Abstract
Nasal natural killer/T-cell lymphoma (NNKTL) is associated with Epstein–Barr virus and has a poor prognosis because of local invasion and/or multiple dissemination. Various chemokines play a role in tumor proliferation and invasion, and chemokine receptors including the C-C chemokine receptor 4 (CCR4) are recognized as potential targets for treating hematologic malignancies. The aim of the present study was to determine whether specific chemokines are produced by NNKTL. We compared chemokine expression patterns in culture supernatants of NNKTL cell lines with those of other lymphoma or leukemia cell lines using chemokine protein array and ELISA. Chemokine (C-C motif) ligand (CCL) 17 and CCL22 were highly produced by NNKTL cell lines as compared to the other cell lines. In addition, CCL17 and CCL22 were readily observed in the sera of NNKTL patients. The levels of these chemokines were significantly higher in patients than in healthy controls. Furthermore, we detected the expression of CCR4 (the receptor for CCL17 and CCL22) on the surface of NNKTL cell lines and in tissues of NNKTL patients. Anti-CCR4 monoclonal antibody (mAb) efficiently induced antibody-dependent cellular cytotoxicity mediated by natural killer cells against NNKTL cell lines. Our results suggest that CCL17 and CCL22 may be important factors in the development of NNKTL and open up the possibility of immunotherapy of this lymphoma using anti-CCR4 mAb.
Keywords: CCR4, CCL17, CCL22, Nasal NK/T-cell lymphoma, Chemokine
Introduction
Nasal natural killer/T-cell lymphoma (NNKTL) has characteristic clinical and histological features and is characterized by a poor prognosis and progressive necrotic lesions with the infiltration of tumor and inflammatory cells in the nasal cavity, nasopharynx, and/or palate [1]. Epidemiologically, NNKTL is more common in Asian countries and Latin America than in the USA and Europe [1]. Previous studies clearly suggest that NNKTL is derived from natural killer cell (NK cell) or γδT-cell lineages and expresses CD56, a known NK-cell marker [2–4]. Etiologically, we first reported the presence of Epstein–Barr virus (EBV) DNA, EBV-derived oncogenic proteins, and the clonotypic EBV genome in tissues of NNKTL patients, suggesting that EBV plays an important role in NNKTL tumorigenesis [2, 5, 6]. We previously reported that several cytokines such as interleukin (IL)-9, IL-10, and interferon-gamma-inducible protein-10 (IP-10) are produced by NNKTL cell lines and modulate both proliferation and invasion of tumor cells [7–9]. We also showed that NNKTL cells produce soluble intercellular adhesion molecule-1, which acts as an autocrine factor for lymphoma progression [10]. Furthermore, we recently demonstrated that high expression of CD70 and down-regulation of micro-RNA (miR)-15a are implicated in the pathogenesis of NNKTL by mediating the proliferation of tumor cells [11, 12]. Thus, although NNKTL is a relatively rare disease, its complex biological features are gradually being identified.
Recent work has demonstrated that although chemokines are known to regulate cell migration of various leukocytes, they also favor the growth and metastasis of tumors by promoting cell proliferation, migration, and/or neovascularization in tumor tissue [13, 14]. Chemokines can also have beneficial rather than unfavorable effects for tumor progression, especially for EBV-related hematologic malignancies such as Hodgkin’s lymphoma [15, 16], suggesting that they may be pharmacological therapeutic targets or diagnostic markers for EBV-related hematologic malignancies. Furthermore, the use of therapeutic monoclonal antibodies (mAbs) specific for chemokine receptors could constitute a promising therapeutic approach for the treatment of malignant diseases. For example, the C-C chemokine receptor 4 (CCR4) has been recently recognized as a crucial target molecule for some hematologic malignancies and mogamulizumab; a humanized IgG1 anti-CCR4 mAb has demonstrated meaningful antitumor activity in adult T-cell leukemia/lymphoma (ATLL) [17–20]. It is therefore of great interest to determine the expression patterns of chemokines and chemokine receptors for other hematologic malignancies such as NNKTL.
In order to determine which chemokines are preferentially produced by NNKTL, we compared the chemokine profiles of NNKTL cell line culture supernatants with those of other cell lines. We found that chemokine (C-C motif) ligand (CCL) 17 (also known as thymus and activation-regulated chemokine, TARC), and CCL22 (also known as macrophage-derived chemokine, MDC), was upregulated in NNKTL cell lines and in the sera of NNKTL patients. Expression of the CCL17 and CCL22 receptor CCR4 [21, 22] was observed on NNKTL cell lines and in tissues of NNKTL patients. Moreover, an anti-CCR4 mAb was able to induce antibody-dependent cellular cytotoxicity (ADCC) mediated by NK cells against NNKTL. The overall results indicate that anti-CCR4 mAb may be a novel treatment for patients with NNKTL.
Materials and methods
Patients
Twelve NNKTL patients were analyzed in this study and were diagnosed according to the World Health Organization classification of hematologic malignancies [23] at the Department of Otolaryngology-Head and Neck Surgery, Asahikawa Medical University (Hokkaido, Japan) between 2000 and 2012. Ten healthy volunteers were also analyzed as a control. All patients and volunteers signed appropriate informed consent forms. This study was organized with the approval of the Institutional Review Board at Asahikawa Medical University.
