Abstract
Therapeutic vaccines to induce anti-tumor CD8 T cells have been used in clinical trials for advanced melanoma patients, but the clinical response rate and overall survival time have not improved much. We believe that these dismal outcomes are caused by inadequate number of antigen-specific CD8 T cells generated by most vaccines. In contrast, huge CD8 T cell responses readily occur during acute viral infections. High levels of type-I interferon (IFN-I) are produced during these infections, and this cytokine not only exhibits anti-viral activity but also promotes CD8 T cell responses. The studies described here were performed to determine whether promoting the production of IFN-I could enhance the potency of a peptide vaccine. We report that cyclic diguanylate monophosphate (c-di-GMP), which activates the stimulator of interferon genes, potentiated the immunogenicity and anti-tumor effects of a peptide vaccine against mouse B16 melanoma. The synergistic effects of c-di-GMP required co-administration of costimulatory anti-CD40 antibody, the adjuvant poly-IC, and were mediated in part by IFN-I. These findings demonstrate that peptides representing CD8 T cell epitopes can be effective inducers of large CD8 T cell responses in vaccination strategies that mimic acute viral infections.
Keywords: Peptide vaccines, Immunotherapy, CD8 T cells, Type-I interferon, STING
Introduction
Malignant melanoma accounts for 76,000 new cases and about 10,000 deaths every year in the USA, and the 5-year survival rate for patients with advanced melanoma remains <20 % [1, 2]. Although CD8 T cell-based vaccines can increase clinical response rates, the median overall survival time for metastatic patients is only 11–25 months [3, 4]. In many of these clinical studies, antigen-specific CD8 T cells can hardly be detected in the blood of vaccinated patients, with frequencies of <1 % [3, 5]. We believe that these disappointing results can be partially explained by the use of poorly immunogenic vaccine formulations that utilize weak immunological adjuvants (e.g., incomplete Freund’s adjuvant) and by suboptimal modes of vaccine administration.
Type-I interferon (IFN-I) plays an important role in both innate and adaptive anti-tumor immune responses, including (a) the activation of professional antigen-presenting cells (APCs) such as dendritic cells; (b) serving as a growth/survival factor for activated CD8 T cells [6]; (c) helping in overcoming tumor-specific T cell tolerance [7]; and (d) promoting autophagy of cancer cells [8, 9]. Moreover, IFN-I has been shown to improve disease-free survival and is being recommended for the treatment for stage IIB-V melanoma patients [10]. In view of the above, we believe that an effective therapeutic vaccination strategy for melanoma would be to design vaccine formulations that result in high levels of IFN-I production. Cyclic diguanylate monophosphate (c-di-GMP) is a bacterial second messenger that activates the stimulator of interferon genes (STING) [11, 12], resulting in increased IFN-I secretion [13–15], and thus may function as a potent adjuvant for melanoma immunotherapy. In addition, STING ligands activate canonical and non-canonical NF-kB signaling pathways [16] that are likely to enhance vaccine-induced T cell priming and expansion.
For >12 years, our laboratory has focused on optimizing peptide vaccines to induce robust anti-tumor CD8 T cell responses [17]. We have recently described an improved peptide vaccination strategy that generated up to 80 % epitope-specific cells of all the CD8 T cells in the blood of mice after two consecutive immunizations (prime/boost). This strategy called TriVax consists of a mix of synthetic peptide corresponding to a CD8 T cell epitope, poly-IC adjuvant, and costimulatory anti-CD40 antibodies. TriVax is administered intravenously in order to mimic a systemic infection and recruit most of the naïve CD8 T cell precursors [18, 19]. However, not all the peptides so far tested have been efficient in generating strong immune responses when administered using TriVax indicating the need of further optimization. As we reported previously, the human hgp10025-33 (hgp100) heteroclitic epitope, used to study the immunity against B16 mouse melanoma, is unable to induce endogenous antigen-specific tumor-reactive CD8 T cells in mice after TriVax immunization [20]. However, hgp100-TriVax was very effective at stimulating potent T cell responses when used after adoptive cell transfers (ACT) of Pmel-1 TCR transgenic CD8 T cells [20]. To improve the generation of endogenous anti-tumor CD8 T cell responses to peptide vaccination, we have enhanced the immunogenicity of synthetic peptides by increasing the amphiphilicity of the minimal epitopes, which is achieved by the addition of two palmitic acid chains (Pam2) to the epitope’s amino terminus end using a KMFV linker (Pam2KMFV-), resulting in a substantially more immunogenic constructs as compared to the minimal epitopes [21]. Moreover, in many instances, the heteroclitic peptide constructs are efficient in generating strong CD8 T cell responses when administered systemically (i.v.) with poly-IC alone, in the absence of anti-CD40 antibody (BiVax) [21].
In the experiments described herein, we attempted to induce endogenous tumor-reactive antigen-specific CD8 T cell responses against B16 tumors by combining Pam2KMFV-hgp100 TriVax with c-di-GMP. Our results show that this vaccination strategy generated substantial numbers of antigen-specific CD8 T cells that were capable of recognizing B16 tumor cells, resulting in remarkable therapeutic anti-tumor effects in mice. These results show that the additional administration of c-di-GMP further enhanced the anti-tumor effects of the TriVax vaccine.
