Abstract
Background
Antiretroviral drugs (ARV), specifically nucleoside analogs, are toxic to mitochondrial oxidative phosphorylation (OXPHOS). Other metabolic pathways, such as fatty acid oxidation, organic acid metabolism and amino acid metabolism, are dependent on normal OXPHOS but remain unexamined as potential points of ARV toxicity.
Methods
We analyzed newborn screening data from New York and compared proportions of abnormal newborn metabolic screens in HIV antibody screen-positive and HIV screen-negative neonates. Subsequently, we compared acylcarnitine levels in ARV-exposed (n=16) and ARV-unexposed (n=14) HIV-exposed infants to assess for dysfunctional fatty and organic acid metabolism.
Results
The rate of abnormal newborn metabolic screens in HIV screen-positive infants was higher than that in the general population (2.2% versus 1.2%, p=0.00025), most of which were for disorders of mitochondria-related metabolism. Abnormal acylcarnitine levels occurred more frequently in ARV-exposed compared to ARV-unexposed infants (43% versus 0%, p=0.02).
Conclusions
A higher proportion of positive metabolic screens in HIV screen-positive neonates suggests that HIV or ARV exposure is associated with dysfunctional intermediary metabolism in newborns. Abnormal acylcarnitine levels were more frequent in ARV-exposed infants suggesting that ARV may perturb normal fatty acid oxidation in some infants. Studies designed to validate and determine the clinical significance of these findings are warranted.
Keywords: HIV/AIDS, mitochondrial toxicity, newborn screening, metabolism
While antiretroviral drugs (ARV) are generally considered safe, they have been associated with mitochondrial toxicity in experimental and clinical studies 1–4 and they have been linked to a wide range of clinical problems in children including lactic acidosis, acute liver injury and myopathy5. The focus of mitochondrial toxicity investigations has been on the effects of nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) effects on oxidative phosphorylation (OXPHOS) through inhibition of the mitochondrial polymerase gamma6. In children, there is evidence that exposure to NRTIs in utero and in the newborn period causes lactic acidosis and abnormal mitochondria number and function7 and that dysfunction may persist after the exposure ends, potentially affecting growth and development8. Despite the fact that many pathways of intermediary metabolism (including fatty acid oxidation, organic acid metabolism and amino acid metabolism) are dependent on normal OXPHOS and dysfunction of these steps can lead to clinical problems such as those seen in ARV-exposed patients 9,10, there is little known in humans about whether these pathways are points of ARV toxicity.
In the last decade, the number of metabolic disorders (or inborn errors of metabolism) included in newborn screening (NBS) panels in the United States has increased dramatically due to the implementation of tandem mass spectrometry (MS/MS) 11. MS/MS is a sensitive and specific method for the quantitation of the substrates and products of intermediary metabolism, including acylcarnitines and amino acids, abnormal levels of which indicate dysfunctional fatty acid oxidation/organic acid metabolism or amino acid metabolism, respectively12. Levels of acylcarnitines and amino acids can also be abnormal in patients with inherited mitochondrial diseases representing secondary dysfunction in metabolic pathways biochemically related to OXPHOS 13.
We hypothesized that HIV/ARV-exposed children would have disruption of metabolic pathways that are normally dependant on functional OXPHOS leading to a greater incidence of positive newborn metabolic screens compared to the general newborn population. We then specifically assessed fatty acid oxidation/organic acid metabolism in a group of HIV/ARV-exposed infants and a group of HIV-exposed/ARV-unexposed infants by measuring acylcarnitine levels in plasma.
METHODS
New York State Newborn Screening
New York State screens all neonates for HIV (by ELISA) and metabolic disorders (by MS/MS). We analyzed New York State newborn screening data to determine the frequencies of positive newborn screens for metabolic disorders in HIV screen-positive and HIV screen-negative infants. In collaboration with NY State Newborn Screening Program, we retrospectively reviewed newborn screening data from 2005–2008, inclusive. We defined an abnormal newborn metabolic screen as any result above the laboratory’s cut-off value that necessitated collection of a repeat sample or prompted referral to a state-designated Specialty Treatment Center. For the period of this study we assumed that virtually all infants exposed to HIV in New York State would have also been exposed to antiretroviral drugs (including peripartum and postnatal AZT) in the perinatal period since NY State mandates first trimester HIV screening in all pregnant women and recommends third trimester screening. Individual exposures will have varied depending on maternal ARV regimen and adherence.
