Abstract
Bariatric surgery is usually successful for treatment of severe obesity. The mechanisms of weight loss after bariatric surgery and the role of central energy homeostatic pathways in this weight loss are not well understood. The study of individuals with complete loss of function of important genes in the leptin-melanocortin system may help establish the importance of these pathways for weight loss after bariatric surgery. We describe the outcome of bariatric surgery in an adolescent with compound heterozygosity and complete functional loss of both alleles of the melanocortin 4 receptor (MC4R). The patient underwent laparoscopic adjustable gastric banding and truncal vagotomy at 18 8/12 yrs of age, which resulted in initial, but not long-term weight loss. Our experience with this patient suggests that complete MC4R deficiency impairs response to gastric banding and results in poor weight loss after this surgery.
Keywords: melanocortin-4 receptor, adolescent, obesity, gastric banding, vagotomy, bariatric surgery
Introduction
Bariatric surgery is currently the most effective therapy for severe obesity. Adjustable gastric banding is safe and effective in adolescents who have not succeeded in losing weight through medical and behavioral therapy (1). How bariatric surgery alters the compensatory response to weight loss, allowing patients to maintain a state of negative energy balance is not well understood. Specifically, whether the integrity of central neuroendocrine systems regulating energy homeostasis is required for the success of bariatric surgery is unknown.
The major neuroendocrine pathway involved in long-term energy homeostasis is the leptin-melanocortin system. This system integrates information about peripheral energy stores, relayed primarily by the adipocyte-secreted hormone leptin, leading to adaptive changes in food intake and energy expenditure. The description of severe cases of human obesity due to mutations in genes of the leptin-melanocortin axis has validated its essential role in the regulation of long-term energy homeostasis in humans. (2). Bariatric surgery lowers both adiposity and leptin levels, inferring improved leptin sensitivity post-operatively.
A major mediator of the central effect of leptin is the melanocortin 4 receptor (MC4R). MC4R is a G-protein-coupled receptor (GPCR) expressed in neurons of the paraventricular nucleus of the hypothalamus (PVN) (3), which regulates food intake and maintains long-term energy homeostasis by integrating signals provided by its agonist (α-MSH) and antagonist (AgRP) from leptin sensitive neurons within the arcuate nucleus (4). Heterozygous mutations in MC4R are the most common monogenic form of obesity, found in 2.5% of severely obese children and adults (5, 6). These patients present with very early onset severe obesity and hyperphagia without other physical, hormonal or developmental consequences (5). In contrast, patients with homozygous or compound heterozygous MC4R mutations are extremely rare with fewer than ten cases described (7-10). The study of MC4R null mutants has highlighted the role of the melanocortin system and its role in energy homeostasis. The clinical outcome of such patients after bariatric surgery reflects the importance of the leptin melanocortin pathway to weight loss observed after bariatric surgery. We describe the first patient with complete MC4R deficiency, who underwent bariatric surgery.
Subject and Methods
Case report
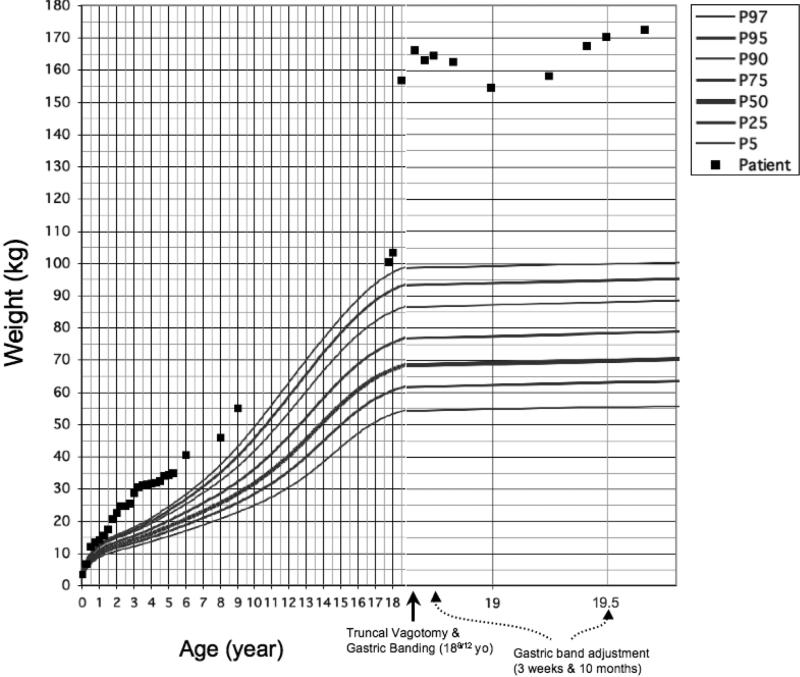
A 17-year-old Caucasian male with severe obesity was referred for evaluation of hyperphagia and abnormal weight gain since infancy. At presentation, his weight was 103.4 kg (>99th %ile for age), height was 175 cm (50th %ile for age) and BMI was 33.8 (> 99th %ile for age). At 18 8/12 y/o his weight was 166.2 kg and BMI was 54.27 (Fig. 1).
