Abstract
Background
Mitochondrial dysfunction in adipose tissue has been implicated as a pathogenic step in the development of type 2 diabetes mellitus (T2DM). In adipose tissue, chronic nutrient overload results in mitochondria driven increased reactive oxygen species (ROS) leading to carbonylation of proteins that impair mitochondrial function and downregulation of key genes linked to mitochondrial biogenesis. In patients with T2DM, Roux-en-Y gastric bypass (RYGB) surgery leads to improvements in glycemic profile prior to significant weight loss. Consequently, we hypothesized that improved glycemia early after RYGB would be paralleled by decreased protein carbonylation and increased expression of genes related to mitochondrial biogenesis in adipose tissue.
Methods
To evaluate this hypothesis, 16 obese individuals were studied before and 7–8 days following RYGB and adjustable gastric banding (AGB). Subcutaneous adipose tissue was obtained pre- and post-bariatric surgery as well as from eight healthy, non-obese individual controls.
Results
Prior to surgery, adipose tissue expression of PGC1α, NRF1, Cyt C, and eNOS (but not Tfam) showed significantly lower expression in the obese bariatric surgery group when compared to lean controls (p<0.05). Following RYGB, but not after AGB, patients showed significant decrease in HOMA-IR, reduction in adipose protein carbonylation, and increased expression of genes linked to mitochondrial biogenesis.
Conclusions
These results suggest that rapid reduction in protein carbonylation and increased mitochondrial biogenesis may explain postoperative metabolic improvements following RYGB.
Keywords: Protein carbonylation, Mitochondrial biogenesis, Roux-en-Y gastric bypass, Adjustable gastric banding, Adipose tissue
Introduction
The last 30 years have witnessed a worldwide epidemic of type 2 diabetes mellitus (T2DM) and T2DM-linked diseases associated primarily with increasing rates of obesity. Despite advances in medicine and nutrition, bariatric surgery, in particular the Roux-en-Y gastric bypass (RYGB), and more recently the sleeve gastrectomy are important tools for achieving weight loss in obese patients [1]. Improvements in fasting glucose and insulin levels in patients with T2DM occur rapidly following the RYGB and prior to any significant weight loss [2]. However, immediate improvement in glycemia is not typically described in patients undergoing adjustable gastric banding (AGB), which produces little in the way of gastric restriction early after placement. Extreme caloric restriction is a hallmark following the RYGB with a dramatic reduction in endogenous glucose production indicative of improved hepatic insulin resistance [3]. Similar changes have been shown following extreme caloric restriction (600 cal/day) in the non-surgical setting [4].
Often overlooked is the critical role that white adipose tissue (WAT) has in the pathogenesis of T2DM. WAT is a highly active endocrine organ tasked with storing and releasing lipids in response energy excess and demand [5]. At the center of the pathogenesis of T2DM is adipocyte dysfunction, which impacts whole body insulin sensitivity [6]. In particular, adipocyte mitochondrial dysfunction is emerging as a key component of insulin resistance and is thought to be an early pathologic step in the development of insulin resistance [7]. Given the low mitochondrial yield of WAT, few studies have investigated the impact of obesity and T2DM on the oxidative capacity of mitochondria [5]. However, focus has shifted, and has been placed on the role of mitochondrial biogenesis, as humans with insulin resistance (IR) have reduced WAT mitochondrial content and function [8].
A complex regulatory network coordinates mitochondrial biogenesis and is regulated by a variety of physiological processes. Regulatory proteins include the transcription factors peroxisome proliferator-activated receptor coactivator-1 alpha (PGC-1α) and its downstream effects on nuclear respiratory factors 1 and 2 (NRF-1 and NRF-2) and mitochondrial transcription factor A (Tfam) [7, 9]. Nutrient excess in conjunction with hyperinsulinemia mediates the activation of the glucosamine pathway decreasing PGC-1α expression and other genes involved in oxidative phosphorylation leading to reduced oxidative capacity [10]. On the other hand, a potent stimulus for PGC-1α is caloric restriction mediated in part by endothelial nitric oxide synthase (eNOS) [11]. Nitric oxide (NO) generated by eNOS increases mitochondrial biogenesis and enhances respiration and ATP content in various mammalian cells. Body mass index and waist circumference are inversely related to eNOS expression [11]. Moreover, it has previously been shown that there is a differential expression of eNOS in subcutaneous adipose tissue from obese and non-obese humans [11, 12].
Excess glucose metabolism leads to chronic increases in FADH2 and NADH within the mitochondrial electron transport chain and to a concomitant increase in reactive oxygen species (ROS) production generally referred to as oxidative stress [13, 14]. Low levels of ROS play a key physiologic role in inducing peroxisome proliferator-activated receptor γ (PPARγ) [15]. However, increased ROS levels, particularly hydroxyl radicals, are associated with oxidative modification of DNA, protein, and lipid [16]. One of the most common post-translational modifications of proteins is carbonylation [17]. Protein carbonylation refers to the covalent adduction of reactive lipid aldehydes to the side chains of cysteine, histidine, and lysine residues. Cellular synthesis of reactive lipid aldehydes occurs via hydroxyl radical mediated lipid peroxidation and is markedly increased under conditions of increased oxidative stress. Protein carbonylation often leads to enzyme inactivation since the commonly modified side chains are frequently components of enzyme active sites or participate in protein-protein interactions [17, 18]. A number of proteins have been characterized as carbonylation targets including brain Na+-K+-ATPase, glucose transporter (GLUT3), NADP+ dependent isocitrate dehydrogenase, and the adipocyte and epithelial fatty acid-binding proteins [18]. Proteomic analysis of carbonylated proteins from aged skeletal muscle identified various proteins involved in oxidative phosphorylation such as NADH dehydrogenase (complex I), succinate dehydrogenase (complex II), cytochrome c reductase (complex III), various subunits of ATP synthase (complex V), pyruvate dehydrogenase, and thiorexodin two suggesting that the mitochondrion is a “hot spot” for carbonylation [19].
