Abstract
Fluoroquinolone resistance in Salmonella has become increasingly prevalent in recent years. To probe the molecular basis of this phenomenon, the genetic and phenotypic features of fluoroquinolone resistant Salmonella strains isolated from food samples were characterized. Among the 82 Salmonella strains tested, resistance rate of the three front line antibiotics of ceftriaxone, ciprofloxacin and azithromycin was 10%, 39% and 25% respectively, which is significantly higher than that reported in other countries. Ciprofloxacin resistant strains typically exhibited cross-resistance to multiple antibiotics including ceftriaxone, primarily due to the presence of multiple PMQR genes and the blaCTX-M-65, blaCTX-M-55 blaCMY-2 and blaCMY-72 elements. The prevalence rate of the oqxAB and aac(6’)-Ib-cr genes were 91% and 75% respectively, followed by qnrS (66%), qnrB (16%) and qnrD (3%). The most common PMQR combination observable was aac(6’)-Ib-cr-oqxAB-qnrS2, which accounted for 50% of the ciprofloxacin resistant strains. Interestingly, such isolates contained either no target mutations or only a single gyrA mutation. Conjugation and hybridization experiments suggested that most PMQR genes were located either in the chromosome or a non-transferrable plasmid. To summarize, findings in this work suggested that PMQRs greatly facilitate development of fluoroquinolone resistance in Salmonella by abolishing the requirement of target gene mutations.
Foodborne salmonellosis is one of the leading causes of foodborne illnesses worldwide. Although antimicrobial treatment is usually not necessary due to the self-limiting nature of salmonellosis, it can be lifesaving in cases of invasive infections1, with ceftriaxone and ciprofloxacin being the key drugs of choice2. Resistance to ceftriaxone or other extended spectrum beta-lactams is usually due to intracellular production of extended spectrum β-lactamases (ESBLs) such as the CTX-M group and AmpC β-lactamase, including the CMY-2 enzyme, which are usually located on transmissible plasmids that tend to disseminate among members of Enterobacteriaceae3,4. Prevalence of resistance to ceftriaxone in Salmonella appears to be slowly increasing, reaching a rate of around 3 ~ 4% at present5. However, the rate of resistance to ciprofloxacin has increased dramatically both in clinical and food isolates around the world, in particular China and the adjacent areas6. Ciprofloxacin resistance is mainly attributed to double mutations in the gyrA gene and single mutation in the parC gene in Salmonella7,8. Efflux pumps and the presence of plasmid-mediated quinolone resistance (PMQR) determinants have also been regarded as contributive factors of development of low level resistance to nalidixic acid. At least three types of PMQRs have been reported so far including (i) the Qnr types, which are pentapeptide repeat proteins that bind to DNA gyrase by mimicking double stranded DNA, preventing fluroquinolone binding to gyrase, (ii) Aac(6′)-Ib-cr, a modified aminoglycoside acetyltransferase that hydrolyzes fluoroquinolones and (iii) the efflux pumps QeqA, and OqxAB. Unlike E. coli and various other members of Enterobacteriaceae, the development of mutations in the gyrA and parC genes in Salmonella is known to be a very slow event, resulting in an unusually low level of ciprofloxacin resistance in Salmonella. On the other hand, although PMQRs were commonly detectable in Enterobacteriaceae, in particular E. coli, prevalence of PMQRs in Salmonella remains extremely low. To date, a few types of PMQRs including qnrA, qnrB, qnrD, and qnrS alleles have been reported in a limited number of studies9,10,11,12,13,14. Recently, a new PMQR gene, oqxAB, which was originally identified on a plasmid (pOLA52) recoverable from E. coli, was first reported in Salmonella isolates of food origin. The mobile efflux pump OqxAB belongs to the RND-family and shares up to 40% homology with other RND- type efflux systems such as AcrAB in E. coli and MexAB in Pseudomonas aeruginosa15. Exhibiting the ability to enhance the MICs of olaquindox, ampicillin, quinolones and chloramphenicol6,16, this element was subsequently found to be increasingly prevalent among Salmonella isolates recoverable from different sources after the year 20066,17. The oqxAB operon was suggested to play a functional role which helps accelerate the development of ciprofloxacin resistance in Salmonella, and was hence considered to be responsible for causing the recent dramatic increase of ciprofloxacin resistance in clinical Salmonella strains18.
The combination of PMQR such as oqxAB and a single target gene mutation, in particular in the gyrA gene, could possibly mediate development resistance to ciprofloxacin in Salmonella, and dramatically reduced the time required for the development of a resistance phenotype associated with generation of double gyrA mutations and single parC mutation18. This idea is supported by the observation of an increasing prevalence of different PMQR genes in various species of Enterobacteriaceae, and the emergence of ciprofloxacin-resistant E. coli and Salmonella strains carrying multiple PMQRs without target mutations19,20. In this study, we reported the high prevalence of ciprofloxacin resistant Salmonella strains in food samples, most of which were found to harbor either only a single mutation in gyrA, or no mutation in both target genes. However, the strains commonly contained multiple PMQR genes, with the most prevalent being the oqxAB and aac(6′)-Ib-cr elements. Discovery of this novel phenomenon, in which one bacterial resistance mechanism promotes the onset of another, signals a risk of aggravation of the clinical problem of ciprofloxacin resistance in Salmonella. The current situation warrants a need for continuous surveillance of the prevalent mechanisms of ciprofloxacin resistance in Salmonella in order to better understand the genetic background of this new category of resistant organisms.
