Abstract
Vitamin A deficiency leads to increased susceptibility to a spectrum of infectious diseases. The studies presented dissect the intrinsic role of each of the retinoic acid receptor (RAR) isoforms in the clonal expansion, differentiation, and survival of pathogen-specific CD8 T cells in vivo. The data show that RARα is required for the expression of gut-homing receptors on CD8+ T cells and survival of CD8+ T cells in vitro. Furthermore, RARα is essential for survival of CD8+ T cells in vivo following Listeria monocytogenes infection. In contrast, RARβ deletion leads to modest deficiency in Ag-specific CD8+ T cell expansion during infection. The defective survival of RARα-deficient CD8+ T cells leads to a deficiency in control of L. monocytogenes expansion in the spleen. To our knowledge, these are the first comparative studies of the role of RAR isoforms in CD8+ T cell immunity.
Vitamin A deficiency (VAD) undermines the ability of the host to mount protective immune response to a wide spectrum of pathogens. There is increased morbidity and mortality associated with respiratory (1) and gastrointestinal infections (2) among young children with VAD. The underlying causes of this increased susceptibility need to be fully resolved, but the important role of the active metabolite of vitamin VA, retinoic acid (RA), in maintaining the integrity of the mucosal tissues and immune homeostasis is of critical importance.
RA is a powerful immunoregulatory mediator that impacts on leukocyte homing (3, 4) and can increase immune suppression and modulate inflammation (5–7), depending on the immunologic context. RA was shown to induce conversion of naive CD4+ T cells to adaptive regulatory T cells (Tregs) (5–7), as well as induce (8) or inhibit (9) Th17 differentiation, depending on the concentration. Beyond its role in altering the fate of differentiating T cells, RA also was shown to be essential for the development of adaptive immune responses. Using genetically engineered mouse models in which T cell–restricted RA signaling was blocked by RA receptor (RAR)α deletion or conditional overexpression of dominant-negative RARα, studies demonstrated that RA signaling is required for CD4+ T cell proliferation and effector cell generation in both Toxoplasma gondii infection (10) and an allogenic skin transplantation model (11). Furthermore, we (12) showed that T cell–restricted RA signaling is required for Ag-specific effector CD8+ T cell survival in both tumor models and models using neoantigen. Our study also showed that RA signaling is required for short-lived effector CD8+ T cell differentiation but inhibits effector memory CD8+ T cell differentiation in the context of vaccinia virus infection (13).
Although it is clear that RARs play a critical role in controlling adaptive immunity, illuminating the role of each RAR would profoundly advance our ability to strategically target each of these receptors for immune regulation. RA binds to three RARs: RARα, RARβ, and RARγ (14–17). Studies using RAR-specific antagonists/agonists suggested that RARα is the isoform involved in Treg conversion, Th17 inhibition (18), and the regulation of gut homing (19). Although our previous studies revealed the essential role for T cell–intrinsic RA signaling in controlling both CD4+ cell (11) and CD8+ T cell differentiation (12, 13), no study has comprehensively evaluated the intrinsic role of each RAR in T cell immunity. As such, clearly defining the role of RAR isoforms will provide a better understanding of how different RARs may regulate CD8+ T cell immunity in different diseases.
The use of mice in which each RAR can be conditionally deleted in specific cell lineages offers the opportunity to incisively evaluate the roles of RARα, RARβ, and RARγ in T cell immunity. In this study, the functions of each RAR in CD8+ T cell responses to Listeria monocytogenes were assessed. Mice in which each RAR was conditionally deleted from the T cell lineage were produced and evaluated. We show that RARα, but not RARβ or RARγ, regulates RA-induced upregulation of the gut-homing receptor α4β7 and CCR9 on CD8+ T cells in vitro. Furthermore, RARα also controls CD8+ T cell survival upon activation in vitro and in vivo. In contrast, RARβ seems to modestly affect Ag-specific CD8+ T cell accumulation in response to L. monocytogenes infection in vivo. However, the lack of RARγ did not lead to any apparent deficient CD8+ T cell responses under the conditions investigated. To our knowledge, these are the first studies to provide insight into the essential function of each RAR in CD8+ T cell–dependent immunity in infectious diseases.
Materials and Methods
Animals
C57BL/6 mice were purchased from the National Cancer Institute. CD4Cre mice were from The Jackson Laboratory. RARαL2/L2 (20), RARβL2/L2 (21), and RARγL2/L2 (22) mice are as previously described. All animals were maintained in a pathogen-free facility at Geisel School of Medicine at Dartmouth.
mAbs
The following FITC-, PE-, PerCP-, allophycocyanin-Cy7–, or allophycocyanin-conjugated Abs were used: anti-CD8 (53-6.7), anti-CD25 (PC61), anti–TNF-α (MP6-XT22), anti–granzyme B (GB11), anti-CD44 (IM7), anti-MHCII (M5), anti–IFN-γ (XMG1.2), anti-CD69 (H1.2F3), anti-CCR9 (242503), and anti-α4β7 (DATK32). All Abs were purchased from Bio-Legend, with the exception of anti-α4β7 (BD Biosciences). A LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit (Invitrogen) was used to exclude dead cells in FACS analysis.
T cell activation in vitro
CD8+ T cells were purified from RARαfl/fl mice, RARβfl/fl mice, RARγfl/fl mice, and control mice by MicroBeads (Miltenyi Biotec) selection. For α4β7 and CCR9 measurement, CD8+ T cells were activated with plate-bound anti-CD3 (2C11; 10 μg/ml) and anti-CD28 (PV1; 1 μg/ml) for 48 h in RPMI 1640 and then restimulated in fresh RPMI 1640 with 100 U/ml human IL-2 for an additional 48 h. All-trans retinoic acid (ATRA) was supplemented at the indicated concentrations. Cultured cells were harvested for analysis by 96 h in culture. For the cell-viability assay, CD8+ T cells were cultured under the same condition without exogenous ATRA. Cells were harvested at different time points for the analysis of CD25 and CD69 expression. CountBright Absolute Counting Beads (Invitrogen) were used in FACS analysis for assessment of live CD8+ T cells.
