ABSTRACT
Sharp wave ripples (SPW‐Rs) represent the most synchronous population pattern in the mammalian brain. Their excitatory output affects a wide area of the cortex and several subcortical nuclei. SPW‐Rs occur during “off‐line” states of the brain, associated with consummatory behaviors and non‐REM sleep, and are influenced by numerous neurotransmitters and neuromodulators. They arise from the excitatory recurrent system of the CA3 region and the SPW‐induced excitation brings about a fast network oscillation (ripple) in CA1. The spike content of SPW‐Rs is temporally and spatially coordinated by a consortium of interneurons to replay fragments of waking neuronal sequences in a compressed format. SPW‐Rs assist in transferring this compressed hippocampal representation to distributed circuits to support memory consolidation; selective disruption of SPW‐Rs interferes with memory. Recently acquired and pre‐existing information are combined during SPW‐R replay to influence decisions, plan actions and, potentially, allow for creative thoughts. In addition to the widely studied contribution to memory, SPW‐Rs may also affect endocrine function via activation of hypothalamic circuits. Alteration of the physiological mechanisms supporting SPW‐Rs leads to their pathological conversion, “p‐ripples,” which are a marker of epileptogenic tissue and can be observed in rodent models of schizophrenia and Alzheimer's Disease. Mechanisms for SPW‐R genesis and function are discussed in this review. © 2015 The Authors Hippocampus Published by Wiley Periodicals, Inc.
Keywords: memory, imagining, planning, epilepsy, learning
INTRODUCTION
Since the early days of human neurophysiology, scientists have been fascinated by the variety of mesoscopic events that can be recorded from the scalp as EEG and directly from within the brain as local field potential (LFP; cf., Lopes da Silva, 2013). Many of these events are periodic and the different frequency bands identified over the years are designated by Greek letters (δ, θ, α/μ, β, γ, ɛ; Steriade et al., 1990; Lopes da Silva, 2013). The various rhythms interact with each other through hierarchical “cross‐frequency coupling” and the ensuing interference is the basis for the perpetually changing electrical landscape of the brain (Buzsáki and Draguhn, 2004). Many of these network oscillations are present in the hippocampus as well. In the waking rat, LFP activity of the CA1 region is dominated by theta oscillations (6–10 Hz) during preparatory behaviors, such as ambulation, exploration, rearing and sniffing (Vanderwolf, 1969). In contrast, during consummatory behaviors, such as immobility, drinking, eating and grooming, theta is replaced by irregularly occurring sharp waves (SPW; Fig. 1) (Buzsáki et al., 1983). Historically, non‐theta states have been also referred to as large amplitude irregular activity (LIA, Vanderwolf, 1969), mainly because, in addition to SPWs or LIA spikes, other events such as slow and spindle oscillations are mixed with SPWs (Jouvet et al., 1959; Vanderwolf, 1969; Hartse et al., 1979). However, in the alert but still animal, SPWs represent the only large‐amplitude events. Gamma oscillations (30–120 Hz) or “fast waves” (Stumpf, 1965) are present during both theta state and SPW state, but gamma amplitude is small although its amplitude variability is much larger in the absence of theta (Csicsvari et al., 2003a, 2003b).
Figure 1.
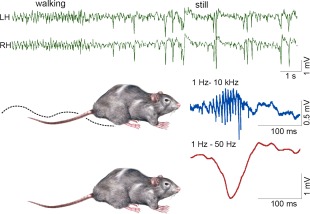
Behavior‐dependence of hippocampal LFP activity. Top, LFP recorded from symmetric locations of the left (LH) and right (RH) dorsal CA1 str. radiatum during locomotion—immobility transition. Note regular theta waves during locomotion and large amplitude, bilaterally synchronous negative waves (sharp waves, SPW) during immobility. Below, SPWs recorded from str. radiatum (red) and ripple recorded from the CA1 pyramidal layer. Reproduced from Buzsáki et al. (1992).
SPWs are large amplitude negative polarity deflections (40–100 ms) in CA1 stratum radiatum that are most often, but not always, associated with a short‐lived fast oscillatory pattern of the LFP in the CA1 pyramidal layer, known as “ripples” (110–200 Hz; O'Keefe, 1976; O'Keefe and Nadel, 1978; Buzsáki et al., 1983, 1992; Buzsáki 1986; Suzuki and Smith, 1987, 1988a,b; Kanamori 1985).1 SPWs and coupled ripples form the sharp wave‐ripple complex (SPW‐R; Table 1). SPW‐Rs have been observed in the hippocampus of every species investigated so far (Fig. 2), including humans (Bragin et al., 1999a; Le Van Quyen et al., 2010). The existence of SPW‐Rs in non‐mammalian species is debated. Although SPW‐like events and associated spike bursts have been reported in the zebrafish (Vargas et al., 2012), neither SPW‐Rs in the hippocampus analog structure nor spindles in the caudolateral nidopallium have been found in birds (Rattenborg et al., 2011).
Table 1.
SPW‐R‐Related Terms and Definitions
| SPWs |
| SPWs are characterized by amplitude or sink magnitude, probability of occurrence (or incidence; numbers per second) and inter‐SPW intervals (ms) |
| SPW cluster (or burst) refers to temporally close SPWs |
| Ripples |
| Individual ripple waves are characterized by duration (ms) and amplitude (µV) |
| Ripple events (or ripples) consist of a series of ripple waves (typical range 3–9) |
| Ripple events are described by magnitude (expressed as amplitude or power µV2), duration (ms), number of ripple waves per event, intra‐ripple frequency (Hz), probability of occurrence (or incidence; number per second) and inter‐ripple event intervals (ms) |
| Ripple/SPW‐R cluster or ripple burst refers to temporally close ripple/SPW‐R events |
Figure 2.
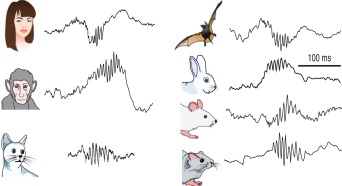
Preservation of SPW‐Rs in mammals. Illustrative traces of ripples recorded from various species. Reproduced from Buzsáki et al., (2013).
In addition to its preservation during mammalian evolution, SPW‐Rs have a number of remarkable features. First, SPW‐Rs are emergent population events; the temporal coordination of firing across many neurons transpose the spiking of many neurons into a powerful population event during SPW‐Rs (Buzsáki et al., 1992). Second, SPW‐Rs are the most synchronous events in the mammalian brain, associated with a robustly enhanced transient excitability in the hippocampus and its partner structures (Buzsáki 1986; Chrobak and Buzsáki, 1994; Csicsvari et al., 1999b). The synchronous, regenerative population events underlying SPW‐Rs are volatile as small alterations of the recruitment process can transform the physiological event into pathological interictal epileptic discharges (Suzuki and Smith, 1988d; Buzsáki et al., 1989a, 1989b) and associated “fast ripples,” considered as biological markers of epileptic propensity of brain networks (Bragin et al., 1999a). Finally, perhaps the most important feature of SPW‐Rs is its spike content. Despite its super‐synchronous appearance, neurons participating in SPW‐R events are sequentially organized and the orderly structure of these events reflects a temporally compressed version of the sequential neuronal firing patterns in the waking animal (Wilson and McNaughton, 1994; Skaggs and McNaughton, 1996; Nadasdy et al., 1999; Lee and Wilson, 2002). These unusual features of SPW‐Rs led to the hypothesis that the organized neuronal assemblies can serve as a mechanism to transfer compressed spike sequences from the hippocampus to the neocortex for long‐term storage when the brain is disengaged from environmental stimuli (SPW‐R‐Supported Memory Consolidation section). In its simplest version, this “two‐stage” memory consolidation model posits that during learning the neocortex provides the hippocampus with novel information leading to transient synaptic reorganization of its circuits (theta phase), followed by the transfer of the modified hippocampal content to the neocortical circuits (SPW‐R state; Buzsáki, 1989). Over the past decades, these ideas have been greatly expanded by new observations in behaving animals, human patients, in vitro slice preparations and computational modeling (Buzsáki and Silva, 2012). The SPW‐R has become the first definite biomarker for cognitive operations. Recently, several novel observations have been reported and the retrospective role of SPW‐Rs has been supplemented with ideas about their prospective roles. The goal of this review is twofold. First, it aims to overview the various physiological facets of hippocampal SPW‐Rs (see from Behavioral Correlates and Mechanisms of SPW Generation section to Mechanisms of Ripple Generation sections) and considers the evidence and caveats for their role in memory. The second goal is to discuss multiple novel predictive functions of SPW‐Rs in light of more recent discoveries (see Constructive Role of SPW‐R section). Both the physiological and behavioral/cognitive sections are followed by extensive summaries, which highlight the main points of progress and novel insights (see Physiological Mechanisms of SPW‐Rs‐Summary section and Retrospective, Prospective, Constructive, and Maintenance Roles of SPW‐Rs—A New Synthesis section). Modification of SPW‐Rs and Other Forms of Fast Rythms in Epilepsy section and P‐Ripples in Non‐Epileptic Disease section discuss the role of SPW‐Rs in disease.
BEHAVIORAL CORRELATES AND MECHANISMS OF SPW GENERATION
SPW is the most prominent self‐organized event in the hippocampus and occurs when suppressing extrahippocampal inputs are withdrawn (Buzsáki et al., 1983). Thus, the default pattern of hippocampal circuits is a population burst of neurons associated with the LFP SPW.
SPW‐Rs Define Consummatory Brain States
A main goal of directly monitoring brain activity is to unveil and understand the mechanisms of “covert” or hidden behaviors, such as cognition. The first step in this program is to identify correlations between brain activity and observable, overt behaviors. Because animals are usually separated from motivational stimuli by distance and time, they must allocate considerable resources toward stimulus‐seeking behavior. Such behavior can be characterized as an on‐going sequence of “preparatory” (appetitive) actions leading to “consummatory” events (Woodworth, 1918; Konorski, 1976). The term “consummatory” refers to consummation (and not consumption), meaning to complete or to finish a planned action. The dichotomy of preparatory and consummatory has reappeared multiple times in the history of animal behavior in various forms such as Jackson's separation of voluntary versus involuntary actions (Jackson, 1875), seeking versus taking or motivated versus automatic behaviors (Cofer and Appley, 1964). Preparatory actions are related to foraging, exploratory, goal‐directed, or planned behaviors. Once the motivational goals are reached, the brain switches to another fundamental type of behavior, referred to as consummatory, involuntary or automatic, including immobility and sleep since they also terminate explorative ambulation.
Hippocampal theta oscillation versus SPW‐R dichotomy reliably reflects the brain‐state categories of voluntary‐preparatory and consummatory‐terminal behaviors (Fig. 1) (Vanderwolf, 1969; Buzsáki, 2005). The theta‐SPW‐R electrophysiological dichotomy is also present during sleep; REM sleep is characterized by theta (Grastyán and Karmos, 1961; Jouvet, 1999), whereas non‐REM sleep (i.e., slow wave sleep) by SPW‐Rs. In short, SPW‐Rs and hippocampal theta oscillations are competing mechanisms (Buzsáki et al., 1983).
Properties of SPW‐Rs during waking and exploration are different from those of sleep. This is perhaps not surprising since while in the sleeping animal activity of subcortical neuromodulators is considerably more strongly attenuated than in the waking immobile animal. SPW‐Rs during transient immobility epochs of exploration are expected to be different from prolonged still periods (O'Neill et al., 2006; Csicsvari et al., 2007; Karlsson and Frank, 2009) since neuromodulator levels in the hippocampus, such as acetylcholine, vary slower than motor behavior (Hobson and Pace‐Schott, 2002), thus subcortical neuromodulators can be still be present during transient but not prolonged immobility. SPW‐Rs occur most frequently during non‐REM sleep, followed by prolonged immobility periods and least often when ambulation comes to a transient halt. SPW sinks in str. radiatum and ripple power in the CA1 pyramidal layer are largest during non‐REM sleep and are somewhat smaller during prolonged immobility periods and during brief pauses of ambulation. SPW‐R clusters (i.e., events with <100 ms intervals between them; Fig. 3) are more common during brief pauses and almost half of them can be classified as bursts of two or more clustered events, while only 20% of SPW‐Rs are bursts during awake prolonged immobility or sleep in the home cage. The frequency of ripples is faster during transient waking immobility than during non‐REM sleep (Fig. 4) (Ponomarenko et al., 2008). While the amplitude of SPWs and the magnitude and peak frequency of SPW‐Rs in the CA1 pyramidal layer are correlated during non‐REM sleep and prolonged immobility, this relationship largely disappears during exploratory pauses (Fig. 4). During REM sleep and very rarely during exploratory ambulation, SPW‐Rs are occasionally embedded in the stream of theta waves. However, these rare “theta‐associated” ripples can be distinguished from their surrounding theta waves by their association with distinct SPWs in CA1 str. radiatum and the CA3 region (Fig. 5).
Figure 3.
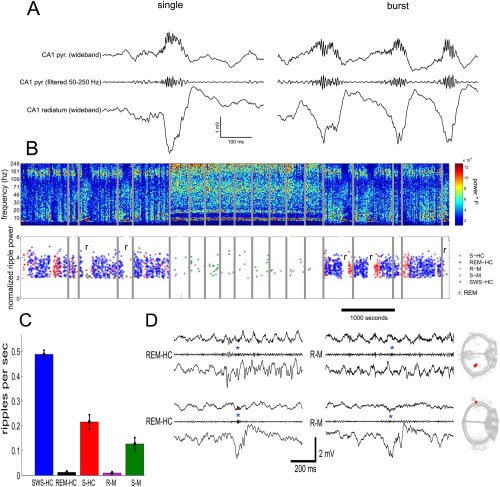
SPWs and ripples in the CA1 region during sleep and stillness on the maze. (A) Raw traces of wide‐band LFP (1–625 Hz) recorded simultaneously from the CA1 pyramidal layer and the mid str. radiatum, together with a band‐pass filtered (50–250 Hz) trace of the pyramidal layer signal. An isolated single and a cluster of SPW‐Rs (burst) are shown. (B) Top, time‐frequency spectrogram (whitened; log scale) of several hours of recording on the same day from the CA1 region in the home cage (left and right) and maze (middle sessions). Gray lines, gaps in recordings. Note increased theta and gamma power during maze sessions. Bottom, SPW‐R events as a function of time/behavior. Each asterisk is a single SPW‐R event. r, REM sleep episodes of sleep. SWS‐HC, events detected during slow wave (non‐REM) sleep in the home cage; S‐HC, events detected during quiet wakefulness in the home cage; REM‐HC, events detected during REM sleep in the home cage; S‐M, events detected during immobility, drinking in the maze; R‐M, events detected during runs in the maze. (C) Incidence of SPW‐Rs during different behaviors. Same code as in (B). (D) Examples of true SPW‐Rs during REM‐HC (left), and false SPW‐R events during R‐M (right). The bottom REM‐HC panel shows a true SPW, embedded in a stream of theta waves. The rat's trajectory for the whole R‐M session in shown gray; the rat's position during the R‐M spurious SPW‐R is shown in red (Sullivan and Buzsáki, unpublished data).
Figure 4.
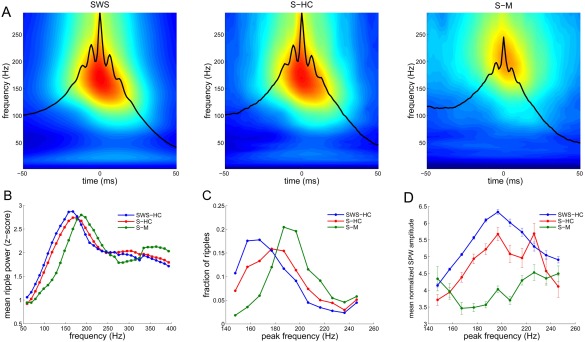
Properties of SPW‐Rs during sleep and waking are different. (A) Average wavelet spectrograms of SPW‐R‐centered epochs from the CA1 pyramidal layer recorded during slow wave (non‐REM) sleep in the home cage (SWS‐HC), quiet wakefulness in the home cage (S‐HC), and immobility, drinking in the maze (S‐M). (B) Average power spectrum of SPW‐Rs during SWS‐HC, S‐HC, and S‐M (mean ± SEM; n = 9 rats). (C) Average histogram of the peak spectral frequency (calculated via FFT) of SPW‐R events in different brain states. Modal frequency of SPW‐Rs during SWS‐HC: 167 Hz, S‐HC: 177 Hz and S‐M: 187 Hz (P < 0.00001; Kruskal‐Wallis test). (D) Relationship between SPW amplitude and peak ripple frequency (mean ± SEM; n = 9 rats). Note ripple frequency at 200 Hz during maximum SPW amplitude during SWS‐HC and S‐HC but not S‐M (Sullivan and Buzsáki, unpublished data).
Figure 5.
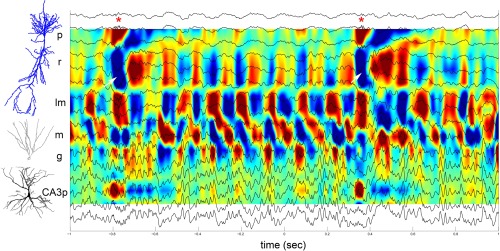
SPW‐Rs embedded in a stream of theta waves. Two‐second epoch of activity during REM sleep recorded with a linear silicon probe, covering the CA1‐CA3‐axis. LFP traces (16 sites) are superimposed on the CSD map. Two SPW‐Rs were detected (asterisks). Note that in both cases, ripples in the CA1 pyramidal layer are coupled with a large SPW in str. radiatum (white arrows) and a large source in the CA3 pyramidal layer. P, CA1 pyramidal layer,; r, str. radiatum; lm, str. lacunosum‐moleculare; m, dentate molecular layer; g, granule cell layer; CA3p, hilus, pyramidal layer of CA3c subregion (Sullivan and Buzsáki, unpublished data).
Depth Versus Amplitude Profiles of SPWs Identify the CA3 Region as the Generator
LFP SPWs have a characteristic depth versus voltage profile and current sink‐source distributions in all hippocampal regions (Buzsáki et al., 1983; Buzsáki, 1986; Sullivan et al., 2011) with the largest sinks in the mid‐apical dendritic layers of the CA1 and CA3 regions and the supragranular layer of the dentate gyrus, coupled with respective return current sources in the cell body layers (Fig. 6). These sink‐source distributions are strongly correlated spatially with the evoked LFP responses of electrically evoked discharges of CA3 pyramidal neurons (Fig. 6B), the relationship suggests that SPWs reflect the excitatory depolarization of the apical dendrites of CA1, CA3 pyramidal neurons by the synchronously bursting of CA3 pyramidal cells (Buzsáki et al., 1983; Buzsáki, 1986; Suzuki and Smith, 1988b; Sullivan et al., 2011). Dentate granule cells might be affected indirectly by the CA3 pyramidal neuron‐mossy cell disynaptic connection (Li et al., 1994). In support of the critical role of the CA3 input to SPW generation, long‐term potentiation of the Schaffer collateral‐CA1 synapses results in a parallel increase of the amplitude of both evoked responses and spontaneously occurring SPWs (Buzsáki, 1984).
Figure 6.
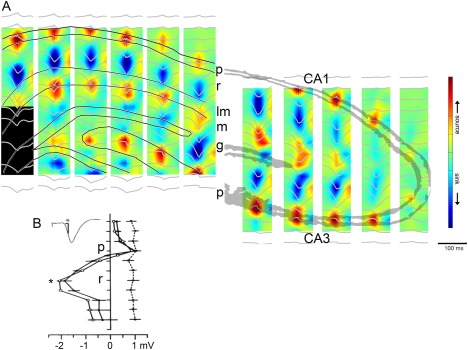
CA3 afferents drive SPWs. (A) Regional distribution of SPW currents. CSD maps (1 Hz to 10 kHz) in two different animals with the average SPW waveforms superimposed (gray traces). Note strong SPWs (sinks) in the stratum radiatum of CA1 and CA3 and the inner molecular layer of the dentate gyrus. Sinks in the inner molecular layer of the dentate gyrus possibly reflect activation of excitatory inputs from hilar mossy cells. Eight‐shank × 16 recording/site per shank probes were used to record LFP simultaneously. (B) Depth versus amplitude profiles of SPWs (filled circles) derived from a movable microelectrode (solid line) and a stationary electrode in CA1 pyramidal layer (dashed line). Each point is an average of 30 SPWs recorded concurrently with the two electrodes. Horizontal bars indicate standard error of the mean. Also shown is the depth versus amplitude profile of the simultaneously recorded field potential in response to stimulation of the Schaffer collaterals (inset, empty circles). Ordinate: 166 µm intervals. Peak amplitude of SPW (asterisk) occurs in the mid stratum radiatum. p, pyramidal layer; r, stratum radiatum, m, molecular layer; g, granule cell layer. A, reproduced after Sullivan et al. (2011). B, reproduced from Buzsáki et al. (1983).
Emergent Properties of SPW Bursts
The SPW‐R is a cooperative emergent event. Observing spikes or spike bursts of individual pyramidal cells does not allow for the identification of SPWs no matter how long the observation period is. This is because no special change takes place in the firing patterns of individual pyramidal neurons; they either fire a spike or a spike burst or remain silent. However, the LFP SPW or ripple is a telltale that a large fraction of neurons fire together and, therefore, the LFP events allow for the quantification of the relationship between individual neurons and population behavior. The correlation between SPW‐R events and participation of single neurons in those events varies from 0 to 40–50% (Ylinen et al., 1995). The relationship between firing of single cells and population events can be estimated by quantifying the proportion of spikes during SPW‐Rs (i.e., the number of spikes during SPW‐Rs divided by the number of all spikes during immobility of non‐REM sleep), the proportion of SPW‐Rs in which a single neuron fires at least one spike (see also Okun et al., 2015) and the mean number of spikes per SPW‐R. Each of these quantities shows a strongly skewed, long‐tailed distribution with several orders of magnitude span, which typically take the form of a lognormal distribution (Fig. 7) (Mizuseki and Buzsáki, 2013). A small minority (1.5%) of CA1 pyramidal cells participates in half of SPW‐R events, whereas half of all neurons fire in less than 10% of SPW‐Rs. The fraction of SPWs in which a pyramidal neuron fires is positively correlated with the overall firing rate of that neuron during both sleep and waking and the number of place fields (O'Keefe and Nadel, 1978) of the neuron during spatial navigation. Burst firing (defined either as a series of three or more spikes with <8 ms interspike intervals; Ranck, 1973; Harris et al., 2001; Mizuseki et al., 2012) during SPW‐Rs shows a similarly skewed lognormal distribution (Misuzeki and Buzsáki, 2013). The incidence of spike bursts of both CA1 and CA3 pyramidal neurons appears to be high during SPW‐Rs, giving the impression that bursts have a particular association with SPW‐Rs (Buzsáki, 1986; Buzsáki et al., 1996 ; Kamondi et al., 1998a). Indeed, this is the case in the CA3 region (Mizuseki et al., 2012). However, in the CA1 region bursts of neurons pairs are not strongly correlated (Mizuseki et al., 2012) and the expected probability of spike bursts during SPW‐Rs actually decreases from what might be predicted from the overall increase of spike emission of hippocampal neurons during SPW‐Rs (Stark et al., 2014; English et al., 2014). In summary, spiking and bursting of pyramidal cells during SPW‐Rs display a spectrum with a wide dynamic range, spanning from vast numbers of silent or very slow‐firing neurons to a very small fraction of highly active and super‐bursting “champion” cells.
Figure 7.
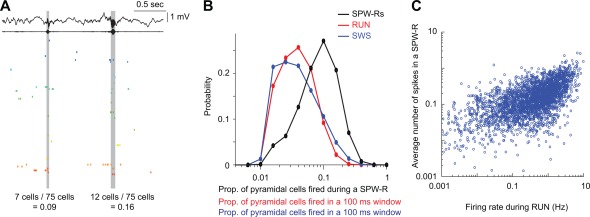
Skewed distribution of the magnitude of population synchrony during ripples and other brain states. (A) Wide‐band and ripple band (140–230 Hz) filtered LFP (top) and spiking activity of simultaneously recorded 75 CA1 pyramidal cells. Two ripple events with relatively low (0.09) and high (0.16) fractions of neurons firing synchronously during ripples. (B) Distribution of the synchrony of CA1 pyramidal cells’ firing during ripples of sleep and in 100 ms time windows during non‐REM (slow wave sleep, SWS) and exploration (RUN). (C) Correlation between firing rates of single CA1 pyramidal cells during ambulation in a maze (RUN) and SPW‐Rs of sleep. Note that high firing rate of neurons in the waking brain are more constant participants in SPW‐Rs than slow firing neurons. A and B are reproduced from Mizuseki and Buzsáki (2013).
In contrast to single neurons, recording from large cell assemblies can instantaneously identify SPW‐Rs because of the strong synchrony of neurons. The population cooperation underlying SPW‐Rs is perhaps the clearest experimental support for the idea of the “temporal code” because it is the magnitude of synchrony of the member neurons, as opposed to their individual firing rate changes, that matters for the downstream “observer‐classifier” network (Buzsáki et al., 1983, 1992). These properties identify the SPW‐R as an emergent event characterized by qualitatively different features resulting from cooperative behavior compared with the properties of the contributing individual neurons (Traub and Wong, 1982). The magnitude of synchrony can be measured as the spiking fraction of all simultaneously recorded neurons during each population burst or as the strength of co‐firing of neuron pairs during SPW‐R. The distribution of the fraction of CA1 and CA3 pyramidal neurons that fire during SPW‐Rs of immobility and non‐REM sleep is strongly skewed and follows a lognormal‐like pattern: strongly synchronized (i.e., very large) rare events are interspersed among many medium‐ and small‐sized events (Fig. 7). In addition, the synchrony of spike bursts across cells also increases in both CA3 and CA1 and can reach a sixfold increase over non‐SPW‐R epochs in CA3 pyramidal cells during SPW‐Rs. Population synchrony of neurons is strongly correlated with the power of the LFP ripples (Csicsvari et al., 2000; Schomburg et al., 2012), qualifying the LFP measures for monitoring pyramidal cell synchrony. Because of the lognormal nature of the ripple magnitude distribution, there is no objectively definable minimum threshold. Instead, in practice the threshold for detection of ripples and population bursts is determined by an arbitrary magnitude of standard deviation from the background activity (Csicsvari et al., 1999a, 1999b). The arbitrary nature of the threshold, in turn, has a consequence on the estimation of SPW‐R rates in different studies. Whereas in early experiments SPW rates of 2‐5 per min were reported (Buzsáki et al., 1983), more recent estimates are higher (non‐REM sleep: 2–4/s; waking immobility: 1–2/s; Csicsvari et al., 1999a, 1999b; Sullivan et al., 2011) with the majority of SPW‐Rs being small amplitude events. In summary, collective synchronous firing of hippocampal neurons can significantly deviate from the baseline during SPW‐Rs, increasing the signal‐to‐noise ratio and making the hippocampal output effectively “heard” by the downstream paleo‐neocortical and subcortical targets.
Generation of SPW bursts—Key properties of the CA3 recurrent network
What induces a SPW burst? The similarity of the spatial distribution of the spontaneous SPWs and Schaffer collateral‐evoked responses in the hippocampus suggested that the source of the SPW burst is the CA3 region (Fig. 6) (Buzsáki et al., 1983; Sullivan et al., 2011). Lesion experiments also support this view since SPWs survive de‐neocortication (Jouvet et al., 1959; Buzsáki et al., 1983; Suzuki and Smith, 1988c), unilateral hippocampal lesion (Suzuki and Smith, 1988c), entorhinal cortex lesion (Ylinen et al., 1995), septal and fimbria‐fornix lesions (Buzsáki et al., 1983). In fact, several of these manipulations typically increased their incidence and magnitude. Thus, it appears that the intrinsic circuits of the isolated hippocampus are sufficient to give rise to SPW‐Rs. Indeed, fetal hippocampal tissue derived from either rat or human brain and transplanted into a vascularized cavity above the thalamus continue to generate SPW‐like population bursts for several months (Buzsáki et al., 1987a, 1987b, 1987c; 1989a; 1989b; Buzsáki and Gage, 1988). Furthermore, surgically isolated hippocampal CA3 region in vitro also generates SPW‐like events (see Generation of SPW‐R Bursts In Vitro section).
Networks with recurrent excitation, in general, inevitably give rise to synchronized population bursts. The CA3 hippocampal region may represent the largest recurrent‐associational system in the brain (Wittner et al., 2007). The strongly recurrent, excitatory collateral system of the CA3 pyramidal cells is, thus, an ideal substrate for the generation of regenerative population bursts (Traub and Wong, 1982). CA3 pyramidal neurons give rise to extensive axon collaterals that project to both CA3 and CA1 regions, and the synapses they form represent the overwhelming majority of intrahippocampal connections (Amaral and Witter, 1989). Both in vitro and in vivo intracellular markers have shown extensive axon collaterals of CA3 neurons (Fig. 8) (Finch et al., 1983; Ishizuka et al., 1990; Li et al., 1994; Sik et al., 1993; Tamamaki et al. 1988; Wittner et al., 2007). The axon arbors of CA3 pyramidal cells are very large and cover from one to two thirds of the septo‐temporal extent of the rat hippocampus, terminating in 15,000 to 40,000 boutons (Li et al., 1994; Wittner et al., 2007). The 250,000 CA3 pyramidal cells in rats give rise to a total of 60 to 100 km of axon collaterals and an estimated 5 to 10 billion boutons (i.e., potential synaptic connections; Wittner et al., 2007) in the 10 mm long banana‐shape rat hippocampus. Typically, more than one axon collateral projects to any of the target dendrites with overlapping projection fields and these multiple routes can converge onto the same target population (Sorra and Harris, 1993; Shepherd and Harris, 1998 ; Wittner et al., 2007). The terminals are distributed relatively homogeneously so that CA3 pyramidal neurons contact nearby and more distantly located target CA3 and CA1 neurons with more or less the same probability. This relatively constant probability of connections can be viewed as a large random graph (Muller et al. 1996) comprising a single giant cortical module (Wittner et al., 2007). Although precise numbers are not available, recordings from cell pairs indicate that each pyramidal cell connects to ∼5 to 10% of the potential targets (Miles, 1990). However, the CA3 connectivity matrix, while widespread, is not homogeneous; the pattern of the recurrent collaterals varies systematically along the CA3a, b, c axis. Whereas neurons in the CA2, CA3a and the more distal CA3b subregions give rise to extensive recurrent collaterals that are confined largely to the CA3 region, pyramidal cells in the more proximal CA3b (i.e., close to the hilus) and CA3c subregions send their axons mainly to the CA1 region (Ishizuka et al., 1990; Li et al., 1994; Wittner et al., 2007). In addition to CA3 and CA1 projections, a small fraction of collaterals returns back to the dentate gyrus, mainly the supragranular molecular layer, especially in the ventral hippocampus (Ishizuka et al., 1990; Li et al., 1994). In addition to such direct recurrent excitation, CA3 pyramidal cells also excite mossy cells (Scharfman et al., 1994) and, in turn, mossy cell axon arbors can extend through more than 50% of the total septotemporal length of the hippocampus. The density of the mossy cell axon terminals in the inner molecular layer of the dentate gyrus is relatively weak at the level of the soma and increases with distance from it in both septal and temporal directions. The majority of axon collaterals of the 20,000 mossy cells (in rat; Buckmaster et al., 1996) are concentrated in the inner one‐third of the molecular layer and an estimated 90% of the boutons terminate on the spines of proximal dendrites of presumed granule cells (Buckmaster et al., 1996).
Figure 8.
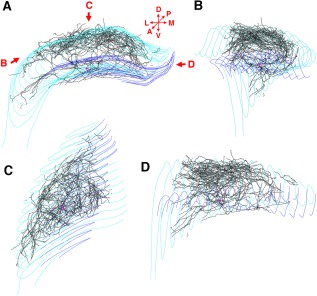
Complete reconstruction of the axon arbor of an in vivo recorded and filled CA3b pyramidal cell. (A) Coronal view of the entire axon arbor (black lines). Red triangle shows the location of the cell body. Dentate granule cell layer is marked with blue lines, CA1‐3 pyramidal cell layer is marked with light blue lines. D: dorsal, V: ventral, L: lateral, M: medial, A: anterior, P: posterior. (B) This rotated view shows that CA3 pyramidal cell axon collaterals follow the curve of the cornu Ammonis. (C) View of the axon arbor from dorsal direction. (D) View from medial direction. Reproduced from Wittner et al. (2007).
Propagation of excitation in the CA3 recurrent system is limited by a number of mechanisms, including inhibition and presynaptic control of glutamate release. During theta oscillation‐associated behaviors, the excitatory spread in the recurrent system is suppressed by activation of the presynaptic cholinergic‐muscarinergic receptors (Hounsgaard, 1978; Dutar and Nicoll, 1988; Hasselmo, 1995; Hasselmo, 1999, 2006) and as well as cannabinoid (CB1) receptors (Robbe et al., 2006). In support of the hypothesis of the cholinergic suppression of recurrent excitation, SPW‐Rs are strongly suppressed by selective optogenetic activation of the medial septal cholinergic neurons (Vandecasteele et al., 2014). On the other hand, when the suppressing effects of the subcortical neuromodulators are removed, as is the case during consummatory behaviors and non‐REM sleep, recurrent excitation can proceed and can give rise to population bursts underlying SPWs. In other words, SPW bursts are not induced but “released” in the absence of suppression mechanisms because the default mode of the CA3 recurrent system is burst generation (Buzsáki et al., 1983). The avalanche of excitation may be initiated by a relatively small group of highly active and presumably well‐connected seed cells with strong synaptic connections and progress to the rest of the population. For example, in the disinhibited hippocampal slice preparation in vitro, current induced burst discharge of just a single pyramidal neuron was sufficient to induce or bias the timing of population burst occurrence (Miles and Wong, 1986; Menendez de la Prida et al., 2006), although it is not known whether similar efficacy is present in vivo (but see Li et al., 2009). The speed and magnitude of the recruitment process is controlled by a variety of finely tuned interneuron classes (see Discharge Patterns of Inhibitory Neurons During SPW‐Rs section) to limit both the fraction of the participating neurons and their temporal sequence in the SPW‐R event.
The strongly skewed distribution of inter‐SPW‐R intervals (Axmacher et al., 2008; Sullivan et al., 2011; Schlingloff et al., 2014) and the lognormal statistics of the magnitude of SPW‐Rs (Mizuseki and Buzsáki, 2013) suggest that the population burst generation is a stochastic process. Patch clamp measurements in vitro show that the incidence of both EPSCs and IPSCs begin to increase 50 to 100 ms before the SPW‐R event with increasing amplitude of EPSCs and these smooth changes are then replaced by periodic and phase‐shifted oscillations of EPSCs and IPSCs, signifying the ripple (Schlingloff et al., 2014). The pre‐SPW‐R changes are also evident in the ramp‐like increase of multiple unit discharges before the onset of the LFP ripple (Fig. 9). Thus, the ripple oscillation is an expression of the network's solution to counter the increasing excitatory gain because balance is easier to achieve with oscillatory than with steady state mechanisms (Buzsáki, 2006).
Figure 9.
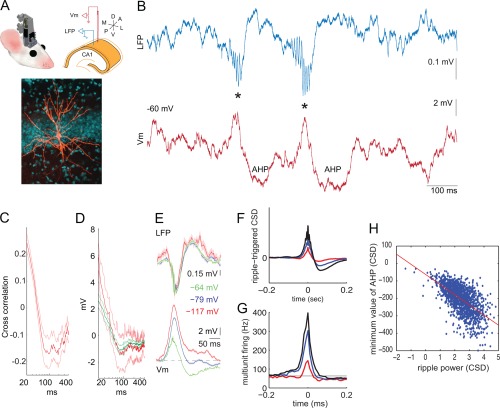
SPW‐Rs are terminated by hyperpolarization. (A) Top: cartoon of a microdrive and scheme of the electrode arrangement in the dorsal CA1 pyramidal layer for intracellular recording in freely moving mice. Bottom: intracellular filled and labeled pyramidal cell. (B) LFP trace (blue) showing two SPW‐R events (asterisks) and simultaneously recorded membrane potential (V m, red). (C) Correlation between V m ripple power (100–200 Hz) and postripple V m as a function of time; red is mean; light red lines are SEM. (D) LFP ripple‐triggered average of post‐ripple V m. Ripples with spikes: mean (red) and SEM (light red); ripples with no spikes: mean (dark green) and SEM (green); (E) LFP ripple‐triggered average of LFP and V m at rest (−64 mV) and during two levels of negative current injection (resulting in −79 mV, −117 mV). (F) SPW‐R peak‐triggered average LFP, using three different thresholds (<3 SD, 3–5 SD and >5 SD of background power in the 100–200 Hz band). (G) Multiple unit firing during the corresponding three averages. Note ramp‐like elevation of multiple unit discharge preceding the SPW‐R and the decreased (below baseline) activity after the SPW‐R. For these analyses only isolated SPW‐Rs were selected. (H) Correlation between ripple current power (CSD) and the magnitude of post‐ripple negativity (“AHP”) in the CA1 pyramidal layer. Note that larger amplitude ripples are followed by larger post‐ripple negativity (as in F). A–E are reproduced from English et al. (2014). F–H are unpublished findings by Sullivan and Buzsáki.
In summary, SPW‐Rs are the default, intrinsic events in the hippocampus, arising from the extensive CA3 collateral system. The synchronous event is an emergent phenomenon, not triggered but “released” when subcortical neuromodulators decrease their suppressive effects on the excitability of the hippocampus. Neuronal participation in SPW‐Rs is strongly skewed, with most pyramidal neurons participating very rarely, while a small minority nearly half of the time. Up to 50% of the pyramidal neurons can be recruited for a large SPW event, although in most SPWs only a smaller fraction is active (Ylinen et al., 1995; Csicsvari et al., 1999a, 1999b; Mizuseki and Buzsáki, 2013).
Triggering/releasing SPW bursts—Role of inhibition
While a main role of inhibition is to protract the recruitment process of pyramidal neurons during SPW‐Rs (Csicsvari et al., 1999a, 1999b; Klausberger et al., 2003), interneurons have been suggested to also assist in triggering SPW bursts. For example, Schlingloff et al. (2014) have shown that in the hippocampal slice preparation, strong optogenetic stimulation of parvalbumin‐expressing (PV) interneurons was sufficient to trigger SPW‐Rs. Another suggested mechanism of synchrony is silencing of selected pyramidal cells followed by their rebound activation (Papatheodoropoulos, 2010). In line with such reasoning, Ellender et al. (2010) demonstrated that current pulse activation of intracellularly recorded CA3 basket neurons in vitro leads to local inhibition followed by a transient increase in excitation over inhibition up to several hundred milliseconds. In this postinhibitory high‐excitatory gain epoch, the probability of occurrence of SPW bursts is significantly increased in the axonal territory of the stimulated single interneuron. These results suggest that spiking activity of perisomatic targeting interneurons under the right circumstances can facilitate the generation of SPWs by providing a temporary inhibition within a subpopulation of pyramidal neurons, and in the wake of inhibition rebound synchronization of a critical number of pyramidal neurons may ignite a population burst (Ellender et al., 2010). Rebound excitation of pyramidal cells by inhibitory interneurons has been shown in the hippocampus both in vitro (Cobb et al., 1995) and by optogenetic activation of parvalbumin (PV)‐expressing neurons in behaving mice (Stark et al., 2013). Although inhibition may contribute to the emergence of population events, the source and mechanism of basket cell activation that would lead to SPW‐Rs remains to be identified.
Yet another potential mechanism by which interneurons can contribute to the ignition or maintenance of a population burst is by their transient silencing. One such candidate for this job is the chandelier or axo‐axonic interneuron (Somogyi, 1977). Axo‐axonic neurons in both CA1 and CA3 regions decrease their firing rates during SPW‐R (Klausberger et al., 2003; Viney et al., 2013). Viney et al. (2013) identified a subset of GABAergic medial septal cells, which project to the CA3 region and selectively innervate axo‐axonic cells. This subset of GABAergic medial septal cells increases their firing discharge during SPW‐Rs. On the basis of these anatomical and physiological findings, Somogyi et al. (2014) and Viney et al. (2013) put forward the hypothesis that SPWs may be triggered by the increased discharge of this special group of septal GABAergic neurons, which, in turn, silences CA3 axo‐axonic cells, resulting in the disinhibition of the axon initial segment of the pyramidal neurons they innervate. This hypothesis can also explain why activity of transplanted hippocampal pyramidal neurons, which lack axo‐axonic neurons (Freund and Buzsáki, 1988), is dominated by robust population bursts. However, the activation source and mechanism of the SPW‐R‐coupled medial septal neurons remain to be identified. Furthermore, silencing of the axo‐axonic neurons during SPW‐Rs may also be accomplished by PV‐basket cells and bistratified interneurons (Buhl et al., 1994), which robustly increase their discharges during SPW‐Rs (Ylinen et al., 1995; Klausberger et al., 2003; Varga et al., 2014). Because SPW‐Rs can emerge also in the subcortically denervated hippocampus (Buzsáki et al., 1983), interneuron mechanisms should be regarded as an ancillary rather than primary force of SPW bursts. However, they may contribute to the emergence of self‐organized population bursts initiated mainly by the widespread recurrent axon collaterals of the CA3 pyramidal cells (Buzsáki et al., 1983; Buzsáki, 1986). In turn, the cooperative discharge of CA3 neurons can broadcast their collective excitation over a large volume of the CA1 region (Ylinen et al., 1995).
Termination of SPW‐Rs
A prominent feature of SPW‐R is its transient nature (mode: 50 ms in the rat; Nguyen et al., 2009; Sullivan et al., 2011). After the transient ripple and associated increase of population synchrony, SPW‐Rs are terminated by a hyperpolarization, which imposes a short refractory period after each event. Intracellular depolarization‐evoked spikes given during the refractory period are strongly attenuated, suggesting that it is not only the lack of synaptic drive but likely an intrinsic event, possibly a GABAB receptor‐mediated K+ channel conductance increase that is responsible for the refractoriness (Fig. 9) (English et al., 2014). In support of this hypothesis, the power of the ripple oscillation in the membrane potential is correlated with the magnitude of the post‐ripple hyperpolarization, irrespective whether the neuron spiked or not during the ripple (Fig. 9D). The post‐ripple hyperpolarization reverses at ∼80 mV, suggesting that an active (possibly K+) current rather than a decrease in excitation is responsible.
The silent period after the SPW‐R is also visible in the LFP as a negative polarity event (especially in the more localized current source density traces) and this epoch is associated with decreased multiple unit neuronal activity. Similar to the intracellular observations, the magnitude of the extracellular ripple power is correlated with the magnitude of the negative potential (more precisely the minimum current source density value) following the ripple (Fig. 9F–H). Further explorations are needed to understand the precise mechanisms underlying the termination of SPW‐R and especially to account for the occurrence of SPW‐R doublets and triplets in the face of the post‐ripple silence.
SPW‐R‐Like Events in Other Brain Structures
Synchronous population patterns, reminiscent of SPW‐Rs, have been observed in several extrahippocampal areas. Although many of these are clearly distinct from the strongly synchronous and powerful SPW‐Rs in the hippocampus, it is worth examining how population synchrony emerges in other structures.
SPWs and ripples in piriform cortex and amygdala
The structural organization of the anterior piriform cortex shares many properties with the single layer hippocampus (Manabe et al., 2011). SPWs with similar duration and behavioral correlates occur in the olfactory cortex (Manabe et al., 2011; Narikiyo et al., 2014; Barnes and Wilson, 2014). The large SPWs in the dendritic layer are often associated with enhanced population spiking of neurons and even LFP ripples in the cell body layer. The strong population burst can discharge deep layer neurons in the olfactory tubercle (Narikiyo et al., 2014) and olfactory cortex SPW‐Rs occur together with SPWs in the olfactory bulb. Olfactory cortex sharp waves occur largely independently from hippocampal SPW‐Rs, although some coupling has been described. Similarly to the behavioral functions of the hippocampal SPW‐Rs (see Ripples and Fast Gamma/Epsilon Oscillations section), sharp waves in the olfactory system may represent a key offline mechanism to consolidate olfactory memory (Wilson, 2010; Narikiyo et al., 2014). Ponomarenko et al. (2003a, 2003b) described ripple‐like oscillations in the basolateral amygdala and adjacent dorsal endopiriform nucleus. Units from both structures were phase locked to the local LFP ripple. The duration of ripples were shorter than in the hippocampus and the ripples in the two structures did not show synchrony.
Neocortical K‐complex
A neocortical population event with similarities to hippocampal SPWs is the K complex (Loomis et al., 1938). The complex consists of a sharp scalp surface positive, depth negative wave. K complexes can occur at the DOWN‐UP transition of slow oscillation (Amzica and Steriade, 1997; Steriade and Amzica, 1998) or in response to external stimuli such as an unexpected, startling noise (Loomis et al., 1938; Halasz et al., 1985). The synchronous cortical discharge associated with the K wave can often activate the GABAergic neurons of the reticular thalamic nucleus and induce a sleep spindle (Steriade et al., 1993a, 1993b, 1993c). The recruitment speed of the K complex and its duration (∼70 ms in the rat; Luczak et al., 2007) and the excitatory gain at the beginning of the UP state (Shu et al., 2003) are comparable with those of SPWs. A main difference is the sustained activity of neocortical activity after the K wave, in contrast to the silence following SPW‐Rs in the hippocampus. The K wave and UP state is generated mainly by layer 5 neurons, and it is possible that the spread of activity into the superficial layers in the neocortex is responsible for the prolongation of activity in the neocortex.
The synchronous activity underlying the K wave often triggers sleep spindles spindle (Steriade et al., 1993). An excessive variant of sleep spindles is known as high voltage spindles (Kandel and Buzsáki, 1997). The spike and wave components of high voltage spindles correspond to the UP and DOWN state of slow oscillations and have identical phase profiles. Such shortening of the UP state may be due to a functional suppression of acitivity in the superficial cortical layers. Both K waves and sleep spindles are associated with fast LFP oscillations varying from 150‐300 Hz (during sleep spindles), which can accelerate to 300‐500 Hz during high voltage spindles (Kandel and Buzsáki, 1997). These fast oscillations resemble hippocampal ripples, but it remains to be shown whether hippocampal and neocortical ripples are generated by similar or different mechanisms. It appears though that strong, transient optogenetic activation of cortical pyramidal cells is sufficient to induce ripple‐like oscillations (Stark et al., 2014).
Rotational waves
Another neocortical population burst event with similarities to SPWs is the “rotational waves” recently described in the motor cortex of monkeys during movement preparation (Churchland et al., 2012; Shenoy et al., 2013). The firing rates of neurons during the rotational waves strongly, and rapidly deviate from baseline, thus strongly increasing the signal‐to‐noise ratio in the network response. The motor cortical network can become highly excitable from a large set of states and those states produce responses that are distinguishable from one another and effectively describe unique movement trajectories (Hennequin et al., 2014). As will be discussed in Travel of SPW‐Rs in the Septotemporal Axis section, the spike sequences during SPW‐Rs can also specifically predict the future movement trajectories of the animal, although at a longer time scale. These observations indicate that the necessary anatomical substrate needed to generate transient and robust increases in population activity is present in both hippocampus and neocortex, perhaps with shared dynamical mechanisms.
Fast oscillations in thalamus
Fast oscillations (∼300 Hz) were observed in the subthalamic nucleus in Parkinson patients (Foffani et al., 2003). Originally condidered a disease condition, its power increased with dopaminergic medication and symptom reduction (Foffani et al., 2006). Subsequent work using microelectrode recordings from the subthalamic nucleus in non‐Parkinson patients demonstrated similar high frequency rhythms in the range of 300 to 600 Hz, suggesting that such fast rhythms are normal physiological patterns (Danish et al., 2007). Similarly in mice, recordings from anterodorsal thalamic nucleus in normal mice showed sustained oscillations exceeding 200 Hz among coactive head‐direction coding neurons. Moreover, such high frequency oscillations were also present between anterodorsal neurons and their target neurons in the prosubiculum provided that they shared the same preferred direction as their presynaptic thalamic neuron (Peyrache et al., 2015). These finding demonstrate that the fast rhythms are not necessarily confined to small anatomical regions but are determined by connectivity and the ability of the partner neurons to interact with each other at a fast scale.
In summary, the overviewed studies in different parts of the brain reveal that fast oscillations are ubiquitous and hippocampal SPW‐Rs show the most synchronous and largest amplitude variant of all known fast rhythms. SPW‐Rs are distinct from many of these patterns in their short duration. Understanding the mechanisms responsible for terminating SPW‐Rs may offer clues why in neocortical circuits synchronous K‐complexes are followed by sustained activity (UP state; Steriade et al., 1993b) rather than silence.
Generation of SPW‐Rs In Vitro
Persistence of SPW‐Rs after subcortical and entorhinal deafferentation and numerous experiments supports the view that it is a true intrinsic event of the CA3‐CA1 hippocampal regions. Thus, the necessary and sufficient conditions for its generation are expected to be present also in the isolated hippocampal slice in vitro. The slice method creates favorable technical conditions and offers unique advantages of experimental control to study the cellular and network mechanisms underlying distinct and isolated elements of cooperative population activities (Traub et al., 2004). Yet, the slice method does not allow drawing a precise correspondence between vitro and in vivo events (Traub et al., 2004). Rodent slice preparations exhibiting in vitro correlates of SPW‐Rs provided an excellent tool to examine cellular and network properties of this population activity (Kubota et al., 2003; Maier et al., 2002, 2003; Colgin et al., 2004; Behrens et al., 2005; Nimmrich et al., 2005; Wu et al., 2005a; Foffani et al., 2007; Ellender et al., 2010; Schlingloff et al., 2014). However, an active debate persists whether in vitro events capture the essential features of in vivo SPW‐Rs or largely reflect epileptic or other pathological events (Fig. 10) (Karlocai et al., 2014).
Figure 10.
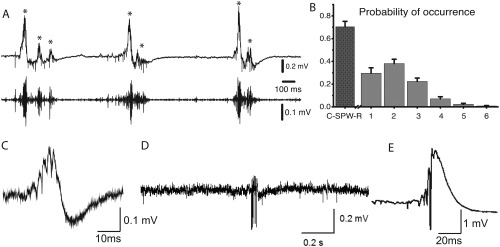
(A) Spontaneous SPW‐Rs recorded from transverse ventral hippocampal slices. A. Typical field recording of SWR events from the CA1 pyramidal cell layer. The original trace (top) and the band‐pass filtered sweep disclosing the ripple oscillation (bottom) are shown. Three episodes of clustered SPW‐Rs containing two to three events are presented. (B) The histogram of probabilities of occurrence of SPW‐R clusters containing one or more events (gray bars). (C) Wide‐band recorded SPW‐R in the CA1 pyramidal cell layer. (D) Wide‐band recorded SPW‐R induced by repeated high frequency stimulation in area CA3. (E) SPW‐R in CA1; (A, B), reproduced from Papatheodoropoulos and Koniaris (2011); (C) reproduced from Maier et al. (2003); (D) Reproduced from Bukalo et al. (2013); (E) Reproduced from Behrens et al. (2011). Note highly variable in vitro ripple patterns from various laboratories.
There are multiple ways to create SPW‐R‐like events in vitro by changing the composition of the extracellular ionic milieu. Perhaps the first in vitro model of spontaneously recurring events was demonstrated Schwartzkroin and Haglund (1986), described as spontaneous rhythmic synchronous events (SRSEs) in slices taken from human epileptic temporal lobe and normal monkey hippocampus that were blocked by the GABAA receptor antagonist bicuculline. The burst discharges were induced by large depolarization and shared similarities with both physiological SPW‐Rs and interictal epileptic spikes. Wu et al. (2002) used a whole hippocampus preparation from mice, rats and gerbils and showed rhythmically occurring large amplitude field potentials often crowned with population spikes that propagated along the ventro‐dorsal hippocampal axis. Since nearly all pyramidal cells spiked in correlation with the LFP events, this model may be more relevant to epileptic activity than to SPW‐Rs. Subsequently, in vivo‐like sharp wave events were described in the ventral hippocampus of the rat which occurred spontaneously in artificial cerebrospinal fluid (ACSF), while under the same conditions slices from the dorsal hippocampus (350 µm thick) did not give rise to SPW‐Rs (Papatheodoropoulos and Kostopoulos, 2002a, 2002b,c; Kubota et al., 2003; Colgin et al., 2004). SPW‐Rs occurred in the CA3 region relatively irregularly with an average frequency of ∼4 Hz and persisted for hours and were not accompanied by aberrant discharges (Kubota et al., 2003). The weaker synaptic inhibition in the ventral hippocampus could account for the dorsal‐ventral difference (Buzsáki et al., 1990; Papatheodoropoulos et al., 2002a,b,c). Another potential explanation for this difference is that slicing the brain may sever axon collaterals of CA3 pyramidal neurons, which are critical for the emergence of population bursts (Buzsáki and Chrobak, 2005). Indeed, it was calculated from the reconstruction of in vivo filled CA3 neurons that from 60 to 93% of the recurrent collaterals are lost in a 300‐µm thick slice from the dorsal hippocampus (Li et al., 1994). Therefore, the remaining collaterals may not have the necessary strength to initiate spreading population recruitment. Thicker slices may contain larger circuits. While 350 µm thick slices only rarely show spontaneous SPW‐Rs, most of the 400‐600 µm slices of mouse hippocampus do (Schlingloff et al., 2014). In further support of this hypothesis, when the synaptic strengths are enhanced by repeated tetanic (repetitive electrical) stimulation which brings about long‐term potentiation of CA3‐CA1 and CA3‐CA3 synapses, SPW‐Rs emerge even from regular dorsal hippocampal slices of rats in vitro (Behrens et al., 2005). Furthermore, spontaneously occurring SPW‐Rs have been also identified from coronal slices of the dorsal hippocampus (Yanovsky et al., 1995) and horizontal slices from the mid‐to‐ventral portion of the hippocampus of the mouse (Maier et al., 2002, 2003), perhaps because more extensive connectivity can be preserved in the same volume of tissue in a smaller brain. Alternative ways to induce SPW‐R‐like events include changing the ionic composition of ACSF (Table 1; Maier et al., 2012; Aivar et al., 2014), local pressure ejection of hypertonic solutions or solutions with elevated concentration of K+ that may bring about sufficient depolarization of pyramidal cells or affect electrical coupling between neurons (Bennett and Verselis, 1992). Selective depolarization of pyramidal cells and interneurons can be also evoked by optogenetic means, as demonstrated in the intact brain (Stark et al., 2014; 2015) and brain slices (Schlingloff et al., 2014).
Many factors can affect the emergence and various aspects of SPW‐Rs in vitro, including species differences, age of the animal, slice cuts from the dorsal or ventral hippocampus, horizontal, transverse or coronal slices, temperature, and ionic composition of the bathing solution, slice thickness, the use of interface or submerged chamber (Hajos et al., 2009; Maier et al., 2012; Aivar et al., 2014). While in standard submerged‐type chambers exceptionally few slices show spontaneous SPW‐Rs, specialized chambers with “ideal” flow‐profile conditions, small‐volume to enhance oxygen supply of the slices, increased ACSF flow and pre‐incubation conditions can dramatically improve the recording conditions and detectability of SPW‐Rs (Hájos et al., 2009; Maier et al., 2012). Using mouse hippocampal slices cut at thicknesses ≥400 μm can also improve the yield of slices with spontaneous events compared with thinner slices (Yanovsky et al., 1995; Wu et al., 2002). Temperature is another critical condition that can affect SPW‐Rs. Typically no SPW‐Rs are observed in slices at room temperature (Maier et al., 2012). The intra‐ripple frequency, but not the rate or magnitude of SPW‐Rs, changes linearly with temperature monotonically within the 27 and 37°C range (Wu et al., 2005; Papatheodoropoulos, 2007). These experimental factors can determine the similarities and differences between the in vivo and in vitro situations and the frequent dissimilarities of the in vitro features of SPW‐R‐like events across laboratories, which vary from in vivo‐comparable patterns to overt epileptic spikes (Fig. 10).
Similar to the in vivo situation, in vitro SPW‐Rs are typically initiated in the CA3 region and spread to CA1 and subiculum (Papatheodoropoulos and Kostopoulos, 2002a; Kubota et al., 2003; Maier et al., 2003; Kano et al., 2005; Nimmrich et al., 2005; Wu et al., 2006). The depth profiles of SPWs and ripples in both CA1 and CA3 region are similar to those in the intact brain (Maier et al., 2003; Kubota et al., 2003). Hájos et al. (Hájos et al., 2013; Hofer et al., 2015) measured the layer‐by‐layer LFP gradient by placing a laminar multielectrode array (24 channels, 50 µm intercontact distance) on the surface of the hippocampal slice, perpendicularly to the pyramidal or granule cell layer. Current source density analysis of SPW‐Rs showed remarkable similarities to in vivo current distributions (Buzsáki, 1986; Ylinen et al., 1995; Sullivan et al., 2011) with a large SPW sink in CA1 str. radiatum and a source in the pyramidal layer, and large ripple wave sinks in the pyramidal layer coincident with spiking, supporting the view that the origin of SPW‐Rs is the CA3 recurrent collateral system, that can bring about rapid synchronized activation (Miles and Wong, 1983), with possible contribution of gap junctions (Ylinen et al., 1995; Traub, 1995, 2001; Avoli et al., 1998; see Gap Junction‐Based Model of Ripple Generation section).
In several in vitro models, SPW‐Rs could not be detected in the isolated CA1 region (Colgin et al., 2004; Wu et al., 2005, 2006; Foffani et al., 2007; Ellender et al., 2010; Hofer et al., 2015), while in others very small CA1 islands generated ripple‐like events (Maier et al., 2003; Nimmrich et al., 2005). In some preparations, ripple events were detected in the dentate gyrus (Maier et al., 2003; Colgin et al., 2004, 2005; Hofer et al., 2015) but not in others (Wu et al., 2005). Ripple amplitude, ripple frequency, the magnitude of spike synchrony, participation probability of pyramidal cells in SPW‐R events and their probability of occurrence and rhythmicity vary extensively across the various in vitro models, ranging from values comparable to the in vivo situation or more silent pyramidal cell activity (Hajos et al., 2013; Schlingloff et al., 2014) to super‐synchronous, large‐amplitude, and high‐frequency events (Behrens et al., 2007), more reminiscent to interictal spikes than physiological SPW‐Rs (Wu et al., 2002; Maier et al., 2003; Liotta et al., 2011). In most in vitro studies, events are present or studied only in the CA3 region, yet their relationship to SPW‐Rs is not always clear (Hofer et al., 2015). In vitro, both depolarizing and hyperpolarizing components occur in principal neurons during SPW‐Rs (Maier et al., 2003; Behrens et al., 2005; Wu et al., 2005; Colgin et al., 2005), although in CA3 pyramidal cells some authors have observed only depolarizing potentials (Wu et al., 2005). This is in contrast to the drug‐free, in vivo situation SPW‐R participating neurons typically show depolarization with concurrent, strong inhibition, which prevents spiking in most pyramidal neurons (Maier et al., 2011; English et al., 2014).
An objective comparison between SPW‐Rs in vivo and SPW‐R‐like events in vitro is difficult because of the high variability of the patterns among the in vitro models. A major difference between the in vitro and in vivo situations is the high coherence and tight coupling between CA3‐CA1 ripples in slices (Behrens et al., 2005, 2007; Both et al., 2008), in contrast to the different frequency of fast oscillation patterns in these regions in vivo (Buzsáki 1986; Ylinen et al., 1995; Sullivan et al., 2011). In the intact brain, CA1 pyramidal neurons are not phase‐locked to the LFP fast oscillations in CA3, although a minority of interneurons can be entrained (Sullivan et al., 2011). One possible mechanism of fast inter‐regional entrainment of spikes (spike‐spike coherence) in vitro is the strong activation of perisomatic interneurons by the synchronous CA3 output and consequent phase‐entrainment of CA1 pyramidal neurons by the synchronously spiking interneurons (Csicsvari et al., 2003a, 2003b). Alternatively, strong CA3 activation may induce dendritic spikes in their partner CA1 pyramidal neurons (Magee and Carruth, 1999), which, in turn, can propagate and induce precisely timed somatic spikes. The peak frequency of ripple in vivo is ∼140 Hz during sleep and 160 Hz during waking immobility (Fig. 4) (Suzuki and Smith, 1987; Ponomarenko et al., 2008; Sullivan et al., 2011), whereas in vitro ripples are typically above 180 Hz (see Generation of SPW‐R Bursts In Vitro section). The fraction of active and bursting neurons also differ between in vivo and in vitro situations; in some slice models only exceptionally few pyramidal cells spike are active, whereas in others the majority of them fire. The spiking synchrony is also reflected by the LFP ripple magnitude and waveform (Schomburg et al., 2012). Inter‐SPW‐R intervals in vitro can be highly regular (Behrens et al., 2007) or can show a Poisson‐like distribution (Papatheodoropoulos 2010; Schlingloff et al., 2014), similar to the distribution in the intact brain (Sullivan et al., 2011). The method of induction of spontaneous population bursts can also explain why the pharmacological responses differ across the in vitro models and between in vitro and in vivo situations (Table 2). Similarly, the specific contributions of interneurons also vary across models (see Discharge Patterns of Inhibitory Neurons During SPW‐Rs section).
Table 2.
Features of SPW‐Rs in Vitro Depends on Numerous Conditions
| Laboratory | Article | PMDI | Species | Age | Slice width (µm) | Slice recovery/ Recording | KCl | KH2PO4 | NaH2PO4 | MgSO4 | MgCl2 | CaCl2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Both M. | Viereckel et al. 2013 | 23460368 | C57 m | 4–8 w | 450 | interface/interface | 3 | 1.25 | 1.8 | 1.6 | ||
| Hippocampus | ||||||||||||
| Draguhn A. | Maier et al. 2003 | 12807984 | C57 m | 4–12 w | 450 | interface/interface | 3 | 1.25 | 1.8 | 1.6 | ||
| J Physiol | ||||||||||||
| Gulyas A | Karlocai et al. 2014 | 24390441 | CD1 & BI6 mice | 19–40 d | 200 or 450 | Interface/submerged | 3.5 | 1.25 | 1.2 | 1.6 | ||
| Brain | ||||||||||||
| Hajos N. | Holderith et al. 2011 | 21859823 | CD1 m | 14–26 d | 350‐400 | interface/submerged | 2.5 | 1.25 | 2 | 2 | ||
| J Physiol | ||||||||||||
| Heinemann U. | Behrens et al. 2005 | 16222227 | W rat | 5–8 w | 400 ‐ 12º | interface/? | 3 | 1.25 | 1.8 | 1.6 | ||
| Nat Neurosci | ||||||||||||
| Ikegaya Y. | Sun et al. 2012 | 22293299 | C57 m | 3–5 w | 400 ‐ 12.7º | submerged/subm | 3.5 | 1.24 | 1.3 | 2 | ||
| J Pharmacol Sci | ||||||||||||
| Ikegaya Y. | Norimoto et al. 2012 | 22608077 | C57 m | 400 ‐ 12.7º | submerged/subm | 3.5 | 1.24 | 1.2 | 2 | |||
| Brain Res | ||||||||||||
| Lynch G. | Kubota et al. 2003 | 12522161 | SD rat | 4 w | 350 | interface/interface | 3 | 1.25 | 1 | 3 | ||
| J Neurophysiol | ||||||||||||
| Lynch G. | Colgin et al. 2004 | 15194734 | SD rat | 4–5 w | 350 | interface/interface | 3 | 1.25 | 3 | 1 | ||
| J Physiol | ||||||||||||
| Menendez de la Prida L. | Foffani et al. 2007 | 17880896 | SD rat | 6–7 w | 350 | submerged/interface | 4 | 1.2 | 1 | 1 | ||
| Neuron | ||||||||||||
| Menendez de la Prida L. | Aivar et al. 2014 | 24553939 | W rat | 17–21 d | 400 | interface/interface | 4.25 | 1.2 | 1 | 1‐3 | ||
| J Neuroscience | ||||||||||||
| Menendez de la Prida L & Miles.R | Alvarado‐Rojas et al. 2015 | 25448920 | Human TLE | 18–52 y | 400 | interface/interface | 4 | 2 | 2 | |||
| Annals Neurol | ||||||||||||
| Papatheo doropoulos C. | Papatheodoropoulos et al. 2008 | 18938226 | W rat | 4–6 w | 500–550 | interface/interface | 4 | 1.25 | 2 | 2 | ||
| Neuroscience | ||||||||||||
| Patrylo P.R. | Kanak et al. 2013 | 23593474 | C57 m | 4–21 m | 400 | interface/interface | 3 | 1.4 | 1.3 | 2.5 | ||
| Plos One | ||||||||||||
| Paulsen O. | Hájos et al. 2009 | 19200237 | W rat /CD1 m | 14–20 d | 400–450 | interface & submerged/same | 3 | 1.25 | 1 | 3 | ||
| Eur J. Neurosci | ||||||||||||
| Paulsen O. | Ellender et al. 2010 | 20427657 | W rat | 14–24 d | 400 | interface/submerged | 3 | 1.25 | 1 | 3 | ||
| J. Neuroscience | ||||||||||||
| Rho J. M. | Simeone et al. 2013 | 23466697 | C3HeB/FeJ m | 30–45 d | 350 | ?/submerged | 3 | X | 1.25 | 2.5 | X | 2.4 |
| Neurobiol Disease | ||||||||||||
| Schmitz D. | Maier et al. 2009 | 19738897 | C57 m | 4–8 w | 400 | submerged/subm | 2.5 | 1 | 1.3 | 2.5 | ||
| PLoS One | ||||||||||||
| Schmitz D. | Maier et al. 2012 | 21853502 | C57 m | 4–8 w | 400 | interface/submerged | 2.5 | 1 | 1.3 | 2.5 | ||
| Hippocampus | ||||||||||||
| Zhang L. | Wu et al. 2009 | 18785213 | C57Bl/6 mice | 21d – 4 m | 500 | submerged/subm | 3.5 | 1.25 | 1.3 | 2 | ||
| Hippocampus |
Species: m, mouse; Age: w, weeks; d, days; m, months. NaCl concentration ranged between 124‐129 mM. Glucose concentration was 10 mM in all cases. NaHCO3 concentration ranged between 21‐26 mM. TLE, temporal lobe epilepsy patient
Table modified after Aivar et al., 2014 (courtesy of Liset Menendez de la Prida)
In summary, in vitro models can capture various aspects of in vivo SPW‐Rs and continue to be a powerful approach for studying the biophysical, pharmacological and other aspects of SPW‐R generation, which are quite challenging in the intact hippocampus. However, the model‐specific differences should be carefully considered for interpreting the observations and applying them to the in vivo situation (see Modification SPW‐Rs and Other Forms of Fast Rhythms in Epilepsy section). A largely unexplored area in the slice preparation is whether sequential activity of neurons during SPW‐Rs is random or consistent from event to event or how these patterns can be altered by various targeted manipulations.
PHARMACOLOGICAL CONTROL OF SPW‐R
Most pharmacological experiments on SPW‐Rs have been performed in vitro. SPW‐Rs offer a test bed for examining the effects of various drugs not only on synaptic transmission of single cells but assessing the effects on network excitability. The in vitro slice preparation offers unique advantages of experimental control for studying the distinct pharmacological, neurotransmitter/modulator mechanisms of SPW‐R control (Papatheodoropoulos, 2007). Since a delicate balance of multiple different neurotransmitters and modulators influences network excitability, it does not come as a surprise that many neurotransmitters and drugs exert various impacts on SPW‐Rs.
Glutamate Receptors
The hypothesis that SPW‐related population bursts emerge as the result of recurrent excitation of CA3 pyramidal neurons is supported by numerous experiments in which pharmacologic blockade of AMPA and kainate type of glutamate receptors in vitro eliminated or reduced SPW‐Rs (Papatheodoropoulos et al., 2002a,b,c; Wu et al., 2002; Maier et al., 2003; Kubota et al., 2003; Colgin et al., 2004; Behrens et al., 2005; Wu et al., 2005b; Ellender et al., 2010; Hofer et al., 2015).
The role of NMDA glutamate receptors in SPW‐R generation needs further clarification. In most studies, modulating NMDA receptor activity did not alter the fundamental features of SPW‐Rs (Maier et al., 2003; Behrens et al., 2005; Wu et al., 2005a,b; Hofer et al., 2015), although in ventral slices of rats NMDA receptor antagonists increased the size of both SPWs and ripples (Colgin et al., 2005; Ellender et al., 2010). This effect may have involved decreased Ca2+ influx through NMDA receptors and a subsequent reduction in the activation of SK2 Ca2+‐activated potassium channels in pyramidal cells (Colgin et al., 2005). SPW‐Rs often occur in clusters, repeated at 70‐150 ms intervals, and NMDA receptors appear important in clustering SPW‐Rs (Papatheodoropoulos, 2010). Clusters of double and triple SPW‐Rs occur in vivo (Fig. 3), where they typically align in time with sleep spindles (Sirota et al., 2003). However, clusters of SPW‐Rs are often present in the waking animal (Fig. 3) and in the ventral hippocampus of rats in vitro (Papatheodoropoulos, 2010), indicating that intrahippocampal mechanisms might contribute to the clustering. NMDA receptor antagonists reversibly abolished clustered SPW‐Rs without affecting isolated SPW‐Rs. Such cluster formation of SPW‐Rs may be important for learning, since SPW‐R bursts (doublets and triplets) may link various parts of the environment into single trajectory (see Constructive Role of SPW‐R section; Davidson et al., 2009; Wu and Foster, 2014).
Trains of high‐frequency stimulation at the Schaffer collaterals that induce long‐term potentiation of the evoked field response enhance both the amplitude of SPWs (Buzsáki, 1984) and the incidence of SPW clusters, and these effects are NMDA receptor dependent (Papatheodoropoulos, 2010). The occurrence SPW‐R clusters is also sensitive to pharmacological manipulation of the GABAA receptor‐mediated transmission, indicating that precise levels of GABAergic transmission are required for the cluster generation of SPW‐R clusters. This GABAergic effect may be indirect, since NMDA receptor‐mediated currents are suppressed by GABAA receptor‐mediated tonic inhibition (Mann and Mody, 2010). In addition or alternatively, inhibition may induce rebound excitation (Cobb et al., 1995; Stark et al., 2013), which may trigger transient increase in the network excitability required for the induction of additional SPW‐Rs (Papatheodoropoulos, 2010).
GABA Receptors
GABA receptors can affect SPW‐Rs in multiple ways, including regulating their incidence, the frequency of ripples and recruitment of pyramidal cells and interneurons in both SPWs and ripples. Given the central role of inhibitory interneurons in controlling the recruitment of pyramidal cells into the SPW‐R burst, the effect of GABAergic drugs, affecting both GABAA or GABAB receptors have been tested in numerous experiments. GABAA receptors are heteropentameric ligand‐gated chloride‐ion channels composed of different subunits. The distinct combinations of subunits form different subtypes of GABAA receptors with distinct cellular domain localization (Farrant and Kaila, 2007) and they have distinct physiological and pharmacological properties (Korpi et al., 2002; Sieghart, 2006). Synaptic GABAA receptors mediate transient effects through phasic actions, whereas extrasynaptic GABAA receptors produce more prolonged (tonic) changes in the membrane potential and conductance, thereby affecting excitability (Semyanov et al., 2004; Kullmann et al., 2005; Farrant and Kaila, 2007). The different subtypes are involved in distinct brain functions (Rudolph and Mohler, 2006), and they affect different aspects of SPW‐Rs as well.
Investigation of the effect of GABAA receptor blockers is complicated because strong disinhibition of the pyramidal neuron population often induces epileptic discharges. At subepileptic doses (1–6 mg/kg, i.p.) the GABA antagonist bicuculline enhances dramatically the amplitude of SPWs and SPW‐concurrent population burst discharges and converts the typically single event to large amplitude bursts (Buzsáki et al., 1983; Suzuki and Smith, 1988d). The anesthetic pentobarbital (a GABAA receptor agonist) decreases the probability of SPW‐R occurrence or abolishes them (Suzuki and Smith, 1988d). Experiments with low drug concentrations show that both stimulus‐induced and spontaneous SPW‐Rs can be transiently suppressed by the GABAA receptor antagonists bicuculline or gabazine (SR‐95531) in vitro (Maier et al., 2003; Nimmrich, et al., 2005; Behrens et al., 2007) and in vivo (Stark et al., 2014). Prolonged application of these drugs can convert ripples into pathological events (200–400 Hz “fast” ripples; Bragin et al., 1999a). On the other hand, activation of GABAA receptors by the barbiturate pentobarbital reduces the probability of occurrence or abolishes SPW‐Rs both in vitro and in vivo at pre‐anesthetic or anesthetic doses, respectively (Suzuki and Smith, 1988d; Papatheodoropoulos et al., 2007). The general anesthetics methohexital, ether and halothane exert similar effects on SPW‐Rs (Suzuki and Smith, 1988d; Ylinen et al., 1995; Wu et al., 2005). These volatile anesthetics also have GABA‐mimetic effect and suppress the Ca2+‐influx into pre‐synaptic terminals resulting in a depression of excitatory synaptic transmission (Krnjevic, 1992). The GABAA‐receptor agonist thiopental reduces the rate of SPW‐Rs in vitro in a dose‐dependent manner and prolongs their duration (Papatheodoropoulos et al., 2007) at concentrations that produce sedation (∼50–150 μM) and anesthesia (>150 μM; Franks and Lieb, 1994; MacIver et al., 1996).
The role of inhibition in ripple pacing has been debated despite the repeated observation that PV basket neurons fire phase‐locked to ripple cycles and often at ripple frequency both in vivo (Buzsáki et al., 1992; Ylinen et al., 1995; Csicsvari et al., 1999a, 1999b; Klausberger et al., 2003; Klausberger and Somogyi, 2008; Rácz et al., 2009; Varga et al., 2012) and in vitro (Maier et al., 2003, 2011; Bähner et al., 2011; Hajos et al., 2013; Karlocai et al., 2014) and this effect is likely mediated through α1 subunit‐containing GABAA receptors (Somogyi et al., 1996; Thomson, 2000). Genetic down‐regulation of fast excitatory synaptic transmission onto PV+ interneurons in the hippocampus results in enhanced phase‐locking of CA1 pyramidal cells to ripple waves, leading to network hyper‐synchronization (Rácz et al., 2009). These authors suggest that their findings argue against the role of PV+ interneurons in ripple generation. Alternatively, the findings may demonstrate that the pyramidal‐interneuron loop is not critical for ripple generation and, in fact, its weakening can somehow enhance PV‐PV interactions, increasing the amplitude of LFP ripples and associated neuronal entrainment.
Two in vitro studies dismissed the importance of inhibition (Draguhn et al., 1998; Maier et al., 2011), because ripple‐like fast oscillations in the dentate gyrus and CA3 could be reinstated by local puff of KCl (Nimmrich et al., 2005) and because perfusion of hippocampal slices by GABAA receptor blockers, barbiturates, GABA reuptake inhibitor or the GABAA‐receptor‐positive allosteric modulator diazepam did not affect ripple frequency (Liotta et al., 2011; Viereckel et al., 2013). These studies suggested that phasic inhibition is not responsible for setting ripple frequency. However, KCl‐induced oscillations in those studies were very fast (>200 Hz), unlike naturally occurring ripples. In other studies, thiopental reduced the rate of SPW‐R activity and the frequency of ripples by enhancing tonic inhibition and prolonging IPSPs in pyramidal neurons (Papatheodoropoulos et al., 2002a,b,c). Thiopental also slowed or disrupted gamma frequency oscillations (Whittington et al., 1996; Faulkner et al., 1998; Dickinson et al., 2003). The low concentration of thiopental that already interfered with SPW‐Rs is similar to the dose shown to affect explicit memory in human subjects (Veselis et al., 1997). Furthermore, systemic injection of the GABAA receptor agonists diazepam and zolpidem in sleeping rats reduced the oscillation frequency of ripples (Ponomarenko et al., 2008), suggesting that inhibition plays a role in pacing ripple frequency. Subsequent in vivo studies demonstrate that GABAA receptor‐mediated inhibition is critical for ripple generation since focal application of the GABAA receptor‐antagonist picrotoxin into the CA1 pyramidal layer of anesthetized mice fully abolished optogenetically induced ripples in the drug‐perfused volume (Stark et al., 2014). In addition, picrotoxin infusion also decreased ripple coherence between the perfused and non‐perfused locations. Local puffing of gabazine in slices also eliminated LFP ripples locally (Schlingloff et al., 2014). Furthermore, selective optogenetic activation of PV interneurons brought about ripple‐frequency patterning of interneurons and pyramidal cell spikes, implying that ripple timing can be set by inhibition among PV interneurons (Stark et al., 2014).
