Abstract
Background
The optimal use of bevacizumab in recurrent glioblastoma (GBM), including the choice of monotherapy or combination therapy, remains uncertain. The purpose of this study was to compare combination therapy with bevacizumab monotherapy.
Methods
This was a 2-part randomized phase 2 study. Eligibility criteria included recurrent GBM after radiotherapy and temozolomide, no other chemotherapy for GBM, and Eastern Cooperative Oncology Group performance status 0–2. The primary objective (Part 1) was to determine the effect of bevacizumab plus carboplatin versus bevacizumab monotherapy on progression-free survival (PFS) using modified Response Assessment in Neuro-Oncology criteria. Bevacizumab was given every 2 weeks, 10 mg/kg; and carboplatin every 4 weeks, (AUC 5). On progression, patients able to continue were randomized to continue or cease bevacizumab (Part 2). Secondary endpoints included objective radiological response rate (ORR), quality of life, toxicity, and overall survival (OS).
Results
One hundred twenty-two patients (median age, 55y) were enrolled to Part 1 from 18 Australian sites. Median follow-up was 32 months, and median on-treatment time was 3.3 months. Median PFS was 3.5 months for each arm (hazard ratio [HR]: 0.92, 95% CI: 0.64–1.33, P = .66). ORR was 14% (combination) versus 6% (monotherapy) (P = .18). Median OS was 6.9 (combination) versus 7.5 months (monotherapy) (HR: 1.18, 95% CI: 0.82–1.69, P = .38). The incidence of bevacizumab-related adverse events was similar to prior literature, with no new toxicity signals. Toxicities were higher in the combination arm. Part 2 data (n = 48) will be reported separately.
Conclusions
Adding carboplatin resulted in more toxicity without additional clinical benefit. Clinical outcomes in patients with recurrent GBM treated with bevacizumab were inferior to those in previously reported studies.
Clinical trials registration nr
ACTRN12610000915055.
Keywords: bevacizumab, carboplatin, glioblastoma
Glioblastoma (GBM) has a universally poor prognosis. Its incidence is low compared with other malignancies, but with one of the highest average years of life lost for any malignancy,1–3 it carries a high morbidity and mortality burden and a high social burden for the cancer sufferer and carer. There is no standard management for recurrent disease. Traditionally used chemotherapy drugs have included carboplatin, lomustine, carmustine, and temozolomide in various schedules, with typical response rates less than 20%, 6-month progression-free survival (6PFS) around 15%, and overall survival (OS) generally less than 6 months, although results across studies are somewhat heterogeneous.4–6
In recent years, the vascular endothelial growth factor (VEGF) inhibitor bevacizumab has emerged as a promising agent, effective both as monotherapy and in combination with traditional chemotherapy drugs, in early-phase clinical trials in the setting of recurrent GBM. Encouraging results from phase 2 studies in recurrent disease were reported in 2007 and 2009, with response rates up to 50% and progression-free survival (PFS) up to 9 months, representing a substantial improvement on historical data for chemotherapy alone.7,8 This led to US FDA approval in 2009 for use of bevacizumab in recurrent GBM and widespread uptake of the drug on that continent.9
Despite this, there remain several unanswered questions, which include the use of bevacizumab as monotherapy versus in combination with chemotherapy, the potential utility of continuing bevacizumab beyond disease progression, and comparison of the recently developed Response Assessment in Neuro-Oncology (RANO) guidelines10 with the Macdonald criteria used to interpret MRI changes in earlier studies.11 RANO criteria place emphasis on fluid attenuated inversion recovery (FLAIR) sequence abnormalities, which were not considered in the traditional Macdonald criteria and may be more relevant in the setting of antiangiogenic agents (eg, bevacizumab) that may affect T1 contrast enhancement. In addition, the effect of bevacizumab on quality of life (QOL) and neurocognitive function (NCF) has been questioned, with limited existing information at the time of study design and now controversial findings in the first-line setting for bevacizumab use in GBM.12–14
We report the primary endpoint for Part 1 of “A randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma multiforme” (CABARET) in which we compared the effect of bevacizumab plus carboplatin with bevacizumab monotherapy on progression-free survival (PFS) in patients with recurrent GBM. At the time of protocol development, carboplatin was a commonly used second-line chemotherapy drug for recurrent GBM in Australia based on previous studies showing modest benefit, with response or stabilization of disease in approximately 50% of patients and median time to progression of 19–26 weeks.15,16 Carboplatin had also been used in combination with other cytotoxics and bevacizumab in 2 single-arm phase 2 studies.17,18 At the time of study design, chemotherapy alone was regarded as standard therapy for recurrent GBM in Australia; while bevacizumab monotherapy was approved for use in this context, it was not funded by the Australian Pharmaceutical Benefits Scheme (PBS) and thus was not standard second-line therapy. A control arm using chemotherapy alone was not included in the trial design due to lack of funding support for this comparator.
While efficacy was the primary endpoint, important secondary endpoints, including toxicity and QOL, were evaluated to help determine whether the combination of bevacizumab and carboplatin warranted further evaluation.
Materials and Methods
Study Objectives
The primary objective was to determine the effect of bevacizumab plus carboplatin versus bevacizumab monotherapy on PFS in patients with recurrent GBM, using modified RANO criteria. Secondary objectives included objective radiological response rate, neurocognitive function, health-related QOL, corticosteroid use, toxicity, OS, and time to treatment failure (TTF). In Part 2, we aimed to determine the effect of continuing or stopping bevacizumab after disease progression on the above parameters and on subsequent PFS.
Exploratory objectives included correlation between steroid dose and clinical outcome, correlation of MRI response at 4 weeks with clinical outcome, comparison between Macdonald and modified RANO criteria for assessment of disease response or progression, documenting the location and type of radiological progression on and after bevacizumab discontinuation, and correlation between blood and tissue biomarkers and clinical outcome.
