Introduction
Asthma is a chronic disease with a prevalence of 7% in US adults (1). It is a multifactorial disease in which patients exhibit various degrees of asthma control and responses to therapy. Management of asthma involves the use of β2-agonists, often in combination with corticosteroids for patients with persistent symptoms. However, 10-25% of asthmatics are resistant to steroid treatment (2). Th2 cells are the predominant inflammatory cell type in asthmatic airways biopsy and bronchoalveolar lavage (BAL) and studies found that these cells exhibit higher levels of IL-3, IL-4, IL-5 and IL-13 mRNA than cells from healthy controls. Reduced levels of the Th1 cytokines IFN-α2 and IFN-γ in PBMCs and BAL are associated with asthma exacerbations (3-5). However, as the disease becomes more severe the T cell population becomes a mixed Th2/ Th1 phenotype (6;7). Potential serum immunological biomarkers of asthma control have yet not been identified.
Previous studies evaluated cytokine profiles in asthma patient bronchoalveolar lavage fluid (8) or induced sputum (9). Bronchoalveolar lavage is an invasive and time consuming technique, and induced sputum measurements can lack reproducibility. Few studies have reported serum cytokine levels. Huang et al. (10) found no statistically significant differences between serum IL-5 levels in asthmatic children with acute exacerbations and stable condition . However IL-5 levels were higher than normal controls. Joseph et al. (11) found elevated serum IL-5 levels in mild and moderate persistent asthmatics which were not affected by corticosteroid use. Since persistent asthma involves several factors including airway inflammation and airway remodeling, the overall immunological profile tends to be a complex amalgam of cytokines, chemokines and growth factors (12). A more sophisticated approach such as the multiplex assay that allows detection of multiple analytes in a single well of a 96-well plate might be a suitable approach.
This study is the first attempt to determine protein profiles of cytokines, chemokines, growth factors, adhesion molecules and cytokine receptors in asthmatic patients’ serum using the multiplex assay in which 50 different analytes were analyzed simultaneously, correlated with measures of asthma control and quality of life.
Methods
Patient Selection
This study used samples from a subgroup of patients enrolled in a prospective cohort study of inner-city asthmatics. Patients were enrolled over a 19 month period in the general internal medicine clinic of Mount Sinai Hospital, located in East Harlem, NY. Additional details of the design of the parent study are reported elsewhere (13-17). This study was limited to non smoking asthmatics 18 to 55 years of age, with mild persistent, moderate persistent or severe disease (18) Non-asthmatic controls (NAC) were also recruited at Mount Sinai Hospital. Controls were non-smokers greater than 18 years of age with a history and spirometry test to rule out physician diagnosis of asthma.
As part of the interview of patients with asthma, we collected data on sociodemographic characteristics, asthma history, self-management behaviors, and asthma treatment. Asthma control was assessed using the Asthma Control Questionnaire (ACQ) (19), a validated instrument that includes information about wheezing, shortness of breath, awakening during the night, limitation of daily activities, severity of morning symptoms, and bronchodilator use. All questions were scored on a 7-point Likert scale with higher scores indicating worse asthma control. This instrument has been shown to have good responsiveness, reliability, and is one of the most commonly used methods of evaluating asthma control (20). Additional measures of health care services used at baseline included frequency of emergency department (ED) visits and hospitalizations for asthma during the previous year.
Asthma-related quality of life was assessed with the Mini-Asthma Quality of Life Questionnaire (AQLQ) (21). The Mini-AQLQ is a 15-item questionnaire that assesses respondents’ asthma-related quality of life in four domains: symptoms, emotional function, environmental stimuli, and activity limitation. Responses for these items were recorded on a seven point Likert scale (1 = all of the time to 7 = none of the time). The validity and reliability of the instrument has been demonstrated and this is one of the most frequently used instruments to measure asthma related quality of life (22).
From the study cohort, we randomly selected 14 samples from asthmatics classified as poorly controlled (PC), and 14 samples from patients classified as well controlled (WC) (13-17). For the purpose of this study, PC asthma was defined as an ACQ score >4 and Mini-AQLQ score ≤2.5 and/or history of ≥1 ED visit or hospitalization for asthma during the prior year. Patients with ACQ scores between 1- 2.5 were categorized as WC asthmatics. Additionally, sera of 14 NACs were analyzed. The study protocol was approved by the Mount Sinai School of Medicine Institutional Review Board, and all patients gave informed consent.
Multiplex assay for detection of cytokines, chemokines, growth factors and adhesion molecules
Serum protein levels of cytokines, chemokines, growth factors and adhesion molecules were evaluated using 23 and 27-Plex panels of Bio-Plex human cytokine assays (Bio-Rad laboratories, CA, USA) according to the manufacturer's instructions. Briefly, 50μl per well of capture antibody coated beads were added to a 96 well filter plate. The plate was washed between steps with a vacuum manifold and 50μl per well of serum samples at a dilution of 1:4 or standards were incubated with the beads for 30 mins at room temperature with constant shaking at 300rpm. Thereafter the plate was washed as in the earlier step and 25μl per well of detection antibody was added and incubated with shaking for 30 minutes at room temperature. After washing, streptavidin-phycoerythrin was added and incubated for 10 minutes at room temperature with shaking. The plate was washed and 125μl of Bio-Plex assay buffer was added. After rapid shaking for 30 seconds the plate was read using the Bio-Plex 200 Suspension Array System (Bio-Rad). Concentration of various analytes was calculated using the Bio-Plex Manager software version 4.1 (Bio-Rad). Low PMT settings were used for 23-plex and high PMT settings were used for 27-plex. Detectable limits of analytes were variable and in the range of 0.1-7pg/mL.
Statistical Analysis
Data were analyzed by using the SigmaStat statistical software package (SPSS Inc, Chicago, Ill). As the data distribution was not normal, the three groups were compared for each analyte using the Kruskal-Wallis test followed by pairwise comparisons between groups using Student-Newman-Keuls correction. The Chi-square and t-test were used for demographic data analysis. The relationship between the expression of different analytes and asthma control score and quality of life measurements were assessed with the Spearman's Rank correlation coefficient using SAS statistical software (SAS, Cary, NC).
