Abstract
In this paper, we have synthesized BSA protected gold nanoclusters (BSA Au nanocluster) and studied the effect of quencher, protein denaturant, pH and temperature on the fluorescence properties of the tryptophan molecule of the BSA Au nanocluster and native BSA. We have also studied their effect on the peak emission of BSA Au nanoclusters (650 nm). The phtophysical characterization of a newly developed fluorophore in different environments is absolutely necessary to futher develop their biomedical and analytical applications. It was observed from our experiments that the tryptophan in BSA Au nanoclusters is better shielded from the polar environment. Tryptophan in native BSA showed a red shift in its peak emission wavelength position. Tryptophan is a highly polarity sensitive dye and a minimal change in its microenvironment can be easily observed in its photophysical properties.
Keywords: BSA Au nanoclusters, tryptophan emission, fluorescence, quenching, denaturants
1. Introduction
Noble metal nanomaterials with impressive size dependent optical and chemical properties have attained great attention in the last few years. The sizes of these nanoclusters are usually below 2 nm, comparable to the Fermi wavelength of the electron. They bridge the gap between atoms and metallic nanoparticles. The size of the core metal can be controlled and hence a desired emission wavelength can be obtained. These noble metal nanoclusters can be synthesized using various ligands like amino acids, peptides, DNA, proteins and dendrimers [1–9].
Protein protected noble metal nanoclusters are known to have numerous optical and bioimaging applications. In the literature, various protein matrices have been reported for the synthesis of fluorescent metal nanoclusters. Different research groups have used proteins like BSA, HSA, lysozyme, trypsin and ferratin family of proteins for the synthesis of these fluorescent metallic nanoclusters [10–12]. Xie et al. first introduced the use of BSA as a ligand to produce red emitting Au nanoclusters [9]. They introduced an easy directed synthesis for protein protected gold nanoclusters. Zhag et al. recently studied the temperature induced fluorescence properties and conformation change in BSA protected gold nanoclusters [13]. Raut et al. studied the synthesis, polarization properties, two photon induced luminescence properties, its application as a time gated intensity probe and the energy transfer from tryptophan to BSA protected Au nanoclusters [14–17].Yu et al. studied the interaction between BSA Au nanocluster and metallic ions(Hg2+ and Cu2+). They observed that, Hg 2+ quenches the delayed fluorescence of BSA Au25 nanocluster via triplet state electron transfer through metallic bond. However, the Cu2+ do not alter the delayed fluorescence in BSA Au25 [18]. There are other numerous reports on the BSA Au clusters for spectroscopic investigations and biochemical applications [2,3,19–23].
Understanding the effect of environment on the formation of gold cluster in protein templates and its effect on the tryptophan residues in that protein is an essential information that should be studied. While studying the properties of the protein templated gold clusters, tryptophan emission and effect of surrounding environment on its properties appears to be neglected in the literature. Raut et al. recently studied the energy transfer from tryptophan to gold clusters formed within BSA and HSA proteins, However, that just provided information on the interaction of tryptophan with gold cluster. Moreover, certain questions that we need to answer about these novel flurophores: Is it possible to quench the fluorescence of gold cluster with classic quenchers like Potassium Iodide? What will be the effect on the tryptophan emission? Will temperature, pH and denaturing compounds will denature these proteins in different manner compared to its native form? These kinds of questions can be answered by probing the tryptophan emission from these protein protected clusters along with its native counterpart. BSA contains two tryptophan molecules at position 134 and 213. The tryptophan at position 213 is more deeply buried compared to tryptophan at position 134. The position of the emission spectrum of tryptophan depicts the average environment of both residues. Tryptophan is highly polarity sensitive and shows a red shift in emission peak, decrease in quantum yield and fluorescence lifetime when exposed to polar environment.
Therefore in this report, we are studying the effect of quenchers, protein denaturants, temperature and pH on the fluorescence properties of BSA protected gold nanoclusters. We also studied their effect on the tryptophan emission for BSA Au nanoclusters and compared it with the fluorescent properties of native BSA molecules.
2. Material and Methods
Bovine serum albumin (BSA), gold (III) chloride hydrate, potassium iodide (KI), urea and guanidine hydrochloride were purchased from Sigma Aldrich (St Louis, MO, USA).
2.1. Synthesis of BSA Au nanoclusters
The BSA Au nanoclusters were prepared using an approached mentioned in our previous paper [14]. Typically, 5 mL of 10 mM HAuCl4 was mixed with 5 mL of 50 mg /mL BSA followed by addition of 1 mL of 1 M NaOH and kept at 37°C overnight in an incubator. The light brown solution of clusters was further dialyzed (2000 MWCO membrane) against de-ionized water for at least 12 hours with periodic change of water to remove any small impurities. The dialyzed cluster solution was filtered using a 0.02 mm syringe filter and used for subsequent measurements.
2.2. Spectroscopic measurements
Absorption spectra of native BSA and BSA Au nanocluster were measured using Carry 50 Bio UV-Vis spectrophotometer. Fluorescence emission spectra were obtained using the Carry Eclipse spectrofluorometer (Varian Inc.). All measurements were done in 1cm × 1cm cuvette. Emission from the tryptophan of native BSA and BSA Au nanocluster were measured using 280 nm excitation. To measure the peak emission of the BSA Au nanoclusters a 360 nm excitation was used. Both measurements were done using appropriate filers on the emission side.
Time resolved intensity decay were measured using the FluoTime 200 (PicoQuant, GmbH, Berlin, Germany) time resolved spectrofluorometer. This instrument contains a multichannel plate detector (Hamamatsu, Japan) and a 290 nm LED was used as an excitation source to measure intensity decay for tryptophan in native BSA and BSA Au nanocluster. To measure the peak emission (650 nm) of BSA Au nanocluster, a 375 nm laser diode was used. The fluorescence intensity decays were measured in magic angle conditions and data was analyzed with FluoFit version 4.5.3 software (PicoQuant GmbH, Berlin, Germany) using the exponential reconvolution procedure using non-liner regression (multiexponential deconvolution model).
