Abstract
Isocitrate dehydrogenase-1 (Idh1) is an important metabolic enzyme that produces NADPH by converting isocitrate to α-ketoglutarate. Idh1 is known to reduce reactive oxygen species (ROS) induced in cells by treatment with lipopolysaccharide (LPS) in vitro. Here, we used Idh1-deficient knockout (Idh1 KO) mice to investigate the role of Idh1 in antioxidant defense in vivo. Idh1 KO mice showed heightened susceptibility to death induced by LPS and exhibited increased serum levels of inflammatory cytokines such as tumor necrosis factor-α and interleukin-6. The serum of LPS-injected Idh1 KO mice also contained elevated levels of AST, a marker of inflammatory liver damage. Furthermore, after LPS injection, livers of Idh1 KO mice showed histological evidence of elevated oxidative DNA damage compared with livers of wild-type (WT) mice. Idh1 KO livers showed a faster and more pronounced oxidative stress than WT livers. In line with that, Idh1 KO hepatocytes showed higher ROS levels and an increase in the NADP+/NADPH ratio when compared with hepatocytes isolated from WT mice. These results suggest that Idh1 has a physiological function in protecting cells from oxidative stress by regulating the intracellular NADP+/NADPH ratio. Our findings suggest that stimulation of Idh1 activity may be an effective therapeutic strategy for reducing oxidative stress during inflammatory responses, including the early stages of septic shock.
There are five isocitrate dehydrogenase (Idh) genes in mammals: Idh1, Idh2 and Idh3α, β and γ. The Idh3 proteins form a heterotrimeric enzyme, are localized in the mitochondria, use NAD+ as a coenzyme and function in the tricarboxylic acid cycle.1 The Idh2 enzyme is a homodimer and is also localized in mitochondria but uses NADP+ as a coenzyme.1 The Idh1 enzyme is also a homodimer, is located in the cytosol and peroxisomes, and uses NADP+ as its coenzyme. All Idh enzymes catalyze the oxidative decarboxylation of isocitrate to α-ketoglutarate (αKG), but Idh1 and Idh2 produce one molecule of NADPH in the process, whereas Idh3 produces NADH.1
NADPH is a substrate for many metabolic redox reactions, and a sufficient supply of reducing equivalents of NADPH is required for the detoxification of the reactive oxygen species (ROS) that routinely accumulate in normal cells.2 One major source of NADPH is the glycolytic enzyme glucose-6-phosphate dehydrogenase (G6PDH). Lipopolysaccharide (LPS) induces G6PDH expression and activity, resulting in elevated NADPH production and protection of the cell against death induced by oxidative stress.3 As Idh1 activity also generates reducing equivalents of NADPH, we postulated that Idh1 might have an important role in resolving cytosolic oxidative stress.
Although excessive ROS accumulation induces apoptosis, moderate ROS generation is a critical component of the inflammation characteristic of the innate immune response.4 Indeed, previous reports have shown that, at least in vitro, Idh1 contributes to reducing the ROS induced in cells by treatment with lipopolysaccharide (LPS) or H2O2.5, 6 Expression of Idh1 is induced in RAW 264.7, which is derived from murine macrophages, by LPS treatment and overexpression of Idh1 in these cells enhances their survival upon exposure to H2O2.5 These observations prompted us to investigate the role of Idh1 in murine host defense and activation of the innate immune system in vivo.
LPS, which is derived from Gram-negative bacteria, is the prototypical stimulus for triggering an inflammatory cascade both in vitro and in vivo. Mice challenged with LPS quickly mount an inflammatory response that may start as fever but can progress rapidly to septic shock or disseminated intravascular coagulation. This excessive inflammation, which is mediated by tumor necrosis factor-α (TNF-α) and several other inflammatory cytokines, can cause damage to multiple organs, including the liver.7 TNF-α is a pleiotropic cytokine that induces a plethora of biological effects, including the production of other inflammatory cytokines and chemokines, upregulation of adhesion molecules, and cell proliferation, differentiation or death.8 TNF-α is produced mainly by macrophages and also by a variety of other cell types, including hepatocytes.9
In the liver, TNF-α has an important role in maintaining the balance between hepatocyte cell death and proliferation.10, 11, 12 TNF-α causes ROS generation in hepatocytes and induces apoptosis.13 During milder inflammatory insults, hepatocytes upregulate their oxidative stress defense mechanisms to maintain viablity.14 This mechanism relies mainly on Nrf2,15 a master transcription factor for cytoprotective and antioxidant genes. Nrf2 directly regulates the production of glutathione, through the transactivation of glutamine cysteine ligase catalytic subunit (GCLC) and glutamine cysteine ligase modifier subunit (GCLM). GSH exists in both reduced (GSH) and oxidized (GSSG) states. GSH is one of the most important scavengers of ROS, and its ratio with GSSG is a marker of oxidative stress.16 To maintain sufficient levels of GSH, de novo GSH synthesis is required, which involves the GCLC and the GCLM. Reduction of GSSG to GSH by the enzymes GSH reductase also aids in maintaining levels of GSH.17 Both of these pathways require NADPH to provide reducing power. Because Idh1 is highly expressed in the liver18 and is an important source of cytosolic NADPH, we hypothesized that Idh1 might contribute to host antioxidant defense by producing NADPH capable of protecting hepatocytes from ROS-induced death.
In this study, we evaluated the response of Idh1-deficient (Idh1 KO) mice to LPS-induced systemic inflammation. Idh1 KO mice were more sensitive to LPS-induced sepsis, as evidenced by their elevated serum levels of TNF-α. LPS-induced mortality in Idh1 KO mice was attributed to massive hepatocyte apoptosis or cytotoxicity. Freshly isolated Idh1-deficient hepatocytes increased oxidative stress upon LPS treatment and exhibited altered upregulation of antioxidant response genes. Importantly, primary hepatocytes isolated from Idh1 KO mice at steady state also contained increased ROS compared with steady-state wild-type (WT) hepatocytes, suggesting a constitutive role for Idh1 in regulating hepatocyte responses to oxidative stress. Modulation of intracellular Idh1 levels may therefore represent a therapeutic opportunity to target oxidative stress in patients suffering from various inflammatory conditions.