Acylcarnitine profiles in ARV-exposed and ARV-unexposed infants
To explore the relationship between abnormal intermediary metabolism and ARV exposure we compared acylcarnitine levels from a random selection of HIV/ARV-exposed infants to those in HIV-exposed/ARV-unexposed infants. Samples were from New York University’s (NYU) Department of Pediatrics, Division of Immunology and Infectious Diseases and were stored at −80°C since collection. Plasma samples collected at or near birth as well as at or near 2 months of age were obtained from 14 randomly selected HIV-exposed subjects who had no documented exposure to ARV and 16 subjects who were exposed to AZT pre- and postnatally. Blood specimen volume requirements of the parent studies ensured the exclusion of subjects born at gestational age less than 37 weeks. The plasma samples were de-identified and Mayo Medical Laboratories measured acylcarnitine levels using MS/MS as previously described14. The “average of the averages” for each acylcarnitine species were analyzed for direct comparison between groups although an individual acylcarnitine profile was called abnormal only if at least one metabolite was >99th percentile compared to age-matched references (Mayo Medical Laboratories). Institutional Review Board exemption was obtained at participating institutions before initiation of the study.
Newborn screening data and subsequent acylcarnitine profiles in the NYU samples were summarized as proportions and means (± standard deviation [SD]), respectively. Numbers of subjects positive for newborn metabolic screening were compared between HIV screen-positive and HIV screen-negative groups (Table 1) using two-sided Fisher’s exact test. Mean acylcarnitine levels (Figure 1) were compared by ARV exposure status using the t-test. Statistical tests were performed using SAS/STAT software v.9 (SAS Institute, Inc., Cary, NC).
Table 1.
Proportions of positive newborn screens for metabolic disorders in HIV screen-positive and HIV screen-negative neonates in New York State (2005–2008).
| POSITIVE NBS FOR: | HIV NBS + (N=2371) | HIV NBS − (N=1,003,954) | P value |
|---|---|---|---|
| Any metabolic disorder | 53 (2.2%) | 11,949 (1.2%) | 2.5 × 10−5 |
| Mitochondrial metabolic disorder* | 42 (1.8%) | 8118 (0.8%) | 5.0 × 10−6 |
| Urea cycle disorder | 3 (0.1%) | 1310 (0.1%) | 0.99 |
| Organic Acidemia | 14 (0.6%) | 1220 (0.1%) | 2.2 × 10−6 |
| Fatty acid oxidation disorder† | 20 (1.0%) | 4130 (0.4%) | 0.001 |
| MCADD/MADD | 5 (0.2%) | 923 (0.09%) | 0.07 |
| CPT1/2 | 8 (0.3%) | 471 (0.05%) | 2.3 × 10−5 |
| Carnitine deficiency | 9 (0.4%) | 1869 (0.2%) | 0.05 |
| Amino acid disorder | 11 (0.5%) | 3831 (0.4%) | 0.5 |
| PKU | 8 (0.3%) | 1027 (0.1%) | 0.004 |
| MSUD | 1(0.04%) | 916 (0.09%) | 0.65 |
| Tyrosinemia | 1(0.04%) | 497 (0.04%) | 0.76 |
| Homocystinuria | 1(0.04%) | 900(0.03%) | 0.67 |
Includes Urea cycle disorders, Organic acidemias, Fatty acid oxidation disorders.
Two newborns were positive for both MCADD/MADD and CPT1/2. NBS, Newborn screen; MCADD, Medium chain acyl-CoA dehydrogenase deficiency; MADD, Multiple acyl-CoA dehydrogenase deficiency (Glutaric aciduria, type 2); CPT, Carntine palmitoyltransferase; PKU, Phenylketonuria; MSUD, maple syrup urine disease.
