Abstract
Objective
To determine if rapamycin inhibits the growth, function, and metabolism of human laryngotracheal stenosis (LTS)–derived fibroblasts.
Study Design
Controlled in vitro study.
Setting
Tertiary care hospital in a research university.
Subjects and Methods
Fibroblasts isolated from biopsies of 5 patients with laryngotracheal stenosis were cultured. Cell proliferation, histology, gene expression, and cellular metabolism of LTS-derived fibroblasts were assessed in 4 conditions: (1) fibroblast growth medium, (2) fibroblast growth medium with dimethylsulfoxide (DMSO), (3) fibroblast growth medium with 10−10 M (low-dose) rapamycin dissolved in DMSO, and (4) fibroblast growth medium with 10−9 M (high-dose) rapamycin dissolved in DMSO.
Results
The LTS fibroblast count and DNA concentration were reduced after treatment with high-dose rapamycin compared to DMSO (P = .0007) and normal (P = .0007) controls. Collagen I expression decreased after treatment with high-dose rapamycin versus control (P = .0051) and DMSO (P = .0093) controls. Maximal respiration decreased to 68.6 pMoles of oxygen/min/10 mg/protein from 96.9 for DMSO (P = .0002) and 97.0 for normal (P = .0022) controls. Adenosine triphosphate (ATP) production decreased to 66.8 pMoles from 88.1 for DMSO (P = .0006) and 83.3 for normal (P = .0003) controls. Basal respiration decreased to 78.6 pMoles from 108 for DMSO (P = .0002) and 101 for normal (P = .0014) controls.
Conclusions
Rapamycin demonstrated an anti-fibroblast effect by significantly reducing the proliferation, metabolism, and collagen deposition of human LTS fibroblast in vitro. Rapamycin significantly decreased oxidative phosphorylation of LTS fibroblasts, suggesting at a potential mechanism for the reduced proliferation and differentiation. Furthermore, rapamycin’s anti-fibroblast effects indicate a promising adjuvant therapy for the treatment of laryngotracheal stenosis.
Keywords: human, trachea, laryngotracheal stenosis, rapamycin, fibroblasts, collagen
Introduction
Laryngotracheal stenosis (LTS) is a condition characterized by a buildup of pathologic fibrotic tissue in the airway leading to a narrowing in the tracheal opening. LTS can adversely affect voice and breathing and can progress to life-threatening airway compromise, necessitating tracheostomy or multiple surgical procedures.1 LTS has multiple etiologies, including iatrogenic tracheostomy tube trauma or long-term endotracheal tube intubation, autoimmune disease, radiation, and idiopathic.1–4 It has been hypothesized that LTS may be propagated by an abnormal immune response in which injury to the epithelium and lamina propria of the subglottis and trachea spurs subepithelial leukocyte activity, edema, and chronic inflammation.5,6 The sustained inflammation results in fibroblast proliferation, collagen deposition, and ultimately the formation of scar tissue in the airway.5–7
Current medical therapies are not successful in halting the development of laryngotracheal scar tissue. Surgical procedures, including endoscopic excision and dilation, tracheal/cricotracheal resection, and tracheostomy remain mainstay therapy, with adjuvant therapies including local and systemic steroid treatment, Mitomycin C, antibiotics, and anti-inflammatory medication.8–12 Evidence for each of these medical therapies is inconsistent, demonstrating the need for rational new therapies to address scar tissue formation in the larynx and trachea.
Rapamycin (sirolimus, rapamine) is a macrocyclic antibiotic produced by the bacteria streptomyces hygroscopicus that functions as an immunosuppressive medication.13–15 Rapamycin complexes with another protein to inhibit the mammalian target of rapamycin (mTor) and prevents activation of lymphocytes and other immune cells, thereby inhibiting the normal immune response.13,16,17 It is an FDA-approved drug that is frequently used as an antirejection agent for transplants as well as in coronary stents to reduce restenosis and accelerated arteriopathy.14,18,19 There is also evidence to suggest that rapamycin is effective in reducing fibrosis and proliferation in a variety of different cell tissue types, including dermal, hepatic, and renal fibroblasts.20–22
The aim of this study was to determine whether rapamycin could inhibit human LTS fibroblast proliferation in vitro. Fibroblast proliferation is a crucial step for development of LTS, and collagen is usually implicated in deposition of scar tissue and in fibrosis. Therefore, we hypothesized that rapamycin would decrease LTS fibroblast proliferation and metabolism and reduce collagen production. We characterized the effect of rapamycin on fibroblasts through growth kinetics, morphology, gene expression, and cellular metabolism profile.