Cytokine secretion in vitro
CD8+ T cells were cultured in RPMI 1640 with or without 10 μg/ml anti–CD3 and with or without 1 μg/ml anti-CD28 for 24 h. Brefeldin A was applied during the final 10 h of culture. IL-2 and IFN-γ production by CD8+ T cells was assessed by intracellular staining, according to the protocol described previously (23).
Infection
Mice were infected i.v. with 5 × 104 CFU L. monocytogenes engineered to overexpress OVA (L. monocytogenes–OVA). In all infection experiments, CD4+ T cells were depleted by i.p. injection of 200 μg anti-CD4 (GK1.5; Bio × Cell) on day 0 prior to infection. Bacterial load in spleen was measured as previously described (24). In brief, a single-cell suspension was prepared on day 5 postinfection, incubated with 1% paraformaldehyde for 30 min, and plated (10 μl 1:10- and 1:100-diluted cells) onto BHI media with erythromycin. Colonies were counted 2 d later.
Analysis of MHC class I tetramer, granzyme B, IFN-γ, and TNF-α by flow cytometry
MHC class I tetramer, IFN-γ, and TNF-α staining was performed as previously described (23). Splenocytes were not restimulated ex vivo for granzyme B staining but were restimulated with 10 μg/ml SIINFEKL in the presence of 10 U/ml human IL-2 for 5 h prior to IFN-γ and TNF-α staining. Five-color FACS data were collected on a BD FACSCalibur flow cytometer and analyzed using FlowJo software.
Statistical analysis
Graphs were made using GraphPad Prism software, and data are expressed as mean ± SEM or mean ± SD. Differences for data with one grouping variable were analyzed by the Student t test (two groups).
Results
Phenotypic analysis of T cells in mice with conditional deletion of RARα, RARβ, or RARγ
The expression of RARα, RARβ, and RARγ mRNA in naive and anti-CD3–activated CD8+ T cells was evaluated. As shown in Supplemental Fig. 1A, all three RARs are expressed in naive CD8+ T cells, as well as CD8+ T cells activated for 48 or 72 h. To define the different functions of RARα, RARβ, and RARγ in determining various aspects of CD8+ T cell function, RARαL2/L2-transgenic (20), RARβL2/L2-transgenic (21), and RARγL2/L2-transgenic (22) mice were crossed with CD4Cre mice to generate mice deleted of RARα, RARβ, or RARγ in T cells. These mice are known as RARαfl/fl, RARβfl/fl, and RARγfl/fl, respectively. In all experiments, littermate controls were used bearing alleles of RARαL2/L2, RARβL2/L2, or RARγL2/L2 but not CD4Cre, hereafter referred to as “control.” Specific RAR deletion in CD8+ T cells was confirmed by both the detection of the Cre-dependent null allele in the genome and the absence of corresponding receptor mRNA in RARαfl/fl, RARβfl/fl, and RARγfl/fl mice. In this context, naive CD8+ T cells were purified from spleen of these mice, and PCR was performed with previously published primers to detect the null allele of RARα in RARαfl/fl mice (20), RARβ in RARβfl/fl mice (21), and RARγ in RARγfl/fl mice (22). As shown in Supplemental Fig. 1B, the null allele of RARα was detectable in CD8+ T cells from RARαfl/fl mice but not in their counterparts from RARβfl/fl, RARγfl/fl, or control mice. The same is true for the specific deletion of RARβ and RARγ in CD8+ T cells from RARγfl/fl and RARγfl/fl mice, respectively. Specific deletion by evaluation of the three isotypes expressed in naive CD8+ T cells was quantified by real-time PCR. As shown in Supplemental Fig. 1C, RARα mRNA was undetectable in CD8+ T cells of RARαfl/fl mice, but it was found at comparable levels in CD8+ T cells of RARβfl/fl, RARγfl/fl, and control mice. Similarly, RARβ and RARγ are specifically deleted only in CD8+ T cells of RARβfl/fl and RARγfl/fl mice, respectively. The presented data confirmed the deletion of each specific RAR in each strain and provided the opportunity to define distinct functions for RARα, RARβ, and RARγ in various aspects of CD8+ T cell development and immunity.
Further studies were conducted to determine whether the deletion of specific receptors impairs T cell development under steady-state. No drastic differences among RARαfl/fl, RARβfl/fl, RARγfl/fl, and control mice were observed with regard to the proportion and total number of CD8+ T cells in the thymus, spleen, and peripheral lymph node (Supplemental Fig. 2A–C). However, a difference in the proportion of memory-like CD8+ CD44high T cells was observed across the mutant strains: RARαfl/fl (26.52 ± 2.23%), RARβfl/fl (10.13 ± 2.70%), RARγfl/fl (16.39 ± 1.8%), and control (12.93 ± 1.61%) mice (Supplemental Fig. 2D, 2E). Similarly, no dramatic differences were observed in CD4+ T cells, with the exception of a slightly lower proportion of CD4+ T cells and fewer total Tregs in the peripheral lymph node of RARαfl/fl mice compared with RARβfl/fl, RARγfl/fl, and control mice (Supplemental Fig. 2). Overall, the analysis indicated no overt T cell development deficiency due to the deletion of RARα, RARβ, or RARγ in T cells.