3. Experimental section
3.1. Effect of KI
Solution of native BSA and BSA Au nanoclusters were treated with different concentrations of potassium iodide (KI), which is a known quencher for fluorophores. We measured the effect of KI on the fluorescence lifetime of tryptophan of native BSA and BSA Au nanocluster when excited at tryptophan excitation wavelength. We also measured the effect of potassium iodide on the fluorescence lifetime of BSA Au nanocluster when clusters alone were excited away from tryptophan excitation. A 290 nm LED was used for the tryptophan excitation and a 375 nm laser diode was used as an excitation source to measure the fluorescence lifetime of BSA Au nanocluster. Stern –Volmer quenching data for tryptophan lifetime and fluorescence lifetime of BSA Au nanoclusters were obtained using different concentration of KI. The time resolved intensity decays for all the samples were analyzed using the exponential reconvolution procedure using non-liner regression (multiexponential deconvolution model). The fluorescence intensity decay for tryptophan was analyzed using:
The fluorescence intensity decay for BSA Au nanocluster was analyzed using:
Where IRF (t’) is the instrument response function at time t', α is the amplitude of the decay of the ith component at time t and τi is the lifetime of the ith component
3.2. Effect of Denaturant
We have used guanidine hydrochloride and urea to check the effect of protein denaturant on the tryptophan emission from both native BSA and BSA Au nanoclusters. We also checked their effect on the peak emission of BSA Au nanoclusters (650 nm). For this experiment we have added 0M to 6M of guanidine hydrochloride with an increment of 1M to both native BSA and BSA Au nanoclusters. It is known from the literature that 6M guanidine is enough to completely denature the structure of proteins like BSA [24]. Emission of tryptophan from both native BSA and BSA Au nanoclusters were measured following a 280 nm excitation. The peak emission of BSA Au nanoclusters was measured using a 360 nm excitation. Steady state emission intensity and peak emission wavelength was noted as a function of guanidine concentration.
3.3. Effect of Temperature
We also measured the effect of temperature on the fluorescence emission of tryptophan in native BSA and BSA Au nanoclusters and the peak emission of BSA Au nanoclusters. Native BSA and BSA Au nanoclusters were subjected to different temperatures ranging from 20°C to 70°C. The temperature in these solutions were maintained using a temperature controlled cuvette holder. It is known from the literature that the thermal denaturation of BSA starts at 58°C. BSA does not denature upto 40°C. Irreversible unfolding of α- helices starts occurring around 52–60°C. From 60°C onwards, unfolding of BSA progresses and β aggregation of the molecule begins. Above 70°C, the gel formation by unfolding of BSA molecule continues. Therefore, we have covered the entire range of temperature (20–70°C) to study the effect of temperature on the BSA template of BSA Au nanocluster [25,26]. Emission from the tryptophan was measured using a 280 nm excitation and the peak emission of BSA Au nanoclusters was measured using a 360 nm excitation. Emission intensity and the peak emission wavelength were measured as a function of change in the temperature.
3.4. Effect of pH
To check the effect of change in the pH, both native BSA and BSA Au nanoclusters were added to the premade water with pH djusted using sodium chloride (NaOH) or hydrochloric acid (HCL). The conformation of BSA can undergo various structural changes with change in pH of their environment. The native form is called “N” which predominate at neutral pH.. The basic form occurring around pH 8 is called “B”. “F” is for fast migrating form present at pH below 4.3, while “A” is for aged form produced at pH higher than 10. All these conformational state have their own molecular dimension and shape [27,28].
For native BSA and BSA Au nanocluster, emission intensity and emission wavelength from tryptophan was measured following a 280 nm excitation after adding the sample to the solutions of different pH. For BSA Au nanoclusters, the peak emission intensity and the peak emission wavelength were measured using a 360 nm excitation.
4. Results and Discussion
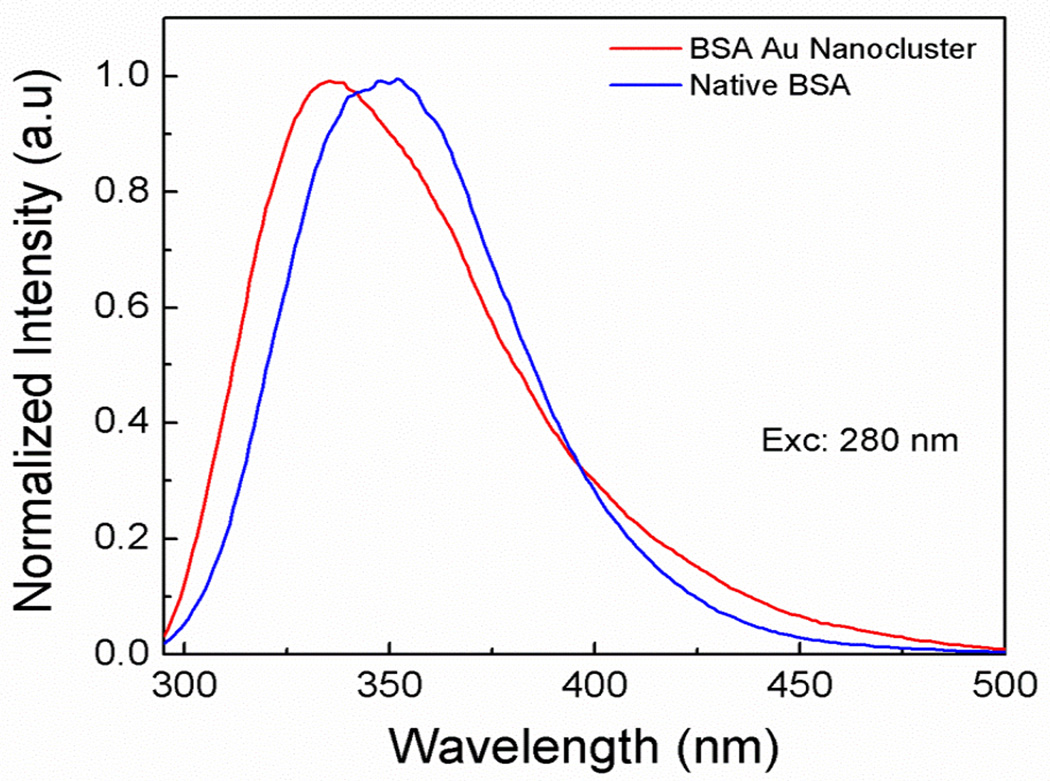
The absorption spectra of Native BSA and BSA Au nanocluster are shown in supplementary figure S1. As mentioned earlier, tryptophan is a polarity sensitive fluorophore and shows a red shift in the emission peak when exposed to the polar environment [29]. We observed that the fluorescence emission peak for the native BSA after a 280 nm excitation was at 345 nm whereas; the tryptophan fluorescence emission from the BSA Au nanoclusters shows a peak at 335 nm (figure 1). This shows that the tryptophans in the BSA Au nanoclusters are better shielded and is present in a more hydrophobic environment compared to the native BSA. This change change in tryptophans position/environment inside BSA occurred during formation of gold clusters and it’s stabilization with thiol bonds from BSA suggesting change in native BSA structure. Mohanty et al. has shown the changes in alpha helix content of BSA Au nanocluster compared to native BSA using CD spectroscopy. The result shows the alpha helix content to be 13.8% in BSA Au nanocluster compared to 64.5% in native BSA [30].