Materials and methods
Cells and mice
Murine melanoma B16F10 and EL4 thymoma were purchased from the American Type Culture Collection (Manassas, VA), and C57BL/6 (B6) of 8–10 weeks of age were from the National Cancer Institute/Charles River program. IFNαβ receptor-deficient (IFNαβR−/−) mice [22] (B6 background) and Pmel-I mice (TCR transgenic specific for the melanoma gp10025-33 CD8 epitope [23] were bred in our animal facility using mice originally obtained from Dr. P. Marrack (National Jewish Medical and Research Center, Denver, CO) and The Jackson Laboratory (Bar Harbor, ME), respectively. Animal care and experiments were performed following the Institutional Animal Care and Use Committee Guidelines.
Reagents and antibodies
Poly-IC (high molecular weight) and c-di-GMP were purchased from InvivoGen (San Diego, CA). Synthetic peptides representing H-2Kb-restricted CD8 T cell epitopes, Ovalbumin257-264 (Ova, SIINFEKL) the H-2Db-restricted human gp10025-33 (hgp100, KVPRNQDWL), mouse gp10025-33 (mgp100, EGSRNQDWL), and the amphiphilic lipopeptide (Pam)2-KMFVKVPRNQDWL (Pam-hgp100) were ordered from A&A Laboratories (San Diego, CA). The purity and identity of peptides were determined by high-performance liquid chromatography and mass spectrometry analysis by the vendor. Monoclonal anti-mouse CD40 (FGK45.5) antibody was purchased from BioXcell (West Lebanon, NH). PE-labeled H-2Kb/SIINFEKL and H-2Db/KVPRNQDWL tetramers were purchased from MBL International (Woburn, MA) All other fluorochrome-labeled antibodies were from eBioscience, Inc. (San Diego, CA).
Immunizations
All vaccines were administered intravenously (200 μl/injection in the tail vein). Mice were usually given two sequential vaccinations 9 days apart (prime and boost). The following vaccine formula was used (unless otherwise noted): In prime, TriVax consisted of a mixture of 120 μg Pam-hgp100, 100 µg hgp100 or 100 µg Ova, 50 μg anti-CD40 antibody, and 25 μg Poly-IC. For the boost, the anti-CD40 antibody dose was reduced to 25 μg. In some experiments, the mice also received 100 µg of c-di-GMP intravenously both in the prime and boost.
Assessment of immune responses
Antigen-specific CD8 T cells were assessed by tetramer staining using peripheral blood samples or spleen cells at the termination of the experiments. Cells were stained with fluorescein isothiocyanate–anti-MHC class II, PerCP Cy5.5-anti-CD8a, and PE-conjugated tetramers for 40 min in ice. Fluorescence was measured using an LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (Ashland, OR). For the detection of IFNγ-secreting CD8 T cells, enzyme-linked immunosorbent spot (EliSpot) and ELISAs were performed as previously described [18, 19, 24] using purified CD8 T cells (Miltenyi Biotec). The number of CD8 T cells per well was determined by the flow tetramer staining results, to ensure that 1 × 105 tetramer-positive cells were in each well. The CD8 T cells were incubated with 1 × 105 target cells (EL4, EL4 plus mgp100 peptide, and B16F10 cells) for 18 h at 37 °C. When Pmel-1 cells were used, positively selected CD8 T cells were first activated by incubating at 3 × 104/well in 96-well plates with CD3/CD28 Dynabeads (Life Technologies) at a 1:1 ratio. Seven days later, the Pmel-1 CD8 T cells were harvested and incubated at 1 × 105 per well with the same numbers of target cells. Then, the cultures were developed as described by the EliSpot kit manufacturer (Mabtech, Inc., Mariemont, OH), and spot counting was done with an ImmunoSpot System (Cellular Technology Ltd, Cleveland, OH).
Flowcytometry-based cytotoxicity assay
A cell-mediated assay was performed using fluorescent beads to enable quantitative analysis of the number of live CFSE-labeled target cells [25–27]. Briefly, target cells (EL4, peptide-pulsed EL4, or B16F10) were resuspended at 1 × 106/ml and labeled with 1.5 µM CFSE (Life Technologies) for 15 min at 37 °C. Then, target cells were incubated at 1 × 105 per well with 1 × 105 CD8 T cells in 96-well, round-bottom plates at 37 °C. Eighteen hours later, cells were harvested and suspended in 200 µl of PBS per well. To stain dead cells, 7-AAD (BD, San Diego, CA) was added at 15 µl/ml. 30 µl of fluorescent beads (Flow-Count Fluorospheres, Coulter Corporation, Miami, FL) were added into each tube just before flow analysis. For each sample, 2000 fluorescent beads were acquired, allowing the calculation of the numbers of surviving live target cells (CFSE-positive and 7-AAD-negative/2000 bead count). B16 Survival Rate = (Live target cells with CD8 T cells/Live target cells alone) × 100.
Anti-tumor effects
Mice (five per group) were injected subcutaneously with 4 × 105 B16F10 cells in a shaved rear flank, and 8 days later, mice received the indicated vaccine. Boosters were administered on day 17, and tumor growth was monitored every 2–3 days in individually tagged mice by measuring two opposing diameters with a set of calipers. Mice were euthanized when the tumor area reached >400 mm2. Results are presented as the mean tumor size (area in mm2) ±SD for every treatment group at various time points until the termination of the experiment.