Assessment of RARα, RARβ, and RARγ in T cell activation, proliferation, and survival in vitro
To elucidate the role of each RAR in T cell activation, a series of in vitro studies was executed with each of the conditionally deleted RARs mutants. The use of T cells in which there is conditional deletion only in the T cell compartment avoids any significant issues with isolating T cells from a severely compromised host in which RAR is universally deleted. RARα-knockout mice present with significant morbidities and mortality (25). First, the expression of early activation markers CD25 and CD69 was measured at different time points postactivation. As shown in Fig. 1A, at 18 h postactivation, CD8+ T cells from all RAR-deficient groups showed upregulation of CD25 and CD69 in response to anti-CD3 + anti-CD28 stimulation, which was indistinguishable from controls. We observed no significant differences in mean fluorescence intensity of CD25 and CD69 on CD8+ T cells at 18 h (Fig. 1B) or other time points we measured (data not shown). This indicated that early T cell activation in the absence of RARα, RARβ, or RARγ was intact. Such intact early T cell activation excluded the possibility of any deficiency caused by purification of T cells from different hosts per se. Second, CD8+ T cells from RARαfl/fl, RARβfl/fl, RARγfl/fl, and control mice were activated in vitro with anti-CD3, with or without anti-CD28, and the proliferation was evaluated by CFSE dilution. No differences were observed in the proliferation profile of live CD8+ T cells post–72 h activation in vitro (Fig. 1C, 1D). No difference in CD25 or CD69 upregulation or proliferation profile was observed among CD8+ T cells from RARαfl/fl, RARβfl/fl, RARγfl/fl, and control mice when soluble anti-CD3 was used with CD11c+ dendritic cells as APCs (data not shown). In addition, we observed no deficiency in CD25 and CD69 upregulation or proliferation of CD8+ T cells when exogenous RA (100 nM) was supplemented during activation (data not shown). However, we observed reduced cell survival in the RARαfl/fl group.
FIGURE 1.
Analysis of activation and proliferation of RARαfl/fl, RARβfl/fl, RARγfl/fl, and control CD8+ T cells in vitro. (A) Representative graph of CD25 (upper panels) and CD69 (lower panels) in activated CD8+ T cells at 18 h postactivation. Filled: naive control. Blue line: activated CD8+ T cell. (B) Mean fluorescence intensity of CD25 (left panel) and CD69 (right panel) of CD8+ T cells at 18 h postactivation. Horizontal lines stand for the mean value of mean fluorescence intensity in indicated strain. (C and D) CD8+ T cells were purified from spleen and peripheral lymph node of RARαfl/fl, RARβfl/fl, RARγfl/fl, and control mice and activated with plate-bound anti-CD3, with or without anti-CD28, at the indicated concentrations for 72 h before analysis. (C) Representative CFSE profile of CD8+ T cells 72 h postactivation. Data are gated on live CD8+ T cells. (D) Quantification of the proportion of CD8+ T cells undergoing proliferation in various concentrations of anti-CD3, with or without anti-CD28. Shown is the percentage of CFSElow cells of live CD8+ T cells. Data shown are representative of at least four experiments (one mouse/group in triplicate wells in each experiment) with similar results. Data shown are mean ± SD.
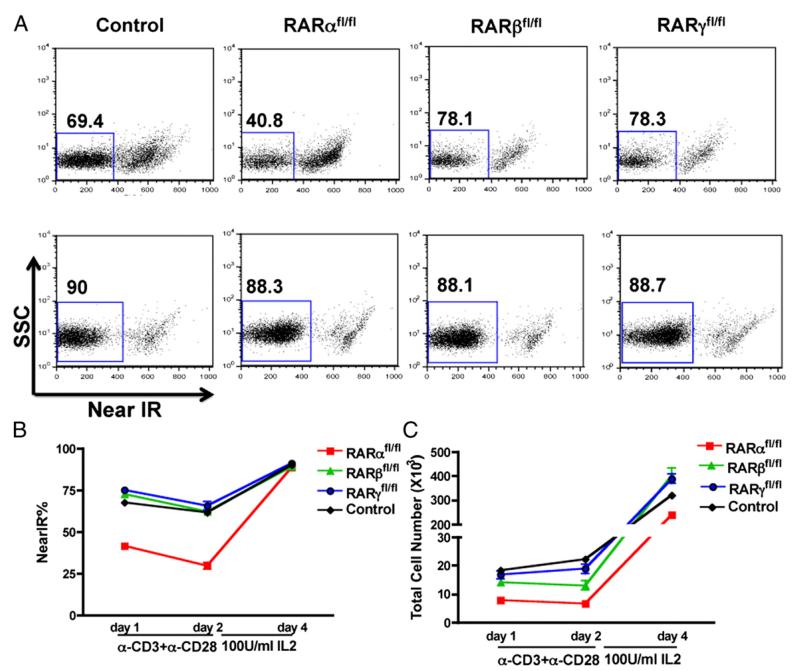
To gain further insight into a possible role for RARα in CD8 survival, viable cell numbers were monitored following anti-CD3 + anti-CD28 stimulation of CD8+ T cells from RARαfl/fl, RARβfl/fl, RARγfl/fl, and control mice in vitro. As shown in Fig. 2A and 2B, after 24 h of stimulation there was a 50% reduction in the percentage of viable CD8+ cells, which decreased even further at 48 h in RARα-deficient CD8+ T cells (Fig. 2B, 2C). To determine whether cytokines could rescue the apparent anti-CD3–associated defect in RARα-deficient T cells, T cells were put into 2-d culture in fresh media with high exogenous IL-2 supplementation (100 U/ml) without anti-CD3 or anti-CD28. IL-2 rescued the viability of the RARα-deficient CD8+ T cells to the same level as their counterparts from other groups, even though the total cell number did not reach the same level (Fig. 2). Our study also showed that 10 U/ml exogenous IL-2 in culture from day 0 can sustain a high viability of RARαfl/fl CD8+ T cells (data not shown).
FIGURE 2.
Impaired survival of RARαfl/fl, but not RARβfl/fl, RARγfl/fl, or control, CD8+ T cells when activated in vitro. CD8+ T cells were purified from spleen and peripheral lymph node of RARαfl/fl, RARβfl/fl, RARγfl/fl, and control mice, activated with plate-bound anti-CD3 (10 μg/ml) + anti-CD28 (1 μg/ ml) for 48 h, washed, resuspended in fresh media with 100 U/ml IL-2 for an additional 48 h, and analyzed at the indicated time points. (A) Representative near-infrared staining of CD8+ T cells on day 1 (upper panels) and day 4 (lower panels) postactivation. (B) Proportion of live CD8+ T cells measured by Live/Dead Near-IR staining. (C) Total live CD8+ T cell number at indicated time points. Data shown are representative of at least five experiments (one mouse/group in triplicate wells in each experiment) with similar results. Data shown are mean ± SD.
