise behavior between sessions is an important limitation. In addition, the reliability and validity of the TLFB for assessing levels of daily exercise over extended periods of time, as utilized in this study, has only recently been examined (Panza, et al., 2012). Future studies should consider supplementing TLFB assessments using objective monitoring devices, such as pedometers or accelerometers. It should also be noted that while the AE condition included an incentive system and BA-E participants were paid non-contingently, the average amount earned by AE participants in a prior pilot study, the amount paid to BA-E participants ended up being about $30 more that the average amount received by AE participants. Although somewhat unlikely, there is no way to determine whether this had an effect on study outcomes. Finally, since adherence to treatment was not manipulated in this study, we are unable to rule out the possibility that a third variable (e.g., personality trait) associated with both treatment adherence and primary alcohol outcome is driving the findings. As such, inference about causal relations should be tempered.
Given the positive findings from the current preliminary trial, a larger study evaluating the efficacy of the AE intervention as an adjunct to outpatient alcohol treatment is warranted. Future studies may also choose a comparison group that allows investigation of the effects of exercise on alcohol use such as a contact control non-exercise group. Unfortunately, our comparison group did not allow us to truly examine the treatment effects on alcohol consumption through levels of exercise since those in the comparison group reported equivalent increases in levels of exercise as those in the active condition. In order to develop effective interventions, it is important to distinguish the beneficial effects of exercise (e.g., amount and quality) on alcohol recovery from those non-specific aspects of a group intervention. Finally, only a few purported processes underlying the treatment effects were included in this study. Future studies should examine alternative mechanisms through which an exercise intervention may reduce alcohol use.
ACKNOWLEDGMENTS
The current study was conducted in the United States.
Supported in part by grant AA13418 from the National Institute on Alcoholism and Alcohol Abuse to Dr. Richard A. Brown.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Richard A. Brown, Addictions Research, Alpert Medical School of Brown University/Butler Hospital, Providence, RI 02906.
Ana M. Abrantes, Addictions Research, Alpert Medical School of Brown University/Butler Hospital, Providence, RI 02906
Haruka Minami, Addictions Research, Alpert Medical School of Brown University/Butler Hospital, Providence, RI 02906
Jennifer P. Read, University at Buffalo, The State University of New York Buffalo, NY 14260
Bess H. Marcus, Department of Family and Preventive Medicine, University of California San Diego
John M. Jakicic, University of Pittsburgh
David R. Strong, Department of Family and Preventive Medicine, University of California San Diego, La Jolla, CA 92093
Mary Ella Dubreuil, Alpert Medical School of Brown University/Butler Hospital, Providence, RI 02906.
Alan A. Gordon, Alpert Medical School of Brown University/Butler Hospital, Providence, RI 02906
Susan E. Ramsey, Alpert Medical School of Brown University/Rhode Island Hospital, Providence, RI 02903
Christopher W. Kahler, Center for Alcohol and Addiction Studies, Brown University, Providence, RI 02903
Gregory L. Stuart, Department of Psychology, University of Tennesee-Knoxville, Knoxville, TN 37996
REFERENCES
- Agne C, Paolucci K. A holistic health approach to an alcoholic treatment program. Journal of Drug Education. 1982;12:137–145. [Google Scholar]
- American College of Sports Medicine. Guidelines for exercise testing and prescription. 2000 doi: 10.2165/00007256-200030040-00003. [DOI] [PubMed] [Google Scholar]
- Annesi JJ. Effects of computer feedback on adherence to exercise. Perceptual and motor skills. 1998;87(2):723–730. doi: 10.2466/pms.1998.87.2.723. [DOI] [PubMed] [Google Scholar]
- Annis HM, Graham JM. Situational Confidence Questionnaire (SCQ-39) User’s Guide. 1988 [Google Scholar]
- Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, Krishnan KR. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosomatic Medicine. 2000;62(5):633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Broocks A, Bandelow B, Pekrun G, George A, Meyer T, Bartmann U, Ruther E. Comparison of aerobic exercise, clomipramine, and placebo in the treatment of panic disorder. American Journal of Psychiatry. 1998;155(5):603–609. doi: 10.1176/ajp.155.5.603. [DOI] [PubMed] [Google Scholar]
- Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, Gordon AA. Aerobic exercise for alcohol recovery: Rationale, program description, and preliminary findings. Behavior Modification. 2009;33(2):220–249. doi: 10.1177/0145445508329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Monti PM, Myers MG, Martin RA, Rivinus T, Dubreuil ME, Rohsenow DJ. Depression among cocaine abusers in treatment: relation to cocaine and alcohol use and treatment outcome. The American journal of psychiatry. 1998;155(2):220–225. doi: 10.1176/ajp.155.2.220. [DOI] [PubMed] [Google Scholar]
- Brown SA, Schuckit MA. Changes in depression among abstinent alcoholics. Journal of studies on alcohol. 1988;49(5):412–417. doi: 10.15288/jsa.1988.49.412. [DOI] [PubMed] [Google Scholar]
- Brownell KD, Marlatt GA, Lichtenstein E, Wilson GT. Understanding and preventing relapse. The American psychologist. 1986;41(7):765–782. doi: 10.1037//0003-066x.41.7.765. [DOI] [PubMed] [Google Scholar]
- Burling TA, Reilly PM, Moltzen JO, Ziff DC. Self-efficacy and relapse among inpatient drug and alcohol abusers: a predictor of outcome. Journal of studies on alcohol. 1989;50(4):354–360. doi: 10.15288/jsa.1989.50.354. [DOI] [PubMed] [Google Scholar]
- Cannistra LB, Balady GJ, O’Malley CJ, Weiner DA, Ryan TJ. Comparison of the clinical profile and outcome of women and men in cardiac rehabilitation. The American journal of cardiology. 1992;69(16):1274–1279. doi: 10.1016/0002-9149(92)91220-x. [DOI] [PubMed] [Google Scholar]
- Carek PJ, Laibstain SE, Carek SM. Exercise for the treatment of depression and anxiety. International Journal of Psychiatry in Medicine. 2011;41(1):15–28. doi: 10.2190/PM.41.1.c. [DOI] [PubMed] [Google Scholar]
- Carroll KM. Relapse prevention as a psychosocial treatment: A review of controlled clinical trials. Experimental and Clinical Psychopharmacology. 1996;4:46–54. [Google Scholar]
- Chinn DJ, White M, Harland J, Drinkwater C, Raybould S. Barriers to physical activity and socioeconomic position: implications for health promotion. Journal of epidemiology and community health. 1999;53(3):191–192. doi: 10.1136/jech.53.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Coleman KJ, Raynor HR, Mueller DM, Cerny FJ, Dorn JM, Epstein LH. Providing sedentary adults with choices for meeting their walking goals. Preventive medicine. 1999;28(5):510–519. doi: 10.1006/pmed.1998.0471. [DOI] [PubMed] [Google Scholar]
- Craft LL, Landers DM. The effect of exercise on clinical depression and depression resulting from mental illness: A meta-analysis. Journal of Sport and Exercise Psychology. 1998;20:339–357. [Google Scholar]
- DeBusk RF, Stenestrand U, Sheehan M, Haskell WL. Training effects of long versus short bouts of exercise in healthy subjects. The American journal of cardiology. 1990;65(15):1010–1013. doi: 10.1016/0002-9149(90)91005-q. [DOI] [PubMed] [Google Scholar]
- Dinas PC, Koutedakis Y, Flouris AD. Effects of exercise and physical activity on depression. Irish Journal of Medical Science. 2011;180(2):319–325. doi: 10.1007/s11845-010-0633-9. [DOI] [PubMed] [Google Scholar]
- Dishman RK. Exercise Adherence: Its Impact on Public Health. 1988 [Google Scholar]
- Driver HS, Taylor SR. Exercise and sleep. Sleep medicine reviews. 2000;4(4):387–402. doi: 10.1053/smrv.2000.0110. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, O’Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Medicine and science in sports and exercise. 2001;33(6 Suppl):S587–597. doi: 10.1097/00005768-200106001-00027. discussion 609-510. [DOI] [PubMed] [Google Scholar]
- Gill A, Womack R, Safranek S. Clinical Inquiries: Does exercise alleviate symptoms of depression? Journal of Family Practice. 2010;59(9):530–531. [PubMed] [Google Scholar]
