Abstract
OBJECTIVE
Patients with brain arteriovenous malformation (AVM) are at life-threatening risk of intracranial hemorrhage (ICH). Identification of genetic variants associated with increased new ICH risk would facilitate risk stratification and guide therapeutic intervention.
METHODS
Brain AVM patients evaluated at University of California, San Francisco or Kaiser Permanente Northern California were followed longitudinally. Primary outcome was new ICH after diagnosis; censoring events were any AVM treatment or last follow-up examination. The association of ApoE ε2 and ε4 genotype with new ICH was evaluated by Kaplan-Meier survival analysis and further characterized via a Cox proportional hazards model.
RESULTS
We genotyped 284 brain AVM patients (50% women; 57% Caucasian; median follow-up time, 0.3 yr) including 18 patients with a history of new ICH). ApoE ε2, but not ApoE ε4 genotype, was associated with new ICH (P = 0.0052). ApoE ε2 carriers had fivefold increased risk of new ICH (hazard ratio, 5.09; 95% confidence interval, 1.46–17.7; P = 0.010; Cox proportional hazards model adjusting for race/ethnicity and clinical presentation). Subset analysis in the largest homogenous ethnic subcohort (Caucasians) confirmed the increased risk of new ICH in ApoE ε2 carriers (hazard ratio, 8.71; 95% confidence interval, 1.4–53.9; P = 0.020; multivariate model adjusting for clinical presentation).
CONCLUSION
ApoE genotype may influence the risk of ICH in the natural course of brain AVM. The identification of genetic predictors of ICH risk may facilitate estimation of AVM natural history risk and individualize clinical decision-making and therapeutic recommendations.
Keywords: Cerebral hemorrhage, Genetic epidemiology, Vascular malformations
Patients with brain arteriovenous malformations (AVMs) are at life-threatening risk of intracranial hemorrhage (ICH) (4, 7). Clinical presentation of a brain AVM with ICH is associated with increased risk of new ICH in the natural course (8). Additional predictors of future ICH risk, particularly in brain AVM patients with unruptured lesions, would facilitate ICH risk stratification and therapeutic decision-making.
Genetic variation may influence the clinical course of AVMs. We previously reported that the −174 G>C promoter polymorphism of interleukin 6 (IL6) is associated with presenting ICH at diagnosis (20). A promoter polymorphism in another inflammatory cytokine, tumor necrosis factor α (TNFα), may be associated with risk of new ICH in the natural course of AVM (1).
Apolipoprotein E (ApoE) genotype has been implicated in many human disease phenotypes including ICH and subarachnoid hemorrhage (6, 15, 18). Presence of the ApoE ε2 or ε4 variant increases recurrent lobar ICH risk in patients with cerebral amyloid angiopathy (19, 22). In the present study, we investigated whether ApoE genotype is associated with risk of new ICH in the natural course of brain AVM.
PATIENTS AND METHODS
Patients
Brain AVM patients enrolled at the University of California San Francisco (UCSF) (10, 20) and Kaiser Permanente Northern California (KPNC) provided informed consent for genotyping (9). Demographic and clinical data (initial presentation, treatment history, follow-up and outcome, including ICH occurring after initial diagnosis) were collected. AVM size and venous drainage pattern were classified using standard guidelines (13).
The primary outcome was occurrence of new ICH, defined as evidence of new hemorrhage as a symptomatic event with signs of new intracranial blood on computed tomographic or magnetic resonance imaging, after initial presentation but prior to any intervening treatment. Clinical presentation leading to diagnosis was coded dichotomously as hemorrhagic (ruptured) or non-hemorrhagic (unruptured). The period at risk for analyses was defined from date of initial AVM diagnosis to date of an event, i.e., onset of new (first or subsequent) ICH or censoring due to initiation of first AVM treatment (surgery, embolization, or radiosurgery) or loss to follow-up (using date of last available follow-up). We also examined time to first AVM treatment, which was the major reason for censoring in the survival analysis.