Impaired differentiation of RARα- and RARβ-deficient CD8+ T cells in vitro
The prior data clearly indicated that T cell activation was not impaired in any of the RAR mutant T cells by virtue of cell activation markers or proliferation. However, RARα appeared to impact on the survival of T cells in vitro. To determine whether any of the RAR-deficient CD8+ T cells were impaired in their differentiation to effector cells, the production of effector cytokines was measured in vitro. IL-2 and IFN-γ production was measured by intracellular cytokine staining of activated CD8+ T cells from control, RARαfl/fl, RARβfl/fl, and RARγfl/fl mice. There was a significantly lower proportion of IL-2 producers in RARαfl/fl and RARβfl/fl CD8+ T cells than in control and RARγfl/fl CD8+ T cells when activated by anti-CD3 + anti-CD28. The same reduction due to RARα or RARβ deficiency also was observed when activated by anti-CD3 alone, even if not statistically significant (Fig. 3A, 3C). A reduction in IFN-γ production was observed in RARαfl/fl CD8+ T cells, but not in other groups of CD8+ T cells, when activated by anti-CD3 alone (Fig. 3B, 3C). The decrease in IFN-γ production was not observed in the presence of anti-CD28 (Fig. 3B, 3C). We also measured IL-2 and IFN-γ production by CD8+ T cells stimulated with soluble anti-CD3 in the presence of CD11c+ dendritic cells. Under this condition, we also observed the decrease in IL-2 and IFN-γ production by RARα-deficient CD8+ T cells. Furthermore, exogenous RA in culture showed no impact on cytokine production (data not shown). Therefore, RARαfl/fl CD8+ T cells activated in vitro produce fewer effector molecules (IL-2 and IFN-γ). The reduced production of IL-2 likely impacts on the impaired survival of RARα-deficient CD8+ T cells in vitro. This was consistent with the fact that the short-term stimulation-induced cell death in RARα-deficient CD8+ T cells was rescued by high IL-2 (Fig. 2). Further studies are needed to elucidate the mechanism of IL-2 regulation by RARα.
FIGURE 3.
Impaired IL-2 and IFN-γ production by RARαfl/fl CD8+ T cells upon activation. Representative FACS plots of IL-2 (A) and IFN-γ (B) in CD8+ T cells by intracellular staining at 24 h postactivation with no stimulation (left panels), anti-CD3 stimulation (middle panels), or anti-CD3 + anti-CD28 stimulation (right panels). Data shown are gated on live CD8+ T cells. Numbers in boxes indicate percentage of IL2+ (A) or IFNγ+ (B) CD8+ T cells. (C) Quantification of percentage of IL2+ (left panel) or IFNγ+ (right panel) among CD8+ T cells. Data shown are mean ± SEM pooled from two experiments with n = 5–6 mice/group.
RARα is required for RA-induced gut-homing receptor α4β7 and CCR9 expression on activated CD8+ T cells
One important function that has been ascribed to RA is the imprinting of T cells to express the gut-homing receptors α4β7 and CCR9 (3, 26). Previous studies using different receptor-specific agonists/antagonists (3) and transgenic mice (27) showed that RARα is required for RA-induced α4β7 and CCR9 upregulation in CD4+ T cells upon activation. To address whether RARβ or RARγ influences RA-induced gut imprinting of CD8+ T cells, RA-induced α4β7 and CCR9 expression was evaluated in RARαfl/fl, RARβfl/fl, RARγfl/fl, and control CD8+ T cells. To this end, CD8+ T cells from different strains were stimulated in vitro with anti-CD3 + anti-CD28 for 48 h, rested with fresh media containing 100 U/ml IL-2 for another 48 h, and analyzed at 96 h postculture. ATRA was supplemented in the culture at various concentrations. This culture regimen efficiently induced α4β7 and CCR9 expression on activated CD8+ T cells in vitro (28), as well as overcame the lower viability shown in RARαfl/fl CD8+ T cells (Fig. 2). The expression of α4β7 and CCR9 was analyzed at 96 h post-activation. As shown in Fig. 4, although RA induced significant upregulation of α4β7 (Fig. 4A, 4C) and CCR9 (Fig. 4B, 4C) in control, RARβfl/fl, and RARγfl/fl CD8+ T cells, there was very low, if any, expression of α4β7 and CCR9 in RARαfl/fl CD8+ T cells. This is true across a range of RA concentrations (1–100 nM) tested. This observation extended the prior studies showing that neither RARβ nor RARγ contributes to RA-induced imprinting and that only RARα is critical for this induction.
FIGURE 4.
Analysis of RA-induced α4β7 and CCR9 expression in activated CD8+ T cells from RARαfl/fl, RARβfl/fl, RARγfl/fl, and control mice. CD8+ T cells from different strains were activated as in Fig. 2, except that RA was in culture at various concentrations until analysis at 96 h. Representative α4β7 (A) and CCR9 (B) expression on activated CD8+ T cells in the presence of 0 nM (top panels) or 100 nM (bottom panels) RA. Data shown are gated on live CD8+ T cells. Numbers indicate percentage of α4β7+ (A) and CCR9+ (B) CD8+ T cells. (C) Quantification of α4β7 (left panel) and CCR9 (right panel) on CD8+ T cells from different strains at various RA concentrations. Data shown are representative of four experiments (one mouse/group in triplicate wells in each experiment) with similar results. Data shown are mean ± SD.
The essential requirement of RARα, but not RARβ or RARγ, for CD8+ T cell survival and bacterial clearance
To examine whether the impact of RAR deficiency that was manifested in vitro was recapitulated in vivo, CD8+ T cell responses were tracked and analyzed in each mutant RAR mouse strain. To this end, we analyzed the primary CD8+ T cell response to systemic infection with L. monocytogenes–OVA. CD8+ T cell responses in RARαfl/fl, RARβfl/fl, RARγfl/fl, and control mice were monitored by measurement of OVA-MHC I tetramer+ CD8+ T cell expansion and differentiation.
