Abstract
Background
The prognosis of glioblastoma (GBM) remains poor; therefore, effective therapeutic strategies need to be developed. CD40 is a costimulatory molecule whose agonistic antibody has been shown to activate antitumor effects. Recently, CD40 has been extensively targeted for immunotherapeutic purposes.
Methods
Expressions of CD40/CD40L mRNAs were examined in 86 cases of World Health Organization grade IV GBM and 36 cases of grade III gliomas and correlated with outcomes. CD40 signaling was employed to augment the efficacy of immunotherapy against gliomas. The efficacy of FGK45, an agonistic antibody for CD40, was examined by adding it to a tumor lysate–based subcutaneous vaccination against a GL261 glioma model and an NSCL61 glioma-initiating cell–like cell tumor model.
Results
We demonstrated for the first time using quantitative PCR that grade III gliomas express higher levels of CD40/CD40L than does grade IV GBM. The higher expression of CD40/CD40L was associated with good prognoses in patients with GBM. Addition of FGK45 to the subcutaneous tumor cell lysate–based vaccination significantly prolonged survival in both tumor models. However, the efficacy was modest in NSCL61-model mice. Therefore, we established combination immunotherapeutic strategies using FGK45 and OX86, an agonistic antibody for OX40. Combination immunotherapy significantly prolonged survival with synergistic effects. Apoptosis increased and proliferation decreased in tumors treated with combination immunotherapy.
Conclusions
The high expression of CD40/CD40L can be used as a biomarker for better prognoses in patients with gliomas. Immunotherapy using FGK45 significantly prolonged survival and represents a potential therapeutic strategy for gliomas including glioma-initiating cells.
Keywords: CD40, CD40 ligand, glioma, immunotherapy, prognosis
Glioblastoma (GBM), classified as grade IV glioma by the World Health Organization (WHO), are highly malignant primary brain tumors in adults. The current standard of care for patients with GBM consists of maximal resection followed by fractionated radiation therapy and the administration of temozolomide.1 Despite recent extensive research and the current therapeutic interventions, the prognoses of patients with GBM remain poor, with median survival of between 12 and 15 months.1,2 The resistance of malignant gliomas to various therapies has recently been attributed to cancer-initiating cells (CICs). CICs have the ability to perpetuate themselves through self-renewal and generate amplifying cancer cells.3 Thus, the development of CIC targeting therapy is critical to improve the outcomes of patients with malignant gliomas. Many novel strategies to improve treatments against GBM are currently being investigated. Immunotherapy-based strategies are now expected to be effective approaches in the treatment of cancer.4–7
With the aim of reinforcing tumor-specific immunity, we focused our research on cluster of differentiation (CD)40, a costimulatory molecule. CD40 is a member of the tumor-necrosis factor receptor family and is expressed on the surface of immune cells, including B cells, monocytes, macrophages, dendritic cells (DCs), and non-immune cells such as endothelial cells and epithelial cells, as well as malignant tumors.8,9 The cognate ligand, CD40 ligand (CD40L), is expressed on activated T cells, platelets, and macrophages. Cross-linking of CD40 with CD40L has been shown to induce B cells to proliferate, differentiate, and form germinal centers.8 Interactions between CD40 and CD40L activate B cells and DCs. The agonistic anti-CD40 antibody, FGK45, was previously reported to activate antigen-presenting cells and promote antitumor T-cell responses.10 Thus, CD40L has been extensively targeted for immunotherapeutic purposes. In the present study, we first examined the expressions of CD40/CD40L in glioma tissue and correlated them to the outcomes of patients with GBM. We conducted a retrospective study analysis of patients with GBM. They were divided into 2 groups—high and low expression levels of CD40 mRNA—and each subgroup was investigated regarding associated prognostic factors. The factors analyzed included age, sex, preoperative KPS, MGMT promoter methylation, Ki-67 labeling index, alteration of 7p (EGFR), 9p (CDKN2A), and 10q (PTEN), all of which are known prognostic factors of high-grade gliomas. Molecular analysis: MGMT promoter methylation, 7q amplification, 10q Loss, 9p deletion analyses of MGMT promoter methylation, 7q (EGFR) amplification, 10q (PTEN) loss, and 9p (CDKN2A) deletion were performed as described in our previous study (see Supplementary data).11 Subsequently the same analyses were again performed by dividing patients into 2 groups: high and low expression levels of CD40L mRNA.
Then, in vivo immunotherapy was conducted involving the addition of FGK45 to a tumor cell lysate–based subcutaneous vaccination against the GBM cell line GL261 or the glioma-initiating cell (GIC)–like cell line NSCL61, an intracranial isografted tumor model. In order to enhance antitumor efficacy and establish potent immunotherapy, even against GICs, combination therapies using FGK45, DCs, and an agonistic antibody for another costimulatory molecule, OX40, were examined using the NSCL61 mouse model.
Materials and Methods
Patients, Tumor Specimens, and Cell Lines
From January 1999 to January 2010, 86 patients with GBM underwent gross total resection in our department. Patients with GBM consisted of 47 males and 39 females, with a median age of 55.7 years (range, 11–79). Clinical patient profiles were obtained from medical records. All patients with GBM underwent surgery followed by nitrosourea-based chemotherapy (nimustine hydrochloride [ACNU] or temozolomide) and radiation therapy (total dose, 60 Gy). Median follow-up was 22.2 months (range, 3–65). Their tissue specimens were stored at −80°C until use in experiments. Informed consent was obtained from all patients, and the ethics committee of Tohoku University Graduate School of Medicine approved the study.