Statistical analyses
Unpaired Student’s t tests were used to determine statistical significance of differences in numbers of antigen-specific CD8 T cells. Tumor sizes between two populations throughout time were analyzed for significance using two-way ANOVA. Log-rank test was used to compare the survival rate of tumor-bearing mice. All analyses and graphics were done using Prism 5.01 software (GraphPad). P values <0.05 were considered to be statistically significant. Most experiments were repeated 2–3 times with nearly identical findings.
Results
C-di-GMP enhances TriVax-induced immune responses to melanoma
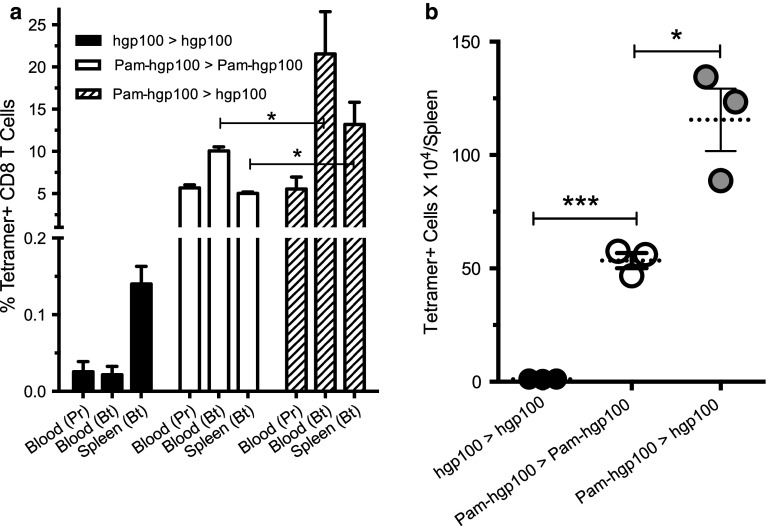
We previously reported that TriVax immunization using the minimal hgp100 peptide epitope (KVPRNDQWL) could activate and induce the large expansion of adoptively transferred TCR transgenic Pmel-1 cells resulting in significant anti-tumor effects. However, the same vaccination strategy was inefficient in producing anti-tumor effects and generating endogenous antigen-specific, CD8 T cell responses [20]. In other studies, we observed that modification of some minimal T cell epitopes to create amphiphilic peptides dramatically increased their immunogenicity [21]. Thus, we first tested whether the amphiphilic peptide Pam-hgp100 would be capable of inducing endogenous CD8 T cell responses using the TriVax immunization strategy (prime–boost, 9 days apart). The results shown in Fig. 1a, b demonstrate that TriVax using the minimal epitope hgp100 failed to produce any substantial antigen-specific (tetramer+) CD8 T cell responses. On the other hand, a TriVax prime–boost protocol using Pam2hgp100 was quite effective in generating a substantial CD8 T cell response. Interestingly, prime vaccination with Pam-hgp100, followed by an hgp100 minimal epitope boost, was even more effective, doubling the response observed using Pam-hgp100 for both the prime and boost. In view of these results, we utilized a Pam2hgp100 prime, hgp100 boost protocol for the remaining experiments.
Fig. 1.
Heterologous Pam-hgp100 prime, hpg100 boost induces potent immune responses to a melanoma CD8 epitope. Mice (three per group) received homologous or heterologous prime > boost TriVax vaccines (9 days apart) with the minimal hgp100 and Pam-hgp100 peptides as indicated. a Percentages of tetramer-positive CD8 T cells were measured in blood 7 days after the prime (Pr) and in blood and spleens 7 days after the boost (Bt). b Total numbers of tetramer-positive CD8 T cells in individual spleens showing averages (dotted lines) and SD (error bars) (* P < 0.05; *** P < 0.005). Similar results obtained in a repeat experiment
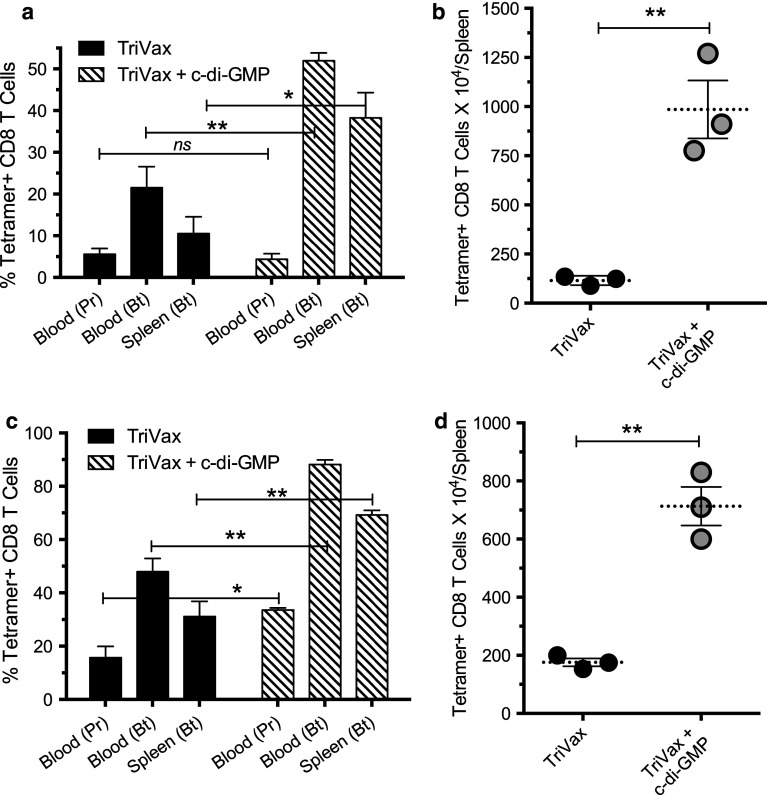
Next, we assessed whether the STING activator c-di-GMP, a potent IFN-I inducer [11], would further enhance the immune response to TriVax. As shown in Fig. 2a, TriVax in combination with c-di-GMP induced significantly higher numbers of antigen-specific CD8 T cells as compared to TriVax w/o c-di-GMP. The differences between TriVax and TriVax plus c-di-GMP were even more apparent when quantifying the total numbers of antigen-specific CD8 T cells in spleen (Fig. 2b). The additive effects of c-di-GMP on the immune responses to TriVax were also observed using the Ova peptide (SIINFEKL) in a protocol where both prime and boost were performed with the minimal epitope (Fig. 2c, d). These results indicate that the administration of c-di-GMP is effective in potentiating the magnitude of the immune responses generated by TriVax.
