Abstract
Current topical treatments for papillomas use ablative, cytotoxic and immunomodulating strategies and reagents. However, the effectiveness of topical treatments using different formulations has not been examined in preclinical models or clinical trials. The purpose of this study was to determine whether formulation of the small molecule acyclic nucleoside, cidofovir (CDV), could lead to improved therapeutic endpoints following topical treatment of papillomas using the cottontail rabbit papillomavirus (CRPV)/rabbit model. Different formulations with a set dose of 1% cidofovir were tested to establish comparative data.
The results demonstrated that anti-papilloma treatments with topical CDV were greatly enhanced when formulated versus unformulated. Best results were obtained with CDV formulated in cremophor, then in carbomer 940, and then in DMSO. Further studies indicated that effective formulations led to complete cures of papillomas at dilutions less than 0.3% CDV. These studies together with previous observations demonstrated that unformulated CDV under the same treatment regime required doses of 2% to achieve cures demonstrating that much less compound can be used when properly formulated.
Introduction
Currently approved topical treatments for warts include a variety of strategies such as ablation, cytotoxic reagents and immunomodulators. Ablative techniques involve curettage with scalpel, lazer or freezing (Ferenczy, Behelak et al., 1995; Wollina, Konrad et al., 2001; Oni & Mahaffey, 2011; Khandelwal, Bumb et al., 2013); topical cytotoxic treatments include salicylic acid, trichloroacetic acid, acyclic nucleosides, podophyllotoxin, and photodynamic treatments (Snoeck, 2006); immunomodulators include interferon A, contact sensitizers such as dichlorobenzene and innate immune activators such as imiquimod (Schofer, Van et al., 2006; Gallagher & Derkay, 2009). In general, the treatments show modest levels of efficacy (clinical outcomes summarized recently in (Kwok, Gibb et al., 2012)), and include several side effects as well as recurrences (Gye, Nam et al., 2013). Improved outcomes are noted in combination treatment approaches (Kaspari, Gutzmer et al., 2002; Lu, Yang et al., 2012; Xu, Xiang et al., 2013). Thus, preclinical models to assess new and improved therapies for the treatment of HPV-associated diseases are needed, despite the existence of effective prophylactic vaccines (Kwok, Gibbs et al., 2012; Coremans & Snoeck, 2009).
Preclinical model systems to compare various anti-virals and improved formulations are lacking, with the exception of the cottontail rabbit papillomavirus (CRPV) cutaneous wart model (Ostrow, Forslund et al., 1992; Bodily, Hoopes et al., 1999; Christensen, 2005), the canine oral papillomavirus model (Chambers & Evans, 1959; Nicholls & Stanley, 1999) and the multimammate rat model (Amtmann, Volm et al., 1984; Nafz, Schafer et al., 2008). We and others have used the cutaneous CRPV rabbit model extensively to examine anti-viral activities (Duan, Paris et al., 2000; Kreider, Christensen et al., 1992; Christensen, 2005), prophylactic and therapeutic vaccines (Breitburd, Kirnbauer et al., 1995; Jensen, Selvakumar et al., 1997; Leachman, Shylankevich et al., 2002) and virological studies (Hu, Cladel et al., 2007). In general, the observations obtained in the rabbit model show general correlations with clinical studies (Christensen, 2005), including the phenomenon of post-treatment recurrences (Christensen, Han et al., 2001).
Clinical trials with cidofovir (CDV) have demonstrated effectiveness against vaginal warts, skin warts and laryngeal papillomas (Van, Snoeck et al., 1995; De, 1996; Safrin, Cherrington et al., 1997; Davis, Gostout et al., 2000; Snoeck, Andrei et al., 2001; Snoeck, Bossens et al., 2001; Stragier, Snoeck et al., 2002; DeRossi & Laudenbach, 2004; Silverman & Pitman, 2004). The delivery strategies included topical applications in saline or gel, as well as intralesional injections. The observation of clinical recurrences after treatments and additional local and systemic side-effects has limited the use of this compound as a general anti-papillomavirus clinical strategy. Some of these treatment failures however may be attributable to inadequate delivery of cidofovir and the potentially short “window” of the treatments when unformulated (Snoeck, Andrei et al., 2001). Preclinical models provide opportunities to directly compare various treatment strategies that could improve clinical outcomes. Despite the existence of an effective prophylactic vaccine against several HPV types, this vaccine does not induce a post-infection therapeutic response (Munoz, Manalastas, Jr. et al., 2009). There continues to be an unmet need for effective anti-papillomavirus treatments for existing infections and for those patients that do not receive the prophylactic vaccine. In addition, a combination of anti-viral and therapeutic T-cell based vaccines may ultimately be the best strategy to cure persistent papillomavirus infections and HPV-associated precancerous lesions.
Materials and Methods
1. Inoculation of rabbits with CRPV viral DNA
The studies were reviewed and approved by the Penn State University (PSU), College of Medicine IACUC, and the PSU Biological Safety and Recombinant DNA Committee. Rabbits were inoculated at 4 back sites with CRPV viral DNA using our recent delayed-scarification protocol that greatly improves the efficiency of infection with both viral DNA and infectious virions (Cladel, Hu et al., 2008). Our standard anti-viral testing protocol (Christensen, 2005) is to establish two sites on the right (R) and left (L) side of rabbits with wild-type CRPV DNA (wtCRPV) and two sites on the right and left side of the rabbits with E8 mutant CRPV DNA (mE8-CRPV) (Hu, Han et al., 2002). The latter viral genome develops papillomas that are substantially attenuated such that the papillomas are small, slow-growing and better mimic the clinical tumor mass and size of human warts. In contrast, the wtCRPV-induced sites grow rapidly and reach a diameter of 15-20 cm in 8-10 weeks (Hu, Han et al., 2002), such that these lesions represent a significant challenge to anti-viral treatments.