Results
Idh1-deficient mice exhibit increased susceptibility to septic shock
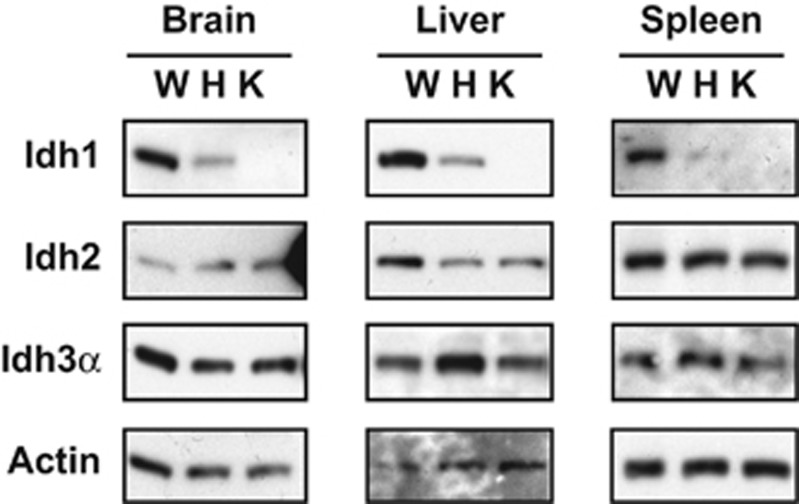
To study the role of Idh1 in antioxidant defense, we used an Idh1 LSL/LSL mouse strain (C57BL/6 background) generated previously in our laboratory in the course of our studies of Idh1 mutations in hematopoietic and neuronal cells.19, 20 In the absence of Cre recombinase, this mutant is null for Idh1 in all tissues and so constitutes an Idh1 KO mouse (Figure 1). Idh1 KO mice showed normal development and fertility. To determine if Idh1 has a role in preventing septic shock, we administered a lethal dose of LPS (100 μg per mouse) to one cohort of WT and Idh1 KO mice, and a sublethal dose of LPS (50 μg per mouse) to a second cohort, and monitored mouse survival over 5 days. About 60% of WT mice survived the sublethal LPS treatment, whereas only 10% of their Idh1 KO counterparts survived (Figure 2a). These data indicate that effective host defense against LPS-induced septic shock requires Idh1.
Figure 1.
Confirmation of Idh1 deletion in Idh1 LSL/LSL (Idh1 KO) mice. Immunoblot analysis to detect Idh1, Idh2 and Idh3 proteins in extracts of brain, liver and spleen from WT Idh1 wt/wt (W), heterozygous Idh1 wt/LSL (H) and Idh1 LSL/LSL Idh1 KO (K) mice. Actin, loading control. Results are representative of three mice per group
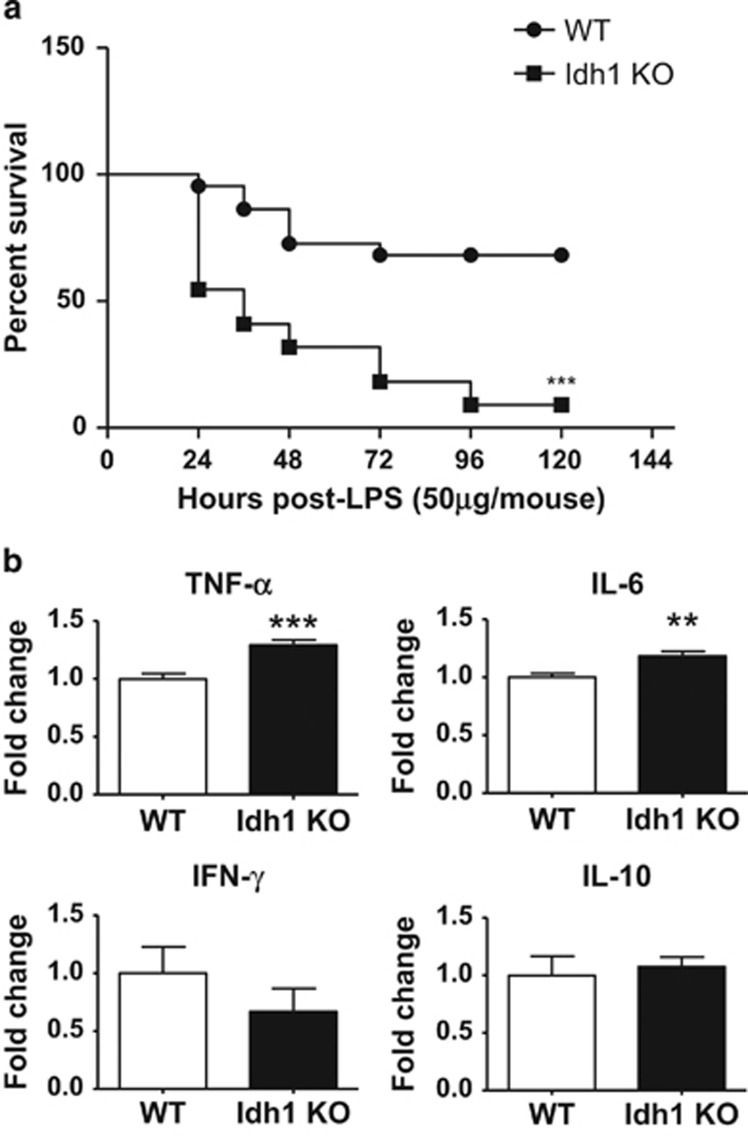
Figure 2.
Increased in vivo susceptibility of Idh1 KO mice to LPS-induced septic shock. (a) Kaplan–Meier analysis of the survival of Idh1 KO and WT mice (n=22 per group) that received a sublethal dose of LPS (50 μg) and were monitored for 5 days. Data are expressed as percent survival. ***P<0.001. (b) Quantitation by ELISA of serum levels of the indicated cytokines in the mice in (a) at 2 h after LPS injection. Data are expressed as the fold change relative to values in LPS-treated WT mice and are the mean±S.D. (n=22). **P<0.01 and ***P<0.001
We next analyzed levels of various inflammatory cytokines in the serum of WT and Idh1 KO mice at 2 h after LPS (50 μg per mouse) treatment. TNF-α and interleukin -6 (IL-6) are the major effectors of LPS-induced cytotoxicity in vivo.21 As expected, TNF and IL-6 were not detectable in the serum of either WT or Idh1 KO mice at steady state. Upon LPS administration, both these cytokines were significantly increased in the serum of Idh1 KO mice compared with WT controls (Figure 2b, top). In contrast, levels of IL-10, an anti-inflammatory cytokine, and interferon-γ (IFN-γ), an inflammatory cytokine, were comparable between these groups (Figure 2b, bottom). It has been shown that LPS treatment also induces the production of type I IFN, such as IFN-α and IFN-β. However, there was no significant difference between the level of these cytokines in the serum of LPS-administered WT and Idh1 KO mice (Supplementary Figure 1).