Fig. 2.
Enhancement of CD8 T cell responses to TriVax by c-di-GMP. Mice (three per group were vaccinated with heterologous Pam-hgp100 > hgp100 prime/boost (a, b) or with homologous Ova minimal epitope (c, d) administered with or w/o c-di-GMP. Vaccinations and immune response determinations were performed as described in Fig. 1 legend after the vaccine prime (Pr) and boost (Bt) (ns not significant, * P < 0.05; ** P < 0.01; *** P < 0.005)
Endogenous CD8 T cells generated by TriVax recognize B16 melanoma cells
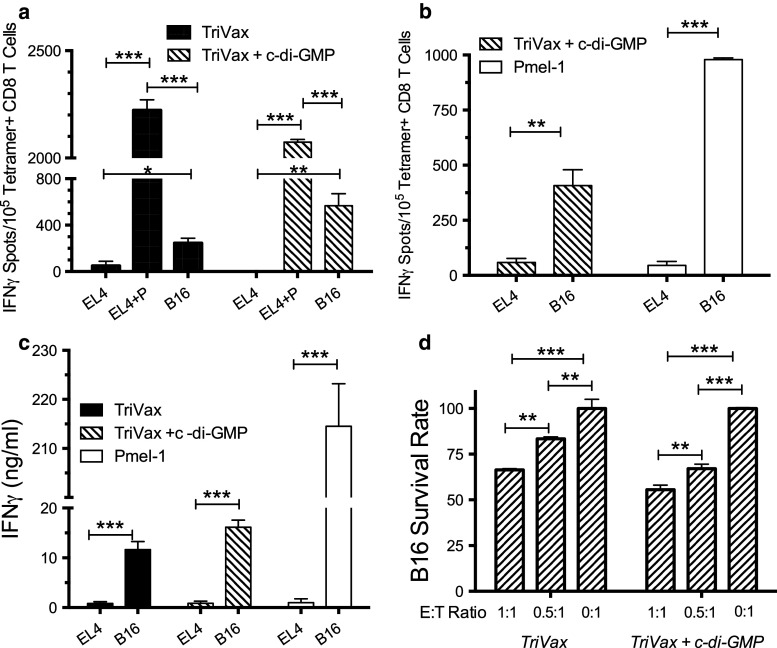
In many instances, especially with peptide-based vaccines, the resulting epitope-specific CD8 T cells are not capable of recognizing tumor cells, which naturally process and express the corresponding epitope. In most cases, these results are due to the generation of low-avidity T cells that require a high density of peptide/MHC complexes on the APC surface. Another possibility when using modified peptides such as the hgp100 epitope (KVPRNDQWL) is that the resulting CD8 T cells may not be able to cross-react with the mouse mgp100 epitope (EGSRNDQWL), which is the one naturally processed and expressed on the B16 melanoma cells. Thus, we performed various immunological assays to assess whether the CD8 T cells generated by TriVax against the hgp100-based vaccine would be capable of recognizing the mgp100 peptide. The EliSpot assay results shown in Fig. 3a indicate that endogenous CD8 T cells generated by TriVax were capable of recognizing mgp100-pulsed target (EL4) cells and more importantly, the B16 melanoma cells. Moreover, the addition of c-di-GMP to TriVax increased the efficiency of the T cells to recognize the B16 tumor cells. However, these results also indicate that a substantially larger number of CD8 T cells recognized the mgp100-pulsed EL4 cells as compared to B16 cells, suggesting that low-avidity T cells are also generated in the vaccinated mice. The side-by-side comparison of endogenous CD8 T cells generated by the combination of TriVax and c-di-GMP with the high-avidity TCR transgenic Pmel-1 T cells shown in Fig. 3b agrees with this assumption. Similar findings were observed in separate experiments, where the T cell responses were measured using IFNγ release ELISA and cytotoxicity assays (Fig. 3c, d).
Fig. 3.