Five human glioma cell lines—U87, U251, U373, T98, A172—and the mouse lung cancer cell line LLC12 were used for mRNA analyses of CD40/CD40L and OX40/OX40L (see Supplementary data). The mouse glioma cell line GL26113 and the mouse GIC-like cell line NSCL6114 were used for the mouse intracranial tumor models.
RNA Isolation and Quantitative Real-Time PCR
Total RNA was extracted from frozen human glioma primary specimens using the RNeasy Lipid Tissue Mini Kit (Qiagen Science) (see Supplementary data). The mRNA expression of CD40, CD40L, and the internal control, β-actin, was measured using probes purchased from the TaqMan Gene Expression Assays library (Applied Biosystems). Relative mRNA levels were calculated based on cycle threshold (Ct) values and were corrected for the expression of β-actin according to the following equation15: 2−Δ(ΔCt) [Δ(ΔCt) = ΔCt(CD40, CD40L, OX40, OX40L) − ΔCt(β-actin)].
Ki-67 Labeling Index
The resected human GBM specimens were examined by immunohistochemical staining for Ki-67 antigen (Ki-67 antibody, Dako). Each slide was individually reviewed and scored by one neuropathologist (M.W.) (see Supplementary data).
Immunohistochemistry
Paraffin-embedded sections of human GBM tissue were used for CD40/CD40L staining (see Supplementary data). In the immunohistochemical study of CD4 and CD8, sections were incubated with either an anti-CD4 (1:500) or an anti-CD8a (1:200) antibody (eBioscience). For Ki-67 staining, slices were incubated with an SP6 rabbit anti-mouse Ki-67 antibody (1:200) (Nichirei Biosciences). To detect apoptotic cells, sections were stained with terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) using the In Situ Cell Death Detection Kit (Roche) in accordance with the manufacturer's instructions.
Mouse Strains
Six- to 8-week-old C57BL/6 female mice and 7- to 8-week-old BALB/c male nude mice were purchased from SLC Japan (see Supplementary data). The protocols used in the animal studies were approved by the Institute for Animal Experimentation of the Tohoku University Graduate School of Medicine. CD40 agonistic antibody, FGK45 was refined using FGK45 hybridoma cells from 7- to 8-week-old BALB/c male nude mice (see Supplementary data).
Brain Tumor Models
GL261 and NSCL61 cell lines were used as a mouse intracranial tumor model (see Supplementary data). A total of 2 × 105 GL261 cells or 1 × 104 NSCL61 cells in 2 µL phosphate buffered saline was then injected into the right striatum using a 2-µL Hamilton syringe with a 26-gauge needle.
Dendritic Cell Culture
C57BL/6 mouse bone marrow–derived DCs were harvested and cultured (see Supplementary data). These cells were then cultured with the tumor lysates (TLs) of 2 × 105 GL261 and 1 × 104 NSCL61 for 3 h and again collected for use in the vaccination therapy.
Vaccination Therapy
Heavily irradiated tumor cells were used as TLs. Irradiation of 5000 rad was administered for 2 × 105 GL261 cells and 7000 rad for 1 × 104 NSCL61 cells. In the standard vaccination, mice received a subcutaneous injection of TLs in combination with 250 μg of an immunoglobulin G (IgG) control antibody. To observe the additive effects of triggering OX40 or CD40, 250 μg OX86, an anti-OX40 agonistic antibody, or 100 μg FGK45 was injected instead of the IgG antibody. Vaccinations were administered twice at a 5-day interval. To assess the tumor therapy used, each tumor model—GL261 and NSCL61—was treated with TLs in combination with FGK45, DCs, OX86, or the IgG antibody. Mice were monitored daily for survival and general health.
Enzyme-Linked Immunosorbent Assay
Lymphocytes were harvested from the spleens of mice vaccinated with FGK45, OX86, DCs, and IgG (see Supplementary data). A total of 1 × 105 CD4 T cells were cultured for 24 h, and the supernatants were used for an enzyme-linked immunosorbent assay (ELISA) to detect mouse interferon (IFN)-γ (BD OptEIA, BD Biosciences).
To determine the levels of transforming growth factor (TGF)–β1, 1 × 107 NSCL61 cells or 1 × 107 GL261 cells were cultured. The supernatant (n = 4) was collected and subjected to evaluation using an ELISA kit (R&D Systems) according to the manufacturer's instructions.
Microscopy and Image Capture
Regarding optical and fluorescence microscopy, sections were imaged with a BZ9000 microscope (Keyence) (see Supplementary data).
Statistical Analyses
In the rodent study, data were collected from independent experiments of 10 mice each. Significance was determined using the Mann–Whitney U test and Fisher's exact test for comparison between 2 groups. Comparison among more than 3 groups was determined using one-way ANOVA. For multivariate analysis, factors achieving P< .20 in univariate analysis were used in backward stepwise Cox regression analysis for estimating hazard ratios (HRs) and 95% CIs. The log-rank test was used for the analysis of Kaplan–Meier survival curves (see Supplementary data).