2. Treatments
The topical treatments were conducted daily for 5 days per week by applying compound in 100 ul doses onto the left-side only papillomas. Treatments began at either week two, three or four depending upon the study, and the duration of the treatments ranged from two to five weeks. The right-side papillomas were untreated and represent internal controls for the treated papillomas for each rabbit, and were compared to the placebo-treated papillomas in the control group to determine whether topically applied compounds have systemic effects. The compounds were delivered via 1ml syringes without needles, and if formulated into a gel were gently spread over the surface of the infected area (prior to papilloma appearance) or over the surface of the papilloma (if apparent). Skin tattoo spots next to the sites of infection were used as guides to locate the original site of the viral infection for early treatments and to positionally identify sites of cures.
3. Formulations
Compounds were formulated as described below. A 2% stock of cidofovir in saline was prepared from which the final formulations were developed. The 1% cidofovir in saline formulation was prepared by diluting the 2% stock 1:2 with saline to make a final 1% solution. The 1% cidofovir in 10% DMSO formulation was prepared by adding the appropriate amount of DMSO and saline to achieve a final concentration of 1% cidofovir in 10% DMSO. A 2% stock of Carbomer 940 was prepared using saline, and diluted with 2% cidofovir in saline to achieve a final gel containing 1% cidofovir in 1% Carbomer 940. The final formulation was a 50:50 emulsion containing 2% cidofovir in saline and cremophor. The emulsion was prepared by mixing the solutions together using two glass syringes and a luer-lock device in a procedure often used to develop emulsions for antigen-adjuvants that use oil-based formulations. All formulations were taken up in 1ml syringes and stored at 4°C prior to use. Each syringe was used once for each daily group treatment.
4. Statistical analyses
Students t-test and Mann-Whitney Rank Sum test were conducted on mean papilloma sizes for treated versus untreated sites for wtCRPV and mE8-CRPV at weekly time points to determine whether significant (p < 0.05) differences in papilloma size were observed. The Mann-Whitney Rank Sum test was used when the data sets failed the Normality Test (Shapiro-Wilk) for the t-test. Graphics and statistics were conducted using SigmaPlot 11.0 software (Systat Software Inc., San Jose, CA).
Results
A series of experiments were conducted to test various formulations of cidofovir in the CRPV rabbit model. Cidofovir was chosen as the candidate compound as we and others have shown efficacy in the rabbit model even in the absence of any formulation (Duan, Paris et al., 2000; Christensen, Pickel et al., 2000). We have observed significant activity of cidofovir in saline when daily treatments of 2% cidofovir were used topically (Christensen, Pickel et al., 2000). We have also obtained therapeutic clearance of CRPV-induced papillomas by intralesional delivery of cidofovir in saline (Christensen, Han et al., 2001). However, we noted that recurrences were common (Christensen, Han et al., 2001), as also found in clinical treatments of both genital (Snoeck, Bossens et al., 2001) and laryngeal warts (Gallagher & Derkay, 2009).
1. Different Formulations of cidofovir establish different levels of anti-papilloma activity
We first conducted a comparative study in which the same concentration of cidofovir (1%) was tested topically in different formulations. The comparative study included cidofovir unformulated (saline), formulated in 10% DMSO, formulated in 2% Carbomer 940, and formulated as a 50:50 emulsion in cremophor. We chose 1% cidofovir as in past studies, because unformulated cidofovir at 1% showed only little to modest therapeutic effects (Christensen, Pickel et al., 2000). Six groups of rabbits were infected with wtCRPV and mE8-CRPV as described in the methods section. On day 19, the topical treatments were initiated at a time when papillomas were not clinically evident or were very small, so as to maximize the therapeutic potential of the topical treatments. Control groups contained treatments with the formulations without cidofovir. The outcome of these treatments is presented in Table 1 and Figures 1-3.
Table 1.
Outcome of treatments for individual sites in the formulation experiment.
| Group and treatments | Papillomas induced with 5 ug wtCRPV | Papillomas induced with 5 ug mE8-CRPV | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Growth inhibitiona | Curesb | Recurrencesc | Growth inhibitiona | Curesb | Recurrencesc | |
| Group A (1% cidofovir in saline) | 1/4 | 0/4 | 0/0 | 1/4 | 0/4 | 0/0 |
| Group B (1% cidofovir in 10% DMSO) | 2/4 | 1/4 | 0/1 | 3/4 | 1/4 | 0/1 |
| Group C (1% cidofovir in carbomer 940) | 0/4 | 2/4 | 1/2 | 0/4 | 4/4 | 1/4 |
| Group D (1% cidofovir in cremophor) | 0/4 | 4/4 | 0/4 | 0/4 | 4/4 | 0/4 |
| Group E (saline) | 0/2 | 0/2 | 0/0 | 0/2 | 0/2 | 0/0 |
| Group F (10% DMSO) | 0/2 | 0/2 | 0/0 | 0/2 | 0/2 | 0/0 |
| Group G (carbomer 940) | 0/2 | 0/2 | 0/0 | 0/2 | 0/2 | 0/0 |
| Group H (cremophor) | 0/2 | 0/2 | 0/0 | 0/2 | 0/2 | 0/0 |
Papillomas per infected sites.
