Abstract
Genetic strategies such as linkage analysis and quantitative trait locus (QTL) mapping have identified a multitude of loci implicated in the pathogenesis of hypertension in the spontaneously hypertensive rat (SHR). While several candidate genetic regions have been identified in the SHR and its control, the Wistar–Kyoto rat (WKY), systematic follow-up of candidate identification with polymorphism discovery has not been widespread. In the current report, we develop a data-mining strategy to identify candidate genes for hypertension in the SHR, and then sequence each gene in the SHR and WKY strains. We integrate blood pressure QTL data, microarray data and data-mining methods. First, we determined the set of genes differentially expressed in SHR and WKY adrenal glands. Next, the chromosomal position of all differentially expressed genes was compared with peak marker position of all reported SHR blood pressure QTLs. We also identified the set of differentially expressed genes with the most extreme fold-change. Finally, the QTL positional candidates and the genes with extreme differential expression were proposed as candidate genes if they had biologically plausible roles in hypertensive pathology. We identified seven candidate genes that merit resequencing (catechol-O-methyltransferase [Comt], chromogranin A [Chga], dopamine beta-hydroxylase [Dbh], electron transferring flavoprotein dehydrogenase [Etfdh], endothelin receptor type B [Ednrb], neuropeptide Y [Npy] and phenylethanolamine-N-methyltransferase [Pnmt]), and then discovered polymorphism in four of these seven candidate genes. Chga is proposed as the strongest candidate for additional functional investigation. Our method for candidate gene identification is portable and can be applied to microarray data from any tissue, in any disease model with a QTL database.
Keywords: Essential hypertension, polymorphism, sequencing, SHR rat strain, WKY rat strain
Introduction
The spontaneously hypertensive rat (SHR) and its control, the Wistar–Kyoto rat (WKY), form the most widely studied inbred, rodent model of genetic hypertension. Though development of these strains began in 1963 (1), understanding of specific genetic and molecular mechanisms of hypertension pathogenesis in the SHR remains incomplete. Tools customarily used to dissect the genetic basis of hypertension in the SHR include linkage analysis and quantitative trait locus (QTL) mapping(2), consomic and congenic line development (3), recombinant inbred strain creation (4), and genetical genomic strategies (5). These approaches have generated a multitude of candidate loci and genes [as many as 105 SHR blood pressure QTLs have been reported and deposited in the Rat Genome Database (RGD), as of April 12, 2010 (6)], yet a limited number of these candidates have been examined for DNA sequence variation (5,7–16). In addition, few investigations of hypertension in the SHR have coupled polymorphism discovery to functional testing of newly identified mutations in order to elucidate genetic and molecular mechanisms of disease processes (5,7). It is expected that the number of reported polymorphisms within SHR hypertension candidate genes will rise with the increasing accessibility and affordability of DNA sequencing technology. Ultimately, a complete map of the SHR and WKY genomes and a catalog of all genetic variation between these strains will become available.
The recent publication of the SHR genome (SHR/OlaIpcv; 10.7-fold coverage), and determination and cataloging of polymorphisms present in comparison with the Brown Norway (BN) reference genome, was a significant advancement in the field of SHR genetics (17). Indeed, a subset of the 3.6 million single nucleotide polymorphisms (SNPs), 343,243 short indels (insertion/deletions), and other types of polymorphisms that differentiate the SHR from BN, was predicted to result in potentially significant functional effects (such as gain or loss of stop codons, frameshifts or non-synonymous coding mutations resulting in amino acid change) and provided meaningful insight into the molecular and genetic basis of disease in the SHR. Researchers as the National Bio Resource Project for the Rat in Japan (NBRP-Rat) genotyped 357 simple sequence length polymorphisms (SSLPs) in 122 inbred rat strains and mapped the phylogenetic relationship between the strains (18). The 91–92% sequence divergence of the SHR and BN genomes and 55–56% divergence of the SHR and WKY genomes, as reported by the NBRP-Rat, revealed that the SHR and WKY genomes are more similar than the SHR and BN genomes. Perhaps this is not surprising, since both SHR and WKY were originally derived simultaneously from the same stock of outbred Wistar rats (1). Polymorphism discovery between SHR and WKY is likely to bolster identification of specific mutations underlying the genetic basis of disease in the SHR.
In the current report, we propose a novel data-mining strategy to identify candidate genes for hypertension in the SHR. Our starting point was genome-wide microarray analysis of adrenal glands in SHR and WKY, and we integrated these data with the wealth of SHR blood pressure QTLs available at the RGD. The adrenal gland is a logical and intriguing tissue for study in hypertension because its secretory products, both cortical and medullary, can directly influence cardiovascular, endocrine and sympathetic function. Adrenocortical mineralocorticoid hormones regulate the reabsorption and secretion of sodium and potassium and, therefore, also modulate blood pressure. Medullary epinephrine and norepinephrine act through G-protein-coupled adrenergic receptors to control sympathetic function, such as the force of contraction of the heart or constriction of blood vessels. We identified seven candidate genes that merit resequencing in the SHR and WKY strains, and discovered polymorphism in four of these seven candidate genes. Chromogranin A (Chga) is proposed as the strongest candidate for continued investigation.
Methods
Transcriptome-wide gene expression analysis by microarray
Age-matched young (4-week-old) SHR (n=3) and WKY (n=3) male rats were obtained from colonies at the University of California, San Diego, in La Jolla, CA, USA. Total RNA was extracted from isolated adrenal glands of the SHR and WKY rats by the RNAzol (guanidinium thiocyanate) method (TelTest, Friendswood, TX, USA), followed by RNAse-free DNAse I (Qiagen, Valencia, CA, USA) treatment to eliminate residual genomic DNA. Integrity of the RNA was confirmed through 28S and 18S rRNA profiles on Agilent (Palo Alto, CA, USA) columns and ethidium bromide-stained gels.
Gene expression in the adrenal gland of each animal (n=3 SHR, n=3 WKY) was measured using Affymetrix RG-U34A rat genome GeneChips and standard protocols, as previously described (19). The RG-U34A chip contained 8740 probe sets (excluding quality controls) corresponding to all full length, annotated rat gene clusters (~6000) from the UniGene database (Build 34) as well as ~3000 expressed sequence tag (EST) clusters. Tab-delimited text files of all chip spot features and probe design information are publicly available on the Affymetrix website: http://www.affymetrix.com. In accordance with MIAME guidelines (http://www.mged.org) (20), the microarray data and a detailed description of experimental conditions and parameters are available at the NCBI Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo) under the following accession numbers: GSE1675, GDS1464. We previously used quantitative real-time-polymerase chain reaction (Q-RT-PCR) to confirm the fidelity of gene expression as determined by Affymetrix GeneChips (19,21).
Candidate gene identification strategy: Differential expression versus positional
Criterion 1
Initially, genes were considered candidates for hypertension and resequencing if they were differentially expressed in SHR and WKY adrenal glands. Adrenal differential expression was determined by two methods: (i) microarray analysis and (ii) literature review. After a comprehensive list of differentially expressed genes was compiled, the list was parsed for genes previously reported as candidates for hypertension. Such genes were identified as candidates for hypertension and resequencing in the SHR. Two other criteria were also imposed on the list of differentially expressed genes to determine additional candidates.