Figure 1.
Tryptophan emission spectra in native BSA and BSA Au nanoclusters using a 280 nm excitation.
4.1. Effect of quenchers
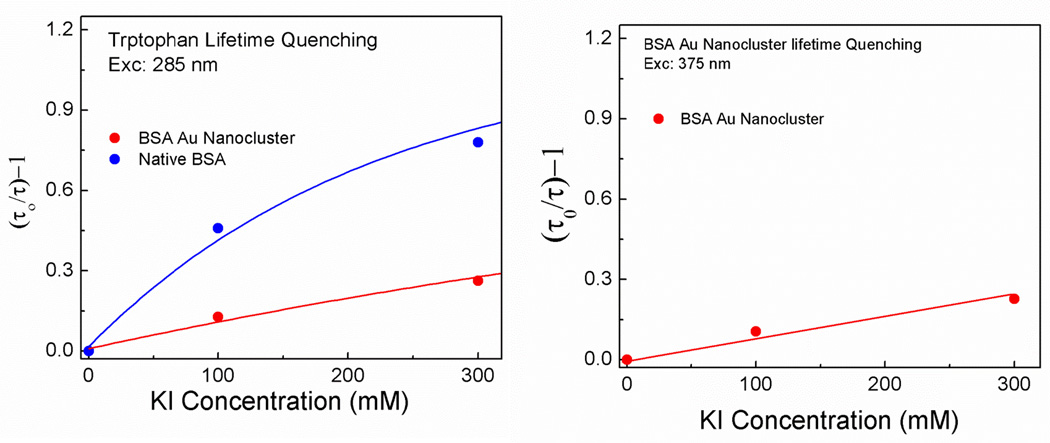
BSA contains two tryptophan residues that are located in two distinct environments. Each residue is differently accessible to the quenchers (potassium iodide) which affect its photophysical properties [31,32]. In figure 2 left panel, the Stern-Volmer plot of tryptophan lifetime for the native BSA and BSA Au nanoclusters after treatment with potassium iodide are shown (lifetime decay in supplementary data, figure S2). Two components were needed to fit the fluorescence decay of native BSA, on the other hand three components were needed to fit the data for BSA Au nanocluster. It is clear from the plot that potassium iodide (KI) efficiently quenches the tryptophan emission in native BSA compared to the tryptophan emission from the BSA Au nanoclusters suggesting that the tryptophan is well shielded inside the BSA gold cluster complex and can not be accessed easily. In figure 2 right panel, the Stern-Volmer plot for the emission intensity (650 nm) of BSA Au nanoclusters is shown (fluorescence decay in supplementary data, figure S3). Three components were needed to fit the lifetime decay of BSA Au nanocluster at its peak emission. It can be observed from the graph that the KI has a very little effect on the peak emission intensity of BSA Au nanoclusters due to the protein corona covering the gold clusters tightly. The steady state intensity change in tryptophan emission of the native BSA and BSA Au nanoclusters and the peak emission (650 nm) of the BSA Au nanoclusters are shown in supplementary data (Figure S4, S5 and S6). This experiment further confirms the shielding of tryptophan along with gold clusters inside BSA and increases the probability of tryptophan residues being present in close proximity to the gold atoms.
Figure 2.
Left panel- Stern-Volmer plot showing tryptophan quenching in native BSA and BSA Au clusters. Right panel- Stern- Volmer showing quenching of peak emission (650nm) of BSA Au nanoclusters.
4.2. Effect of denaturants
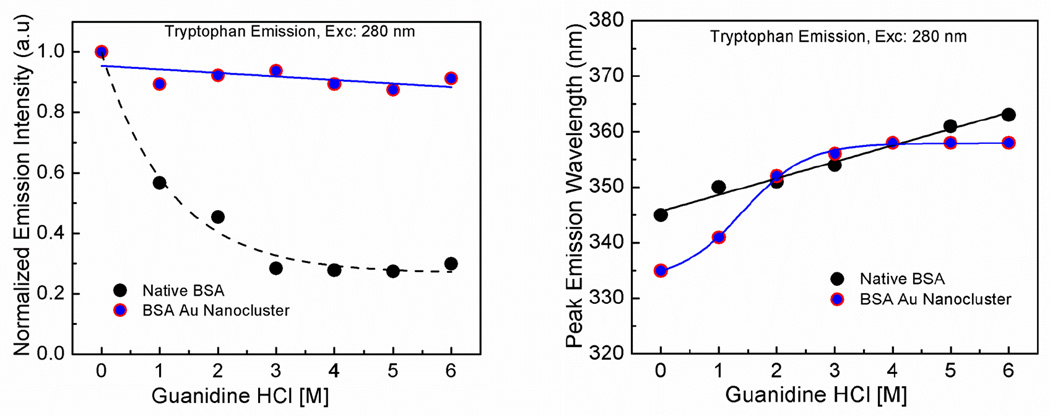
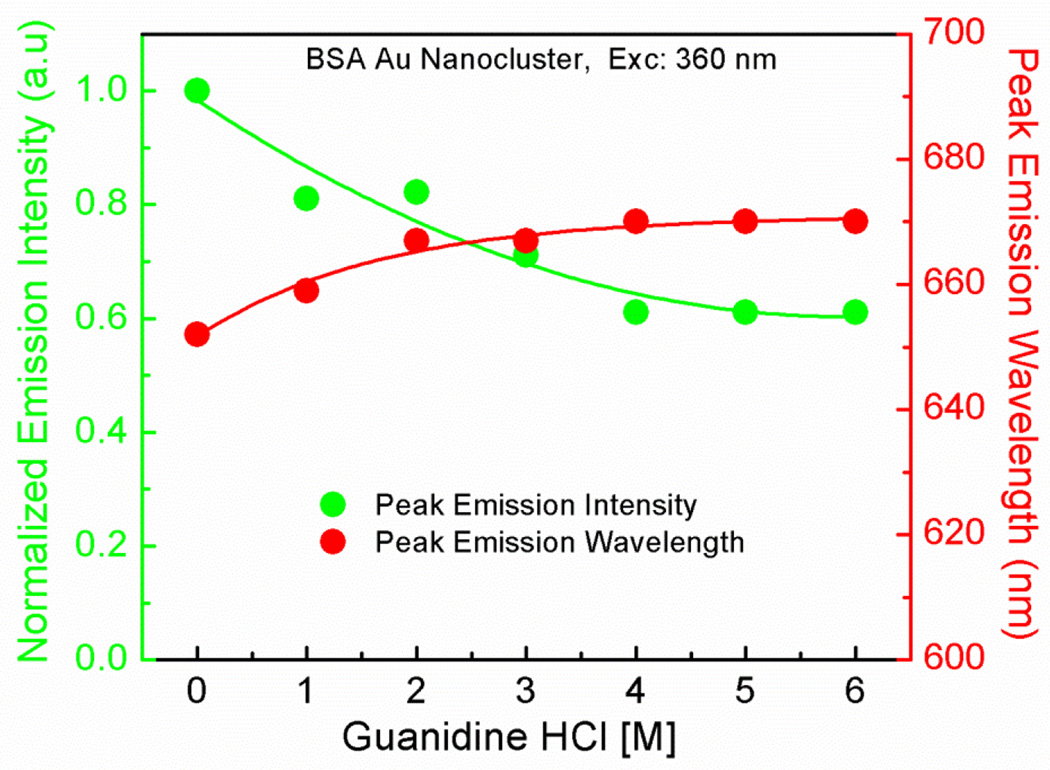
Guanidine hydrochloride is a known denaturant for proteins and hence widely used to study the chemical and physical properties of folded and unfolded proteins. Guanidine hydrochloride has the ability to weaken the non-covalent bonds and hence alter proteins structure and stability. It is assumed that the guanidine hydrochloride binds to the peptide bonds as the protein unfolds, more number of