Abstract
The focus of study for nearly two centuries1, fossils of early gnathostomes—or jawed vertebrates—yield key clues about the evolutionary assembly of the bodyplan common to the group, as well the divergence of the two living gnathostome lineages: the cartilaginous and bony fishes2,3. A series of remarkable new palaeontological discoveries4-10, analytical advances and innovative reinterpretations of old fossils11-14 have fundamentally altered a decades-old consensus on the relationships of extinct gnathostomes15,16, delivering a new evolutionary framework3,6,10-14 for exploring major questions which remain unanswered, including the origin of jaws17-19.
Jawed vertebrates (gnathostomes) comprise more than 99% of living vertebrate species, including humans. This diversity is built upon features including jaws, teeth, paired appendages, and specialised embryonic and skeletal tissues (Box 1); centuries of research have attempted to explain their origins17,18,20-24. In particular, jaws and paired appendages have become flagship systems in the study of evolutionary novelty23,24—a key research programme in evolutionary biology25.
Box 1. Crowns, stems and the characters of jawed vertebrates.
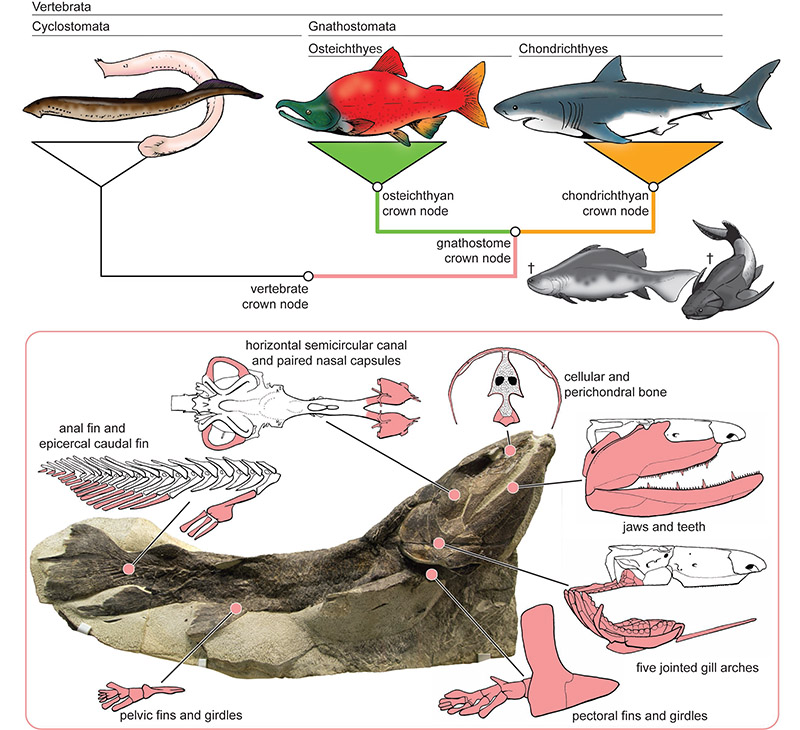
Crown-, total- and stem-group concepts provide a useful framework for navigating evolutionary trees that include fossils. The tree shown here reflects the most basic splits among living vertebrates. Crown groups comprise the last common ancestor of a group of living species plus all of its descendants, both fossil and modern. The gnathostome crown group includes the last common ancestor of osteichthyans (represented by a salmon) and chondrichthyans (represented by a shark) plus all of its descendants, and comprises all the green and orange parts of the tree. Total groups include the crown group of interest plus all extinct forms more closely related to that lineage than any other living species. Here, the gnathostome total group is represented by all coloured parts of the tree. Stem groups are equal to a clade’s total group minus its crown group, shown here by the pink lineage connecting the vertebrate and gnathostome crown nodes. Jawed vertebrates include all of the gnathostome crown, and the upper reaches of the gnathostome stem. The lower part of the gnathostome stem is populated by jawless ostracoderms, which are more closely related to jawed vertebrates than they are to modern jawless fishes. The principal task faced by palaeontologists is to fit fossil groups (like acanthodians and placoderms; ‘†’ indicates they are extinct) within the genealogical framework for modern species. Monophyly of jawed vertebrates is evidenced by a series of shared morphological specializations including, but not limited to, jaws. Key gnathostome features are illustrated here for Eusthenopteron (Cleveland Museum of Natural History CMNH 8158, courtesy of D. Chapman), an osteichthyan and relative of land vertebrates. These traits must have evolved along the gnathostome stem lineage, but without fossils it is impossible to determine either the order in which—or when—they arose.
The deepest split in the modern gnathostome tree is that between the chondrichthyans (sharks, rays, and chimaeras) and the osteichthyans (bony fishes and tetrapods). This divergence occurred in the Palaeozoic Era, at least 423 million years ago8, leaving a vast temporal and evolutionary gulf between modern lineages, with ample time for new innovations to overwrite primitive conditions. These complexities compel researchers to turn to the Palaeozoic fossil record to elucidate the origin of jawed vertebrates. A few well-preserved fossil taxa from a handful of Silurian-Permian sites in Europe and North America1 shaped late 19th and early 20th century hypotheses of gnathostome evolution17,26,27(Fig. 1). Many of these narratives persist to this day, either implicitly or explicitly. However, fossils once hailed as avatars for scenarios of jaw27,28 or fin17,29 origins often turn out to be specialized rather than primitive upon phylogenetic investigation30,31.
Figure 1. Fossils relevant to early jawed vertebrate evolution derive from major fossil sites in North America and Europe, and increasingly China and Australia.
Discs mark palaeogeographic positions of early jawed vertebrate localities characterized by abundant fossils, high fidelity preservation, or both. Palaeogeographic reconstructions by R. Blakey (Colorado Plateau Geosystems, Inc.). Taxonomic breakdown of gnathostome diversity within sites indicated by associated pie charts (size scaled to reported species richness). Localities are: 1, Yulungssu Formation, China; 2, Miakao Formation, China; 3, Kuanti Formation, China; 4, Anderson River, Canada; 5, MOTH, Canada; 6, Belén Formation, Bolivia; 7, Turin Hill/Tillywhandland, UK; 8, Hunsrück Slate; 9, Xitun and Guijiatun formations, China; 10, Pongsongchong and Xujiachong formations, China; 11, Wee Jasper/Burrinjuck, Australia; 12, Orcadian Basin, UK; 13, Wood Bay Group, Spitsbergen; 14, Cleveland Shale, USA; 15, Aztec Siltstone, Antarctica; 16, Mt Howitt, Australia; 17, Gogo, Australia; 18, Wildungen, Germany; 19, Bergisch-Gladbach, Germany; 20, Miguasha, Canada. Vignettes depict scenes based on key fossil sites: Gogo, Australia (left) and Cleveland Shale, USA (right) in the late Middle-Late Devonian; the Xitun Formation, China (left) and Orcadian Basin, UK (right) in the Early-early Middle Devonian; and the Kuanti Formation, China (left and right). Paintings by B. Choo (Flinders University).
Until they are placed in a phylogenetic tree, Palaeozoic fossils are mute on the question of gnathostome origins. In this review, we examine the progress made in the past two decades on the study of early gnathostome interrelationships, focusing on key fossil discoveries that have prompted a renewed intensity of phylogenetic investigation. Although tremendous advances have been made, much work remains before this research can deliver finely atomised transformational hypotheses like those available for mammals32, birds33, and early tetrapods34.