Patient Eligibility
Eligible participants were adults >aged18 years with Eastern Cooperative Oncology Group (ECOG) performance status ≤2 and a histological diagnosis of GBM (WHO grade IV glioma) following resection or biopsy, who had received treatment with both radiotherapy and temozolomide (concurrently and/or sequentially). Patients with first or subsequent recurrences were eligible to participate, provided that prior therapy had only included radiotherapy and temozolomide. This was to enable inclusion of the patients with recurrent GBM often seen in routine practice with more than one recurrence, who would potentially benefit from bevacizumab therapy, recognizing that other prominent contemporary studies such as the BRAIN trial also permitted patients beyond first recurrence to participate.8 The prior dosing schedule of temozolomide was not stipulated, and prior metronomic temozolomide was permitted (including in the recurrent setting). At least 12 weeks must have elapsed since the cessation of radiotherapy. Recurrent or progressive disease had to be confirmed by MRI showing measurable disease according to RANO criteria10 or surgical resection of recurrent disease. The baseline or eligibility MRI was performed within 14 days prior to randomization. The craniotomy or biopsy site had to be adequately healed. Other key inclusion criteria were adequate renal function (including <2+ urine protein on dipstick or urine protein/creatinine ratio ≤1.0) and adequate hematological parameters (including neutrophil count ≥1.5 × 109/L and platelets ≥100 × 109/L). Anticoagulation was permitted if required; low molecular-weight heparin was the preferred approach.
Exclusion criteria included prior chemotherapy other than temozolomide, prior bevacizumab or other investigational agent for the treatment of glioma, surgery within 4 weeks before treatment commencement, evidence of recent hemorrhage on MRI with the exception of asymptomatic punctate hemorrhage or resolving postsurgical change, inability to undergo MRI, inadequately controlled hypertension, clinically significant cardiovascular disease, history of coagulation disorder, prior or concurrent malignancy (except nonmelanomatous skin cancer or malignancy treated and disease-free for >5 years), pregnancy or lactation, or other concurrent physical, psychological, or sociological condition that could jeopardize patient safety or compliance.
Study Design
This was a multicenter, sequential, stratified, nonblinded, randomized phase 2 study in 2 parts, recruiting from 18 Australian sites (Supplementary Fig. S1). Eligible patients were randomized 1:1 to receive bevacizumab 10 mg/kg IV every 2 weeks plus carboplatin AUC 5 every 4 weeks (4 weeks was deemed to be the length of one cycle), or bevacizumab monotherapy at the same dose (Part 1). Study therapy continued until progressive disease, unacceptable toxicity, participant withdrawal, noncompliance with protocol guidelines, or death. Following disease progression, participants considered suitable for further treatment, and who consented to further treatment on the trial, were then randomized to cease or continue bevacizumab using the same dose and schedule, in addition to further chemotherapy dependent on clinician preference (Part 2). Details and results from Part 2 will be reported separately as part of a planned and separate analysis of the 48 participants randomized to Part 2.
Dose Modification
The causative drug was discontinued for any grade 3 or 4 hypersensitivity reaction. No bevacizumab dose reductions were permitted at any time. For grade 2 neutropenia, bevacizumab was continued, but carboplatin was withheld until resolution to grade 1. For grade ≥3 neutropenia, both drugs were withheld until resolution to grade 1 and then resumed with a carboplatin dose reduction to AUC 4. For grade 2 thrombocytopenia, carboplatin was withheld until platelet counts improved to ≥100 × 109/L and recommenced at the same dose; bevacizumab was continued. For grade 3 thrombocytopenia, once platelet counts were ≥100 × 109/L, carboplatin was restarted at AUC 4; for grade 4, carboplatin was permanently discontinued. For both grade 3 and 4, bevacizumab was restarted once platelet counts were ≥75 × 109/L. For grade 2–3 increase in ALT or AST, carboplatin was withheld until grade ≤1 and restarted at the same dose (for grade 2) or AUC 4 (for grade 3); bevacizumab was withheld until grade ≤2. Both drugs were discontinued if AST or ALT toxicity was grade 4. Bevacizumab was discontinued for grade 4 hypertension and delayed for grade 2–3 hypertension until blood pressure was ≤150/100 mmHg. Bevacizumab was also discontinued for any grade CNS hemorrhage (with the exception of clinically asymptomatic hemosiderin or punctate hemorrhage) and for nephrotic syndrome. For grade 3 proteinuria, bevacizumab was delayed until grade ≤2. Bevacizumab was delayed if a grade 3–4 venous thromboembolic event occurred and was restarted once resolution or full-dose anticoagulation was established. A maximum of 8 weeks delay was permissible for either drug.
Response Evaluation and Radiological Assessments
The primary criterion for assessment of efficacy was PFS. PFS was defined as time from randomization to disease progression based on centrally reviewed modified RANO criteria or death from any cause. OS was defined as the time of randomization to the date of death from any cause. Participants who were alive at their last follow-up were censored at that date. Both PFS and OS were estimated using the Kaplan-Meier method.
Response evaluation was determined by MRI, clinical and neurological examination, and steroid use, which are incorporated in the RANO criteria. The primary endpoint, as well as the secondary and exploratory radiological endpoints, were assessed by blinded central radiology review. Cerebral MRI including pre- and postgadolinium T1, T2/FLAIR was performed at baseline and then every 8 weeks or more frequently if clinically indicated during study treatment. An additional MRI was performed 4 weeks after randomization for an exploratory endpoint but was not used by site investigators for decision-making except when safety concerns arose.