Cell lines
The EBV-positive NNKTL cell lines (SNK-6, SNT-8, and SNK-1) were kindly provided by Dr. Norio Shimizu of Tokyo Medical and Dental University [4, 24]. SNK-6 and SNK-1 exhibit an NK-cell phenotype, whereas SNT-8 has a γδT-cell phenotype. The EBV-positive NK-cell leukemia cell line YT [25] was a gift from Prof. Eva Klein of the Karolinska Institute (Stockholm, Sweden). KHYG-1, established from patients with NK-cell leukemia [26], is an EBV-negative NK-cell line and was purchased from the Health Science Research Resources Bank (Osaka, Japan). The T-cell leukemia cell lines Jurkat [27] and MOLT-4 [28] were purchased from the American Type Culture Collection (Manassas, VA). PEER, derived from T-cell leukemia [29], was purchased from the Health Science Research Resources Bank. The EBV-transformed lymphoblastoid cell line (LCL) was produced from peripheral blood mononuclear cells (PBMCs) using culture supernatant from the EBV-producing B95-8 cell line, obtained from the American Type Culture Collection. The Hodgkin’s lymphoma cell line HDLM-2 [30] was purchased from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). SNK-6, SNT-8, and SNK-1 were cultured in RPMI 1640 supplemented with 10 % heat-inactivated human serum and 700 units/mL recombinant human IL-2. KHYG-1 was cultured in RPMI 1640 supplemented with 10 % fetal bovine serum (FBS) and 100 units/mL recombinant human IL-2. YT, Jurkat, MOLT-4, PEER, and LCL were cultured in RPMI 1640 supplemented with 10 % FBS. HDLM-2 was cultured in RPMI 1640 supplemented with 20 % FBS. All cell lines were incubated in a 5 % CO2 atmosphere at 37 °C.
Human protein chemokine array
The RayBio Human Chemokine Antibody Array I (RayBiotech) was used according to the instruction manual. Briefly, SNK-6, SNT-8, SNK-1, and KHYG-1 cells (2.5 × 105/mL) were cultured in 24-well plates, and supernatants of the cell cultures were collected after 72 h. Membranes were blocked with a blocking buffer, completely covered with 1 mL of supernatant, and then incubated at room temperature for 2 h. After washing, membranes were incubated with 1 mL of primary biotin-conjugated antibodies at room temperature for 2 h and were then incubated with 2 mL of 1000-fold diluted horseradish peroxidase-conjugated streptavidin at room temperature for 2 h. Membranes were developed, and signal intensities were quantified using Image J software. A positive control was applied to normalize the results from different membranes that were being compared.
Measurement of chemokines by ELISA
CCL17 and CCL22 levels in cell culture supernatants and sera were quantified using the ELISA kits, Quantikine Human CCL17/TARC, and CCL22/MDC (R&D Systems), respectively. Cell lines (2.5 × 105/mL) were cultured in 96-well round-bottomed plates, and supernatants were collected after 24, 48, and 72 h. All sera were taken at diagnosis and were frozen at −80 °C. The capture antibody was coated onto the bottom of supplied 96-well ELISA plates and was incubated overnight at 4 °C. Cell culture supernatants (100 μL) or sera (50 μL) were added to each well. The plates were washed after 2 h of incubation at room temperature, and biotinylated anti-chemokine antibody (detection antibody) was added to each well. After 2 h, the plates were washed again and streptavidin-labeled horseradish peroxidase (1:200) was added for 20 min. A substrate solution was added after washing and incubated for 20 min in the dark, and the reaction was stopped by adding 2 N H2SO4. The absorbance of each well was determined at 450 nm using a microplate reader. For cell culture supernatants, measurements were done in triplicate. The results correspond to means ± SD.
Flow cytometric analysis
Cell lines were washed in cold phosphate-buffered saline (PBS), centrifuged, and resuspended in an appropriate volume of fluorescence-activated cell sorting staining buffer (PBS containing 0.1 % NaN3 and 2 % FBS). Cells were incubated with mouse antihuman CCR4 mAb conjugated with FITC (DAKO) for 60 min at 4 °C. FITC-conjugated mouse IgG1 (DAKO) was used as an isotype control. Fluorescence-activated cell sorting, scanning, and data analysis were carried out using the Becton–Dickinson FACScan and the accompanying CellQuest software according to the manufacturer’s protocols.
Immunohistochemical staining
Formalin-fixed, paraffin-embedded samples were obtained from pretreatment biopsy tissues of NNKTL patients and were cut in 4-μm-thick sections. For CCR4 staining, we used POTELIGEO® TEST IHC (Kyowa Hakko Kirin Co.), which is a companion diagnostic test used with mogamulizumab, according to the instruction manual. For CD56 staining, we used a mouse antihuman CD56 mAb (Novocastra) diluted 1:50 as the primary antibody and the Envision HRP System (DAKO) for visualization. Before staining for CD56, the sections were boiled in 0.01 M citrate buffer (pH 6.0) for 15 min in a microwave oven to retrieve the antigen. Serial sections were used for CCR4 and CD56 staining. We considered a case as CCR4-positive if >10 % of tumor cells were CCR4-positive.
ADCC assays
NK cells were isolated for ADCC assay. Briefly, PBMCs were isolated from healthy donors by using a Ficoll-Conray gradient, followed by NK-cell isolation using a CD56 NK-cell isolation kit and a MACS LS column (Miltenyi Biotech) according to the manufacturer’s instructions. ADCC activity of NK cells was measured using a colorimetric CytoTox 96 assay (Promega). This system quantifies the release of lactate dehydrogenase (LDH) from target cells. NK cells were mixed with 2 × 104 target cells with or without mogamulizumab (2 μg/mL, antihuman CCR4 humanized mAb, Kyowa Hakko Kirin Co.) or cetuximab (2 μg/mL, antihuman epidermal growth factor receptor chimeric mAb, Merck AG) at different effector-to-target (E:T) ratios in 96-well round-bottomed plates. After 6 h incubation at 37 °C, 50 μL supernatants were collected and incubated with coloring agent at room temperature for 30 min. LDH density was measured using a fluorescence plate reader (490 nm).
Statistical analysis
The Mann–Whitney U test was used to compare chemokine values between NNKTL patients and healthy controls. The Spearman rank correlation was used to compare serum levels of chemokines. P < 0.05 was considered statistically significant. All analyses and graphics were done using GraphPad Prism 5 (GraphPad Software).