Genotyping
The two single nucleotide polymorphisms in ApoE, Cys112Arg (T>C) and Arg158Cys (C>T) that determine ApoE ε2/ε3/ε4 genotype (6) were genotyped by template-directed dye-terminator incorporation assay (12, 20). Analyses compared ApoE ε2 carriers (ApoE ε2+) against all other genotypes (ApoE ε2−), or ε4 carriers (ApoE ε4+) against all other genotypes (ApoE ε4−).
Statistical Analysis
We examined the association between incidence rate of new hemorrhage in natural course and ApoE genotype by Kaplan-Meier survival analysis and log rank test. Genotypes significantly associated with new ICH incident rate after Bonferroni adjustment for 2 tests (P = 0.05/2 = 0.025) were selected for Cox regression analysis. Association of ApoE genotype with initial presentation with ICH was evaluated by univariate logistic regression, with odds ratio reported.
We ran a full multivariate model including all measured covariates: ApoE genotype, age at diagnosis (yr), sex, race/ethnicity (Caucasian versus non-Caucasian), initial presentation (presenting ICH versus other), venous drainage pattern (exclusively deep versus other), and AVM size (largest dimension < 3 cm versus ≥3 cm). We also examined the effects of ApoE genotype and the previously reported TNFα −238G>A genotype (1) together in a multivariate model. The final multivariate model was selected for maximum parsimony and included ApoE genotype, race/ethnicity and initial presentation. Hazard ratios (HR) and confidence intervals (CI) were reported. The primary analyses were repeated within each racial subgroup to investigate the relationship between ethnicity and the effect of ApoE genotype.
RESULTS
We genotyped ApoE in 284 patients with brain AVM (50% women; 57% Caucasian; median follow-up time, 0.3 yr [25–75 percentile; 0.04–1.4 yr]), including 18 patients with new ICH. New ICH was not associated with any demographic and clinical AVM characteristics (Table 1), nor with cohort (UCSF versus KPNC) (log rank test, P = 0.12). The majority of patients were censored because of treatment after diagnosis (median time to treatment, 0.32 yr among patients presenting with ICH, 0.68 yr among patients presenting without ICH).
TABLE 1.
Association of new arteriovenous malformation hemorrhage with demographic and arteriovenous malformation characteristics and Apolipoprotein E genotypea
| New ICH events | No new ICH events | Total | New ICH (%) | P-value | |
|---|---|---|---|---|---|
| Race | |||||
| Caucasian | 11 | 151 | 162 | 6.8 | |
| African American | 2 | 10 | 12 | 16.7 | |
| Asian/Pacific Islander | 4 | 58 | 62 | 6.5 | |
| Hispanic | 1 | 35 | 36 | 2.8 | |
| Other/unknown | 0 | 12 | 12 | 0.0 | |
| Total | 18 | 266 | 284 | 6.3 | 0.72 |
| Sex | |||||
| Female | 9 | 134 | 143 | 6.3 | |
| Male | 9 | 132 | 141 | 6.4 | |
| Total | 18 | 266 | 284 | 6.3 | 0.98 |
| Age (yr) | |||||
| Mean ± SD | 36.8 ± 16.8 | 36.3 ± 17.3 | 36.3 ± 17.2 | ||
| No. | 18 | 266 | 284 | 0.17 | |
| Size of AVM | |||||
| < 3 cm | 5 | 104 | 109 | 4.6 | |
| > 3 cm | 10 | 98 | 108 | 9.3 | |
| Total | 15 | 202 | 217 | 6.9 | 0.19 |
| Venous drainage | |||||
| Any superficial | 12 | 183 | 195 | 6.2 | |
| Deep only | 2 | 38 | 40 | 5.0 | |
| Total | 14 | 221 | 235 | 6.0 | 0.45 |
| Presentation | |||||
| ICH | 6 | 109 | 115 | 5.2 | |
| Non-ICH | 12 | 157 | 169 | 7.1 | |
| Total | 18 | 266 | 284 | 6.3 | 0.49 |
| ApoE ε2 | |||||
| ε2− | 14 | 234 | 248 | 5.6 | |
| ε2+ | 4 | 32 | 36 | 11.1 | |
| Total | 18 | 266 | 284 | 6.3 | 0.0052 |
| ApoE ε4 | |||||
| ε4− | 13 | 197 | 210 | 6.2 | |
| ε4+ | 5 | 69 | 74 | 6.8 | |
| Total | 18 | 266 | 284 | 6.3 | 0.57 |
ICH, intracranial hemorrhage; SD, standard deviation; AVM, arteriovenous malformation; ApoE, Apolipoprotein E. Baseline characteristics are compared between patients suffering new ICH in the natural course and those who did not have a new ICH in longitudinal follow-up. Some subgroups do not sum to 284 because of missing data. P, log rank test of incident rate with new AVM hemorrhage for categorical variables, log rank test for race/ethnicity (Caucasian versus non-Caucasian), Cox regression for continuous variables.