Criterion 2a
The gene was a positional candidate for a blood pressure QTL in the SHR. Positional candidates for SHR blood pressure QTLs were identified through alignment of the chromosomal position of genes differentially expressed in the SHR adrenal gland with the chromosomal position of peak markers for all available SHR blood pressure QTLs. A flat file containing an annotated list of current (as of April 7, 2004) Rattus norvegicus QTLs was downloaded from the RGD (6) FTP server (http://rgd.mcw.edu/pub/) and parsed to identify the peak markers for all SHR or SHR-stroke prone (SHRSP) blood pressure QTLs (79 were identified). Next, the chromosomal position (in base pairs) of the QTL peak markers was determined using the University of California, Santa Cruz, rat genome browser (http://genome.ucsc.edu). Similarly, the chromosomal position of genes differentially expressed in the SHR adrenal gland was determined using Affymetrix RG-U34A microarray annotation (date=December 15, 2003; downloaded from http://www.affymetrix.com) or the University of California, Santa Cruz, rat genome browser. A gene differentially expressed in the SHR adrenal gland was identified as a positional candidate for a SHR blood pressure QTL if its chromosomal base pair position was close (within ~5 Mb) to the chromosomal position of a QTL peak marker and the gene was also a biologically plausible candidate for hypertensive pathology.
Criterion 2b
The gene was among the most extreme in terms of fold-change [i.e. highly underexpressed (<0.25-fold) or highly overexpressed (>4-fold)] and also had a biologically plausible role in hypertensive pathology.
Criteria 2a and 2b were applied independently to form two separate lists of candidate genes. These two lists were then merged to form the final list of candidate genes.
Genomic DNA sequencing across candidate loci
The nucleotide sequence of each candidate gene was downloaded from the University of California, Santa Cruz, Rattus norvegicus genome browser (http://genome.ucsc.edu) (22), June 2003, Baylor 3.1/rn3 assembly. Genomic alignment and annotation of mRNA and coding regions in the genomic assembly was derived from the following reference sequences (RefSeqatNCBI):Chga(NM_021655.1,GI:11527393), Comt(NM_012531.1,GI:6978680),Ednrb(S65355.1, GI:410692), Etfdh (NM_198742.2, GI:52138634), Npy (NM_012614.1, GI:6981285). Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) (23) was used to design polymerase chain reaction (PCR) primers to amplify the 5′-flanking sequence (~1500 base pairs, bp, of proximal promoter), exons (including 5′- and 3′- untranslated regions; UTR), and intron/exon border regions of the candidate genes from genomic DNA of male SHR and WKY rats (from Charles River Laboratories, Wilmington, MA, USA). Primers were designed to produce 500–700 bp amplicons (Table I). Genomic DNA PCR was performed using HotStar Master Mix (Qiagen, Valencia, CA, USA) and 25 ng of genomic DNA. Shrimp Alkaline Phosphatase (Promega, Madison, WI, USA) and Exonuclease I (Fermentas, Hanover, MD, USA) were used to purify genomic DNA PCR amplicons. Sequencing PCR was performed using Big Dye Terminator Version 3.1 (Applied Biosystems, Foster City, CA, USA). Sequencing PCR products were column-purified using multi-screen 96-well plates (Fisher Scientific) and Sephadex G-50 Fine DNA grade beads (Sigma-Aldrich), and then sequenced with the Applied Biosystems 3100 Genetic Analyzer. Polymorphisms were identified and visually confirmed in the raw sequencing data using EditView 1.0.1 software for Mac OS 9 (Applied Biosystems).
Table I.
Candidate gene polymerase chain reaction and sequencing primers.
| Primer pair name | Forward primer sequence (5′→3′) | Reverse primer sequence (5′→3′) | Gene target region |
|---|---|---|---|
| Catechol-O-methyltransferase (Comt) | |||
| Comt_Pa | GTTTCCATGTCTGCTCAGCTC | GCTGGGTGAGCTCATGTGTA | Promoter |
| Comt_Pb | CCCAGTTAGATCCTGGGTTG | GAGATCTCTGTGTCCTTTCTCCT | Promoter |
| Comt_Pc | CAGGTTGATAATAGAGGTTAGTGGT | TGAGGAGGTCCAAGGTTCAG | Promoter |
| Comt_E1 | AAGTGACACCACCATCACGA | TCCTACAAGGACTGCCATACC | Exon 1 |
| Comt_E2 | GGTCAGGGACATGAGAGGAG | CAGGTGCTTAGTGGCTGACA | Exon 2 |
| Comt_E3 | CTCCAGAGCCCCAAAGAGAT | TAAGAGGCCCAAGCTCAGTG | Exon 3 |
| Comt_E4a | GGTTTGCCAAGCCTTCCT | CACTGAAACCCCGTGAAGAT | Exon 4 |
| Comt_E4b | GAGCCCACTATGCAAAATCA | TGCAGAGTAACAGCAGTGTGG | Exon 4 |
| Chromogranin A (Chga) | |||
| Chga_1 | CGGACTCTGAAACTTGTGGTG | TGTACACTATGCTGGGTCATGG | Promoter |
| Chga_2 | CGAGATGGTATTTTGGAGACAG | AGCTGGATATTTTGGGTGTGAG | Promoter |
| Chga_3 | AGTTTCTCATTTAGGGGCATGA | TTCTCTTGATTTCACTCGGTTG | Promoter |
| Chga_4 | GCACACATTGAACTTGTGTGAA | ACAGCAGAAGCGCCAAAG | Promoter |