Results
NNKTL cell lines produce CCL17 and CCL22
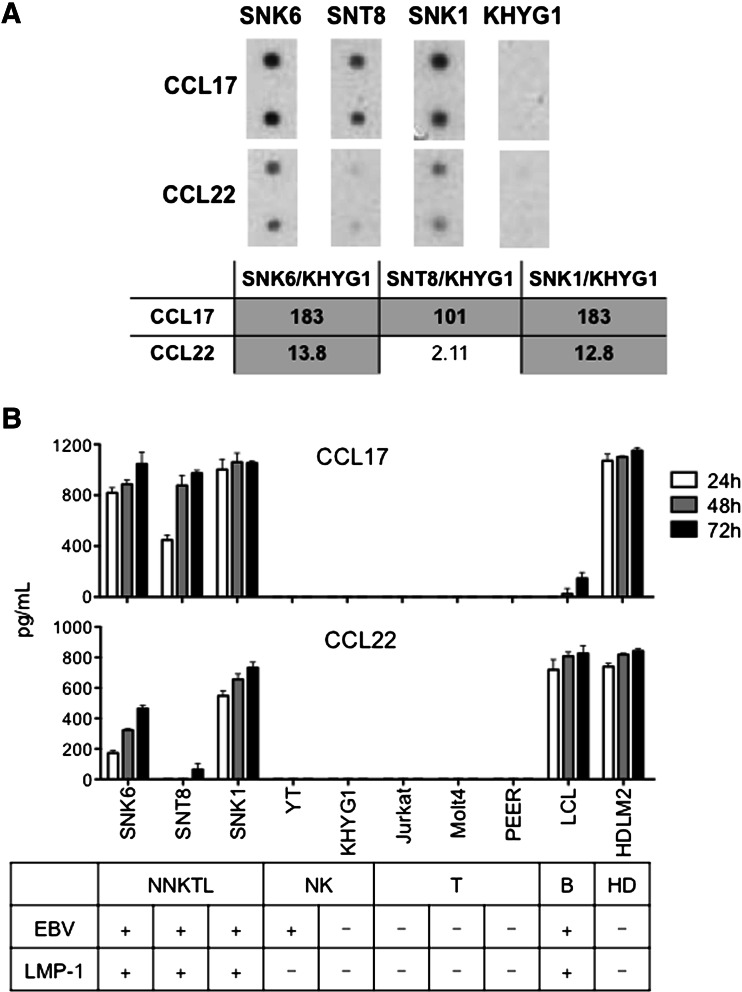
To identify the chemokines preferentially produced by NNKTL, the chemokine expression patterns of EBV-positive NNKTL cell lines (SNK-6, SNT-8, and SNK-1) were compared with that of KHYG-1 (an EBV-negative NK-cell lymphoma) using a chemokine protein array. The levels of CCL17 were at least 100-fold higher in all 3 NNKTL cell lines, and the levels of CCL22 were at least ten-fold higher in 2/3 NNKTL cell lines, as compared to KHYG-1 cells (Fig. 1a). To validate these observations, the levels of these chemokines were measured by ELISA in culture supernatants of the above 3 NNKTL cell lines, 2 NK-cell leukemia cell lines (YT and KHYG-1), 3 T-cell leukemia cell lines (Jurkat, MOLT-4, and PEER), LCL, and one Hodgkin’s lymphoma cell line (HDLM-2). As shown in Fig. 1b, a time-dependent accumulation of CCL17 was observed in the culture supernatants of the EBV-positive NNKTL cell lines SNK-6, SNT-8, and SNK-1 cells. Furthermore, 2 NNKTL lines, SNK-6 and SNK-1, also produced CCL22 in a time-dependent manner, while SNT-8 produced a smaller amount of this chemokine. As reported previously [31, 32], CCL17 and CCL22 were observed in culture supernatants of LCL and HDLM-2 cell lines. In contrast, even after 72 h in culture, CCL17 and CCL22 were barely detected in the culture supernatants of the other cell lines tested. Previously, we investigated various EBV characteristics, including the expression of latent membrane protein-1 (LMP-1) in the cell lines used in the present study [10–12]. When we examined whether LMP-1 expression correlated with chemokine production, we found that the NNKTL cell lines that were positive for EBV and LMP-1 were CCL17 and CCL22 producers, while the YT (EBV-positive but LMP-1-negative) and KHYG-1 (EBV and LMP-1-negative) cell lines did not secrete these chemokines (Fig. 1b). These results suggest that the expression of CCL17 and CCL22 in NK-cell malignancies may be somehow regulated by LMP-1.
Fig. 1.
Expression of CCL17 and CCL22 in NNKTL cell lines. a Chemokine protein array analysis of CCL17 and CCL22 expression in NNKTL cell lines (SNK-6, SNT-8, and SNK-1) and KHYG-1 cells. The table shows the NNKTL cell/KHYG-1 expression ratios, which were calculated as spot intensity of NNKTL cells divided by spot intensity of KHYG-1 cells. The production of CCL17 was 183-fold higher in SNK-6, 101-fold higher in SNT-8, and 183-fold higher in SNK-1 compared with KHYG-1. The production of CCL22 was 13.8-fold higher in SNK-6, 2.11-fold higher in SNT-8, and 12.8-fold higher in SNK-1 compared with KHYG-1. b Supernatants from the indicated cell cultures (2.5 × 105/mL) in 96-well round-bottomed plates were collected after 24, 48, and 72 h, and CCL17 and CCL22 production was assessed using ELISA. Columns, means of triplicate determinations; bars, SD
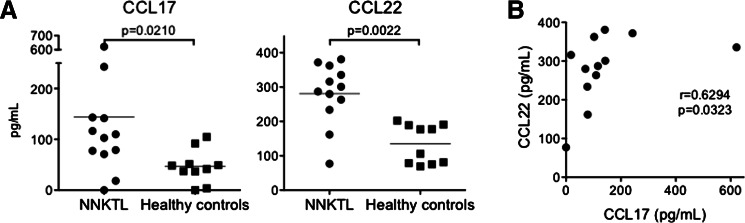
Detection of CCL17 and CCL22 in the sera of NNKTL patients
Previous studies reported that increased serum CCL17 and CCL22 levels were observed in Hodgkin’s lymphoma patients [33]. It was therefore possible that these chemokines might also be detected in the sera of patients with NNKTL. Thus, we measured the levels of CCL17 and CCL22 in the sera of NNKTL patients and control healthy volunteers. As shown in Fig. 2a, CCL17 and CCL22 serum levels were significantly increased in NNKTL patients as compared to healthy individuals. Furthermore, the CCL17 and CCL22 levels correlated with each other in the NNKTL patients (Fig. 2b).