Genotype distributions of the two single nucleotide polymorphisms that make up the ApoE ε2/ε3/ε4 genotypes (see above in the Methods section) were consistent with Hardy-Weinberg equilibrium among all race-ethnic subgroups (P > 0.15).
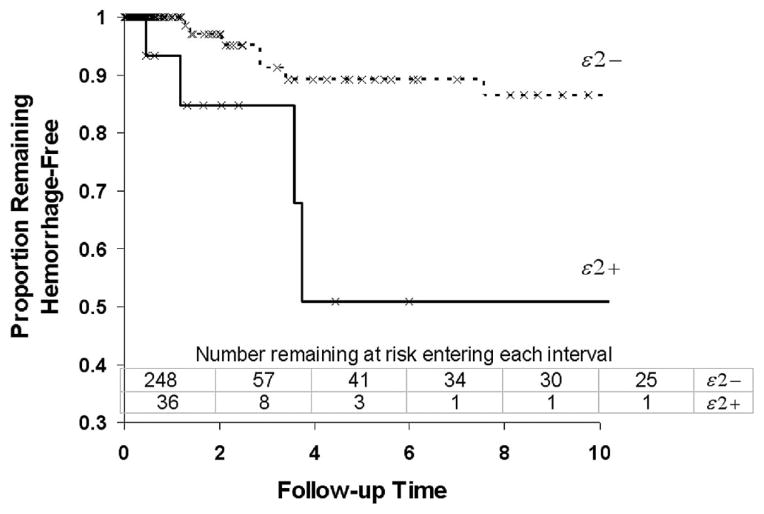
Kaplan-Meier survival analysis revealed that presence of the ApoE ε2 allele (ApoE ε2+, either one or two copies), was associated with new ICH (P = 0.0052, log-rank test, Table 1 and Fig. 1). No significant effect was observed for ApoE ε4 (Table 1). Neither ApoE ε2 nor ApoE ε4 was significantly associated with ICH diagnosis on initial presentation (univariate logistic regression, ApoE ε2+: odds ratio, 1.37; P = 0.38; ApoE ε4+: odds ratio, 0.80; P = 0.42).
FIGURE 1.
Kaplan-Meier survival analysis of new ICH during AVM clinical course before treatment, by ApoE ε2 carrier status. ApoE ε2 carriers are at greater risk for subsequent hemorrhage than non-carriers (log rank, P = 0.0052). Censored events (treatment, death, or loss to follow-up) are indicated by tick marks on survival curves.
Multivariate Cox proportional-hazards regression analysis controlling for race/ethnicity and presenting ICH revealed that ApoE ε2 carriers were at a fivefold increased risk of new ICH (HR, 5.09; 95% CI, 1.46–17.7; P = 0.010; Table 2).
TABLE 2.