| Chga_5 | ATGACGTAATTTCCTGGGTGTG | GAGTGCAGAGCTGAAATCAAGTT | Exon 1 |
| Chga_6 | AACTATAGAGCCTGACCCAACC | CTTTCTGCAGTTGCCTAAGGAC | Exon 2 |
| Chga_7 | ATTCGATTGGCCCACAGTAAC | TTGGAAAGGTGTGGTCTTTCTT | Exon 3 |
| Chga_8 | GGGACCCTGAGGTTTGTAGACT | AAGTTCCTTCAGCAAATTCTGG | Intron C |
| Chga_9 | GCAGTAGGAAGGTGATGGACAC | TTAATCTCTTGGGGGCAAGTTA | Exon 4 |
| Chga_10 | CCTCTGGTGTCTTGGACAGATA | GACTGTTGGGAACTGGTCTTTC | Exon 5 |
| Chga_11 | TGAGTGGGTAACTTCAATCCTT | CCACTCATCTTTCACGGTCAT | Intron E |
| Chga_12 | AGAAGGCTGGGCCTAAAGAAGT | ACCGGTCAGGTCATCTTCC | Exon 6 |
| Chga_13 | GTGTGCTTGGCCTTAGAGGTAG | CCTAAGAGGCAAGTCCTGCTAA | Exon 7 |
| Chga_14 | TACAGCGTCCTAGCATTACTGG | ACCCAGCCCAGTGTAGAAATC | Intron G |
| Chga_15 | AGATTCTTTCTCGGAACACAGG | TTTCCAAATTGGGCCTAAGAC | Exon 8 |
| Chga_16 | CTCCTGGACTGTCCCCTAGTTA | ACGTTTAGCATCACCATCTCCT | Exon 8 |
| Chga_17 | CACCACCCAACTTTCCTTTTTA | GACGTCATACAGGTGTCTCCAC | 3′ downstream |
| Chga_18 | AAGAGTCCTCGTCTCCAATGTG | TGCAGGACATAGGAGATGTTTC | 3′ downstream |
| Electron-transferring-flavoprotein dehydrogenase (Etfdh) | |||
| Etfdh_Pa | CCTGACCTACACGCAGAACC | GGGAGAGCGTTGTGGAATAA | Promoter |
| Etfdh_Pb | TGAGCTGAGCTGACAGAACAT | AGGGCTAGCTGGTGATGCTA | Promoter |
| Etfdh_Pc | TGTTGGGAGTCAGGAAGTGA | TCTTCATCGTCCCTCAGCAT | Promoter |
| Etfdh_Pd | GCCCTCAACTCCAAGAACTG | CGTGCGCACTAGAAGCATAG | Promoter |
| Etfdh_Pe | GCCCCATCTTCTCGTTTGT | TCTGTCAGCCTCTGGGATCT | Promoter |
| Etfdh_E1 | TCCCCGCTATGCTTCTAGTG | GTGTTTGCAGTACCCCAGGT | Exon 1 |
| Etfdh_E2 | AACATTCCATTTAGATTTGTGTCAA | TCTGGGATATATATTGGATGCTTT | Exon 2 |
| Etfdh_E3 | TGGTCCTATTAATCCCAGAGTTG | GAGACAGCATAGATTAGACCTTGTG | Exon 3 |
| Etfdh_E4 | TCAGAGGCATATTCACCCAAC | AAATAAAGTCAATATTAAAGCCTGAAA | Exon 4 |
| Etfdh_E5 | TGCAGCAGTACACACTGGTT | TTCAATTCCTCTTTGGATTAGCA | Exon 5 |
| Etfdh_E6 | CCCACCCTTCTTGCTTCA | TGTGCAGTTTGTAGGAAGACCT | Exon 6 |
| Etfdh_E7 | AGTTTTCCTTCCAGTACATAGGTC | GGCAATCATTTGACCTGTTTTAG | Exon 7 |
| Etfdh_E8 | AATTTTGTGCTGCTTTCATGT | CATGCTGGTAAGTTCAAAAGTCA | Exon 8 |
| Etfdh_E9 | TGCCAATTATCCTTTTGCTT | TGTTAAGCTCCTTAAAGTTTTGTCC | Exon 9 |
| Etfdh_E10 | CCCTTTTCCAGGCGTTTACT | ATGTGCTGAAAGGGGACATC | Exon 10 |
| Etfdh_E11 | ATGATTCTGCATGGGTCCAC | GGGGAACAACCCTACAACAA | Exon 11 |
| Etfdh_E12 | ATGAGCTGCCTGTATCACCA | TGAACTGGGAAATTGTTAAATGT | Exon 12 |
| Etfdh_E13 | AAGGAAGGGCTGGAGTCAAT | GGAGCTTAGTAGCACAAGTTTCTGT | Exon 13 |
| Endothelin receptor, type B (Ednrb) | |||
| Ednrb_Pa | AACCTTGAGCACCCGTAATG | CCCTGTGCCCAATATAGAAC | Promoter |
| Ednrb_Pb | GTCATTGGCCCTTCTGACAA | TGTTAGGTGATGTTTTCCCTTTC | Promoter |
| Ednrb_Pc | CAGTAGAAAAGAACACAGGAAAAGTG | TGGGAAGAAAGAAATGTTTATGA | Promoter |
| Ednrb_E1 | AATTTTGCTCAGCTGCCTACA | CAGCAGAAGGCAATGATTCTC | Exon 1 |
| Ednrb_E2 | CATGATCCCTAGCGATTTTAGG | GGGAGTCTTAATTGGCCTCTG | Exon 2 |
| Ednrb_E3 | GGTGCTTTACAGGCAAATCG | TGATCAAGTCTAATTGTATCGGTGA | Exon 3 |
| Ednrb_E4a | AGAGGGGGACATGGAAAGAG | GCTTTCCCGAGGCTTCAT | Exon 4 |
| Ednrb_E4b | TCCTCATCGTGGACAGATAGC | TCAAACATCCAGGCTGTGC | Exon 4 |
| Neuropeptide Y (Npy) | |||
| Npy_Pa | AGACCGGTGCTTTGAATGAC | TGAACACCAATATCCCATCC | Promoter |
| Npy_Pb | TGACCGATGTTACTCCCTGA | TTAAAAGACCAACGCCACTG | Promoter |
| Npy_Pc | CATCCCTATTTAAACAATGCACA | GCAGTCGAGCAAGGTTTTTC | Promoter |
| Npy_Pd | AGTGTTCATTCGGGCGTTAG | GTCTGGAGCCACCCACAC | Promoter |
| Npy_E1 | GCTCCCCAAGTACAGTGTCTG | GGGTCGAACCAGAGTCCA | Exon 1 |
| Npy_E2 | GCCCTCTGCTTCTCACTAGG | TATCCAGTTTGTGGCGTGTG | Exon 2 |
| Npy_E3 | TGAGAATACTTATTAGCTCATGAACAG | CCTTGAAAGTTGAGATTTGCTG | Exon 3 |
| Npy_E4 | GGCAAAAGCTGATGAACTGG | TCATCCACTCATGCCTGCTA | Exon 4 |
Sequencing data was deposited in the Gen-Bank database at the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov) under the following accession numbers: Chga (HM443078, HM443079), Comt (HM443074, HM443075), Ednrb (HM443076, HM443077), Etfdh (HM443072,HM443073) and Npy (HM443070, HM443071).
Results
Candidate gene identification: Differential expression versus positional identification
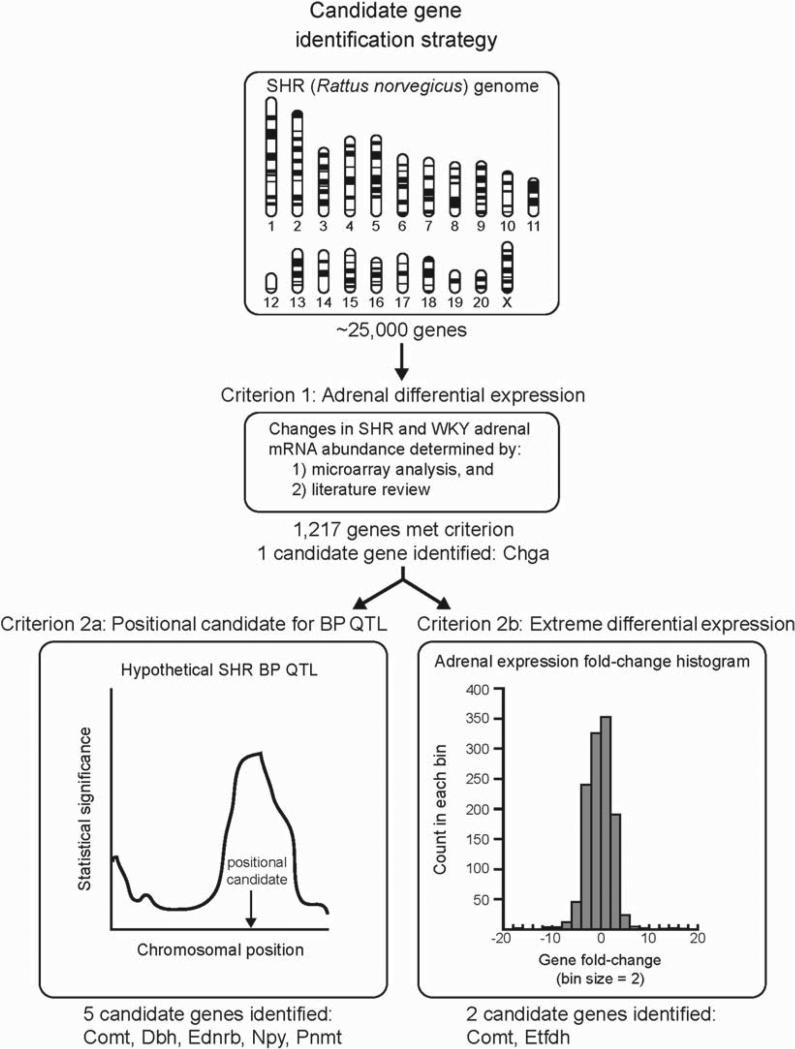
The strategy to identify candidate genes for hypertension and resequencing in the SHR consisted of sequential application of selection criteria (Figure 1). The starting set of genes from which to select candidates for hypertension consisted of the 1217 genes differentially expressed (in terms of mRNA abundance) in SHR and WKY adrenal glands, as determined by review of the scientific literature and analysis of our microarray experiments. From this list of 1217 genes, we selected Chga as a candidate gene since it was overexpressed 1.73-fold (p<0.05) in SHR adrenal gland (24) and substantial evidence has been presented implicating the gene in the pathogenesis of human essential hypertension (25).