Concomitant with increased carbonylation is the decrease in overall activity of respiratory chain complexes I–IV in isolated mitochondria in subcutaneous WAT depots from obese humans [20]. In the visceral fat depot, downregulation of several genes of the electron transport chain has also been described [21]. The mechanisms responsible for the diminished mitochondrial oxidative capacity remains poorly described, but may comprise several pathways, including WAT inflammation, endoplasmic reticulum stress, and reduced mitochondrial biogenesis and impaired dynamics [5, 22]. Furthermore, the reversal of mitochondrial dysfunction following bariatric surgery remains unknown.
Chief among proteins that are carbonylated is fatty acid binding protein 4 (FABP4), which has garnered considerable interest recently as a main biologic target for the treatment of T2DM. Targeted deletions impact IR and connection with insulin secretion. While FABP4 continues to be characterized with regards to its multifaceted function, it seems to have a critical role in fatty acid efflux. Indeed, mouse knockout models are protected from diet-induced insulin resistance and hyperglycemia [23].
The purpose of this study is to examine the effects of RYGB on the expression of genes linked to mitochondrial biogenesis and protein carbonylation during the early postoperative stage before any significant weight loss has been achieved. We will use adjustable banding (AGB) as a surgical control. We report herein that increased expression of mitochondrial biogenesis genes as well as reduction in protein carbonylation occurs within days after RYGB but not in those individuals undergoing laparoscopic adjustable gastric banding.
Methods
Subjects
All investigations were approved by the University of Minnesota Institutional Review Board, and informed consent was obtained from participants. Eight non-obese (BMI <30 kg/m2), healthy patients undergoing elective surgery were recruited as the healthy control group. Sixteen obese patients with planned bariatric surgery were recruited for this study at baseline and 7–8 days following surgery. Eight of those patients underwent RYGB and the remainder eight underwent AGB.
Clinical Data
Demographic data on sex, age, and history of T2DM were collected at the time of surgery for all patients (non-obese and obese) and 7–8 days following bariatric surgery in the obese group. Weight and height were measured immediately prior to surgery (pre op) and during the postoperative visit. Body mass index (BMI) was calculated as weight (kg) divided by height (m2).
Assessment of Insulin Resistance
Fasting plasma glucose (FPG) and fasting plasma insulin (FPI) levels were obtained at the time of surgery and 1 week after bariatric surgery. In each subject, the degree of insulin resistance was estimated by Homeostasis Model of Assessment for Insulin Resistance (HOMA-IR) according to the method described by Matthews et al. [24]. Low HOMA-IR values indicate high insulin sensitivity, whereas high HOMA-IR values indicate low insulin sensitivity (insulin resistance). In previous studies, the reliability of HOMA-IR was evaluated by comparison with euglycemic-hyperinsulinemic clamp. HOMA-IR was able to explain 65 % of insulin sensitivity (IS) measured by glucose clamp and a misclassification of subjects according to insulin resistance virtually never occurred. This holds true in both non-diabetic and type 2 diabetic subjects [25]. However, acutely after RYGB, there is discordance with the fall in HOMA and an unchanged whole body IS as measured by clamp studies. However, the favorable reduction in elevated hepatic glucose production (sine quo non for T2DM) directionally follows the pattern of reduction in HOMA [3]. Glycated hemoglobin or hemoglobin A1c (HbA1c) levels were determined preoperatively in all patients with T2DM. Use of oral medication and insulin treatment for type 2 diabetes mellitus was also recorded before and 1 week after undergoing bariatric surgery.
Adipose Tissue Sampling
Subcutaneous adipose tissue samples were obtained from all patients from initial incision edge at time of surgery. Incision for non-obese patients was supraumbilical midline (open cases) and supraumbilical port site (laparoscopic cases). For the bariatric patients, incision was made in the supraumbilical area for laparoscopic port placement. Seven to eight days after bariatric surgery, under standard sterile technique and local anesthesia (1 % lidocaine with epinephrine), one of the incisions previously used for the surgery reopened and adipose tissue was excised in an area deep to and remote from the actual skin incision. Approximately 2–5 g of fat was obtained from each subject. The specimens were dissected to remove stromal tissue, immediately frozen with liquid nitrogen in 0.1–0.2 g aliquots, and stored at −80 ° C until further study.
Roux-en-Y Gastric Bypass Operation
The laparoscopic RYGB is a restrictive and malabsorptive (due to intestinal rearrangement) weight loss surgery and performed as described previously [26]. Briefly, a 15–30 mL gastric pouch is constructed with a 10–12 mm gastrojejunal anastomosis. The Roux limb, or malabsorptive limb, measures 75–150 cm in length and the biliopancreatic limb measures 75–100 cm in length.
Adjustable Gastric Banding Operation
The laparoscopic AGB is a pure restrictive procedure without intestinal rearrangement, performed using a pars flaccida approach. The band is tightened 6 to 8 weeks after placement [27] and thus is still in the initial loosened state at the 1-week follow-up visit.
RNA Extraction, Reverse Transcription, and Real-Time PCR
Total RNA was extracted from about 0.3 g of adipose tissue using Trizol© (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. After DNase treatment, cDNA was synthesized using iScript cDNA synthesis kit (BioRad). Relative quantification of mRNAs was performed by RT-PCR using iQ SYBR green Supermix and the MyiQ detection system (BioRad). Human primers for the target genes were PGC-1α: forward 5′-AGG TGA AAG TGT AAT ACT GTT GGT TGA-3′ and reverse 5′-CAT GTA GAA TTG GCA GGT GGA A-3′; NRF1: forward 5′-GGG AAG ACC TTT TGT ATG CCT TTG-3′ and reverse 5′-TGA GAC AGT GCC ATC AGG GTT AC-3′; Tfam: forward 5′-GAT TGC TGG AGT TGT GAT TTG CC-3′ and reverse 5′-GCC AAG ACA GAT GAA AAC CAC CTC-3′; eNOS forward 5′-AAG ACA AGG CAG CAG TGG AAA TC-3′ and reverse 5′-CGG GGA CAG GAA ATA GTT GAC C-3′; cytochrome c oxidase (Cyt C) forward 5′-TGG CCC CTC CCATCTACA C-3′ and reverse 5′-ATCCTTGGCTATCTGGGACATG-3′; and the reference gene human TATA binding protein (hTBP): forward 5′-AGC GGT TTG CTG CGG TAA TC-3′ and reverse 5′-ACT GTT CTT CAC TCT TGG CTC CTG-3′. Gene expression data are expressed as arbitrary units normalized to the reference gene hTBP.