Impact of ApoE ε2+ genotype on risk of new intracranial hemorrhage in the natural course of arteriovenous malformations
| Cox proportional hazard estimates | Variables in model | P-value | HR | 95% CI |
|---|---|---|---|---|
| Univariate | ApoE ε2+ | 0.012 | 4.97 | 1.43–17.3 |
| Multivariate | ApoE ε2+ | 0.010 | 5.09 | 1.46 –17.7 |
| Caucasian race | 0.80 | 0.88 | 0.32–2.42 | |
| Presentation with ICH | 0.50 | 1.46 | 0.49 – 4.40 |
HR, hazard ratio; CI, confidence interval; ApoE, Apolipoprotein E; ICH, intracranial hemorrhage. Multivariate model includes effect of ApoE ε2+ controlling for race/ethnicity (Caucasian versus non-Caucasian) and initial presentation (ICH versus other). Data for all fields were complete (n = 284), see Table 1 for frequencies.
Subset analysis in patients of Caucasian race/ethnicity (the largest homogenous ethnic subcohort) confirmed the increased risk of new ICH in ApoE ε2 carriers (univariate: HR, 9.44; 95% CI, 1.53–58.1; P = 0.016; multivariate controlling for initial presentation: HR, 8.71; 95% CI, 1.4–53.9; P = 0.020). Although there were not enough patients to achieve significance, the direction and magnitude of the effect of ApoE ε2 on risk of new ICH in Hispanics was comparable to Caucasians (multivariate model, data not shown). We did not have enough patients of African-American or Asian ethnicity and ApoE ε2 genotype to evaluate the effect of ApoE ε2 within those subgroups.
We previously reported that the AG genotype of the TNFα −238 G>A polymorphism is associated with new ICH in brain AVM patients (1). To examine the combined effect of the two polymorphisms and new ICH, we entered both risk genotypes in a multivariate model. Both ApoE ε2+ and TNFα −238 AG remained significantly associated with new ICH in a multivariate model, suggesting that the two polymorphisms are independent predictors of ICH risk. However, with the small number of new ICH patients, we did not have the power to fully characterize a possible interaction.
DISCUSSION
We report for the first time an association between ApoE genotype and the clinical behavior of brain AVMs. In our study, carriers of the ApoE ε2 allele were at a fivefold in-creased risk of new ICH in the natural course of AVM. This association may be of use in developing more sophisticated risk-stratification methods for balancing the risks and benefits of surgical therapy.
ApoE is involved in several signaling cascades, including cholesterol transport, lipoprotein metabolism, and neuronal sprouting (6, 14). Numerous studies have implicated the ApoE ε4 allele in poor ICH outcomes (14, 15, 18). Both ε2 and ε4 were associated with increased risk of recurrent ICH in lobar amyloid angiopathy (19, 22). In contrast, in our study of AVM patients, only ApoE ε2 was found to have a significantly increased new ICH risk. This may reflect insufficient power to detect an association with ApoE ε4; another possibility is that ApoE ε2-specific mechanisms influence AVM hemorrhage, although such mechanisms remain speculative at this point.
For example, ApoE interacts with the plasminogen activation cascade in an allele-specific manner. The addition of exogenous ApoE ε2 can enhance tissue plasminogen activator (tPA)-induced clot lysis, whereas ε4 decreases clot lysis and ε3 has no effect (3, 5). Supplemental ApoE ε2 reduces clotting even in the absence of tPA (2). tPA and ε2 form a tight quaternary structure distinct from a looser tPA-ε4 complex and a non-specific tPA-ε3 complex, and these interactions modulate tPA proteolytic activity (2). Thus, enhanced proteolytic activity in ApoE ε2 carriers might contribute to AVM bleeding.
Through its effects on the plasminogen activation system, ApoE ε2 may also influence activation of the matrix metalloproteinase (MMP) cascade, resulting in increased MMP9 activity (16). MMP9 levels and activity are higher in AVM vessels compared with normal brain vessels, (11) consistent with abnormal vascular remodeling as the underlying pathological mechanism. Thus, although ApoE genotype likely influences AVM ICH through nonspecific mechanisms relevant to all brain hemorrhage, it may also exert AVM-specific effects via the MMP pathway.