Figure 1.
Candidate gene identification strategy. The strategy to select candidate genes for hypertension and resequencing in the spontaneously hypertensive rat (SHR) is depicted. The initial set of genes from which candidates were selected consisted of 1217 genes showing mRNA differential expression between SHR and Wistar–Kyoto (WKY) adrenal glands. One candidate, chromogranin A (Chga), was selected from this set of 1217 genes because substantial evidence suggests it plays a role in pathogenesis of human essential hypertension. Six additional genes were identified as candidates since they were biologically interesting positional candidates for previously published SHR blood pressure (BP) quantitative trait loci (QTL) and/or were among the most highly overexpressed or underexpressed genes with biologically plausible roles in hypertension pathology. A histogram of the fold-change for the 1217 differentially expressed genes is presented (positive fold change indicates overexpression in SHR and was calculated as SHR signal/WKY signal; negative fold-change indicates underexpression in SHR and was calculated as –WKY signal/SHR signal). Examination of the histogram revealed that most differentially expressed genes (~92%) showed a fold change between –4 and +4.
Application of criterion 2a (the gene was a positional candidate for a blood pressure QTL in the SHR) to the set of 1217 differentially expressed genes identified 5 candidates: catechol-O-methyltransferase (Comt), dopamine beta-hydroxylase (Dbh), endothelin receptor type B (Ednrb), neuropeptide Y (Npy), and phenylethanolamine-N-methyltransferase (Pnmt) (Table II). Comt was overexpressed 37.39-fold in SHR adrenal gland and lies within 5.4 Mb (megabases; 1 × 106 bases) of the Bp104 QTL peak (26). Dbh was underexpressed 0.39-fold in SHR and lies within 0.3 Mb of the Bp15 QTL peak (27). Ednrb was overexpressed 2.09-fold in SHR and lies within 0.1 Mb of the Bp126 QTL peak (28). Npy was underexpressed 0.67-fold in SHR and lies within 0.1 Mb of the Bp135 QTL peak (29). Pnmt was underexpressed 0.67-fold in SHR and lies within 13.6 Mb of the Bp1 QTL peak (2).
Table II.
Positional candidates for spontaneously hypertensive rat (SHR) blood pressure quantitative trait loci (QTLs).
| Blood pressure QTL | Cross | Chr | QTL peak marker | Peak marker position (Mb) | Positional candidate | Positional candidate position (Mb) | Positional candidate fold change (SHR/WKY) |
|---|---|---|---|---|---|---|---|
| Bp104 | SHR × Wild | 11 | Sst | 79.2 | Comt | 84.6 | 37.39 |
| Bp15 | SHRSP × WKY | 3 | D3Mgh16 | 6.3 | Dbh | 6.0 | 0.39 |
| Bp126 | SHRSP × WKY | 15 | Ednrb | 87.9 | Ednrb | 87.9 | 2.09 |
| Bp135 | SHR × WKY | 4 | Npy | 78.3 | Npy | 78.3 | 0.67 |
| Bp1 | SHRSP × WKY | 10 | Gh1 | 95.7 | Pnmt | 82.1 | 0.67 |
Differentially expressed genes in the SHR adrenal gland that were positional candidates for SHR blood pressure quantitative trait loci (QTL) are listed. SHRSP, spontaneously hypertensive rat stroke-prone. Column headers: Cross, the rat strains used to generate the QTL; Chr, chromosomal location of the QTL; Peak marker position (Mb), physical position of the QTL peak marker in units of mega-bases (1×106 bases); Positional candidate, symbol of the SHR adrenal differentially expressed gene that was proposed as a positional candidate for the QTL; Positional candidate fold change (SHR/WKY), the adrenal mRNA fold change of the positional candidate.
Application of criterion 2b (the gene was among the most extreme in terms of mRNA fold-change and had a biologically plausible role in hypertension pathology) to the set of 1217 differentially expressed genes identified 2 candidates: Comt and Etfdh (Table III). Etfdh showed a dramatic 0.02-fold (−50-fold) underexpression in SHR adrenal gland and its biological function, transfer of electrons from flavin-containing dehydrogenases to the electron transport chain of the mitochondria, makes it a logical and compelling candidate for the oxidative stress component of hypertensive pathology (30).
Table III.
Candidate genes for hypertension and resequencing in the spontaneously hypertensive rat (SHR).
| Candidate gene | Criterion 1: adrenal mRNA differential expression | Criterion 2a: Positional candidate for SHR BP QTL | Criterion 2b: Extreme mRNA fold-change (SHR/WKY) |
|---|---|---|---|
| Chga | Yes (p=0.017) | No | No (1.73-fold) |
| Comt | Yes (p=4.69E-7) | Yes (Bp104) | Yes (37.39-fold) |
| Dbh | Yes (p=8.32E-6) | Yes (Bp15) | No (0.39-fold) |
| Ednrb | Yes (p=0.035) | Yes (Bp126) | No (2.09-fold) |
| Etfdh | Yes (p=4.76E-9) | No | Yes (0.02-fold) |
| Npy | Yes (p=0.023) | Yes (Bp135) | No (0.67-fold) |
| Pnmt | Yes (p=0.005) | Yes (Bp1) | No (0.67-fold) |
Our selection strategy identified seven genes as candidates for hypertension and resequencing in the SHR. Chga, chromogranin A; Comt, catechol-O-methyltransferase; Dbh, dopamine beta-hydroxylase; Ednrb, endothelin receptor type B; Etfdh, electron transferring flavoprotein dehydrogenase; Npy, neuropeptide Y; Pnmt, phenylethanolamine-N-methyltransferase. All genes were differentially expressed in SHR and Wistar-Kyoto (WKY) adrenal glands and, therefore, satisfied criterion 1. Chga was selected as a candidate since it met criterion 1 and substantial evidence indicates it plays a role in the pathogenesis of human essential hypertension. Comt, Dbh, Ednrb, Npy and Pnmt were selected as candidate genes since they satisfied criterion 2 [they were positional candidates for SHR blood pressure (BP) quantitative trait loci (QTLs)]. Comt and Etfdh were selected as candidate genes since they satisfied criterion 2b [they were among the most highly differentially expressed genes (in terms of fold-change) in the SHR adrenal gland]. Bold text indicates that a candidate gene met a specific criterion.