Materials and Methods
Laryngotracheal Fibroblast Isolation and Culture
Biopsy of human laryngotracheal stenosis was performed during routine suspension microlaryngoscopy, bronchoscopy, excision, and dilation of subglottic/tracheal stenosis in 5 patients. Informed consent was obtained from all patients to join this study, which was approved by the Johns Hopkins Institutional Review Board (NA_00078310). Immediately after excision, tissue samples were placed in phosphate-buffered saline (PBS; Gibco Life Technologies by Invitrogen, Grand Island, New York, USA) supplemented with 5% penicillin/streptomycin (Gibco). Biopsy specimens were minced, and the tissue fragments were spread out evenly across uncoated plastic tissue culture flasks (BD Biosciences, San Jose, California, USA) and suspended in fibroblast growth medium at 37°C in 5% CO2-humidified atmosphere. Fibroblast growth medium consisted of Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (FBS; HyClone by Thermo Scientific, Logan, Utah, USA), 100 U/mL penicillin (Gibco), 100 ug/mL streptomycin (Gibco), and 100× nonessential amino acids (NEAA; Gibco). Once cells were confluent (between 20–30 days), short-term 0.25% Trypsin-EDTA treatment (Gibco) proved effective in releasing only fibroblasts from the flasks, which were then plated into new flasks for expansion. Passage 2 or 3 of these primary fibroblast cell lines were used in the following experiments. Fibroblast presence was confirmed in culture with positive immunohistochemical staining of the anti-fibroblast antibody ER-TR7 (Santa Cruz Biotechnology, Inc, Dallas, Texas, USA) and negative staining for epithelial cells using the anti-human panCytokeratin antibody AF488 (Becton-Dickenson, Franklin Lakes, New Jersey, USA).
Cell Proliferation by Cell Count and DNA Assay
After reaching confluence, the adherent LTS fibroblasts were trypsinized and passaged into 12-well culture plates (Falcon by BD Biosciences) containing fibroblast growth medium and seeded with 20,000 cells per well. Medium was aspirated and replaced daily with the following conditions: (1) control, normal fibroblast growth medium; (2) control-vehicle, medium containing vehicle solution, 1% dimethylsulfoxide (DMSO); (3) rapamycin-1, medium containing rapamycin (Sigma-Aldrich, St. Louis, Missouri, USA) dissolved in DMSO at a concentration of 1ng/mL or 10−10 M; (4) rapamycin-10, medium containing rapamycin dissolved in DMSO at a concentration of 10 ng/mL or 10−9 M. These 2 concentrations were chosen due to their low and inhibitory doses in previous reports and order of magnitude difference between the doses.23,24 All conditions were grown in triplicate. Cells were harvested on the sixth day of incubation. Prior to DNA assay, cell counts were performed on the aforementioned cells; twice on each well of trypsinized LTS fibroblasts. Then the LTS fibroblasts of each well were lysed (10 mM Tris, 1 mM EDTA, 0.1% Triton X-100 and 0.1 mg/ml Proteinase K), and DNA concentration was determined using the PicoGreen fluorescent method,25 adapted from the manufacturer’s protocol (Invitrogen Quant-iT PicoGreen dsDNA Reagent and Kits Protocol, Molecular Probes P11496). Each sample was run in duplicate for the assay. Fluorescence was read using the BioTek Synergy 2 Microplate Reader (BioTek Instruments, Winooski, Vermont, USA).
Cell Staining and Histology
LTS fibroblasts were seeded in the 4 medium conditions on microscope cover glass slips (Fisher Scientific, Hampton, New Hampshire, USA) that were pretreated with 0.01% gelatin solution, dried, and sterilized. After 6 days, the LTS fibroblast glass cover slips were stained with Masson’s Trichrome in order to visually assess differences in collagen deposition among the 4 medium conditions. The fibroblast glass cover slips were fixed with 10% formalin and stained following the manufacturer’s instructions (Sigma-Aldrich #HT15 kit). Microscopic images of the stained sections were taken and analyzed with a Zeiss AX10 microscope (20× magnification, Carl Zeiss, Oberkochen, Germany).
