INTRODUCTION
Severe sepsis and septic shock are increasing in incidence and are associated with high morbidity and mortality[1]. The development of respiratory failure and acute respiratory distress syndrome (ARDS) are common manifestations of sepsis-associated organ dysfunction[2,3]. Progression to ARDS during the course of sepsis is associated with an increase in mortality and organ failure[2,4]. As such, prevention has become a central focus of reducing the public health burden of ARDS in critical illness[5,6].
ARDS is caused by vascular permeability leading to non-cardiogenic alveolar edema. Multiple studies have demonstrated an association between positive fluid balance and ARDS incidence[7–10]. Quantitative resuscitation strategies, conversely, are typically associated with larger fluid resuscitation volumes. This approach may serve to worsen alveolar edema in the setting of systemic inflammation, yet quantitative resuscitation reduces inflammatory biomarkers and improves clinical outcomes even in mechanically ventilated ARDS patients[11–14]. This suggests that early reversal of global tissue hypoxia promotes pulmonary integrity at an endothelial and epithelial level and that quantitative resuscitation strategies may decrease ARDS incidence.
Data from the emergency department (ED) suggest that modifiable factors (e.g. shock, high tidal volume ventilation) could be targeted to decrease the incidence of ARDS[4,5]. In the setting of sepsis, evidence of hypoperfusion (elevated lactate level) has also been directly associated with ARDS progression in ED patients[15]. Lactate clearance is associated with improved outcome in critically ill patients, and serial lactate monitoring has been shown to be a strategy associated with improved outcomes[16–19]. These data provide rationale for serial lactate monitoring as a possible therapeutic target for ARDS prevention[19]. However, the impact of serial lactate monitoring on the incidence of ARDS has not been investigated.
In this study, we aimed to determine whether serial lactate monitoring in the ED was associated with a reduction in pulmonary complications (e.g. ARDS, new respiratory failure) for patients with severe sepsis and septic shock. We hypothesized that serial lactate monitoring would be associated with a decrease in incidence of pulmonary complications when compared to patients resuscitated without the use of serial lactate monitoring.
MATERIALS AND METHODS
This was a retrospective observational cohort study and preplanned secondary analysis of previously published data, reported in accordance with The Strengthening the Reporting of Observational Studies in Epidemiology Statement: Guidelines for Reporting Observational Studies [19,20]. This study was approved by the Human Research Protection Office at the principal investigator’s institution with waiver of informed consent, approval number 201206130.
This study was conducted at a university-affiliated, 1250-bed urban teaching hospital with an annual ED census of 95,000 patients. The total study period was sixteen months (January – December, 2011; December 2012 – March 2013). Adult patients with severe sepsis or septic shock and an initial ED lactate level ≥ 4 mmol/L were eligible for inclusion[1]. Patients were excluded for ED length of stay < 2 hours, do not resuscitate (DNR)/do not intubate (DNI) status, and transfer outside of hospital network.
Qualifying patients with severe sepsis or septic shock and lactate ≥ 4 mmol/L were identified by query of the electronic medical record. To ensure uniform data collection and accuracy, all variables were defined prior to data extraction and placed in a standardized format during the data collection process. Regular meetings and monitoring of data collection were performed. Data were cross-checked for accuracy with the electronic medical record prior to final data entry.
Baseline patient characteristics included: age, gender, race, weight, height, predicted body weight (PBW), body mass index, comorbidities, vital signs, laboratory values, sequential organ failure assessment (SOFA) score, suspected source of infection, and ED length of stay. PBW in kilograms (kg) was calculated according to the formula: males, 50 + 2.3 [height (inches) – 60]; females, 45.5 + 2.3 [height (inches) – 60].
ED process of care variables included time to antibiotics, intravenous crystalloid volume administered, vasopressor use, packed red blood cell transfusion, corticosteroids, and use of mechanical ventilation. Ventilator-related variables included tidal volume, tidal volume indexed to PBW, peak pressure, and inspiratory plateau pressure.