- Greenfield SF, Hufford MR, Vagge LM, Muenz LR, Costello ME, Weiss RD. The relationship of self-efficacy expectancies to relapse among alcohol dependent men and women: a prospective study. Journal of Studies on Alcohol. 2000;61(2):345–351. doi: 10.15288/jsa.2000.61.345. [DOI] [PubMed] [Google Scholar]
- Group PMR. Matching alcoholism treatments to client heterogeneity: Project MATCH posttreatment drinking outcomes. Journal of studies on alcohol. 1997;58:7–29. [PubMed] [Google Scholar]
- Heinz A, Dufeu P, Kuhn S, Dettling M, Graf K, Kurten I, Schmidt LG. Psychopathological and behavioral correlates of dopaminergic sensitivity in alcohol-dependent patients. Archives of general psychiatry. 1996;53(12):1123–1128. doi: 10.1001/archpsyc.1996.01830120061011. [DOI] [PubMed] [Google Scholar]
- Ilgen M, Tiet Q, Finney J, Moos RH. Self-efficacy, therapeutic alliance, and alcohol-use disorder treatment outcomes. Journal of studies on alcohol. 2006;67(3):465–472. doi: 10.15288/jsa.2006.67.465. [DOI] [PubMed] [Google Scholar]
- Irons JG, Pope DA, Pierce AE, Van Patten RA, Jarvis BP. Contingency management to induce exercise among college students. Behaviour Change. 2013;30(2):84–95. [Google Scholar]
- Irvin JE, Bowers CA, Dunn ME, Wang MC. Efficacy of relapse prevention: a meta-analytic review. Journal of Consulting and Clinical Psychology. 1999;67(4):563–570. doi: 10.1037//0022-006x.67.4.563. [DOI] [PubMed] [Google Scholar]
- Jakicic JM, Wing RR, Butler BA, Robertson RJ. Prescribing exercise in multiple short bouts versus one continuous bout: effects on adherence, cardiorespiratory fitness, and weight loss in overweight women. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1995;19(12):893–901. [PubMed] [Google Scholar]
- Kelly K. Patient compliance: the final frontier of diabetes care. Practical Diabetology. 1995;14:20–23. [Google Scholar]
- King AC, Pruitt LA, Woo S, Castro CM, Ahn DK, Vitiello MV, Bliwise DL. Effects of moderate-intensity exercise on polysomnographic and subjective sleep quality in older adults with mild to moderate sleep complaints. The journals of gerontology. Series A, Biological sciences and medical sciences. 2008;63(9):997–1004. doi: 10.1093/gerona/63.9.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. British Medical Journal. 2001;322(7289):763–767. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Jensen BE, Oberman A, Fletcher GF, Fletcher BJ, Raczynski JM. Adherence in the training levels comparison trial. Medicine and science in sports and exercise. 1996;28(1):47–52. doi: 10.1097/00005768-199601000-00013. [DOI] [PubMed] [Google Scholar]
- Lejoyeux M. Use of serotonin (5-hydroxytryptamine) reuptake inhibitors in the treatment of alcoholism. Alcohol and alcoholism. 1996;1:69–75. [PubMed] [Google Scholar]
- Linke SE, Gallo LC, Norman GJ. Attrition and adherence rates of sustained vs. intermittent exercise interventions. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2011;42(2):197–209. doi: 10.1007/s12160-011-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JS. Regression Models for Categorical and Limited Dependent Variables. 1997 [Google Scholar]
- Macfarlane DJ, Taylor LH, Cuddihy TF. Very short intermittent vs continuous bouts of activity in sedentary adults. Preventive medicine. 2006;43(4):332–336. doi: 10.1016/j.ypmed.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Maisto SA, Connors GJ, Zywiak WH. Alcohol treatment, changes in coping skills, self-efficacy, and levels of alcohol use and related problems 1 year following treatment initiation. Psychology of Addictive Behaviors. 2000;14(3):257–266. doi: 10.1037//0893-164x.14.3.257. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Donovan DM. Relapse Prevention: Maintenance strategies in the treatment of addictive behaviors. Second Edition. 2005. p. 416. [Google Scholar]
- Marlatt GA, Witkiewitz K. Relapse Prevention: Maintenance strategies in the treatment of addictive behaviors. 2nd Edition. 2005. Relapse prevention for alcohol and drug problems; pp. 1–44. [Google Scholar]
- Mead GE, Morley W, Campbell P, Greig CA, McMurdo M, Lawlor DA. Exercise for depression. Cochrane database of systematic reviews. 2009;(3):CD004366. doi: 10.1002/14651858.CD004366.pub4. [DOI] [PubMed] [Google Scholar]