Cured papillomas per infected sites
Recurrences per cured papillomas
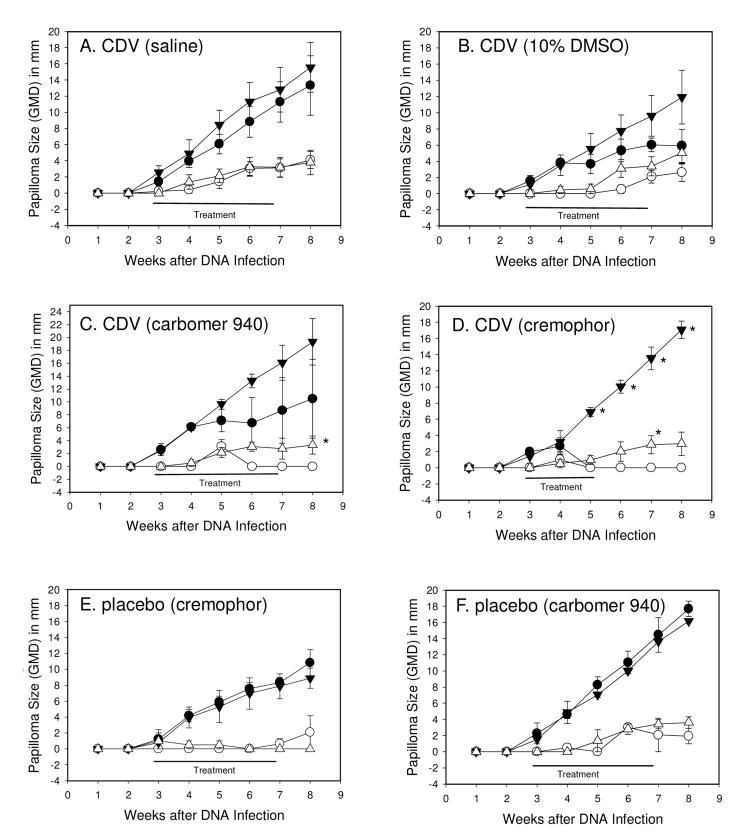
Figure 1.
Mean ± SEM papilloma size of wtCRPV induced left-side (L) papillomas treated with compound (●) or right-side (R) papillomas that were untreated (▼); mE8-CRPV induced papillomas treated with compound (○) or untreated (▽). Each point represents weekly measurements of 4 papillomas on 4 rabbits (cidofovir treatments) or 2 papillomas on 2 rabbits (formulations without cidofovir). Topical treatments began at day 19, and were daily applications for 5 days per week. Treatments were 1% cidofovir in saline (A), 1% cidofovir in 10% DMSO (B), 1% cidofovir in Carbomer 940 (C), 1% cidofovir in cremophor (D), cremophor alone (E) and carbomer 940 alone (F). Statistics (p<0.05 marked with asterisk): (Graphs A, B, E and F) no significant differences; (Graph C) Mann-Whitney Rank Sum test p<0.05 for L2 versus R2, week 8; (Graph D) Mann-Whitney Rank Sum test p<0.05 for L1 versus R1, weeks 5-8, and Mann-Whitney Rank Sum test p<0.05 L2 versus R2, week 7.
Figure 3.
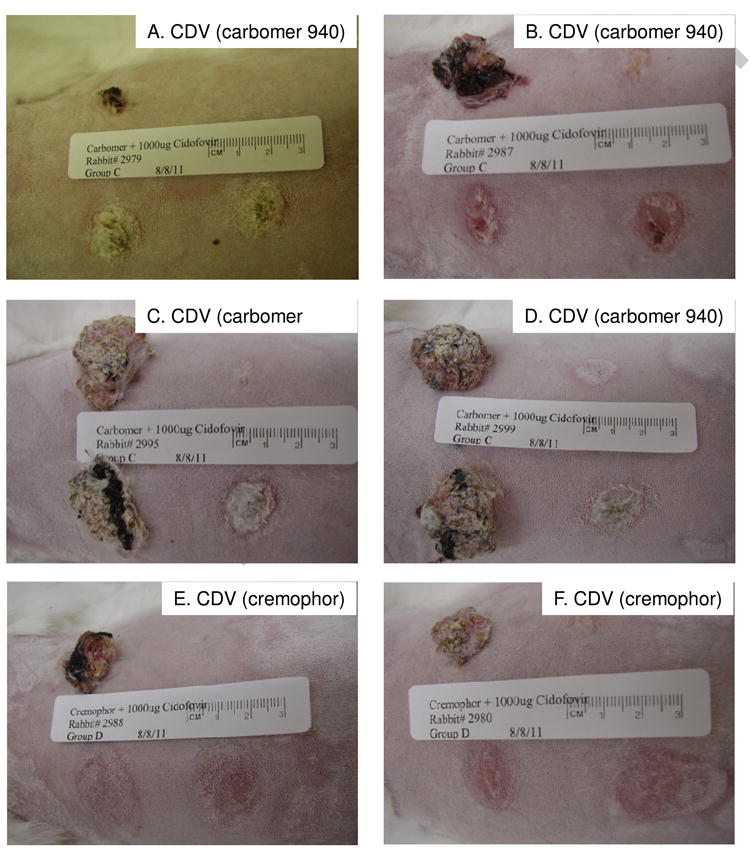
Rabbits topically treated with 1% cidofovir in Carbomer 940 (A-D) and 1% cidofovir in cremophor (E-F) at week 8 for rabbits shown in Figure 2. The head is to the left and the lower sites representing the left side are the treated sites.