Results
Expression of CD40/CD40L in Glioma Tissues and Its Association With Outcomes of Gliomas
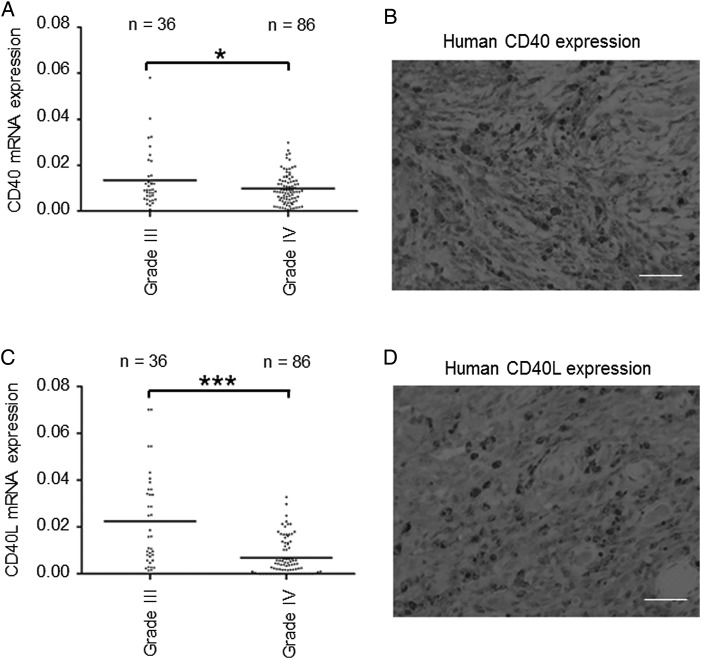
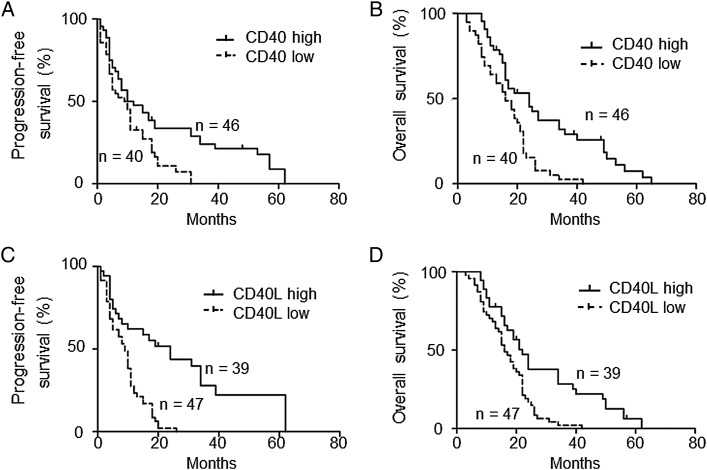
The frozen tissue samples of 122 patients with WHO grade III (n = 36) or IV (n = 86) gliomas were collected retrospectively. The expression levels of human CD40/CD40L mRNA were investigated by quantitative PCR. The expression of CD40/CD40L by grade III gliomas was significantly higher than that by grade IV GBM (Fig. 1A and C). The expression of the CD40/CD40L proteins was confirmed with immunohistochemical staining in high mRNA–expressing tissues (Fig. 1B and D). We subsequently evaluated the relationship between the mRNA expression levels of CD40/CD40L and progression-free survival (PFS) and overall survival (OS). We subdivided patients with GBM into a high CD40 (CD40L) expression group and a low CD40 (CD40L) expression group. A CD40 value higher than 0.01 (relative mRNA level) was defined as high expression and a lower value as low expression. Similarly, a CD40L value higher than 0.001 (relative mRNA level) was defined as high expression and a lower value as low expression. The higher expression of CD40/CD40L correlated with prolonged PFS (Fig. 2A and C) and OS (Fig. 2B and D). These results suggested that the high expression of CD40/CD40L could be used as a prognostic factor of GBM. We next validated the expression of CD40/CD40L and survival using data from The Cancer Genome Atlas (TCGA).16 PFS of cases without alteration in the CD40 gene was significantly longer than that of cases with alteration in the CD40 gene (P = .0248; Supplementary Fig. 1A). OS of cases without alteration in the CD40 gene was significantly longer than that of cases with alteration in the CD40 gene (P = .0474; Supplementary Fig. 1B). PFS of cases without alteration in the CD40L gene, designated as CD40LG in TCGA, was longer but not significantly so compared with that of cases with alteration in the CD40L gene (P = .658; Supplementary Fig. 2A). OS of cases without alteration in the CD40L gene was significantly longer than that of cases with alteration in the CD40L gene (P = .0437; Supplementary Fig. 2B).
Fig. 1.
CD40/CD40L gene expression and immunohistochemistry in glioma tissues. (A) CD40 gene expression analyzed by quantitative PCR in glioma tissues. Expression of CD40 was significantly higher in 36 cases of grade III gliomas than in 86 cases of grade IV glioblastomas (GBM) (*P = .0334). (B) Representative immunohistochemical image of CD40 in GBM tissues that revealed the high expression of CD40 by quantitative PCR. CD40-positive GBM cells were detected. Scale bar, 100 μm. (C) CD40L gene expression analyzed by quantitative PCR. Expression of CD40L was significantly higher in grade III gliomas than in grade IV GBM (*P< .0001). (D) Representative immunohistochemical image of CD40L in GBM tissues that revealed the high expression of CD40L by quantitative PCR. CD40L-positive GBM cells were detected. Scale bar, 100 μm.
Fig. 2.
Kaplan–Meier survival curves of patients with GBM. (A, B) High expression levels of CD40 (2−Δ(ΔCt) > .01; n = 46) in patients with GBM who underwent gross total resection of the tumor were associated with longer PFS and OS compared with low expression levels (2−Δ(ΔCt) < .01; n = 40). (C, D), High expression levels of CD40L (2−Δ(ΔCt) > .001; n = 39) in patients with GBM who underwent gross total resection of the tumor were also associated with longer PFS and OS compared with low expression levels (2−Δ(ΔCt) < .001; n = 47).