Definitions
Sepsis was defined as previously described[1]. Suspected source of infection was extracted from the inpatient medical record. The serial lactate cohort (SL) was defined as patients who had a second lactate checked while in the ED. The no serial lactate cohort (NL) was defined as patients that did not have a second lactate checked while in the ED. Lactate clearance was calculated as a percentage and defined as: initial lactate value minus second value divided by initial lactate, then multiplied by 100 [(lactateinitial – lactatesecond)/lactateinitial) × 100]. Lactate clearance was defined as a decrease in lactate of ≥ 20% between the two measured lactate values.
SOFA score was assessed as previously described[21]. A modified SOFA score was used, which omits the neurologic function component of the score[22]. When more than one value was present, SOFA scores were calculated from the most abnormal value. For the calculation of initial SOFA score, if a value was not measured in the ED, then the first value after hospital admission was used to calculate initial SOFA score (only applicable to the bilirubin component of the score). DNR/DNI was defined as documentation of “DNR,” “DNI,” or “comfort care” in the ED record.
Outcomes
The primary outcome of interest was a composite of major pulmonary complications: the development of ARDS or respiratory failure requiring mechanical ventilation after hospital admission. ARDS was defined according to the Berlin definition[23]. Respiratory failure was defined as the initiation of mechanical ventilation after hospital admission. Outcomes were assessed over the first five days after admission. This was done to establish a temporal trend relative to admission from the ED, and previous data indicate that pulmonary complications occur early during intensive care unit stay[24]. An a priori subgroup of interest consisted of patients mechanically ventilated in the ED.
Each investigator reviewed a set of training radiographs prior to the ARDS adjudication process[25]. Study radiographs were then reviewed and categorized as consistent, inconsistent, or equivocal for the diagnosis of ARDS. When agreement existed between investigators, the patient was then deemed acceptable for ARDS adjudication status. When disagreement existed, a third investigator (the study primary investigator) further reviewed the images independently, and agreement was made by consensus. To limit ascertainment bias, each investigator was blinded to all other ARDS adjudication decisions.
Analysis
Descriptive statistics, including mean [± standard deviation (SD)], median [interquartile range (IQR)], and frequency distributions were used to assess the characteristics of the patient cohort. Normality of distribution was tested for by inspection of histograms, testing for skewness and kurtosis in the data, and the Kolmogorov-Smirnov test. Continuous and categorical data were compared using an unpaired t-test, Mann-Whitney U test (for non-normally distributed data), chi-square test, or Fisher’s exact test as appropriate.
To test predictors of the primary outcome, continuous and categorical variables were compared using an unpaired t-test, Mann Whitney U test, chi-square test, or Fisher’s exact test, as appropriate. A multivariable model was created to assess predictors of outcome. A priori variables selected for the model included: 1) those related to illness severity; and 2) those shown in prior studies to be predictors of ARDS in critically ill ED patients. These variables included SOFA score, lactate, vasopressor use, and body mass index [4,15]. Other candidate variables for inclusion in the backward stepwise, multivariable, logistic regression analysis included biologically plausible variables without missing data and statistically significant in univariable analysis at a p≤0.05 level. The stepwise regression method selected variables for inclusion or exclusion from the model in a sequential fashion based on a significance level of 0.05 for entry and 0.05 for removal. Goodness-of-fit of the model was assessed with the Hosmer-Lemeshow test, and residual statistics were examined. Statistical interactions were assessed. Collinearity diagnostics were assessed to test the assumption of no multicollinearity. The model used variables that contributed information that was statistically independent of the other variables in the model. Adjusted odds ratios (aORs) and corresponding 95% confidence intervals (CIs) are reported for variables in the multivariable model, adjusted for all variables in the model. The analysis of mechanical ventilator variables as predictors was restricted to the subgroup patients that were mechanically ventilated in the ED. All tests were two-tailed and a p-value <0.05 was considered statistically significant. As this was a preplanned secondary analysis of previously reported data, we began with a sample size of 243 patients. From previous data, we expected an event rate of approximately 30% for the primary outcome[4,15]. We aimed for an absolute reduction in the primary outcome from 10% to 15%. Therefore, with a total sample size of 243 patients, we were reasonably assured of an adequate sample. Analyses were performed using IBM SPSS Statistics for Windows, Version 21.0 (Armonk, NY: IBM Corp).