Responses were defined by modified RANO criteria,10 and any response needed to be sustained at the subsequent scan for the purpose of confirmation. In the setting of resected recurrent disease with no baseline measurable disease, the best response was stable disease. Because the existing RANO criteria are not specific regarding extent of FLAIR changes warranted to be labeled as progressive disease, a novel 5-point scale was devised by several neuroradiologists and neuro-oncologists for the purpose of this study, in order to quantify T2/FLAIR abnormality, and added to the existing RANO criteria; hence, modified RANO criteria were used (Supplementary Table S1). Further details about the use of the 5-point scale and its use compared with standard RANO criteria, as well as a comparison between RANO and Macdonald criteria for disease assessment on this trial, will be the subject of a separate paper.
Site investigators assessed disease progression for the purpose of eligibility for continuing participation in Part 1 of the study. For the purpose of trial reporting, the date of the MRI at which the central radiology review detected progression was used as the progression date. Participants were censored if they commenced any new anticancer treatment.
Clinical assessments, including QOL and NCF testing for those participants able to complete them, were performed at the start of each 4-week cycle. Laboratory assessments, including urinalysis for patients receiving bevacizumab, were performed every 2 weeks. All participants were assessed at the cessation of study treatment and then every 4 weeks until death, loss to follow-up, or withdrawal of consent. QOL was measured using the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life questionnaire (QLQ-C30) and BN20 validated measurement tools. The EQ-5D health outcome measure was also obtained. Neurocognitive function testing was measured by the Mini-Mental status examination and CogState neurocognitive function testing, and will be reported separately for the subset of patients able to participate in this testing modality beyond baseline.
Safety
The National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0 was used to classify and grade adverse events.19 Safety data were collected for all participants until at least 30 days after their last study drug dose.
Study Oversight
The study protocol was written by members of the trial management committee and approved by the relevant human research ethics committees for participating sites. All participants provided written informed consent before commencement of study procedures. Data for each participant were collected using an InForm clinical trial database (Oracle). The study was conducted under the auspices of the Cooperative Trials Group for Neuro-Oncology (COGNO), coordinated at the National Health and Medical Research Council (NHMRC) Clinical Trials Centre, University of Sydney. The Clinical Trials Centre was responsible for the collection, maintenance, integrity, and confidentiality of all data. The trial management committee was responsible for all aspects of the conduct of the study. An independent data safety monitoring committee (IDSMC) monitored the progress of all safety aspects of the study. The statistical analysis was performed at the Clinical Trials Centre. While Roche Products, Pty Limited (Australia) provided funding for the trial and access to bevacizumab, the company was not involved in data monitoring, analysis, or manuscript preparation.
Statistical Analysis
The intention-to-treat population of all randomly assigned participants was used for survival analysis. Toxicities, treatment details, and QOL were reported for participants receiving at least one dose of study treatment.
For Part 1 sample size calculations, 6PFS was assumed to be 35% for bevacizumab monotherapy and 50% for the combination of bevacizumab and carboplatin, based on data for the bevacizumab-irinotecan combination.8 (At the time of protocol writing, only retrospective data existed for the bevacizumab-carboplatin combination.20) We sought to detect a HR of approximately 0.6 to consider the combination clinically significantly different from monotherapy.
The sample size of 120 participants was chosen to provide 70% power at 2-sided alpha = 0.1 to detect a HR of 0.62. Time to progression, OS, and time on treatment were measured from the date of randomization, estimated using the nonparametric Kaplan-Meier method including 95% CIs, and proportional-hazards regression was used to compare the 2 arms of the study. Randomized treatment arms were compared for overall response and best response using chi-square tests. No interim analyses were planned or conducted, with the exception of safety monitoring.
Patient randomization for Part 1 used the method of minimization, stratified by site, sex, age >65 years, and ECOG performance status. For Part 2, the same factors plus previous treatment in Part 1 were used to stratify the participants.
Bevacizumab-related adverse events, as well as hematologic adverse events, were specifically reported. No formal statistical comparisons between arms were made for adverse events. All reporting of adverse events and QOL included participants who continued to receive Part 1 treatment on the basis of site radiology and clinical reviews, even if central radiology review had deemed progression to be earlier.
Results
Patient Baseline Characteristics
Characteristics of the 122 participants enrolled in the study between November 2010 and March 2012 are summarized in Table 1. The median time from initial GBM surgery to randomization was 11 months for both arms. Most participants (87%, n = 106) had an initial diagnosis of GBM; the remainder had been diagnosed with an earlier grade I-III glioma that had subsequently progressed to histologically confirmed GBM. Sixty-six percent (n = 80) were enrolled at first disease recurrence. Forty-four percent (n = 54) had undergone surgery for recurrent disease. Baseline demographic data were comparable between the 2 groups (Table 1).
Table 1.
Baseline characteristics of participants; n (%) or median (range)
| Characteristic | Bevacizumab + Carboplatin (N = 60) | Bevacizumab (N = 62) |
|---|---|---|
| Age (y) | 55 (32–79) | 55 (25–82) |
| Sex | ||
| Female | 26 (43%) | 29 (47%) |
| Male | 34 (57%) | 33 (53%) |
| ECOG performance status | ||
| 0 | 7 (12%) | 11 (18%) |
| 1 | 35 (58%) | 35 (56%) |
| 2 | 18 (30%) | 16 (26%) |
| KPS | ||
| 90–100 | 21 (35%) | 22 (35%) |
| 70–80 | 28 (47%) | 28 (45%) |
| <70 | 11 (18%) | 10 (16%) |
| Not done | 0 (0%) | 2 (3%) |
| Prior diagnosis of grade I-III glioma | ||
| No | 54 (90%) | 52 (84%) |
| Yes | 6 (10%) | 10 (16%) |
| Recurrence | ||
| First | 39 (65%) | 41 (66%) |
| Second or more | 21 (35%) | 19 (31%) |
| Unknown | 0 (0%) | 2 (3%) |
| Initial surgery | ||
| Biopsy | 6 (10%) | 9 (15%) |
| Debulking | 21 (35%) | 16 (26%) |
| Resection | 33 (55%) | 37 (60%) |
| Surgery for recurrent disease | ||
| Unknown | 0 (0%) | 2 (3%) |
| No | 37 (62%) | 29 (47%) |
| Yes | 23 (38%) | 31 (50%) |
| Corticosteroid use at baseline | ||
| No | 10 (17%) | 16 (26%) |
| Yes | 50 (83%) | 46 (74%) |
| Months from last radiotherapy to randomization | 9 (3–61) | 9 (3–101) |
| Months from initial glioblastoma surgery to randomization | 11 (1–48) | 11 (1–43) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; y, years.