CD40/CD40L Expression Levels and Other Prognostic Factors
CD40/CD40L expression levels were analyzed and divided into subgroups of high and low expression level. Age, sex, Ki-67 labeling index, 7p (EGFR) amplification, 10q (PTEN) loss, MGMT gene promoter methylation, and 9p (CDKN2A) deletion were not correlated with CD40/CD40L status (Supplementary Tables 1 and 2). Next, we assessed PFS and OS in relation to other parameters. In univariate analysis, factors associated with prolonged PFS were Ki-67 labeling index <30% (P = .038), retained 10q (PTEN; P = .024), high expression levels of CD40 (P = .0085), and high expression levels of CD40L (P = .0006, Supplementary Table 3). Factors associated with prolonged OS were KPS ≥80% (P = .032), high expression levels of CD40 (P = .0027), and high expression levels of CD40L (P = .0001). In multivariate analysis for OS, independent good prognostic factors were Ki-67 labeling index <30% (HR, 1.19; 95% CI, 0.76–1.96; P = .045), high expression levels of CD40 (HR, 2.04; 95% CI, 1.28–3.26; P = .0028), and high expression levels of CD40L (HR, 2.13; 95% CI, 1.32–3.51; P = .0019; Supplementary Table 4).
Expression of CD40/CD40L in Tumor Cell Lines
We assessed the expression levels of CD40/CD40L in 5 human glioma cell lines (A172, U87, U251, U373, T98), 2 mouse glioma cell lines (GL261, NSCL61), and a lung cancer cell line (LLC). We detected CD40 expression in A172, U87, GL261, NSCL61, and LLC (Supplementary Fig. 3A). U87 and LLC cells showed significantly higher expression of CD40 compared with the others. GL261 cells showed significantly higher expression of CD40 compared with NSCL61 cells (P = .0079; Supplementary Fig. 3C). CD40L expression was not found in these cell lines (Supplementary Fig. 3A). Additionally, we assessed OX40/OX40L expression in the same tumor cell lines (Supplementary Fig. 3B). Only LLC cells expressed OX40, whereas OX40L was expressed in A172, U87, U251, U373, T98, and LLC cells. A172 cells showed significantly higher OX40L expression compared with the others (Supplementary Fig. 3B).
Effects of CD40 Signaling on the GL261 Glioma Cell Line
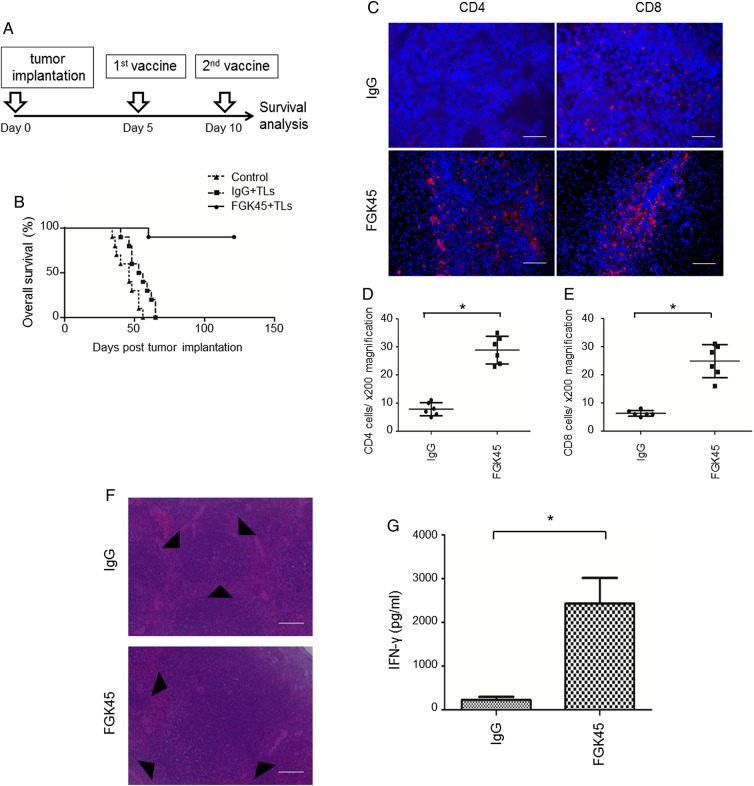
Since the higher expression of CD40/CD40L correlated with prolonged OS and PFS, we hypothesized that the stimulation of CD40 signaling could induce stronger antitumor immunity against gliomas. To investigate the effects of CD40 signaling on the survival of an intracranial mouse glioma model, we established such a model using the GL261 glioma cell line. At first, mice were randomly divided into 3 groups. One of these 3 groups was observed as the nontreated group. For the other 2 groups, starting from 5 days after the inoculation of tumor cells, FGK45 only or FGK45 with irradiated GL261 TLs was administered subcutaneously as vaccines twice at a 5-day interval (Supplementary Fig. 4A). Although the FGK45-treated group survived significantly longer than the nontreated group (P = .0072), the group treated with FGK45 plus TLs survived significantly longer than the group treated with FGK45 alone (P = .0301). Subsequently, tumor-bearing mice were randomly divided into 3 groups. One of these 3 groups was observed as the nontreated group. For the other 2 groups, starting from 5 days after the inoculation of tumor cells, we administered IgG, a control, or FGK45 subcutaneously with TLs as vaccines twice at a 5-day interval (Fig. 3A). Mice vaccinated with IgG and TLs survived longer than the control nontreated group (P = .0162; Fig. 3B). However, efficacy was modest. Mice vaccinated with FGK45 and TLs survived significantly longer than mice vaccinated with IgG and TLs (P < .0001; Fig. 3B). This result suggested that the administration of TLs and FGK45 induced significant antitumor effects.