RESULTS
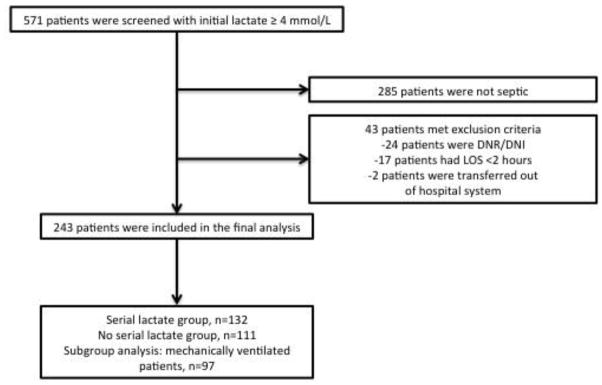
Five hundred seventy-one patients with a lactate of ≥4 mmol/L were screened for inclusion in the study (Figure 1). Two hundred forty-three patients were included in the final analysis. Table 1 shows the baseline characteristics and process of care variables for the study population. Baseline characteristics were similar except for a higher rate of chronic obstructive pulmonary disease (COPD) (22% vs. 9%, p<0.01) and pulmonary source of infection (25% vs. 14%, p=0.05) in the NL group. The NL group received less intravenous crystalloid (2.5 vs. 3.6 liters, p<0.01) and more mechanical ventilation (49.5% vs. 31.8%, p<0.01) when compared to the SL group. Ninety-seven (40%) patients underwent mechanical ventilation in the ED. Median tidal volume in this group was 7.9 mL/kg PBW (IQR 7.1–9.1).
Figure 1.
Table 1.
Baseline characteristics of the study cohort
| Entire cohort (n=243) | Serial Lactate Group (SL, n=132) | No Serial Lactate Group (NL, n=111) | p | |
|---|---|---|---|---|
|
| ||||
| Baseline characteristics | ||||
|
| ||||
| Age, yr | 61.1 (16.7) | 61.6 (15.8) | 60.5 (17.8) | 0.59 |
|
| ||||
| Female, n (%) | 106 (43.6) | 58 (44) | 48 (43) | 0.91 |
|
| ||||
| Race, n (%) | 0.89 | |||
| - Caucasian | 102 (42.0) | 55 (42) | 47 (42) | |
| - Other | 141 (58.0) | 77 (58) | 64 (58) | |
|
| ||||
| Weight (kg) | 77.1 (63.5–90.7) | 77.9 (63.7–81.6) | 77.1 (63.5–95.3) | 0.44 |
|
| ||||
| Height (in) | 67.0 (63.0–70.0) | 67.0 (63.0–70.0) | 66.0 (63.0–70.0) | 0.65 |
|
| ||||
| PBW (kg) | 62.6 (54.7–73.0) | 61.6 (54.7–73.0) | 62.6 (52.4–73.0) | 0.65 |
|
| ||||
| BMI | 26.1 (21.3–31.3) | 25.7 (21.1–29.7) | 27.2 (21.9–32.9) | 0.11 |
|
| ||||
| Comorbidities, n (%) | ||||
|
| ||||
| CHF | 34 (14.0) | 15 (11) | 19 (17) | 0.20 |
|
| ||||
| Diabetes Mellitus | 79 (32.5) | 37 (28) | 42 (38) | 0.10 |
|
| ||||
| COPD | 37 (15.2) | 12 (9) | 25 (22) | <0.01 |
|
| ||||
| ESRD | 18 (7.4) | 8 (6) | 10 (9) | 0.38 |
|
| ||||
| Liver Disease | 32 (13.2) | 17 (13) | 15 (13) | 0.88 |
|
| ||||
| Malignancy | 43 (17.7) | 25 (19) | 18 (16) | 0.58 |
|
| ||||
| Immunosuppression | 24 (9.9) | 13 (9.8) | 11 (9.9) | 0.99 |
|
| ||||
| Alcohol | 21 (8.6) | 15 (11.4) | 6 (5.4) | 0.10 |
|
| ||||
| Temperature, C | 36.7 (1.6) | 36.7 (1.6) | 36.7 (1.5) | 1.0 |
|
| ||||
| SBP | 83.2 (25.2) | 81.5 (23.6) | 85.2 (27.0) | 0.26 |
|
| ||||
| HR | 123.6 (24.5) | 122.4 (24.5) | 125.1 (24.5) | 0.39 |
|
| ||||
| SpO2, (%) | 95.0 (93.0–98.0) | 95.0 (93.0–98.0) | 94.0 (93.0–98.0) | 0.39 |
|
| ||||
| WBC | 14.2 (9.9–19.6) | 14.0 (9.2–18.9) | 14.5 (10.7–20.0) | 0.24 |
|
| ||||
| Hemoglobin | 11.5 (3.0) | 11.6 (2.9) | 11.5 (3.1) | 0.92 |