peptide bonds becomes accessible to the denaturant and hence they tend to lose their tertiary structure [33–37]. Here in this experiment, we have tried to observe the effect of guanidine hydrochloride on the fluorescence properties of native BSA and BSA Au nanoclusters. In figure 3 left panel, it can be observed that the native BSA shows about an 80% decrease in the peak emission intensity of the tryptophan due to significant unfolding and exposure of tryptophans to the aqueous environment whereas; there is only a slight change in the emission intensity of the tryptophan of the BSA Au nanoclusters due to the strong interaction of gold surface and thiol groups of the protein which resist the complete unfolding and may result in small structural changes in protein conformation. Figure 3 right panel shows the effect of guanidine on the peak emission wavelength of the tryptophan from the native BSA and BSA Au nanoclusters. As shown in the figure that both native BSA and BSA Au nanoclusters showed a small red shift when exposed to guanidine. This bathochromic shift in the tryptophan emission is due to the exposure of tryptophan molecule to a more polar environment [38]. In figure 4, we observed the effect of guanidine on the peak emission intensity and wavelength of BSA Au nanoclusters after 360 nm excitation. It was observed that there is 40% decrease in the peak emission intensity of BSA Au nanoclusters and a small red shift in the peak emission wavelength. This drop in emission intensity is due to partial unfolding of the BSA which will expose the gold cluster to surrounding solvent and oxygen dissolved in it. The drop in staeady state intensity conforms with the observation made by Mali et al. They had shown similar results in the fluorescence lifetime of BSA Au nanoclusters. They could see a 44% decrease in the fluorescence lifetime of BSA Au nanocluster with 8M guanidinium chloride suggesting the simultaneous change in fluorescence lifetime and qantum yeild [31]. Similar experiment was performed using urea. The data for the denaturant effect of urea is provided in the supplementary data (figure S7 and S8).
Figure 3.
Left panel- Emission intensity of tryptophan in native BSA and BSA Au nanoclusters as a function of guanidine concentration. Right Panel- Peak emission wavelength tryptophan in native BSA and BSA Au clusters as a function of guanidine concentration. Both measurements were done using a 280 nm excitation.
Figure 4.
Normalized emission intensity and Peak emission wavelength of BSA Au nanoclusters as a function of guanidine concentration using a 360 nm excitation.