The effects of GABAA receptor targeting drugs also depend on the subunit composition of the receptor. Zolpidem and diazepam differ in their selectivity for the various subtypes of GABAA receptors. Zolpidem preferentially binds to α1‐containing receptors whereas diazepam, but not zolpidem, also activates α5‐GABAA receptors (Sieghart, 2006). Remarkably, zolpidem enhanced whereas diazepam reduced the probability of occurrence of SPW‐Rs in vitro. Further diazepam, but not zolpidem, produces dissociation between ripples and SPWs. Both drugs suppress the generation of SPW‐R clusters (Koniaris et al., 2011). One of the most abundant subtypes of GABAA receptors in the hippocampus contains the α5 subunit on pyramidal neurons (Sur et al., 1998; Sieghart, 2006). A large percentage of extrasynaptic GABAA receptors contain coassembled α5 and ß3 subunits (Sur et al., 1998). Activation of α5 subunit‐containing GABAA receptors brings about tonic inhibition and dampens CA1 pyramidal cell excitability (Glykys and Mody, 2006; Prenosil et al., 2006; Bonin et al., 2007). Etomidate and L‐655,708 are substances that display opposite effects on the α5 subunit‐containing GABAA receptor. Etomidate is a positive allosteric modulator at GABAA receptor (Evans and Hill, 1978) and α5 subunit‐containing GABAA receptors are highly sensitive to etomidate, producing a strong increase in tonic but not phasic inhibition (Caraiscos et al., 2004). L‐655,708 is an inverse agonist with 50‐ to 100‐fold higher functional affinity for the a5 subunit‐containing GABAA receptors compared with receptors containing the α1, α2 or α3 subunit (Quirk et al., 1996; Sur et al., 1998; Atack et al., 2006). Etomidate reduced (0.1 mM) or abolished (1 mM) the probability of occurrence of SPW‐Rs and reduced SPW‐R clusters in vitro, while L‐655,708 had the opposite effects. Etomidate decreased while L‐655,708 increased ripple power, duration and the number of ripple waves per ripple events. L‐655,708 also robustly increased the probability of occurrence of SWP‐R clusters (Papatheodoropoulos and Koniaris, 2011). These findings illustrate the importance of tonic GABA effects on various parameters of SPW‐Rs and may explain, at least partly, the memory decreasing and enhancing effects of etomidate and L‐655,708, respectively (Chambers et al., 2003; Cheng et al., 2006; Atack et al., 2006; Martin et al., 2009). Since different interneuron types exert their effects via GABAA receptors with different subunit compositions (Freund, 2003), these findings demonstrate that the different interneuron types may affect specific aspects of SPW and ripple generation (see Discharge Patterns of Inhibitory Neurons During SPW‐Rs section).
In addition to GABAA receptors, GABA can also activate both postsynaptic and presynaptic GABAB receptors (Raiteri, 2008). GABAB receptors are G‐protein coupled heptahelical transmembrane proteins which form dimers when activated. They exert their postsynaptic effects via GIRK channels, which mediate a slow hyperpolarization (Konnerth and Heinemann, 1983; Kuner et al., 1999). When acting presynaptically, GABAB receptors reduce presynaptic calcium entry and thereby reduce both GABA release and glutamate release (Konnerth and Heinemann, 1983). SPW‐Rs induced in the CA3 region by tetanic stimulation (Behrens et al., 2005) in slices from the dorsal hippocampus of rats were reversibly blocked by application of the GABAB receptor agonist baclofen. The effect of baclofen was prevented by the GABAB receptor antagonist CGP55846 (Hollnagel et al., 2014). However, CGP55846 alone did not increase the incidence of SPW‐Rs, suggesting that at least in vitro the interstitial GABA acting on GABAB receptors is not strongly involved in regulating the incidence of SPW‐Rs. Baclofen did not affect the duration of ripples but it reduced its amplitude. The SPW‐R blocking effect of baclofen was associated with membrane hyperpolarization and a change in input resistance (Connors et al., 1988), and such postsynaptic effects may partly explain the amplitude and incidence reduction of SPW‐Rs by baclofen. Because baclofen also suppresses P/Q‐ and N‐type Ca2+currents (Lei and McBain, 2003), and since these currents underlie presynaptic Ca2+dependent transmitter release, weakening of transmitter release in the CA3 recurrent excitatory network might be another mechanism of the drug for reducing the incidence of SPW‐Rs (Hollnagel et al., 2014). In turn, interference with SPW‐R generation can explain the spatial memory deficit brought about by intrahippocampal infusion of baclofen (Arolfo et al., 1998).
Acetylcholine and Histamine
Acetylcholine activates metabotropic muscarinic and ionotropic nicotinic receptors, both of which are abundant in the hippocampus (Drever et al., 2011). Acting on these receptors, acetylcholine can have profound effects on synaptic transmission, plasticity, firing patterns of neurons and oscillatory network activity (Hounsgaard, 1978; Dutar and Nicoll, 1988; Hasselmo, 2006; Drever et al., 2011). In mouse hippocampus in vitro remarkably stable repetitive activation of neuronal assemblies characterize SPW‐Rs. When the slice is exposed to the cholinergic‐muscarinergic agonist carbachol, SPW‐Rs are replaced with gamma oscillation and in the recovery period the waveform of SPW‐Rs is significantly altered, implying that the spike assembly content of SPW‐Rs depends on the firing patterns of neurons in the pre‐SPW‐R epochs (Zylla et al., 2013; Hofer et al., 2015; see also Kubota et al., 2003), mimicking the in vivo situation where assembly events in the waking animal predict the spike sequences during subsequent SPW‐Rs (Buzsáki, 1989; Wilson and McNaughton, 1994; Taxidis et al., 2015).
Following its release into the extracellular space from the presynaptic terminal, acetylcholine is split into choline and acetate by cholinesterase and choline is transported back into the terminal for re‐synthesis of acetylcholine (Sarter and Parikh, 2005). The local elevation of the precursor choline can selectively activate α7‐ containing nicotinic acetylcholine receptors, which are widely expressed in hippocampal interneurons, especially those in stratum lacunosum‐moleculare (Alkondon et al., 1999; Alkondon and Albuquerque, 2001, 2002). In addition, choline can suppress excitatory synaptic transmission in CA1 via facilitation of inhibition by nicotinic receptors (Mielke et al., 2011). In mouse hippocampal slices, choline efficiently suppresses spontaneously occurring SPW‐Rs and can induce gamma oscillations. Local application of choline decreased the rate of SPW‐Rs to ∼60% of the baseline values in the CA1 region. These effects were only partially blocked by muscarinic receptor blocker atropine, whereas a mixture of both muscarinic and nicotinic receptor antagonists exerted a full block. In addition, choline reduced synaptic transmission between hippocampal subfields CA3 and CA1, as demonstrated by the decrease of evoked responses in response to stimulation of the Schaffer collaterals. The effects of choline were mediated by stimulation of both α7‐containing nicotinic acetylcholine receptors and muscarinic‐1 acetylcholine receptors (Fischer et al., 2014). The muscarinic effects may have been mediated by activation of leak K+ currents, M currents, Ca2+‐dependent K+ currents (Benardo and Prince, 1982; Halliwell and Adams, 1982; Cole and Nicoll, 1983) or reduction of excitatory synaptic transmission (Hounsgaard, 1978; Dutar and Nicoll, 1988; Hasselmo and Schnell, 1994; Hasselmo, 1995; Egorov et al., 1999). It remains to be investigated whether endogenous levels of choline can influence cholinergic activation in the intact brain. Nevertheless, support for the role of muscarinic cholinergic mechanisms in suppressing SPW‐Rs comes from experiments in which the muscarinic agonists drug pilocarpine and carbachol disrupted SPW‐R both in vivo and in vitro in the CA1 region of mice, and the in vitro effects were reversed by blocking muscarinic cholinergic receptors by atropine (Norimoto et al., 2012).
The in vitro findings demonstrate that acetylcholine interferes with several mechanisms required for SPW‐R generation. In the behaving mouse, the most prominent and consistent effect of optogenetic stimulation of MS cholinergic neurons is decreasing the probability of occurrence of SPW‐Rs recorded in the CA1 pyramidal layer even at low stimulation intensities, which do not induce theta oscillations (Vandecasteele et al., 2014). Conversely, systemic application of atropine (50–100 mg/kg) increases the amplitude SPW‐Rs in intact rats (Buzsáki, 1986) and affects the incidence of SPW‐Rs (Suzuki and Smith, 1988d). However, the drug‐induced locomotor hyperactivity under the influence of atropine is a potential confound which should be considered for in the evaluation of the drug effect. Overall, these findings support the antagonistic relationship between SPW‐Rs and theta oscillations (Buzsáki et al., 1983).
The effect of histamine on SPW‐Rs share many similarities with acetylcholine. Intracerebroventricularly administered histamine suppresses SPW‐Rs (Knoche et al., 2003). Administration of H1‐antagonists pyrilamine and ketotifen strongly increases their incidence, while the H2‐ and H3‐antagonists cimetidine and thioperamide are ineffective.
These findings show that histamine released from the axon terminals of the tuberomammillary neurons (Mochizuki et al., 1991) exerts a tonic suppression influence on SPW‐Rs. The effect may occur in the hippocampus or mediated by histaminergic excitation of septal cholinergic neurons (Knoche et al., 2003).
The effect of serotonin on SPW‐R has been little studied. Wang et al. (2015) recorded from median raphe neurons in freely moving mice. They found a negative relationship between the firing of putative serotoninergic neurons and hippocampal SPW‐Rs, with serotoninergic neurons decreasing their activity just before occurrence of SPW‐Rs.
Catecholamines
Norepinephrine (NE; 10–50 μM) and phenylephrine (100 μM) dose‐dependently and reversibly suppressed the generation of SPW‐Rs in vitro via activation of α1 adrenoreceptors (Ul Haq et al., 2012). In contrast, the nonspecific β adrenoreceptor agonist isoproterenol (2 μM) significantly increased the incidence of SPW‐Rs within the CA3 network. Suppression of SPW‐Rs by NE was associated with a moderate hyperpolarization in the majority of CA3 pyramidal cells and with a reduction of presynaptic Ca2+ influx and consequent decrease of glutamate release from terminals in stratum radiatum. In the presence of NE, repeated high frequency stimulation failed to induce SPW‐Rs, although SPW‐Rs appeared following washout of NE (Ul Haq et al., 2012). These data indicate that the NE‐mediated suppression of hippocampal SPW‐Rs depends on α1 adrenoreceptor activation, while their expression and activity‐dependent induction is facilitated via β1‐adrenoreceptors.
The effects of NE and SPW‐Rs are reciprocally related. NE neurons in the locus ceruleus are usually quiescent during non‐REM sleep. However, following odor‐reward association learning or extinction, locus ceruleus neurons emit prolonged discharge (Eschenko and Sara, 2008) at a frequency usually associated with wakefulness. Such pairing of increased NE release and SPW‐Rs during sleep may be important for assisting SPW‐R‐related depolarization and plasticity in target circuits. The relationship between SPW‐R density and enhanced activity of locus ceruleus neurons remains to be studied.
The effect of dopamine on SPW‐Rs is not intensely investigated. Miyawaki et al. (2014) demonstrated that a brief bath‐application of dopamine to mouse hippocampal slices induced a long‐lasting increase in the probability of SPW‐Rs events, which was mediated by dopamine D1/D5 receptor activation. The D1/D5 receptor activation did not increase ripple power or the number of CA1 neurons that were activated in single SPW‐Rs but the drug perfusion reorganized combinations of neurons co‐participating in SPW‐R events. The dopaminergic effects may also be related to the increased participation of CA3 pyramidal cells in SPW‐Rs when rewarded in a novel environment (Singer and Frank, 2009).
Adenosine, Cannabinoids
Activation of cannabinoid receptor (Freund et al., 2003) reduces the probability of occurrence SPW‐Rs in rats (Robbe et al., 2006) and mice (Maier et al., 2012; Sun et al., 2012) without affecting ripple frequency. Similar suppression occurs in vitro, where suppression was paralleled by a selective reduction of SPW‐R‐associated inward but not outward charge transfer, suggesting a reduction of glutamate release (Maier et al., 2012). In contrast to subcortical neuromodulators, which also suppress SPW‐Rs but promote gamma oscillations, CB1 receptor agonists suppress gamma oscillations as well. The hypothesized presynaptic mechanisms of CB1 agonists affecting transmitter release is supported by the finding that adenosine acting on presynaptic A1 receptors mimicked and occluded the effects of cannabinoids on SPW‐Rs (see also Colgin et al., 2004). Inhibition of glutamatergic excitation can contribute to cannabinoid‐induced impairment of hippocampus‐dependent memory (Robbe et al., 2006; Robbe and Buzsáki, 2009).
Opioids
The hippocampus contains relatively high levels of μ‐opioid receptors (Zastawny et al., 1994) that are most strongly expressed in perisomatic interneurons (Drake and Milner, 2002). Nanomolar concentrations of the μ‐opioid receptor agonists (e.g., morphine, DAMGO, and fentanyl) increase the occurrence and amplitude of SPW‐Rs in ventral hippocampal slices of rats in vitro. The low concentrations of fentanyl are comparable to the endogenous μ‐opioids in the rat brain (Lam et al., 2008). At large (µM) concentrations the effects were reversed. All drug‐induced effects were reversed by the μ‐opioid receptor antagonist naloxone (Giannopoulos and Papatheodoropoulos, 2013). Two mechanisms may contribute to the increased network excitability underlying SPW‐R potentiation. First, μ‐opioids can increase the excitability of the pyramidal cells (Zieglgansberger et al., 1979; Masukawa and Prince, 1982). Second, μ‐opioids reduce synaptic inhibition in pyramidal neurons by hyperpolarizing GABAergic interneurons especially those that project to the perisomatic domain of principal neurons (Madison and Nicoll, 1988; McQuiston, 2001). μ‐opioids are implicated in memory consolidation (Izquierdo, 1979).
HCN, Calcium Channels, and CNP
Hyperpolarization‐activated cyclic nucleotide‐gated (HCN) channels regulate cellular excitability and synaptic signal integration (Biel et al., 2009) and subunits HCN1–HCN4 are differentially expressed in various brain regions (Lörincz et al., 2002; Notomi and Shigemoto, 2004). HCN blockers ZD 7288, cilobradine, and ivabradine, when bath applied to mouse hippocampal slices, reduce the probability of occurrence of SPW‐Rs. ZD7288 and cilobradine, increase SPW amplitudes and the number of ripple waves per SPW. However, the HCN‐blocking agents do not affect ripple frequency or spike coupling to ripples (Kranig et al., 2013). The results are similar when the drug is applied to CA3 but not to CA1 region. One potential mechanism of HCN channel blockers is reducing hyperpolarization‐induced rebound excitability of CA3 neurons (Cobb et al., 1995; Stark et al., 2013). HCN channel manipulations on SPW‐Rs are independent of potential side effects of these drugs on T‐type calcium channels, since more specific T channel blockers, ethosuximide and mifrebradil, reduce the amplitude of SPW‐R (Kranig et al., 2013). The two major types of soma‐targeting interneurons are the PV and CCK basket cells and GABA release from their terminals is regulated by P/Q and N‐type Ca2+ channels, respectively (Wilson et al., 2001; Hefft and Jonas, 2005; Szabo et al., 2014). Blockade of N‐type channels by Ω‐conotoxin has no effect on SPW‐Rs in the CA3 region in vitro, whereas when Ω‐agatoxin (a P/Q channel blockers) is infused locally into the pyramidal layer, the amplitude and rhythmicity of ripples are reduced (Schlingloff et al., 2014). These findings support the view that PV but not CCK basket neurons are critical for ripple frequency pacing of the pyramidal cells (Klausberger et al., 2005; Lasztóczi et al., 2011; Hájos et al., 2013).
C‐type natriuretic peptide (CNP) is highly expressed in the hippocampal regions CA1–CA3 (Herman et al., 1996). Bath application of CNP reduces the incidence of SPW‐Rs, an effect that might be related to the hyperpolarization of CA3 pyramidal cells but it does not affect the frequency or duration of ripples or GABAergic inhibition during SPW‐Rs (Decker et al., 2009). CNP effects anxiety behavior (Biró and Telegdy, 1996; Montkowski et al., 1998) as well as learning and memory processes (Telegdy and Nyerges, 1999).
Overall, the above‐discussed experiments demonstrate that a great deal has been uncovered about the potential role various neurotransmitters and neuromodulators in controlling various aspects of SPW‐Rs. All behaviorally arousing subcortical neurotransmitters and drugs tend to decrease SPW‐Rs and shift the brain state from SPW‐Rs to gamma oscillations. Many drugs that affect SPW‐Rs also affect memory. However, since most experiments have been conducted on in vitro models and because there is a large variability of the in vitro models of SPW‐Rs across laboratories, caution should be exercised for the interpretation of the exact mechanisms of drugs on SPW‐Rs in the intact brain. In vivo, an additional complication is that drugs which tend to generate locomotion and general “arousal” can block SPW‐Rs through hidden, intermediate variables. Therefore, in the evaluation of any drug or transmitter effect, it is of utmost importance to “clamp” behavior so that the comparison is made under identical motoric conditions.
SPW‐Rs and Drug Discovery
The SPW‐R is a discrete, physiologically distinct event in the hippocampal system. It is a physiologically well‐characterized, well‐understood robust phenotype, embedded in a memory circuit. The SPW‐R is a “built‐in” sensor of hippocampal excitability and as such it is an easily quantifiable mesoscopic phenotype to screen and test drug effects. Since it is critically involved in the consolidation of episodic memory (see SPW‐R‐Supported Memory Consolidation section), SPW‐Rs lend themselves for testing the potential physiological effects of drugs on memory stabilization. Overall, the pharmacological experiments both in vivo and in vitro demonstrate that neurotransmitters, neuromodulators and drugs that reduce glutamatergic transmission directly or indirectly and/or hyperpolarize pyramidal neurons reduce the probability of occurrence of SPW‐Rs and may affect other features of SPW‐Rs. Many of the same neurotransmitters/modulators and drugs in the same doses promote theta and gamma oscillations. Although such effects are usually interpreted as beneficial for enhancing learning, they may impair memory consolidation. Indeed, the effects of drugs on memory have to be regarded separately for the acquisition, consolidation, and recall phase and for different memory systems (Olton et al., 1991). For example, the cholinomimetic physostigmine (which increases acetylcholine levels by blocking its breakdown) has long been used clinically to enhance memory function in amnesic syndromes (Peters and Levin, 1977), yet it can impair memory consolidation of paired‐associate wordlists when the drug is given before sleep (Gais and Born, 2004). One of the possible mechanisms of such brain state‐dependent effect of the drug is the suppression of SPW‐Rs due to cholinergic presynaptic inhibition of glutamate transmission at CA3 collaterals (Hasselmo, 1995; Hasselmo, 1999). These experiments illustrate that drug effects are strongly brain state‐dependent and appropriate timing within the sleep‐wave cycle can enhance (or deteriorate) the memory‐enhancing effects of drugs.
DISCHARGE PATTERNS OF INHIBITORY NEURONS DURING SPW‐RS
SPW‐Rs are delicately controlled by inhibitory interneurons. Inhibitory interneurons represent a minority (∼11%) of the total CA1 neuron population, and parvalbumin (PV) cells comprise approximately a quarter of the interneurons in the rat (Bezaire and Soltész, 2013). There are many different families of interneurons in the cortex (Freund and Buzsáki, 1996; McBain and Fisahn, 2001; Soltész, 2005; Klausberger and Somogyi, 2008; Fishell and Rudy, 2011; Kepecs and Fishell, 2014) and hippocampal interneurons show diverse firing patterns during SPW‐Rs from potent increase to silence. Classification of hippocampal interneurons exploits their firing relationship to SPW‐Rs (Csicsvari et al., 1999a, 1999b; Klausberger et al., 2003, 2004, 2005). Interneurons contribute to both the time‐protracted recruitment of pyramidal cells during SPWs and the temporal patterning of the sequentially active pyramidal cell assemblies during ripples.
CA1 Interneurons In Vivo Under Anesthesia
Perhaps the most critical interneuron type in the control of ripple generation is the PV type of basket cell, although the role of other interneurons, to date, is less explored. In vivo intracellular recording from a histologically identified PV basket interneuron under urethane anesthesia showed large amplitude depolarizations during SPW‐Rs and high frequency spiking with spikes coinciding with most or all extracellularly recorded ripples waves (Ylinen et al., 1995). When the spike was absent, ripple wave‐related depolarization was still prominent. Combined with results from extracellularly recorded fast spiking interneurons, it was hypothesized that basket cells provide the critical timing signal for the CA1 pyramidal neurons during ripple oscillations (Ylinen et al., 1995). The first systematic analysis of the roles of histologically identified interneurons in SPW‐Rs was performed in an elegant series of studies by Somogyi, Klausberger et al., using juxtacellular recordings in urethane‐ketamine anesthetized rats (Klausberger et al., 2003, 2004, 2005; cf. Klausberger and Somogyi, 2008). These studies showed that CA1 PV‐expressing basket and bistratified cells fired time‐locked to SPW‐Rs, whereas PV‐expressing axo‐axonic (AAC, chandelier) and O‐LM cells were suppressed (Fig. 11). The suppression of AAC neurons during SPW‐Rs is likely advantageous for information transmission, because their silence can increase the excitability of pyramidal cells so that, in turn, the population discharge of pyramidal cells can powerfully impact downstream neurons.
Figure 11.
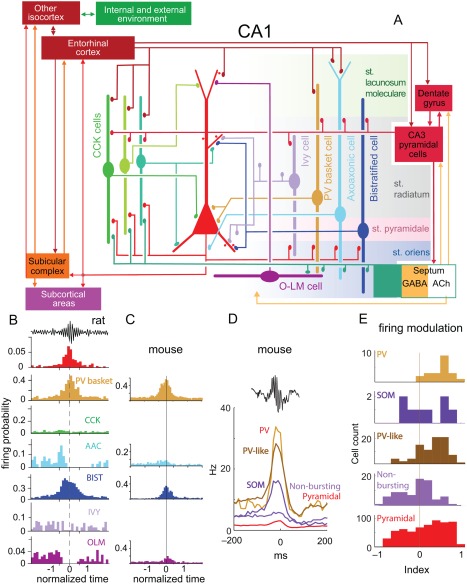
SPW‐R‐related firing patterns of interneurons. (A) Schematic of the main synaptic connections of pyramidal cells (red, middle), three types of CCK‐expressing cells (basket cell, perforant path‐associated cell, Schaffer collateral‐associated cell), ivy cells and PV‐expressing basket, axo‐axonic, bistratified and O‐LM interneurons. Connections among interneurons are not shown. (B) Firing probability histograms; averages from several cells of the same type recorded in anaesthetized rats. Note different scales for the y‐axis. (C) Firing probability histograms for non‐anesthetized mice. In B and C all neurons were labeled juxtacellularly and identified histologically. (D) Firing patterns of optogenetically identified PV‐expressing and SOM‐expressing interneurons during SPW‐Rs. Also shown are putative, physiologically characterized interneurons. Note that almost all PV and PV‐like neurons were robustly active during ripples, whereas the firing behavior of SOM, non‐bursting and pyramidal cells was variable. (E) Distribution of ripple modulation index in the five cell groups. The bimodal behavior of SOM neurons may represent a mixture of O‐LM (ripple‐activated) and bistratified (ripple suppressed) interneurons. Modulation index: the mean firing rate between −150 and −50 ms is subtracted from the mean rate between −50 and 50 ms and normalized. Positive (negative) indexes indicate an increase (suppression) of firing during SPW‐Rs. A and B, reproduced from Somogyi et al., (2014). C, Reproduced from Varga et al. (2012). D, E, reproduced from Royer et al. (2012).
Several interneuron types have most of their dendritic arbors in the axon termination zone of CA3 pyramidal cells and may also receive recurrent input from CA1 pyramidal cells, yet they do not appear to be strongly affected by SPW‐Rs. One of these is the inhibitory “ivy” cell with fine dense axons that innervate mostly basal and oblique pyramidal cell dendrites, and whose slow discharge activity appears undisturbed by SPW‐Rs (Fuentealba et al., 2008b, 2010 2010; Lapray et al., 2012). A subset of basket cells expressing the peptide cholecystokinin (CCK) innervate the same perisomatic domain as do the fast firing PV‐expressing basket cells but most anatomically verified CCK interneurons fire at a low rate during non‐theta states and also show little change in discharge frequency during SPW‐Rs under anesthesia in both CA1 (Klausberger et al., 2005) and CA3 (Lasztóczi et al., 2011) regions. It is not clear why CCK neurons remain silent during SPW‐Rs but their suppressed activity may contribute to disinhibition of both pyramidal neurons and PV basket cells, which they innervate (Freund and Katona, 2007; Karson et al., 2009). Nerve terminals of CCK‐expressing GABAergic neurons are richly endowed with CB1 cannabinoid receptors (Katona et al., 1999), and by a retrograde process they mediate the suppression of GABA release upon strong depolarization by their target pyramidal cells. The same retrograde mechanism is in action also for dendrite‐targeting CCK‐expressing interneurons. Since strongly bursting pyramidal neurons can, by this mechanism, potentially induce their own disinhibition in the entire somato‐dendritic domain (Freund, 2003; Freund et al., 2003; Freund and Katona, 2007; Katona and Freund, 2008), understanding the role of CCK interneurons in the auto‐disinhibition of pyramidal neurons is an important task for future research in the drug‐free animal. Their interaction with somatostatin (SOM)‐expressing interneurons, such as the O‐LM cells, is critical, since SOM‐immunoreactive neurons can strongly attenuate bursting of pyramidal cells in both the hippocampus (Royer et al., 2012; Lovett‐Barron et al., 2012) and neocortex (Gentet et al., 2012). It should be noted that auto‐disinhibition of active/bursting pyramidal neurons is also possible by different mechanisms of retrograde signaling. Nitric oxide (NO)‐sensitive guanylyl cyclase (NOsGC) is present in the presynaptic boutons of PV‐expressing interneurons and, to a lesser extent, in the terminals for SOM‐immunoreactive interneurons (Szabadits et al., 2007). On the postsynpatic side of the synapse in pyramidal neurons, Ca2+/calmodulin‐dependent enzyme nNOS and Ca2+‐permeable NMDARs are present in the postsynaptic zone (Szabadits et al., 2007, 2011). Such retrograde signaling, in principle, would be an effective mechanism to release neurons from perisomatic and dendritic inhibition while suppressing the surrounding competing neuronal assemblies. Whether auto‐disinhibition of highly active pyramidal neurons occurs during SPW‐Rs has to be demonstrated in future experiments.
Another type of GABAergic interneuron, which shows suppressed firing during SPW‐Rs is a small group of endogenous opioid encephalin (ENK)‐expressing cells (Fuentealba et al., 2008a). The cell bodies and most dendrites of ENK‐expressing cells are in the stratum radiatum, while their axons arborize in both stratum radiatum and oriens/alveus, innervating mainly PV but not SOM calbindin‐, or CCK‐expressing interneurons. Some branches also enter the subiculum where they exclusively target interneurons. While ENK interneurons are silenced during SPW‐Rs, they exhibit rebound activity of high‐frequency spike bursts, presumably causing peptide release and may contribute to the suppressed activity of both interneurons and CA1 pyramidal cells during the post‐ripple silence (Fuentealba et al., 2008a; English et al., 2014).
Interneurons with long‐range axons, such as backprojection cells (Sik et al., 1994, 1995), hippocampo‐septal cells (Toth et al., 1993; Gulyas et al., 1999), and double projection interneurons (Jinno et al., 2007) also change their firing patterns during SPW‐Rs, some of them with increased firing rates and coupled to individual ripple cycles (Jinno et al., 2007; Fuentealba et al., 2008a). Since long‐range interneurons innervate the basal and proximal apical dendritic region of CA1 pyramidal cells, they may act in concert with bistratified cells and, at the same time, suppress their mostly interneuron targets in the septum, hippocampus, subiculum and other retrohippocampal areas (Klausberger and Somogyi, 2008). Trilaminar interneurons (Sik et al., 1995) fire high frequency burst of action potentials and are consistently active during SPW‐Rs in anesthetized (Ferraguti et al., 2005) and in freely moving rats (Linda Katona and Peter Somogyi, personal communication).
Interneurons in CA2‐3 Regions
Similar to the CA1 region, most interneurons in the CA3 region are robustly coupled to SPWs and local ripples, but not to CA1 ripples, which demonstrates that ripple events are expressed locally in each area of the temporal lobe (Buzsáki, 1986; Ylinen et al., 1995; Chrobak and Buzsáki, 1996; Sullivan et al., 2011). However, the firing relationship of interneuron types to SPW‐Rs in the CA3 region in vivo is poorly understood. PV‐immunoreative basket cells in CA2‐CA3 regions fire phase‐coupled to local ripples in CA3 but not to CA1 ripples in urethane‐ketamine anesthetized rats (Tukker et al., 2013). As in CA1, the discharge frequency of AAC is decreased during SPWs in the CA3 region (Viney et al., 2013), leading to the hypothesis that silencing of CA3 AAC neurons contributes to SPW burst generation (Somogyi et al., 2014).
Interneurons in Behaving Animals
There are two direct approaches to identify GABAergic interneuron classes in the behaving animal. The first is the extension of juxtracellular labeling for drug‐free behaving animals, followed by histological analysis. The advantage of this method is that only a single neuron is labeled in a given hippocampus, thus the soma and dendrites of the same cell can be labeled by multiple antibodies and that the labeled axon collaterals may identify the precise somadendritic targets (Lapray et al., 2012). However, this method can typically label only a single neuron per animal and long‐term recordings are rarely possible. Waking, head‐fixed preparations simplify the recording gear (Varga et al., 2012), although head restraint may affect the quality of sleep, which may be detrimental in sleep analysis of SPW‐Rs. The second method is a combination of recording and optogenetic techniques (Boyden et al., 2005). This latter approach provides a solution to identify specific genetically defined neuronal subtypes in blind extracellular recordings by expressing light‐sensitive opsins in a given neuronal population (Roux et al., 2014). The optogenetic technique also allows several neurons to be simultaneously activated or inactivated, thus allowing probing the functional contribution of specific neuron classes. A disadvantage of the optogenetic method is that transgenic lines often label multiple overlapping classes of neurons. This can be overcome by generating novel specific lines in the future or combining optogenetics with intracellular or juxtacellular labeling methods, allowing for targeted search for the desired neuron type and labeling of single neurons (Muñoz et al., 2014).
PV‐expressing interneurons
Since firing rates and patterns of interneurons are strongly affected by anesthetics, it is not surprising that differences of SPW‐R‐related discharges have been discovered between anesthetized and waking preparations, along with numerous similarities. PV basket cells in freely behaving rats showed robust discharge during SPW‐Rs, whereas firing rates of ivy interneurons were not significantly modulated (Lapray et al., 2012), although the low overall firing rates of the ivy neurons make it difficult to unambiguously identify lack of suppression during SPW‐Rs. Optogenetically identified PV interneurons in both head‐fixed (Royer et al., 2012; Varga et al., 2012) and freely behaving/sleeping mice (Stark et al., 2014) fire robustly and at high frequency during SPW‐Rs. However, even within the PV group, sublaminar differences in the CA1 pyramidal layer were noted. The deep and superficial sub‐strata of the CA1 pyramidal neurons have different physiological features (Mizuseki et al., 2011). Superficial (bordering str. radiatum) pyramidal neurons are more likely to be entrained by SPW‐Rs compared with deep (bordering str. oriens) pyramidal cells (Stark et al., 2014). Conversely, a larger fraction of deep than superficial interneurons was entrained during SPW‐Rs. Superficial (bordering str. radiatum) pyramidal cells and interneurons fired earlier than deep (bordering str. oriens) cells during the ripple cycle (Stark et al., 2014). These SPW‐R‐related differences can be explained by related findings by Lee et al. (2014), who reported stronger excitation of PV basket cells by superficial pyramidal cells and, conversely, stronger PV basket cell‐mediated inhibition of deep layer pyramidal cells, indicating the PV basket cells contribute to the functional segregation of deep and superficial CA1 pyramidal neurons. Intracellular recordings in vivo support this framework by showing preferential depolarization in superficial and preferential hyperpolarization in deep layer CA1 pyramidal cells and CA2 pyramidal neurons (Valero et al., 2015).
The contribution of AAC interneurons to SPW‐Rs is not well understood. AAC cells innervate the axon initial segment of pyramidal cells and comprise a small fraction of hippocampal PV interneurons (Freund and Buzsáki, 1996; Klausberger and Somogyi, 2008). Similar to the anesthetized rat (Somogyi et al., 2013), a CA1 and a CA2 AAC cell were suppressed during SPW‐Rs recorded in the naturally sleeping rat (Viney et al., 2013). In contrast, some AAC neurons in the waking mouse increased, rather than decreased their firing rates (Varga et al., 2014). The seemingly contradictory findings may be explained by the differential innervation of subgroups of AAC neurons. AAC neurons in the CA1 pyramidal cell layer were either not influenced at all or decreased their discharge rates during SPW‐Rs. In contrast, two juxtacellularly recorded, biocytin‐labeled and histologically verified AAC neurons in str. oriens showed increased discharge during SPW‐Rs. The responding AACs fired phase‐locked to the ripple waves ∼1.4 ms after the basket cells (Varga et al., 2014). These anatomical location‐based differences in firing patterns may be explained by the higher density of basket cell terminals in the pyramidal layer so that AAC neurons in the pyramidal layer can be more strongly inhibited by the PV basket cells than AAC neurons residing in the str. oriens. However, direct evidence for the hypothesized basket neuron innervation of AAC cells is still missing. In addition, a subgroup of AAC neurons may receive differential inhibition from bistratified interneurons or the medial septal GABAergic projection (Viney et al., 2013), and such difference may explain their differential involvement in SPW‐Rs. It remains uncertain whether these subcircuit‐specific firing patterns of AAC interneurons also contribute to the differential firing patterns of deep and superficial layer pyramidal cells (Mizuseki et al., 2011). Another insight for explaining the variable firing patterns of AAC across and within experiments comes from observations in the barrel cortex (Zhu et al., 2004). During single whisker stimulation, many interneuron types, including basket cells, net basket cells, double bouquet cells, bitufted cells and neurogliaform cells, respond to whisker stimulation, whereas the same weak stimulation evokes an IPSP‐EPSP sequence but no firing in AAC interneurons. In contrast, strong stimulation of multiple whiskers induces stronger discharge in AAC cells than in the other interneurons, even though the spontaneous firing of AAC interneurons is low (Zhu et al., 2004). These observations in the neocortex indicate that AAC interneurons have a high and nonlinear activation threshold. Assuming that similar mechanisms are at work in the hippocampus, AAC interneurons would be silenced during small and intermediate size SPW‐Rs but fire multiple spikes during large amplitude SPW‐Rs associated with a high fraction of active pyramidal cells.
The third group of PV interneurons, the bistratified cells (Klausberger and Somogyi, 2008) strongly increase their discharge rates during SPW‐Rs in both waking mice (Varga et al., 2014) and rats (Katona et al., 2014). These interneurons also have at least two subgroups, one with dendrites in both str. radiatum and oriens, whereas dendrites of the other subgroup are confined to str. oriens, with members of the latter group firing at higher frequency during SPW‐Rs. These discharge rate differences are similar to those of the subgroups of basket cells, which are also characterized by the presence or absence of dendritic branches in str. radiatum (Varga et al., 2014). Thus, the discharges by the three cardinal PV interneuron classes are organized to generate a spatiotemporally expanding pattern, from the somata/proximal dendrites to the axon initial segment and mid‐level dendrites by fast GABAergic inhibition in a short (<1.5 ms) time window (Varga et al., 2014). Increased activity of SOM‐expressing, putative bistratified interneurons can suppress spike bursts (Royer et al., 2012). Indeed, during SPW‐Rs bistratified are active and spike burst probability in CA1 pyramidal neurons is reduced relative to the level expected by the increased spike rate (Stark et al., 2014).
O‐LM interneurons
O‐LM interneurons express both PV and SOM (Klausberger et al., 2004). In relation to SPW‐Rs, SOM‐expressing interneurons in the CA1 are bimodally distributed: approximately half increasing and half decrease their discharge rates (Fig. 11) (Royer et al., 2012). Based on their firing relationships to SPW‐Rs, it is tempting to identify these subgroups with the SOM‐expressing bistratified and O‐LM interneurons, respectively (Klausberger and Somogyi, 2008). However, although O‐LM cells decrease their firing rates during SPW‐Rs under anesthesia (Somogyi et al., 2013), in head‐fixed waking mice (Varga et al., 2012) and freely moving rats (Katona et al., 2014) an increase was observed although to a much lesser extent than those of PV basket cells. The differences between anesthetized and waking preparations are important: O‐LM cells receive excitatory inputs from local CA1 pyramidal cells with minimal input from CA3 pyramidal cell axon collaterals (Takács et al., 2012) and they can control the major direct entorhinal input onto CA1 pyramidal cells. Recordings from non‐anesthetized animals suggest that this input is suppressed during SPW‐Rs, rather than disinhibited. However, the differential effect may not only be between anesthesia and its absence because the same O‐LM interneurons occasionally showed increased firing during waking SPW‐Rs while their activity was suppressed during SPW‐Rs of slow wave sleep (Katona et al., 2014). Furthermore, SPW‐Rs recorded in non‐anesthetized mice may correspond to waking ripples or shallow sleep since sleep in head‐fixed animals is not natural. The overall picture is complicated by the traveling nature of SPW‐Rs (Patel et al., 2013). At a given septotemporal level, O‐LM interneurons may be silenced, while their activity in the surrounding segments is enhanced. Only high‐resolution, simultaneous recordings from the long axis of the hippocampus can properly resolve these issues. Alternatively, the pattern of activity in neuronal assembly may determine whether O‐LM cells (and other interneurons) are activated or suppressed. If O‐LM neurons are activated by a subgroup of synchronously firing pyramidal cells, they can laterally inhibit the surrounding population of neurons. Yet another explanation for the activity‐dependent recruitment of O‐LM interneurons is the strongly potentiating nature of the pyramidal cell – O‐LM interneuron synapse (Ali and Thomson, 1998). Thus, while the majority of SPW‐Rs with relatively low fraction of spiking pyramidal cells may fail to activate O‐LM cells, a minority of SPW‐Rs with strongly synchronous and prolonged activity (Mizuseki and Buzsáki, 2013) can effectively recruit them.
Overall, these recent findings demonstrate the vital importance of micro‐circuit wiring patterns in the functional control of network excitability. Further work is required to refine the anatomical connections and relate them quantitatively to the firing patterns of the individual network components in drug‐free behaving animals. SPW‐Rs in the waking and sleeping animal may be performed by different interneuronal activity patterns. Such detail will simplify our understanding of the dynamics underlying SPW‐Rs. A particularly puzzling issue is the role of O‐LM interneurons—why is it advantageous disinhibiting the entorhinal input and facilitating action potential backpropagation during a CA3‐triggered population event. Alternatively, O‐LM interneurons may serve to segregate inputs from lateral and medial entorhinal cortex or from layer 3 and layer 2 inputs (Kitamura et al., 2014). Further work is needed to elucidate the role of other types of interneurons in segregating pyramidal cell assemblies (see SPW‐R‐Supported Memory Consolidation section) during the course of SPW‐Rs.
Interneuron firing during SPW‐rs in humans
The firing patterns and timing of different neurons during SPW‐Rs in non‐rodent species is much less studied. In humans (Le van Quyen et al., 2008), spectral analyses of SPW‐R show that ripple frequency is slower (80 and 160 Hz, with a mode at 100 Hz) than in rats and mice and closer to the 100–120 Hz oscillations observed in monkeys (Skaggs et al., 2007; Logothetis et al., 2012). Pyramidal cells fired maximally at the peak of the SPW‐R event and later than the peak firing of putative interneurons. A large proportion of pyramidal neurons and putative interneurons were phase‐locked to the ripple oscillations and the preferred phase of discharge of interneurons followed the maximum discharge probability of pyramidal neurons (Fig. 12). Thus, the physiological features of SPW‐Rs are qualitatively similar to those in vivo in rodents (Le van Quyen et al., 2008), and imply similar underlying mechanisms are also similar.
Figure 12.
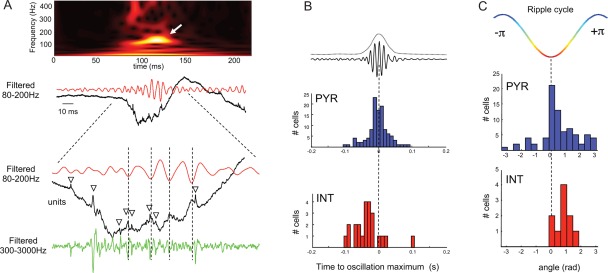
Neuronal correlates of SPW‐R in the human hippocampus. (A) Single SPW‐R (black trace), its filtered version (red trace) and time–frequency representation (top). The expanded trace (bottom panel) shows spikes (triangles) during the oscillations, mostly occurring around the negative peak of ripple cycle. (B) Temporal relationship between the maximal neuronal discharge (red, interneurons; blue, pyramidal cells) and the highest peak of the ripple power. (C) Cumulative histogram of preferred firing phase for pyramidal cells (blue) and interneurons (red). Reproduced from Le van Quyen et al. (2008).
Interneurons In Vitro
In contrast to the in vivo investigations, most work on hippocampal interneurons in vitro has been performed in the CA3 region. Hajos et al. (2013) comprehensively studied the role of interneurons in SPW‐R generation in CA3 region of mouse hippocampal slices. They investigated the firing patterns of 8 different types of interneurons, including PV‐expressing neurons, CB1 receptor‐expressing (CCK) neurons, AAC cells, O‐LM cells, bistratified (oriens‐radiatum) interneurons, ivy cells, oriens‐oriens cells (O‐O; with both dendrites and axons confined to str. oriens) and radiatum interneurons (RAD; with both dendrites and axons confined to str. radiatum). Virtually all interneurons increased discharge rates during SPW‐Rs although to different degrees. Nearly two thirds of the recorded interneurons fire maximally before the peak of the LFP SPW. PV basket cells are the most active neurons during SPW‐Rs, followed by O‐LM interneurons; ivy cells were silent. Patch clamp analysis of the synaptic currents during SPW‐Rs uncovered that the dominant synaptic input to most pyramidal cell is inhibitory, whereas spiking interneurons receive larger synaptic excitation than inhibition. The discharge frequency and timing of the interneurons is determined by the magnitude of synaptic excitation. As suspected from in vivo data, AAC received the strongest inhibitory conductances, although their excitatory conductances during SPW‐Rs resemble those of PV basket cells. These findings suggest that AACs receive more numerous and/or stronger inhibitory synaptic inputs from PV basket cells than basket cells from each other (Zhu et al., 2004). RAD and CCK interneurons received both weak excitatory and inhibitory drive and, accordingly, their spike contribution to SPW‐Rs was also weak. These physiological observations echo the circuit embedding of these interneurons, since PV basket cells are innervated by three times more excitatory, but the same number of inhibitory synapses, than CCK basket cells (Gulyas et al., 1999; Matyas et al., 2004). The relative magnitude of discharge activity of O‐LM cells was comparable to that reported in the CA1 region of head‐fixed mice (Varga et al., 2012). The ratio of inhibition and excitation was strongest in CA3 pyramidal cells and, as a result, most of them were silent during SPW‐Rs (Fig. 13).
Figure 13.
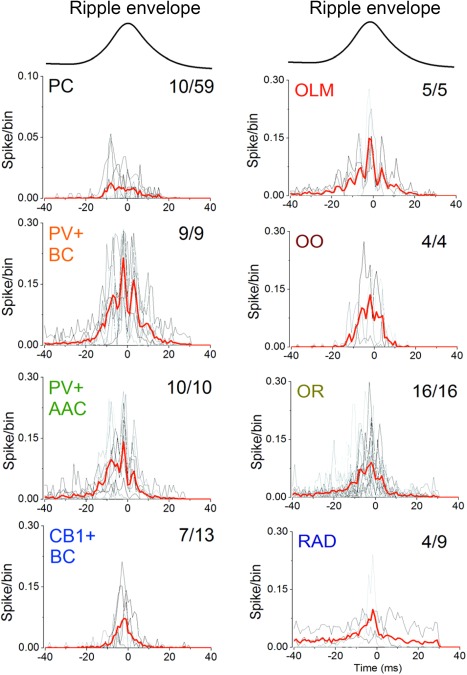
Spike distribution histograms of CA3 neurons during SPW‐Rs in vitro. (A) Spike distribution histograms shown for individual neurons (gray) and their average (red) relative to the peak of the SPW‐R envelop. Numbers in the top‐right indicate the number of neurons that discharged during SPW‐Rs from all recorded and anatomically identified neurons. Note that all interneurons increase their discharge rates during SPW‐R. Reproduced after Hájos et al. (2013).
Another key finding from of Hájos et al. (2013) concerns temporal dynamics of synaptic inputs experienced by the different interneurons. While the ratio of excitation and inhibition during the course of SPW‐Rs is comparable in several interneuron types, AAC neurons are more strongly driven before the peak of the SPW‐R event than after the peak, suggesting that they are excited mainly by the early discharging pyramidal cells or that the excitatory synapses onto AAC neurons are depressing (Zhu et al., 2004). By contrast, inhibition is stronger in the late part of the SPW‐R, likely mediated by maximally firing PV basket cells.
The finding that O‐LM interneurons receive a higher ratio of excitation to inhibition during SPW‐Rs was confirmed in the CA1 region of the mouse (Pangalos et al., 2013). Excitation in O‐LM neurons was phase‐locked to the LFP ripples. Among O‐LM neurons, ∼50% spiked during SPW‐Rs and the spikes were delayed by several milliseconds compared with the LFP ripple trough. Spike probability correlated with the magnitude of the respective excitatory input, and, as the magnitude of excitation was larger in activated vs. silent cells. In addition, ripple‐related inhibition is more pronounced in nonspiking vs. discharging O‐LM cells. These findings corroborate observations in head‐fixed, nonanesthetized mice (Varga et al., 2012a) and freely moving rats (Katona et al., 2014).
Quantitative analysis of the firing patterns and timing of interneurons is essential to understand how coordinated inhibition is orchestrated along the somadendritic domains of pyramidal neurons during SPW‐Rs. Describing the precise wiring of the interneuron types is an important step in understanding the initiation SPWs and the mechanisms controlling excitation and inhibition during ripples. Since SPW‐R magnitude can vary by orders of magnitude with very large range of participation probability and spike bursting of single neurons, future studies need to address how interneuron recruitment and timing relate to the size of the LFP SPW‐R. Maintaining stability during the non‐linearly growing recruitment of neurons into the population burst, each pyramidal neuron must be controlled by appropriately choreographed, individualized inhibition (see Large Dynamic Range and Individualized Inhibition section). A goal is to precisely map the spatio‐temporal evolution of the events that make up the SPW‐R (Klausberger and Somogyi, 2008) and identify the role of interneurons in organizing neuronal assembly sequences. The differences between in vivo and in vitro observations described above likely reflect the larger magnitude and faster frequency of the SPW‐Rs in the slice preparation and the regional differences (CA3 vs. CA1) rather than a fundamental difference of SPW‐ripple generation.
RIPPLES AND FAST GAMMA/EPSILON OSCILLATIONS
In the absence of theta oscillation‐related behaviors, SPW‐Rs as well as other distinct events occur in the hippocampus. This mixed variety inspired Vanderwolf (1969) to refer to this non‐theta event as large (amplitude) irregular activity (LIA). In the waking immobile rodent, LFP events most related to ripples are the bursts of gamma/epsilon waves. Gamma oscillations are supported by multiple mechanisms (Bartos et al., 2007; Buzsáki and Wang, 2012) and in the hippocampus they occur in several varieties: low‐frequency (or “slow”; 30–80 Hz), mid‐frequency (60–120 Hz), and fast or “high” (>100 Hz; epsilon) gamma oscillations (Csicsvari et al., 1999a, 2003b; Canolty et al., 2006; Belluscio et al., 2012). These “gammas” not only occupy largely different frequency bands but are also segregated by the phase of hippocampal theta waves (Colgin et al., 2009; Belluscio et al., 2012; Schomburg et al., 2014) and possibly use different mechanisms (Csicsvari et al., 1999b, 2003a, 2003b; Mann et al. 2005; Whittington et al., 2011). During theta oscillations, packets of gamma waves recur regularly, are phase‐modulated by theta and have low coefficient of variation of their amplitudes. Without theta, the amplitude variability of gamma is high (Bragin et al., 1995a; Csicsvari et al., 2003a, 2003b).
Coordination of Fast Oscillations in CA3 and CA1 Regions
Although CA1 ripples and epsilon bursts are distinct, they share physiological mechanisms and anatomical substrate. In the CA1 region, the frequency distribution of the fast LFP oscillatory episodes during non‐REM sleep is characterized by a definable dip between 130 and 150 Hz, surrounded by distinct peaks at 170–180 and 110 Hz (Fig. 14). An additional dip at 80–90 Hz is also present, reflecting a putative boundary between gamma and epsilon oscillations (Sullivan et al., 2011). The distribution of peak frequencies in CA3 has a similar bimodality, but the main peak occurs in the epsilon oscillation band, rather than at ripple frequency. While both patterns are associated with SPW sinks in the str. radiatum, an indication of the critical involvement of the CA3 region, both ripple and epsilon waves have low coherence (<0.2) between CA3 and CA1, indicating that timing of events are established largely independently in the two regions. Ripples differ from epsilon bursts in their coupling to larger magnitude of SPWs. More precisely, the magnitude of the driving excitatory “force” and oscillatory frequency show an inverted‐U relationship in CA1. Small‐amplitude SPWs are associated with epsilon oscillations, and medium‐size SPWs with either 140–170 or >190 Hz ripples, whereas the largest‐amplitude SPWs consistently co‐occur with 170–180 Hz ripples. The SPW magnitude‐dependence of the oscillation frequency is much weaker in the CA3 region.
Figure 14.
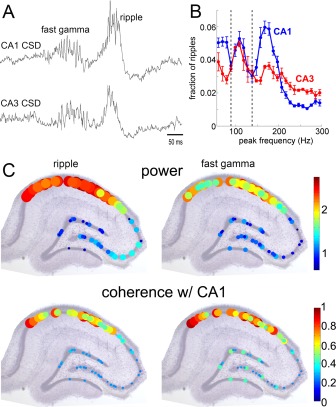
Qualitatively similar and quantitatively distinct features of ripple and fast gamma oscillations. (A) Simultaneously recorded current source density (CSD) traces from CA1 and CA3 pyramidal layers. (B) Normalized power distribution of ripples (measured at 175 Hz) and fast gamma oscillations (110 Hz) in the CA1 and CA3 cell body layers (n = 10 rats). (C) Regional distributions of power within the cell body layers (top) and phase coherence (bottom) with the most medial CA1 pyramidal layer site as the reference. The size and color of circles indicate the magnitude of power and coherence. From Sullivan et al. (2011).
The following hypothesis may explain these complex relationships (Sullivan et al., 2011). CA1 and CA3 networks act as voltage‐controlled oscillators, where SPWs provide the depolarization force and both networks can resonate at 110 Hz or >140 Hz. The converging excitatory drive to CA1 is stronger than the drive brought about by the CA3 collaterals within CA3; therefore under physiological conditions the two networks oscillate typically at different frequencies. Weaker excitation generates ∼110 Hz oscillations in both regions. Because the oscillations can emerge independently in CA1 and CA3, their frequencies might not perfectly match and show low CA3–CA1 coherence. Yet, because of the small frequency difference, some neurons in CA3 and CA1 show phase‐entrainment (Csicsvari et al., 1999b; Sullivan et al., 2011). At stronger excitation, the CA1 network responds with faster (ripple frequency) oscillation than CA3, because of its steeper input‐excitation versus frequency gain in the CA1 region. In fact, during most SPW‐R events, the CA3 region is involved in lower (gamma) frequency activity (Ylinen et al., 1995). Carr et al. (2012) suggest that slow frequency gamma (20‐50 Hz) in the CA3 region is responsible of synchronizing SPW‐Rs bursts in the CA3 region of the two hemipheres, and, in turn synchronizing cell sequentially firing cell assemblies bilaterally in the CA3‐CA1 regions (Pfeiffer and Foster, 2015).
A variation of the above scenario is that fast oscillations in recurrent systems are strongly limited in space by the axon conduction delays (Jahnke et al., 2014). As a result, local population bursts of various magnitude and frequency emerge in multiple “islands” of the CA3 recurrent system. When these events occur simultaneously, their interactions induce an intermediate global ripple frequency. An unexplored hypothesis for the alternation and competition of ripples and epsilon band oscillations is that the fluctuation of the concentrations of subcortical neuromodulators determines which of the two frequencies prevails.
Both anatomical and physiological data support the double‐resonance hypothesis. As discussed in Generation of SPW Bursts—Key Properties of the CA3 Recurrent Network section, the axon collaterals of all pyramidal neurons of the CA3a, CA3b, and CA3c subregions target both CA3 and CA1 populations. In contrast, the CA3c subregion sends only limited numbers of collaterals to other CA3 neurons (Ishizuka et al., 1990; Li et al., 1994; Wittner et al., 2007). Because of the stronger convergence of CA3 afferents, CA1 neurons are more strongly excited during a SPW burst than CA3 neurons themselves. The notion of the stronger excitatory gain in the CA1 region during SPW‐Rs is supported by the relatively higher fraction of pyramidal cell versus interneuron spikes in CA1 compared with the CA3 region, subicular complex and layers of the entorhinal cortex (see Fig. 20) (Mizuseki et al., 2009). Part of the gain increase reflects the weakening of pyramidal cell‐interneuron spike transfer: increasing synchrony of the pyramidal cells limits interneuron spiking. Parallel with the decreasing gain in these networks, the oscillation frequency of SPW‐Rs also decreases from CA1 to entorhinal cortex (Chrobak and Buzsáki, 1996). The double‐resonance framework predicts that sufficiently strong enough excitation of the CA3 network can generate ripples at the same frequency as in CA1. This has been shown in vitro, where both CA3 and CA1 regions generate ripple or higher frequency oscillations (Draguhn et al., 1998; Bragin et al., 1999a, 1999b, 2000 2000; Dzhala and Staley, 2004; Menendez de la Prida and Gal, 2004; Behrens et al., 2005; Both et al., 2008; Liotta et al., 2011). Indeed, CA3 ripples (>150 Hz) generated in vitro (Generation of SPW‐R Bursts In Vitro section) and optogenetic induction of bona fide ripples in multiple hippocampal networks and deep layers of the neocortex (Stark et al., 2014) support this prediction. Computational modeling also demonstrates that propagation of activity is facilitated by resonance, especially in the 150 to 250 Hz range (Jahnke et al., 2014).
Figure 20.
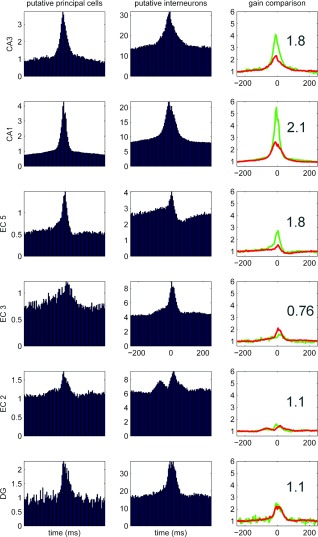
Gain and loss of excitation in different hippocampal‐entorhinal regions during SPW‐Rs. Population means of ripple‐unit cross‐correlograms in CA1, CA3 and dentate gyrus (DG) of the hippocampus and layers II, III, and V of the entorhinal cortex (EC2, EC3, EC5). Principal cells and putative interneurons are shown in the left and middle columns, respectively. Peak of the ripple episode is time 0. Right column, Relative increase of neuronal discharge, normalized to baseline (−200 to 200 ms) for both pyramidal cells (pyr, green line) and interneurons (int, red line). The ratio between the relative peaks of pyramidal cells and interneurons is defined as “gain.” Note largest excitatory gain in CA1, flowed by CA3 and EC5. Gain is balanced in DG and EC2, whereas in EC3 inhibition dominates. Data from Mizuseki et al. (2009).
Two Types of Epsilon Bursts (“Ripples”) in the CA3 Region
The recurrent system of the CA3 region can generate self‐organized population bursts. However, both the timing and, possibly, the spike content of these bursts may be modified by extrahippocampal inputs (see Modulation of SPW‐Rs by Subcortical and Neocortical Inputs section). The CA3 region is controlled by the dentate gyrus output and fast, epsilon band oscillations generated in the dentate‐hilus circuits (Bragin et al., 1995b), can also entrain CA3 neurons. Thus, without simultaneous recordings from dentate, CA3 and CA1 regions, it remains ambiguous whether fast oscillations bursts take part in SPW‐R generation or represent a response to the dentate input. Ripples in the CA3 region can reflect either SPW‐triggered or “dentate spike” (Bragin et al., 1995b)‐induced fast oscillatory events.
Hofer et al. (2015) described two types of ripples (∼200 Hz) in the CA3 regions in vitro that differ mainly in the depth distribution of the currents and sinks. Although typically one type dominated in a particular slice, in a small fraction of slices both events were present. Different sets of pyramidal cells and interneurons were active in the two versions of CA3 ripples. Type 1 ripples had a strong sink in the stratum lucidum, the termination zone of mossy fibers of granule cells and electrical stimulation of mossy fibers could trigger comparable events to type 1 ripples. Yet, blocking mossy fiber activity pharmacologically or cutting the connection between the dentate gyrus and the CA3 region did not abolish type 1 ripples. The possibility still remains though that spontaneous release of neurotransmitter(s) from the mossy terminals (Rex et al., 2009) is critical to induce a type 1 CA3 ripple. In vivo studies are needed to establish firmly whether the CA3 region generates multiple types of fast oscillatory events and examine their relationship to bona fide SPW‐Rs.
TRAVEL OF SPW‐RS IN THE SEPTOTEMPORAL AXIS
In Behavioral Correlates and Mechanisms of SPW Generation section, the author has discussed that the fraction of co‐firing neurons during SPW‐Rs shows very large variability and the distribution of the participating fractions follows a lognormal form. Thus, there is no “typical” size SPW‐R. The estimate of simultaneously active neurons relies on experiments in small volumes, limiting such estimates. Since SPW‐Rs are often local but can also invade large volumes of tissue, exceptionally the entire hippocampus, synchrony estimation only applies to the studied tissue. The emergence of spatially distinct SPW‐Rs is especially important in light of the known topographical organization between the septotemporal segments of the hippocampus and their entorhinal–neocortical targets (Witter et al., 1989; Petrovich et al., 2001; Amaral and Lavanex, 2007). Converging evidence from anatomical, lesion, physiological, and fMRI studies suggests two main communication streams between hippocampus and neocortex (Suzuki and Amaral, 1994; Dolorfo and Amaral, 1998; Ranganath and Ritchey, 2012). The septal third/half is more strongly connected to the dorsomedial entorhinal cortex and the postrhinal cortex (rodent homolog of the parahippocampal cortex in primates) and forms one stream, whereas the ventral third, lateral entorhinal cortex, and perirhinal cortex form another stream (Ranganath and Ritchey, 2012). In turn, the parahippocampal cortex communicates mainly with the “default network” (Raichle et al., 2001), while the perirhinal cortex has stronger connections to the lateral orbitofrontal cortex and the anterior ventrolateral temporal cortex (Ranganath and Ritchey, 2012). The medial and lateral streams have been hypothesized to mediate non‐egocentric and egocentric types of information (Lisman, 2007). It is easy to see how such anatomically segregated streams can differentially affect SPW‐R‐mediated communication between different segments of the hippocampus and these different, although overlapping, neocortical domains.
Despite these topographic relationships, surprisingly few studies examined how SPW‐Rs emerge at different segments of the septotemporal axis of the hippocampus. Simultaneous recordings from the CA1 pyramidal layer in rats indicate that qualitatively similar ripples emerge from the septal, intermediate, and temporal segments of the long hippocampal axis (Fig. 15) (Patel et al., 2013). The probability of SPW‐R occurrence is similar along the entire extent of the septotemporal axis, indicating that each segment of the hippocampus can equally support both SPWs and ripples. However, ripple power is lower in the temporal segment, which can be explained by decreased activation or synchrony of pyramidal cell spikes and/or the lower density of neurons in the temporal hippocampus (Schomburg et al., 2012).
Figure 15.
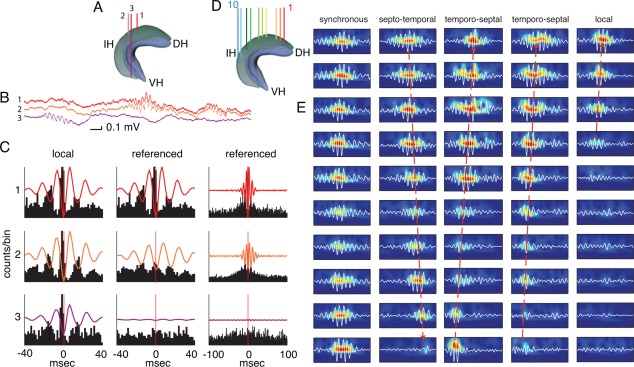
Ripples occur along the entire septotemporal axis of CA1. (A) Diagram indicating the locations of the recording electrodes in the septal and temporal segments of the hippocampus. SH, Septal hippocampus; IH, intermediate hippocampus; TH, temporal hippocampus. (B) LFP traces recorded from the three sites shown in A. (C) Ripple trough‐triggered LFP (100–250 Hz bandpass) and correlated multiple unit activity detected on each electrode (same color code as in A). Middle and right columns show averaged LFP and MUA, but the ripple event times were taken from the most septal reference site (1; reference). The two time scales emphasize ripple wave (middle) and ripple event (right) relationships. Note absence of ripple wave coherence between the septal and temporal segments. (D and E) Examples of single ripple events and their wavelet maps recorded from 10 sites in the septal and intermediate CA1 segments (as in D). Ripple events can spread in a septotemporal or temporoseptal direction (long dashed arrows), but multiple other forms, such as synchronous, or locally confined events are also present. After Patel et al. (2013).
SPW‐Rs can remain local or can invade large segments of the CA1 region. From the local seed, ripples can travel septally or temporally at a speed of ∼0.35 m/s. The extent of the spatial spread depends largely on the magnitude of SPW‐R. SPW‐Rs propagate smoothly across the septal and intermediate segments, but SPW‐Rs in the temporal segment often remained isolated, reflecting functional segregation of the ventral segment and the dorsal/intermediate segments (Royer et al., 2010; Hinman et al., 2011; Patel et al., 2012, 2013). Individual SPW‐R events can display various patterns of propagation (Fig. 15), including locally confined events, sweeping patterns from single or multiple locations, collisions, and reflections, similar to propagating events described previously in the neocortex (Arieli et al., 1996; Roland et al., 2006; Benucci et al., 2007; Xu et al., 2007). Each SPW is a sweep in the longitudinal axis and supported by the spread of excitation in the recurrent collaterals of the CA3 neurons (Ishizuka et al., 1990; Li et al., 1994). In contrast, phase coherence of SPW‐induced ripple waves is confined to a much smaller volume (Fig. 15) because fusion of neighboring ripples is supported by local CA1 inhibitory mechanism.
The conditions that confine or allow for the propagation of ripple events along the septotemporal axis are largely unknown (Ylinen et al., 1995; Chrobak and Buzsáki, 1996; Csicsvari et al., 2000; Patel et al., 2013). Preliminary observations indicate that the dominant direction of propagation of SPW‐Rs during sleep depends on the nature of prior waking experience (Patel et al., 2013; see SPW‐R‐Supported Memory Consolidation section). Projections from different segments of the hippocampus communicate with different structures, such as the subiculum, amygdala, and different territories of the neocortex via the entorhinal cortex (cf. Amaral and Lavenex, 2007; Ranganath and Ritchey, 2012). SPW‐Rs that emerge from the septal, intermediate, and temporal segments of the CA1 region may broadcast different types of information to their targets at the same or different times. The relative functional isolation of the temporally emerging SPW‐Rs is likely important because in primates the temporal segment grows disproportionally (uncus and body of the hippocampus) and communicate with the expanded associational (e.g., prefrontal) cortices and amygdala (Amaral and Lavenex, 2007; Royer et al., 2010; Ranganath and Ritchey, 2012). In summary, SPW‐Rs do not engage and synchronize the entire hippocampus. Instead, the SPW sweeps through focal segments of the hippocampus and induce ripples locally, which in turn can be synchronized by GABAergic interneurons. The behavioral conditions and mechanisms that determine the direction of propagation of SPW‐Rs remain undetermined. Given the topographic relationship between the different septotemporal segments of the hippocampus/entorhinal cortex and neocortex, the dominance of spatially confined SPW‐Rs indicate that the different segments of the hippocampus can send messages to different cortical targets and at different times.
MODULATION OF SPW‐RS BY SUBCORTICAL AND NEOCORTICAL INPUTS
Most of our knowledge about the influence of extrahippocampal structures on SPW‐Rs is indirect. Subcortical neuromodulators promote theta and slow/mid gamma oscillations (see Pharmacological Control of SPW‐R section; Bland, 1986; Buzsáki, 2002; Vertes et al., 2004; Vertes and Kocsis, 1997), and these oscillations appear mutually exclusive with hippocampal SPW‐Rs (Buzsáki et al., 1983). Only when the suppressing effects of the subcortical neuromodulators are removed can recurrent excitation in the CA3 recurrent collateral system proceed and release a SPW‐population burst (Buzsáki et al., 1983). In agreement with the correlational data, SPW‐Rs persist after lesion of the medial septum or fimbria‐fornix that abolishes theta oscillations and they are still suppressed during theta‐associated behaviors (walking, rearing head movement, REM sleep (Buzsáki et al., 1983; Suzuki and Smith, 1988c). However, during immobility, SPW‐R probability decreases after medial septum damage. SPW‐Rs also survive following large bilateral lesions of the entorhinal cortex and, again, keep their normal behavioral correlations (Buzsáki et al., 1983; Bragin et al., 1995b). After combined entorhinal‐medial septum lesions, SPW‐Rs persist during slow wave sleep and immobility and are suppressed during movement (Suzuki and Smith, 1988c). These observations are the basis for the theta‐SPW or theta‐LIA dichotomy (Vandervolf, 1969; Buzsáki et al., 1983). More direct evidence comes from optogenetic stimulation of channelrhodopsin (ChR2)‐expressing cholinergic neurons in the medial septum of anesthetized and freely moving mice. Such stimulation strongly suppresses SPW‐Rs even at low intensities but do not alter theta/gamma power (Fig. 16) (Vandecasteele et al., 2014). Activation of other subcortical neuromodulators can exert a similar effect on SPW‐Rs (Wang et al., 2015).
Figure 16.
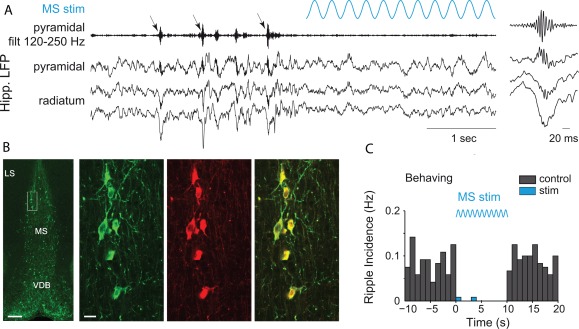
Medial septum stimulation suppresses hippocampal SPW‐Rs. (A) Hippocampal LFP in the CA1 pyramidal layer and stratum radiatum displaying SPW‐R (arrows) before the onset of optogenetic stimulation. (Inset) Expanded time scale of a detected ripple. (B) Left‐most panel: YFP‐positive immunostaining in a coronal section of the MS in a ChATChR2‐YFP hybrid transgenic mouse. LS, lateral septum; VDB, ventral diagonal band. (Scale bar: 200 μm.) Right panels: higher magnification of the MS (rectangle in left panel), double immunostaining of YFP (green, left), and ChAT (red, middle), showing their colocalization. (Scale bar: 10 μm.) (C) Perievent time histogram of SPW‐R occurrence, before, during, and after 10 s sine stimulations. Reproduced from Vandecasteele et al. (2014).
The distribution of inter‐SPW‐R intervals is irregular and strongly skewed both in vivo and in in vitro models (Papatheodoropoulos and Kostopoulos, 2002a, 2002b,c; Schlingloff et al., 2014), although their occurrence can be super‐regular in some slice preparations (Maier et al., 2003; Colgin et al., 2005; Behrens et al., 2007; Foffani et al., 2007). The causes for the skewed distribution of the SPW‐R intervals can be both intra‐ and extra‐hippocampal. The stochastic nature of SPW‐Rs is attributed to the population burst in the CA3 region emerging from a randomly fluctuation of synchrony with critical threshold of coincidental firing neurons (Traub and Wong, 1982; Buzsáki et al., 1983; Menendez de la Prida et al., 2006; Schlingloff et al., 2014). However, in the intact brain the exact timing of the population burst may also be influenced by the irregular occurrence of extrahippocampal inputs such as neocortical slow oscillations and sleep spindles (see Modulation of SPW‐Rs by Subcortical and Neocortical Inputs section). The rhythmic recurrence of in vitro SPW‐Rs in the CA3 network may reflect an underlying slow relaxation oscillator with a duty phase followed by a refractory‐recovery period, in which the occurrence of the SPW burst can be advanced or delayed by weak inputs in a time and input‐magnitude‐dependent manner (Buzsáki, 2006). Both neocortical events are correlated, at least weakly and on seconds time scale, with SPW‐Rs (Siapas and Wilson, 1998; Sirota et al., 2003; Battaglia et al., 2004; Isomura et al., 2006; Mölle et al., 2006, 2009; Clemens et al., 2011).
Effect of Cortical Slow Oscillation on SPW‐Rs
The most prominent excitability changes in the neocortex occur during slow oscillations (0.5–2 Hz) of non‐REM sleep, due to the synchronized membrane potential changes of mainly deep layer pyramidal cells from a hyperpolarized (DOWN) to a depolarized (UP) level (Steriade et al., 1993a, 1993b, 1993c). In contrast to neocortical and entorhinal neurons, hippocampal CA3 and CA1 pyramidal cells do not possess bimodal distribution of their membrane potential during non‐REM sleep (Isomura et al., 2006). Yet, the widespread excitability changes in the cerebral cortex and subcortical areas can affect the probability of occurrence of hippocampal SPW‐Rs (Sirota et al., 2003; Battaglia et al., 2004; Isomura et al., 2006; Mölle et al., 2006, 2009; Clemens et al., 2011). This correlation may be due to a common subcortical mechanism that affects both neocortex and hippocampus or spread of the slow oscillation from the neocortex to entorhinal cortex. In support of the latter mechanism, co‐occurrence of SPW‐Rs with entorhinal cortex UP states is much stronger than with UP states recorded in the neocortex (Sirota et al., 2003; Isomura et al., 2006; Ji and Wilson, 2007). During slow wave sleep, the distribution of granule cells’ membrane potentials is skewed, likely driven by the layer II entorhinal input, but is not bimodal (Isomura et al., 2006; Wolansky et al., 2006; Hahn et al., 2007; Nir et al., 2011). The synchronous discharge of neocortical neurons during the DOWN‐UP transition of the slow oscillation can be a timed trigger for SPW‐Rs (Fig. 17). Irrespective of the route of excitation, the Poisson‐like distribution of the intervals between the UP states of cortical slow oscillation (Johnson et al., 2010) and DOWN states in the dentate gyrus (Sullivan et al., 2011) may contribute to the irregular temporal distribution of SPW‐Rs in vivo. The surge of activity from the entorhinal cortex during UP states can either advance or delay the next SPW‐R event, depending both on timing and the relative dominance of excitation versus inhibition. Such external perturbations can effectively generate irregular events because of the interference between the hypothesized SPW‐pacing oscillator in the CA3 network and the entorhinal cortex‐mediated inputs.
Figure 17.
Influence of entorhinal input on CA1 SPW‐R. A. Short epochs of wide‐band CSD traces (raw, 1 Hz to 5 kHz) and their filtered derivatives recorded simultaneously from the CA1 pyramidal layer (CA1 PYR), outer molecular layer (DG OML), and granule cell layer (DG GCL) of the dentate gyrus. The horizontal bar indicates a DOWN state. B. Relationship between DOWN‐UP transition of population firing in the entorhinal cortex (EC, top) and the occurrence of hippocampal SPW‐R (bottom). Reproduced after Isomura et al. (2006).
Effect of Sleep Spindles on SPW‐Rs
The sleep spindle is a waxing–waning 12–18 Hz oscillatory, short duration event (0.4–1 s) that is most frequent in the superficial (early) stages of sleep (Steriade et al., 1993a; Bremer, 1935) and especially during intermediate sleep (Gottesmann, 1973). Sleep spindles are generated by the interaction between the GABAergic neurons of the thalamic reticular nucleus and the thalamic nuclei (Steriade et al., 1993b, 1993c; von Krosigk et al., 1993; Steriade, 2001; Halassa et al., 2011) and are synchronized across the multiple thalamic nuclei via neocortical feedback (Contreras et al., 1996). Sleep spindles can reach the hippocampus by the neocortical–entorhinal cortex path (Isomura et al., 2006; Wolansky et al., 2006; Hahn et al., 2007; Sullivan et al., 2014). The direct projection from thalamic nucleus reuniens to the distal dendrites of CA1 pyramidal neurons (Herkenham, 1978; Wouterlood et al., 1990) is an alternative route, but it remains uncertain if neurons in the nucleus reuniens are phase‐locked to sleep spindles. Both current source density analysis and neuron spike entrainment studies demonstrate that thalamocortical spindles, as the theta rhythm, phase‐modulate both principal cells and interneurons in the CA1, CA3, and dentate gyrus subregions of the hippocampus and layers 2, 3, and 5 of medial entorhinal cortex as the theta rhythm (Sirota et al., 2003; Sullivan et al., 2014). Yet, the organization of neuronal firing patterns during spindles and theta oscillations are fundamentally different. While in the exploring animal the entire phase range of theta waves are used by cell assembly sequences, entorhinal‐hippocampal neurons are strongly phase‐locked to the local LFP spindle (Sullivan et al., 2014).
There is a loose temporal relationship between SPW‐Rs and thalamocortical spindles (Fig. 18) (Siapas and Wilson, 1998; Sirota et al., 2003; Eschenko et al., 2006; Isomura et al., 2006; Mölle et al., 2006; Clemens et al., 2007, 2011; Johnson et al., 2010; Peyrache et al., 2011; Sullivan et al., 2011) but their exact physiological relationship remains to be clarified. Two aspects of this relationship are important: the time window of correlations and the neocortical areas whose activity is correlated with hippocampal SPW‐Rs. On a larger (seconds) timescale, SPW‐Rs typically precede spindles (Siapas and Wilson, 1998), possibly because of common modulation of both events by the slow oscillation, as discussed above. At the tens of milliseconds timescale, SPW‐Rs are often phase‐locked to spindle cycles (Sirota et al., 2003; Mölle et al., 2006; Clemens et al., 2007; Wierzynski et al., 2009), an indication of thalamocortical influence on the timing of SPW‐Rs. The synchronous DOWN‐UP transitions may be a common driving force for both spindles and SPW‐Rs. However, SPW‐Rs also are more probable at the end of the UP state (Sirota et al., 2003; Peyrache et al., 2011). This delayed relationship may result from the traveling nature of the slow oscillations and sleep spindles across the cortical surface (Massimini et al., 2004). A hitherto unexplored factor in the relationship between SPW‐Rs and spindles is topography. This may be important since prefrontal slower (9–14 Hz) spindles may be generated by a different mechanism than parietal‐posterior faster (12–18 Hz) spindles (De Gennaro and Ferrara, 2003; Andrillon et al., 2011). Spindles recorded from the somatosensory and visual areas tend to entrain SPW‐Rs (Sirota et al., 2003; Sirota and Buzsáki, 2005; Ji and Wilson, 2007), whereas SPW‐Rs typically precede prefrontal slow spindles (Peyrache et al., 2011). Similar to sleep spindles, high‐voltage spindles (HVS) can also entrain hippocampal neurons, possibly through the same pathways as spindles. Yet, the incidence of SPW‐Rs decreases with the onset of HVSs (Haggerty and Ji, 2014), an indication of impaired communication between neocortex and hippocampus. The lack of appropriate information exchange between these structures may also explain why “replay” of waking neuronal sequences in the prefrontal cortex is also impaired during HVSs (Johnson et al., 2010).
Figure 18.
Hippocampal‐neocortical cross‐frequency interactions during sleep. (A) Hippocampal SPW‐Rs can induce prefrontal delta wave and sleep spindle. Both spindles and ripples then travel from their source location. (B) Traces of neocortical layer V and hippocampal CA1 (filtered between 140 and 240 Hz and rectified) LFP in the rat. (Inset) Filtered ripple at a faster time scale. Dots: peak of delta wave, troughs of sleep spindle and hippocampal ripple waves. (C) Hippocampal ripple peak‐triggered neocortical spectrogram. Power spectrograms, centered on ripples (time 0 s), were averaged and normalized by the mean power over the entire recording session and log transformed. Note increased correlation of power in the slow oscillation (delta; 0.5–4 Hz) and sleep spindle (10–18 Hz) bands with hippocampal ripples. *Slow (∼0.1 Hz) comodulation of neocortical and hippocampal activity. Reproduced from Sirota et al. (2003).