Figure 1.
Patient's weight growth curve from ages 0 to 18 y/o and post-bariatric surgery weight from baseline weight prior to surgery until 12 months of follow-up.
The patient's parents are not consanguineous. His birth weight was 3.25 kg. His parents report he had insatiable hunger since 2 months of age. He gained 11 pounds between 3 and 5 months of age. He progressed through puberty normally, but had a history of depression, gastroesophageal reflux disease and obstructive sleep apnea requiring tonsillectomy and adenoidectomy. At 18 years his serum leptin (37.9 ng/ml) was appropriate for his body fat, his ghrelin was 452 pg/ml, fasting LDL 141 mg/dl, total cholesterol 210 mg/d, triglycerides 160 mg/dl, insulin 50.3 U/L, glucose 85 mg/dl, aspartate aminotransferase 29 U/L and alanine transaminase 58 U/L.
Genetic studies
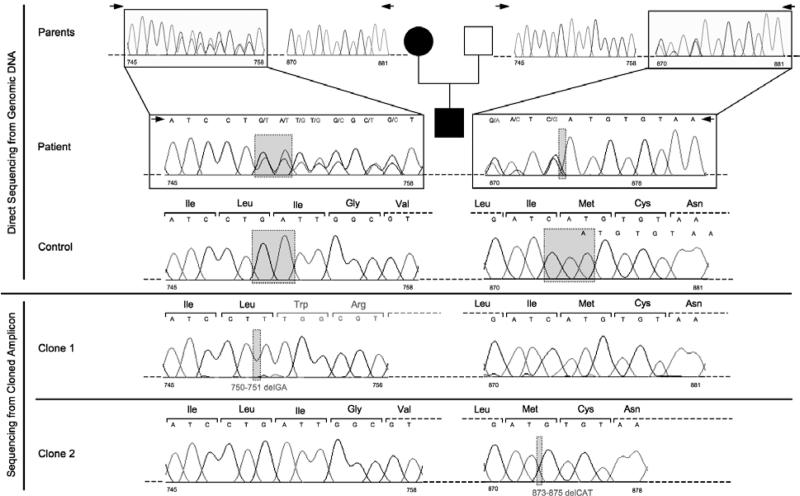
With informed consent, the patient's genomic DNA was extracted from white blood cells using standard methods. Forward (5'-ATCAATTCAGGGGGACACTG-3') and reverse (5'-TGCATGTTCCTATATTGCGTG-3') primers were used to amplify the coding region of MC4R as previously described (11). PCR products were sequenced using the above and two internalprimers: 5'-TGTAGCTCCTTGCTTGCATC-3' and 5'-GGCCATCAGGAACATGTGGA-3'. Sequencing was performed on an ABI PRISM 3700 automated DNA sequencer using a BigDye terminator kit according to the manufacturer's protocol (Applied Biosystems, Foster City, CA, USA). Sequencing of the MC4R gene from the patient and his parents showed that the patient was compound heterozygous for 750-751delGA (Ile251fsX34), inherited from his mother, and 873-875delCAT (Ile291del), inherited from his father (Fig. 2).
Figure 2.