4.3. Effect of temperature
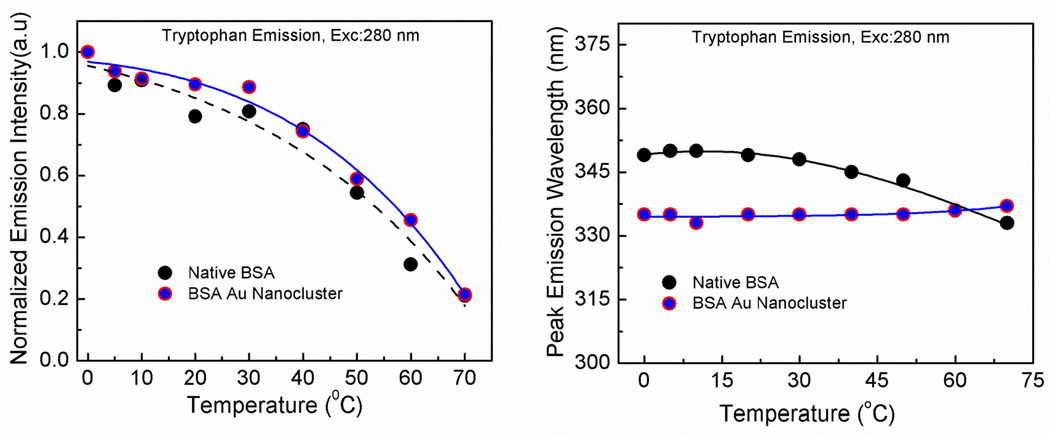
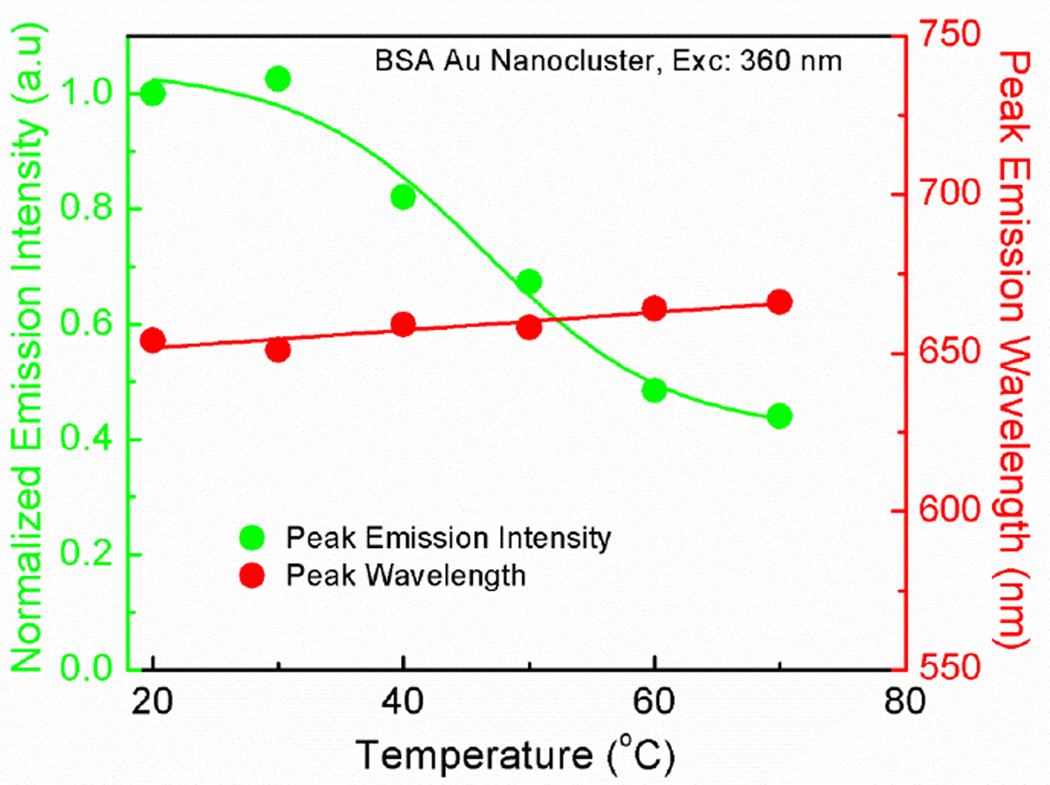
Temperature is known to produce a local change in the protein microenvironment. It is known that the heat disrupts the hydrogen bonds and the non-polar hydrophobic interactions, which can produce a local change in the tryptophan microenvironment and affects its spectroscopic properties [39,40]. In figure 5 left panel, we can observe the effect of increasing temperature on the emission intensity of the tryptophan from both native BSA and BSA Au nanoclusters. The emission intensity for the native BSA is decreased by about 80% along with a blue shift in the peak emission wavelength (figure 5 right panel). This blue shift and low intensity is due the change in the local hydrophobicity along with decrease in quantum yield (due to intramolecular quenching) with an increasing temperatures. We also measured the effect of temeperature on the fluorescence lifetime of tryptophan in native BSA and BSA Au nanocluster (Supplimentary data, figure S9). Three components were needed to fit the lifetime decay of tryptophan for both native BSA and BSA Au nanocluster. A decrease in fluorescence lifetime with increasing temperature was observed for both native BSA and BSA Au nanocluster. In case of BSA Au nanoclusters, it appears that the emission intensity of the tryptophan decreases with an increase in temperature. The peak emission wavelength of the tryptophan from BSA Au nanoclusters showed a small red shift suggesting temperature induced small conformational change. In figure 6, we observed the effect of the temperature on the peak emission intensity and wavelength following a 360 nm excitation. It is observed from the figure that with an increase in temperature, there is a decrease in the emission intensity whereas, peak emission wavelength remained constant. This decrease in emission intensity is due to the decrease in the quantum yield with an increasing temperature. To confirm this, we also measured the effect of increasing temperature on the fluorescence lifetime of BSA Au nanocluster. It was observed that with increasing temperature, there was a decrease in fluorescence lifetime (Supplementary data, figure S10). Three components were needed to fit the fluorescence decay of BSA Au nanocluster when observed at peak. Similar result was reported by Zhang et al. in which they have shown the temperature induced changes on the steady state emission intensity of BSA Au nanocluster following an excitation at 500 nm however they did not measure the lifetimes. However, there was no report on the tempetature induced effect on the tryotophan emission of these BSA Au nanoclusters [41]. Wen et al. had shown the fluorescence origin of BSA Au nanocluster using temperature dependent fluorescence. They proved that the structure of BSA Au nanocluster consistes of two bands. The band I originates from the icosahedral core of 13 Au(0) atoms. A red shift in emission upon increasing temperature is due to electron-phonon and surface scattering. The band II arise from the [-S-Au(I)-S-Au(I)-S] semirings [42,43].
Figure 5.
Left panel- Emission intensity change as a function of temperature for tryptophan in native BSA and BSA Au nanoclusters. Right panel- Peak wavelength change as a function of temperature for the tryptophan in native BSA and BSA Au nanoclusters.
Figure 6.
Emission intensity change and the peak emission wavelength as a function of temperature for tryptophan in BSA Au nanoclusters.