Phylogeny of extant gnathostomes
From the perspective of modern lineages alone, deep vertebrate phylogeny is well resolved and there is little disagreement about the branching patterns surrounding the gnathostome crown node (Box 1). Morphological3 and molecular2 data unambiguously indicate that chondrichthyans and osteichthyans are each monophyletic sister taxa. Together, they form a clade to the exclusion of the cyclostomes: hagfishes and lamprey (Box 1). Molecular evidence strongly supports the monophyly of living agnathans with respect to jawed vertebrates. The long-standing morphological hypothesis indicated monophyly of lampreys and gnathostomes to the exclusion of hagfishes1,35, but re-appraisal of traits in living species36-38 and existing datasets39 have exposed its weaknesses.
These established relationships put study of early gnathostome evolution at an advantage. Modern taxa can be organized into a set of crown groups delimiting three stem branches: the respective branches subtending Osteichthyes and Chondrichthyes, and the branch subtending their last common ancestor (Box 1). The palaeontological problem is reduced to phylogenetic placement of Palaeozoic fossils within this three-branch framework.
Palaeozoic jawed vertebrates and their phylogeny
Diversity of Palaeozoic jawed vertebrates
Putative examples date to the Ordovician40-42, but the first definitive jawed vertebrate remains are of early Silurian age43. Early Devonian (419 Ma) mandibulate gnathostomes were already ecologically diverse44 and, by the close of the Devonian (360 Ma), the first tetrapods and many of their adaptations for terrestriality had emerged34.
Early jawed fishes are divided into four broad categories: ancient representatives of chondrichthyans and osteichthyans, along with two exclusively extinct assemblages: acanthodians and placoderms. The early chondrichthyan record is dominated by isolated denticles (scales), teeth, and spines. The oldest records of scales attributed to chondrichthyans are earliest Silurian in age (ca. 443 Ma)40, such as mongolepids45. Sinacanthids, represented by isolated spines that share histological similarities with chondrichthyans46, are also known from the early Silurian (ca. 438 Ma)43. The oldest universally accepted chondrichthyans are substantially younger, represented by Early Devonian body fossils (ca. 400 Ma; Fig. 2e). Some of these specimens derive from the “Malvinokaffric Realm”, a cold-water Southern Hemisphere palaeobiogeographic province first identified by invertebrate distributions, which yield distinctive jawed vertebrate faunas composed almost exclusively of acanthodians and chondrichthyans47. Articulated chondrichthyans remain rare throughout the Devonian, with most specimens known from the exceptional latest Devonian Cleveland Shale Lagerstätte (Fig. 1).
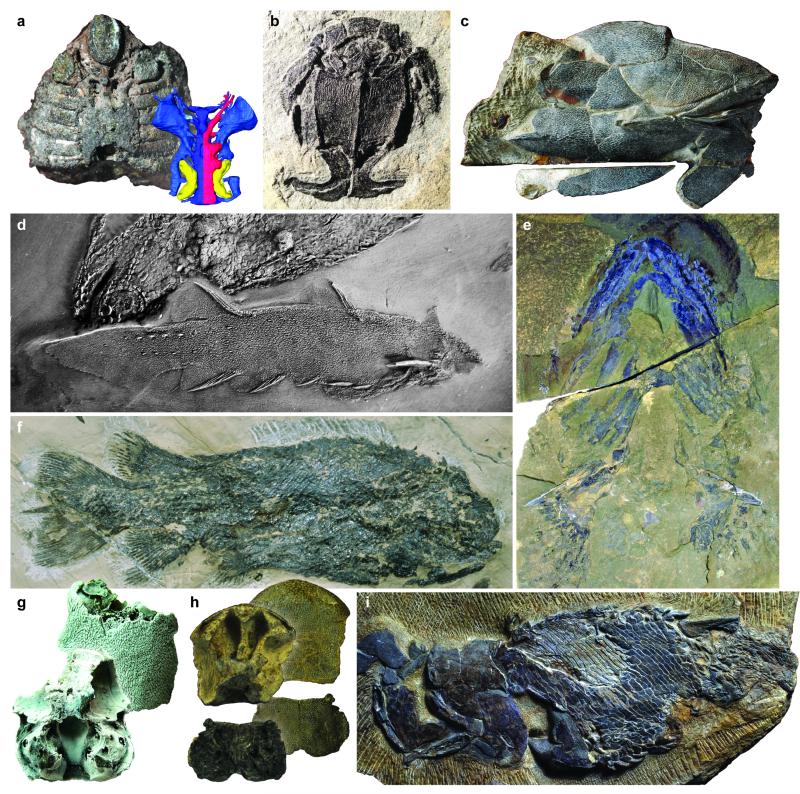
Figure 2. Discoveries over the past two decades provide new clues about the evolution of early jawed vertebrates and their kin.
a, high-fidelity virtual models of the Silurian galeaspid Shuyu reveal cranial architecture in jawless relatives of jawed vertebrates. b, claspers in most placoderm groups, including antiarchs like Microbrachius shown here, raise questions about placoderm relationships and the evolution of vertebrate reproductive strategies. c, osteichthyan-like pattern of bones in the Silurian placoderm Entelognathus suggest that the last common ancestor of all modern jawed vertebrates was clad in a bony-fish-like skeleton. d, stunningly preserved acanthodians from the Early Devonian MOTH locality of Canada challenge their monophyly, suggesting affinities with chondrichthyans. e, pectoral-fin spines and tooth whorls with fused bases in the Early Devonian chondrichthyan Doliodus are features typically associated with acanthodians. f, the Early Devonian osteichthyan Dialipina shows a puzzling combination of traits despite being initially identified from isolated scales as a ray-finned fish. g, Early Devonian braincase attributed to the osteichthyan Ligulalepis. h, braincase of Psarolepis, an Early Devonian lobe-finned osteichthyan from China represented by isolated bones including spines of the kind associated with chondrichthyans, placoderms and acanthodians. i, the surprising reconstruction of Psarolepis was corroborated by the discovery of the more complete and even more ancient Guiyu, from the late Silurian of China.
The late Silurain-Devonian osteichthyan record is considerably better than that of chondrichthyans due to the armour of dermal plates and ossified endoskeleton typical of bony fishes. Consequently, osteichthyans have been intensively studied, with particular emphasis on sarcopterygians (lobe-finned fishes) reflecting their significance in reconstructing early stages of tetrapod evolution34,48,49. Lobe-fins are known from the late Silurian (ca. 423 Ma)8, but the earliest definitive remains of the other division of modern bony fish radiation— actinopterygians—are from the latest Early or earliest Middle Devonian, some 30 million years later50. Some scales and other skeletal detritus of late Silurian-Early Devonian age (ca. 427-400 Ma) are traditionally aligned with actinopterygians51,52. However, many—or perhaps all—of these taxa could represent stem osteichthyans53,54 or even stem gnathostomes14(Fig. 3). As with chondrichthyans, early osteichthyans show some striking distributional patterns, including the conspicuous concentration of early members of major lobe-fin lineages in the latest Silurian and earliest Devonian of the South China Block43(Fig. 1). Outside of this restricted area, coeval bony fishes are limited to a handful of mostly fragmentary examples.
Figure 3. Time-calibrated phylogeny of early jawed vertebrates and their immediate jawless relatives, showing minimum times of divergence based on fossil evidence.