Fig. 3.
Effects of triggering CD40 by FGK45 in the GL261 tumor model. (A) Schedule of vaccination therapy. (B) Intracranial glioma model established using the GL261 glioma cell line was randomly divided into 3 groups. One of these 3 groups was observed as a nontreated group. For the other 2 groups, IgG or FGK45 was administered s.c. with irradiated GL261 TLs as vaccines. Mice vaccinated with IgG and TLs (n = 10) survived longer than the nontreated group (n = 10, P = .0162). However, efficacy was modest. Mice vaccinated with FGK45 and TLs (n = 10) survived significantly longer than mice vaccinated with IgG and TLs (P < .0001). (C) Mouse CD4- and CD8-positive T cells (red) in GL261 tumor models were counterstained with 4′,6′-diamidino-2-phenylindole (blue). Scale bars, 100 μm. Immunohistochemistry showed the stronger infiltration of CD4- and CD8-positive T cells in GL261 tumor models 4 days after the second vaccination with FGK45. (D, E) The cells that were positively stained for CD4 or CD8 were counted under ×400 magnification. Bars indicate the mean ± SD (*P < .05). (F) Hematoxylin and eosin stain of mouse spleens from the IgG vaccination group and FGK45 vaccination group. The size of germinal centers (black triangles) in the FGK45 vaccination group was larger than that in the IgG vaccination group. Scale bars, 100 μm. (G) Production of the INF-γ protein by mouse CD4-positive T cells after the second s.c. vaccination with IgG + TLs or FGK45 + TLs. Bars indicate the mean ± SD.
Efficacy of Stimulating CD40 Signaling in Tumor Immunotherapy
To investigate the mechanism underlying the antitumor effects of the FGK45-mediated vaccination, brain sections from mice 4 days after the treatment (ie, second vaccination) were stained immunohistochemically with an anti-CD4, anti-CD8 antibody (Fig. 3C–E). The infiltration of CD4 and CD8 T cells in the FGK45-vaccinated group was significantly stronger than that in the IgG-treated group. Furthermore, the size of germinal centers in the spleen was larger in the FGK45-vaccinated group than in the IgG-treated group (Fig. 3F). This result suggested that the strong immune stimulatory effects of FGK45 induced the formation of large germinal centers in the spleen. Using ELISA, we also demonstrated that the FGK45 vaccination induced significantly larger amounts of IFN-γ proteins than did the IgG vaccination (Fig. 3G).
Combination Immunotherapy in Mice in a Glioma-Initiating Cell Model
The same treatment was tested in the NSCL61 tumor model. Starting from 5 days after the inoculation of tumor cells, we administered IgG or FGK45 with irradiated NSCL61 TLs subcutaneously as vaccines twice at a 5-day interval, similar to the study using GL261 (Fig. 3A). At first, we analyzed the survival of NSCL61-model mice. An intracranial glioma model was established using the NSCL61 cell line. Mice were randomly divided into the following 3 groups: control nontreated (n = 10), IgG + TLs vaccinated (n = 10), and FGK45 + TLs vaccinated (n = 10) (Supplementary Fig. 4B). Treatment was given in the same method as Fig. 3A. The survival times of the control and IgG + TLs groups were not different (P = .179). The FGK45 + TLs-vaccinated mice survived significantly longer than the IgG + TLs-vaccinated mice (P = .0001; Supplementary Fig. 4B).
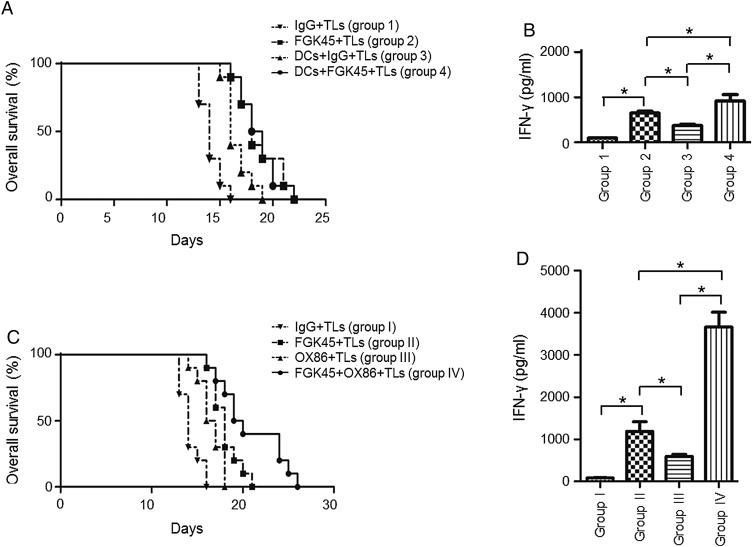
However, efficacy was not as prominent as that observed in the GL261 model. Therefore, a combination treatment with DC vaccination was performed. An intracranial glioma model was again established using the NSCL61 cell line. Mice were randomly divided into the following 4 groups: with the same method as Fig. 3A, group 1 received IgG and TLs as subcutaneous vaccines; group 2 received FGK45 and TLs as subcutaneous vaccines; group 3 received DCs, IgG, and TLs as subcutaneous vaccines; group 4 received DCs, FGK45, and TLs as subcutaneous vaccines. The survival of intracranial model mice was longer in groups 2, 3, and 4 (P < .0001, P = .0001, and P < .0001 for each group versus group 1; Fig. 4A). The prolongation of survival in the combination group (ie, group 4) was significantly different from that in the DCs group, but not different from that in the FGK45 group (ie, group 2) (P = .0083 and .8559; Fig. 4A). The production of IFN-γ from CD4 lymphocytes harvested from the spleens of treated mice was synergistically induced in the combination group (Fig. 4B). Subsequently, we tested the combination of FGK45 and OX86, an agonistic antibody for another costimulatory molecule, OX40. Intracranial isografted tumor model mice were randomly divided into 4 groups. With the same method as Fig. 3A, group I received vaccination of IgG and TLs as subcutaneous vaccines; group II received FGK45 and TLs as subcutaneous vaccines; group III received OX86 and TLs as subcutaneous vaccines; group IV received FGK45, OX86, and TLs as subcutaneous vaccines. Survival was prolonged in both group II and group III (P = .0001 and .0013 for each group vs group I in the log-rank test; Fig. 4C). Furthermore, combination therapy, group IV, significantly prolonged survival compared with group II (P = .0382) and group III (P = .0313) (Fig. 4C). We also observed the synergistic induction of IFN-γ from CD4 lymphocytes harvested from the spleens of treated mice (Fig. 4D).