Results
High prevalence of antimicrobial resistance in Salmonella food isolates
A total of 82 Salmonella strains were isolated from chicken and pork samples purchased from supermarkets and wet-markets in Shenzhen, China during the period of November 2012 to June 2013. These Salmonella strains were subjected to further characterization of their antimicrobial resistance to various antibiotics (Table 1), and the underlying resistance mechanisms. Overall, these strains exhibited a very high rate of resistance to most of the antibiotics tested. The resistance rate of the three most important front line antibiotics (ceftriaxone, ciprofloxacin and azithromycin) were respectively 10%, 39% and 25%, which is significantly higher than that reported in other countries. Salmonella strains isolated from pork samples exhibited a higher rate of resistance to most of the antibiotics tested when compared to Salmonella chicken isolates, in particular ciprofloxacin (Table 1). Surprisingly, chicken Salmonella isolates exhibited a much higher rate of resistance to ceftriaxone (35%) than that of the pork isolates (11%). This phenomenon is probably due to the high rate of resistance to ceftriaxone in S. Indiana. Among the different serotypes tested, S. Indiana also exhibited the highest rate of resistance to most antibiotics, including the three front line drugs of ceftriaxone, ciprofloxacin and azithromycin. Such phenotype has only been reported in S. Typhimurium and S. Kentucky previously6,21. S. Typhimurium and S. Derby also exhibited a very high resistance rate except that S. Typhimurium did not exhibit resistance to ceftriaxone. Two serotypes, namely S. Heidelberg and S. Rosentha, exhibited an intermediate rate of resistance to the test antibiotics. On the other hand, resistance was less commonly observed among S. Enteritidis. Another important observation is that, among 32 ciprofloxacin-resistant strains, all were resistant to ampicillin, nalidixic acid, kanamycin, streptomycin, chloramphenicol, tetracycline and sulfamethoxazole; furthermore, the MIC of olaquindox was generally 32 mg/L or higher for these strains. We also observed that, among such isolates, up to 84% were resistant to gentamicin, 25% were resistant to azithromycin (MIC ≥ 32 mg/L), and 13% were also resistant to ceftriaxone (Table 2).
Table 1. Prevalence of antimicrobial resistance in different Salmonella serotypes.
| Antimicrobials | % of Resistance |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 82) | Chicken isolates (n = 29) | Pork isolates (n = 53) | S. Derby (n = 32) | S. Typhimurium (n = 16) | S. Heidelberg (n = 8) | S. Rosentha (n = 8) | S. Indiana (n = 4) | S. Enteritidis (n = 4) | |
| Ampicillin | 68 | 62 | 72 | 69 | 100 | 100 | 63 | 100 | 25 |
| Cefotaxime | 10 | 21 | 4 | 3 | 0 | 25 | 0 | 100 | 25 |
| Ceftriaxone | 10 | 21 | 4 | 3 | 0 | 25 | 0 | 100 | 25 |
| Chloramphenicol | 74 | 69 | 75 | 83 | 79 | 100 | 50 | 75 | 50 |
| Gentamicin | 40 | 19 | 47 | 52 | 43 | 25 | 0 | 100 | 0 |
| Kanamycin | 48 | 27 | 55 | 69 | 64 | 25 | 25 | 100 | 0 |
| Streptomycin | 50 | 42 | 53 | 59 | 57 | 63 | 25 | 100 | 0 |
| Nalidixic acid | 63 | 46 | 68 | 72 | 57 | 50 | 38 | 100 | 25 |
| Ciprofloxacin | 39 | 17 | 51 | 50 | 57 | 0 | 0 | 75 | 0 |
| Sulfamethoxazole | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Tetracycline | 65 | 42 | 75 | 76 | 79 | 63 | 63 | 100 | 0 |
| Amikacin | 4 | 4 | 4 | 0 | 0 | 0 | 0 | 75 | 0 |
| Azithromycin | 25 | 23 | 31 | 38 | 6 | 13 | 25 | 75 | 0 |
| Olaquidox | 51 | 35 | 58 | 90 | 71 | 50 | 25 | 75 | 25 |
Table 2. Phenotypic and genotypic characteristics of Salmonella strains isolated from retail meat products.