Study Treatment
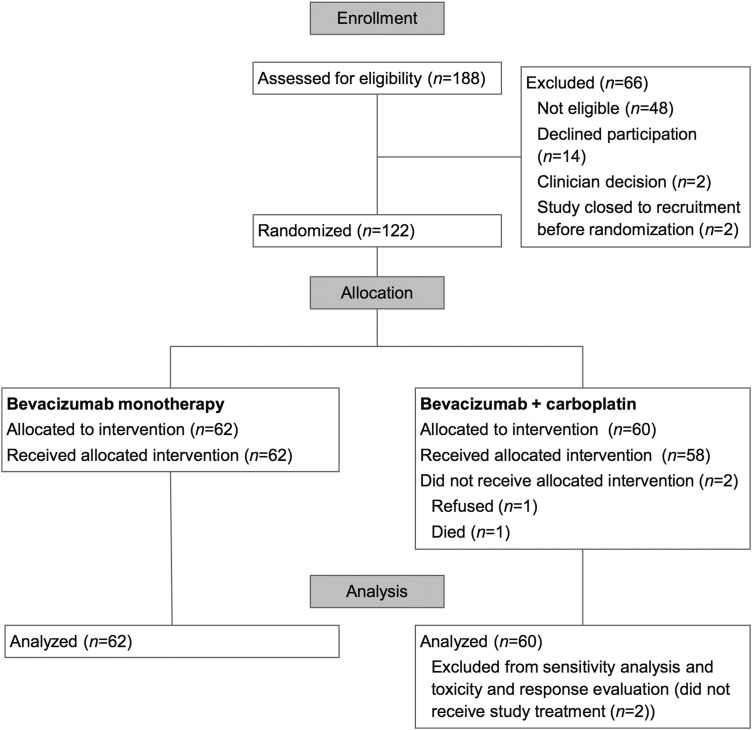
One hundred twenty-two participants were registered and randomized to the trial (enrolled population) (Fig. 1). In total, 120 participants received at least one dose of study treatment (toxicity-evaluable population). Two participants, both assigned to bevacizumab plus carboplatin, did not receive study treatment. These participants were included in the survival analysis as part of the intention-to-treat population; sensitivity analyses excluding both patients did not change the primary outcome. The study was closed on December 5, 2014. At the time of study closure, 2 participants were receiving Part 1 study treatment, none were receiving Part 2 study treatment, 2 participants (2%) were in follow-up; 117 (96%) were deceased, and one (<1%) had withdrawn consent to follow-up.
Fig. 1.
CONSORT diagram (Part 1 of the study).
The median number of treatment cycles per participant was 4 (range: 1–40) for the combination arm and 4 (range: 1–35) for the monotherapy arm. Carboplatin dose reductions were required in 21 of 58 participants (36%). In the combination arm, 12 of 58 participants (21%) ceased carboplatin during the trial but were able to continue bevacizumab; and one participant (2%) ceased bevacizumab but continued carboplatin.
Among the 118 participants who received at least one dose of treatment and were off-study at the time of analysis, Part 1 study treatment was discontinued because of disease progression (as determined by the local investigator) or death in 102 participants (86%); adverse events in 9 participants (8%, 5 in the combination arm and 4 in the monotherapy arm); participant preference in 5 participants (4%, all in the combination arm); and clinician preference in 2 participants (2%, 1 from each arm).