|
| ||||
| Platelet | 203.0 (137.0–301.0) | 193.0 (125.0–296.0) | 213.0 (146.0–302.0) | 0.49 |
|
| ||||
| Bilirubin | 0.7 (0.4–1.6) | 0.8 (0.4–1.4) | 0.6 (0.3–1.7) | 0.85 |
|
| ||||
| Creatinine | 1.3 (0.9–2.4) | 1.4 (1.0–2.8) | 1.3 (0.9–1.7) | 0.09 |
|
| ||||
| INR | 1.4 (1.2–1.7) | 1.4 (1.2–1.7) | 1.4 (1.1–1.7) | 0.49 |
|
| ||||
| pH | 7.28 (0.14) | 7.28 (0.13) | 7.28 (0.15) | 0.73 |
|
| ||||
| Albumin | 3.1 (0.8) | 3.1 (0.8) | 3.1 (0.7) | 0.81 |
|
| ||||
| Lactate, (mmol/L) | 5.7 (4.6–8.1) | 5.8 (4.6–8.3) | 5.6 (4.7–7.0) | 0.51 |
|
| ||||
| SOFA* | 4.0 (2.0–7.0) | 4.0 (2.0–7.0) | 4.0 (2.0–8.0) | 0.79 |
|
| ||||
| Source of infection, n (%) | ||||
|
| ||||
| Pulmonary | 44 (18.1) | 18 (13.6) | 26 (23.4) | 0.05 |
|
| ||||
| Urinary | 47 (19.3) | 28 (21.2) | 19 (17.1) | 0.42 |
|
| ||||
| Intra-abdominal | 17 (7.0) | 11 (8.3) | 6 (5.4) | 0.37 |
|
| ||||
| Skin/soft tissue | 13 (5.3) | 5 (3.8) | 8 (7.2) | 0.24 |
|
| ||||
| Blood | 22 (9.0) | 13 (9.8) | 9 (8.1) | 0.64 |
|
| ||||
| ED LOS (min) | 407 (298.0–570.0) | 458 (332.0–660.5) | 359 (240.0–472.0) | <0.01 |
|
| ||||
| Process-of-care variables | ||||
|
| ||||
| Time to antibiotics, min** | 145.0 (91.0–247.0) | 135.0 (87.0–258.5) | 150.0 (100.0–243.0) | 0.44 |
|
| ||||
| Intravenous crystalloid, L | 3.1 (1.8) | 3.6 (1.7) | 2.5 (1.8) | <0.01 |
|
| ||||
| Vasopressor use, n (%) | 85 (34.5) | 44 (33) | 41 (37) | 0.77 |
|
| ||||
| pRBC transfusion, n(%) | 44(18.1) | 24 (18) | 20 (18) | 0.97 |
|
| ||||
| Corticosteroids, n (%) | 25 (10.3) | 17 (13) | 8 (7.4) | 0.15 |
|
| ||||
| Mechanical ventilation, n (%) | 97 (39.9) | 42 (31.8) | 55 (49.5) | <0.01 |
|
| ||||
| Tidal volume, mL | 500 (450–550) | 500 (450–500) | 500 (450–562.5) | 0.10 |
|
| ||||
| Tidal volume, mL/kg PBW | 7.9 (7.1–9.1) | 7.7 (7.0–8.9) | 8.4 (7.1–9.4) | 0.14 |
|
| ||||
| Peak pressure, cmH2O (n=81) | 25.0 (22.0–33.5) | 25.0 (22.0–32.5) | 26.5 (21.0–33.5) | 0.80 |
|
| ||||
| Plateau pressure, cm H20 (n=74) | 18.5 (16.0–24.0) | 17.5 (15.0–22.5) | 19.0 (16.0–24.0) | 0.22 |
Continuous variables are reported as mean (standard deviation), and median (interquartile range). PBW: predicted body weight; BMI: body mass index; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; ESRD: end stage renal disease; SBP: systolic blood pressure; HR: heart rate; SpO2: peripheral oxygen saturation; WBC: white blood count; INR: international normalized ratio; SOFA: sequential organ failure assessment; ED: emergency department; LOS: length of stay, pRBC: packed red blood cell
Modified score, which excludes Glasgow Coma Score
All patients in the study received antimicrobial therapy.