Gene Expression Analysis by Real-time Polymerase Chain Reaction
LTS fibroblasts were seeded in 6-well culture plates (Falcon by BD Biosciences) with 300,000 cells per well and grown in the 4 medium conditions in duplicate for 6 days. The RNeasy Micro Kit (Qiagen, Valencia, California, USA) was used for RNA extraction of each well, and the RNA was quantitated using the nanodrop 2000 spectrophotometer (Thermo Scientific, Waltham, Massachusetts, USA). The iScript cDNA synthesis kit (BioRad, Hercules, California, USA) was used for cDNA synthesis according to the manufacturer’s protocol. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed and monitored using the SYBR Green PCR Mastermix (Life Tech, Carlsbad, California, USA) on the StepOnePlus Real Time PCR System (Life Tech). Reaction mixtures were incubated for 10 minutes at 95°C followed by 40 cycles of 15 seconds at 95°C, 1 minute at 60°C, and finally 15 seconds at 95°C, 1 minute at 60°C, and 15 seconds at 95°C. For each sample, gene expression was corrected against Beta Actin level. All samples were run in duplicate. The level of expression of the target gene was calculated as 2ΔΔCt as previously described.26 The control LTS fibroblasts grown in normal growth medium were used for reference. Error was calculated by the standard deviation of ΔΔCT.27 The fibrosis marker, collagen I (Col I), was assessed as previously described.28
Cellular Metabolism Using Oxygen Consumption Rate Measurements
Bioenergetic analysis of oxygen consumption rate (OCR) provides a measurement of overall metabolic status of the cell.29 OCR measurements of LTS fibroblasts were completed using a Seahorse XF24 Flux Analyzer (Seahorse Bioscience, Billerica, Massachusetts, USA) at 4 × 104 cells per well. They were grown in 3 medium conditions for 2 days post seeding with respective medium changed daily. Due to constraints with the Seahorse experimental plate, only 3 conditions could be run simultaneously with sufficient replicates. The 3 medium conditions tested were the 2 control conditions and high-dose rapamycin. The assay used XF minimal assay medium supplemented with 25 mM glucose (Sigma), 1X GlutaMax (Invitrogen), and 1X NEAA (Invitrogen). Prior to the stress test, the cells were washed twice with this medium and incubated for 1 hour at 37°C in a humidified atmosphere without CO2. In order to evaluate the mitochondrial respiratory capacity, Oligomycin (an adenosine triphosphate [ATP] coupler), Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP, an ATP uncoupler), Rotenone and Antimycin (mitochondrial inhibitors) at 10 uM, 4 uM, and 20 uM concentration, respectively, were injected into the medium at 34, 72, and 110 minutes, respectively. PicoGreen DNA quantization assay was performed on each well in order to normalize the OCR. DNA content was correlated with the total protein amount per well. Basal metabolic rate, ATP production, and maximal respiratory capacity were measured.
Statistical Analysis