Efficacy
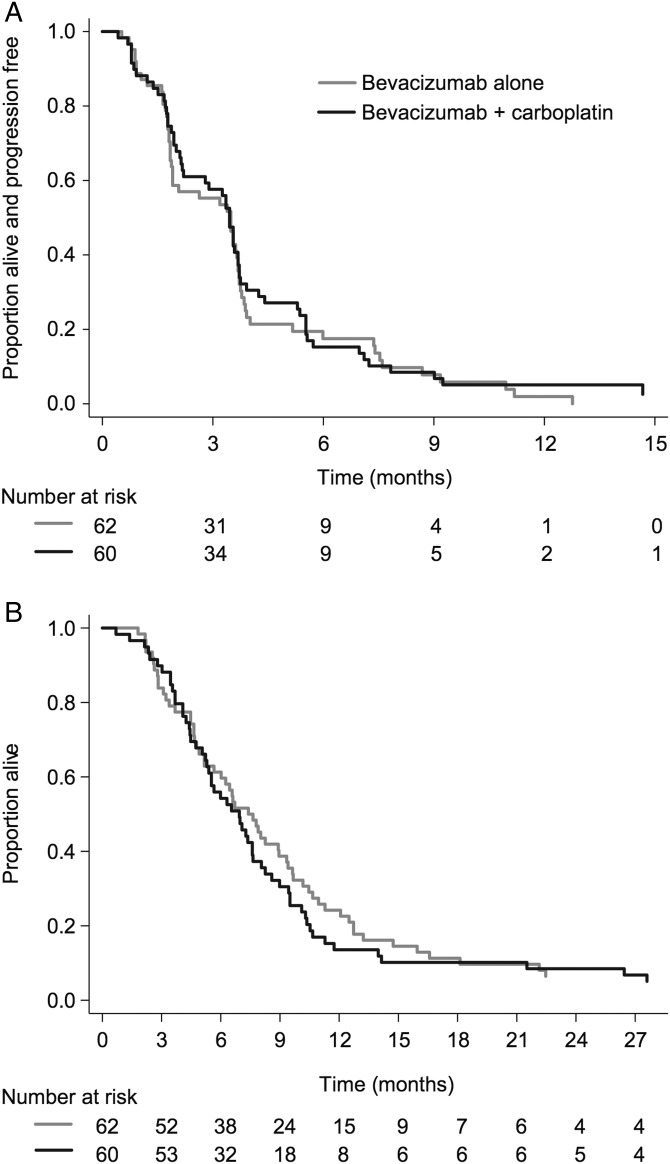
The median follow-up was 32 months (Fig. 2). The central radiology review-determined endpoint of 6PFS for Part 1 was 15% (combination) and 18% (monotherapy). Median PFS was 3.5 months (95% CI: 2.2–3.7 mo) (combination) and 3.5 months (95% CI: 1.9–3.7 mo) (monotherapy), (HR: 0.92, 95% CI: 0.64–1.33, P = .66) (Fig. 2A). Progression was determined clinically for 30 of the 118 participants who had completed Part 1 (25%) without radiological confirmation at the time of progression. For the remaining participants, central radiological confirmation of disease progression included increased enhancement on the postcontrast T1-weighted images, T2/FLAIR increase, a new lesion, or a combination of these radiologic findings, with no single imaging technique predominating in terms of determining disease progression (Supplementary Table S2). In particular, T2/FLAIR changes alone were the stated reason for progression in only 11.6% (n = 14) of participants.
Fig. 2.
(A) Progression-free survival. (B) Overall survival.
No participant had a complete response (Table 2). Of the 120 participants who received at least one dose of study treatment, 8 (14%) in the combination arm and 4 (6%) in the monotherapy arm had a RANO-defined partial response. Overall, 44 participants (76%) in the combination arm and 40 (65%) in the monotherapy arm had an initial response or stable disease before progressing. Median OS was 6.9 months (combination) versus 7.5 months (monotherapy), (HR: 1.18, 95% CI: 0.82–1.69, P = .38) (Fig. 2B). Comparison between participants who participated in CABARET with first versus second or subsequent recurrence did not show any statistically significant difference in PFS or OS outcomes.
Table 2.
Response rates (n (%)) by treatment group
| Response | Bevacizumab + Carboplatin (N = 58) | Bevacizumab (N = 62) | P |
|---|---|---|---|
| Objective response (complete or partial response) | 8 (14%) | 4 (6%) | .18 |
| Best response | .23 | ||
| Partial response | 8 (14%) | 4 (6%) | |
| Stable disease | 36 (62%) | 36 (58%) | |
| Progressive disease | 14 (24%) | 22 (35%) |
Quality of Life Analysis
Analysis of QOL data comparing change scores (mean across all treatment cycles minus baseline score, using a 0–100 scale transformation) for the QLQ-C30 overall QOL responses during Part 1 treatment indicated no significant differences between arms. The mean of the change scores was −0.2 for the combination arm and −5.2 for bevacizumab monotherapy (difference between arms −5.0, 95% CI: −14.1 to 4.1, P = .28). More detailed QOL analyses will be reported separately.
Safety
Safety data are summarized in Tables 3 and 4. Events are presented here as the combination versus monotherapy arm, but the groups were not statistically compared. The most common adverse events (all grades) included fatigue, neurological symptoms or signs, hypertension, nausea and vomiting, thrombocytopenia, and constipation. Hematologic adverse events were more common in the combination arm. In addition to the grade 3 events documented in Tables 3 and 4, several other grade 3 events were reported, including headache, seizures, weight gain, dyspnea, and joint pain.
Table 3.
Adverse events (Number [%] of participants experiencing adverse event, by treatment group)a
| Adverse Event | Grade | Bevacizumab + Carboplatin (N = 58) | Bevacizumab (N = 62) |
|---|---|---|---|
| Anemia | All grades | 16 (28%) | 6 (10%) |
| Grade ≥3 | 0 (0%) | 0 (0%) | |
| Febrile neutropenia | All grades | 0 (0%) | 0 (0%) |
| Grade ≥3 | 0 (0%) | 0 (0%) | |
| Neutrophil count decreased (without fever) | All grades | 14 (24%) | 4 (6%) |
| Grade ≥3 | 4 (7%) | 0 (0%) | |
| Thrombocytopenia | All grades | 32 (55%) | 14 (23%) |
| Grade ≥3 | 9 (16%) | 2 (3%) | |
| Nausea and vomiting | All grades | 29 (50%) | 24 (39%) |
| Grade ≥3 | 0 (0%) | 2 (3%) | |
| Diarrhea | All grades | 15 (26%) | 15 (24%) |
| Grade ≥3 | 1 (2%) | 0 (0%) | |
| Constipation | All grades | 26 (45%) | 18 (29%) |
| Grade ≥3 | 0 (0%) | 0 (0%) | |
| Fatigue | All grades | 50 (86%) | 52 (84%) |
| Grade ≥3 | 5 (9%) | 4 (6%) | |
| Any adverse event | Grade ≥3 | 37 (64%) | 36 (58%) |
| Causing death | 2 (3%) | 0 (0%) |
aFor the 120 participants who had at least one dose of study medication, from the first treatment dose through to 30 days after the last treatment dose on Part 1 of the study.
Table 4.