- Miller WR, Walters ST, Bennett ME. How effective is alcoholism treatment in the United States. Jounal of Studies on Alcohol. 2001;62:211–220. doi: 10.15288/jsa.2001.62.211. [DOI] [PubMed] [Google Scholar]
- Miller WR, Wilbourne PL. Mesa Grande: a methodological analysis of clinical trials of treatments for alcohol use disorders. Addiction. 2002;97(3):265–277. doi: 10.1046/j.1360-0443.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- Moore SM, Ruland CM, Pashkow FJ, Blackburn GG. Women’s patterns of exercise following cardiac rehabilitation. [Research Support, U.S. Gov’t, P.H.S.] Nursing research. 1998;47(6):318–324. doi: 10.1097/00006199-199811000-00005. [DOI] [PubMed] [Google Scholar]
- Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction. 2006;101(2):212–222. doi: 10.1111/j.1360-0443.2006.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TJ, Pagano RR, Marlatt GA. Lifestyle modification with heavy alcohol drinkers: effects of aerobic exercise and meditation. Addictive behaviors. 1986;11(2):175–186. doi: 10.1016/0306-4603(86)90043-2. [DOI] [PubMed] [Google Scholar]
- Oldridge NB. Compliance and dropout in cardiac exercise rehabilitation. Journal of Cardiopulmonary Rehabiltation. 1984;4:166–177. [Google Scholar]
- Osgood DW. Poisson-based Regression Analysis of Aggregate Crime Rates. Journal of Quantitative Criminology. 2000;16:21–44. [Google Scholar]
- Panza GA, Weinstock J, Ash GI, Pescatello LS. Psychometric evaluation of the Timeline Followback for Exercise among college students. Psychology of Sport and Exercise. 2012;13:779–788. doi: 10.1016/j.psychsport.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternoster R, Brame R. Multiple routes to delinquency? A test of developmental and general theories of crime. Criminology. 1997;35:45–84. [Google Scholar]
- Peluso MA, Guerra de, Andrade LH. Physical activity and mental health: the association between exercise and mood. Clinics. 2005;60(1):61–70. doi: 10.1590/s1807-59322005000100012. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Current opinion in psychiatry. 2005;18(2):189–193. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- Perraton LG, Kumar S, Machotka Z. Exercise parameters in the treatment of clinical depression: a systematic review of randomized controlled trials. Journal of Evaluation in Clinical Practice. 2010;16(3):597–604. doi: 10.1111/j.1365-2753.2009.01188.x. [DOI] [PubMed] [Google Scholar]
- Piazza-Gardner AK, Barry AE. Examining physical activity levels and alcohol consumption: are people who drink more active? [Review] American journal of health promotion. 2012;26(3):e95–104. doi: 10.4278/ajhp.100929-LIT-328. [DOI] [PubMed] [Google Scholar]
- Pop-Eleches C, Thirumurthy H, Habyarimana JP, Zivin JG, Goldstein MP, de Walque D, Bangsberg DR. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS. 2011;25(6):825–834. doi: 10.1097/QAD.0b013e32834380c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. Summer. [Google Scholar]
- Read JP, Kahler CW, Stevenson JF. Bridging the gap between alcoholism treatment and practice: What works and why. Professional Psychology: Research and Practice. 2001;32:227–238. [Google Scholar]
- Reed J, Ones DS. The effect of acute aerobic exercise on positive activated affect: A meta-analysis. Psychology of Sport and Exercise. 2006;7(5):477–514. [Google Scholar]
- Rodrigues R, Shet A, Antony J, Sidney K, Arumugam K, Krishnamurthy S, DeCosta A. Supporting adherence to antiretroviral therapy with mobile phone reminders: results from a cohort in South India. PloS one. 2012;7(8):40723. doi: 10.1371/journal.pone.0040723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseeuw PJ, Yohai V. Robust Regression by Means of S-estimators. In: Franke J, Härdle W, Martin RD, editors. Robust and Nonlinear Time Series Analysis. NY: Springer; 1984. pp. 256–272. [Google Scholar]
- Rychtarik RG, Prue DM, Rapp SR, King AC. Self-efficacy, aftercare and relapse in a treatment program for alcoholics. Journal of studies on alcohol. 1992;53(5):435–440. doi: 10.15288/jsa.1992.53.435. [DOI] [PubMed] [Google Scholar]