Topology based on Giles et al.14, with some taxa pruned for clarity and modifications showing presumed phylogenetic positions of key extant lineages. Also shown are key early jawed vertebrates or putative jawed vertebrates with uncertain affinities to the crown group. The minimum age of the gnathostome crown could be profoundly recalibrated if Skiichthys41 is confirmed as a crown-group gnathostome. Ages shown across the top are in hundreds of millions of years.
Several extinct groups join the familiar modern jawed vertebrate lineages. Armoured jawless fishes (ostracoderms) that are most often implicated as a jawed vertebrate sister group include: thelodonts, Middle Ordovician-Late Devonian (467-370 Ma), encompassing dorsoventrally flattened to cigar-shaped to deep-bodied forms55 and bearing a shark-like shagreen of tiny scales; galeaspids, bottom-dwelling early Silurian-Late Devonian (439-370 Ma) fishes with flattened headshields that assume a bewildering variety of shapes, found only in Chinese and Vietnamese deposits56,57; and osteostracans, another benthic group with spade-shaped headshields, restricted to the middle Silurian-Late Devonian (433-372 Ma) of today’s northern landmasses57,58. Two extinct jawed groups join this ostracoderm parade: placoderms, a species-rich and anatomically heterogeneous early Silurian-Late Devonian (435-360 Ma) assemblage, characterized by heavy head and trunk armour and bony jaw plates59; and acanthodians, covered in tiny scales and bearing well-developed spines along the leading edges of nearly all of their fins1 that together inspire the moniker ‘spiny sharks’. The earliest fossils interpreted as acanthodians are isolated scales from the latest Ordovician (ca. 444 Ma)40, but their record extends to early Permian deposits (ca. 295 Ma) that yield the best-known and last-surviving genus: Acanthodes11,15.
The evolution of gnathostome phylogeny
The current picture of Palaeozoic gnathostome relationships is the product of three phases of study. Throughout, researchers have benefitted from high-quality data, thanks to the early application of physical tomography by Erik Stensiö and the ‘Stockholm School’60-62, followed by the maturation of acid-preparation techniques in the middle of the 20th century9,63-65 and the non-destructive computed tomography of the past decade and a half13,14,66-68.
The modern phase of research into gnathostome relationships began with the introduction of phylogenetic systematics to vertebrate palaeontology, which had previously focused on linking species from successive geological strata as an approximate ancestor-descendant chain. Monophyly of the major taxonomic divisions of early gnathostomes was assumed, and their relative relationships were largely inferred using evidence from European and North American fossils. Within a decade of the initial application of cladistics to early vertebrates, an imperfect consensus emerged that acanthodians were a clade of stem osteichthyans15 and that placoderms were the immediate sister group of crown gnathostomes69. This framework would persist for more than 30 years1, despite the intervening discovery and detailed description of fossils from Australia63,65,70, China43,71 and northern Canada72 that provided fresh morphological information beyond the stagnating stable of classic Euramerican taxa (see below).
The second phase began in the 1980s with a cladistic reinterpretation of the ostracoderms. Detailed anatomical reinvestigations of ostracoderm sub-lineages and numerical phylogenetic analysis resulted in the recognition of this assemblage as a paraphyletic gnathostome stem group73-77. Reconfiguration of the agnathan menagerie permitted reconstructions of evolutionary patterns in fin morphology and skeletal hard tissues, and identified the extinct jawless sister group of jawed vertebrates. Although many ostracoderm lineages have been considered contenders, anatomical evidence overwhelmingly supports osteostracans. Like jawed vertebrates, but unlike other agnathans, osteostracans bear developed pectoral fins with associated girdles, a hypercercal tail, and perichondral and cellular bone (Box 1).
The third and ongoing phase is the detailed scrutiny of the pioneering cladistic framework relating acanthodians and placoderms to modern jawed vertebrate lineages. Traction on this problem arose indirectly, beginning around the turn of the century with the development of expanded numerical phylogenetic analyses targeting relationships within osteichthyans7,78-80 and chondrichthyans81-83, but employing acanthodian and placoderm ougroups. These studies introduced the use of increasingly large datasets, and provided the character information that would seed analyses targeting not individual lineages, but early jawed vertebrates as a whole. At the same time, a series of new fossil discoveries (outlined below) revealed unexpected anatomical combinations that raised serious questions about the coherence of acanthodians and placoderms. This set in motion a series of refined analyses of early jawed vertebrates bent on testing the supposed monophyly of these groups6,11-14. This final phase is a live debate and the setting for the following discussion.
New fossil discoveries and their significance
Here we highlight key finds since the 1980s that have challenged embedded perceptions and explain their significance in light of what is or was known about early jawed vertebrate evolution. Presented in approximate phylogenetic order, ascending from jawless members of the stem lineage, to placoderms, to members of the gnathostome crown, these discoveries provide a broad summary of the emerging picture of major evolutionary patterns in early gnathostomes. Detailed accounts of character transformation are provided elsewhere3.
Shuyu and Romundina and their noses for success
The neurocranium or braincase is a primitively cartilaginous structure that houses the brain and paired sensory organs in vertebrates. When coated with a mineralized rind, structurally complex braincases can be preserved as fossils and are a key source of phylogenetic information. Discriminating between specialized and primitive features in jawed vertebrates demands comparison with jawless fishes, but knowledge of internal anatomy in ostracoderm lineages lacking endoskeletal mineralization is rudimentary1,73. By contrast, a thin coat of bone surrounds the cartilage forming the consolidated braincase and supports for the gills and pectoral fins of osteostracans. This permitted the first detailed reconstructions of osteostracan brains, cranial vessels and nerves nearly a century ago60,61. Galeaspids too bear a mineralized endoskeleton, but interpretations of their neurocranial structure have long been sketchy. High resolution synchrotron scanning of the early galeaspid Shuyu66 reinforced past identifications of widely separated, anterolaterally placed nasal capsules76,84 that open medially into a central, dorsally directed duct that is also joined by the hypophysis (Fig. 2a). Thus, galeaspids show a tantalizing mosaic of cyclostome-like (nasal capsules located well behind the front of the head and opening into a common nasohypophysial duct) and crown gnathostome-like (broad separation of nasal capsules) traits in the anterior region of the skull, and suggest that the cyclostome-like geometry of the better known osteostracans might be secondary. These features are more than just anatomical arcana: broad separation of nasal capsules is interpreted as a developmental necessity for the origin of jaws, as the median nasohypophyseal placode of cyclostomes obstructs anterior growth of neural crest cells that contribute substantially to mandibles19,37,66. It appears that restructuring of the anterior portion of the head continued after the origin of jaws. Posteriorly placed, separate nasal capsules resembling those of galeaspids characterize many early diverging placoderms like antiarchs, Brindabellaspis, and Romundina, but these share with other jawed vertebrates a hypophysis that opens into the mouth, rather than a common nasohypophysial duct as in agnathans13. In contrast, placoderms like arthrodires, with their anteriorly placed nasal capsules, broadly resemble crown gnathostomes. These major architectural changes reflect a key piece of evidence for placoderm paraphyly6,11-13,80, but ambiguities in the relationships among placoderms do not provide a consistent picture for the evolution of skull geometry in this crownward segment of the gnathostome stem.