4.4. Effect of pH
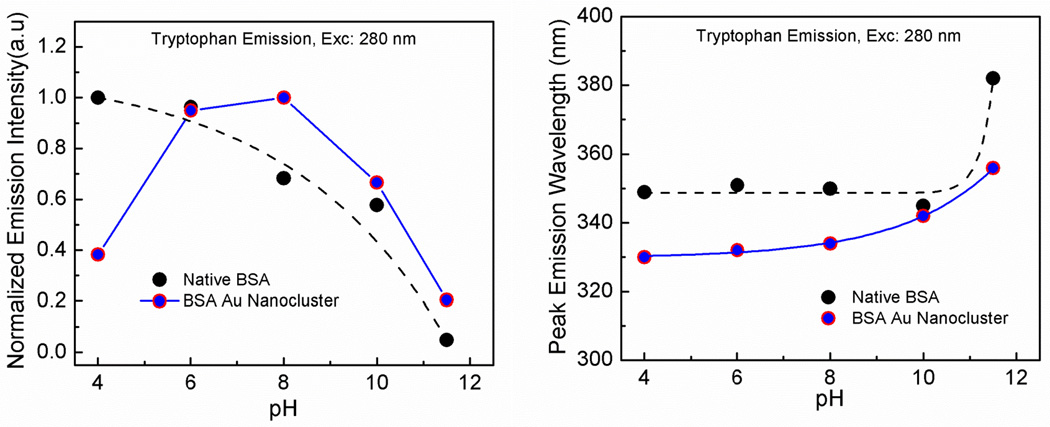
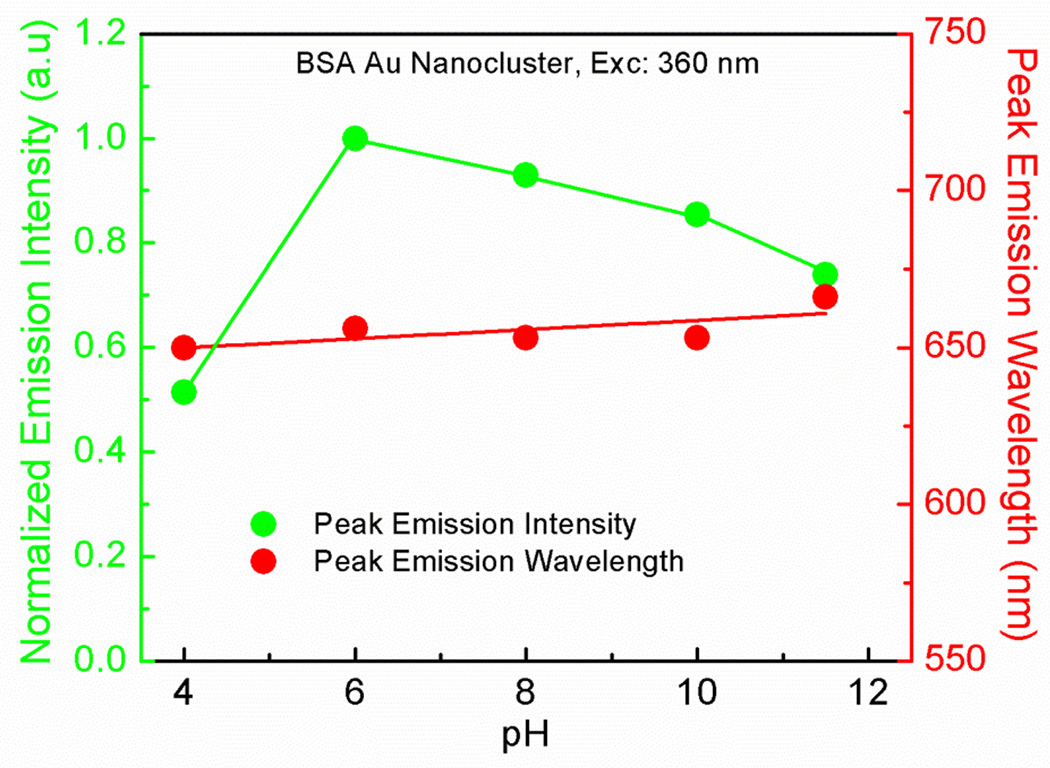
It is known that the pH of the solution has a significant role in maintaining the structural integrity of the proteins [44]. Therefore, we measured the effect of pH on the emission profile of tryptophan of native BSA and BSA Au nanoclusters following a 280 nm exciation light. We also observed the effect of the pH on the peak emission intensity and wavelength of BSA Au nanoclusters after 360 nm excitation. In figure 7 left panel, it can be seen that there is a change in the emission intensity of the tryptophan from both native BSA and BSA Au nanoclusters with an increase in pH. Native BSA showed a decrease in the emission intensity with an increase in pH. In case of BSA Au nanoclusters there was a decrease in the emission intensity of the tryptophan from pH 6 to 12. Lower intensity at pH 4, is due to the visible aggregation of the protein in the solution as isoelectric point of the BSA lies very close to this pH. In fig 7 right panel, for native BSA, it was observed that there was no change in the peak emission wavelength until pH 10 and after that there was a sudden red shift suggesting protein unfolding and exposure of the tryptophan to a more polar/aqueous environment. In case of BSA Au nanoclusters there was a red shift with an increasing pH for the peak tryptophan emission wavelength after 280 nm excitation pointing towads reshuffling of amino acids chains making tryptophan accessible for polar environment. Figure 8 shows the effect of pH on the peak emission intensity and wavelength of BSA Au nanoclusters after 360 nm excitation. Emission intensity decreases slightly from pH 6 to 12 whereas there was no significant change in the peak emission wavelength. Cao et al. reported a similar decrease in the tryptophan emission with an increasing pH. However, they reported an increase in the peak emission intensity of BSA Au nanocluster following excitation at 500nm for pH 2–11 which is not in accordance with our results [45]. One would expect a decrease in the fluorescence intensity of the BSA Au nanocluster with increasing pH suggesting protein unfolding at higher pH. Wen et al. reported quantum confined stark effect in Au8 and Au25 nanocluster and suggested that ultrasmall size gold nanocluster can be used for probing electric field or pH sensing in microenvironment of biological systems[46] and hence this information is useful.
Figure 7.
Left panel- Emission intensity change as a function of pH for tryptophan in native BSA and BSA Au nanoclusters using a 280 nm excitation. Right panel- Peak wavelength change as a function of pH for tryptophan in native BSA and BSA Au clusters.
Figure 8.
Emission intensity change and change in peak emission wavelength as a function of pH for BSA Au nanoclusters.
5. Conclusions
In this paper we have experimentally measured the effect of quenchers, denaturants, temperature and pH on the emission intensity and emission wavelength of native BSA and BSA Au nanoclusters. Native BSA showed drop in tryptophan fluorescence and red shifted emission at higher pH, denaturant concentration, temperature, guanidine concentrations due to unfolding of the protein. However, all these effects were less prominent in case of BSA Au cluster suggesting the strong interaction of thiol bonds with gold surface. We have also observed some changes in the the peak emission intensity and emission wavelength of BSA Au Nanocluster following a 360 nm excitation. Robustness of such hybrid flurophores to the changes in its environment is an important information in exploring the biochemical applications of such flurophores.