In summary, the interactions between hippocampal SPW‐Rs and the two major neocortical events, slow oscillations and sleep spindles, reflect important physiological mechanisms for the transfer and exchange of neuronal information during sleep. Hippocampal SPW‐Rs and sleep spindles have a nuanced relationship and the direction of communication may depend on sleep state, target structure and previous experience. Such bidirectional communication may be important for exchanging critical neuronal information. When a neocortical spindle successfully invades the entorhinal‐hippocampal networks, it can trigger a SPW‐R. In turn, the hippocampal output can “readdress” the activity of those very same neurons that initiated the neocortical‐hippocampal dialogue by enhancing and prolonging sleep spindles. Because the SPW‐R is a punctuate event whereas the sleep spindle is temporally more protracted, the hippocampal output can be directed to the still active neocortical assemblies (Sirota and Buzsáki, 2005; Mölle et al., 2009; Sullivan et al., 2011).
Influence of Dentate Gyrus Activity on SPW‐Rs
While the CA3‐CA1 system is engaged in SPW‐Rs in the absence of theta oscillations, activity of the dentate gyrus also shows characteristic changes. Gamma oscillations persist in the dentate even after complete subcortical denervation of the hippocampus (Buzsáki et al., 1987a, 1987b, 1987c). However, compared with theta states the coefficient of variation of the gamma waves increases considerably (Csicsvari et al., 2003a, 2003b). Based on amplitude criteria, two events were isolated and termed dentate LFP spike 1 and 2 (DS1 and DS2; Bragin et al., 1995b). DS 1 is defined as a ∼8 to 30 ms large wave (>2.5 mV) surrounded by smaller amplitude waves, and can be viewed as a brief “gamma burst.” The current sink‐source distribution of DS1 shows a negative polarity in the molecular layer and positive polarity in the granule cell layer and hilus. Intracellular recordings in urethane anesthetized rats showed large depolarization of spiking during DS1. In addition, several intracellularly identified interneuron types, some with axons extending into the CA3b region and the contralateral hippocampus, fire robustly during the LFP spike (Penttonen et al., 1997). Similar events can be induced by transient optogenetic activation of CaMKII‐expressing excitatory neurons (Stark et al., 2014). DS2 is a more isolated event with an identical voltage versus depth profile to the entorhinal cortex evoked responses. The onset of DS2 is preceded by a large decrease of neuronal firing. DS2s are more prominent in older animals. DS2 likely reflects the invasion of HVSs to the dentate gyrus. The probability of occurrence of SPW‐Rs is reliably decreased after DS2 (Bragin et al., 1995b), in agreement with other observations showing that the occurrence of HVSs decreases the incidence of SPW‐Rs (Haggerty and Ji, 2014). Both DS1 and DS2 virtually disappear after surgical removal of the entorhinal cortex, coinciding with an increased incidence of SPW‐Rs (Bragin et al., 1995b).
The power of dentate gyrus gamma has an inverted U relationship with SPW‐Rs, an indication of stochastic resonance. Both reduced gamma power, largely reflecting the DOWN state, and high gamma power, present during theta oscillations, are associated with the absence or low incidence of SPW‐Rs and the highest probability of SPW‐Rs occur at an intermediate level of gamma power (Sullivan et al., 2011). Dentate fast gamma oscillations (90–120 Hz) may also recruit CA3 neurons. As a result CA3 “ripples” can have two origins (Yuta Sensai and Buzsáki, unpublished observations), one is associated with CA1 SPW‐Rs, whereas the other may reflect the invasion of the dentate activity (“dentate spikes,” Bragin et al., 1995b). This distinction should be considered when CA3 activity is used to predict CA1 ripples. Overall, these experiments illustrate that in the intact brain the emergence of SPW‐Rs can be influenced by many areas, including the subcortex, entorhinal and dentate gyrus.
IMPACT OF SPW‐R OUTPUT ON CORTICAL AND SUBCORTICAL TARGETS
Large Excitatory Gain Characterizes SPW‐Rs
SPW‐Rs are characterized by the largest transient increase of excitation and inhibition of any known physiological events. Although both pyramidal cells and interneurons show a robust synchrony, the speed of pyramidal cell recruitment is faster than that of the interneurons, resulting in a two to threefold excitatory gain over inhibition in the CA1 (Csicsvari et al., 1999a, 1999b). This large gain may derive, paradoxically, from the highly reliable EPSCs and spike transfer between pyramidal cells and interneurons (Gulyas et al., 1993; Csicsvari et al., 1998). Because pyramidal neurons fire highly synchronously in a given ripple wave and because even a single pyramidal neuron spike can discharge fast spiking interneurons, spiking of many additional pyramidal cells within the spike refractory period of the interneuron (1–2 ms) cannot recruit more spikes and more inhibition. This refractoriness may be the key mechanisms for the excitatory gain during SPW‐Rs.
Impact of SPW‐Rs on Cortical Targets
The strong excitatory gain during SPW‐Rs can depolarize the target regions of CA1, including the subicular complex, deep layers of the entorhinal cortex, medial prefrontal cortex and subcortical structures (Fig. 19). In the hippocampus‐subicular complex‐entorhinal loop, the super‐synchronous output can induce local ripples (Chrobak and Buzsáki, 1994, 1996), presumably by the same mechanisms as in CA1 (Stark et al., 2014), and the excitatory gain is dissipated by the increased recruitment of inhibition. The excitatory gain is largest in CA1, followed by CA3, subiculum, and the deep layers of the entorhinal cortex; the impact of excitation dissipates strongly in dentate gyrus and superficial layers of the entorhinal cortex (Fig. 20) (Chrobak and Buzsáki, 1994; Mizuseki et al., 2009).
Figure 19.
Self‐organized burst of activity in the CA3 region produces a SPW sink (negative wave) in the apical dendrites of CA1 pyramidal neurons and also discharge interneurons. The interactions between the discharging pyramidal cells and interneurons give rise to a short‐lived fast oscillation (“ripple”; 140–200 Hz), which can be detected as a fast LFP oscillation in the CA1 pyramidal layer. The CA1 population burst, in turn, brings about synchronized activity in the target populations of parahippocampal structures as well (Sub, subiculum; Para, parasubiculum; EC, entrorhinal cortex. These parahippocampal ripples are slower and less synchronous, compared with CA1 ripples. Reprinted from Buzsáki and Chrobak (2005).
In the prefrontal cortex, another monosynaptic target of the mid‐ and ventral CA1 region, Wierzynski et al. (2009) found strong and short latency (10–20 ms) responses of neurons and increased spindle power following hippocampal SPW‐Rs. Peyrache et al. (2011) found that superficial neurons were more strongly phase‐locked to local LFP spindles than deep layer neurons, whereas the opposite relationship was observed in response to SPW‐Rs. These findings suggest that the stronger direct projections of ventral CA1 and subicular pyramidal neurons to the deep layers (Swanson, 1981; Thierry et al., 2000). The influence of SPW‐R on the discharge of deep prefrontal neurons was stronger without sleep spindles, indicating that spindles and SPW‐Rs compete with each other, perhaps mediated through the recruitment of inhibitory neurons (Peyrache et al., 2011). This process may be more complex though since Wierzynski et al. (2009) also showed that only strong ripple bursts were correlated with prefrontal spindles, whereas weak ripples affected only single neuron events, as in the Peyrache et al. (2011) study.
The exact impact of the CA1 output is hard to assess without knowledge of the anatomical connections of the recorded neurons. Hippocampal output during SPW‐Rs may also affect the phase of slow oscillations. However, such functional link appears too weak to switch the DOWN state to an UP state. Intracellular recordings in layer 5 entorhinal cortical neurons show that SPW‐Rs can depolarize and discharge these neurons in both UP and DOWN states (Chrobak and Buzsáki, 1996). However, when a SPW‐R occurs during the DOWN state, the membrane potential of the depolarized target entorhinal neuron returns quickly to the DOWN state (Isomura et al., 2006). These findings suggest that, at least under anesthesia, SPW‐Rs do not routinely bias the phase of cortical slow oscillations.
The impact of SPW‐Rs on neocortical activity is hard to assess quantitatively because of the functional topographical organization between the hippocampus and neocortex (Royer et al., 2010). CA1 ripples are strongly localized (Chrobak and Buzsáki, 1996; Csicsvari et al., 2003a, 2003b; Patel et al., 2013) and, therefore, ripples, e.g., in the ventral hippocampus may not affect neurons in the dorsolateral entorhinal cortex, a region that does not receive inputs from the ventral hippocampus. Conversely, ripples confined to the dorsal hippocampus may not strongly affect neurons in the prefrontal cortex since hippocampo‐frontal cortex afferents arise from the posterior‐ventral segments of the hippocampus. Only high‐resolution, yet large spatial coverage of the neocortex together with multiple site recording of SPW‐Rs along the longitudinal axis of the CA1 region can address the relevance of topographical issues and quantify the impact of SPW‐Rs on cortical circuits.
Impact of SPW‐Rs on Subcortical Targets
The subcortical impact of SPW‐Rs is rarely considered, although subcortical structures (e.g., lateral septum, hypothalamus and nucleus accumbens) are major targets of the CA3‐CA1‐subicular systems (Swanson and Cowan, 1975; Swanson et al., 1981). To survey the brain‐wide impact of SPW‐Rs, Logothetis et al. (2012) combined physiological recordings of SPW‐Rs in the monkey with functional magnetic resonance imaging (fMRI) method, quantifying fractional modulated volume and response‐dynamics in each region is of interest. This correlational analysis in both anesthetized and awake monkeys showed a surprising modulation dichotomy between cortical and subcortical areas. SPW‐Rs are associated with robust BOLD activations of the neocortex and limbic cortex as expected. These increases are concurrent with robust negative BOLD responses in subcortical thalamic, associational (e.g., basal ganglia, cerebellum) and midbrain‐brainstem neuromodulatory structures (Fig. 21). Such downregulation of many different subcortical structures during SPW‐R occurrence may be due to a state‐transition favoring optimal hippocampal–cortical communication. Logothetis et al. (2012) interpret the negative BOLD signal as suppression of neuronal spiking activity and suggest that SPW‐R‐induced suppression of thalamic‐subcortical inputs can ensure a higher quality communication between the hippocampus and neocortex by suppressing the potentially interfering inputs from subcortical sites.
Figure 21.
SPW‐R‐triggered multi‐structure BOLD activity. (A) Time–frequency representation of the SPW‐R events in the macaque monkey under anesthesia. (B) Average activation maps. Positive BOLD response is observed in the neocortex and limbic cortex, while activity suppression of BOLD signal is seen in the diencephalon, mesencephalon and metencephalon. (C) Time courses of each group each region of interest (ROI). Note the sign change in the transition from cortical to subcortical areas and the differences in response onset. (D) Fractions of activated voxels for each region of interest. The blanket reduction of the BOLD signal in subcortical structures, however, do not mean suppression of spiking activity (see text). Reproduced from Logothetis (2015).
While the large‐scale brain wide sampling of BOLD activity help to quantify the topographic relationship between hippocampal‐neocortical communication, the fMRI data conflicts with physiological observations. Neurons in the primary visual cortex are co‐activated with hippocampal SPW‐Rs (Ji and Wilson, 2007), yet this cortical area was the only one where BOLD was reduced. Further, concurrent recordings of LFP and spikes from the hippocampus and subcortical structures show robust increase, rather than decrease, of spiking activity in the medial septum (Dragoi et al., 1999), lateral septum, triangular nucleus, interfimbrial nucleus (Carpi et al., 1997), ventral striatum (Pennartz et al., 2004; Lansink et al., 2009), various thalamic nuclei (Peyrache and Buzsáki, unpublished data; Logothetis, 2015) and hypothalamus (Carpi and Buzsáki, unpublished data). In many of these structures, the dominant cell type is inhibitory, and the BOLD signal may not reliably report inhibition or spiking of inhibitory neurons (Buzsáki et al., 2007). An alternative explanation for the discrepancy between neurophysiological and imaging data is that the short SPW‐Rs are followed by a post‐SPW‐R silence (English et al., 2014) and the integrated spiking activity in the wider time windows needed to sample the BOLD signal can be lower compared with the background activity (Csicsvari et al., 1999a, 1999b).
The physiological importance of SPW‐Rs on their subcortical structures is not well understood. Many of the subcortical targets of the hippocampus are peptide‐ and hormone‐releasing GABAergic neurons; release of peptide and/or hormone from these neurons is facilitated by the strong and synchronous hippocampal output during SPW‐Rs. Several hormones are released mainly during slow wave sleep when SPW‐R density is largest. For example, growth hormones can affect the protein synthesis and may assist with neuronal plasticity. Long‐term potentiation (LTP)‐induced plasticity consists of several stages (cf. Bliss and Collingridge, 1993), the longest form of which depends on activation of immediate early genes, transcription and protein synthesis (Bading et al., 1993). This step introduces a problem for the synaptic specificity of LTP since the newly synthesized proteins must be transported back to the synapses, which originally gave rise to the nuclear activation (Frey and Morris, 1997). SPW‐R bursts that occur hours after the learning events may provide such a guiding mechanism. When learning‐associated cellular‐synaptic events are “replayed” during SPW‐Rs (see SPW‐R‐Supported Memory Consolidation section), the reactivated synapses may serve to direct the somadendritic flow of proteins and ensure that they are incorporated into the relevant synapses (Buzsáki, 1998). This speculation implies that SPW‐Rs may coordinate both neocortical and subcortical mechanisms required for synaptic plasticity.
MECHANISMS OF RIPPLE GENERATION
Populations of both pyramidal cells and interneurons reach their highest synchrony during hippocampal SPW bursts (Buzsáki et al., 1983). In response to the strong input supplied most often by the CA3 region, the emerging ripple oscillation may help the CA1 neuronal network achieve a balance between such strong competition of excitation and inhibition. However, ripples are local events and can be induced by any input that can provide sufficient level of excitation. Understanding ripple generation requires answers to four mechanisms: (a) generating the oscillating LFP (current generation), (b) supporting the transient rhythm (rhythm generation), (c) spatial coordination of ripples and (d) mechanisms that terminate SPW‐Rs.
Various Inputs Can Induce CA1 Ripples
SPW bursts most often emerge from the CA2‐CA3a subnetworks, spread to CA3b and c subregions to eventually give rise to the depolarization of the apical dendrites of CA1 pyramidal neurons by the synchronous CA3c output. This mechanism represents the most ubiquitous form of SPW‐Rs with a large negative wave in the CA1 str. radiatum. In a minority of cases, the CA2 neurons directly depolarize the basal dendrites of CA1 pyramidal cells, as reflected by a sink in the str. oriens and a passive return source (positive wave) in str. radiatum (A. Gonzales, A. Fernández‐Ruiz, A. Berénnyi, G. Buzsáki, unpublished observations). Under altered conditions, the entorhinal cortex and other inputs can serve as triggers. Nakashiba et al. (2009) examined ripple activity in the CA3‐TeTX transgenic mouse, in which CA3 output can be specifically and inducibly controlled. After blockade of the CA3 output, ripples persisted. However, the mean frequency of the “mutant ripples” (or epsilon bursts) was reduced to 110 Hz. Also, both coordination of reactivation of CA1 cell pairs during post‐experience sleep and memory performance were impaired. One potential explanation for ripple persistence is that interneurons that innervate the distant dendrites in CA1 stratum lacunosum‐molecure are activated by the CA3 pyramidal cells in the intact brain and reduce the effectiveness of the entorhinal cortex. Without CA3 excitation, the impact of the entorhinal input is more efficient and triggers ripples in the CA1 circuit.
Using Ca2+ imaging in hippocampal slices, Norimoto et al. (2013) found that some subicular neurons were active before the LFP ripples detected in the CA1 pyramidal layer. Unfortunately, no electrical recordings were made from the subiculum so it remains to be investigated whether the Ca2+ signal measured there corresponded to ripples. In contrast, simultaneous recordings from both CA1 and subiculum show a time lag of both LFP ripples (Chrobak and Buzsáki, 1996) and spike cross‐correlations (Sam McKenzie and Buzsáki, unpublished data), as expected on the basis of anatomical connections. The subgroup whose Ca2+ activity preceded CA1 ripples remained active after the boundary between CA1 and subiculum was surgically severed (Norimoto et al. 2013). The authors concluded that a fraction of the ripple events were initiated in the entorhinal cortex, which induced ripples in both subiculum and CA1. However, the ripple‐triggering role of the layer 3 entorhinal input in the intact brain is uncertain since superficial entorhinal neurons are either not active during CA1 ripple (Chrobak et al., 1994) or typically fire after CA1 ripples (Fig. 20) (Mizuseki et al., 2009). Nevertheless, multiple superimposed subcircuits may exist between the entorhinal cortex and subiculum.
Generation of LFP Ripples: Current Sources
In general, increasing frequency of brain rhythms correlates with a decrease in signal power (Buzsáki and Draguhn, 2004). SPW‐Rs in this regard are an anomaly and show a larger power within the ripple frequency band compared with the gamma band. This “anomaly” is due to the strongly synchronous nature of neuronal firing during SPW‐Rs. When neurons are synchronized in short time windows, the temporal summation of the action potentials can become the dominant source of the measured extracellular current. Due to the significant contribution of the spikes, the largest amplitude ripples occur in the middle of the CA1 pyramidal layer (Mizuseki et al., 2011). The shape of the LFP ripple event depends strongly on how many nearby pyramidal neurons discharge concurrently within the ripple cycles. These superimposed spikes represent “mini population spikes” (Buzsáki 1986), making ripples appear spiky. In addition to spikes, somatic IPSCs are another source of current that contributes to the LFP ripple. Thus, the negative polarity troughs of the ripple largely represent spikes, whereas the positive polarity peaks mainly derive from the transmembrane currents of somatic IPSCs (Ylinen et al., 1995; Schomburg et al., 2012; English et al., 2014). The relative contribution of spikes and IPSCs to the LFP rhythm in the epsilon and ripple bands is controversial (Reichinnek et al., 2010; Schomburg et al., 2012) and depends on factors including the size and position of the recording electrode, frequency and, importantly, the nature of the preparation (in vivo vs. in vitro; Aivar et al., 2014).
Evidence supports the role of pyramidal cell spikes in the LFP ripple (see Fig. 31) (Schomburg et al., 2012). The power of ripples correlates with the summed firing rate of CA1 pyramidal cells (Csicsvari et al., 1999a). The discharge probability curve of pyramidal cells matches the ripple power curve more precisely than the discharge probabilities of the interneurons (Csicsvari et al., 1999a) and ripple power is more strongly correlated with the spatial coherence of CA1 pyramidal cell firing than the spatial coherence of interneuron firing (Csicsvari et al., 2000). Moreover, the spatiotemporal patterns of SPW‐Rs reliably reflect spatial constellations of spiking cells that encode activation of local place cell ensembles during replays of waking spike sequences (see Section on Constuctive Role of SPW‐R). With sufficiently large numbers of spatially distributed recording sites, the ordered activation of place cell ensembles of waking experience can be reconstructed from the unique spatiotemporal features of the LFP ripple (Taxidis et al., 2015).
Figure 31.
Synchronous spikes from distant neurons can contribute substantially to LFP ripple oscillations. (A) The locations of neurons that spike during ripple periods are indicated by triangles in a top‐down view of the pyramidal layer (left), with colors indicating the 50‐µm wide rings from which the spikes originate. Voltage, Ve, traces are colored correspondingly, with contributions from each ring of cells adding cumulatively from the outside in. Stacked histograms above the potential traces show spike times. Averaged power spectra of the stratum pyramidale Ve from each individual ring. The insets indicate the proportions of the total Ve power at 150 Hz generated by each ring‐ or disk‐shaped subpopulation (i.e., the peak values of the power spectra, normalized by the power at 150 Hz in the full population). Reproduced after Schomburg et al. (2012).
Spiking is a substantial but incomplete source of the LFP ripple (Schomburg et al., 2012). Many interneuron types fire strongly in phase with the LFP ripple cycles (see Discharge Patterns of Inhibitory Neurons During SPW‐Rs section), including PV‐expressing basket cells, which induce rhythmic outward current in pyramidal cells both in vivo (Ylinen et al., 1995) and in vitro (Hajos et al., 2013). The amplitude of intracellular ripple is smallest between −70 and −80 mV with a phase‐reversal of the intracellular oscillation relative to the LFP ripple in this voltage range. In addition, after elevation of intracellular Cl− ions in experiments with KC1 electrodes, the voltage dependence of the intracellular oscillation near resting potential is abolished, indicating that the intracellular ripple is GABAA receptor‐mediated. PV basket cells extend their axons 400 to 800 µm in both the transverse and the longitudinal directions and contact more than a thousand pyramidal cells (Gulyas et al., 1993; Buhl et al., 1994; Sik et al., 1995). In vitro, discharge of single interneurons can bring about measurable extracellular voltage, corresponding to ∼15 μV amplitude, with a 1.2 ms rise time and exponential decay with 6.6 ms time constant (Glickfeld et al., 2009; Bazelot et al., 2010). In slices, synchronized GABAA receptor‐mediated currents during ripples give rise to a major component of the LFP (Schlingloff et al., 2014). In their experiments, optogenetic stimulation of PV‐positive interneurons induced LFP ripples even after both fast and slow glutamatergic receptors were blocked pharmacologically, demonstrating that inhibitory perisomatic currents contribute to the LFP ripple in the pyramidal layer. On the other hand, local application of the GABAA receptor blocker gabazine strongly reduced LFP ripples locally. In slice preparations, CA1 pyramidal cells receive fast synchronous glutamatergic inputs from their upstream CA3 neurons, which may also contribute to the LFP in stratum radiatum (Maier et al., 2011). However, these dendritic layer oscillations cohere with the pacing frequency of its input neurons rather than with the ripple‐rhythmic firing of the CA1 population (Schönberger et al., 2014; Buzsáki and Schomburg, 2015). Given the different frequencies of the CA3 and CA1 oscillations during SPW‐Rs in in vivo (Sullivan et al., 2011), EPSCs in LFP ripple generation in the intact brain are likely negligible. EPSCs in interneurons are phase‐locked with the LFP ripple but since most of the pyramidal‐interneuron synapses are located outside the CA1 pyramidal layer their contribution is small. Finally, the synchronously discharging GABAergic boutons of the PV basket cells generate an inward (negative) current in the pyramidal layer at the time of the wave (positive) component of the LFP ripple. However, computer simulations suggest that axonal spikes contribute little to the LFP (Schomburg et al., 2012).
In summary, the spatiotemporal summation of active outward currents (IPSCs) in the somata of regularly arranged CA1 pyramidal cells is a significant source of the extracellularly recorded oscillatory field potential. In addition, the temporal overlap of action potentials around the trough of the ripple waves (2.5–3 ms; “mini” population spikes) and the spike‐associated active inward currents in the cell bodies of neighboring pyramidal cells are another significant source of extracellular currents underlying the extracellular ripples (Ylinen et al., 1995; Schomburg et al., 2012).
Generation of LFP Ripples: Rhythm Sources
In contrast to the consensus on the current generators of the LFP, the physiological origin of the ripple frequency pacing is uncertain (cf., Traub et al., 2004). Controversy may reflect differences between the mechanisms of generating oscillations in the intact brain and in vitro, and the large variance among the in vitro models. Various ideas have been put forward to explain the mechanisms of the rhythm generation. A simple one is a short‐lived mutual interaction between pyramidal cells and interneurons (PYR‐INT; Fig. 30) (Buzsáki et al., 1992; Ylinen et al., 1995; Brunel and Wang, 2003; Klausberger et al., 2003; Memmesheimer 2010; Maier et al., 2011). Another is the excitatory barrage by the discharging CA3 and CA1 pyramidal neurons transiently depolarizing and activating voltage‐dependent channels in target interneurons and inducing rhythmic oscillations (Llinas, 1988). The relatively unequal discharge frequency of perisomatic interneurons would generate only transient coherent coupling of their spikes, corresponding to the duration of the ripple (Ylinen et al., 1995). A variation of this model is that some mechanism, e.g., mutual synaptic inhibition or gap junction coupling among the activated interneurons, produces the fast temporal coordination of the spiking interneurons (P‐I‐I model; Fig. 30). In this hypothesized scenario, rhythmic firing of perisomatic intemeurons is the primary event and the time‐locked firing of the pyramidal neurons results from fast coordinated inhibition (Buzsáki et al., 1992; Traub et al., 1996b, 2012; Brunel and Hakim, 1999; Geisler et al., 2005; Rácz et al., 2009; Taxidis et al., 2012; Stark et al., 2014; Schlingloff et al., 2014). A variation of this model posits that PV basket cells are endowed with intrinsic resonant properties to fire preferentially at ripple frequency (Chiovini et al., 2014). Radically different models of ripple rhythm generation postulate that pyramidal cells excite each other both antidromically and orthodromically by an electrically coupled axonal plexus and that the connectivity graph and speed of propagation in the axonal network set the ripple frequency (Fig. 30). Thus, the primary source of rhythm generation is the pyramidal cell population and interneurons simply inherit the rhythm (Draguhn et al., 1998; Traub and Bibbig, 2000; Schmitz et al., 2001; Maier et al., 2003, 2011; Bähner et al., 2011; Traub et al., 2012).
Figure 30.
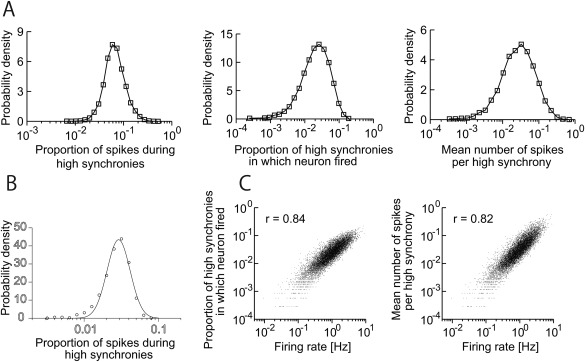
Model of SPW‐Rs built from skewed statistics of firing rates, bursts and synaptic weights. (A) Proportion of spikes during highly synchronous events, the proportion of highly synchronous events in which the neuron fires two or more events, and the mean number of spikes per highly synchronous events. Note lognormal distributions. (B) The proportion of pyramidal neurons that fire at least once during a “SPW‐R” synchronous event is distributed lognormally. (C) Correlation between firing rate and proportion of synchronous events in which the neuron fired (left) and between firing rate and mean number of spikes per “SPW‐R” synchronous events. Reproduced from Omura et al. (2015).
Pyramidal‐interneuron interactions
Pyramidal‐interneuron interactions and somatic inhibition of pyramidal cells can help explain the ripple‐locked discharge of pyramidal cells (Buzsáki et al., 1992; Ylinen et al., 1995; Csicsvari et al., 1999a, 1999b; Klausberger et al., 2003; Maier et al., 2003, 2011; Klausberger and Somogyi, 2008; Rácz et al., 2009; Bähner et al., 2011; Varga et al., 2012; Hajos et al., 2013; Karlocai et al., 2014). Blockade of either AMPA receptors or GABAA receptors abolishes ripple oscillations (see Pharmacological Control of SPW‐R section). PV interneurons increase their discharge rates and are phase‐locked to the network rhythm (Ylinen et al., 1995; Klausberger et al., 2003). The frequency of the intracellular ripple in CA1 pyramidal cells is not voltage dependent, indicating that the fast oscillation of membrane voltage in pyramidal cells does not emerge as a result of intrinsic, voltage‐dependent ionic mechanisms in these neurons (Ylinen et al., 1995). When a few local CA1 pyramidal cells discharge in high synchrony by chance in response to the excitatory SPW input, they can drive PV basket cells, which in turn pace action potentials in a larger number of pyramidal cells for a few cycles. This two‐neuron pool (PYR‐INT) model of rhythm generation does not require interactions among either pyramidal cells or interneurons. The frequency of the rhythm is set by the repetitive fast action potentials of the basket interneurons or by the delay between excitation and inhibition between the two pools of neurons. The fast excitation and the delayed feedback inhibition alternate, and at appropriate strength of excitation and inhibition, cyclic behavior may persist for a while. This scenario predicts that the first wave of the ripple is the largest and the amplitude and frequency of the ringing response dissipates quickly over time. However, such descrescendo LFP ripples are rarely observed.
Optogenetic techniques allow for selective manipulations of circuit components (Boyden et al., 2005) to address questions about the generation of SPW‐Rs. In rats and mice, Stark at al. (2014) applied brief localized optogenetic depolarization of CAG::ChR2‐expressing pyramidal cells and interneurons with a half‐sine waveform, designed to mimic the SPW envelope‐induced spiking. Transient activation of the local neuronal population results in high‐frequency oscillations resembling spontaneous ripples recorded at the same site (Fig. 23). Simultaneous direct optogenetic activation of interneurons is not necessary, since ripples are also readily induced when ChR2 was expressed only in pyramidal cells (CaMKII::ChR2 animals). Only a few dozens of pyramidal cells have to be discharged to induce a local ripple (Stark et al., 2014). The correlation between the magnitude of optogenetic excitation and the amplitude and frequency of the induced fast rhythm share strong similarities with the correlation between SPW amplitude and ripple amplitude/frequency in the intact brain (Sullivan et al., 2011; Patel et al., 2013; Stark et al., 2014). The importance of pyramidal cell spiking is further supported by ripple‐triggered closed loop photo‐illumination of pyramidal neurons prolonging ripple frequency oscillations locally, while leaving firing rate and ripple power unaffected at nonilluminated shanks. Conversely, ripple‐contingent closed loop optogenetic silencing of pyramidal cells or optogenetic activation of either PV or SOM‐expressing interneurons can terminate LFP ripples (Fig. 24) (Stark et al., 2014). Thus, even artificial spiking activity of a small pyramidal cell network can induce and maintain local ripples, while silencing pyramidal cells either directly or indirectly via interneuron‐mediated inhibition aborts spontaneously generated ripples. The hypothesis that the minimal circuitry to induce ripples requires only excitatory and inhibitory basket cells is also supported by optogenetic induction of local ripples in CaMKII::ChR2 mice in the dentate gyrus, CA3 region and deep layers of the somatosensory cortex (Stark et al., 2014). However, neither the correlational nor the perturbation experiments can perfectly differentiate between models in which ripple timing is determined by the PYR‐INT loop or interactions among interneurons.
Figure 23.
Local activation of pyramidal cells induces ripples. (A) Schematic of diode‐probe shanks overlaid on histological image. (B) Spontaneous ripple (top trace) and optogenetically induced ripple (PYR activation) recorded by the same electrode in a mouse. Stimulus waveform is in blue. Right: time‐frequency decomposition of the ripple event. (C) Left: induced LFP traces during individual pulses (50 ms) of increasing intensity. Right: time‐frequency decomposition. Weak light only induces spiking, whereas ripple oscillations of increasing amplitude and frequency are induced with stronger light. After Stark et al. (2014).
Figure 24.
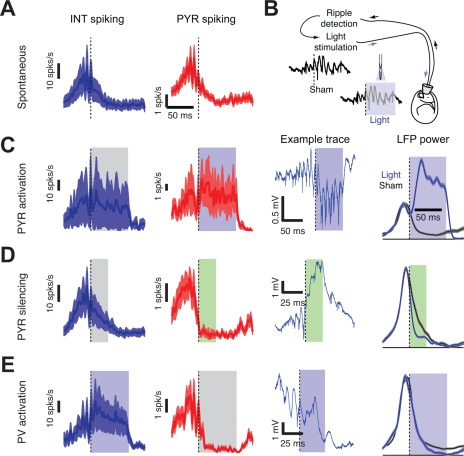
Prolongation and truncation of ripples. (A–E) Perievent histograms of ensemble spiking activity of interneurons (INT, blue) and pyramidal neurons (PYR, red; mean ± SEM) during spontaneous SPW‐Rs (A) and optogenenetic closed‐loop stimulation (C–E). (B) Ripples are detected in real‐time about three cycles after onset, and the detection triggers light stimulation. (C) Ripple‐contingent activation of pyramidal cells drives PYR and increases duration of spontaneously occurring ripples. Example wide‐band trace shows a single event. (D) Direct silencing of pyramidal cells shortens spontaneously occurring ripples. (E) Indirect silencing of pyramidal cells via PV interneuron activation shortens ripples. After Stark et al. (2014).
The PYR‐INT model is also often used to explain the emergence of gamma oscillations (cf., Whittington et al., 2000, Tiesinga and Sejnowski, 2009; Buzsáki and Wang, 2012), raising the issue whether gamma and ripple oscillations are paced by the same mechanisms. However, it is unlikely that simple strengthening of the PYR‐INT‐PYR loop is sufficient to shift from gamma to ripples. The nonlinearity in frequency preference suggests that some non‐linear shift is in action, perhaps favoring the INT‐INT over PYR‐INT transmission at high frequencies. In support of this reasoning, the ratio of the excitatory and inhibitory synaptic conductances during gamma oscillations, measured in pyramidal neurons, is 0.23, while this ratio almost doubles (0.47) during SPW‐Rs, reflecting much stronger excitation during SPW‐Rs (Hajos et al., 2004, 2013; Oren et al., 2006). Genetic reduction of AMPA receptor‐mediated excitation onto parvalbumin (PV)‐positive interneurons exerts differential effects gamma and ripple oscillations: gamma power remains intact whereas ripple amplitude and ripple‐related synchronization of pyramidal cells and interneurons are enhanced (Rácz et al., 2009). Computational models also suggest that interneuron interactions are better suited to pace faster oscillations than PYR‐INT loops (see Models of Ripple Oscillations section).
Gap junctions for pacing and synchrony
As an alternative to the PYR‐INT loop or INT‐INT synaptic interactions as timers of intra‐ripple frequency, electrical coupling of neurons through gap junctions emerged as a possible mechanism. The potential role of gap junctions in SPW‐Rs was first suggested by the observation that ripples were abolished by halothane, a potent gap junction blocker (Dermietzel and Spray, 1993) but not by urethane or ketamine anesthesia (Ylinen et al., 1995). Channel‐forming proteins (connexins) among neurons can support electrical communication between neurons (Kosaka, 1983; Bennett and Verselis, 1992; Fukuda and Kosaka, 2000; Elias and Kriegstein, 2008). Connexin36 is a major neuron‐specific gap‐junction protein, which is located on interneurons in mature animals (Condorelli et al., 2000; Venance et al., 2000). Electrical communication between perisomatic PV interneurons would be particularly suitable because gap junction coupling between them is strong (0.7–1.6 nS) and can synchronize the spikes of similarly activated neurons (Gibson et al., 1999; Galarreta and Hestrin, 2001). However, recordings from connexin36 −/− knockout mice in vitro and in vivo revealed no significant differences in frequency, power, duration or incidence of SPW‐Rs (Hormuzdi et al., 2001; Maier et al., 2002; Buhl et al. 2003). The lack of impact on SPW‐Rs in the knockout animals may result from Connexin45, functionally replacing Connexin36 channels, as demonstrated in the retina (Frank et al., 2010). This possibility, however, is refuted by the observation that although no impairment of SPW‐Rs is present in the knockout mice in vivo, gamma oscillations in the same animals are affected (Buhl et al., 2003). Critically, SPW‐Rs appear normal in the double knockout Connexin36/45 mouse (Fig. 25), indicating these neuronal gap junctions between hippocampal neurons are not essential for SPW‐Rs.
Figure 25.
Lack of neuronal connexins does not noticeably affect SPW‐Rs. (A) Average LFP ripples from Cx36 knockout (n = 3) and Cx36‐Cx45 double knockout (n = 4) mice. (B) Distribution of inter‐SPW‐R intervals. (C) Distribution of peak frequency of ripples (n = ∼10,500 ripples in Cx36 and 12,000 in Cx36‐Cx45 mice) (Vandecasteele M, Menzies, AS, Creese, I, Paul DL, Buzsaki G. 2008. Persistence of hippocampal oscillations in connexin 36, 45 double knock‐out mice. Society for Neuroscience Abstracts. 435.4/H9).
Traub and his colleagues (Draguhn et al., 1998; Traub and Bibbig, 2000; Schmitz et al., 2001; Traub et al., 1999a,b, 2002, 2003, 2004, 2012) propose that electrical communication between axons of pyramidal cells set ripple frequency. A major motivation of this model is based on experiments in vitro, which indicate that fast (∼200 Hz) network oscillations can occur without phasic synaptic inhibition (Draguhn et al., 1998; Jones and Barth, 2002; Nimmrich et al., 2005; Maier et al., 2011). Thus, according to the model, inhibition cannot be responsible for adjusting the periodic discharge of pyramidal cells during ripples. A key assumption of the model is that spikes emerge spontaneously in the sparsely electrically connected matrix of axons of pyramidal cells (Draguhn et al., 1998; Traub and Bibbig, 2000; Schmitz et al., 2001; Traub et al., 2001, 2012), and the excitability in the axon matrix is enhanced by GABA, acting via GABAA receptors (Avoli et al., 1996; Traub et al., 2001; Bähner et al., 2011). These ectopic axonal spikes, in turn, can occasionally invade the soma of a few pyramidal cells and generate an antidromic spikelet or spike. Since propagation of spikes across the postulated axonal gap junction and back to the cell bodies takes time, this delay (5 ms) is then the key limiting pacemaker of the ripple frequency. Thus, “the frequency is determined by network topology and not by the intrinsic or synaptic time constants” (Draguhn et al., 2000). Since ripple frequency varies little despite large changes in brain volume across species, on the basis of this model one needs to assume that the density of axonal matrix remains invariant in different animals.
Several in vitro experiments support the axon electric coupling model. First, “spikelets” in intracellular recordings (Knowles and Schwartzkroin, 1981) are regarded as either dendritic spikes (Spencer and Kandel, 1961; Kamondi et al., 1998a; Gasparini et al., 2004 ) or antidromic spikes. Ectopic, antidromic spikes were initially described under conditions of reduced GABAergic inhibitions, such as the penicillin model of epilepsy (Gutnick and Prince, 1972) or when 4‐aminopyridine is applied to the perfusion solution (Traub et al., 1999b). Schmitz et al. (2001) used antidromic stimulation to demonstrate spikelets in hippocampal pyramidal neurons in low [Ca2+] media, combined with dual patch recordings and found dye coupling between axons. These and other data (Bukalo et al., 2013) suggest that spikelets arise from strong electrical coupling between axons. Second, spikelets occur phase‐locked to the LFP ripples in vitro (Draguhn et al., 1998). Third, ripples are abolished by gap junction blockers halothane, octanol and carbenoloxone (Draguhn et al., 1998; Schmitz et al., 2001; Maier et al., 2003). Fourth, in some in vitro models of SPW‐Rs, all spikes during ripples show features of antidromic spikes without prior depolarization of the soma, in contrast to action potentials outside ripples that ride on preceding V m depolarization (Papatheodoropoulos, 2008; Bähner et al., 2011; Bukalo et al., 2013). In addition, a freeze–fracture electronmicroscopic examination suggested gap junctions on CA3 pyramidal cells in the guinea pig, although not between their axons (Schmalbruch and Jahnsen, 1981).
Albeit attractive, experimental support for several key assumptions of the axon junction model is lacking. First, connexins cannot be the proper conduit for spikelets because spikelets persist in pyramidal neurons in Connexin36 knockout mice (Pais et al., 2003). To date, there is no molecular or anatomical evidence for the presence of connexins among CA1 pyramidal neurons. Second, dye coupling cannot affirm electrical junction. Dye coupling of biocytin has never been reported between verified gap junction‐connected interneurons (Galarreta and Hestrin, 2001; Gibson et al., 1999). On the other hand, spurious electrical junctions between dendrites and axons occur in vitro by membrane fusion due to the slicing procedure, osmotic shock, pH or ion alterations between the intracellular and extracellular compartments (Gutnick et al., 1985; Church and Baimbridge, 1991). Third, gap junction blockers are non‐specific and even at low concentration can affect other processes, such as GABAA receptor‐mediated inhibition. While several gap junction blockers abolish ripples in vitro, mefloquine, which is known to interfere with Cx36, Cx 43, and Cx50 gap junctions, failed to block ripples induced with tetanic stimulation of the Schaffer collaterals in rat slices (Behrens et al., 2011). Fourth, in vivo sharp electrode recordings from the soma of CA1 pyramidal cells failed to observe evidence for spikelets in healthy neurons, and the waveforms of the action potentials both in and outside ripples, were absent of signs of axonal origin, including the critical pre‐spike period (Fig. 26) (Ylinen et al., 1995; English et al., 2014; but see Spencer and Kandel, 1961 and Epsztein et al., 2010 with patch electrodes), although spikelets are routinely observed in thin dendrites (Kamondi et al., 1998a, 1998b). Spikelets observed in the behaving rat can have spatial (place) correlates (Epsztein et al., 2010), a finding more compatible with their synaptically regulated control than with stochastic ectopic axonal origin. Finally, extracellular recording and comparison of spike waveforms in a large group of pyramidal cells found no significant difference in the waveforms of spikes in ripples and those out of ripples (English et al., 2014). These findings indicate that spikes in CA1 pyramidal cells are triggered orthodromically during SPW‐Rs. Independent of the problem of ripple‐pacing mechanisms, the axon gap junction model of ectopic spike generation poses a serious challenge to the neuronal “doctrine.” Action potentials are regarded as a fundamental physiological mechanism to broadcast the activity of neurons in a directed way from a presynaptic to a postsynaptic neuron (Bullock et al., 2005). If spikes can emerge “ectopically” in a hypothetical axon mesh, the spikes emerging in the axonal mesh would collide with orthodromic spikes and the direction of neuron‐to‐neuron communication would no longer be guaranteed by the unidirectional presynaptic‐postsynaptic direction. Ectopic spikes in peripheral nerves often occur after injury and contribute to neuropathic dysesthesia and chronic pain (Amir et al., 2005). It is thus possible that ectopic action potentials in hippocampal slices emerge as a result of the slicing procedure.
Figure 26.
Action potential properties in and outside of SPW‐Rs. (A) Left, Example waveforms of average action potential during ripples (green) and nonripple periods (blue) from one neuron. Right, Expanded view of the spike pair at left to illustrate the difference in threshold. SPW‐R periods are defined from the times at the peaks of the SPW‐Rs detected in the LFP. Shaded area is SEM. (B) Phase plane plot of average action potential waveforms. Each color is one neuron; solid lines are outside of ripples; dashed lines are during ripples. (C) Expanded view of section phase plane plot with spike amplitude normalized across neurons to illustrate the shape near threshold. After English et al. (2014).
Ripple timing is set by PV interneurons
According to the PYR‐INT‐INT model, the frequency of ripple oscillations is set by the interactions among interneurons, and the phase‐locked timing of pyramidal cells is a secondary event. Because isolation of such a mechanism in vivo is not straightforward, previous evidence in favor of the PYR‐INT‐INT framework was supported by only correlation studies, computational modeling (see Computational Models of SPW‐Rs section) and in vitro pharmacological manipulations (Whittington et al., 1996). However, optogenetic experiments support the critical role of PV interneurons in pacing pyramidal cells at ripple frequency.
While PV‐expressing interneuron activation alone (i.e., without pyramidal cell activation) cannot generate LFP ripples in vivo, optogenetic activation of PV interneurons brings about ripple‐frequency patterning of interneuron and pyramidal cell spikes. Conversely, optogenetic silencing of PV‐positive cells prevents the occurrence of spontaneous SPW‐Rs. In addition, focal application of the GABAA receptor‐antagonist picrotoxin in vivo fully abolishes the induction of LFP ripples. These observations support the hypothesis that ripple timing can be set by interactions among PV interneurons (Fig. 27). Optogenetic experiments in vitro are in line with the in vivo observations (Schlingloff et al., 2014). Optogenetic stimulation of PV‐positive cells induces fast LFP oscillations and IPSC‐EPSC sequences intracellularly in pyramidal cells. Blocking AMPA and NMDA receptor‐mediated fast excitatory synaptic transmission by applying NBQX and AP‐5 in the bath completely abolishes spontaneous SPW‐Rs, yet optogenetic excitation of PV neurons continues to induce LFP ripples, albeit with a smaller amplitude and without a spiky appearance of the negative phases (Fig. 27). Furthermore, when the GABAA‐receptor blocker gabazine is puffed onto the somata of post hoc identified PV basket cells recorded in a loose‐patch configuration, the firing rate of the neuron is increased but its ripple‐phase modulation is eliminated. These findings offer direct support for the critical role of phasic inhibition in ripple frequency pacing of PV basket cells (Schlingloff et al., 2014). A number of differences between the in vivo and in vitro situations may explain why LFP oscillations are not observed by focal activation of PV interneurons in the behaving animal. First, in vivo light was delivered locally, activating perhaps a dozen or so PV interneurons, whereas a large area of the slice was illuminated. Second, the excitability of pyramidal neurons in vitro may be elevated. Third, in vivo GABAA‐receptor activation of the pyramidal cells during SPW‐Rs has a strong shunting component, which does not induce transmembrane currents (English et al., 2014).
Figure 27.
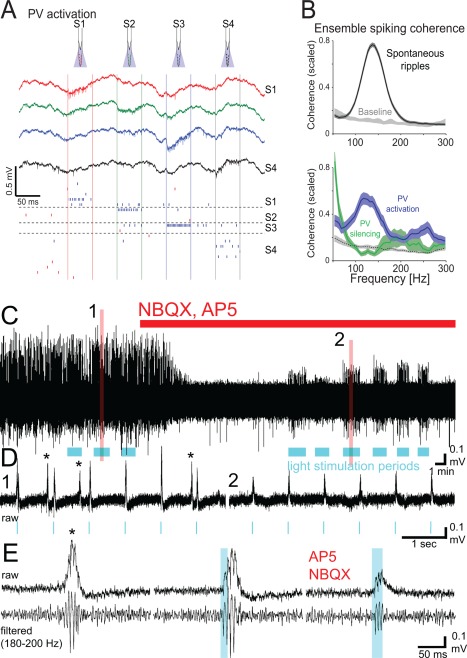
Tonically driven PV interneurons induce coherent spiking at ripple frequency and can pace ensemble spiking. (A) Optogenetic activation of PV interneurons in a behaving PV::ChR2 mouse. Wide‐band traces recorded at 200 µm intervals during sequential illumination (square pulses) of the CA1 pyramidal layer. Vertical colored lines delimit illumination on each shank, and horizontal dashed lines separate units recorded on distinct shanks (S1 to S4). Red/blue ticks indicate pyramidal and interneuron spike times, respectively. Each row corresponds to a single unit. Note locally induced interneuron spiking but no visible LFP ripples. (B) Ensemble spiking coherence. Coherence between aggregated pyramidal neurons recorded on different shanks. Cross‐shank spiking coherence was computed between agglomerated spike trains during spontaneous ripples (top, black trace), during PV activation (bottom, blue) or PV silencing (C, green). Note increased and decreased spike coherence during PV activation and inactivation, respectively. (C) Induced ripples by optogenetic driving of PV interneurons in vitro during blockade of ionotropic excitatory synaptic transmission (NBQX and AP5). Blue bars, light stimulation periods. (D) Zoomed traces from C. Asterisks, spontaneous ripples. A–B, reprinted with permission from Stark et al. (2014). D–E, reprinted with permission from Schlingloff et al. (2014).
In summary, local drug applications and optogenetic studies in vivo and in vitro lend strong support to the hypothesis that the activity of PV‐positive basket neurons appears both necessary and possibly sufficient for ripple‐frequency pacing of pyramidal neurons. A potential mechanism responsible for the timing could be the synaptic time constant of GABAA‐receptors between PV‐expressing neurons, most likely basket cells. Coordinated inhibition is often discounted as a timer of fast oscillatory events based on the argument that the time constant of GABAA receptor‐mediated inhibition is too slow to pace >200 Hz patterns. However, assuming that multiple time‐shifted cell assemblies are superimposed during ripples, the individual assemblies may be active only once in a given event and the partner interneurons of the current assembly can time the next assembly by the fast rising slope of inhibition without the need for the IPSP to relax rapidly. In addition to synaptic mechanisms, dendritic “hot spots” with high‐density voltage‐gated Na+ channels in PV interneurons may provide ripple frequency resonance and, thus, assist in timing of action potentials during SPW‐Rs (Chiovini et al., 2014). The findings also support the hypothesis that pyramidal cells, while critical in providing tonic excitation, are not essential for ripple precision timing of interneurons.
Temporal Coordination of Spatially Distributed Ripples
The optogenetic ripple‐induction experiments indicate that only a few dozen of pyramidal cells are needed to bring about ripple‐frequency oscillations in pyramidal cells and that the frequency of ripple oscillation is set by the interactions among PV‐expressing interneurons. If multiple ripples emerge at different locations along the septo‐temporal axis (see Modulation of SPW‐Rs by Subcortical and Neocortical Inputs section), how are they linked together into a coherent pattern? While small amplitude ripples often remain local, large amplitude coherent ripple events can span the entire septal and intermediate segments of the hippocampus (Ylinen et al., 1995). In the intact brain, the sweep of the SPW in the septo‐temporal or temporo‐septal direction and the consequent excitation of CA1 neurons is a coarse but efficient coordinating mechanism (Patel et al., 2013). However, because different levels of excitation by the CA3‐induced SPWs generate different frequency local ripples, additional timing mechanisms should be at play to provide frequency coherence for ripples that occupy a wider spatial area. Such spatial coordination at the ripple frequency scale is likely supported by interneuron‐mediated inhibitory synchronization. PV basket cells are the most likely candidates since they form mutually interconnected inhibitory networks in the hippocampus (Sik et al., 1995; Fukuda et al., 1996) and because they communicate with each other through fast synaptic inhibition (Bartos et al., 2007).
Optogenetically induced ripples at different locations of the CA1 axis can differ in power, frequency, and phase. During single‐site illumination, the LPF ripple power declines progressively with distance and at >400 µm the induced power is indistinguishable from baseline. LFP ripples induced by threshold‐level single‐site illumination involve a smaller network than typical spontaneous ripples, indicating that the coherence of LFP ripples across multiple sites observed during spontaneous ripples is not a volume‐conducted effect but rather an outcome of a dedicated temporal coordination mechanism.
Similarly to the spontaneous ripple events, when multiple CA1 locations are concurrently activated by light, the local LFP events become coherent and the frequency and phase differences between different sites are reduced. Consistent with the LFP results, optogenetic recruitment of pyramidal cells tapers off quickly with distance. In contrast, interneurons remain phase‐locked to the focal light‐induced LFP oscillations even at those sites several hundred µm away where pyramidal cells are not directly activated by light and no LFP oscillations are detected (Fig. 29) (Stark et al., 2014). The above‐discussed experiments demonstrate that optogenetic induction of LFP ripples at multiple sites can phase‐lock local events and form a single global oscillator. When the GABAA receptor blocker picrotoxin is infused locally, the surrounding sites continue to generate LFP ripples but their phase coherence is deteriorated (Stark et al., 2014). In vitro experiments also support the role of inhibition in temporal coupling of spatially separate CA3 ripple events (Schlingloff et al., 2014). When ripples are induced optogenetically by illuminating wide areas of the slice, the coherence between two recording sites placed ∼200 µm apart is high. However, when a small surgical cut is made in stratum oriens, the pyramidal layer and stratum lucidum between the two recording sites, the LFP ripple and related multiple unit oscillations remain unaffected but ripple correlations between multiple units across the cut disappear (Schlingloff et al., 2014). This observation also argues against the axonal gap junction hypothesis since the cut did not affect the axonal arbors of pyramidal cells.
Figure 29.
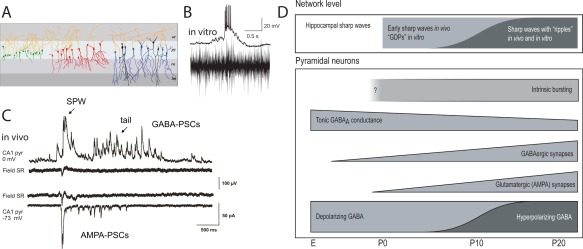
SPW‐Rs in early development. (A) Three different type of CA1 pyramidal cells are encountered at birth: cells that are synaptically silent, cells with only GABAergic synaptic activity and cells with both GABAergic and glutamatergic synaptic activity. (B) Simultaneous recordings of a CA3 pyramidal cell and extracellular multiple unit burst in CA3 pyramidal cell layer in postnatal day 6 hippocampal slice during GDP. (C) Intracellular correlates of SPWs (GDP) in vivo. Intracellular (CA1 pyramidal cell, whole‐cell) and extracellular (stratum radiatum, SR) recordings during a SPW event in a postnatal day 5 pup. Upper pair: intracellular voltage clamp at glutamate reversal potential (0 mV), showing presumed GABAA receptor‐mediated postsynaptic currents (upward deflections). Lower pair: intracellular voltage clamp at GABAA reversal potential (−73 mV), showing presumed glutamate receptor‐mediated postsynaptic currents (downward deflections). (D) Developmental changes of hippocampal network events and single neuron properties. Already at the embryonic stage, immature pyramidal neurons have a large tonic GABAergic conductance. GABAergic synapses are functional before glutamatergic ones, but GDPs/SPWs are first seen only after the establishment of functional glutamatergic synapses. CA3 pyramidal neurons generate intrinsic burst activity throughout postnatal development. In vitro, the probability of GDP occurrence decreases as the GABAA‐receptor mediate action shifts from depolarizing to hyperpolarizing. In vivo, SPWs are the first endogenous event of activity seen during ontogeny but ripple oscillation emerge at an older age. The approximate developmental time scale shows the late embryonic period (E) and the postnatal period from P0 (postnatal day 0; time of birth) to P20 in rats. A and B, reproduced from Ben‐Ari (2001), C, reproduced from Leinegukel et al. (2002), D, reproduced from Sipilä and Kaila (2007).
Overall, these experiments show that in the intact brain, the CA3‐generated SPW sweep, which induces local ripple oscillations in CA1, is assisted by local inhibitory neurons interacting with each other through GABAA receptors at the temporal resolution of a single ripple cycle. Interneurons also assist in combining multiple local events into a spatially coherent single oscillatory event. Reciprocal connections between PV‐expressing interneurons cells are essential in both ripple‐timing and supporting spatial synchrony. Finally, the discussed findings argue against the critical role of gap junctions (interneuronal or pyramidal cell axon) in determining the frequency of ripples and their phase coordination across neuronal space in vivo.
DEVELOPMENT OF SHARP WAVES AND RIPPLES
Development of SPWs and ripples provides an additional demonstration that the two events are independent since their maturation follows different time courses. While SPWs represent the first organized events in the hippocampus, ripples emerge late during the ontogenetic development of the brain.
Emergence of SPWs in the Developing Hippocampus
One of the most remarkable features of early cortical activity in humans and other animals is its discontinuous temporal organization. Bursts of activity are separated by periods of “flat” LFP (or “tracé discontinue” in scalp EEG in premature human babies) that can last for seconds or even tens of seconds before mid‐gestation (Dreyfus‐Brisac et al., 1956; Stockard‐Pope et al., 1992). Rats and mice are “premature” at birth and their first postnatal week roughly corresponds to the third trimester of pregnancy in humans. At this early stage, both hippocampal and neocortical events are characterized by long silence, interrupted by transient population bursts of neurons (Khazipov et al., 2004; Khazipov and Luhmann, 2006). In the hippocampus, the earliest organized network event is a SPW‐burst (Leinekugel et al., 2002). As early as postnatal day 3, LFP SPWs and associated synchronous unit discharges are present mainly during immobility periods, sleep, and feeding, while they are rare during crawling. Amplitude versus‐depth profiles of SPWs show a phase‐reversal just below the CA1 pyramidal layer and the largest amplitude negative deflection in the middle of stratum radiatum. The amplitude‐versus‐depth distribution of SPWs is largely identical with the depth profile of the evoked potentials in response to stimulation of the ventral hippocampal commissure (Leinekugel et al., 2002; Karlsson and Blumberg, 2004). These observations suggest that early SPWs and associated bursts of CA1 neurons are brought about by population bursts of the CA3 region and conveyed by the glutamatergic commissural collaterals to the apical and basal dendrites of CA1 pyramidal cells, similar to the SPWs in adults (Buzsáki et al., 1983).
At least three features distinguish early SPWs from the adult form. First, LFP SPWs in the newborn are most often followed by a “tail” of multiple unit firing lasting for 0.5 to 3 s, which is occasionally shaped into gamma frequency rhythm (Fig. 30) (Leinekugel et al., 2002; Karlsson et al., 2006). Second, early SPWs are not associated with LFP “ripples” in the CA1 pyramidal layer in neonates (Leinekugel et al., 2002). Third, rat pups often display myoclonic twitches of the limbs and such twitches can trigger neocortical spindles and hippocampal SPWs (Khazipov et al., 2004; Karlsson et al., 2006; Mohns and Blumberg, 2008). The trigger for SPWs is likely the entorhinal cortex (Mohns and Blumberg, 2010), since entorhinal‐hippocampal projections are already present prenatally (Super and Soriano, 1994). In adults, movement never triggers SPW‐Rs.
The synaptic origin of early SPWs was investigated with combined extracellular and patch‐clamp recordings in urethane‐anesthetized pups between postnatal days 3 and 6 (Leinekugel et al., 2002). LFP SPWs were associated with large complex synaptic events in CA1 pyramidal cells (Fig. 30). In voltage clamp mode, large GABAA receptor–mediated postsynaptic currents were observed at glutamate reversal potential (0 mV) and, conversely, glutamatergic postsynaptic currents were identified at GABAA reversal potential (−70 mV). The prominent glutamatergic component of SPWs most likely reflects an excitatory drive mediated by Schaffer collaterals, consistent with the extracellular LFP and unit firing observations.
Overall, the above‐discussed results suggest that hippocampal SPW bursts are the main hippocampal population events during the first postnatal days in rodents. Because of the dominance of these events in early life, they may play a major role in the maturation and maintenance of hippocampal‐entorhinal circuits in the newborn (Leinekugel et al., 2002; Khazipov et al., 2004; Karlsson et al., 2006; Mohns and Blumberg, 2008, 2010).
Giant Depolarizing Potentials (GDP) are In Vitro Counterparts of In Vivo SPWs
SPW bursts in the developing hippocampus in vitro have a special name: Giant Depolarizing Potential or GDP, a term coined by Ben‐Ari (Ben‐Ari et al., 1989; Ben‐Ari, 1997). GDP refers to the large intracellular depolarization of pyramidal cells as well as a population event, even though the two can be dissociated. Other terms were also introduced, such as giant GABArgic potentials (Strata et al., 1997), Early Network Oscillations (Garaschuk et al., 1998), Fast Network Oscillations (Palva et al., 2000) or simply Population Bursts (Lamsa et al., 2000) but GDP persisted. It is not fully understood whether the various in vitro events observed in different laboratories, preparations, and particular conditions correspond to distinct classes of naturally occurring events in the healthy developing brain or if they represent particular aspects of the same basic physiological event. I take the view here that GDPs represent an in vitro variant of the early form of in vivo SPWs, especially since SPWs are the only population burst events in the intact brain during the first week of life (Leinekugel et al., 2002; for a more extensive review of this topic, see Sipilä and Kaila, 2007).
GDPs were first described in intracellular recordings from CA3 pyramidal neurons in vitro during the first eight days of postnatal life (Ben‐Ari et al., 1989). GDPs are CA3 network‐driven large excitatory synaptic events (hence the term GDP) and constitute the first synchronized activity in the newborn rodents and late in utero life of the rhesus macaque fetus (Khazipov et al., 2001). Their spatial propagation along the septotemporal axis has been studied in whole (in toto) hippocampus preparations in vitro using multisite extracellular and whole cell recordings (Khalilov et al., 1997; Leinekugel et al., 1998). These experiments show that spontaneous GDPs can be initiated at multiple locations along the CA3 axis but most often they emerge in the septal end and propagate temporally at a speed of 7 to 10 mm/s. This septotemporal dominance of travel is explained by the similar gradient of the incidence of GDPs in isolated slices cut from the different segments of the hippocampus. Similar to in vivo SPWs, GDPs occur synchronously in the left and right hippocampi in in toto preparations as long as the ventral hippocampal commissure is kept intact (Leinekugel et al., 1998). Although GDPs emerge spontaneously, their occurrence can be timed by stimulation of the dentate hilus (Ben‐Ari et al., 1989). Depending on the exact circumstances and in vitro conditions, GDPs can be observed in mini‐slices of CA3 and CA1 regions in both rats and rabbits (Khazipov et al., 1997; Garaschuk et al., 1998; Menendez de la Prida et al., 1998), although in some preparations their occurrence is abolished by cutting the connections between dentate and CA3 (Strata et al., 1997). However, in most experiments the GDPs are initiated in the CA3 region and spread to CA1 (Fig. 30; Ben‐Ari, 2001).
Early experiments on GDPs suggested that they were driven exclusively by the depolarizing and excitatory effect of GABA via GABAA receptors in immature pyramidal cells, based largely on the observations that both bicucullin and picrotoxin can attenuate or abolish GDPs (Ben‐Ari et al., 1989; Leinekugel et al., 1998) and their alleged transient appearance is thought to reflect the maturation of the postsynaptic GABAA receptor‐mediated depolarizing action to hyperpolarization (Khazipov et al., 2004). GABA‐induced depolarization of immature pyramidal neurons can activate voltage‐gated Ca2+ channels, which leads to intracellular Ca2+ transients and to the activation of various kinds of intracellular signaling cascades (Leinekugel et al., 1995, 1997; Khazipov et al., 1997). The trophic actions of GABA can contribute to the morphological maturation and differentiation of interneurons and synaptogenesis (Marty et al. 1996, 2000).
The depolarizing action of GABAA in immature neurons is due to active uptake of Cl– by NKCC1, a secondarily active Na‐K‐2Cl cotransporter (Marty et al. 2002; Sipilä et al. 2006). The outwardly‐directed Cl– electrochemical gradient generated by NKCC1 provides the driving force of inward (i.e., depolarizing) GABAergic Cl– currents. At this stage of development, expression of functionally‐active KCC2, the main neuronal K‐Cl cotransporter which extrudes Cl–, is low (Sipilä et al. 2006). During neuronal maturation, the functionality of KCC2 progressively increases and the depolarizing actions are gradually replaced by hyperpolarizing IPSPs ( Hubner et al. 2001; Rivera et al. 1999; Zhu et al. 2005). In line with this explanation, GDPs are blocked by the NKCC1 inhibitor, bumetanide (Sipilä et al. 2006). In addition to Cl–, GABAA receptor channels are permeable to , which produces depolarization (Kaila et al. 1994). In both adult and immature brains, activity‐dependent accumulation of Cl– in pyramidal neurons can increase permeability and, thus, depolarization (Staley et al., 1995; Kaila et al. 2014). It is possible, although not demonstrated explicitly, that during the SPW‐Rs, the equilibrium potential of GABAergic currents (EGABA) shifts in a positive direction and, consequently, the efficacy of inhibition may show an activity‐dependent decrease, particularly in the superficial calbindin‐immonoreactive neurons (Valero et al., 2015). This hypothetical mechanism may contribute to the large excitatory gain during SPW‐Rs (see Section on Physiological Mechanisms of SPW‐Rs). Both types of depolarizing actions of GABA, the one based on NKCC1 in immature neurons and the other dependent on HCO3−, work in synergy with NMDA receptors by reducing the voltage‐dependent Mg2+ block (Leinekugel et al., 1997). In addition, depolarization also facilitates the action of other voltage‐gated Ca2+ channels (Leinekugel et al., 1995; Ben‐Ari et al., 1997). More recent experiments, on the other hand, emphasize that GABA can exert a dual excitatory‐inhibitory effect on pyramidal cells. The GABAA‐receptor‐activated Cl– conductance is more negative (−35 mV) than the reversal potential of the glutamate‐mediated currents (∼0 mV) even in young animals. Therefore, when AMPA, NMDA, and GABAA receptors are activated synchronously, GABAA receptor‐mediated conductance can shunt the excitatory effects of glutamate (Khazipov et al., 1997; Khalilov et al., 1999; Bolea et al., 1999; Palva et al., 2000; Wells et al., 2000), as in the adult hippocampus (English et al., 2014). In addition, hyperpolarizing effects, mediated by GABAA receptors, may also be present in a fraction of neurons from early postnatal days (Wong et al., 2005). Finally, other experiments emphasize that glutamate excitation of both interneurons and pyramidal cells is an equally important mechanism in the induction of GDPs in both rats (cf. Menendez de la Prida and Sanchez‐Andres, 2000; Ben‐Ari, 2001) and mice (Wong et al., 2005).
The duration of GDPs is typically longer (Fig. 30; 200–1,000 ms) than the duration of adult SPWs and GDPs have been suggested to be terminated by the activation of presynaptic GABAB receptors acting on the terminals of glutamatergic neurons (Caillard et al., 1998). However, SPWs in the early postnatal days in rat pups are also longer than in adults (Leinekugel et al., 2002). While the early postnatal depolarizing action of GABA is undisputable, it may not fully be responsible for the population bursts of CA3 pyramidal cells underlying GDPs. GDPs/SPWs are associated with both strong GABA currents and pronounced postsynaptic glutamatergic response (Khazipov et al., 1997; Leinekugel et al., 2002). A bath‐applied cocktail of AMPA‐kainate antagonists (e.g., CNQX, NBQX) and an NMDA antagonist fully abolishes GDPs, while NMDA‐receptor antagonists alone have only a small effect on GDP frequency (Ben‐Ari et al., 1989; Gaiarsa et al., 1991; Bolea et al., 1999; Lamsa et al., 2000; Khazipov et al., 2001). Furthermore, upon application of glutamate blockers, the cross‐correlation between interneuronal activity and LFP GDPs is abolished (Sipilä et al., 2005). The absence of glutamate‐receptor mediated responses in a subpopulation of pyramidal neurons, especially during early postnatal days, is not surprising since they may have only functional GABAergic synapses but no glutamatergic ones (Tyzio et al., 1999). However, this observation does not preclude the explanation that the majority of neurons are recruited by glutamatergic excitation into the population bursts underlying GDPs (Menendez de la Prida and Sanchez‐Andres, 2000; Leinekugel et al., 2002). In summary, the available evidence indicates that excitatory glutamatergic transmission, mediated by AMPA receptors, is of primary importance for the generation of GDPs, similar to SPWs in the intact brain. Another similarity is the buildup dynamics that precede both GDPs (Menendez de la Prida et al., 1999, Menendez de la Prida and Sanchez‐Andres, 2000) and SPW‐Rs (Fig. 9g; Schlingloff et al., 2014).
The disappearance of GDPs in vitro during the second week of life in slice preparations can be explained by the reduced excitability of the more matured hippocampus and/or the severed excitatory connections in the CA3 recurrent system during slicing, whose importance becomes more critical at a later stage of development for the emergence of population bursts than the early GABAA receptor‐mediated excitation. In mice, CA3‐initiated population bursts in in toto hippocampal preparations persist up to at least two weeks after birth (Wong et al., 2005). Furthermore, improvement of in vitro conditions has been shown to facilitate the spontaneous emergence of SPWs also in adult slices (Hajos et al., 2013).
Large‐scale interactions among CA3 neurons during GDPs were recently investigated using multibeam two‐photon Ca2+ imaging in 5‐ to 7‐day‐old mice (Bonifazi et al., 2009). Although temporal resolution was limited to 50 to 150 ms time windows, the temporal correlations of many neuron pairs could be studied in the slice. Neurons with high probability of coactivation with other neurons (dubbed “hub cells”) were then subsequently patched and stimulated. Induction of bursts of action potentials in this highly connected small minority, but not in those with few partners, increased the probability of occurrence of GDPs or trigger GDPs. Most but not all hub cells were GABAergic interneurons with dense local axon trees, had low threshold for action generation, received high frequency EPSPs and they were frequent and early participants in spontaneous GDPs, suggesting that these special neurons play an active role in initiating GDPs, similar to a subgroup of SPW‐triggering fast‐spiking GABAergic interneurons in adults (Ellender et al., 2010). Interestingly, stimulation of some hub cells could also reduce, delay or eliminate the occurrence of GDPs, perhaps due to their shunting action that prevents synchronization of pyramidal cells. The intereneurons in this subgroup tended to have long projecting axons. One can speculate that the highly active and strongly interconnected hub cells may come from the earliest born, highly active group of both excitatory and inhibitory neurons, which may form a strongly interconnected (“rich club”) backbone of circuit organization. Subsequently born, less active, less bursting neurons then would populate the developing circuits, establish greater autonomy and expand the dynamic range of hippocampal networks so that their firing rates, burst probability and affiliations to population bursts form a lognormal distribution in the matured brain (Buzsáki and Mizuseki, 2014). Members at the two ends of this skewed distribution may then be called “choristers” and “soloists” (Okun et al., 2015) and may correspond to neurons with different developmental history. In summary, these findings indicate that GDPs are the in vitro counterparts of SPWs in the intact hippocampus, with possibly similar functions. It remains to be verified though whether the population burst‐initiating/participating interneurons in the newborn are transiently expressed phenotypes or whether they persist as burst initiating cells in the adults, and whether they use the same or different mechanisms to induce bursts.
Emergence of Ripples in the Developing Hippocampus
Following the appearance of SPWs, several other organized events emerge during early development. Gamma oscillations first appear as the “tail” of SPWs (Leinekugel et al., 2002) and begin to persist independently after postnatal days 5 to 6 in the rat (Karlsson and Blumberg, 2004; Karlsson et al., 2006; Mohns et al., 2006), along with the emergence of dentate spikes (Leinekugel et al., 2002). Short bouts of theta oscillation can be detected also around this age, while clear continuous theta, associated with ambulation and REM sleep, appear at postnatal day 8 (Leblanc and Bland, 1979; Lahtinen et al., 2002; Mohns et al., 2006). Although cholinergic and GABAergic septohippocampal projections are already present before birth, their rapid growth in the second week of life (Bender et al., 1996) and the proliferation of perisomatic interneuronal synapses (Danglot et al., 2006) may be the main “drivers” of theta and gamma oscillations. Ripples, however, take the longest time to emerge (Fig. 30D).
Ripple‐like events have been reported in the hippocampus in vitro right after birth. However, these spiking events may not correspond to true ripples, since they do not show the characteristic spindle shape in the LFP and because they also occur when all chemical synaptic transmission is blocked (Palva et al., 2000). Recordings of LFP and unit firing from the CA1 region in both freely moving and head‐fixed pups from postnatal day 12 to 20, show prominent SPWs and associated firing of neurons. However, SPW‐associated fast‐field oscillations do not begin to emerge until the end of the second postnatal week and grow to near adult‐like ripple oscillations by postnatal day 20 (Buhl and Buzsáki, 2005). Interestingly, once the fast oscillations become detectable, their frequency corresponds to the ripple frequency in adult animals (120–180 Hz with peak power at 140 Hz).
These developmental observations also place constraints on the relevance of gap junctions in the control of ripples. If gap junctions are critical in the emergence of ripple oscillations, their developmental profile is expected to match that of the physiological events underlying ripple generation. However, most gap junctions are thought to be strong early in development and often disappear later in life (Yuste et al., 1995). However, no ripples are present during the first week of life in rodents even though large SPWs are already present from birth. Non‐connexin type electrical junctions, such as Pannexin 1 and 2 (Bruzzone et al., 2003) may, in principle, play a role but, to date, their physiological role in the hippocampus is unknown. The developmental time course of ripples somewhat parallels the switch in the GABAA receptor‐mediated signaling from depolarization to hyperpolarization (Ben‐Ari, 1997). However, the GABA switch may occur several days earlier and can correspond better to the emergence of gamma oscillations than that of ripples (Khazipov and Luhmann, 2006). Another candidate to be investigated is the kinetics of the GABAA receptor between PV‐expressing interneurons, which are critical in setting the frequency of ripple oscillations in adults.
COMPUTATIONAL MODELS OF SPW‐RS
Modeling Collective Behavior of Neurons—SPW Bursts
As discussed earlier, SPW‐associated population bursts are not triggered or induced by an external drive but they are “released” when subcortical suppressive mechanisms are suspended. SPWs are self‐organized emerging phenomena, brought about by the rapid spread of activity in the excitatory recurrent CA3 network. Recurrent excitation‐based population bursts likely follow the same basic mechanisms, independent of the exact substrate and other details. Computer models, capturing the essential aspects of such population bursts, have provided mechanistic insights into emergent phenomena. Traub and Wong (1982) constructed the first detailed computer model of population bursting, using 100 CA3 compartmental neurons, each capable of intrinsic bursting and randomly interconnected by excitatory chemical synapses. Bursting in single neurons is induced by strong depolarization. Weakening inhibition facilitates the recruitment of pyramidal neurons into population bursts. The synaptic strengths in the model are set so that bursting in one neuron can evoke bursting in postsynaptic follower neurons. This may not be the case in the CA3 network when inhibition is intact but each model neuron can be conceived as a small group of synchronously firing units. As a result of the efficient spread of excitation, new neurons are recruited until the majority of the population fires in a short time window. The event is terminated by a refractory process. Although this early model was designed to mimic the population bursts underlying epileptic spikes, it captures the essential process of SPW‐related neuronal recruitment as well. The main difference is that during SPW‐Rs the recruitment is protracted in time and it involves only a minor fraction of the entire CA3 pyramidal neuron population. Another difference is that in the model, all neurons have similar properties, whereas in real networks the participation probability of individual neurons is strongly skewed so that a highly active small minority dominates the majority of the events (see Behavioral Correlates and Mechanisms of SPW Generation section). Dur‐e‐Ahmad et al. (2012) also used bursting as the main communication mechanism in generating population bursts in networks of up to 10,000 model neurons.
An extension of the Traub network model is made by Taxidis et al. (2012, 2013). This model is a one‐dimensional array of 1,000 pyramidal cells and 100 interneurons. Pyramidal cells are modeled by the two‐compartmental Pinsky‐Rinzel model (Pinsky and Rinzel, 1994), and interneurons are modeled by the single‐compartment Wang‐Buzsáki model (1996). Pyramidal cells are connected sparsely (10.6%) and randomly. Spike bursts are initiated in nonspecific locations and quickly spread throughout the whole network via recurrent excitatory connections with a propagation velocity of 0.145 m/s. The SPW population burst lasts for 20 to 80 ms and is terminated by interneuronal inhibition and afterhyperpolarization of pyramidal cells. Model pyramidal cells fire rarely and mostly in spike bursts. Each population burst in CA3 produces a corresponding burst of activity in CA1 by exciting both pyramidal cells and interneurons through the Schaffer collaterals. The majority of CA1 neurons receive weak connections while a small minority receives many more synapses than average, yielding a subset of CA1 pyramidal cells that will be driven by much stronger input from CA3 than the rest, mimicking the skewed distribution of synaptic strengths in real networks and forming a strongly driven subset. In another variation (Taxidis et al., 2013), CA3 neurons receive inputs from model granule cells, while CA1 pyramidal cells are also excited by entorhinal afferents, to mimic the role of slow cortical oscillations on triggering SPW‐Rs. As expected from the network design, the overall amount of CA1 spiking in relation to UP/DOWN states is affected by the excitation‐to‐inhibition ratio induced via the entorhinal input. Similarly, the ratio of excitation and inhibition conveyed by the mossy terminals will bias the probability of occurrence of SPW bursts in CA3.
While the above models successfully describe certain specific features of SPWs, they do not replicate the in vivo observed distributions of SPW magnitude. Similar to the lognormal distribution of synaptic weights, firing rates and spike burst, the magnitude of SPW variations also shows a lognormal form (Mizuseki and Buzsáki, 2013). However, the links between these skewed distributions across various levels of neuronal organization are not known. Omura et al. (2015) constructed a computation model of SPWs with a recurrent neural network in which the weights of recurrent excitatory connections are distributed lognormally and neurons fire both single spikes and bursts. To model spike bursts in single neurons, they employed a multi‐timescale adaptive threshold (MAT) neuron. Because the fraction of very strong synapses in the lognormal network is small, single pyramidal cells acquire very strong synapses only when they receive a large number of synapses, that is, when the recurrent network is sufficiently large. In the model with recurrently connected MAT neurons with long‐tail ends, lognormal distributions naturally emerged in firing rates, burstiness and the magnitude of population synchrony (Fig. 30). The model of Omura et al. (2015) also shows that the recurrent lognormal network efficiently broadcasts spike bursts multisynaptically to distant neurons through strongly connected pathways. Interestingly, the strongly activated neurons during SPWs were not necessarily those neurons that had high spontaneous high firing rates in the absence of SPWs, because many of these neurons were actually inhibited during the synchronous SPW events, similar to what is observed during waking and SPW‐R‐replay (see SPW‐R‐Supported Memory Consolidation section). Although not all aspects of the model reflect physiological realism (e.g., bimodal distribution of spikes and bursts), the model captures numerous features of the SPW dynamics from the skewed distribution of synaptic weights to the long‐tail distribution of neuronal synchrony.