Claspers and their evolutionary implications
The ptyctodontid placoderms have long been known to possess claspers85, intromittent organs associated with the pelvic fins and evidence of internal fertilization. This trait factored in early cladistic investigations of placoderm intra- and interrelationships, tying placoderms to chondrichthyans62 and fuelling arguments that ptyctodonts are the sister group of all other placoderms1. The discovery of arthrodire embryos within adult specimens prompted renewed investigation of this group where long-overlooked evidence of claspers was finally discovered4,86,87, followed by the realization that antiarchs too possessed these structures10(Fig. 2b). The palaeobiological and reproductive significance of claspers has been well considered10,86, but their full phylogenetic significance is unresolved. Current phylogenetic consensus does not regard placoderm and chondrichthyan claspers to be homologous3, but the homology of claspers within placoderms seems likely. Placoderm paraphyly demands the loss of internal fertilization before the origin of crown gnathostomes, signalling an unprecedented shift in reproductive biology within vertebrates10. Thus, we face two problematic alternatives: either internal fertilization was lost in a crownward segment of the gnathostome stem, defying observational data on the reproductive biology of living vertebrates10, or placoderms with claspers form a clade, contradicting apparent signal of other traits13.
Entelognathus reframes ancestral conditions
Perceived ‘primitiveness’ of chondrichthyan anatomy entrenched in many general introductions to vertebrate biology has deep pre-Darwinian roots. Faced only with living species, this view seems reasonable enough: with their shagreen of tiny scales and cartilaginous internal skeletons, chondrichthyans seem tailor-made morphological intermediates between the naked hagfishes and lampreys on one hand and the internally and externally bony osteichthyans on the other. The fossil record subverts this tidy picture by showing that both large dermal plates and a bony internal skeleton are innovations that arose long before the divergence of osteichthyans and chondrichthyans35,74,75,77,88. However, the condition of the skeleton in the last common ancestor of jawed vertebrates has remained controversial thanks to two mutually reinforcing phenomena: reluctance to make explicit comparisons between the bony plates of osteichthyans and placoderms, and repeated interpretations of at least some acanthodians as early osteichthyan relatives11,12,15,54,80. Together these factors paint a picture of an ancestral crown gnathostome covered in a ‘micromeric’ outer skeleton of tiny scales, with a ‘macromeric’ skeleton composed of large plates re-appearing in the osteichthyan lineage. This view was turned on its head by the discovery of the late Silurian Entelognathus in China6 (ca. 423 Ma; Fig. 2c). Although Entelognathus broadly resembles a standard-issue placoderm, its cheek and upper and lower jaws are covered with bones that match the pattern seen in osteichthyans, rather than other placoderms. This remarkable correspondence suggests evolutionary continuity between the large dermal plates of placoderms and those of bony fishes6,13,14.
MOTH brings acanthodians into the light
The Man on the Hill (MOTH) locality in the Northwest Territories of Canada is an Early Devonian (ca. 419 Ma) Konservat Lagerstätte yielding articulated early vertebrates. Originally discovered in the 1970s72, new collections and advances in chemical preparation have since revealed exquisitely preserved fossils (Fig. 2d). Jawed vertebrates from MOTH are mostly acanthodians (Fig. 1), providing important anatomical detail on this enigmatic assemblage. Previously, the record of complete acanthodian fossils was mostly restricted to crudely prepared specimens from low-diversity, fluvial-lacustrine Early Devonian deposits of the UK27. By contrast, acid-prepared acanthodians from the species-rich marine MOTH locality reveal crisp anatomical details. In particular, a host of these species have umbellate and denticle-like scales like those found in chondrichthyans89-92. Perhaps more importantly, the MOTH fauna include examples of acanthodian-like fishes covered in scales with growth patterns and structure previously known only from isolated fragments but traditionally assigned to chondrichthyans91. This simultaneously suggests a position for acanthodians in the jawed vertebrate tree whilst undermining confidence that they comprise a natural group.
The inside story on acanthodian morphology
Several early placoderms, osteichthyans and chondrichthyans yield detailed braincases1,62,93, but acanthodian examples are rare. Subject to many re-interpretations over the past 100 years11,15,27, the neurocranium of the Permian Acanthodes is central to debates on the evolutionary affinities of acanthodians. Various authors have been impressed by what they perceived as either particularly osteichthyan-12,15,54 or chondrichthyan-like11,62 features of Acanthodes, triggering contrasting views on the placement of acanthodians as a whole. The Early Devonian (ca. 419 Ma) Ptomacanthus also preserves a braincase, although detail is obscure to the degree that this structure was initially ignored. Re-examination of Ptomacanthus revealed a neurocranium with a gross architecture more similar to that of placoderms or chondrichthyans than Acanthodes and osteichthyans, providing key evidence in the first explicit argument for acanthodian paraphyly12.
A sneak peek at early shark anatomy
With a sparse early record, interpretation of primitive chondrichthyan conditions drew heavily on body fossils from the latest Devonian26 and even younger braincases93, all of which are likely highly specialized. This all changed with two stunning finds in the early 2000s. First was the discovery of more complete neurocrania of Pucapampella from the Early Devonian of Bolivia83 and a similar South African form94. Previously named on the basis of an isolated neurocranial base, Pucapampella bears a chondrichthyan-specific hard tissue (prismatic calcified cartilage) in combination with a ventral fissure: a persistent division between two embryonic braincase components. Absent in ostracoderms, placoderms and other chondrichthyans, but present in Acanthodes and bony fishes, the ventral fissure was long considered key evidence for a close relationship between acanthodians and osteichthyans15. Pucapampella suggests this trait is a general feature of crown-group jawed vertebrates. Subsequent discoveries provided additional anatomical details for Pucapampella, revealing peculiar teeth and jaws to accompany its unanticipated neurocranial architecture47. Hot on the heels of Pucapampella came the discovery of the oldest articulated chondrichthyan. Doliodus, from the Early Devonian of New Brunswick5, was long known for more than a century by isolated teeth assigned to acanthodians. Recovery of an articulated head and forequarters revealed the signature chondrichthyan trait of prismatic calcified cartilage occurring in a fish with stubby spines along the leading edges of its pectoral fins (Fig. 2e), casting further doubt on acanthodian monophyly. Subsequent analysis of the braincase67 and dentition68,95 of Doliodus revealed primitive character states, such as fused tooth bases, not widely seen in crown chondrichthyans and certainly absent in modern sharks and rays, but common to acanthodians and early osteichthyans.
Rosetta stones for fragmentary bony fish remains
Fossil bony fishes have conventionally been deposited in one of the two living divisions: actinopterygians or sarcopterygians. This leaves the osteichthyan stem bereft of fossils that document the origin of this enormously successful clade. A series of isolated scales of late Silurian-Early Devonian age were loosely tethered to actinopterygians as their representatives51,52, but the discovery of more complete material attributed to Dialipina96(Fig. 2f) and Ligulalepis9,64(Fig. 2g) raised questions about their actinopterygian affinities, and the significance of scale-based characters used to identify ray-finned fishes54,80. The braincase aligned with the scale-taxon Ligulalepis shows evidence of an eyestalk9,64, a cartilaginous plinth that supports the eye in chondrichthyans and placoderms but absent in modern osteichthyans. This might suggest ‘Ligulalepis’ is a stem osteichthyan, but reports of eyestalks in early sarcopterygians79 argue for parallel loss in the two bony fish divisions. Complete specimens of Dialipina are even more puzzling, marrying a tail geometry found only in lobe-finned fishes with a cheek comprising tiny bones that bear no clear resemblance to the large plates of other osteichthyans or even Entelognathus. ‘Ligulalepis’ and Dialipina vacillate between Actinopterygii and the osteichthyan stem in many analyses6,14, and solid placements are likely to be elusive until these taxa are more completely documented.