Susceptibility of Idh1-deficient mice to LPS treatment correlates with a defect in non-hematopoietic cells
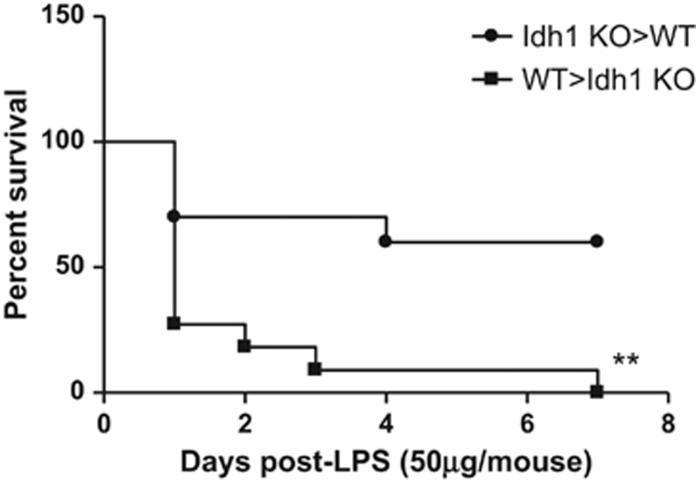
Because of the marginal increase in TNF and IL-6 compared with the WT, we speculated that non-hematopoietic cells might be responsible for LPS sensitivity of Idh1 KO mice. TNF and IL-6 are produced mainly by hematopoietic cells. To determine whether the increased mortality of Idh1 KO mice following LPS injection was because of alterations in the hematopoietic or the non-hematopoietic cell compartment, we generated reciprocal bone marrow (BM) chimeras using BM cells from either WT or Idh1 KO mice. Interestingly, Idh1 KO recipients of WT BM cells were more sensitive to LPS treatment than WT recipients of Idh1-deficient BM cells (Figure 3). However, there were no significant differences in serum levels of TNF between WT and KO recipients (Supplementary Figure 2), which is in line with our previous experiments. These data suggest that it is a defect in non-hematopoietic cells, rather than hematopoietic cells, that enhances LPS-induced mortality in Idh1 KO mice and points to a crucial of Idh1 in the regulation of septic shock responses.
Figure 3.
Idh1 in non-hematopoietic cells protects against LPS-induced septic shock. Kaplan–Meier analysis of the survival of lethally irradiated WT mice (n=11) that had been reconstituted with Idh1 KO BM cells (KO>WT, ●), as well as lethally irradiated Idh1 KO mice (n=10) that had been reconstituted with WT BM cells (WT>KO, ▪). Mice received a sublethal LPS dose at 10 weeks after reconstitution and survival was monitored for 7 days. Data are expressed as percent survival. **P<0.01
Idh1-deficient mice show increased liver injury
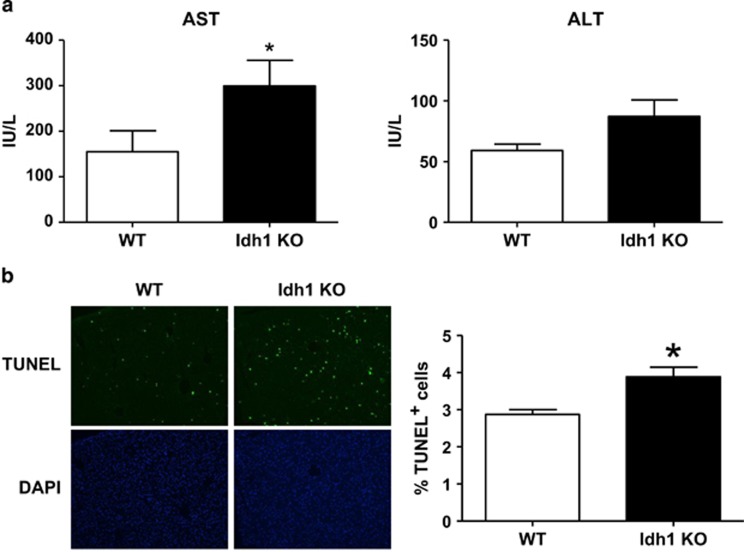
Because Idh1 is highly expressed in the liver,18 and the mortality of our Idh1 KO mice exposed to LPS was linked to non-hematopoietic cells, we examined the effects of Idh1 deficiency on the liver during LPS-induced inflammation in vivo. We collected blood from the tail veins of WT and Idh1 KO mice treated with a sublethal dose of LPS and evaluated serum levels of alanine aminotransferase (ALT) and aspartate transaminase (AST), which are two markers of hepatocellular injury or necrosis.22, 23 AST was significantly elevated in WT and Idh1 KO mice after 6 h of LPS treatment, confirming liver injury (Figure 4a). On the other hand, ALT was unaffected. However, there were no remarkable findings on H&E staining during WT and Idh1 mice (Supplementary Figure 3). To examine directly liver damage, we subjected liver sections of our LPS-treated mice to TUNEL staining to detect hepatocyte apoptosis. In line with their elevated levels of AST, the number of TUNEL+ hepatocytes was significantly increased in livers of LPS-treated Idh1 KO mice compared with livers of LPS-treated WT mice (Figure 4b). These results indicate that a higher degree of LPS-induced liver inflammation and LPS-induced hepatocyte death is enhanced in the absence of Idh1.
Figure 4.