Results
Patient characteristics
Baseline characteristics of the study cohort are described in Table 1. The mean age of the patients in three groups was not statistically different. All groups had a high percentage of females, PC (93%), WC (79%) and NAC (71%); however there was no significant difference between groups. High percentage of women asthmatics in the study is consistent with the epidemiology of urban asthma population. The study population was predominantly Hispanic, with PC (57%), WC (57%) and NAC (50%). Although the number of patients in this study is small to investigate any statistical correlations regarding ethnicity, the parent study showed a high percentage of asthmatics to be low-income Hispanics and African Americans which is consistent with epidemiology of inner-city asthmatics. There were no Whites in the PC group and no Blacks in the NAC group. ICS treatment in PC patients was 79%, in WC patients was 86%, and other patients were on β2-agonists. ACQ score (p < 0.001) and AQLQ score (p < 0.001) were significantly different among PC and WC groups.
Table 1.
Baseline characteristics of study subjects
| Characteristics | PC | WC | NACs | P value |
|---|---|---|---|---|
| Demographics | 14 | 14 | 14 | |
| Number of subjects | 37 ± 5 | 43 ± 9 | 42 ± 15 | 0.28 |
| Age, years (mean ± SD) | ||||
| Female, N (%) | 13 (93) | 11 (79) | 10 (71) | 0.33 |
| Race/ ethnicity, N (%) | 0.69 | |||
| White | 0 | 2 (14) | 4 (29) | |
| Black | 5 (36) | 3 (21) | 0 | |
| Hispanic | 8 (57) | 8 (57) | 7 (50) | |
| Others | 1 (7) | 1 (7) | 3 (21) | |
| Asthma history | ||||
| Age of onset, years (mean ± SD) | 14 ± 12 | 20 ± 16 | NA | 0.31 |
| Duration of asthma (mean ± SD) | 23 ± 10 | 23 ± 15 | NA | 0.89 |
| PEF predicted, (mean ± SD) | 294 ± 160 | 371 ± 61 | NA | 0.1 |
| ICS treatment, N (%) | 11 (79) | 12 (86) | NA | 0.62 |
| Outpatient visits in past year (%) | 12 (86) | 9 (64) | NA | 0.19 |
| ED visits in the past year (%) | 14 (100) | 0 | NA | .000 |
| Hospitalized in the past year (%) | 10 (71) | 9 (64) | NA | 0.69 |
| Ever intubated (%) | 11 (79) | 10 (71) | NA | 0.63 |
| Required steroids in the past year (%) | 2 (14) | 0 | NA | 0.14 |
| ACQ scores | 4.86 | 1.77 | NA | <0.001 |
| AQLQ scores | 2.75 | 4.8 | NA | <0.001 |
| Indoor allergen sensitization (%)* | ||||
| Dermatophagoides pteronyssinus- dust mite, N (%) | 4 (36) | 5 (36) | NA | 0.97 |
| Dermatophagoides farinae- dust mite, N (%) | 3 (27) | 5 (36) | NA | 0.65 |
| Cat, N (%) | 5 (46) | 4 (29) | NA | 0.38 |
| Mouse, N (%) | 3 (27) | 2 (14) | NA | 0.42 |
| Blatella germanica- cockroach, N (%) | 7 (64) | 10 (71) | NA | 0.68 |
| Periplaneta americana- cockroach, N (%) | 2 (18) | 3 (21) | NA | 0.84 |
| Aspergillus fumigatus- mold, N (%) | 3 (27) | 1 (7) | NA | 0.17 |
| Alternaria alternata- mold, N (%) | 2 (18) | 4 (29) | NA | 0.55 |
| Sensitized to at least 1 allergen, N (%) | 9 (82) | 10 (71) | NA | 0.55 |
PC = Poorly Controlled, WC = Well Controlled, NAC = Non-Asthmatic Controls, ICS = Inhaled Corticosteroids, ACQ = Asthma Control Questionnaire and AQLQ = Asthma Quality of Life Questionairre.
Patients were classified as sensitized if serum IgE titers were > 0.35kU/L.
Cytokine levels in PC, WC and NAC sera and correlations with ACQ and AQLQ scores
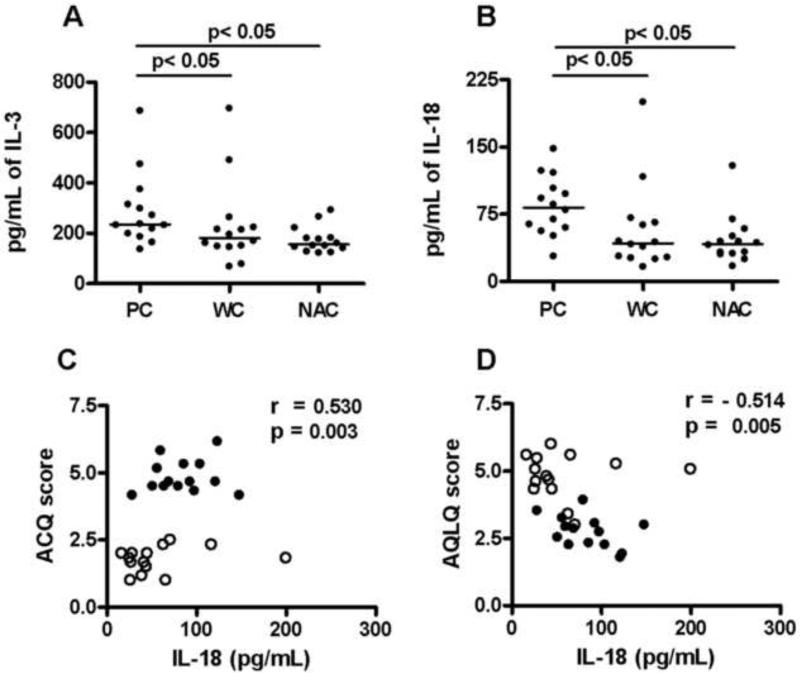
Eighteen of 29 cytokines tested were detectable. The levels of 15 cytokines differed significantly between groups. Among these, IL-1ra, IL-2, IL-6, IL-7, IL-9, IL-17, GM-CSF, MIF, Trail and TNF-α levels were significantly higher in sera from both PC and WC than NAC (Table 2). IFN-γ and IFN-α2 levels were significantly lower in PC and WC sera than NAC sera (Table 2, p < 0.05). However there were no significant differences between these cytokine levels in the PC and WC groups. IL-3 and IL-18 serum levels were significantly higher only in the PC group (p < 0.05), and were significantly higher than in WC (p < 0.05 Table 2 and Figure 1 A and B). Only IL-18 was positively correlated with the ACQ scores (r = 0.530; p = 0.003; Figure 1C) and negatively correlated with AQLQ (r = −0.514; p = 0.005; Figure 1D).