Psarolepis and Guiyu encapsulate the revolution
Perhaps more than any other discovery, Psarolepis represents the principal instigator of the current revolution in early jawed vertebrate systematics. Recovered from late Silurian and very Early Devonian rocks of China, it is one of the earliest bony fishes (Fig. 1). First identified as a stem lungfish on the basis of jaw and braincase material97, subsequent investigation of Psarolepis and the discovery of isolated cheek and shoulder bones highlighted more interesting affinities7. Psarolepis exhibits two hallmarks of the lobe-finned fishes: a braincase divided into front and hind units by an articulating joint and a pore-canal complex in its dermal bones (Fig. 2h). However, the cleaver-shaped cheek and maxilla (upper external jaw bone) bears an uncanny resemblance to early ray-finned fishes, suggestive of a shared primitive condition for bony fishes. More surprisingly, Psarolepis bristled with spines: the shoulder girdle bears a pronounced spine over the fin articulation area, reminiscent of acanthodians and some placoderms, while the dorsal fins were preceded by spines like those of chondrichthyans and acanthodians. Psarolepis is most reasonably interpreted as a stem-group sarcopterygian8,79,80, and thus an early example of the bony fish lineage that would give rise to tetrapods. However, it is held in this position by such a small number of traits, and retains so many plesiomorphies, that some analyses have recovered it as a stem-group osteichthyan7,78. This shook confidence in the seemingly stable, decades old sets of attributes characterizing major early vertebrate groups1. However, the disarticulated nature of these fossils raised the troubling possibility that the combination of characters in Psarolepis was chimeric; parts of different species misattributed to a single one. This concern was rejected, albeit indirectly, by the discovery of Guiyu8 (Fig. 2j). Broadly similar to Psarolepis but from even older Silurian rocks in China (ca. 423 Ma), Guiyu provides exceptional corroboration that traits like a jointed braincase occurred in the same animal as pectoral- and dorsal-fin spines, and delivers further surprises including the presence of placoderm-like external pelvic girdles98. Interpreted as an early sarcopterygian, Guiyu also shows that the last common ancestor of all modern osteichthyans arose no later than the Silurian, before the Devonian ‘Age of Fishes’.
The re-shaping of early jawed vertebrate phylogeny
This panoply of new taxa and unexpected character distributions fuelled doubts about the status of classic early jawed vertebrate catagories5,99, but early studies did not match these queries with cladistic tests. In the past five years, the field has witnessed a spate of numerical analyses giving rise to rapidly shifting perspectives on phylogenetic relationships8,10-14. However, some stable patterns are apparent and key areas of ongoing debate are now coming into focus.
The monophyly of fossil osteichthyans and chondrichthyans is universally supported. Placoderms are repeatedly recovered as stem-group gnathostomes and acanthodians are generally agreed to be members of the gnathostome crown, with some noteworthy exceptions11. Major differences with previous hypotheses stem from important shifts in approach, such as abandoning prior assumptions of placoderm and acanthodian monophyly. In all cases to date, the monophyly of placoderms has been rejected and, in all but one 13, acanthodian monophyly has also been rejected.
In the earliest iterations, acanthodians were inferred to be massively paraphyletic, with some members associated with chondrichthyan, osteichthyan, and gnathostome stem branches11,13. This configuration helped explain the odd conjunctions of osteichthyan, chondrichthyan and more primitive characters found in acanthodians. Furthermore, it implied an acanthodian-like appearance of the gnathostome crown ancestor: a small fusiform fish, covered in a denticle shagreen, a skull composed of mostly undifferentiated plates, with spines preceding the fins. The unfortunate complication of this hypothesis was that it implied non-homology of osteichthyan and placoderm armoured exoskeletons. Similarities between osteichthyan and placoderm skulls and shoulder girdles had not gone unnoticed7,78,100, but were matched by dismissals citing ‘fundamental differences’ in construction16. The discovery of Entelognathus (discussed above) deals a blow to the latter perspective. Phylogenetic analysis accompanying the discovery6 unsurprisingly led to a wholesale shift of acanthodian-type taxa to the chondrichthyan total group. Every subsequent analysis has corroborated this outcome10,13,14. This key rearrangement eliminates the need to invoke convergence between placoderm and osteichthyan exoskeletons. By viewing the fragmented dermal skeletons of chondrichthyans and acanthodians as a derived condition, no special sister group relationship between osteichthyans and placoderms is implied, as had been done in the past100.
Current analyses universally reject placoderm monophyly, with arthrodires (and similar forms like Entelognathus) resolved closest to the gnathostome crown (Fig. 3). This arrangement suggests resemblances between arthrodires and modern gnathostomes are homologous—a point reinforced by the arthrodire gestalt of Entelognathus. Likewise, it suggests the similarities between the more flat-headed and presumably benthic placoderms, such as antiarchs and petalichthyids, and jawless outgroups reflect a shared primitive condition1,3,12. This has the convenient effect of stretching the placoderms into an array of jaw-bearing stem gnathostomes, although mandibles remain unknown in forms such as Brindabellaspis and petalichthyids.
The consistency of placoderm paraphyly across recent analyses3,6,10-14,80 suggest this is well supported. However, available solutions are not wholly independent, with each dataset incrementally updated from a core original study12. Perhaps significantly, the addition of taxa and characters has not increased support for the paraphyletic placoderm backbone. Instead, successive analyses have seen a winnowing of branch support for the deepest divergences among jaw-bearing stem gnathostomes, coupled with inconsistent arrangements of major placoderm lineages crownward of the deeply diverging antiarchs and Brindabellaspis. This instability, combined with potential placoderm synapomorphies like pelvic claspers10 and a persistent fissure between the nasal capsules and the remainder of the braincase3, indicate that the ‘placoderm problem’ is far from resolved. A satisfactory resolution of the relationships of placoderms will have profound consequences for our understanding of the origin of modern jawed vertebrates.
Conclusions
Early jawed vertebrate phylogenetics is in a state of infancy, but rapid progress is being made. Present discourse on early jawed vertebrate phylogenetics is marked by a growth of healthy debate and a relative lack of the kind of dogmatism that held back the field for nearly half a century. The question of the origin of jaws themselves remains open. To date, the problem has been debated in terms of highly idealised archetypal scenarios, such as the transformation of gill arches into jaws17. From both palaeontological and neontological perspectives, this scenario has proved deficient1,18,19. Little direct evidence of the visceral skeleton of fossil jawless fishes is known; even the proximate outgroups of the jawed vertebrates—osteostracans and galeaspids—are presumed to have been jawless, but remains of the oral skeleton remain absent. What is known of the oral regions of osteostracans and galeaspids suggests they possessed mouths that were specialised relative to the branchial arches, a condition consistent with modern jawless fishes1. Placoderm paraphyly raises some hope that relevant data could be sourced from this assemblage (e.g. Brindabellaspis or petalichthyids). The discovery of additional fossils will hopefully help fill these gaps, but they will not be sufficient by themselves. Rigorous phylogenetic analysis must accompany these new finds to avoid simply shoehorning fossils into appealing narratives27.
Acknowledgements
We thank H. Gee for the invitation to contribute this review, which benefitted from the comments of two anonymous reviewers. This work was supported by the Philip Leverhulme Prize and John Fell Fund, both to M.F., and the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement number 311092 to M.D.B.