CD4+ T cell help was shown to be required for optimal CD8+ T cell response against L. monocytogenes–OVA infection (29), but our preliminary data showed measurable CD8+ T cell response in CD4+ T cell–depleted hosts at 5 × 104 CFU infection, even if at lower magnitude than CD8+ T cell response elicited in CD4+ T cell–sufficient hosts (data not shown). Because we wanted to unequivocally evaluate the intrinsic requirement for each RAR in the control of CD8+ T cell responses without the potential interference from possibly defective CD4+ T cell help, we depleted CD4+ T cells systemically prior to infection. RARαfl/fl, RARβfl/fl, RARγfl/fl, and control mice were infected with 5 × 104 CFU L. monocytogenes–OVA systemically. Complete systemic CD4+ T cell depletion was confirmed on day 5 postinfection (data not shown). Under this condition, peak accumulation of OVA-specific CD8+ T cell was observed on day 5 postinfection (data not shown). Thus, the following analyses were performed on day 5 postinfection. The Ag-specific CD8+ T cell response and bacterial load were analyzed. The proportion and total CD8+ T cell number in the spleen of infected RARαfl/fl mice was ~2-fold lower compared with other groups of mice, indicating CD8+ T cell death due to infection (Fig. 5A, 5B). The lower CD8+ T cell number in RARαfl/fl hosts is consistent with in vitro results (Fig. 2A, 2B), suggesting an essential role for RARα in control of overall, polyclonal CD8+ T cell survival. The frequency and total number of OVA-specific CD8+ T cells were measured in the spleen by MHC I–OVA–tetramer staining. There was a significantly lower number of total CD44highOVA-tet+ CD8+ T cells, despite the slightly higher frequency of CD44highOVA-tet+ cells among CD8+ T cells in infected RARαfl/fl mice, as a result of massive CD8+ T cell loss in these hosts (Fig. 5A, 5C). There was a significant, but modest, reduction in both the frequency (4.481 ± 0.6829% versus 8.806 ± 0.8455%, p = 0.0008) and total number (25.74 ± 4.276 × 104 versus 46.17 ± 3.185 × 104, p = 0.0008) of CD44highOVA-tet+ CD8+ T cells in infected RARβfl/fl mice compared with control mice, indicating modestly defective Ag-specific CD8+ T cell expansion in response to L. monocytogenes–OVA infection. On the contrary, RARγfl/fl mice did not show any significant differences from the control group with regard to either the frequency or total number of OVA-specific CD8+ T cells in these studies, with the exception of a slight increase in the percentage of CD8+ T cells (Fig. 5A–C). Our measurement of CD8+ T cell response in the liver revealed the same decrease in OVA-specific CD8+ T cells in infected RARαfl/fl mice, suggesting a systemic deficiency in Ag-specific CD8+ T cell accumulation (data not shown).
FIGURE 5.
Defective CD8+ T cell response against L. monocytogenes–OVA infection and bacterial burden control in RARαfl/fl mice. (A) Representative FACS plots of MHC-I OVA-tetramer staining in naive and infected control, RARαfl/fl, RARβfl/fl, and RARγfl/fl mice splenocytes. Representative profile of CD8+MHCII− T cells gated on live cells (upper panels). Representative profile of CD44 and OVA-tet on gated CD8+MHCII− T cells (lower panels). Gating strategy was determined with naive mice as negative control. (B) Quantification of proportion (left panel) and total number (right panel) of CD8+ T cells in spleens of infected mice on day 5 postinfection. (C) Proportion of CD44highOVA-tet+ cells among CD8+ T cells (left panel) and total number of CD44highOVA-tet+ CD8+ T cells (right panel) in the spleen of infected mice on day 5. (D) Total number of CD8+granzyme B+ (upper left panel), CD8+IFNγ+ (upper right panel), and CD8+TNFα+ (lower left panel) T cells in the spleen of infected mice on day 5. (E) Bacterial load in spleen of day-5 infected mice. Data shown in (B), (C), (D), and (E) are pooled from four independent experiments (n ≥ 11 mice/group). Horizontal lines in (B)–(D) stand for mean value in corresponding strain. Dashed lines in (E) indicate lowest number of bacteria that can be detected in our system.
The ability of CD8+ T cells to produce effector molecules was measured by intracellular staining of granzyme B (without ex vivo stimulation), IFN-γ, and TNF-α (post–ex vivo stimulation with SIINFEKL). Given the lower number of polyclonal CD8+ T cells in RARαfl/fl mice, there were fewer total IFN-γ–, granzyme B–, and TNF-α–producing CD8+ T cells in RARαfl/fl mice compared with the control group (Fig. 5D, Supplemental Fig 3). In agreement with previous results, the overall expansion of Ag-specific CD8+ T cells and the accumulation of IFN-γ–producing CD8+ T cells do not always mirror each other during infection (30). A modest decrease in the number of granzyme B–producing, but not IFN-γ– or TNF-α–producing, CD8+ T cells was observed in infected RARβfl/fl mice (Fig. 5D, Supplemental Fig. 3), despite the reduced accumulation of CD44highOVA-tet+CD8+ T cells (Fig. 5C). On the contrary, infected RARγfl/fl mice showed no deficiency in the production of any effector molecule, as evaluated above, suggesting that CD8+ T cell–intrinsic RARγ is dispensable for Ag-specific CD8+ T cell expansion and differentiation in our studies.
Earlier studies (31) showed that CD8+ T cells are required for full clearance of L. monocytogenes–OVA from the infected spleen. Therefore, the bacterial burden in different groups of mice post-infection was measured. Although L. monocytogenes–OVA was completely cleared from the spleen of RARβfl/fl, RARγfl/fl, and control mice on day 5 postinfection, RARαfl/fl mice failed to clear the bacteria completely (Fig. 5E). However, the decrease in only granzyme B production in RARβfl/fl mice kept the host intact in bacterial clearance. Consistent with the dominant role of innate immunity in L. monocytogenes clearance of liver (32), L. monocytogenes–OVA were cleared from the liver in all strains of mice at the same level on day 5 postinfection, despite the slightly dampened CD8+ T cell response in the liver of RARαfl/fl mice (data not shown).