Large dynamic range and individualized inhibin
Recurrent neural networks, such as those supporting SPW‐Rs, face the problem of an effective balance between amplification and stability, because the positive feedback increases the potential for chaotic instability (van Vreeswijk and Sompolinsky, 1996). While chaotic states can be advantageous because even very minor perturbations get amplified leading to diverging trajectories of network activity, stabilizing chaos without losing the rich spatio‐temporal structures has been a persistent challenge (Sussillo and Abbott, 2009; Laje and Buonomano, 2013). Gerstner et al. have recently provided a potential solution (Hennequin et al., 2014). Their model implemented a plasticity rule on inhibitory synapses and adjusted the pattern of inhibition on individual neurons to achieve network stability, yet allowed multiphasic transient population bursts. In contrast to global feedback inhibition, the precise arrangement of inhibitory connections onto every neuron can provide network stability while allowing the evolution of high‐dimensional “nonnormal” amplification. Although the model network was designed to mimic the “rotational” population structure observed in motor cortical areas (Shenoy et al., 2013), the ensuing dynamic is reminiscent of that of SPW‐Rs. The spirit of the model also provides insights about the need and advantages of the spatio‐temporally orchestrated inhibitory mechanisms that characterize the SPW‐R dynamic for the coordination of sequential recruitment of pyramidal neurons and helps explain why recruitment of neurons in SPW‐Rs are self‐terminating under physiological conditions.
Models of Ripple Oscillations
Optogenetic experiments provided evidence that direct activation of a handful of pyramidal neurons is sufficient for inducing ripples (Stark et al., 2014). Therefore, local mechanisms should be responsible for determining the frequency of the fast oscillation. Local interactions between PV interneurons (Stark et al., 2014; Schlingloff et al., 2014), pyramidal cells and interneurons, and pyramidal‐pyramidal neuronal interactions contribute to controlling both the frequency and the period over which ripples occur. Computational models have the advantage of examining each of these contributions in isolation.
Modeling extracellular currents of LFP ripples
Computer simulations with spiking neurons examined the contribution of spikes and other processes responsible for the extracellular pattern of ripples (Fig. 32). Utilizing detailed biophysical models of pyramidal and interneuron populations of the hippocampus (Gold et al., 2006), Schomburg et al. (2012) demonstrated that the magnitude of the LFP ripple could be accounted for by a combination of the sizes of the recorded action potentials recorded by the electrode and the number of neurons firing. Neurons in a ring of 50 to 100 µm from the electrode contributed approximately half of the LFP ripple power, since this range has the most effective combination of spike count and spike amplitude, although more distant but more numerous neurons also contribute significantly (Fig. 31). This is consistent with the in vivo observation that LFP ripple amplitude is often similar regardless of whether or not it contains recognizable spikes. The simulations also demonstrate that the contribution of spiking interneurons is negligible, mainly due to their small numbers in the pyramidal layer.
Figure 32.
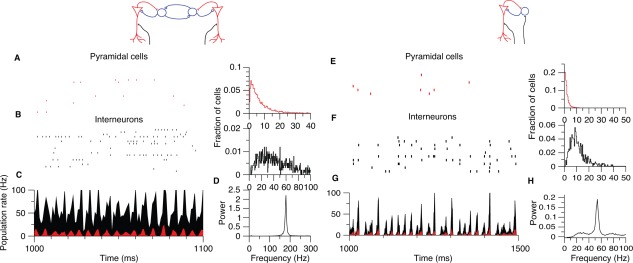
Fast oscillations supported by chemical synapses in a model. (A–C) Ripple frequency oscillations in a strongly driven network with pyramidal cells and interneurons, but without pyramid‐to‐pyramid connections (such as CA1). (A) Pyramidal population rastergram and (B) interneuron population rastergram. Right: strongly skewed distribution of firing rates. (C) Instantaneous population firing rate of pyramidal cells (red) and interneurons. (E–H) Same network as in the left but with slow time constants, added recurrent excitatory connections between pyramidal cells and weaker external noise drive (mimicking the CA3 region). Note slow gamma oscillation frequency in the power spectrum. Reproduced from Brunel and Wang (2003).
Although spiking is a substantial source of the LFP ripple, spikes do not account for all the ripple power (Schomburg et al., 2012). PV‐expressing basket cells fire strongly in phase with the LFP ripple cycles (see Discharge Patterns of Inhibitory Neurons During SPW‐Rs section), which bring about rhythmic outward current in pyramidal cells both in vivo (Ylinen et al., 1995) and in vitro (Maier et al., 2011; Hajos et al., 2013). These inhibitory perisomatic currents are also an important contributor of the LFP ripple in the pyramidal layer.
Local synaptic network models of ripple generation
To date, the most systematic model that has examined the individual mechanisms of ripple generation in isolation is by Brunel and Wang (2003). These authors used sparsely and randomly connected, leaky integrate‐and‐fire excitatory and inhibitory neurons with membrane time constants of 20 ms (pyramidal cells) and 10 ms (interneurons) and absolute refractory periods of 2 ms (pyramidal cells) and 1 ms (interneurons). Below is an intuitive description of firing behavior of neurons connected in various configurations. In the simplest possible scenario, various fractions of unidirectionally and bidirectionally connected fast‐spiking interneurons are driven by strong external noise (Traub et al., 1999a) so that their firing rate distributions are skewed (Wang and Buzsáki, 1996). Without inhibitory connections among neurons, the firing rates of the individual neurons and synchrony increase relatively linearly with the strength of their excitatory inputs. However, when they are linked by inhibition, the distribution of their firing frequencies is a product of their interactions (i.e., skewed) and population synchrony takes a sigmoid curve: at low level of excitation firing is largely asynchronous, at mid level it is maximum and above a certain excitatory level, synchrony tapers off. At the asynchrony‐synchrony transition, the population firing assumes a sinusoid pattern, indicating the emergence of oscillation. With the above parameters, population oscillation occurs between 150 and 200 Hz and is independent of single cell firing rate and depends only weakly on the magnitude of external drive. Oscillation frequency is largely determined by the features of synaptic connections, including the latency of mutual inhibition and rise time constant of synaptic inhibition but much less so by the decay time constant. Numerical simulations also show that as the network frequency decreases, its coherence increases (Brunel and Hakim, 1999). At very strong levels of excitation the firing frequency of neurons becomes comparable to the network frequency and the network enters the regime of coupled oscillators (Brunel and Wang, 2003). If noise‐driven pyramidal neurons are added to the interneuron network and interneurons inhibit pyramidal cells, the entire population fires in perfect synchrony since all neurons are inhibited at the same time.
An alternative to the interneuron network model, local loops of pyramidal‐to‐interneuron excitation and interneuron‐to‐pyramidal inhibition can also generate fast oscillations (Fig. 32) (Freeman, 1975; Leung, 1982; Fisahn et al., 1998; Whittington et al., 2000). In this pyramidal‐interneuron loop model of leaky intergrate‐and‐fire neurons, population frequency is largely determined by the sum of excitatory and inhibitory synaptic phase delays. Because in this model interneurons are mainly driven by the CA1 pyramidal cells, the additional pyramidal‐interneuron spike transmission delay leads to a decrease of the population frequency, compared with the interneuron network model. If synaptic time scales of excitation and inhibition are similar, interneurons lag pyramidal cells by 90°, and the oscillation frequency decreases to half of the frequency of the purely interneuronal network (Brunel and Wang, 2003), i.e., slower than the frequency of ripples.
If both types of connections (i.e., connections between pyramidal cells and interneurons and among interneurons) are implemented in the model network, such changes tend to settle into a rhythm that is a compromise between the two scenarios described above with a frequency and phase lag that are intermediate between these two extremes. The phase lags and, therefore, the frequency of the rhythm, are then determined by the relative strength of the pyramidal‐interneuron and interneuron‐interneuron connections. With a strong external drive, a coherent fast oscillation emerges in the model network, so that the interneurons lag behind pyramidal cells by ∼90°. Both pyramidal cells and interneurons fire intermittently and with broad distribution of firing rates across individual cells, spanning several orders of magnitude, similar to the CA1 ripple network (Buzsáki et al., 1992; Csicsvari et al., 1999a; Mizuseki and Buzsáki, 2013). Without local pyramidal‐interneuron feedback, the oscillation frequency is faster, demonstrating that the pyramidal‐interneuron excitatory loop is responsible for slowing down the oscillation. Thus, in this hybrid network, the population oscillation frequency is determined by both the pyramidal cell‐interneuron‐pyramidal cell loop and the interactions among interneurons (Brunel and Wang, 2003; Geisler et al., 2005).
Adding recurrent excitation among pyramidal neurons to the hybrid networks brings about further modifications, because the additional excitation tends to decrease the phase shift between excitatory and inhibitory populations (Fig. 32). When the balance between inhibition and excitation is equal in both pyramidal cells and interneurons, the time lag between spikes of pyramidal cells and interneurons can become zero and the frequency of the network oscillations is slow. Such zero‐time lag synchrony has been reported experimentally in the carbachol model of gamma oscillations in vitro (Fisahn et al., 1998). Overall, the Brunel and Wang (2003) model provides an intuitive explanation for the essential mechanisms underlying oscillations in networks supported by only fast excitatory and inhibitory synaptic interactions. In accordance with the model predictions, networks with extensive recurrent excitatory connections, such as the CA3 region, give rise to lower frequency gamma oscillations (slow gamma, 30‐80 Hz) than those with sparse connections (60–120 Hz; e.g., CA1 and entorhinal cortex; Bragin et al., 1995a; Belluscio et al., 2012; Schomburg et al., 2014). When the external excitatory drive is strong, the impact of local excitation diminishes and the oscillation frequency is set largely by the interactions among interneurons (i.e., ripple frequency generation; see Mechanisms of Ripple Generation section). Thus, the same exact network can give rise to oscillations at different frequencies, and the properties of the rhythm are determined essentially by the ratio of time scales of excitatory and inhibitory currents and the balance between recurrent excitation and inhibition. Contrary to intuition, local excitation and higher excitation/inhibition ratio tend to decrease the oscillation frequency because of the increasing dependence of cycle duration on the pyramidal‐interneuron loops.
A conceptually similar model of ripple generation was put forward by Taxidis et al. (2012). It differs from the Brunel‐Wang model in two aspects. First, the model of Taxidis et al. (2012) is a combination of population burst generator mechanisms residing in the CA3 recurrent excitatory system and a ripple generator in the CA1 inhibitory network. Second, in the new model pyramidal cells are modeled by two‐compartmental Pinsky‐Rinzel neurons (Pinsky and Rinzel, 1994). The firing rate of Pinsky‐Rinzel model neurons also scales linearly with increasing current but at large depolarization a burst is produced, followed by tonically firing spikes. The quasi‐synchronous population bursts in the CA3 region produce a strong depolarizing input to both pyramidal cells and interneurons in the CA1 region and the strong excitatory AMPA‐synaptic currents generated in the dendrites of both cell types produce an LFP SPW in the apical dendritic layer of CA1. The fast spiking model interneurons, aimed to mimic PV basket cells, are strongly excited by the CA3 output and fire intrinsically (i.e., without inhibitory synaptic connections) in a wide range of frequencies (∼100–400 Hz). The strong recurrent inhibitory connectivity among the interneurons, in combination with the fast timescales of interneuron IPSPs, reduces the firing frequency and confines it within the range of the ripple (150–200 Hz). Recurrent inhibition also causes interneurons to skip ripple cycles, resulting in a firing rate which is lower than the average membrane potential oscillation frequency of interneurons. CA1 pyramidal cells have a secondary role during the ripple episode. Only a small minority of CA1 pyramidal cells, which receive super‐strong CA3 inputs, can overcome the created strong feed‐forward inhibition in the model, and fire during ripples. These spikes contribute further to the excitation of the neighboring CA1 interneurons but influence their timing only to a small extent. The basic model has been extended to incorporate other interneurons (Cutsuridis and Taxidis, 2013) and examined the role of the entorhinal input and dentate gyrus in SPW‐R induction (Taxidis et al., 2013). A main caveat of the model is that it does not reproduce the consistent time delay between the firing of CA1 pyramidal cells and PV interneurons, a reproducable feature of the ripple cycle in vivo (see Discharge Patterns of Inhibitory Neurons During SPW‐Rs section).
While mathematical analysis of rhythm generation in networks of leaky integrate‐and‐fire and Pinsky‐Rinzel neurons captures many features of in vivo oscillations, there are shortcomings. First, in the integrate‐and‐fire model (Wang and Buzsáki, 1996; Brunel and Wang, 2003), both pyramidal cells and interneurons fire sparsely and their autocorrelograms do not reveal the network rhythm. In contrast, autocorrelograms of PV‐expressing basket and bistratified interneurons in vivo often show peaks in their spike autocorrelograms at ripple frequency (Ylinen et al., 1995; Klausberger et al., 2003). This may be important for sustaining fast oscillations in the ripple band, since when interneuron firing rates are strongly heterogeneous in the model, the emergent oscillation is less pronounced and stable (Wang and Buzsáki, 1996). Second, connectivity in the computer network is typically random. However, this assumption may not hold in the brain since most inhibitory interactions are local. Third, while leaky integrate‐and‐fire and Pinsky‐Rinzel model neurons have a fixed action potential threshold, in real neurons spike threshold and timing is variable (Azouz and Gray, 2000; Henze and Buzsáki, 2001; English et al., 2014) and an additional delay of 0.2–1.5 ms is added by the rise time of the action potential to reach its peak. In contrast, the spiking output of Hodgkin‐Huxley conductance‐based neurons and real hippocampal neurons strongly depends on the stimulus frequency of the afferents (Fourcaud‐Trocme et al., 2003; Vaidya and Johnston, 2013). Not surprisingly, when networks are built from conductance‐based neurons, the membrane time constant of single neurons strongly affects the frequency of network oscillations (Geisler et al., 2005; Schlingloff et al., 2014). To achieve a 200 Hz oscillation, the effective membrane time constant needs to be <5 ms and it is not known whether the membrane time constant can be reduced to this level even during the high conductance state of CA1 pyramidal cells during ripples. In support of this possibility, larger amplitude ripples, expected to be associated with higher membrane conductance, are coupled with faster ripples (Sullivan et al., 2011; Stark et al., 2014).
Another model also exploits active properties of pyramidal neurons for ripple generation (Memmesheimer, 2010). The key assumption in this model is that the sparse recurrent collaterals of CA1 neurons (Deuchars and Thomson, 1996) can lead to supralinear dendritic amplification and Na+ spikes in the basal dendrites of CA1 pyramidal cells (Ariav et al., 2003; Gasparini et al., 2004). The active dendritic amplification assures that the number of recruited postsynaptic neurons increases supralinearly. Periodicity in the model arises from two components. First, there is a delay time constant of τ ≈ 5 ms that results from adding the axonal and synaptic delays and the latencies of the dendritic spike and the somatic action potential initiation. Second, the first‐discharging neurons of the population event become refractory and the continued CA3 excitation recruits another group of CA1 pyramidal cells limited by the delay time constant. In essence, the CA3 input during SPWs initiates a short‐lived Markovian chain of propagating, enhanced synchrony and the sequentially recruited neurons manifest in an oscillation frequency of ∼200 Hz. If the strength of the driving excitation decreases or sufficient magnitude of inhibition builds up, the chain extinguishes. The saltatoric recruitment feature and the refractory periods of the model can effectively explain the sequential activation of CA1 neurons during SPW‐Rs without resorting to a precise inheritance of sequential patterns from the upstream CA3 neurons (Cutsuridis and Hasselmo, 2012). A key component of the Markovian model of ripple generation is recurrent excitation among principal cells. However, ripple frequency oscillations can also be generated optogenetically in the dentate gyrus (Stark et al., 2014) where granule cells do not communicate with each other, implying that recurrent excitation is not an obligatory ingredient of ripple oscillations in vivo.
Gap junction‐based model of ripple generation
Intuitively, gap junctions may boost local synchrony but it is not clear how they regulate the frequency of an oscillation. Yet, Traub et al. argue that electrical communication among pyramidal cell axons is, in fact, the rhythm‐generating mechanism of ripples. Although various iterations of this basic model have been presented (Draguhn et al., 1998; Traub et al., 1999a, 2011; Traub and Bibbig, 2000; Schmitz et al., 2001; Traub et al., 2010; Traub et al., 2010; Cunningham et al., 2012; Simon et al., 2014), the common feature of all models is that ectopic action potentials spreading across hypothetical gap junctions between axons of the principal neurons act as a high‐frequency network oscillator. This network of pyramidal axons acts as a “signal generator” (Traub et al., 1999b; Traub and Bibbig, 2000). The excitatory input from CA3 allows ectopic spikes to propagate and multiply in the axonal net. The antidromic and orthodromic spikes depolarize pyramidal cells and interneurons and induce a transient rhythmic network activity. In the formal model, synchronized population oscillations arise when the following conditions are met: action potentials propagate across axon gap junctions (typically in 0.5 ms), gap junctions are sparse (2.5–3.0 gap junctions per axon) and sufficient level of ectopic spikes arise spontaneously so that a sufficient number of pyramidal cells are recruited (Traub and Bibbig, 2000). The resulting population oscillations then occur at 150 Hz or faster, and the frequency is determined exclusively by topological structure of the network rather than by synaptic time constants and ripple frequency oscillation can be induced and maintained without any firing by the pyramidal cell somata. The cycle time of the rhythm is determined by the time for a spike in one axon to induce a spike in a connected axon. However, it is not clear what happens when spikes occur randomly at multiple gap junction‐connected axons.
The current generation of LFP ripples in the axon gap junction model is the same as in other models. Ectopic spikes can invade the somata and generate full spikes and the output of the axonal signal generator phasically excites interneurons. The resulting synchronous discharge of pyramidal cells (representing the trough of the ripple) alternate with the inhibition‐induced hyperpolarization of the pyramid cell membrane, which is responsible for the positive portions of the LFP ripples. An explicit prediction of the model is the presence of antidromic spikes during ripples. The axo‐axonic electrical junction hypothesis can explain fast oscillations, which persist in the presence of GABAA receptor blockers and it has gained support from several in vitro models of ripple‐like oscillations. However, in vivo experiments failed to observe antidromic spikes and provided strong support for the need of synaptic communication among PV neurons for the rhythm generation of ripples. Overall, while the different models capture various and multiple features of ripple dynamics and each have their own merits, no current model fully accounts for all aspects of in vivo SPW‐Rs.
SPW‐induced ephaptic effects affect spike timing
Neurons are embedded in an electrically conducting medium, the extracellular fluid, which, in principle, allows the extracellular activity of one cell to be perceived by its surrounding cells (Jefferys and Haas, 1982; Gold et al., 2006; Buzsáki et al., 2012). A long‐standing question has been whether LFPs are simply an epiphenomenon of neuronal signaling (but still useful to experimenters) or whether they have a direct functional significance. SPWs are large amplitude LFPs and, importantly, show a large voltage change across the CA1 pyramidal cell layer. The estimated voltage gradient during a medium size SPW is ∼15 mV/mm along the somatodendritic axis of pyramidal neurons in rats (Ylinen et al., 1995). Experiments applying external electric fields have shown that as small as 1 to 2 mV/mm voltage gradients are sufficient to affect spike timing of neurons both in vitro and in vivo (Chan and Nicholson, 1986; Ozen et al., 2010). It is thus expected that the electric fields generated by the synchronously active CA1 pyramidal neurons are large enough to provide a feedback ephaptic effect to bias spike threshold and timing of pyramidal neurons whose afferent excitation would otherwise remain subthreshold. Indeed, computational modeling demonstrates that relatively small (in amplitude) and inhomogeneous (in space), extracellular electric fields can exert relatively large effects on the excitability of morphologically reconstructed passive pyramidal neurons (Anastassiou et al., 2010). Time‐dependent simulations of a spatially inhomogeneous external potential demonstrate that ephaptic events within the physiologically measured range persist for relatively high temporal frequencies (<200 Hz). Thus, in addition to synaptic and gap junction‐mediated effects, ephaptic action of voltage should also be considered in the mechanisms of neuronal synchrony during SPW‐Rs in vivo.
PHYSIOLOGICAL MECHANISMS OF SPW‐RS—SUMMARY
SPW‐Rs are the most prominent self‐organized events in the hippocampal system. These super‐synchronous bursts arise when release of subcortical neuromodulators in the hippocampus is decreased, as is the case during consummatory behaviors and slow wave sleep. SPW‐R is a complex of two independent events, the sharp wave‐related population burst, which emerges in the strongly recurrent system of the CA3 region and the fast ripple oscillation, which is dominant in the CA1 circuit. The synchronous discharge of CA3 pyramidal cells excite primarily the mid‐apical dendrites of the CA1 region and the inward currents brought about by the transient depolarization process (40–150 ms) are manifested extracellularly as the LFP SPW, which can exceed 2.5 mV. During SPW events, 0 to 40% of pyramidal neurons discharge in different hippocampal regions. The probability of pyramidal neuron participation and the fraction of neurons firing in each SPW event show a strongly skewed, lognormal‐like distribution. During a typical SPW event, 50,000 to 150,000 neurons discharge in the CA3‐CA1‐subicular complex and entorhinal cortex. SPW bursts rarely invade the entire septo‐temporal length of the hippocampus. Instead, most events are local and spread to either the rostral or caudal direction. SPWs represent a transient but very large gain of excitation, from a more than two‐fold gain in CA1 to decreasing levels in CA3, dentate gyrus, subiculum and deep layers of the entorhinal cortex. Neurons in the superficial entorhinal layers can also be recruited occasionally but the ratio of excitation and inhibition during hippocampal SPW‐Rs is already balanced in these circuits. The large excitatory gain can explain why SPW‐Rs can drive neurons in many primary and even secondary targets of the hippocampal complex. While SPW bursts can emerge in the isolated hippocampus, in the intact brain multiple input pathways and events can bias their occurrence, including neocortical slow oscillations, thalamocortical spindles and influence from the septum and possibly other subcortical areas.
The CA1 circuit's compensatory solution to a strong SPW‐associated excitatory input from CA3 is a fast oscillatory balancing act between principal cells and inhibitory interneurons, resulting in the LFP ripple. Ripples are local circuit phenomena, and their genesis depends on the interactions between pyramidal cells and perisomatic interneurons. They can occur in virtually any circuit with these two cell types as long as pyramidal neurons are strongly excited, for example by local optogenetic stimulation. The LFP ripple has two major components. The negative peaks represent largely the superimposed action potentials of the synchronously discharging neurons, whereas the positive portions mainly reflect outward membrane currents (i.e., sources) from the somata of pyramidal neurons, due to the hyperpolarizing action of actively discharging PV basket and other interneurons. Current knowledge indicates that the main source of the periodicity of ripples (i.e., its rhythm generator) is the time constant of the GABAA‐receptors between connected PV‐expressing basket interneurons, driven mainly by CA1 but also CA3 pyramidal cells.
The temporal choreography of recruitment of the different types of neurons into SPW‐Rs is not well understood. Nearly all interneuron types, innervated by CA1 pyramidal cells can fire phase‐locked to the ripple, albeit with very different probabilities during the SPW‐R event and some types are actively suppressed. Basket cells appear to be the most important interneuron type, since their interactions set the frequency of the ripples, they fire robustly and are strongly phase‐locked to the ripple cycle, following the discharge of pyramidal neurons by ∼90°. Bistratified interneurons are also strongly active during SPW‐Rs and are phase‐locked to the ripple cycles. Both PV‐basket cells and bistratified neurons are excited by both CA3 and CA1 neurons, which may explain their high firing rates during SPW‐Rs. Dendritic inhibition of pyramidal cells by the bistratified interneuron group during SPW‐Rs can reduce the bursting ability of CA1 pyramidal cells and the emergence of dendritic Ca2+ spikes. Axo‐axonic cells show a large variability in their SPW‐R‐related activity. Many of them may be suppressed whereas a subgroup may fire in the early part of the SPW‐R event and be silenced later. Yet another small subgroup can increase its activity. Their variable firing patterns may be explained by their differential inhibition from basket cells, bistratified interneurons and/or the medial septal GABAergic projection. O‐LM interneurons and CCK basket cells also show large variability in their responses but the majority appears to be suppressed during SPW‐Rs, likely inhibited by the active PV basket and bistratified interneurons. Several interneuron types can be also affected by the entorhinal and septal afferents and it is not clear currently whether their role is only secondary or they play a more leading role in governing the population events. Overall, the complex chronoanatomical organization of SPW‐Rs implies that the delicate control of the different somatodendritic domains of pyramidal cells is of high priority for the selection of cell assemblies and affecting plastic processes for the maintenance of their alliance. Unfortunately, to date, most of our knowledge about timing and participation probability of interneurons and pyramidal cells comes from averages of large numbers of successive events. Proper understanding of the assembly organization will require monitoring large numbers of simultaneously recorded and identified neurons and documenting their sequential firing patterns within single SPW‐R events.
While numerous experiments illustrate the role and impact of hippocampal SPW‐Rs in affecting their target structures, particularly the prefrontal cortex, the exact importance of the fast ripple oscillation remains to be explained. It is not clear presently whether the ripple simply reflects the transient tug‐of‐war between excitation and inhibition or whether the fast oscillation plays a critical role in e.g., tetanizing hippocampal targets or whether the spike sequences phase‐locked to the ripple waves are utilized in any special way by the downstream “reader” mechanisms. These caveats notwithstanding, SPW‐Rs play important roles in shaping experience‐related information (see SPW‐R‐Supported Memory Consolidation and Constructive Role of SPW‐R sections).
COMPUTING WITH SPW‐RS
The CA3‐derived excitatory terminals make up 80% of all intrahippocampal synapses (Amaral and Witter, 1989) and approximately half of these terminals remain within the confine of the CA3 region (Lorente de Nó, 1934; Lebovitz et al., 1971; Ishizuka et al. 1990; Li et al., 1994; Wittner et al., 2007). With the exception of the lateral septum and other subcortical projections, all targets of CA3 pyramidal neurons are intrahippocampal (Amaral and Lavenex, 2007). In contrast to the modularly organized neocortex, the CA3‐CA1 neurons form a single large three‐dimensional graph (Muller et al., 1996) in which each CA3 pyramidal cell can address its peers with relatively similar probability, and the pathlength between any two pyramidal cells is only one or two synapes (Wittner et al., 2007). This strongly connected recurrent graph formed by the CA3 neurons can be conceptualized as a “hidden” layer, sandwiched between the lamellarly organized granule cell inputs (Andersen et al., 1971) and the very sparely recurrent output CA1 region (Fig. 33). Thus, all computation performed by the CA3 regions needs to be “translated” and “re‐interpreted” by the CA1 region before transferring the results to the paleo‐neocortex. Computational structures with these formal features (segregated inputs and outputs with a hidden recurrent network) are used to perform sequential pattern segregation and pattern integration, the two critical ingredients of memory storage and recall (Treves and Rolls, 1994; Lörincz and Buzsáki, 2000).
Figure 33.
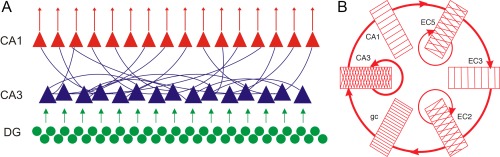
The CA fields of the hippocampus form a random graph. (A) The dentate gyrus (green)—as input region of the hippocampus—gives local parallel connections to the CA3 region. The CA3 region (blue) forms a strongly connected recurrent axonal graph, in addition to forwarding the information to the CA1 region. The output CA1 region (red) also shows mainly local parallel connections. (B) Multisynaptic, reverberatory path in the entorhinal‐hippocampal system. Segregation and intergration can be iteratively performed by the largely unidirectionally connected layers with alternating parallel and recurrent organization.
Before discussing how SPWs can contribute to unique computation, it is useful to summarize how computation is implemented in artificial recurrent neural networks. Learning in these networks is typically achieved by backpropagation, which is an error correction procedure (Rumelhart et al., 1986). Similar to the largely unidirectionally coupled entorhinal‐hippocampal regions, backpropagation neural networks have multiple “layers.” Various patterns are presented simultaneously to the input layer, which filters or segregates items in the incoming stream by some rule and presents the segregated information to the middle hidden layer(s) where training takes place by changing the connection weights. Once a desirable output (a “goal”) appears at the output layer, the constellation of synaptic weights leading to the goal is strengthened, leading to a more efficient generation of the desired output when a similar input pattern is presented at another occasion (called “error” reduction or correction). After numerous repetitions (“supervised teaching”), a “global minimum” or an “attractor state” (Hopfield, 1982) is reached, which is the theoretical solution with the lowest possible error (Rojas, 1996). The goal of the algorithm is to find a function that best links a set of inputs to its correct output, for example to classify input features (e.g., fragments of a face) to provide the most likely answer (e.g., the complete face). The attractor can be conceived of as a memory, which is distributed in the overall activation “state” of the network rather than in individual components and it is only an approximation of the desired item rather than its identical replica. Because neural networks are trained to be “good enough” to segregate items, they are not precise. On the other hand, they can effectively recognize regularities and similarities and have a high tolerance to error.
The hidden layer(s) are often trained to identify specific features, and multiple features can be differentiated because recurrent networks can have multiple attractor states (Hopfield, 1982). The output layer can then chunk or combine the hidden layer activations, and this integration process is often referred to as “pattern completion” in neuroscience (Marr, 1971; McNaughton and Morris, 1987; Mizumori et al., 1989). By analogy to artificial neural networks, separate types of activity in CA3 and CA1 regions can simplify the overall computation. The CA3 region has been suggested to operate as an attractor or autoassociative network for memory functioning (Treves and Rolls, 1992; De Almeida et al., 2007). In turn, the output CA1 layer can assign a “meaning” (such as position coordinates in the environment or elements of an episode) to the more fragmented representations in the (hidden) CA3 region.
However, these conceptualizations of computational operations in the hippocampus and analogies to multilayer recurrent neural networks have several limitations. Artificial neural networks are considered “supervised” because training the network by backward propagation of errors requires a known, desired output for each input value. Errors in the largely feedforward networks of the hippocampus cannot backpropagate although, in principle, long‐range inhibitory feedback from CA1 acting on the upstream CA3 and dentate regions may be exploited for such function (Sik et al., 1994; Jinno et al., 2007; Melzer et al., 2012). In the recurrent Hopfield network, all connections are symmetric which is not the case in the hippocampus. But perhaps the most fundamental difference is that neural networks require moderate to extensive training to tune the synaptic matrix of the hidden layer(s) in stark contrast to the hippocampus (Treves and Rolls, 1992, 1994; Hasselmo, 1995; Hasselmo and Wyble, 1997), which can generate an episodic memory after just a single exposure (Tulving, 1985, 2002). The solution might be provided by a two‐stage process (Marr, 1971; Buzsáki, 1989), which involves a self‐organized repetition of an episode or its fragments. SPW‐Rs, which become activated in the offline states of the brain, might appear ideal for this task. The episodic event in the waking state may occur only once but the numerous repetitions needed for adjusting the synaptic weights in the hidden layer are performed by the repeated and compressed replay of the waking content during SPW‐Rs (see below; Buzsáki, 1989; McClelland et al., 1995; Lörincz and Buzsáki, 2000). Terminal (i.e., consummatory) behaviors leading to either success or failure, such as immobility eating and drinking, are typically associated with SPW‐Rs (see Behavioral Correlates and Mechanisms of SPW Generation section). Hundreds to thousands of SPW‐Rs may occur during sleep after a learning experience. SPW‐R‐induced replay may allow access to episodes stored in the hippocampus, and SPW‐Rs may integrate them with old neocortical representations (Kali and Dayan, 2004). For an alternative view for the role of SPW‐R in the learning process (“matching” experience with pre‐existing patterns), see Retrospective, Prospective, Contructive, and Maintenance Roles of SPW‐Rs—A New Synthesis section.
SPW‐R AND SYNAPTIC PLASTICITY
A lot of enthusiasm was generated about the discovery of long‐term potentiation (LTP; Bliss and Lomo, 1973) and kindling (Goddard and Douglas, 1975) as models of synaptic and cellular plasticity, largely because they appeared to satisfy most psychological constructs of information storage (Morris et al., 1986; Teyler and DiScenna, 1987). Early experiments demonstrated in the anesthetized rat that stimulation protocols that involve stimulation of presynaptic axons at 100 Hz to 400 Hz brought about highly synchronous discharge of postsynaptic targets and induced the strongest LTP effect (Goddard and Douglas, 1975; McNaughton et al., 1978). Furthermore, the LTP‐inducing stimulus can be viewed as a “supervisor” or “teacher” to enhance the paired weak (“pupil”) synapses to satisfy the associative requirement of learning (McNaughton et al., 1978; Levy and Steward, 1979; McNaughton and Morris, 1987). However, the conditions under which LTP occurs in the brain remains to be established.
SPW‐Rs appear to satisfy several requirements of LTP induction, since they represent the most synchronous pattern with the largest excitatory gain of any known event in the mammalian brain (Buzsáki et al., 1983, 1992; Buzsáki, 1985, 1989; Csicsvari et al., 1999a, 1999b), can co‐activate large numbers of neurons in multiple brain regions, and during SPWs neurons are organized into a fast oscillatory event mimicking a tetanic train. Furthermore, the probability of co‐occurrence of complex spike bursts (with <6 ms interspike intervals) in CA3 pyramidal cells pairs increases eight‐fold during SPW‐Rs (Mizuseki and Buzsáki, 2013), and such synchronous bursts may be especially efficient at inducing plastic changes in their targets (Lisman, 1997).
The idea that CA3 population bursts can induce LTP was first examined in the hippocampal slice preparation in vitro (Fig. 34) (Buzsáki et al., 1987d) under the hypothesis that the “mini” population spike series during the SPW can serve as an effective tetanic stimulus train. Single pulse stimuli were delivered to the Schaffer collaterals throughout the experiment, which evoked antidromic spikes in CA3 pyramidal cells and orthodromically activated CA1 neurons. After baseline recording, bicuculline was locally applied to the CA3a,b subregion for 5 min. As a result of the partial disinhibition of the CA3 circuit, the antidromic stimulation induced bursts of diminishing amplitude population spikes instead of a single antidromic spike present during the control and washout periods. The pairing of single pulses and CA3 population bursts, mimicking SPW bursts in vivo, induced an enhancement of both the simulation‐evoked CA1 postsynaptic potential and population spike. This was a form of LTP because it persisted after drug removal and lasted for ∼60 min (Buzsáki et al., 1987d). In another experiment, LTP was induced by high‐strength single volleys in hippocampal slices, in which GABAA receptors were blocked by picrotoxin. The authors (Abraham et al., 1986) noted the presence of repetitive population spikes and found that these were prerequisite for the induction of LTP. In a complementary experiment, LTP was induced in the CA1 commissural/Schaffer afferents by tetanic (200 Hz) bursts of electrical pulses in the behaving rat. Both the stimulus‐evoked responses and the amplitude of the spontaneously occurring SPWs were enhanced (Buzsáki, 1984). By analogy, one can hypothesize that the repetitive mini‐population spikes of SPW‐Rs can be analogous to tetanic stimulation and bring about long‐term synaptic, and possibly other intracellular, changes.
Figure 34.
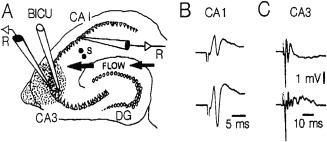
SPW‐mimicking bursts induce long‐term plasticity. A: diagram of a hippocampal slice showing the location of the stimulating electrode (S), the recording electrodes (R), and the bicuculline‐containing pipette (BICU). The hatched area shows the typical extent of bicuculline/methylene blue diffusion. B: averages of 8 evoked responses in the pyramidal layer of CA 1 before (above) and 20 min after (below) transient (5 min) bicuculline application to the CA 3 region. Note enhancement of the evoked response after the washout of the drug. C: antidromic responses in CA 3 before (above) and during (below) bicuculline application near the recording electrode in CA3 pyramidal layer. Reproduced from Buzsáki et al. (1987d).
In a complimentary set of experiments, spontaneously occurring SPW‐Rs were used to assess the input–output relationship between the CA3 population input and the response of single CA1 pyramidal neurons (King et al., 1999). SPW‐Rs were detected and single neurons were depolarized by intracellular current injection in a closed‐loop configuration in anesthetized rats or by extracellular current pulses in behaving animals. The pairing of SPW‐Rs and the induced excess spiking of arbitrarily selected CA1 pyramidal cells resulted in a long‐lasting (>1 h) enhancement of the SPW‐R‐participation of the neurons, compared with unpaired cells. These experiments demonstrate that pairing the CA3‐induced SPW input with concurrent and consistent discharge of CA1 pyramidal cells increases their participation probability in future SPW‐R events (King et al., 1999). An implication of these findings is that SPW‐Rs can function as a teaching pattern, which can potentiate appropriately timed weaker inputs, e.g., from the entorhinal cortex.
Using a different approach, Ishikawa et al. (2014) paired large amplitude intracellular EPSCs in CA1 pyramidal neurons in head‐fixed immobile mice with rewarding lateral hypothalamic stimulation. Within 15 min, most mice learned to increase the frequency of the large amplitude EPSCs in neurons with relatively high firing baseline rates. Similarly, when bursts of spikes were paired with the rewarding stimulation, the incidence of bursting increased. In contrast, when bursts were explicitly unpaired with hypothalamic stimulation, their incidence decreased. The conditioning effects depended on activation of both NMDA receptors and dopaminergic D1 receptors. Since in the immobile animals most large EPSCs and bursts of spikes occur during SPW‐Rs, Ishikawa et al. (2014) also paired SPW‐Rs with rewarding stimulation and demonstrated a 50 to 100% increase in SPW‐R incidence in 20 min (Fig. 35). The authors suggest that reinforcement acted through network reorganization of the CA3 network via an NMDA receptor‐mediated plasticity. In addition, NMDA receptors might be needed for increasing the efficacy of the lateral hypothalamus to drive dopaminergic ventral tegmental (VTA) neurons. The association between VTA activity and SPW‐R‐related reactivation of the memory trace was further explored by McNamara et al. (2014).
Figure 35.
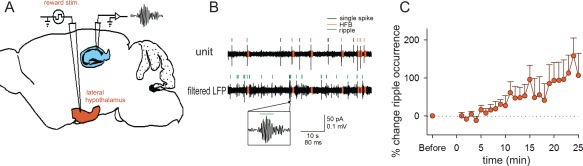
Closed‐loop enhancement of the incidence of SPW‐Rs. (A) Experimental arrangement. LFP recording from CA1 pyramidal cell layer and SPW‐R‐contingent stimulation of the lateral hypothalamus. (B) Representative traces of cell‐attached unit recording and LFP recording. A ripple event is magnified in the inset. (C) Time course of the percent change in the probability of occurrence of SPW‐Rs during 25 min of conditioning. Reproduced from Ishikawa et al. (2014).
In a conceptually similar manner to the design of Ishikawa et al. (2014), SPW‐R was used as a contingent signal to deliver an auditory conditional stimulus (CS) and airpuff to the cornea (an aversive signal; US) in rabbits (Nokia et al., 2010). A yoked rabbit also received CS‐US presentations at the same time, thus regardless of the occurrence of SPW‐Rs in its own hippocampus. SPW‐R‐contingent training resulted in accelerated learning and slower extinction of the CS‐induced nictitating membrane response compared with the control rabbits.
While the above studies suggest that SPW‐Rs are effective for changing synaptic strengths among neurons, findings from other laboratories argue that brain states associated with SPW‐Rs are not ideal to support plasticity or, in fact, may bring about depression of activity. Comparison of the magnitude of tetanic train‐induced LTP in the dentate gyrus showed that trains applied during slow wave sleep were less effective and more variable than the same trains in the waking rat or during REM sleep (Leonard et al., 1987). Tetanic stimulation of the entorhinal input to granule cells during slow wave sleep rarely induced LTP. Yet, if a tetanic train failed at one time, the same train later during sleep could induce LTP. Sometimes, potentiation of the extracellular synaptic potential slope was not affected but the population spike was nevertheless enhanced (Bramham and Srebro, 1987, 1989). Other studies argue in favor of plasticity and LTP during non‐REM sleep (Blanco et al., 2015). It remains to be demonstrated though whether variability in the inducibility of LTP depends on the cyclic changes of UP and DOWN states or the presence or absence of SPW‐Rs at the time of the applied tetanus. Commissural responses in the CA1 region show several‐fold enhancement during SPW‐Rs, while the responses between SPW‐Rs are smaller than during theta‐associated behaviors (Buzsáki, 1986). Thus, it is possible that the momentary brain state during which the stimulation is applied determines whether plastic changes are induced or not. Another possibility is that during SPW‐Rs a different type of plasticity is at work. Indeed, SPW‐Rs per se are not dependent of NMDA receptors, and NMDA receptor‐independent plasticity has been described in multiple brain regions (Johnston et al., 1992; Weisskopf et al., 1999) and different forms of plasticity may be present in the CA3 and CA1 hippocampal regions (Kanterewicz et al., 2000; Mitra et al., 2011).
Irrespective whether SPW‐Rs themselves can modify synaptic weights, manipulations that affect synaptic strength distributions in hippocampal networks can have a profound impact on the neuronal membership of spontaneous network events, such as SPW‐Rs. Figure 36 illustrates such a scenario. Following subcortical denervation of the hippocampus (fimbria‐fornix lesion), SPW‐Rs were converted into large amplitude interictal spikes (Buzsáki et al., 1989b). Single pulse stimulation of the entorhinal inputs in such preparations evoked a unique spatio‐temporal event of the trisynaptic responses with multiple population spikes at the different CA1 recording locations. Following tetanic stimulation of the entorhinal input, the spatial pattern of the evoked responses was modified, as expected. In addition, the LTP‐train also induced large amplitude “exaggerated” SPW‐Rs or more appropriately called interictal LFP spikes. Remarkably, the spatio‐temporal distribution of the induced spontaneous events was virtually identical to that of the evoked responses with the exception that no dentate responses (black triangle in Fig. 36) preceded the spontaneous CA1 events. Stimulation of a different set of afferent axons in the perforant path could induce another unique spatial‐temporal constellation of the spontaneous events. The implication of these findings is that the neuronal composition of the SPW‐Rs is biased by the recent past of the hippocampal network. Once the new event emerges it repeats spontaneously numerous times after the tetanic stimulation. These experiments (Buzsáki, 1989) provided firm evidence that (a) the pattern of the entorhinal inputs can modify intrahippocampal synaptic connections, (b) the modified connections are reflected in the spontaneous, self‐organized population events, and (c) the hippocampal output can address its downstream partners in a topographically specific way. One interpretation of these experiments is that the LTP‐inducing trains modify the excitability of a subgroup of CA3 pyramidal neurons, which can be selectively reactivated in subsequent spontaneous events and repeatedly send out unique hippocampal messages to entorhinal‐neocortical targets. Extrapolating from these obviously artificial conditions to the intact hippocampus, the hypothesis was put forward that synaptic‐cellular modification of neurons during learning can be read out at a later stage from the spontaneously emerging neuronal activity and, specifically, that SPW‐Rs in the intact brain can support memory‐related functions. The LTP‐induction and expression of spontaneous population events can be conceptualized as learning‐induced modification and memory‐related replay of the modified hippocampal circuit, respectively (Buzsáki, 1989, 1994, 1996).
Figure 36.
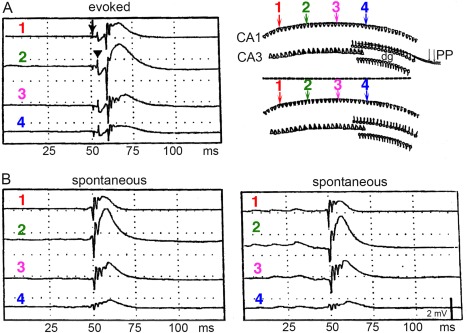
Input‐specific events of spontaneous population events in the hippocampus. Recording electrodes (1–4) were placed in the CA1 pyramidal layer along the septotemporal axis of rats in which the fimbria‐fornix was previously removed. Interelectrode distance: 0.5 mm. (A) Single pulse stimulation of the perforant path (PP; arrow) evoked monosynaptic population spike in the dentate gyrus (DG; visible as a volume‐conducted negative spike in CA1; triangle) and multiple population spikes in CA1. (B) Following tetanic stimulation of the perforant path input, spontaneous LFP events (“exaggerated SPWs”) emerged. Two spontaneous events are shown. Note striking similarity between the spatially distinct evoked and spontaneous events and the absence of the dentate component in the spontaneous events. Top right in A, Hypothesis: tetanic stimulation induces synaptic strengthening among a subset of activated CA3 pyramidal neurons (triangles, top). Bottom, during spontaneous events, the same neurons that were activated during stimulation become the initiators and participants of the spontaneous events. Reproduced after Buzsáki (1989).
SPW‐R‐SUPPORTED MEMORY CONSOLIDATION
It is generally thought that events and behavioral acts are remembered because they are reinforced one way or another (Skinner, 1938; Thorndike, 1905; Sutton and Barto, 1998). Most reinforcers (reward or punishment) must be “taken in” or experienced in some way in order to be effective. A long‐lasting dilemma that has persisted in psychology is the identification of brain mechanisms by which reinforcement can act retroactively and cement associations and behaviors since the traces of preparatory actions are vanished by the time the reinforcement exerts its effect during consummatory brain states. One potential solution is that some neuronal trace representing previous perceptions and responses persists until the reinforcement can make its impact and stabilize the neuronal events leading to reinforcement. Whether reward and punishment as positive and negative reinforcements work the same way or different ways has been strongly debated (Craik, 1943). The consummatory response model of reinforcement asserts that it is the act of eating, drinking, immobility, freezing and sleep that gives rise to the reinforcing effect (Sheffield et al., 1954; Glickman and Schiff, 1967). As discussed earlier in this review, the preparatory—consummatory behavioral distinction reliably maps onto the dichotomy of theta—SPW‐R brain states (see Behavioral Correlates and Mechanisms of SPW Generation section). SPW‐Rs thus may be a mechanism that allows cementing learned associations. Fueled largely by the observations that the entorhinal input can modify the intra‐SPW‐R membership of hippocampal neurons and that SPW‐Rs can exert the needed impact on downstream targets, a neurophysiology‐based, two‐stage model of memory formation was put forward (Buzsáki, 1989, 1994, 1996, 1998; Chrobak and Buzsáki, 1994). The hypothesized two‐stage routine is this:
Labile form of memory trace. During learning, associated with theta brain state, afferent activity from the neocortex/entorhinal cortex brings about a transient change of synaptic strengths in the CA3 hippocampal regions, where the learned information is temporarily held.
Long‐lasting form of memory trace. During consummatory behaviors, including slow wave sleep, spontaneous SPW bursts are initiated in the CA3 recurrent network and the recurring SPW‐Rs transfer the newly acquired hippocampal information to the neocortex and the repeating SPW‐Rs continue to potentiate those same synapses which gave rise to the synaptic changes during the learning process.
According to the model, a single experience (“incidental” learning) can be remembered because “the SPW event would in essence compress time and allow temporally distinct neuronal representations to be combined into a coherent whole” and SPW‐Rs repeatedly “reactivate the same subset of neurons in CA3 and CA1 precisely determined by the recent past of the neural network” (Buzsáki et al., 1994; p. 168). The model made several predictions:
Participation of neurons in SPW bursts is not random
The constellation of neuronal events during SPW bursts is a consequence of learning in the waking brain
The learned sequences of neuronal discharges are replayed during SPWs in a time‐compressed manner and integrated with preexisting knowledge
SPW‐Rs have a brain‐wide impact
Cortical circuits can be modified by the SPW‐Rs
Consolidation of the memory trace can occur during both waking consummatory behavior and non‐REM sleep, brain states rich with SPW‐Rs
SPW‐Rs are relevant to behavior and their modification should affect memory performance
The notion that sleep can contribute to memory preservation and consolidation is old (see SPW‐R‐Supported Memory Consolidation section). The contribution of the two‐stage model to the memory‐sleep debate is two‐fold. First, it shifted the focus and emphasis from REM sleep and dreaming in memory consolidation to the more extensive non‐REM stage of sleep. Second, it offered a well‐circumscribed and understood specific neurophysiological event, the SPW‐R, as the potential mechanism by which memories can be transferred from the hippocampus to permanent storage in the neocortex. Over the years, SPW‐R has become a de facto “biomarker” event for memory transfer and consolidation. Several predictions of the two‐stage model have been tested additional modifications were added to the basic model and new findings expanded it in several important ways. The supportive evidence falls into three categories. First, spike sequences within SPW‐Rs are related to the sequences present during waking experience. Second, consolidation of episodic memories is correlated with several parameters of SPW‐Rs, and perturbation of sleep events rich in SPW‐Rs impairs memories in both humans and other animals. Third, specific and selective perturbation of SPW‐Rs affects hippocampus‐dependent memories. In addition, numerous recent experiments have also demonstrated that in addition to a retroactive, memory‐assisting role, SPW‐Rs also play a prospective role that may assist in route planning, recall and decision‐making, perhaps due to the preexistence of a rich variety of SPW‐R events before experience.
Consolidation of Memory
The concept of memory consolidation (“Konsolidierung”) was introduced by Georg Elias Müller and his student Alfons Pilzecker in their classic monograph (Müller and Pilzecker, 1900). They noticed that volunteers in their experiments often reported a strong tendency for syllable pairs to come to mind repeatedly between training sessions. Müller and Pilzecker called this process “perseveration” and suggested that memories are not imprinted in the mind instantly but repetition or perseveration of the learned material may be needed for strengthening the associations between items (McGaugh, 2000). The modern day extension of these ideas is based on experiments and case reports, starting with severe anterograde and temporally limited retrograde amnesia following bilateral removal of medial temporal lobe structures (Scoville and Milner, 1957). This and related findings ultimately led to the identification of the hippocampus and perihippocampal cortical structures of the medial temporal lobe as components of a memory system that are essential for the formation of long‐term memory (Squire and Zola‐Morgan, 1991). Damage to these structures causes retrograde amnesia, which can extend across several years in humans (Russel and Nathan, 1946; Squire et al., 1975) and across weeks in rodents (for review, see Milner et al., 1998). Overall, these findings led to the proposal that medial temporal lobe structures contribute to the prolonged and gradual consolidation of memory over extended time. More specifically, the hippocampus induces a gradual reorganization and stabilization of representations in the neocortex, which is distinct from the process by which synaptic efficacy is altered during the early phases of learning (for reviews, see Squire and Alvarez, 1995; Redish and Touretzky, 1998; Knowlton and Fanselow, 1998; Frankland and Bontempi, 2005).
Although memory consolidation is a century‐old concept, it still lacks a clear definition. The early term “Konsolidierung” referred to all types of memories without specification and was meant to be a relatively short process (tested for only a few hours). Thus, it is far from clear whether the mechanisms involved in this early, interference‐prone process are the same or related to the consolidation process that involves months and years. Is the consolidation process the same or different during sleep and waking? Since sleep involves different stages, do the various stages contribute equally or differentially? Finally, do the different types of memories utilize similar or different consolidation mechanisms and sleep stages? Although these are important questions, I do not attempt to comprehensively discuss the vast literature on the link between memory and sleep here. Instead, I restrict the discussion to topics relevant to hippocampal SPW‐Rs.
Memories that can be consciously perceived and verbally declared are distinguished from non‐declarative or procedural forms (such as habits and skills) of memories. Consciously declarable memories are also often called hippocampus‐dependent memories, since they depend on the integrity of the hippocampal system (Squire, 1992; Eichenbaum, 2000). In humans, episodic memories represent unique personal experiences (“autobiographic” memories) embedded in space and time (Tulving, 2002). Because a strict definition of episodic memory involves autonoetic consciousness during recollection (Tulving, 2002), the term episodic‐like memory is more appropriate in other animals. In contrast, semantic memories (objects, places, concepts, names, facts) compose a knowledgebase about the world, free from spatial and temporal contextual information (i.e., they are explicit). Firing fields of place cells in the hippocampus (O'Keefe and Nadel, 1978) or neurons coding for specific words, objects or scenes in humans (Heit et al., 1988; Quiroga et al., 2005) exemplify such explicit information. It remains to be clarified whether mechanisms underlying consolidation serve both subtypes of declarative memory, i.e., episodic and semantic. Alternatively, semantic memories may arise from the repeated encoding or activation of overlapping episodic memories and in the process they become stripped of their specific spatial, temporal and emotional context (Buzsáki, 2005; Buzsáki and Moser, 2013). This transformation process can be viewed as consolidation or a gradual redistribution of the hippocampal content of episodes to neocortical structures (McClelland et al., 1995).
Computational considerations also point out the advantages of coupling a fast temporary storage network with limited capacity with a slower long‐term storage space with large capacity (Marr, 1971; Hasselmo and Bower, 1993; McClelland et al., 1995; Redish and Touretzky, 1998; Kali and Dayan, 2004; Samsonovich and Ascoli, 2005). In these models, the flow of incoming information is first fed into a temporary store and some selected information is gradually integrated with preexisting knowledge and becomes resistant to interference from other sources. Once a representation has been redistributed to the long‐term neocortical store, its trace is cleared from the hippocampus, making room for new information (McClelland et al., 1995, Frankland and Bontempi, 2005). An alternative view is that episodic, autobiographic memories never become completely independent from hippocampal function (Nadel and Moscovitch, 1997; Winocur et al., 2010) and the hippocampus continues to function as an index for neocortical circuits that encode semantic features of the episode (Teyler and Discenna, 1987). The two‐stage model is compatible with both single‐trace (i.e., consolidated neocortical trace) or dual‐trace (i.e., SPW‐Rs induce plastic changes in the hippocampus and transfer information to neocortex) models of memory consolidation.
Sleep and Memory
The consolidation process continues after the initial experience and may include both waking and sleep periods. The relationship between sleep and memory has a long history. Early links are based on the conjecture that dreams serve to repeat fragments of waking experience (Ladd, 1892). De Manacéine (1897) noted that dreams “have a direct salutary influence insofar as they serve to exercise regions of the brain which in the waking state remain unemployed” (cited in Kavanau, 2000).
Perhaps the first dedicated experiment to address the role of sleep was performed by Jenkins and Dallenbach (1924), although these investigators were preoccupied mainly with the problem of forgetting. Their subjects were given a list of nonsense syllables to recall and they were tested after intervals of 1, 2, 4, or 8 h for recall performance. Subjects who slept demonstrated a decline in recall only for the first 2 h after which performance remained stable. In contrast, subjects who remained awake demonstrated a monotonous decrease in recall over the 8‐h interval. The overall conclusion of this landmark study was that subjects who sleep following learning remember more than subjects who are awake for the same length of time (but see criticism in Grosvenor and Lack, 1984).
Sleep can be useful for memory consolidation process in two different ways. First, because the memory trace is labile in its early period, novel information may interfere with it. Therefore, sleep as an unperturbed brain state can offer a protective role. From this perspective, the role of sleep is passive and its benefit comes from preventing new information to perturb the consolidation process. Second, sleep can play an active role so that its presence is more beneficial than the same amount of waking time without additional learning (for an extensive coverage of this subject, see reviews by Stickgold, 2005; Diekelmann and Born, 2010).
The discovery by Dement and Kleitman (1957) that sleep can be divided into stages with distinctive physiological characteristics provided a major impetus to a re‐investigation of the relationship between sleep and memory. Early on, Roffwarg et al. (1962) suggested that REM sleep is critical in the developing brain to maintain brain functions, while Moruzzi (1966) also speculated that sleep can repeat learned acts, although these sleep researchers did not specifically test memory performance. REM sleep is characterized by rapid conjugate eye movements, loss of muscle tone, “desynchronized” neocortical EEG and continuous hippocampal theta oscillations (Jouvet, 1999, 2004). During non‐REM sleep, muscle tone is reduced, neocortical EEG is dominated by sleep spindles and slow oscillations and hippocampal activity is rich in SPW‐Rs. In humans, the first 4 h of sleep is largely a deepening process of non‐REM sleep, whereas during the second 4 h shallow non‐REM and REM episodes alternate. These differences in sleep stages have been utilized in attempts to discriminate between the importance of non‐REM and REM‐rich parts of sleep for theories of forgetting and, more recently, memory consolidation.
In the face of numerous conjectures that it is the REM sleep stage and dreaming that is important for rehearsing and consolidating experience, Fowler et al. (1973), Yaroush et al. (1971), and Barrett and Ekstrand (1972) reported that learning followed by the first half of the night's sleep led to much better memory of neutral material than did learning followed by the second half of the night's sleep. These experimenters therefore concluded that non‐REM sleep following learning was more beneficial to memory than REM‐rich sleep. A major caveat in these studies, however, is that retention over the second half‐night's sleep may have been detrimentally affected by non‐REM sleep immediately before learning. Indeed, Stones (1973), Idzikowski (1978), and Ekstrand et al. (1977) have demonstrated that sleep with sleep lengths from 0.5 h to as long as 6 h of prior sleep has a detrimental effect on long‐term recall, although it has no effect on rate of learning or immediate recall of items. Furthermore, a valid criticism of several previous studies is that they could not disambiguate the loss of function due to sleep deprivation from fatigue and circadian effects nor did they separate an enduring effect on memory consolidation from impaired retrieval (Plihal and Born, 1997; Siegel, 2001; Smith, 1985, 2001; Vertes, 2004).
More recent studies consider the effects of both prior sleep and the effect of the circadian cycle and systematically compare subjects that sleep or stay awake during the first or second half of the night (Plihal and Born, 1997). In addition, many contemporary studies employ scalp recordings of EEG and correlate sleep patterns with memory performance, although the first learning‐dependent brain re‐activation study during sleep used PET imaging (Maquet, 2000; Peigneux et al., 2003). Jan Born and colleagues have produced a long series of elegant experiments on the subject and provided compelling support for the view that “offline neuronal reactivation during sleep may work as a principal mechanism to form any kind of memory, i.e., as a mechanism that serves to abstract temporally stable invariants from a complex stream of inputs that is dynamic and only structured in time” (Inostroza and Born, 2013). Since their accomplishments and those of others have been reviewed extensively (Hasselmo, 1999; Stickgold et al., 2000; Stickgold, 2005; Walker and Stickgold, 2006; Born et al., 2006; Marshall and Born, 2007; Diekelmann and Born, 2010; Payne and Kensinger, 2011; Lewis and Durrant, 2011; Rasch and Born, 2013), I will only discuss a small sample of this large and rapidly growing literature, insofar as it is relevant to SPW‐Rs.
Comparison of the effects of the first and second halves of a night sleep suggests that non‐REM sleep is more important for strengthening declarative memory than is REM sleep (Plihal and Born, 1997). In addition to simple associations and learning lists, several studies suggest that sleep is more important in strengthening of contextual and relational features of an episode than learning of items and details of the episode (Davachi, 2006; Tambini and Davachi, 2013). A short nap can selectively enhance the context in which lists of words are embedded, without affecting item memory. Importantly, context memory is correlated with the amount of non‐REM sleep and the density of sleep spindles during the nap (van der Helm et al., 2011). When a what‐where‐when task is memorized, during which participants learn two lists of nouns (items) one after the other (when), with the words written either on the top or on the bottom of a page (where), sleep enhances context memory without any impact on the word list, which is not hippocampal dependent (Rauchs et al., 2004). Sleep can also strengthen memory of learning temporal sequences of word‐triplets (Drosopoulos et al., 2007) and temporal order in picture sequences (Griessenberger et al., 2012). Because the formation of contextual learning is hippocampus‐dependent, these observations support the view that the benefit of sleep on memory performance is largely contributed by (non‐monitored) hippocampal mechanisms. Comparisons of explicit recall and familiarity‐based judgments for words and pictures shows that sleep improves mainly explicit recollection of memories, without affecting familiarity‐based judgments (Rauchs et al., 2004, Drosopoulos et al., 2005, Daurat et al., 2007, Atienza and Cantero, 2008). The integration of newly learned spoken words with existing knowledge can also be boosted by sleep. Words learned before sleep in the evening do not induce competition effects immediately but do so after a night's sleep. In contrast, words learned in the morning do not show such effects immediately or after 12 h of wakefulness, but show the effect 24 hrs later, after sleep has occurred (Dumay and Gaskell, 2007). In an extension of this latter study, participants recalled more words and recognized them faster after a polysomnographically monitored night of sleep, whereas in the wake group such changes were not observed until the final test one week later. Importantly, high spindle activity was associated with overnight lexical integration in the sleep group, but not with gains in recall rate or recognition speed of the novel words themselves (Tamminen et al., 2010). Sleep can also exert an impact on insight, problem solving and creativity by changing the representational structure of memories (Stickgold, 2005; Buckner, 2010). In the experiments of Wagner et al. (2004) subjects were shown an arithmetic problem that required a series of digits to be sequentially transformed into a new pattern. Performance could improve abruptly after gaining insight into a hidden abstract rule underlying all sequences. Subjects that had 8 h of nocturnal sleep recognized the hidden rule twice as frequently as control subjects with nocturnal wakefulness, or daytime wakefulness. Fischer et al. (2006) used a serial reaction time task paradigm to examine whether sleep can support explicit knowledge on an implicitly acquired skill. In the implicit task, grammatically incorrect target positions were occasionally interspersed with grammatically correct and incorrect target positions. To assess explicit sequence knowledge, subjects were instructed to predict the sequential target positions. Performance in subjects who had slept in the retention interval was significantly better than in the wake group. These results indicate a selective enhancement of explicit memory formation during sleep, and suggest an interaction between implicit and explicit memory systems in the off‐line, sleeping brain. In another serial reaction time task (Drosopoulos et al., 2011), half of the participants were informed afterwards that there was some regularity in the underlying sequence while the other half was not informed. Subgroups in each group slept the night after training or remained awake and all subjects were tested after a second night of (recovery) sleep. Both “sleep” and “awareness” improved generation task performance, but the two factors did not interact. Making inference from relations can also benefit from sleep (Ellenbogen et al., 2007). Even a short nap containing solely non‐REM sleep may be sufficient to reorganize discrete memory traces into flexible relational memory networks (Lau et al., 2011). A brief daytime nap benefited learning paired associates but not mirror tracing performance (Tucker et al., 2006) and a positive correlation was found between the duration of non‐REM sleep and recognition memory performance for landscape photographs (Takashima et al., 2006).
Using sensory cues associated with learning during post‐learning sleep is an effective way of testing the significance of context. In a study by Rasch et al. (2007), the participants learned card‐pair locations in the presence of a pleasant odor (scent of roses), which was re‐exposed during subsequent non‐REM, REM sleep or while the subjects were awake. Odors were used because olfactory information bypasses the thalamus and can have direct access to the hippocampus during sleep. Memory performance for card locations was significantly better after re‐exposure of the odor during non‐REM sleep compared with REM or waking conditions. In another experiment, odor‐induced reactivations during sleep facilitated the induction of creative solutions to the presented problem before sleep (Ritter et al., 2012). Sensory cues presented during sleep can affect non‐contextual aspects of the task as well. In the study of Rudoy et al. (2009) subjects heard sounds during sleep that had earlier been associated with objects at specific spatial locations. When tested in the waking state, they recalled these locations more accurately than other locations for which no reminder cues were provided. This latter study is complemented by the findings of Bendor and Wilson (2012) who trained rats on an auditory‐spatial association task while recording from neuronal ensembles in the hippocampus. The task‐related auditory cue (go right or go left) biased reactivation events toward replaying the spatial memory associated with that cue.
Overall, the reviewed studies provide convincing evidence that several aspects of declarative memory encoding can benefit from sleep. However, the magnitude of the effect is consistently small and often only one of many parameters of memory performance is affected. Although many of the human studies are largely driven by the general model that SPW‐Rs, neocortical slow oscillations and sleep spindles form a triad of events that are critically involved in memory consolidation (Siapas and Wilson, 1998; Sejnowski and Destexhe, 2000; Sirota et al., 2003; Battaglia et al., 2004; Isomura et al., 2006; Mehta 2007; Stickgold and Walker, 2005; Dudai 2012), the correlation between behavioral performance and physiological changes is rarely studied. Axmacher et al. (2008) reported that SPW‐Rs detected with depth microelectrodes in the hippocampus and the rhinal cortex in epileptic patients had a similar frequency composition and they were strongly correlated. SPW‐Rs occurred with the highest incidence during periods when subjects lay awake during the nap time and, importantly, the number of SPW‐Rs in the rhinal cortex, but not hippocampus, was correlated with the number of successfully recalled items during the post‐nap period learned before sleep (Fig. 37). Exploiting the correlation between neocortical slow oscillations and hippocampal SPW‐Rs, Marshall et al. (2006) used transcranial electrical stimulation (0.75 Hz) in human subjects during non‐REM sleep to increase the regularity and power of slow oscillations. This artificial manipulation improved the retention of hippocampus‐dependent declarative memories, while stimulation at 5 Hz left declarative memory unchanged. Supporting these studies in humans, entraining slow oscillations by transcranial electrical stimulation in rats was also shown to improve memory consolidation of maze learning tasks (Binder et al., 2012, 2014). Although it is tempting to conclude that replay of hippocampal cell assemblies during SPW‐Rs of non‐REM sleep was responsible for improved recall and facilitating the qualitative restructuring of the memory representation into a prospectively adaptive form, direct proof of such relationship is still missing. It is also not clear what mechanisms can be responsible to the constructive role of sleep, in addition to its benefit to memory consolidation.
Figure 37.
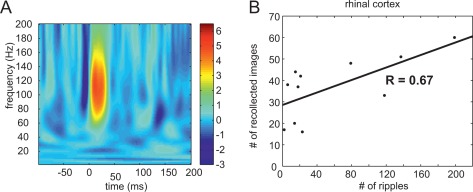
Memory consolidation and increased incidence of SPW‐Rs in humans. (A) Averaged SPW‐Rs recorded in the rhinal cortex in a representative patient. (B) Correlation between the number of SPW‐Rs during rest/sleep period in the rhinal cortex after leaning and memory performance tested after sleep. Reproduced from Axmacher et al. (2008).
Effect of Experience on SPW‐Rs
While SPW‐Rs may assist in the hippocampal‐neocortical transfer of memories and their consolidation, waking experience is needed to affect the cell assembly configuration of subsequent SPW‐Rs (see SPW‐R‐Supported Memory Consolidation section). In addition to spike content, increased post‐learning incidence and other features of SPW‐Rs can be viewed as further correlational support for the role of SPW‐Rs in memory.
In the waking rat, SPW‐R rates depend on ongoing behavior (see Behavioral Correlates and Mechanisms of SPW Generation section). In a fixed interval lever‐pressing task, a positive correlation was observed between trial number and SPW‐R incidence (Buzsáki, 1985), indicating a possible relationship between motor activity, satiation and incidence of SPW‐Rs (Jackson et al., 2006). SPW‐R occurrence during non‐REM sleep is proportional to preceding waking duration (Ponomarenko et al., 2003a, 2003b), which can be interpreted either as a learning‐induced or homeostatic effect of SPW‐Rs on neuronal excitability (Grosmark et al., 2012). In an odor‐reward association task, rats had to dig in odor‐impregnated gravel to obtain food reward. Control rats foraged to find the randomly distributed reward. The experimental group showed sustained increase in SPW‐R activity and large amplitude ripples after the learning session, lasting at least one hour. In control animals only a small increase was present, limited to the first 30 min of non‐REM sleep after the learning session (Eschenko et al., 2008). In a follow up study, recording of sleep for 2 h before training served to normalize the increased incidence of SPW‐Rs after learning on the maze. Again, increased incidence of SPW‐Rs was observed in the experimental group compared with the rats of the control group (Ramadan et al., 2009). In a spatial discrimination study, each daily session consisted of five trials in which both groups were required to move about in the maze to obtain the rewards located at the end of the maze arms. Each trained rat was yoked to a pseudo‐trained rat to assure that locomotor activity was the same in each group and sleep was recorded in a room different from the one in which conditioning took place. While initially the SPW‐R incidence during sleep was comparable in the two groups, it decreased to lower levels in the pseudo‐conditioned animal after 6 days of training, whereas increased incidence of SPW‐Rs was sustained in the experimental group (Ramadan et al., 2009). Three further studies show that SWP‐R‐associated replay occurs more frequently after exploration of a novel rather than a familiar environment (O'Neill et al., 2008; Cheng and Frank, 2008; Grosmark et al., 2014). The experience‐dependence of ripples is also supported by the correlation between length of the replay involving several arms of the maze and the number of ripple cycles (Davidson et al., 2009). In human patients, learning is also followed by an increased probability of occurrence of SPW‐Rs in the rhinal cortex, although not in the hippocampus (Axmacher et al., 2008). Finally, the direction of travel of SPW‐Rs along the long axis of the hippocampus can be biased by previous learning experience (Patel et al., Society for Neuroscience Abstract, 2013). Despite these encouraging experiments, the learning‐related specificity of SPW‐Rs needs to be demonstrated more rigorously because elevated firing rates have been reported after waking exploration even in a familiar maze (Kudrimoti et al., 1999) and such rate increases may confound the correlation measures.
Overall, the above results indicate that the incidence of SPW‐Rs increases following learning, and aborting them interferes with memory consolidation. One might argue that sleep architecture is altered after novel learning or even after exploration in a familiar environment, and that the increased incidence of SPW‐Rs is simply a consequence of a global change in sleep. Experiments by Girardeau et al. (2014) indicate that this explanation may not be entirely correct. They used closed‐loop stimulation to interfere with SPW‐Rs. When the rats were trained on a radial arm maze to learn a spatial reference task (the position of 3 baited arms), the incidence of SPW‐Rs (i.e., the hippocampus’ attempts to produce them) was higher than in the stimulation‐control group where the electrical pulses occurred randomly relative to the SPW‐R occurrence. Importantly, such compensatory increase of SPW‐Rs did not occur following random foraging in a familiar environment in which no learning was required, indicating that the increased SPW‐R incidence was dependent on previous learning per se. The authors tested this hypothesis by blocking NMDA receptors. Systemic injection of MK‐801 during learning abolished the post‐learning elevation of SPW‐Rs but the same drug injection did not affect SPW‐R incidence or waveform when given before sleep. These findings complement previous results, which demonstrate that NMDA receptor blockade during learning impairs subsequent replay of place cell sequences during sleep, but it is without an effect when given after learning (Dupret et al., 2010). Unfortunately, it was not analyzed whether the learning‐induced excess of SPW‐Rs were due to the higher occurrence of double or clustered SPW‐Rs, since clustered SPW‐Rs have been implicated in combining various aspects of learning (Davidson et al., 2009; Wu and Foster, 2014). Girardeau et al. (2014; see also Girardeau and Zugaro, 2011) interpret their observations by suggesting that the increased drive for SPW‐Rs after learning results from an NMDA receptor‐dependent plasticity during learning, which sets the stage for subsequent consolidation during consummatory behaviors, including rest and sleep.
Replay of Waking Experience During SPW‐Rs—Sleep Replay
Pavlides and Winson (1989) were the first investigators to note that if a rat is confined to a particular location, the firing rates and burst probability of the associated place cells are more elevated during the subsequent sleep episode compared with place cells which were not activated before sleep. This relationship may reflect a learning effect but because pre‐experience sleep was not examined, it may also reflect a preconfigured firing rate‐dependent relationship (see Constructive Role of SPW‐R section). In a subsequent study, neuronal firing during SPW‐Rs was examined in two non‐REM sleep episodes in the rat's home cage, separated by explorative activity in a novel environment. The most consistent partners of SPW‐Rs in the second sleep episode were those neurons that were most active during waking even if they fired relatively rarely during the pre‐exploration sleep (Buzsáki, 1986). Although these findings are compatible with mnemonic functions, the coherent representations of the preceding experience (i.e., memories) could not be convincingly demonstrated without monitoring a representative fraction of the neuronal population. This was accomplished by a groundbreaking study by Wilson and McNaughton (1994). Spike‐train cross‐correlations in 100 ms windows (“coactivations”) were computed between pairs of CA1 pyramidal cells during the pre‐behavioral sleep (PRE), ambulation on familiar open field or linear track (RUN), and post‐behavioral sleep (POST) periods, each lasting ∼20 min. During RUN, neurons with overlapping place fields exhibited highly positively correlated activity, whereas in pairs with non‐overlapping fields no reliable temporal correlations were observed, as expected. Importantly, neuron pairs, which showed overlapping place fields and strong correlations during RUN continued to display higher correlations during the POST sleep compared with PRE sleep (Fig. 38). The authors also noted that the strength of the pair‐wise correlations was several‐fold higher during SPW‐Rs than between them. However, increased RUN‐POST correlations were true only for a small fraction of pairs only, whereas the correlations for the entire population were rather modest (0.017 and 0.069 for two rats). The RUN‐induced selective reactivation of correlated states declined during POST sleep with a time constant of ∼12 min. Wilson and McNaughton (1994) suggested that the RUN‐induced increased positive correlations during the POST sleep epoch reflected increased synaptic connectivity in the CA3 region and these correlations were inherited by the recorded CA1 neurons. They also hypothesized that neuronal states encoded within the hippocampus are played back as part of a consolidation process by which the hippocampus can provide spatial contextual information for other elements of the experience and bind all aspects together in the neocortex. In a follow up study, Skaggs and McNaughton (1996; see also Qin et al., 1997) extended these observations by demonstrating that the temporal order in which neuron pairs fire during waking experience is preserved in the subsequent (POST) non‐REM sleep episode. Since in these studies the data were arbitrarily partitioned into weak and strong groups, in their next set of experiments, McNaughton et al. (Kudrimoti et al., 1999) searched for a more quantitative statistical estimator of neuronal event reactivation. It should also be noted that in the previous experiments no new learning took place during RUN since the rats were tested in their already familiar testing environments for several days to weeks. In the experiments of Kudrimoti et al. (1999), the rats were trained on a triangular maze, a Figure 8 maze or a linear track. A subset of three rats were first trained on one side of the Figure 8 maze but were also recorded from subsequently during sleep after exploring the remaining part of the maze (POST “novel”). In addition to calculating the correlation between the magnitude of rate co‐activation of place cells and their activity during sleep, they calculated a measure called “explained variance” (r RUN‐POST/PRE), which is a correlation between RUN and POST after controlling for the linear effects of the PRE correlations. Using this novel measure, they could explain ∼15% of the variance of firing rate correlations in POST sleep when tested after RUN in the highly familiar environment but only ∼5% when the rats explored the novel arms of the Figure 8 maze. The magnitude of the explained variance was substantially larger during SPW‐Rs than in their absence, although this difference may be due to the low firing probabilities between the SPW‐R events. As in the previous experiments, only a small minority of neuron pairs showed moderate correlations between RUN and POST sleep, with the majority showing weak correlations (overall mean r = ∼0.035). In the familiar RUN experiments, they also found a weak but reliable correlation between RUN and PRE, which the authors interpreted as representation of the residual traces from the previous days of training. In addition to changes in neuronal coactivation measures, this study also reported increased firing rates of the maze‐active neurons during POST sleep.
Figure 38.
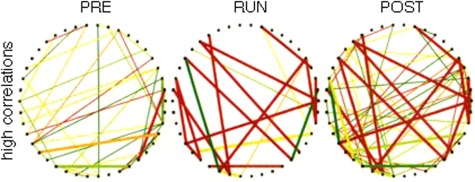
First compelling demonstration of experience‐dependent replay of hippocampal activity. Diagram of the co‐activation matrix of 42 neurons (dots around the perimeter of the circle) recorded from as single rat. Lines indicate a small subset of all positive correlation (>0.2) between the pairs, with color reflecting the magnitude of the correlation (red, high; green, low). Bold lines indicate cell pairs that were correlated during waking activity (RUN) and also correlated during either PRE‐RUN or POST‐RUN sleep. Note that most of the highly correlated pairs that are present during RUN are also present in the POST phase but less frequently during PRE phase. Reproduced from Wilson and McNaughton (1994).
In support of the studies by McNaughton et al., Hirase et al. (2001b) also reported stronger coactivation of spikes across CA1 pyramidal neuron pairs between RUN (exploration and wheel running) and POST sleep, compared with RUN and PRE sleep for the subset of neurons which were active during RUN. The discharge frequencies of individual pyramidal neurons were robustly correlated across PRE and POST sleep and between both RUN‐PRE and RUN‐POST comparisons. When tested in the familiar apparatus, the RUN‐PRE rate correlations were stronger than RUN‐POST correlations. However, when the rats were tested in a novel apparatus (but in the same room), both the PRE‐POST and the RUN‐PRE rate correlations decreased substantially. These findings suggest that exposure to novelty can induce lasting changes in both firing rates and correlated firing in the small activated subset of neurons. The observations also imply that increased rates of a selected small fraction of neurons are associated with a commensurate homeostatic decrease in the discharge activity of the remaining neurons so that the overall firing rate of the entire population remains stable.