References
- 1.Janvier P. Early vertebrates. Clarendon Press; 1996. [A masterful summary—rather than original piece of research—providing a window on the ‘state of the art’ immediately preceding the major changes to our understanding of relationships among early gnathostomes that took place over the past two decades; still an indispensible and accessible resource.] [Google Scholar]
- 2.Chen M, Zou M, Yang L, He S. Basal jawed vertebrate phylogenomics using transcriptomic data from Solexa sequencing. Plos One. 2012;7:e36256. doi: 10.1371/journal.pone.0036256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brazeau MD, Friedman M. The characters of Palaeozoic jawed vertebrates. Zool. J. Linn. Soc. 2014;170:779–821. doi: 10.1111/zoj.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahlberg P, Trinajstic K, Johanson Z, Long J. Pelvic claspers confirm chondrichthyan-like internal fertilization in arthrodires. Nature. 2009;460:888–889. doi: 10.1038/nature08176. [The direct evidence of claspers in arthrodires renewed the palaeobiological significance of placoderms regarding internal fertilisation, but potentially weakens the case for their paraphyly.] [DOI] [PubMed] [Google Scholar]
- 5.Miller RF, Cloutier R, Turner S. The oldest articulated chondrichthyan from the Early Devonian period. Nature. 2003;425:501–504. doi: 10.1038/nature02001. [Oldest record of an articulated chondrichthyan and first example with paired fin spines, initiating the dissolution support for acanthodian monophyly.] [DOI] [PubMed] [Google Scholar]
- 6.Zhu M, et al. A Silurian placoderm with osteichthyan-like marginal jaw bones. Nature. 2013;502:188–193. doi: 10.1038/nature12617. [Of the many remarkable early gnathostome fossils to emerge from China, few have shifted the evolutionary paradigm as much as Entelognathus, a placoderm-like creature with jaw bones resembling those of bony fishes.] [DOI] [PubMed] [Google Scholar]
- 7.Zhu M, Yu X, Janvier P. A primitive fossil fish sheds light on the origin of bony fishes. Nature. 1999;397:607–610. [The bizarre combination of traits reported for Psarolepis highlighted weaknesses in existing phylogenies of early jawed vertebrates, and triggered a resurgence in systematic studies.] [Google Scholar]
- 8.Zhu M, et al. The oldest articulated osteichthyan reveals mosaic gnathostome characters. Nature. 2009;458:469–474. doi: 10.1038/nature07855. [DOI] [PubMed] [Google Scholar]
- 9.Basden AM, Young GC, Coates MI, Richtie A. The most primitive osteichthyan braincase? Nature. 2000;408:185–188. doi: 10.1038/35003183. [DOI] [PubMed] [Google Scholar]
- 10.Long JA, et al. Copulation in antiarch placoderms and the origin of gnathostome internal fertilization. Nature. 2015;517:196–199. doi: 10.1038/nature13825. [DOI] [PubMed] [Google Scholar]
- 11.Davis SP, Finarelli JA, Coates MI. Acanthodes and shark-like conditions in the last common ancestor of modern gnathostomes. Nature. 2012;486:247–250. doi: 10.1038/nature11080. [DOI] [PubMed] [Google Scholar]
- 12.Brazeau MD. The braincase and jaws of a Devonian ‘acanthodian’ and modern gnathostome origins. Nature. 2009;457:305–308. doi: 10.1038/nature07436. [The first study to rigorously test—and, in doing so, reject—placoderm and acanthodian monophyly, this analysis provides the empirical core for most subsequent phylogenetic investigations of early gnathostomes.] [DOI] [PubMed] [Google Scholar]
- 13.Dupret V, Sanchez S, Goujet D, Tafforeau P, Ahlberg PE. A primitive placoderm sheds light on the origin of the jawed vertebrate face. Nature. 2014;507:500–503. doi: 10.1038/nature12980. [DOI] [PubMed] [Google Scholar]
- 14.Giles S, Friedman M, Brazeau MD. Osteichthyan-like cranial conditions in an Early Devonian stem gnathostome. Nature. 2015 doi: 10.1038/nature14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miles RS. In: Interrelationships of Fishes. Greenwood PH, Miles RS, Patterson C, editors. Academic Press; 1973. pp. 63–103. [A first-generation application of cladistic methodology to early jawed vertebrates placing the ‘spiny sharks’ as early relatives of bony fishes, a perspective that profoundly influenced perceptions of the ancestral crown gnathostome for over 40 years.] [Google Scholar]
- 16.Young GC. The relationships of the placoderm fishes. Zool. J. Linn. Soc. 1986;88:1–57. [Provided an explicit argument for the status of placoderms as stem gnathostomes that has not been seriously challenged in the following three decades.] [Google Scholar]
- 17.Gegenbaur C, Bell FJ, Lankester ER. Elements of Comparative Anatomy. Macmillan and Co.; 1878. [Google Scholar]
- 18.Kuratani S. Evolution of the vertebrate jaw: comparative embryology and molecular developmental biology reveal the factors behind evolutionary novelty. J. Anat. 2004;205:335–347. doi: 10.1111/j.0021-8782.2004.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuratani S. Evolution of the vertebrate jaw from developmental perspectives. Evol. Dev. 2012;14:76–92. doi: 10.1111/j.1525-142X.2011.00523.x. [DOI] [PubMed] [Google Scholar]
- 20.Balfour FM. On the development of the skeleton of the paired fins of Elasmobranchii, considered in relation to its bearings on the nature of the limbs of the vertebrata. Proc. Zool. Soc. Lond. 1881;49:656–670. [Google Scholar]
- 21.de Beer G. The Development of the Vertebrate Skull. Oxford University Press; 1937. [Google Scholar]
- 22.Reif W-E. Evolution of dermal skeleton and dentition in vertebrates. Evol. Biol. 1982;15:287–368. [Google Scholar]
- 23.Shubin NH. Origin of evolutionary novelty: examples from limbs. J. Morphol. 2002;252:15–28. doi: 10.1002/jmor.10017. [DOI] [PubMed] [Google Scholar]
- 24.Shigetani Y, Sugahara F, Kuratani S. A new evolutionary scenario for the vertebrate jaw. Bioessays. 2005;27:331–338. doi: 10.1002/bies.20182. [DOI] [PubMed] [Google Scholar]
- 25.Wagner GP, Lynch VJ. Evolutionary novelties. Curr. Biol. 2010;20:R48–52. doi: 10.1016/j.cub.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Dean B. Contributions to the morphology of Cladoselache (Cladodus) J. Morphol. 1894;9:87–114. [Google Scholar]
- 27.Watson DMS. The acanthodian fishes. Philosophical Transactions of the Royal Society of London. 1937;228:49–146. [Google Scholar]
- 28.Zangerl R, Williams ME. New evidence on the nature of the jaw suspension in Palaeozoic acanthous sharks. Palaeontology. 1975;18:333–341. [Google Scholar]