Patient's pedigree showing direct MC4R sequencing from parents (A) and patient (B). The mutant genes were cloned into pcDNA 3.1: Clone 1, MC4R 750-751 delGA; Clone 2, MC4R 873-875 delCAT.
WT and mutant alleles of MC4R were amplified and cloned into the vector pcDNA 3.1 (Invitrogen, San Diego, CA, USA) directly from the genomic DNA of the patient and his parents. All expression vectors were sequenced to establish the presence of the mutation and the absence of any induced mutations.
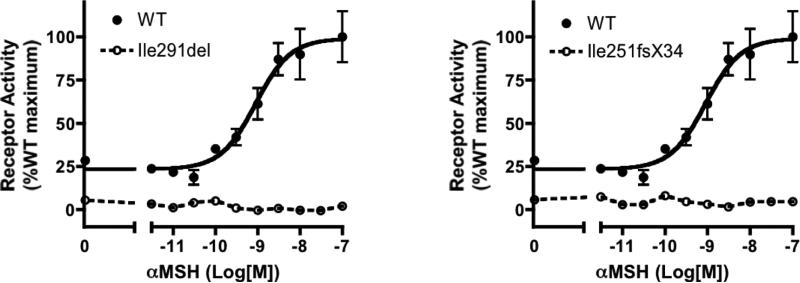
The maternal del750-751GA frameshift mutation, which results in a receptor that is truncated at the sixth transmembrane domain with 33 additional amino acids, has been previously described (8). The paternal 873-875delCAT mutation, which results in an in-frame deletion of isoleucine 291, is novel. To assess the function of this novel mutant, we expressed it in transfected cells and assayed its ability to be activated by α-MSH; both mutations resulted in proteins that were devoid of measurable activity (Fig. 3).
Figure 3.
Functional analysis of mutant MC4Rs. Dose-response to α-MSH in mutant and wild-type receptor. Data points represent means ± SEM of at least 3 experiments performed in triplicate. The data are expressed as a percentage of the maximal wild-type activity.
MC4R functional studies
HEK293 cells stably expressing the luciferase reporter under the control of a cAMP− responsive promoter (12, 13) were maintained in alpha-MEM supplemented with 10% calf serum (Invitrogen, San Diego, CA, USA), L-glutamine, non-essential amino acids and penicillin/streptomycin. Transfection and dose response assays were performed using α-MSH (Sigma, St Louis, MO, USA) added to the medium at the desired concentrations (12, 13). Luciferase activity, representing cAMP production through MC4R activation, was assessed using the Steady-Glo Luciferase Assay System (Promega, Madison, WI, USA) and a microplate luminescence counter (Packard Instrument, Downers Grove, IL, USA). Luciferase activity upon MC4R activation was normalized for transfection efficiency by co-transfection with Renilla luciferase, and the results were expressed as percentage of maximum stimulation of the WT receptor.
Surgery and outcome
The patient underwent laparoscopic truncal vagotomy and laparoscopic adjustable gastric banding (LAGB) at 18 8/12 yrs. The vagotomy was performed as part of a research protocol approved by the committee on human research at UCSF. The anterior and posterior trunks of the vagus nerve were identified posterior to the esophagus and transected below the diaphragm. Subsequently, the gastric band was sutured between the seromuscular layers of the stomach. The patient tolerated the procedure well, and was discharged 24h later in good condition.
The patient initially lost 11.6 kg over 4 months following surgery (Fig. 1), an excess weight lost (EWL) of 12%. However, by 12 months after the surgery he had regained his pre-operative weight and gained additional 6.5 kg (EWL of −7%). Previous reports in obese adolescent males have shown an average of 59% EWL and 40% EWL 2 and 3 years after LAGB, respectively. (14, 15).
Our patient still describes insatiable hunger. He has not followed a caloric restriction program after the surgery. He missed several of his follow-up appointments in our bariatric surgery clinic, and only had the band adjusted two times, at 3 weeks and 10 months after surgery (Fig.1).
Discussion
Our patient is unique because of his compound heterozygosity for two null MC4R mutations. Two non-functioning MC4R alleles likely compromise his satiety response significantly, and therefore, affect his response to bariatric surgery. However, other factors such as non-compliance with follow-up appointments and poor adherence to diet and physical activity modifications may also have contributed to the weight loss failure after bariatric surgery in this patient. Prior to surgery, our patient had abnormally excessive weight gain, which stabilized post-operatively.