Table 2.
Cytokine Levels Among Poorly Controlled, Well Controlled and Non- Asthmatic Control groups
| Cytokines | PC Median (IQR) | WC Median (IQR) | NAC Median (IQR) |
P value |
||
|---|---|---|---|---|---|---|
| PC vs NAC | WC vs NAC | PC vs WC | ||||
| IL-1α | ND | ND | ND | – | – | – |
| IL-1β | ND | ND | ND | – | – | – |
| IL-1ra | 190 (128-382) | 207 (149-281) | 109 (102-144) | p < 0.05 | p < 0.05 | NS |
| IL-2 | 7 (6-15) | 9 (6-11) | 0 | p < 0.05 | p < 0.05 | NS |
| IL-3 | 235 (203-311) | 182 (149-222) | 156 (142-182) | p< 0.05 | NS | p < 0.05 |
| IL-4 | ND | ND | ND | – | – | – |
| IL-5 | ND | ND | ND | – | – | – |
| IL-6 | 8 (6-10) | 8 (5-10) | 2 (2-3) | p < 0.05 | p < 0.05 | NS |
| IL-7 | 13 (10-16) | 16 (11-19) | 3 (2-4) | p < 0.05 | p < 0.05 | NS |
| IL-9 | 26 (12-69) | 27 (14-58) | 6 (4-11) | p < 0.05 | p < 0.05 | NS |
| IL-10 | ND | ND | ND | – | – | – |
| IL-12 (p40) | ND | ND | ND | – | – | – |
| IL-12 (p70) | ND | ND | ND | – | – | – |
| IL-13 | 7 (6-7) | 8 (5-15) | 7 (5-13) | NS | NS | NS |
| IL-15 | ND | ND | ND | – | – | – |
| IL-16 | 390 (319-434) | 365 (189-410) | 189 (137-223) | p < 0.05 | p < 0.05 | NS |
| IL-17 | 18 (12-26) | 17 (14-23) | 0 | p < 0.05 | p < 0.05 | NS |
| IL-18 | 83 (61-102) | 43 (27-65) | 42 (32-49) | p < 0.05 | NS | p < 0.05 |
| G-CSF | 12 (11-19) | 13 (10-16) | 18 (15-20) | NS | NS | NS |
| GM-CSF | 11 (5-29) | 7 (3-18) | 0 | p < 0.05 | p < 0.05 | NS |
| IFN-α2 | ↓ 45 (40-52) | ↓ 42 (40-46) | 51 (47-54) | p < 0.05 | p < 0.05 | NS |
| IFN-γ | ↓ 56 (48-89) | ↓ 56 (50-75) | 107 (104-122) | p < 0.05 | p < 0.05 | NS |
| LIF | ND | ND | ND | – | – | – |
| M-CSF | ND | ND | ND | – | – | – |
| MIF | 374 (257-746) | 268 (198-351) | 74 (38-122) | p < 0.05 | p < 0.05 | NS |
| SCF | 91 (80-123) | 92 (62-105) | 109 (89-117) | NS | NS | NS |
| TNF-α | 52 (38-80) | 40 (35-57) | 0 | p < 0.05 | p < 0.05 | NS |
| TNF-β | ND | ND | ND | – | – | – |
| Trail | 132 (89-169) | 106 (66-154) | 53 (36-76) | p < 0.05 | p < 0.05 | NS |
Data are expressed as median in pg/mL with interquartile range in paranthesis. One Way analysis of variance, with Student-Newman-Keuls correction for pair-wise comparisons, was used to assess statistical significance between groups and p < 0.05 was considered statistically significant. IL: Interleukin, IL-1ra: Interleukin 1 receptor antagonist, G-CSF: Granulocyte colony-stimulating factor, GM-CSF: Granulocyte macrophage colony-stimulating factor, IFN-α2 and γ: Interferon alpha 2 and gamma, LIF: Leukemia inhibitory factor, M-CSF: Macrophage colony-stimulating factor, MIF: Macrophage migration inhibitory factor, SCF: Stem cell factor, TNF-α:Tumor necrosis factor- alpha, TNF-β:Tumor necrosis factor- beta, Trail: tumor necrosis factor-related apoptosis-inducing ligand. PC = Poorly Controlled, WC = Well Controlled, NAC = Non-Asthmatic Controls ↓ = values lower in asthmatics compared to NAC. Detectable limits for 23-plex (Low PMT) in pg/mL were; IL-1α: 1.3, IL-3: 1.9, IL-12(p40): 1.6, IL-16: 0.9, IL-18:1.8, IFN-α2:0.3, LIF: 1.5, M-CSF: 2.1, MIF: 1.6, SCF: 1.7, TNF-β: 1.6, Trail: 2.6 and for 27-plex (high PMT) were; IL-1β: 0.3, IL-1ra: 0.1, IL-2: 0.1, IL-4: 0.1, IL-5: 0.2, IL-6: 0.2, IL-7: 0.3, IL-9: 0.1, IL-10: 0.2, IL-12(p70): 0.3, IL-13: 0.1, IL-15: 0.1, IL-17: 0.2, G-CSF: 0.2, GM-CSF: 0.1, IFN-γ: 0.1 and TNF-α: 0.5.
Figure 1. Serum cytokine Levels of IL-3, IL-18, and correlation of IL-18 with asthma control and quality of life.
Serum IL-3 and IL-18, levels were compared in poorly controlled (PC), well controlled (WC) and non-asthmatic controls (NAC) by multiplex assay (A-B). A One Way Anova on ranks with Student-Newman-Keuls correction was used to calculate statistical significance between the three groups and a p< 0.05 was considered statistically significant. Spearman's Rank correlation method was used to calculate the correlation coefficient ‘r’ value. A positive ‘r’ value shows increase in cytokine level correlated with increase in ACQ score (poor control of asthma) and a negative value shows increase in cytokine level correlated with decrease in AQLQ score (poor quality of life). A p< 0.05 value was considered significant. C) Correlation between serum IL-18 levels vs. ACQ score. D) Correlation between IL-18 levels and AQLQ score. ● - PC and ○- WC.