Results were expressed as averages ± standard error of the mean. Statistical analysis was performed using the Wilcoxin rank-sum test. To minimize type I error, we performed a Bonferonni correction and calculated our corrected alpha level to be 0.05/3 groups = 0.017, with P values < .05 considered significant (STATA; StataCorp LP; College Station, Texas, USA).
Results
Rapamycin Decreases LTS Fibroblast Proliferation
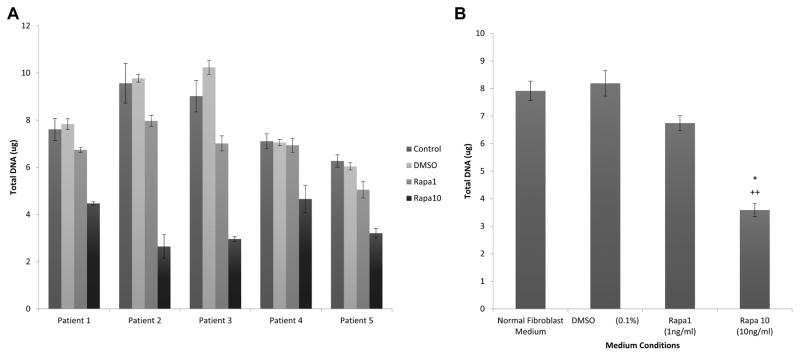
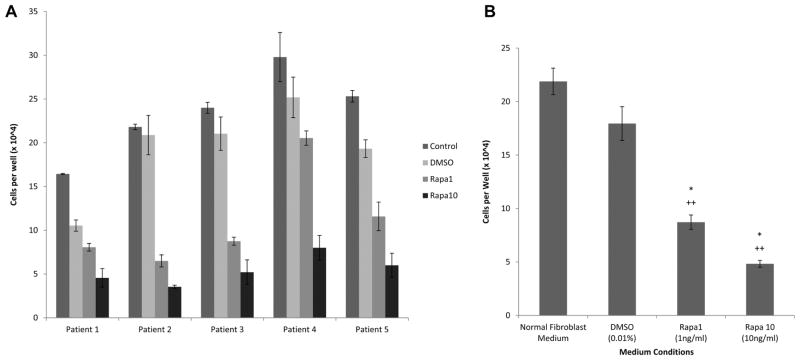
Increased rapamycin dosing showed a decreased LTS fibroblast growth rate in all patients (Figures 1A, 2A) as well as in the mean (Figures 1B, 2B) for all patients. Fibroblasts proliferated in all medium conditions, with fibroblasts cultured in normal medium (mean cell count at day 6 = 21.9 × 104, SD = 3.9 × 104) growing at a faster rate than fibroblasts cultured in DMSO (mean = 17.9 × 104, SD = 5.0 × 104), low-dose rapamycin (mean = 8.7 × 104, SD = 2.1 × 104) and high-dose rapamycin (mean = 4.8 × 104, SD = 1.0 × 104). This represented a significant decrease in individual and average LTS fibroblast count cultured with high-dose rapamycin versus DMSO vehicle control (P = .0007) and normal (P = .0007) medium. Low-dose rapamycin also demonstrated a significant reduction in fibroblast count when compared to control (P = .0008) and normal (P = .0007) medium. There was also a significant decrease in DNA concentration of individual (Figure 1A) and average (Figure 1B) LTS fibroblasts cultured with high-dose rapamycin versus DMSO vehicle control (P = .0007) and normal (P = .0007) medium. Furthermore, there was a significant reduction in DNA concentration for fibroblasts grown in low-dose rapamycin versus normal (P = .0026) and vehicle medium (P = .0015). The DNA per cell was not significantly different in fibroblasts cultured in different cell medium types.
Figure 1.
Rapamycin inhibits fibroblast proliferation. Rapamycin significantly reduced DNA in laryngotracheal stenosis (LTS)–derived fibroblasts (A) compared to controls. When averaged (B), DNA was reduced for low- and high-dose rapamycin versus normal (P = .0026, P = .0007) and dimethylsulfoxide (DMSO) vehicle (P = .0015, P = .0007) controls.
Figure 2.
Rapamycin reduces fibroblast count. Fibroblasts were significantly lower after rapamycin treatment (A) compared with controls. Average cell count (B) was reduced for low- and high-dose rapamycin versus normal (P = .0001, P = .0001) and dimethylsulfoxide (DMSO) (P = .0001, P = .0001) controls.
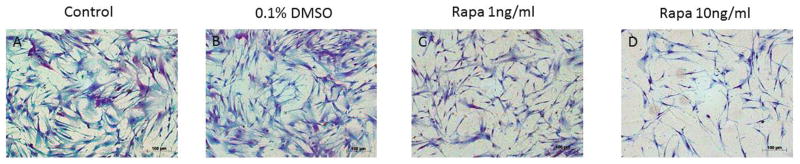
Rapamycin-treated Fibroblasts Demonstrate Reduced Collagen Staining
LTS fibroblasts were stained with Masson’s Trichrome in order to visualize collagen deposition, which stains blue. Histological evaluation of LTS cultured cells after treatment of rapamycin is shown in Figure 3.
Figure 3.
Fibroblast morphology. (A) Normal and (B) dimethylsulfoxide (DMSO) vehicle controls demonstrate confluent fibroblasts with intense blue staining for collagen compared with fibroblast proliferation and collagen deposition after treatment with (C) low- and (D) high-dose rapamycin.