Fig. 2.
Levels of CCL17 and CCL22 in sera. a CCL17 and CCL22 levels in the sera from 12 NNKTL patients and 10 healthy volunteers were measured using ELISA. The horizontal lines indicate mean values. Statistical significance was determined using the Mann–Whitney U test. b There was a significant correlation between the CCL17 levels and the CCL22 levels in the sera from NNKTL patients. Statistical significance was determined using the Spearman rank correlation
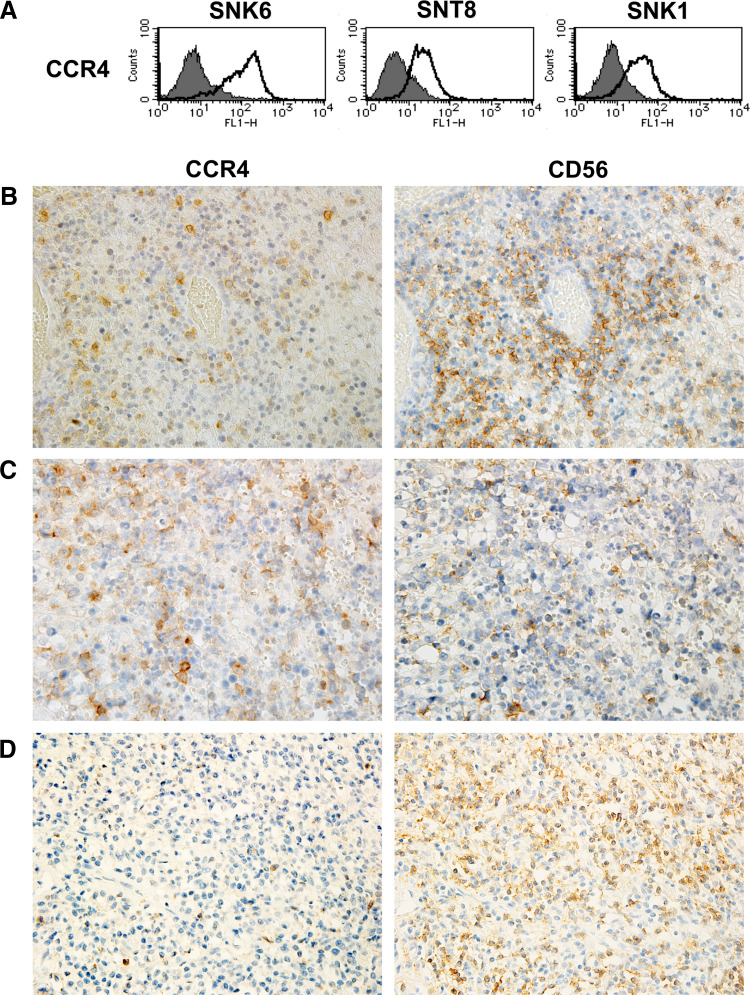
CCR4 is expressed on NNKTL cell lines and in NNKTL tissues
Next, we determined whether NNKTL cell lines expressed CCR4, the receptor for CCL17 and CCL22 [21, 22]. Flow cytometric analysis revealed that CCR4 was expressed on the surface of 3 NNKTL cell lines (Fig. 3a). To confirm the expression of CCR4 in NNKTL tissues, immunohistochemistry was performed using serial sections of biopsy samples collected from five patients. The neoplastic cells of all samples were positive for CD56. In two of five patients, >10 % of the CD56-positive tumor cells were CCR4-positive (Fig. 3b–d).
Fig. 3.
Expression of CCR4 in NNKTL. a Flow cytometric analysis of the surface expression of CCR4 in NNKTL cell lines. Cells were stained with FITC-conjugated anti-CCR4 mAb (thick lines). Filled histograms, cells stained with isotype control Ab. b–d Immunohistological analysis of CCR4 and CD56 expression using serial sections of biopsy samples from NNKTL patients. b and c, 2 cases showing that >10 % of CD56-positive tumor cells are CCR4 positive. d representative case showing negative staining for CCR4. Original magnification, ×400
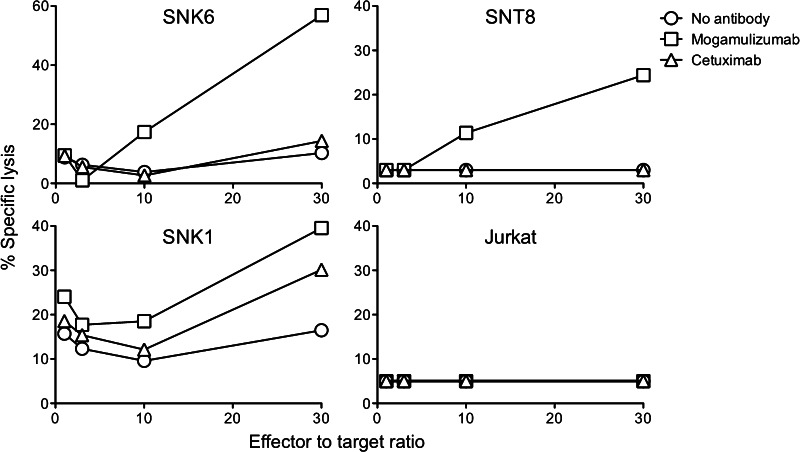
Anti-CCR4 mAb induces ADCC activity against NNKTL cell lines
It was recently shown that mogamulizumab, a humanized mAb against CCR4, exhibits strong cytotoxicity for ATLL cells via ADCC [19, 20]. We therefore examined whether mogamulizumab could mediate cytolysis of CCR4-expressing NNKTL cell lines by NK cells. NNKTL cells were cocultured with normal human NK cells with or without mogamulizumab, and tumor cell death was measured using an LDH cytotoxicity assay. As shown in Fig. 4, mogamulizumab induced robust ADCC against NNKTL cell lines as compared to no antibody or cetuximab (epidermal growth factor receptor antagonistic antibody, used as a negative control), whereas ADCC was not observed in the CCR4-negative Jurkat cell line. These findings suggest that CCR4 might be a targeted molecule for the treatment of NNKTL.