Loss of Idh1 exacerbates LPS-induced liver injury. (a) Quantitation of serum levels of (top) AST and (bottom) ALT in Idh1 KO and WT mice that received a sublethal LPS dose. Blood samples were collected at 6 h after LPS injection. Data are expressed as the fold change relative to values in LPS-treated WT mice and are the mean±S.D. (n=6). *P<0.05. (b) TUNEL staining of apoptotic cells in the livers of the mice in (a), which were killed at 12 h after LPS injection. Top: TUNEL staining; bottom: DAPI (4',6-diamidino-2-phenylindole). Magnification × 10. Quantitation of apoptotic cells in the livers was shown on the right. Data were collected as described in Materials and Methods and are the mean percentage of TUNEL+ cells±S.D. *P<0.05
Endotoxin treatment induces excessive oxidative stress in livers of Idh1-deficient mice
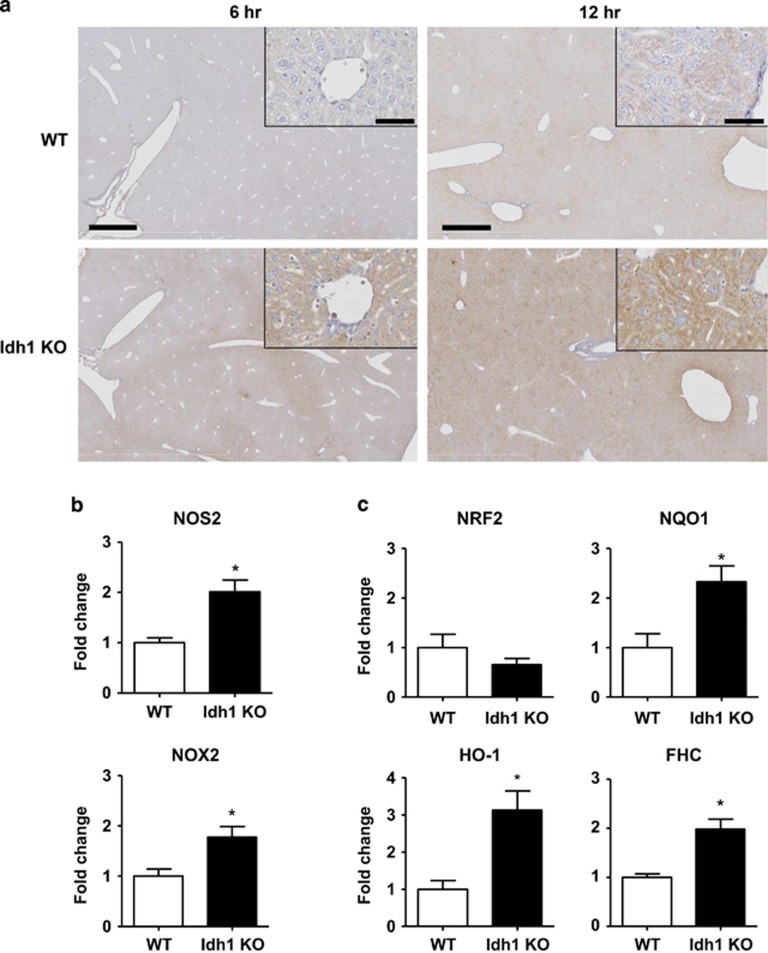
It has been reported that Idh1 is crucial for suppressing intracellular ROS levels.5 To assess ROS accumulation in the livers of our LPS-treated WT and Idh1 KO mice, we stained liver sections to detect 8-hydroxydeoxyguanosine (8-OHdG), a readout of oxidative DNA damage.24 Analyses of liver revealed higher levels of 8-OHdG in LPS-treated Idh1 KO mice, indicating a higher level of oxidative damage compared with LPS-treated WT mice (Figure 5a). This elevation of 8-OHdG staining observed in Idh1 KO liver appeared as early as 6 h after LPS treatment, a time when 8-OHdG was undetectable in the liver of LPS-treated WT mice (Figure 5a). The liver of Idh1 KO mice clearly showed a temporal response with 8-OHdG staining intensity increasing between 6 and 12 h after LPS injection. Most of this early response appears to reflect stress-related effects on mitochondria of liver parenchymal cells (hepatocytes). It is worth noting that while there was some nuclear staining present, this appeared to be in non-parenchymal cells (Figure 5, enlarged box). These results of early activation of 8-OHdG are supported by previous reports in the literature of an early stress response in mitochondria of hepatocytes induced by LPS,25 as well as stress induced by TNF-α.26
Figure 5.
Loss of Idh1 induces excessive ROS accumulation in the liver. (a) Histological staining to detect the oxidative stress marker 8-OHdG (brown) in livers of Idh1 KO and WT mice that received sublethal LPS injection and were killed 6 h or 12 h later, as indicated. Results presented are representative of two sections per mouse in two mice per group at 6 h and the average of five sections per mouse in six mice per group at 12 h. Scale bars, 1 mm (6 h), 0.5 mm (12 h) and 50 μm (insets). (b and c) Quantitative PCR analysis of mRNA levels of the indicated ROS-inducing (b) and antioxidant (c) genes in livers of Idh1 KO and WT mice that received a sublethal LPS dose. Liver extracts were prepared at 6 h after LPS injection. Data are expressed as the fold change relative to values in LPS-treated WT mice and are the mean±S.D. (n=3). *P<0.05
We used quantitative PCR to analyze the expression of genes related to the induction and control of oxidative stress in the liver. Compared with LPS-treated WT controls, mRNA levels of oxidative stress-inducing genes such as Nos2 and Nox2 were significantly upregulated in livers of Idh1 KO mice following LPS injection (Figure 5b), as were mRNA levels of the antioxidant genes NAD(P)H quinone oxidoreductase-1 (Nqo1), heme oxygenase-1 (Ho-1) and ferritin heavy chain (Fhc) (Figure 5c). These results suggest that hepatocytes of Idh1 KO mice succumb to death induced by excessive ROS accumulation, and that this level of ROS cannot be overcome despite a concomitant increase in antioxidant gene expression.
Idh1-deficient mice have a high basal level of ROS
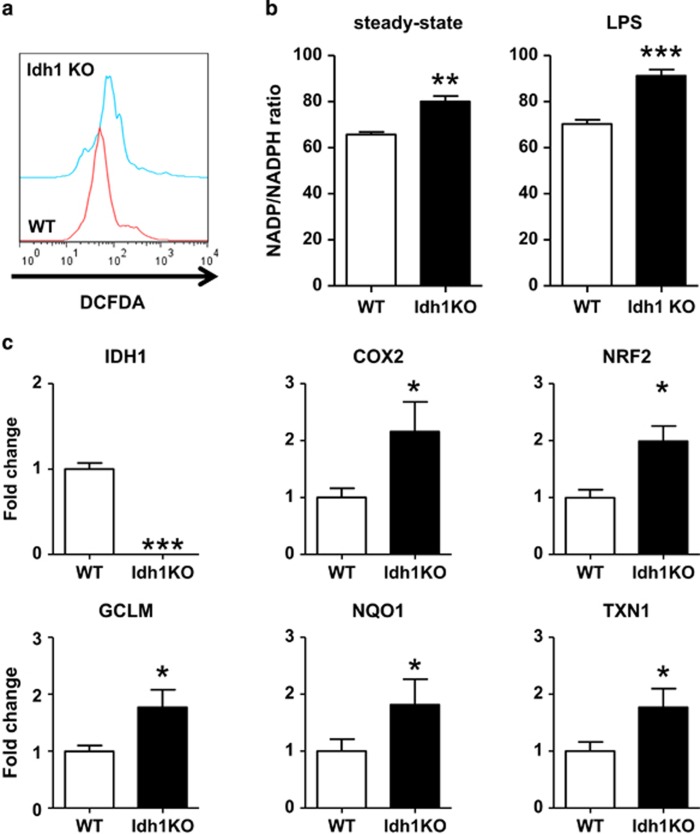
In light of the accumulation of relatively high levels of ROS in the LPS-treated Idh1 KO mice early after LPS administration, we wondered whether ROS level were already increased in Idh1 KO mice at steady state. To investigate this hypothesis, we isolated hepatocytes from the livers of untreated WT and Idh1 KO mice and measured intracellular ROS levels. Indeed, Idh1 KO hepatocytes showed increased levels of ROS compared with WT hepatocytes as measured by the CM-H2DCFDA (DCFDA) assay (Figure 6a), indicating a fundamental abnormality in the oxidative stress response in these mutant cells, which is not limited to states of inflammation.