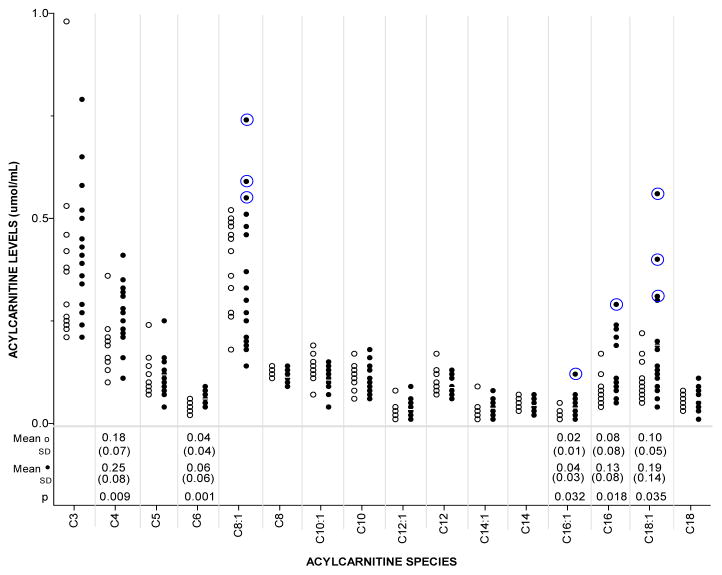
Figure 1.
Acylcarnitine levels in ARV-unexposed (○) and ARV-exposed (●) infants. Each acylcarnitine species (C3 through C18) corresponds to a substrate for a step of either fatty acid oxidation or organic acid catabolism. Group means, standard deviations and p-values for significant differences are listed under corresponding species. Circled measurements represent values of birth sample and/or 2 month sample that were >99th percentile of age-matched references.
RESULTS
New York State Newborn Screening
Of 1,006,325 newborns who underwent mandated screening, 11,949 had at least one abnormal newborn screen for an IEM as measured by MS/MS and 2371 had a positive ELISA for HIV antibody. The average gestational age for the HIV screen-positive population (data collected from 2007–2009) and the general population of newborns was 37 weeks and 39 weeks, respectively. The average birth weight for the HIV screen-positive population and the general population of newborns was 2894 grams and 3311 grams, respectively.
There was a significantly (p=0.000025) higher proportion of HIV screen-positive/ARV-exposed newborns who also screened positive for any inborn error of metabolism (2.2% of 2371) compared to the proportion with such abnormal metabolic screens in the general population of New York State newborns (1.2% of 1,003,953) born between 2005 and 2008(Table 1). Mean carnitine levels were 26.92 +/− 10.71 μmol/L in the HIV screen positive group and 28.56 +/− 10.27 μmol/L in the general newborn population (p<0.0001). Only one of the HIV/IEM screen-positive newborns was confirmed through subsequent testing to meet diagnostic criteria for a metabolic disorder (inherited form of 3-methylcrotonyl carboxylase deficiency).
Acylcarnitine profiles in ARV exposed and ARV-unexposed infants
The mean plasma free carnitine levels in the ARV-exposed infants and in the ARV-unexposed infants were not significantly different [10.99 μmol/mL vs 12.91 μmol/mL p=0.89]. Of the 42 acylcarnitine species that were analyzed as part of an acylcarnitine profile, mean levels of 7 species were significantly different between the groups. At least one acylcarnitine level was abnormal (>99th percentile for age) in 7 of 16 (43%) ARV-exposed infants while no abnormal acylcarnitine level was detected in the 14 ARV-unexposed infants (p=0.02). Figure 1 shows acylcarnitine levels for clinically relevant species and indicates individuals who had at least one acylcarnitine level >99th percentile of age-matched reference values for at least one of their two samples. Mean levels of butyryl/isobutyryl- (C4), hexanoyl- (C6), palmitoleyl- (C16:1), palmitoyl- (C16) and oleoyl- (C18:1) carnitine were significantly higher in the ARV-exposed group. In the ARV-exposed group, there were 10 measurements spread over 7 subjects that were > 99th percentile for age-matched reference values for 5 different acylcarnitine species (octenolycarnitine (C8:1), palmitoleylcarnitine (C16:1), palmitoylcarnitine (C16) oleoylcarnitine (C18:1) and linoleylcarnitine (C18:2)). Of these, abnormal levels of C8:1 (3 subjects) and C18:1 (3 subjects) represented the most consistent abnormalities detected. All acylcarnitine species that were abnormal corresponded to fatty acid oxidation (even-carbon number) and not organic acid metabolism (short-chain, odd-carbon number).