| Strain # | Isolation date | Sources | Serotypes | PFGE | Resistance Profiles* | CIP | CIP/PAβN | PMQRs | Mutations in gyrA | Mutations in parC |
|---|---|---|---|---|---|---|---|---|---|---|
| S3a | 12/12/12 | P | Derby | DER1 | Gen | 16 | 2 | aac-oqxAB-qnrS2 | — | — |
| S24 | 01/12/13 | P | Derby | DER3 | Gen | 4 | 2 | oqxAB | S83I | |
| S35 | 01/26/13 | P | Derby | DER5 | Gen | 4 | 4 | aac-oqxAB-qnrS2 | S83T | — |
| S36 | 01/26/13 | P | Derby | DER5 | Gen-Azi | 8 | 2 | aac-oqxAB-qnrS2 | — | — |
| S37 | 01/26/13 | P | Derby | DER5 | Gen | 4 | 2 | aac-oqxAB-qnrS2 | — | — |
| S38a | 01/26/13 | P | Derby | DER5 | Gen-Azi | 2 | 2 | aac-oqxAB-qnrS2 | N78H | — |
| S39 | 01/26/13 | P | Derby | DER5 | Gen | 8 | 4 | aac-oqxAB-qnrS2 | — | — |
| S40 | 01/26/13 | P | Derby | DER5 | Gen-Azi | 8 | 4 | aac-oqxAB-qnrS2 | — | — |
| S41 | 01/26/13 | P | Derby | DER5 | Gen-Azi | 4 | 4 | aac-oqxAB-qnrS2 | — | — |
| S42 | 01/26/13 | P | Derby | DER5 | Gen | 4 | 2 | aac-oqxAB-qnrS2 | — | — |
| S44 | 01/26/13 | P | Derby | DER5 | Gen | 4 | 4 | aac-oqxAB-qnrS2 | — | — |
| S4a | 12/12/12 | P | Derby | DER6 | Gen | 2 | 2 | aac-oqxAB-qnrS2-qnrB8 | — | — |
| S9 | 12/12/12 | P | Derby | DER6 | 4 | 0.5 | aac-oqxAB-qnrS2 | S83T | — | |
| S54 | 03/13/13 | C | Derby | DER8 | 2 | 0.12 | oqxAB-qnrS8-qnrB | S83T | ||
| S49 | 03/03/13 | P | Derby | DER10 | Gen-Azi | >32 | >8 | oqxAB-qnrS1-qnrB | S83T | |
| S48 | 03/03/13 | C | Derby | DER11 | Gen | 2 | 0.5 | qnrS1 | S83T | — |
| S7a | 12/12/12 | P | Typhimurium | TR1 | Gen | 4 | 0.25 | aac-oqxAB | D87N | — |
| S8 | 12/12/12 | P | Typhimurium | TR1 | 4 | 0.25 | oqxAB | D87N | — | |
| S11 | 12/12/12 | P | Typhimurium | TR1 | Gen | 2 | 0.25 | aac-oqxAB | D87N | — |
| S79 | 05/17/13 | P | Typhimurium | TR2 | Gen | 4 | 0.12 | oqxAB | S83F | — |
| S65 | 03/21/13 | P | Typhimurium RH2 | TRH1 | Gen | 2 | 0.5 | aac-oqxAB-qnrS1 | D87N | — |
| S66 | 03/21/13 | P | Typhimurium RH2 | TRH2 | Gen | 4 | 0.5 | aac-oqxAB-qnrS1 | — | — |
| S71a | 05/01/13 | P | Typhimurium RH2 | TRH2 | Gen | >32 | >8 | aac-oqxAB-qnrS1 | D87N | — |
| S6 | 12/12/12 | P | Typhimurium RH2 | TRH2 | Gen | 4 | 0.25 | aac-oqxAB-qnrS1 | — | — |
| S20 | 01/12/13 | C | Typhimurium RH2 | TRH3 | Gen | 2 | 0.5 | aac-qnrB | D87N | — |
| S13 | 12/25/12 | P | Indiana | I1 | Gen-Azi-Cro | >32 | >8 | aac-oqxAB | S83F, D87N | — |
| S14 | 12/25/12 | C | Indiana | I1 | Gen-Azi-Cro | >32 | >8 | aac-oqxAB-qnrB | S83F, D87N | S80 R |
| S16 | 12/25/12 | P | Indiana | I1 | Gen-Azi-Cro | >32 | >8 | aac-oqxAB | S83F, D87N | C72G, S80 R |
| S27 | 01/19/13 | P | Rissen | R1 | 8 | 0.12 | oqxAB | H80N, S83T | Q91H | |
| S59 | 03/16/13 | C | London | L1 | 2 | 0.12 | D87N | |||
| S2 | 12/12/12 | P | Sanferberg | S1 | Gen | 4 | 4 | aac-oqxAB-qnrS2 | S83T | — |
| S45 | 02/22/13 | P | Virchow | V2 | Gen | 4 | 4 | aac-oqxAB-qnrS8-qnrD | — | — |
*All isolates were resistant to the antibiotic profile of Amp-Cip-Nal-Kan-Str-Chl-Tet-Sul-Ola (olaquindox); specific strains were also resistant to Gen, gentamicin; Azi, azithromycin; and Cro, ceftriaxone. PaβN, Phenylalanine-arginine β-naphthylamide. C, Chicken; P, Pork; aac, aac(6′)-Ib-cr;
aselected for S1-PFGE and Southern hybridization.
Diverse mechanisms of ceftriaxone resistance in Salmonella food isolates
Mechanisms of resistance in selected strains, in particular those mediating resistance to the front line antibiotics such as ceftriaxone and ciprofloxacin, were investigated. Resistance to ceftriaxone was detectable in 8 Salmonella isolates including 4 S. Indiana, 2 S. Heidelberg, 1 S. Enteritidis and 1 S. Derby. These 8 strains were examined for their ability to produce Extended-spectrum β-lactamases and AmpC β-lactamases, with results showing that diverse resistance mechanisms were observable. Three out of the four S. Indiana strains were found to contain blaCTX-M-65, with the fourth one harboring the blaCMY-2 gene. The blaCTX-M-55 gene was detectable in one S. Enteritidis and one S. Derby strain. For the two S. Heidelberg isolates, the blaCMY-2 and blaCMY-72 genes were each detectable in one strain (Table 2).