Protein Carbonylation
Adipose tissue was minced and homogenized on ice in coupling buffer (100 mM sodium acetate at pH 5.5, 20 mM NaCl and 0.1 mM EDTA supplemented with 0.1 mM PMSF, 2 mg/mL pepstatin, 2 mg/mL aprotinin, 2 mg/mL leupeptin) [28]. Homogenates were centrifuged at 600×g at 4 ° C for 5 min to separate the lipid cake, the infranatant was removed, and sodium dodecyl sulfate was added to a final concentration of 1 %. The lysate was centrifuged at 100,000×g for 1 h at 4 ° C to remove insoluble residue. The supernatant was removed and assayed for protein content (BCA assay; Sigma). A total of 25 μg of soluble protein was coupled with EZ-link biotin hydrazide (Pierce) to covalently label free aldehydes as previously described [17]. Proteins were separated using a precast 10–20 % polyacrylamide Tris–HCl gel (Bio-rad), transferred to PVDF membranes, and blocked in Odyssey® Blocking Buffer. Biotinylated proteins were detected with DyLight™ 800 Conjugated Streptavidin and visualized using a LiCor Odyssey® Infrared Imager. Both total protein carbonylation as well as carbonylation of the FABP band were determined by quantification of LiCor signal intensity.
Statistical Analysis
The data are expressed as means±SE. Statistical analysis was performed using unpaired Student’s t test when comparing between groups and paired Student’s t test to compare pre- and post-surgical measures within groups. Statistical differences in gender between groups were calculated using chi-square. P values less than 0.05 were considered statistically significant differences.
Results
Subject Characteristics Preoperatively
In order to study the effect of obesity and weight loss surgery on gene expression linked to mitochondrial biogenesis and oxidative stress, 16 obese patients, 14 female and two male, were recruited for this study (Table 1). Eight patients underwent RYGB and the remainder eight underwent AGB. Six female and two male non-obese patients undergoing elective surgery (kidney donor extraction, ventral hernia repair, and hiatal hernia repair) were recruited as controls. The mean age was not different between the obese and lean groups (46.3±2.8 vs. 44.7±5.5 years, respectively; p=0.81). As expected, bariatric patients had significantly higher BMI than non-obese controls (average BMI being 40.6±1.2 vs. 23.9±0.9 kg/m2, respectively; p<0.001). Fasting plasma glucose (FPG) was normal in control patients (82.5±2.2 mg/dL). Bariatric patients had significantly elevated FPG (138.4±12.8 mg/dL) relative to lean controls (p=0.001). At the time of surgery, ten out of the 16 bariatric patients had a diagnosis of T2DM (seven in the RYGB group and three in the AGB group) with a mean HbA1c of 7.86 %. All patients diagnosed with T2DM were under current treatment with either metformin or sulfonylureas, and six patients (five undergoing RYGB and one undergoing AGB) were on insulin therapy as well.
Table 1.
Physical and clinical characteristics among eight lean and 16 obese individuals undergoing adipose biopsy
| Lean Mean±SD or N (%) |
Obese | P valuea | |
|---|---|---|---|
| N | 8 | 16 | |
| Age (years) | 44.5±5.5 | 46.3±2.8 | 0.80 |
| Sex (female) | 6 (75 %) | 14 (88 %) | 0.44 |
| BMI (kg/m2) | 23.9±0.9 | 40.6±1.2 | <0.001 |
| FPG (mg/dL) | 82.5±2.2 | 138.4±12.8 | <0.001 |
| HbA1c | – | 7.8±1.3 | – |
BMI bodymass index, FPG fasting plasma glucose, HbA1C glycosylated hemoglobin A1c
P values represent significance level of difference between lean and obese by Student t test for all values except gender, which is determined by chi-square
Patient Characteristics 1 Week After Bariatric Surgery
Seven to eight days following bariatric surgery, there was no statistically significant weight loss in any of the surgical groups (Table 2). The mean body weight in the RYGB group went from 249.5±40.6 to 232.8±37.9 lb (p=0.59) while the mean weight in the AGB group went from 233.4±30.2 to 221.7±17.3 lb (p=0.63). HOMA-IR levels were significantly improved in patients with T2DM who underwent RYGB (n=7) but not AGB (n=3). HOMA-IR did not change in the non-diabetic AGB patients. HOMA-IR went from 4.2±0.5 to 0.8±0.1 in patients undergoing RYGB with diagnosis of T2DM (p<0.001) and from 3.8±1.0 to 3.3±0.2 in the three patients with T2DM undergoing AGB (p=0.61). Of all the patients undergoing RYGB with T2DM, five of seven discontinued the use of all medications by 1 week after surgery and only two remained on long acting insulin alone. On the other hand, following AGB, medication use for the three patients with T2DM remained unchanged.
Table 2.