The final set of candidates for hypertension and resequencing in SHR contained seven genes: Chga, Comt, Dbh, Ednrb, Etfdh, Npy and Pnmt (Table III).
Candidate gene systematic polymorphism discovery
Targeted resequencing of the proximal promoter (~1500 bp), exons (including 5′- and 3′- untranslated regions), and intron/exon borders (splice junctions) of each candidate gene was performed in the SHR and WKY strains (Table IV). A total of 20 polymorphisms were discovered in the promoter, coding region and 3′-UTR of the Chga gene. Five polymorphic sites were discovered in the Chga promoter: −1694 single-base In/Del (insertion/deletion) (WKY allele=deletion, SHR allele=G); A-1616T (WKY allele=A, SHR allele=T); –753 4-bp In/Del (WKY allele=11 consecutive A, SHR allele=15 consecutive A); C-177T (WKY allele=C, SHR allele=T); and C-59T (WKY allele=C, SHR allele=T). Two polymorphisms were identified in the open reading frame (ORF): +6361 24-bp In/Del (exon 5; WKY allele=16 tri-nucleotide “CAG” repeats, or 20 glutamine repeat; SHR allele=8 tri-nucleotide “CAG” repeats, or 12 glutamine repeat); and +8093 In/Del (exon 6; WKY allele= “GAG” , or 16 glutamic acid repeat; SHR allele=deletion, or 15 glutamic acid repeat). One SNP was discovered in the 3′-UTR: G +11177T (or G +174T, with respect to the start of the 3′-UTR) (WKY allele=G, SHR allele=T). Twelve polymorphisms were identified in the introns of Chga: T +413C, C +885G, A + 1113G, A + 1196T, G + 3033T, C + 3168T, + 3386 1-bp In/Del (WKY allele=C, SHR allele=deletion), C + 3863T, T + 3961C, C + 6587T, A + 8388G, and G + 10882A.
Table IV.
Polymorphism discovery in candidate genes.
| Gene | Promoter | 5′-UTR | Exons (ORF) | 3′-UTR | Introns |
|---|---|---|---|---|---|
| Chga | −1694 In/Del A-1616T-753 In/Del C-177T C-59T | None | +6361 In/Del+8093In/Del | G+11177T | T+413C, C+885G, A+1113G, A+1196T, G+3033T, C+3168T, +3386 In/Del, C+3863T, T+3961C, C +6587T, A+8388G, G +10882A (3775/9465 bp; 39.9%) |
| Comt | None | None | None | None | None (297/3470 bp; 8.6%) |
| Ednrb | None | None | None | None | None (958/4589 bp; 20.9%) |
| Etfdh | None | None | None | None | None (1953/19,833 bp, 9.8%) |
| Npy | −1025 In/Del | None | None | None | None (765/6639 bp, 11.5%) |
UTR, untranslated region; ORF, open reading frame; Chga, chromogranin A; Comt, catechol-O-methyltransferase; Ednrb, endothelin receptor type B; Etfdh, electron transferring flavoprotein dehydrogenase; Npy, neuropeptide Y. Polymorphisms discovered in the promoter, 5′-UTR, exons (ORF), 3′-UTR, and introns of Chga, Comt, Ednrb, Etfdh and Npy are listed. Multiple polymorphisms were discovered throughout the Chga locus. No polymorphisms were detected in Comt, Ednrb and Etfdh. One polymorphism was discovered in the Npy locus. Introns were not specifically targeted for polymorphism discovery and the sequencing coverage is indicated in parentheses in the final column (bp sequenced/total bp in all introns; % coverage). Polymorphism nomenclature: [Wistar-Kyoto (WKY) allele] [base pair position] [spontaneously hypertensive rat (SHR) allele]; promoter polymorphism position is indicated in terms of base pairs upstream of the transcriptional start site (5′-cap site); exonic (UTR and ORF) and intronic polymorphism position is indicated in terms of base pairs downstream from the transcriptional start site (5′-cap site); In/Del, insertion/deletion; A, adenine, G, guanine; C, cytosine; T, thymine.
No polymorphisms were detected in Comt, Ednrb or Etfdh. One polymorphism was detected in Npy (promoter polymorphism -1025 3-bp In/Del; WKY allele=23 “TC” di-nucleotide repeats, SHR allele=22 “TC” di-nucleotide repeats). Polymorphisms were also detected in Dbh and Pnmt, and we recently published an in-depth investigation of the functional effects these polymorphisms and their role in abnormal catecholamine biosynthesis and the pathogenesis of hypertension in the SHR (5).
Discussion
Overview
Traditional linkage analysis and QTL mapping of blood pressure in the SHR has implicated many loci in the pathogenesis of the hypertension in this strain. Indeed, as many as 105 SHR blood pressure QTLs have been reported and deposited in the RGD (6) (as of April 12, 2010). Generation of these QTLs is a significant achievement and follow-up investigation of positional candidates for the QTLs holds the potential to yield insight into mechanisms of hypertension. Several investigations have reported polymorphism discovery in candidate genes for hypertension in the SHR (5,7–16), and with the recent increases in accessibility and affordability of DNA sequencing, this number is likely to increase. A limited number of investigations are at the forefront of candidate gene exploration where polymorphism discovery is coupled to functional testing of newly discovered mutations in order to elucidate genetic and molecular processes underlying disease (5,7).
Candidate gene identification
We designed and employed a method to select candidate genes for hypertension in the SHR that utilizes and integrates expertise in sympathoadrenal biology and pathophysiology, with both microarray technology and the RGD repository of SHR blood pressure QTLs. In employing our method, we made several assumptions: 1) genetic mutations contributing to hypertension in the SHR act in cis to alter mRNA transcript abundance; 2) these cis-acting mutations cause mRNA expression changes in the adrenal gland; and 3) the largest adrenal gene expression changes (in terms of fold-change) make the most significant contributions to disease pathophysiology. It is unlikely that all pathogenic genes for hypertension in the SHR satisfy these assumptions; however, these constraints proved valuable in identifying a specific subset of candidate genes with potential for successful follow-up experiments. Since mRNA transcript abundance is arguably the most accurate and direct reflection of cis-acting mutations, our requirement for adrenal mRNA differential expression likely enriched our set of candidates for genes that contain cis-acting regulatory polymorphisms. In this case, polymorphism discovery in the proximal promoter and 5′- and 3′- UTRs was important. Our selection strategy was not designed to identify genes with qualitative changes (e.g. non-synonymous coding polymorphisms resulting in amino acid substitution) nor trans-acting contributors.