Fig. 4.
Effects of the combined immunotherapy of FGK45, DCs, and OX86 in the GIC model mouse. (A) Survival analysis of NSCL61-model mice treated with an s.c. injection of irradiated NSCL61 cells as TLs and IgG (group 1), TLs and FGK45 (group 2), TLs, DCs, and IgG (group 3), and TLs, DCs, and FGK45 (group 4). FGK45 therapy (group 2), DC therapy (group 3), and combination therapy (group 4) significantly prolonged survival compared with group 1 (P < .0001, P = .0001, P < .0001). The synergistic effects of the combination therapy (group 4) were detected compared with DC therapy alone (group 3; P = .0083), while not detected compared with FGK45 therapy alone (group 2; P = .8559). (B) Production of the IFN-γ protein by mouse CD4-positive T cells after the second s.c. vaccination as determined by ELISA. Comparisons among groups 1, 2, 3, and 4. Bars indicate the mean ± SD. (C) Survival analysis of NSCL61-model mice treated with an s.c. injection of irradiated NSCL61 cells as TLs and IgG (group I), TLs and FGK45 (group II), TLs and OX86 (group III), and TLs, OX86, and FGK45 (group IV). FGK45 therapy (group II), OX86 therapy (group III), and combination therapy (group IV) significantly prolonged survival compared with group I (P = .0001, P = .0013, P < .0001). Synergistic effects of the combination therapy (group IV) were detected compared with FGK45 therapy alone (group II; P = .0382) and OX86 therapy alone (group III; P = .0313). (D) Production of the IFN-γ protein by mouse CD4-positive T cells after the second s.c.vaccination as determined by ELISA. Comparisons among groups I, II, III, and IV. Bars indicate the mean ± SD.
Immunotherapy Downregulated Proliferation and Promoted Apoptosis of Tumor Cells
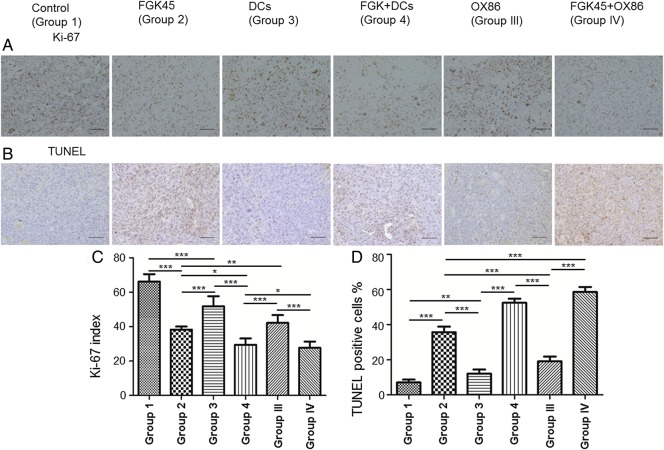
Proliferation and cell death were evaluated in mouse brains harvested 4 days after the second vaccination. The proliferation of tumor cells was evaluated by Ki-67 staining (Fig. 5A). The percentage of Ki-67–positive cells in the FGK45 group, the FGK45 + DCs group, and the FGK45 + OX86 group was significantly lower than that in the control group (P < .05, P < .01, P < .0001; Fig. 5C). This result suggested that CD40 signaling significantly inhibited the proliferation of tumors in the NSCL61 model. To investigate the mechanism underlying the antitumor effects of the FGK45-mediated vaccination, brain sections from mice after the treatment were stained using the TUNEL method. The FGK45 vaccination induced markedly larger numbers of apoptotic cells than the IgG or OX86 vaccination (Fig. 5B). A significant difference was observed in the percentage of TUNEL-positive NSCL61 between these groups. The percentage of TUNEL-positive cells was significantly higher in the FGK45 group, the FGK45 + DCs group, and the FGK45 + OX86 group than in the control group (P < .05, P < .01, P < .0001; Fig. 5D). This result suggested that immunotherapy augmented by stimulation of CD40 signaling significantly induced apoptosis in the NSCL61 model.
Fig. 5.