The results of the univariate analysis for the primary outcome are presented in e-Table 1. The primary outcome occurred in 28 patients (21.2%) in the SL group and 37 (33.3%) in the NL group, p=0.03. The multivariable analysis for the primary outcome is presented in Table 2. The NL group demonstrated increased pulmonary complications (aOR 2.1, CI 1.15–3.78). Of the SL group (n=132), ninety (68.2%) achieved lactate clearance while in the ED. Lactate clearance was not associated with a decreased incidence of the primary outcome [17 patients (18.9%) vs. 11 patients (26.2%), p=0.34].
Table 2.
Multivariate analysis for factors associated with the development of major pulmonary complications and ARDS
| Outcome Variable | ||||
|---|---|---|---|---|
| Primary composite outcome | Predictor Variable | aOR | 95% CI | p |
| Lactate group | 2.1 | 1.15–3.78 | 0.02 | |
| Albumin | 0.59 | 0.40–0.88 | 0.01 | |
| Initial lactate | 1.1 | 1.01–1.19 | 0.03 | |
| ARDS | Predictor Variable | aOR | 95% CI | p |
| ED mechanical ventilation | 3.5 | 1.80–7.0 | <0.01 | |
| Albumin | 0.6 | 0.40–0.97 | 0.04 | |
ARDS: acute respiratory distress syndrome; ED: emergency department
ARDS developed within the first five days after admission from the ED in 18 patients (13.6%) in the SL group, compared with 28 patients (25.2%) in the NL group, p=0.02. Multivariable logistic regression analysis (Table 2) demonstrated that mechanical ventilation in the ED was independently associated with development of ARDS (aOR 3.5, 1.8–7.0).
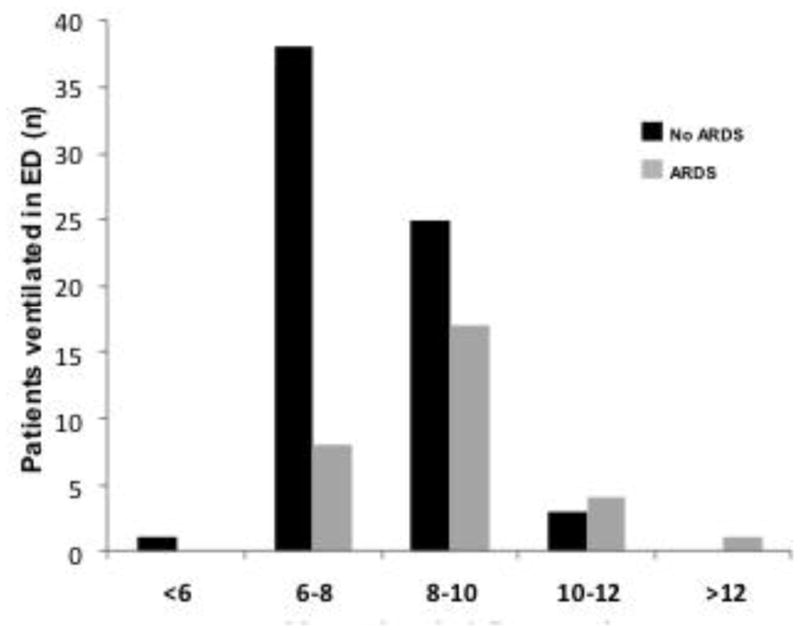
The a priori subgroup was patients receiving mechanical ventilation in the ED (n=97). Patients that developed ARDS received higher tidal volumes compared to patients that did not develop ARDS [8.7 mL/kg PBW (IQR 7.6–9.5) vs. 7.6 (IQR 6.8–9.0), p<0.01]. After multivariable analysis, this was the only independent predictor for ARDS in this subgroup of patients (aOR 1.7, 1.2–2.5) (Figure 2).