Endogenous hgp100-specific CD8 T cells can recognize B16 melanoma cells in vitro. Mice (three per group) were vaccinated as described in Figs. 1 and 2, and spleen cells were harvested 8 days after the boost. a EIiSpot assay performed (as described in “Materials and methods”). Peptide-pulsed EL4 cells (EL4+P), un-pulsed (EL4), or B16 cells were used as stimulator cells. b EliSpot assay to compare responses of endogenous TriVax + c-di-GMP CD8 T cells with Pmel-1 CD8 T cells against B16 melanoma cells and un-pulsed EL4 cells (negative controls). c IFNγ ELISA comparing responses of endogenous TriVax + c-di-GMP CD8 T cells with Pmel-1 CD8 T cells. d Flowcytometry-based cytotoxicity assay measuring the remaining viable B16 cells after 18-h co-incubation with purified CD8 T cells from vaccinated mice at the indicated effector to target (E:T) ratios. B16 survival rate = (live B16 cells with CD8 T cells/Live B16 cells alone) X 100 (* P < 0.05; ** P < 0.01; *** P < 0.005)
Anti-tumor effects of TriVax and c-di-GMP
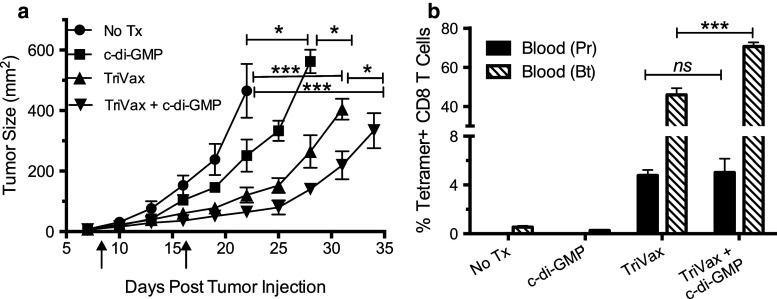
Next, we evaluated the therapeutic effects of TriVax alone or in combination with c-di-GMP against 8-day established subcutaneous B16 melanoma tumors. As shown in Fig. 4a, tumor growth in mice receiving TriVax was significantly lower as compared to the untreated group. Moreover, the addition of c-di-GMP to TriVax further increased the therapeutic effect of TriVax. Interestingly, mice treated with c-di-GMP alone exhibited a small, but significant therapeutic anti-tumor effect, which could be the result of the cytostatic effects of IFN-I. The frequencies of antigen-specific CD8 T cells in blood of these mice correlated with the magnitude of the anti-tumor effects (Fig. 4b). These results indicate that the endogenous CD8 T cells generated by TriVax using the hgp100 epitope are capable of slowing tumor growth, and that co-administration of c-di-GMP enhances this therapeutic effect.
Fig. 4.
c-di-GMP increases the therapeutic anti-tumor effect of TriVax against established B16. B6 mice (five per group) were inoculated subcutaneously on day 0 with 4 × 105 live B16 cells and immunized on days 8 (prime) and 17 (boost) with TriVax, with or without c-di-GMP (vertical arrows). Controls included no treatment” (No Tx) and c-di-GMP-alone-treated groups. a Tumor sizes (areas), measured every 3 days. b Immune responses were measured 7 days after the TriVax prime (Pr) and boost (Bt) in blood samples (ns not significant; * P < 0.05; *** P < 0.005)
Role of IFN-I in the synergistic effects of TriVax and c-di-GMP
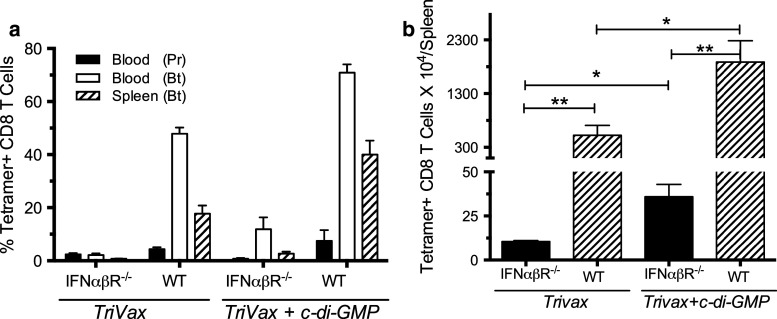
Next, we evaluated the participation of IFN-I in the effects of TriVax and c-di-GMP. Thus, we compared the immune responses generated by TriVax alone and TriVax plus c-di-GMP in type-I IFN receptor-deficient (IFNαβR−/−) mice with those generated in wild-type (WT) mice. As presented in Fig. 5a, immune responses observed in IFNαβR−/− mice to TriVax alone and TriVax plus c-di-GMP were drastically reduced, as compared to those observed in WT mice. Interestingly, the addition of c-di-GMP to TriVax resulted in an approximately 2.5- to 3-fold increase in the total number of antigen-specific T cells obtained in the spleens of both the WT and IFNαβR−/− mice (Fig. 5b).
Fig. 5.