Mean and standard deviation values of physical and clinical characteristics among eight patients undergoing RYGB and eight patients undergoing AGB
| Roux-en Y gastric bypass |
Lap adjustable gastric band |
P valueb RYGB pre vs. AGB pre | |||||
|---|---|---|---|---|---|---|---|
| Mean±SD or N (%) | P valuea | Mean±SD or N (%) | P valuea | ||||
|
|
|
||||||
| Pre op | Post op | Pre vs. post | Pre op | Post op | Pre vs. post | ||
| Total N | 8 | – | 8 | – | |||
| N with T2DM | 7 | – | 3 | – | |||
| Age (years) | 49.7±3.1 | – | 42.2±4.5 | – | 0.23 | ||
| Sex (female) | 6 (75 %) | – | 8 (100 %) | – | 0.36 | ||
| BMI (kg/m2) | 40.3±1.7 | 39.2±1.6 | 0.64 | 40.9±2.0 | 40.3±1.9 | 0.83 | 0.82 |
| FPG (mg/dL) | 170.1±13.6 | 102.8±5.0 | <0.001 | 106.7±15.1 | 110.6±9.5 | 0.32 | 0.007 |
| FPI (mU/L) | 10.3±1.3 | 3.3±0.6 | <0.001 | 5.8±1.9 | 5.8±1.9 | 0.50 | 0.09 |
| HOMA-IR | 4.2±0.5 | 0.8±0.1 | <0.001 | 1.8±0.6 | 1.6±0.5 | 0.80 | <0.05 |
BMI body mass index, FPG fasting plasma glucose, FPI fasting plasma insulin, HOMA-IR homeostasis model of assessment for insulin resistance
P values represent significance level of difference between pre op and post op in each surgery group
P values represent significance level of difference between RYGB pre op and AGB pre op. All p values were determined by Student t test except gender, which is determined by chi-square
Mitochondrial Related Gene Expression in Lean vs. Obese Patients
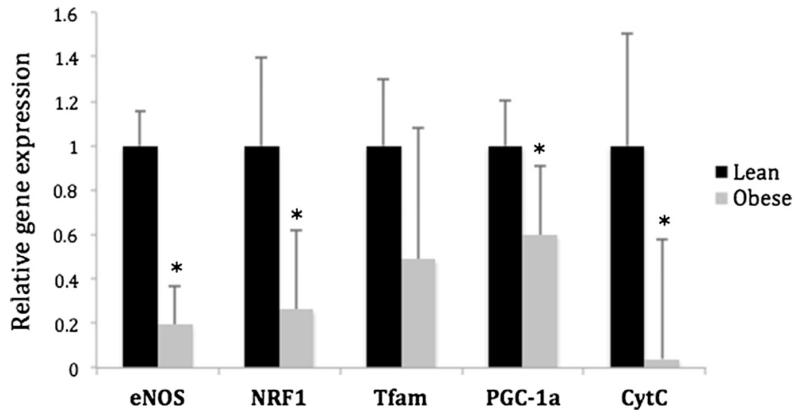
In order to assess mitochondrial capacity in the adipose tissue, the expression of genes linked to mitochondrial biogenesis and oxidative stress was measured. In this small cohort of patients, there were significant differences between non-obese and obese cohorts (Fig. 1). All the genes measured, except Tfam, showed significantly lower expression in the obese, bariatric surgery cohort when compared to non-obese controls (p<0.05).
Fig. 1.
Relative expression of genes in subcutaneous adipose tissue from lean vs. obese patients. eNOS, NRF1, Tfam, PGC-1α, and Cyt C were significantly reduced in the obese (n=8) group when compared to lean (n=8). Gene expression was normalized to the expression in lean patients (*p<0.05). eNOS endothelial nitric oxide synthase, NRF1 nuclear respiratory factor 1, Tfam mitochondrial transcription factor A, PGC-1α peroxisome proliferator-activated receptor coactivator-1 alpha, Cyt C cytochrome c oxidase
Effects of Bariatric Surgery on Expression of Genes Involved in Mitochondrial Biogenesis
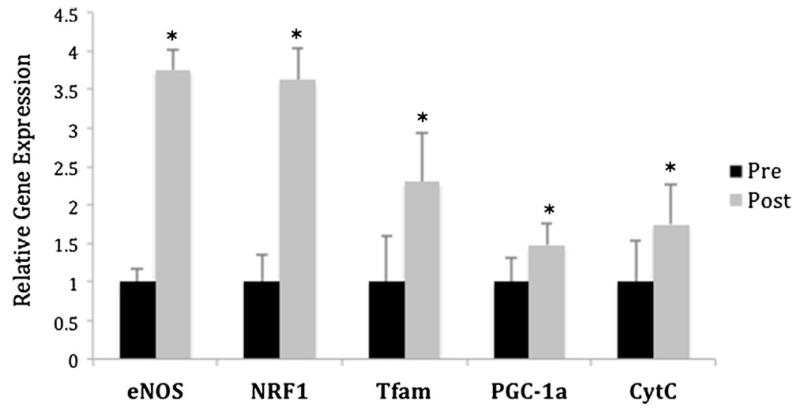
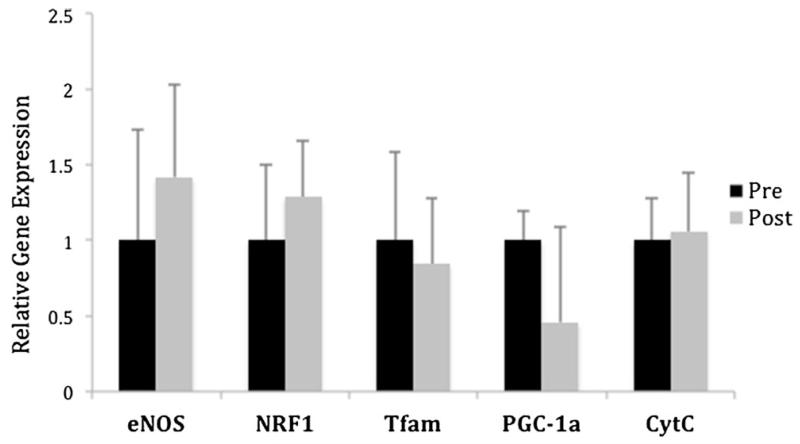
One week following RYGB, the expression of genes linked to mitochondrial biogenesis were reassessed and compared to baseline expression. Despite showing no significant weight loss at that early time point, there was a significant increase in the expression of all genes affecting mitochondrial biogenesis. NRF1 and eNOS expression increased significantly in all the patients studied by an average of almost 4-fold (p<0.01). Tfam, PGC-1α, and Cyt C also showed a significant improvement that reached on average a 2-fold increase in expression (p<0.05) (Fig. 2). In contrast, when gene expression was assessed before and after laparoscopic AGB, no significant changes were observed among any of the genes evaluated (Fig. 3).
Fig. 2.
Effect of RYGB on relative expression of mitochondrial genes in subcutaneous adipose tissue. eNOS, NRF1, Tfam, PGC-1α, and Cyt C were significantly upregulated 1 week after undergoing RYGB (n=8). Gene expression was normalized to the preoperative expression (*p<0.05). RYGB Roux-en Y gastric bypass, pre Op before surgery, post Op 1 week following surgery, eNOS endothelial nitric oxide synthase, NRF1 nuclear respiratory factor 1, Tfam mitochondrial transcription factor A, PGC-1α peroxisome proliferator-activated receptor coactivator-1 alpha, Cyt C cytochrome c oxidase
Fig. 3.