Rapamycin-treated Fibroblasts Have Reduced Collagen I Expression
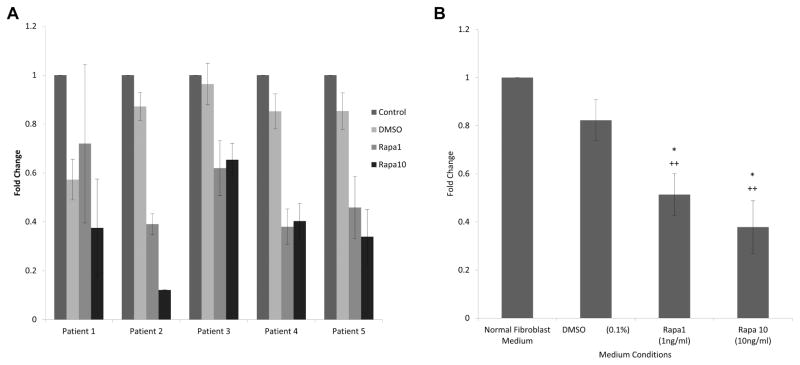
RT-PCR demonstrated decreased Col I gene expression in rapamycin-treated LTS fibroblasts compared to control LTS fibroblasts in all 5 patients (Figure 4A). The average of all 5 patients also showed a linear trend in which increasing rapamycin concentration resulted in a decrease in Col I gene expression (Figure 4B). There was a significant decrease in Col I expression between LTS fibroblasts grown in high-dose 10 ng/mL rapamycin and in control fibroblast medium (P = .0054) and in DMSO vehicle medium (P = .0102). There was a significant decrease in Col I expression in LTS fibroblasts grown in low-dose rapamycin versus normal medium (P = .0074), though not when compared with the vehicle medium (P = .0784) control.
Figure 4.
Rapamycin inhibits collagen 1 expression. (A) Four patients had significantly reduced collagen 1 expression after rapamycin treatment. When averaged (B), high-dose rapamycin reduced collagen 1 expression compared to normal (P = .0054) and dimethylsulfoxide (DMSO) (P = .0102) controls.
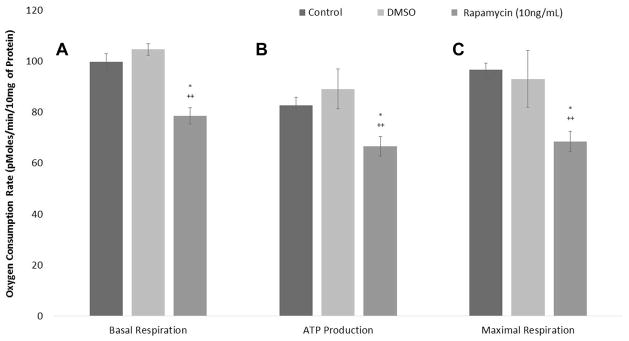
Rapamycin Decreases the Metabolic Rate of LTS Fibroblasts
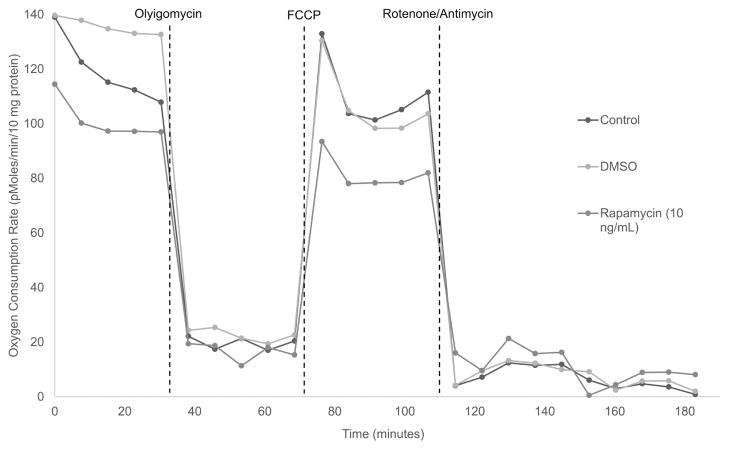
LTS patient-derived fibroblasts were less metabolically active than those grown in normal and vehicle medium controls (Figures 5, 6). When averaged together, there were significant decreases in basal respiration (Figure 5A), ATP production (Figure 5B), and maximal respiration (Figure 5C) between LTS fibroblasts treated with high-dose rapamycin and those cultured in DMSO control and fibroblast growth medium. Maximal respiration decreased to 68.6 pMoles of oxygen/min/10 mg of protein units from 96.9 pMoles for DMSO medium (P = .0002) and from 97.0 pMoles for the normal medium control (P = .0022). Similarly, ATP production decreased to 66.8 pMoles of oxygen/min/10 mg of protein units from 88.1 pMoles for the DMSO control (P = .0006) and from 83.3 for the normal medium control (P = .0003). Lastly, basal respiration decreased to 78.6 pMoles of oxygen/min/10 mg of protein units from 108 pMoles for DMSO medium (P = .0002) and from 101 pMoles in the normal medium control (P = .0014). Figure 6 demonstrates the difference in a representative patient’s fibroblast oxygen consumption rate between the 3 medium conditions: normal medium control, DMSO control, and high-dose rapamycin. High-dose rapamycin decreased the basal metabolic rate (difference in peak OCR prior to Oligomycin and lowest OCR post Rotenone/Antimycin) and ATP production (difference in OCR prior to and post Oligomycin injection) and decreased maximal respiratory capacity (difference between peak OCR post FCCP injection and the nonmitochondrial respiration) in LTS fibroblasts.