Discussion
To our knowledge, this is the first report to elucidate the distinct functions of each RAR isoform in the control of CD8+ T cell immunity through conditional deletion of specific RAR isoforms in T cells. The key findings are that RA signaling is not required for CD8+ T cell activation and proliferation; RA signaling mediated by RARα, but not RARβ or RARγ, is required for the up-regulated expression of the gut-homing receptors α4β7 and CCR9 on activated CD8+ T cells; RARα is required for CD8+ T cell survival and expression of effector molecules; RARβ may be involved in Ag-specific CD8+ T cell expansion in response to L. monocytogenes infection; and RARαfl/fl mice are incompetent in bacterial burden control.
Previous studies (3, 26) revealed the role of RA in imprinting the gut-homing receptors α4β7 and CCR9 on activated CD8+ T cells. In this study, the role of each RAR isoform in the intrinsic control of α4β7 and CCR9 upregulation was addressed. The exclusive requirement of RARα in the control of gut-homing receptor expression supports the following conclusions. First, the functional distinction between RARs is not determined solely by the expression pattern, as indicated in other studies (reviewed in Ref. 33), because all three RARs are expressed in CD8+ T cells. Second, the exclusive requirement of RARα in control of α4β7 and CCR9 argues against the well-established notion of functional redundancy among RARs. Numerous studies (34–36) suggested redundancy among these receptors because of the need to produce double- or triple-receptor knockout to recapitulate (or add to) VAD phenotype. Our data also corroborated the recent finding that BATF is required for normal α4β7 and CCR9 expression in CD4+ T cells by regulation of RARα binding to the regulatory regions of Itg-α4 and ccr9 genes (37).
Previous studies (12) showed that RA signaling is required for proliferating CD8+ T cell survival at the effector phase in both tumor and anti-CD40/TLR agonist immunization-induced CD8+ T cell responses without any impact on early cell division per se. Using both in vitro and in vivo CD8+ T cell–activation studies, we found that RARα-deficient, but not RARβ– or RARγ-deficient, CD8+ T cells showed survival deficiency upon activation. The significantly lower IL-2 production may partially lead to the defective viability of RARα-deficient CD8+ T cells in vitro. This essential role for RARα-mediated IL-2 production in CD8+ T cell survival also was supported by the fact that the short-term anti-CD3 + anti-CD28 stimulation-induced cell death in RARα-deficient CD8+ T cells was rescued by high IL-2 in the following culture. Further studies are needed to elucidate the molecular mechanism of IL-2 regulation by RARα.
Early non-Ag–specific T cell depletion in lymphoid organs was reported during the acute phase of L. monocytogenes infection (38, 39). Clearly, deletion of RARα, but not RARβ or RARγ, exacerbated the activation-induced cell death seen upon L. monocytogenes infection. The unaltered proportion of Ag-specific CD8+ T cells in these hosts indicated that the reduced number of total CD8+ T cells may be due to deficiency in overall survival instead of Ag-specific expansion during the priming phase. The reduced CD8+ T cell survival following L. monocytogenes infection in RARαfl/fl mice is consistent with in vitro results, confirming the essential role of RARα in control of CD8+ T cell survival. Further studies are needed to elucidate how RARα regulates CD8+ T cell survival during L. monocytogenes infection.
Our data showed a significant, but modest, decrease in Ag-specific CD8+ T cell expansion in the absence of RARβ. No other dramatic biological impact of RARβ deficiency has been noted either in vitro or in vivo. Furthermore, the reduced expansion of L. monocytogenes–specific CD8 T cells had no impact on L. monocytogenes clearance or burden control, despite decreased granzyme B production. Although primary clearance of L. monocytogenes is not solely dependent on Ag-specific CD8+ T cell expansion, clearance in response to bacterial challenge is heavily influenced by Ag-specific CD8+ T cells (40). Whether RARβ deficiency impacts on memory responses to L. monocytogenes is currently being examined. Nonetheless, the data clearly demonstrate the contrasting roles of RARα and RARβ in CD8+ T cell function.
Cytokines produced by polyclonally activated CD8+ T cells facilitate innate immunity–mediated clearance of L. monocytogenes during primary infections (41, 42). The number and frequency of L. monocytogenes-induced effector CD8+ T cells were reduced in RARαfl/fl mice. Thus, the lower number of activated CD8+ T cells and, consequently, fewer effector molecule–producing CD8+ T cells may directly render RARαfl/fl hosts unable to facilitate bacterial clearance during primary infection. Various effector molecules are redundant in bacterial clearance. Previous studies demonstrated that CD8+ T cells lacking either IFN-γ (43) or TNF-α (44) were equally potent in protection against L. monocytogenes as their wild-type counterparts. Therefore, unsurprisingly, a decrease in granzyme B production alone by CD8+ T cells still allowed for intact bacterial clearance in RARβfl/fl hosts.
This study extends our understanding of RA signaling in the control of Ag-specific CD8+ T cell survival during expansion (12) and differentiation in tumor (12) and vaccinia virus infection (13) by showing the exclusive control of polyclonal CD8+ T cell survival by RARα both in vitro and during intracellular pathogen infection. In addition, our study clearly demonstrated the requirement of CD8+ T cell–intrinsic RARα for primary L. monocytogenes infection clearance, as opposed to a previous study (45) illustrating the requirement of RARγ for proinflammatory cytokine production by macrophages and T cells, without any impact on disease control. Together, these studies enable us to better understand how RA and RAR(s) may regulate adaptive immunity directly or indirectly in different disease models.