Following our sequential strategy to indentify genes for resequencing and polymorphism discovery, we identified 1217 genes as differentially expressed in SHR and WKY adrenal glands (Figure 1). From this set of 1217 genes, we selected Chga as a candidate gene since substantial evidence exists for a role of the gene in the pathogenesis in human essential hypertension (25). In addition, we constrained this set of 1217 differentially expressed genes to those that were positional candidates for SHR blood pressure QTLs (Figure 1, Table II) or to those that showed the most extreme differential expression (i.e. the most highly underexpressed or overexpressed genes). Application of these restraints identified 6 additional candidate genes (Figure 1). In total, seven genes (Chga, Comt, Dbh, Ednrb, Etfdh, Npy and Pnmt) were selected for resequencing and polymorphism discovery (Table III).
Candidate gene polymorphisms
Each candidate gene was resequenced in the SHR and WKY rat strains in order to identify cis-acting polymorphisms within the gene that could contribute to its adrenal mRNA differential expression in vivo, and potentially to hypertension pathophysiology. The proximal promoter and exons (especially the 5′-UTR and 3′-UTR) are likely locations for polymorphisms that alter transcription and mRNA abundance, but it is also conceivable that intronic polymorphisms could affect mRNA levels. For the purposes of the current investigation, however, only the proximal promoter (~1500 bp), exons (including 5′- and 3′- untranslated regions), and intron/exon borders (splice junctions) were targeted for resequencing. A small portion of intronic sequence was obtained incidental to resequencing of exons and exon/intron borders (Table IV); however, introns were neither specifically targeted nor completely sequenced.
Our candidate gene selection strategy was successful: four of the seven candidate genes (Chga, Dbh, Npy and Pnmt) contained polymorphisms in the regions targeted for resequencing (proximal promoter, 5′-UTR, exons, intron/exon borders and 3′-UTR). Publication bias against negative results hinders accurate assessment of the total number of candidate genes for hypertension in the SHR that have been resequenced but lack polymorphism. Nonetheless, a 57% (four of seven) success rate suggests that our strategy was indeed effective in identifying polymorphism-containing candidate genes.
Chga is of particular interest as a candidate gene since accumulating evidence indicates it has a pathogenic role in human essential hypertension (25), and its molecular and physiological functions and mechanisms are well-studied (31). The multiple polymorphisms discovered in the promoter, ORF, and 3′-UTR might impact the quantity and/or the functionality of gene product (mRNA and protein). Changes in the amount or function of Chga protein could impact blood pressure through alterations in catecholamine storage and release (32,33), glucose homeostasis (34) or the inflammatory response (35). In addition to the 1.73-fold elevation of adrenal Chga mRNA in SHR, 2.21-fold elevation of adrenal Chga protein (in SHR vs WKY) has previously been reported (24). Elucidation of the role of the newly discovered polymorphisms in elevation of adrenal Chga mRNA and protein, and ultimately in hypertension, prompts further investigation.
Npy is a biologically intriguing candidate for hypertension in the SHR, since it has diverse effects on the cardiovascular, immune, and central and peripheral nervous systems (36). For example, Npy protein can modulate vasoconstriction and vascular smooth muscle cell proliferation (36). The SHR Npy gene contained one polymorphism, –1025 In/Del in the promoter. It is conceivable that the mutation affects Npy transcription, Npy mRNA abundance, and, ultimately, blood pressure. Additional investigation is required to test the function of this polymorphism and determine if it plays a role in hypertension in the SHR.
Dbh and Pnmt have long been investigated in hypertension pathophysiology since these enzymes catalyze the final two steps in the catecholamine biosynthetic pathway: conversion of dopamine to norepinephrine (Dbh), and norepinephrine to epinephrine (Pnmt). These enzymes have been implicated in development of hypertension in multiple species (e.g. in human patients with essential hypertension (37–41), in the SHR rat (42–45) and in the BPH mouse (19,21)), suggesting a universal role for catecholamines in disease pathology. We recently published an extensive study of the heritability, genetic control, and functional effects of regulatory polymorphisms present in the Dbh and Pnmt genes in the SHR, using the recombinant inbred (RI) strain framework(5). A previous investigation of the Pnmt locus in SHRSP and WKY reported an absence of polymorphisms in the coding, and 5′- and 3′-flanking regions (16).
No polymorphisms were discovered in the Comt, Ednrb and Etfdh genes. Nonetheless, these genes remain interesting for the study of hypertension pathology because of their role in catecholamine metabolism (Comt) (46,47), vasoconstriction (Ednrb) (48), and electron transport and mitochondrial function (Etfdh) (49).
Conclusions and perspectives
Our strategy to identify candidates for hypertension in the SHR identified seven genes: Chga, Comt, Dbh, Ednrb, Etfdh, Npy and Pnmt. While the relationship between genetic contributors to hypertension in the SHR and hypertension susceptibility genes in humans remains incompletely understood, six of the seven genes we identified as candidates in the SHR have been reported to contain polymorphisms that associate with hypertension in humans [Chga (25), Comt (50–52), Dbh (37,38), Ednrb (53), Npy (54) and Pnmt (40,41); none has been reported in Etfdh]. Study of these genes in the SHR might provide insight into the genetic and molecular basis of human hypertension. Polymorphism discovery in these genes in the SHR and WKY rat strains revealed mutations throughout the Chga locus and highlighted the value of resequencing in candidate gene investigation. Whereas genes such as Comt were strong candidates for hypertension in the SHR, lack of polymorphism in these loci suggests they do not play a germ-line pathogenic role. It is possible, however, that important, functional polymorphisms were present outside of the regions specifically targeted for resequencing (here: ~1500 bp of proximal promoter, exons-ORF and UTR, and intron/exon borders). Chga emerged as the strongest candidate for continued investigation, since it contained multiple polymorphisms and can modulate several hypertensive disease phenotypes. Our method for candidate gene identification is portable and easily applied to microarray data from any tissue, in any disease model with an available database of QTLs. Polymorphism identification is a crucial step in determining if candidate genes have pathogenic roles in disease with genetic underpinnings. Functional testing of discovered polymorphisms and elucidation of their genetic and molecular mechanisms remains at the forefront of candidate gene investigation.
Acknowledgments
We thank Theodore W. Kurtz for providing us with SHR and WKY genomic DNA (originally from Charles River Laboratories, Wilmington, MA, USA). Funding was provided by the National Center for Research Resources, M01RR 000827, the Comprehensive Research Center of Excellence in Minority Health and Health Disparities (CRCHD), MD00020, the NIH/NIDDK DK007671 Nephrology Training Grant, Department of Veterans Affairs (DTO), and National Institutes of Health (DK 60702 and P01 HL58120 to DTO).
Footnotes
Declaration of interest: The authors declare no conflicts of interest.