Figure 6.
Idh1 KO hepatocytes accumulate ROS even at steady state. (a) Flow cytometric determination of ROS levels in steady-state WT and Idh1 KO hepatocytes that were stained with DCFDA. Data are representative of three cultures per group and three experiments. (b) Quantitation of the intracellular NADP+/NADPH ratio in steady-state hepatocytes (left) and sublethal LPS-injected hepatocytes at 6 h (right) of each WT and Idh1 KO mice determined as described in Materials and Methods. *P<0.05 and ***P<0.001. (c) Quantitative PCR analysis of mRNA levels of the indicated ROS-related genes in steady-state WT and Idh1 KO hepatocytes. Data are expressed as the fold change relative to values in WT hepatocytes and are the mean±S.D. (n=3). *P<0.05; **P<0.01; ***P<0.001
The intracellular ratio of NADP+ to NADPH is believed to be a critical regulator of the oxidative stress response.27 As NAPDH is produced from NADP+ upon Idh1-mediated conversion of isocitrate to αKG, we speculated that levels of NAPDH might be compromised in our Idh1 KO mice even in the steady state. Consistent with this hypothesis, Idh1 KO hepatocytes exhibited a significantly higher intracellular NADP+/NADPH ratio than WT hepatocytes (Figure 6b, left). We also examined the intracellular ratio of NADP+ to NADPH in hepatocytes derived from LPS-treated mice. Idh1 KO hepatocytes showed a significant increase in the intracellular NADP+/NADPH ratio compared with WT hepatocytes (Figure 6b, right).
Elevated intracellular ROS activate a number of antioxidant and oxidative stress response genes.28 Quantitative RT-PCR analysis of untreated Idh1-deficient hepatocytes revealed significantly greater mRNA levels of the antioxidant genes Nrf2, Gclm, Nqo1 and thioredoxin-1 (Txn1) compared with untreated WT hepatocytes (Figure 6c). In addition, Idh1 KO hepatocytes showed elevated mRNA expression of Cox2, which generates ROS.29 These results suggest that, even in the absence of inflammation, hepatocytes lacking Idh1 accumulate excessive ROS despite attempting to mount an antioxidant response. Thus, Idh1 has an important role in maintaining baseline antioxidant defense as well as during inflammation.
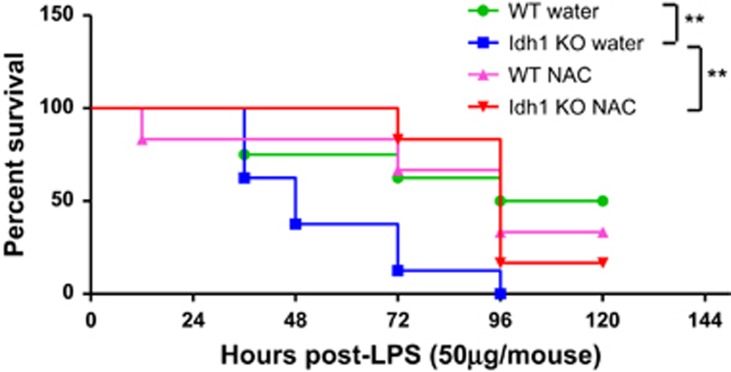
To investigate whether the elevated ROS level in Idh1 KO mice was functionally important for the increased sensitivity to LPS-induced septic shock, Idh1 WT and KO mice were treated with or without the antioxidant, N-acetyl cysteine (NAC), followed by a sublethal dose of LPS. The increased LPS sensitivity of Idh1 KO mice was largely rescued by NAC administration (Figure 7). The survival of the NAC-treated Idh1 KO mice was similar to Idh1 WT animals, showing that increased sensitivity to LPS in Idh1 KO mice is primarily due to the effects of elevated ROS.
Figure 7.
NAC-treated Idh1 KO mice increase survival. Kaplan–Meier analysis of the survival of LPS-injected Idh1 KO mice and WT mice treated with (n=6 for each genotype) or without (n=8 for each genotype) NAC included in drinking water. These mice received a sublethal dose of LPS (50 μg) and were monitored for 5 days. Data are expressed as percent survival. **P<0.01
Discussion
We previously reported the in vivo function of mutant IDH1 in neuronal development and hematopoiesis using conditional Idh1 KI mice.19, 20 However, the in vivo biological functions of endogenous WT Idh1 are not fully understood. Here, we provide the first report of the in vivo function of WT Idh1 using a KO mouse model. Although Idh1 KO mice were viable, fertile and displayed no gross pathological phenotype, we found that loss of Idh1 increased mortality caused by endotoxin-induced inflammation, and demonstrated that this mortality was linked to a defect in non-hematopoietic cells. Moreover, compared with WT controls, hepatocytes from Idh1 KO mice exhibited increased oxidative stress both at steady state and following LPS exposure. These findings confirm the importance of Idh1 function in the liver to host antioxidant defense and in limiting liver inflammation.
We found that Idh1 KO mouse hepatocytes accumulated ROS and ROS-induced damage, even in the absence of inflammation, and that the loss of Idh1 in these mice enhanced their susceptibility to LPS-induced shock. These data indicate that Idh1 KO hepatocytes were able to overcome their excessive ROS accumulation at steady state, but could not stave off lethality in the face of robust inflammation. We also examined Idh2 expression during LPS treatment as a potential mechanism of adaptation to loss of Idh1, but its expression was unaffected (Supplementary Figure 4). Thus, Idh1 has a physiological function in suppressing inflammation and protecting against septic shock by inhibiting ROS accumulation.