Effect of laparoscopic AGB on relative expression of mitochondrial genes in subcutaneous adipose tissue. No significant differences were observed in the relative expression of mitochondria-related genes 1 week after undergoing laparoscopic AGB (n=8). Gene expression was normalized to the preoperative expression. AGB adjustable gastric banding, pre Op before surgery, post Op 1 week following surgery, eNOS endothelial nitric oxide synthase, NRF1 nuclear respiratory factor 1, Tfam mitochondrial transcription factor A, PGC-1α peroxisome proliferator-activated receptor coactivator-1 alpha, Cyt C cytochrome c oxidase
Protein Carbonylation
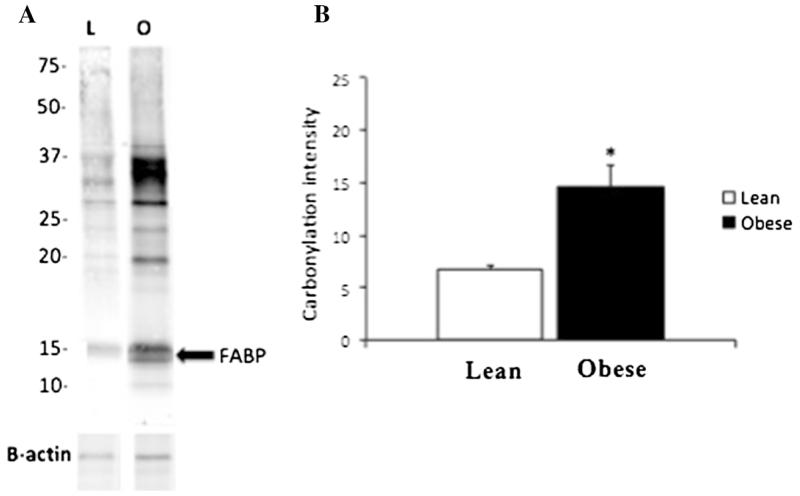
Consistent with an increase in oxidative stress, adipose tissue from obese individuals exhibits greater levels of protein carbonylation than that from lean patients (Fig. 4). For each individual, total adipose tissue carbonylation was quantified by analysis of the total signal intensity (excluding the 67-kDa albumin band) while fatty acid binding protein (FABP) carbonylation (as identified previously using two-dimensional gel electrophoresis and monospecific antibodies) [13] was determined by intensity of the 15-kDa band. Total protein carbonylation and specifically FABP carbonylation were significantly higher in the obese group (p<0.05).
Fig. 4.
Protein carbonylation comparison between lean and obese patients. Panel (a) shows a representative blot from one lean to one obese individual where the fatty acid binding protein band is denoted by an arrow. b For each individual, total carbonylation was quantified by analysis of the signal intensity for the entire lane (excluding the 67-kDa albumin band). On average, the obese group (n=5) showed significantly higher carbonylation when compared to the lean cohort of patients (n=5) (*p<0.05). FABP fatty acid binding protein
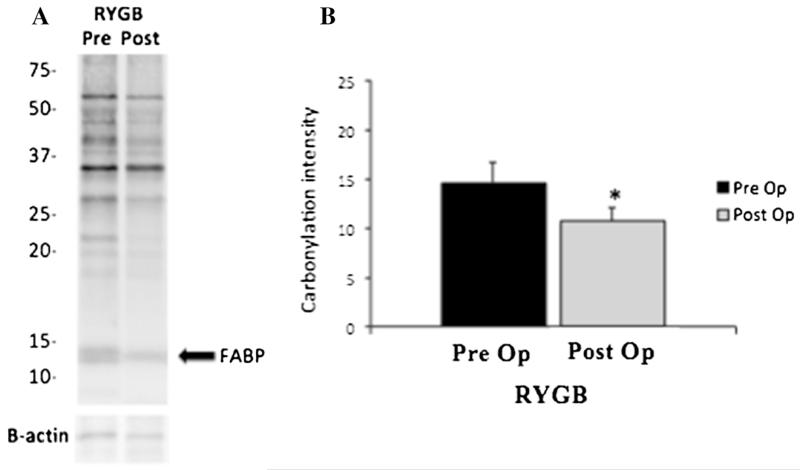
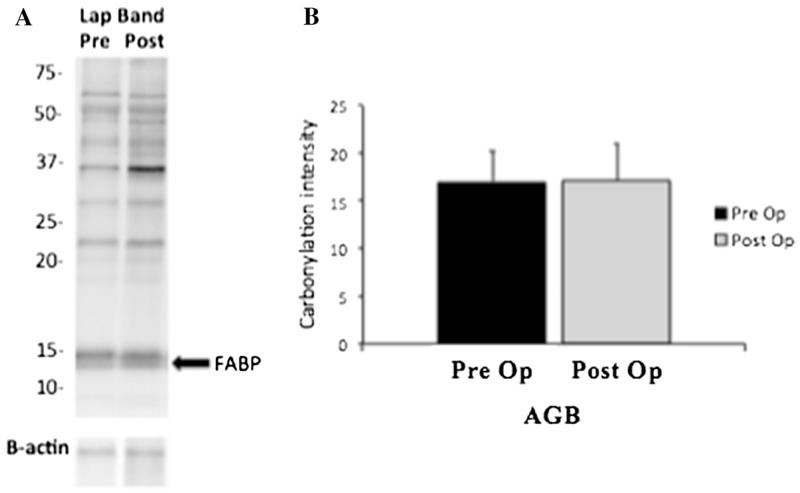
Subcutaneous adipose tissue protein carbonylation was assessed in a cohort of five representative patients with T2DM undergoing RYGB and the three patients with T2DM undergoing laparoscopic AGB. Carbonylation of total proteins as well as FABP-specific carbonylation were significantly decreased after RYGB (Fig. 5). On the other hand, no significant changes were observed in adipose tissue protein carbonylation from obese individuals with T2DM undergoing laparoscopic adjustable gastric banding (Fig. 6).
Fig. 5.