Fig. 4.
Mogamulizumab-induced ADCC activity against NNKTL cell lines. ADCC activity against NNKTL cell lines with mogamulizumab (2 μg/mL, squares), cetuximab (control, 2 μg/mL, triangles), or no antibody (circles) was measured using an LDH cytotoxic assay in the presence of effector NK cells obtained from a healthy donor. Points, means of triplicate determinations. Points without bars had a SD of <10 % the value of the mean. Results are representative of three separate experiments
Discussion
Chemokines have been shown to play pleiotropic roles in promoting tumor invasion, migration, and vascularization [13, 14]. In the present study, we showed that CCL17 and CCL22 were highly upregulated in NNKTL cell lines and patient sera. Furthermore, serum CCL17 levels were significantly correlated with serum CCL22 levels in NNKTL patients. We also found that CCR4, the receptor for CCL17 and CCL22, was expressed on NNKTL cell lines and in NNKTL tissues, indicating that these chemokines might exert their activity through an autocrine mechanism. We previously reported that IP-10 and the IP-10 receptor CXCR3 are coexpressed by NNKTL cell lines and that IP-10 enhances cell invasion via an autocrine mechanism [9]. However, using cell invasion assays, neither administration of exogenous recombinant protein nor the addition of CCL17 or CCL22 neutralizing antibodies influenced cell invasion (data not presented). Both CCL17 and CCL22 have been reported to be highly expressed in some hematologic malignancies such as Hodgkin’s lymphoma and B cell lymphoma [33, 34]. Epigenetic modification is considered to affect CCL17 expression in Hodgkin’s lymphoma, but the precise mechanism through which this occurs in Hodgkin’s lymphoma remains unknown [35]. Similar to the results of this study, in Hodgkin’s lymphoma, these chemokines show significantly increased levels in sera, and CCL17 levels are significantly correlated with CCL22 levels [33]. Because CCR4 is expressed by regulatory T-cells (Tregs), CCL17 and CCL22 produced by Hodgkin’s lymphoma cells are thought to play a role in immune evasion by attracting Tregs to the tumor microenvironment [36–38]. Thus, a similar mechanism involving CCL17 and CCL22 might be operative for immune evasion in NNKTL. We recently demonstrated that CD14-positive monocytes enhance the proliferation of NNKTL cell lines through cell-to-cell contact in vitro and contacted with CD56-positive lymphoma cells in tissues of patients with NNKTL, suggesting that a positive feedback loop between monocytes and NNKTL cells contributes to tumor progression in the tumor microenvironment [39]. Since CD14-positive monocytes express CCR4 [40], it is therefore possible that the CCL17 and CCL22 produced by NNKTL cells attract CD14-positive monocytes, which then enhance the proliferation of NNKTL cells. We found that CCL17 expression was higher than CCL22 expression in culture supernatants of NNKTL cell lines (Fig. 1b), but on the other hand, CCL22 expression was higher than CCL17 expression in patient sera (Fig. 2). Because the CCL22 level was higher than that of CCL17 even in healthy donors as we and another group have reported [31], this finding might indicate that cells other than NNKTL cells are responsible for CCL22 production in healthy or pathological conditions. Since monocyte-derived cells can produce CCL22 as well as NNKTL cells [41], future studies will be required to elucidate the functional roles of CCL17 and CCL22 in NNKTL tissue consisting of tumor cells and surrounding cells such as Tregs and monocytes.
LMP-1, an EBV-encoded gene product, has been considered to be the main viral oncogene that promotes cell growth, metastasis, apoptotic resistance, and immune modulation in EBV-related malignancies including NNKTL [42]. A previous study showed that LMP-1 can induce CCL17 and CCL22 production by EBV-infected B cells via activation of NF-κB [31]. We found here that NNKTL cell lines, which were infected with EBV and expressed LMP-1, secreted CCL17 and CCL22, while YT cells, which were infected with EBV but did not express LMP-1, could not secret these chemokines. Thus, LMP-1 might be responsible for the induction of CCL17 and CCL22 in NNKTL.
CCR4 is under consideration as a target molecule for the therapy of some hematologic malignancies that express CCR4. In particular, a humanized anti-CCR4 mAb, mogamulizumab, is undergoing clinical testing in ATLL patients, and these trials have been showing favorable results [20]. Defucosylation of this mAb enhances ADCC activity of NK cells against CCR4-expressing cells [43]. Furthermore, it is possible that an anti-CCR4 mAb could not only kill the CCR4-expressing tumor cells directly, but also inhibit the suppressive function of CCR4-expressing Tregs in the tumor microenvironment [44]. In the present study, we provide evidence that mogamulizumab can induce ADCC activity of NK cells against NNKTL cells. More recently, Kanazawa et al. also demonstrated that CCR4 is expressed on EBV-positive T and NK-cell lines and that mogamulizumab induces ADCC activity against these cell lines [45]. Our results and the data reported by Kanazawa et al. may open the door to the development of a new therapeutic strategy for EBV-associated T/NK-cell malignancies. Since anti-CCR4 mAb is currently being developed for clinical use, we believe that molecular-targeted therapy and the blockade of CCL17/CCR4 and CCL22/CCR4 pathways using anti-CCR4 mAb may be a potential approach for NNKTL therapy.