We also observed that the 8-OHdG staining observed was predominantly cytoplasmic in both WT and Idh1 KO hepatocytes after LPS treatment, although there was some nuclear staining in non-parenchymal cells (Figure 5, enlarged box). Consistent with our data, LPS/TNF-induced ROS production in hepatocytes results in relatively rapid oxidative damage of mitochondrial, rather than nuclear DNA.25, 26 These observations strongly suggest that the early cytoplasmic 8-OHdG staining in the Idh1 KO hepatocytes is due to enhanced oxidative damage to mitochondrial DNA.
Although TNF-α and IL-6 production were increased in sera of Idh1 KO mice compared with sera of WT mice following LPS exposure, we found no significant differences in serum levels of TNF-α between WT and Idh1 KO BM recipient mice. However, macrophages and neutrophils in Idh1 KO mice did not show increased TNF-α production compared with those from WT animals. Because TNF-α is produced by both hematopoietic and non-hematopoietic cells, it is still unclear which cells contribute to the observed increase in TNF-α levels.
Idh1 KO recipient mice reconstituted with WT BM cells were just as susceptible to LPS-induced mortality as intact Idh1 KO mice, without an increase in TNF-α production, ruling out a role for Idh1 in the hematopoietic system in this phenotype. This result suggests that the mortality of Idh1 KO mice is due to an enhanced sensitivity to this inflammatory cytokine rather than to a direct effect of its absolute level or an increased activity of cytokine-secreting cells.
Our demonstration that Idh1 deletion induces ROS accumulation in hepatocytes at steady state supports a previous report in which overexpression of Idh1 inhibited ROS accumulation induced in RAW264.7 cells by H2O2 treatment.5 Furthermore, our finding that Idh1 KO hepatocytes have an increased NADP+/NADPH ratio is consistent with the drop in NADPH expected in the absence of Idh1 owing to a failure to convert isocitrate to αKG. As noted earlier, one of the major producers of cytosolic NADPH is G6PDH. Similar to our Idh1 KO mice, G6PDH KO mice show heightened susceptibility to LPS and increased production of inflammatory cytokines, particularly TNF-α and IL-6.30 Taken together, these observations establish that the mechanism underlying LPS susceptibility involves an increase in the intracellular NADP+/NADPH ratio.
At an early stage following LPS injection, Idh1 KO mice exhibited significant ROS accumulation accompanied by elevated Nox2 mRNA expression. These results suggest that the heightened ROS level present after LPS treatment is due not only to the deficit in NADPH and GSH production caused by the absence of Idh1 (i.e. not due only to the inability of Idh1-deficient cells to suppress ROS) but also to enhanced production of ROS by the mitochondria or by cytosolic NADPH oxidases. Furthermore, at steady state, Idh1 KO hepatocytes already contained elevated ROS levels, even though they also showed upregulation of several antioxidant genes, including Nrf2, Gclm, Nqo1 and Txn1. However, despite these abnomalies, steady-state Idh1 KO mice were healthy, fertile, maintained a normal weight and showed no inflammatory symptoms. These observations were initially puzzling because other mutants with impaired antioxidant responses have abnormal phenotypes caused by ROS accumulation. For example, steady-state Nrf2 KO mice display various inflammatory disorders,31, 32, 33, 34 Gclc KO mice are lethal,35 and Gclm KO mice exhibit weight loss.36 In addition, a previous report showed that Idh-mediated NADPH production cannot compensate for the loss of the NADPH supply in G6PDH-deficient mice.37 We believe our steady-state Idh1 KO mice show non-impaired survival because they upregulate antioxidant genes and limit ROS accumulation to a level lower than occurs in these other mutants. However, when robust inflammation occurs, such as that induced by LPS, the levels of NADPH production and antioxidant gene expression in Idh1 KO hepatocytes are insufficient to counteract their excessive ROS accumulation, leading to mouse mortality. We performed rescue experiments by adenoviral expression of WT Idh1 and by treatment with NAC to define the mechanism of the observed phenotypes (Figure 7). We were not able to achieve WT expression levels of Idh1 via the adenoviral system (Supplementary Figure 5), and thus we could not rescue the phenotype. However, NAC treatment produced a significant survival advantage in Idh1 KO mice after LPS challenge. These results indicated that antioxidant treatment might compensate for loss of Idh1.
We found that Idh1-deficient hepatocytes at steady state contained high levels of ROS, and that further accumulation was promoted by vigorous inflammation. Such abnormally high ROS concentrations not only inflict tissue damage but may also introduce gene mutations. When ROS production exceeds a cell's ability to metabolize these radicals and overwhelms antioxidant defenses, DNA lesions leading to carcinogenesis can be induced.38 Compared with the frequency of Idh1 gain-of-function mutations that result in altered Idh1 activity and lead to acute myeloid leukemia or glioblastoma,39, 40, 41 the frequency of cancers with complete loss of Idh1 function is low (Supplementary Figure 6). It is unclear whether inflammation contributed to tumorigenesis in these patients. However, our study suggests that the Idh1 deletion in the cancer cells of these patients might have been a contributing factor to their malignancies. It will be interesting to examine ROS levels in the tumor cells of these Idh1-deleted cancer patients.
In conclusion, our work has demonstrated a role for Idh1 in mitigating damage inflicted by intracellular oxidative stress. It is therefore possible that stimulation of Idh1 activity might be a beneficial therapeutic strategy for inflammatory disorders, including the early stages of septic shock.
Materials and Methods
Mice
Idh1 LSL/LSL (Idh1 KO) mice have been described previously.20 WT mice were standard C57BL/6 animals. All mice were maintained at the Ontario Cancer Institute Animal Facility in compliance with the regulations of the Animal Ethics and Animal Care Committees at the Princess Margaret Cancer Centre, Toronto, Ontario. These bodies approved all experiments.
Immunoblotting
Cells were lysed in 0.5% Triton X-100 lysis buffer (20 mM Tris-HCl at pH 7.4, 150 mM NaCl, 10% glycerol) containing protease inhibitor (Roche, Indianapolis, IN, USA; catalog no. 1183617001) for 30 min on ice. Cell lysates (50 μg) were subjected to standard SDS-PAGE and immunoblotting using the primary Abs; Idh1 (Santa Cruz, Dallas, TX, USA; cat. no.: sc-49996), Idh2 (Abcam, Cambridge, MA, USA; AB55271) and Idh3α (Abcam; AB5864) and actin (Sigma-Aldrich, St. Louis, MO, USA; A2066).
Septic shock induction
Idh1 KO and WT mice (10 weeks old; n=4–8 per group) were intravenously injected with a sublethal dose of LPS (50 μg in 300 μl PBS per mouse; Sigma-Aldrich). Mouse survival was monitored for 5 days.