Figure 2.
DISCUSSION
In a previous investigation, serial lactate monitoring in patients with severe sepsis and septic shock was associated with improved mortality outside the setting of a randomized trial[19]. The use of serial lactate monitoring seemed to be a surrogate for a “more aggressive” acute resuscitation, as there was a greater use of central venous catheters, as well as monitoring of central venous pressure and mixed venous oxygenation. The results of the current study extend these findings by examining pulmonary complications associated with serial lactate monitoring in the ED.
One of the main findings is that the use of serial lactate monitoring in ED patients with severe sepsis and septic shock is associated with a decrease in major pulmonary complications, including ARDS. Serial lactate monitoring was associated with additional administration of 1.1 liters of intravenous fluid. This would seem to place the SL group at higher risk for pulmonary complications, as several studies have shown an association between positive fluid balance and ARDS incidence, and an association between conservative fluid management and improved outcome in patients with established ARDS[7–10, 26]. The exact opposite was seen in this study, as the SL group had decreased pulmonary complications.
Several hypotheses could explain these findings. Serial lactate monitoring could represent a more targeted resuscitation early in the course of septic shock. Early and aggressive fluid administration could ameliorate global tissue hypoxia, reversing organ failure at the pulmonary level. This is supported by a cohort study of patients with sepsis-associated ARDS which showed that early adequate fluid resuscitation reduced mortality and a randomized trial showing that quantitative resuscitation reduced the need for mechanical ventilation[12,14]. Second, inflammation and increased vascular permeability are pathologic features of both sepsis and ARDS[27,28]. The degree of this systemic inflammation is associated with both progression to ARDS in mechanically ventilated patients, as well as higher lactate levels[13,29]. Lactate clearance has been associated with a decrease in inflammatory biomarkers and improved organ function[13, 30]. It is therefore possible that mitigation of inflammation improved pulmonary function and prevented ARDS and respiratory failure in this study.
The other major finding of the current study is the influence of ED mechanical ventilation on the incidence of ARDS. Forty-six (18.9%) patients in the entire cohort progressed to ARDS after hospital admission. Existing studies demonstrate that mechanical ventilation promotes injury and pulmonary edema [ventilator-associated lung injury (VALI)][31]. In the current study, mechanical ventilation in the ED was an independent risk factor for ARDS, suggesting VALI was present in the ED. Previous research has shown that tidal volume in mechanically ventilated ED patients is higher than recommended[4,5]. In the 97 mechanically ventilated patients in the ED, tidal volume was the only independent risk factor for ARDS progression. This adds to the body of literature pointing to initial tidal volume as a risk factor for ARDS, and extends that finding to the ED[32,33]. Severe sepsis and septic shock are associated with perhaps the highest risk of lung injury, therefore it is of paramount importance to mitigate “second hits” which may contribute to the development of ARDS in this group[3,26]. It appears that the mechanical ventilator may have served as the “second hit” in this cohort.
This study has several implications. By reducing the incidence of major pulmonary complications, serial lactate monitoring may serve a preventive role when implemented in the ED. Our study also suggests that while pulmonary edema is the final common pathway of ARDS, early fluid administration (driven by serial lactate monitoring) may actually serve to prevent pulmonary complications in hypoperfused septic patients. Finally, lung-protective ventilation strategies initiated in the ED may reduce the incidence of ARDS by mitigating early VALI.