Chemokine levels in PC, WC and NAC and correlations with ACQ and AQLQ scores
Of the 12 chemokines tested (Table 3), IL-8, MCP-1, MIP-1α, MIP-1β and MCP-3 were significantly elevated in both PC and WC sera vs. NAC. IL-8 and MCP-1 levels were not detectable in NAC sera. CTACK levels were significantly higher in PC sera than in NAC. However there were no significant differences in IL-8, MCP-1, MIP-1α, MIP-1β and MCP-3 and CTACK between PC and WC groups. RANTES levels in all groups were above the highest accurately measurable level (>2330 pg/mL). There was no significant correlation between IL-8, MCP-1, MIP-1α, MIP-1β, MCP-3 and CTACK levels and ACQ or AQLQ scores.
Table 3.
Chemokine Levels Among Poorly Controlled, Well Controlled and Non-Asthmatic Control groups
| Chemokines | PC Median (IQR) | WC Median (IQR) | NAC Median (IQR) |
P value |
||
|---|---|---|---|---|---|---|
| PC vs NAC | WC vs NAC | PC vs WC | ||||
| CTACK | 594 (490-700) | 478 (391-681) | 439 (366-532) | p < 0.05 | NS | NS |
| Eotaxin | 56 (32-107) | 83 (63-105) | 91 (34-139) | NS | NS | NS |
| GRO-α | 369 (9102-730) | 136 (79-195) | 102 (67-157) | NS | NS | NS |
| IL-8 | 6 (5-13) | 8 (5-17) | 0 | p < 0.05 | p < 0.05 | NS |
| IP-10 | 356 (261-974) | 280 (189-465) | 352 (231-619) | NS | NS | NS |
| MCP-1 (MCAF) | 41 (31-47) | 60 (36-81) | 0 | p < 0.05 | p < 0.05 | NS |
| MIG | 354 (259-470) | 396 (269-482) | 263 (249-468) | NS | NS | NS |
| MIP-1α | 4 (3-8) | 4 (3-7) | 8 (8-9) | p < 0.05 | p < 0.05 | NS |
| MIP-1β | 108 (63-165) | 132 (96-165) | 54 (42-84) | p < 0.05 | p < 0.05 | NS |
| MCP-3 | 33 (28-49) | 29 (24-35) | 6 (0-14) | p < 0.05 | p < 0.05 | NS |
| RANTES | >2330 | >2330 | >2330 | – | – | – |
| SDF-1α | 745 (503-1089) | 409 (325-784) | 470 (381-556) | NS | NS | NS |
Data are expressed as median in pg/mL with interquartile range in paranthesis. One Way analysis of variance, with Student-Newman-Keuls correction for pair-wise comparisons, was used to assess statistical significance between groups and p < 0.05 was considered statistically significant. CTACK: Cutaneous T-cell attracting chemokine, GRO-α: Growth regulated protein- alpha; IL: Interleukin, IP-10: Interferon gamma inducible Protein-10, MCP-1 (MCAF) and 3: Macrophage chemoattractant protein-1 (monocyte chemotactic and activating factor) and 3, MIG: Monokine induced interferon gamma, MIP-1α and β: Macrophage inflammatory protein-1 alpha and beta, RANTES: Regulated on activation, normal T expressed and secreted, SDF-1α: Stromal cell derived factor-1 alpha. PC = Poorly Controlled, WC = Well Controlled, NAC = Non-Asthmatic Controls. Detectable limits for 23-plex (Low PMT) in pg/mL were; CTACK: 2.1, GRO-α: 1.7, MIG: 0.2, MCP-3: 1.1, SDF-1α: 2.4 and for 27-plex (high PMT) were; Eotaxin: 0.1, IL-8: 0.1, IP-10: 0.4, MCP-1: 0.2, MIP-1α: 0.1, MIP-1β: 0.2 and RANTES: 0.1.
Growth factor levels in PC, WC and NAC sera, and correlation with ACQ and AQLQ scores
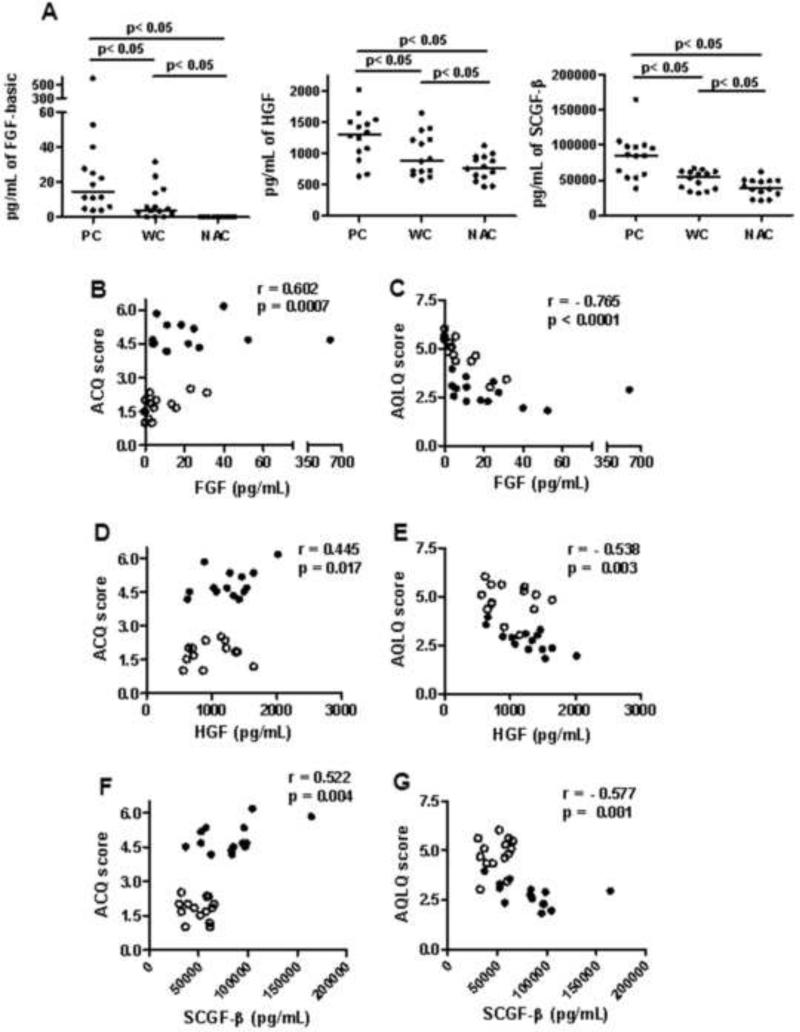
Five growth factors were tested. PDGF-BB and VEGF were significantly higher in both PC and WC sera than NAC sera (p < 0.05, Table 4), but PC and WC levels did not differ. Three growth factors- FGF-basic, Hepatocyte Growth Factor (HGF) and Stem Cell Growth Factor–beta (SCGF-β) were significantly higher in PC than WC and NAC groups (p < 0.05, Fig 3A). FGF-basic (r = 0.602, p = 0.0007), HGF (r = 0.445, p = 0.017) and SCGF-β (r = 0.522, p = 0.004) levels showed a positive correlation with ACQ scores (Fig. 3B, D and F). Similarly, FGF-basic (r = −0.765, p < 0.0001), HGF (r = −0.538, p = 0.003) and SCGF-β (r = −0.577, p = 0.001) showed a negative correlation with AQLQ scores (Fig. 3C, E and G).