Protein carbonylation before and 1 week after RYGB. Panel (a) shows a representative blot from one obese individual before and 1 week following RYGB operation. An arrow denotes fatty acid binding protein band. b For each individual, total carbonylation was quantified by analysis of the signal intensity for the entire lane (excluding the 67-kDa albumin band). On average, there was a significant reduction in protein carbonylation 1 week after undergoing RYGB on five patients with T2DM (*p<0.05). FABP fatty acid binding protein, RYGB roux-en Y gastric bypass, T2DM type 2 diabetes mellitus
Fig. 6.
Protein carbonylation before and 1 week after AGB. Panel (a) shows a representative blot from one obese individual before and 1 week following AGB operation. An arrow denotes fatty acid binding protein band. b For each individual, total carbonylation was quantified by analysis of the signal intensity for the entire lane (excluding the 67-kDa albumin band). There were no changes in protein carbonylation 1 week following AGB on three patients with T2DM. FABP fatty acid binding protein, AGB adjustable gastric banding, T2DM type 2 diabetes mellitus
Discussion
It is well known that bariatric surgery produces a dramatic improvement in blood glucose profile and is associated with significant weight loss. This effect appears to be durable up to 14 years from the time of surgery but may be affected with weight regain [29]. Improved glycemic control occurs rapidly following RYGB but prior to weight reduction suggesting that there are metabolic changes linked to the surgical procedure or concomitant caloric reduction that affect insulin sensitivity independently of those signals that regulate insulin action affected by obesity [30]. Insulin sensitivity is improved as early as 6 days following RYGB as measured by intravenous glucose tolerance tests and HOMA-IR [31], but not when evaluated with insulin clamp studies [32]. In contrast to RYGB, patients undergoing laparoscopic AGB exhibited no amelioration of diabetes until a significant weight loss was achieved [33]. However, when similar weight loss is achieved 3 months following either RYGB or AGB, glycemic control improvement is comparable between both procedures [34]. In our study, only patients undergoing RYGB had significant improvement of HOMA-IR levels 7–8 days following surgery when compared to patients undergoing AGB.
The biologic underpinnings of improved glucose homeostasis following gastric bypass remain enigmatic. Two broad concepts involving either incretin biology and/or caloric restriction are most frequently cited. Isbell et al. demonstrate that reduced caloric intake alone accounts for the rapid improvement in glycemia after surgery [2]. The authors compared the metabolic effects of caloric restriction before and 4 days after undergoing RYGB plus diet and pre-post matched RYGB diet alone. All patients had a significant improvement of fasting plasma glucose levels although patients undergoing surgery had increased levels of glucagon-like peptide-1 (GLP-1) following RYGB when compared to diet alone. However, increased GLP-1 levels were not associated with increased insulin secretion [2]. Despite the fact that incretin function is changed postprandially after surgery, studies of duodenal bypass in rodent models suggest that incretin functions may not be mechanistically linked to changes in insulin resistance [35]. More interestingly, changes in incretin hormone secretion after RYGB cannot completely explain decreased fasting glucose and fasting insulin levels.
Skeletal muscle represents an important regulator of whole body insulin resistance, being the principal recipient of a postprandial glucose load [36-39]. Mitochondria represent an important link both causally and contributory to insulin resistance. Compromise of muscle mitochondrial fatty acid oxidation (FAO) and accumulation of acyl carnitines can occur in the setting of increased oxidative stress and damage to electron transport complexes due to overnutrition [36, 37]. Sarcopenia associated with aging and low physical activity also contribute to impaired mitochondrial function. Lipid moieties such as diacylglycerol and ceramide from adipose tissue not cleared by fatty acid oxidation are known to impair muscle insulin signaling [38]. Diet-induced weight loss favorably improves muscle IS by reducing ceramide levels. Caloric restriction is known to improve muscle mitochondrial biogenesis programs. Interestingly, there is a paradox between obese subjects and fit subjects relative to the amount of intramyocellular fat content suggesting improved FAO in the latter group [40]. The acute post-gastric bypass state may be similar to the “starved” state with increased lipolysis and ectopic fat deposition in the setting of marked reduction in insulin levels. An important common denominator of muscle mitochondrial function is adipocyte function. Adipose tissue performs critical functions related to metabolic health [2, 6]. Obesity is characterized by increased fat mass and altered adipose tissue function, including cellular stress responses, changes in extra-cellular matrix, chronic inflammation, and impaired adipokine secretion. Mitochondrial metabolism is also essential in maintaining many of the physiological functions in human adipose tissue. Although there is no clear consensus [7, 10], it has been postulated that adipocyte insulin resistance is a result of mitochondrial dysfunction and reduced mitochondrial density [21]. Less is known about adipose tissue mitochondrial function and its effect on insulin sensitivity. Decreased expression of genes linked to oxidative respiration was measured in visceral adipose tissue from obese patients when compared to healthy patients [41]. In our study, we were able to show a significant reduction in the expression of genes (PGC-1α, NRF-1, Tfam, eNOS, and Cyt C) encoding key transcription factors and/or regulatory proteins in subcutaneous adipose tissue from obese patients when compared to healthy lean subjects. Moreover, expression of such genes was upregulated following RYGB, but not AGB.
Bariatric surgery has been shown to induce several-fold changes in expression of genes related to mitochondrial function and biogenesis in muscle and adipose tissue 1 year following biliopancreatic diversion (BPD) and duodenal switch (DS) [41]. At that time point, patients had already lost most of their excess weight. Other studies were able to show similar results as early as 6 months following BPD [42]. To our knowledge, our study is the first to show early improvement of mitochondrial-related gene expression. Within 7–8 days after RYGB, patients with uncontrolled T2DM and BMI greater than 35 kg/m2 had upregulated expression of all the key transcriptional regulatory factors for mitochondrial biogenesis. On the other hand, patients undergoing a purely restrictive surgery, such as AGB, did not show any changes in mitochondria gene expression following surgery.
Studies in mice have shown that the level of carbonylation is significantly associated with obesity and insulin resistance [15]. Carbonylation may also lead to decreased mitochondrial biogenesis as evidenced by changes in key factors in adipocytes at both the mRNA and protein levels [13] or there may be direct carbonylation of mitochondrial proteins, causing dysfunction or targeting for degradation. Our group has shown in the past that there is a strong relationship between adipose tissue protein carbonylation and obesity in humans. Multiple protein targets are modified by reactive aldehydes in human adipose tissue and carbonylation occurs to a greater degree in the adipose tissue of obese relative to lean individuals [28]. In the present study, we were able to replicate the differences in protein carbonylation between lean and obese individuals. Furthermore, we have shown for the first time that protein carbonylation in human obesity can be significantly reduced early after RYGB but not after AGB.