Novel mechanisms of fluoroquinolone resistance in Salmonella food isolates
A total of 32 ciprofloxacin-resistant Salmonella strains were subjected to investigation of the mechanisms involved. Contrary to the resistance mechanisms commonly observable in clinical ciprofloxacin resistant strains, in which double and single mutations often occur in the gyrA and parC genes respectively, most of the 32 ciprofloxacin resistant Salmonella strains tested in this work were found to contain either only a single mutation in gyrA, with S83T, S83F, and D87N being the most common amino acid changes, or no mutation in both target genes (Table 2). The few exceptions were all S. Indiana isolates which harbored the double gyrA mutations S83F and D87N, with or without the single parC mutation S80R. It should also be noted that a pair of novel double gyrA mutations which resulted in the H80N and S83T changes, and single parC mutation causing the Q91H substitution, were detectable in a S. Rissen isolate; however, the roles of such mutations in development of Salmonella fluoroquinolone resistance are not well defined at present. Other less common mutations that were detectable include the C72G change in the parC gene product of a S. Indiana strain, and a S83I change in the GyrA protein of a S. Derby isolate. The nature of contribution of these novel mutations to the development of ciprofloxacin resistance in Salmonella needs further investigation. No mutations were detected in gyrB and parE.
The presence of PMQR genes in ciprofloxacin resistant Salmonella isolates were also screened by PCR and sequencing (Table 2). Surprisingly, all isolates were found to carry PMQRs, with oqxAB and aac(6′)-Ib-cr, the most prevalent elements, reaching a rate of 91% and 75% respectively. Other PMQR genes detectable included qnrS (66%), qnrB (16%) and qnrD (3%). The most common PMQR combination observable was aac(6′)-Ib-cr-oqxAB-qnrS2, which accounted for 50% of all the ciprofloxacin resistant Salmonella strains tested. To determine if other resistance mechanisms such as efflux activities contribute to ciprofloxacin resistance in such isolates, the MIC of ciprofloxacin against these isolates was determined in presence and absence of the efflux pump inhibitor, Phenylalanine-arginine β-naphthylamide (PAβN). The results showed that PAβN caused a mild reduction in the MIC level, suggesting that drug efflux only played a partial role in ciprofloxacin resistance development in these organisms (Table 2). Detailed analysis of the relative roles of PMQRs and target gene mutations in conferment of ciprofloxacin resistance phenotypes suggested that several PMQRs including aac(6′)-Ib-cr, oqxAB and qnrS, alone or in combination, could mediate ciprofloxacin resistance development in Salmonella isolates which did not contain target gene mutations. In particular, the presence of four different PMQRs, such as the aac(6′)-Ib-cr-oqxAB-qnrS2-qnrB8 and aac-oqxAB-qnrS2-qnrD combinations, was consistently observable in ciprofloxacin-resistant Salmonella isolates without any target mutations, suggesting that effects of such elements in conferring antibiotic resistance in Salmonella are additive or synergistic in nature (Table 2). It should also be noted that PMQR-mediated ciprofloxacin resistance is commonly associated with a MIC level of 4 to 8 μg/ml, which is comparable to those conferred by target mutations.
Conjugation experiments were performed on these 32 ciprofloxacin-resistant Salmonella isolates to confirm if PMQRs were readily transferable to other Enterobacteriaceae species, using the E. coli J53 strain as recipient. Surprisingly, none of the ciprofloxacin resistance phenotypes tested could be transferred to E. coli, suggesting these PMQR genes may be present on a non-conjugative plasmid or the chromosomal DNA of Salmonella. To test these possibilities, five representative Salmonella strains including S. Derby strains S3, S4 and S38, and S. Typhimurium strains S7 and S71, all exhibiting different PFGE types and harboring different PMQRs, were selected for S1-PFGE and southern hybridization analysis (Fig. 1). Among these isolates, the aac(6′)-Ib-cr gene was shown to be located in both chromosomal DNA and a ~200 kb size plasmid of the S. Derby strain S38, in the chromosome of the S. Derby strain S3 and S. Typhimurium strain S71, and in a ~200 kb plasmid in the S. Derby strain S4 and S. Typhimurium strain S7 (Fig. 1). The oqxAB gene was found to be located in the chromosome of all the three S. Derby strains and the S. Typhimurium strain S71, as well as in the same ~200 kb plasmid of S. Typhimurium strain S7 which also harbored the aac(6′)-Ib-cr gene as aforementioned. The qnrS element was shown to be located in the chromosomal DNA of all S. Derby strains and the S. Typhimurium strain S71, but not in strain S7. Such findings are consistent with the PCR screening results (Fig. 1, Table 2). Hybridization was also performed to probe the location of qnrB that was present in S. Typhimurium strain S71. However the hybridization experiment was not successful even though the presence of qnrB in this strain has been confirmed by both PCR and sequencing.
Figure 1. Analysis of genetic location of specific PMQRs in specific ciprofloxacin resistant strains by S1-PFGE and Southern hybridization.
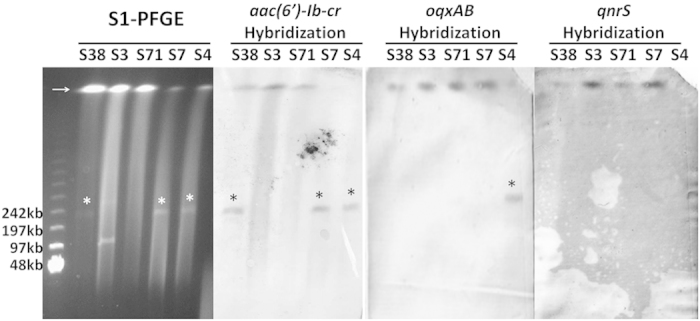
The arrow denotes the position of chromosomal DNA. An asterisk denotes plasmid band in which positive hybridization signal was detectable.