LIMITATIONS
This study has important limitations. This is a retrospective observational study and therefore can only examine associations. This study must rely on previous investigations to infer possible mechanisms of benefit and cannot answer why the SL group experienced fewer complications. Reversal of global tissue hypoxia is one hypothesis, yet there was no association between lactate clearance and the primary outcome in this study. This finding is hindered by a small sample, as a 7.3% absolute risk reduction associated with lactate clearance and the primary outcome is an encouraging effect size. The higher event rate for the primary outcome may have been a reflection of imbalance in clinical severity; the NL group was ventilated more and had a pulmonary source of sepsis more frequently. These two facts may have put the NL group at higher risk for pulmonary complications when compared to the SL group. However, other indicators of severity, such as SOFA score, were well balanced, and pulmonary source of infection and COPD failed to show independent association with the primary outcome. While sepsis is by nature a heterogeneous critical care syndrome, this study is composed of a relatively narrow cohort of patients. As such, our results may not be generalizable beyond patients with severe sepsis and septic shock and a lactate level of ≥4 mmol/L. Finally, while the assessment of new respiratory failure was very objective (i.e. need for endotracheal intubation and mechanical ventilation), we do not know why these patients required mechanical ventilation. It is possible that new respiratory failure did not represent worsening pulmonary function secondary to lung injury and inflammation, and that this outcome was an epiphenomenal byproduct of other clinical variables.
CONCLUSION
Serial lactate monitoring and mechanical ventilation seem to be important determinants of major pulmonary complications, including ARDS, in ED patients with severe sepsis and septic shock. These data provide two potential targets for the prevention of pulmonary complications in future studies.
Supplementary Material
Acknowledgments
MRD was supported by the Emergency Medicine Foundation (EMF)/Emergency Medicine Residents’ Assocation (EMRA) Resident Critical Care Research Grant. NMM was supported by the Emergency Medicine Foundation Research Fellowship. BMF was supported by the Washington University Emergency Care Research Core, which receives funding from the Barnes-Jewish Hospital Foundation, as well as the Washington University Institute of Clinical and Translational Sciences grants UL1 TR000448 and KL2 TR000450 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or any of the other supporting bodies.
ABBREVIATIONS
- ARDS
acute respiratory distress syndrome
- aOR
adjusted odds ratio
- COPD
chronic obstructive pulmonary disease
- CI
confidence interval
- DNI
do not intubate
- DNR
do not resuscitate
- ED
emergency department
- IQR
interquartile range
- NL
no serial lactate monitoring
- PBW
predicted body weight
- SL
serial lactate monitoring
- SOFA
sequential Organ Failure Assessment
- VALI
ventilator-associated lung injury
Footnotes
Author contributions: MRD and BMF conceived study design, performed data analysis and interpretation, ARDS adjudication, and writing of the manuscript. NMM contributed to ARDS adjudication and writing of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Matthew R. Dettmer, Email: dettmer-matthew@cooperhealth.edu, Department of Medicine, Division of Critical Care Medicine, Cooper Medical School of Rowan University, Cooper University Hospital, One Cooper Plaza, Camden, NJ 08103.
Nicholas M. Mohr, Email: nicholas-mohr@uiowa.edu, Departments of Emergency Medicine and Anesthesia, Division of Critical Care, Roy J. and Lucille A. Carver College of Medicine, University of Iowa, 200 Hawkins Drive, 1008 RCP, Iowa City, IA 52242.
Brian M. Fuller, Email: fullerb@wusm.wustl.edu, Departments of Emergency Medicine and Anesthesiology, Division of Critical Care, Washington University School of Medicine in St. Louis, St. Louis, MO 63110.