BM chimeras
BM chimeras was performed as described previously.42
Serum components
Serum samples were obtained from tail veins of untreated or LPS-treated mice using microtainer serum separator tubes (BD and Company, Franklin Lakes, NJ, USA). Serum levels of TNF-α, IL-6, IFN-γ and IL-10 were determined by ELISA using the appropriate kits (BD Biosciences, San Jose, CA, USA) according to the manufacturer's instructions. Serum levels of ALT and AST were determined using a test devised by IDEXX Laboratory Inc. (Markham, ON, Canada) and performed by the Animal Health Laboratory of the University of Guelph (Guelph, ON, Canada).
Apoptosis
TUNEL assays were performed using the In Situ Cell Death detection kit (Roche) according to the manufacturer's instructions. Visiopharm Image Analysis software (Visiopharm, Denmark) was used to count the number of TUNEL+ cells per field as well as the total number of DAPI+ (Molecular Probes, Eugene, OR, USA) nuclei per field. Ten contiguous fields per liver were counted at × 10 magnification for WT (n=3) and Idh1 KO (n=3) mice. The results were expressed as the ratio of TUNEL+ cells to total number of cells (DAPI+ nuclei) for each liver.
Histological oxidative stress determinations
Liver samples were fixed in 4% paraformaldehyde and embedded in paraffin, and tissue sections of 5 μm were cut. Oxidative DNA damages by oxidative stress were detected using mouse anti-8-OHdG antibody (Abcam; AB62623) as the primary antibody. Sites of anti-8-OHdG Ab binding were visualized using 3, 3-diamino-benzidine tetrahydrochloride (Vector Labs, Burlingame, CA, USA; SK-4100) as the substrate and hematoxylin as the counterstain. Stained slides were analyzed using an Olympus Nano Zoomer Pathology Slide Scanner (2.0HT) (Hamamatsu, Bridgewater, NJ, USA).
Real-time RT-PCR
Total RNA was extracted using the NucleoSpin RNA II Kit (Clontech, Palo Alto, CA, USA) and reverse-transcribed using iScript (Bio-Rad, Hercules, CA, USA). The resulting cDNAs were diluted to 1:5–10 and served as templates for real-time PCR using Power SYBR Green PCR Master Mix (Applied Biosystems, Burlington, ON, Canada) and 500 nM forward primer plus 500 nM reverse primer on an ABI 7700 instrument (Applied Biosystems). All procedures were performed according to the manufacturer's instructions. The primer sequences used for real-time RT-PCR are listed in Supplementary Table 1. Each sample was assayed in triplicate, and data were normalized to the housekeeping gene 18 S ribosomal RNA. Results were calculated using the comparative threshold cycle method (2−ΔΔCt).
Hepatocyte isolation
Hepatocytes were isolated from WT and Idh1 KO mice (10 weeks old) by in situ digestion of the liver using collagenase type II perfusion, as described previously.43 Following perfusion, livers were immediately transferred to a sterile 6 cm dish and minced, and hepatocytes were dispersed by aspiration with a large-bore pipette. Dispersed hepatocytes were filtered through a 100-μm cell strainer (BD Falcon, San Jose, CA, USA) to remove tissue debris. After washing two times with cold DMEM and centrifuging at 50 × g for 3 min at 4 ºC, an aliquot of freshly isolated hepatocytes was placed in a hemocytometer and stained with trypan blue to evaluate cell viability and determine total cell number.
NADP+/NADPH ratio
Hepatocytes (5 × 105) isolated from WT or Idh1 KO mice as above were lysed in the NADP Extraction Buffer supplied with the Fluoro NADP/NADPH Detection Kit (Cell Technology, Fremont, CA, USA). Extracts were analyzed to quantitate the NADP+/NADPH ratio according to the manufacturer's instructions.
Flow cytometric ROS determinations
To measure intracellular ROS in hepatocytes, hepatocytes were incubated with 300 nM DCFDA (Invitrogen, Carlsbad, CA, USA) for 10 min at 37 °C. DCFDA fluorescence was analyzed by flow cytometry using a FACSCalibur instrument (BD and Company) and FlowJo software (Tree Star, Ashland, OR, USA).
Statistics
The statistical significance of most differences between groups was calculated using Prism software (GraphPad, San Diego, CA, USA). Differences in mouse survival were assessed by the Kaplan–Meier method. In both cases, differences with P-values of <0.05 were considered statistically significant.
Acknowledgments
This study is supported by the CHIR (ID: 288190). DB is supported by the ATTRACT program of the Luxembourg National Research Fund (FNR).