Our patient underwent vagotomy in combination with LAGB for treatment of his obesity. LAGB is a gastric restrictive procedure that lacks a neurohormonal mechanism to alter satiation (16). However, LAGB is a well-established procedure for the treatment of obesity in adolescent patients (14, 15). Vagotomy, as a surgical treatment for obese patients, is currently performed as a research protocol. The vagus nerve is downstream of the MC4R, has many functions that mediate satiety, and is critically important for energy storage (17). A recent study comparing 46 adult patients (23 with LAGB and vagotomy, 23 with LAGB without vagotomy) found a significantly greater weight loss in the vagotomy group at 6 months follow up (18). Further clinical trials are needed to determine the role of vagotomy as a surgical treatment for obese patients.
The leptin-melanocortin system is influenced by signals mediated through peptides made in the gut (19). Appetite and gut hormone changes are reported within days following roux-en-Y gastric bypass (20). As a result, patients have long-term decreased appetite after gastric bypass. Our patient might have had a better response to gastric bypass, given that the weight loss after gastric banding is mainly dependent on mechanical restriction with no additional effect on neurohormonal appetite regulation (although the vagotomy was attempted to improve satiety). However, it may be argued that an individual with a compromised ability to signal satiety and maintain energy balance may not benefit from the neurohormonal changes effected by gastric bypass any better than from gastric banding.
Our case report suggests that complete MC4R deficiency impairs the response to gastric banding. This could provide insight into the underlying physiology of the therapeutic effects of bariatric surgery. Larger studies will be required to draw clear conclusions about the role of the leptin-melanocortin system in the response to bariatric surgery. Unfortunately, because the number of homozygous MC4R null patients is small, a case-control study in humans with homozygous MC4R mutations is not feasible. Animal studies using homozygous MC4R knockout mice are needed to understand if MC4R plays a role in resetting the metabolic state after bariatric surgery.
Acknowledgements
This publication was supported by NIH/NCRR UCSF-CTSI UL1 RR024131. IA is supported by ADA mentor-based postdoctoral fellowship award, American Heart Association Post-doctoral fellowship and Genentech clinical fellowship. SAR was supported by Pediatric Endocrinology Training Grant T32-DK07161. CV is supported by NIH RO1 DK DK60540 and DK068152 as well as an established investigator award from the American Heart Association: AHA#0740041N. The authors are indebted to the subjects who participated in this study.
Footnotes
Conflict of Interest Statement: None declared.
Contributor Information
Ivy Aslan, Department of Pediatrics, Division of Endocrinology, University of California San Francisco, San Francisco, California 94143.
Sayali A. Ranadive, Department of Endocrinology, Children's Hospital and Research Center Oakland, Oakland, California 94609
Baran A. Ersoy, Department of Medicine and Diabetes Center, University of California San Francisco, San Francisco, California 94143
Stanley J. Rogers, Department of Surgery, University of California San Francisco, San Francisco, California 94143
Robert H. Lustig, Department of Pediatrics, Division of Endocrinology, University of California San Francisco, San Francisco, California 94143
Christian Vaisse, Department of Medicine and Diabetes Center, University of California San Francisco, San Francisco, California 94143.