Acknowledgments
The authors thank Dr. Shimizu N (Tokyo Medical and Dental University) and Prof. Klein E (Karolinska Institute) for generously providing cell lines. This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI [Grant Numbers 24791735 (Kumai T), 23791869 (Nagato T), 25460430 (Kobayashi H), and 24390385 (Harabuchi Y)] and by National Institutes of Health (NIH) Grants [Grant Numbers R01CA136828 and R01CA157303 (Celis E)].
Conflict of interest
The authors have no financial conflict of interest.
Abbreviations
- ADCC
Antibody-dependent cellular cytotoxicity
- ATLL
Adult T-cell leukemia/lymphoma
- CCL
Chemokine (C-C motif) ligand
- CCR4
C-C chemokine receptor 4
- EBV
Epstein–Barr virus
- FBS
Fetal bovine serum
- IL
Interleukin
- IP-10
Interferon-gamma-inducible protein-10
- LDH
Lactate dehydrogenase
- LMP-1
Latent membrane protein-1
- mAbs
Monoclonal antibodies
- MDC
Macrophage-derived chemokine
- miR
Micro-RNA
- NK cell
Natural killer cell
- NNKTL
Nasal natural killer/T-cell lymphoma
- PBMCs
Peripheral blood mononuclear cells
- PBS
Phosphate-buffered saline
- TARC
Thymus and activation-regulated chemokine
Footnotes
Takumi Kumai and Toshihiro Nagato contributed equally to this work.
Contributor Information
Toshihiro Nagato, Phone: +81-166-68-2554, Email: rijun@asahikawa-med.ac.jp.
Hiroya Kobayashi, Phone: +81-166-68-2381, Email: hiroya@asahikawa-med.ac.jp.
References
- 1.Harabuchi Y, Takahara M, Kishibe K, Moriai S, Nagato T, Ishii H. Nasal natural killer (NK)/T-cell lymphoma: clinical, histological, virological, and genetic features. Int J Clin Oncol. 2009;14:181–190. doi: 10.1007/s10147-009-0882-7. [DOI] [PubMed] [Google Scholar]
- 2.Harabuchi Y, Yamanaka N, Kataura A, Imai S, Kinoshita T, Mizuno F, Osato T. Epstein–Barr virus in nasal T-cell lymphomas in patients with lethal midline granuloma. Lancet. 1990;335:128–130. doi: 10.1016/0140-6736(90)90002-M. [DOI] [PubMed] [Google Scholar]
- 3.Emile JF, Boulland ML, Haioun C, Kanavaros P, Petrella T, Delfau-Larue MH, Bensussan A, Farcet JP, Gaulard P. CD5-CD56+ T-cell receptor silent peripheral T-cell lymphomas are natural killer cell lymphomas. Blood. 1996;87:1466–1473. [PubMed] [Google Scholar]
- 4.Nagata H, Konno A, Kimura N, Zhang Y, Kimura M, Demachi A, Sekine T, Yamamoto K, Shimizu N. Characterization of novel natural killer (NK)-cell and gammadelta T-cell lines established from primary lesions of nasal T/NK-cell lymphomas associated with the Epstein–Barr virus. Blood. 2001;97:708–713. doi: 10.1182/blood.V97.3.708. [DOI] [PubMed] [Google Scholar]
- 5.Harabuchi Y, Imai S, Wakashima J, Hirao M, Kataura A, Osato T, Kon S. Nasal T-cell lymphoma causally associated with Epstein–Barr virus: clinicopathologic, phenotypic, and genotypic studies. Cancer. 1996;77:2137–2149. doi: 10.1002/(SICI)1097-0142(19960515)77:10<2137::AID-CNCR27>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Minarovits J, Hu LF, Imai S, Harabuchi Y, Kataura A, Minarovits-Kormuta S, Osato T, Klein G. Clonality, expression and methylation patterns of the Epstein–Barr virus genomes in lethal midline granulomas classified as peripheral angiocentric T cell lymphomas. J Gen Virol. 1994;75(Pt 1):77–84. doi: 10.1099/0022-1317-75-1-77. [DOI] [PubMed] [Google Scholar]
- 7.Nagato T, Kobayashi H, Kishibe K, Takahara M, Ogino T, Ishii H, Oikawa K, Aoki N, Sato K, Kimura S, Shimizu N, Tateno M, Harabuchi Y. Expression of interleukin-9 in nasal natural killer/T-cell lymphoma cell lines and patients. Clin Cancer Res. 2005;11:8250–8257. doi: 10.1158/1078-0432.CCR-05-1426. [DOI] [PubMed] [Google Scholar]
- 8.Takahara M, Kis LL, Nagy N, Liu A, Harabuchi Y, Klein G, Klein E. Concomitant increase of LMP1 and CD25 (IL-2-receptor alpha) expression induced by IL-10 in the EBV-positive NK lines SNK6 and KAI3. Int J Cancer. 2006;119:2775–2783. doi: 10.1002/ijc.22139. [DOI] [PubMed] [Google Scholar]
- 9.Moriai S, Takahara M, Ogino T, Nagato T, Kishibe K, Ishii H, Katayama A, Shimizu N, Harabuchi Y. Production of interferon-{gamma}-inducible protein-10 and its role as an autocrine invasion factor in nasal natural killer/T-cell lymphoma cells. Clin Cancer Res. 2009;15:6771–6779. doi: 10.1158/1078-0432.CCR-09-1052. [DOI] [PubMed] [Google Scholar]
- 10.Takahara M, Nagato T, Komabayashi Y, Yoshino K, Ueda S, Kishibe K, Harabuchi Y. Soluble ICAM-1 secretion and its functional role as an autocrine growth factor in nasal NK/T cell lymphoma cells. Exp Hematol. 2013;41:711–718. doi: 10.1016/j.exphem.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Yoshino K, Kishibe K, Nagato T, Ueda S, Komabayashi Y, Takahara M, Harabuchi Y. Expression of CD70 in nasal natural killer/T cell lymphoma cell lines and patients; its role for cell proliferation through binding to soluble CD27. Br J Haematol. 2013;160:331–342. doi: 10.1111/bjh.12136. [DOI] [PubMed] [Google Scholar]