Role of IFN-I in the effects of TriVax and c-di-GMP. IFNαβR−/− mice and B6 WT mice (3 per group) were vaccinated with TriVax on days 0 and 9 with or w/o c-di-GMP. a Immune responses were measured 7 days after prime (Pr) and boost (Bt). b Average numbers of tetramer-positive CD8 T cells in spleens (SD, error bars; * P < 0.05; ** P < 0.01; *** P < 0.005)
C-di-GMP alone cannot function as a potent immune adjuvant
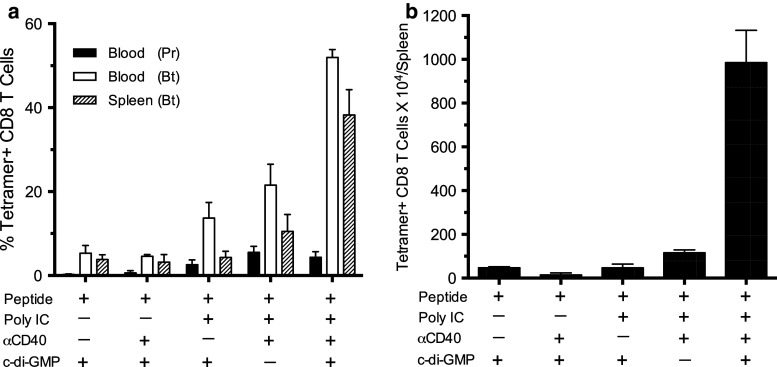
Given the fact that c-di-GMP is an IFN-I stimulator [11, 28], and that poly-IC, which is a component of TriVax, also induces IFN-I production (by stimulating TLR3 and MDA5), we evaluated whether c-di-GMP might function as an adjuvant on its own or in combination with anti-CD40 antibodies in our peptide vaccination approach. The results in Fig. 6 indicate that peptide vaccination using c-di-GMP alone or in combination with anti-CD40 antibodies generated much lower immune responses as compared to those obtained when poly-IC was included in the vaccine formulation. More importantly, the best responses (as measured by the overall expansion of antigen-specific CD8 T cells that were harvested from the spleens of vaccinated mice, Fig. 6b) were obtained when both poly-IC and c-di-GMP were used, demonstrating that these effects are synergistic and not simply additive.
Fig. 6.
c-di-GMP w/o poly-IC does not function as an immune adjuvant. B6 mice received heterologous peptide vaccination in various combinations of poly-IC, anti-CD40 antibody (αCD40), and c-di-GMP as noted. a Immune responses were measured 7 days after prime (Pr) and boost (Bt). b Average numbers of tetramer-positive CD8 T cells in spleens (SD, error bars)
Discussion
The development of effective immunotherapy to treat cancer continues to be a top research priority. Unfortunately, up to now the results obtained with therapeutic vaccines including those using peptides as immunogens are far from optimal and contrast with the amazing effects observed with ACT using T lymphocytes. One of the possible explanations for the contrasting effects observed between vaccines and ACT could be that most vaccines tested in the clinic generate very low numbers of tumor-reactive T cells [3, 5, 29, 30], while huge numbers of adoptively transferred tumor-reactive T cells are infused and persist in lymphodepleted patients treated with ACT [31]. Thus, we strongly believe that the suboptimal clinical outcomes of most vaccines can be attributed to their weak immune responses and hypothesize that by mimicking a viral infection, a subunit, non-infectious vaccine may be able to achieve what ACT does.
Viral infections are notorious for generating huge T cell responses and high levels of IFN-I by the stimulation of the innate immune system via numerous pattern recognition receptors (PRRs). At the same time, it has been reported that IFN-I promotes CD8 T cell expansion and memory development [6, 32]. Over the past few years, our laboratory has developed several vaccination strategies that utilize synthetic peptides that are administered in combination with PRR agonists. In our hands, poly-IC, a PPR agonist that stimulates the production of IFN-I through the activation of endosomal TLR3 and cytoplasmic MDA5, has been shown to function as an extremely potent immune adjuvant with peptide vaccines administered systemically (i.v.) with anti-CD40 antibodies (TriVax) and w/o this costimulatory antibody (BiVax) [18, 20, 21]. It should be noted that in addition to the work presented here, in many of our previous studies in mice [18–21, 24], we have utilized a systemic route (i.v.) of vaccine administration with the purpose of disseminating the antigen and adjuvants throughout the lymphoid organs to recruit and stimulate the majority of naïve T cell precursors. We are aware that when translating this mode of vaccine administration to humans this may pose some safety concerns. Nevertheless, we have observed that intramuscular administration of BiVax is almost as effective as i.v. administration [21].
In the present study, we first compared the immunogenicity of the minimal hgp100 peptide epitope with its amphiphilic peptide variant, Pam-hgp100, after a prime–boost vaccination protocol. The results showed that the minimal hgp100 was not as effective for priming the response, as compared to Pam-hgp100. On the other hand, the minimal hgp100 peptide was more effective for boosting the response as compared to Pam-gp100 (Fig. 1). It has been advocated for more than a decade that vaccines using minimal peptide epitopes are not immunogenic because they generate peptide/MHC-I complexes in non-professional APCs, resulting in immune tolerance and/or T cell anergy [33]. However, most of these studies were done with suboptimal immune adjuvants such as incomplete Freund’s adjuvant. Using TriVax, we have observed that many minimal CD8 T cell peptide epitopes are highly immunogenic at inducing endogenous CD8 T cell responses [18], and as shown here, the minimal Ova peptide was quite successful in generating these responses (Fig. 2c, d). One of the exceptions is hgp100, which is inefficient at priming endogenous immune responses when administered as a minimal epitope TriVax (Fig. 1) but was effective at priming responses when Pmel-1 cells are adoptively transferred into B6 hosts [20]. We have observed that TriVax priming with hgp100 elicits low-avidity endogenous CD8 T cell responses that cannot bind tetramer but respond to peptide stimulation in intracellular cytokine staining assays and recognize peptide-pulsed target cells in EliSpot assays ** (E. Celis, unpublished). We believe that the low-avidity T cells compete and interfere with the expansion of high-avidity T cells, and that when artificially increasing the numbers of high-avidity CD8 T cells by Pmel-1 ACT, these cells are able to expand and overcome the competition of the endogenous low-avidity T cells. Thus, the use of Pam-hgp100 during priming may preferentially stimulate high-avidity CD8 T cells by forcing antigen presentation by professional APCs at relatively low peptide/MHC complex densities that would be ignored by low-avidity T cells.