Ki-67 and TUNEL staining in NSCL61-model mice treated with either IgG, FGK45, DCs, OX86, DCs + FGK45, or OX86 + FGK45. (A) Ki-67 staining. Scale bars, 100 μm. (B) TUNEL staining. Scale bars, 100 μm. (C) The cells that positively stained for Ki-67 were counted under ×400 magnification. Bars indicate the mean ± SD. *P < .05, **P < .01, ***P < .0001. (D) The cells that positively stained with TUNEL were counted under ×400 magnification. Bars indicate the mean ± SD. *P < .05, **P < .01, ***P < .0001.
Discussion
Malignant gliomas are the most common type of primary brain tumor and still have a poor prognosis. In the present study, we demonstrated for the first time that the expression of CD40/CD40L was associated with the outcomes of gliomas. Grade III gliomas expressed higher levels of CD40/CD40L than grade IV gliomas, GBM (Fig. 1A and C). The high expression levels of CD40/CD40L were also associated with better PFS and OS of GBM patients who underwent gross total removal of the tumor (Fig. 2A–D). These results suggested that CD40/CD40L could be useful biomarkers for gliomas. Also, in multivariate analysis for OS, independent good prognostic factors were Ki-67 labeling index <30% (HR, 1.19; 95% CI, 0.76–1.96; P = .045), high expression levels of CD40 (HR, 2.04; 95% CI, 1.28–3.26; P = .0028), and high expression levels of CD40L (HR, 2.13; 95% CI, 1.32–3.51; P = .0019; Supplementary Table 4). We additionally validated the relationship of expression of CD40/CD40L and survival using TCGA data.16 Findings here were almost the same as our findings except for the significance of PFS and CD40L expression. It was significant in our data but not in those of TCGA. We collected only patients in whom gross total removal was achieved. This might be the reason for this slight difference.
In vitro, glioma cell lines expressed CD40 but not CD40L (Supplementary Fig. 3A). However, judging from the immunohistochemical images of human tumor specimens, glioma cells likely express both CD40 and CD40L (Fig. 1B and D). Therefore, there is a possibility of autocrine stimulation. However, CD40 signaling is known to stimulate immune cells as well as directly kill tumor cells by apoptosis.8,9 So, the benefit of autocrine stimulation requires further research. Anyway, as the higher expression of CD40/CD40L in GBM was associated with improved survival, we hypothesized that triggering CD40/CD40L induces immune stimulation and therefore antitumor effects.
In order to develop effective treatments based on this hypothesis, FGK45 was added to TLs vaccination against a rodent glioma model. Immunotherapy using FGK45 significantly induced a larger amount of IFN-γ (Fig. 3G) from CD4 T cells than that using IgG and showed prolonged survival of the GL261 model (Fig. 3B) as well as the NSCL61 GIC-like cell model (Fig. 4A and C).
Although vaccination using FGK45 markedly prolonged the survival of the GL261 glioma model, its efficacy against NSCL61 was not as strong.
This might be because TLs were prepared from fewer cells in the NSCL61 model: 1 × 104 cells compared with 2 × 105 cells in the GL261 model. However, TLs from 1 × 104 cells and 2 × 105 cells demonstrated no difference in efficacy when combined with FGK45 as a vaccine against the NSCL61 model (Supplementary Fig. 4C). To determine the difference of antitumor effects against GL261 cells and NSCL61 cells, the levels of TGF-β1 were analyzed. TGF-β1 is a central mediator of GBM, maintains cancer cell stemness, and induces profound immunosuppression in the host.17 NSCL61 cells released a significantly higher amount of TGF-β1 than GL261 cells (Supplementary Fig. 5). Additionally, the expression levels of CD40 mRNA of NSCL61 cells were significantly lower compared with GL261 (Supplementary Fig. 3C). Partly from these mechanisms, the NSCL61 tumor model was not as susceptible to treatment with vaccination and CD40 signaling as the GL261 model. We therefore attempted to augment its efficacy by combining it with the other immunostimulants. Antigen-presenting cells, DCs, and another costimulation molecule, OX40, were selected as candidates. We initially used a vaccination based on FGK45 and DCs. Combination immunotherapy induced large amounts of IFN-γ from CD4 T cells (Fig. 4B). However, the effects of the combination immunotherapy were not significantly different from those of the FGK45-treated group. We then used a combination of FGK45 and OX86. The combination immunotherapy induced a large amount of IFN-γ from CD4 T cells (Fig. 4D) and prolonged survival with significant synergistic effects (Fig. 4C). Ki-67 staining revealed that immunotherapy inhibited the proliferation of tumor cells, especially when CD40 was stimulated (Fig. 5A and C). TUNEL staining showed that immunotherapy induced apoptosis against tumor cells (Fig. 5B and D). Furthermore, the stimulation of CD40 signaling significantly induced apoptosis against tumor cells (Fig. 5B and D).
CD40 signaling has been shown to stimulate B cells, monocytes, macrophages, and DCs,8 while OX40 signaling stimulates CD4 and CD8 T cells and natural killer T cells.18,19 Therefore, it is likely that combination immunotherapy using FGK45 and OX86 stimulates a broad range of molecules that mediate antitumor effects and result in enhanced efficacy. In addition, IFN-γ was previously shown to enhance the expression of CD40 in glioma cells.9 Since the significant induction of IFN-γ was observed in CD4 lymphocytes following the combined treatment with FGK45 and OX86, combination immunotherapy appeared to potently induce synergistic effects.