Our studies focused on the different functions of RARα, RARβ, and RARγ in the control of CD8+ T cell immunity via genetic deletion of each specific isoform. An RA-independent impact of RAR deletion was noted previously in a number of in vivo models, in particular in neutrophil development (46–48), and, furthermore, with other nuclear receptors, such as thyroid receptor (49). A recent study (50) suggested that RAR also may control the CpG methylation status of specific promoter regions in a ligand-independent manner. The above findings suggest that caution is needed because RAR knockout mice may reveal both RA-dependent and -independent function of RARs. Studies are underway to dissect such differences.
In summary, our study revealed for the first time, to our knowledge, the essential requirement of RARα, but not RARβ or RARγ, for CD8+ T cell gut imprinting, survival, and bacterial clearance. Given the increased susceptibility to infectious disease among children with VAD, this is important for specific targeting of each receptor for more efficacious vaccines against infection.
Supplementary Material
Acknowledgments
We thank Dr. Pierre Chambon for providing RARαL2/L2, RARβL2/L2, and RARγL2/L2 mice.
This work was supported by the National Institutes of Health (Grant R01AT005382) and the National Institute for Health Research Biomedical Research Centre (Grant MR/J006742/1) based at Guy’s and St. Thomas’ National Health Service Foundation Trust, Medical Research Council Centre for Transplantation, King’s College London.
Abbreviations used in this article
- ATRA
all-trans retinoic acid
- RA
retinoic acid
- RAR
RA receptor
- Treg
regulatory T cell
- VAD
vitamin A deficiency
Footnotes
Disclosures
The authors have no financial conflicts of interest.
The online version of this article contains supplemental material.
References
- 1.Cameron C, Dallaire F, Vezina C, Muckle G, Bruneau S, Ayotte P, Dewailly E. Neonatal vitamin A deficiency and its impact on acute respiratory infections among preschool Inuit children. Can. J. Public Health. 2008;99:102–106. doi: 10.1007/BF03405454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.el Bushra HE, Ash LR, Coulson AH, Neumann CG. Interrelationship between diarrhea and vitamin A deficiency: is vitamin A deficiency a risk factor for diarrhea? Pediatr. Infect. Dis. J. 1992;11:380–384. doi: 10.1097/00006454-199205000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 5.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 7.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Kang SG, HogenEsch H, Love PE, Kim CH. Retinoic acid determines the precise tissue tropism of inflammatory Th17 cells in the intestine. J. Immunol. 2010;184:5519–5526. doi: 10.4049/jimmunol.0903942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, et al. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34:435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pino-Lagos K, Guo Y, Brown C, Alexander MP, Elgueta R, Bennett KA, De Vries V, Nowak E, Blomhoff R, Sockanathan S, et al. A retinoic acid-dependent checkpoint in the development of CD4+ T cell-mediated immunity. J. Exp. Med. 2011;208:1767–1775. doi: 10.1084/jem.20102358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Y, Pino-Lagos K, Ahonen CA, Bennett KA, Wang J, Napoli JL, Blomhoff R, Sockanathan S, Chandraratna RA, Dmitrovsky E, et al. A retinoic acid–rich tumor microenvironment provides clonal survival cues for tumor-specific CD8(+) T cells. Cancer Res. 2012;72:5230–5239. doi: 10.1158/0008-5472.CAN-12-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allie SR, Zhang W, Tsai CY, Noelle RJ, Usherwood EJ. Critical role for all-trans retinoic acid for optimal effector and effector memory CD8 T cell differentiation. J. Immunol. 2013;190:2178–2187. doi: 10.4049/jimmunol.1201945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zelent A, Krust A, Petkovich M, Kastner P, Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989;339:714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- 15.Taneja R, Roy B, Plassat JL, Zusi CF, Ostrowski J, Reczek PR, Chambon P. Cell-type and promoter-context dependent retinoic acid receptor (RAR) redundancies for RAR beta 2 and Hoxa-1 activation in F9 and P19 cells can be artefactually generated by gene knockouts. Proc. Natl. Acad. Sci. USA. 1996;93:6197–6202. doi: 10.1073/pnas.93.12.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lohnes D, Kastner P, Dierich A, Mark M, LeMeur M, Chambon P. Function of retinoic acid receptor gamma in the mouse. Cell. 1993;73:643–658. doi: 10.1016/0092-8674(93)90246-m. [DOI] [PubMed] [Google Scholar]
- 17.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 18.Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O’Shea JJ. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J. Immunol. 2007;179:3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 20.Chapellier B, Mark M, Garnier JM, LeMeur M, Chambon P, Ghyselinck NB. A conditional floxed (loxP-flanked) allele for the retinoic acid receptor alpha (RARalpha) gene. Genesis. 2002;32:87–90. doi: 10.1002/gene.10071. [DOI] [PubMed] [Google Scholar]
- 21.Chapellier B, Mark M, Bastien J, Dierich A, LeMeur M, Chambon P, Ghyselinck NB. A conditional floxed (loxP-flanked) allele for the retinoic acid receptor beta (RARbeta) gene. Genesis. 2002;32:91–94. doi: 10.1002/gene.10073. [DOI] [PubMed] [Google Scholar]
- 22.Chapellier B, Mark M, Garnier JM, Dierich A, Chambon P, Ghyselinck NB. A conditional floxed (loxP-flanked) allele for the retinoic acid receptor gamma (RARgamma) gene. Genesis. 2002;32:95–98. doi: 10.1002/gene.10072. [DOI] [PubMed] [Google Scholar]