Using BiVax, we observed that while T cell priming depends heavily on TLR3 stimulation, the dramatic expansion of CD8 T cells occurring after the second, booster immunization relies mostly in MDA5 activation [21]. The ability of poly-IC to stimulate MDA5 indicates that this large, synthetic double-stranded RNA mimic must somehow gains access to the APC’s cytoplasm. The escape of endocytosed nucleic acids into the cytoplasm is probably not an efficient process and may require disruption of endosomal membranes. Thus, we evaluated whether other compounds capable of stimulating IFN-I production could enhance T cell responses to peptide vaccination. Since STING activation using di-cyclic nucleotides results in the production of vast amounts of IFN-I [11, 15], we assessed whether co-administration of c-di-GMP with TriVax would further promote CD8 T cell responses and anti-tumor immunity to melanoma in mice. Indeed, our results show that T cell responses and corresponding anti-tumor effects induced with TriVax using an amphiphilic peptide containing a CD8 T cell epitope for the melanoma antigen gp100 were significantly increased by the addition of c-di-GMP to the vaccination regimen (Figs. 2, 4). The synergistic effect of c-di-GMP with TriVax was more apparent after the booster immunization, indicating that the effect of c-di-GMP was more critical for the expansion of the T cells as compared to their priming. The important role that IFN-I plays in the CD8 T cell responses to TriVax and TriVax + c-di-GMP was demonstrated when these vaccination regimens were less effective in IFNαβR-deficient mice as compared to WT animals (Fig. 5a). However, it was surprising that c-di-GMP was able to increase the overall CD8 T cell response to TriVax even in the IFNαβR-deficient mice (Fig. 5b), suggesting that this compound may have a T cell potentiating activity independent of IFN-I. Thus, the synergistic effects of c-di-GMP are probably not mediated just by an increase in IFN-I mediated by STING activation and probably other participants such as pro-inflammatory cytokines induced by NF-kB activation by STING [16] play a role in enhancing the immune responses to TriVax. Lastly, our results show that the peptide vaccine formulations containing c-di-GMP without poly-IC were ineffective for inducing CD8 T cell responses (Fig. 6). Thus, poly-IC is probably mediating additional critical signals that allow effective T cell priming and boosts in addition to inducing some IFN-I production. Nevertheless, our results clearly demonstrate that concurrent TLR3/MDA5/STING activation using peptide vaccines generates CD8 T cell levels that are rarely observed with non-infectious immunogens. We advocate that effective anti-tumor responses will require the generation of vast numbers of antigen-specific T cells, such as those that we observe using this vaccination strategy.
Acknowledgments
This work was supported by grants from the National Cancer Institute of the National Institutes of Health, R01CA136828 and R01CA157303, and by start-up funds from Georgia Regents University Cancer Center and the Georgia Research Alliance (GRA). We thank Ms. Diane Addis for the valuable technical expertise provided to this project and the GRU Cancer Center Flow Cytometry Core for services provided.
Conflict of interest
Esteban Celis has filed patent applications based on the use of synthetic peptides and poly-IC combinatorial vaccines. The rights of the patent applications have been transferred to the Moffitt Cancer Center (Tampa, FL). Zili Wang declares no conflict of interest.
Abbreviations
- ACT
Adoptive cell therapy
- APC
Antigen-presenting cell
- Bt
Boost
- c-di-GMP
Cyclic diguanylate monophosphate
- DC
Dendritic cell
- ELISA
Enzyme-linked immune assay
- EliSpot
Enzyme-linked immunospot
- IFNαβR
Interferon alpha–beta receptor
- IFN-I
Type-I interferon
- IFNγ
Interferon gamma
- i.v.