- Schneider SH, Khachadurian AK, Amorosa LF, Clemow L, Ruderman NB. Ten-year experience with an exercise-based outpatient life-style modification program in the treatment of diabetes mellitus. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] Diabetes care. 1992;15(11):1800–1810. doi: 10.2337/diacare.15.11.1800. [DOI] [PubMed] [Google Scholar]
- Sinyor D, Brown T, Rostant L, Seraganian P. The role of a physical fitness program in the treatment of alcoholism. Journal of studies on alcohol. 1982;43(3):380–386. doi: 10.15288/jsa.1982.43.380. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers self-reports of drinking behavior. Behaviour Research and Therapy. 1979;17(2):157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Convergent validity: An approache to increasing confidence in treatment outcome conclusions with alcohol and drug abusers. In: Sobell LC, Sobell MB, Ward E, editors. Evaluating alcohol and drug abuse treatment efectivness:Recent advances. Elmsford, NY: Pergamon Press; 1980. pp. 177–183. [Google Scholar]
- Spielberger M, Aigner F, Schmid T, Bosmuller C, Konigsrainer A, Margreiter R. Long-term results of cadaveric renal transplantation after conversion from cyclosporine to azathioprine: a controlled randomized trial. Transplantation proceedings. 1988;20(3 Suppl 3):169–170. [PubMed] [Google Scholar]
- Suter M, Strik W, Moggi F. Depressive symptoms as a predictor of alcohol relapse after residential treatment programs for alcohol use disorder. Journal of substance abuse treatment. 2011;41(3):225–232. doi: 10.1016/j.jsat.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Sallis JF, Needle R. The relation of physical activity and exercise to mental health. Public Health Reports. 1985;100(195–201) [PMC free article] [PubMed] [Google Scholar]
- Tkachuk GA, Martin GL. Exercise therapy for patients with psychiatric disorders: Research and clinical implications. Professional Psychology: Research and Practice. 1999;30(3):275–282. [Google Scholar]
- Trivedi MH, Greer TL, Church TS, Carmody TJ, Grannemann BD, Galper DI, Blair SN. Exercise as an augmentation treatment for nonremitted major depressive disorder: a randomized, parallel dose comparison. The Journal of clinical psychiatry. 2011;72(5):677–684. doi: 10.4088/JCP.10m06743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDHHS. Physical activity and health: A report of the Surgeon General. Atlanta, GA: Centers for Disease Control; 1996. [Google Scholar]
- Ussher M, Sampuran AK, Doshi R, West R, Drummond DC. Acute effect of a brief bout of exercise on alcohol urges. Addiction. 2004;99(12):1542–1547. doi: 10.1111/j.1360-0443.2004.00919.x. [DOI] [PubMed] [Google Scholar]
- Vielva I, Iraurgi I. Cognitive and behavioural factors as predictors of abstinence following treatment for alcohol dependence. Addiction. 2001;96(2):297–303. doi: 10.1046/j.1360-0443.2001.96229713.x. [DOI] [PubMed] [Google Scholar]
- Wei I. Application of an urn model to the design of sequential controlled clinical trials. Journal of the American Statistical Association. 1978;73:559–563. [Google Scholar]
- Weisner C, Matzger H, Kaskutas LA. How important is treatment? One-year outcomes of treated and untreated alcohol-dependent individuals. Addiction. 2003;98(7):901–911. doi: 10.1046/j.1360-0443.2003.00438.x. [DOI] [PubMed] [Google Scholar]
- West AP, Gocka EF. Self-ratings of typical mood and behavior during recovery from alcohol abuse. A multivariate study of alcoholic men. Psychopathology. 1986;19(6):303–308. doi: 10.1159/000284453. [DOI] [PubMed] [Google Scholar]
- Williams P, Lord SR. Effects of group exercise on cognitive functioning and mood in older women. Australian and New Zealand Journal of Public Health. 1997;21(1):45–52. doi: 10.1111/j.1467-842x.1997.tb01653.x. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: that was Zen, this is Tao. The American psychologist. 2004;59(4):224–235. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]
- Woodard CM, Berry MJ. Enhancing adherence to prescribed exercise: structured behavioral interventions in clinical exercise programs. Journal of cardiopulmonary rehabilitation. 2001;21(4):201–209. doi: 10.1097/00008483-200107000-00002. [DOI] [PubMed] [Google Scholar]