Despite their success, these early pioneering studies have shortcomings. A confound of increased coactivation in POST relative to PRE sleep may be a correlated discharge rate change since correlation is proportional to the cells’ firing rates (de la Rocha et al., 2007). Furthermore, pairwise cross‐correlograms do not sufficiently define the exact temporal structure of neuronal sequences, especially when higher‐order connections are also involved. A sequence of A‐B‐C place cells may represent a simple “synfire” chain (Abeles et al., 1993), with no interaction present between A and C. If place cell B also participates in sequence M‐B‐N in addition to sequence A‐B‐C, activity of place cell B would predict not only cell C but also N, representing different places (Nadasdy, 2000). Such ambiguities or corruptions can be avoided if sequences are represented by sequential activation of neuronal assemblies (Hebb, 1949), of which the recorded neurons may be representative members. To examine sequential activation of neurons more directly, Nádasdy et al. (1999) applied template‐matching and joint probability map methods to search for repeating spike sequences in excess of chance occurrences. Reliably repeating spike sequences were found in both waking and sleeping animals in excess of what was predicted by random coincidences. Importantly, the spike sequences observed in waking while the rat explored a novel environment were “replayed” at several times faster timescale during SPW‐Rs of non‐REM sleep compared with waking. Furthermore, the incidence of wake‐detected sequences (RUN) was considerably more frequently expressed in POST compared with RPE sleep, supporting the suggestion that time‐compressed neuronal patterns during SPW‐Rs are a consequence of firing patterns learned during waking exploration. Lee and Wilson (2002) introduced a behavioral event‐based template‐matching method where the sequences of smoothed place fields of CA1 pyramidal cells during RUN on an elevated track defined the template (a “neuronal word”; Fig. 39). Using novel combinatorial decoding statistics in which the distribution of uninterrupted sequences of neuronal activity during sleep was matched to the sequence of their place fields on a linear track, they showed that the matching between POST and the RUN sequences cannot be explained by nonspecific increases of firing rates or random chaining together of shorter pair sequences. Sequences during POST sleep occurred intermittently in brief (∼100 ms) time windows of the SPW‐Rs, i.e., 10 to 20 times faster than in the behaviorally defined template sequence, i.e., the time elapsed between place field peaks. However, it should be emphasized that this impressively large compression is not specific for SPW‐Rs, since compression of behavioral time‐scale sequences is also present in theta cycles (Skaggs et al., 1996; Dragoi and Buzsáki, 2006). In fact, SPW‐Rs represent only a 1.3‐fold compression relative to theta waves (Diba and Buzsáki, 2007). Despite the elegance of matching statistics introduced by Lee and Wilson (2002), it has drawbacks. First, while it tolerates missing spikes from the sequence, an “intruder” spike from a non‐template neuron will terminate the sequence, making it shorter than expected. Second, computation of significance on the basis of a null hypothesis that assumes the lack of any common sequence content across population activity events may bias the results (Diba and Buzsáki, 2007; Pfeiffer and Foster, 2013).
Figure 39.
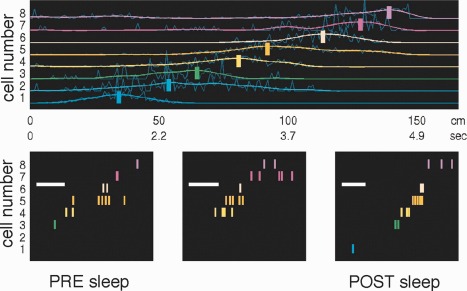
Replay of waking neuronal spike sequences during sleep in hippocampus. Smoothed place fields (colored lines) of eight place cells during runs from left to right on a track (average of 30 trials). Vertical bars mark the positions of the normalized peaks of the smoothed fields. Nonuniform time axis below shows time within an average lap when above positions were passed. Bottom panels: three SPW‐R‐related sequences from slow‐wave sleep after the waking session. Note similar sequences during SPW‐Rs and run. Note also difference in timescale. The scale bar represents 50 ms. Reproduced from Lee and Wilson (2002).
What exactly is being replayed during SPW‐Rs? Is recent experience as critical as conceived by the two‐stage model? SPW‐R‐related activity of neurons may reflect partial reactivation of the global cognitive map (Shen and McNaughton, 1996), short or long locomotion trajectories of the animal, discrete places the animal visited before falling asleep, a combination of old and recent experience or even a novel combination of preexisting knowledge and recently learned novel information. According to a simple synaptic connectivity or cellular excitability framework, SPW‐R sequences should recapitulate the temporal order in which place cells are activated during waking exploration, assuming that all place cells and their connections are equal. However, this assumption may not hold since the distribution of within‐field and peak firing rates of place cells show a strongly skewed, lognormal distribution (Mizuseki and Buzsáki, 2013), implying that the probability and temporal position of a given neuron during SPW‐R is not determined by recent experience alone. Furthermore, more strongly activated neurons may bring about stronger connections compared with slowly firing neurons and, consequently, affect the neurons’ tendency to discharge during SPW‐Rs. Csicsvari et al. devoted a series of elegant experiments to address some of these issues (O'Neill et al., 2006, 2008; Csicsvari et al., 2007; Dupret et al., 2010; cf., O'Neill et al., 2010; Csicsvari et al., 2014; Csicsvari and Dupret, 2014). After exploration of a familiar open field, cell pairs representing the most visited regions of the platform show the strongest PRE‐POST sleep increases during SPW‐R. The co‐firing probability depended on the number of times the cells fired together with short latencies (<50 ms) during exploration. In contrast, cells firing at non‐overlapping locations reduced their co‐firing in proportion to the number of times that they fired independently. Furthermore, the change in coactivation was larger for novel than familiar environments (O'Neill et al., 2008). To examine the role of learning, Dupret et al. (2010) designed a spatial memory task in which rats learned and sub sequently recalled the locations of three randomly selected food wells out of the numerous possible food locations on a large cheeseboard maze. As these baited locations changed from day to day but stayed fixed within a given session, this “matching‐to‐multiple‐places” procedure required frequent updating of the memory for goal locations in an otherwise unchanging environment. Place‐related firing patterns in CA1, but not CA3, were reorganized daily after just a handful of initial trials to represent new goal locations. Importantly, these experiments demonstrated a critical relationship between SPW‐Rs and remembering goal locations. Neuronal population activity in most SPW‐Rs during POST sleep represented one of the three goal locations, and the number of times a given goal location was reactivated predicted how well that location was subsequently remembered, as expressed by rat's memory performance. However, such enhanced representation of goal locations by assembly firing during SPW‐R was not seen when NMDA receptors were attenuated by peripheral injection of CPP. Importantly, this drug treatment also deteriorated memory performance. These experiments, therefore, demonstrate that the stabilization of new place fields surrounding novel goal locations depends on NMDA receptors even though NMDA receptors are not critical for SPW‐R (see Pharmacological Control of sPW‐R section). However, when NMDA receptors are blocked during learning, replay of neuronal activity during SPW‐Rs is dominated largely by already consolidated events, which can interfere with the activity of neurons representing new places and goals.
Cheng and Frank (2008) compared SPW‐R‐related firing patterns of CA1 pyramidal cells on novel and familiar arms of a radial arm maze. The probability of occurrence of SPW‐Rs and the firing rates of the neurons were higher on the novel arm (see also Csicsvari et al., 2007). Furthermore, coactivation of neuron pairs in the novel arm was significantly stronger than in the familiar arms. Even when SPW‐Rs occurred in the familiar arms, coactivation of place cell pairs in the novel arm was more strongly represented than those of the familiar arm. Similar to O'Neill et al. (2006), Cheng and Frank (2008) suggest a Hebbian learning rule in which pairing SPW input with increased firing of CA1 neurons induce synaptic strengthening (King et al., 1999) and effective incorporation of newly formed place cells into SPW‐R population activity.
SPW‐Rs Assist with System Level Consolidation During Sleep
As discussed above, converging findings from multiple experiments and laboratories provide convincing support for a special role of SPW‐Rs in the learning‐consolidation process. However, all previous experiments have been confined to examining activity within the hippocampus without addressing the critical issue of whether and how SPW‐Rs exert an impact on target cortical structures (Frankland and Bontempi, 2005). If SPW‐R‐related replay is involved in systems consolidation, a temporal coordination should be present between the hippocampus and behaviorally relevant cortical areas. Ji and Wilson (2007) were the first to examine this conjecture. They trained rats in an eight‐shape maze and recorded neuronal activity simultaneously from CA1 pyramidal cells and visual cortical neurons. They exploited the fact that place cells in the maze often coincided with activation of particular neurons in the visual cortex, presumably by their unique visual drive in some parts of the maze. On the basis of sequential activation of hippocampal place cells and visual cortical neurons, they constructed templates of ordered activity and searched for similar sequences during sleep. They observed that the UP‐DOWN state changes of cortical neuronal populations during slow oscillations of non‐REM sleep preceded similar groupings of activity in the hippocampus by ∼50 ms. Most critically they established that matching patterns to the waking templates were observed during non‐REM sleep in excess of what was expected by chance and more frequently during POST than PRE experience sleep, at least in the hippocampus. Ji and Wilson (2007) concluded their study by hypothesizing that hippocampal sequences are initiated in the neocortex (Sirota et al., 2003) that is in the same temporal order as during waking experience and that such repeated reactivations are responsible for long‐term memory storage. However, a caveat in this study is that the physiological testing was carried out after the task has already been well practiced, thus presumably after memory consolidation and transfer of contextual hippocampal information to the visual cortex. Furthermore, it remains to be elucidated whether the neocortical‐entorhinal inputs “impose” the sequence on the hippocampus or whether the cortical inputs only serve as pointers to select a particular initial condition, after which the hippocampal neuronal trajectories take their self‐organized course.
Bendor and Wilson (2012) also examined systems level consolidation. They trained rats on an auditory go‐left, go‐right discrimination task. Since travels to the right or left from the reward area were associated with different hippocampal sequences of place cells, the two different firing pattern templates could be used to examine whether SPW‐R‐related sequences during POST sleep represented right or left trajectory. When the left and right travel‐related auditory cues were played out during sleep, the investigators observed that the sound could effectively bias the reactivation of the spatial sequence associated with that sound cue. These results support the view that sensory inputs can reach the hippocampus during sleep and affect the selections of SPW‐R‐related sequential patterning of hippocampal neurons.
Another experiment focused on the problem of whether new learning rules introduced during the waking state can influence hippocampal‐neocortical interactions during sleep. In the experiments by Peyrache et al. (2009), rats had to learn to select the rewarded arm of a Y maze using one of four possible rules (left arm, right arm, illuminated arm, and non‐illuminated arm). During each trial, one target arm was illuminated at random. After the rat achieved criterion performance according to the current rule, the rule was changed without providing any information to the animal and the rat had to infer the new rule from the pattern of rewarded and non‐rewarded arms. The authors used a principal component analysis‐based method to reveal the replay of rule learning‐specific changes in the medial prefrontal cortex. With this novel method, a quantifiable “replay” value of cell assemblies could be assigned to each activity time‐bin analyzed during sleep. Replay events were largely brief, lasting 100 ms or less, and were most often associated with the UP state of slow oscillation. Virtually all prefrontal assembly reactivation events occurred in concert with hippocampal SPW‐Rs and more often during POST than PRE sleep epochs (Fig. 41). Prefrontal activation typically occurred 40 ms after hippocampal SPW‐R, thus in the opposite temporal order than in the Ji and Wilson (2007) study where recordings were made in the primary visual cortex. The SPW‐R‐to‐prefrontal reactivation delay is likely due to the direct monosynaptic connections between CA1 hippocampal neurons and the recipient prefrontal cortex (Swanson, 1981). Importantly, assembly replays in the prefrontal cortex during hippocampal SPW‐Rs recurred mainly after the acquisition of the new rule. Critically, when the rat adhered to the newly acquired rule, the reactivation patterns that contributed the most to memory replay were those that appeared at the position at which the rat committed to choosing a goal arm. The summary message from these sets of experiments from different laboratories is that system‐wide consolidation of learned information, including that of learning rules, takes place mainly during SPW‐Rs.
Figure 41.
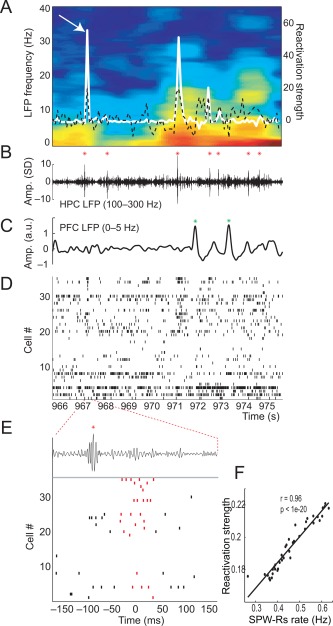
Cell assemblies in the medial prefrontal cortex (mPFC) are reactivated by hippocampal SWP‐Rs during sleep. (A) Reactivation strength (white traces, right axis) of the signal component superimposed on the mPFC LFP spectrogram (left axis). The black dashed line represents the normalized population firing rate. (B) The bandpass‐filtered hippocampal LFP (100–300 Hz) shows ripple events (red asterisks). (C) Bandpassfiltered (0–5 Hz) PFC LFP. Delta waves are denoted by green asterisks. (D) Raster plot of spike trains from the mPFC cells sorted by principal component weight magnitude. (E) Expansion of the 300 ms surrounding the peak indicated by an arrow in A. Red rasters represent spikes occurring in the bin of peak reactivation strength. (F) Relationship of reactivation strength and SPW‐R occurrence. Reproduced from Peyrache et al. (2009).
The Role of Waking SPW‐Rs
Most early works on the relationship between memory consolidation and SPW‐Rs examined SPW‐R firing patterns during sleep. However, the two‐stage model emphasizes the importance and continuity of SPW‐Rs immediately after experience in the waking animal and those occurring subsequently during sleep. In its initial formulation it assumed a hierarchy of sequential activity of pyramidal neurons so that the “most excitable cells fire first followed by less excitable ones, that is in the reverse order to that in which they were potentiated during exploration” (Fig. 42) (Buzsáki, 1989). This hypothesis was based on the contiguity theory of reinforcement in experimental psychology, postulating that reinforcement works backward in time, thus events and locations spatially and temporally immediate to the preparatory‐consummatory brain state shift are remembered best followed by a backward gradient of previous events and places (Craik 1943; Guthrie, 1952).
Figure 42.
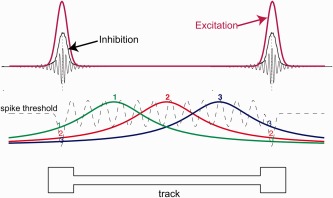
A hypothetical place‐field model proposed to account for forward and reversed SPW‐R sequences. Place‐related inputs for three neurons are indicated in color. Spiking threshold is shown with a dashed line. On the track, this threshold is theta‐modulated. On the reward platforms, during immobility, a transient decrease in the threshold during SPW‐Rs causes cells to fire outside of their classical place‐fields. Due to hypothetical subthreshold place fields, sequence of firing is forward (1, 2, 3) before and reverse (3, 2, 1) after a rightward journey. The top panel illustrates the transient rise in global excitation (and inhibition), deduced from population spiking activity during immobility ripples (Csicsvari et al., 1999a). Similar models (place field ‘tail’ hypothesis) have been put forward by Buzsáki (1989), Foster and Wilson (2006), and O'Neill et al. (2006). Reproduced from Diba and Buzsaki (2008).
Reverse replay during SPW‐R
The first experimental support for the conjecture of reverse replay was provided by the pioneering study of Foster and Wilson (2006). They trained rats to run from one end of a linear track to the other and back again. Within a given lap, the animal stopped at each end to consume food from a food well and waited of its own accord while grooming, whisking or being still. Place fields of CA1 pyramidal neurons were ordered according to the position of the field peaks in order to generate a sequence, which was then used as a template to examine the neuronal patterns during SPW‐Rs at the end of the tracks. The match between the template and firing during SPW‐Rs was assessed by quantifying the rank‐order correlation between cell number and time, together with a probability. Remarkably, within the replay events, the sequence of neuron spikes was in reverse order with respect to the probe sequence, and spanned virtually the equivalent of the entire track, on a timescale of hundreds of milliseconds. Reverse replay was observed even after the first lap on a novel track. This reverse replay in the waking animal was contrasted to the typically forward sequences documented during non‐REM sleep in previous studies. Foster and Wilson (2006) suggested that “awake replay represents efficient use of hard‐won experience” by virtue of its precise temporal relation to a current, anchoring event, whereas the role of sleep replay may be in chaining learned events. They suggested that reverse replay is the norm in the waking animal, in contrast to the forward replay during sleep, implying a fundamental difference how activity patterns during SPW‐Rs are organized in different brain states. The robust nature of the reverse replay of place cell sequences was immediately replicated in other laboratories in both CA1 and CA3 regions (Jackson et al., 2006; Csicsvari et al., 2007; Diba and Buzsáki, 2007).
There are reasons to expect that sleep replay and awake replay are different. In the waking state, sensory stimuli can affect network activity in contrast to the isolated state of sleep. Furthermore, SPW‐Rs occurring during eating drinking and perhaps freezing may co‐occur with surges/decrease of activity in VTA, lateral habenula, hypothalamus and amygdala and while such co‐occurrences are likely absent during sleep. O'Neill et al. (2006) have noted some special properties of waking SPW‐Rs. In addition to immobility and reward‐related SPW‐Rs, they distinguished exploration‐related SPW‐Rs as well. These latter events occur during pauses in locomotion at the border of theta oscillations (see Fig. 5), thus the spike content of SPW‐Rs can be strongly affected by synaptic changes brought about during theta‐related exploration‐ambulation. O'Neill et al. (2006) hypothesized that firing patterns of neurons during SPW‐Rs should differ depending on whether the rat stops within or outside the place field. Indeed, this was a fundamental observation. Within the place field, the SPW‐R‐related firing had heavy tails surrounding the event, indicating that its baseline is influenced by the environmental factors that determined the “placeness” of the neuron (Fig. 40). Furthermore, the peak firing of the neuron during SPW‐R was also higher within than outside the field and cross‐correlations of neurons with overlapping place fields gave higher peaks when the rat stopped within the overlapping part of the place fields. Further, SPW‐R‐related peak firing rate inside the place field was higher than the sum of the baseline activity plus the peak firing rate outside the place field of the neuron, indicating a supralinear summation of environmental and internally generated inputs. The authors suggested that the coupling of the SPW input from CA3 with place‐selective firing during exploratory SPW‐Rs facilitates initial associations between neurons with similar place fields and enables the formation of place‐related ensembles. Once incorporated into the SPW‐R, the same cell assembly can be reactivated during subsequent SPW‐Rs in any part of the environment.
Figure 40.
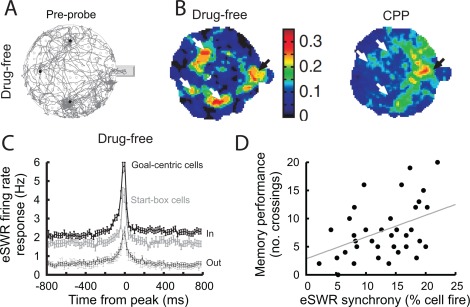
Relationship between SPW‐R parameters and memory performance. (A) Representative example of an animal's path (gray lines) in a cheeseboard task where food is hidden at three locations (black dots). (B) Color‐coded maps illustrating the post‐probe spatial distribution of CA1 place fields in the drug‐free and the CPP‐treated conditions. In the drug‐free condition a higher proportion of cells was associated with goal locations (white arrows). (C) SPW‐R firing rate histograms of CA1 “goal‐centric” and “start‐box” cells inside (In) and outside (Out) their place fields. Note higher firing rates at the tail of the SPW‐R histogram when the rat was sitting within the place field of that neuron. (D) Scatter plot shows post‐probe memory performance (number of crossings near the food locations) as a function of “eSPW‐R synchrony” (percentage of CA1 pyramidal cells that fired during exploratory SPW‐R) after learning. Reproduced from Dupret et al. (2010).
Forward and reverse replays during SPW‐R
Numerous experiments in humans have shown that memory of a temporal sequence is characterized by both forward and backward associations among the stored items of the sequence, with forward associations showing stronger connections (Kahana, 1996; Howard et al., 2005; Drosopoulos et al., 2007; Griessenberger et al., 2012). Thus, the demonstration of reverse replay during waking together with the well‐established forward replay during sleep (Foster and Wilson, 2006) was a welcome development since it provided putative mechanisms for bidirectional chaining of sequential events. However, it was not clear why such processes should be separated in both space and time, involving different brain states. For establishing bidirectional connections, it appears more advantageous to establish forward and reverse links in the same behavioral context. This turned out to be the case. Diba and Buzsáki (2007) replicated the experiments of Foster and Wilson (2006), using the same template matching method. During drinking and immobility at the end of the track following the run, the same neurons fired again but in a temporally compressed manner and in the reverse temporal order as in the Foster and Wilson (2006) study. In addition, the same neurons fired in the forward temporal order during immobility before running across the track (Fig. 43). Approximately twice as many forward replay events were detected as reverse replay events (see also Davidson et al., 2009). Importantly, the majority forward event occurred at the start end of the track before running, whereas the reverse replay events occurred mostly at the other end following the run. Vector distances between the place fields were faithfully preserved in the temporal structure of the SPW‐R compressed events. Thus, in the waking animal, two mechanisms appear to favor forward associations: time compression of neuronal sequences within the timescale of theta waves (Skaggs et al., 1996; Dragoi and Buzsáki, 2006; Johnson and Redish, 2007) and SPW‐Rs. The stronger forward associations may explain why forward associations are favored as compared with backward associations during free recall (Kahana, 1996). In sum, these experiments suggest that forward replay “events play a role in ‘planning’ upcoming trajectories” (Diba and Buzsáki, 2007) and the passed routes are recapitulated during reverse replay events.
Figure 43.
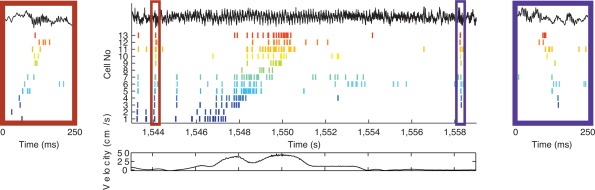
Place cell sequences experienced during behavior are replayed in both the forward and reverse direction during awake SPW‐Rs. Spike trains for place fields of 13 CA3 pyramidal cells on the track are shown before, during and after a single traversal. Sequences that occur during track running are reactivated during SPW‐Rs both before and after the run, when the rat stays immobile. Forward replay (left inset, red box) occurs before traversal of the environment and reverse replay (right inset, blue box) after. The CA1 local field potential is shown on top and the animal's velocity is shown below. Reprinted from Diba and Buzsáki (2007).
The hypothesis that the “tail” of place‐fields is responsible for reverse replay (O'Neill et al., 2006; Csicsvari et al., 2007) applies also to the forward replay (Fig. 42). Csicsvari et al. (2007) defined the boundary of a place‐field by the location of a 95% drop in its mean firing rate. Using these criteria, they showed that as long as the animal was within this boundary for a given place‐field while staying still, the cell fired in inverse relation to the distance from the place‐field center during SPW‐Rs. They suggested that a gradient from the center of the place field represents a potential mechanism for replay. Diba and Buzsáki (2007, 2008) also examined whether the spatial proximity of the place field representation played a role in the sequence replay (O'Neill et al., 2006; Csicsvari et al., 2007). However, including only neurons with ≥10 cm outside of the boundaries of their place‐fields did not notably affect either forward or reverse correlations. Thus, sensory inputs alone and the place field “tail” hypothesis cannot explain replay sequences under all conditions. It also cannot explain why the tail of a place field would sometimes initiate a reverse while other times forward replay. Yet, the place field tail observations clearly indicate that the firing patterns during SPW‐Rs are influenced by the current or immediately preceding perceptual inputs.
Experiments by Karlsson and Frank (2009) also cast doubt on the place field tail hypothesis as an exclusive mechanism for replay. They trained rats to run on two similar but differently oriented W mazes in the same room and generated separate place cell sequence templates in each of the maze. Next, they examined whether SPW‐Rs replay mainly sequences that occurred on the same maze where the spatial‐temporal context can effectively shape SPW‐R content (Csicsvari et al., 2007) or whether SPW‐Rs also reactivate stored representations of remote experiences at one place while being awake in a different place. Although SPW‐R replay sequences were often related to local spatial input at the rat's location, the spike content of SPW‐Rs also reflected place field sequences of the other maze or either mazes when the rat was tested in a holding box. Thus, awake and remote replay continues long after the initial experience vanishes and can be as common as local replay of the current environment. Karlsson and Frank (2009) also demonstrated that remote replay was more robust when the rat had recently been in motion than after extended periods of quiescence even in the holding box. This may reflect the slow decay of neuromodulatory drive after exploratory‐theta state. Overall, these observations suggest that SPW‐Rs during brief pauses in explorative behavior can lead to repeated and accurate reactivations of remote memories in the midst of an ongoing experience, potentially combining multiple distinct events across long spans of time. The mechanisms that determine the direction of replay (forward versus reverse) remain to be elucidated.
Selective Perturbation of SPW‐Rs Affects Memory Consolidation
While correlative studies indicate a link between hippocampal SPW‐Rs and memory consolidation, they do not necessarily prove that their organized spike content has functional relevance. A more direct approach to establish a causal relationship requires selective manipulations of SPW‐R events without affecting sleep architecture and other parameters. To this end, Girardeau et al. (2009) trained rats in a hippocampus‐dependent, spatial‐reference memory task. After each day's training session, SPW‐Rs were detected online during a one‐hour POST‐task sleep session and each detected event was truncated by closed‐loop weak, single pulse electrical stimulation of the ventral hippocampal commissure (Fig. 44). The stimulation silenced hippocampal networks and prevented potential replay of place cell sequences previously activated during learning. Uncontrolled effects of the stimulation were ruled out by including a control group with the same number of weak stimulations delivered outside SPW‐R events. These control subjects underwent same stimulation and transient silencing of hippocampal networks (50–200 ms), except that a random delay was introduced between SPW‐R detection and stimulation, ensuring that the stimulations occurred mainly outside of the SPW‐R events. Learning performance of the stimulation‐only control group was comparable to that of an unimplanted group. In contrast, elimination of SPW‐Rs and associated neuronal replay resulted in deterioration of memory consolidation in test animals (Fig. 44). These findings therefore indicate that SPW‐Rs are critical for memory performance, possibly because of the consistent and compressed replay of cell assembly sequences brought about by maze learning.
Figure 44.
Disruption of SWP‐Rs affects memory performance. (A) Training and recording protocol. Rats were allowed to perform three trials each day with the same three arms baited once per trial with chocolate cereal (left, red dots). After the third trial the rat was allowed to rest/sleep in the flowerpot for one hour during which stimulations were triggered, either during (test rats, middle) or outside SPW‐R (stimulation control rats, right). (B) Test rats were significantly impaired in the radial maze task compared with control rats. Grey shading indicates the chance zone. Although performance increased in all groups, rats with ripple suppression took more days to perform above upper chance level and their performance remained consistently below that of the control groups. (C) SPW‐R disruption in waking rats causes a specific impairment in the spatial working memory component in a W‐track task. Proportion correct versus day number for outbound trials is shown. Horizontal dotted line represents chance‐level performance of 0.5. Control rats received stimulation irrespective of SPW‐R occurrence. A and B, Reproduced after Girardeau et al. (2009): C, Reproduced after Jadhav et al. (2012).
Another study by Ego‐Stengel and Wilson (2010) used a modified radial arm maze (“wagon wheel” maze) to test the impact of SPW‐Rs during post‐learning sleep on spatial memory. Each of the five rats was trained daily on two halves of the maze in separate daily sessions and each session was followed by a POST sleep session. During randomly chosen alternate POST sessions, SPW‐Rs were detected online and interrupted by stimulation of the ventral hippocampal commissure. Disruption of SPW‐Rs during POST sleep resulted a slower learning in the corresponding half of the maze. Since no stimulation control was used in this study, it remained an open question whether the stimulation‐induced memory performance deficit was due to the interruption of SPW‐R‐related replay and hippocampo‐cortical transfer of the memory traces or other non‐specific effects of the stimulation. However, since weak control stimulation in the Girardeau et al. (2009) study did not affect behavior, the learning impairment reported in the Ego‐Stengel and Wilson (2010) study was also likely due to selective elimination of SPW‐Rs.
The findings in the POST sleep SPW‐R perturbation paradigm are further supported by results in the waking animal. Jadhav et al. (2012) used the same closed‐loop method to erase SPW‐Rs and SPW‐R‐yoked random stimulation in control rats as in Girardeau et al. (2009). The rats were trained to alternate between two arms of a W maze task (outbound travels) and return to the base of the central arm (inbound travels). SPW‐Rs were disrupted across 8 days of learning. The close‐loop truncation of waking SPW‐Rs while performing the task resulted in increased choice errors on the outbound (spatial working memory) component of the task, without increasing errors on inbound travels. All SPW‐R‐disrupted animals learned more slowly than control rats, and all of them had lower performance on the last days of training than the combined group of unstimulated and randomly stimulated animals (Fig. 44). When highly performing animals were switched to closed‐loop elimination of SPW‐Rs, their performance also declined, indicating that interference with waking SPW‐Rs likely affected a working memory component. SPW‐R‐disruption in the waking rat did not affect reactivation of cell pair correlations in the POST training rest period.
In another closed‐loop experiment, rabbits were trained in a trace eyeblink conditioning task (sound followed by an air puff after a delay), a hippocampus‐dependent associative learning task. For the experimental rabbits a bright light flash was presented during the inter‐trial intervals, triggered by the spontaneous occurrence of SPW‐Rs and the same light was presented to a yorked rabbit, i.e., irrespective of its brain state. Learning progressed significantly slower in the experimental rabbits compared with yoked controls. The light itself did not interrupt SPW‐Rs since the delay from the visual input to the hippocampus was longer than the duration of the ripple. Instead, the light stimulus evoked a short sequence of theta‐wave like pattern (Nokia et al., 2012). Thus, the impairment of learning in this paradigm is not due to aborting SPW‐R sequences but to the fast switching back from SPW‐R to theta state and perhaps preventing neocortical activity from processing the SPW‐R content properly. At present it is not clear why the post‐SPW‐R silent period is important for memory. One possibility is that memory consolidation requires multiple, coupled SPW‐Rs (Davidson et al., 2009; Wu and Foster, 2014), and the needed SPW‐R clusters were prevented by the light stimulus.
Since SPW‐Rs are suppressed by subcortical neuromodulators (see Pharmacological Control of SPW‐R section), another mechanism by which memory consolidation can be affected is to manipulate one of the several subcortical nuclei. Wang et al. (2015) observed that the firing rates of both putative serotoninergic and glutamatergic neurons in the medial raphe were decreased before the occurrence of SPW‐Rs. Optogenetic activation of median raphe neurons suppressed SPW‐Rs. Importantly, the same optogenetic manipulation administered after fear conditioning disrupted the acquisition of the conditioned fear response.
In addition to aborting and abolishing SPW‐Rs, partial functional “deafferentation” of the CA1 region can also affect learning and memory consolidation. Nakashiba et al. (2009) recorded SPW‐R activity in the CA3‐TeTX transgenic mouse, in which CA3 output can be specifically and inducibly controlled. As a result of transient removal of the CA3 input to CA1, normal ripples disappeared and, instead, large “mutant ripples” (or epsilon bursts) at ∼110 Hz emerged. The memory impairment they observed could reflect either the absence of normal communication between CA3 and CA1 regions (loss of function) or the abnormal CA1 output that was broadcasted to cortical regions.
In addition to loss‐of‐function experiments, gain‐of‐function approach also supports the role of SPW‐Rs in learning. Maingret et al. (2013) hypothesized that enhancement of the functional link between hippocampal SPW‐Rs and neocortical slow oscillations and sleep spindles, the three candidate biomarkers of memory consolidation, can increase memory performance. Using online detection of SPW‐Rs, they applied single electrical pulses to the prefrontal cortical networks to induce/reset DOWN states of slow oscillation and sleep spindles during POST‐learning sleep. During training, rats were exposed to two identical objects located in two adjacent corners of a familiar arena (encoding phase) for a short time. In the recall phase 24 h later, one of the objects was displaced to the opposite corner, and the animals were allowed to explore the arena for 5 min. Due to the short exposure to the task, unstimulated and stimulated control rats performed at chance level. However, experimental rats with closed‐loop coupling between SPW‐Rs and neocortical slow oscillations/spindles preferentially explored the displaced object, demonstrating that the hippocampal‐neocortical coupling during SPW‐Rs can enhance consolidation of the weak memory traces.
Overall, the selective SPW‐R‐manipulation experiments provide strong support for a critical role of SPW‐Rs in consolidating hippocampus‐dependent memories and assisting the modification of place cell sequences.
CONSTRUCTIVE ROLE OF SPW‐R
Forward replay of upcoming place cell sequences is an indication for a prospective, constructive role of SPW‐Rs (Diba and Buzsáki, 2007; Johnson and Redish, 2007; Karlsson and Frank, 2009; Davidson et al., 2009; Pfeiffer and Foster, 2013). Davidson et al. (2009) introduced a Bayesian decoding algorithm to estimate posterior probabilities of the rat's position on the track during strong synchronous spiking of the CA1 pyramidal cell population (SPW‐R). Based on posterior probabilities, SPW‐R events were segmented in position and time into trajectory‐specific subregions of the track. The use of a Bayesian method allowed for the inclusion of poorly clustered units as well and the increased number of spatially active units/multiple units improved trajectory reconstruction. Using such a combined approach, Davidson et al. (2009) showed that behavioral sequences spanning long sections of a track (10 m) could be re‐expressed during replay. Consistent with previous observations, they found that the start but not the end locations of the neuronal replay trajectories were strongly biased toward the rat's current location (“local replay”), as if the hippocampal dynamics are triggered by local inputs (Csicsvari et al., 2007) and expanded towards multiple possible directions. However, when the rat stopped on the track, they also observed numerous replay events which started at least 1 m away from the animal's current location, indicating that the replay is not limited to the animal's current location (Fig. 41) (see also Johnson and Redish, 2007; Karlsson and Frank, 2009). Davidson et al. (2009) also demonstrated that the length of replay trajectory is correlated with the duration of the replay event. This relationship implies a characteristic speed of replay (∼8 m/s), which is 10 to 20 faster than the average travel speed by which the rat traverses through the place fields (Nadasdy et al., 1999; Lee and Wilson, 2002). In contrast to the rats’ irregular behavior during exploration, the constant speed of replay may indicate that SPW‐R‐related activity reflects the places visited and sequential behaviors rather than the details of particular locomotor trajectories. Based on the constant‐speed replay and the frequent occurrence of long (>400 ms) sequences, spanning several SPW‐R events, Davidson et al. (2009) hypothesized that a single ripple‐associated sequence is limited to roughly the spatial scale of a typical‐size place field of dorsal hippocampal CA1 pyramidal cells and that extended sequences reflect chains of short subsequences of single SPW‐R events. Plastic chaining of multiple SPW‐Rs for representing long sequences and higher‐order relationships can explain why SPW‐R clusters are dependent on NMDA receptors (see Pharmacological Control of sPW‐R section). In many of these studies, the spike sequences often began earlier and lasted longer than the duration of the LFP ripple (Fig. 46). This “discrepancy” between LFP and spiking may arise from two sources. First, the LFP ripple largely reflects synchronized spikes of pyramidal neurons (Schomburg et al., 2012) and it builds up gradually. Thus, the few “initiator” neuron(s) might not generate sufficiently large extracellular currents to be detected as LFP. The second and related cause is that SPW‐Rs travel and their magnitude can vary substantially at any given location (Patel et al., 2013). Thus, the sequential firing of neurons may correspond to sequential recruitment of neurons in anatomical space but not with the same magnitude of neuronal involvement. Thus, the temporally longer neuronal sequences relative to the LFP ripple can be explained by simple physiological mechanisms that underly LFP generation (Buzsáki et al., 2012).
Figure 46.
Construction of novel shortcuts by SPW‐Rs. Examples of trajectories never directly experienced by the rat. In the bottom panels, spikes are plotted by ordered place field center for both left and right loops over the same 0.5 s period. The gray vertical lines mark the beginning and end of the shortcut sequence and capture the exact same period of time on both left and right loop raster plots (as can also be seen in the repeated LFP trace). Diamond, position of the rat during SPW‐R replay. Color coding represents the trajectory of the animal. Spikes plotted on the 2D maze (top panels) to visualize the shortcut trajectories spanning the top of the maze. Note that the “shotcut” replays are not smooth but are potentially composed of an initial reverse replay of one segment followed by a forward replay of another segment of the maze (arrows). Reproduced from Gupta et al. (2010).
The study of Gupta et al. (2010) compliments the above studies. These investigators trained rats to run two distinct sequences (A and B) on a large track. They observed both forward and backward replays of B trajectories even when rats had been performing A for >10 min, challenging the idea that sequence activation during SPW‐Rs is a simple replay of recent experience. Moreover, while the animals ran the maze in one direction only, forward and reverse replay events were observed with relatively similar proportion. Gupta et al. (2010) interpreted their findings by suggesting that the critical role of forward and reverse sequences is the maintenance of the cognitive map and allowing for flexible and goal‐driven behaviors. They also reported rare occurrence of SPW‐R constructed trajectories in the maze that have never been experienced by the rat (Fig. 46). These “invented” trajectories began close to the animal's location on one side of the maze and pointed to the reward location on the opposite side. An alternative interpretation of “shotcuts” of never crossed paths is that the shortcut representations are in fact not single smooth trajectories but are constructed from the concatenation of two sequences, the first of which is a reverse sequence of a visited segment linked to a forward sequence of another visited segment (see also Wu and Foster, 2014).
In the above studies, SPW‐R replay was probed by a template of place cell sequences on a linear track. These experiments therefore could not address the question whether SPW‐R sequences reflected the rat's choice among alternatives. This question was examined by Singer et al. (2013). In their task, the neuronal trajectories reactivated during SPW‐Rs preceding correct trials were biased toward representing sequences that proceeded away from the animal's current location. Correct trials were generally preceded by multiple SPW‐Rs, and spike patterns represented in these events often included both the upcoming correct outer arm of the maze as well as the other, incorrect, outer arm. The coactivation probability of neuron pairs during SPW‐Rs was stronger than would be expected from the activity of the individual place cells and what was observed during SPW‐Rs preceding incorrect trials. While this study is indicative of the choice‐specific spike content of SPW‐Rs, it remains to be specifically demonstrated that spike sequences during SPW‐Rs predict the place cell sequences of the chosen arm.
Spatial navigation and planning require not only recalling previous memories but also evaluating alternative scenarios and calculating optimal choices. Forward replay is compatible with such requirements but previous studies did not convincingly demonstrate the crucial role of forward replay in navigational planning. To this end, Pfeiffer and Foster (2013) recorded from rats in a foraging task while the animals were exploring a large open arena with numerous wells. In the first phase of the task, food was hidden in a randomly changing well, whereas in the second phase in the same well. Therefore, the investigators could examine neuronal correlates of both memory for goal location and flexible planning of a novel route to get there. Neuronal sequences were identified in population bursts (SPW‐Rs) during periods of momentary immobility while the rat performed the task. The Bayesian decoding algorithm applied to the SPW‐Rs revealed temporally compressed, two‐dimensional “trajectory events” across the environment. Before goal‐directed motor ambulation, most brief events encoded travel trajectories of the animals from the rat's current position to several possible directions but most often leading to the goal location. Thus, in this two‐dimensional exploration task neuronal trajectories matched the future path more often than the past path. Overall, the findings demonstrate that hippocampal SPW‐R‐associated neuronal sequences predict immediate future navigational behavior, even in cases in which the combination of start and goal locations is novel. These experiments using a two‐dimensional navigation task therefore extend previous observations on linear tracks and provide strong support for the involvement of hippocampal SPW‐Rs not only in memory consolidation and reporting past experiences but also in planning future actions.
Various aspects of the above‐described experiments are synthesized by the work of Wu and Foster (2014) (Fig. 47). Hippocampal activity was recorded while rats navigated in a Y maze with arms separated by 120°. One short arm was chosen to be the central arm; the other two arms were the “alternating arms.” One of the alternating arms was twice as long (145 cm) as the others. Baits were available at the ends of the arms. Given that there were separate arms that could be joined for longer trajectories on a given run, the investigators examined how the sequential activation of neurons during SPW‐Rs reconstructed the travel trajectories of the animal. As expected from previous experiments, the firing rates and place fields of the majority of neurons in a given arm were identical, irrespective of the left or right choice after the bifurcation point. The majority of SPW‐R sequence replays spun two segments (“joint replays”; e.g., central—left arm, central—right arm; right arm—left arm) and started from the current position of the animal. However, sequence replay events could be clearly segmented into subsequences during distinct SPW‐Rs, corresponding to the route the rat chose, by switching the direction of replay (as shown in Fig. 47), suggesting that SPW‐Rs can “stitch together” fragments to represent joined parts of the environment. Furthermore, a distinct organizational pattern was observed so that first segments of joint replays tended to be reverse, while forward replays dominated the second segments of joint replays. Overall, the findings of Wu and Foster (2014) imply that reverse replay represents a recapitulation of the immediate past trajectory, and forward replay reflects the exploration of alternative options, supporting the hypothesis that forward replays serve planning future or imagined behavioral routes (Diba and Buzsáki, 2007; Davidson et al., 2009; Karlsson and Frank, 2009; Gupta et al., 2010; Pfeiffer and Foster, 2013). Each SPW‐R can be conceptualized as an attractor and each attractor corresponds to a single location. It has been shown recently that the representation of the location during SPW‐Rs can sharpen over the course of several milliseconds and the reactivation can transition rapidly from immobility at one location to another spatially discontiguous location (Pfeiffer and Foster, 2015). The transitions may be discretized by the CA3‐driven slow‐gamma (25 to 50 Hz) rhythm (Carr et al., 2012; Pfeiffer and Foster, 2015).
Figure 47.
Joint replay directionality. (A) The junction of the three arms (C, central; R, right; L, left) is the choice point. Running toward the choice point is “inbound” (In) and running away is “outbound” (Out). (B–E) Examples of joint replay sequences with different combinations of directionalities from a representative rat. Horizontal dashed lines indicate arm boundaries. Black diamond shapes mark the location of the rat when each replay occurred. The color scale is set so that maximally saturated colors correspond to the highest position probability of each replay. (B) A consistent reverse replay of CR. Below (E), raw LFP recording from one selected tetrode channel. Note the presence of double ripple, each corresponding to the replay of one arm segment (C and R). Reproduced from Wu and Foster (2014).
Preplay of Unvisited Routes During SPW‐R
A consistent observation is that during SPW‐Rs many more neurons fire and many more neuronal sequences are present than can be accounted for by the sequentially active neurons on linear tracks or two‐dimensional open field environments. One may consider that the majority of the patterns present during SPW‐Rs are simply noise (Lubenov and Siapas, 2008). However, as the number of simultaneously recorded neurons increases with the steady improvement of recording technologies, more and more ‘noisy’ action potentials can be accounted for by matching them to sequences. An alternative view is that the hippocampus can a priori generate numerous self‐organized sequences and that over the lifetime of the animal, many of these events “match” with accumulating discrete experiences, while the remaining ones are available for future experiences (Buzsáki, 2006, 2010; Dragoi and Tonegawa, 2014). In line with such reasoning, neuronal sequences that predict the place cell sequences on never crossed paths or in novel environments have been detected during both waking (Gupta et al., 2010) and sleep (Dragoi and Tonegawa, 2011) before travels of novel routes. Dragoi and Tonegawa (2011) recorded ensembles of CA1 pyramidal cells in mice during sleep/rest sessions in a sleep box after they repeatedly explored a familiar L‐shaped track but before they were allowed to explore a novel arm that was linked to the L track (Fig. 48). During SPW‐Rs, a small fraction (15%) of sequential neuronal firing events (trajectories) corresponded to place cell sequences in the familiar L track, as expected. Unexpectedly, a small fraction (∼10%) of neuronal sequences during SPW‐Rs, consisting of a mix of previously active place cells and silent cells, correlated significantly with future firing order of place cells on a novel linear track. The authors termed the predictive temporal sequences that preceded the matching place cell sequences on the novel track “preplay.” Preplay sequences occurred more frequently when the mouse was resting at spatial locations adjacent to the novel track compared with the more remote locations, indicating that in the awake resting state, the external cues from the environment may influence, but not completely determine, selection of neurons and their sequential firing on not‐yet‐explored tracks. Consistent with this interpretation, Ólafsdóttir et al., (2015) reported that viewing the delivery of food to an unvisited portion of an environment was necessary and sufficient for pre‐activation of place cell sequences corresponding to that space. Such ‘preplay’ was not observed for an unrewarded but otherwise similar portion of the environment. Such goal‐biased preplay may support preparation for future experiences in novel environments.
Figure 48.
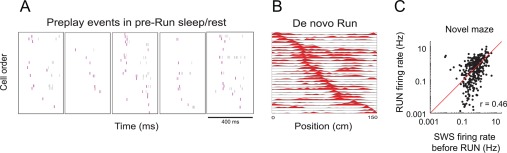
Prelay of neuronal spike sequences. (A) Examples of forward preplay of a future novel place cell sequence using template matching method. (B) Sequential firing of place cells on the novel track (C) Comparison between firing rates during exploration of a novel maze (RUN) and SWS in the home cage either before or after the maze session. (A, B) reproduced after Dragoi and Tonegawa (2011). (C), reproduced from Mizuseki and Buzsáki (2013).
SPW‐R‐related preplay of future sequential activity of neuronal firing in novel environment was also demonstrated in the rat. In this experiment, Dragoi and Tonegawa (2013) allowed naïve rats to explore three contiguous novel linear tracks that were each 1.5 m long and attached in a U shape. After first exploring track 1, they had access to each of the other parallel tracks (tracks 2 and 3). A unique sequence of place cells was detected on each of the three tracks, which could be used as templates for searching for matching temporal sequences during sleep recorded several hours earlier. Although many individual neurons were active on more than one track in at least one direction of travel, the place cell sequences were distinct on the three tracks and these sequences had significant preplay matching sequences (both forward and reverse) during SPW‐R in the PRE rest/sleep session. The authors conclude that the basic unit that specifically represents different novel spatial experiences is the sequence of place cell firing rather than the identity of individual cells.
Previous experiments have already reported reliable correlations of both firing rates and pairwise correlations between PRE and maze RUN (Wilson and McNaughton, 1994; Kudrimoti et al., 1999), although in those experiments the PRE correlations could have been interpreted as persisting changes of the synapses from previous experience in a familiar environment. Kudrimoti et al. (1999) also examined the relationship between pairwise correlation during PRE and RUN on novel tracks of the maze. While they found significant correlations for coactivation of neuron pairs in half of their PRE vs. RUN data sets when the rat ran on the familiar tracks of the maze, no significant correlations was observed in any of their six PRE vs. RUN data sets when the rat was allowed to explore the previously unvisited parts of the maze. One possible reason for this difference is that while Kudrimoti et al. (1999) used pairwise comparisons of neuron coativations, Dragoi and Tonegawa (2011, 2013) used template matching and Bayesian decoding methods. In support of the idea of preexisting cell assemblies (Samsonovich and McNaughton, 1997; McNaughton et al., 2006; Dragoi and Tonegawa, 2015), Hirase et al. (2001a) also found a significant correlation of both firing rates and pairwise co‐activations between PRE sleep and RUN in a novel testing apparatus. Although preplay of future place cell sequences is surprising from a tabula rasa framework (Dragoi and Tonegawa, 2015), it may be considered as a natural consequence of the log‐dynamic organization of hippocampal networks (Fig. 48) (see Behavioral Correlates and Mechanisms of SPW Generation section; Buzsáki and Mizuseki, 2014; Buzsáki, 2015).
Is PRE Versus POST Sleep Comparison Reliable for the Assessment of learning‐Related Sequence Replay During SPW‐R?
Assuming a tabula rasa brain, the typical RUN‐POST correlations, normalized by the PRE sleep condition should provide a reliable index of learning‐induced experience (Dragoi and Tonegawa, 2015). However, several recently uncovered findings make this approach less than ideal. The first “complication” is the strongly skewed, lognormally distributed nature of synaptic weights, firing rates and spike bursts of hippocampal pyramidal neurons. Log‐firing rates and bursts are strongly correlated across brain states and environmental situations (Mizuseki and Buzsáki, 2013). Comparison of PRE sleep and subsequent waking firing patterns (rate and bursts) in a completely novel environment provides significant statistical correlations on a log scale. Highly active neurons form an active partnership and their relative excitability relationships can “drive” sequential activity, potentially assisted by their effectively driven interneurons. This “preformed” relationship may be a reason for the experimentally observed “preplay” phenomenon. A second complication is that firing rate distributions after waking experience shift to the right (Vyazovskiy et al., 2008; Kudrimoti et al., 1999; Grosmark et al., 2012). It is not known though whether such change affects the high firing minority or slow firing majority of neurons but increased rates should impact coactivations of neuron pairs and the probability of neuronal sequences (de la Rocha et al., 2007). A third problem is that in most replay experiments only short PRE and POST sleep epochs are recorded. The consequence of this practice is that the comparison is often made between the end of a sleep epoch (or an unknown part of it) during PRE and the beginning of sleep or just simple rest during POST sleep. This is problematic since large changes in both rates and co‐firing take place during the course of sleep, even within just a single non‐REM epoch and such changes may differ during SPW‐Rs and in the inter‐SPW‐R periods (Grosmark et al., 2012). As a result, PRE‐POST sleep changes may reflect not only experience‐induced processes but also homeostatic changes during sleep. The final complication is that the PRE vs. POST changes are often evaluated at the population level. This practice is based on the assumption that learning affects large assemblies of neurons and their synapses and such changes are distributed relatively equally across a homogenous population. However, the fraction of the affected neurons is not quantified and it remains unknown whether learning‐related changes occur largely in neurons at the left or right ends of the distributions. To address the above issues, novel methods are needed that allow the characterization of each neuron's true contribution to experience‐induced effects on SPW‐R‐related population activity.
A seemingly more direct way of exploring the experience‐dependent changes of the affected neurons and avoiding the statistical problems is to compare the physiological properties of active and non‐participant neurons after the learning process. In an elegantly performed study, Mizunuma et al. (2014) recorded from SPW‐R‐associated neurons in ex‐in vivo slice preparation after the animal's previous experience. Using the promoter for the immediate‐early gene Arc, they probed active neurons in vivo with dVenus, a modified yellow fluorescent protein, in Arc‐dVenus transgenic mice (Eguchi and Yamaguchi, 2009). dVenus expression was correlated with the magnitude of behavioral experience of the mice, which showed a four to eightfold increase after exploration in cages less‐enriched and enriched with objects, respectively, relative to home‐cage baseline controls. dVenus‐expressing neurons that had been activated while mice explored the enriched cages were preferentially reactivated during spontaneous SPW‐Rs in subsequent hippocampal slice experiment in vitro. Importantly, the EPSC‐to‐IPSC ratios of the mean charges were significantly higher in SPW‐R‐participating dVenus‐expressing neurons than those of unlabeled neurons firing at low rates outside SPW‐Rs. These observations are compatible with the hypothesis that waking active and SPW‐participating neurons are more excitable. However, whether the responsible synapses were enhanced by behavioral exploration or pre‐existed before exploration has yet to be elucidated (Mizuseki and Buzsáki, 2013; Mizunuma et al., 2014).
RETROSPECTIVE, PROSPECTIVE, CONTRUCTIVE AND MAINTENANCE ROLES OF SPW‐Rs—A NEW SYNTHESIS
The super‐synchronous nature and the large impact on the rest of the brain make it difficult to dismiss SPW‐Rs as a simple epiphenomenon of hippocampal dynamics. SPW‐Rs occur in the right behavioral context to perform computation that is beneficial for maintaining and selectively modifying relevant brain circuits. What makes SPW‐Rs attractive for cognitive functions is their spike content: the orderly neuronal sequences are strongly related to the sequential activity of the same neurons during waking performance (SPW‐R‐Supported Memory Consolidation section). Hippocampus‐dependent, rapid and often single trial learning (Morris, 2001) may occur because SPW‐R‐related sequence reactivations in the hippocampal and neocortical circuits are repeated from hundreds to thousands of times after the initial experience has already vanished. This function of SPW‐Rs alone may justify their importance. Yet, more recent experiments demonstrate that the spike content of SPW‐Rs does not always reflect copies of past experience. For example, when the rat sits in the home cage or the home base of a training apparatus, spike sequences often correspond to cell assemblies that were active in a different environment. Many more sequences occur in SPW‐Rs than could be accounted for by the forward or reverse sequences of experienced place cells or episode cells. SPW‐R sequences that predict the place cell sequences on not‐yet experienced tracks are often present during both waking and sleep before traversing the novel routes. Such SPW‐R‐generated novel combinations of neuronal paths indicate that SPW‐Rs may also play a constructive role (see Constructive Role of SPW‐R section). The constructive or anterograde role of SPW‐Rs is also expected from the early presence of SPWs in ontogenetic development (see Development of Sharp Waves and Ripples section), before the emergence of hippocampal place cells and entorhinal grid cells (Wills et al., 2010; Langston et al., 2010). The special ability of the hippocampus to construct multiple possible routes, shortcuts and detours is served by the extraordinarily large graph organization of the CA3 recurrent collateral system (see Fig. 8). Once the main landmarks of an environment are mapped onto neuronal assembly representations, all possible combinations (routes) can be computed with ease (Muller et al., 1996). It has been postulated that SPW‐Rs are exploited for this purpose (Samsonovich and Ascoli, 2005) and possibly the same mechanisms can be used to generate novel solutions to non‐navigational problems (Buzsáki, 2005; Buzsáki and Moser, 2013).
Reminiscence, remembering, recollection, recall, recapitulation, retrieval and replay are often used as synonyms and refer to mechanisms that allow for accessing memories from long‐term stores. Since fragments of neuronal sequences of waking experience are replayed in a compressed format during SPW‐Rs, it is tempting to conclude that SPW‐Rs, at least in the waking brain, are an appropriate conduit for memory recall (Carr et al., 2011; Roumis and Frank, 2015). However, because recall of declarative memories is defined as an active, autonoetic conscious process (Tulving, 2002) and mental travel into the past proceeds in real time, that is at a similar speed as the experience of the event itself (Suddendorf and Corballis, 2007), the time‐compressed sequences during SPW‐Rs may not well serve conscious recall. Furthermore, remembering requires an attentive, conscious state (Tulving, 2002) and presumably deploys theta mechanisms, especially since “conscious experience” in humans has a minimum duration requirement for a sustained attentive state (0.5 s “mind time”; Libet, 2005) and activation of widespread brain areas (Dehaene and Changeux, 2011; Tononi 2012). On the other hand, there are multiple unexplained subconscious aspects of memory recall in which SPW‐Rs may play an important role, as discussed in the “Subconscious Priming” of Recall, Planning, and Creative Thoughts by SPW‐Rs section below.
Generating and Reading SPW‐R Sequences
The biological “meaning” of a sequential pattern translates to the utilization of that pattern by downstream reader neurons (Buzsáki, 2010). Therefore, “the information” embedded in the spike sequences does not lie in the rich variety of SPW‐R sequences constantly produced by the hippocampus but is determined solely by the ability of hippocampal targets to differentiate among those sequences and utilize them for guiding behavior. This sender‐reader relationship may explain why the neocortex‐to‐hippocampus volume ratio increased several folds from rodent to primates (Amaral and Lavenex, 2007). Such disproportional scaling may occur because the large CA3 recurrent system even of a small size hippocampus in the rodent is capable of generating an extraordinarily large repertoire of sequences. However, these events become meaningful only if they are effectively segregated and utilized by downstream reader mechanisms. Thus, the usefulness of the preexisting rich repertoire of hippocampal events is determined largely by the expanse of neocortical “readers” because it is the experience of the organism that matches preexisting events with appropriate behavioral outcomes.
The large repertoire of firing sequence events (trajectories) can emerge even in the absence of experience. This hypothesis is supported by the observation that large variations of events and lengths are also evident in the transplanted fetal hippocampus with no or very little communication with the host brain (Buzsáki et al., 1987a, 1987b, 1987c; 1989a). Presumably, such variable population events arise from the internal dynamics of the isolated hippocampal tissue rather than from cumulative, experience‐dictated buildup of diversification. Furthermore, SPWs are the earliest organized event in the developing hippocampus (see Development of Sharp Waves and Ripples section) and can generate neuronal sequences before any explorative experience. In the adult hippocampus, many more sequences occur during SPW‐Rs than can be accounted for by the forward or reverse sequences of experienced place cells (Lubenov and Siapas, 2008; Gupta et al., 2010). The physiological‐anatomical foundation of such rich repertoires may be the consequence of the strongly skewed distribution of multiple features of hippocampal organization. Distributions of synaptic weights among pyramidal cells and between pyramidal cells and interneurons, firing rates, bursting probability and the fraction of SPW‐R‐active neurons typically span several orders of magnitude and follow a lognormal form (Mizuseki and Buzsáki, 2013; Buzsáki and Mizuseki, 2014). These features, in turn, may relate to the strongly skewed distribution of information content of spike rates of place cells, place field size, the number of place fields, the fraction of neurons active in increasing size environments and multiple rooms and our skewed perception of space (Mizuseki et al., 2012; Alme et al., 2014; Rich et al., 2014; Buzsáki, 2015). The skewed nature of synaptic weight distributions and the large differences in the biophysical properties of neurons at the tails of the distribution imply that the “replay” sequences are largely constrained by the internal dynamics of the hippocampus. While the majority of CA1 pyramidal cells participate in less than 10% of SPW‐Rs, a small minority fires in half of SPW‐R events, generating mostly bursts of spikes (Mizuseki and Buzsáki, 2013). Furthermore, optogenetic activation of CA1 pyramidal neurons can generate local ripple events (see Mechanisms of Ripple Generation section) and, importantly, the discharge probability and to some extent the ordered sequences of the participating pyramidal cells and interneurons in such synthetic events are correlated with “native” sequences present during both spontaneously occurring SPW‐Rs and theta oscillations (Stark et al., 2015) (Fig. 49). Importantly, both forward and reverse sequences can be induced by optogenetic stimulation of CA1 pyramidal neurons, indicating that perhaps the spiking history in the local network and/or the specific initiating inputs addressing a particular set of CA1 neurons is critical in determining the direction of activity flow and the sequence generation is facilitated by local processes. These findings indicate that CA1 neurons are not strictly driven spike‐by‐spike by their upstream CA3 partners but their participation in SPW‐Rs is also influenced by their intrinsic properties and local circuit constraints. Importantly, the highly active minority does not necessarily represent recently activated neurons by immediate experience. Instead, these “generalizer” (Mizuseki and Buzsáki, 2013) or “chorister” (Okun et al., 2015) neurons may be part of stable subnetworks and can serve to chain multiple SPW‐R events and generalize across situations. The firing dynamics that arise from the skewed distribution of synaptic weights and firing rates and the a priori self‐organized sequences that emerge from such skewed dynamics might explain SPW‐R preplay of place cell sequences in not‐yet‐visited places (see Constructive Role of SPW‐R section; Dragoi and Tonegawa, 2011, 2013, 2015). In short, self‐organized sequential activation can emerge at multiple correlated time scales and can be considered as a ‘default’ circuit mechanism in cortical circuits.
Figure 49.
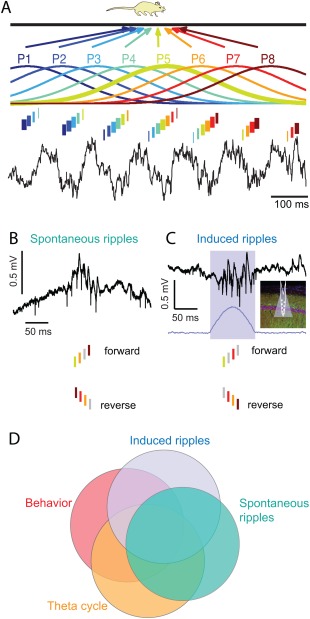
Temporal correlation of spike sequences at multiple time scales. (A) Gaussians indicate idealized, smoothed sequences of place fields of CA1 place cells P1 to P8 on the track. Ticks within theta cycles represent spikes. The width of the bars indicates firing intensity. Theta timescale temporal differences related to their respective distance representations. While the rat moves left to right, place fields (P1 to P8) shift together in time and sustain a temporal order relationship with each other so that the place cell that fires on the earliest phase represents a place field whose center the animal traverses first. By this temporal compression mechanism distances are translated into time. Reproduced from Dragoi and Buzsáki (2006). (B) During immobility periods at the beginning or the end of the track, place cell sequences are also replayed during SPW‐Rs in a forward or reverse manner, respectively. (C) Optogenetically induced ripples also generate organized firing sequences whose temporal order correlates with the order of firing during both SPW‐Rs and theta waves. Reproduced from Stark et al. (2015). (D) Venn diagram indicating partial correlations across various conditions. Part of the correlations may emerge by local mechanisms while other parts can be inherited from upstream (e.g., CA3) regions. Figure courtesy of Lisa Roux.
An often‐cited problem of incorporating new knowledge into memory networks is “catastrophic interference,” that is, the forgetting or corrupting of previously learned information upon learning new information (McClelland et al., 1995). In a preconfigured network with self‐generated multitudes of sequences, interference is much less of a problem since most sequences are constructed from preexisting neural word sequences in an already balanced system. When new episodic information enters the hippocampal networks, e.g., after visiting a novel environment, the ensuing episodes may select from the existing repertoire of sequences and preexisting maps (Samsonovich and McNaughton, 1997; Dragoi and Tonegawa, 2015) to gain “meaning,” rather than synthesize new events de novo. Under the hypothesis of largely preconfigured neuronal sequences, learning is a synthesis of a matching process between preexisting neuronal events and their abstractions (“schemas”; Tse et al., 2011). Therefore, accumulating discrete experiences may only modestly affect network dynamics.
Preexisting Knowledge and Novel Experience are Reflected by the Spike Content of SPW‐R
Based on observations in the adult brain, the correlation between neuronal activity in the behaving and sleeping animal can be interpreted by assuming that it is the waking experience that modifies synaptic connections and such changes are inevitably re‐expressed during sleep due to the dynamical interactions among neuronal populations. Thus, sleep patterns provide a stable and reliable readout of the waking‐induced alterations of neuronal activity because sleep‐related neuronal activity is driven exclusively by the internal dynamics set by synaptic weights and firing patterns. From this perspective, the number of replay events during SPW‐Rs should be related to the number of episodes we have experienced in our lifetime if all SPW‐R sequences are brought about learning. This experience‐dependent Aristotelian tabula rasa view has influenced thinking in Christian and Persian philosophy, British empiricism, the Marxist doctrine and it has become the leading thought in cognitive and social sciences (Popper, 1959).
But is it really true that the number of SPW‐R‐related neuronal sequences scales with the amount of experience of the individual? The first organized population event in the hippocampus is a SPW burst before any spatial experience and numerous variations of SPWs with presumably different spike contents occur in the newborn rodent (see Development of Sharp Waves and Ripples section). It remains to be demonstrated whether the spike contents of early SPWs are stereotypical, random or adult‐like with multitudes of sequences. It is also unknown whether spike sequences are more diverse in animals raised in enriched environments compared with those in impoverished environments. While it is unquestionable that experience does exert an impact on brain circuits, the exact mechanisms are not understood. Given the present uncertainty about the origin of self‐organized neuronal sequences, one can take an entirely different approach from the tabula rasa view by assuming that the hippocampus can generate very large numbers of sequences even in an inexperienced brain. The internally formed sequences can be viewed as a preconfigured vocabulary from which very large sets of complex events can be generated by combinatorial linking of the vocabulary elements. Such internally generated SPW‐R events, therefore, may bias the likelihood of firing probability and their ordered patterns in the waking state. In turn, in any given awake situation, the evolving patterns of neuronal firing reflect the corresponding most likely state of the hippocampal network, which can be regarded as the brain's “best guess.” If the played out sequential pattern in a novel situation consistently leads to the same shift from preparatory to terminal behavior (e.g., due to reinforcement), it may lead to an increased probability of re‐occurrence of that event during both theta oscillation and SPW‐R replays. From this perspective, a large part of learning is a “matching process,” rather than a de novo synthesis, utilizing pre‐existing elements of neuronal vocabulary (Tsodyks et al., 1999; Kenet et al., 2003; Buzsáki, 2006, 2010; Luczak et al., 2009; Dragoi and Tonegawa, 2015). In this process, one of the many possible events acquires “meaning” from a behavioral point of view and thus become to “represent” a particular constellation of the environment or a relationship. In turn, this selected trajectory is refined further by newly recruited, typically low firing and plastic pyramidal cells.
The multiple ripple cycles of a SPW‐R event largely correspond a sequential series of cell assemblies, each of which can be conceptualized as a “letter” of the hypothetical neuronal vocabulary, and the multiple cycles form neuronal “words.” The word content of SPW‐Rs then can be chained together to represent “sentences” or extended sequences of discrete segments of travel paths or events. From a coding perspective, the SPW to ripple relationship is analogous to the assembly‐sequence relationship of theta‐nested gamma waves (Lisman and Idiart, 1995; Buzsáki, 2010). Recent experiments provide support for a vocabulary‐based construction role of SPW‐Rs (SPW‐R‐Supported Memory Consolidation section). For example, SPW‐R events in rats exhibit a characteristic substructure that maps onto the maze topology. Individual SPW‐R sequences largely correspond to discrete arms of the maze. The rat's choice of an arm is predicted by chained SPW‐Rs representing the current and chosen arm representations (Wu and Foster, 2014). Furthermore, the length of the visited or to‐be‐visited arm correlates with the duration of the SPW‐R replay sequence, suggesting a constant‐speed replay (Davidson et al., 2009). Such constant‐speed replay in humans may explain the correlation between distance traveled mentally across an imagined map and response times (Kosslyn et al., 1978). The relationship between replay length and multiple differing aspects of the environment may explain the strongly skewed distribution of SPW‐R duration, especially when the content of adjacent SPW‐R events is fused into a single long replay sequence. Yet, even within such continuously appearing sequences, the discrete maze arm representations can be deciphered from the switch between reverse and forward sequences of adjacent arms (Wu and Foster, 2014), supporting the largely discrete “word” representation nature of SPW‐Rs. Importantly, linkage between arm representations can emerge during the first travel through maze and may reflect pre‐existence of preferred chains, as exemplified by the SPW‐R preplay of never visited places (Dragoi and Tonegawa, 2011, 2013). The implication of these observations and conjectures is that place assembly sequences coding for distances are also quantal, conforming limited lengths of neural words. Overall, the currently available findings support a “chunking” or parsing role of SPW‐Rs, which, combined with chaining of multiple SPW‐R contents, allows the flexible generation of a large number of combinations from a finite number of preformed neuronal words (Buzsáki, 2010).
How does the hypothetical neuronal vocabulary arise and what is the relationship between the hypothesis of pattern matching by experience and the traditional view of experience‐constructed sequences? Recent experiments performed in novel environments offer some clues (A. Grosmark and Buzsáki. Replay of familiar and novel aspects of experience by subpopulation of hippocampal neurons during sleep. Society for Neuroscience Meeting. Chicago, 2015). These experiments show that only a small fraction of mainly slow firing neurons is affected by novel experience. During the initial exposure, a minority of fast firing neurons instantaneously forms a sequence. These neurons have low place specificity, multiple place fields, their firing rates and sequential activation patterns during SPW‐Rs are preserved between PRE and POST sleep conditions and are less plastic (Dragoi et al., 2003). This fast firing “rigid” minority represents the generalized familiar aspects of novel environments from previous experiences (Buzsáki, 2015). In support of this hypothesis, fast firing neurons have multiple place fields, fire in multiple corridors of a maze (Mizuseki et al., 2012; Rich et al., 2014), are active in multiple rooms (Alme et al., 2014) and project axons to multiple targets (Ciocchi et al., 2015). Furthermore, they preserve their firing patters from PRE to POST experience sleep. In contrast, members of the slow firing majority have stronger plastic properties (Dragoi et al., 2003) and lend themselves to modifications in novel situations. This subset gains place specificity over multiple trials during maze exploration, shifts its relative position within the lognormal distribution of the population from PRE to POST sleep and increases its association with SPW‐Rs and bursting ability during POST‐experience sleep. Thus, one can speculate that the physiological correlates of learning and experience reflect a combination of a rapid matching process between the familiar aspects of the environment based on a preexisting dynamics of a fast firing minority group of neurons and a gradual refinement of the firing patterns of a subgroup drawn from the slow firing larger reserve to the novel aspects of the situation. Under this hypothesis, when an animal is exposed to a novel situation, a crude place sequence (a “protomap”) is instantaneously induced from a preexisting minority of fast firing neurons, which reflects the brain's best guess about the newly faced situation. With further exploration, plastic neurons from the large reserve of the slow firing majority are added to the protomap. This learning‐induced refinement, in turn, is reflected by the increased SPW‐R‐membership of the newly recruited small subset of neurons (Grosmark and Buzsaki, 2015).
Prospective Coding by SPW‐R Assembly Sequences
The future begins in the past. This truism is amply supported by recent findings in several laboratories, which indicate that brain structures that have been traditionally viewed as part of a memory system are inseparable parts of planning, imagining, and decision making systems (Eichenbaum, 2004; Buckner and Carroll, 2007; Schacter and Addis, 2007; Lisman and Redish, 2009). Buckner (2010) suggests that a fundamental role of the hippocampus and allied structures is to facilitate predictions about the future: “the capture of associations that define event sequences is adaptive because these sequences can be reassembled into novel combinations that anticipate and simulate future events” and that “the functional role of the hippocampus is nonetheless best understood from an adaptive, forward‐oriented perspective.” Planning for the future and making right decisions must rely heavily on the brain's ability to manipulate stored representations of past experiences (Cohen and Eichenbaum, 1993).
The sequential organization of theta‐nested cell assemblies (Lisman and Idiart, 1995) is a prime example of how retrospective and prospective information is brought together at a physiological scale. As the rat traverses the maze, evolving unique combinations of hippocampal pyramidal cells are active in successive theta cycles. The most active group at the trough of the theta cycle defines the current location, flanked by spikes of other neurons on the descending and ascending phases of theta, representing past and future locations, respectively (Dragoi and Buzsáki, 2006). By this mechanism, the sequentially ordered firing patterns representing experienced and expected future places by the animal during navigation can be compressed into single theta cycles. In effect, every theta cycle is a quick sweep through space and time beginning with position representations behind the animal (“look back”) and ending with representations of possible paths ahead of the animal (“look ahead”; Wood et al., 2000; Ferbinteanu and Shapiro, 2003; Dragoi and Buzsáki, 2006; Johnson and Redish, 2007; Wikenheiser and Redish, 2015). The theta compression mechanism thus binds a current item in the context of the past and future representations (Wood et al., 2000; Ferbinteanu and Shapiro, 2003; Buzsáki, 2005; Hasselmo, 2005; Dragoi and Buzsáki, 2006; Foster and Wilson, 2006). At a longer, behavioral time scale, internally organized cell assembly sequences in the hippocampus during the delay period of a memory task can correctly predict the rat's future choice in the maze with high accuracy, including commission errors (Pastalkova et al., 2008), indicating that the action outcome is the consequence of the brain's interpretation of the past experience under recurring similar conditions. These results demonstrate that internally organized hippocampal activity predicts planned future decisions many seconds in advance. Similar prediction mechanisms are at work in the prefrontal (Fujisawa et al., 2008) and parietal (Harvey et al., 2012) cortices.
Experiments in related fields also show that the hippocampus and its partner structures are critical for the conscious exploration of the world (Buckner and Carroll, 2007). Imaging experiments in humans found repeatedly that imagining and planning invariably activate structures previously categorized as parts of the episodic memory system (Buckner and Carroll, 2007; Schacter and Addis, 2007; Buckner, 2010). The meta‐analysis by Svoboda et al. (2006) demonstrates that within the hippocampal‐cortical system the same regions are activated during envisioning the future as remembering autobiographical episodes. Psychiatric patients with memory problems and patients with hippocampal damage suffer not only from the difficulty of recalling past memories but also from their inability to imagine upcoming events and chose from distant options (Tulving, 1983; Hassabis et al., 2007; D'Argembeau et al., 2008). Patients with documented hippocampal amnesia show profound deficits in their ability to flexibly recombine the remnants of past events into useful future actions or combine events that depend on an imagined scene (Hassabis et al., 2007). Overall, there is a consensus that hippocampal amnesia is associated with deficits in the ability to envision future‐oriented actions (cf., Buckner, 2010).
Internally generated sequences in the hippocampus can serve as flexible physiological mechanisms to replay past events and combine them to construct “what if” scenarios and anticipate the possible consequences of alternative actions without actually testing them. The flexible use of the internally generated vocabulary of assembly sequences would allow the brain to create new knowledge not only by interacting with the outside world, but also through “vicarious” (imagined) experience (Buzsáki et al., 2015).
“Subconscious Priming” of Recall, Planning, and Creative Thoughts by SPW‐Rs
The prospective role of SPW‐Rs is amply demonstrated by their preplay of upcoming place sequences and planned routes, foreshadowing future decisions made by the animal (Diba and Buzsáki, 2007; Davidson et al., 2009; Karlsson and Frank 2009; Pfeiffer and Foster, 2013; Yu and Frank, 2015). SPW‐R sequences predicting future actions or problem solutions can be induced by chaining together clusters of SPW‐Rs, where the initiating event(s) begins recalling past information and serves to trigger subsequent sequence(s) representing potential routes of real or mental navigation. In the process, multiple “what if” scenarios can be played out by preconscious computation mediated by SPW‐clusters until an optimum solution pops up.
To a large extent, the SPW‐nested ripple relationship is analogous to theta‐nested gamma waves. Both mechanisms combine assemblies into discrete neural “words” and compress behaviorally relevant longer time scale sequences, such as place field sequences. However, there are also differences between the two mechanisms. Theta compression begins in the past (and behind the back of the animal) and ends with possible paths ahead of the animal, so that the “here and now” can be placed in appropriate context to influence vicarious representation of possible choices. In contrast, SPW representations typically begin with the current position of the animal and move either forward or back in time and space (SPW‐R‐Supported Memory Consolidation section). SPW‐R clusters and theta waves can weave neural words into contiguous and overlapping chunks, respectively. Thus, even though the mechanisms supporting theta oscillations and SPW‐Rs are antagonistic toward each other because they compete largely for the same anatomical resources, the forward and reverse sequences allowed by the SPW‐Rs, together with the strengthening of forward associations during theta oscillations, can explain the relatively flexible yet forwardly biased associations during free or cued recall (Kahana, 1996; Howard et al., 2005).
The conscious process (in humans) is only a small part of brain computation and the larger part goes on subconsciously even in the waking brain. Conscious effort often only sets the start and the end of a goal but the computation needed to bridge the start and end is not monitored consciously. It is possible that the phylogenetically evolved dichotomy between theta and non‐theta brain states forms the neurophysiological foundation of the conscious versus non‐conscious distinction. If the conscious effort needed to retrieve information from long‐term storage to working memory involves theta brain state, what is the role SPW‐Rs? Since the SPW‐R is a search mechanism in the hippocampal system, it may efficiently bias subsequent conscious recall by priming (i.e., potentiating) relevant circuits.
An often‐experienced brain state is the “feeling of knowing” (Hart, 1965), i.e., the subjective confidence that an item is (or is not) in long‐term storage and could be recognized if seen or cued. Even if the searched‐for item is not accessible immediately, it can pop up with some delay without any additional effort or cue. One can hypothesize that the perpetual subconscious search by SPW‐Rs can prime the appropriate assembly sequences needed for facilitating the item to enter into conscious, active memory. SPW‐Rs are particularly suited for this function since they can compress large chunks of information and have the needed strong excitation to bias intrahippocampal connections and downstream reader neurons and circuits.
Intrusive, involuntary thoughts of episodic or semantic information can occur throughout the day but most often before going to sleep (“mind pops”; Gordon, 2013), a state rich with SPW‐Rs. These functions of the mind are beyond conscious control and SPW‐Rs may be the physiological substrate for priming such events. This view is compatible with the idea that a main function of SPW‐Rs is to maintain the “matches” between world events and neuronal sequences (Gupta et al., 2010) and in the process snippets of brain computation breach into consciousness.
In addition to maintaining learned information, SPW‐Rs can also assist in bringing about novel solutions. Creative thoughts oftentimes surface in the subliminal, transition state of drowsiness between waking and sleep. Interviews with creative individuals reveal that such states are often intentionally sought after or created by people in search for novel ideas (Andreasen, 2005). Sleep itself can also serve to incubate and mix previously experienced events and link them into insightful solutions to solve previously faced puzzles (Wagner et al., 2004; Verleger et al., 2013). Both drowsiness and non‐REM sleep are rich in SPW‐Rs and their chained sequence‐generating ability is likely critical for mixing seemingly unrelated past experiences to provide novel solutions to unsolved problems. The hypothesized “creating power” of SPW‐Rs may derive from its ability to flexibly mix recently acquired information with large chunks of pre‐existing knowledge. Alternatively, the new synthesis is a result of mixing novel information conveyed by the SPWs to the recipient, interpreting neocortical targets which hold the pre‐existing knowledge.