Intravenous
- MDA5
Melanoma differentiation-associated protein 5
- Ova
Ovalbumin
- Pam
Palmitic acid
- Poly-IC
Polyinosinic-polycytidylic acid
- Pr
Prime
- PRR
Pattern recognition receptor
- STING
Stimulator of interferon genes
- TLR3
Toll-like receptor 3
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith JW, 2nd, Walker EB, Fox BA, et al. Adjuvant immunization of HLA-A2-positive melanoma patients with a modified gp100 peptide induces peptide-specific CD8+ T-cell responses. J Clin Oncol. 2003;21:1562–1573. doi: 10.1200/JCO.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunological Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 7.Horkheimer I, Quigley M, Zhu J, Huang X, Chao NJ, Yang Y. Induction of type I IFN is required for overcoming tumor-specific T-cell tolerance after stem cell transplantation. Blood. 2009;113:5330–5339. doi: 10.1182/blood-2008-05-155150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuertes MB, Woo SR, Burnett B, Fu YX, Gajewski TF. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 2013;34:67–73. doi: 10.1016/j.it.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmeisser H, Fey SB, Horowitz J, Fischer ER, Balinsky CA, Miyake K, Bekisz J, Snow AL, Zoon KC. Type I interferons induce autophagy in certain human cancer cell lines. Autophagy. 2013;9:683–696. doi: 10.4161/auto.23921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coit DG, Andtbacka R, Anker CJ, et al. Melanoma, version 2.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11:395–407. doi: 10.6004/jnccn.2013.0055. [DOI] [PubMed] [Google Scholar]
- 11.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parvatiyar K, Zhang Z, Teles RM, et al. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol. 2012;13:1155–1161. doi: 10.1038/ni.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karaolis DK, Means TK, Yang D, et al. Bacterial c-di-GMP is an immunostimulatory molecule. J Immunol. 2007;178:2171–2181. doi: 10.4049/jimmunol.178.4.2171. [DOI] [PubMed] [Google Scholar]
- 14.McWhirter SM, Barbalat R, Monroe KM, et al. A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J Exp Med. 2009;206:1899–1911. doi: 10.1084/jem.20082874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shu C, Yi G, Watts T, Kao CC, Li P. Structure of STING bound to cyclic di-GMP reveals the mechanism of cyclic dinucleotide recognition by the immune system. Nat Struct Mol Biol. 2012;19:722–724. doi: 10.1038/nsmb.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abe T, Barber GN. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-kappaB activation through TBK1. J Virol. 2014;88:5328–5341. doi: 10.1128/JVI.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buteau C, Markovic SN, Celis E. Challenges in the development of effective peptide vaccines for cancer. Mayo Clin Proc. 2002;77:339–349. doi: 10.4065/77.4.339. [DOI] [PubMed] [Google Scholar]
- 18.Cho HI, Celis E. Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Cancer Res. 2009;69:9012–9019. doi: 10.1158/0008-5472.CAN-09-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrios K, Celis E. TriVax-HPV: an improved peptide-based therapeutic vaccination strategy against human papillomavirus-induced cancers. Cancer Immunol Immunother. 2012;61:1307–1317. doi: 10.1007/s00262-012-1259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho HI, Reyes-Vargas E, Delgado JC, Celis E. A potent vaccination strategy that circumvents lymphodepletion for effective antitumor adoptive T-cell therapy. Cancer Res. 2012;72:1986–1995. doi: 10.1158/0008-5472.CAN-11-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho HI, Barrios K, Lee YR, Linowski AK, Celis E. BiVax: a peptide/poly-IC subunit vaccine that mimics an acute infection elicits vast and effective anti-tumor CD8 T-cell responses. Cancer Immunol Immunother. 2013;62:787–799. doi: 10.1007/s00262-012-1382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 23.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho HI, Lee YR, Celis E. Interferon gamma limits the effectiveness of melanoma peptide vaccines. Blood. 2011;117:135–144. doi: 10.1182/blood-2010-08-298117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fortier MH, Caron E, Hardy MP, Voisin G, Lemieux S, Perreault C, Thibault P. The MHC class I peptide repertoire is molded by the transcriptome. J Exp Med. 2008;205:595–610. doi: 10.1084/jem.20071985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jedema I, van der Werff NM, Barge RM, Willemze R, Falkenburg JH. New CFSE-based assay to determine susceptibility to lysis by cytotoxic T cells of leukemic precursor cells within a heterogeneous target cell population. Blood. 2004;103:2677–2682. doi: 10.1182/blood-2003-06-2070. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Chahroudi A, Silvestri G, et al. Visualization and quantification of T cell-mediated cytotoxicity using cell-permeable fluorogenic caspase substrates. Nat Med. 2002;8:185–189. doi: 10.1038/nm0202-185. [DOI] [PubMed] [Google Scholar]
- 28.Jin L, Hill KK, Filak H, Mogan J, Knowles H, Zhang B, Perraud AL, Cambier JC, Lenz LL. MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP. J Immunol. 2011;187:2595–2601. doi: 10.4049/jimmunol.1100088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker JC, Andersen MH, Hofmeister-Muller V, et al. Survivin-specific T-cell reactivity correlates with tumor response and patient survival: a phase-II peptide vaccination trial in metastatic melanoma. Cancer Immunol Immunother. 2012;61:2091–2103. doi: 10.1007/s00262-012-1266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dangoor A, Lorigan P, Keilholz U, et al. Clinical and immunological responses in metastatic melanoma patients vaccinated with a high-dose poly-epitope vaccine. Cancer Immunol Immunother. 2010;59:863–873. doi: 10.1007/s00262-009-0811-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunological Rev. 2014;257:56–71. doi: 10.1111/imr.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 33.Zwaveling S, Ferreira Mota SC, Nouta J, Johnson M, Lipford GB, Offringa R, van der Burg SH, Melief CJ. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J Immunol. 2002;169:350–358. doi: 10.4049/jimmunol.169.1.350. [DOI] [PubMed] [Google Scholar]