Adverse events (Number [%] of participants experiencing a bevacizumab-related adverse event, by treatment group)a
| Adverse Event | Any |
Grade ≥3 |
||
|---|---|---|---|---|
| Bevacizumab + Carboplatin (N = 58) | Bevacizumab (N = 62) | Bevacizumab + Carboplatin (N = 58) | Bevacizumab (N = 62) | |
| Gastrointestinal perforation | 1 (2%) | 0 | 1 (2%) | 0 |
| CNS hemorrhage | 3 (5%) | 3 (5%) | 1 (2%) | 0 |
| Bleeding (other) | 17 (29%) | 16 (26%) | 1 (2%) | 1 (2%) |
| Deep vein thrombosis | 4 (7%) | 6 (10%) | 2 (3%) | 0 |
| Pulmonary embolus | 2 (3%) | 0 | 2 (3%) | 0 |
| Deep vein thrombosis and pulmonary embolus | 0 | 1 (2%) | 0 | 1 (2%) |
| Thromboembolic other | 1 (2%) | 1 (2%) | 0 | 0 |
| Wound healing complication | 1 (2%) | 1 (2%) | 0 | 0 |
| Proteinuria | 7 (12%) | 4 (6%) | 0 | 2 (3%) |
| Hypertension | 36 (62%) | 51 (82%) | 10 (17%) | 10 (16%) |
| Abscesses or fistulae | 1 (2%) | 1 (2%) | 1 (2%) | 1 (2%) |
aFor the 120 participants who had at least one dose of study medication, from the first treatment dose through to 30 days after the last treatment dose on Part 1 of the study.
There was one death related to CNS hemorrhage and one death related to bowel perforation; both participants were receiving bevacizumab and carboplatin. One suspected unexpected serious adverse reaction (SUSAR) occurred in a male patient receiving bevacizumab monotherapy who developed acute renal failure and biopsy-proven acute interstitial nephritis. Four months earlier, this participant had also commenced carbamazepine, which is known to be associated with this complication, and it could not be discerned whether bevacizumab was the causative agent.
Discussion
In this large, multicenter randomized phase 2 study, no obvious clinically significant benefit for the combination of bevacizumab and carboplatin was detected, and CABARET provides no support for further study of this combination. The efficacy of bevacizumab in both arms was lower than in previous reports available at the time of trial design.8,21
Previous studies of bevacizumab monotherapy or bevacizumab plus chemotherapy in recurrent GBM have resulted in somewhat varied findings, with 6PFS ranging from 19% to 50%. Further, combination therapy does not appear superior to monotherapy in cross-trial comparisons but does seem to result in greater toxicity.8,21–23 At the time the CABARET trial was designed, the only evidence that combining bevacizumab with chemotherapy might improve outcomes relative to bevacizumab alone came from the BRAIN study, published in 2009.8 In this trial, bevacizumab plus irinotecan resulted in 6PFS of 50% versus 43% for monotherapy and median PFS of 5.6 versus 4.2 months.8
More recently, the BELOB randomized phase 2 study results showed 6PFS of 42% for bevacizumab plus lomustine versus 16% for bevacizumab monotherapy and 13% for lomustine monotherapy.24 The BELOB study has been the first and only prospective trial to date to show a potential survival advantage of combination therapy over bevacizumab monotherapy or chemotherapy alone. Following on from the BELOB study, the EORTC 26101 randomized phase 3 clinical trial compares bevacizumab + lomustine combination therapy with lomustine monotherapy; the trial is now closed, and results are being eagerly awaited as to whether the promising results from the combination in the BELOB phase 2 study will be sustained in the larger phase 3 clinical trial design setting. This will help to definitively determine the role of bevacizumab in the setting of recurrent glioblastoma.
In the CABARET trial, adding carboplatin to bevacizumab did not provide additional efficacy when compared with bevacizumab monotherapy. The combination of carboplatin and bevacizumab in recurrent glioma has been reported in small retrospective series with 6PFS rates up to 50% and median OS up to 40 weeks.20,25 A prospective cohort study of 61 participants, 7 of whom had carboplatin and bevacizumab for recurrent GBM, reported a median PFS of 5 months and OS of 9 months with no significant differences between treatment groups, although the small sample size and nonrandomized design preclude robust conclusions.26 Recommended practice in Australia does not include carboplatin in the GBM management algorithm, with the drug not being listed in the Australian eviQ Cancer Treatments Online options for management of GBM. Both irinotecan and lomustine, when used in combination with bevacizumab, have resulted in better efficacy outcomes than those in the CABARET study.7,8,24 However, irinotecan is not routinely available in Australia for recurrent GBM, and the BELOB trial, which included lomustine monotherapy as a comparator arm, also did not suggest that lomustine monotherapy was particularly efficacious with 6PFS of only 13%.24 Again, results from the EORTC 26101 study will be extremely informative.
The fact that a third (33%) of our participants were enrolled at their second or subsequent recurrence may have also impacted the response rates and survival outcomes, as these patients were further down the disease pathway and were more heavily pretreated. In the BRAIN study, only 19% of participants were enrolled at second/subsequent recurrence.8 The higher number of multiple progressions included in the CABARET study may partly account for the lower OS compared with prior studies.