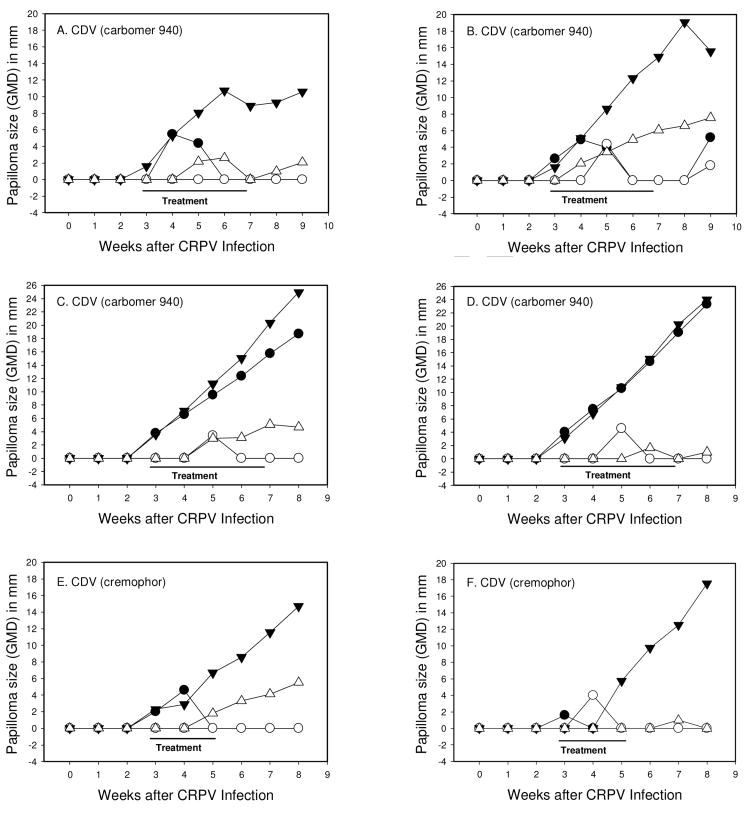
Strong anti-papilloma activity against wtCRPV-induced infections was observed with cidofovir formulated in carbomer 940 leading to 2 of 4 sites cured in 2 of the 4 treated rabbits (Table 1, Figures 2 and 3, rabbits A-D). However, mean papilloma size differences were not significantly different using Student t-test and Mann-Whitney Rank Sum tests. In contrast, cidofovir formulated in cremophor lead to complete cures of all sites treated (Figs 1C, D and Figs 2 and 3, rabbits E-F). The treatment period for cidofovir formulated in cremophor was reduced to only a two-week period because complete cures were obtained, and because there were some local redness and slight blistering at the treatment sites at the end of week two. Cidofovir in saline had no effect, and when formulated in 10% DMSO showed weak effects (1/4 sites were cured; Fig 1A, B) in which mean papilloma sizes compared with untreated sites were statistically nonsignificant, but some growth inhibition was observed. Cremophor and Carbomer 940 formulated in saline had no effect on papilloma growth rates, and no cures were observed (Fig 1E, F). The number of sites that were cured or showed reduced growth rates are summarized in Table 1.
Figure 2.
Papilloma growth on individual rabbits topically treated with 1% cidofovir in Carbomer 940 (A-D) and 1% cidofovir in Cremophor (E-F). Symbols represent weekly GMDs of wtCRPV induced papillomas treated with compound (●) or left untreated (▼); mE8-CRPV induced papillomas treated with compound (○) or left untreated (▽). Left (L) sites were treated and right (R) sites were untreated. The four rabbits in graphs A-D demonstrate 2 successful treatment responders (A and B) and two treatment non-responders (C and D). Two representative examples are shown for 1% cidofovir in cremophor (E and F) showing complete treatment responders.
When treatments against the clinically smaller papillomas initiated with mE8-CRPV were analyzed, the following observations were obtained:
There were no cures using cidofovir in saline; 1 of 4 sites were cured with cidofovir in 10% DMSO; all sites treated with cidofovir formulated in Carbomer 940 and cremophor were cured (Table 1).
No sites treated with saline, Carbomer 940 or cremophor were cured or showed growth inhibition.
2. Titration of formulated cidofovir
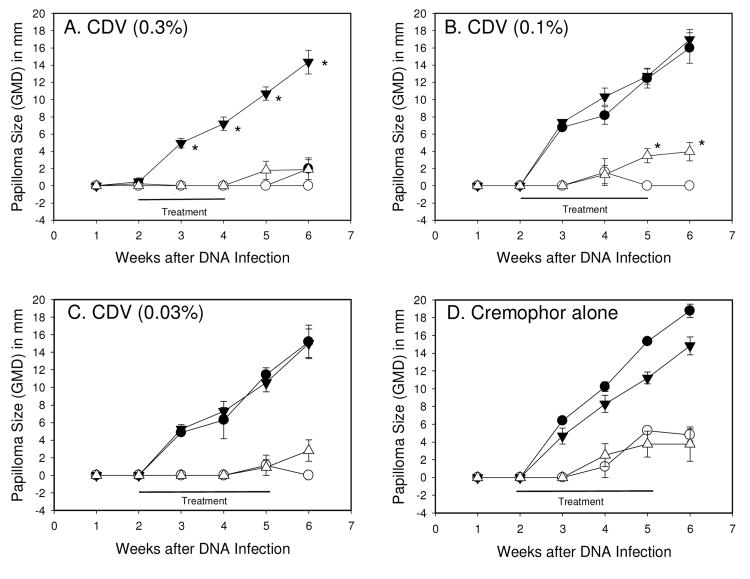
In the first set of experiments, cidofovir formulated in cremophor showed the strongest anti-viral activity, and complete cures were observed using 1% formulated cidofovir (Fig 1D). The next set of experiments was designed to determine the lowest dose of formulated cidofovir that showed anti-papilloma activity. Three doses of formulated cidofovir were tested, including 0.3%, 0.1% and 0.03%, beginning at week two after infection. The data showed that complete cures were established using 0.3% formulated cidofovir against wtCRPV infections (Fig 4A) and complete cures against mE8-CRPV-induced lesions for all three doses (Fig 4). Cremophor alone had no anti-papilloma activity (Fig 4D). For the 0.3% formulated treatment groups, we noted that there were some recurrences after treatment cessation, a phenomenon that we have observed previously (Christensen, Han et al., 2001).