Salmonella isolates that exhibited resistance to both ceftriaxone and ciprofloxacin included three S. Indiana strains, S13, S14 and S16. Again, conjugation experiments failed to transfer either the ciprofloxacin or ceftriaxone resistance phenotype to E. coli J53. Southern hybridization was performed on strains S13 and S14 to determine the genetic location of the blaCTX-M-65 element and the PMQR genes. Our data demonstrated that the blaCTX-M-65, oqxAB and aac(6′)-Ib-cr elements were all located on the same ~200 kb plasmid (Fig. 2). However, hybridization experiment performed to confirm the genetic location of the qnrB gene in strain S14 was not successful even though it was proven to be present in the isolate by PCR.
Figure 2. Analysis of genetic location of the blaCTX-M-65 element and specific PMQRs in specific ciprofloxacin and ceftriaxone resistant strains by S1-PFGE and Southern hybridization.
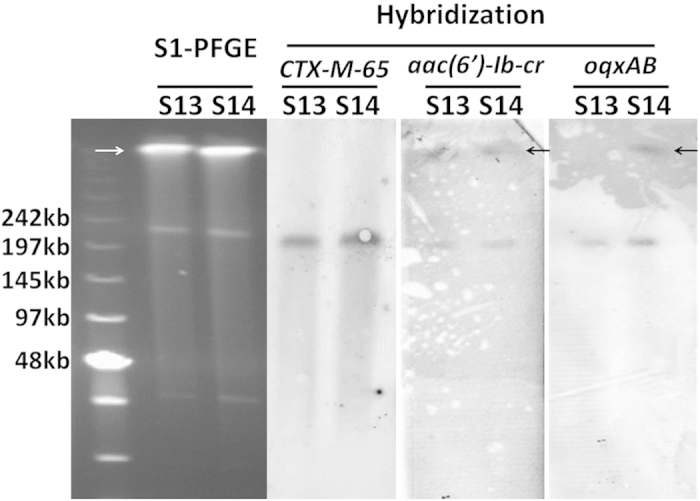
The arrow denotes the position of chromosomal DNA. An asterisk denotes plasmid band in which positive hybridization signal was detectable.
Discussion
PMQRs play an important role in the development of fluoroquinolone resistance in Enterobacteriaceae18,19. These elements have mainly been reported in E. coli strains isolated from various sources and their prevalence has been shown to increase dramatically in recent years. In contrast, PMRQs have only been recoverable from Salmonella since 2005; nevertheless, their prevalence remains extremely low in Salmonella until the emergence of a new PMQR determinant, namely oqxAB, which encoded an efflux pump mediating resistance to olaquindox, chloramphenicol, nalidixic acid and elevated MICs of other antimicrobial reagents including ampicillin and gentamicin16. The oqxAB operon was first found to be present in an IncX1 type plasmid designated as pOLA52, which was recoverable from swine Escherichia coli isolates15,22. More recently, oqxAB was reported to be prevalent in organisms isolated from pork as well as pig farms in China23,24,25. In fact, various lines of evidence suggest that this mobile resistance element already existed in poultry E. coli isolates as early as 199423. On the other hand, oqxAB has not been found in clinical isolates until recently, when it became detectable in clinical strains of E. coli and Klebsiella pneumoniae26,27,28,29.
In Salmonella, oqxAB was first found to be present in the chromosomal DNA of two S. Derby strains of food origin in 201317. Retrospective study of clinical isolates of Salmonella in China revealed that oqxAB could be detected in Salmonella as early as 20066, and that it was often genetically associated with the aac(6′)-Ic-br element, contributing to transmission of drug resistant organisms in clinical setting in both clonal and non-clonal manner. Further studies showed that oqxAB and aac(6′)-Ic-br could greatly facilitate development of fluoroquinolone resistance by abolishing the requirement of target gene mutations, thereby potentially causing a dramatic increase of fluoroquinolone resistance in Salmonella18. Our data confirmed that this is indeed the case, and suggested that by further acquiring other PMQRs in Salmonella strains which already harbored the oqxAB and aac(6′)-Ib-cr elements, fluoroquinolone resistance at a level comparable to that conferred by target mutations is consistently achievable in organisms that do not even harbor a single gyrA mutation. The finding that multiple PMQR elements can simultaneously or synergistically produce a fluoroquinolone resistance phenotype via the mechanisms of enzymatic inactivation, drug efflux, and competitive inhibition of drug binding is intriguing. Since oqxAB or other PMQR determinants that confer reduced susceptibility of the host organism to fluoroquinolones may also enhance the rate of mutational changes in the drug target gene (12), the phenomenon of rapid transmission of PMQR elements among members of Enterobacteriaceae is alarming.