Figure 5.
Rapamycin decreases the metabolic rate of laryngotracheal stenosis (LTS) fibroblasts. (A) Mean basal respiration, (B) adenosine triphosphate (ATP) production, and (C) maximal respiration significantly decreased with high-dose rapamycin when compared to normal medium and vehicle medium controls.
Figure 6.
Oxygen consumption rate in representative patient-derived fibroblasts. LTS-derived fibroblasts were seeded in FGM, FGM + DMSO, and FGM + DMSO + rapamycin (10 ng/mL) medium. ATP production, basal, and FCCP-coupled OCR were significantly reduced with rapamycin treatment. ATP, adenosine triphosphate; DMSO, dimethylsulfoxide; FCCP, Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone; FGM, fibroblast growth medium; OCR, oxygen consumption rate.
Discussion
The results of this in vitro study suggest rapamycin as a potential anti-fibroblast therapy for LTS. As shown, we observed a decrease in LTS fibroblast proliferation, function, and metabolism with rapamycin. High-dose rapamycin demonstrated a significant reduction in every outcome measured as compared to vehicle medium and normal medium controls in the cells from the 5 patients included in this study. While the 10 ng/mL rapamycin concentration represented the “high dose” in this study, it is relatively low compared to other reports.23,24 Doses as high as 10 ug/mL inhibit fibroblast proliferation with minimal toxicity.23 The results in these 5 LTS patients are supported by rapamycin’s effect on fibroblasts in other organ systems, including the lung, liver, and skin, by decreasing expression and deposition of collagen type I and reducing cell proliferation.21,22,24 This study suggests at a potential mechanism by assessing cellular respiration in LTS fibroblasts, which showed a reduced mitochondrial metabolic rate after treatment with rapamycin.
Bioenergetic analysis is a novel technique that monitors the oxygen consumption and extracellular acidification at a cellular level.30–32 The key parameters of basal respiration, ATP production, and respiratory capacity may be measured to assess mitochondrial metabolism.30,32 Importantly, maximal respiratory capacity was significantly reduced in rapamycin-treated fibroblasts when nonmitochondrial respiration levels were normalized between control and rapamycin-treated groups. As seen in this study, the decreased basal respiration, decreased ATP production, and maximal respiratory capacity after treatment suggest that rapamycin interferes with mitochondrial energy production, resulting in decreased fibroblast function and collagen proliferation.
In clinical application for the treatment of LTS, we expect rapamycin to exert an immunosuppressive effect in addition to antiproliferative effects. Rapamycin has FDA approval for immunosuppression to prevent rejection in renal transplant patients and is used to coat coronary stents to reduce inflammation and collagen synthesis.15,33 Furthermore, rapamycin was shown to disrupt mTOR signaling in fibroblasts and macrophages and reduce kidney fibrosis.20 Therefore, we are encouraged by the potential of rapamycin treatment as adjuvant therapy for LTS. Modulating the immune response may be more effective than current medical therapies. In topical application, rapamycin could assist in suppressing the inflammatory response and fibroblast proliferation and collagen synthesis seen in LTS while avoiding side effects seen with systemic therapy. Ultimately, a rapamycin-eluting stent could direct sustained local immunotherapy and anti-fibroblast effect at the area of fibrosis while providing mechanical forces on the tracheal wall. Further in vivo investigation is required to support our data and hypothesis.
While this study demonstrates the benefits of rapamycin on human LTS cells, there are several limitations. It is an in vitro study of human cells, and the behavior of the LTS fibroblasts on culture dishes may not be representative of cells in vivo. While only passage 2 and 3 cells were used in experiments, the repeated passaging and freeze-thawing may have an effect on cell phenotype. Lastly, it is important to note that there was variability among LTS fibroblasts in the 5 different patients (Figures 1A, 2A, 4A, and 6) with varying response to rapamycin appreciated. This patient-specific variability in therapeutic response may be encountered with any clinical treatment and may be due to the heterogeneity of the disease process or possibly genetic differences in wound healing. We are encouraged by the significant anti-fibroblast effect of rapamycin when the results from the 5 patients with LTS were averaged.