Of interest, we observed lower than expected response rates, PFS, and OS for bevacizumab in patients compared with several previous trials. Vredenburgh, in the first prospective clinical trial of bevacizumab in GBM, reported 46% 6PFS and 9.7 month median OS in a single-arm phase 2 study of bevacizumab plus irinotecan.7 The BRAIN study had similar outcomes.8 More recently though, lower response rates and survival outcomes have been noted. The BELOB study documented only 16% 6PFS with bevacizumab monotherapy.24 A single-arm bevacizumab monotherapy phase 2 trial in 2010 reported 6PFS of 25%, which was also lower than expected.27 In our study, the 6 PFS was 15%–18%, in keeping with these more recent trials. The lower apparent benefits in more recent years could be attributable to several variables including a better appreciation of the significance of T2/FLAIR abnormalities on radiological imaging, how heavily patients had been pretreated, and performance status inclusion criteria among others. ECOG performance status is a relatively subjective criterion; we allowed patients with ECOG 2 or better into the study, but assigning a PS of 2 is always subject to investigator discretion, and it is possible that some participants were more unwell than those recruited to earlier studies. It is possible that more contemporary studies, such as CABARET and BELOB, provide a more accurate representation of disease progression times for this cohort of patients compared with older studies. Notably though, fewer than 12% of the participants were deemed to have progressed on T2/FLAIR signal change alone, suggesting that the incorporation of T2/FLAIR in RANO criteria for disease assessment may not have substantially affected the PFS endpoint of this trial compared with the more traditional approach of measuring contrast enhancement alone.
Since the CABARET trial did not include a chemotherapy-alone arm, it does not provide any direct evidence of the effectiveness of bevacizumab compared with chemotherapy, as the BELOB study did. While our study has shown that neither PFS nor OS is improved when carboplatin is added to bevacizumab, it did not explore whether bevacizumab results in greater benefit than carboplatin monotherapy. To date, aside from the BELOB study, no other clinical trial in recurrent GBM can answer the question of whether bevacizumab is truly superior to chemotherapy, aside from historical comparisons.
Where should bevacizumab sit in the recurrent glioblastoma setting? Recent nonrandomized cohort studies have suggested no disadvantage in introducing bevacizumab later, after initial chemotherapy for disease progression or recurrence.28,29 Whether chemotherapy alone, followed by subsequent bevacizumab, is an acceptable strategy has not been addressed by CABARET with the lack of a chemotherapy-only arm. At present the most common time to deliver bevacizumab, assuming it is available, is at first recurrence after temozolomide therapy since this is the setting of the majority of available clinical trial evidence.
In summary, we did not find that the combination of bevacizumab and chemotherapy resulted in additional PFS or OS benefit compared with bevacizumab monotherapy in recurrent GBM. Hematologic toxicities were more common in the combination arm but were generally manageable, and preliminary analysis of QOL data suggests no differences between arms while patients are on treatment. Overall response rates and survival outcomes, using modified RANO criteria, were somewhat inferior to those in several previously reported studies. Despite this, a small proportion of patients clearly responded, and some derived prolonged clinical benefit from therapy. We await biomarker studies, which will search for signals to discern the patients who are most likely to benefit from bevacizumab.
Supplementary Material
Funding
Investigator-driven study funded by Roche Products Australia Pty Ltd.
Supplementary Material
Acknowledgments
This trial was conducted under the auspices of the Cooperative Trials Group for Neuro-Oncology (COGNO), coordinated at the NHMRC Clinical Trials Centre, University of Sydney, supported by Roche Products Pty Limited (Australia).
Trial Management Committee: K. Field (Chair), J. Simes, E. Hovey, A. Nowak, L. Cher, H. Wheeler, C. Brown, E. Barnes, K. Sawkins, A. Livingstone, and M. Rosenthal.
Independent Central Radiological Review Committee: P. Phal, G. Fitt, and C. Goh.
Independent Data Safety Monitoring Committee: M. Tattersall (Chair), P. Kelly, and A. Hayden.
Clinical Trials Centre: K. Sawkins, C. Brown, L. Barnes, A. Livingstone, D. Winter, B. Tomes, R. Pike, and J. Simes.
The following study sites participated in the CABARET study and randomized at least one patient (principal investigator and site coordinator):
Monash Medical Centre, Victoria: R. Freilich and I. Arzhintar (17); Royal Melbourne Hospital, Victoria: K. Field, M. Rosenthal, and L. Garrett (16); Royal Prince Alfred Hospital, New South Wales: J. Simes and A. Byrne (13); St Vincent's Hospital, Victoria: A. Dowling and N. Ranieri (11); Epworth HealthCare Richmond, Victoria: R. Jennens and F. Osmond (9); The Queen Elizabeth Hospital, South Australia: W.K. Patterson and A. Phay (8); Calvary Mater Newcastle, New South Wales: F. Abell and L. Plowman (7); Austin Hospital, Victoria: L. Cher and J. Flynn (7); Prince of Wales Hospital, New South Wales: E. Hovey and H. Kilsby (6); Royal North Shore Hospital, New South Wales: H. Wheeler and S. Kirby-Lewis (6); Royal Adelaide Hospital, South Australia: N. Singhal, S. Smith, and M. Whelan (5); Royal Brisbane and Women's Hospital, Queensland: P. Inglis and A. Ives (5); Sir Charles Gairdner Hospital, Western Australia: A. Nowak and S. Lobb (5); Port Macquarie Base Hospital, New South Wales: S. Begbie and P. Williams (4); Mater Adult Hospital, Queensland: Z. Lwin, N. Woodward, and G. Crosbie (1); Royal Hobart Hospital, Tasmania: R. Harrup and L. Pyszkowski (1); Launceston General Hospital, Tasmania: S. Gauden and A. Neville (1).
This trial has previously been reported at the American Society of Clinical Oncology Annual Meeting (2013), the Society for Neuro-Oncology Annual Meeting (2012), the Australian Cooperative Trials Group for Neuro-Oncology Annual Meetings (2012–2014), the European Association for Neuro-Oncology and the European Society of Medical Oncology Annual Meetings (2012).
Conflict of interest statement. K.F. has received conference travel grants and honoraria from Roche for speaking invitations. E.H. has been a member of a Roche Advisory Board 2009–2013. A.N. has been a member of a Roche Advisory board 2013 and received honoraria from Roche for speaking invitations. M.R. has been a member of a Roche Advisory Board. J.S. has received research funding from Roche. H.W. has received research funding from Roche and has been a member of a Roche Advisory board. E.B., G.F., P.P., K.S., C.B., A.L., and L.C. declare no conflict of interest. There is no stated conflict of interest for R.F.