- 23.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J. Exp. Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner MJ, Jellison ER, Lingenheld EG, Puddington L, Lefrançois L. Avidity maturation of memory CD8 T cells is limited by self-antigen expression. J. Exp. Med. 2008;205:1859–1868. doi: 10.1084/jem.20072390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub MP, LeMeur M, Chambon P. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc. Natl. Acad. Sci. USA. 1993;90:7225–7229. doi: 10.1073/pnas.90.15.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svensson M, Johansson-Lindbom B, Zapata F, Jaensson E, Austenaa LM, Blomhoff R, Agace WW. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunol. 2008;1:38–48. doi: 10.1038/mi.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohoka Y, Yokota A, Takeuchi H, Maeda N, Iwata M. Retinoic acid-induced CCR9 expression requires transient TCR stimulation and cooperativity between NFATc2 and the retinoic acid receptor/retinoid X receptor complex. J. Immunol. 2011;186:733–744. doi: 10.4049/jimmunol.1000913. [DOI] [PubMed] [Google Scholar]
- 29.Obar JJ, Molloy MJ, Jellison ER, Stoklasek TA, Zhang W, Usherwood EJ, Lefrançois L. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc. Natl. Acad. Sci. USA. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaiss DM, Sijts AJ, Mosmann TR. Enumeration of cytotoxic CD8 T cells ex vivo during the response to Listeria monocytogenes infection. Infect. Immun. 2008;76:4609–4614. doi: 10.1128/IAI.00563-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 32.Cousens LP, Wing EJ. Innate defenses in the liver during Listeria infection. Immunol. Rev. 2000;174:150–159. doi: 10.1034/j.1600-0528.2002.017407.x. [DOI] [PubMed] [Google Scholar]
- 33.Dollé P. Developmental expression of retinoic acid receptors (RARs) Nucl. Recept. Signal. 2009;7:e006. doi: 10.1621/nrs.07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendelsohn C, Lohnes D, Décimo D, Lufkin T, LeMeur M, Chambon P, Mark M. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- 35.Lohnes D, Mark M, Mendelsohn C, Dollé P, Dierich A, Gorry P, Gansmuller A, Chambon P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- 36.Subbarayan V, Kastner P, Mark M, Dierich A, Gorry P, Chambon P. Limited specificity and large overlap of the functions of the mouse RAR gamma 1 and RAR gamma 2 isoforms. Mech. Dev. 1997;66:131–142. doi: 10.1016/s0925-4773(97)00098-1. [DOI] [PubMed] [Google Scholar]
- 37.Wang C, Thangamani S, Kim M, Gu BH, Lee JH, Taparowsky EJ, Kim CH. BATF is required for normal expression of gut-homing receptors by T helper cells in response to retinoic acid. J. Exp. Med. 2013;210:475–489. doi: 10.1084/jem.20121088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merrick JC, Edelson BT, Bhardwaj V, Swanson PE, Unanue ER. Lymphocyte apoptosis during early phase of Listeria infection in mice. Am. J. Pathol. 1997;151:785–792. [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang J, Lau LL, Shen H. Selective depletion of nonspecific T cells during the early stage of immune responses to infection. J. Immunol. 2003;171:4352–4358. doi: 10.4049/jimmunol.171.8.4352. [DOI] [PubMed] [Google Scholar]
- 40.Lauvau G, Vijh S, Kong P, Horng T, Kerksiek K, Serbina N, Tuma RA, Pamer EG. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science. 2001;294:1735–1739. doi: 10.1126/science.1064571. [DOI] [PubMed] [Google Scholar]
- 41.Sasaki T, Mieno M, Udono H, Yamaguchi K, Usui T, Hara K, Shiku H, Nakayama E. Roles of CD4+ and CD8+ cells, and the effect of administration of recombinant murine interferon gamma in listerial infection. J. Exp. Med. 1990;171:1141–1154. doi: 10.1084/jem.171.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mielke ME, Niedobitek G, Stein H, Hahn H. Acquired resistance to Listeria monocytogenes is mediated by Lyt-2+ T cells independently of the influx of monocytes into granulomatous lesions. J. Exp. Med. 1989;170:589–594. doi: 10.1084/jem.170.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harty JT, Schreiber RD, Bevan MJ. CD8 T cells can protect against an intracellular bacterium in an interferon gamma-independent fashion. Proc. Natl. Acad. Sci. USA. 1992;89:11612–11616. doi: 10.1073/pnas.89.23.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White DW, Badovinac VP, Kollias G, Harty JT. Cutting edge: antilisterial activity of CD8+ T cells derived from TNF-deficient and TNF/perforin double-deficient mice. J. Immunol. 2000;165:5–9. doi: 10.4049/jimmunol.165.1.5. [DOI] [PubMed] [Google Scholar]
- 45.Dzhagalov I, Chambon P, He YW. Regulation of CD8+ T lymphocyte effector function and macrophage inflammatory cytokine production by retinoic acid receptor gamma. J. Immunol. 2007;178:2113–2121. doi: 10.4049/jimmunol.178.4.2113. [DOI] [PubMed] [Google Scholar]
- 46.Tsai S, Bartelmez S, Sitnicka E, Collins S. Lymphohematopoietic progenitors immortalized by a retroviral vector harboring a dominant-negative retinoic acid receptor can recapitulate lymphoid, myeloid, and erythroid development. Genes Dev. 1994;8:2831–2841. doi: 10.1101/gad.8.23.2831. [DOI] [PubMed] [Google Scholar]
- 47.Tsai S, Collins SJ. A dominant negative retinoic acid receptor blocks neutrophil differentiation at the promyelocyte stage. Proc. Natl. Acad. Sci. USA. 1993;90:7153–7157. doi: 10.1073/pnas.90.15.7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kastner P, Lawrence HJ, Waltzinger C, Ghyselinck NB, Chambon P, Chan S. Positive and negative regulation of granulopoiesis by endogenous RARalpha. Blood. 2001;97:1314–1320. doi: 10.1182/blood.v97.5.1314. [DOI] [PubMed] [Google Scholar]
- 49.Hashimoto K, Curty FH, Borges PP, Lee CE, Abel ED, Elmquist JK, Cohen RN, Wondisford FE. An unliganded thyroid hormone receptor causes severe neurological dysfunction. Proc. Natl. Acad. Sci. USA. 2001;98:3998–4003. doi: 10.1073/pnas.051454698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laursen KB, Wong PM, Gudas LJ. Epigenetic regulation by RARα maintains ligand-independent transcriptional activity. Nucleic Acids Res. 2012;40:102–115. doi: 10.1093/nar/gkr637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.