Supplementary Material
Highlights.
Tryptophan is easily accessible in native BSA compared to BSA Au nanoclusters.
Guanidine HCL denatures native BSA more compared to BSA Au nanoclusters.
High temperature decreases the quantum yield of tryptophan and BSA Au nanocluster.
Emission wavelength of BSA Au nanoclusters remains constant with increasing pH.
BSA Au nanoclusters are robust to the changes in their environments.
ACKNOWLEDGEMENTS
This work was supported by the NIH grant R01EB12003 (Z.G) and NSF grant CBET-1264608 (I.G).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen L, Wang C, Yuan Z, Chang H. Fluorescent Gold Nanoclusters: Recent Advances in Sensing and Imaging. Anal. Chem. 2014 doi: 10.1021/ac503636j. [DOI] [PubMed] [Google Scholar]
- 2.Chevrier DM, Chatt A, Zhang P. Properties and applications of protein-stabilized fluorescent gold nanoclusters: short review. Journal of Nanophotonics. 2012;6(1) 064504,1-064504-16. [Google Scholar]
- 3.Lin CJ, Lee C, Hsieh J, Wang H, Li JK, Shen J, et al. Review: Synthesis of fluorescent metallic nanoclusters toward biomedical application: recent progress and present challenges. J Med Biol Eng. 2009;29(6):276–283. [Google Scholar]
- 4.Söptei B, Nagy LN, Baranyai P, Szabó I, Mező G, Hudecz F, et al. On the selection and design of proteins and peptide derivatives for the production of photoluminescent, red-emitting gold quantum clusters. Gold Bulletin. 2013;46(3):195–203. [Google Scholar]
- 5.Wen X, Yu P, Toh Y, Hsu A, Lee Y, Tang J. Fluorescence Dynamics in BSA-Protected Au25 Nanoclusters. The Journal of Physical Chemistry C. 2012;116(35):19032–19038. [Google Scholar]
- 6.Wilcoxon J, Abrams B. Synthesis, structure and properties of metal nanoclusters. Chem. Soc. Rev. 2006;35(11):1162–1194. doi: 10.1039/b517312b. [DOI] [PubMed] [Google Scholar]
- 7.Xavier PL, Chaudhari K, Baksi A, Pradeep T. Protein-protected luminescent noble metal quantum clusters: an emerging trend in atomic cluster nanoscience. Nano reviews. 2012;3:14767_1–14767_16. doi: 10.3402/nano.v3i0.14767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu H, Suslick KS. Water-Soluble Fluorescent Silver Nanoclusters. Adv Mater. 2010;22(10):1078–1082. doi: 10.1002/adma.200904199. [DOI] [PubMed] [Google Scholar]
- 9.Xie J, Zheng Y, Ying JY. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009;131(3):888–889. doi: 10.1021/ja806804u. [DOI] [PubMed] [Google Scholar]
- 10.Chen T, Tseng W. (Lysozyme Type VI)-Stabilized Au8 Clusters: Synthesis Mechanism and Application for Sensing of Glutathione in a Single Drop of Blood. Small. 2012;8(12):1912–1919. doi: 10.1002/smll.201102741. [DOI] [PubMed] [Google Scholar]
- 11.Hu L, Han S, Parveen S, Yuan Y, Zhang L, Xu G. Highly sensitive fluorescent detection of trypsin based on BSA-stabilized gold nanoclusters. Biosensors and Bioelectronics. 2012;32(1):297–299. doi: 10.1016/j.bios.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Le Guével X, Daum N, Schneider M. Synthesis and characterization of human transferrin-stabilized gold nanoclusters. Nanotechnology. 2011;22(27):275103. doi: 10.1088/0957-4484/22/27/275103. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Zhang M, Wu Y. Temperature-induced optical property and conformational change of BSA-protected gold nanoclusters. J. Mol. Struct. 2014;1069:245–250. [Google Scholar]
- 14.Raut S, Chib R, Rich R, Shumilov D, Gryczynski Z, Gryczynski I. Polarization properties of fluorescent BSA protected Au 25 nanoclusters. Nanoscale. 2013;5(8):3441–3446. doi: 10.1039/c3nr34152f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raut S, Chib R, Butler S, Borejdo J, Gryczynski Z, Gryczynski I. Evidence of energy transfer from tryptophan to BSA/HSA protected gold nanoclusters. Methods and Applications in Fluorescence. 2014;2(3):035004. doi: 10.1088/2050-6120/2/3/035004. [DOI] [PubMed] [Google Scholar]
- 16.Raut SL, Shumilov D, Chib R, Rich R, Gryczynski Z, Gryczynski I. Two photon induced luminescence of BSA protected gold clusters. Chemical Physics Letters. 2013;561:74–76. doi: 10.1016/j.cplett.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raut S, Fudala R, Rich R, Kokate R, Chib R, Gryczynski Z, et al. Long lived BSA Au clusters as a time gated intensity imaging probe. Nanoscale. 2014;6(5):2594–2597. doi: 10.1039/c3nr05692a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu P, Wen X, Toh Y, Huang J, Tang J. Metallophilic Bond-Induced Quenching of Delayed Fluorescence in Au25@ BSA Nanoclusters. Particle & Particle Systems Characterization. 2013;30(5):467–472. [Google Scholar]
- 19.Hu D, Sheng Z, Gong P, Zhang P, Cai L. Highly selective fluorescent sensors for Hg2 based on bovine serum albumin-capped gold nanoclusters. Analyst. 2010;135(6):1411–1416. doi: 10.1039/c000589d. [DOI] [PubMed] [Google Scholar]
- 20.Le Guével X, Hötzer B, Jung G, Hollemeyer K, Trouillet V, Schneider M. Formation of fluorescent metal (Au, Ag) nanoclusters capped in bovine serum albumin followed by fluorescence and spectroscopy. The Journal of Physical Chemistry C. 2011;115(22):10955–10963. [Google Scholar]
- 21.Lin CJ, Yang T, Lee C, Huang SH, Sperling RA, Zanella M, et al. Synthesis, characterization, and bioconjugation of fluorescent gold nanoclusters toward biological labeling applications. ACS nano. 2009;3(2):395–401. doi: 10.1021/nn800632j. [DOI] [PubMed] [Google Scholar]