References
- 1.Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- 2.Jacob HJ Lindpaintner K, Lincoln SE, Kusumi K, Bunker RK, Mao YP, et al. Genetic mapping of a gene causing hypertension in the stroke-prone spontaneously hypertensive rat. Cell. 1991;67:213–224. doi: 10.1016/0092-8674(91)90584-l. [DOI] [PubMed] [Google Scholar]
- 3.Graham D, McBride MW, Brain NJ, Dominiczak AF. Congenic/consomic models of hypertension. Methods Mol Med. 2005;108:3–15. doi: 10.1385/1-59259-850-1:003. [DOI] [PubMed] [Google Scholar]
- 4.Printz MP, Jirout M, Jaworski R, Alemayehu A, Kren V. Genetic models in applied physiology. HXB/BXH rat recombinant inbred strain platform: A newly enhanced tool for cardiovascular, behavioral, and developmental genetics and genomics. J Appl Physiol. 2003;94:2510–2522. doi: 10.1152/japplphysiol.00064.2003. [DOI] [PubMed] [Google Scholar]
- 5.Jirout ML, Friese RS, Mahapatra NR, Mahata M, Taupenot L, Mahata SK, et al. Genetic regulation of catecholamine synthesis, storage and secretion in the spontaneously hypertensive rat. Hum Mol Genet. 2010;19:2567–2580. doi: 10.1093/hmg/ddq135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Twigger SN, Shimoyama M, Bromberg S, Kwitek AE, Jacob HJ. The Rat Genome Database, update 2007 – Easing the path from disease to data and back again. Nucleic Acids Res. 2007;35:D658–D662. doi: 10.1093/nar/gkl988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woon PY, Kaisaki PJ, Bragança J, Bihoreau MT, Levy JC, Farrall M, et al. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci USA. 2007;104:14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yagisawa H, Tanase H, Nojima H. Phospholipase C-delta gene of the spontaneously hypertensive rat harbors point mutations causing amino acid substitutions in a catalytic domain. J Hypertens. 1999;9:997–1004. doi: 10.1097/00004872-199111000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Klett CP, Printz MP. Is the M1-muscarinic receptor a candidate gene for hypertension in the spontaneously hypertensive rat? Clin Exp Pharmacol Physiol Suppl. 1995;22:S4–S6. doi: 10.1111/j.1440-1681.1995.tb02962.x. [DOI] [PubMed] [Google Scholar]
- 10.Huang H, Pravenec M, Wang JM, Kren V, St Lezin E, Szpirer C, et al. Mapping and sequence analysis of the gene encoding the beta subunit of the epithelial sodium channel in experimental models of hypertension. J Hypertens. 1995;13:1247–1251. doi: 10.1097/00004872-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Pausova Z, Sedova L, Berube J, Hamet P, Tremblay J, Dumont M, et al. Segment of rat chromosome 20 regulates diet-induced augmentations in adiposity, glucose intolerance, and blood pressure. Hypertension. 2003;41:1047–1055. doi: 10.1161/01.HYP.0000064347.49341.0B. [DOI] [PubMed] [Google Scholar]
- 12.McBride MW, Brosnan MJ, Mathers J, McLellan LI, Miller WH, Graham D, et al. Reduction of Gstm1 expression in the stroke-prone spontaneously hypertension rat contributes to increased oxidative stress. Hypertension. 2005;45:786–792. doi: 10.1161/01.HYP.0000154879.49245.39. [DOI] [PubMed] [Google Scholar]
- 13.Clemitson JR, Dixon RJ, Haines S, Bingham AJ, Patel BR, Hall L, et al. Genetic dissection of a blood pressure quantitative trait locus on rat chromosome 1 and gene expression analysis identifies SPON1 as a novel candidate hypertension gene. Circ Res. 2007;100:992–999. doi: 10.1161/01.RES.0000261961.41889.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham D, McBride MW, Gaasenbeek M, Gilday K, Beattie E, Miller WH, et al. Candidate genes that determine response to salt in the stroke-prone spontaneously hypertensive rat: Congenic analysis. Hypertension. 2007;50:1134–1141. doi: 10.1161/HYPERTENSIONAHA.107.095349. [DOI] [PubMed] [Google Scholar]
- 15.Johnson MD, He L, Herman D, Wakimoto H, Wallace CA, Zidek V, et al. Dissection of chromosome 18 blood pressure and salt-sensitivity quantitative trait loci in the spontaneously hypertensive rat. Hypertension. 2009;54:639–645. doi: 10.1161/HYPERTENSIONAHA.108.126664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koike G, Jacob HJ, Krieger JE, Szpirer C, Hoehe MR, Horiuchi M, et al. Investigation of the phenylethanolamine N-methyltransferase gene as a candidate gene for hypertension. Hypertension. 1995;26:595–601. doi: 10.1161/01.hyp.26.4.595. [DOI] [PubMed] [Google Scholar]
- 17.Atanur SS, Birol I, Guryev V, Hirst M, Hummel O, Morrissey C, et al. The genome sequence of the spontaneously hypertensive rat: Analysis and functional significance. Genome Res. 2010;20:791–803. doi: 10.1101/gr.103499.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mashimo T, Voigt B, Tsurumi T, Naoi K, Nakanishi S, Yamasaki K, et al. A set of highly informative rat simple sequence length polymorphism (SSLP) markers and genetically defined rat strains. BMC Genet. 2006;7:19. doi: 10.1186/1471-2156-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friese RS, Mahboubi P, Mahapatra NR, Mahata SK, Schork NJ, Schmid-Schönbein GW, et al. Common genetic mechanisms of blood pressure elevation in two independent rodent models of human essential hypertension. Am J Hypertens. 2005;18:633–652. doi: 10.1016/j.amjhyper.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 20.Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, et al. Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat Genet. 2001;29:365–371. doi: 10.1038/ng1201-365. [DOI] [PubMed] [Google Scholar]
- 21.Fries RS, Mahboubi P, Mahapatra NR, Mahata SK, Schork NJ, Schmid-Schoenbein GW, et al. Neuroendocrine transcriptome in genetic hypertension: multiple changes in diverse adrenal physiological systems. Hypertension. 2004;43:1301–1311. doi: 10.1161/01.HYP.0000127708.96195.E6. [DOI] [PubMed] [Google Scholar]
- 22.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 24.O'Connor DT, Takiyyuddin MA, Printz MP, Dinh TQ, Barbosa JA, Rozansky DJ, et al. Catecholamine storage vesicle protein expression in genetic hypertension. Blood Press. 1999;8:285–295. doi: 10.1080/080370599439508. [DOI] [PubMed] [Google Scholar]
- 25.Sahu BS, Sonawane PJ, Mahapatra NR. Chromogranin A: A novel susceptibility gene for essential hypertension. Cell Mol Life Sci. 2010;67:861–874. doi: 10.1007/s00018-009-0208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kloting I, Kovacs P, van den Brandt J. Quantitative trait loci for body weight, blood pressure, blood glucose, and serum lipids: linkage analysis with wild rats (Rattus norvegicus). Biochem Biophys Res Commun. 2001;284:1126–1133. doi: 10.1006/bbrc.2001.5091. [DOI] [PubMed] [Google Scholar]
- 27.Clark JS, Jeffs B, Davidson AO, Lee WK, Anderson NH, Bihoreau MT, et al. Quantitative trait loci in genetically hypertensive rats. Possible sex specificity. Hypertension. 1996;28:898–906. doi: 10.1161/01.hyp.28.5.898. [DOI] [PubMed] [Google Scholar]