DISCUSSION
Our analysis of newborn screening data reveals a higher proportion of positive metabolic screens in HIV-exposed newborns compared to the HIV-screen negative population. Furthermore, we found that full term, ARV-exposed infants were more likely than ARV-unexposed infants to have fatty acid oxidation dysfunction as measured by acylcarnitne analysis. Together, these results suggest that ARV may negatively affect intermediary energy metabolism, particularly fatty acid oxidation.
Taken as a group, the frequency of positive screens (Table 1) for the disorders that correspond to reactions that take place either completely in the mitochondria (fatty acid oxidation), partially in the mitochondria (urea cycle) or produce substrate for the citric acid cycle (organic acid metabolism), was significantly higher in the HIV screen-positive group. In contrast, the frequency of positive screens for amino acid disorders, the corresponding reactions for which occur in the cytosol, was not significantly higher in the HIV screen-positive group. This apparent dichotomy suggests a mechanism of generalized mitochondrial dysfunction possibly due to NRTI-induced disruption of OXPHOS (see Figure, Supplemental Digital Content 1, http://links.lww.com/INF/B339).
A more detailed comparison reveals that positive NBS for long-chain fatty acid abnormalities (i.e., CPT1/2) and carnitine deficiency predominated in the HIV screen-positive newborn population. Moreover, mean carnitine levels for the HIV screen-positive population were significantly lower than those observed in the general population, a phenomenon previously described15. Mitochondrial fatty acid oxidation, which depends on normal carnitine metabolism, is the primary cellular means of catabolizing free fatty acids for energy and is an important source of cellular fuel during times of metabolic stress, fasting and moderate to vigorous exercise10. In the extreme, inherited disorders of fatty acid oxidation present in childhood during times of catabolic stress and manifest as hypoglycemia, hypoketonemia, acute liver injury and myopathy. There are also less severe variants of fatty acid oxidation disorders that may present similarly in late childhood or adulthood when there is sufficient catabolic stress16. Intact fatty acid oxidation is essential for normal health and development throughout childhood, and there is evidence that abnormal levels of acylcarnitines, particularly the long-chain species, may themselves be toxic.17. The second most common abnormality seen in the HIV screen-positive population were for analytes used to screen for organic acidemias. The majority of the positive screens were for 3-methylcrotonyl carboxylase deficiency, a biochemical finding of unclear clinical significance18. Phenylketonuria (PKU) was the only disorder of amino acid metabolism that was significantly more common in the HIV screen-positive group than the general population. While elevated phenylalanine levels have been observed previously in HIV infected patients19, phenylalanine metabolism is dependent on hepatocyte function and immune system activation, normalizing after effective antiretroviral treatment20.
Besides direct effects on neonatal metabolism, the increased frequency of abnormal metabolic screens in the HIV screen-positive newborn population may be due to the effects of HIV and/or ARV treatment on maternal intermediary metabolism. It is known that some analytes that are detected on newborn screen cross the placenta. Indeed, there are several reports of asymptomatic metabolic disorders being diagnosed in mothers of infants with positive newborn screens but no overt disease21–23. Other maternal factors that may have contributed to our observations include renal function, liver health 24,25 and possibly immunologic/virological status. Because there is little known about the effects of HIV or ARV on adult intermediary metabolism alone or maternal-fetal intermediary metabolism during pregnancy, future studies would ideally include concomitant analysis of biomarkers and clinical data from mother and infant.
Because the average gestational age and birth weight was lower for HIV screen-positive infants it is possible that “metabolic immaturity” may, at least in part, be responsible for an increase in false-positive screens for some metabolic disorders. While it is known that there is an inverse correlation between birth weight and false-positive newborn screens, one study found that this correlation did not hold for C4 and long-chain acylcarnitine species (C14 and C16 were considered in this study)26 and was most pronounced for VLBW infants (<1000 grams). So while there remains the possibility that immature metabolic pathways play a role in an increased false-positive rate for disorders of amino and organic acid metabolism, it may not fully explain the difference in positive screens for fatty acid oxidation disorders.