In summary, rapamycin demonstrated an anti-fibroblast effect by reducing the proliferation, metabolism, and collagen deposition of human LTS fibroblast in vitro. The bioenergetic effects of rapamycin demonstrated a significant decrease in oxidative phosphorylation of LTS fibroblasts, suggesting at a potential mechanism for the reduced proliferation and differentiation. Rapamycin’s anti-fibroblast effects combined with its immunosuppressive action suggest at a promising adjuvant therapy for the treatment of laryngotracheal stenosis. Further studies are underway to compare the metabolism of normal and scar fibroblasts isolated from patients with LTS as well as to assess rapamycin’s effect on laryngotracheal stenosis in an in vivo model.
Footnotes
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: None.
No sponsorships or competing interests have been disclosed for this article.
Author Contributions
Daryan R. Namba, acquisition, drafting, approval, agreement; Garret Ma, acquisition, revising, approval, agreement; Idris Samad, acquisition, revising, approval, agreement; Dacheng Ding, acquisition, revising, approval, agreement; Vinciya Pandian, acquisition, revising, approval, agreement; Jonathan D. Powell, concept, revising, approval, agreement; Maureen R. Horton, concept, revising, approval, agreement; Alexander T. Hillel, concept, drafting, approval, agreement.
References
- 1.Hseu AF, Benninger MS, Haffey TM, Lorenz R. Subglottic stenosis: a ten-year review of treatment outcomes. Laryngoscope. 2014;124(3):736–741. doi: 10.1002/lary.24410. [DOI] [PubMed] [Google Scholar]
- 2.Cotton RT. Pediatric laryngotracheal stenosis. J Pediatr Surg. 1984;19:699–704. doi: 10.1016/s0022-3468(84)80355-3. [DOI] [PubMed] [Google Scholar]
- 3.Halmos GB, Schuiringa FS, Palinko D, van der Laan TP, Dikkers FG. Finding balance between minimally invasive surgery and laryngotracheal resection in the management of adult laryngotracheal stenosis. Eur Arch Otorhinolaryngol. 2014;271:1967–1971. doi: 10.1007/s00405-014-2901-1. [DOI] [PubMed] [Google Scholar]
- 4.Herrington HC, Weber SM, Andersen PE. Modern management of laryngotracheal stenosis. Laryngoscope. 2006;116:1553–1557. doi: 10.1097/01.mlg.0000228006.21941.12. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh A, Malaisrie N, Leahy KP, et al. Cellular adaptive inflammation mediates airway granulation in a murine model of subglottic stenosis. Otolaryngol Head Neck Surg. 2011;144:927–933. doi: 10.1177/0194599810397750. [DOI] [PubMed] [Google Scholar]
- 6.Minnigerode B, Richter HG. Pathophysiology of subglottic tracheal stenosis in childhood. Prog Pediatr Surg. 1987;21:1–7. doi: 10.1007/978-3-642-71665-2_1. [DOI] [PubMed] [Google Scholar]
- 7.Hillel AT, Namba D, Ding D, Pandian V, Elisseeff JH, Horton MR. An in situ, in vivo murine model for the study of laryngotracheal stenosis. JAMA Otolaryngol Head Neck Surg. 2014;140:961–966. doi: 10.1001/jamaoto.2014.1663. [DOI] [PubMed] [Google Scholar]
- 8.Croft CB, Zub K, Borowiecki B. Therapy of iatrogenic subglottic stenosis: a steroid/antibiotic regimen. Laryngoscope. 1979;89:482–489. doi: 10.1288/00005537-197903000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh A, Philiponis G, Lee JY, et al. Pulse steroid therapy inhibits murine subglottic granulation. Otolaryngol Head Neck Surg. 2013;148:284–290. doi: 10.1177/0194599812466533. [DOI] [PubMed] [Google Scholar]
- 10.Kil HK, Alberts MK, Liggitt HD, Bishop MJ. Dexamethasone treatment does not ameliorate subglottic ischemic injury in rabbits. Chest. 1997;111:1356–1360. doi: 10.1378/chest.111.5.1356. [DOI] [PubMed] [Google Scholar]
- 11.Li NY, Chen F, Dikkers FG, Thibeault SL. Dose-dependent effect of mitomycin C on human vocal fold fibroblasts. Head Neck. 2014;36:401–410. doi: 10.1002/hed.23310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Supance JS. Antibiotics and steroids in the treatment of acquired subglottic stenosis. A canine model study. Ann Otol Rhinol Laryngol. 1983;92:377–382. doi: 10.1177/000348948309200417. [DOI] [PubMed] [Google Scholar]