While current experiments are compatible with the hypothesis that SPW‐Rs are preconscious precursors of future plans and decisions performed subsequently in the theta state, currently this idea is based solely on correlation data. To provide direct support for the predictive, constructive role of SPW‐Rs, they need to be disrupted or enhanced specifically and confronted with the behavioral consequences of such manipulations. In the experiments of Jadhav et al. (2012), aborting SPW‐Rs in the waking animals resulted in deterioration of cognitive performance and the outcome was attributed to spatial working memory problems. However, choice errors made after the interruption of SPW‐Rs can be also interpreted as an interference of the prospective route‐priming role of SPW‐Rs. In addition to such loss‐of‐function tests, a gain‐of‐function approach would be more convincing to demonstrate that induced synthetic neuronal sequences during SPW‐Rs can exert a precise bias on influencing the animal's subsequent decision in theta brain state. Furthermore, since the spike content of SPW‐Rs can be influenced by neocortical events (see Modulation of SPW‐Rs by Subcortical and Neocortical Inputs section), weak sensory stimuli applied during sleep can be used to bias SPW‐R content (Rasch et al., 2007; Bendor and Wilson, 2012; Ritter et al., 2012) and affect not only consolidation of learned information but also to prompt future decisions, facilitate problem solving and, potentially, induce creative thoughts. Overall, SPW‐Rs that dominate “off‐line” computation of the brain may represent an evolutionally adaptive, vicarious mechanism to optimize subsequent overt behavior by computing favorable outcomes of actions without testing each.
SPW‐R AND SLEEP HOMEOSTASIS
Although the high gain of excitation during SPW‐Rs can be very effective in enhancing synaptic strengths, there must be a compensatory process, which prevents the runaway increase of global excitability during self‐organized population events. When such self‐regulatory mechanisms fail, SPW‐Rs are converted into interictal epileptic spikes (see Modification of SPW‐Rs and Other Forms of Fast Rhythms in Epilepsy section). Unfortunately, these hypothetical regulatory mechanisms are not well understood. According to the two‐stage model of memory consolidation, recently and actively used synapses and neurons are selectively reactivated. If SPW‐Rs differentially increase experience‐related activity patterns, this can occur only at the expense of downregulating the remaining population. Such homeostatic mechanisms may be operating within or outside SPW‐Rs. Under the hypothesis of Hebbian spike timing‐dependent plasticity (STDP; Markram et al., 1997), both potentiation and depression can take place within the time window of SWP‐Rs. Early firing “leader” neurons within the population burst can receive depolarizing inputs from the rest of the SPW‐R‐participating neurons and such single spike‐population burst pairing might enhance the efficacy of inputs which brought about the spike in the leader cells. In contrast, the connections between late firing neurons in the SPW‐R should undergo depression, according to the STDP rule. Lubenov and Siapas (2008) speculate that perhaps the entire SPW‐R‐associated neuronal population undergoes a synaptic weakening process, and SPW‐Rs serve to decorrelate neuronal activity during sleep. This conclusion is based on the assumption that activity during SPW‐Rs builds up randomly within the network. Under such hypothesized random buildup conditions, any given connection contributes only occasionally, and therefore weakly, to the firing of the postsynaptic neuron, a conclusion supported by the broad, zero time peaks of pair‐wise cross‐correlograms (O'Neill et al., 2006). Since under randomized conditions different synapses are responsible for depolarizing the postsynaptic neuron at different times during and across SWP‐R bursts, SPW‐R burst should de‐correlate, rather than strengthen, neuronal connections (for counter arguments, see Buzsáki, 1998; Roberts and Bell, 2002; Morrison et al., 2007). A computation model by Battaglia and Pennartz (2011) also assumes that sleep replay is fundamentally noisy but suggests that such noise is in fact useful for constructing semantic memories in the necortex.
The core argument of Lubenov and Siapas (2008) is that repeated sequential activity of neurons during SPW‐Rs is extremely rare but, instead, SPW‐Rs largely reflect a randomly firing and ever‐changing population of neurons. In contrast to their hypothesis, several recent papers examining ever increasing numbers of pyramidal neurons demonstrate robust and frequently repeating sequences of experience‐related activity during SPW‐Rs (Foster and Wilson, 2006; Diba and Buzsáki, 2007; Davidson et al., 2009; Pfeiffer and Foster, 2013). Thus, it is possible that sequentially replayed neuronal assemblies, even if they represent only a small minority of SPW‐R‐recruited neurons, selectively strengthen their connections, while the majority of the connections remain unaffected or undergo compensatory depression. It should be noted though that STDP typically refers to potentiation of subthreshold EPSPs and other models indicate that once a weak synapse becomes suprathreshold, the teaching/potentiating effect of the strong input is suspended (Buzsáki et al., 2002). According to this latter framework, synaptic connections between neurons that are strong enough to discharge the postsynaptic cell may not be modified anymore. Overall, the available empirical data on the impact of SPW‐Rs in synaptic plasticity is very limited and this area of research could much benefit from well‐designed targeted experiments and specific models.
Experiments indicate that the total synaptic weight in a given network remains constant over time, irrespective of the synaptic rules. Tetanic stimulation of the commissural/Schaffer collaterals induced spatially localized LTP of the evoked responses, brought about novel place fields and made some place fields disappear in CA1 neurons (Dragoi et al., 2003), perhaps due to competition among different inputs to the same pyramidal cells (De Almeida et al., 2007) or re‐routing activity by inhibition. On the one hand, these findings demonstrate that synaptic strengths are critical in selecting which neuron fires at a particular location. LTP typically affects neurons with relatively low within‐field firing rates, whereas the minority of neurons with high in‐field discharge rates might not be strongly affected. On the other hand, they also demonstrate that the LTP‐induced rearrangement of spatial representation in the neuronal population can occur without altering the global firing rate of the network (Dragoi et al., 2003). Given the unchanged global firing rates and the preservation of the overall network excitability, one may assume that the global synaptic weight in the hippocampus also remains stable even after artificial rearrangement of the synaptic strengths. Experiments performed on amygdalar slices in vitro further corroborate the idea that homeostatic mechanisms can reconcile the apparently opposite requirements of plasticity and stability (Royer and Pare, 2003). The STDP regimen of potentiation or depression of particular glutamatergic inputs lead to opposite changes in the strength of other inputs innervating other dendritic sites. As a result, no change in total synaptic weight occurred, even though the relative strength of some inputs was strongly enhanced.
A related idea to explain synaptic weight conservation was put forward by Lisman and Morris (2001). They suggest that the reason for slow consolidation in the neocortex (assuming similar LTP/LTD mechanisms in the hippocampus and cortex) is that consolidation involves the process of random disconnection of old synapses, the random formation of new synapses, and SPW‐R replay‐induced stabilization of new synapses that happen to be appropriate for the specific memory that is replayed by the hippocampus. At the heart of this idea is that there are not enough axon connections in the cortex to code for all possible associations of a lifetime. Instead, the appropriate mechanism is to mix and match axon to target connections during sleep until, by chance, the needed one is transiently formed, after which LTP processes can stabilize it. Thus, new learning is a reorganization of the existing connections, rather than the establishing of new ones.
Global firing rates and synaptic strengths, however, can change across brain states. Such gradual change is an essential part of the wake‐sleep cycle. According to a prominent model for sleep‐wake regulation, sleep and wakefulness are driven by an interplay between circadian and homeostatic processes (Borbely, 1982). The circadian mechanism promotes sleep and largely determines the timing and duration of the sleep period (Czeisler et al., 1980). Superimposed on this circadian mechanism is a homeostatic mechanism, which can affect sleep propensity and quality due to the accumulation of a hypothetical S (sleep) factor during time spent awake. A more recent reincarnation and refinement of this two‐process model is the synaptic homeostasis hypothesis (Tononi and Cirelli, 2003, 2014). In its original formulation, the synaptic homeostasis model states that wakefulness is associated with cumulative synaptic potentiation of cortical circuits, whereas non‐REM sleep is associated with synaptic downscaling. The stated goal of the global sleep‐homeostatic process is to avoid overuse and runaway excitation due to the continual buildup of excitation in the waking brain (Tononi and Cirelli, 2003). According to the model, the total synaptic weight in cortical circuits would progressively return to a baseline level after a good sleep, thus sleep offers a restorative homeostasis. The synaptic homeostasis is also manifested at the level of firing rates (Vyazovskiy et al., 2008).
The original form of the global “homeostasis” model explicitly argues against the ability of sleep to bring about differential and continued strengthening of neuronal connections active during recent experience: “Since downscaling would affect all synapses in a similar manner, it would not require any fine‐tuning at the level of the individual synapse. By contrast, selective potentiation or depression of specific synapses would require carefully titrated synaptic activations, which would not be easy to achieve considering that neural activity during sleep is by and large intrinsically generated” (Tononi and Cirelli, 2003). However, the model does not specify the mechanism(s) by which the synaptic or firing rate downscaling could be accomplished nor does it consider whether specific network events such as SPW‐Rs, slow oscillations or sleep spindles are uniquely involved or not. Subsequent modifications of the homeostasis model acknowledge that sleep is not a simple globally controlled state. In fact, several experiments demonstrate that slow oscillations, sleep spindles and SPW‐Rs are most often confined to restricted anatomical regions at a given time (Huber et al., 2004; Sirota and Buzsáki, 2005; Nir et al., 2011; Patel et al., 2013) and that extensive and specific motor learning in the waking brain can differentially affect sleep physiology in the involved brain region (Huber et al., 2004, 2006). Imaging experiments in mice show that sleep is specifically involved in modifying those very same spines, which were active in waking training (Cichon and Gan, 2015). Similarly, numerous experiments demonstrate the beneficial and selective role of sleep in the maturation of the visual system (Frank et al., 2001).
The memory consolidation model is preoccupied with the mechanisms by which new information enters into long‐term storage and simply assumes that previously active synapses get strengthened at the expense of other synapses so that the total summed synaptic weight in the hippocampal‐cortical areas involved remain constant. On the other hand, there is no doubt that total synaptic weight and global firing rates increase during waking and undergo global decrease during sleep (Vyazovskiy et al., 2008; Kudrimoti et al., 1999; Grosmark et al., 2012). A recent modification of the sleep homeostasis framework acknowledges that the “downsize all” framework is oversimplified and entertains, although does not demonstrate, the possibility that previously used and unused synapses are differentially affected during sleep (Tononi and Cirelli, 2014). With this modification, the homeostasis and memory consolidation models have become compatible. While the memory consolidation framework emphasizes that transfer of information from the hippocampus to neocortex occurs during sleep packaged in the spike content of SPW‐Rs, the homeostasis model emphasizes the need for a widespread downscaling of total synaptic strength in cortical networks based on metabolic considerations. A main difference, however, persists: under the framework of the consolidation model wake‐active synapses are preferentially strengthened, whereas the homeostasis model maintains that the wake‐used synapses should undergo larger changes than weaker ones during sleep since they were more extensively used during waking.
A challenge to both models is posed by recent observations that the distributions of synaptic weights, firing rates and spike bursts of cortical principal neurons are strongly skewed and log‐firing rates and bursts remain correlated across brain states and environmental situations (Mizuseki and Buzsáki, 2013; Buzsáki and Mizuseki, 2014). Highly active neurons form partnership with other active neurons and their rate change may be determined by such relationship (Okun et al., 2015). Given the strongly skewed distribution of synaptic weights and firing rates, it is not clear whether either the learning‐induced effect or the sleep‐induced downscaling is proportional or whether the left and right tails of the distribution are differentially affected. Addressing these issues quantitatively will require methods that can characterize firing pattern changes individually in single neurons (see Pre‐Existing Knowledge and Novel Experience are Reflected by the Spike Content of SPW‐R section) and perturbation methods to artificially change firing rates and bursting of single neurons in isolation, independent of the global network changes.
MODIFICATION SPW‐RS AND OTHER FORMS OF FAST RHYTHMS IN EPILEPSY
SPW‐Rs are the most synchronous physiological events in the mammalian brain with a high excitatory gain. These features make SPW‐Rs an efficient mechanism to transfer hippocampal events to the neocortex. However, there is a high price to pay for such synchronous carrier patterns since even minor perturbations of the hippocampal circuits can turn SPW‐Rs into pathological events. The existence of high‐risk SPW‐Rs may explain why the hippocampus is the most epileptogenic structure in the brain. Increased synchrony in SPW can manifest as epileptic spikes, while ripples can turn into pathological high frequency oscillations with more strongly synchronized population spikes (Bragin et al., 1999a; Staba and Bragin, 2011; Staba et al., 2012) or pathological ripples (p‐ripples). Many authoritative reviews have been written about the genesis, mechanisms and clinical importance of p‐ripples in recent years (Draguhn et al., 2000; Traub et al., 2002; Engel et al., 2009; Roopun et al., 2010; Köhling and Staley, 2011; Jiruska and Bragin, 2011; (Menendez de la Prida and Trevelyan, 2011); Worrell and Gotman, 2011; Jacobs et al., 2012; Jefferys et al., 2012; Zijlmans et al., 2012; Gulyás and Freund, 2015).
Definition of Pathological Events
Analogous to the SPW‐ripple dichotomy/coupling, interictal epileptiform discharges (IED) have a similar relationship to p‐ripples. The two events are often coupled but both IEDs and p‐ripples can occur independent from each other (Alvarado‐Rojas et al., 2015). IEDs in the hippocampus can assume at least two different forms (Wadman et al., 1983). Type 1 IEDs can be considered as “exaggerated SPWs” because their depth profiles are virtually identical and because they are also induced by epileptiform population bursts in the CA3 region (Buzsáki et al., 1989b, 1991a). In contrast, type 2 IEDs have positive polarity in the CA1 stratum radiatum and they are typically initiated by the population discharge of CA2/CA3a neurons whose axon collaterals target mainly the basal dendrites of CA1 pyramidal cells (Li et al., 1994). Finally, p‐ripples can also occur without IED.
In the intact rat brain, the duration of SPW and associated spike burst activity varies between 30 and 150 ms and its amplitude never exceeds 3 mV in any layer (Buzsáki et al., 1983; Suzuki and Smith, 1987). Given this well‐defined amplitude‐duration range, any LFP event in the CA3‐CA1 region shorter that 30 ms in duration and larger than 3 mV in size should be considered as non‐physiological (Fig. 50A–C). However, this definition is fully inclusive since more complex IEDs can overlap with the physiological range in both amplitude and duration. More objective definition of IEDs and p‐ripples therefore may require multiple measures that may include brain state‐affiliation, spatial‐temporal distribution, relationship to spikes of principal cells and interneurons, pharmacological responses, and intracellular behavior (Engel and da Silva, 2012).
Figure 50.
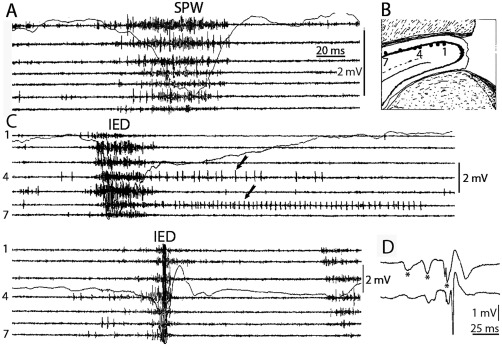
SPWs and interictal epileptic discharges (IEDs). (A) Neuronal synchrony along the longitudinal axis of the CA1 pyramidal layer during a sharp‐wave burst (SPW) in an intact animal. (B) Hippocampus disconnected from its subcortical connections (fimbria‐fornix lesion). Placement of electrodes 1–7. (C) Type 1 IEDs in lesioned rats (middle and bottom). Note tighter synchrony of population bursts and larger amplitude of the field responses during IEDs. Note different amplitude calibration in the intact and lesioned rats. Arrows, prolonged, post‐IED activity of two putative interneurons. (D) Type 1 IED. Note reverberation of activity in the entorhinal cortex‐hippocampus‐entorhinal cortex and amplification of neuronal activity. Top trace, the dentate molecular layer (DG mol). Note entorhinal input‐induced responses (asterisks). Bottom trace, CA1 pyramidal layer trace. Reverberation in was terminated by the appearance of a large population spike. Reproduced from Buzsáki et al. (1991).
The oscillatory interplay between pyramidal cells and interneurons during the SPW burst can be viewed as a dissipative mechanism to decelerate and limit population synchrony of pyramidal cells (Ylinen et al., 1995). Blockade of GABAergic inhibition or surgical removal of subcortical inputs to the hippocampus results in significantly faster recruitment of a larger number of pyramidal cells as reflected by large‐amplitude (>5 mV) and short‐duration (∼20 ms) “augmented” SPWs, corresponding to type 1 IEDs (Buzsáki et al., 1989b, 1991a). Such short, synchronized field events in CA1 often have the appearance of a stimulation‐evoked response with one or several large population spikes in the pyramidal layer (Buzsáki et al., 1989b, 1991a). Depending on the size of the population spike and the magnitude of the recruited inhibition, the CA1 output can initiate multiple cycles of reverberation of excitatory activity in the hippocampal‐entorhinal loop (Fig. 50D).
P‐ripples have been described first in the epileptic human hippocampus (Fig. 51) (Bragin et al., 1999a) and a large body of our knowledge about p‐ripples derives from research on humans. While there is no good definition of p‐ripples, and the distinction between physiological and p‐ripples cannot be made from either amplitude or frequency criteria only (Jacobs et al., 2012; Jefferys et al., 2012), fast ripples (>200 Hz in the hippocampus), ripples with large‐amplitude population spikes (>1 mV) and ripples in structures which normally do not generate such events (such as the dentate gyrus; Bragin et al., 2004) can be safely regarded as p‐ripples in every species. However, many p‐ripples are lower in frequency and can overlap with physiological ripples. A main practical problem is that the exact layer and subregion of the recording sites in clinical studies are rarely known, therefore amplitude criteria are difficult to apply. Small amplitude p‐ripples can either represent small clusters of synchronously discharging pyramidal cells or events recorded further away from the recording site. Duration criteria are also not reliable since the physiological ripples vary from 30 to 150 ms (occasionally longer with a skewed distribution) in both rats (Csicsvari et al., 1999a, 1999b; Patel et al., 2013) and humans (Le van Quyen et al., 2008). Neocortical ripples can exceed 300 Hz during sleep spindles, especially during high‐voltage spindles (Kandel and Buzsáki, 1997) and strong sensory stimulation (Curio, 1999; Jones and Barth, 1999), and these events show a frequency overlap with p‐ripples (Grenier et al., 2001, 2003; Timofeev et al., 2002; Jacobs et al., 2012). Certain thalamic nuclei can display sustained oscillations exceeding 200 Hz in the intact brain (Peyrache et al., 2015) and Parkinson Disease (Danish et al., 2007). Increasing the accuracy of separation between normal and pathological fast oscillations in clinical settings will require recording probes with high spatial resolution, unit recordings and precise anatomical localization of the recording sites.
Figure 51.
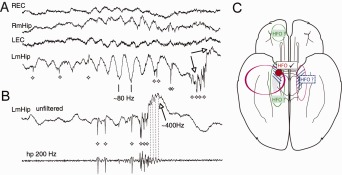
P‐ripples in epileptic patients. (A) Coexistence of ripples and p‐ripples in human entorhinal cortex and hippocampus of an epileptic patient. Note bilateral occurrence of the ripple but unilateral confinement of the p‐ripple (arrows). (B) Another p‐ripple (arrow) in the left medial hippocampus accompanied by high frequency unit discharges. Unfiltered data are shown in the top. The bottom trace is high‐pass filtered (200 Hz). Diamonds mark action potentials. LEC, REC: left and right entorhinal cortex. LmHip, RmHip: left and right medial hippocampus. Reproduced from Bragin et al., 1999. (C) P‐ripples (high frequency oscillations, HFO) are able to identify the seizure zone (in red). Correlation between removal of HFO‐generation tissue removal and post‐surgical seizure outcome indicate the epileptogenic area (blue dashed lines). Whether HFOs outside the epileptogenic zone are present and contribute to neuropsychological deficit (green areas) needs to be investigated further. Reproduced from Jacobs et al. (2012).
Pathologial Oscillations in Chronic Models of Epilepsy
Experimentally induced p‐ripples were first described in the kainic acid rat model (Ben‐Ari et al., 1980, 1997) of chronic epileptogenesis (Bragin et al., 1999b). Kainic acid induces status epilepticus and consequent cell loss, followed by a latent period of days to several weeks before spontaneously recurring seizures start. In the “incubation” period and thereafter, multiple abnormal electric events are present, the most characteristic of which are p‐ripples (Bragin et al., 1999b, 1999c, 2000, 2004, 2005, 2007; Lévesque et al., 2011, 2012). Bragin et al. called these epileptic events “fast ripples” (Bragin et al., 1999a, 1999b) because fast intermittent oscillatory events, ranging from 200 to 500 Hz and 10 to 100 ms in duration were frequently encountered in kainic acid‐treated rats, similar to epileptic humans (Bragin et al., 1999a). P‐ripples were found adjacent to the epileptogenic zone, including the hippocampus proper, dentate gyrus and entorhinal cortex and they are typically unilateral, whereas SPW‐Rs occur largely synchronously in homologous areas of the hippocampus bilaterally in both rodents (Buzsáki, 1989; Buzsáki et al., 2003) and humans (Bragin et al., 1999a). P‐ripples are typically associated with synchronous burst discharge of putative pyramidal cells. Importantly, p‐ripples coexist with normal SPW‐Rs. They both occur with maximum probability during non‐REM sleep and behavioral immobility but p‐ripples invade a smaller neuronal volume than do SPW‐Rs and can occur also in the absence of accompanying large IED in the dendritic layers. Bragin et al. put forward the hypothesis that p‐ripples are the pathological versions of SPW‐Rs and reasoned that they play a role in seizure initiation. Furthermore, since p‐ripples were observed only in pathological tissue with overt structural damage, they suggested that their preoperative or intraoperative “identification could be a powerful functional indicator of the epileptic region in patients evaluated for surgical treatment” (Bragin et al., 1999b).
Several subsequent experiments supported these early views and expanded the initial findings in several important ways. First, p‐ripples have been observed in several forms of seizure‐prone brain tissue. Lévesque et al. (2011, 2012) have described two categories of p‐ripples (ripples in the 80–200 Hz band and fast ripples in the 250–500 Hz band) in the pilocarpine model, which also depends on the establishment of a substantial hippocampal lesion akin to hippocampal sclerosis (Foffani et al., 2007). Similar to human seizures and chronic kainic acid‐induced seizures in rodents, the onset of spontaneous seizures in the pilocarpine model show two variations, beginning either with low‐voltage fast activity or hypersynchrony (Velasco et al., 2000; Bragin et al., 2005; Ogren et al., 2009). The occurrence of 250–500 Hz p‐ripples was more frequent compared with 80–200 Hz oscillations before the onset of seizures with synchronous onset, whereas 80–200 Hz oscillations were the dominant preictal event preceding seizures with low‐voltage fast activity onset, implying differential involvement of these possible different types of p‐ripples in seizure genesis (Lévesque et al., 2012). Second, neuronal damage is not a requirement for the emergence of p‐ripples. Similar to the chronic seizures that erupt long after drug‐induced status epilepticus, spontaneous, recurring seizures can also be induced by intrahippocampal tetanus without any overt neuronal damage toxin (Hawkins and Mellanby, 1987). P‐ripples (250–800 Hz) in the tetanus model were observed in all subregions, including the dentate gyrus, CA3 and CA1 areas either before seizure onset or in the interictal periods (Jiruska and Bragin, 2011). P‐ripples were more reliably observed in the primary epileptogenic zone than either interictal discharges or ripples (100–250 Hz), supporting the view that mapping p‐ripples is potentially valuable in the pre‐surgical workup in both lesional and nonlesional temporal lobe epilepsy. Subcortical deafferentation is yet another model of chronic epilepsy without cell loss (Buzsáki et al., 1989b, 1991a). In this model, seizures are rare, yet IEDs and fast oscillations are present. Third, following repeated kindling, another model of epileptic seizures without neuronal loss (Goddard, 1967), p‐ripples develop gradually while normal ripples decrease over time as behavioral seizures emerge. Fourth, the onset of p‐ripples after status epilepticus or other perturbations in the hippocampus correlates well with the eruption of the first spontaneous seizure. Finally, the earlier p‐ripples occur, the more severe the subsequent spontaneous seizures become (Bragin et al., 2004).
P‐ripple‐generating locations remain spatially stable over days to months before the emergence of spontaneous seizures in kainic acid‐treated rats (Bragin et al., 2003), suggesting that p‐ripples are good predictors of epileptic activity. In the kainic acid‐induced model, p‐ripples were observed only in the dentate gyrus adjacent to the lesion and in the ipsilateral entorhinal cortex (Bragin et al, 1999b). In the tetanus toxin model, p‐ripples could be detected bilaterally but the rate of occurrence and frequency of p‐ripples were higher in the toxin‐injected hippocampus and the majority of spontaneous seizures were initiated in the areas that generate p‐ripples (Jiruska et al., 2010a). These experimental findings indicate that p‐ripples can provide information about the lateralization and localization of the epileptogenic zone (Fig. 51).
P‐Ripples In Vitro and In Silico
As discussed in Generation of SPW‐R Bursts In Vitro section, many in vitro models devoted to the study of physiological ripples may, in fact, better represent p‐ripples. The majority of dedicated in vitro studies on p‐ripples have been undertaken in slices from normal animals and fast oscillations are produced by either pharmacologic means or changing the composition of the perfusion fluid (Table 2), for example low‐calcium, high‐potassium, low‐magnesium perfusion solution (Dzhala and Staley, 2004; D'Antuono et al., 2005; Jiruska et al., 2010a; Karlocai et al., 2014; Aivar et al., 2014). P‐ripples can occur in each of the major hippocampal subregions, subiculum or entorhinal cortex but their main properties (mean frequency, shape, amplitude, spatial distribution, and cellular mechanisms) vary from preparation to preparation. Typically, such experiments examine high‐frequency oscillations before the emergence of seizures (Bikson et al., 2003; Jiruska et al., 2010a) and more rarely isolated p‐ripples (Menendez de la Prida, 2006, 2011; Foffani et al., 2007).
Adding the GABAA receptor blocker penicillin or picrotoxin to the artificial cerebrospinal fluid can readily induce epileptic high frequency activity (Wong and Traub, 1983). Similar to SPW‐Rs, p‐ripples under disinhibited conditions originate in the CA2/CA3a region with high bursting propensity of single neurons, from where the population bursts spread to CA3b and c and by way of these subregions eventually to CA1. P‐ripples, consisting of three to nine population spikes at 300 to 500 Hz can be elicited in surgically isolated CA2 slabs by a stimulus applied locally and direct activation of a small number of pyramidal cells by pressure ejection of K+ produce a synchronized burst. CA1 pyramidal cells show large‐amplitude excitatory postsynaptic potentials and burst firing, and the spikes of the bursts coincide with the extracellularly recorded population spikes. Similar high‐frequency LFP oscillations with multiple population spikes have also been described in bicuculline‐perfused slices of the dentate gyrus in vitro (Bragin et al., 2002a).
P‐ripples in the disinhibited slice differ from in vivo ripples in several major ways. First, the “dome‐like” positive waves characteristic of CA1 ripples in vivo are absent, likely due to the absence of IPSPs in pyramidal cells. Second, a very high fraction of the pyramidal cells participate in each p‐ripple event and they tend to fire bursts of spikes. Third, each spike of the spike burst coincides in time with the negative peaks of LFP population spikes of the p‐ripple. Essentially, the LFP p‐ripple corresponds to superimposed spike bursts of many pyramidal cells. Nearly identical p‐ripples can be induced by local application of picrotoxin in vivo (Fig. 52) (Stark et al., 2014).
Figure 52.
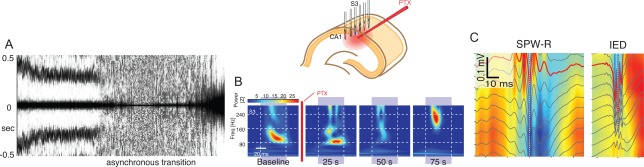
Conversion of SPW‐Rs to p‐ripples may be separated by an asynchronous transition epoch. (A) Time‐binned autocorrelogram of multi‐unit activity showing that the synchrony of firing during SPW‐Rs, transition period and preictal p‐ripples (24 min sweep). Epileptiform event was induced by high K+ in vitro. (B) Abolishing SPW‐Rs in vivo by local infusion of picrotoxin (PTX) and induction of p‐ripples. Note long transition period of no‐SPW‐Rs at 50s. Both SPW‐Rs and fast p‐ripples were induced by focal light stimulation of CA1 pyramidal cells in a CaMKII::ChR2 mouse. Panels show the time‐frequency decomposition of the pyramidal layer CSD traces. (C) LFP traces at different depths across the CA1 pyramidal layer at 50 µm steps (grey lines) and current source density (color maps) of average SPW‐Rs (100 ms sweep) and PTX‐induced p‐ripples (50 ms sweep). (A) Reproduced from Karlocai et al., 2014; (B, C) Reproduced from Stark et al., 2014.
Disinhibition‐induced p‐ripples may represent at least one major class of p‐ripples, since similar events are observed in slices obtained from chronically epileptic rats (e.g., pilocarpin or kainic acid) without pharmacological blockade of inhibition (Sanabria et al., 2001; Foffani et al., 2007). Whereas only a small percent of CA1 pyramidal cells fire spike bursts from control animals, neurons in almost half of the slices from pilocarpine animals generate high‐frequency bursts of three to six spikes in response to threshold depolarizations, and many of these neurons also display spike bursts spontaneously. Burst firing in most cases can be completely blocked by adding the Ca2+ channel blocker Ni2+ to, or removing Ca2+ from, the ACSF, but not by intracellular application of the Ca2+ chelater 1,2‐bis(o‐aminophenoxy)ethane‐N,N,N',N'‐tetra‐acetic acid (BAPTA), suggesting spike bursts are driven by a Ca2+ current (Sanabria et al., 2001). However, other mechanisms should also be considered since changes in firing dynamics and conversion from dominantly single spike firing pyramidal neurons to bursters may also be facilitated by an increase in persistent sodium currents, a decrease in dendritic A‐type potassium channels and perhaps by molecular changes in Ca2+ channels (Azouz et al., 1996; Bernard et al., 2004). P‐ripples in most preparations are abolished by adding CNQX to the ACSF, indicating that fast synaptic excitation is critical for the generation of these events.
While p‐ripples induced by blockade of GABAA receptors may form a specific group, studies of ex‐in vivo tissue from patients with pharmacoresistant epilepsy paint a more complex picture (Cohen et al., 2002; Alvarado‐Rojas et al., 2015). Combined intra‐ or juxtacellular and extracellular recordings of p‐ripples in slices of the subiculum of epileptic patients often show spontaneous IEDs and p‐ripples as well as physiological SPW‐Rs. SPW‐Rs were associated with rhythmic GABAergic and glutamatergic synaptic potentials and moderate spiking. In contrast, p‐ripples were characterized by depolarizing synaptic inputs frequently reaching the threshold for bursting in most pyramidal cells. Intracellular recordings revealed depolarizing GABAA receptor‐mediated postsynaptic events, indicating a perturbed Cl‐ homeostasis in a fraction of subicular pyramidal neurons. Double in situ hybridization investigation demonstrated that mRNA for KCC2 was dramatically reduced in these neurons, whereas neurons that were hyperpolarized during p‐ripples were immunopositive for KCC2. Bumetanide, at doses that selectively block the chloride‐importing potassium‐sodium‐chloride cotransporter NKCC1, produced a hyperpolarizing shift in GABAA reversal potentials and suppressed p‐ripple activity (Huberfeld et al., 2007). The critical role of Cl− homeostasis in p‐ripples was further demonstrated in slices resected from patients with gliomas (Pallud et al., 2014). Tissue surrounding glioma infiltration showed a high incidence of p‐ripples, which depended on both glutamatergic AMPA receptor‐mediated transmission and depolarizing GABAergic signaling. The perturbed chloride homeostasis could be explained by the reduced expression of KCC2 and increased NKCC1 (Na‐K‐2Cl cotransporter 1). The effect of chloride dysregulation was recently modeled experimentally in the mouse by artificially loading Halorhodopsin‐expressing pyramidal cells with Cl− (Alfonsa et al., 2015). Small positive shifts in EGABA produced a transient rise in network excitability, with many distinctive features of p‐ripples (Ibarz et al., 2010) but without triggering ictal events. Since dysregulation of both glutamate and cellular Cl− homeostasis has been implicated in oncogenesis, pharmacological control of chloride in neurons and glioma cells may provide a therapeutic target for patients with epileptogenic gliomas (Pallud et al., 2014).
Mechanisms and Degeneracy of p‐Ripples
Several strikingly different structural, molecular, pharmacological and ionic composition changes can give rise to seemingly similar oscillatory events lumped under the umbrella of LFP “p‐ripple,” demonstrating degeneracy of the inducing mechanisms. Conversely, the same mechanisms can give rise to multiple forms of p‐ripples depending on brain state and other subtle mechanisms. Presumably, degeneracy also applies to p‐ripples observed in epileptic patients and chronic models of epilepsy.
Analysis of voltage‐depth profiles of p‐ripples in vivo in rats showed the largest amplitude within pyramidal and granular layers (Bragin et al., 2007a,b). The depth versus voltage profiles were quite similar to the population spikes evoked in response to electrical stimulation of the CA3 or entorhinal input, respectively, implying that p‐ripples correspond to bursts of population spikes in the upstream regions (Bragin et al., 2007a,b; Schomburg et al., 2012). Indeed, there is a consensus that p‐ripples largely reflect synchronous spiking activity (Jefferys et al., 2012) but it is not well understood how such local synchrony is brought about. P‐ripples can be induced by acute manipulations without invoking anatomical changes or alteration of intrinsic neuronal properties. On the other hand, chronic changes in anatomical connectivity, synaptic strength distributions, loss of inhibitory neurons, changes in glia‐neuron relationship, reduction of the extracellular space and alterations of selected biophysical properties of neurons due to various acquired “channelopathies” (Yaari and Beck, 2002; Dudek and Sutula, 2007) can be equally important in the induction of abnormal neuronal events.
P‐ripples are consistently present in hippocampal slices obtained from pilocarpine treated epileptic animals (Foffani et al., 2007). Oftentimes, the oscillations are much faster than the intervals of spikes in bursts (300–400 Hz), leading to the suggestion that multiple, phase‐shifted populations of synchronously discharging neurons can give rise to the superfast LFP oscillations. Cell loss and/or sprouting in the epileptic tissue may bring about out‐of‐phase firing (Foffani et al., 2007; Ibarz et al., 2010) or uncoordinated increased spiking which will manifest as a broad increase in the high frequency end of the power spectrum (Ibarz et al., 2010; Jiruska et al., 2010b). Neuronal loss can be a contributor since in the pilocarpine model of epilepsy, hippocampal volume is negatively correlated with incidence of p‐ripples. Coordination of enhanced bursting (Sanabria et al., 2001; Dzhala and Staley, 2004) in chronic models can be facilitated by shared inhibition, gliosis which shrinks the extracellular space and enhances ephaptic effects (Jefferys, 1995; Anastassiou et al., 2010), altered local ionic homeostasis, expression of gap junctions (Traub et al., 2004) and other channelopathies as well as other hitherto unidentified causes. Since analogous forms of p‐ripples of chronically epileptic preparations can also be induced acutely both in vitro and in vivo, other mechanisms may also be at action in the production of p‐ripples.
Type 1 IED and SPWs utilize largely the same anatomical substrate and physiological mechanisms (Buzsáki et al., 1989a, 1989b, 1991a,b) and the strong drive of IEDs may be responsible for at least some forms of p‐ripples. However, ripples and p‐ripples are different in multiple aspects, as discussed above. Importantly, ripples are not simply converted into faster p‐ripples but are separated in time by an interim transition period of elevated asynchronous activity of neurons in vitro (Fig. 52A) (Karlocai et al., 2014), in vivo (Stark et al., 2014) and in silico (Brunel and Wang, 2003; Geisler et al., 2005) models. In contrast to these acute models, in epileptic patients and chronic epilepsy models in experimental animals ripples and p‐ripples coexist and are often in close temporal proximity (Fig. 51) (Bragin et al., 1999a, 1999b), suggesting that an asynchronous “incubation” period is not an absolute requirement for interictal p‐ripples.
Although blockade of GABAA receptor‐mediated inhibition can induce p‐ripples, fast inhibition may play various roles in other forms of p‐ripples. Karlocai et al. (2014) have systematically compared the firing patterns of in vitro ripples and p‐ripples induced by bath application of high K+, zero Mg2+, 4‐aminopyridine and gabazine in the CA3 region. PV‐expressing basket cells, axo‐axonic interneurons and dendrite‐targeting interneurons all increased their firing rates during the initial part of the p‐ripple, often tens of milliseconds before the LFP p‐ripple. As the population burst of pyramidal neurons built up, basket cells and axo‐axonic interneurons transiently entered into a phase of depolarization blockade and their disinhibition of pyramidal neurons is postulated to be a permissive action for the strong synchrony of bursting pyramidal neurons, as was also shown in in vivo models of epilepsy (Bragin et al., 1999b, 2002a,b). Patch clamp experiments in CA3 pyramidal neurons also showed that in the high K+ model, fast inhibition is compromised and integrative properties of pyramidal cells are reduced, adding further potential causes of increased excitation during p‐ripples and faster propagation of p‐ripples, as is the case in zero Mg2+ model (Trevelyan et al., 2006, 2007). The increased ratio of excitatory and inhibitory transmission is also assumed to be the explanation for the emergence of p‐ripple in low Ca2+ (Aivar et al., 2014). However, in slices where p‐ripples are induced by the GABAA receptor blocker gabazine, depolarization blockade of basket cells are not observed, yet the main features of p‐ripples are remarkably similar to those present in the other forms of epilepsy models.
While gap junctions may not be critical for physiological ripples (see Mechanisms of Ripple Generation section), in chronic models of epilepsy they may be expressed between pyramidal cells and can contribute effectively to the fast synchronization of neurons (Traub et al., 1999a, 1999b). Ephaptic effects induced by the large voltage gradients brought about by IEDs can provide further facilitative mechanisms for population synchrony (Jefferys, 1995; Anastassiou et al., 2010; Jiruska et al., 2010b). Overall, the available research, to date, paints a very complex picture of the mechanisms involved in the generation of p‐ripples. Some forms may be induced by distorting the contributing mechanisms of physiological ripples, whereas other forms may be triggered by a diverse family of novel pathological mechanisms, determined largely by the nature of the epileptic features of the neuronal tissue.
P‐Ripples in Epileptic Patients can Identify Seizure Zone
The hypothesized importance of p‐ripples is twofold. First, they may be directly involved in epileptogenesis and second, their localization can be used to identify the seizure locus even if they are only interictal epiphenomena of seizures (Engel et al., 2009). In case of pharmacologically intractable seizures, surgical removal of the epileptic tissue is often the only available treatment. The outcome of such invasive intervention depends largely on the precise localization of the hypothetical epileptogenic zone, often defined as that volume of neuronal tissue necessary and sufficient for initiating seizures. If all of this volume is removed, seizures, in principle, should disappear completely. Traditionally, the seizure zone is identified by the earliest onset of seizures when multiple recording electrodes are available. However, this is a slow and not always reliable process since it depends on the presence of rare overt seizures and success depends on the appropriate electrode coverage of potential seizure zones. In line with the experiments on epileptic rodents, a body of work from retrospective patient studies indicates that, to date, mapping of p‐ripple locations is a promising technique to localize seizure‐prone tissue and to achieve postsurgical seizure freedom by removing the minimum critical brain tissue.
A pioneering clinical study (Staba et al., 2004) reported that the incidence of ripples was similar in the epileptogenic and non‐epileptogenic temporal lobe (hippocampus, subiculum, and entorhinal cortex), whereas p‐ripples were dominant in epileptogenic areas, suggesting that primary seizure‐generating areas are also specific locations for p‐ripple induction during the interictal periods. The highest p‐ripple to ripple ratio was observed in the subicular cortex, where bursting propensity of pyramidal neurons is highest (Stewart and Wong, 1993; Staba et al., 2002) and seizure threshold is lowest (Huberfeld et al., 2011; Toyoda et al., 2013). Both ripples and p‐ripples showed highest rates of occurrence during non‐REM sleep. During REM sleep, ripples were virtually absent, whereas p‐ripples remained elevated and were comparable to rates observed during waking. Because hippocampal sclerosis can promote p‐ripples and because reduced neuronal density and decreased hippocampal volume are correlated with the rate of occurrence of p‐ripples (Staba et al., 2007; Ogren et al., 2009), it was initially thought that neuronal lesions were prerequisite for the emergence of p‐ripples (Bragin et al., 2003). Yet, in patients with focal cortical dysplasia and nodular heterotopias, conditions that often give rise to chronic seizures, p‐ripples were more strongly linked to locations of seizure onset than to the anatomical lesion, suggesting a fundamental link between p‐ripples and epileptogenecity (Jacobs et al., 2009). Similarly, a microelectode study in patients with temporal lobe epilepsy (Worrell et al., 2008) also showed that the incidence of p‐ripples is higher inside than outside the seizure onset zone. Using high‐density (0.4 mm), two‐dimensional electrode arrays (“Utah arrays”) implanted in or near the focal epilepsy zone in the neocortex, Schevon et al. (2009) reported that p‐ripples were most often generated locally and they could occur in association with IEDs or independent of them. No p‐ripples were found in one patient in whom the electrode array was implanted outside the epileptogenic zone. In the remaining subjects, the majority of p‐ripples were limited to a single channel, indicating localization to a cortical region < 400 x 400 µm. However, a minority of p‐ripples was present at numerous nearby sites. These rare but prominent p‐ripple events may explain why p‐ripples can also be detected even by large electrodes. Removal of areas generating high rates of p‐ripples and ‘ripples on a flat background activity’ showed a significant correlation with a seizure‐free outcome. In contrast, removal of high rates of ‘ripples’ or ‘ripple patterns in a continuously oscillating background’ was not significantly associated with seizure outcome (Kerber et al., 2014).
The most convincing support for the correlation between p‐ripples and the seizure zone is demonstrated by the relationship between the surgical removal of p‐ripple‐generating tissue and a good post‐surgical outcome (Jacobs et al., 2012). Independent studies using different recording methods and patient populations show a reliable correlation between the volume of removed p‐ripple‐generating tissue and clinical improvement (Ochi et al., 2007; Jacobs et al., 2010a, 2010b; Akiyama et al., 2011). Wu et al. (2010) described a patient in whom seizures continued after the first surgery. However, complete removal of the p‐ripple‐generating zone in the second surgery resulted in seizure freedom.
IEDs show a less reliable relationship with the epileptic substrate than p‐ripples (Jacobs et al., 2008). However, p‐ripples, like physiological ripples, may show a lognormal‐like distribution with rare large events interspersed among numerous small p‐ripples. Only large p‐ripples may be accompanied by large amplitude LFP IEDs. This is somewhat paradoxical since, IEDs, like SPWs, often give the necessary driving input to induce local oscillations. Several studies suggest that IEDs are either not predictive or negatively correlated with seizure incidence (Engel and Ackerman, 1980; Avoli et al., 2005). In contrast, other experiments and observations indicate that IEDs, similar to p‐ripples, increase tissue excitability and can trigger seizures (Staley et al., 2005; White et al., 2010; Worrell and Gotman, 2011). However, brief type 1 IED events may only generate a single large population spike in the pyramidal layer (Fig. 50D) rather than a fast ripple, followed by a strong hyperpolarization and such IEDs may decrease rather than increase the probability of seizures. Indeed, electrical stimulation of CA1 neurons at low frequency can suppress 4‐aminopyridine‐induced ictal activity in the entorhinal cortex in combined hippocampal‐entorhinal slices in vitro (Barbarosie et al., 2000). On the other hand, other forms of IEDs, which can induce p‐ripples can actively contribute to the generation and spread of neocortical seizures (Urrestarazu et al., 2006, 2007). Worrell et al. (2008) showed that p‐ripples are tightly related to the IEDs recorded by depth microelectrodes in limbic structures but clinical macroelectrodes often detect only IEDs without p‐ripples, perhaps because the size of the macroelectrodes is larger than the focal volume that generates p‐ripples. It is therefore possible that using high spatial resolution electrodes, better classification of IEDs and a quantitative characterization of the nature of the ensuing seizures and their initiation mechanisms will resolve the current controversy and clarify the relationship between ripples and p‐ripples.
The summarized clinical observations and experiments on rodents provide a strong link between p‐ripples and epileptogenicity. Yet, more work is required to use p‐ripple localization in the clinical decision with high confidence, especially in hippocampal‐entorhinal seizures where mapping of electrical activity is limited by the number of depth electrodes. The first step is to establish a firm relationship between p‐ripples and the suspected epilepsy‐generating zone. High‐density, spike detecting, ultra‐conformable, and biocompatible NeuroGrids (Khodagholy et al., 2014) may be used preoperatively and intraoperatively in prospective studies to provide high‐resolution maps of pathological activity, both ictal and interictal. The preoperative physiological measures in the epileptogenic zone can then be compared quantitatively with the postoperative outcome. If interictal p‐ripple mapping proves as reliable as recording seizures, such novel methods would greatly reduce the risks and costs of invasive studies (Staba and Bragin, 2011; Jacobs et al., 2012).
Ideally, ripples and p‐ripples should be detected in a non‐invasive manner. The synchronously discharging neurons involving IEDs and SPW‐Rs can activate neocortical neuronal assembles and the induced transmembrane potentials may be recovered by high‐density EEG or MEG recordings (Jirsch et al., 2006; Blanco et al., 2010). Since IEDs and SPW‐Rs are sparse events, novel compressed sensing methods, such as Bayesian decoding or independent component analysis, may be deployed in attempts to recover sparse signals even from mixed and subsampled measurements using both supervised (when intracranial electrodes are available to obtain “ground truth” data) and unsupervised techniques (Agarwal et al., 2013). However, small amplitude, localized p‐ripples may remain inaccessible by distant recordings if the number of neurons involved in their generation is too low to exert a detectable impact on neocortical targets. Yet, compressed sensing methods may prove invaluable to detect neocortical p‐ripples from scalp recordings.
In summary, p‐ripples may be involved in at least some forms epileptogenesis and further research is needed to establish a more reliable relationship between p‐ripples and seizure induction. Irrespective of their role in seizures, the precise spatial localization of p‐ripples is a pragmatic goal since extensive research supports their specific role as a biomarker in epileptogenicity. High‐resolution spatial mapping of p‐ripples, in turn, can be a useful tool for guiding effective yet restricted surgeries to achieve seizure freedom. Finally, p‐ripples may also be valuable in the prognosis and treatment of patients with medically refractory epilepsy as input signals to closed‐loop therapies (Krook‐Magnuson et al., 2015).
P‐Ripples, IEDs, and Memory Impairment
One of the most common comorbidities in patients with temporal lobe epilepsy is cognitive decline. It has been long recognized that IEDs are not simply a harmless byproduct of the disease but they may be causal to cognitive impairment (Holmes and Lenck‐Santini, 2006). Specifically, IEDs may substantially contribute to the memory impairments observed in temporal lobe epilepsy patients and animal models of temporal lobe epilepsy, regardless of the severity of the less frequently reoccurring seizures (Brinciotti et al., 1989; Krauss et al., 1997; Binnie, 2003; Weglage et al., 1997). In epileptic human subjects, even single IEDs can transiently disrupt local cortical computation and affect reaction time (Shewmon and Erwin, 1988). Yet, how IEDs affect neural networks involved in memory processes is unclear.
In a study by Kleen et al. (2010), the memory effects of hippocampal IEDs were tested in delayed matching to sample task in pilocarpine‐induced epileptic rats. Memory retrieval was strongly impaired in epileptic animals compared with controls and also slowed down response latency, adding approximately a half second to the time taken to respond. To separate the causal effects of IEDs and other possible contributing mechanisms due to cell loss, sprouting and other consequences of pilocarpine‐induced status epilepticus and subsequent spontaneous seizures, Shatskikh et al. (2006) mimicked the effect of IEDs by stimulating the ventral hippocampal commissure. Large population spike events were induced using a series of strong electrical pulses and the evoked population discharges in the CA1 region resembled naturally occurring IEDs in epileptic rats. The rats were tested in memory tasks, including the Morris water maze, radial arm maze and object recognition tasks. Rats that received induced spikes took longer to reach the escape platform in the water maze trials, had significantly more reference errors and required more trials to complete the radial arm maze task. They also displayed lower investigation ratios in the object recognition task. Overall, these results indicate that induction of large amplitude and complex population spikes, mimicking intermittently occurring IEDs in the hippocampus, results in impairment of both spatial reference and nonspatial object recognition memory. While a clear limitation of this study is that induced population spikes in healthy rats do not fully mimic the epileptic encephalopathies, the findings convincingly demonstrate that even the large population synchrony‐inducing effects of IEDs are sufficient to induce memory impairment. A leading and early symptom of Alzheimer's disease is memory impairment and a large fraction of Alzheimer's disease patients show abnormal electric events and an estimated 10 to 20% develop seizures (Amatniek et al., 2006). Overall, the summarized results indicate that altered SPW‐Rs may contribute to memory impairment and imply that treatment of interictal events, such as IEDs and p‐ripples, may improve memory and prevent cognitive decline in patients with epilepsy. Yet, how such abnormal events can lead to behavioral deterioration is not clear.
IEDs and p‐ripples can contribute to memory impairment in multiple ways. Learning is a protracted process, which allows for modification of the memory trace during consolidation (SPW‐R‐Supported Memory Consolidation section). Three prominent oscillatory brain events have been linked to consolidation: hippocampal sharp wave ripples (SPW‐R), slow oscillations of the neocortex and thalamocortical sleep spindles. Of these, the causal role of SPW‐Rs in memory is best understood since selective elimination of SPW‐Rs severely impairs memory performance in rodents. The temporal coupling of ripples, slow oscillations and spindles is hypothesized to facilitate information exchange between the hippocampus and mPFC, leading to memory consolidation (cf., Diekelmann and Born, 2010). Although both spindles and slow oscillations are correlated with hippocampal SPW‐Rs, it is not clear whether these neocortical events exert their beneficial effects independently or whether their temporal coordination with SPW‐Rs is essential for extracting hippocampal information. A recent study shed light on these hypothesized links (Gelinas J, Khodagholy D, Buzsáki G. unpublished findings). IEDs, induced during the course of daily electrical kindling of the hippocampus, were found to effectively phase‐reset slow oscillations and consistently induce sleep spindles in the medial prefrontal cortex in rats. Remarkably, IEDs induced large delta waves (DOWN state of the slow oscillation; Steriade et al., 1983a) and spindles also during REM sleep and waking, when these events are not observed normally. Similar to rats, patients with frontotemporal focal seizures also exhibited strong correlation between IEDs and spindles recorded in frontal areas (Gelinas et al., 2014). These findings suggest that IEDs can hijack physiological coupling mechanisms, such as the weak coupling between ripples, slow oscillations and spindles between structures critical for memory processes (cf., Diekelmann and Born, 2010). Since action potentials associated with IEDs likely emerge from abnormal internal excitability as opposed to learning induced changes, they may broadcast “nonsense” information to the neocortex. In turn, hippocampal IEDs reset the neocortical oscillations and induce spindles at times when the prefrontal areas are not in the “ready” state and therefore the neurons that are recruited into such induced events likely do not carry learning‐related information. Overall, all three hypothesized pillars of memory consolidation, hippocampal ripples, neocortical slow oscillations and spindles, may be aberrant in the epileptic brain. Because delta waves and spindles induced by hippocampal IEDs can also be recorded by scalp electrodes, the presence of such events in the waking state and REM sleep can be indicative of pathological events in deep temporal lobe structures. In addition, given that hippocampal IED‐triggered spindle oscillations occur at a relatively long latency after an IED, this time window presents an opportunity for closed‐loop therapeutics aimed at improving memory dysfunction in epilepsy (Krook‐Magnuson et al., 2015).
P‐RIPPLES IN NON‐EPILEPTIC DISEASES
The term “interictal” in case of IEDs and p‐ripples may be misleading. It presupposes that seizures are a prerequisite for their emergence and they should occur exclusively in between seizures. However, abnormally synchronous, highly localized and temporally coordinated events may also emerge in the absence of overt seizures. For example, following traumatic head injury, ischemia, or experimental epilepsy paradigms, LFP events are often indistinguishable from IEDs or p‐ripples and may emerge before overt seizures and, in fact, may contribute to the eventual eruption of seizures. In the subcortically denervated hippocampus IEDs persist for months, even though overt seizures may never take place (Buzsáki et al., 1989b, 1991a). In the absence of epileptic seizures, abnormal neuronal synchrony is not classified as epileptic (most often not even diagnosed) yet its genesis may be identical to that of IEDs and p‐ripples. Furthermore, they themselves are sufficient to bring about deterioration of cognitive function, as discussed above. The importance of this insight is that numerous symptoms of “psychiatric” diseases may well be induced by abnormal patterns of neuronal cooperativity. It follows then that drugs effective in the treatment of epilepsy may be useful in other cognitive disorders as well. In line with this reasoning, mainstream antiepileptic drugs, such as valproate, gabapentin, vigabatrin, felbamate, carbamazepine, lamotrigine, levetiracetam, and topiramate, have been routinely and successfully used for the treatment of depression, bipolar disorder, schizophrenia, anxiety and post‐traumatic stress disorder, in the past two decades (Ketter et al., 1999; Boylan et al., 2002; Ovsiew, 2004).
There could be multiple reasons for such an overlap for the therapeutic efficacy of these drugs in diseases with different names, one of which is that the underlying mechanisms are in fact similar. P‐ripples in the hippocampus, for example, will go undetected by all currently available non‐invasive diagnostic tools yet they may be the core problem of some symptoms. This hypothesis may be exemplified by the comorbidity of epilepsy and bipolar disease and the improvement of both epileptic and depressive symptoms by users of lamotrigine (Edwards et al., 2001). Abnormal transformation of physiological events, such as p‐ripples, may contribute to symptoms in several cognitive disorders. Currently, such hypothetical link can be demonstrated directly only in animal models since detection of subtle yet significant changes of local neuronal excitability can be detected only by depth electrodes. Yet, as discussed above p‐ripples can arise as a consequence of dysfunctional Cl‐ homestasis and bumetanide can improve NKCC1 function and reduce the occurrence of p‐ripples. It has been suggested the bumetanide therapy may be effective in ameliorating some cognitive deficits in autistic children by reducing abnormal synchronous patterns (Tyzio et al., 2014).
P‐Ripples in Schizophrenia and Depression
There is widespread support for the notion that abnormal and quantifiable physiological changes in neuronal cooperation underlie several symptoms of schizophrenia. A key pathological alternation in both the hippocampus and prefrontal cortex is the decreased expression of parvalbumin in PV interneurons (Lewis et al., 2005) and a well‐documented consequence of such change is the alteration of gamma oscillations (Gonzalez‐Burgos et al., 2015), in which PV‐interneurons play a key regulatory role (Buzsáki et al., 1983; Buzsáki and Wang, 2012). Since PV basket cells are the key players of physiological SPW‐Rs (see Discharge Patterns of Inhibitory Neurons During SPW‐Rs and Mechanisms of Ripple Generation sections), it is expected that they are also altered in schizophrenia patients. Such hypothesized p‐ripples can affect physiological operations in at least two different ways. First, p‐ripples can compete and interfere with the native events locally and add arbitrary sets of neurons, which do not represent the previously experienced events of activity to SPW‐Rs. Second, p‐ripples can broadcast an arbitrary neuron content to wide areas of the brain and may create hallucinations, delusional and paranoid memories, symptoms also observed in patients with complex partial seizures in the interictal periods (Elliott et al., 2009). In an early study, using sphenoidal electrode recordings, a significant preponderance of temporal medio‐basal spikes was observed in patients with complex partial seizures who developed paranoid/hallucinatory psychosis but not in complex partial seizure patient without such symptoms, an indication that abnormal interictal events in the temporal lobe may be a contributing factor to the pathogenesis of psychosis (Kristensen and Sindrup, 1978).
Animal studies support the view that ripples and hippocampal‐neocortical coordination of activity can contribute to cognitive impairment underlying schizophrenia (Gardner et al., 2014), possibly by impairment of SPW‐R‐related sequence reactivation. Suh et al. (2013) recorded neural activity in the hippocampus of mice that had a forebrain‐specific knockout of the synaptic plasticity‐mediating phosphatase calcineurin, a genetic model that recapitulates some symptoms of schizophrenia (Zeng et al., 2001). Calcineurin knockout mice showed enhanced power in the ripple band and a 2.5‐fold increase in the probability of occurrence of SPW‐Rs during awake resting periods. Pyramidal neurons participated more frequently and fired more action potentials in SPW‐Rs in the knockout animals compared with control mice. While spatial properties of place cells appeared normal, sequential reactivation of place cells during SPW‐Rs was completely abolished in knockout mice. Assuming a similar impairment of SPW‐R‐related spike sequence coordination in schizophrenic subjects, such a deficit may contribute to their cognitive impairments.
Phillips et al. (2012) studied the coordination between hippocampal SPW‐R and slow oscillations/sleep spindles in the prelimbic region of the prefrontal cortex in a rat model of schizophrenia (Moore et al., 2006). In this model, a mitotoxin MAM (methylazoxymethanol‐acetate) is administered to pregnant rats at the time of hippocampal and prefrontal cortical neurogenesis in the fetus to induce a neurodevelopmental disruption of limbic‐cortical circuits (Lodge and Grace, 2009). While hippocampal SPW‐Rs appeared normal in the adult animal, the temporal coordination between SPW‐Rs and prefrontal cortical spindles during NREM sleep was severely impaired in the experimental animals. The authors hypothesized that the decreased coordination may be responsible for the spatial working memory (Gourevitch et al., 2004) and reversal learning (Moore et al., 2006) in this model of schizophrenia.
Ishikawa et al. (2014) used the spontaneous occurrence of large intracellular EPSCs or complex spike burst in CA1 pyramidal neurons, underlying SPW‐Rs, as trigger for rewarding lateral hypothalamic stimulation. Control mice quickly learned to increase the incidence of SPW‐Rs events to obtain rewarding stimulations. In contrast, mice that were tested after placing them in a cylinder filled with water for 5 min on 2 consecutive days failed to initiate such changes in SPW‐Rs. The forced swim test is an often‐used model of depression (Porsolt et al., 1977). In summary, these findings indicate that SPW‐Rs can be altered in various forms of disease and their impairment may be an important contributor of various cognitive symptoms, particularly memory deficit.
P‐Ripples in Aging and Alzheimer's Disease
Aging and particularly Alzheimer's disease and fronto‐temporal dementia are conditions in which learning and memory are severely compromised. Yet, very little is known about the possible connections between dementia‐related pathology and physiological changes underlying the cognitive deficits. The accumulation of β‐amyloid in cortical networks and tau proteins in subcortical structures are hallmarks of several forms of dementias, including Alzheimer's disease (Spires‐Jones and Hyman, 2014). β‐amyloid burden in medial prefrontal cortex inversely correlates with the power of slow oscillations in non‐REM sleep and can lead to non‐REM sleep fragmentation (Roh et al., 2012). In turn, the deterioration of slow oscillations can predict the magnitude of the failure of hippocampus‐dependent memory consolidation (Mander et al., 2015). In the reverse direction, both clinical and experimental observations suggest that non‐REM sleep disruption promotes the buildup of β‐amyloid (Kang et al., 2009). It is possible, but not yet demonstrated, that the mechanism of hippocampal‐dependent memory impairment is due to SPW‐R impairment or its altered coordination with neocortical slow oscillations and sleep spindles. In support of this hypothesis, SPW‐R‐related reactivation of place cell sequences was markedly impaired in old rats compared with young controls, commensurate with deterioration of spatial memory in the aged group (Gerrard et al., 2008).
The rTg4510 mouse, a rodent model of dementia, overexpresses a mutant (P301L) form of the microtubule associated protein tau, develops neurofibrillary tangles and displays neurodegeneration with associated age‐dependent cognitive‐behavioral deficits (Ramsden et al., 2005). Witton et al. (2014) used rTg4510 mice to explore the physiological changes in the hippocampus in this model. At 7‐ to 8‐month old, after the advanced pathological changes and cognitive decline in the mouse, SPW‐Rs were significantly lower in amplitude and had an altered temporal structure in rTg4510 mice compared with control animals, although the probability of occurrence and duration of the SPW‐Rs were relatively unaltered (Fig. 53). Putative pyramidal neurons showed increased phase‐locking to SPW‐Rs, whereas putative interneurons displayed significantly decreased phase‐locking. In another study using rTg4510 mice, hippocampal neurons did not fire at specific locations, yet displayed firing sequences as animals ran along familiar or novel trajectories, suggesting that the sequences were not primarily driven by environmental stimuli but by internally generated brain activities (Cheng and Ji, 2013). Witton et al. (2014) suggest that the reduced inhibitory control of hippocampal network and SPW‐R alterations may contribute to impairments in memory consolidation in this animal model of dementia and, by extension, in the human forms of dementia, perhaps in tandem with the reduced K complexes and sleep spindles in patients (Reynolds et al., 1985). Neuronal circuits vulnerable to Alzheimer's Disease are also affected in human amyloid precursor protein (hAPP) transgenic mice. hAPP mice with high levels of amyloid‐beta peptides in the brain develop several cognitive abnormalities and develop non‐convulsive seizures and IEDs in hippocampal and cortical circuits both of which can be attenuated by reducing excitation (Palop et al., 2007).
Figure 53.
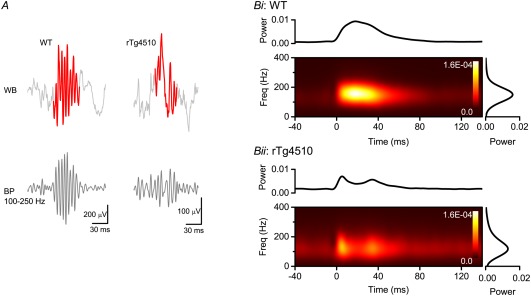
Altered SPW‐Rs in (Alzheimer's Disease model) rTg4510 mice. (A) Example SPW‐R detected from wild type (left) and rTg4510 (right) mice. The detected ripples are highlighted on the wide band (WB) trace. The band‐pass (100–250 Hz) filtered signal is shown below. Scale bars: WT, 200 μV, 30 ms; rTg4510, 100 μV, 30 ms. (B) Mean normalized short‐time Fourier analyses of SPW‐Rs in wild type and rTg4510 mice. The graph above the spectrogram shows the total power with respect to time, whilst the graph to the right shows the total power with respect to frequency. Reproduced from Witton et al. (2014).
Figure 22.
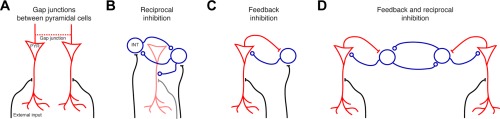
Network models of ripple oscillations. (A) Axonal net. The axons of pyramidal neurons (PYRs) are assumed to be connected via electrical synapses (gap junctions). Upon external input during a CA3‐generated SPW (black), orthodromic spikes generated by one PYR also propagate antidromically to synchronize with other PYR; the rhythm frequency may be determined by the sparseness of the gap junctions. (B) Mixed model of pyramidal cells and interneurons. (C) Pacing by feedback inhibition. Both pyramidal cells and interneurons receive external input, and the rhythm is dictated by the time constants of synaptic interaction between the two populations. (D) PYR‐INT‐INT model. Pyramidal cells receive tonic external input that activates both pyramidal cells and the reciprocally connected inhibitory network. Reciprocal inhibition paces the excited pyramidal cells, which in turn generate an LFP ripple. After Stark et al. (2014).
FUTURE TASKS
Discovery is largely serendipity. It is hard to predict the potential sources and impact of future insights. Yet, on the basis of our current knowledge, several questions can be asked about SPW‐Rs, answers to which will accelerate understanding and progress.
Do SPW‐Rs affect synaptic plasticity in the hippocampus or in downstream structures?
Does experience enrich the number of SPW‐R‐related neuronal trajectories?
What are the critical differences between waking and sleep SPW‐Rs?
What mechanisms are responsible for forward and reverse SPW‐R replays? To what extent do the CA3 and CA1 regions contribute to these sequences?
Can the downstream “reader” neurons distinguish between forward and reverse neuronal sequences?
Can elimination of SPW‐Rs in different segments of the hippocampus along its long axis teach us about the behavioral/cognitive contributions of those segments?
Ripples are largely generated locally but can also spread along the long axis of the hippocampus. What mechanisms determine the direction and extent of the travel? What are the advantages of local ripple generation and the sequestrated broadcasting of local CA1 events to different cortical targets?
Under what conditions do neurons in the dorsal and ventral CA1 region mix their activity during SPW‐Rs? Do these spatially synchronized SPW‐Rs reflect CA3‐mixed combinatorial events?
Does the speed of SPW‐R travel in the hippocampus match the travel speed of activity in topographically‐related neocortical areas?
Do superficial and deep layer CA1 pyramidal neurons generate distinct ripples?
What is the relationship between the lognormally distributed firing rates/bursts of neurons and their participation probability in SPW‐Rs?
Are the high firing, frequently participating neurons born earlier in the ontogenesis? Do the early‐born pyramidal neurons form special circuits and possess their “private” interneurons?
Do preplay neurons belong to the early born and perhaps less plastic subpopulation?
What is the role of SPW‐Rs on subcortical targets of the CA1‐subicular output?
What is the role of the CA2 subregion in SPW and ripple generation?
Does each SPW‐R contain a neuronal “word” and cover distinct segments of the environments, representing distinct objects and events?
Alternatively, if all trajectories pre‐exist independent of experience, is learning indeed a matching process between preformed trajectories and novel experiences?
Can synthetic ripples induce false memories and prime future behaviors?
Can manipulating content of SPW‐Rs or interactions with target structures enhance memories and affect decisions?
What is the significance of SPW‐R clusters and associated long replay sequences for cognition?
Are SPW‐R‐related neuronal sequences “consciously perceived” by the rest of the brain as active recall or do they strictly reflect subconscious preprocessing/priming of the to‐be‐recalled items?
Is the SPW‐R part of the preconscious creative process by linking never‐before associated events?
Can p‐ripples be responsible for multiple symptoms in cognitive diseases and can they be targeted for improving cognitive performance?
Over the past three decades, a number of laboratories interested in SPW‐Rs have made remarkable progress in understanding their mechanisms as well as discovered important links with both navigation and memory. The unique advantages of the SPW‐R are that it is an endogenous event of the hippocampus, its spike content can be manipulated, and its output can be measured effectively. SPW‐Rs of the hippocampus therefore lend themselves to an opportunity to quantify neuronal input‐output transformations and understand the mechanisms supporting such transformations in an anatomically well‐defined system. Therefore, the SPW‐R presents an opportunity of being the first brain network event to be fully understood. “Understanding” will require developing computational models that incorporate existing experimental observations and produce an output whose validity can be verified against further empirical measurements (Marr, 2010).
Figure 28.
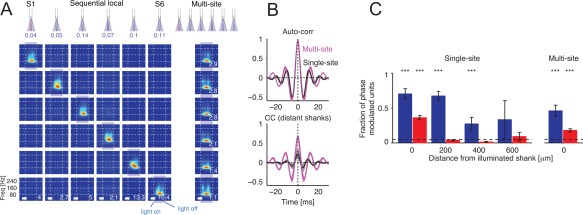
Independent ripple oscillators become coherent by simultaneous activation. (A) Simultaneous multi‐site illumination generates phase‐coherent ripples at higher amplitude (and lower frequency) as compared with sequential, single‐site illumination. Spectrograms show time‐frequency decomposition of the CA1 pyramidal layer LFP traces. Calibration bars: 20 ms. Right: auto‐correlation (same site) and cross‐correlation (distant‐sites: separation >400 μm) of LFP traces. (B) Fraction of phase‐modulated neurons during single site (S1–S6) or simultaneous multiple site (multi‐site) optogenetic stimulation of pyramidal cells (red) and putative interneurons (blue). Note long‐distance entrainment of interneurons but not pyramidal cells. After Stark et al. (2014).
Figure 45.
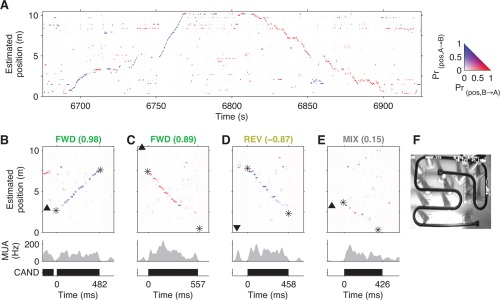
Forward and reverse extended replay. (A) Joint reconstruction of position and running direction (500 ms bins). Color indicates estimated running direction (color mapping on the right). Direction is correctly estimated for both the A/B (6750–6770 s) and B/A directions (6,820–6,850 s). (B–F) Examples of forward (FWD), reverse (REV), and mixed (MIX) replay from a representative rat, each labeled with its replay order score. Joint position and direction estimates (20 ms bins). Black triangles indicates animal's position and facing direction. Asterisks indicate start and end of detected replay trajectory. (Middle) Multiunit activity. (Bottom) Extent of a replay event. B. Forward replay in the A/B direction proceeding ahead of the animal. (C) Forward replay in the B/A direction, starting 2 m behind the animal and proceeding behind the animal. (D) Reverse replay, starting remotely and proceeding toward the animal. Trajectory is similar to (C), but this is a reverse‐ordered replay because the estimated running direction (i.e., A/B [blue]) does not agree with the direction in which the replay proceeds (i.e., from B/A). E. Top view of the 10.3 m long track. Reproduced from Davidson et al. (2009).
Acknowledgments
The author thanks John Lisman for reading and providing constructive feedback on the entire manuscript, Jan Born, Anatol Bragin, Hanna Buzsáki, Orrin Devinsky, Andreas Draguhn, Loren Frank, Attila Gulyas, Michael Hasselmo, Kai Kaila, Szabolcs Káli, Rustem Khazipov, Albert Lee, Liset Menendez de la Prida, Costas Papatheodoropoulos, Adrien Peyrache, Lisa Roux, Iván Soltész, Péter Somogyi, Eran Stark, David Tingley, Roger D. Traub and John Tukker for their comments on various parts of the manuscript.
Footnotes
SPWs can be recognized in early publications of Jouvet et al. (1959). The authors used the density of “EEG spikes” recorded from the ventral hippocampus of cats to identify non‐REM sleep (see also Hartse et al., 1979). Similar patterns were observed in presumed hippocampal recordings from a chimpanzee during simulated space flight (Freemon et al., 1969) and humans (Freemon and Walter, 1970). Kanamori (1985) used the term “hippocampal spindles” or mini‐spindles in cats that are homologous to ripples (see also Eguchi and Satoh, 1987). Buzsáki (1986) referred to them as “mini population spikes.” Vanderwolf (1969) et al. (Kramis et al., 1975) described “LIA spikes,” some of which were likely bona fide SPWs (Buzsáki et al., 1983; Suzuki and Smith, 1987a,b) but the rest could be dentate spikes (Bragin et al., 1995b). After characterizing the relationship between SPWs and the fast oscillatory events in the CA1 pyramidal layer (Buzsáki et al., 1992), I adopted the term “ripple” (despite the objection of one reviewer who felt that ripples reminded her/him of ice cream striped with colored raspberry syrup) to honor O'Keefe's early observations (1976, p. 97; see also O'Keefe and Nadel, 1978) who coined the term.