In order to determine whether the increased frequency of abnormal metabolic screens in HIV screen-positive newborns was associated with HIV or ARV exposure we next compared mean values of acylcarnitine species in ARV-exposed and ARV-unexposed infants who were born at ≥ 37 weeks gestation and who were all HIV-exposed. In this study, we observed higher mean levels of some short- and long-chain acylcarnitine species in the ARV-exposed subjects (Figure 1). Some of the ARV-exposed newborns had acylcarnitine levels outside the normal range and those who did tended to have abnormalities in similar species, including C8:1 and C18:1. While C8:1 is not currently associated with any clinical disorder, C18:1 elevation is used as a marker for carnitine palmitoyltransferase 2 (CPT2) or carnitine-acylcarnitine translocase (CACT) deficiency. This finding agrees with the increased frequency of CPT1/2-positive newborn screens in HIV screen-positive infants described earlier. Moreover, there is experimental evidence of a direct effect of NRTIs on FAO27,28, including a negative affect on CPT2.
It is biochemically plausible that fatty acid oxidation dysfunction is secondary to NRTI-associated OXPHOS dysfunction. Indeed, acylcarnitine profiles are recommended as a screening test for those suspected of inherited OXPHOS disorders29 and can be abnormal in those with established mitochondrial disease13. There is a known biochemical link between fatty acid oxidation and OXPHOS through the former’s production of acetyl-CoA for the Krebs cycle and the donation of reducing equivalents to complexes I and III of the respiratory transport chain. While HIV itself has been shown to adversely affect mitochondria, it is through influence over the mitochondrial apoptotic pathway and not through its effects on OXPHOS30 or related metabolic systems.
Possible explanations for why some of the ARV-exposed infants had acylcarnitine levels above the 99th percentile and others did not include variations in maternal ARV regimen and timing of exposure to ARV. It is also possible that individual genetic variation in one or multiple genes regulating fatty acid oxidation and/or OXPHOS renders some but not all susceptible to dysfunction in these pathways.
The abnormalities of intermediary metabolism described herein, especially those of fatty acid oxidation, suggest that metabolic toxicity in HIV and ARV-exposed children extends beyond mitochondrial DNA abnormalities and OXPHOS dysfunction. Validation of these observations is necessary in other populations and future studies will need to define the clinical ramifications of such biochemical abnormalities, especially in the context of worldwide efforts to scale up access to ARV in pregnant women and 1.49 million pregnancies each year potentially at risk31. It is important that we determine the full scope and depth of ARV effects on energy metabolism and that studies be conducted in children who depend on all aspects of normally functioning metabolism for optimum growth and development.
Supplementary Material
Potential effects of NRTIs on intermediary energy metabolism. NRTIs, nucleoside reverse transcriptase inhibitors; POL-G, polymerase gamma; OXPHOS, oxidative phosphorylation.
Acknowledgments
Funding: NIH T32 Grant, New York State (Empire Clinical Research Investigator Program), Thrasher Research Fund
Footnotes
None of the authors above have any conflicts of interest to declare.