References
- 1.O'Brien PE, Sawyer SM, Laurie C, Brown WA, Skinner S, Veit F, et al. Laparoscopic adjustable gastric banding in severely obese adolescents: a randomized trial. JAMA. 2010 Feb 10;303(6):519–26. doi: 10.1001/jama.2010.81. [DOI] [PubMed] [Google Scholar]
- 2.Ranadive SA, Vaisse C. Lessons from extreme human obesity: monogenic disorders. Endocrinol Metab Clin North Am. 2008 Sep;37(3):733–51. x. doi: 10.1016/j.ecl.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994 Oct;8(10):1298–308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000 Apr 6;404(6778):661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 5.Lubrano-Berthelier C, Dubern B, Lacorte JM, Picard F, Shapiro A, Zhang S, et al. Melanocortin 4 receptor mutations in a large cohort of severely obese adults: prevalence, functional classification, genotype-phenotype relationship, and lack of association with binge eating. J Clin Endocrinol Metab. 2006 May;91(5):1811–8. doi: 10.1210/jc.2005-1411. [DOI] [PubMed] [Google Scholar]
- 6.Calton MA, Ersoy BA, Zhang S, Kane JP, Malloy MJ, Pullinger CR, et al. Association of functionally significant Melanocortin-4 but not Melanocortin-3 receptor mutations with severe adult obesity in a large North-American case control study. Hum Mol Genet. 2008 Dec 17; doi: 10.1093/hmg/ddn431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003 Mar 20;348(12):1085–95. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 8.Lubrano-Berthelier C, Le Stunff C, Bougneres P, Vaisse C. A homozygous null mutation delineates the role of the melanocortin-4 receptor in humans. J Clin Endocrinol Metab. 2004 May;89(5):2028–32. doi: 10.1210/jc.2003-031993. [DOI] [PubMed] [Google Scholar]
- 9.Dubern B, Bisbis S, Talbaoui H, Le Beyec J, Tounian P, Lacorte JM, et al. Homozygous null mutation of the melanocortin-4 receptor and severe early-onset obesity. J Pediatr. 2007 Jun;150(6):613–7. 7, e1. doi: 10.1016/j.jpeds.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi H, Ogawa Y, Shintani M, Ebihara K, Shimodahira M, Iwakura T, et al. A Novel homozygous missense mutation of melanocortin-4 receptor (MC4R) in a Japanese woman with severe obesity. Diabetes. 2002 Jan;51(1):243–6. doi: 10.2337/diabetes.51.1.243. [DOI] [PubMed] [Google Scholar]
- 11.Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest. 2000 Jul;106(2):253–62. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lubrano-Berthelier C, Durand E, Dubern B, Shapiro A, Dazin P, Weill J, et al. Intracellular retention is a common characteristic of childhood obesity-associated MC4R mutations. Hum Mol Genet. 2003 Jan 15;12(2):145–53. doi: 10.1093/hmg/ddg016. [DOI] [PubMed] [Google Scholar]
- 13.Lubrano-Berthelier C, Cavazos M, Le Stunff C, Haas K, Shapiro A, Zhang S, et al. The human MC4R promoter: characterization and role in obesity. Diabetes. 2003 Dec;52(12):2996–3000. doi: 10.2337/diabetes.52.12.2996. [DOI] [PubMed] [Google Scholar]
- 14.Dolan K, Fielding G. A comparison of laparoscopic adjustable gastric banding in adolescents and adults. Surg Endosc. 2004 Jan;18(1):45–7. doi: 10.1007/s00464-003-8805-6. [DOI] [PubMed] [Google Scholar]
- 15.Angrisani L, Favretti F, Furbetta F, Paganelli M, Basso N, Doldi SB, et al. Obese teenagers treated by Lap-Band System: the Italian experience. Surgery. 2005 Nov;138(5):877–81. doi: 10.1016/j.surg.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, Livingston EH, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005 Apr 5;142(7):547–59. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 17.Hellstrom PM, Geliebter A, Naslund E, Schmidt PT, Yahav EK, Hashim SA, et al. Peripheral and central signals in the control of eating in normal, obese and binge-eating human subjects. Br J Nutr. 2004 Aug;92(Suppl 1):S47–57. doi: 10.1079/bjn20041142. [DOI] [PubMed] [Google Scholar]
- 18.Earle KR, Martin MB, Newman DH, Hoxworth BT. Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) Poster Abstract P042; Philadelphia, Pensylvania: 2008. Laparoscopic Adjustable Gastric Banding with Truncal Vagotomy: Does this augment weight loss? [Google Scholar]
- 19.Ellacott KL, Halatchev IG, Cone RD. Interactions between gut peptides and the central melanocortin system in the regulation of energy homeostasis. Peptides. 2006 Feb;27(2):340–9. doi: 10.1016/j.peptides.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 20.Pournaras DJ, le Roux CW. Obesity, gut hormones, and bariatric surgery. World J Surg. 2009 Oct;33(10):1983–8. doi: 10.1007/s00268-009-0080-9. [DOI] [PubMed] [Google Scholar]