Figure 4.
Mean ± SEM papilloma size of wtCRPV induced papillomas treated with compound (●) or left untreated (▼); mE8-CRPV induced papillomas treated with compound (○) or left untreated (▽). Each point represents weekly measurements of 4 papillomas on 4 rabbits. Topical treatments began at day 14 and were daily applications for 5 days per week. Treatments were 0.3% cidofovir formulated in cremophor (A), 0.1% cidofovir formulated in cremophor (B), 0.03% cidofovir formulated in cremophor (C) or cremophor alone (D). Left (L) sites were treated and right (R) sites were untreated. Statistics (p<0.05 marked with asterisk): (Graph A) T-test p<0.05 for L1 versus R1, weeks 3-6; (Graph B) Mann-Whitney Rank Sum test p<0.05 for L2 versus R2, weeks 5-6; (Graphs C and D) no significant differences between treated versus untreated sites for any week.
3. Time course treatments with formulated cidofovir
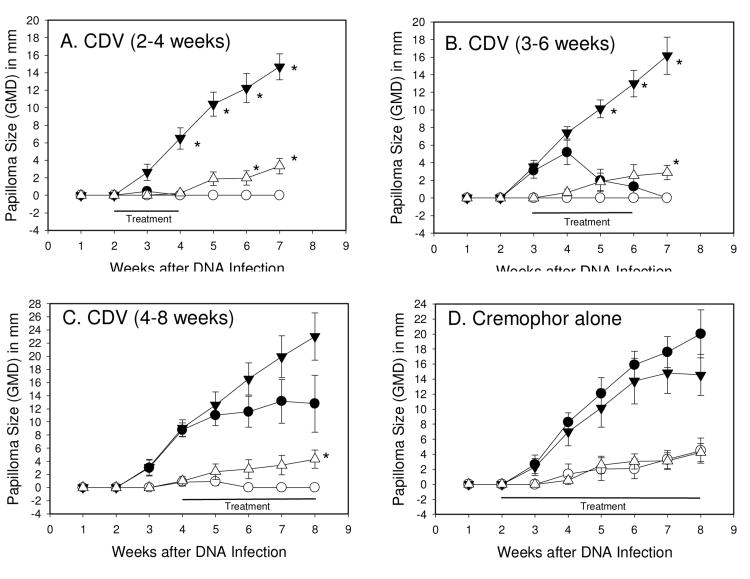
The previous treatments were begun at week two, or between weeks 2 and 3, at a time prior to the clinical appearance of papillomas. The next set of experiments was designed to determine the effectiveness of topical formulated cidofovir on existing papillomas to establish a more clinically relevant test. The experiment was designed to begin treatments at different time points beginning at week 2, 3 and 4, and the data are shown in Figure 5. We also set the formulation at 1% cidofovir in cremophor to ensure a strong anti-viral dosing schedule. The data demonstrated that complete cures against wtCRPV-induced lesions can be achieved when the treatments begin at week 3, at a time when papillomas are evident. Even delaying the start of treatments until week 4 showed strong anti-viral activity in some instances, especially against the smaller papillomas induced by mE8-CRPV (Fig 5C). As observed previously, cremophor alone had no effect on papilloma growth (Fig 1D). Frequencies of papilloma cures and growth reductions are presented in Table 2.
Figure 5.
Mean + SEM papilloma size of wtCRPV induced papillomas treated with compound (●) or left untreated (▼); mE8-CRPV induced papillomas treated with compound (○) or left untreated (▽). Each point represents weekly measurements of 5 papillomas on 5 rabbits. Topical treatments began at day 14 (A) and (D), day 21 (B) or day 28 (C) and were daily applications for 5 days per week. Treatments were 1% cidofovir formulated in cremophor (A, B, C) and cremophor alone (D). Left (L) sites were treated and right (R) sites were untreated. Statistics (p<0.05 marked with asterisk): (Graph A) T-test <0.05 for L1 versus R1, weeks 4-7, and L2 versus R2, weeks 6-7; (Graph B) T-test <0.05 for L1 versus R1, weeks 5-7, and Mann-Whitney Rank Sum test <0.05 for L2 versus R2, week 7; (Graph C) Mann-Whitney Rank Sum test p<0.05 L2 versus R2, week 7; (Graph D) no significant differences for any week between treated versus untreated sites.
Table 2.
Outcome of treatments for individual sites in the time-course treatment with formulated cidofovir.
| Group and treatments | Papillomas induced with 5 ug wtCRPV | Papillomas induced with 5 ug mE8-CRPV | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Growth inhibitiona | Curesb | Recurrencesc | Growth inhibitiona | Curesb | Recurrencesc | |
|
| ||||||
| Group A (weeks 2-4) | 0/5 | 5/5 | 0/5 | 0/5 | 5/5 | 0/5 |
| Group B (weeks 3-5) | 0/5 | 5/5 | 0/5 | 0/5 | 5/5 | 0/5 |
| Group C (weeks 4-8) | 4/5 | 0/5 | 0/0 | 0/5 | 5/5 | 0/5 |
| Group D (cremophor alone) | 0/5 | 0/5 | 0/0 | 0/5 | 0/5 | 0/5 |
cidofovir.
Papillomas per infected sites.
Cured papillomas per infected sites.
Recurrences per cured papillomas.