References
- 1.Dagan T, Sable C, Bray J, Gerschenson M. Mitochondrial dysfunction and antiretroviral nucleoside analog toxicities: what is the evidence? Mitochondrion. 2002 May;1(5):397–412. doi: 10.1016/s1567-7249(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 2.Lewis W, Day BJ, Copeland WC. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nat Rev Drug Discov. 2003 Oct;2(10):812–822. doi: 10.1038/nrd1201. [DOI] [PubMed] [Google Scholar]
- 3.Divi RL, Leonard SL, Kuo MM, et al. Transplacentally exposed human and monkey newborn infants show similar evidence of nucleoside reverse transcriptase inhibitor-induced mitochondrial toxicity. Environ Mol Mutagen. 2007 Apr-May;48(3–4):201–209. doi: 10.1002/em.20201. [DOI] [PubMed] [Google Scholar]
- 4.Arnaudo E, Dalakas M, Shanske S, Moraes CT, DiMauro S, Schon EA. Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine-induced myopathy. Lancet. 1991 Mar 2;337(8740):508–510. doi: 10.1016/0140-6736(91)91294-5. [DOI] [PubMed] [Google Scholar]
- 5.Foster C, Lyall H. HIV and mitochondrial toxicity in children. Journal of Antimicrobial Chemotherapy. 2007;61(1):8–12. doi: 10.1093/jac/dkm411. [DOI] [PubMed] [Google Scholar]
- 6.Lewis W, Kohler JJ, Hosseini SH, et al. Antiretroviral nucleosides, deoxynucleotide carrier and mitochondrial DNA: evidence supporting the DNA pol gamma hypothesis. Aids. 2006 Mar 21;20(5):675–684. doi: 10.1097/01.aids.0000216367.23325.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alimenti A, Burdge DR, Ogilvie GS, Money DM, Forbes JC. Lactic acidemia in human immunodeficiency virus-uninfected infants exposed to perinatal antiretroviral therapy. Pediatr Infect Dis J. 2003 Sep;22(9):782–789. doi: 10.1097/01.inf.0000086400.93257.74. [DOI] [PubMed] [Google Scholar]
- 8.Blanche S, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. The Lancet. 1999;354(9184):1084–1089. doi: 10.1016/S0140-6736(99)07219-0. [DOI] [PubMed] [Google Scholar]
- 9.Krivitzky L, Babikian T, Lee HS, Thomas NH, Burk-Paull KL, Batshaw ML. Intellectual, adaptive, and behavioral functioning in children with urea cycle disorders. Pediatr Res. 2009 Jul;66(1):96–101. doi: 10.1203/PDR.0b013e3181a27a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilcken B. Fatty acid oxidation disorders: outcome and long-term prognosis. J Inherit Metab Dis. 2010 Oct;33(5):501–506. doi: 10.1007/s10545-009-9001-1. [DOI] [PubMed] [Google Scholar]
- 11.Therrell BL, Johnson A, Williams D. Status of newborn screening programs in the United States. Pediatrics. 2006 May;117(5 Pt 2):S212–252. doi: 10.1542/peds.2005-2633C. [DOI] [PubMed] [Google Scholar]
- 12.Wilcken B, Wiley V, Hammond J, Carpenter K. Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N Engl J Med. 2003 Jun 5;348(23):2304–2312. doi: 10.1056/NEJMoa025225. [DOI] [PubMed] [Google Scholar]
- 13.Sim K. Acylcarnitine profiles in fibroblasts from patients with respiratory chain defects can resemble those from patients with mitochondrial fatty acid [beta ]-oxidation disorders. Metabolism. 2002;51(3):366–371. doi: 10.1053/meta.2002.30521. [DOI] [PubMed] [Google Scholar]
- 14.Rinaldo P, Cowan TM, Matern D. Acylcarnitine profile analysis. Genet Med. 2008 Feb;10(2):151–156. doi: 10.1097/GIM.0b013e3181614289. [DOI] [PubMed] [Google Scholar]
- 15.Ilias I, Manoli I, Blackman MR, Gold PW, Alesci S. L-Carnitine and acetyl-L-carnitine in the treatment of complications associated with HIV infection and antiretroviral therapy. Mitochondrion. 2004 Jul;4(2–3):163–168. doi: 10.1016/j.mito.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Raymond K, Bale AE, Barnes CA, Rinaldo P. Medium-chain acyl-CoA dehydrogenase deficiency: sudden and unexpected death of a 45 year old woman. Genet Med. 1999 Sep-Oct;1(6):293–294. doi: 10.1097/00125817-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Sewell AC, Bohles HJ. Acylcarnitines in intermediary metabolism. Eur J Pediatr. 1995 Nov;154(11):871–877. doi: 10.1007/BF01957495. [DOI] [PubMed] [Google Scholar]