Table 4.
Serum Growth Factors Levels Among Poorly Controlled, Well Controlled and Non- Asthmatic Control groups
| Growth factors | PC Median (IQR) | WC Median (IQR) | NAC Median (IQR) |
P value |
||
|---|---|---|---|---|---|---|
| PC vs NAC | WC vs NAC | PC vs WC | ||||
| β-NGF | ND | ND | ND | – | – | – |
| FGF-basic | 15 (7-27) | 4 (2-12) | 0 | p < 0.05 | p < 0.05 | p < 0.05 |
| PDGF-BB | 6713 (5890-9701) | 7783 (4831-8752) | 3495 (3005-4082) | p < 0.05 | p < 0.05 | NS |
| VEGF | 78 (54-159) | 109 (75-138) | 0 | p < 0.05 | p < 0.05 | NS |
| HGF | 1312 (1046-1493) | 894 (716-1224) | 771 (630-934) | p < 0.05 | p < 0.05 | p < 0.05 |
| SCGF-β | 84965 (59179-97272) | 55278 (37776-61429) | 38530 (30466-48560) | p < 0.05 | p < 0.05 | p < 0.05 |
Data are expressed as median in pg/mL with interquartile range in paranthesis. One Way analysis of variance, with Student-Newman-Keuls correction for pair-wise comparisons, was used to assess statistical significance between groups and p < 0.05 was considered statistically significant. β–NGF: Nerve growth factor beta, FGF-basic: fibroblast growth factor, PDGF-BB: Platelet derived growth factor, VEGF: Vascular endothelial growth factor, HGF: Hepatocyte growth factor, SCGF-β: Stem cell growth Factor beta. PC = Poorly Controlled, WC = Well Controlled, NAC = Non-Asthmatic Controls. Detectable limits for 23-plex (Low PMT) in pg/mL were; β-NGF: 1.1, HGF: 2.1, SCGF-β: 7 and for 27-plex (high PMT) were; FGF-basic: 0.1, PDGF-BB: 0.2 and VEGF: 0.2.
Adhesion molecules and IL-2ra receptor levels in PC, WC and NAC sera
No significant differences were found in levels of the two adhesion molecules VCAM-1, and ICAM-1 and IL-2ra receptor between groups (Table 5).
Table 5.
Adhesion Molecules And Receptor Levels Among Poorly Controlled, Well Controlled and Non- Asthmatic Control groups
| Adhesion molecules & receptor | PC Median (IQR) | WC Median (IQR) | NAC Median (IQR) |
P value |
||
|---|---|---|---|---|---|---|
| PC vs NAC | WC vs NAC | PC vs WC | ||||
| VCAM-1 | 105459 (91186-267674) | 90301 (66182-141906) | 81736 (54050-97599) | NS | NS | NS |
| ICAM-1 | 220129 (103039-565058) | 182711 (78336-279389) | 120446 (73567-193757) | NS | NS | NS |
| IL-2ra | 93 (82-141) | 70 (58-99) | 98 (86-110) | NS | NS | NS |
Data are expressed as median in pg/mL with interquartile range in paranthesis. One Way analysis of variance, with Student-Newman-Keuls correction for pair-wise comparisons, was used to assess statistical significance between groups and p < 0.05 was considered statistically significant. VCAM-1: Vascular cell adhesion molecule, ICAM-1: Intercellular adhesion molecule, IL-2ra: Interleukin 2 receptor. PC = Poorly Controlled, WC = Well Controlled, NAC = Non-Asthmatic Controls. Detectable limits for 23-plex (Low PMT) in pg/mL were; VCAM-1: 1.4, ICAM-1: 1.8 and IL-2ra: 0.6.
Discussion
Asthma is a heterogeneous disease, and it is well known that reduction of asthma symptoms in response to specific pharmacological interventions, such as corticosteroids, varies widely between individuals. Although the National Asthma Education and Prevention Program (NAEPP) guideline-based asthma controller therapy has been in place for 20 years, clinical outcomes remain suboptimal. Nearly 1.8 million asthma patients were treated in emergency departments in 2005 (23) and asthma accounts for 10.1 million missed work days annually (24). There are several different factors that may influence asthma control including inability to avoid indoor-outdoor allergens, smoking, viral infections, genetic makeup, treatment resistance, poor treatment adherence, and under treatment. All these factors individually or in combination can influence the patients’ serum immunological profile. Certain underlying defects in the innate immune system of the healthy individuals could make them susceptible to developing an asthmatic phenotype. Studies have shown impaired induction of virus-induced interferon secretion in asthmatics which could be correlated with severity of virus-induced asthma exacerbations (25). Our observation in the current study is in agreement with this study. In the current study we observed a statistically significant decrease in IFN-α2 and IFN-γ levels in asthmatics as compared to non asthmatic controls. Several previous studies investigated possible correlations between IL-5 and other cytokines and asthma control status but found no association between asthma exacerbation and IL-5 levels (10). In our study IL-4 and IL-5 were not detectable and IL-13 although detectable was not different among the 3 groups. The Th1/Th2 imbalance at the cytokine level leads to airway tissue damage prompting the immune system to respond with a repair process, airway remodeling- a hallmark feature of asthma, which involves several other cytokines and growth factors (26). Due to the ensuing complexity of the disease the mechanisms underlying asthma symptom control outcomes are not fully elucidated. Our study is the first to use multiplex technique to determine an array of cytokines, chemokines, growth factors, adhesion molecules and receptors in sera from patients with poorly and well controlled asthma and compare the results to non asthmatic controls.