Although our data showed that the PMQRs detectable in Salmonella were often found to be located in chromosome or plasmids that could not be transferred to other bacterial through conjugation, these elements must have been, at some stages, harbored by mobile elements that are capable of transferring its contents to the chromosome of the host strain via transposition events. This was evidenced by the observation that the oqxAB element can be recoverable in both chromosome and mobile elements containing the IS26 element17,18. With the fast progress of oqxAB-associated PMQR evolution in Salmonella, transmission of plasmids mediating fluoroquinolone resistance among Salmonella, or between Salmonella and other bacterial species, may become even more efficient, posing a huge threat to Salmonella infection control in clinical settings. Plasmids carrying the blaCTX-M-65 gene and multiple PMQR cassettes are of particular concern, despite the fact that they are currently restricted to specific strains such as S. Indiana.
Olaquindox has been a widely used growth enhancer in the pig-raising industry since the 1970s30,31. Its antibiotic activity can be attributed to its ability to inhibit DNA synthesis. This agent was previously considered safe since they were not structurally related to any human drug. Findings in this work constitute part of the evidence that the use of olaquindox as growth promoter in the swine industry has resulted in some unexpected consequences, the impact of which only became evident decades later. First, our recent study confirmed that oqxAB actually originated as a chromosomal efflux pump gene of Klebsiella pneumoniae, which was picked up and incorporated into a mobile element by IS26-mediated transposition, presumably under the selection pressure of olaquindox. These events resulted in constitutive expression of the plasmid-borne oqxAB operon. Second, the process of inter-species transmission from E. coli to Salmonella occurred over a period of at least a decade, during which oqxAB was not detectable in clinical Salmonella strains until 2006. Third, amplification of an oqxA –positive S. Typhimurium strain resulted in a sharp increase in the prevalence of oqxAB-borne clinical Salmonella isolates in subsequent years. Finally, our data demonstrated that co-existence of the oqxAB genes with other PMQR elements has become commonplace, leading to emergence of a new category of fluoroquinolone-resistant organisms that exhibit selective advantages in both the environment and clinical settings where antibiotic selection pressure is high. At present, Salmonella strains harboring multiple PMQR/oqxAB elements appear to be confined to zoonotic organisms but the risk of these strains causing human infections is apparently increasing rapidly. To conclude, findings in this work highlight a need to devise specific infection control measures to halt further transmission of the oqxAB/PMQR–borne resistant Salmonella strains, and investigate the impact of other animal growth promoters in selection of both bacterial resistance and virulence determinants in a wide range of foodborne and zoonotic pathogens.
Materials and Methods
Salmonella isolation from retail meat products
Salmonella were isolated from retail meat samples including chicken and pork from supermarkets and wet markets in Shenzhen, China from October 2012 to June 201332. Food samples were collected aseptically in plastic bags and transported on ice to the laboratory for isolation of Salmonella within 6 h. Twenty-five grams of meat samples were placed in a stomacher bag with 100-ml Buffered Peptone Water (BPW) (Difco, Detroit, MI) which was subjected to homogenization for 5 min. The homogenate was incubated at 35 °C for 24 h. One ml aliquot of pre-enriched homogenate was transferred to 10 mL of Tetrathionate broth (Difco) and incubated at 42 °C for 24-h. A loopful of the enriched content was streaked on XLT4 agar and incubated for 24 h to 48 h at 37 °C. One typical Salmonella strain recovered from each sample was purified and subjected to species identification by detection of the invA gene and 16S RNA sequencing. All isolates were serotyped according to the Kauffmann-White scheme, using commercial antiserum (Difco, Detroit).
Antimicrobial susceptibility tests
Confirmed S. Typhimurium isolates were subjected to antimicrobial susceptibility testing using the agar-dilution method, and the results were interpreted according to the CLSI guidelines33. Fourteen antimicrobial agents were tested: ampicillin, cefotaxime, ceftriaxone, amoxicillin/clavulanic acid, sulfamethoxazole, kanamycin, amikacin, gentamicin, tetracycline, chloramphenicol, ciprofloxacin, nalidixic acid, streptomycin, and olaquindox. E.coli strains ATCC 25922 and 35218, Enterococcus faecalis strain ATCC 29212, Staphylococcus aureus strain ATCC 29213 and Pseudomonas aeruginosa ATCC 27853 were used as quality control.
Screening of target gene mutations, oqxAB, and other PMQR genes
The QRDR regions of the gyrA, gyrB, parC and parE genes were amplified by PCR as previously described34, followed by determination of their nucleotide sequences and comparison to the wild-type Salmonella Typhimurium LT2 strain for identification of target gene mutations in the test strains. The presence of the PMQR genes qnrA, qnrB, qnrC, qnrD, qnrS, qepA, oqxAB and aac(6′)Ib-cr, was determined by PCR using primers described previously34,35.
Molecular typing
Clonal relationship between representative Salmonella isolates was examined by pulsed-field gel electrophoresis (PFGE) according to the PulseNet PFGE protocol for Salmonella36. S1-PFGE was conducted to determine the size of large plasmids. Briefly, agarose-embedded DNA was digested with S1 nuclease (New England Bio-Lab) at 37 °C for 1 hr. The restriction fragments were separated by electrophoresis in 0.5 Tris-borate-EDTA buffer at 14 °C for 18 h using a Chef Mapper electrophoresis system (Bio-Rad, Hercules, CA) with pulse times of 2.16 to 63.8 S. Phage Lambda PFGE ladder (New England Biolab) was used as DNA size marker. The gels were stained with GelRed, and DNA bands were visualized with UV transillumination (Bio-Rad). Southern blot hybridization was carried out by following the manufacturer’s instructions of the DIG-High Prime DNA Labeling and Detection Starter Kit II (Roche Diagnostics), using the different PMQR gene and blaCTX-M-64 digoxigenin-labeled probes.