- 29.Gregory WK. Further observations on the pectoral girdle and fin of Sauripterus taylori Hall, a crossopterygian fish from the Upper Devonian of Pennsylvania, with special reference to the origin of the pentadactylate extremities of Tetrapoda. Proc. Am. Phil. Soc. 1935;75:673–690. [Google Scholar]
- 30.Miles RS. A reinterpretation of the visceral skeleton of Acanthodes. Nature. 1964;206:524–525. [Google Scholar]
- 31.Davis MC, Shubin N, Daeschler EB. A new specimen of Sauripterus taylori (Sarcopterygii, Osteichthyes) from the Famennian Catskill Formation of North America. J. Vert. Paleontol. 2004;24:26–40. [Google Scholar]
- 32.Kemp TS. The Origin and Evolution of Mammals. Oxford University Press; 2005. [Google Scholar]
- 33.Makovicky PJ, Zanno LE. In: The Evolutionary History of Modern Birds. Dyke G, Kaiser G, editors. John Wiley & Sons; 2011. pp. 9–29. [Google Scholar]
- 34.Clack JA. Gaining Ground. Indiana University Press; 2012. [Google Scholar]
- 35.Donoghue PC, Forey PL, Aldridge RJ. Conodont affinity and chordate phylogeny. Biol. Rev. Camb. Philos. Soc. 2000;75:191–251. doi: 10.1017/s0006323199005472. [DOI] [PubMed] [Google Scholar]
- 36.Ota KG, Fujimoto S, Oisi Y, Kuratani S. Identification of vertebra-like elements and their possible differentiation from sclerotomes in the hagfish. Nature Comm. 2011;2:373. doi: 10.1038/ncomms1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oisi Y, Ota KG, Kuraku S, Fujimoto S, Kuratani S. Craniofacial development of hagfishes and the evolution of vertebrates. Nature. 2013;493:175–180. doi: 10.1038/nature11794. [DOI] [PubMed] [Google Scholar]
- 38.Ota KG, Kuraku S, Kuratani S. Hagfish embryology with reference to the evolution of the neural crest. Nature. 2007;446:672–675. doi: 10.1038/nature05633. [DOI] [PubMed] [Google Scholar]
- 39.Heimberg AM, Cowper-Sal-lari R, Semon M, Donoghue PC, Peterson KJ. microRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:19379–19383. doi: 10.1073/pnas.1010350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karatajute-Talimaa V, Predtechenskyj N. The distribution of the vertebrates in the Late Ordovician and Early Silurian palaeobasins of the Siberian Platform. Bull. Mus. natl. Hist. nat. C. 1995;17:39–55. [Google Scholar]
- 41.Smith MM, Sansom IJ. Exoskeletal micro-remains of an Ordovician fish from the Harding Sandstone of Colorado. Palaeontology. 1997;40:645–658. [Google Scholar]
- 42.Sansom IJ, Davies NS, Coates MI, Nicoll RS, Ritchie A. Chondrichthyan-like scales from the Middle Ordovician of Australia. Palaeontology. 2012;55:243–247. [Google Scholar]
- 43.Zhao W-J, Zhu M. Siluro-Devonian vertebrate biostratigraphy and biogeography of China. Palaeoworld. 2010;19:4–26. [Google Scholar]
- 44.Anderson PS, Friedman M, Brazeau MD, Rayfield EJ. Initial radiation of jaws demonstrated stability despite faunal and environmental change. Nature. 2011;476:206–209. doi: 10.1038/nature10207. [DOI] [PubMed] [Google Scholar]
- 45.Karatajute-Talimaa VN, Novtistkaya LI, Rozman KS, Sodov J. Mongolepis, a new genus of Elasmobranchii from the Lower Silurian of Mongolia. Paleontol. Zh. 1990;1:76–86. [Google Scholar]
- 46.Sansom IJ, Wang N-Z, Smith M. The histology and affinities of sinacanthid fishes: primitive gnathostomes from the Silurian of China. Zool. J. Linn. Soc. 2005;144:379–386. [Google Scholar]
- 47.Janvier P, Maisey JG. In: Morphology, Phylogeny and Paleobiogeography of Fossil Fishes. Elliott DK, Maisey JG, Yu X, Miao D, editors. Verlag Dr Freidrich Pfeil; 2010. pp. 431–459. [Google Scholar]
- 48.Panchen AL, Smithson TR. Character diagnosis, fossils and the origin of tetrapods. Biol. Rev. Camb. Philos. Soc. 1987;62:341–436. [Google Scholar]
- 49.Ahlberg PE, Johanson Z. Osteolepiforms and the ancestry of tetrapods. Nature. 1998;395:792–794. [Google Scholar]
- 50.Lukševičs E, Lebedev OA, Zakharenko GV. Palaeozoogeographical connections of the Devonian vertebrate communities of the Baltica Province. Part I. Eifelian-Givetian. Palaeoworld. 2010;19:94–107. [Google Scholar]
- 51.Schultze H-P. Palaeoniscoidea-Schuppen aus dem Unterdevon Australiens und Kanadas und aus dem Mitteldevon Spitzbergens. Bulletin of the British Museum (Natural History): Geology. 1968;16:343–376. [Google Scholar]
- 52.Gross W. Fragliche Actinopterygier-Schuppen aus dem Silur Gotlands. Lethaia. 1968;1:184–218. [Google Scholar]
- 53.Botella H, Blom H, Dorka M, Ahlberg PE, Janvier P. Jaws and teeth of the earliest bony fishes. Nature. 2007;448:583–586. doi: 10.1038/nature05989. [DOI] [PubMed] [Google Scholar]
- 54.Friedman M, Brazeau MD. A reappraisal of the origin and basal radiation of the Osteichthyes. J. Vert. Paleontol. 2010;30:36–56. [Google Scholar]
- 55.Märss T, Turner S, Karatajute-Talimaa V. In: Handbook of Paleoichthyology Volume 1B. Schultze H-P, editor. Verlag Dr Friedrich Pfeil; 2007. [Google Scholar]
- 56.Zhu M, Gai Z-K. Phylogenetic relationships of galeaspids (Agnatha) Vertebrata PalAsiatica. 2006;44:1–27. [Google Scholar]
- 57.Sansom RS. Endemicity and palaeobiogeography of the Osteostraci and Galeaspida: a test of scenarios of gnathostome evolution. Palaeontology. 2009;52:1257–1273. [Google Scholar]
- 58.Sansom RS. Phylogeny, classification and character polarity of the Osteostraci (Vertebrata) J. Syst. Paleontol. 2009;7:95–115. [Google Scholar]
- 59.Young GC. Placoderms (armoured fish): dominant vertebrates of the Devonian period. Ann. Rev. Earth Planet. Sci. 2010;38:523–550. [Google Scholar]
- 60.Stensiö EA. The Devonian and Downtonian vertebrates of Spitsbergen. 1. Family Cephalaspidae. Srift. Svalbard Ishavet. 1927;12:1–212. [Google Scholar]
- 61.Stensiö EA. The Cephalaspids of Great Britain. British Museum (Natural History); 1932. [Google Scholar]
- 62.Jarvik E. Basic structure and evolution of vertebrates. Academic Press; 1980. [Google Scholar]
- 63.White EI. The larger arthrodiran fishes from the area of the Burrinjuck Dam, N.S.W. Tranactions of the Zoological Society of London. 1978;34:149–262. [Google Scholar]
- 64.Basden AM, Young GC. A primitive actinopterygian neurocranium from the Early Devonian of Southeastern Australia. J. Vert. Paleontol. 2001;21:754–766. [Google Scholar]