In the present study, we found ApoE ε2 to be associated with new ICH in the natural course of AVM, but not with ICH presentation. It may be that there is an interaction between effect of ApoE genotype and disease progression; it is also possible that this study was underpowered to detect a smaller effect of ApoE genotype on ICH presentation. The small number of new ICH events is a limitation of our study, and constrains our ability to assess confounding effects through more complex models. Nevertheless, the effect of ApoE ε2 in predicting new ICH is not confounded by clinical and demographic factors, and its magnitude and significance level remain consistent under different sensitivity analyses, while the confidence interval, though wide, bounds the ApoE ε2 HR at greater than 1.40.
Finally, a large proportion of patients come to treatment early, within the first year. This is the main reason for censoring and contributes to the small number of new ICH events. However, because this censoring occurs very early in the follow-up period, we do not think it introduces a significant bias to the results reported.
Selecting which patients to recommend for AVM resection is one of the most difficult clinical challenges in their management, demanding a careful evaluation of the AVM anatomy: the patient’s presentation, neurological condition, expectations, and emotions; the neurosurgeon’s technical skills and experience; and the results of the multidisciplinary treatment team. At the most basic level, it requires an evaluation of the likelihood of AVM hemorrhage in a patient’s lifetime to determine the risk of conservative management, and an evaluation of possible complications associated with each element of treatment (embolization, surgical resection, and/or radiosurgery) to determine the risk of aggressive management. A comparison of these risks then leads to a recommendation. The risks associated with therapeutic interventions have been carefully analyzed; for example, tools like the Spetzler-Martin grading system provide some estimation of surgical risk.
Whereas surgical risk can be stratified by Spetzler-Martin grade, no such analysis has been published for natural history risk, although there are clearly subgroups of AVM patients that are at higher risk for future spontaneous hemorrhage; the strongest predictor is clinical presentation with bleeding (9, 17). Clinicians are left estimating a patient’s life expectancy from actuarial tables, selecting an aggregate annual rate of hemorrhage between 2 and 4%, and then calculating a cumulative lifetime risk of harm using crude tables and simplified formulas. This approach to risk prediction is not ideal, particularly when the magnitude of this clinical decision is considered.
The identification of genetic polymorphisms offers an appealing strategy for furnishing risk information that is potentially more robust with less inter-observer variance than traditional radiographic, morphological descriptors. Screening for polymorphisms might refine the process of estimating an AVM’s natural history risk and individualize this part of clinical decision-making. Clinicians will be able to recommend treatment with confidence in patients who harbor ominous markers, and patients will undoubtedly make better, more informed choices regarding therapy. Patients with these polymorphisms might be more comfortable with intervention and its attendant risks, while those without these polymorphisms might be more assured that observation is an appropriate course. Genetic testing could thereby transform the process of patient selection into a more rational process. In a similar manner, stratification of hemorrhage risk has facilitated the management of other hemorrhagic lesions in the brain, like aneurysms and their size (21) and dural arteriovenous fistulae and their Borden classification.
Acknowledgments
The following members of the UCSF Brain Arteriovenous Malformation Study Project (<http://avm.ucsf.edu>) contributed to portions of this work: Nancy Quinnine, Guo-Yuan Yang, Yongmei Chen, Frankye Pang, Tomoki Hashimoto, Eric Theise, Brad Dispensa, Van V. Halbach, Randall T. Higashida, and Christopher F. Dowd. This study was supported in part by PHS grants: R01 NS34949, R01 NS41877, P01 NS44155.