REFERENCES
- Abeles M, Bergman H, Margalit E, Vaadia E. 1993. Spatiotemporal firing patterns in the frontal cortex of behaving monkeys. J Neurophysiol 70:1629–1638. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Gustafsson B, Wigstrom H. 1986. Single high strength afferent volleys can produce long‐term potentiation in the hippocampus in vitro. Neurosci Lett 70:217–222. [DOI] [PubMed] [Google Scholar]
- Adey WR, Kado RT, Rhodes JM. 1963. Sleep: Cortical and subcortical recordings in the chimpanzee. Science 141:932–933. [DOI] [PubMed] [Google Scholar]
- Agarwal G, Stevenson IH, Berenyi A, Mizuseki K, Buzsáki G, Sommer FT. 2014. Spatially distributed local fields in the hippocampus encode rat position. Science 344:626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aivar P, Valero M, Bellistri E, Menendez de la Prida L. 2014. Extracellular calcium controls the expression of two different forms of ripple‐like hippocampal oscillations. J Neurosci 34:2989–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, McCoy B, Go CY, Ochi A, Elliott IM, Akiyama M, Donner EJ, Weiss SK, Snead OC III, Rutka JT, Drake JM, Otsubo H. 2011. Focal resection of fast ripples on extraoperative intracranial EEG improves seizure outcome in pediatric epilepsy. Epilepsia 52:1802–1811. [DOI] [PubMed] [Google Scholar]
- Alfonsa H, Merricks EM, Codadu NK, Cunningham MO, Deisseroth K, Racca C, Trevelyan AJ. 2015. The contribution of raised intraneuronal chloride to epileptic network activity. J Neurosci 35:7715–7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AB, Thomson AM. 1998. Facilitating pyramid to horizontal oriens‐alveus interneurone inputs: Dual intracellular recordings in slices of rat hippocampus. J Physiol 507(Pt 1):185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. 2001. Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol 86:3043–3055. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. 2002. A non‐alpha7 nicotinic acetylcholine receptor modulates excitatory input to hippocampal CA1 interneurons. J Neurophysiol 87:1651–1654. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. 1999. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci 19:2693–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alme CB, Miao C, Jezek K, Treves A, Moser EI, Moser MB. 2014. Place cells in the hippocampus: Eleven maps for eleven rooms. Proc Natl Acad Sci USA 111:18428–18435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado‐Rojas C, Huberfeld G, Baulac M, Clemenceau S, Charpier S, Miles R, Menendez de la Prida L, Le Van Quyen M. 2015. Different mechanisms of ripple‐like oscillations in the human epileptic subiculum. Ann Neurol 77:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D, Lavenex P. 2007. Hippocampal neuroanatomy In: Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus Book. New York: University Oxford Press. [Google Scholar]
- Amaral DG, Witter MP. 1989. The three‐dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience 31:571–591. [DOI] [PubMed] [Google Scholar]
- Amatniek JC, Hauser WA, DelCastillo‐Castaneda C, Jacobs DM, Marder K, Bell K, Albert M, Brandt J, Stern Y. 2006. Incidence and predictors of seizures in patients with Alzheimer's disease. Epilepsia 47:867–872. [DOI] [PubMed] [Google Scholar]
- Amir R, Kocsis JD, Devor M. 2005. Multiple interacting sites of ectopic spike electrogenesis in primary sensory neurons. J Neurosci 25:2576–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amzica F, Steriade M. 1997. The K‐complex: Its slow (<1‐Hz) rhythmicity and relation to delta waves. Neurology 49:952–959. [DOI] [PubMed] [Google Scholar]
- Anastassiou CA, Montgomery SM, Barahona M, Buzsáki G, Koch C. 2010. The effect of spatially inhomogeneous extracellular electric fields on neurons. J Neurosci 30:1925–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Bliss TV, Skrede KK. 1971. Lamellar organization of hippocampal pathways. Exp Brain Res 13:222–238. [DOI] [PubMed] [Google Scholar]
- Andreasen N. 2005. The Creating Brain: The Neuroscience of Genius. New York: Dana Press. [Google Scholar]
- Andrillon T, Nir Y, Staba RJ, Ferrarelli F, Cirelli C, Tononi G, Fried I. 2011. Sleep spindles in humans: Insights from intracranial EEG and unit recordings. J Neurosci 31:17821–17834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariav G, Polsky A, Schiller J. 2003. Submillisecond precision of the input‐output transformation function mediated by fast sodium dendritic spikes in basal dendrites of CA1 pyramidal neurons. J Neurosci 23:7750–7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arieli A, Sterkin A, Grinvald A, Aertsen A. 1996. Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science 273:1868–1871. [DOI] [PubMed] [Google Scholar]
- Arolfo MP, Zanudio MA, Ramirez OA. 1998. Baclofen infused in rat hippocampal formation impairs spatial learning. Hippocampus 8:109–113. [DOI] [PubMed] [Google Scholar]
- Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR. 2006. L‐655,708 enhances cognition in rats but is not proconvulsant at a dose selective for alpha5‐containing GABAA receptors. Neuropharmacology 51:1023–1029. [DOI] [PubMed] [Google Scholar]
- Atienza M, Cantero JL. 2008. Modulatory effects of emotion and sleep on recollection and familiarity. J Sleep Res 17:285–294. [DOI] [PubMed] [Google Scholar]
- Avoli M, Methot M, Kawasaki H. 1998. GABA‐dependent generation of ectopic action potentials in the rat hippocampus. Eur J Neurosci 10:2714–2722. [DOI] [PubMed] [Google Scholar]
- Avoli M, Louvel J, Pumain R, Kohling R. 2005. Cellular and molecular mechanisms of epilepsy in the human brain. Prog Neurobiol 77:166–200. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Elger CE, Fell J. 2008. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain 131:1806–1817. [DOI] [PubMed] [Google Scholar]
- Azouz R, Gray CM. 2000. Dynamic spike threshold reveals a mechanism for synaptic coincidence detection in cortical neurons in vivo. Proc Natl Acad Sci USA 97:8110–8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz R, Jensen MS, Yaari Y. 1996. Ionic basis of spike after‐depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J Physiol 492:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bading H, Ginty DD, Greenberg ME. 1993. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science 260:181–186. [DOI] [PubMed] [Google Scholar]
- Bähner F, Weiss EK, Birke G, Maier N, Schmitz D, Rudolph U, Frotscher M, Traub RD, Both M, Draguhn A. 2011. Cellular correlate of assembly formation in oscillating hippocampal networks in vitro. Proc Natl Acad Sci USA 108:E607–E616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarosie M, Louvel J, Kurcewicz I, Avoli M. 2000. CA3‐released entorhinal seizures disclose dentate gyrus epileptogenicity and unmask a temporoammonic pathway. J Neurophysiol 83:1115–1124. [DOI] [PubMed] [Google Scholar]
- Barnes DC, Wilson DA. 2014. Sleep and olfactory cortical plasticity. Front Behav Neurosci 8:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett TR, Ekstrand BR. 1972. Effect of sleep on memory. 3. Controlling for time‐of‐day effects. J Exp Psychol 96:321–327. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. 2007. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8:45–56. [DOI] [PubMed] [Google Scholar]
- Battaglia FP, Pennartz CM. 2011. The construction of semantic memory: Grammar‐based representations learned from relational episodic information. Front Comput Neurosci 5:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia FP, Sutherland GR, McNaughton BL. 2004. Hippocampal sharp wave bursts coincide with neocortical “up‐state” transitions. Learn Mem 11:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazelot M, Dinocourt C, Cohen I, Miles R. 2010. Unitary inhibitory field potentials in the CA3 region of rat hippocampus. J Physiol 588:2077–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens CJ, van den Boom LP, de Hoz L, Friedman A, Heinemann U. 2005. Induction of sharp wave‐ripple complexes in vitro and reorganization of hippocampal networks. Nat Neurosci 8:1560–1567. [DOI] [PubMed] [Google Scholar]
- Behrens CJ, van den Boom LP, Heinemann U. 2007. Effects of the GABA(A) receptorantagonists bicuculline and gabazine on stimulus‐induced sharp wave‐ripplecomplexes in adult rat hippocampus in vitro. Eur J Neurosci 25:2170–2181. [DOI] [PubMed] [Google Scholar]
- Behrens CJ, Ul Haq R, Liotta A, Anderson ML, Heinemann U. 2011. Nonspecific effects of the gap junction blocker mefloquine on fast hippocampal network oscillations in the adult rat in vitro. Neuroscience 192:11–19. [DOI] [PubMed] [Google Scholar]
- Belluscio MA, Mizuseki K, Schmidt R, Kempter R, Buzsáki G. 2012. Cross‐frequency phase‐phase coupling between theta and gamma oscillations in the hippocampus. J Neurosci 32:423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Ari Y. 2001. Developing networks play a similar melody. Trends Neurosci 24:353–360. [DOI] [PubMed] [Google Scholar]
- Ben‐Ari Y, Tremblay E, Ottersen OP. 1980. Injections of kainic acid into the amygdaloid complex of the rat: An electrographic, clinical and histological study in relation to the pathology of epilepsy. Neuroscience 5:515–528. [DOI] [PubMed] [Google Scholar]
- Ben‐Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. 1989. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol 416:303–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. 1997. GABAA, NMDA and AMPA receptors: A developmentally regulated ‘menage a trois'. Trends Neurosci 20:523–529. [DOI] [PubMed] [Google Scholar]
- Benardo LS, Prince DA. 1982. Ionic mechanisms of cholinergic excitation in mammalian hippocampal pyramidal cells. Brain Res 249:333–344. [DOI] [PubMed] [Google Scholar]
- Bender R, Plaschke M, Naumann T, Wahle P, Frotscher M. 1996. Development of cholinergic and GABAergic neurons in the rat medial septum: Different onset of choline acetyltransferase and glutamate decarboxylase mRNA expression. J Comp Neurol 372:204–214. [DOI] [PubMed] [Google Scholar]
- Bendor D, Wilson MA. 2012. Biasing the content of hippocampal replay during sleep. Nat Neurosci 15:1439–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MV, Verselis VK. 1992. Biophysics of gap junctions. Semin Cell Biol 3:29–47. [DOI] [PubMed] [Google Scholar]
- Benucci A, Frazor RA, Carandini M. 2007. Standing waves and traveling waves distinguish two circuits in visual cortex. Neuron 55:103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D. 2004. Acquired dendritic channelopathy in temporal lobe epilepsy. Science 305:532–535. [DOI] [PubMed] [Google Scholar]
- Bezare MJ, Soltész I. 2013. Quantitative assessment of CA1 local circuits: Knowledge base for interneuron‐pyramidal cell connectivity. Hippocampus 23:751–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel M, Wahl‐Schott C, Michalakis S, Zong X. 2009. Hyperpolarization‐activated cation channels: From genes to function. Physiol Rev 89:847–885. [DOI] [PubMed] [Google Scholar]
- Bikson M, Fox JE, Jefferys JG. 2003. Neuronal aggregate formation underlies spatiotemporal dynamics of nonsynaptic seizure initiation. J Neurophysiol 89:2330–2333. [DOI] [PubMed] [Google Scholar]
- Binder S, Baier PC, Mölle M, Inostroza M, Born J, Marshall L. 2012. Sleep enhances memory consolidation in the hippocampus‐dependent object‐place recognition task in rats. Neurobiol Learn Mem 97:213–219. [DOI] [PubMed] [Google Scholar]
- Binder S, Rawohl J, Born J, Marshall L. 2014. Transcranial slow oscillation stimulation during NREM sleep enhances acquisition of the radial maze task and modulates cortical network activity in rats. Front Behav Neurosci 7:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnie CD. 2003. Cognitive impairment during epileptiform discharges: Is it ever justifiable to treat the EEG? Lancet Neurol 2:725–730. [DOI] [PubMed] [Google Scholar]
- Biró E, Tóth G, Telegdy G. 1996. Effect of receptor blockers on brain natriuretic peptide and C‐type natriuretic peptide caused anxiolytic state in rats. Neuropeptides 30:59–65. [DOI] [PubMed] [Google Scholar]
- Blanco JA, Stead M, Krieger A, Viventi J, Marsh WR, Lee KH, Worrell GA, Litt B. 2010. Unsupervised classification of high‐frequency oscillations in human neocortical epilepsy and control patients. J Neurophysiol 104:2900–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco W, Pereira CM, Cota VR, Souza AC, Renno‐Costa C, Santos S, Dias G, Guerreiro AM, Tort AB, Neto AD, Ribeiro S. 2015. Synaptic homeostasis and restructuring across the sleep‐wake cycle. PLoS Comput Biol 11:e1004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland BH. 1986. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol 26:1–54. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. 1993. A synaptic model of memory: Long‐term potentiation in the hippocampus. Nature 361:31–39. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. 1973. Long‐lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232:331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolea S, Avignone E, Berretta N, Sanchez‐Andres JV, Cherubini E. 1999. Glutamate controls the induction of GABA‐mediated giant depolarizing potentials through AMPA receptors in neonatal rat hippocampal slices. J Neurophysiol 81:2095–2102. [DOI] [PubMed] [Google Scholar]
- Bonifazi P, Goldin M, Picardo MA, Jorquera I, Cattani A, Bianconi G, Represa A, Ben‐Ari Y, Cossart R. 2009. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science 326:1419–1424. [DOI] [PubMed] [Google Scholar]
- Bonin RP, Martin LJ, MacDonald JF, Orser BA. 2007. Alpha5GABAA receptors regulate the intrinsic excitability of mouse hippocampal pyramidal neurons. J Neurophysiol 98:2244–2254. [DOI] [PubMed] [Google Scholar]
- Borbely AA. 1982. A two process model of sleep regulation. Hum Neurobiol 1:195–204. [PubMed] [Google Scholar]
- Born J, Rasch B, Gais S. 2006. Sleep to remember. Neuroscientist 12:410–424. [DOI] [PubMed] [Google Scholar]
- Both M, Bähner F, von Bohlen und Halbach O, Draguhn A. 2008. Propagation of specific network patterns through the mouse hippocampus. Hippocampus 18:899–908. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. 2005. Millisecond‐timescale, genetically targeted optical control of neural activity. Nat Neurosci 8:1263–1268. [DOI] [PubMed] [Google Scholar]
- Boylan LS, Devinsky O, Barry JJ, Ketter TA. 2002. Psychiatric uses of antiepileptic treatments. Epilepsy Behav 3:54–59. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nádasdy Z, Hetke J, Wise K, Buzsáki G. 1995a. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci 15:47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nádasdy Z, van Landeghem M, Buzsáki G. 1995b. Dentate EEG spikes and associated interneuronal population bursts in the hippocampal hilar region of the rat. J Neurophysiol 73:1691–1705. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J Jr, Wilson CL, Fried I, Buzsáki G. 1999a. High‐frequency oscillations in human brain. Hippocampus 9:137–142. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J Jr, Wilson CL, Fried I, Mathern GW. 1999b. Hippocampal and entorhinal cortex high‐frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid‐treated rats with chronic seizures. Epilepsia 40:127–137. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J Jr, Wilson CL, Vizentin E, Mathern GW. 1999c. Electrophysiologic analysis of a chronic seizure model after unilateral hippocampal KA injection. Epilepsia 40:1210–1221. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Engel J Jr. 2000. Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: A hypothesis. Epilepsia 41(Suppl 6):S144–S152. [DOI] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J Jr. 2002a. Local generation of fast ripples in epileptic brain. J Neurosci 22:2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, Engel J Jr. 2002b. Interictal high‐frequency oscillations (80–500 Hz) in the human epileptic brain: Entorhinal cortex. Ann Neurol 52:407–415. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Engel J. 2003. Spatial stability over time of brain areas generating fast ripples in the epileptic rat. Epilepsia 44:1233–1237. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J Jr. 2004. High‐frequency oscillations after status epilepticus: Epileptogenesis and seizure genesis. Epilepsia 45:1017–1023. [DOI] [PubMed] [Google Scholar]
- Bragin A, Azizyan A, Almajano J, Wilson CL, Engel J Jr. 2005. Analysis of chronic seizure onsets after intrahippocampal kainic acid injection in freely moving rats. Epilepsia 46:1592–1598. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Engel J Jr. 2007. Voltage depth profiles of high‐frequency oscillations after kainic acid‐induced status epilepticus. Epilepsia 48(Suppl 5):35–40. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Srebro B. 1987. Induction of long‐term depression and potentiation by low‐ and high‐frequency stimulation in the dentate area of the anesthetized rat: Magnitude, time course and EEG. Brain Res 405:100–107. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Srebro B. 1989. Synaptic plasticity in the hippocampus is modulated by behavioral state. Brain Res 493:74–86. [DOI] [PubMed] [Google Scholar]
- Bremer F. 1935. Cerveau “isole” et physiologie du sommeil. C R Séances Soc Biol Fil 118:1235–1241. [Google Scholar]
- Brinciotti M, Matricardi M, Paolella A, Porro G, Benedetti P. 1989. Neuropsychological correlates of subclinical paroxysmal EEG activity in children with epilepsy. 1: Qualitative features (generalized and focal abnormalities). Funct Neurol 4:235–239. [PubMed] [Google Scholar]
- Brunel N, Hakim V. 1999. Fast global oscillations in networks of integrate‐and‐fire neurons with low firing rates. Neural Comput 11:1621–1671. [DOI] [PubMed] [Google Scholar]
- Brunel N, Wang XJ. 2003. What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation‐inhibition balance. J Neurophysiol 90:415–430. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Hormuzdi S, Barbe MT, Herb A, Monyer H. 2003. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci USA 100:13644–13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Wenzel HJ, Kunkel DD, Schwartzkroin PA. 1996. Axon arbors and synaptic connections of hippocampal mossy cells in the rat in vivo. J Comp Neurol 366:271–292. [DOI] [PubMed] [Google Scholar]
- Buckner RL. 2010. The role of the hippocampus in prediction and imagination. Annu Rev Psychol 61:27–48, C21–28. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. 2007. Self‐projection and the brain. Trends Cogn Sci 11:49–57. [DOI] [PubMed] [Google Scholar]
- Buhl DL, Buzsáki G. 2005. Developmental emergence of hippocampal fast‐field “ripple” oscillations in the behaving rat pups. Neuroscience 134:1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl EH, Halasy K, Somogyi P. 1994. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature 368:823–828. [DOI] [PubMed] [Google Scholar]
- Buhl DL, Harris KD, Hormuzdi SG, Monyer H, Buzsáki G. 2003. Selective impairment of hippocampal gamma oscillations in connexin‐36 knock‐out mouse in vivo. J Neurosci 23:1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukalo O, Campanac E, Hoffman DA, Fields RD. 2013. Synaptic plasticity by antidromic firing during hippocampal network oscillations. Proc Natl Acad Sci USA 110:5175–5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock TH, Bennett MV, Johnston D, Josephson R, Marder E, Fields RD. 2005. Neuroscience. The neuron doctrine, redux. Science 310:791–793. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. 1984. Long‐term changes of hippocampal sharp‐waves following high frequency afferent activation. Brain Res 300:179–182. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. 1985. What does the “LTP model of memory” model?In: Will GE, Schmitt P, Dalrymple‐Alford JC, editors. Brain Plasticity, Learning and Memory. New York: Plenum Press; pp 157–166. [Google Scholar]
- Buzsáki G. 1986. Hippocampal sharp waves: Their origin and significance. Brain Res 398:242–252. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. 1989. Two‐stage model of memory trace formation: A role for “noisy” brain states. Neuroscience 31:551–570. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. 1996. The hippocampo‐neocortical dialogue. Cereb Cortex 6:81–92. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. 1998. Memory consolidation during sleep: A neurophysiological perspective. J Sleep Res 7(Suppl 1):17–23. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. 2002. Theta oscillations in the hippocampus. Neuron 33:325–340. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. 2005. Theta rhythm of navigation: Link between path integration and landmark navigation, episodic and semantic memory. Hippocampus 15:827–840. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. 2006. Rhythms of the Brain. New York: Oxford University Press. [Google Scholar]
- Buzsáki G. 2010. Neural syntax: Cell assemblies, synapsembles, and readers. Neuron 68:362–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. 2015. Neuroscience. Our skewed sense of space. Science 347:612–613. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Chrobak JJ. 2005. Synaptic plasticity and self‐organization in the hippocampus. Nat Neurosci 8:1418–1420. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. 2004. Neuronal oscillations in cortical networks. Science 304:1926–1929. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Gage FH. 1988. Neural grafts: Possible mechanisms of action In: Petit TL, Ivy GO, editors. Neural Plasticity: A Lifespan Approach. New York: Alan R. Liss, Inc. [Google Scholar]
- Buzsáki G, Mizuseki K. 2014. The log‐dynamic brain: How skewed distributions affect network operations. Nat Rev Neurosci 15:264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Moser EI. 2013. Memory, navigation and theta rhythm in the hippocampal‐entorhinal system. Nat Neurosci 16:130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Schomburg EW. 2015. What does gamma coherence tell us about inter‐regional neural communication? Nat Neurosci 18:484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Silva FL. 2012. High frequency oscillations in the intact brain. Prog Neurobiol 98:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Wang XJ. 2012. Mechanisms of gamma oscillations. Annu Rev Neurosci 35:203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Leung LW, Vanderwolf CH. 1983. Cellular bases of hippocampal EEG in the behaving rat. Brain Res 287:139–171. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Czopf J, Kondakor I, Bjorklund A, Gage FH. 1987a. Cellular activity of intracerebrally transplanted fetal hippocampus during behavior. Neuroscience 22:871–883. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Gage FH, Czopf J, Bjorklund A. 1987b. Restoration of rhythmic slow activity (theta) in the subcortically denervated hippocampus by fetal CNS transplants. Brain Res 400:334–347. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Gage FH, Kellenyi L, Bjorklund A. 1987c. Behavioral dependence of the electrical activity of intracerebrally transplanted fetal hippocampus. Brain Res 400:321–333. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Haas HL, Anderson EG. 1987d. Long‐term potentiation induced by physiologically relevant stimulus patterns. Brain Res 435:331–333. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Bayardo F, Miles R, Wong RK, Gage FH. 1989a. The grafted hippocampus: An epileptic focus. Exp Neurol 105:10–22. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Ponomareff GL, Bayardo F, Ruiz R, Gage FH. 1989b. Neuronal activity in the subcortically denervated hippocampus: A chronic model for epilepsy. Neuroscience 28:527–538. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Chen LS, Gage FH. 1990. Spatial organization of physiological activity in the hippocampal region: Relevance to memory formation. Prog Brain Res 83:257–268. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Hsu M, Slamka C, Gage FH, Horvath Z. 1991a. Emergence and propagation of interictal spikes in the subcortically denervated hippocampus. Hippocampus 1:163–180. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Masliah E, Chen LS, Horvath Z, Terry R, Gage FH. 1991b. Hippocampal grafts into the intact brain induce epileptic patterns. Brain Res 554:30–37. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Horvath Z, Urioste R, Hetke J, Wise K. 1992. High‐frequency network oscillation in the hippocampus. Science 256:1025–1027. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Bragin A, Chrobak JJ, Nádasdy Z, Sik A, Hsu M, Ylinen A. 1994. Oscillatory and intermittent synchrony in the hippocampus: Relevance to memory trace formation In: Buzsáki G, Llinas R, Singer W, Berthoz A, Christen Y, editors. Temporal Coding in the Brain. Berlin: Springer. [Google Scholar]
- Buzsáki G, Penttonen M, Nádasdy Z, Bragin A. 1996. Pattern and inhibition‐dependent invasion of pyramidal cell dendrites by fast spikes in the hippocampus in vivo. Proc Natl Acad Sci USA 93:9921–9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Csicsvari J, Dragoi G, Harris K, Henze D, Hirase H. 2002. Homeostatic maintenance of neuronal excitability by burst discharges in vivo. Cereb Cortex 12:893–899. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Buhl DL, Harris KD, Csicsvari J, Czeh B, Morozov A. 2003. Hippocampal network patterns of activity in the mouse. Neuroscience 116:201–211. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Kaila K, Raichle M. 2007. Inhibition and brain work. Neuron 56:771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C. 2012. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat Rev Neurosci 13:407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Logothetis N, Singer W. 2013. Scaling brain size, keeping timing: Evolutionary preservation of brain rhythms. Neuron 80:751–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Peyrache A, Kubie J. 2014. Emergence of cognition from action. Cold Spring Harb Symp Quant Biol 79:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillard O, McLean HA, Ben‐Ari Y, Gaiarsa JL. 1998. Ontogenesis of presynaptic GABAB receptor‐mediated inhibition in the CA3 region of the rat hippocampus. J Neurophysiol 79:1341–1348. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. 2006. High gamma power is phase‐locked to theta oscillations in human neocortex. Science 313:1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You‐Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. 2004. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit‐containing gamma‐aminobutyric acid type A receptors. Proc Natl Acad Sci USA 101:3662–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpi D, Dragoi G, Benuck M, Buzsáki G. 1997. Neuronal activity of lateral septum during hippocampal theta and sharp waves. Soc Neurosci (Abst) 23:484. [Google Scholar]
- Carr MF, Jadhav SP, Frank LM. 2011. Hippocampal replay in the awake state: A potential substrate for memory consolidation and retrieval. Nat Neurosci 14:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MF, Karlsson MP, Frank LM. 2012. Transient slow gamma synchrony underlies hippocampal memory replay. Neuron 75:700–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers MS, Atack JR, Broughton HB, Collinson N, Cook S, Dawson GR, Hobbs SC, Marshall G, Maubach KA, Pillai GV, Reeve AJ, MacLeod AM. 2003. Identification of a novel, selective GABA(A) alpha5 receptor inverse agonist which enhances cognition. J Med Chem 46:2227–2240. [DOI] [PubMed] [Google Scholar]
- Chan CY, Nicholson C. 1986. Modulation by applied electric fields of Purkinje and stellate cell activity in the isolated turtle cerebellum. J Physiol 371:89–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Frank LM. 2008. New experiences enhance coordinated neural activity in the hippocampus. Neuron 57:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Ji D. 2013. Rigid firing sequences undermine spatial memory codes in a neurodegenerative mouse model. Elife 2:e00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng VY, Martin LJ, Elliott EM, Kim JH, Mount HT, Taverna FA, Roder JC, Macdonald JF, Bhambri A, Collinson N, Wafford KA, Orser BA. 2006. Alpha5GABAA receptors mediate the amnestic but not sedative‐hypnotic effects of the general anesthetic etomidate. J Neurosci 26:3713–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiovini B, Turi GF, Katona G, Kaszas A, Palfi D, Maak P, Szalay G, Szabo MF, Szabo G, Szadai Z, Kali S, Rozsa B. 2014. Dendritic spikes induce ripples in parvalbumin interneurons during hippocampal sharp waves. Neuron 82:908–924. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsáki G. 1994. Selective activation of deep layer (V‐VI) retrohippocampal cortical neurons during hippocampal sharp waves in the behaving rat. J Neurosci 14:6160–6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsáki G. 1996. High‐frequency oscillations in the output networks of the hippocampal‐entorhinal axis of the freely behaving rat. J Neurosci 16:3056–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church J, Baimbridge KG. 1991. Exposure to high‐pH medium increases the incidence and extent of dye coupling between rat hippocampal CA1 pyramidal neurons in vitro. J Neurosci 11:3289–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Cunningham JP, Kaufman MT, Foster JD, Nuyujukian P, Ryu SI, Shenoy KV. 2012. Neural population dynamics during reaching. Nature 487:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichon J, Gan WB. 2015. Branch‐specific dendritic Ca(2+) spikes cause persistent synaptic plasticity. Nature 520:180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Passecker J, Malagon‐Vina H, Mikus N, Klausberger T. 2015. Brain computation. Selective information routing by ventral hippocampal CA1 projection neurons. Science 348:560–563. [DOI] [PubMed] [Google Scholar]
- Clemens Z, Mölle M, Eross L, Barsi P, Halasz P, Born J. 2007. Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain 130:2868–2878. [DOI] [PubMed] [Google Scholar]
- Clemens Z, Mölle M, Eross L, Jakus R, Rasonyi G, Halász P, Born J. 2011. Fine‐tuned coupling between human parahippocampal ripples and sleep spindles. Eur J Neurosci 33:511–520. [DOI] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. 1995. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 378:75–78. [DOI] [PubMed] [Google Scholar]
- Cofer CN, Appley MH. 1964. Motivation: Theory and Research. New York: Wiley. [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. 2002. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science 298:1418–1421. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. 1993. Memory, Amnesia, and the Hippocampal System. Cambridge, MA: MIT Press. [Google Scholar]
- Cole AE, Nicoll RA. 1983. Acetylcholine mediates a slow synaptic potential in hippocampal pyramidal cells. Science 221:1299–1301. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Kubota D, Jia YS, Rex CS, Lynch G. 2004. Long‐term potentiation is impaired in rat hippocampal slices that produce spontaneous sharp waves. J Physiol 558:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Jia Y, Sabatier JM, Lynch G. 2005. Blockade of NMDA receptors enhances spontaneous sharp waves in rat hippocampal slices. Neuroscience letters 385:46–51. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. 2009. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 462:353–357. [DOI] [PubMed] [Google Scholar]
- Condorelli DF, Belluardo N, Trovato‐Salinaro A, Mudo G. 2000. Expression of Cx36 in mammalian neurons. Brain Res Brain Res Rev 32:72–85. [DOI] [PubMed] [Google Scholar]
- Connors BW, Malenka RC, Silva LR. 1988. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor‐mediated responses in neocortex of rat and cat. J Physiol 406:443–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Destexhe A, Sejnowski TJ, Steriade M. 1996. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science 274:771–774. [DOI] [PubMed] [Google Scholar]
- Craik K. 1943. The Nature of Explanation. Cambridge: Cambridge University Press. [Google Scholar]
- Csicsvari J, Dupret D. 2014. Sharp wave/ripple network oscillations and learning‐associated hippocampal maps. Philos Trans R Soc Lond Ser B Biol Sci 369:20120528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Buzsáki G. 1998. Reliability and state dependence of pyramidal cell‐interneuron synapses in the hippocampus: An ensemble approach in the behaving rat. Neuron 21:179–189. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsáki G. 1999a. Fast network oscillations in the hippocampal CA1 region of the behaving rat. J Neurosci 19:RC20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsáki G. 1999b. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving Rat. J Neurosci 19:274–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Mamiya A, Buzsáki G. 2000. Ensemble patterns of hippocampal CA3‐CA1 neurons during sharp wave‐associated population events. Neuron 28:585–594. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Henze DA, Jamieson B, Harris KD, Sirota A, Bartho P, Wise KD, Buzsáki G. 2003a. Massively parallel recording of unit and local field potentials with silicon‐based electrodes. J Neurophysiol 90:1314–1323. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsáki G. 2003b. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron 37:311–322. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, O'Neill J, Allen K, Senior T. 2007. Place‐selective firing contributes to the reverse‐order reactivation of CA1 pyramidal cells during sharp waves in open‐field exploration. Eur J Neurosci 26:704–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MO, Roopun A, Schofield IS, Whittaker RG, Duncan R, Russell A, Jenkins A, Nicholson C, Whittington MA, Traub RD. 2012. Glissandi: Transient fast electrocorticographic oscillations of steadily increasing frequency, explained by temporally increasing gap junction conductance. Epilepsia 53:1205–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curio G. 1999. High frequency (600 Hz) bursts of spike‐like activities generated in the human cerebral somatosensory system. Electroencephalogr Clin Neurophysiol Suppl 49:56–61. [PubMed] [Google Scholar]
- Cutsuridis V, Hasselmo M. 2012. GABAergic contributions to gating, timing, and phase precession of hippocampal neuronal activity during theta oscillations. Hippocampus 22:1597–1621. [DOI] [PubMed] [Google Scholar]
- Cutsuridis V, Taxidis J. 2013. Deciphering the role of CA1 inhibitory circuits in sharp wave‐ripple complexes. Front Syst Neurosci 7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, Weitzman E, Moore‐Ede MC, Zimmerman JC, Knauer RS. 1980. Human sleep: Its duration and organization depend on its circadian phase. Science 210:1264–1267. [DOI] [PubMed] [Google Scholar]
- D'Antuono M, de Guzman P, Kano T, Avoli M. 2005. Ripple activity in the dentate gyrus of dishinibited hippocampus‐entorhinal cortex slices. J Neurosci Res 80:92–103. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Raffard S, Van der Linden M. 2008. Remembering the past and imagining the future in schizophrenia. J Abnorm Psychol 117:247–251. [DOI] [PubMed] [Google Scholar]
- Danglot L, Triller A, Marty S. 2006. The development of hippocampal interneurons in rodents. Hippocampus 16:1032–1060. [DOI] [PubMed] [Google Scholar]
- Danish SF, Moyer JT, Finkel LH, Baltuch GH, Jaggi JL, Priori A, Foffani G. 2007. High‐frequency oscillations (>200 Hz) in the human non‐parkinsonian subthalamic nucleus. Brain Res Bull 74:84–90. [DOI] [PubMed] [Google Scholar]
- Daurat A, Terrier P, Foret J, Tiberge M. 2007. Slow wave sleep and recollection in recognition memory. Conscious Cogn 16:445–455. [DOI] [PubMed] [Google Scholar]
- Davachi L. 2006. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol 16:693–700. [DOI] [PubMed] [Google Scholar]
- Davidson TJ, Kloosterman F, Wilson MA. 2009. Hippocampal replay of extended experience. Neuron 63:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida L, Idiart M, Lisman JE. 2007. Memory retrieval time and memory capacity of the CA3 network: Role of gamma frequency oscillations. Learn Mem 14:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M. 2003. Sleep spindles: An overview. Sleep Med Rev 7:423–440. [DOI] [PubMed] [Google Scholar]
- de la Rocha J, Doiron B, Shea‐Brown E, Josic K, Reyes A. 2007. Correlation between neural spike trains increases with firing rate. Nature 448:802–806. [DOI] [PubMed] [Google Scholar]
- De Manacéine M. 1897. Sleep: Its Physiology, Pathology, Hygiene, and Psychology. New York: Charles Scribner's Sons. [Google Scholar]
- Decker JM, Wojtowicz AM, Ul Haq R, Braunewell KH, Heinemann U, Behrens CJ. 2009. C‐type natriuretic peptide decreases hippocampal network oscillations in adult rats in vitro. Neuroscience 164:1764–1775. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP. 2011. Experimental and theoretical approaches to conscious processing. Neuron 70:200–227. [DOI] [PubMed] [Google Scholar]
- Dement W, Kleitman N. 1957. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr Clin Neurophysiol 9:673–690. [DOI] [PubMed] [Google Scholar]
- Dermietzel R, Spray DC. 1993. Gap junctions in the brain: Where, what type, how many and why? Trends Neurosci 16:186–192. [DOI] [PubMed] [Google Scholar]
- Deuchars J, Thomson AM. 1996. CA1 pyramid‐pyramid connections in rat hippocampus in vitro: Dual intracellular recordings with biocytin filling. Neuroscience 74:1009–1018. [DOI] [PubMed] [Google Scholar]
- Diba K, Buzsáki G. 2007. Forward and reverse hippocampal place‐cell sequences during ripples. Nat Neurosci 10:1241–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diba K, Buzsáki G. 2008. Hippocampal network dynamics constrain the time lag between pyramidal cells across modified environments. J Neurosci 28:13448–13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson R, Awaiz S, Whittington MA, Lieb WR, Franks NP. 2003. The effects of general anaesthetics on carbachol‐evoked gamma oscillations in the rat hippocampus in vitro. Neuropharmacology 44:864–872. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. 2010. The memory function of sleep. Nat Rev Neurosci 11:114–126. [DOI] [PubMed] [Google Scholar]
- Dolorfo CL, Amaral DG. 1998. Entorhinal cortex of the rat: Topographic organization of the cells of origin of the perforant path projection to the dentate gyrus. J Comp Neurol 398:25–48. [PubMed] [Google Scholar]
- Dragoi G, Buzsáki G. 2006. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron 50:145–157. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Tonegawa S. 2011. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature 469:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G, Tonegawa S. 2013. Distinct preplay of multiple novel spatial experiences in the rat. Proc Natl Acad Sci USA 110:9100–9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G, Tonegawa S. 2014. Selection of preconfigured cell assemblies for representation of novel spatial experiences. Philos Trans R Soc Lond Ser B Biol Sci 369:20120522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G, Carpi D, Recce M, Csicsvari J, Buzsáki G. 1999. Interactions between hippocampus and medial septum during sharp waves and theta oscillation in the behaving rat. J Neurosci 19:6191–6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G, Harris KD, Buzsáki G. 2003. Place representation within hippocampal networks is modified by long‐term potentiation. Neuron 39:843–853. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Traub RD, Schmitz D, Jefferys JG. 1998. Electrical coupling underlies high‐frequency oscillations in the hippocampus in vitro. Nature 394:189–192. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Traub RD, Bibbig A, Schmitz D. 2000. Ripple (approximately 200‐Hz) oscillations in temporal structures. J Clin Neurophysiol 17:361–376. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA. 2002. Mu opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus 12:119–136. [DOI] [PubMed] [Google Scholar]
- Drever BD, Riedel G, Platt B. 2011. The cholinergic system and hippocampal plasticity. Behav Brain Res 221:505–514. [DOI] [PubMed] [Google Scholar]
- Dreyfus‐Brisac C, Fischgold H, Samson‐Dollfus D, Saint‐Anne Dargassies S, Ziegler T, Monod N, Blanc C. 1956. Veille sommeil et reactivite sensorielle chez le premature et le nouveau‐ne. Electroencephalogr Clin Neurophysiol 6:418–440. [Google Scholar]
- Drosopoulos S, Wagner U, Born J. 2005. Sleep enhances explicit recollection in recognition memory. Learn Mem 12:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosopoulos S, Windau E, Wagner U, Born J. 2007. Sleep enforces the temporal order in memory. PloS One 2:e376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosopoulos S, Harrer D, Born J. 2011. Sleep and awareness about presence of regularity speed the transition from implicit to explicit knowledge. Biol Psychol 86:168–173. [DOI] [PubMed] [Google Scholar]
- Dudai Y. 2012. The restless engram: Consolidations never end. Annu Rev oNeurosci 35:227–247. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Sutula TP. 2007. Epileptogenesis in the dentate gyrus: A critical perspective. Prog Brain Res 163:755–773. [DOI] [PubMed] [Google Scholar]
- Dumay N, Gaskell MG. 2007. Sleep‐associated changes in the mental representation of spoken words. Psychol Sci 18:35–39. [DOI] [PubMed] [Google Scholar]
- Dupret D, O'Neill J, Pleydell‐Bouverie B, Csicsvari J. 2010. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci 13:995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dur‐e‐Ahmad M, Nicola W, Campbell SA, Skinner FK. 2012. Network bursting using experimentally constrained single compartment CA3 hippocampal neuron models with adaptation. J Comput Neurosci 33:21–40. [DOI] [PubMed] [Google Scholar]
- Dutar P, Nicoll RA. 1988. Pre‐ and postsynaptic GABAB receptors in the hippocampus have different pharmacological properties. Neuron 1:585–591. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Staley KJ. 2004. Mechanisms of fast ripples in the hippocampus. J Neurosci 24:8896–8906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KR, Sackellares JC, Vuong A, Hammer AE, Barrett PS. 2001. Lamotrigine monotherapy improves depressive symptoms in epilepsy: A double‐blind comparison with valproate. Epilepsy Behav 2:28–36. [DOI] [PubMed] [Google Scholar]
- Ego‐Stengel V, Wilson MA. 2010. Disruption of ripple‐associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus 20:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorov AV, Gloveli T, Muller W. 1999. Muscarinic control of dendritic excitability and Ca(2+) signaling in CA1 pyramidal neurons in rat hippocampal slice. J Neurophysiol 82:1909–1915. [DOI] [PubMed] [Google Scholar]
- Eguchi K, Satoh T. 1987. Relationship between positive sharp wave bursts and unitary discharges in the cat hippocampus during slow‐wave sleep. Phys Behav 40:497–499. [DOI] [PubMed] [Google Scholar]
- Eguchi M, Yamaguchi S. 2009. In vivo and in vitro visualization of gene expression dynamics over extensive areas of the brain. NeuroImage 44:1274–1283. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. 2000. A cortical‐hippocampal system for declarative memory. Nat Rev Neurosci 1:41–50. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. 2004. Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron 44:109–120. [DOI] [PubMed] [Google Scholar]
- Ekstrand BR, Barrett T, West IN, Maier WG. 1977. The effect of sleep on human long‐term memory In: Drucker‐Colin RR, McGaugh JL, editors. Neurobiology of Sleep and Memory. New York: Academic Press; pp 419–438. [Google Scholar]
- Elias LA, Kriegstein AR. 2008. Gap junctions: Multifaceted regulators of embryonic cortical development. Trends Neurosci 31:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen JM, Hu PT, Payne JD, Titone D, Walker MP. 2007. Human relational memory requires time and sleep. Proc Natl Acad Sci USA 104:7723–7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellender TJ, Nissen W, Colgin LL, Mann EO, Paulsen O. 2010. Priming of hippocampal population bursts by individual perisomatic‐targeting interneurons. J Neurosci 30:5979–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott B, Joyce E, Shorvon S. 2009. Delusions, illusions and hallucinations in epilepsy: 2. Complex phenomena and psychosis. Epilepsy Res 85:172–186. [DOI] [PubMed] [Google Scholar]
- Engel J Jr, Ackermann RF. 1980. Interictal EEG spikes correlate with decreased, rather than increased, epileptogenicity in amygdaloid kindled rats. Brain Res 190:543–548. [DOI] [PubMed] [Google Scholar]
- Engel J Jr, da Silva FL. 2012. High‐frequency oscillations—Where we are and where we need to go. Prog Neurobiol 98:316–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J Jr, Bragin A, Staba R, Mody I. 2009. High‐frequency oscillations: What is normal and what is not? Epilepsia 50:598–604. [DOI] [PubMed] [Google Scholar]
- English DF, Peyrache A, Stark E, Roux L, Vallentin D, Long MA, Buzsáki G. 2014. Excitation and inhibition compete to control spiking during hippocampal ripples: Intracellular study in behaving mice. J Neurosci 34:16509–16517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epsztein J, Lee AK, Chorev E, Brecht M. 2010. Impact of spikelets on hippocampal CA1 pyramidal cell activity during spatial exploration. Science 327:474–477. [DOI] [PubMed] [Google Scholar]
- Eschenko O, Sara SJ. 2008. Learning‐dependent, transient increase of activity in noradrenergic neurons of locus coeruleus during slow wave sleep in the rat: Brain stem‐cortex interplay for memory consolidation? Cereb Cortex 18:2596–2603. [DOI] [PubMed] [Google Scholar]
- Eschenko O, Mölle M, Born J, Sara SJ. 2006. Elevated sleep spindle density after learning or after retrieval in rats. J Neurosci 26:12914–12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenko O, Ramadan W, Mölle M, Born J, Sara SJ. 2008. Sustained increase in hippocampal sharp‐wave ripple activity during slow‐wave sleep after learning. Learn Mem 15:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RH, Hill RG. 1978. GABA‐mimetic action of etomidate. Experientia 34:1325–1327. [DOI] [PubMed] [Google Scholar]
- Farrant M, Kaila K. 2007. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog Brain Res 160:59–87. [DOI] [PubMed] [Google Scholar]
- Faulkner HJ, Traub RD, Whittington MA. 1998. Disruption of synchronous gamma oscillations in the rat hippocampal slice: A common mechanism of anaesthetic drug action. Br J Pharmacol 125:483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbinteanu J, Shapiro ML. 2003. Prospective and retrospective memory coding in the hippocampus. Neuron 40:1227–1239. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Klausberger T, Cobden P, Baude A, Roberts JD, Szucs P, Kinoshita A, Shigemoto R, Somogyi P, Dalezios Y. 2005. Metabotropic glutamate receptor 8‐expressing nerve terminals target subsets of GABAergic neurons in the hippocampus. J Neurosci 25:10520–10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch DM, Nowlin NL, Babb TL. 1983. Demonstration of axonal projections of neurons in the rat hippocampus and subiculum by intracellular injection of HRP. Brain Res 271:201–216. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. 1998. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature 394:186–189. [DOI] [PubMed] [Google Scholar]
- Fischer S, Drosopoulos S, Tsen J, Born J. 2006. Implicit learning—Explicit knowing: A role for sleep in memory system interaction. J Cogn Neurosci 18:311–319. [PubMed] [Google Scholar]
- Fischer V, Both M, Draguhn A, Egorov AV. 2014. Choline‐mediated modulation of hippocampal sharp wave‐ripple complexes in vitro. J Neurochem 129:792–805. [DOI] [PubMed] [Google Scholar]
- Fishell G, Rudy B. 2011. Mechanisms of inhibition within the telencephalon: “Where the wild things are”. Annu Rev Neurosci 34:535–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foffani G, Priori A, Egidi M, Rampini P, Tamma F, Caputo E, Moxon KA, Cerutti S, Barbieri S. 2003. 300‐Hz subthalamic oscillations in Parkinson's disease. Brain 126:2153–2163. [DOI] [PubMed] [Google Scholar]
- Foffani G, Ardolino G, Egidi M, Caputo E, Bossi B, Priori A. 2006. Subthalamic oscillatory activities at beta or higher frequency do not change after high‐frequency DBS in Parkinson's disease. Brain Res Bull 69:123–130. [DOI] [PubMed] [Google Scholar]
- Foffani G, Uzcategui YG, Gal B, Menendez de la Prida L. 2007. Reduced spike‐timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron 55:930–941. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA. 2006. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440:680–683. [DOI] [PubMed] [Google Scholar]
- Fourcaud‐Trocme N, Hansel D, van Vreeswijk C, Brunel N. 2003. How spike generation mechanisms determine the neuronal response to fluctuating inputs. J Neurosci 23:11628–11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler MJ, Sullivan MJ, Ekstrand BR. 1973. Sleep and memory. Science 179:302–304. [DOI] [PubMed] [Google Scholar]
- Frank MG, Issa NP, Stryker MP. 2001. Sleep enhances plasticity in the developing visual cortex. Neuron 30:275–287. [DOI] [PubMed] [Google Scholar]
- Frank M, Eiberger B, Janssen‐Bienhold U, Muller LPD, Tjarks A, Kim JS, Maschke S, Dobrowolski R, Sasse P, Weiler R, Fleischmann BK, Willecke K. 2010. Neuronal connexin‐36 can functionally replace connexin‐45 in mouse retina but not in the developing heart. J Cell Sci 123:3605–3615. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. 2005. The organization of recent and remote memories. Nature Rev Neurosci 6:119–130. [DOI] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. 1994. Molecular and cellular mechanisms of general anaesthesia. Nature 367:607–614. [DOI] [PubMed] [Google Scholar]
- Freeman WJ. 1975. Mass action in the Nervous System: Examination of the Neurophysiological Basis of Adaptive Behavior Through the EEG. New York: Academic Press. [Google Scholar]
- Freemon FR, Walter RD. 1970. Electrical activity of human limbic system during sleep. Compr psychiatry 11:544–551. [DOI] [PubMed] [Google Scholar]
- Freemon FR, McNew JJ, Adey WR. 1969. Sleep of unrestrained chimpanzee: Cortical and subcortical recordings. Exp Neurol 25:129–137. [DOI] [PubMed] [Google Scholar]
- Freund TF. 2003. Interneuron diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci 26:489–495. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. 1988. Alterations in excitatory and GABAergic inhibitory connections in hippocampal transplants. Neuroscience 27:373–385. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. 1996. Interneurons of the hippocampus. Hippocampus 6:347–470. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. 2007. Perisomatic inhibition. Neuron 56:33–42. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. 2003. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83:1017–1066. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. 1997. Synaptic tagging and long‐term potentiation. Nature 385:533–536. [DOI] [PubMed] [Google Scholar]
- Fuentealba P, Begum R, Capogna M, Jinno S, Marton LF, Csicsvari J, Thomson A, Somogyi P, Klausberger T. 2008a. Ivy cells: A population of nitric‐oxide‐producing, slow‐spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron 58:295–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba P, Tomioka R, Dalezios Y, Marton LF, Studer M, Rockland K, Klausberger T, Somogyi P. 2008b. Rhythmically active enkephalin‐expressing GABAergic cells in the CA1 area of the hippocampus project to the subiculum and preferentially innervate interneurons. J Neurosci 28:10017–10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba P, Klausberger T, Karayannis T, Suen WY, Huck J, Tomioka R, Rockland K, Capogna M, Studer M, Morales M, Somogyi P. 2010. Expression of COUP‐TFII nuclear receptor in restricted GABAergic neuronal populations in the adult rat hippocampus. J Neurosci 30:1595–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa S, Amarasingham A, Harrison MT, Buzsáki G. 2008. Behavior‐dependent short‐term assembly dynamics in the medial prefrontal cortex. Nat Neurosci 11:823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Kosaka T. 2000. Gap junctions linking the dendritic network of GABAergic interneurons in the hippocampus. J Neurosci 20:1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Aika Y, Heizmann CW, Kosaka T. 1996. Dense GABAergic input on somata of parvalbumin‐immunoreactive GABAergic neurons in the hippocampus of the mouse. Neurosci Res 26:181–194. [DOI] [PubMed] [Google Scholar]
- Gaiarsa JL, Corradetti R, Cherubini E, Ben‐Ari Y. 1991. Modulation of GABA‐mediated synaptic potentials by glutamatergic agonists in neonatal CA3 rat hippocampal neurons. Eur J Neurosci 3:301–309. [DOI] [PubMed] [Google Scholar]
- Gais S, Born J. 2004. Low acetylcholine during slow‐wave sleep is critical for declarative memory consolidation. Proc Natl Acad Sci USA 101:2140–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. 2001. Spike transmission and synchrony detection in networks of GABAergic interneurons. Science 292:2295–2299. [DOI] [PubMed] [Google Scholar]
- Garaschuk O, Hanse E, Konnerth A. 1998. Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J Physiol 507(Pt 1):219–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RJ, Kersante F, Jones MW, Bartsch U. 2014. Neural oscillations during non‐rapid eye movement sleep as biomarkers of circuit dysfunction in schizophrenia. Eur J Neurosci 39:1091–1106. [DOI] [PubMed] [Google Scholar]
- Gasparini S, Migliore M, Magee JC. 2004. On the initiation and propagation of dendritic spikes in CA1 pyramidal neurons. J Neurosci 24:11046–11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler C, Brunel N, Wang XJ. 2005. Contributions of intrinsic membrane dynamics to fast network oscillations with irregular neuronal discharges. J Neurophysiol 94:4344–4361. [DOI] [PubMed] [Google Scholar]
- Geisler C, Robbe D, Zugaro M, Sirota A, Buzsáki G. 2007. Hippocampal place cell assemblies are speed‐controlled oscillators. Proc Natl Acad Sci USA 104:8149–8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet LJ, Kremer Y, Taniguchi H, Huang ZJ, Staiger JF, Petersen CC. 2012. Unique functional properties of somatostatin‐expressing GABAergic neurons in mouse barrel cortex. Nat Neurosci 15:607–612. [DOI] [PubMed] [Google Scholar]
- Gerrard JL, Burke SN, McNaughton BL, Barnes CA. 2008. Sequence reactivation in the hippocampus is impaired in aged rats. J Neurosci 28:7883–7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannopoulos P, Papatheodoropoulos C. 2013. Effects of μ‐opioid receptor modulation on the hippocampal network activity of sharp wave and ripples. Br J Pharmacol 168:1146–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. 1999. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402:75–79. [DOI] [PubMed] [Google Scholar]
- Girardeau G, Zugaro M. 2011. Hippocampal ripples and memory consolidation. Curr Opin Neurobiol 21:452–459. [DOI] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. 2009. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci 12:1222–1223. [DOI] [PubMed] [Google Scholar]
- Girardeau G, Cei A, Zugaro M. 2014. Learning‐induced plasticity regulates hippocampal sharp wave‐ripple drive. J Neurosci 34:5176–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickfeld LL, Roberts JD, Somogyi P, Scanziani M. 2009. Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nature Neurosci 12:21–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman SE, Schiff BB. 1967. A biological theory of reinforcement. Psychol Rev 74:81–109. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. 2006. Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABA A receptor alpha5 subunit‐deficient mice. J Neurophysiol 95:2796–2807. [DOI] [PubMed] [Google Scholar]
- Goddard GV. 1967. Development of epileptic seizures through brain stimulation at low intensity. Nature 214:1020–1021. [DOI] [PubMed] [Google Scholar]
- Goddard GV, Douglas RM. 1975. Does the engram of kindling model the engram of normal long term memory? Can J Neurol Sci 2:385–394. [DOI] [PubMed] [Google Scholar]
- Gold C, Henze DA, Koch C, Buzsáki G. 2006. On the origin of the extracellular action potential waveform: A modeling study. J Neurophysiol 95:3113–3128. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Burgos G, Cho RY, Lewis DA. 2015. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry 77:1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon B. 2013. Can We Control Our Thoughts? Why Do Thoughts Pop into My Head as I'm Trying to Fall Asleep? Sci Am. Available at: http://www.scientificamerican.com/article/can-we-control-our-thoughts/. [Google Scholar]
- Gottesmann C. 1973. [Intermediate stages of sleep in the rat]. Rev Electroencephalogr Neurophysiol Clin 3:65–68. [DOI] [PubMed] [Google Scholar]
- Gourevitch R, Rocher C, Le Pen G, Krebs MO, Jay TM. 2004. Working memory deficits in adult rats after prenatal disruption of neurogenesis. Behav Pharmacol 15:287–292. [DOI] [PubMed] [Google Scholar]
- Grastyán E, Karmos G. 1961. A study of a possible “dreaming” mechanism in the cat. Acta Physiol Acad Sci Hung 20:41–50. [PubMed] [Google Scholar]
- Grenier F, Timofeev I, Steriade M. 2001. Focal synchronization of ripples (80–200 hz) in neocortex and their neuronal correlates. J Neurophysiol 86:1884–1898. [DOI] [PubMed] [Google Scholar]
- Grenier F, Timofeev I, Steriade M. 2003. Neocortical very fast oscillations (ripples, 80–200 Hz) during seizures: Intracellular correlates. J Neurophysiol 89:841–852. [DOI] [PubMed] [Google Scholar]
- Griessenberger H, Hoedlmoser K, Heib DP, Lechinger J, Klimesch W, Schabus M. 2012. Consolidation of temporal order in episodic memories. Biol Psychol 91:150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosmark AD, Mizuseki K, Pastalkova E, Diba K, Buzsáki G. 2012. REM sleep reorganizes hippocampal excitability. Neuron 75:1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosvenor A, Lack LC. 1984. The effect of sleep before or after learning on memory. Sleep 7:155–167. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Freund TT. 2015. Generation of physiological and pathological high frequency oscillations: The role of perisomatic inhibition in sharp‐wave ripple and interictal spike generation. Curr Opin Neurobiol 31:26–32. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Miles R, Sik A, Toth K, Tamamaki N, Freund TF. 1993. Hippocampal pyramidal cells excite inhibitory neurons through a single release site. Nature 366:683–687. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Megias M, Emri Z, Freund TF. 1999. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci 19:10082–10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AS, van der Meer MA, Touretzky DS, Redish AD. 2010. Hippocampal replay is not a simple function of experience. Neuron 65:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie ER. 1952. The Psychology of Learning. New York: Harper and Row. [Google Scholar]
- Gutnick MJ, Prince DA. 1972. Thalamocortical relay neurons: Antidromic invasion of spikes from a cortical epileptogenic focus. Science 176:424–426. [DOI] [PubMed] [Google Scholar]
- Gutnick MJ, Lobel‐Yaakov R, Rimon G. 1985. Incidence of neuronal dye‐coupling in neocortical slices depends on the plane of section. Neuroscience 15:659–666. [DOI] [PubMed] [Google Scholar]
- Haggerty DC, Ji D. 2014. Initiation of sleep‐dependent cortical‐hippocampal correlations at wakefulness‐sleep transition. J Neurophysiol 112:1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn TT, Sakmann B, Mehta MR. 2007. Differential responses of hippocampal subfields to cortical up‐down states. Proc Natl Acad Sci USA 104:5169–5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N Hájos, TJ Ellender, R Zemankovics, EO Mann, R Exley, SJ Cragg, TF Freund, O Paulsen. 2009. Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. Eur J Neurosci 29:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Mody I. 2009. Establishing a physiological environment for visualized in vitro brain slice recordings by increasing oxygen supply and modifying aCSF content. J Neurosci Methods 183:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Palhalmi J, Mann EO, Nemeth B, Paulsen O, Freund TF. 2004. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci 24:9127–9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Karlocai MR, Nemeth B, Ulbert I, Monyer H, Szabo G, Erdelyi F, Freund TF, Gulyás AI. 2013. Input‐output features of anatomically identified CA3 neurons during hippocampal sharp wave/ripple oscillation in vitro. J Neurosci 33:11677–11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Siegle JH, Ritt JT, Ting JT, Feng G, Moore CI. 2011. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nat Neurosci 14:1118–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halász P, Pál I, Rajna P. 1985. K‐complex formation of the EEG in sleep. A survey and new examinations. Acta Phys Hung 65:3–35. [PubMed] [Google Scholar]
- Halliwell JV, Adams PR. 1982. Voltage‐clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res 250:71–92. [DOI] [PubMed] [Google Scholar]
- Harris KD, Hirase H, Leinekugel X, Henze DA, Buzsáki G. 2001. Temporal interaction between single spikes and complex spike bursts in hippocampal pyramidal cells. Neuron 32:141–149. [DOI] [PubMed] [Google Scholar]
- Hart JT. 1965. Memory and the feeling‐of‐knowing experience. J Educ Psychol 56:208–216. [DOI] [PubMed] [Google Scholar]
- Hartse KM, Eisenhart SF, Bergmann BM, Rechtschaffen A. 1979. Ventral hippocampus spikes during sleep, wakefulness, and arousal in the cat. Sleep 1:231–246. [DOI] [PubMed] [Google Scholar]
- Harvey CD, Coen P, Tank DW. 2012. Choice‐specific sequences in parietal cortex during a virtual‐navigation decision task. Nature 484:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. 2007. Using imagination to understand the neural basis of episodic memory. J Neurosci 27:14365–14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. 1995. Neuromodulation and cortical function: Modeling the physiological basis of behavior. Behav Brain Res 67:1–27. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. 1999. Neuromodulation: Acetylcholine and memory consolidation. Trends Cogn Sci 3:351–359. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. 2006. The role of acetylcholine in learning and memory. Curr Opin Neurobiol 16:710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Bower JM. 1993. Acetylcholine and memory. Trends Neurosci 16:218–222. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Schnell E. 1994. Laminar selectivity of the cholinergic suppression of synaptic transmission in rat hippocampal region CA1: Computational modeling and brain slice physiology. J Neurosci 14:3898–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Wyble BP. 1997. Free recall and recognition in a network model of the hippocampus: Simulating effects of scopolamine on human memory function. Behav Brain Res 89:1–34. [DOI] [PubMed] [Google Scholar]
- Hawkins CA, Mellanby JH. 1987. Limbic epilepsy induced by tetanus toxin: A longitudinal electroencephalographic study. Epilepsia 28:431–444. [DOI] [PubMed] [Google Scholar]
- Hefft S, Jonas P. 2005. Asynchronous GABA release generates long‐lasting inhibition at a hippocampal interneuron‐principal neuron synapse. Nature Neurosci 8:1319–1328. [DOI] [PubMed] [Google Scholar]
- Heit G, Smith ME, Halgren E. 1988. Neural encoding of individual words and faces by the human hippocampus and amygdala. Nature 333:773–775. [DOI] [PubMed] [Google Scholar]
- Hennequin G, Vogels TP, Gerstner W. 2014. Optimal control of transient dynamics in balanced networks supports generation of complex movements. Neuron 82:1394–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Buzsáki G. 2001. Action potential threshold of hippocampal pyramidal cells in vivo is increased by recent spiking activity. Neuroscience 105:121–130. [DOI] [PubMed] [Google Scholar]
- Herkenham M. 1978. The connections of the nucleus reuniens thalami: Evidence for a direct thalamo‐hippocampal pathway in the rat. J Comp Neurol 177:589–610. [DOI] [PubMed] [Google Scholar]
- Herman JP, Dolgas CM, Rucker D, Langub MC Jr. 1996. Localization of natriuretic peptide‐activated guanylate cyclase mRNAs in the rat brain. J Comp Neurol 369:165–187. [DOI] [PubMed] [Google Scholar]
- Hinman JR, Penley SC, Long LL, Escabi MA, Chrobak JJ. 2011. Septotemporal variation in dynamics of theta: Speed and habituation. J Neurophysiol 105:2675–2686. [DOI] [PubMed] [Google Scholar]
- Hirase H, Leinekugel X, Csicsvari J, Czurko A, Buzsáki G. 2001a. Behavior‐dependent states of the hippocampal network affect functional clustering of neurons. J Neurosci 21:RC145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase H, Leinekugel X, Czurko A, Csicsvari J, Buzsáki G. 2001b. Firing rates of hippocampal neurons are preserved during subsequent sleep episodes and modified by novel awake experience. Proc Natl Acad Sci USA 98:9386–9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson JA, Pace‐Schott EF. 2002. The cognitive neuroscience of sleep: Neuronal systems, consciousness and learning. Nat Rev Neurosci 3:679–693. [DOI] [PubMed] [Google Scholar]
- Hofer KT, Kandracs A, Ulbert I, Pal I, Szabo C, Heja L, Wittner L. 2015. The hippocampal CA3 region can generate two distinct types of sharp wave‐ripple complexes, in vitro. Hippocampus 25:169–186. [DOI] [PubMed] [Google Scholar]
- Hollnagel JO, Maslarova A, Haq RU, Heinemann U. 2014. GABAB receptor dependent modulation of sharp wave‐ripple complexes in the rat hippocampus in vitro. Neurosci Lett 574:15–20. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Lenck‐Santini PP. 2006. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav 8:504–515. [DOI] [PubMed] [Google Scholar]
- Hopfield JJ. 1982. Neural networks and physical systems with emergent collective computational abilities. Proc Natl Acad Sci USA 79:2554–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormuzdi SG, Pais I, LeBeau FE, Towers SK, Rozov A, Buhl EH, Whittington MA, Monyer H. 2001. Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36‐deficient mice. Neuron 31:487–495. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J. 1978. Presynaptic inhibitory action of acetylcholine in area CA1 of the hippocampus. Exp Neurol 62:787–797. [DOI] [PubMed] [Google Scholar]
- Howard MW, Fotedar MS, Datey AV, Hasselmo ME. 2005. The temporal context model in spatial navigation and relational learning: Toward a common explanation of medial temporal lobe function across domains. Psychol Rev 112:75–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. 2004. Local sleep and learning. Nature 430:78–81. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. 2006. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci 9:1169–1176. [DOI] [PubMed] [Google Scholar]
- Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, Rivera C. 2007. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci 27:9866–9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberfeld G, Menendez de la Prida L, Pallud J, Cohen I, Le Van Quyen M, Adam C, Clemenceau S, Baulac M, Miles R. 2011. Glutamatergic pre‐ictal discharges emerge at the transition to seizure in human epilepsy. Nat Neurosci 14:627–634. [DOI] [PubMed] [Google Scholar]
- Hubner CA, Stein V, Hermans‐Borgmeyer I, Meyer T, Ballanyi K, Jentsch TJ. 2001. Disruption of KCC2 reveals an essential role of K‐Cl cotransport already in early synaptic inhibition. Neuron 30:515–524. [DOI] [PubMed] [Google Scholar]
- Ibarz JM, Foffani G, Cid E, Inostroza M, Menendez de la Prida L. 2010. Emergent dynamics of fast ripples in the epileptic hippocampus. J Neurosci 30:16249–16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzikowski C. 1978. Sleep and memory in humansIn: Gruneberg MM, Morris PE, Sykes RN, editors. Practical Aspects of Memory. London: Academic Press; pp 313–314. [Google Scholar]
- Inostroza M, Born J. 2013. Sleep for preserving and transforming episodic memory. Annu Rev Neurosci 36:79–102. [DOI] [PubMed] [Google Scholar]
- Ishikawa D, Matsumoto N, Sakaguchi T, Matsuki N, Ikegaya Y. 2014. Operant conditioning of synaptic and spiking activity patterns in single hippocampal neurons. J Neurosci 34:5044–5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. 1990. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol 295:580–623. [DOI] [PubMed] [Google Scholar]
- Isomura Y, Sirota A, Ozen S, Montgomery S, Mizuseki K, Henze DA, Buzsáki G. 2006. Integration and segregation of activity in entorhinal‐hippocampal subregions by neocortical slow oscillations. Neuron 52:871–882. [DOI] [PubMed] [Google Scholar]
- Izquierdo I. 1979. Effect of naloxone and morphine on various forms of memory in the rat ‐ possible role of endogenous opiate mechanisms in memory consolidation. Psychopharmacology 66:199–203. [DOI] [PubMed] [Google Scholar]
- Jackson J. 1875. Clinical and Physiological Researches on the Nervous System. No. 1. On the Localisation of Movements on the Brain. London: J. and A. Churchill. [Google Scholar]
- Jackson JC, Johnson A, Redish AD. 2006. Hippocampal sharp waves and reactivation during awake states depend on repeated sequential experience. J Neurosci 26:12415–12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. 2008. Interictal high‐frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia 49:1893–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Levan P, Chatillon CE, Olivier A, Dubeau F, Gotman J. 2009. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain 132:1022–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Zijlmans M, Zelmann R, Chatillon CE, Hall J, Olivier A, Dubeau F, Gotman J. 2010a. High‐frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol 67:209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Zijlmans M, Zelmann R, Olivier A, Hall J, Gotman J, Dubeau F. 2010b. Value of electrical stimulation and high frequency oscillations (80–500 Hz) in identifying epileptogenic areas during intracranial EEG recordings. Epilepsia 51:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Staba R, Asano E, Otsubo H, Wu JY, Zijlmans M, Mohamed I, Kahane P, Dubeau F, Navarro V, Gotman J. 2012. High‐frequency oscillations (HFOs) in clinical epilepsy. Prog Neurobiol 98:302–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Kemere C, German PW, Frank LM. 2012. Awake hippocampal sharp‐wave ripples support spatial memory. Science 336:1454–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnke S, Memmesheimer RM, Timme M. 2014. Oscillation‐induced signal transmission and gating in neural circuits. PLoS Comput Biol 10:e1003940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JG. 1995. Nonsynaptic modulation of neuronal activity in the brain: Electric currents and extracellular ions. Phys Rev 75:689–723. [DOI] [PubMed] [Google Scholar]
- Jefferys JG, Haas HL. 1982. Synchronized bursting of CA1 hippocampal pyramidal cells in the absence of synaptic transmission. Nature 300:448–450. [DOI] [PubMed] [Google Scholar]
- Jefferys JG, Menendez de la Prida L, Wendling F, Bragin A, Avoli M, Timofeev I, Lopes da Silva FH. 2012. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol 98:250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins JG, Dallenbach KM. 1924. Obliviscence during sleep and waking. Am J Psychol 35:605–612. [Google Scholar]
- Ji D, Wilson MA. 2007. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci 10:100–107. [DOI] [PubMed] [Google Scholar]
- Jinno S, Klausberger T, Marton LF, Dalezios Y, Roberts JD, Fuentealba P, Bushong EA, Henze D, Buzsáki G, Somogyi P. 2007. Neuronal diversity in GABAergic long‐range projections from the hippocampus. J Neurosci 27:8790–8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. 2006. High‐frequency oscillations during human focal seizures. Brain 129:1593–1608. [DOI] [PubMed] [Google Scholar]
- Jiruska P, Bragin A. 2011. High‐frequency activity in experimental and clinical epileptic foci. Epilepsy Res 97:300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiruska P, Csicsvari J, Powell AD, Fox JE, Chang WC, Vreugdenhil M, Li X, Palus M, Bujan AF, Dearden RW, Jefferys JG. 2010a. High‐frequency network activity, global increase in neuronal activity, and synchrony expansion precede epileptic seizures in vitro. J Neurosci 30:5690–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiruska P, Powell AD, Chang WC, Jefferys JG. 2010b. Electrographic high‐frequency activity and epilepsy. Epilepsy Res 89:60–65. [DOI] [PubMed] [Google Scholar]
- Johnson A, Redish AD. 2005. Hippocampal replay contributes to within session learning in a temporal difference reinforcement learning model. Neural Netw 18:1163–1171. [DOI] [PubMed] [Google Scholar]
- Johnson A, Redish AD. 2007. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci 27:12176–12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Euston DR, Tatsuno M, McNaughton BL. 2010. Stored‐trace reactivation in rat prefrontal cortex is correlated with down‐to‐up state fluctuation density. J Neurosci 30:2650–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Williams S, Jaffe D, Gray R. 1992. NMDA‐receptor‐independent long‐term potentiation. Annu Rev Physiol 54:489–505. [DOI] [PubMed] [Google Scholar]
- Jones MS, Barth DS. 1999. Spatiotemporal organization of fast (>200 Hz) electrical oscillations in rat Vibrissa/Barrel cortex. J Neurophysiol 82:1599–1609. [DOI] [PubMed] [Google Scholar]
- Jones MS, Barth DS. 2002. Effects of bicuculline methiodide on fast (>200 Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol 88:1016–1025. [DOI] [PubMed] [Google Scholar]
- Jouvet M. 1999. The Paradox of Sleep: The Story of Dreaming. Cambridge: MIT Press. [Google Scholar]
- Jouvet M. 2004. How sleep was dissociated into two states: Telencephalic and rhombencephalic sleep? Arch Ital Biol 142:317–326. [PubMed] [Google Scholar]
- Jouvet M, Michel F, Courjon JL. 1959. L'activite electrique du rhinencephale au cours du sommeil chez le chat. C R Soc Biol (Paris) 153:101–105. [PubMed] [Google Scholar]
- Kahana MJ. 1996. Associative retrieval processes in free recall. Mem Cogn 24:103–109. [DOI] [PubMed] [Google Scholar]
- Kaila K. 1994. Ionic basis of GABAA receptor channel function in the nervous system. Prog Neurobiol 42:489–537. [DOI] [PubMed] [Google Scholar]
- Kaila K, TJ Price, JA Payne, M Puskarjov, J Voipio. 2014. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci 15:637–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kali S, Dayan P. 2004. Off‐line replay maintains declarative memories in a model of hippocampal‐neocortical interactions. Nat Neurosci 7:286–294. [DOI] [PubMed] [Google Scholar]
- Kamondi A, Acsády L, Buzsáki G. 1998a. Dendritic spikes are enhanced by cooperative network activity in the intact hippocampus. J Neurosci 18:3919–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamondi A, Acsády L, Wang XJ, Buzsáki G. 1998b. Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: Activity‐dependent phase‐precession of action potentials. Hippocampus 8:244–261. [DOI] [PubMed] [Google Scholar]
- Kanamori N. 1985. A spindle‐like wave in the cat hippocampus: A novel vigilance level‐dependent electrical activity. Brain Res 334:180–182. [DOI] [PubMed] [Google Scholar]
- Kandel A, Buzsáki G. 1997. Cellular‐synaptic generation of sleep spindles, spike‐and‐wave discharges, and evoked thalamocortical responses in the neocortex of the rat. J Neurosci 17:6783–6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. 2009. Amyloid‐beta dynamics are regulated by orexin and the sleep‐wake cycle. Science 326:1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano T, Inaba Y, Avoli M. 2005. Periodic oscillatory activity in parahippocampal slices maintained in vitro. Neuroscience 130:1041–1053. [DOI] [PubMed] [Google Scholar]
- Kanterewicz BI, Urban NN, McMahon DB, Norman ED, Giffen LJ, Favata MF, Scherle PA, Trzskos JM, Barrionuevo G, Klann E. 2000. The extracellular signal‐regulated kinase cascade is required for NMDA receptor‐independent LTP in area CA1 but not area CA3 of the hippocampus. J Neurosci 20:3057–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlocai MR, Kohus Z, Kali S, Ulbert I, Szabo G, Mate Z, Freund TF, Gulyás AI. 2014. Physiological sharp wave‐ripples and interictal events in vitro: What's the difference? Brain 137:463–485. [DOI] [PubMed] [Google Scholar]
- Karlsson KA, Blumberg MS. 2004. Temperature‐induced reciprocal activation of hippocampal field activity. J Neurophysiol 91:583–588. [DOI] [PubMed] [Google Scholar]
- Karlsson MP, Frank LM. 2009. Awake replay of remote experiences in the hippocampus. Nat Neurosci 12:913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MA Karson, AH Tang, TA Milner, BE Alger. 2009. Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J Neurosci 29:4140–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KA, Mohns EJ, di Prisco GV, Blumberg MS. 2006. On the co‐occurrence of startles and hippocampal sharp waves in newborn rats. Hippocampus 16:959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Freund TF. 2008. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med 14:923–930. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. 1999. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 19:4544–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona L, Lapray D, Viney TJ, Oulhaj A, Borhegyi Z, Micklem BR, Klausberger T, Somogyi P. 2014. Sleep and movement differentiates actions of two types of somatostatin‐expressing GABAergic interneuron in rat hippocampus. Neuron 82:872–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanau JL. 2000. Mental malfunction and memory maintenance mechanisms. Med Hypotheses 54:678–683. [DOI] [PubMed] [Google Scholar]
- Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A. 2003. Spontaneously emerging cortical representations of visual attributes. Nature 425:954–956. [DOI] [PubMed] [Google Scholar]
- Kepecs A, Fishell G. 2014. Interneuron cell types are fit to function. Nature 505:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- K Kerber, M Dümpelmann, B Schelter, P Le Van, R Korinthenberg, A Schulze-Bonhage, J Jacobs. 2014. Differentiation of specific ripple patterns helps to identify epileptogenic areas for surgical procedures. Clin Neurophysiol 125:1339–1345. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Post RM, Theodore WH. 1999. Positive and negative psychiatric effects of antiepileptic drugs in patients with seizure disorders. Neurology 53:S53–67. [PubMed] [Google Scholar]
- Khalilov I, Esclapez M, Medina I, Aggoun D, Lamsa K, Leinekugel X, Khazipov R, Ben‐Ari Y. 1997. A novel in vitro preparation: The intact hippocampal formation. Neuron 19:743–749. [DOI] [PubMed] [Google Scholar]
- Khalilov I, Dzhala V, Ben‐Ari Y, Khazipov R. 1999. Dual role of GABA in the neonatal rat hippocampus. Developmental neuroscience 21:310–319. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Luhmann HJ. 2006. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci 29:414–418. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Leinekugel X, Khalilov I, Gaiarsa JL, Ben‐Ari Y. 1997. Synchronization of GABAergic interneuronal network in CA3 subfield of neonatal rat hippocampal slices. J Physiol 498(Pt 3):763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Esclapez M, Caillard O, Bernard C, Khalilov I, Tyzio R, Hirsch J, Dzhala V, Berger B, Ben‐Ari Y. 2001. Early development of neuronal activity in the primate hippocampus in utero. J Neurosci 21:9770–9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben‐Ari Y, Buzsáki G. 2004. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature 432:758–761. [DOI] [PubMed] [Google Scholar]
- Khodagholy D, Gelinas JN, Thesen T, Doyle W, Devinsky O, Malliaras GG, Buzsáki G. 2015. NeuroGrid: Recording action potentials from the surface of the brain. Nat Neurosci 18:310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Henze DA, Leinekugel X, Buzsáki G. 1999. Hebbian modification of a hippocampal population pattern in the rat. J Physiology 521(Pt 1):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Pignatelli M, Suh J, Kohara K, Yoshiki A, Abe K, Tonegawa S. 2014. Island cells control temporal association memory. Science 343:896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. 2008. Neuronal diversity and temporal dynamics: The unity of hippocampal circuit operations. Science 321:53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsáki G, Somogyi P. 2003. Brain‐state‐ and cell‐type‐specific firing of hippocampal interneurons in vivo. Nature 421:844–848. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, Baude A, Roberts JD, Magill PJ, Somogyi P. 2004. Spike timing of dendrite‐targeting bistratified cells during hippocampal network oscillations in vivo. Nat Neurosci 7:41–47. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, O'Neill J, Huck JH, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, Csicsvari J, Somogyi P. 2005. Complementary roles of cholecystokinin‐ and parvalbumin‐expressing GABAergic neurons in hippocampal network oscillations. J Neurosci 25:9782–9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleen JK, Scott RC, Holmes GL, Lenck‐Santini PP. 2010. Hippocampal interictal spikes disrupt cognition in rats. Ann Neurol 67:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoche A, Yokoyama H, Ponomarenko A, Frisch C, Huston J, Haas HL. 2003. High‐frequency oscillation in the hippocampus of the behaving rat and its modulation by the histaminergic system. Hippocampus 13:273–280. [DOI] [PubMed] [Google Scholar]
- Knowles WD, Schwartzkroin PA. 1981. Local circuit synaptic interactions in hippocampal brain slices. J Neurosci 1:318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ, Fanselow MS. 1998. The hippocampus, consolidation and on‐line memory. Curr Opin Neurobiol 8:293–296. [DOI] [PubMed] [Google Scholar]
- Köhling R, Staley K. 2011. Network mechanisms for fast ripple activity in epileptic tissue. Epilepsy Res 97:318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koniaris E, Drimala P, Sotiriou E, Papatheodoropoulos C. 2011. Different effects of zolpidem and diazepam on hippocampal sharp wave‐ripple activity in vitro. Neuroscience 175:224–234. [DOI] [PubMed] [Google Scholar]
- Konnerth A, Heinemann U. 1983. Effects of GABA on presumed presynaptic Ca2+ entry in hippocampal slices. Brain Res 270:185–189. [DOI] [PubMed] [Google Scholar]
- Konorski J. 1976. Integrative Activity of the Brain. An Interdisciplinary Approach. Chicago: University of Chicago Press. [Google Scholar]
- Korpi ER, Grunder G, Luddens H. 2002. Drug interactions at GABA(A) receptors. Prog Neurobiol 67:113–159. [DOI] [PubMed] [Google Scholar]
- Kosaka T. 1983. Gap junctions between non‐py ramidal cell dendrites in the rat hippocampus (CAI and CA3 regions). Brain Res 271:157–161. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Ball TM, Reiser BJ. 1978. Visual images preserve metric spatial information: Evidence from studies of image scanning. J Exp Psychol Hum Percept Perform 4:47–60. [DOI] [PubMed] [Google Scholar]
- Kramis R, Vanderwolf CH, Bland BH. 1975. Two types of hippocampal rhythmical slow activity in both the rabbit and the rat: Relations to behavior and effects of atropine, diethyl ether, urethane, and pentobarbital. Exp Neurol 49:58–85. [DOI] [PubMed] [Google Scholar]
- Kranig SA, Duhme N, Waldeck C, Draguhn A, Reichinnek S, Both M. 2013. Different functions of hyperpolarization‐activated cation channels for hippocampal sharp waves and ripples in vitro. Neuroscience 228:325–333. [DOI] [PubMed] [Google Scholar]
- Krauss GL, Summerfield M, Brandt J, Breiter S, Ruchkin D. 1997. Mesial temporal spikes interfere with working memory. Neurology 49:975–980. [DOI] [PubMed] [Google Scholar]
- Kristensen O, Sindrup EH. 1978. Psychomotor epilepsy and psychosis. II. Electroencephalographic findings (sphenoidal electrode recordings). Acta Neurol Scand 57:370–379. [PubMed] [Google Scholar]
- Krnjevic K. 1992. Cellular and synaptic actions of general anaesthetics. Gen Pharmacol 23:965–975. [DOI] [PubMed] [Google Scholar]
- Krook‐Magnuson E, Gelinas JN, Soltész I, Buzsáki G. 2015. Neuroelectronics and biooptics: Closed‐loop technologies in neurological disorders. JAMA Neurol 72:823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota D, Colgin LL, Casale M, Brucher FA, Lynch G. 2003. Endogenous waves in hippocampal slices. J Neurophysiol 89:81–89. [DOI] [PubMed] [Google Scholar]
- Kudrimoti HS, Barnes CA, McNaughton BL. 1999. Reactivation of hippocampal cell assemblies: Effects of behavioral state, experience, and EEG dynamics. J Neurosci 19:4090–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. 2005. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: Where and why? Prog Biophys Mol Biol 87:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner R, Kohr G, Grunewald S, Eisenhardt G, Bach A, Kornau HC. 1999. Role of heteromer formation in GABAB receptor function. Science 283:74–77. [DOI] [PubMed] [Google Scholar]
- Ladd GT. 1892. Contribution to the psychology of visual dreams. Mind 1:299–304. [Google Scholar]
- Lahtinen H, Palva JM, Sumanen S, Voipio J, Kaila K, Taira T. 2002. Postnatal development of rat hippocampal gamma rhythm in vivo. J Neurophysiol 88:1469–1474. [DOI] [PubMed] [Google Scholar]
- Laje R, Buonomano DV. 2013. Robust timing and motor patterns by taming chaos in recurrent neural networks. Nat Neurosci 16:925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MP, Marinelli PW, Bai L, Gianoulakis C. 2008. Effects of acute ethanol on opioid peptide release in the central amygdala: An in vivo microdialysis study. Psychopharmacology 201:261–271. [DOI] [PubMed] [Google Scholar]
- Lamsa K, Palva JM, Ruusuvuori E, Kaila K, Taira T. 2000. Synaptic GABAA activation inhibits AMPA‐kainate receptor‐mediated bursting in the newborn (P0‐P2) rat hippocampus. J Neurophysiol 83:359–366. [DOI] [PubMed] [Google Scholar]
- Langston RF, Ainge JA, Couey JJ, Canto CB, Bjerknes TL, Witter MP, Moser EI, Moser MB. 2010. Development of the spatial representation system in the rat. Science 328:1576–1580. [DOI] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CM. 2009. Hippocampus leads ventral striatum in replay of place‐reward information. Plos Biol 7:e1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapray D, Lasztoczi B, Lagler M, Viney TJ, Katona L, Valenti O, Hartwich K, Borhegyi Z, Somogyi P, Klausberger T. 2012. Behavior‐dependent specialization of identified hippocampal interneurons. Nat Neurosci 15:1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasztóczi B, Tukker JJ, Somogyi P, Klausberger T. 2011. Terminal field and firing selectivity of cholecystokinin‐expressing interneurons in the hippocampal CA3 area. J Neurosci 31:18073–18093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau H, Alger SE, Fishbein W. 2011. Relational memory: A daytime nap facilitates the abstraction of general concepts. PloS one 6:e27139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Van Quyen M, Bragin A, Staba R, Crepon B, Wilson CL, Engel J Jr. 2008. Cell type‐specific firing during ripple oscillations in the hippocampal formation of humans. J Neurosci 28:6104–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Van Quyen M, Staba R, Bragin A, Dickson C, Valderrama M, Fried I, Engel J. 2010. Large‐scale microelectrode recordings of high‐frequency gamma oscillations in human cortex during sleep. J Neurosci 30:7770–7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc MO, Bland BH. 1979. Developmental aspects of hippocampal electrical activity and motor behavior in the rat. Exp Neurol 66:220–237. [DOI] [PubMed] [Google Scholar]
- Lebovitz RM, Dichter M, Spencer WA. 1971. Recurrent excitation in the CA3 region of cat hippocampus. Int J Neurosci 2:99–107. [DOI] [PubMed] [Google Scholar]
- Lee AK, Wilson MA. 2002. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36:1183–1194. [DOI] [PubMed] [Google Scholar]
- Lee SH, Marchionni I, Bezaire M, Varga C, Danielson N, Lovett‐Barron M, Losonczy A, Soltész I. 2014. Parvalbumin‐positive basket cells differentiate among hippocampal pyramidal cells. Neuron 82:1129–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, McBain CJ. 2003. GABA B receptor modulation of excitatory and inhibitory synaptic transmission onto rat CA3 hippocampal interneurons. J Physiol 546:439–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinekugel X, Tseeb V, Ben‐Ari Y, Bregestovski P. 1995. Synaptic GABAA activation induces Ca2+ rise in pyramidal cells and interneurons from rat neonatal hippocampal slices. J Physiol 487(Pt 2):319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinekugel X, Medina I, Khalilov I, Ben‐Ari Y, Khazipov R. 1997. Ca2 + oscillations mediated by the synergistic excitatory actions of GABA(A) and NMDA receptors in the neonatal hippocampus. Neuron 18:243–255. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Khalilov I, Ben‐Ari Y, Khazipov R. 1998. Giant depolarizing potentials: The septal pole of the hippocampus paces the activity of the developing intact septohippocampal complex in vitro. J Neurosci 18:6349–6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinekugel X, Khazipov R, Cannon R, Hirase H, Ben‐Ari Y, Buzsáki G. 2002. Correlated bursts of activity in the neonatal hippocampus in vivo. Science 296:2049–2052. [DOI] [PubMed] [Google Scholar]
- Leonard BJ, McNaughton BL, Barnes CA. 1987. Suppression of hippocampal synaptic plasticity during slow‐wave sleep. Brain Res 425:174–177. [DOI] [PubMed] [Google Scholar]
- Leung LS. 1982. Nonlinear feedback model of neuronal populations in hippocampal CA1 region. J Neurophysiol 47:845–868. [DOI] [PubMed] [Google Scholar]
- Lévesque M, Bortel A, Gotman J, Avoli M. 2011. High‐frequency (80‐500 Hz) oscillations and epileptogenesis in temporal lobe epilepsy. Neurobiol Dis 42:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque M, Salami P, Gotman J, Avoli M. 2012. Two seizure‐onset types reveal specific patterns of high‐frequency oscillations in a model of temporal lobe epilepsy. J Neurosci 32:13264–13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy WB, Steward O. 1979. Synapses as associative memory elements in the hippocampal formation. Brain Res 175:233–245. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Durrant SJ. 2011. Overlapping memory replay during sleep builds cognitive schemata. Trends Cogn Sci 15:343–351. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. 2005. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6:312–324. [DOI] [PubMed] [Google Scholar]
- Li XG, Somogyi P, Ylinen A, Buzsáki G. 1994. The hippocampal CA3 network: An in vivo intracellular labeling study. J Comp Neurol 339:181–208. [DOI] [PubMed] [Google Scholar]
- Li CY, Poo MM, Dan Y. 2009. Burst spiking of a single cortical neuron modifies global brain state. Science 324:643–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libet B. 2005. Mind Time: The Temporal Factor in Consciousness. Cambridge, MA: Harvard University Press. [Google Scholar]
- Liotta A, Çalışkan G, ul Haq R, Hollnagel JO, Rösler A, Heinemann U, Behrens CJ. 2011. Partial disinhibition is required for transition of stimulus‐induced sharp wave–ripple complexes into recurrent epileptiform discharges in rat hippocampal slices. J Neurophysiol 105:172–187. [DOI] [PubMed] [Google Scholar]
- Lisman JE. 1997. Bursts as a unit of neural information: Making unreliable synapses reliable. Trends Neurosci 20:38–43. [DOI] [PubMed] [Google Scholar]
- Lisman JE. 2007. Role of the dual entorhinal inputs to hippocampus: A hypothesis based on cue/action (non‐self/self) couplets. Prog Brain Res 163:615–625. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Idiart MA. 1995. Storage of 7 +/− 2 short‐term memories in oscillatory subcycles. Science 267:1512–1515. [DOI] [PubMed] [Google Scholar]
- Lisman J, Morris RG. 2001. Memory. Why is the cortex a slow learner? Nature 411:248–249. [DOI] [PubMed] [Google Scholar]
- Lisman J, Redish AD. 2009. Prediction, sequences and the hippocampus. Philos Trans R Soc Lond Ser B Biol Sci 364:1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR. 1988. The intrinsic electrophysiological properties of mammalian neurons—Insights into central nervous‐system function. Science 242:1654–1664. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. 2009. Gestational methylazoxymethanol acetate administration: A developmental disruption model of schizophrenia. Behav Brain Res 204:306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. 2015. Neural‐Event‐Triggered fMRI of large‐scale neural networks. Curr Opin Neurobiol 31:214–222. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Eschenko O, Murayama Y, Augath M, Steudel T, Evrard HC, Besserve M, Oeltermann A. 2012. Hippocampal‐cortical interaction during periods of subcortical silence. Nature 491:547–553. [DOI] [PubMed] [Google Scholar]
- Loomis AL, Harvey EN, Hobart GA. 1938. Distribution of disturbance‐patterns in the human electroencephalogram, with special reference to sleep. J Neurophysiol 1:413–430. [Google Scholar]
- Lopes da Silva F. 2013. EEG and MEG: Relevance to neuroscience. Neuron 80:1112–1128. [DOI] [PubMed] [Google Scholar]
- Lorente de Nó R. 1934. Studies of the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J Psychol Neurol 46:113–177. [Google Scholar]
- Lörincz A, Buzsáki G. 2000. Two‐phase computational model training long‐term memories in the entorhinal‐hippocampal region. Ann N Y Acad Sci 911:83–111. [DOI] [PubMed] [Google Scholar]
- Lörincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z. 2002. Polarized and compartment‐dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci 5:1185–1193. [DOI] [PubMed] [Google Scholar]
- Lovett‐Barron M, Turi GF, Kaifosh P, Lee PH, Bolze F, Sun XH, Nicoud JF, Zemelman BV, Sternson SM, Losonczy A. 2012. Regulation of neuronal input transformations by tunable dendritic inhibition. Nat Neurosci 15:423–430, S421–423. [DOI] [PubMed] [Google Scholar]
- Lubenov EV, Siapas AG. 2008. Decoupling through synchrony in neuronal circuits with propagation delays. Neuron 58:118–131. [DOI] [PubMed] [Google Scholar]
- Luczak A, Barthó P, Marguet SL, Buzsáki G, Harris KD. 2007. Sequential structure of neocortical spontaneous activity in vivo. Proc Natl Acad Sci USA 104:347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak A, Barthó P, Harris KD. 2009. Spontaneous events outline the realm of possible sensory responses in neocortical populations. Neuron 62:413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver MB, Mandema JW, Stanski DR, Bland BH. 1996. Thiopental uncouples hippocampal and cortical synchronized electroencephalographic activity. Anesthesiology 84:1411–1424. [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. 1988. Enkephalin hyperpolarizes interneurones in the rat hippocampus. J Physiol 398:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Carruth M. 1999. Dendritic voltage‐gated ion channels regulate the action potential firing mode of hippocampal CA1 pyramidal neurons. J Neurophysiol 82:1895–1901. [DOI] [PubMed] [Google Scholar]
- Maier N, Guldenagel M, Sohl G, Siegmund H, Willecke K, Draguhn A. 2002. Reduction of high‐frequency network oscillations (ripples) and pathological network discharges in hippocampal slices from connexin 36‐deficient mice. J Physiol 541:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier N, Nimmrich V, Draguhn A. 2003. Cellular and network mechanisms underlying spontaneous sharp wave‐ripple complexes in mouse hippocampal slices. J Physiol 550:873–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier N, Tejero‐Cantero A, Dorrn AL, Winterer J, Beed PS, Morris G, Kempter R, Poulet JF, Leibold C, Schmitz D. 2011. Coherent phasic excitation during hippocampal ripples. Neuron 72:137–152. [DOI] [PubMed] [Google Scholar]
- Maier N, Morris G, Schuchmann S, Korotkova T, Ponomarenko A, Böhm C, Wozny C. 2012. Schmitz D Cannabinoids disrupt hippocampal sharp wave‐ripples via inhibition of glutamate release. Hippocampus 22:1350–1362. [DOI] [PubMed] [Google Scholar]
- Maingret N, Girardeau G, Goutierre M, Zugaro MB. Selective reinforcement of hippocampo‐cortical interactions during sleep potentiates memory consolidation. Federation of European Neuroscience Societies Meeting 2013. Poster 326.24/G47.