- 13.Cardenas ME, Heitman J. FKBP12-rapamycin target TOR2 is a vacuolar protein with an associated phosphatidylinositol-4 kinase activity. EMBO J. 1995;14:5892–5907. doi: 10.1002/j.1460-2075.1995.tb00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saunders RN, Metcalfe MS, Nicholson ML. Rapamycin in transplantation: a review of the evidence. Kidney Int. 2001;59:3–16. doi: 10.1046/j.1523-1755.2001.00460.x. [DOI] [PubMed] [Google Scholar]
- 15.Sehgal SN. Rapamune (sirolimus, rapamycin): an overview and mechanism of action. Ther Drug Monit. 1995;17:660–665. doi: 10.1097/00007691-199512000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Brown EJ, Albers MW, Shin TB, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 17.Granger DK, Cromwell JW, Chen SC, et al. Prolongation of renal allograft survival in a large animal model by oral rapamycin monotherapy. Transplantation. 1995;59:183–186. [PubMed] [Google Scholar]
- 18.Suzuki T, Kopia G, Hayashi S, et al. Stent-based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation. 2001;104:1188–1193. doi: 10.1161/hc3601.093987. [DOI] [PubMed] [Google Scholar]
- 19.Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 20.Chen G, Chen H, Wang C, et al. Rapamycin ameliorates kidney fibrosis by inhibiting the activation of mTOR signaling in interstitial macrophages and myofibroblasts. PLoS One. 2012;7:e33626. doi: 10.1371/journal.pone.0033626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamaki Z, Asano Y, Kubo M, et al. Effects of the immunosuppressant rapamycin on the expression of human alpha2(I) collagen and matrix metalloproteinase 1 genes in scleroderma dermal fibroblasts. J Dermatol Sci. 2014;74:251–259. doi: 10.1016/j.jdermsci.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Zhu J, Wu J, Frizell E, et al. Rapamycin inhibits hepatic stellate cell proliferation in vitro and limits fibrogenesis in an in vivo model of liver fibrosis. Gastroenterology. 1999;117:1198–1204. doi: 10.1016/s0016-5085(99)70406-3. [DOI] [PubMed] [Google Scholar]
- 23.Milani BY, Milani FY, Park DW, et al. Rapamycin inhibits the production of myofibroblasts and reduces corneal scarring after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2013;54:7424–7430. doi: 10.1167/iovs.13-12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poulalhon N, Farge D, Roos N, et al. Modulation of collagen and MMP-1 gene expression in fibroblasts by the immunosuppressive drug rapamycin. A direct role as an antifibrotic agent? J Biol Chem. 2006;281:33045–33052. doi: 10.1074/jbc.M606366200. [DOI] [PubMed] [Google Scholar]
- 25.Ahn SJ, Costa J, Emanuel JR. PicoGreen quantitation of DNA: effective evaluation of samples pre- or post-PCR. Nucleic Acids Res. 1996;24:2623–2625. doi: 10.1093/nar/24.13.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hillel AT, Varghese S, Petsche J, Shamblott MJ, Elisseeff JH. Embryonic germ cells are capable of adipogenic differentiation in vitro and in vivo. Tissue Eng Part A. 2009;15:479–486. doi: 10.1089/ten.tea.2007.0352. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Hillel AT, Unterman S, Nahas Z, et al. Photoactivated composite biomaterial for soft tissue restoration in rodents and in humans. Sci Transl Med. 2011;3:93ra67. doi: 10.1126/scitranslmed.3002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuh RA, Clerc P, Hwang H, et al. Adaptation of microplate-based respirometry for hippocampal slices and analysis of respiratory capacity. J Neurosci Res. 2011;89:1979–1988. doi: 10.1002/jnr.22650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh J, Giri S. Loss of AMP-activated protein kinase in X-linked adrenoleukodystrophy patient-derived fibroblasts and lymphocytes. Biochem Biophys Res Commun. 2014;445:126–131. doi: 10.1016/j.bbrc.2014.01.126. [DOI] [PubMed] [Google Scholar]
- 32.Wu M, Neilson A, Swift AL, et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292:C125–C136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- 33.Costa MA, Simon DI. Molecular basis of restenosis and drug-eluting stents. Circulation. 2005;111:2257–2273. doi: 10.1161/01.CIR.0000163587.36485.A7. [DOI] [PubMed] [Google Scholar]