As mentioned previously, one particularly highly abundant and carbonylated protein is FABP4, of which carbonylation significantly improves after RYGB. The impact of FABP4 has yet to be evaluated in the gastric bypass population. Its impairment may cause disruption of intracellular free fatty acid transport resulting in changes in local free fatty acid concentration impacting inflammation. Recently, our group has extended our observations to indicate a direct relationship between FABP4 in its regulation of intracellular free fatty acid levels to indirectly control the expression of Uncoupling Protein 2 (UCP2) to alleviate endoplasmic reticulum stress and decrease inflammation [43].
One limitation of this study is the use of subcutaneous WAT given evidence suggesting that visceral adipose tissue is perhaps more critical to the development of metabolic disease [44]. However, subcutaneous WAT remains approximately 10 times more in volume than visceral adipose tissue, and maintains a critical and dynamic function. In this study, we correlated subcutaneous WAT gene expression patterns and carbonylation to whole body measures of insulin sensitivity. Thus, although we are not evaluating visceral depot functions, we have assessed outcomes. A second limitation is that although we did not measure the length of the small bowel, the impact on each patient will likely be longer term. Regardless, the importance of this cannot be discounted.
Conclusion
Our findings suggest that induction of mitochondrial biogenesis by RYGB occurs early before any significant weight loss has been achieved correlating with improvements of HOMA-IR levels in the same cohorts of patients. Lack of improvement of glycemic control as well as mitochondrial gene expression following AGB may suggest a differential regulation of mitochondrial function in response to these two types of bariatric procedures. Further studies to define early effects of caloric restriction alone or in combination with weight loss surgery may yield important insights into the mechanism by which bariatric surgery affects mitochondrial function.
Acknowledgments
This work was supported by the American Diabetes Association (ADA 7-11-ST-01), The Minnesota Obesity Center (NIH DK050456) and NIH DK084669. We thank the members of the Bernlohr laboratory for helpful comments and suggestions during the preparation of this manuscript. We also thank transplant surgeons Drs Ty Dunn, Raja Kandaswamy, Erik B. Finger, David E.R. Sutherland, and Rajinder Singh for their kind assistance in these studies.
Footnotes
We declare that the authors do not have any conflict of interests.
Disclosure of Potential Conflict of Interest Dr. Ikramuddin serves on boards for Novo Nordisk, Medica, and OptumHealth, consults for Metamodix Inc. and Covidien, and has research grants from USGI Medical Inc., Enteromedics, Covidien, and ReShape Medical. Dr. Bernlohr is SAB member for Celladon Corp. For the remaining authors, no conflicts were declared.
Statement of Informed Consent Informed consent was obtained from all individual participants included in the study.
Statement of Human Rights All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309(21):2240–9. doi: 10.1001/jama.2013.5835. doi:10.1001/jama.2013.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isbell JM, Tamboli RA, Hansen EN, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33(7):1438–42. doi: 10.2337/dc09-2107. Epub 2010 Apr 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunn JP, Abumurad NN, Breitman I, et al. Hepatic and peripheral insulin sensitivity and diabetes remission at 1 month after Roux-en-Y gastric bypass surgery in patients randomized to omentectomy. Diabetes Care. 2012;35:137–42. doi: 10.2337/dc11-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malandrucco I, Pasqualetti P, Giordani I, et al. Very-low-calorie diet: a quick therapeutic tool to improve b cell function in morbidly obese patients with type 2 diabetes. Am J Clin Nutr. 2012;95:609–13. doi: 10.3945/ajcn.111.023697. [DOI] [PubMed] [Google Scholar]
- 5.Boudina S, Graham TE. Mitochondrial function/dysfunction in white adipose tissue. Exp Physiol. 2014;99(9):1168–78. doi: 10.1113/expphysiol.2014.081414. doi:10.1113/expphysiol.2014.081414. Epub 2014 Aug 15. [DOI] [PubMed] [Google Scholar]
- 6.De Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54(6):945–55. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- 7.Abdul-Ghani MA, DeFronzo RA. Mitochondrial dysfunction, insulin resistance, and type 2 diabetes mellitus. Curr Diab Rep. 2008;8(3):173–8. doi: 10.1007/s11892-008-0030-1. [DOI] [PubMed] [Google Scholar]
- 8.Viera VJ, Valentine RJ. Mitochondrial biogenesis in adipose tissue: can exercise make fat cells ‘fit’? J Physiol. 2009;587(14):3427–8. doi: 10.1113/jphysiol.2009.175307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mootha VK, Lindgren CM, Eriksson K, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–73. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 10.Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100(14):8466–71. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elizalde M, Rydén M, van Harmelen V, et al. Expression of nitric oxide synthases in subcutaneous adipose tissue of nonobese and obese humans. J Lipid Res. 2000;41(8):1244–51. [PubMed] [Google Scholar]
- 12.Hickner RC, Kemeny G, Stallings HW, et al. Relationship between body composition and skeletal muscle eNOS. Int J Obes. 2006;30(2):308–12. doi: 10.1038/sj.ijo.0803134. [DOI] [PubMed] [Google Scholar]
- 13.Curtis JM, Grimsrud PA, Wright WS, et al. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes. 2010;59(5):1132–42. doi: 10.2337/db09-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demozay D, Mas J, Rocchi S, et al. FALDH reverses the deleterious action of oxidative stress induced by lipid peroxidation product 4-hydroxynonenal on insulin signaling in 3T3-L1 adipocytes. Diabetes. 2008;57(5):1216–26. doi: 10.2337/db07-0389. [DOI] [PubMed] [Google Scholar]