Glossary
- αKG
α-ketoglutarate
- G6PDH
glucose-6-phosphate dehydrogenase
- GCLM
glutamine cysteine ligase modifier subunit
- GSH
glutathione
- Idh
isocitrate dehydrogenase
- LPS
lipopolysaccharide
- NADP
nicotinamide adenine dinucleotide phosphate
- ROS
reactive oxygen species
- TNF-α
tumor necrosis factor-α
- ALT
alanine aminotransferase
- AST
aspartate transaminase
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited RA Knight
Supplementary Material
References
- 1Cairns RA, Mak TW. Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer Discov 2013; 3: 730–741. [DOI] [PubMed] [Google Scholar]
- 2Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 2010; 48: 749–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Garcia-Nogales P, Almeida A, Fernandez E, Medina JM, Bolanos JP. Induction of glucose-6-phosphate dehydrogenase by lipopolysaccharide contributes to preventing nitric oxide-mediated glutathione depletion in cultured rat astrocytes. J Neurochem 1999; 72: 1750–1758. [DOI] [PubMed] [Google Scholar]
- 4Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 2004; 4: 181–189. [DOI] [PubMed] [Google Scholar]
- 5Maeng O, Kim YC, Shin HJ, Lee JO, Huh TL, Kang KI et al. Cytosolic NADP(+)-dependent isocitrate dehydrogenase protects macrophages from LPS-induced nitric oxide and reactive oxygen species. Biochem Biophys Res Commun 2004; 317: 558–564. [DOI] [PubMed] [Google Scholar]
- 6Kim JY, Shin JY, Kim M, Hann SK, Oh SH. Expression of cytosolic NADP(+)-dependent isocitrate dehydrogenase in melanocytes and its role as an antioxidant. J Dermatol Sci 2012; 65: 118–125. [DOI] [PubMed] [Google Scholar]
- 7Ghosh S, Latimer RD, Gray BM, Harwood RJ, Oduro A. Endotoxin-induced organ injury. Crit Care Med 1993; 21: S19–S24. [DOI] [PubMed] [Google Scholar]
- 8Mallick AA, Ishizaka A, Stephens KE, Hatherill JR, Tazelaar HD, Raffin TA. Multiple organ damage caused by tumor necrosis factor and prevented by prior neutrophil depletion. Chest 1989; 95: 1114–1120. [DOI] [PubMed] [Google Scholar]
- 9Spencer NY, Zhou W, Li Q, Zhang Y, Luo M, Yan Z et al. Hepatocytes produce TNF-alpha following hypoxia-reoxygenation and liver ischemia–-reperfusion in a NADPH oxidase- and c-Src-dependent manner. Am J Physiol Gastrointest Liver Physiol 2013; 305: G84–G94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Papa S, Bubici C, Zazzeroni F, Franzoso G. Mechanisms of liver disease: cross-talk between the NF-kappaB and JNK pathways. Biol Chem 2009; 390: 965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol 2006; 290: G583–G589. [DOI] [PubMed] [Google Scholar]
- 12Wullaert A, Heyninck K, Beyaert R. Mechanisms of crosstalk between TNF-induced NF-kappaB and JNK activation in hepatocytes. Biochem Pharmacol 2006; 72: 1090–1101. [DOI] [PubMed] [Google Scholar]
- 13Czaja MJ. Induction and regulation of hepatocyte apoptosis by oxidative stress. Antioxid Redox Signal 2002; 4: 759–767. [DOI] [PubMed] [Google Scholar]
- 14Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J Gastroenterol Hepatol 2011; 26: 173–179. [DOI] [PubMed] [Google Scholar]
- 15Klaassen CD, Reisman SA. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol 2010; 244: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Chen Y, Dong H, Thompson DC, Shertzer HG, Nebert DW, Vasiliou V. Glutathione defense mechanism in liver injury: insights from animal models. Food Chem Toxicol 2013; 60: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Lushchak VI. Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids 2012; 2012: 736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Jennings GT, Sechi S, Stevenson PM, Tuckey RC, Parmelee D, McAlister-Henn L. Cytosolic NADP(+)-dependent isocitrate dehydrogenase. Isolation of rat cDNA and study of tissue-specific and developmental expression of mRNA. J Biol Chem 1994; 269: 23128–23134. [PubMed] [Google Scholar]
- 19Sasaki M, Knobbe CB, Itsumi M, Elia AJ, Harris IS, Chio II et al. D-2-hydroxyglutarate produced by mutant IDH1 perturbs collagen maturation and basement membrane function. Genes Dev 2012; 26: 2038–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Sasaki M, Knobbe CB, Munger JC, Lind EF, Brenner D, Brustle A et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature 2012; 488: 656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21DeForge LE, Remick DG. Kinetics of TNF, IL-6, and IL-8 gene expression in LPS-stimulated human whole blood. Biochem Biophys Res Commun 1991; 174: 18–24. [DOI] [PubMed] [Google Scholar]
- 22Clermont RJ, Chalmers TC. The transaminase tests in liver disease. Medicine (Baltimore) 1967; 46: 197–207. [DOI] [PubMed] [Google Scholar]
- 23Kaplowitz DE N, Yamada T. Biochemical tests for liver disease. In: Zakim D, Boyer TD (eds). Hepatology, A Textbook of Liver Disease. Saunders: Philadelphia, PA, 1982, pp 583–612. [Google Scholar]
- 24Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J 1996; 313: 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Bartz RR, Suliman HB, Fu P, Welty-Wolf K, Carraway MS, MacGarvey NC et al. Staphylococcus aureus sepsis and mitochondrial accrual of the 8-oxoguanine DNA glycosylase DNA repair enzyme in mice. Am J Respir Crit Care Med 2011; 183: 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Nagakawa Y, Williams GM, Zheng Q, Tsuchida A, Aoki T, Montgomery RA et al. Oxidative mitochondrial DNA damage and deletion in hepatocytes of rejecting liver allografts in rats: role of TNF-alpha. Hepatology 2005; 42: 208–215. [DOI] [PubMed] [Google Scholar]
- 27Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal 2008; 10: 179–206. [DOI] [PubMed] [Google Scholar]
- 28Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal 2008; 10: 1343–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res 2011; 21: 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Wilmanski J, Villanueva E, Deitch EA, Spolarics Z. Glucose-6-phosphate dehydrogenase deficiency and the inflammatory response to endotoxin and polymicrobial sepsis. Crit Care Med 2007; 35: 510–518. [DOI] [PubMed] [Google Scholar]
- 31Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J 2004; 18: 1258–1260. [DOI] [PubMed] [Google Scholar]
- 32Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med 2005; 202: 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW et al. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest 2006; 116: 984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Wang J, Fields J, Zhao C, Langer J, Thimmulappa RK, Kensler TW et al. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med 2007; 43: 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Dalton TP, Dieter MZ, Yang Y, Shertzer HG, Nebert DW. Knockout of the mouse glutamate cysteine ligase catalytic subunit (Gclc) gene: embryonic lethal when homozygous, and proposed model for moderate glutathione deficiency when heterozygous. Biochem Biophys Res Commun 2000; 279: 324–329. [DOI] [PubMed] [Google Scholar]
- 36Kendig EL, Chen Y, Krishan M, Johansson E, Schneider SN, Genter MB et al. Lipid metabolism and body composition in Gclm(−/−) mice. Toxicol Appl Pharmacol 2011; 257: 338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Filosa S, Fico A, Paglialunga F, Balestrieri M, Crooke A, Verde P et al. Failure to increase glucose consumption through the pentose-phosphate pathway results in the death of glucose-6-phosphate dehydrogenase gene-deleted mouse embryonic stem cells subjected to oxidative stress. Biochem J 2003; 370: 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Ziech D, Franco R, Pappa A, Panayiotidis MI. Reactive oxygen species (ROS)-induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res 2011; 711: 167–173. [DOI] [PubMed] [Google Scholar]
- 39Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med 2009; 361: 1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008; 321: 1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med 2009; 360: 765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Afzal S, Hao Z, Itsumi M, Abouelkheer Y, Brenner D, Gao Y et al. Autophagy-independent functions of UVRAG are essential for peripheral naive T-cell homeostasis. Proc Natl Acad Sci USA 2015; 112: 1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Klaunig JE, Goldblatt PJ, Hinton DE, Lipsky MM, Chacko J, Trump BF. Mouse liver cell culture. I. Hepatocyte isolation. In Vitro 1981; 17: 913–925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.