Additional Information
How to cite this article: Lin, D. et al. Increasing prevalence of ciprofloxacin-resistant food-borne Salmonella strains harboring multiple PMQR elements but not target gene mutations. Sci. Rep. 5, 14754; doi: 10.1038/srep14754 (2015).
Acknowledgments
This work was supported by the Chinese National Key Basic Research and Development 973 Program (2013CB127200) and the Health and Medical Research Fund of the Food and Health Bureau, Hong Kong (12111612, 13121412 to SC).
Footnotes
The authors declare no competing financial interests.
Author Contributions D.C.L. performed experiments, analyzed the data and wrote manuscript; K.C.C. performed experiments; E.W.C.C. analyzed the data and edited the manuscript; S.C. designed the experiments, analyzed the data, wrote manuscript and coordinated the whole project.
References
- Gomez T. M., Motarjemi Y., Miyagawa S., Kaferstein F. K. & Stohr K. Foodborne salmonellosis. World health statistics quarterly. Rapport trimestriel de statistiques sanitaires mondiales 50, 81–89 (1997). [PubMed] [Google Scholar]
- Hohmann E. L. Nontyphoidal salmonellosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 32, 263–269, 10.1086/318457 (2001). [DOI] [PubMed] [Google Scholar]
- White D. G. et al. The isolation of antibiotic-resistant salmonella from retail ground meats. N Engl J Med 345, 1147–1154, 10.1056/NEJMoa010315 (2001). [DOI] [PubMed] [Google Scholar]
- Romero L. et al. Characterization of the first CTX-M-14-producing Salmonella enterica serotype Enteritidis isolate. J Antimicrob Chemother 53, 1113–1114, 10.1093/jac/dkh246 (2004). [DOI] [PubMed] [Google Scholar]
- Zhao S. et al. beta-Lactam resistance in salmonella strains isolated from retail meats in the United States by the National Antimicrobial Resistance Monitoring System between 2002 and 2006. Appl Environ Microbiol 75, 7624–7630, 10.1128/AEM.01158-09 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. H., Yan M., Chan E. W., Biao K. & Chen S. Emergence of clinical Salmonella enterica serovar Typhimurium isolates with concurrent resistance to ciprofloxacin, ceftriaxone, and azithromycin. Antimicrobial agents and chemotherapy 58, 3752–3756, 10.1128/AAC.02770-13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C. Emerging mechanisms of fluoroquinolone resistance. Emerging infectious diseases 7, 337–341, 10.3201/eid0702.700337 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. et al. Characterization of multiple-antimicrobial-resistant salmonella serovars isolated from retail meats. Appl Environ Microbiol 70, 1–7 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmolka A. et al. First report on IncN plasmid-mediated quinolone resistance gene qnrS1 in porcine Escherichia coli in Europe. Microbial drug resistance 17, 567–573, 10.1089/mdr.2011.0068 (2011). [DOI] [PubMed] [Google Scholar]
- Gunell M. et al. Mechanisms of resistance in nontyphoidal Salmonella enterica strains exhibiting a nonclassical quinolone resistance phenotype. Antimicrobial agents and chemotherapy 53, 3832–3836, 10.1128/AAC.00121-09 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari R. et al. Plasmid-mediated quinolone resistance by genes qnrA1 and qnrB19 in Salmonella strains isolated in Brazil. J Infect Dev Ctries 5, 496–498 (2011). [DOI] [PubMed] [Google Scholar]
- Ceyssens P. J., Mattheus W., Vanhoof R. & Bertrand S. Trends in serotype distribution and antimicrobial susceptibility in Salmonella enterica isolates from humans in Belgium, 2009 to 2013. Antimicrobial agents and chemotherapy 59, 544–552, 10.1128/AAC.04203-14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abgottspon H., Zurfluh K., Nuesch-Inderbinen M., Hachler H. & Stephan R. Quinolone resistance mechanisms in Salmonella enterica serovars Hadar, Kentucky, Virchow, Schwarzengrund, and 4,5,12:i:-, isolated from humans in Switzerland, and identification of a novel qnrD variant, qnrD2, in S. Hadar. Antimicrobial agents and chemotherapy 58, 3560–3563, 10.1128/AAC.02404-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuesch-Inderbinen M., Abgottspon H., Sagesser G., Cernela N. & Stephan R. Antimicrobial susceptibility of travel-related Salmonella enterica serovar Typhi isolates detected in Switzerland (2002-2013) and molecular characterization of quinolone resistant isolates. BMC Infect Dis 15, 212, 10.1186/s12879-015-0948-2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L. H., Johannesen E., Burmolle M., Sorensen A. H. & Sorensen S. J. Plasmid-encoded multidrug efflux pump conferring resistance to olaquindox in Escherichia coli. Antimicrobial agents and chemotherapy 48, 3332–3337, 10.1128/AAC.48.9.3332-3337 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L. H., Jensen L. B., Sorensen H. I. & Sorensen S. J. Substrate specificity of the OqxAB multidrug resistance pump in Escherichia coli and selected enteric bacteria. The Journal of antimicrobial chemotherapy 60, 145–147, 10.1093/jac/dkm167 (2007). [DOI] [PubMed] [Google Scholar]