One potentially important finding is that IL-3 and IL-18 levels were significantly higher in the PC group than in the WC group. IL-3, a cytokine secreted by lymphocytes and basophils, plays an important role in the maturation and priming of basophils, a pathway associated with asthma exacerbations (27;28). IL-18 levels also showed a positive correlation with ACQ scores, and a negative correlation with AQLQ scores in our study. IL-18 is released from activated monocytes, macrophages, airway epithelial cells and has been described as an interferon-gamma-inducing factor (29;30). Therefore IL-18 might be expected to suppress Th2 cytokine secretions. However, Kodama et al. demonstrated that IL-18 deficiency selectively enhances allergen-induced eosinophilia in mice (31). Other studies using murine models reported contradictory data. Hoshino et al. (32) demonstrated that in vivo administration of IL-18 induced IgE production through Th2 cytokine induction and up-regulation of CD40 ligand expression on CD4+ T cells. These controversial findings may be due to the presence or absence of IFN-γ as previously reported (33). IL-18 in the absence of Th1 cytokines skews the response to Th2 type (34-36). Our study found that higher levels of IL-18 in serum from PC was also associated with decreased IFN-γ and elevated IL-3, levels. This combined cytokine profile may prove to be an important marker of PC, however, further investigation is required.
In this study, three growth factors, FGF-basic, HGF and SCGF-β were significantly higher in PC than WC and NAC sera and showed a positive correlation with poor asthma control and poor quality of life scores. FGF-basic is a pleiotropic polypeptide involved in angiogenesis, tissue repair and inflammatory processes and is produced by fibroblasts, endothelial cells, macrophages, T-lymphocytes and mast cells (37-41). Increased levels of FGF-basic have been found in BAL fluids and airway tissue of subjects with bronchial asthma (38;42), however, there are no reports of a correlation between plasma FGF-basic levels and asthma. HGF is a pleiotropic biological component that plays a vital role in angiogenesis, cell-proliferation, anti-apoptosis and anti-fibrosis. It is known to play a role in oncogenesis and cancer metastasis (43). The role of HGF in asthma is controversial. Administration of exogenous HGF in a murine asthma model significantly diminished AHR and inflammatory cytokine levels (44). Nomura et al. (45) found higher levels of HGF in induced sputum of asthmatic patients than normal controls. However HGF serum levels in well and poorly controlled asthma have not been previously reported. SCGF is a cytokine of the C-type lectin family which supports hematopoietic stem/progenitor cell proliferation. Expression of SCGF-β mRNA is restricted to myeloid cells and fibroblasts. SCGF lacks colony-stimulating activity, but has burst-promoting activity for human bone marrow erythroid progenitors, granulocyte/macrophage–promoting activity for GM progenitors and colony forming unit –GM –sustaining or recruiting effect during short-term liquid BM cell culture (46). There have been no previous reports of a role for SCGF-β in asthma. A recent study indicates that pathological mechanisms of asthma involve more than just Th1/Th2 inflammation (47). The role of growth factors in asthma pathological mechanisms has not been extensively studied, but might be associated with airway remodeling and inflammatory cell survival (48-50). Airway remodeling involves thickening of the airways due to increase in airway smooth muscles, myofibroblasts, collagen and angiogenesis. Various cells such as mast cells, eosinophils, macrophages and epithelial cells involved in airway inflammation secrete growth factors which are important for the proliferation of myofibroblasts that further secrete extracellular matrix components. The role of transforming growth factor β (TGF-β), PDGF, insulin like growth factor (IGF)-I, FGF-basic and VEGF have been demonstrated in airway thickening and angiogenesis (38;45;48;49). Hoshino et al. also demonstrated that subepithelial layer thickening plays a role in promoting bronchial responsiveness (48). We did not test (TGF-β) and (IGF)-I but among the growth factors assessed PDGF and VEGF were not significantly different in PC and WC however, FGF-basic, HGF and SCGF-β were important growth factors that were statistically higher in PC as compared to WC. The detection of statistically significant higher levels of growth factors and absence of proinflammatory cytokines in the serum of PC asthmatics suggests that the current therapy may control inflammation however is inadequate in suppressing airway remodeling. To generate biomarkers, the subjects were selected such that there was sharp contrast between poor controlled and well controlled patients so as to avoid overlap between two groups. The bioplex technology is relatively new and expensive. To maintain the cost-effectiveness we used samples from well defined groups which limited the number of qualified patients from the parent study. Due to the small number of patients, the homogeneity in ethnicity is compromised and PC group has no whites and NAC group has no blacks, this factor should be considered while interpreting the results of this study.
In conclusion, this study is the first attempt to use broad array of immunological and growth factor measures to identify possible candidates as markers of asthma control status. The use of sera and multiplex assay deserves additional application. IL-18, FGF-basic, HGF and SCGF-β levels were significantly elevated in the poorly controlled group in our study. Our study has provided important candidates for future studies which may include comparison of BALF and sputum samples. This finding may provide a rationale for further identification of serum biomarkers associated with asthma control status.
Figure 2. Comparison of serum growth factors FGF-basic, HGF and SCGF-β levels in PC, WC and NAC groups and their correlation with ACQ and AQLQ score.
A) Serum growth factors FGF-basic, HGF and SCGF-β levels were compared in poorly controlled (PC), well controlled (WC) and non-asthmatic controls (NAC) by multiplex assay. A One Way Anova with Student-Newman-Keuls correction was used to calculate statistical significance between the three groups and a p< 0.05 was considered statistically significant. Spearman's Rank correlation method was used to calculate the correlation coefficient ‘r’ value. A p< 0.05 value was considered significant. B) Correlation between FGF-basic values and ACQ score. C) Correlation between FGF-basic value and AQLQ score. D) Correlation between HGF value and ACQ score. E) Correlation between HGF value and AQLQ score. F) Correlation between SCGF-β value and ACQ score. G) Correlation between SCGF-β and AQLQ score. ● - PC and ○- WC.
Acknowledgements
We would like to thank Linda Lurslurchachai for administrative support and data management. SP Patil acknowledges the American Academy of Allergy, Asthma & Immunology's Strategic Training in Allergy Research (ST*AR) Award, which allowed the presentation of part of these results as an abstract during the 2008 American Academy of Allergy, Asthma & Immunology annual conference.