Kosaka and colleagues20 recently reported the efficacy of a CD40 agonist against glioma intracranial models. They administered FGK45 intraperitoneally and demonstrated synergistic effects with a cyclooxygenase-2 inhibitor. To the best of our knowledge, no other study has demonstrated the efficacy of CD40 stimulation against gliomas. We here showed the efficacy of the addition of a CD40 agonistic antibody to a vaccine against glioma models. In a previous phase I trial, a cytokine storm was unexpectedly induced by the intravenous systemic administration of an anti-CD28 monoclonal antibody.21 Since CD28 is a costimulatory molecule for T-cell activation, similar to CD40, a similar cytokine reaction may limit the dose of the CD40 agonistic antibody when delivered systemically. From this point of view, subcutaneous administration as an additive to a vaccine may provide a safer strategy when considering clinical development. In this study, we closely monitored neurological deficits, general health conditions, and body weight losses of all mice. We did not observe any side effects with subcutaneous vaccination.
In the present study, we demonstrated for the first time that the expression of CD40/CD40L could be used as a prognostic factor for gliomas and their higher expression correlated with better survival. We then showed that CD40-based immunotherapy was effective against rodent glioma tumor models and a GIC-like cell tumor model. Combination immunotherapy using FGK45 and OX86 further prolonged survival with synergistic effects. Therefore, this combination immunotherapy can be used as an effective treatment strategy, even against GICs. We only vaccinated animals twice at a 5-day interval; therefore, modifying the administration schedule and the amount of antibodies may further induce stronger antitumor immunity. Clinical development is warranted.
Supplementary Material
Funding
This work was supported in part by Grants-in-Aid for Challenging Exploratory Research (#25670613 to R.S.); from the Ministry of Education, Culture, Sports, Science and Technology in Japan; and from the Japan Society for the Promotion of Science.
Supplementary Material
Acknowledgments
We thank Antonius G. Rolink (Basel University, Basel, Switzerland) for providing the FGK45 hybridoma cells, Masahiro Toda (Keio University School of Medicine, Tokyo, Japan) for providing the GL261 cells, and Toru Kondo (Institute for Genetic Medicine, Hokkaido, Japan) for providing the NSCL61 cells. We thank Takuichiro Hide and Tatsuya Takezaki (Kumamoto University, Kumamoto, Japan) for their helpful suggestions regarding the manuscript.
Conflict of interest statement. The authors have no conflict of interest associated with this manuscript.
References
- 1.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 2.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 3.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. [DOI] [PubMed] [Google Scholar]
- 4.Bonavida B. Preface: antibody therapies for cancer. Oncogene. 2007;26(25):3592–3593. [DOI] [PubMed] [Google Scholar]
- 5.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. [DOI] [PubMed] [Google Scholar]
- 6.Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009;373(9668):1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah AH, Bregy A, Heros DO, et al. Dendritic cell vaccine for recurrent high grade gliomas in pediatric and adult subjects: clinical trial protocol. Neurosurgery. 2013;735:863–867. [DOI] [PubMed] [Google Scholar]
- 8.Banchereau J, Bazan F, Blanchard D, et al. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. [DOI] [PubMed] [Google Scholar]
- 9.Wischhusen J, Schneider D, Mittelbronn M, et al. Death receptor–mediated apoptosis in human malignant glioma cells: modulation by the CD40/CD40L system. J Neuroimmunol. 2005;162(1–2):28–42. [DOI] [PubMed] [Google Scholar]
- 10.Khong A, Brown MD, Vivian JB, et al. Agonistic anti-CD40 antibody therapy is effective against postoperative cancer recurrence and metastasis in a murine tumor model. J Immunother. 2013;36(7):365–372. [DOI] [PubMed] [Google Scholar]
- 11.Iizuka Y, Kojima H, Kobata T, et al. Identification of a glioma antigen, GARC-1, using cytotoxic T lymphocytes induced by HSV cancer vaccine. Int J Cancer. 2006;118(4):942–949. [DOI] [PubMed] [Google Scholar]
- 12.Hide T, Takezaki T, Nakatani Y, et al. Sox11 prevents tumorigenesis of glioma-initiating cells by inducing neuronal differentiation. Cancer Res. 2009;69(20):7953–7959. [DOI] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 14.Shibahara I, Sonoda Y, Kanamori M, et al. IDH1/2 gene status defines the prognosis and molecular profiles in patients with grade III gliomas. Int J Clin Oncol. 2012;17(6):551–561. [DOI] [PubMed] [Google Scholar]
- 15.Andarini S, Kikuchi T, Nukiwa M, et al. Adenovirus vector–mediated in vivo gene transfer of OX40 ligand to tumor cells enhances antitumor immunity of tumor-bearing hosts. Cancer Res. 2004;64(9):3281–3287. [DOI] [PubMed] [Google Scholar]
- 16.Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wick W, Naumann U, Weller M. Transforming growth factor–beta: a molecular target for the future therapy of glioblastoma. Curr Pharm Des. 2006;12(3):341–349. [DOI] [PubMed] [Google Scholar]
- 18.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004;4(6):420–431. [DOI] [PubMed] [Google Scholar]
- 19.Gramaglia I, Weinberg AD, Lemon M, et al. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161(12):6510–6517. [PubMed] [Google Scholar]
- 20.Kosaka A, Ohkuri T, Okada H. Combination of an agonistic anti-CD40 monoclonal antibody and the COX-2 inhibitor celecoxib induces anti-glioma effects by promotion of type-1 immunity in myeloid cells and T-cells. Cancer Immunol Immunother. 2014;63(8):847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355(10):1018–1028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.