- Wong M. H. & Chen S. First detection of oqxAB in Salmonella spp. isolated from food. Antimicrobial agents and chemotherapy 57, 658–660, 10.1128/AAC.01144-12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. H., Chan E. W., Liu L. Z. & Chen S. PMQR genes oqxAB and aac(6′)Ib-cr accelerate the development of fluoroquinolone resistance in Salmonella typhimurium. Frontiers in microbiology 5, 521, 10.3389/fmicb.2014.00521 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T. et al. Fluoroquinolone resistance mechanisms in an Escherichia coli isolate, HUE1, without quinolone resistance-determining region mutations. Frontiers in microbiology 4, 125, 10.3389/fmicb.2013.00125 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrois D. et al. Prevalence and characterization of extended-spectrum beta-lactamase-producing clinical Salmonella enterica isolates in Dakar, Senegal, from 1999 to 2009. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases 20, O109–116, 10.1111/1469-0691.12339 (2014). [DOI] [PubMed] [Google Scholar]
- Le Hello S. et al. Highly drug-resistant Salmonella enterica serotype Kentucky ST198-X1: a microbiological study. The Lancet infectious diseases 13, 672–679, 10.1016/S1473-3099 (2013). [DOI] [PubMed] [Google Scholar]
- Hansen L. H., Sorensen S. J., Jorgensen H. S. & Jensen L. B. The prevalence of the OqxAB multidrug efflux pump amongst olaquindox-resistant Escherichia coli in pigs. Microb Drug Resist 11, 378–382, 10.1089/mdr.2005.11.378 (2005). [DOI] [PubMed] [Google Scholar]
- Chen X. et al. Prevalence of qnr, aac(6′)-Ib-cr, qepA, and oqxAB in Escherichia coli isolates from humans, animals, and the environment. Antimicrobial agents and chemotherapy 56, 3423–3427, 10.1128/AAC.06191-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B. T. et al. Plasmid-mediated quinolone resistance determinants oqxAB and aac(6′)-Ib-cr and extended-spectrum beta-lactamase gene blaCTX-M-24 co-located on the same plasmid in one Escherichia coli strain from China. The Journal of antimicrobial chemotherapy 66, 1638–1639, 10.1093/jac/dkr172 (2011). [DOI] [PubMed] [Google Scholar]
- Zhao J. et al. Prevalence and dissemination of oqxAB in Escherichia coli isolates from animals, farmworkers, and the environment. Antimicrobial agents and chemotherapy 54, 4219–4224, 10.1128/AAC.00139-10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J. et al. Prevalence of the oqxAB gene complex in Klebsiella pneumoniae and Escherichia coli clinical isolates. The Journal of antimicrobial chemotherapy 67, 1655–1659, 10.1093/jac/dks086 (2012). [DOI] [PubMed] [Google Scholar]
- Ruiz E. et al. qnr, aac(6′)-Ib-cr and qepA genes in Escherichia coli and Klebsiella spp.: genetic environments and plasmid and chromosomal location. The Journal of antimicrobial chemotherapy 67, 886–897, 10.1093/jac/dkr548 (2012). [DOI] [PubMed] [Google Scholar]
- Park K. S. et al. Prevalence of the plasmid-mediated quinolone resistance genes, aac(6′)-Ib-cr, qepA, and oqxAB in clinical isolates of extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae in Korea. Annals of clinical and laboratory science 42, 191–197 (2012). [PubMed] [Google Scholar]
- Kim H. B. et al. oqxAB encoding a multidrug efflux pump in human clinical isolates of Enterobacteriaceae. Antimicrobial agents and chemotherapy 53, 3582–3584, 10.1128/AAC.01574-08 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronsch K., Schneider D. & Rigal-Antonelli F. [Olaquindox - a new growth promoter in animal nutrition. 1. Effectiveness in raising piglets]. Zeitschrift fur Tierphysiologie, Tierernahrung und Futtermittelkunde 36, 211–221 (1976). [PubMed] [Google Scholar]
- Kirchgessner M. & Roth F. X. [Olaquindox–a new growth promoter in animal nutrition. III. Activity in fattening calves]. Zeitschrift fur Tierphysiologie, Tierernahrung und Futtermittelkunde 38, 23–28 (1977). [PubMed] [Google Scholar]
- Lin D., Yan M., Lin S. & Chen S. Increasing prevalence of hydrogen sulfide negative Salmonella in retail meats. Food microbiology 43, 1–4, 10.1016/j.fm.2014.04.010 (2014). [DOI] [PubMed] [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing. CLSI document M100-S22. (Clinical and Laboratory Standards Institute, 2012).
- Chen S. et al. Contribution of target gene mutations and efflux to decreased susceptibility of Salmonella enterica serovar typhimurium to fluoroquinolones and other antimicrobials. Antimicrob Agents Chemother 51, 535–542, 10.1128/AAC.00600-06 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y. et al. Dissemination of IncFII plasmids carrying rmtB and qepA in Escherichia coli from pigs, farm workers and the environment. Clin Microbiol Infect 17, 1740–1745, 10.1111/j.1469-0691.2011.03472.x (2011). [DOI] [PubMed] [Google Scholar]
- Ribot E. M. et al. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis 3, 59–67, 10.1089/fpd.2006.3.59 (2006). [DOI] [PubMed] [Google Scholar]