Funding: This work was partially supported by the K08 HS13312-01A1 to JP Wisnivesky, 5 R24AG023958-04 to EA Halm and PO1 AT002647 to XM Li by NIH/NCCAM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors have no conflicts of interest.
References
- 1.Pleis JR, Lucas JW. Summary health statistics for U.S. adults: National Health Interview Survey, 2007. Vital Health Stat. 2009;10(240):1–159. [PubMed] [Google Scholar]
- 2.Leung DY, Bloom JW. Update on glucocorticoid action and resistance. J Allergy Clin Immunol. 2003;111(1):3–22. doi: 10.1067/mai.2003.97. [DOI] [PubMed] [Google Scholar]
- 3.Gehlhar K, Bilitewski C, Reinitz-Rademacher K, Rohde G, Bufe A. Impaired virus-induced interferon-alpha2 release in adult asthmatic patients. Clin Exp Allergy. 2006;36(3):331–337. doi: 10.1111/j.1365-2222.2006.02450.x. [DOI] [PubMed] [Google Scholar]
- 4.Renzi PM, Turgeon JP, Marcotte JE, et al. Reduced interferon-gamma production in infants with bronchiolitis and asthma. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1417–1422. doi: 10.1164/ajrccm.159.5.9805080. [DOI] [PubMed] [Google Scholar]
- 5.Tang C, Rolland JM, Ward C, Quan B, Walters EH. IL-5 production by bronchoalveolar lavage and peripheral blood mononuclear cells in asthma and atopy. Eur Respir J. 1997;10(3):624–632. [PubMed] [Google Scholar]
- 6.Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mamessier E, Nieves A, Lorec AM, et al. T-cell activation during exacerbations: a longitudinal study in refractory asthma. Allergy. 2008;63(9):1202–1210. doi: 10.1111/j.1398-9995.2008.01687.x. [DOI] [PubMed] [Google Scholar]
- 8.Brasier AR, Victor S, Boetticher G, et al. Molecular phenotyping of severe asthma using pattern recognition of bronchoalveolar lavage-derived cytokines. J Allergy Clin Immunol. 2008;121(1):30–37. doi: 10.1016/j.jaci.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HB, Kim CK, Iijima K, Kobayashi T, Kita H. Protein microarray analysis in patients with asthma: elevation of the chemokine PARC/CCL18 in sputum. Chest. 2009;135(2):295–302. doi: 10.1378/chest.08-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang CS, Chen SJ, Chung RL, Tang RB. Serum interleukin-5 measurements for monitoring acute asthma in children. J Asthma. 2005;42(4):297–300. doi: 10.1081/jas-200057886. [DOI] [PubMed] [Google Scholar]
- 11.Joseph J, Benedict S, Safa W, Joseph M. Serum interleukin-5 levels are elevated in mild and moderate persistent asthma irrespective of regular inhaled glucocorticoid therapy. BMC Pulm Med. 2004;4:2. doi: 10.1186/1471-2466-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becky Kelly EA, Busse WW, Jarjour NN. A comparison of the airway response to segmental antigen bronchoprovocation in atopic asthma and allergic rhinitis. J Allergy Clin Immunol. 2003;111(1):79–86. doi: 10.1067/mai.2003.28. [DOI] [PubMed] [Google Scholar]
- 13.Wisnivesky JP, Sampson H, Berns S, Kattan M, Halm EA. Lack of association between indoor allergen sensitization and asthma morbidity in inner-city adults. J Allergy Clin Immunol. 2007;120(1):113–120. doi: 10.1016/j.jaci.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 14.Ponieman D, Wisnivesky JP, Leventhal H, Musumeci-Szabo TJ, Halm EA. Impact of positive and negative beliefs about inhaled corticosteroids on adherence in inner-city asthmatic patients. Ann Allergy Asthma Immunol. 2009;103(1):38–42. doi: 10.1016/S1081-1206(10)60141-X. [DOI] [PubMed] [Google Scholar]
- 15.Wisnivesky JP, Kattan M, Evans D, et al. Assessing the relationship between language proficiency and asthma morbidity among inner-city asthmatics. Med Care. 2009;47(2):243–249. doi: 10.1097/MLR.0b013e3181847606. [DOI] [PubMed] [Google Scholar]
- 16.Wisnivesky JP, Lorenzo J, Lyn-Cook R, et al. Barriers to adherence to asthma management guidelines among inner-city primary care providers. Ann Allergy Asthma Immunol. 2008;101(3):264–270. doi: 10.1016/S1081-1206(10)60491-7. [DOI] [PubMed] [Google Scholar]
- 17.Wisnivesky JP, Leventhal H, Halm EA. Predictors of asthma-related health care utilization and quality of life among inner-city patients with asthma. J Allergy Clin Immunol. 2005;116(3):636–642. doi: 10.1016/j.jaci.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 18.National Heart, Lung and Blood Institute . Expert Panel report 2: guidelines for the diagnosis and management of asthma. National Institutes of Health; 1997. [Google Scholar]
- 19.Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 20.de Vries MP, van den BL, Lince S, Muris JW, Thoonen BP, van Schayck CP. Factors associated with asthma control. J Asthma. 2005;42(8):659–665. doi: 10.1080/02770900500264903. [DOI] [PubMed] [Google Scholar]
- 21.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14(1):32–38. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 22.Holbrook JT, Harik-Khan R. Montelukast and emotional well-being as a marker for depression: Results from 3 randomized, double-masked clinical trials. J Allergy Clin Immunol. 2008;122(4):828–829. doi: 10.1016/j.jaci.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schatz M, Rachelefsky G, Krishnan JA. Follow-up after acute asthma episodes: what improves future outcomes? J Allergy Clin Immunol. 2009;124(2 Suppl):S35–S42. doi: 10.1016/j.jaci.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Akinbami L. Asthma prevalence, health care use and mortality: United States 2003-05. CDC National Center for Health Statistics; 2006. [PubMed] [Google Scholar]
- 25.Wark PA, Johnston SL, Bucchieri F, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201(6):937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38(6):872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 27.Kepley CL, McFeeley PJ, Oliver JM, Lipscomb MF. Immunohistochemical detection of human basophils in postmortem cases of fatal asthma. Am J Respir Crit Care Med. 2001;164(6):1053–1058. doi: 10.1164/ajrccm.164.6.2102025. [DOI] [PubMed] [Google Scholar]