- 65.Young GC. A new Early Devonian placoderm from New South Wales, Australia, with a discussion of placoderm phylogeny. Palaeontogr. Abt. A. 1980;167:10–76. [Google Scholar]
- 66.Gai Z, Donoghue PC, Zhu M, Janvier P, Stampanoni M. Fossil jawless fish from China foreshadows early jawed vertebrate anatomy. Nature. 2011;476:324–327. doi: 10.1038/nature10276. [DOI] [PubMed] [Google Scholar]
- 67.Maisey JG, Miller R, Turner S. The braincase of the chondrichthyan Doliodus from the Lower Devonian Campbellton Formation of New Brunswick, Canada. Acta Zool. 2009;90:109–122. [Google Scholar]
- 68.Maisey JG, Turner S, Naylor GJ, Miller RF. Dental patterning in the earliest sharks: Implications for tooth evolution. J. Morphol. 2014;275:586–596. doi: 10.1002/jmor.20242. [DOI] [PubMed] [Google Scholar]
- 69.Schaeffer B. In: Problèmes actuels de paléontologie: evolution des Vertébrés. Lehman J-P, editor. Vol. 218. Colloques internationaux du Centre national de la Recheche scientifique; 1975. pp. 101–109. [Google Scholar]
- 70.Long JA, Trinajstic K. The Late Devonian Gogo Formation Lägerstatte of Western Australia: exceptional early vertebrate preservation and diversity. Ann. Rev. Earth Planet. Sci. 2010;38:255–279. [Google Scholar]
- 71.Zhu M. Catalogue of Devonian vertebrates in China, with notes on bio-events. Cour. Forsch.-Inst. Senckenberg. 2000;223:379–390. [Google Scholar]
- 72.Bernacsek GM, Dineley DL. New acanthodians from the Delorme Formation (Lower Devonian) of N.W.T. Canada. Palaeontogr. Abt A. 1977;158:1–25. [Google Scholar]
- 73.Janvier P, Blieck A. New data on the internal anatomy of the Heterostraci (Agnatha), with general remarks on the phylogeny of the Craniota. Zool. Scr. 1979;8:287–296. [Google Scholar]
- 74.Janvier P. The phylogeny of craniata, with particular reference to the significance of fossil “agnathans”. J. Vert. Paleontol. 1981;1:121–159. [Google Scholar]
- 75.Forey PL. Yet more reflections on agnathan-gnathostome relationships. J. Vert. Paleontol. 1984;4:330–343. [Google Scholar]
- 76.Wang N-Z. In: Early Vertebrates and Related Problems of Evolutionary Biology. Chang M-M, Lui Y-H, Zhang G-R, editors. Science Press; 1991. [Google Scholar]
- 77.Forey PL, Janvier P. Agnathans and the origin of jawed vertebrates. Nature. 1993;361:129–134. [Google Scholar]
- 78.Zhu M, Schultze H-P. In: Major Events in Early Vertebrate Evolution. Ahlberg PE, editor. Taylor & Francis; 2001. pp. 81–84. [Google Scholar]
- 79.Zhu M, Yu X, Ahlberg PE. A primitive sarcopterygian fish with an eyestalk. Nature. 2001;410:81–84. doi: 10.1038/35065078. [DOI] [PubMed] [Google Scholar]
- 80.Friedman M. Styloichthys as the oldest coelacanth: implications for early osteichthyan interrelationships. J. Syst. Palaeontol. 2007;5:289–343. [Google Scholar]
- 81.Coates MI, Sequiera SEK. A new stethacanthid chondrichthyan from the Lower Carboniferous of Bearsden, Scotland. J. Vert. Paleontol. 2001;21:438–459. [Google Scholar]
- 82.Coates MI, Sequiera SEK. In: Major Events in Early Vertebrate Evolution. Ahlberg PE, editor. Taylor & Francis; 2001. pp. 241–262. [Google Scholar]
- 83.Maisey JG. In: Major Events in Early Vertebrate Evolution. Ahlberg PE, editor. Taylor & Francis; 2001. pp. 263–288. [Google Scholar]
- 84.Halstead LB. Internal anatomy of the polybranchiaspids (Agnatha, Galeaspida) Nature. 1979;282:833–836. [Google Scholar]
- 85.Miles RS. Observations on the ptyctodont fish, Rhamphodopsis Watson. Zool. J. Linn. Soc. 1967;47:99–120. [Google Scholar]
- 86.Trinajstic K, Boisvert C, Long J, Maksimenko A, Johanson Z. Pelvic and reproductive structures in placoderms (stem gnathostomes) Biol. Rev. Camb. Philos. Soc. 2014 doi: 10.1111/brv.12118. [DOI] [PubMed] [Google Scholar]
- 87.Long JA, Trinajstic K, Johanson Z. Devonian arthrodire embryos and the origin of internal fertilization in vertebrates. Nature. 2009;457:1124–1127. doi: 10.1038/nature07732. [DOI] [PubMed] [Google Scholar]
- 88.Janvier P. The relationships of the Osteostraci and Galeaspida. J. Vert. Paleontol. 1984;4:344–358. [Established osteostracans and galeaspids as successive outgroups to—and thus important comparative models for—jawed vertebrates, an arrangement that has survived intact for more than three decades.] [Google Scholar]
- 89.Hanke GF, Wilson MVH. In: Recent Advances in the Origin and Early Radiation of Vertebrates. Arratia G, Wilson MVH, Cloutier R, editors. Verlag Dr Friedrich Pfeil; 2004. pp. 189–216. [Google Scholar]
- 90.Hanke GF, Wilson MVH. In: Morphology, Phylogeny and Paleobiogeography of Fossil Fishes. Elliott DK, Maisey JG, Yu X, Miao D, editors. Verlag Dr Friedrich Pfeil; 2010. pp. 149–182. [Google Scholar]
- 91.Hanke GF, Wilson MVH, Saurette F. Partial articulated specimen of the Early Devonian putative chondrichthyan Polymerolepis whitei Karatajūtė-Talimaa, 1968, with an anal fin spine. Geodiversitas. 2013;35:529–543. [Google Scholar]
- 92.Hanke GF, Wilson MVH. Anatomy of the Early Devonian acanthodian Brochoadmones milesi based on nearly complete body fossils, with comments on the evolution and development of paired fins. J. Vert. Paleontol. 2006;26:526–537. [Google Scholar]
- 93.Schaeffer B. The xenacanth shark neurocranium, with comments on elasmobranch monophyly. Bull. Am. Mus. Nat. Hist. 1981;169:1–66. [Google Scholar]
- 94.Maisey JG, Anderson ME. A primitive chondrichthyan braincase from the Early Devonian of South Africa. J. Vert. Paleontol. 2001;21:702–713. [Google Scholar]
- 95.Turner S. In: Recent Advances in the Origin and Early Radiation of Vertebrates. Arratia G, Wilson MVH, Cloutier R, editors. Verlag Dr Friedrich Pfeil; 2004. pp. 67–94. [Google Scholar]
- 96.Schultze H-P, Cumbaa SL. In: Major Events in Early Vertebrate Evolution. Ahlberg PE, editor. Taylor & Francis; 2001. pp. 315–332. [Google Scholar]
- 97.Yu X. A new porolepiform-like fish, Psarolepis romeri, gen. et sp. nov. (Sarcopterygii, Osteichthyes) from the Lower Devonian of Yunnan, China. J. Vert. Paleontol. 1998;18:261–274. [Google Scholar]
- 98.Zhu M, et al. Fossil fishes from China provide first evidence of dermal pelvic girdles in osteichthyans. Plos One. 2012;7:e35103. doi: 10.1371/journal.pone.0035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coates MI. The evolution of paired fins. Theory Biosci. 2003;122:266–287. [Google Scholar]
- 100.Gardiner BG. The relationships of placoderms. J. Vert. Paleontol. 1984;4:375–395. [Google Scholar]