Contributor Information
Collaborators: CABARET/COGNO investigators, K. Field, J. Simes, E. Hovey, A. Nowak, L. Cher, H. Wheeler, C. Brown, E. Barnes, K. Sawkins, A. Livingstone, M. Rosenthal, P. Phal, G. Fitt, C. Goh, M. Tattersall, P. Kelly, A. Hayden, K. Sawkins, C. Brown, L. Barnes, A. Livingstone, D. Winter, B. Tomes, R. Pike, J. Simes, R. Freilich, I. Arzhintar, K. Field, M. Rosenthal, L. Garrett, J. Simes, A. Byrne, A. Dowling, N. Ranieri, R. Jennens, F. Osmond, W.K. Patterson, A. Phay, F. Abell, L. Plowman, L. Cher, J. Flynn, E. Hovey, H. Kilsby, H. Wheeler, S. Kirby-Lewis, N. Singhal, S. Smith, M. Whelan, P. Inglis, A. Ives, A. Nowak, S. Lobb, S. Begbie, P. Williams, Z. Lwin, N. Woodward, G. Crosbie, R. Harrup, L. Pyszkowski, S. Gauden, and A. Neville
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. [DOI] [PubMed] [Google Scholar]
- 2.Schwartzbaum JA, Fisher JL, Aldape KD, et al. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2(9):494–503; quiz 491 p following 516. [DOI] [PubMed] [Google Scholar]
- 3.Australian Cancer Network Adult Brain Tumour Guidelines Working Party, Clinical Practice Guidelines for the Management of Adult Gliomas: Astrocytomas and Oligodendrogliomas. Sydney: Cancer Council Australia, Australian Cancer Network and Clinical Oncological Society of Australia Inc., 2009. [Google Scholar]
- 4.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–2578. [DOI] [PubMed] [Google Scholar]
- 5.Nieder C, Grosu AL, Molls M. A comparison of treatment results for recurrent malignant gliomas. Cancer Treat Rev. 2000;26(6):397–409. [DOI] [PubMed] [Google Scholar]
- 6.Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–1259. [DOI] [PubMed] [Google Scholar]
- 8.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MH, Shen YL, Keegan P, et al. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14(11):1131–1138. [DOI] [PubMed] [Google Scholar]
- 10.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 11.Quant EC, Wen PY. Response assessment in neuro-oncology. Curr Oncol Rep. 2011;13(1):50–56. [DOI] [PubMed] [Google Scholar]
- 12.Henriksson R, Bottomley A, Mason W, et al. Progression-free survival (PFS) and health-related quality of life (HRQoL) in AVAglio, a phase III study of bevacizumab (Bv), temozolomide (T), and radiotherapy (RT) in newly diagnosed glioblastoma [abstract2 2005]. J Clin Oncol. 2013;31(Suppl). [Google Scholar]
- 13.Armstrong T, Won M, Wefel J, et al. Comparative impact of treatment on patient reported outcomes (PROs) in patients with glioblastoma (GBM) enrolled in RTOG 0825 [abstract 2003]. J Clin Oncol. 2013;31(Suppl). [Google Scholar]
- 14.Wefel J, Pugh S, Armstrong T, et al. Neurocognitive function (NCF) outcomes in patients with glioblastoma (GBM) enrolled in RTOG 0825 [ abstract 2004} J Clin Oncol. 2013;31(Suppl). [Google Scholar]
- 15.Yung WK, Mechtler L, Gleason MJ. Intravenous carboplatin for recurrent malignant glioma: a phase II study. J Clin Oncol. 1991;9(5):860–864. [DOI] [PubMed] [Google Scholar]
- 16.Warnick RE, Prados MD, Mack EE, et al. A phase II study of intravenous carboplatin for the treatment of recurrent gliomas. J Neurooncol. 1994;19(1):69–74. [DOI] [PubMed] [Google Scholar]
- 17.Reardon DA, Desjardins A, Peters KB, et al. Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naive, recurrent glioblastoma. J Neurooncol. 2012;107(1):155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francesconi AB, Dupre S, Matos M, et al. Carboplatin and etoposide combined with bevacizumab for the treatment of recurrent glioblastoma multiforme. J Clin Neurosci. 2010;17(8):970–974. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute. Common Terminology Criteria for Adverse Events version 4.0. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm - ctc_40 Accessed July 2014.
- 20.Thompson EM, Dosa E, Kraemer DF, et al. Treatment with bevacizumab plus carboplatin for recurrent malignant glioma. Neurosurgery. 2010;67(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–4729. [DOI] [PubMed] [Google Scholar]
- 22.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desjardins A, Reardon DA, Coan A, et al. Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer. 2012;118(5):1302–1312. [DOI] [PubMed] [Google Scholar]
- 24.Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 25.Mrugala MM, Crew LK, Fink JR, et al. Carboplatin and bevacizumab for recurrent malignant glioma. Oncol Lett. 2012;4(5):1082–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narayana A, Kelly P, Golfinos J, et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg. 2009;110(1):173–180. [DOI] [PubMed] [Google Scholar]
- 27.Raizer JJ, Grimm S, Chamberlain MC, et al. A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer. 2010;116(22):5297–5305. [DOI] [PubMed] [Google Scholar]
- 28.Anderson MD, Hamza MA, Hess KR, et al. Implications of bevacizumab discontinuation in adults with recurrent glioblastoma. Neuro Oncol. 2014;16(6):823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piccioni DE, Lai A. Deferred use of bevacizumab for recurrent glioblastoma is not associated with diminished efficacy. Neuro Oncol. 2014;16(10):1427–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.