Discussion
Preclinical models are needed to compare different formulations and combination treatments for most effective anti-viral treatments against papillomavirus infections. The species and tissue restriction of papillomaviruses prevent direct preclinical testing of HPV infections in vivo in laboratory settings in immunocompetent hosts. The CRPV/rabbit model has been the most effective animal model for testing due to a number of outcomes that are relevant to the clinical situation, especially including cutaneous lesions that are amenable to topical, intralesional and systemic treatments (Kreider, Balogh et al., 1990; Kreider, Christensen et al., 1992; Ostrow, Forslund et al., 1992; Lofgren, Ronn et al., 1994; Shikowitz, Steinberg et al., 1986; Christensen, 2005).
We and others have used the CRPV rabbit model for testing various anti-virals for activity against papillomavirus infections and the model shows correlates with clinical treatments with the same compounds (Kreider, Christensen et al., 1992; Christensen, Pickel et al., 2000). In addition, the CRPV rabbit model has been used to test unformulated cidofovir topically, intralesionally and systemically (Christensen, Pickel et al., 2000; Duan, Paris et al., 2000). The data demonstrate that daily topical and intralesional treatments can be effective when 1% unformulated compound is used although in some studies only partial growth reductions are seen (Christensen, Pickel et al., 2000). In the present study we have noted that when 1% cidofovir was applied daily for 5 (not 7) days per week, there were minimal effects on papilloma growth (Fig 1A). In order to improve clinical outcomes, we tested several different formulations to determine whether the efficacy of 1% cidofovir could be improved, and the studies demonstrated a clear and significant improvement when formulated in cremophor as an emulsion (Fig 1D). The mechanism by which cidofovir acts on papilloma growth inhibition is unclear, but most likely occurs via cellular cytotoxicity at the site of application. Given that cidofovir is a nucleoside analog, and that papillomaviruses replicate via cellular polymerases, the effects on papillomavirus life cycle are likely to be indirect.
Cidofovir has been used successfully to treat various papillomas in clinical settings including laryngeal papillomas, genital and cutaneous warts (De, 1996; Snoeck, Andrei et al., 2001). The outcome of these studies is in general some growth inhibition and some proportion of cures but with recurrences, particularly upon treatment cessation. Most of these treatments included unformulated cidofovir for intralesional injections into laryngeal papillomas (DeRossi & Laudenbach, 2004; Kimberlin, 2004) and cidofovir formulated into a gel for several genital wart treatment studies (Snoeck, Bossens et al., 2001). Comparative and control studies were not generally presented in these trials.
Improved efficacy of formulated cidofovir in the current CRPV/rabbit model studies presented here may be due to a “depot” effect such that the compound has a more sustained and continuous release into the papilloma tissue. However, the formulations themselves using cremophor emulsion and Carbomer 940 showed local physical differences that may help explain the different outcomes. The Carbomer gel treatments tended to dry quickly and establish a crust over the papilloma whereas the cremophor emulsion remained as a thick gel that was absorbed into the papilloma more effectively. DMSO-formulated cidofovir was less viscous and was quickly absorbed into the tissue. These observations may explain the reduced efficacy of the Carbomer formulation in which less penetration of the compound was the likely outcome of the dried crust overlay and reduced time of continuous treatments of the DMSO-formulated treatments.
From these studies we conclude that formulated compounds with potential anti-papilloma activity will benefit from formulation testing in preclinical papilloma models. There is the likelihood that promising anti-viral compounds will be dismissed as inactive if unformulated compounds only are screened for activity.
Highlights.
Anti-papilloma efficacy by topical cidofovir was greatly enhanced when formulated.
Best formulations were cremophor, then Carbomer 940, then DMSO.
Effective formulations led to complete cures using less than 0.3% cidofovir.
Less compound can be used to achieve the same efficacy when properly formulated.
Acknowledgments
This study was supported by NIAID Contract HHSN272201000020I. We thank the veterinary staff of the Department of Comparative Medicine for animal care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Amtmann E, Volm M, Wayss K. Tumour induction in the rodent Mastomys natalensis by activation of endogenous papilloma virus genomes. Nature. 1984;308:291–292. doi: 10.1038/308291a0. [DOI] [PubMed] [Google Scholar]
- Bodily JM, Hoopes DJ, Roeder BL, Gilbert SG, Pettit GR, Herald CL, Rollins DN, Robison RA. The inhibitory effects of bryostatin 1 administration on the growth of rabbit papillomas. Cancer Letters. 1999;136:67–74. doi: 10.1016/s0304-3835(98)00310-3. [DOI] [PubMed] [Google Scholar]
- Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller JT, Lowy DR. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. Journal of Virology. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers VC, Evans CA. Canine oral papillomatosis. I Virus assay and observations of the various stages of the experimental infection. Cancer Research. 1959;19:1188–1195. [PubMed] [Google Scholar]
- Christensen ND. Cottontail rabbit papillomavirus (CRPV) model system to test antiviral and immunotherapeutic strategies. Antivir Chem Chemother. 2005;16:355–362. doi: 10.1177/095632020501600602. [DOI] [PubMed] [Google Scholar]
- Christensen ND, Han R, Cladel NM, Pickel MD. Combination treatment with intralesional cidofovir and viral-DNA vaccination cures large cottontail rabbit papillomavirus-induced papillomas and reduces recurrences. Antimicrob Agents Chemother. 2001;45:1201–1209. doi: 10.1128/AAC.45.4.1201-1209.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen ND, Pickel MD, Budgeon LR, Kreider JW. In vivo anti-papillomavirus activity of nucleoside analogues including cidofovir on CRPV-induced rabbit papillomas. Antiviral Res. 2000;48:131–142. doi: 10.1016/s0166-3542(00)00124-8. [DOI] [PubMed] [Google Scholar]
- Cladel NM, Hu J, Balogh K, Mejia A, Christensen ND. Wounding prior to challenge substantially improves infectivity of cottontail rabbit papillomavirus and allows for standardization of infection. J Virol Methods. 2008;148:34–39. doi: 10.1016/j.jviromet.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coremans G, Snoeck R. Cidofovir: clinical experience and future perspectives on an acyclic nucleoside phosphonate analog of cytosine in the treatment of refractory and premalignant HPV-associated anal lesions. Expert Opin Pharmacother. 2009;10:1343–1352. doi: 10.1517/14656560902960154. [DOI] [PubMed] [Google Scholar]
- Davis MD, Gostout BS, Mcgovern RM, Persing DH, Schut RL, Pittelkow MR. Large plantar wart caused by human papillomavirus-66 and resolution by topical cidofovir therapy. J Am Acad Dermatol. 2000;43:340–343. doi: 10.1067/mjd.2000.100534. [DOI] [PubMed] [Google Scholar]
- De CE. Therapeutic potential of Cidofovir (HPMPC, Vistide) for the treatment of DNA virus (i.e. herpes-, papova-, pox- and adenovirus) infections. Verh K Acad Geneeskd Belg. 1996;58:19–47. [PubMed] [Google Scholar]
- DeRossi SS, Laudenbach J. The management of oral human papillomavirus with topical cidofovir: a case report. Cutis. 2004;73:191–193. [PubMed] [Google Scholar]
- Duan J, Paris W, De Marte J, Roopchand D, Fleet T, Cordingley MG. Topical effects of cidofovir on cutaneous rabbit warts: treatment regimen and inoculum dependence. Antiviral Res. 2000;46:135–144. doi: 10.1016/s0166-3542(00)00080-2. [DOI] [PubMed] [Google Scholar]
- Ferenczy A, Behelak Y, Haber G, Wright TC, Jr, Richart RM. Treating vaginal and external anogenital condylomas with electrosurgery vs CO2 laser ablation. J Gynecol Surg. 1995;11:41–50. doi: 10.1089/gyn.1995.11.41. [DOI] [PubMed] [Google Scholar]
- Gallagher TQ, Derkay CS. Pharmacotherapy of recurrent respiratory papillomatosis: an expert opinion. Expert Opin Pharmacother. 2009;10:645–655. doi: 10.1517/14656560902793530. [DOI] [PubMed] [Google Scholar]
- Gye J, Nam C, Kim JS, Kim JY, Hong SP, Park BC, Kim M. Multiple traumatic neuromas after laser ablation treatment for viral warts. J Dermatol. 2013 doi: 10.1111/1346-8138.12319. [DOI] [PubMed] [Google Scholar]
- Hu J, Cladel NM, Balogh K, Budgeon L, Christensen ND. Impact of genetic changes to the CRPV genome and their application to the study of pathogenesis in vivo. Virology. 2007;358:384–390. doi: 10.1016/j.virol.2006.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Han R, Cladel NM, Pickel MD, Christensen ND. Intracutaneous DNA vaccination with the E8 gene of cottontail rabbit papillomavirus induces protective immunity against virus challenge in rabbits. Journal of Virology. 2002;76:6453–6459. doi: 10.1128/JVI.76.13.6453-6459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen ER, Selvakumar R, Shen H, Ahmed R, Wettstein FO, Miller JF. Recombinant Listeria monocytogenes vaccination eliminates papillomavirus-induced tumors and prevents papilloma formation from viral DNA. Journal of Virology. 1997;71:8467–8474. doi: 10.1128/jvi.71.11.8467-8474.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspari M, Gutzmer R, Kaspari T, Kapp A, Brodersen JP. Application of imiquimod by suppositories (anal tampons) efficiently prevents recurrences after ablation of anal canal condyloma. British Journal of Dermatology. 2002;147:757–759. doi: 10.1046/j.1365-2133.2002.04979.x. [DOI] [PubMed] [Google Scholar]
- Khandelwal K, Bumb RA, Mehta RD, Ghiya BC, Satoskar AR. Long-term efficacy of radiofrequency ablation in treatment of common and palmo-plantar warts. Australas J Dermatol. 2013;54:307–309. doi: 10.1111/j.1440-0960.2012.00966.x. [DOI] [PubMed] [Google Scholar]
- Kimberlin DW. Current status of antiviral therapy for juvenile-onset recurrent respiratory papillomatosis. Antiviral Res. 2004;63:141–151. doi: 10.1016/j.antiviral.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Kreider JW, Balogh K, Olson RO, Martin JC. Treatment of latent rabbit and human papillomavirus infections with 9-(2-phosphonylmethoxy)ethylguanine (PMEG) Antiviral Res. 1990;14:51–58. doi: 10.1016/0166-3542(90)90065-f. [DOI] [PubMed] [Google Scholar]