- 28.Ono E, Taniguchi M, Higashi N, et al. CD203c expression on human basophils is associated with asthma exacerbation. J Allergy Clin Immunol. 2010;125(2):483–489. doi: 10.1016/j.jaci.2009.10.074. [DOI] [PubMed] [Google Scholar]
- 29.Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378(6552):88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 30.Lee KS, Kim SR, Park SJ, et al. Antioxidant down-regulates interleukin-18 expression in asthma. Mol Pharmacol. 2006;70(4):1184–1193. doi: 10.1124/mol.106.024737. [DOI] [PubMed] [Google Scholar]
- 31.Kodama T, Matsuyama T, Kuribayashi K, et al. IL-18 deficiency selectively enhances allergen-induced eosinophilia in mice. J Allergy Clin Immunol. 2000;105(1 Pt 1):45–53. doi: 10.1016/s0091-6749(00)90176-3. [DOI] [PubMed] [Google Scholar]
- 32.Hoshino T, Yagita H, Ortaldo JR, Wiltrout RH, Young HA. In vivo administration of IL-18 can induce IgE production through Th2 cytokine induction and up-regulation of CD40 ligand (CD154) expression on CD4+ T cells. Eur J Immunol. 2000;30(7):1998–2006. doi: 10.1002/1521-4141(200007)30:7<1998::AID-IMMU1998>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12(1):53–72. doi: 10.1016/s1359-6101(00)00015-0. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimoto T, Tsutsui H, Tominaga K, et al. IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc Natl Acad Sci U S A. 1999;96(24):13962–13966. doi: 10.1073/pnas.96.24.13962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshimoto T, Mizutani H, Tsutsui H, et al. IL-18 induction of IgE: dependence on CD4+ T cells, IL-4 and STAT6. Nat Immunol. 2000;1(2):132–137. doi: 10.1038/77811. [DOI] [PubMed] [Google Scholar]
- 36.Wild JS, Sigounas A, Sur N, et al. IFN-gamma-inducing factor (IL-18) increases allergic sensitization, serum IgE, Th2 cytokines, and airway eosinophilia in a mouse model of allergic asthma. J Immunol. 2000;164(5):2701–2710. doi: 10.4049/jimmunol.164.5.2701. [DOI] [PubMed] [Google Scholar]
- 37.Inoue Y, King TE, Jr., Tinkle SS, Dockstader K, Newman LS. Human mast cell basic fibroblast growth factor in pulmonary fibrotic disorders. Am J Pathol. 1996;149(6):2037–2054. [PMC free article] [PubMed] [Google Scholar]
- 38.Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol. 2001;107(2):295–301. doi: 10.1067/mai.2001.111928. [DOI] [PubMed] [Google Scholar]
- 39.Inoue Y, King TE, Jr., Barker E, Daniloff E, Newman LS. Basic fibroblast growth factor and its receptors in idiopathic pulmonary fibrosis and lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2002;166(5):765–773. doi: 10.1164/rccm.2010014. [DOI] [PubMed] [Google Scholar]
- 40.Henke C, Marineili W, Jessurun J, et al. Macrophage production of basic fibroblast growth factor in the fibroproliferative disorder of alveolar fibrosis after lung injury. Am J Pathol. 1993;143(4):1189–1199. [PMC free article] [PubMed] [Google Scholar]
- 41.Blotnick S, Peoples GE, Freeman MR, Eberlein TJ, Klagsbrun M. T lymphocytes synthesize and export heparin-binding epidermal growth factor-like growth factor and basic fibroblast growth factor, mitogens for vascular cells and fibroblasts: differential production and release by CD4+ and CD8+ T cells. Proc Natl Acad Sci U S A. 1994;91(8):2890–2894. doi: 10.1073/pnas.91.8.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redington AE, Roche WR, Madden J, et al. Basic fibroblast growth factor in asthma: measurement in bronchoalveolar lavage fluid basally and following allergen challenge. J Allergy Clin Immunol. 2001;107(2):384–387. doi: 10.1067/mai.2001.112268. [DOI] [PubMed] [Google Scholar]
- 43.Toschi L, Janne PA. Single-agent and combination therapeutic strategies to inhibit hepatocyte growth factor/MET signaling in cancer. Clin Cancer Res. 2008;14(19):5941–5946. doi: 10.1158/1078-0432.CCR-08-0071. [DOI] [PubMed] [Google Scholar]
- 44.Ito W, Kanehiro A, Matsumoto K, et al. Hepatocyte growth factor attenuates airway hyperresponsiveness, inflammation, and remodeling. Am J Respir Cell Mol Biol. 2005;32(4):268–280. doi: 10.1165/rcmb.2004-0058OC. [DOI] [PubMed] [Google Scholar]
- 45.Nomura S, Kanazawa H, Hirata K, Iwao H, Yoshikawa J. Relationship between vascular endothelial growth factor and angiopoietin-2 in asthmatics before and after inhaled beclomethasone therapy. J Asthma. 2005;42(2):141–146. [PubMed] [Google Scholar]
- 46.Hiraoka A, Sugimura A, Seki T, et al. Cloning, expression, and characterization of a cDNA encoding a novel human growth factor for primitive hematopoietic progenitor cells. Proc Natl Acad Sci U S A. 1997;94(14):7577–7582. doi: 10.1073/pnas.94.14.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lloyd CM, Saglani S. Asthma and allergy: the emerging epithelium. Nat Med. 2010;16(3):273–274. doi: 10.1038/nm0310-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoshino M, Nakamura Y, Sim JJ. Expression of growth factors and remodelling of the airway wall in bronchial asthma. Thorax. 1998;53(1):21–27. doi: 10.1136/thx.53.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamashita N, Sekine K, Miyasaka T, et al. Platelet-derived growth factor is involved in the augmentation of airway responsiveness through remodeling of airways in diesel exhaust particulate-treated mice. J Allergy Clin Immunol. 2001;107(1):135–142. doi: 10.1067/mai.2001.111433. [DOI] [PubMed] [Google Scholar]
- 50.Bonini S, Lambiase A, Bonini S, Levi-Schaffer F, Aloe L. Nerve growth factor: an important molecule in allergic inflammation and tissue remodelling. Int Arch Allergy Immunol. 1999;118(2-4):159–162. doi: 10.1159/000024055. [DOI] [PubMed] [Google Scholar]