- 28.Kato N, Mashimo T, Nabika T, Cui ZH, Ikeda K, Yamori Y. Genome-wide searches for blood pressure quantitative trait loci in the stroke-prone spontaneously hypertensive rat of a Japanese colony. J Hypertens. 2003;21:295–303. doi: 10.1097/00004872-200302000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Katsuya T, Higaki J, Zhao Y, Miki T, Mikami H, Serikawa T, et al. A neuropeptide Y locus on chromosome 4 cosegregates with blood pressure in the spontaneously hypertensive rat. Biochem Biophys Res Commun. 1993;192:261–267. doi: 10.1006/bbrc.1993.1408. [DOI] [PubMed] [Google Scholar]
- 30.Suematsu M, Suzuki H, Delano FA, Schmid-Schonbein GW. The inflammatory aspect of the microcirculation in hypertension: Oxidative stress, leukocytes/endothelial interaction, apoptosis. Microcirculation. 2002;9:259–276. doi: 10.1038/sj.mn.7800141. [DOI] [PubMed] [Google Scholar]
- 31.Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 32.Mahapatra NR, O'Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, et al. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahata SK, Mahata M, Fung MM, O'Connor DT. Catestatin: A multifunctional peptide from chromogranin A. Regul Pept. 2010;162:33–43. doi: 10.1016/j.regpep.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang K, Rao F, Wen G, Salem RM, Vaingankar S, Mahata M, et al. Catecholamine storage vesicles and the metabolic syndrome: The role of the chromogranin A fragment pancreastatin. Diabetes Obes Metab. 2006;8:621–633. doi: 10.1111/j.1463-1326.2006.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helle KB. Chromogranins A and B and secretogranin II as prohormones for regulatory peptides from the diffuse neuroendocrine system. Results Probl Cell Differ. 2010;50:21–44. doi: 10.1007/400_2009_26. [DOI] [PubMed] [Google Scholar]
- 36.Pons J, Lee EW, Li L, Kitlinska J. Neuropeptide Y: Multiple receptors and multiple roles in cardiovascular diseases. Curr Opin Investig Drugs. 2004;5:957–962. [PubMed] [Google Scholar]
- 37.Abe M, Wu Z, Yamamoto M, Jin JJ, Tabara Y, Mogi M, et al. Association of dopamine beta-hydroxylase polymorphism with hypertension through interaction with fasting plasma glucose in Japanese. Hypertens Res. 2005;28:215–221. doi: 10.1291/hypres.28.215. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Wen G, Rao F, Zhang K, Wang L, Rodriguez-Flores JL, et al. Human dopamine beta-hydroxylase (DBH) regulatory polymorphism that influences enzymatic activity, autonomic function, and blood pressure. J Hypertens. 2010;28:76–86. doi: 10.1097/HJH.0b013e328332bc87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez-Flores JL, Zhang K, Kang SW, Wen G, Ghosh S, Friese RS, et al. Conserved regulatory motifs at phenylethanolamine N-methyltransferase (PNMT) are disrupted by common functional genetic variation: An integrated computational/experimental approach. Mamm Genome. 2010;21:195–204. doi: 10.1007/s00335-010-9253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui J, Zhou X, Chazaro I, DeStefano AL, Manolis AJ, Baldwin CT, et al. Association of polymorphisms in the promoter region of the PNMT gene with essential hypertension in African Americans but not in whites. Am J Hypertens. 2003;16:859–863. doi: 10.1016/s0895-7061(03)01026-4. [DOI] [PubMed] [Google Scholar]
- 41.Chen A, Chen X, Shi R, Guo Y, Chen L, Xie M, et al. [Association of genetic polymorphism in phenylethanolamine-N-methyl transferase with essential hypertension in Changsha Han people]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2009;34:1120–1125. [PubMed] [Google Scholar]
- 42.Teitelman G, Ross RA, Joh TH, Reis DJ. Differences in utero in activities of catecholamine biosynthetic enzymes in adrenals of spontaneously hypertensive rats. Clin Sci (Lond) 1981;61(Suppl 7):227s–230s. doi: 10.1042/cs061227s. [DOI] [PubMed] [Google Scholar]
- 43.Nagatsu T, Kato T, Numata Y, Ikuta K, Sano M. Norepinephrine-synthesizing enzymes in brain, adrenals and peripheral sympathetic nerves of spontaneously hypertensive rats. Jpn J Pharmacol. 1977;27:531–535. doi: 10.1254/jjp.27.531. [DOI] [PubMed] [Google Scholar]
- 44.Reja V, Goodchild AK, Pilowsky PM. Catecholamine-related gene expression correlates with blood pressures in SHR. Hypertension. 2002;40:342–347. doi: 10.1161/01.hyp.0000027684.06638.63. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen P, Peltsch H, de Wit J, Crispo J, Ubriaco G, Eibl J, et al. Regulation of the phenylethanolamine N-methyltransferase gene in the adrenal gland of the spontaneous hypertensive rat. Neurosci Lett. 2009;461:280–284. doi: 10.1016/j.neulet.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 46.Axelrod J, Tomchick R. Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem. 1958;233:702–705. [PubMed] [Google Scholar]
- 47.Guldberg HC, Marsden CA. Catechol-O-methyl transferase: pharmacological aspects and physiological role. Pharmacol Rev. 1975;27:135–206. [PubMed] [Google Scholar]
- 48.Yasuda H, Kamide K, Takiuchi S, Matayoshi T, Hanada H, Kada A, et al. Association of single nucleotide polymorphisms in endothelin family genes with the progression of atherosclerosis in patients with essential hypertension. J Hum Hypertens. 2007;21:883–892. doi: 10.1038/sj.jhh.1002234. [DOI] [PubMed] [Google Scholar]
- 49.Goodman SI, Binard RJ, Woontner MR, Frerman FE. Glutaric acidemia type II: gene structure and mutations of the electron transfer flavoprotein:ubiquinone oxidoreductase (ETF:QO) gene. Mol Genet Metab. 2002;77:86–90. doi: 10.1016/s1096-7192(02)00138-5. [DOI] [PubMed] [Google Scholar]
- 50.Kamide K, Kokubo Y, Yang J, Matayoshi T, Inamoto N, Takiuchi S, et al. Association of genetic polymorphisms of ACADSB and COMT with human hypertension. J Hypertens. 2007;25:103–110. doi: 10.1097/HJH.0b013e3280103a40. [DOI] [PubMed] [Google Scholar]
- 51.Hagen K, Pettersen E, Stovner LJ, Skorpen F, Holmen J, Zwart JA. High systolic blood pressure is associated with Val/Val genotype in the catechol-O-methyltransferase gene. The Nord-Trondelag Health Study (HUNT). Am J Hypertens. 2007;20:21–26. doi: 10.1016/j.amjhyper.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 52.Annerbrink K, Westberg L, Nilsson S, Rosmond R, Holm G, Eriksson E. Catechol O-methyltransferase val158-met polymorphism is associated with abdominal obesity and blood pressure in men. Metabolism. 2008;57:708–711. doi: 10.1016/j.metabol.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 53.Caprioli J, Mele C, Mossali C, Gallizioli L, Giacchetti G, Noris M, et al. Polymorphisms of EDNRB, ATG, and ACE genes in salt-sensitive hypertension. Can J Physiol Pharmacol. 2008;86:505–510. doi: 10.1139/Y08-045. [DOI] [PubMed] [Google Scholar]
- 54.Bhaskar LV, Thangaraj K, Non AL, Praveen Kumar K, Pardhasaradhi G, Singh L, et al. Neuropeptide Y gene functional polymorphism influences susceptibility to hypertension in Indian population. J Hum Hypertens. 2010;24:617–622. doi: 10.1038/jhh.2009.104. [DOI] [PubMed] [Google Scholar]