- 12.Komabayashi Y, Kishibe K, Nagato T, Ueda S, Takahara M, Harabuchi Y. Downregulation of miR-15a due to LMP1 promotes cell proliferation and predicts poor prognosis in nasal NK/T-cell lymphoma. Am J Hematol. 2014;89:25–33. doi: 10.1002/ajh.23570. [DOI] [PubMed] [Google Scholar]
- 13.Wang JM, Deng X, Gong W, Su S. Chemokines and their role in tumor growth and metastasis. J Immunol Methods. 1998;220:1–17. doi: 10.1016/S0022-1759(98)00128-8. [DOI] [PubMed] [Google Scholar]
- 14.Sarvaiya PJ, Guo D, Ulasov I, Gabikian P, Lesniak MS. Chemokines in tumor progression and metastasis. Oncotarget. 2013;4:2171–2185. doi: 10.18632/oncotarget.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maggio E, van den Berg A, Diepstra A, Kluiver J, Visser L, Poppema S. Chemokines, cytokines and their receptors in Hodgkin’s lymphoma cell lines and tissues. Ann Oncol. 2002;13(Suppl 1):52–56. doi: 10.1093/annonc/13.S1.52. [DOI] [PubMed] [Google Scholar]
- 16.Laurence AD. Location, movement and survival: the role of chemokines in haematopoiesis and malignancy. Br J Haematol. 2006;132:255–267. doi: 10.1111/j.1365-2141.2005.05841.x. [DOI] [PubMed] [Google Scholar]
- 17.Yoshie O, Fujisawa R, Nakayama T, Harasawa H, Tago H, Izawa D, Hieshima K, Tatsumi Y, Matsushima K, Hasegawa H, Kanamaru A, Kamihira S, Yamada Y. Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood. 2002;99:1505–1511. doi: 10.1182/blood.V99.5.1505. [DOI] [PubMed] [Google Scholar]
- 18.Ishida T, Ishii T, Inagaki A, Yano H, Kusumoto S, Ri M, Komatsu H, Iida S, Inagaki H, Ueda R. The CCR4 as a novel-specific molecular target for immunotherapy in Hodgkin lymphoma. Leukemia. 2006;20:2162–2168. doi: 10.1038/sj.leu.2404415. [DOI] [PubMed] [Google Scholar]
- 19.Ishii T, Ishida T, Utsunomiya A, Inagaki A, Yano H, Komatsu H, Iida S, Imada K, Uchiyama T, Akinaga S, Shitara K, Ueda R. Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphoma. Clin Cancer Res. 2010;16:1520–1531. doi: 10.1158/1078-0432.CCR-09-2697. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto K, Utsunomiya A, Tobinai K, Tsukasaki K, Uike N, Uozumi K, Yamaguchi K, Yamada Y, Hanada S, Tamura K, Nakamura S, Inagaki H, Ohshima K, Kiyoi H, Ishida T, Matsushima K, Akinaga S, Ogura M, Tomonaga M, Ueda R. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin Oncol. 2010;28:1591–1598. doi: 10.1200/JCO.2009.25.3575. [DOI] [PubMed] [Google Scholar]
- 21.Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem. 1997;272:15036–15042. doi: 10.1074/jbc.272.23.15036. [DOI] [PubMed] [Google Scholar]
- 22.Nomiyama H, Imai T, Kusuda J, Miura R, Callen DF, Yoshie O. Human chemokines fractalkine (SCYD1), MDC (SCYA22) and TARC (SCYA17) are clustered on chromosome 16q13. Cytogenet Cell Genet. 1998;81:10–11. doi: 10.1159/000015000. [DOI] [PubMed] [Google Scholar]
- 23.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD. The World Health Organization classification of neoplasms of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting—Airlie House, Virginia, November 1997. Hematol J. 2000;1:53–66. doi: 10.1038/sj.thj.6200013. [DOI] [PubMed] [Google Scholar]
- 24.Nagata H, Numata T, Konno A, Mikata I, Kurasawa K, Hara S, Nishimura M, Yamamoto K, Shimizu N. Presence of natural killer-cell clones with variable proliferative capacity in chronic active Epstein–Barr virus infection. Pathol Int. 2001;51:778–785. doi: 10.1046/j.1440-1827.2001.01276.x. [DOI] [PubMed] [Google Scholar]
- 25.Yodoi J, Teshigawara K, Nikaido T, Fukui K, Noma T, Honjo T, Takigawa M, Sasaki M, Minato N, Tsudo M, et al. TCGF (IL 2)-receptor inducing factor(s). I. Regulation of IL 2 receptor on a natural killer-like cell line (YT cells) J Immunol. 1985;134:1623–1630. [PubMed] [Google Scholar]
- 26.Yagita M, Huang CL, Umehara H, Matsuo Y, Tabata R, Miyake M, Konaka Y, Takatsuki K. A novel natural killer cell line (KHYG-1) from a patient with aggressive natural killer cell leukemia carrying a p53 point mutation. Leukemia. 2000;14:922–930. doi: 10.1038/sj.leu.2401769. [DOI] [PubMed] [Google Scholar]
- 27.Schneider U, Schwenk HU, Bornkamm G. Characterization of EBV-genome negative “null” and “T” cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int J Cancer. 1977;19:621–626. doi: 10.1002/ijc.2910190505. [DOI] [PubMed] [Google Scholar]
- 28.Minowada J, Onuma T, Moore GE. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972;49:891–895. [PubMed] [Google Scholar]
- 29.Ravid Z, Goldblum N, Zaizov R, Schlesinger M, Kertes T, Minowada J, Verbi W, Greaves M. Establishment and characterization of a new leukaemic T-cell line (Peer) with an unusual phenotype. Int J Cancer. 1980;25:705–710. doi: 10.1002/ijc.2910250604. [DOI] [PubMed] [Google Scholar]