- 22.Retnakumari A, Setua S, Menon D, Ravindran P, Muhammed H, Pradeep T, et al. Molecular-receptor-specific, non-toxic, near-infrared-emitting Au cluster-protein nanoconjugates for targeted cancer imaging. Nanotechnology. 2010;21(5):055103_1–055103_12. doi: 10.1088/0957-4484/21/5/055103. [DOI] [PubMed] [Google Scholar]
- 23.Durgadas C, Sharma C, Sreenivasan K. Fluorescent gold clusters as nanosensors for copper ions in live cells. Analyst. 2011;136(5):933–940. doi: 10.1039/c0an00424c. [DOI] [PubMed] [Google Scholar]
- 24.Adman E, Jensen L. Structural features of azurin at 2.7 Å resolution. Isr. J. Chem. 1981;21(1):8–12. [Google Scholar]
- 25.Zhang L, Zhang M, Wu Y. Temperature-induced optical property and conformational change of BSA-protected gold nanoclusters. J. Mol. Struct. 2014;1069:245–250. [Google Scholar]
- 26.Murayama K, Tomida M. Heat-induced secondary structure and conformation change of bovine serum albumin investigated by Fourier transform infrared spectroscopy. Biochemistry (N.Y.) 2004;43(36):11526–11532. doi: 10.1021/bi0489154. [DOI] [PubMed] [Google Scholar]
- 27.Sen P, Ahmad B, Khan RH. Formation of a molten globule like state in bovine serum albumin at alkaline pH. European Biophysics Journal. 2008;37(8):1303–1308. doi: 10.1007/s00249-008-0335-7. [DOI] [PubMed] [Google Scholar]
- 28.Curvale RA. Buffer capacity of bovine serum albumin (BSA) J Argent Chem Soc. 2009;97(1):174–180. [Google Scholar]
- 29.Albani J. Origin of tryptophan fluorescence lifetimes. Part 2: Fluorescence lifetimes origin of tryptophan in proteins. J. Fluoresc. 2014;24(1):105–117. doi: 10.1007/s10895-013-1274-y. [DOI] [PubMed] [Google Scholar]
- 30.Mohanty JS, Xavier PL, Chaudhari K, Bootharaju M, Goswami N, Pal S, et al. Luminescent, bimetallic AuAg alloy quantum clusters in protein templates. Nanoscale. 2012;4(14):4255–4262. doi: 10.1039/c2nr30729d. [DOI] [PubMed] [Google Scholar]
- 31.Lehrer S. Solute perturbation of protein fluorescence. Quenching of the tryptophyl fluorescence of model compounds and of lysozyme by iodide ion. Biochemistry (N.Y.) 1971;10(17):3254–3263. doi: 10.1021/bi00793a015. [DOI] [PubMed] [Google Scholar]
- 32.Möller M, Denicola A. Protein tryptophan accessibility studied by fluorescence quenching. Biochemistry and Molecular Biology Education. 2002;30(3):175–178. [Google Scholar]
- 33.Mayo SL, Baldwin RL. Guanidinium chloride induction of partial unfolding in amide proton exchange in RNase A. SCIENCE-NEW YORK THEN WASHINGTON. 1993;262 doi: 10.1126/science.8235609. 873-. [DOI] [PubMed] [Google Scholar]
- 34.Monera OD, Kay CM, Hodges RS. Protein denaturation with guanidine hydrochloride or urea provides a different estimate of stability depending on the contributions of electrostatic interactions. Protein Science. 1994;3(11):1984–1991. doi: 10.1002/pro.5560031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson DR, Jencks WP. The Effect of Compounds of the Urea-Guanidinium Class on the Activity Coefficient of Acetyltetraglycine Ethyl Ester and Related Compounds1. J. Am. Chem. Soc. 1965;87(11):2462–2470. doi: 10.1021/ja01089a028. [DOI] [PubMed] [Google Scholar]
- 36.Roseman M, Jencks WP. Interactions of urea and other polar compounds in water. J. Am. Chem. Soc. 1975;97(3):631–640. [Google Scholar]
- 37.Sułkowska A, Rownicka J, Bojko B, Poźycka J, Zubik-Skupień I, Sułkowski W. Effect of guanidine hydrochloride on bovine serum albumin complex with antithyroid drugs: fluorescence study. J. Mol. Struct. 2004;704(1):291–295. [Google Scholar]
- 38.Lakowicz JR. Principles of Fluorescence Spectroscopy. Third ed. Springer; 2006. [Google Scholar]
- 39.Gally J, Edelman G. The effect of temperature on the fluorescence of some aromatic amino acids and proteins. Biochim. Biophys. Acta. 1962;60(3):499–509. doi: 10.1016/0006-3002(62)90869-7. [DOI] [PubMed] [Google Scholar]
- 40.Demchenko AP, Ladokhin AS. Temperature-dependent shift of fluorescence spectra without conformational changes in protein; studies of dipole relaxation in the melittin molecule. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology. 1988;955(3):352–360. doi: 10.1016/0167-4838(88)90215-4. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Zhang M, Wu Y. Temperature-induced optical property and conformational change of BSA-protected gold nanoclusters. J. Mol. Struct. 2014;1069:245–250. [Google Scholar]
- 42.Wen X, Yu P, Toh Y, Tang J. Structure-correlated dual fluorescent bands in BSA-protected Au25 nanoclusters. The Journal of Physical Chemistry C. 2012;116(21):11830–11836. [Google Scholar]
- 43.Yu P, Wen X, Toh Y, Ma X, Tang J. Fluorescent Metallic Nanoclusters: Electron Dynamics, Structure, and Applications. Particle & Particle Systems Characterization. 2015;32(2):142–163. [Google Scholar]
- 44.Monahan FJ, German JB, Kinsella JE. Effect of pH and temperature on protein unfolding and thiol/disulfide interchange reactions during heat-induced gelation of whey proteins. J. Agric. Food Chem. 1995;43(1):46–52. [Google Scholar]
- 45.Cao X, Li H, Yue Y, Wu Y. pH-Induced conformational changes of BSA in fluorescent AuNCs@ BSA and its effects on NCs emission. Vibrational Spectroscopy. 2013;65:186–192. [Google Scholar]
- 46.Wen X, Yu P, Toh Y, Tang J. Quantum Confined Stark Effect in Au8 and Au25 Nanoclusters. The Journal of Physical Chemistry C. 2013;117(7):3621–3626. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.