- 18.Arnold GL, Salazar D, Neidich JA, et al. Outcome of infants diagnosed with 3-methyl-crotonyl-CoA-carboxylase deficiency by newborn screening. Mol Genet Metab. 2012 Apr 20; doi: 10.1016/j.ymgme.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Ollenschlager G, Jansen S, Schindler J, Rasokat H, Schrappe-Bacher M, Roth E. Plasma amino acid pattern of patients with HIV infection. Clin Chem. 1988 Sep;34(9):1787–1789. [PubMed] [Google Scholar]
- 20.Zangerle R, Kurz K, Neurauter G, Kitchen M, Sarcletti M, Fuchs D. Increased blood phenylalanine to tyrosine ratio in HIV-1 infection and correction following effective antiretroviral therapy. Brain Behav Immun. 2010 Mar;24(3):403–408. doi: 10.1016/j.bbi.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Gibson KM, Bennett MJ, Naylor EW, Morton DH. 3-Methylcrotonyl-coenzyme A carboxylase deficiency in Amish/Mennonite adults identified by detection of increased acylcarnitines in blood spots of their children. J Pediatr. 1998 Mar;132(3 Pt 1):519–523. doi: 10.1016/s0022-3476(98)70032-0. [DOI] [PubMed] [Google Scholar]
- 22.McGoey RR, Marble M. Positive newborn screen in a normal infant of a mother with asymptomatic very long-chain Acyl-CoA dehydrogenase deficiency. J Pediatr. 2011 Jun;158(6):1031–1032. doi: 10.1016/j.jpeds.2011.01.063. [DOI] [PubMed] [Google Scholar]
- 23.Vijay S, Patterson A, Olpin S, et al. Carnitine transporter defect: diagnosis in asymptomatic adult women following analysis of acylcarnitines in their newborn infants. J Inherit Metab Dis. 2006 Oct;29(5):627–630. doi: 10.1007/s10545-006-0376-y. [DOI] [PubMed] [Google Scholar]
- 24.Murphy WJ, Steiber A, Connery GC, Carder J, Spry L, Hoppel C. Altered carnitine metabolism in dialysis patients with reduced physical function may be due to dysfunctional fatty acid oxidation. Nephrol Dial Transplant. 2012 Jan;27(1):304–310. doi: 10.1093/ndt/gfr334. [DOI] [PubMed] [Google Scholar]
- 25.Shneider BL, Rinaldo P, Emre S, et al. Abnormal concentrations of esterified carnitine in bile: A feature of pediatric acute liver failure with poor prognosis. Hepatology. 2005;41(4):717–721. doi: 10.1002/hep.20631. [DOI] [PubMed] [Google Scholar]
- 26.Slaughter JL, Meinzen-Derr J, Rose SR, et al. The effects of gestational age and birth weight on false-positive newborn-screening rates. Pediatrics. 2010 Nov;126(5):910–916. doi: 10.1542/peds.2010-0943. [DOI] [PubMed] [Google Scholar]
- 27.Igoudjil A, Abbey-Toby A, Begriche K, et al. High doses of stavudine induce fat wasting and mild liver damage without impairing mitochondrial respiration in mice. Antivir Ther. 2007;12(3):389–400. [PubMed] [Google Scholar]
- 28.Igoudjil A, Massart J, Begriche K, Descatoire V, Robin M, Fromenty B. High concentrations of stavudine impair fatty acid oxidation without depleting mitochondrial DNA in cultured rat hepatocytes. Toxicology in Vitro. 2008;22(4):887–898. doi: 10.1016/j.tiv.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Haas RH, Parikh S, Falk MJ, et al. The in-depth evaluation of suspected mitochondrial disease. Molecular Genetics and Metabolism. 2008;94(1):16–37. doi: 10.1016/j.ymgme.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacotot E, Ravagnan L, Loeffler M, et al. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J Exp Med. 2000 Jan 3;191(1):33–46. doi: 10.1084/jem.191.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO, UNAIDS, UNICEF. [Accessed August 1, 2011];Estimated number of women living with HIV needing and receiving antiretroviral medicine for PMTCT in low- and middle-income countries. 2010 [WHO website]. Available at: http://www.who.int/hiv/data/Table7_5_full.png.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Potential effects of NRTIs on intermediary energy metabolism. NRTIs, nucleoside reverse transcriptase inhibitors; POL-G, polymerase gamma; OXPHOS, oxidative phosphorylation.