- 15.Tormos KV, Anso E, Hamanaka RB, et al. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011;14(4):537–44. doi: 10.1016/j.cmet.2011.08.007. doi:10.1016/j.cmet.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–8. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 17.Carini M, Aldini G, Facino RM. Mass spectrometry for detection of 4-hydroxy-trans-2-nonenal (HNE) adducts with peptides and proteins. Mass Spectrom Rev. 2004;23(4):281–305. doi: 10.1002/mas.10076. [DOI] [PubMed] [Google Scholar]
- 18.Grimsrud PA, Picklo MJ, Griffin TJ, et al. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Mol Cell Proteomics. 2007;6(4):624–37. doi: 10.1074/mcp.M600120-MCP200. [DOI] [PubMed] [Google Scholar]
- 19.Meany DL, Xie H, Thompson LV, et al. Identification of carbonylated proteins from enriched rat skeletal muscle mitochondria using affinity chromatography-stable isotope labeling and tandem mass spectrometry. Proteomics. 2007;7(7):1150–63. doi: 10.1002/pmic.200600450. [DOI] [PubMed] [Google Scholar]
- 20.Chattopadhyay M, Guhathakurta I, Behera P, et al. Mitochondrial bioenergetics is not impaired in nonobese subjects with type 2 diabetes mellitus. Metabolism. 2011;60(12):1702–10. doi: 10.1016/j.metabol.2011.04.015. doi:10.1016/j.metabol.2011.04.015. Epub 2011 Jun 12. [DOI] [PubMed] [Google Scholar]
- 21.Dahlman I, Forsgren M, Sjogren A, et al. Downregulation of electron transport chain genes in visceral adipose tissue in type 2 diabetes independent of obesity and possibly involving tumor necrosis factor-α. Diabetes. 2006;55(6):1792–9. doi: 10.2337/db05-1421. [DOI] [PubMed] [Google Scholar]
- 22.Valerio A, Cardile A, Cozzi V, et al. TNF-α downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest. 2006;116(10):2791–8. doi: 10.1172/JCI28570. Epub 2006 Sep 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotamisligil GS, Johnson RS, Distel RJ, et al. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274(5291):1377–9. doi: 10.1126/science.274.5291.1377. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Bonora E, Targger G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degree of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 26.Ikramuddin S, Kendrick ML, Kellogg TA, et al. Open and laparoscopic Roux-en-Y gastric bypass: our techniques. J Gastrointest Surg. 2007;11(2):217–28. doi: 10.1007/s11605-006-0028-4. [DOI] [PubMed] [Google Scholar]
- 27.Beitner M, Kurian MS. Laparoscopic adjustable gastric banding. Abdom Imaging. 2012 doi: 10.1007/s00261-012-9864-8. [DOI] [PubMed] [Google Scholar]
- 28.Frohnert BI, Sinaiko AR, Serrot FJ, et al. Increased adipose protein carbonylation in human obesity. Obesity (Silver Spring) 2011;19(9):1735–41. doi: 10.1038/oby.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–256.e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 30.Dankel SN, Staalesen V, Bjørndal B, et al. Tissue-specific effects of bariatric surgery including mitochondrial function. J Obes. 2011;2011:435245. doi: 10.1155/2011/435245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wickremesekera K, Miller G, DeSilva Naotunne T, et al. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15(4):474–81. doi: 10.1381/0960892053723402. [DOI] [PubMed] [Google Scholar]
- 32.Lima MM, Pareja JC, Alegre SM, et al. Acute effect of roux-en-y gastric bypass on whole-body insulin sensitivity: a study with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(8):3871–5. doi: 10.1210/jc.2010-0085. [DOI] [PubMed] [Google Scholar]
- 33.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. J Am Med Assoc. 2008;299(3):316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 34.Campos GM, Rabl C, Peeva S, et al. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14(1):15–23. doi: 10.1007/s11605-009-1060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tammy L, Kindel, Paulo JF, et al. Bypassing the duodenum does not improve insulin resistance associated with diet-induced obesity in rodents. Obesity. 2011;19(2):380–7. doi: 10.1038/oby.2010.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newsholme P, Gaudel C, Krause M. Mitochondria and diabetes. An intriguing pathogenetic role. Adv Exp Med Biol. 2012;942:235–47. doi: 10.1007/978-94-007-2869-1_10. [DOI] [PubMed] [Google Scholar]
- 37.Brands M, Verhoeven AJ, Serlie MJ. Role of mitochondrial function in insulin resistance. Adv Exp Med Biol. 2012;942:215–34. doi: 10.1007/978-94-007-2869-1_9. [DOI] [PubMed] [Google Scholar]
- 38.Martins AR, Nachbar RT, Gorjao R, et al. Mechanisms underlying skeletal muscle insulin resistance induced by fatty acids: importance of the mitochondrial function. Lipids Health Dis. 2012;11:30. doi: 10.1186/1476-511X-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pagel-Langenickel I, Bao J, Pang L, et al. The role of mitochondria in the pathophysiology of skeletal muscle insulin resistance. Endocr Rev. 2010;31-1:25–51. doi: 10.1210/er.2009-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubé JJ, Amati F, Toledo GS, et al. Effects of weight loss and exercise on insulin resistance and intramyocellular triacylglycerol, diacylglyceroland ceramide. Diabetologia. 2011;54:1147–56. doi: 10.1007/s00125-011-2065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dankel SN, Fadnes DJ, Stavrum AK, et al. Switch from stress response to homeobox transcription factors in adipose tissue after profound fat loss. PLoS One. 2010;5-6:e11033. doi: 10.1371/journal.pone.0011033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernandez-Alvarez MI, Chiellini C, Manco M, et al. Genes involved in mitochondrial biogenesis/function are induced in response to bilio-pancreatic diversion in morbidly obese individuals with normal glucose tolerance but not in type 2 diabetic patients. Diabetologia. 2009;52-8:1618–27. doi: 10.1007/s00125-009-1403-y. [DOI] [PubMed] [Google Scholar]
- 43.Xu H, Hertzel AV, Steen KA, et al. Uncoupling lipid metabolism from inflammation through fatty acid binding protein-dependent expression of UCP2. Mol Cell Biol. 2015;35(6):1055–65. doi: 10.1128/MCB.01122-14. doi:10.1128/MCB.01122-14. Epub 2015 Jan 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–7. doi: 10.1038/nature05488. Review. [DOI] [PubMed] [Google Scholar]