- Kreider JW, Christensen ND, Christian CB, Pickel MD. Preclinical system for evaluating topical podofilox treatment of papillomas: dose-response and duration of growth prior to treatment. J Invest Dermatol. 1992;99:813–818. doi: 10.1111/1523-1747.ep12614781. [DOI] [PubMed] [Google Scholar]
- Kwok CS, Gibbs S, Bennett C, Holland R, Abbott R. Topical treatments for cutaneous warts. Cochrane Database Syst Rev. 2012;9:CD001781. doi: 10.1002/14651858.CD001781.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leachman SA, Shylankevich M, Slade MD, Levine D, Sundaram RK, Xiao W, Bryan M, Zelterman D, Tiegelaar RE, Brandsma JL. Ubiquitin-fused and/or multiple early genes from cottontail rabbit papillomavirus as DNA vaccines. Journal of Virology. 2002;76:7616–7624. doi: 10.1128/JVI.76.15.7616-7624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofgren LA, Ronn AM, Abramson AL, Shikowitz MJ, Nouri M, Lee CJ, Batti J, Steinberg BM. Photodynamic therapy using m-tetra(hydroxyphenyl) chlorin. An animal model Archives of Otolaryngology--Head and Neck Surgery. 1994;120:1355–1362. doi: 10.1001/archotol.1994.01880360051010. [DOI] [PubMed] [Google Scholar]
- Lu YG, Yang YD, Wu JJ, Lei X, Cheng QH, He Y, Yang W. Treatment of perianal condyloma acuminate with topical ALA-PDT combined with curettage: outcome and safety. Photomed Laser Surg. 2012;30:186–190. doi: 10.1089/pho.2011.3040. [DOI] [PubMed] [Google Scholar]
- Munoz N, Manalastas R, Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, Clavel C, Luna J, Myers E, Hood S, Bautista O, Bryan J, Taddeo FJ, Esser MT, Vuocolo S, Haupt RM, Barr E, Saah A. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24-45 years: a randomised, double-blind trial. Lancet. 2009;373:1949–1957. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- Nafz J, Schafer K, Chen SF, Bravo IG, Ibberson M, Nindl I, Stockfleth E, Rosl F. A novel rodent papillomavirus isolated from anogenital lesions in its natural host. Virology. 2008;374:186–197. doi: 10.1016/j.virol.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Nicholls PK, Stanley MA. Canine papillomavirus - A centenary review. Journal of Comparative Pathology. 1999;120:219–233. doi: 10.1053/jcpa.1998.0278. [DOI] [PubMed] [Google Scholar]
- Oni G, Mahaffey PJ. Treatment of recalcitrant warts with the carbon dioxide laser using an excision technique. J Cosmet Laser Ther. 2011;13:231–236. doi: 10.3109/14764172.2011.606465. [DOI] [PubMed] [Google Scholar]
- Ostrow RS, Forslund KM, McGlennen RC, Shaw DP, Schlievert PM, Ussery MA, Huggins JW, Faras AJ. Ribavirin mitigates wart growth in rabbits at early stages of infection with cottontail rabbit papillomavirus. Antiviral Res. 1992;17:99–113. doi: 10.1016/0166-3542(92)90045-7. [DOI] [PubMed] [Google Scholar]
- Safrin S, Cherrington J, Jaffe HS. Clinical uses of cidofovir. Rev Med Virol. 1997;7:145–156. doi: 10.1002/(sici)1099-1654(199709)7:3<145::aid-rmv196>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Schofer H, Van OA, Henke U, Lenz T, Eul A. Randomized, comparative trial on the sustained efficacy of topical imiquimod 5% cream versus conventional ablative methods in external anogenital warts. Eur J Dermatol. 2006;16:642–648. [PubMed] [Google Scholar]
- Shikowitz MJ, Steinberg BM, Abramson AL. Hematoporphyrin derivative therapy of papillomas. Experimental study Archives of Otolaryngology--Head and Neck Surgery. 1986;112:42–46. doi: 10.1001/archotol.1986.03780010044007. [DOI] [PubMed] [Google Scholar]
- Silverman DA, Pitman MJ. Current diagnostic and management trends for recurrent respiratory papillomatosis. Curr Opin Otolaryngol Head Neck Surg. 2004;12:532–537. doi: 10.1097/01.moo.0000144392.33250.15. [DOI] [PubMed] [Google Scholar]
- Snoeck R. Papillomavirus and treatment. Antiviral Res. 2006;71:181–191. doi: 10.1016/j.antiviral.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Snoeck R, Andrei G, De CE. Cidofovir in the treatment of HPV-associated lesions. Verh K Acad Geneeskd Belg. 2001;63:93–120. discussion. [PubMed] [Google Scholar]
- Snoeck R, Bossens M, Parent D, Delaere B, Degreef H, Van RM, Noel JC, Wulfsohn MS, Rooney JF, Jaffe HS, De CE. Phase II double-blind, placebo-controlled study of the safety and efficacy of cidofovir topical gel for the treatment of patients with human papillomavirus infection. Clin Infect Dis. 2001;33:597–602. doi: 10.1086/322593. [DOI] [PubMed] [Google Scholar]
- Stragier I, Snoeck R, De CE, Van den Oord JJ, Van RM, De GH. Local treatment of HPV-induced skin lesions by Cidofovir. Journal of Medical Virology. 2002;67:241–245. doi: 10.1002/jmv.2213. [DOI] [PubMed] [Google Scholar]
- Van CE, Snoeck R, Van RM, Fiten P, Opdenakker G, Geboes K, Janssens J, Rutgeerts P, Vantrappen G, De CE. Successful treatment of a squamous papilloma of the hypopharynx-esophagus by local injections of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine. Journal of Medical Virology. 1995;45:230–235. doi: 10.1002/jmv.1890450221. [DOI] [PubMed] [Google Scholar]
- Wollina U, Konrad H, Karamfilov T. Treatment of common warts and actinic keratoses by Er:YAG laser. J Cutan Laser Ther. 2001;3:63–66. doi: 10.1080/146288301753377852. [DOI] [PubMed] [Google Scholar]
- Xu J, Xiang L, Chen J, He Q, Li Q, Li J, Wang J. The combination treatment using CO(2) laser and photodynamic therapy for HIV seropositive men with intraanal warts. Photodiagnosis Photodyn Ther. 2013;10:186–193. doi: 10.1016/j.pdpdt.2012.11.005. [DOI] [PubMed] [Google Scholar]