- 30.Drexler HG, Gaedicke G, Lok MS, Diehl V, Minowada J. Hodgkin’s disease derived cell lines HDLM-2 and L-428: comparison of morphology, immunological and isoenzyme profiles. Leuk Res. 1986;10:487–500. doi: 10.1016/0145-2126(86)90084-6. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama T, Hieshima K, Nagakubo D, Sato E, Nakayama M, Kawa K, Yoshie O. Selective induction of Th2-attracting chemokines CCL17 and CCL22 in human B cells by latent membrane protein 1 of Epstein–Barr virus. J Virol. 2004;78:1665–1674. doi: 10.1128/JVI.78.4.1665-1674.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Stasi A, De Angelis B, Rooney CM, Zhang L, Mahendravada A, Foster AE, Heslop HE, Brenner MK, Dotti G, Savoldo B. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113:6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niens M, Visser L, Nolte IM, van der Steege G, Diepstra A, Cordano P, Jarrett RF, Te Meerman GJ, Poppema S, van den Berg A. Serum chemokine levels in Hodgkin lymphoma patients: highly increased levels of CCL17 and CCL22. Br J Haematol. 2008;140:527–536. doi: 10.1111/j.1365-2141.2007.06964.x. [DOI] [PubMed] [Google Scholar]
- 34.Takegawa S, Jin Z, Nakayama T, Oyama T, Hieshima K, Nagakubo D, Shirakawa AK, Tsuzuki T, Nakamura S, Yoshie O. Expression of CCL17 and CCL22 by latent membrane protein 1-positive tumor cells in age-related Epstein–Barr virus-associated B-cell lymphoproliferative disorder. Cancer Sci. 2008;99:296–302. doi: 10.1111/j.1349-7006.2007.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buglio D, Georgakis GV, Hanabuchi S, Arima K, Khaskhely NM, Liu YJ, Younes A. Vorinostat inhibits STAT6-mediated TH2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood. 2008;112:1424–1433. doi: 10.1182/blood-2008-01-133769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvaro T, Lejeune M, Salvado MT, Bosch R, Garcia JF, Jaen J, Banham AH, Roncador G, Montalban C, Piris MA. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11:1467–1473. doi: 10.1158/1078-0432.CCR-04-1869. [DOI] [PubMed] [Google Scholar]
- 37.Ishida T, Ishii T, Inagaki A, Yano H, Komatsu H, Iida S, Inagaki H, Ueda R. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res. 2006;66:5716–5722. doi: 10.1158/0008-5472.CAN-06-0261. [DOI] [PubMed] [Google Scholar]
- 38.Marshall NA, Christie LE, Munro LR, Culligan DJ, Johnston PW, Barker RN, Vickers MA. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103:1755–1762. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- 39.Ishii H, Takahara M, Nagato T, Kis LL, Nagy N, Kishibe K, Harabuchi Y, Klein E. Monocytes enhance cell proliferation and LMP1 expression of nasal natural killer/T-cell lymphoma cells by cell contact-dependent interaction through membrane-bound IL-15. Int J Cancer. 2012;130:48–58. doi: 10.1002/ijc.25969. [DOI] [PubMed] [Google Scholar]
- 40.Nieto JC, Canto E, Zamora C, Ortiz MA, Juarez C, Vidal S. Selective loss of chemokine receptor expression on leukocytes after cell isolation. PLoS One. 2012;7:e31297. doi: 10.1371/journal.pone.0031297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashimoto S, Nakamura K, Oyama N, Kaneko F, Tsunemi Y, Saeki H, Tamaki K. Macrophage-derived chemokine (MDC)/CCL22 produced by monocyte derived dendritic cells reflects the disease activity in patients with atopic dermatitis. J Dermatol Sci. 2006;44:93–99. doi: 10.1016/j.jdermsci.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Middeldorp JM, Pegtel DM. Multiple roles of LMP1 in Epstein–Barr virus induced immune escape. Semin Cancer Biol. 2008;18:388–396. doi: 10.1016/j.semcancer.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Ito A, Ishida T, Utsunomiya A, Sato F, Mori F, Yano H, Inagaki A, Suzuki S, Takino H, Ri M, Kusumoto S, Komatsu H, Iida S, Inagaki H, Ueda R. Defucosylated anti-CCR4 monoclonal antibody exerts potent ADCC against primary ATLL cells mediated by autologous human immune cells in NOD/Shi-scid, IL-2R gamma(null) mice in vivo. J Immunol. 2009;183:4782–4791. doi: 10.4049/jimmunol.0900699. [DOI] [PubMed] [Google Scholar]
- 44.Ishida T, Ueda R. CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci. 2006;97:1139–1146. doi: 10.1111/j.1349-7006.2006.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanazawa T, Hiramatsu Y, Iwata S, Siddiquey M, Sato Y, Suzuki M, Ito Y, Goshima F, Murata T, Kimura H. Anti-CCR4 monoclonal antibody mogamulizumab for the treatment of EBV-associated T- and NK-cell lymphoproliferative diseases. Clin Cancer Res. 2014;20:5075–5084. doi: 10.1158/1078-0432.CCR-14-0580. [DOI] [PubMed] [Google Scholar]