References
- 1.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferguson ND, Frutos-Vivar F, Esteban A, et al. Clinical risk conditions for acute lung injury in the intensive care unit and hospital ward: a prospective observational study. Crit Care. 2007;11(5):R96. doi: 10.1186/cc6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Fuller B, Mohr NM, Dettmer M, et al. Mechanical ventilation and acute lung injury in emergency department patients with severe sepsis and septic shock: an observational study. Acad Emerg Med. 2013;20(7):659–669. doi: 10.1111/acem.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuller BM, Mohr NM, Miller CN, et al. Mechanical ventilation and acute respiratory distress syndrome in the emergency department: a multi-center, observational, prospective, cross-sectional study. Chest. 2015 Mar 5; doi: 10.1378/chest.14-2476. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spragg RG, Bernard GR, Checkley W, et al. Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med. 2010;181(10):1121–7. doi: 10.1164/rccm.201001-0024WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández-Pérez ER, Sprung J, Afessa B, et al. Intraoperative ventilator settings and acute lung injury after elective surgery: a nested case control study. Thorax. 2009;64(2):121–7. doi: 10.1136/thx.2008.102228. [DOI] [PubMed] [Google Scholar]
- 8.Hughes CG, Weavind L, Banerjee A, Mercaldo ND, Schildcrout JS, Pandharipande PP. Intraoperative risk factors for acute respiratory distress syndrome in critically ill patients. Anesth Analg. 2010;111(2):464–467. doi: 10.1213/ANE.0b013e3181d8a16a. [DOI] [PubMed] [Google Scholar]
- 9.Jia X, Malhotra A, Saeed M, Mark RG, Talmor D. Risk Factors for ARDS in Patients Receiving Mechanical Ventilation for> 48 h. Chest. 2008;133(4):853–861. doi: 10.1378/chest.07-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Licker M, de Perrot M, Spiliopoulos A, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg. 2003;97(6):1558–65. doi: 10.1213/01.ANE.0000087799.85495.8A. [DOI] [PubMed] [Google Scholar]
- 11.Jones AE, Brown MD, Trzeciak S, et al. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med. 2008;36(10):2734–9. doi: 10.1097/CCM.0b013e318186f839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy CV, Schramm GE, Doherty JA, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136(1):102–109. doi: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen HB, Loomba M, Yang JJ, et al. Early lactate clearance is associated with biomarkers of inflammation, coagulation, apoptosis, organ dysfunction and mortality in severe sepsis and septic shock. J Inflamm (Lond) 2010;7(6) doi: 10.1186/1476-9255-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 15.Mikkelsen ME, Shah CV, Meyer NJ, et al. The epidemiology of acute respiratory distress syndrome in patients presenting to the emergency department with severe sepsis. Shock. 2013;40(5):375–381. doi: 10.1097/SHK.0b013e3182a64682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen TC, van Bommel J, Schoonderbeek FJ, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752–761. doi: 10.1164/rccm.200912-1918OC. [DOI] [PubMed] [Google Scholar]
- 17.Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA Investigators EMSRN. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303(8):739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puskarich MA, Trzeciak S, Shapiro NI, et al. Whole blood lactate kinetics in patients undergoing quantitative resuscittyion for severe sepsis and septic shock. Chest. 2013;143(6):1548–53. doi: 10.1378/chest.12-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dettmer M, Holthaus CV, Fuller BM. The impact of serial lactate monitoring on emergency department resuscitation interventions and clinical outcomes in severe sepsis and septic shock: an observational cohort study. Shock. 2015;43(1):55–61. doi: 10.1097/SHK.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 21.Jones AE, Trzeciak S, Kline JA. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med. 2009;37(5):1649. doi: 10.1097/CCM.0b013e31819def97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer M, Rüegg S, Czaplinski A, et al. Inter-rater reliability of the Full Outline of Unresponsiveness score and the Glasgow Coma Scale in critically ill patients: a prospective study. Crit Care. 2010;14(2):R64. doi: 10.1186/cc8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 24.Gajic O, Dabbagh O, Park PK, et al. Early Identification of patients at risk of acute lung injury. Am J Respir Crit Care Med. 2011;183(4):462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 26.Wiedemann H, Wheeler A, Bernard G, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 27.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 28.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 29.Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010;14(1):R1. doi: 10.1186/cc8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen HB, Rivers EP, Knoblich BP, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32(8):1637–1642. doi: 10.1097/01.ccm.0000132904.35713.a7. [DOI] [PubMed] [Google Scholar]
- 31.Dreyfuss D, Saumon G. Ventilator-induced lung injury. Lessons from experimental studies. Am J Respir Crit Care Med. 1998;157(1):294. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 32.Fuller BM, Mohr NM, Drewry AM, Carpenter CR. Lower tidal volume at initiation of mechanical ventilation may reduce progression to acute respiratory distress syndrome-a systematic review. Crit Care. 2013;17(1):R11. doi: 10.1186/cc11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neto AS, Cardoso SO, Manetta JA, et al. Association Between Use of Lung-Protective Ventilation With Lower Tidal Volumes and Clinical Outcomes Among Patients Without Acute Respiratory Distress SyndromeA Meta-analysisProtective Ventilation and Lower Tidal Volumes. JAMA. 2012;308(16):1651–1659. doi: 10.1001/jama.2012.13730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.