- Manabe H, Kusumoto‐Yoshida I, Ota M, Mori K. 2011. Olfactory cortex generates synchronized top‐down inputs to the olfactory bulb during slow‐wave sleep. J Neurosci 31:8123–8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BA Mander, SM Marks, JW Vogel, V Rao, B Lu, JM Saletin, S Ancoli-Israel, WJ Jagust, MP Walker. 2015. β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci 18:1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Mody I. 2010. Control of hippocampal gamma oscillation frequency by tonic inhibition and excitation of interneurons. Nat Neurosci 13:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. 2005. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron 45:105–117. [DOI] [PubMed] [Google Scholar]
- Maquet P. 2000. Sleep on it! Nat Neurosci 3:1235–1236. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. 1997. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275:213–215. [DOI] [PubMed] [Google Scholar]
- Marr D. 1971. Simple memory: A theory for archicortex. Philos Trans R Soc Lond Ser B Biol Sci 262:23–81. [DOI] [PubMed] [Google Scholar]
- Marr D. 2010. VISION: A Computational Investigation into the Human Representation and Processing of Visual Information. San Francisco, CA: W. H. Freeman. [Google Scholar]
- Marshall L, Born J. 2007. The contribution of sleep to hippocampus‐dependent memory consolidation. Trends Cogn Sci 11:442–450. [DOI] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Mölle M, Born J. 2006. Boosting slow oscillations during sleep potentiates memory. Nature 444:610–613. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Oh GH, Orser BA. 2009. Etomidate targets alpha5 gamma‐aminobutyric acid subtype A receptors to regulate synaptic plasticity and memory blockade. Anesthesiology 111:1025–1035. [DOI] [PubMed] [Google Scholar]
- Marty S, Berninger B, Carroll P, Thoenen H. 1996. GABAergic stimulation regulates the phenotype of hippocampal interneurons through the regulation of brain‐derived neurotrophic factor. Neuron 16:565–570. [DOI] [PubMed] [Google Scholar]
- Marty S, Wehrle R, Sotelo C. 2000. Neuronal activity and brain‐derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J Neurosci 20:8087–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. 2004. The sleep slow oscillation as a traveling wave. J Neurosci 24:6862–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masukawa LM, Prince DA. 1982. Enkephalin inhibition of inhibitory input to CA1 and CA3 pyramidal neurons in the hippocampus. Brain Res 249:271–280. [DOI] [PubMed] [Google Scholar]
- Mátyás F, Freund TF, Gulyás AI. 2004. Convergence of excitatory and inhibitory inputs onto CCK‐containing basket cells in the CA1 area of the rat hippocampus. Eur J Neurosci 19:1243–1256. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Fisahn A. 2001. Interneurons unbound. Nat Rev Neurosci 2:11–23. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. 1995. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 102:419–457. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. 2000. Memory—A century of consolidation. Science 287:248–251. [DOI] [PubMed] [Google Scholar]
- McNamara CG, Tejero‐Cantero A, Trouche S, Campo‐Urriza N, Dupret D. 2014. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat Neurosci 17:1658–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcnaughton BL, Morris RGM. 1987. Hippocampal synaptic enhancement and information‐storage within a distributed memory system. Trends Neurosci 10:408–415. [Google Scholar]
- McNaughton BL, Douglas RM, Goddard GV. 1978. Synaptic enhancement in fascia dentata: Cooperativity among coactive afferents. Brain Res 157:277–293. [DOI] [PubMed] [Google Scholar]
- McQuiston AR. 2011. Mu opioid receptor activation normalizes temporo‐ammonic pathway driven inhibition in hippocampal CA1. Neuropharmacology 60:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MR. 2007. Cortico‐hippocampal interaction during up‐down states and memory consolidation. Nat Neurosci 10:13–15. [DOI] [PubMed] [Google Scholar]
- Melzer S, Michael M, Caputi A, Eliava M, Fuchs EC, Whittington MA, Monyer H. 2012. Long‐range‐projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science 335:1506–1510. [DOI] [PubMed] [Google Scholar]
- Memmesheimer RM. 2010. Quantitative prediction of intermittent high‐frequency oscillations in neural networks with supralinear dendritic interactions. Proc Natl Acad Sci USA 107:11092–11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez de la Prida L. 1998. Origin of the synchronized network activity in the rabbit developing hippocampus. Eur J Neurosci 10:899–906. [DOI] [PubMed] [Google Scholar]
- Menendez de la Prida L, Gal B. 2004. Synaptic contributions to focal and widespread spatiotemporal dynamics in the isolated rat subiculum in vitro. J Neurosci 24:5525–5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez de la Prida L, Trevelyan AJ. 2011. Cellular mechanisms of high frequency oscillations in epilepsy: On the diverse sources of pathological activities. Epilepsy Res 97:308–317. [DOI] [PubMed] [Google Scholar]
- Menendez de la Prida L, Bolea S, Sanchez‐Andres JV. 1998. Origin of the synchronized network activity in the rabbit developing hippocampus. Eur J Neurosci 10:899–906. [DOI] [PubMed] [Google Scholar]
- Menendez de la Prida L, Sanchez‐Andres JV. 2000. Heterogeneous populations of cells mediate spontaneous synchronous bursting in the developing hippocampus through a frequency‐dependent mechanism. Neuroscience 97:227–241. [DOI] [PubMed] [Google Scholar]
- Menendez de la Prida L, Huberfeld G, Cohen I, Miles R. 2006. Threshold behavior in the initiation of hippocampal population bursts. Neuron 49:131–142. [DOI] [PubMed] [Google Scholar]
- Mielke JG, Ahuja TK, Comas T, Mealing GA. 2011. Cholinemediated depression of hippocampal synaptic transmission. Nutr Neurosci 14:186–194. [DOI] [PubMed] [Google Scholar]
- Miles R. 1990. Synaptic excitation of inhibitory cells by single CA3 hippocampal pyramidal cells of the guinea‐pig in vitro. J Physiol 428:61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R, Wong RK. 1983. Single neurones can initiate synchronized population discharge in the hippocampus. Nature 306:371–373. [DOI] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER. 1998. Cognitive neuroscience and the study of memory. Neuron 20:445–468. [DOI] [PubMed] [Google Scholar]
- Mitra A, Mitra SS, Tsien RW. 2012. Heterogeneous reallocation of presynaptic efficacy in recurrent excitatory circuits adapting to inactivity. Nat Neurosci 15:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki T, Norimoto H, Ishikawa T, Watanabe Y, Matsuki N, Ikegaya Y. 2014. Dopamine receptor activation reorganizes neuronal ensembles during hippocampal sharp waves in vitro. PloS One 9:e104438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori SJ, McNaughton BL, Barnes CA, Fox KB. 1989. Preserved spatial coding in hippocampal CA1 pyramidal cells during reversible suppression of CA3c output: Evidence for pattern completion in hippocampus. J Neurosci 9:3915–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizunuma M, Norimoto H, Tao K, Egawa T, Hanaoka K, Sakaguchi T, Hioki H, Kaneko T, Yamaguchi S, Nagano T, Matsuki N, Ikegaya Y. 2014. Unbalanced excitability underlies offline reactivation of behaviorally activated neurons. Nat Neurosci 17:503–505. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Buzsáki G. 2013. Preconfigured, skewed distribution of firing rates in the hippocampus and entorhinal cortex. Cell Rep 4:1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K, Sirota A, Pastalkova E, Buzsáki G. 2009. Theta oscillations provide temporal windows for local circuit computation in the entorhinal‐hippocampal loop. Neuron 64:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K, Diba K, Pastalkova E, Buzsáki G. 2011. Hippocampal CA1 pyramidal cells form functionally distinct sublayers. Nat Neurosci 14:1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K, Royer S, Diba K, Buzsáki G. 2012. Activity dynamics and behavioral correlates of CA3 and CA1 hippocampal pyramidal neurons. Hippocampus 22:1659–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T, Yamatodani A, Okakura K, Takemura M, Inagaki N, Wada H. 1991. In vivo release of neuronal histamine in the hypothalamus of rats measured by microdialysis. Naunyn Schmiedebergs Arch Pharmacol 343:190–195. [DOI] [PubMed] [Google Scholar]
- Mohns EJ, Blumberg MS. 2008. Synchronous bursts of neuronal activity in the developing hippocampus: Modulation by active sleep and association with emerging gamma and theta rhythms. J Neurosci 28:10134–10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohns EJ, Blumberg MS. 2010. Neocortical activation of the hippocampus during sleep in infant rats. J Neurosci 30:3438–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M, Yeshenko O, Marshall L, Sara SJ, Born J. 2006. Hippocampal sharp wave‐ripples linked to slow oscillations in rat slow‐wave sleep. J Neurophysiol 96:62–70. [DOI] [PubMed] [Google Scholar]
- Mölle M, Eschenko O, Gais S, Sara SJ, Born J. 2009. The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. Eur J Neurosci 29:1071–1081. [DOI] [PubMed] [Google Scholar]
- Montkowski A, Jahn H, Strohle A, Poettig M, Holsboer F, Wiedemann K. 1998. C‐type natriuretic peptide exerts effects opposing those of atrial natriuretic peptide on anxiety‐related behaviour in rats. Brain Res 792:358–360. [DOI] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. 2006. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: Implications for the neuropathology of schizophrenia. Biol Psychiatry 60:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG. 2001. Episodic‐like memory in animals: Psychological criteria, neural mechanisms and the value of episodic‐like tasks to investigate animal models of neurodegenerative disease. Philos Trans R Soc Lond Ser B Biol Sci 356:1453–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. 1986. Selective impairment of learning and blockade of long‐term potentiation by an N‐methyl‐D‐aspartate receptor antagonist, AP5. Nature 319:774–776. [DOI] [PubMed] [Google Scholar]
- Morrison A, Aertsen A, Diesmann M. 2007. Spike‐timing‐dependent plasticity in balanced random networks. Neural Comput 19:1437–1467. [DOI] [PubMed] [Google Scholar]
- Müller GE, Pilzecker A. 1900. Experimentelle Beiträge zur Lehre vom Gedächtnis. Z Psychol Ergänzungsband 1:1–300. [Google Scholar]
- Muller RU, Stead M, Pach J. 1996. The hippocampus as a cognitive graph. J Gen Physiol 107:663–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz W, Tremblay R, Rudy B. 2014. Channelrhodopsin‐assisted patching: In vivo recording of genetically and morphologically identified neurons throughout the brain. Cell Rep 9:2304–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nádasdy Z. 2000. Spike sequences and their consequences. J Physiol Paris 94:505–524. [DOI] [PubMed] [Google Scholar]
- Nádasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsáki G. 1999. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci 19:9497–9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. 1997. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol 7:217–227. [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Buhl DL, McHugh TJ, Tonegawa S. 2009. Hippocampal CA3 output is crucial for ripple‐associated reactivation and consolidation of memory. Neuron 62:781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narikiyo K, Manabe H, Mori K. 2014. Sharp wave‐associated synchronized inputs from the piriform cortex activate olfactory tubercle neurons during slow‐wave sleep. J Neurophysiol 111:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DP, Kloosterman F, Barbieri R, Brown EN, Wilson MA. 2009. Characterizing the dynamic frequency structure of fast oscillations in the rodent hippocampus. Front Integr Neurosci 3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmrich V, Maier N, Schmitz D, Draguhn A. 2005. Induced sharp wave‐ripple complexes in the absence of synaptic inhibition in mouse hippocampal slices. J Physiol 563:663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Staba RJ, Andrillon T, Vyazovskiy VV, Cirelli C, Fried I, Tononi G. 2011. Regional slow waves and spindles in human sleep. Neuron 70:153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokia MS, Penttonen M, Wikgren J. 2010. Hippocampal ripple‐contingent training accelerates trace eyeblink conditioning and retards extinction in rabbits. J Neurosci 30:11486–11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokia MS, Mikkonen JE, Penttonen M, Wikgren J. 2012. Disrupting neural activity related to awake‐state sharp wave‐ripple complexes prevents hippocampal learning. Front Behav Neurosci 6:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norimoto H, Mizunuma M, Ishikawa D, Matsuki N, Ikegaya Y. 2012. Muscarinic receptor activation disrupts hippocampal sharp wave‐ripples. Brain Res 1461:1–9. [DOI] [PubMed] [Google Scholar]
- Norimoto H, Matsumoto N, Miyawaki T, Matsuki N, Ikegaya Y. 2013. Subicular activation preceding hippocampal ripples in vitro. Sci Rep 3:2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. 2004. Immunohistochemical localization of Ih channel subunits, HCN1‐4, in the rat brain. J Comp Neurol 471:241–276. [DOI] [PubMed] [Google Scholar]
- O'Keefe J. 1976. Place units in the hippocampus of the freely moving rat. Exp Neurol 51:78–109. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. 1978. The Hippocampus as a Cognitive Map. Oxford University Press. [Google Scholar]
- Y Omura, MM Carvalho, K Inokuchi, T Fukai. 2015. A lognormal recurrent network model for burst generation during hippocampal sharp waves. J Neurosci (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J, Senior T, Csicsvari J. 2006. Place‐selective firing of CA1 pyramidal cells during sharp wave/ripple network patterns in exploratory behavior. Neuron 49:143–155. [DOI] [PubMed] [Google Scholar]
- O'Neill J, Senior TJ, Allen K, Huxter JR, Csicsvari J. 2008. Reactivation of experience‐dependent cell assembly patterns in the hippocampus. Nat Neurosci 11:209–215. [DOI] [PubMed] [Google Scholar]
- Ochi A, Otsubo H, Donner EJ, Elliott I, Iwata R, Funaki T, Akizuki Y, Akiyama T, Imai K, Rutka JT, Snead OC III. 2007. Dynamic changes of ictal high‐frequency oscillations in neocortical epilepsy: Using multiple band frequency analysis. Epilepsia 48:286–296. [DOI] [PubMed] [Google Scholar]
- Ogren JA, Wilson CL, Bragin A, Lin JJ, Salamon N, Dutton RA, Luders E, Fields TA, Fried I, Toga AW, Thompson PM, Engel J Jr, Staba RJ. 2009. Three‐dimensional surface maps link local atrophy and fast ripples in human epileptic hippocampus. Ann Neurol 66:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun M, Steinmetz NA, Cossell L, Iacaruso MF, Ko H, Bartho P, Moore T, Hofer SB, Mrsic‐Flogel TD, Carandini M, Harris KD. 2015. Diverse coupling of neurons to populations in sensory cortex. Nature 521:511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olton D, Markowska A, Voytko ML, Givens B, Gorman L, Wenk G. 1991. Basal forebrain cholinergic system: A functional analysis. Adv Exp Med Biol 295:353–372. [DOI] [PubMed] [Google Scholar]
- Oren I, Mann EO, Paulsen O, Hajos N. 2006. Synaptic currents in anatomically identified CA3 neurons during hippocampal gamma oscillations in vitro. J Neurosci 26:9923–9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsiew F. 2004. Antiepileptic drugs in psychiatry. J Neurol Neurosurg Psychiatry 75:1655–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozen S, Sirota A, Belluscio MA, Anastassiou CA, Stark E, Koch C, Buzsáki G. 2010. Transcranial electric stimulation entrains cortical neuronal populations in rats. J Neurosci 30:11476–11485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais I, Hormuzdi SG, Monyer H, Traub RD, Wood IC, Buhl EH, Whittington MA, LeBeau FE. 2003. Sharp wave‐like activity in the hippocampus in vitro in mice lacking the gap junction protein connexin 36. J Neurophysiol 89:2046–2054. [DOI] [PubMed] [Google Scholar]
- Pallud J, Le Van Quyen M, Bielle F, Pellegrino C, Varlet P, Labussiere M, Cresto N, Dieme MJ, Baulac M, Duyckaerts C, Kourdougli N, Chazal G, Devaux B, Rivera C, Miles R, Capelle L, Huberfeld G. 2014. Cortical GABAergic excitation contributes to epileptic activities around human glioma. Sci Transl Med 6:244ra289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien‐Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. 2007. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron 55:697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva JM, Lamsa K, Lauri SE, Rauvala H, Kaila K, Taira T. 2000. Fast network oscillations in the newborn rat hippocampus in vitro. J Neurosci 20:1170–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangalos M, Donoso JR, Winterer J, Zivkovic AR, Kempter R, Maier N, Schmitz D. 2013. Recruitment of oriens‐lacunosum‐moleculare interneurons during hippocampal ripples. Proc Natl Acad Sci USA 110:4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papatheodoropoulos C. 2007. NMDA receptor‐dependent high‐frequency network oscillations (100–300Hz) in rat hippocampal slices. Neuroscience Lett 414:197–202. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C. 2008. A possible role of ectopic action potentials in the in vitro hippocampal sharp wave‐ripple complexes. Neuroscience 157:495–501. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C. 2010. Patterned activation of hippocampal network (approximately 10 Hz) during in vitro sharp wave‐ripples. Neuroscience 168:429–442. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C, Koniaris E. 2011. alpha5GABAA receptors regulate hippocampal sharp wave‐ripple activity in vitro. Neuropharmacology 60:662–673. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C, Kostopoulos G. 2002a. Spontaneous GABA(A)‐dependent synchronous periodic activity in adult rat ventral hippocampal slices. Neuroscience Lett 319:17–20. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C, Kostopoulos G. 2002b. Spontaneous, low frequency (approximately 2–3 Hz) field activity generated in rat ventral hippocampal slices perfused with normal medium. Brain Res Bull 57:187–193. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C, Asprodini E, Nikita I, Koutsona C, Kostopoulos G. 2002. Weaker synaptic inhibition in CA1 region of ventral compared to dorsal rat hippocampal slices. Brain Res 948:117–121. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. 2008. Internally generated cell assembly sequences in the rat hippocampus. Science 321:1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J, Fujisawa S, Berenyi A, Royer S, Buzsáki G. 2012. Traveling theta waves along the entire septotemporal axis of the hippocampus. Neuron 75:410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J, Schomburg EW, Berenyi A, Fujisawa S, Buzsáki G. 2013. Local generation and propagation of ripples along the septotemporal axis of the hippocampus. J Neurosci 33:17029–17041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Winson J. 1989. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J Neurosci 9:2907–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Kensinger EA. 2011. Sleep leads to changes in the emotional memory trace: Evidence from FMRI. J Cogn Neurosci 23:1285–1297. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Destrebecqz A, Collette F, Delbeuck X, Phillips C, Aerts J, Del Fiore G, Degueldre C, Luxen A, Cleeremans A, Maquet P. 2003. Learned material content and acquisition level modulate cerebral reactivation during posttraining rapid‐eye‐movements sleep. NeuroImage 20:125–134. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Lee E, Verheul J, Lipa P, Barnes CA, McNaughton BL. 2004. The ventral striatum in off‐line processing: Ensemble reactivation during sleep and modulation by hippocampal ripples. J Neurosci 24:6446–6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttonen M, Kamondi A, Sik A, Acsády L, Buzsáki G. 1997. Feed‐forward and feed‐back activation of the dentate gyrus in vivo during dentate spikes and sharp wave bursts. Hippocampus 7:437–450. [DOI] [PubMed] [Google Scholar]
- Peters BH, Levin HS. 1977. Memory enhancement after physostigmine treatment in the amnesic syndrome. Arch Neurol 34:215–219. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. 2001. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev 38:247–289. [DOI] [PubMed] [Google Scholar]
- Peyrache A, Khamassi M, Benchenane K, Wiener SI, Battaglia FP. 2009. Replay of rule‐learning related neural patterns in the prefrontal cortex during sleep. Nat Neurosci 12:919–926. [DOI] [PubMed] [Google Scholar]
- Peyrache A, Battaglia FP, Destexhe A. 2011. Inhibition recruitment in prefrontal cortex during sleep spindles and gating of hippocampal inputs. Proc Natl Acad Sci USA 108:17207–17212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A Peyrache, MM Lacroix, PC Petersen, G Buzsáki. 2015. Internally organized mechanisms of the head direction sense. Nat Neurosci 18:569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BE, Foster DJ. 2013. Hippocampal place‐cell sequences depict future paths to remembered goals. Nature 497:74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BE, Foster DJ. 2015. Autoassociative dynamics in the generation of sequences of hippocampal place cells. Science 349:180–183. [DOI] [PubMed] [Google Scholar]
- Phillips KG, Bartsch U, McCarthy AP, Edgar DM, Tricklebank MD, Wafford KA, Jones MW. 2012. Decoupling of sleep‐dependent cortical and hippocampal interactions in a neurodevelopmental model of schizophrenia. Neuron 76:526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky PF, Rinzel J. 1994. Intrinsic and network rhythmogenesis in a reduced Traub model for CA3 neurons. J Comput Neurosci 1:39–60. [DOI] [PubMed] [Google Scholar]
- Plihal W, Born J. 1997. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci 9:534–547. [DOI] [PubMed] [Google Scholar]
- Ponomarenko AA, Korotkova TM, Haas HL. 2003a. High frequency (200 Hz) oscillations and firing patterns in the basolateral amygdala and dorsal endopiriform nucleus of the behaving rat. Behav Brain Res 141:123–129. [DOI] [PubMed] [Google Scholar]
- Ponomarenko AA, Lin JS, Selbach O, Haas HL. 2003b. Temporal pattern of hippocampal high‐frequency oscillations during sleep after stimulant‐evoked waking. Neuroscience 121:759–769. [DOI] [PubMed] [Google Scholar]
- Ponomarenko AA, Li JS, Korotkova TM, Huston JP, Haas HL. 2008. Frequency of network synchronization in the hippocampus marks learning. Eur J Neurosci 27:3035–3042. [DOI] [PubMed] [Google Scholar]
- Popper K. 1959. The Logic of Scientific Discovery. Chicago: Harper & Row. [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. 1977. Depression: A new animal model sensitive to antidepressant treatments. Nature 266:730–732. [DOI] [PubMed] [Google Scholar]
- Prenosil GA, Schneider Gasser EM, Rudolph U, Keist R, Fritschy JM, Vogt KE. 2006. Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J Neurophysiol 96:846–857. [DOI] [PubMed] [Google Scholar]
- Qin YL, McNaughton BL, Skaggs WE, Barnes CA. 1997. Memory reprocessing in corticocortical and hippocampocortical neuronal ensembles. Philos Trans R Soc Lond Ser B Biol Sci 352:1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM. 1996. [3H]L‐655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the alpha 5 subunit. Neuropharmacology 35:1331–1335. [DOI] [PubMed] [Google Scholar]
- Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. 2005. Invariant visual representation by single neurons in the human brain. Nature 435:1102–1107. [DOI] [PubMed] [Google Scholar]
- Rácz A, Ponomarenko AA, Fuchs EC, Monyer H. 2009. Augmented hippocampal ripple oscillations in mice with reduced fast excitation onto parvalbumin‐positive cells. J Neurosci 29:2563–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. 2001. A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiteri M. 2008. Presynaptic metabotropic glutamate and GABAB receptors Handb Exp Pharmacol 373–407. [DOI] [PubMed] [Google Scholar]
- Ramadan W, Eschenko O, Sara SJ. 2009. Hippocampal sharp wave/ripples during sleep for consolidation of associative memory. PloS One 4:e6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K, Guimaraes A, Yue M, Lewis J, Carlson G, Hutton M, Ashe KH. 2005. Age‐dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J Neurosci 25:10637–10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck JB Jr. 1973. Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol 41:461–531. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. 2012. Two cortical systems for memory‐guided behaviour. Nat Rev Neurosci 13:713–726. [DOI] [PubMed] [Google Scholar]
- Rasch B, Born J. 2013. About sleep's role in memory. Physiol Rev 93:681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Buchel C, Gais S, Born J. 2007. Odor cues during slow‐wave sleep prompt declarative memory consolidation. Science 315:1426–1429. [DOI] [PubMed] [Google Scholar]
- Rattenborg NC, Martinez‐Gonzalez D, Roth TC II, Pravosudov VV. 2011. Hippocampal memory consolidation during sleep: A comparison of mammals and birds. Biol Rev Camb Philos Soc 86:658–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauchs G, Bertran F, Guillery‐Girard B, Desgranges B, Kerrouche N, Denise P, Foret J, Eustache F. 2004. Consolidation of strictly episodic memories mainly requires rapid eye movement sleep. Sleep 27:395–401. [DOI] [PubMed] [Google Scholar]
- Redish AD, Touretzky DS. 1998. The role of the hippocampus in solving the Morris water maze. Neural Comput 10:73–111. [DOI] [PubMed] [Google Scholar]
- Reichinnek S, Kunsting T, Draguhn A, Both M. 2010. Field potential signature of distinct multicellular activity patterns in the mouse hippocampus. J Neurosci 30:15441–15449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Colgin LL, Jia Y, Casale M, Yanagihara TK, Debenedetti M, Gall CM, Kramar EA, Lynch G. 2009. Origins of an intrinsic hippocampal EEG pattern. PloS One 4:e7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CF III, Kupfer DJ, Taska LS, Hoch CC, Spiker DG, Sewitch DE, Zimmer B, Marin RS, Nelson JP, Martin D, et al. 1985. EEG sleep in elderly depressed, demented, and healthy subjects. Biol Psychiatry 20:431–442. [DOI] [PubMed] [Google Scholar]
- Rich PD, Liaw HP, Lee AK. 2014. Place cells. Large environments reveal the statistical structure governing hippocampal representations. Science 345:814–817. [DOI] [PubMed] [Google Scholar]
- Ritter SM, Strick M, Bos MW, van Baaren RB, Dijksterhuis A. 2012. Good morning creativity: Task reactivation during sleep enhances beneficial effect of sleep on creative performance. J Sleep Res 21:643–647. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. 1999. The K+/Cl‐ co‐transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397:251–255. [DOI] [PubMed] [Google Scholar]
- Robbe D, Buzsáki G. 2009. Alteration of theta timescale dynamics of hippocampal place cells by a cannabinoid is associated with memory impairment. J Neurosci 29:12597–12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Montgomery SM, Thome A, Rueda‐Orozco PE, McNaughton BL, Buzsáki G. 2006. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat Neurosci 9:1526–1533. [DOI] [PubMed] [Google Scholar]
- Roberts PD, Bell CC. 2002. Spike timing dependent synaptic plasticity in biological systems. Biol Cybern 87:392–403. [DOI] [PubMed] [Google Scholar]
- Roffwarg HP, Dement WC, Muzio JN, Fisher C. 1962. Dream imagery: Relationship to rapid eye movements of sleep. Arch Gen Psychiatry 7:235–258. [DOI] [PubMed] [Google Scholar]
- Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, Holtzman DM. 2012. Disruption of the sleep‐wake cycle and diurnal fluctuation of beta‐amyloid in mice with Alzheimer's disease pathology. Sci Transl Med 4:150ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas R. 1996. Neural networks: A systematic introduction. Berlin: Springer; p 336. [Google Scholar]
- Roland PE, Hanazawa A, Undeman C, Eriksson D, Tompa T, Nakamura H, Valentiniene S, Ahmed B. 2006. Cortical feedback depolarization waves: A mechanism of top‐down influence on early visual areas. Proc Natl Acad Sci USA 103:12586–12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopun AK, Simonotto JD, Pierce ML, Jenkins A, Nicholson C, Schofield IS, Whittaker RG, Kaiser M, Whittington MA, Traub RD, Cunningham MO. 2010. A nonsynaptic mechanism underlying interictal discharges in human epileptic neocortex. Proc Natl Acad Sci USA 107:338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumis DK, Frank LM. 2015. Hippocampal sharp‐wave ripples in waking and sleeping states. Curr Opin Neurobiol 35:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L, Stark E, Sjulson L, Buzsáki G. 2014. In vivo optogenetic identification and manipulation of GABAergic interneuron subtypes. Curr Opin Neurobiol 26:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Pare D. 2003. Conservation of total synaptic weight through balanced synaptic depression and potentiation. Nature 422:518–522. [DOI] [PubMed] [Google Scholar]
- Royer S, Sirota A, Patel J, Buzsáki G. 2010. Distinct representations and theta dynamics in dorsal and ventral hippocampus. J Neurosci 30:1777–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Zemelman BV, Losonczy A, Kim J, Chance F, Magee JC, Buzsáki G. 2012. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat Neurosci 15:769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. 2006. GABA‐based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol 6:18–23. [DOI] [PubMed] [Google Scholar]
- Rudoy JD, Voss JL, Westerberg CE, Paller KA. 2009. Strengthening individual memories by reactivating them during sleep. Science 326:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumelhart DE, Hinton GE, Williams RJ. 1986. Learning representations by back‐propagating errors. Nature 323:533–536. [Google Scholar]
- Samsonovich AV, Ascoli GA. 2005. A simple neural network model of the hippocampus suggesting its pathfinding role in episodic memory retrieval. Learn Mem 12:193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsonovich A, McNaughton BL. 1997. Path integration and cognitive mapping in a continuous attractor neural network model. J Neurosci 17:5900–5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria ER, Su H, Yaari Y. 2001. Initiation of network bursts by Ca2+‐dependent intrinsic bursting in the rat pilocarpine model of temporal lobe epilepsy. J Physiol 532:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V. 2005. Choline transporters, cholinergic transmission and cognition. Nat Rev Neurosci 6:48–56. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. 2007. Constructive memory: The ghosts of past and future. Nature 445:27. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. 1994. Evidence from simultaneous intracellular recordings in rat hippocampal slices that area CA3 pyramidal cells innervate dentate hilar mossy cells. J Neurophysiol 72:2167–2180. [DOI] [PubMed] [Google Scholar]
- Schevon CA, Trevelyan AJ, Schroeder CE, Goodman RR, McKhann G Jr, Emerson RG. 2009. Spatial characterization of interictal high frequency oscillations in epileptic neocortex. Brain 132:3047–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingloff D, Kali S, Freund TF, Hajos N, Gulyás AI. 2014. Mechanisms of sharp wave initiation and ripple generation. J Neurosci 34:11385–11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalbruch H, Jahnsen H. 1981. Gap junctions on CA3 pyramidal cells of guinea pig hippocampus shown by freeze‐fracture. Brain Res 217:175–178. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Schuchmann S, Fisahn A, Draguhn A, Buhl EH, Petrasch‐Parwez E, Dermietzel R, Heinemann U, Traub RD. 2001. Axo‐axonal coupling. A novel mechanism for ultrafast neuronal communication. Neuron 31:831–840. [DOI] [PubMed] [Google Scholar]
- Schomburg EW, Anastassiou CA, Buzsáki G, Koch C. 2012. The spiking component of oscillatory extracellular potentials in the rat hippocampus. J Neurosci 32:11798–11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg EW, Fernandez‐Ruiz A, Mizuseki K, Berenyi A, Anastassiou CA, Koch C, Buzsáki G. 2014. Theta phase segregation of input‐specific gamma patterns in entorhinal‐hippocampal networks. Neuron 84:470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönberger J, Draguhn A, Both M. 2014. Lamina‐specific contribution of glutamatergic and GABAergic potentials to hippocampal sharp wave‐ripple complexes. Front Neural Circuits 8:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin PA, Haglund MM. 1986. Spontaneous rhythmic synchronous activity in epileptic human and normal monkey temporal lobe. Epilepsia 27:523–533. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. 1957. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejnowski TJ, Destexhe A. 2000. Why do we sleep? Brain Res 886:208–223. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. 2004. Tonically active GABA(A) receptors: Modulating gain and maintaining the tone. Trends Neurosci 27:262–269. [DOI] [PubMed] [Google Scholar]
- Shatskikh TN, Raghavendra M, Zhao Q, Cui Z, Holmes GL. 2006. Electrical induction of spikes in the hippocampus impairs recognition capacity and spatial memory in rats. Epilepsy Behav 9:549–556. [DOI] [PubMed] [Google Scholar]
- Sheffield FD, Roby TB, Campbell BA. 1954. Drive reduction versus consummatory behavior as determinants of reinforcement. J Comp Physiol Psychol 47:349–354. [DOI] [PubMed] [Google Scholar]
- Shen B, McNaughton BL. 1996. Modeling the spontaneous reactivation of experience‐specific hippocampal cell assembles during sleep. Hippocampus 6:685–692. [DOI] [PubMed] [Google Scholar]
- Shenoy KV, Sahani M, Churchland MM. 2013. Cortical control of arm movements: A dynamical systems perspective. Annu Rev Neurosci 36:337–359. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Harris KM. 1998. Three‐dimensional structure and composition of CA3‐‐>CA1 axons in rat hippocampal slices: Implications for presynaptic connectivity and compartmentalization. J Neurosci 18:8300–8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewmon DA, Erwin RJ. 1988. The effect of focal interictal spikes on perception and reaction time. I. General considerations. Electroencephalogr Clin Neurophysiol 69:319–337. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. 2003. Turning on and off recurrent balanced cortical activity. Nature 423:288–293. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. 1998. Coordinated interactions between hippocampal ripples and cortical spindles during slow‐wave sleep. Neuron 21:1123–1128. [DOI] [PubMed] [Google Scholar]
- Siegel JM. 2001. The REM sleep‐memory consolidation hypothesis. Science 294:1058–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W. 2006. Structure, pharmacology, and function of GABAA receptor subtypes. Adv Pharmacol 54:231–263. [DOI] [PubMed] [Google Scholar]
- Sik A, Tamamaki N, Freund TF. 1993. Complete axon arborization of a single CA3 pyramidal cell in the rat hippocampus, and its relationship with postsynaptic parvalbumin‐containing interneurons. Eur J Neurosci 5:1719–1728. [DOI] [PubMed] [Google Scholar]
- Sik A, Ylinen A, Penttonen M, Buzsáki G. 1994. Inhibitory CA1‐CA3‐hilar region feedback in the hippocampus. Science 265:1722–1724. [DOI] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Ylinen A, Buzsáki G. 1995. Hippocampal CA1 interneurons: An in vivo intracellular labeling study. J Neurosci 15:6651–6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A, Traub RD, Vladimirov N, Jenkins A, Nicholson C, Whittaker RG, Schofield I, Clowry GJ, Cunningham MO, Whittington MA. 2014. Gap junction networks can generate both ripple‐like and fast ripple‐like oscillations. Eur J Neurosci 39:46–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AC, Frank LM. 2009. Rewarded outcomes enhance reactivation of experience in the hippocampus. Neuron 64:910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AC, Carr MF, Karlsson MP, Frank LM. 2013. Hippocampal SWR activity predicts correct decisions during the initial learning of an alternation task. Neuron 77:1163–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipilä ST, Kaila K. 2008. GABAergic control of CA3‐driven network events in the developing hippocampus. Results Probl Cell Differ 44:99–121. [DOI] [PubMed] [Google Scholar]
- Sipilä ST, Huttu K, Soltész I, Voipio J, Kaila K. 2005. Depolarizing GABA acts on intrinsically bursting pyramidal neurons to drive giant depolarizing potentials in the immature hippocampus. J Neurosci 25:5280–5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipilä ST, Schuchmann S, Voipio J, Yamada J, Kaila K. 2006. The cation‐chloride cotransporter NKCC1 promotes sharp waves in the neonatal rat hippocampus. J Physiol 573:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Buzsáki G. 2005. Interaction between neocortical and hippocampal networks via slow oscillations. Thalamus Relat Syst 3:245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D, Buzsáki G. 2003. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci USA 100:2065–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. 1996. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271:1870–1873. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. 1996. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6:149–172. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Permenter M, Archibeque M, Vogt J, Amaral DG, Barnes CA. 2007. EEG sharp waves and sparse ensemble unit activity in the macaque hippocampus. J Neurophysiol 98:898–910. [DOI] [PubMed] [Google Scholar]
- Skinner BF. 1938. The Behavior of Organisms. New York: Appleton‐Century‐Crofts. [Google Scholar]
- Smith C. 1985. Sleep states and learning: A review of the animal literature. Neurosci Biobehav Rev 9:157–168. [DOI] [PubMed] [Google Scholar]
- Smith C. 2001. Sleep states and memory processes in humans: Procedural versus declarative memory systems. Sleep Med Rev 5:491–506. [DOI] [PubMed] [Google Scholar]
- Soltész I. 2005. Diversity in the neuronal machine. New York: Oxford University Press. [Google Scholar]
- Somogyi P. 1977. A specific ‘axo‐axonal' interneuron in the visual cortex of the rat. Brain Res 136:345–350. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Fritschy JM, Benke D, Roberts JD, Sieghart W. 1996. The gamma 2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the alpha 1 and beta 2/3 subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacology 35:1425–1444. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Katona L, Klausberger T, Lasztoczi B, Viney TJ. 2014. Temporal redistribution of inhibition over neuronal subcellular domains underlies state‐dependent rhythmic change of excitability in the hippocampus. Philos Trans R Soc Lond Ser B Biol Sci 369:20120518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorra KE, Harris KM. 1993. Occurrence and three‐dimensional structure of multiple synapses between individual radiatum axons and their target pyramidal cells in hippocampal area CA1. J Neurosci 13:3736–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer WA, Kandel ER. 1961. Electrophysiology of hippocampal neurons: IV. Fast prepotentials. J Neurophysiol 24:272–285. [DOI] [PubMed] [Google Scholar]
- Spires‐Jones TL, Hyman BT. 2014. The intersection of amyloid beta and tau at synapses in Alzheimer's disease. Neuron 82:756–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. 1992. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev 99:195–231. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. 1995. Retrograde amnesia and memory consolidation: A neurobiological perspective. Curr Opin Neurobiol 5:169–177. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola‐Morgan S. 1991. The medial temporal lobe memory system. Science 253:1380–1386. [DOI] [PubMed] [Google Scholar]
- Squire LR, Slater PC, Chace PM. 1975. Retrograde amnesia: Temporal gradient in very long term memory following electroconvulsive therapy. Science 187:77–79. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Bragin A. 2011. High‐frequency oscillations and other electrophysiological biomarkers of epilepsy: Underlying mechanisms. Biomark Med 5:545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J Jr. 2002. Quantitative analysis of high‐frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol 88:1743–1752. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J Jr. 2004. High‐frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol 56:108–115. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Frighetto L, Behnke EJ, Mathern GW, Fields T, Bragin A, Ogren J, Fried I, Wilson CL, Engel J Jr. 2007. Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia 48:2130–2138. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Ekstrom AD, Suthana NA, Burggren A, Fried I, Engel J Jr, Bookheimer SY. 2012. Gray matter loss correlates with mesial temporal lobe neuronal hyperexcitability inside the human seizure‐onset zone. Epilepsia 53:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KJ Staley, BL Soldo, WR Proctor. 1995. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science 269:977–981. [DOI] [PubMed] [Google Scholar]
- Staley K, Hellier JL, Dudek FE. 2005. Do interictal spikes drive epileptogenesis? Neuroscientist 11:272–276. [DOI] [PubMed] [Google Scholar]
- Stark E, Eichler R, Roux L, Fujisawa S, Rotstein HG, Buzsáki G. 2013. Inhibition‐induced theta resonance in cortical circuits. Neuron 80:1263–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark E, Roux L, Eichler R, Senzai Y, Royer S, Buzsáki G. 2014. Pyramidal cell‐interneuron interactions underlie hippocampal ripple oscillations. Neuron 83:467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark E, L Roux, R Eichler, G Buzsáki. 2015. Local generation of multi-neuronal spike sequences in the hippocampal CA1 region. Proc Natl Acad Sci 112:10521–10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. 2001. Active neocortical processes during quiescent sleep. Arch Ital Biol 139:37–51. [PubMed] [Google Scholar]
- Steriade M, Amzica F. 1998. Slow sleep oscillation, rhythmic K‐complexes, and their paroxysmal developments. J Sleep Res 7(Suppl 1):30–35. [DOI] [PubMed] [Google Scholar]
- Steriade M, Gloor P, Llinas RR, Lopes de Silva FH, Mesulam MM. 1990. Report of IFCN Committee on Basic Mechanisms. Basic mechanisms of cerebral rhythmic activities. Electroencephalogr Clin Neurophysiol 76:481–508. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. 1993a. Thalamocortical oscillations in the sleeping and aroused brain. Science 262:679–685. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. 1993b. Intracellular analysis of relations between the slow ( < 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci 13:3266–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. 1993c. A novel slow ( < 1 Hz) oscillation of neocortical neurons in vivo: Depolarizing and hyperpolarizing components. J Neurosci 13:3252–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M, Wong RK. 1993. Intrinsic properties and evoked responses of guinea pig subicular neurons in vitro. J Neurophysiol 70:232–245. [DOI] [PubMed] [Google Scholar]
- Stickgold R. 2005. Sleep‐dependent memory consolidation. Nature 437:1272–1278. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. 2005. Memory consolidation and reconsolidation: What is the role of sleep? Trends Neurosci 28:408–415. [DOI] [PubMed] [Google Scholar]
- Stickgold R, James L, Hobson JA. 2000. Visual discrimination learning requires sleep after training. Nat Neurosci 3:1237–1238. [DOI] [PubMed] [Google Scholar]
- Stockard‐Pope JE, Werner SS, Bickford RG. 1992. Atlas of Neonatal Electroencelography, 2nd ed. New York: Raven Press. [Google Scholar]
- Stones MJ. 1973. The effect of prior sleep on rehearsal, recoding and memory. Br J Psychol 64:537–543. [DOI] [PubMed] [Google Scholar]
- Strata F, Atzori M, Molnar M, Ugolini G, Tempia F, Cherubini E. 1997. A pacemaker current in dye‐coupled hilar interneurons contributes to the generation of giant GABAergic potentials in developing hippocampus. J Neurosci 17:1435–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf C. 1965. The fast component in the electrical activity of rabbit's hippocampus. Electroencephalogr Clin Neurophysiol 18:477–486. [DOI] [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. 2007. The evolution of foresight: What is mental time travel, and is it unique to humans? Behav Brain Sci 30:299–313; discussion 313–251. [DOI] [PubMed] [Google Scholar]
- Suh J, Foster DJ, Davoudi H, Wilson MA, Tonegawa S. 2013. Impaired hippocampal ripple‐associated replay in a mouse model of schizophrenia. Neuron 80:484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D, Csicsvari J, Mizuseki K, Montgomery S, Diba K, Buzsáki G. 2011. Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: Influence of dentate and entorhinal cortical activity. J Neurosci 31:8605–8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D, Mizuseki K, Sorgi A, Buzsáki G. 2014. Comparison of sleep spindles and theta oscillations in the hippocampus. J Neurosci 34:662–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Norimoto H, Pu XP, Matsuki N, Ikegaya Y. 2012. Cannabinoid receptor activation disrupts the internal structure of hippocampal sharp wave‐ripple complexes. J Pharmacol Sci 118:288–294. [DOI] [PubMed] [Google Scholar]
- Super H, Soriano E. 1994. The organization of the embryonic and early postnatal murine hippocampus. II. Development of entorhinal, commissural, and septal connections studied with the lipophilic tracer DiI. J Comp Neurol 344:101–120. [DOI] [PubMed] [Google Scholar]
- Sur C, Quirk K, Dewar D, Atack J, McKernan R. 1998. Rat and human hippocampal alpha5 subunit‐containing gamma‐aminobutyric AcidA receptors have alpha5 beta3 gamma2 pharmacological characteristics. Mol Pharmacol 54:928–933. [DOI] [PubMed] [Google Scholar]
- Sussillo D, Abbott LF. 2009. Generating coherent patterns of activity from chaotic neural networks. Neuron 63:544–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R, Barto A. 1998. Reinforcement Learning. Cambridge: MIT Press. [Google Scholar]
- Suzuki SS, Smith GK. 1987. Spontaneous EEG spikes in the normal hippocampus. I. Behavioral correlates, laminar profiles and bilateral synchrony. Electroencephalogr Clin Neurophysiol 67:348–359. [DOI] [PubMed] [Google Scholar]
- Suzuki SS, Smith GK. 1988a. Spontaneous EEG spikes in the normal hippocampus. II. Relations to synchronous burst discharges. Electroencephalogr Clin Neurophysiol 69:532–540. [DOI] [PubMed] [Google Scholar]
- Suzuki SS, Smith GK. 1988b. Spontaneous EEG spikes in the normal hippocampus. III. Relations to evoked potentials. Electroencephalogr Clin Neurophysiol 69:541–549. [DOI] [PubMed] [Google Scholar]
- Suzuki SS, Smith GK. 1988c. Spontaneous EEG spikes in the normal hippocampus. IV. Effects of medial septum and entorhinal cortex lesions. Electroencephalogr Clin Neurophysiol 70:73–83. [DOI] [PubMed] [Google Scholar]
- Suzuki SS, Smith GK. 1988d. Spontaneous EEG spikes in the normal hippocampus. V. Effects of ether, urethane, pentobarbital, atropine, diazepam and bicuculline. Electroencephalogr Clin Neurophysiol 70:84–95. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. 1994. Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. J Neurosci 14:1856–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. 2006. The functional neuroanatomy of autobiographical memory: A meta‐analysis. Neuropsychologia 44:2189–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. 1981. A direct projection from Ammon's horn to prefrontal cortex in the rat. Brain Res 217:150–154. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. 1975. Hippocampo‐hypothalamic connections: Origin in subicular cortex, not ammon's horn. Science 189:303–304. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Cowan WM. 1981. Evidence for collateral projections by neurons in Ammon's horn, the dentate gyrus, and the subiculum: A multiple retrograde labeling study in the rat. J Neurosci 1:548–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadits E, Cserép C, Ludányi A, Katona I, Gracia‐Llanes J, Freund TF, Nyiri G. 2007. Hippocampal GABAergic synapses possess the molecular machinery for retrograde nitric oxide signaling. J Neurosci 27:8101–8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadits E, Cserép C, Szönyi A, Fukazawa Y, Shigemoto R, Watanabe M, Itohara S, Freund TF, Nyiri G. 2011. NMDA receptors in hippocampal GABAergic synapses and their role in nitric oxide signaling. J Neurosci 31:5893–5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó GG, Lenkey N, Holderith N, Andrasi T, Nusser Z, Hájos N. 2014. Presynaptic calcium channel inhibition underlies CB1 cannabinoid receptor‐mediated suppression of GABA release. J Neurosci 34:7958–7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takács VT, Klausberger T, Somogyi P, Freund TF, Gulyás AI. 2012. Extrinsic and local glutamatergic inputs of the rat hippocampal CA1 area differentially innervate pyramidal cells and interneurons. Hippocampus 22:1379–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A, Petersson KM, Rutters F, Tendolkar I, Jensen O, Zwarts MJ, McNaughton BL, Fernandez G. 2006. Declarative memory consolidation in humans: A prospective functional magnetic resonance imaging study. Proc Natl Acad Sci USA 103:756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Abe K, Nojyo Y. 1988. Three‐dimensional analysis of the whole axonal arbors originating from single CA2 pyramidal neurons in the rat hippocampus with the aid of a computer graphic technique. Brain Res 452:255–272. [DOI] [PubMed] [Google Scholar]
- Tambini A, Davachi L. 2013. Persistence of hippocampal multivoxel patterns into postencoding rest is related to memory. Proc Natl Acad Sci USA 110:19591–19596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminen J, Payne JD, Stickgold R, Wamsley EJ, Gaskell MG. 2010. Sleep spindle activity is associated with the integration of new memories and existing knowledge. J Neurosci 30:14356–14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxidis J, Coombes S, Mason R, Owen MR. 2012. Modeling sharp wave‐ripple complexes through a CA3‐CA1 network model with chemical synapses. Hippocampus 22:995–1017. [DOI] [PubMed] [Google Scholar]
- Taxidis J, Mizuseki K, Mason R, Owen MR. 2013. Influence of slow oscillation on hippocampal activity and ripples through cortico‐hippocampal synaptic interactions, analyzed by a cortical‐CA3‐CA1 network model. Front Comput Neurosci 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxidis J, CA Anastassiou, K Diba, C Koch. 2015. Local field potentials encode place cell ensemble activation during hippocampal sharp wave-ripples. Neuron 87:590–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telegdy G, Kokavszky K, Nyerges A. 1999. Action of C‐type natriuretic peptide (CNP) on passive avoidance learning in rats: Involvement of transmitters. Eur J Neurosci 11:3302–3306. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, DiScenna P. 1986. The hippocampal memory indexing theory. Behav Neurosci 100:147–154. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Degenetais E, Glowinski J. 2000. Hippocampo‐prefrontal cortex pathway: Anatomical and electrophysiological characteristics. Hippocampus 10:411–419. [DOI] [PubMed] [Google Scholar]
- Thomson AM. 2000. Facilitation, augmentation and potentiation at central synapses. Trends Neurosci 23:305–312. [DOI] [PubMed] [Google Scholar]
- Thorndike E. 1905. The Elements of Psychology. New York: A. G. Seiler. [Google Scholar]
- Tiesinga P, Sejnowski TJ. 2009. Cortical enlightenment: Are attentional gamma oscillations driven by ING or PING? Neuron 63:727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M. 2002. The role of chloride‐dependent inhibition and the activity of fast‐spiking neurons during cortical spike‐wave electrographic seizures. Neuroscience 114:1115–1132. [DOI] [PubMed] [Google Scholar]
- Tononi G. 2012. Phi—A Voyage from the Brain to the Soul. New York: Pantheon Books. [Google Scholar]
- Tononi G, Cirelli C. 2003. Sleep and synaptic homeostasis: A hypothesis. Brain Res Bull 62:143–150. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. 2014. Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81:12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, Borhegyi Z, Freund TF. 1993. Postsynaptic targets of GABAergic hippocampal neurons in the medial septum‐diagonal band of broca complex. J Neurosci 13:3712–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda I, Bower MR, Leyva F, Buckmaster PS. 2013. Early activation of ventral hippocampus and subiculum during spontaneous seizures in a rat model of temporal lobe epilepsy. J Neurosci 33:11100–11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD. 1995. Model of synchronized population bursts in electrically coupled interneurons containing active dendritic conductances. J Comput Neurosci 2:283–289. [DOI] [PubMed] [Google Scholar]
- Traub RD, Bibbig A. 2000. A model of high‐frequency ripples in the hippocampus based on synaptic coupling plus axon‐axon gap junctions between pyramidal neurons. J Neurosci 20: 2086–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Wong RK. 1982. Cellular mechanism of neuronal synchronization in epilepsy. Science 216:745–747. [DOI] [PubMed] [Google Scholar]
- Traub RD, Jefferys JGR, Whittington MA. 1999a. Fast oscillations in cortical circuits. Cambridge, MA: MIT Press. [Google Scholar]
- Traub RD, Schmitz D, Jefferys JG, Draguhn A. 1999b. High‐frequency population oscillations are predicted to occur in hippocampal pyramidal neuronal networks interconnected by axoaxonal gap junctions. Neuroscience 92:407–426. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Buhl EH, LeBeau FE, Bibbig A, Boyd S, Cross H, Baldeweg T. 2001. A possible role for gap junctions in generation of very fast EEG oscillations preceding the onset of, and perhaps initiating, seizures. Epilepsia 42:153–170. [DOI] [PubMed] [Google Scholar]
- Traub RD, Draguhn A, Whittington MA, Baldeweg T, Bibbig A, Buhl EH, Schmitz D. 2002. Axonal gap junctions between principal neurons: A novel source of network oscillations, and perhaps epileptogenesis. Rev Neurosci 13:1–30. [DOI] [PubMed] [Google Scholar]
- Traub RD, Bibbig A, LeBeau FE, Buhl EH, Whittington MA. 2004. Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu Rev Neurosci 27:247–278. [DOI] [PubMed] [Google Scholar]
- Traub RD, Duncan R, Russell AJ, Baldeweg T, Tu Y, Cunningham MO, Whittington MA. 2010. Spatiotemporal patterns of electrocorticographic very fast oscillations ( > 80 Hz) consistent with a network model based on electrical coupling between principal neurons. Epilepsia 51:1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Schmitz D, Maier N, Whittington MA, Draguhn A. 2012. Axonal properties determine somatic firing in a model of in vitro CA1 hippocampal sharp wave/ripples and persistent gamma oscillations. Eur J Neurosci 36:2650–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevelyan AJ, Sussillo D, Watson BO, Yuste R. 2006. Modular propagation of epileptiform activity: Evidence for an inhibitory veto in neocortex. J Neurosci 26:12447–12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevelyan AJ, Sussillo D, Yuste R. 2007. Feedforward inhibition contributes to the control of epileptiform propagation speed. J Neurosci 27:3383–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves A, Rolls ET. 1992. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus 2:189–199. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET. 1994. Computational analysis of the role of the hippocampus in memory. Hippocampus 4:374–391. [DOI] [PubMed] [Google Scholar]
- Tse D, Takeuchi T, Kakeyama M, Kajii Y, Okuno H, Tohyama C, Bito H, Morris RG. 2011. Schema‐dependent gene activation and memory encoding in neocortex. Science 333:891–895. [DOI] [PubMed] [Google Scholar]
- Tsodyks M, Kenet T, Grinvald A, Arieli A. 1999. Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science 286:1943–1946. [DOI] [PubMed] [Google Scholar]
- Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W. 2006. A daytime nap containing solely non‐REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem 86:241–247. [DOI] [PubMed] [Google Scholar]
- Tukker JJ, Lasztoczi B, Katona L, Roberts JD, Pissadaki EK, Dalezios Y, Marton L, Zhang L, Klausberger T, Somogyi P. 2013. Distinct dendritic arborization and in vivo firing patterns of parvalbumin‐expressing basket cells in the hippocampal area CA3. J Neurosci 33:6809–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. 1983. Elements of Episodic Memory. New York: Clarendon. [Google Scholar]
- Tulving E. 2002. Episodic memory: From mind to brain. Annu Rev Psychol 53:1–25. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Represa A, Jorquera I, Ben‐Ari Y, Gozlan H, Aniksztejn L. 1999. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J Neurosci 19:10372–10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S, Khalilov I, Tsintsadze V, Brouchoud C, Chazal G, Lemonnier E, Lozovaya N, Burnashev N, Ben‐Ari Y. 2014. Oxytocin‐mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science 343:675–679. [DOI] [PubMed] [Google Scholar]
- Ul Haq R, Liotta A, Kovacs R, Rösler A, Jarosch M, Heinemann U, Behrens C. 2012. Adrenergic modulation of sharp wave‐ripple activity in rat hippocampal slices. Hippocampus 22:516–533. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Jirsch JD, LeVan P, Hall J, Avoli M, Dubeau F, Gotman J. 2006. High‐frequency intracerebral EEG activity (100‐500 Hz) following interictal spikes. Epilepsia 47:1465–1476. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Chander R, Dubeau F, Gotman J. 2007. Interictal high‐frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain 130:2354–2366. [DOI] [PubMed] [Google Scholar]
- Vaidya SP, Johnston D. 2013. Temporal synchrony and gamma‐to‐theta power conversion in the dendrites of CA1 pyramidal neurons. Nat Neurosci 16:1812–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M Valero, E Cid, RG Averkin, J Aguilar, A Sanchez-Aguilera, TJ Viney, D Gomez-Dominguez, E Bellistri, L Menendez de la Prida. 2015. Determinants of different deep/superficial CA1 pyramidal cell dynamics during sharp-wave-ripples. Nat Neurosci 18:1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Helm E, Gujar N, Nishida M, Walker MP. 2011. Sleep‐dependent facilitation of episodic memory details. PloS One 6:e27421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vreeswijk C, Sompolinsky H. 1996. Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science 274:1724–1726. [DOI] [PubMed] [Google Scholar]
- Vandecasteele M, Varga V, Berenyi A, Papp E, Bartho P, Venance L, Freund TF, Buzsáki G. 2014. Optogenetic activation of septal cholinergic neurons suppresses sharp wave ripples and enhances theta oscillations in the hippocampus. Proc Natl Acad Sci USA 111:13535–13540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH. 1969. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol 26:407–418. [DOI] [PubMed] [Google Scholar]
- Varga C, Golshani P, Soltész I. 2012. Frequency‐invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice. Proc Natl Acad Sci USA 109:E2726–E2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga C, Oijala M, Lish J, Szabo GG, Bezaire M, Marchionni I, Golshani P, Soltész I. 2014. Functional fission of parvalbumin interneuron classes during fast network events. Elife 3:04006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas R, Thorsteinsson H, Karlsson KA. 2012. Spontaneous neural activity of the anterodorsal lobe and entopeduncular nucleus in adult zebrafish: A putative homologue of hippocampal sharp waves. Behav Brain Res 229:10–20. [DOI] [PubMed] [Google Scholar]
- Velasco AL, Wilson CL, Babb TL, Engel J Jr. 2000. Functional and anatomic correlates of two frequently observed temporal lobe seizure‐onset patterns. Neural Plast 7:49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venance L, Rozov A, Blatow M, Burnashev N, Feldmeyer D, Monyer H. 2000. Connexin expression in electrically coupled postnatal rat brain neurons. Proc Natl Acad Sci USA 97:10260–10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verleger R, Rose M, Wagner U, Yordanova J, Kolev V. 2013. Insights into sleep's role for insight: Studies with the number reduction task. Adv Cogn Psychol 9:160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L1 Verret, EO Mann, GB Hang, AM Barth, I Cobos, K Ho, N Devidze, E Masliah, AC Kreitzer, I Mody, L Mucke, JJ Palop. 2012. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149:708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. 2004. Memory consolidation in sleep; dream or reality. Neuron 44:135–148. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Viana Di Prisco G. 2004. Theta rhythm of the hippocampus: Subcortical control and functional significance. Behav Cogn Neurosci Rev 3:173–200. [DOI] [PubMed] [Google Scholar]
- Veselis RA, Reinsel RA, Feshchenko VA, Wronski M. 1997. The comparative amnestic effects of midazolam, propofol, thiopental, and fentanyl at equisedative concentrations. Anesthesiology 87:749–764. [DOI] [PubMed] [Google Scholar]
- Viereckel T, Kostic M, Bahner F, Draguhn A, Both M. 2013. Effects of the GABA‐uptake blocker NNC‐711 on spontaneous sharp wave‐ripple complexes in mouse hippocampal slices. Hippocampus 23:323–329. [DOI] [PubMed] [Google Scholar]
- Viney TJ, Lasztoczi B, Katona L, Crump MG, Tukker JJ, Klausberger T, Somogyi P. 2013. Network state‐dependent inhibition of identified hippocampal CA3 axo‐axonic cells in vivo. Nat Neurosci 16:1802–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Krosigk M, Bal T, McCormick DA. 1993. Cellular mechanisms of a synchronized oscillation in the thalamus. Science 261:361–364. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister‐Genskow M, Faraguna U, Tononi G. 2008. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci 11:200–208. [DOI] [PubMed] [Google Scholar]
- Wadman WJ, Da Silva FH, Leung LW. 1983. Two types of interictal transients of reversed polarity in rat hippocampus during kindling. Electroencephalogr Clin Neurophysiol 55:314–319. [DOI] [PubMed] [Google Scholar]
- Wagner U, Gais S, Haider H, Verleger R, Born J. 2004. Sleep inspires insight. Nature 427:352–355. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. 2006. Sleep, memory, and plasticity. Annu Rev Psychol 57:139–166. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Buzsáki G. 1996. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci 16:6402–6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DV, Yau HJ, Broker CJ, Tsou JH, Bonci A, Ikemoto S. 2015. Mesopontine median raphe regulates hippocampal ripple oscillation and memory consolidation. Nat Neurosci 18:728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Y Wang, S Romani, B Lustig, A Leonardo, E Pastalkova. 2015. Theta sequences are essential for internally generated hippocampal firing fields. Nat Neurosci 18:282–288. [DOI] [PubMed] [Google Scholar]
- Weglage J, Demsky A, Pietsch M, Kurlemann G. 1997. Neuropsychological, intellectual, and behavioral findings in patients with centrotemporal spikes with and without seizures. Dev Med Child Neurol 39:646–651. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Bauer EP, LeDoux JE. 1999. L‐type voltage‐gated calcium channels mediate NMDA‐independent associative long‐term potentiation at thalamic input synapses to the amygdala. J Neurosci 19:10512–10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JE, Porter JT, Agmon A. 2000. GABAergic inhibition suppresses paroxysmal network activity in the neonatal rodent hippocampus and neocortex. J Neurosci 20:8822–8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A, Williams PA, Hellier JL, Clark S, Dudek FE, Staley KJ. 2010. EEG spike activity precedes epilepsy after kainate‐induced status epilepticus. Epilepsia 51:371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Jefferys JG, Traub RD. 1996. Effects of intravenous anaesthetic agents on fast inhibitory oscillations in the rat hippocampus in vitro. Br J Pharmacol 118:1977–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. 2000. Inhibition‐based rhythms: Experimental and mathematical observations on network dynamics. Intern J Psychophysiol 38:315–336. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Cunningham MO, LeBeau FE, Racca C, Traub RD. 2011. Multiple origins of the cortical gamma rhythm. Dev Neurobiol 71:92–106. [DOI] [PubMed] [Google Scholar]
- Wierzynski CM, Lubenov EV, Gu M, Siapas AG. 2009. State‐dependent spike‐timing relationships between hippocampal and prefrontal circuits during sleep. Neuron 61:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AM Wikenheiser, AD Redish. 2015. Hippocampal theta sequences reflect current goals. Nat Neurosci 18:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TJ, Cacucci F, Burgess N, O'Keefe J. 2010. Development of the hippocampal cognitive map in preweanling rats. Science 328:1573–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA. 2010. Single‐unit activity in piriform cortex during slow‐wave state is shaped by recent odor experience. J Neurosci 30:1760–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. 1994. Reactivation of hippocampal ensemble memories during sleep. Science 265:676–679. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. 2001. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron 31:453–462. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M, Bontempi B. 2010. Memory formation and long‐term retention in humans and animals: Convergence towards a transformation account of hippocampal‐neocortical interactions. Neuropsychologia 48:2339–2356. [DOI] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH. 1989. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol 33:161–253. [DOI] [PubMed] [Google Scholar]
- Wittner L, Henze DA, Zaborszky L, Buzsáki G. 2007. Three‐dimensional reconstruction of the axon arbor of a CA3 pyramidal cell recorded and filled in vivo. Brain Struct Funct 212:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witton J, Staniaszek LE, Bartsch U, Randall AD, Jones MW, Brown JT. 2014. Disrupted hippocampal sharp‐wave ripple‐associated spike dynamics in a transgenic mouse model of dementia. J Physiol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolansky T, Clement EA, Peters SR, Palczak MA, Dickson CT. 2006. Hippocampal slow oscillation: A novel EEG state and its coordination with ongoing neocortical activity. J Neurosci 26:6213–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RK, Traub RD. 1983. Synchronized burst discharge in disinhibited hippocampal slice. I. Initiation in CA2‐CA3 region. J Neurophysiol 49:442–458. [DOI] [PubMed] [Google Scholar]
- Wong T, Zhang XL, Asl MN, Wu CP, Carlen PL, Zhang L. 2005. Postnatal development of intrinsic GABAergic rhythms in mouse hippocampus. Neuroscience 134:107–120. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. 2000. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron 27:623–633. [DOI] [PubMed] [Google Scholar]
- Woodworth R. 1918. Dynamic Psychology. New York: Columbia University Press. [Google Scholar]
- Worrell G, Gotman J. 2011. High‐frequency oscillations and other electrophysiological biomarkers of epilepsy: Clinical studies. Biomark Med 5:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, Meyer FB, Marsh R, Litt B. 2008. High‐frequency oscillations in human temporal lobe: Simultaneous microwire and clinical macroelectrode recordings. Brain 131:928–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouterlood FG, Saldana E, Witter MP. 1990. Projection from the nucleus reuniens thalami to the hippocampal region: Light and electron microscopic tracing study in the rat with the anterograde tracer Phaseolus vulgaris‐leucoagglutinin. J Comp Neurol 296:179–203. [DOI] [PubMed] [Google Scholar]
- Wu X, Foster DJ. 2014. Hippocampal replay captures the unique topological structure of a novel environment. J Neurosci 34:6459–6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Shen H, Luk WP, Zhang L. 2002. A fundamental oscillatory state of isolated rodent hippocampus. J Physiol 540:509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Asl MN, Gillis J, Skinner FK, Zhang L. 2005. An in vitro model of hippocampal sharp waves: Regional initiation and intracellular correlates. J Neurophysiol 94:741–753. [DOI] [PubMed] [Google Scholar]
- Wu CP, Huang HL, Asl MN, He JW, Gillis J, Skinner FK, Zhang L. 2006. Spontaneous rhythmic field potentials of isolated mouse hippocampal‐subicular‐entorhinal cortices in vitro. J Physiol 576:457–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Sankar R, Lerner JT, Matsumoto JH, Vinters HV, Mathern GW. 2010. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology 75:1686–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Huang X, Takagaki K, Wu JY. 2007. Compression and reflection of visually evoked cortical waves. Neuron 55:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaari Y, Beck H. 2002. “Epileptic neurons” in temporal lobe epilepsy. Brain Pathol 12:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky Y, Brankack J, Haas HL. 1995. Differences of CA3 bursting in DBA/1 and DBA/2 inbred mouse strains with divergent shuttle box performance. Neuroscience 64:319–325. [DOI] [PubMed] [Google Scholar]
- Yaroush R, Sullivan MJ, Ekstrand BR. 1971. Effect of sleep on memory. II. Differential effect of the first and second half of the night. J Exp Psychol 88:361–366. [DOI] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nádasdy Z, Jando G, Szabo I, Sik A, Buzsáki G. 1995. Sharp wave‐associated high‐frequency oscillation (200 Hz) in the intact hippocampus: Network and intracellular mechanisms. J Neurosci 15:30–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JY, Frank LM. 2015. Hippocampal-cortical interaction in decision making. Neurobiol Learn Mem 117:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Nelson DA, Rubin WW, Katz LC. 1995. Neuronal domains in developing neocortex: Mechanisms of coactivation. Neuron 14:7–17. [DOI] [PubMed] [Google Scholar]
- Zastawny RL, George SR, Nguyen T, Cheng R, Tsatsos J, Briones‐Urbina R, O'Dowd BF. 1994. Cloning, characterization, and distribution of a mu‐opioid receptor in rat brain. J Neurochem 62:2099–2105. [DOI] [PubMed] [Google Scholar]
- Zeng H, Chattarji S, Barbarosie M, Rondi‐Reig L, Philpot BD, Miyakawa T, Bear MF, Tonegawa S. 2001. Forebrain‐specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic‐like memory. Cell 107:617–629. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Stornetta RL, Zhu JJ. 2004. Chandelier cells control excessive cortical excitation: Characteristics of whisker‐evoked synaptic responses of layer 2/3 nonpyramidal and pyramidal neurons. J Neurosci 24:5101–5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Lovinger D, Delpire E. 2005. Cortical neurons lacking KCC2 expression show impaired regulation of intracellular chloride. J Neurophysiol 93:1557–1568. [DOI] [PubMed] [Google Scholar]
- Zieglgansberger W, French ED, Siggins GR, Bloom FE. 1979. Opioid peptides may excite hippocampal pyramidal neurons by inhibiting adjacent inhibitory interneurons. Science 205:415–417. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Jiruska P, Zelmann R, Leijten FS, Jefferys JG, Gotman J. 2012. High‐frequency oscillations as a new biomarker in epilepsy. Ann Neurol 71:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylla MM, Zhang X, Reichinnek S, Draguhn A, Both M. 2013. Cholinergic plasticity of oscillating neuronal assemblies in mouse hippocampal slices. PloS One 8:e80718. [DOI] [PMC free article] [PubMed] [Google Scholar]