Contributor Information
Ludmila Pawlikowska, The Cardiovascular Research Institute, University of California, San Francisco, California
K.Y. Trudy Poon, Center for Cerebrovascular Research, and Department of Anesthesia and Perioperative Care, University of California, San Francisco, California
Achal S. Achrol, Center for Cerebrovascular Research, and Department of Anesthesia and Perioperative Care, University of California, San Francisco, California
Charles E. McCulloch, Department of Epidemiology and Biostatistics, University of California, San Francisco, California
Connie Ha, The Cardiovascular Research Institute, University of California, San Francisco, California
Kristen Lum, The Cardiovascular Research Institute, University of California, San Francisco, California
Jonathan G. Zaroff, Department of Medicine, University of California, San Francisco, California
Nerissa U. Ko, Department of Neurology, University of California, San Francisco, California
S. Claiborne Johnston, Departments of Neurology, and Epidemiology and Biostatistics, University of California, San Francisco, California
Stephen Sidney, Division of Research, Kaiser-Permanente Medical Care Program, Oakland, California
Douglas A. Marchuk, Department of Genetics, Duke University Medical Center, Durham, North Carolina
Michael T. Lawton, Department of Neurological Surgery, University of California, San Francisco, California
Pui-Yan Kwok, The Cardiovascular Research Institute, University of California, San Francisco, California
William L. Young, Center for Cerebrovascular Research, and Departments of Anesthesia and Perioperative Care, Neurological Surgery, and Neurology, University of California, San Francisco, California
References
- 1.Achrol AS, Pawlikowska L, McCulloch CE, Poon KYT, Ha C, Zaroff JG, Johnston SC, Lee C, Lawton MT, Sidney S, Marchuk DA, Kwok PY, Young WL. Tumor necrosis factor-α-238G>A promoter polymorphism is associated with increased risk of new hemorrhage in the natural course of patients with brain arteriovenous malformations. Stroke. 2006;37:231–234. doi: 10.1161/01.STR.0000195133.98378.4b. [DOI] [PubMed] [Google Scholar]
- 2.Biehle SJ, Carrozzella J, Shukla R, Popplewell J, Swann M, Freeman N, Clark JF. Apolipoprotein E isoprotein-specific interactions with tissue plasminogen activator. Biochim Biophys Acta. 2004;1689:244–251. doi: 10.1016/j.bbadis.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Broderick J, Lu M, Jackson C, Pancioli A, Tilley BC, Fagan SC, Kothari R, Levine SR, Marler JR, Lyden PD, Haley EC, Jr, Brott T, Grotta JC. Apolipoprotein E phenotype and the efficacy of intravenous tissue plasminogen activator in acute ischemic stroke. Ann Neurol. 2001;49:736–744. doi: 10.1002/ana.1058. [DOI] [PubMed] [Google Scholar]
- 4.Choi JH, Mohr JP. Brain arteriovenous malformations in adults. Lancet Neurol. 2005;4:299–308. doi: 10.1016/S1474-4422(05)70073-9. [DOI] [PubMed] [Google Scholar]
- 5.Clark JF, Huri DA, Carrozzella J, Jauch EC, Mehta P, Heaton D, Biehle SJ, Broderick JP. Isoforms of apolipoprotein E can modulate tPA-induced clot lysis in vitro. Front Biosci. 2002;7:163–168. doi: 10.2741/A750. [DOI] [PubMed] [Google Scholar]
- 6.Eichner JE, Dunn ST, Perveen G, Thompson DM, Stewart KE, Stroehla BC. Apolipoprotein E polymorphism and cardiovascular disease: A HuGE review. Am J Epidemiol. 2002;155:487–495. doi: 10.1093/aje/155.6.487. [DOI] [PubMed] [Google Scholar]
- 7.Fleetwood IG, Steinberg GK. Arteriovenous malformations. Lancet. 2002;359:863–873. doi: 10.1016/S0140-6736(02)07946-1. [DOI] [PubMed] [Google Scholar]
- 8.Fullerton HJ, Achrol AS, Johnston SC, McCulloch CE, Higashida RT, Lawton MT, Sidney S, Young WL. Long-term hemorrhage risk in children versus adults with brain arteriovenous malformations. Stroke. 2005;36:2099–2104. doi: 10.1161/01.STR.0000181746.77149.2b. [DOI] [PubMed] [Google Scholar]
- 9.Halim AX, Johnston SC, Singh V, McCulloch CE, Bennett JP, Achrol AS, Sidney S, Young WL. Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformation of the brain within a defined population. Stroke. 2004;35:1697–1702. doi: 10.1161/01.STR.0000130988.44824.29. [DOI] [PubMed] [Google Scholar]
- 10.Halim AX, Singh V, Johnston SC, Higashida RT, Dowd CF, Halbach VV, Lawton MT, Gress DR, McCulloch CE, Young WL. Characteristics of brain arteriovenous malformations with coexisting aneurysms: A comparison of two referral centers. Stroke. 2002;33:675–679. doi: 10.1161/hs0302.104104. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto T, Wen G, Lawton MT, Boudreau NJ, Bollen AW, Yang GY, Barbaro NM, Higashida RT, Dowd CF, Halbach VV, Young WL. Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke. 2003;34:925–931. doi: 10.1161/01.STR.0000061888.71524.DF. [DOI] [PubMed] [Google Scholar]
- 12.Hsu TM, Kwok PY. Homogeneous primer extension assay with fluorescence polarization detection. Methods Mol Biol. 2003;212:177–187. doi: 10.1385/1-59259-327-5:177. [DOI] [PubMed] [Google Scholar]
- 13.Joint Writing Group of the Technology Assessment Committee ASoIaTN, Joint Section on Cerebrovascular Neurosurgery, a section of American Association of Neurological Surgeons and Congress of Neurological Surgeons, and Section of Stroke and the Section of Interventional Neurology of the American Academy of Neurology. Reporting terminology for brain arteriovenous malformation clinical and radiographic features for use in clinical trials. Stroke. 2001;32:1430–1442. doi: 10.1161/01.str.32.6.1430. [DOI] [PubMed] [Google Scholar]
- 14.Koistinaho M, Koistinaho J. Interactions between Alzheimer’s disease and cerebral ischemia–focus on inflammation. Brain Res Brain Res Rev. 2005;48:240–250. doi: 10.1016/j.brainresrev.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Leung CH, Poon WS, Yu LM, Wong GK, Ng HK. Apolipoprotein e genotype and outcome in aneurysmal subarachnoid hemorrhage. Stroke. 2002;33:548–552. doi: 10.1161/hs0202.102326. [DOI] [PubMed] [Google Scholar]
- 16.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 17.Mast H, Young WL, Koennecke HC, Sciacca RR, Osipov A, Pile-Spellman J, Hacein-Bey L, Duong H, Stein BM, Mohr JP. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet. 1997;350:1065–1068. doi: 10.1016/s0140-6736(97)05390-7. [DOI] [PubMed] [Google Scholar]
- 18.McCarron MO, Weir CJ, Muir KW, Hoffmann KL, Graffagnino C, Nicoll JA, Lees KR, Alberts MJ. Effect of apolipoprotein E genotype on in-hospital mortality following intracerebral haemorrhage. Acta Neurol Scand. 2003;107:106–109. doi: 10.1034/j.1600-0404.2003.01365.x. [DOI] [PubMed] [Google Scholar]
- 19.O’Donnell HC, Rosand J, Knudsen KA, Furie KL, Segal AZ, Chiu RI, Ikeda D, Greenberg SM. Apolipoprotein E genotype and the risk of recurrent lobar intracranial hemorrhage. N Engl J Med. 2000;342:240–245. doi: 10.1056/NEJM200001273420403. [DOI] [PubMed] [Google Scholar]
- 20.Pawlikowska L, Tran MN, Achrol AS, McCulloch CE, Ha C, Lind DL, Hashimoto T, Zaroff J, Lawton MT, Marchuk DA, Kwok P-Y, Young WL. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke. 2004;35:2294–2300. doi: 10.1161/01.STR.0000141932.44613.b1. [DOI] [PubMed] [Google Scholar]
- 21.Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG, Forbes GS, Thielen K, Nichols D, O’Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 22.Woo D, Sauerbeck LR, Kissela BM, Khoury JC, Szaflarski JP, Gebel J, Shukla R, Pancioli AM, Jauch EC, Menon AG, Deka R, Carrozzella JA, Moomaw CJ, Fontaine RN, Broderick JP. Genetic and environmental risk factors for intracranial hemorrhage: Preliminary results of a population-based study. Stroke. 2002;33:1190–1196. doi: 10.1161/01.str.0000014774.88027.22. [DOI] [PubMed] [Google Scholar]